Abstract
Methylthioadenosine phosphorylase (MTAP) deficiency occurs in various malignancies and is associated with poor survival in cancer patients. However, the mechanisms underlying tumor progression due to MTAP loss are yet to be elucidated. Utilizing integrated analyses of the transcriptome, proteome, and secretome, we demonstrated that MTAP deficiency alters tumor-intrinsic, immune-related pathways and reprograms cytokine profiles toward a tumor-favorable environment. Additionally, MTAP-knockout cells exhibited a marked increase in the immune checkpoint protein PD-L1. Upon co-culturing primary T cells with cancer cells, MTAP loss-mediated PD-L1 upregulation inhibited T cell-mediated killing activity and induced several T cell exhaustion markers. In two xenograft tumor models, we showed a modest increase in average volume of tumors derived from MTAP-deficient cells than that of MTAP-proficient tumors. Surprisingly, a remarkable increase in tumor size was observed in humanized mice bearing MTAP-deficient tumors, as compared to their MTAP-expressing counterparts. Following immunophenotypic characterization of tumor-infiltrating leukocytes by mass cytometry analysis, MTAP-deficient tumors were found to display decreased immune infiltrates with lower proportions of both T lymphocytes and natural killer cells and higher proportions of immunosuppressive cells as compared to MTAP-expressing tumor xenografts. Taken together, our results suggest that MTAP deficiency restructures the tumor immune microenvironment, promoting tumor progression and immune evasion.
Keywords: tumor suppressor, PD-L1, immunosuppression, humanized mice, CyTOF
INTRODUCTION
Advancements in the field of oncoimmunology have revolutionized the progression of cancer research, especially in the areas of delineating tumor heterogeneity and development of innovative treatments. Compared to traditional therapies, immune checkpoint inhibitors (ICIs) targeting the PD-1/PD-L1 axis have demonstrated remarkable success across a broad range of advanced cancer types 1-4 and have been proven to potentiate more long-lasting clinically beneficial effects 4. Despite the pronounced advantages of ICIs, there are still many challenges that impede effective clinical treatment and management of disease. Beyond immune-related adverse events that can occur during treatment, the objective response rates (ORR) for PD-1/PD-L1 inhibitors only fare around 20%-30% 5, 6. This problem is exacerbated by a lack of predictive biomarkers for identifying patients who would most benefit from ICIs. The issue is further marred by the controversy surrounding the use of PD-L1 expression as an ICI biomarker, further obfuscating the ability to optimally select and treat patients with ICIs 7, 8.
One of the potential reasons underlying the low ORR to PD-1/PD-L1 inhibitors may be attributed to the tumor immune microenvironment 9. “Hot” tumors are characterized by high T cell density and IFN-γ level, and this immuno-active phenotype has been leveraged as a biomarker to predict favorable patient response to ICIs. In contrast, “cold” tumors contain low proportions of immune infiltrates, impairing the effective elimination of malignant cells. Such tumors are also immunologically characterized by higher densities of tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), or regulatory T cells (Tregs), and higher secretion of anti-inflammatory cytokines e.g., IL-10 and VEGF. Patients with these types of tumors exhibit poor clinical outcomes 10. The dysregulation of immune responses and recruitment of immune suppressor cells concomitant with cytokine reprogramming promote an immunosuppressive cellular landscape, ultimately contributing to unresponsiveness to immunotherapy 11.
Methylthioadenosine phosphorylase (MTAP) is an enzyme involved in the polyamine biosynthesis pathway, catalyzing the phosphorylation of methylthioadenosine (MTA) for the regeneration of methionine and adenine 12. In addition to its fundamental function in metabolism, MTAP also regulates protein arginine dimethylation through modulation of levels of its substrate metabolite MTA, which has been demonstrated to suppress PRMT5 activity 13-15. Notably, MTAP is frequently deficient in various cancer types 12, 13. In immunogenic malignancies, such as lung cancer and renal cell carcinoma (RCC), MTAP loss was associated with worse prognosis partly through upregulating IGF1R activity and vimentin protein abundance, consequently aggravating tumor progression and malignancy 16, 17. Investigation of the intratumor microenvironment showed elevated level of MTA compared to adjacent non-malignant tissues 18. This elevation was reported to suppress T cell proliferation and effector function, NK cell cytotoxicity, and MHC II transactivation 19-21, implicating a role for MTAP deficiency in tumor immune response. Given the limited information regarding the role of MTAP in the tumor microenvironment (TME), there is a great need to elucidate the mechanism underlying MTAP-mediated tumor immunity.
Herein we characterized the transcriptome and secretome of MTAP-deficient tumor cells to investigate the contribution of MTAP to tumor-intrinsic immune-related pathways. Following co-culture studies of cancer cells with immune cells, we characterized the immune cell population in human tumor xenografts in humanized mice through mass cytometry (CyTOF) analysis. Both our in vitro and in vivo studies suggest the specific contribution of MTAP deficiency in remodeling immune profiles and immune evasion, bridging knowledge gaps in oncoimmunology and aiding better insights into potential therapeutic options for patients with MTAP-deficient tumors.
MATERIALS AND METHODS
Detailed information on reagents and antibodies, cell lines and cell culture, RNA sequencing and pathway analysis, reverse phase protein array, Luminex assays, Western blot assays, immunofluorescent staining, in vivo animal experiments, in vitro PBMC- or T cell-mediated cancer cell killing assays, PBMCs or tumor-infiltrating leukocyte profile analysis by CyTOF or flow cytometry, estimation of infiltrated immune population and statistical analysis are described in the Supplementary Information.
RESULTS
MTAP deficiency alters tumor-intrinsic immune-related pathways
We previously demonstrated the importance of MTAP expression in suppressing epithelial-mesenchymal transition, invasion, and migration of kidney cancer cells 16. A similar suppressive phenomenon was also recapitulated in a MTAP-deficient lung cancer cell line, CL1-5, upon ectopic expression of MTAP. Conversely, aggressive phenotypes were induced after knockout of MTAP in CL1-0 cells, a MTAP-proficient lung cancer cell line (Supplementary Figure S1) 17. To further explore the molecular contribution of MTAP deficiency to these cellular processes, we performed RNA-sequencing on three pairs of cancer cell lines: 1) mock control (Mock) and MTAP-overexpressing (V5-tagged MTAP) CL1-5 lung cancer cells; 2) MTAP-intact (MTAP-WT) and MTAP-knockout (MTAP-KO) CL1-0 lung cancer cells; and 3) MTAP-WT and MTAP-KO 786-O kidney cancer cells, followed by Gene Set Enrichment Analysis (GSEA). Analysis of these datasets identified 20 top hallmark gene sets associated with MTAP status (Figure 1A). Of these gene sets, we noticed several gene sets associated with immune response-regulating signaling such as TNFα signaling via NFκB, inflammatory response, IL6-JAK-STAT3 signaling, interferon γ response, interferon α response, and IL2-STAT5 signaling (Supplementary Figure S2). Pathway enrichment analysis of differentially expressed genes from MTAP-WT versus MTAP-KO CL1-0 cells showed that CXCR4, IL-5 and IFN-γ signaling pathways were significantly enriched with MTAP expression (Figure 1B). Likewise, proteomic network analysis of reverse phase protein array (RPPA) profiles demonstrated that aberrant immune responses mediated by tumor-intrinsic signaling pathways (e.g., PI3K/AKT, MEK/ERK, and JAK/STAT) were noted in MTAP-KO cancer cells (Figure 1C). We further identified several oncogenic molecules with immune modulatory effects as central hubs of MTAP-mediated signaling networks (Figure 1D). These results drew our attention to further investigate the functional ramifications of MTAP deficiency in tumor immunity.
Figure 1. MTAP plays a role in tumor-intrinsic immune signaling.
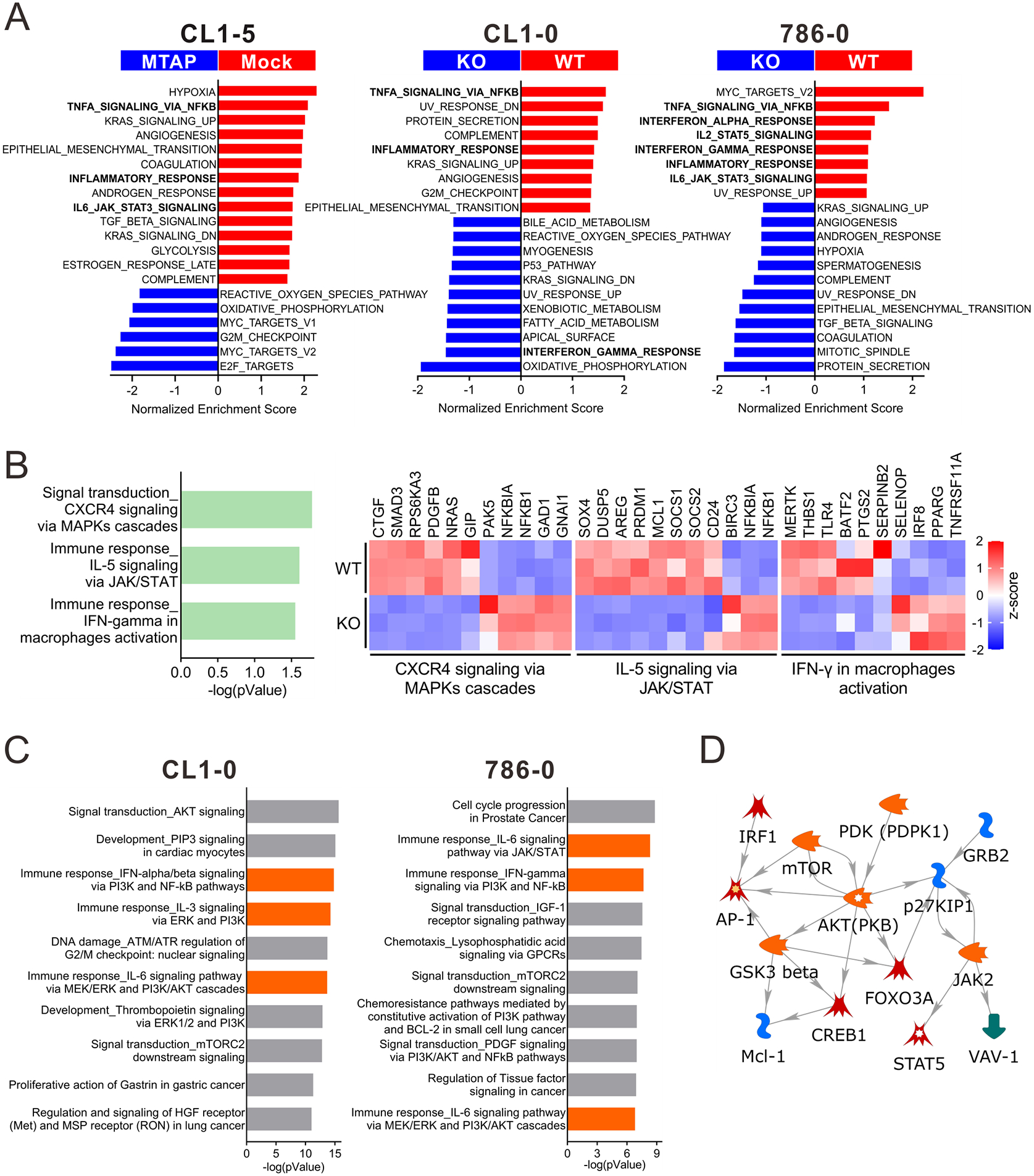
(A) Gene set enrichment analysis (GSEA) was performed on the transcriptomic data of Mock versus MTAP-overexpressing (MTAP) CL1-5 lung cancer cells and MTAP-WT (WT) versus MTAP-KO (KO) CL1-0 lung cancer and 786-O RCC cells using the HALLMARK gene sets. (B) MTAP-altered immune-related pathways in CL1-0 cells were identified through MetaCore analytical suite (version 20.4 build 70300). The differentially expressed genes involved in these pathways were displayed as heatmap graphs (right). (C) Top 10 ranking pathways enriched by differentially expressed proteins measured by Reverse Phase Protein Array (RPPA) in CL1-0 and 786-0 WT/KO cells. Orange bars: immune-related pathways. (D) The signaling direction of the immune-related pathway network objects derived from both RPPA results of CL1-0 and 786-0.
MTAP-deficient cancer cells exert immunosuppressive effects
Tumor-intrinsic signaling pathways have been shown to promote immunosuppression by induction of immunoregulatory cytokines 22. Given this phenomenon, we utilized Luminex assays to compare the expression levels of multiple cytokines secreted by MTAP-WT and MTAP-KO cancer cells. Cytokines with anti-tumor properties including GM-CSF, IL-1α, IL-1β, IL-12, and IFN-γ were abundantly secreted in MTAP-WT cells, while pro-tumoral cytokines such as RANTES, HGF, VEGF, IL-6, IL-8, and IL-9 were more plentiful in tissue culture supernatants produced by MTAP-KO cells (Figure 2A and Supplementary Figure S3A). These taken together suggests that a distinct immunosuppressive cytokine milieu is present with MTAP-deficient cancer cells.
Figure 2. MTAP deficiency enhances tumor immunosuppressive effect.
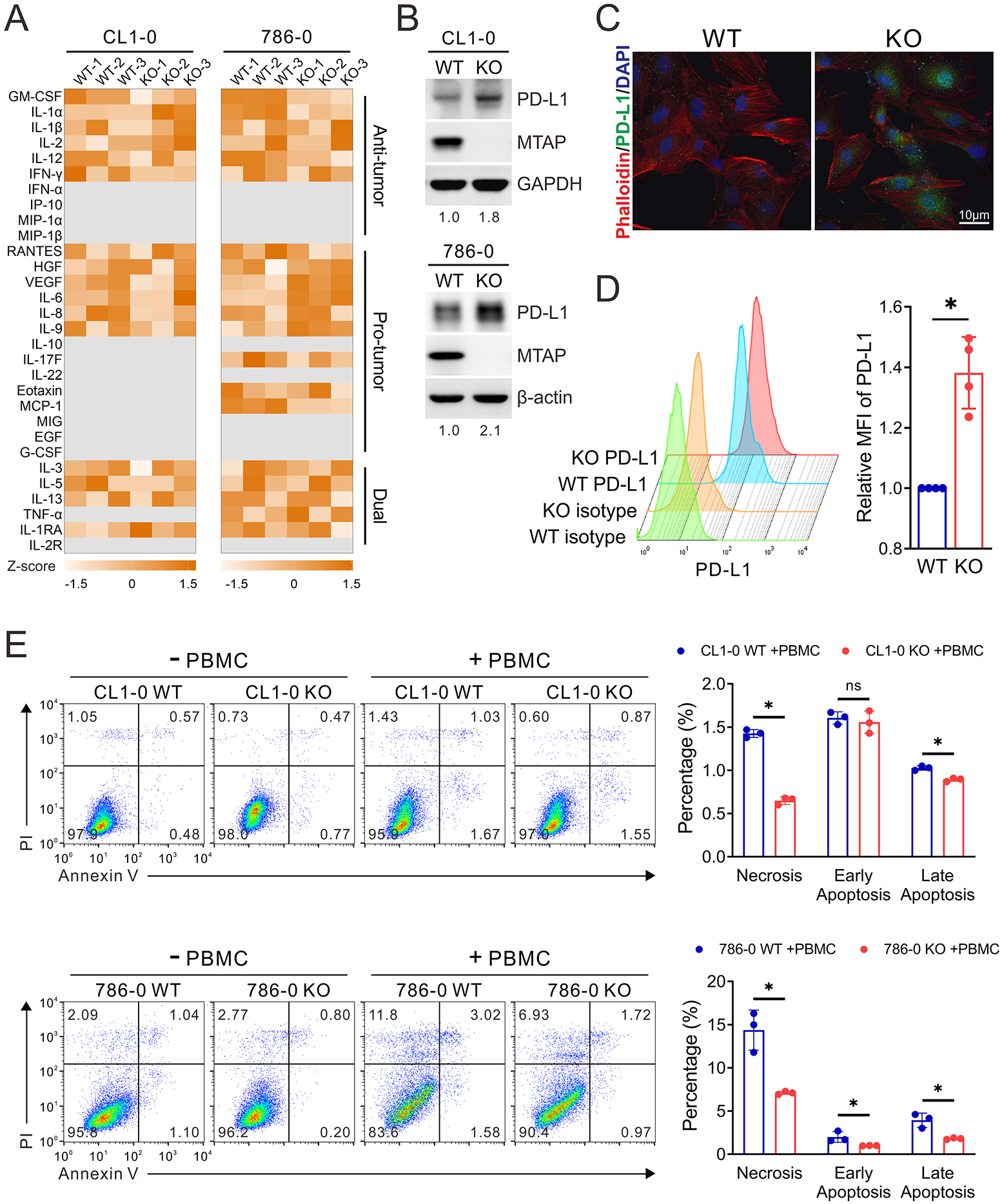
(A) Analysis of cytokines secreted from MTAP-WT and MTAP-KO CL1-0 lung cancer and 786-O RCC cells by Luminex Multiplex Assays. The semiquantitative results of cytokine secretion levels were represented as heatmap graphs (n=3). Gray: below level of detection. (B) Western blot analysis of cellular PD-L1 expression levels in MTAP-WT and MTAP-KO CL1-0 and 786-O cells. The number indicates the quantification of three independent Western blots. (C) Representative immunofluorescent images of cellular PD-L1 expression levels in MTAP-WT and MTAP-KO 786-O RCC cells. (D) Flow cytometric analysis comparing cell surface PD-L1 levels between MTAP-WT and MTAP-KO 786-O cells. The mean fluorescence intensity (MFI) of PD-L1 in each cell population was quantified by FlowJo. Isotype: isotype negative control. (E) The viability of MTAP-WT and MTAP-KO CL1-0 and 786-O cells after being co-cultured with PBMCs for 48 hours. Data are presented as mean ± SD (n=3). *, p < 0.05.
In addition to a shift in cytokine profiles, tumor cells undergo immune escape through upregulating the expression of immune checkpoint inhibitory ligands such as programmed death-ligand 1 (PD-L1). To assess the contribution of PD-L1 on MTAP-mediated immunity, we first analyzed the protein levels of MTAP-WT and MTAP-KO cells. Both Western blot (Figure 2B) and immunofluorescence (Figure 2C and Supplementary Figure S3B) assays demonstrated an elevation of PD-L1 upon MTAP knockout. To confirm that this increased level PD-L1 was functional, PD-L1 cell surface protein expression was detected by flow cytometry. Figure 2D shows that cell surface PD-L1 was more abundant in MTAP-KO cells than that in MTAP-WT cells.
PD-L1 on cancer cells contributes to the hindrance of cytotoxic T cell activation via PD-1 engagement and subsequent deactivation of T cells. With this in mind, we next examined the role of MTAP deficiency in PD-L1-mediated immunosuppression. Peripheral blood mononuclear cells (PBMCs) isolated from healthy donors were treated with anti-CD3/CD28 antibodies to activate T cell populations and then co-cultured with either MTAP-WT or MTAP-KO tumor cells. After co-culturing, the viability of MTAP-KO cells was observed to be higher than that of MTAP-WT cells (Supplementary Figure S3C). In addition to the cancer cells themselves, we also examined the T cells after co-culture. We observed downregulated expression of CD107 and IFN-γ, two T cell activation markers, after incubation with MTAP-KO cells (Supplementary Figure S3D), implying that an interaction with MTAP-KO cells leads to T cell exhaustion. To assess the role of MTAP on cancer cell fitness in the tumor immune microenvironment, MTAP-WT or MTAP-KO cancer cells were co-cultured with unstimulated PBMCs and subjected to flow cytometry for simultaneous assessment of necrosis and apoptosis. Consistently, a significant reduction in both types of cell death was observed in MTAP-deficient cells (Figure 2E), suggesting the regulatory role of MTAP deficiency in immunosuppression.
MTAP deficiency attenuated immune responses
Given our observations in vitro, we next utilized xenograft mouse models to assess the role of MTAP on immunity in vivo. This was accomplished through subcutaneous injections of two pairs of MTAP-expressing and MTAP-deficient lung cancer cells into either immunodeficient NSG-SGM3 mice or human CD34+ hematopoietic stem cell-engrafted NSG-SGM3 mice (NSG-SGM3 humanized mice). The average volume of tumors derived from MTAP-deficient cells injected into NSG-SGM3 mice was shown to be modestly greater than that of MTAP-proficient tumors (Figure 3A and Supplementary Figure S4A). However, a remarkable increase in tumor size was observed in humanized mice bearing MTAP-deficient tumors, as compared to their MTAP-expressing counterparts (Figures 3B and 3C). This observation implies that MTAP-deficient tumors co-opt the immune system to promote tumor growth.
Figure 3. MTAP deficiency promotes tumorigenesis and immune dysfunction.
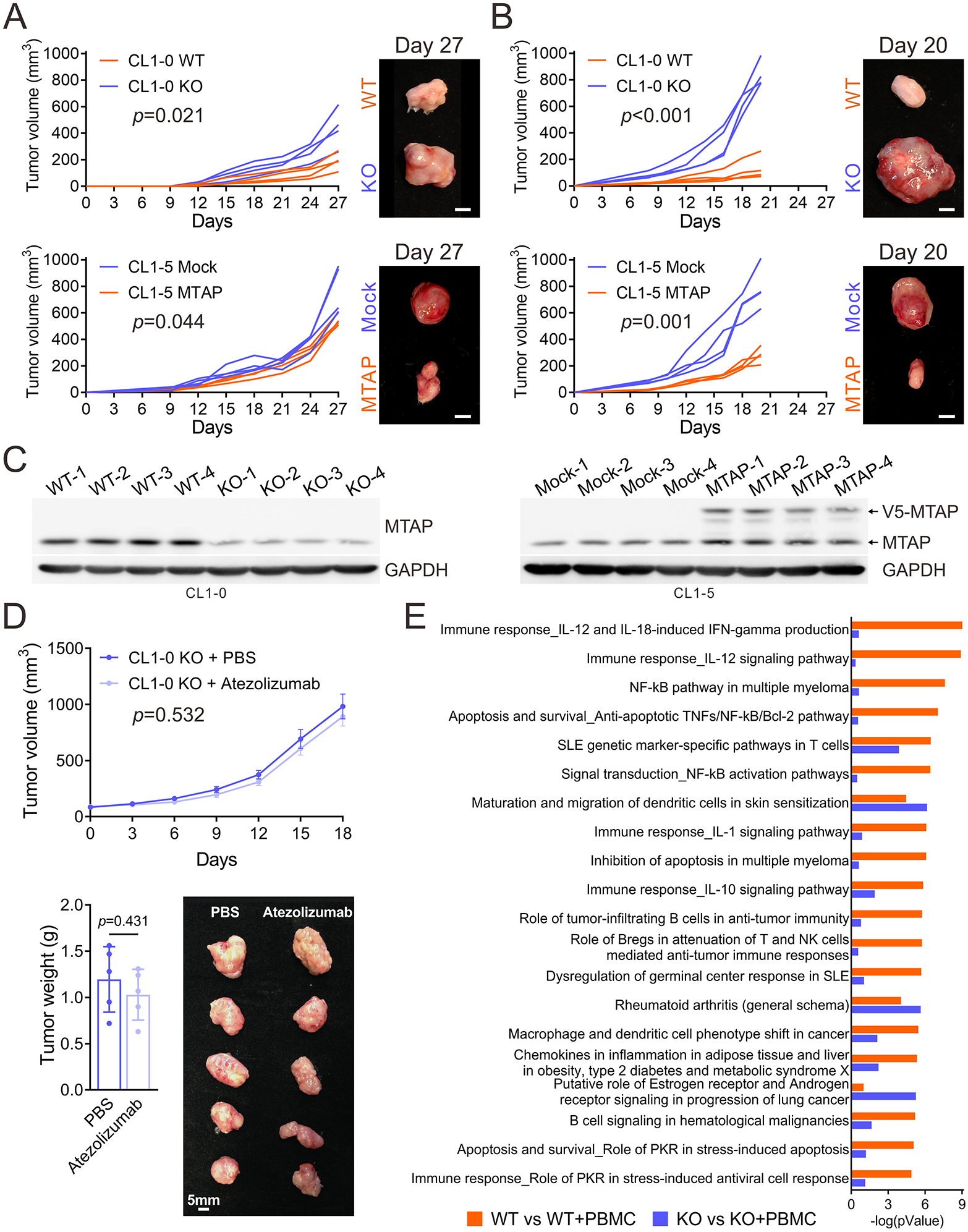
(A, B) Tumor growth of subcutaneously injected MTAP-WT and MTAP-KO CL1-0 and Mock and MTAP-expressing CL1-5 lung cancer cells in NSG-SGM3 humanized mice engrafted without (A) or with (B) human hematopoietic stem cells was measured every three or two days and dissected at Day 27 or 20 (four mice per group). Scale bars: 5 mm. (C) MTAP protein expression levels in tumors isolated from NSG-SGM3 humanized mice were assessed by Western blot assays. (D) Human CD34+ hematopoietic stem cell-engrafted NSG-SGM3 mice bearing subcutaneous tumors derived from MTAP-KO CL1-0 lung cancer cells were treated for 21 days with intraperitoneal injections of PBS or atezolizumab, an anti-PD-L1 antibody. Tumor volumes were measured and data was presented as mean ± SD (n=5, top). Tumor weights (bottom, left) and a representative photo of primary tumors (bottom, right) were shown (n=5, mean ± SE). (E) Top 20 ranking pathways enriched by the 279 genes (orange bars) and 234 genes (purple bars) from the differentially expressed genes (DEGs) with greater or equal to 2-fold change subjected to pathway analysis by the MetaCore analytical suite, as described in Supplementary Figure S3B. The pathway order was ranked by the -log(pValue) of orange bars.
As PD-L1 was upregulated upon MTAP loss (Figure 2) and the dramatic growth of MTAP-deficient tumors in NSG-SGM3 humanized mice, we asked if immune checkpoint inhibitors would be able to effectively target MTAP-deficient tumor growth. To test this, we subcutaneously injected MTAP-KO CL1-0 lung cancer cells into NSG-SGM3 humanized mice. After tumors were palpable in all animals, cohorts of mice were randomly divided and then injected intraperitoneally every 2 days with vehicle or 100 μg atezolizumab, an anti-PD-L1 antibody, for 21 days (n = 5 mice/group). In our study, we did not observe a significant amount of growth inhibition seen in the anti-PD-L1 antibody group compared to the untreated group (Figure 3D), in agreement with previous observations that PD-L1 expression was found to only weakly correlate with response rates to PD-1/PD-L1 inhibitors 7, 8.
Due to our observations that MTAP-deficient cancer cells were less sensitive to anti-tumoral immunity and were able to co-opt the immune system in our animal model, we further investigated the molecular mechanism by which MTAP-deficient cancer cells modulate immune response. MTAP-WT and MTAP-KO CL1-0 lung cancer cells co-cultured with or without unstimulated PBMCs were subjected to RNA-sequencing, and the differentially expressed genes in either MTAP-WT or MTAP-KO cancer cells were further analyzed for enriched pathways respectively (Supplementary Figure S4B). Within MTAP-WT cells co-cultured with PBMCs (WT+PBMC), the top-ranked pathways centered on IL-12 signaling which is exhibited in anti-tumoral immunity 23. In contrast, pathways implicated in cancer progression were enriched in MTAP-KO cells exposed to PBMCs (KO+PBMC, Figure 3E). Looking more in depth at the effect of MTAP in MTAP-WT and MTAP-KO cells through GSEA analysis, MTAP-KO cells exhibited reduced response to type I and type II interferon as well as reduced response to IL-12 (Supplementary Figure S4C). This was also accompanied by downregulation of IFN-γ-mediated signaling (Supplementary Figure S4D), supporting the observations in vivo that MTAP-deficient tumors are able to exploit the immune environment and escape immunosurveillance, thereby promoting progression.
MTAP deficiency reshapes immune cell responses to tumor cells
We next performed Luminex assays to ascertain whether cytokine secretion patterns were altered corresponding to the phenotypes observed in NSG-SGM3 mice after either MTAP-WT or MTAP-KO tumor cells were co-cultured with PBMCs. Compared to the secretion patterns of tumor cells only (Figure 2A and Supplementary Figure S3A), almost all cytokine levels were evidently increased when tumor cells encountered immune cells (Figure 4A and Supplementary Figure S5A), but the magnitude of the increases were distinct between MTAP-WT and MTAP-KO cells. Anti-tumoral IL-1α, IL-1β, IL-12 and IFN-γ as well as IP-10, MIP-1α and MIP-1β levels were higher in WT+PBMC, while pro-tumoral IL-6, IL-8 and IL-9 as well as Eotaxin and IL-22 (specific in lung cancer cells, CL1-0 and H1650) levels were higher in KO+PBMC.
Figure 4. Immune cell responses were reshaped by MTAP-deficient tumor cells.
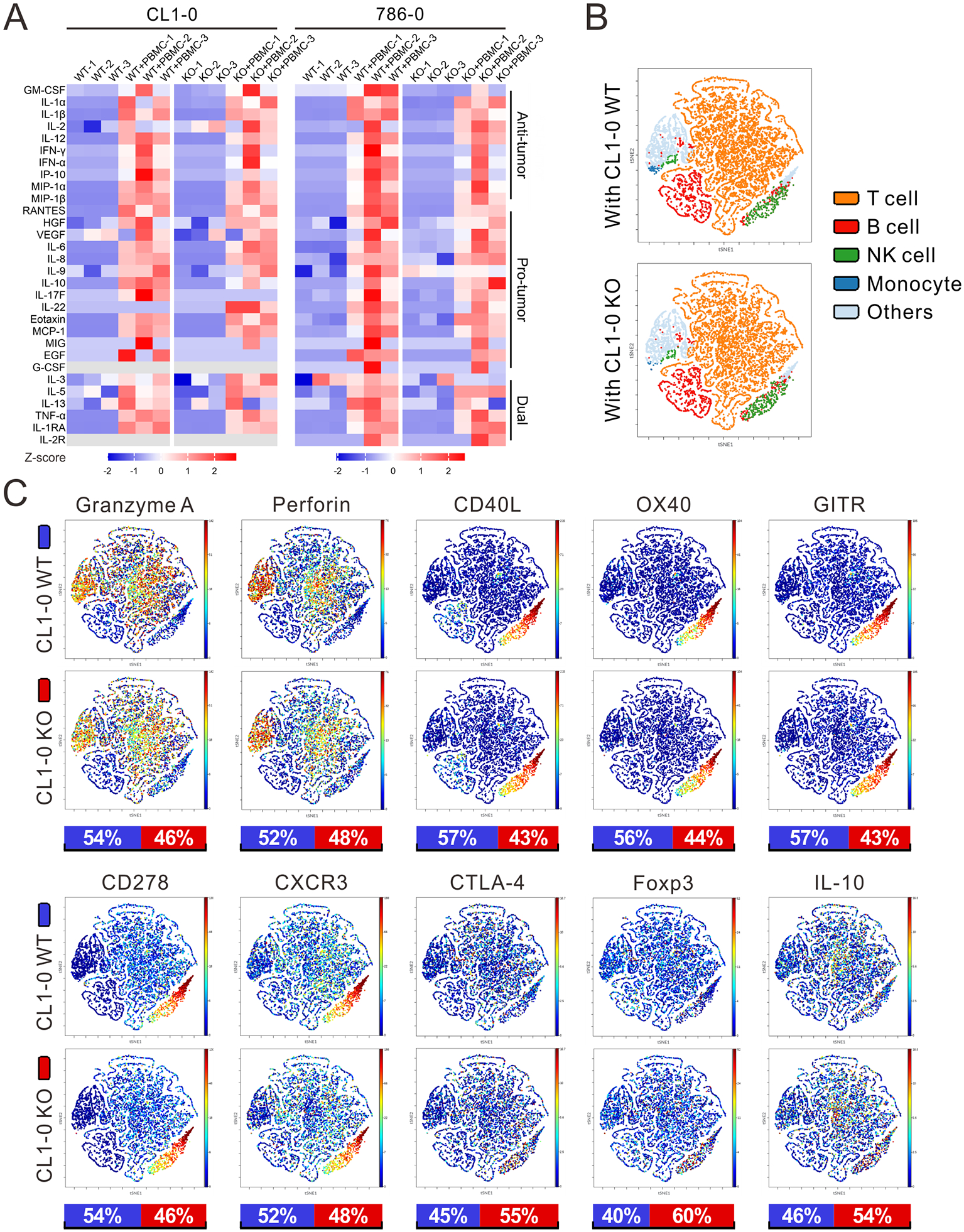
(A) Secreted cytokine levels of MTAP-WT and MTAP-KO CL1-0 lung cancer and 786-O RCC cells co-cultured with or without PBMCs were determined by Luminex Multiplex Assays. The semiquantitative results of cytokine secretion levels were represented as heatmap graphs. PBMCs were isolated from three different donors. Gray: below level of detection. (B) Main sub-populations of CD45+ PBMCs co-cultured with MTAP-WT and MTAP-KO CL1-0 lung cancer cells from CyTOF analysis were shown as t-distributed stochastic neighbor embedding (t-SNE) plots. Others: unspecified CD45+ PBMCs. (C) t-SNE plots displaying the expression levels of immune cell markers in CD45+ PBMCs co-cultured with MTAP-WT or MTAP-KO CL1-0 cells. Bottom bars: the relative intensities of markers in CD45+ PBMCs co-cultured with CL1-0 WT (blue) or KO (red) cells.
As cytokines can be produced by either tumor cells or immune cells and some may be mediated through direct interactions between tumor and immune cells, we designed two modes of tumor cells/PBMCs co-culture, direct and transwell, and collected supernatants from cells in both co-cultured separated conditions respectively for Luminex assays to distinguish the source of cytokines (Supplementary Figure S5B). Although the levels of IL-12, IFN-γ, IL-1α, IL-1β, MIP-1α, MIP-1β and IP-10 were consistently higher in WT+PBMC than in KO+PBMC under direct co-culture condition, the cytokine concentrations under transwell co-culture conditions were relatively low, indicating the importance of cell-cell interaction. After separating tumor cells from PBMCs, most cytokine levels became greatly reduced except for IP-10 which was secreted predominantly by tumor cells (Supplementary Figures S5C-S5I). Overall, these findings suggest that MTAP deficiency may be reprogramming the communication between tumor cells and immune cells toward pro-tumoral state.
To dissect the phenotypic profiles and biological functions of PBMCs influenced by MTAP-deficient tumor cells, PBMCs co-cultured with MTAP-WT or MTAP-KO CL1-0 lung cancer cells were subjected to CyTOF analysis which allows for simultaneous single-cell measurement of multiple immune cell markers. We employed two CyTOF panels (Supplementary Figure S6A) to capture the distribution of immune cell populations in co-cultured PBMCs. The gating strategy for identification of immune cells is shown in Supplementary Figure S6B. Although the difference of each sub-population of immune cells between WT and KO groups was not significant (Figure 4B), we did see moderately higher mean intensities of key marker molecules of immune activation involved in mediating anti-tumor response including Granzyme A, Perforin, CD40L, OX40, Glucocorticoid-induced TNFR-related protein (GITR), CD278 and CXCR3 in CD45+ cells co-cultured with WT cells. Conversely, pro-tumoral immune markers CTLA-4, Foxp3 and IL-10 were induced in CD45+ cells co-cultured with MTAP-KO cells (Figure 4C). Importantly, similar significant findings were detected in PBMCs co-cultured with RCC 786-0 WT/KO cells, suggesting that CD4+ T cells, CD8+ T cells and natural killer (NK) cells become increasingly deactivated/exhausted with less cytotoxicity and as well as an increased population of Tregs (Supplementary Figure S7A). Together, our data revealed that MTAP deficiency overall leads to immunosuppression in vitro through modulating a pro-tumoral immune environment.
MTAP deficiency impairs intratumoral immunity to support tumorigenesis
We further systematically analyzed the tumor-infiltrating leukocyte (TIL) populations from tumors of humanized mice by CyTOF (Supplementary Figures S7B and S7C) and found decreased infiltration of CD45+ cells in MTAP-deficient (CL1-0 KO and CL1-5 Mock) tumors compared to MTAP-expressing (CL1-0 WT and CL1-5 MTAP) tumors (Supplementary Figure S7D). In addition to decreased immune infiltration, CyTOF analysis revealed that the TIL population consisted of decreased T cell, B cell and NK cell sub-populations. The TIL population in MTAP-deficient tumors also comprised of increased dendritic cell (DC) and monocyte/macrophage sub-populations (Figures 5A and 5D). Further profiling of immune cell markers (Figures 5B and 5E) also demonstrated that the expression levels of immune cell-expressed pro-tumoral immune markers PD-L1, Arginase 1, CD34, Nectin-2 and CD47 were elevated in CL1-0 MTAP-KO tumors compared to CL1-0 WT tumors (Figure 5C). The intensities of anti-tumoral immune markers CD169, CXCR3 and OX40L were higher in immune cells isolated from CL1-5 MTAP tumors than in those from CL1-5 Mock tumors (Figure 5F). These results support that MTAP deficiency in tumors could prevent immune cell recruitment and activation, converting tumors to a more immunosuppressive “cold” state.
Figure 5. Impaired intratumoral immunity enhances MTAP-deficient tumor growth.
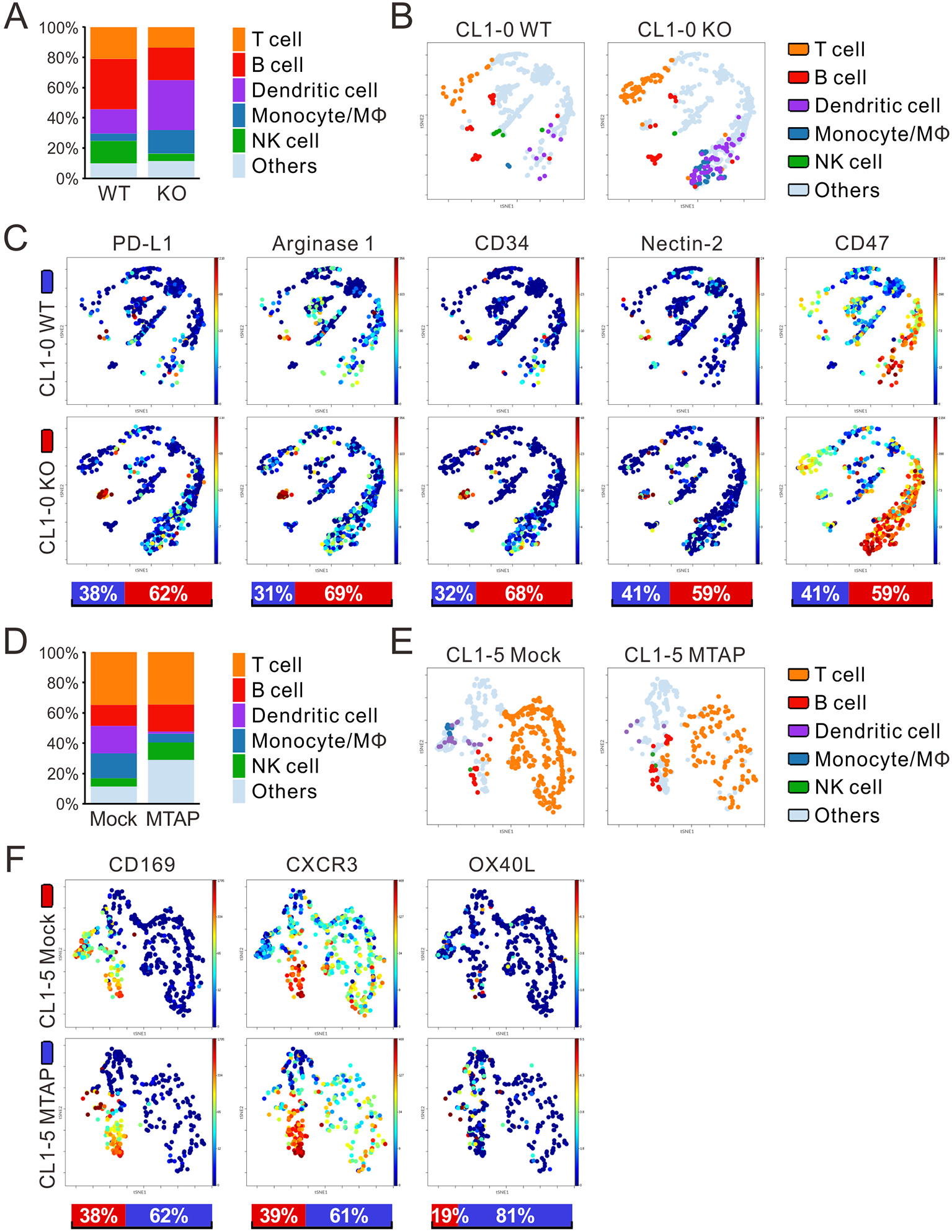
(A, D) Percentage of immune cell populations within CD45+ TILs from CL1-0 WT/KO (A) and CL1-5 Mock/MTAP (D) tumors of humanized mice was assessed by CyTOF analysis (four mice per group). Others: unspecified human CD45+ TILs. (B, E) Main sub-populations of CD45+ TILs from CL1-0 WT/KO (B) and CL1-5 Mock/MTAP (E) tumors of humanized mice derived from CyTOF analysis were shown as t-SNE plots. (C, F) t-SNE plots displaying the expression levels of immune cell markers in CD45+ TILs from CL1-0 WT/KO (C) and CL1-5 Mock/MTAP (F) tumors of humanized mice. Bottom bars: the relative intensities of markers in CD45+ TILs from CL1-0 WT (blue) and KO (red) tumors, or CL1-5 Mock (red) and MTAP (blue) tumors.
Validation of reshaped immune landscape in MTAP-deficient tumors
To validate the differences that we observed in the immune cell infiltrates in response to MTAP alteration from CyTOF profiling, we performed immunofluorescent staining on CL1-5 Mock/MTAP and CL1-0 WT/KO tumors from humanized mice. The immunofluorescence data revealed increased levels of PD-L1, Foxp3 and CD163 in MTAP-deficient tumors (CL1-0 KO and CL1-5 Mock), indicating an increased presence of exhausted T cells, differentiation to Tregs and M2-subtype macrophages respectively. We also noted decreased expression of the NK cell marker CD56 in MTAP-deficient tumors (Figure 6A), corroborating the findings from CyTOF analysis which indicated that NK cells are less prevalent in MTAP-deficient tumors. To further confirm our findings, we turned to the transcriptomic data of cancer patients from The Cancer Genome Atlas and to evaluate the enriched pathways in cancer cells and relative abundance of each immune cell subset in a mixed cell population. This was accomplished through analysis of RNA-sequencing data of lung adenocarcinoma (LUAD) and kidney renal clear cell carcinoma (KIRC) cohorts with GSEA, CIBERSORTx, and xCell software. Data was then segregated into high and low MTAP mRNA expression groups, respectively. GSEA in transcriptomic profiles of cancer patients demonstrated that the immune response-regulating signaling pathways were enriched in high MTAP-expressing tumors (Supplementary Figure S8A), whereas lymphocytes and NK cells were less activated in low MTAP-expressing tumors (Supplementary Figure S8B). Utilizing CIBERSORTx, we noticed decreased proportions of naive B cells, CD8+ T cells (only in LUAD), CD4+ T cells (including memory resting and activated cells), gamma delta T cells, NK cells (including resting and activated) (only in LUAD) and M1 Macrophages in low MTAP-expressing tumors, with concurrent higher levels of regulatory T cells (Tregs), Monocytes and Dendritic cells (resting and activated) (Figure 6B). In xCell analysis, a similar result was also observed with reduced levels in subsets of CD4+ memory T cells, CD4+ T cells, CD8+ T cells, NK cells (only in LUAD) and naive B-cells in low MTAP tumors, concurrently with Monocytes (only in LUAD), M2 Macrophages, and Dendritic cells (only in LUAD) being more abundant in low MTAP expression group (Figure 6C), supporting our findings in humanized mice (Figures 5A and 5D). In summary, we characterized a newfound role of MTAP deficiency in modulating the TME though remodeling immune profiles and contributing to immune evasion.
Figure 6. Comparison of immune cell populations between MTAP-expressing and MTAP-deficient tumors.
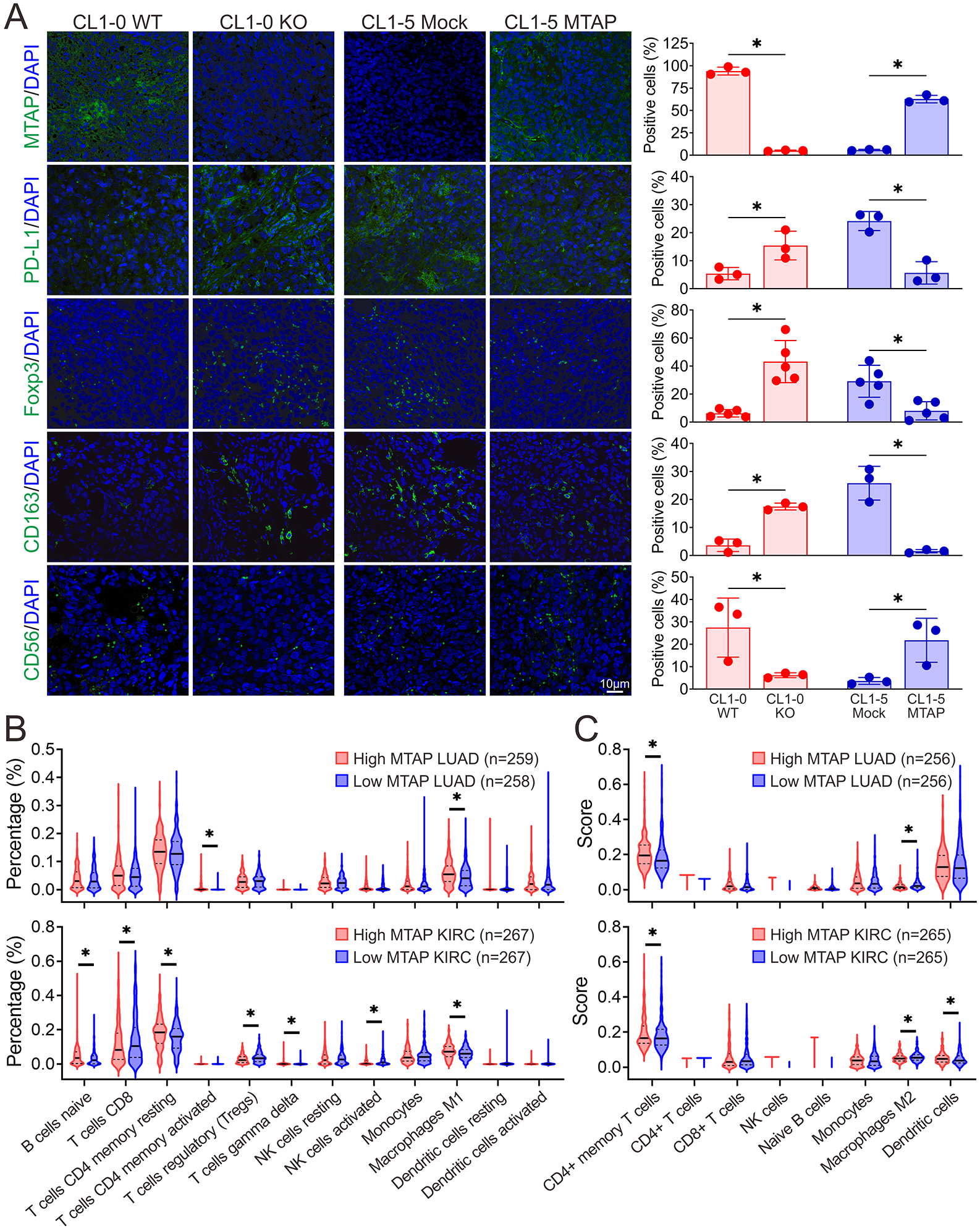
(A) Representative immunofluorescent images of the protein expression patterns of MTAP, T cell exhaustion marker PD-L1, Treg cell marker Foxp3, M2-subtype macrophage marker CD163 and NK cell marker CD56 in tumors from humanized mice. Right: quantification of the positive-stained cells within tumors. Data are from each tumor of humanized mice and shown as mean ± SD. *, p < 0.05. (B, C) Estimated proportion of immune subpopulations in lung adenocarcinoma (LUAD) and kidney renal clear cell carcinoma (KIRC) patients with high or low MTAP mRNA expression from The Cancer Genome Atlas Program was analyzed by computational deconvolution of transcriptomics data using Cibersort (B) and xCell (C). *, p < 0.05.
DISCUSSION
MTAP, the enzyme regulator of polyamine, adenine and methionine metabolic pathways, has been well established as a tumor suppressor 16, 17. Recent studies have further explored the role of MTAP to extend beyond its role in metabolism, with findings suggesting a newfound interaction between proximal cells in the tumor site 19-21. In this study, we define a new role of MTAP in immunomodulation through demonstrating that MTAP-deficient tumors intrinsically exhibit altered immune signaling pathways, extrinsically remodel the immune microenvironment, and subsequently escape immunosurveillance (Figure 7).
Figure 7. Graphical summary for the mechanism of MTAP deficiency-mediated remodeling of the tumor immune landscape.
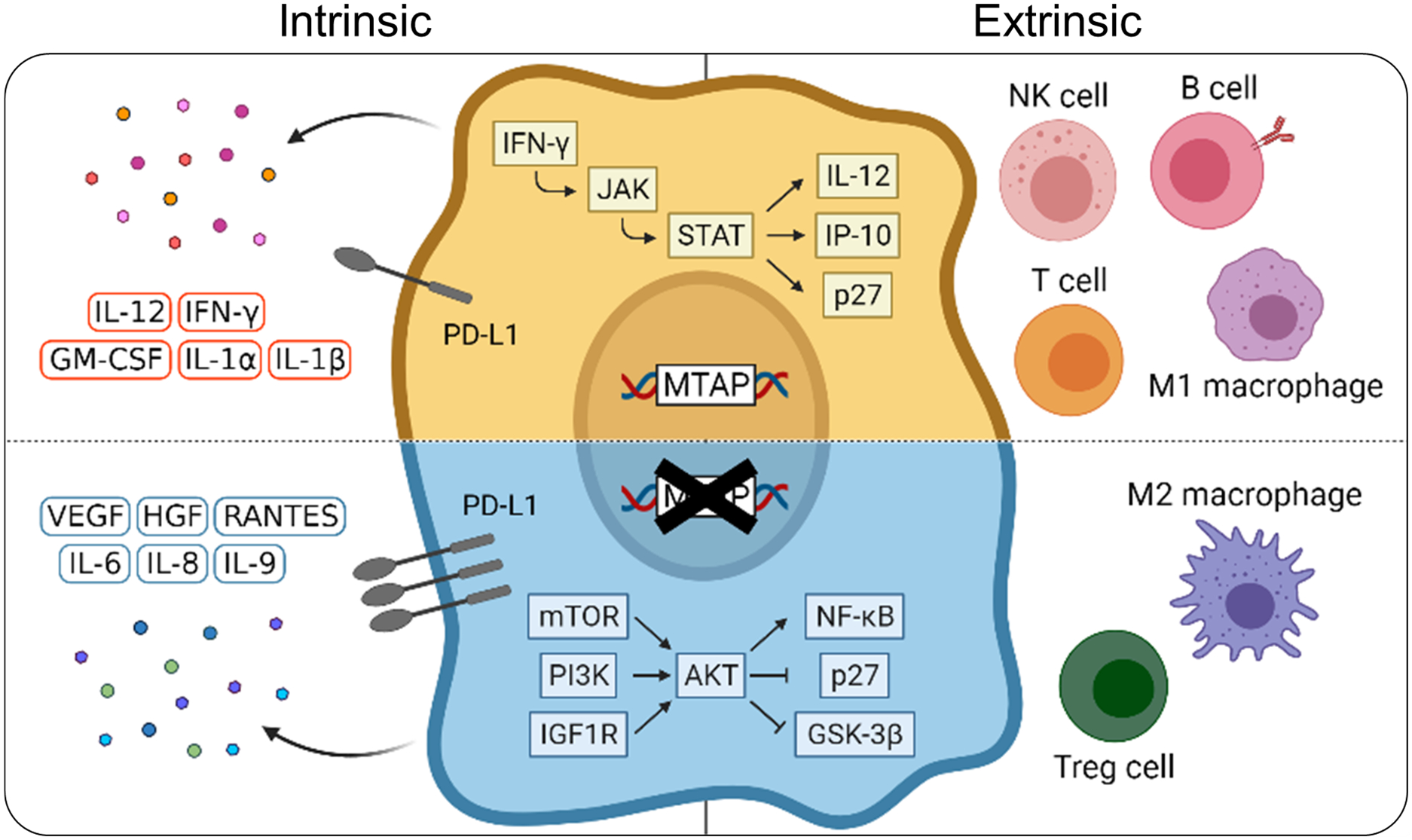
MTAP-deficient progressive tumors tamp down the immune cells’ response via cytokine reprogramming and immune remodeling. Our study sheds new insight on how genetic loss of a metabolic enzyme contributes to immunological modification of the tumor-immune landscape.
Progress in the field of oncoimmunology can be attributed to development of more suitable animal models, through which comprehensive analyses can be accomplished under physiologically relevant conditions. Of particular note, the recent advent of humanized mice has greatly accelerated progress in this field. Compared to other murine models such as syngeneic models, humanized mice have more applicable translational prospects due to their inherent features. We took advantage of these features to assess the role of MTAP in tumor-immune interactions. To the best of our knowledge, we are among the first to study tumor immune microenvironment-related consequences of MTAP loss in vivo using NSG-SGM3 mice. While MTAP-expressing tumors have roughly similar sizes with and without immune interaction, the size of MTAP-deficient tumors is significantly larger in mice with immune cells (Figure 3B) than in those without, suggesting that MTAP, or rather the lack of, significantly contributes to modulating the immune response and drives tumor progression. These results qualitatively depict the distinct tumor landscapes between MTAP-expressing and MTAP-deficient tumors.
Since MTAP deficiency influences both elements of the tumor and the surrounding milieu, another possibility accounting for the immune escape induced by MTAP deficiency may be the result of the differential activity of pathways controlling the tumor immune response 24. Based on our Western blot and CyTOF analyses, the protein levels of co-stimulatory molecules OX40/OX40L, CD40L, GITR and CD278 (ICOS) were reduced in immune cells encountering MTAP-loss cancer cells with a concurrent elevation in co-inhibitory molecules PD-L1 and CTLA-4. Furthermore, we noted weaker T-cell mediated activity (Figure 2E, Figures S3C-D). These taken as a whole demonstrate heightened tumor-induced immunosuppression.
Beyond changes we observed in the immune cells, tumor cells with MTAP deficiency had altered intrinsic cellular activities at transcriptomic and secretomic levels. Of particular interest, the IL-12/IFN-γ/IP-10 axis was distinctly attenuated in MTAP-KO cells. Predominantly produced by antigen-presenting cells (APCs) such as DCs, macrophages and B cells, IL-12 signals through IL-12 receptor (IL-12R). This receptor is mainly expressed on NK cells and activated T cells, allowing downstream JAK2 and TYK2 to facilitate the phosphorylation of STAT4 and subsequent gene transcription of T-bet and IFN-γ 25-27. T-bet, a positive regulator for the differentiation of T helper type 1 (Th1) cells, promotes the expression of anti-tumor cytokines such as IFN-γ, IL-12R, CXCR3, MIP-1α and MIP-1β, and suppresses pro-tumoral Th2, Th17 and Treg cell differentiation through GATA3, RORγt and Foxp3, respectively 26, 27. IFN-γ secreted by IL-12-activated NK cells and T cells further boosts IL-12 production in a positive feedback loop, resulting in abundant cytotoxic NK cells and T cells infiltrating into tumors and secreting perforin and granzyme to induce cancer cell death 26-28. Moreover, IFN-γ contributes to the polarization of macrophages to M1 phenotype and the production of IP-10, which acts on CXCR3 to trigger the recruitment of effector immune cells to tumor sites 28. This antitumor immune response, however, is nearly absent in the TME of MTAP-deficient tumors.
To further characterize the changes in the immune microenvironment, we also analyzed the intratumoral immune cell composition. Here, we noticed significant decreases in total TILs, T cells, B cells, NK cells and increases in monocytes, M2 macrophages, Tregs within MTAP-deficient tumors. DCs, the pivotal initiator and regulator of innate and adaptive immune responses, were surprisingly more abundant in MTAP-deficient tumors. There are several possibilities for this phenomenon. First, there were already plentiful infiltrated DCs in MTAP-deficient tumors, but their antitumor activities are yet to be determined. After sensing environmental antigens, DCs undergo maturation process, expressing chemokine receptors and co-stimulatory molecules and elevating antigen processing for the subsequent T cell priming and activation 29, 30. However, the maturation process can be impaired by tumor-derived VEGF, IL-6, as well as IL-10 from M2 macrophages recruited by tumor-secreted M-CSF (encoded by CSF1, upregulated in CL1-0 KO+PBMC) 30-34, leading to ample but incompetent DCs in MTAP-deficient tumors.
The second possibility is that DCs in the TME of MTAP-deficient tumors fail to migrate to the tumor-draining lymph nodes (TDLNs) to present antigens to T cells, thus accumulating in tumors. The migratory ability of tumor-infiltrating DCs into TDLNs depends on the expression of CCR7 chemokine receptor 35. Ablation of CCR7 expression in DCs resulted in dampened trafficking to TDLNs in melanoma 36. Thus, cancer cells may interfere with DCs migration by regulating the CCR7 expression in DCs. For example, tumor-derived LXR-α has been shown to impede CCR7 expression and CCR7-mediated migration of DCs 37. We did see an upregulation of the LXR-α-coding gene NR1H3 in MTAP-KO cells, suggesting that the LXR-α/CCR7 axis may be contributing to our observation of increased DCs in the tumors in our model.
Lastly, there is a theoretical probability that the state of DCs residing in MTAP-deficient tumors is tolerogenic. Under certain TME conditions, DCs reprogram to regulatory DCs (DCregs), and such DCregs are able to tolerate antigens and induce the differentiation of CD4+ Tregs 38, which are evidently more abundant in MTAP-deficient tumors. The induction of DCregs can be triggered by environmental stimuli e.g. PGE2, TGF-β, VEGF, IL-10, TNF-α 39, all of which may be more abundant in MTAP-deficient tumors in light of the upregulation of the related genes in MTAP-KO cells compared to those in WT cells (Figure 3C). This all implies that MTAP deficiency may induce DCs differentiation from immunogenic to tolerogenic.
An emerging field in cancer immunology concerning cellular metabolism has garnered much interest in recent decades. A growing array of evidence substantiates the role of intrinsic metabolic pathways, metabolites and waste products in shaping both the encompassing microenvironment and immune response. Thus, immunometabolism has emerged as an attractive and crucial prospect in understanding how malignant tumor cells utilize metabolic reprogramming to outsmart the immune system. In our previous finding, we identified several dysregulated metabolites linked to cancer immunity in the methionine and adenine cycles upon MTAP overexpression in lung cancer 17. Methionine is the major source for the production of S-adenosylmethionine (SAM) and its downstream metabolite MTA, both of which participate in methylation reactions and epigenetic regulation. In hepatocellular carcinoma, alteration of methionine metabolism manifested by higher levels of SAM and MTA in tumors which was tightly linked to T cell exhaustion. Exogenous administration of SAM/MTA also resulted in diminished T cell activity by reprogramming chromatin accessibilities 40.
Another recent study also demonstrated that tumor cells are capable of outcompeting T cells for methionine and repress antitumor immunity via lowering the intracellular levels of methionine and expression of H3K79me2 and STAT5 in T cells 41. Increased extracellular adenosine, which is converted from adenine, potentiated the escape of tumor cells from immunosurveillance by inhibiting the functionality of T cells, DCs, NK cells and neutrophils, while promoting the activity of immunosuppressive cell types such as MDSCs and Tregs 42. These reports have suggested that the metabolic requirements result in competition between tumor cells and immune cells and have raised other possibilities for MTAP deficiency-driven immunometabolism. Future research should be directed to solidify a mechanistic understanding of the interchange between the metabolome and immune system and identify molecular targets for the tumor immune microenvironment.
In summary, our studies shed insight on how genetic loss of a metabolic enzyme contributes to immunological modification of the tumor-immune landscape. The substantial changes of the tumor immune microenvironment underscore the dominance of MTAP-deficient tumors, providing a molecular basis to integrate the multi-faceted nature of cancer to develop efficient therapeutics and paving a future avenue to comprehensively study tumor-immune interplay.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the Bioanalytics and Single-Cell (BASiC) Core at UT Health San Antonio (San Antonio, TX, USA) for the technological support as well as Dr. Tsung-Chieh Shih (Department of Biochemistry and Molecular Medicine, UC Davis) and Mr. Sebastian A. Liu (Department of Internal Medicine, UC Davis) for their assistance with animal studies. The results of Figures 6B and 6C here are in whole or part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga. The figure of graphical summary was created with BioRender.com.
Funding statement
This work was supported by the California UCOP grants Tobacco-Related Disease Research Program (T29IR0704), the NIH grant NHLBI R01HL146802 and the DoD KCRP grant W81XWH1910831 (log# KC180170).
Footnotes
Conflict of interest disclosure
The authors declare no conflict of interest.
Data availability statement
The RNA sequencing data in this study are publicly available in the NCBI SRA database (PRJNA720919).
REFERENCES
- 1.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015; 372:2521–32. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387:1540–50. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015; 373:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borcoman E, Kanjanapan Y, Champiat S, Kato S, Servois V, Kurzrock R, et al. Novel patterns of response under immunotherapy. Ann Oncol 2019; 30:385–96. [DOI] [PubMed] [Google Scholar]
- 5.Zhao B, Zhao H, Zhao J. Efficacy of PD-1/PD-L1 blockade monotherapy in clinical trials. Ther Adv Med Oncol 2020; 12:1758835920937612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Man J, Millican J, Mulvey A, Gebski V, Hui R. Response Rate and Survival at Key Timepoints With PD-1 Blockade vs Chemotherapy in PD-L1 Subgroups: Meta-Analysis of Metastatic NSCLC Trials. JNCI Cancer Spectrum 2021; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grossman JE, Vasudevan D, Joyce CE, Hildago M. Is PD-L1 a consistent biomarker for anti-PD-1 therapy? The model of balstilimab in a virally-driven tumor. Oncogene 2021; 40:1393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Durable Complete Response After Discontinuation of Pembrolizumab in Patients With Metastatic Melanoma. J Clin Oncol 2018; 36:1668–74. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017; 168:707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 2019; 18:197–218. [DOI] [PubMed] [Google Scholar]
- 11.Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, et al. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front Immunol 2019; 10:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertino JR, Waud WR, Parker WB, Lubin M. Targeting tumors that lack methylthioadenosine phosphorylase (MTAP) activity: current strategies. Cancer Biol Ther 2011; 11:627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kryukov GV, Wilson FH, Ruth JR, Paulk J, Tsherniak A, Marlow SE, et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science 2016; 351:1214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marjon K, Cameron MJ, Quang P, Clasquin MF, Mandley E, Kunii K, et al. MTAP Deletions in Cancer Create Vulnerability to Targeting of the MAT2A/PRMT5/RIOK1 Axis. Cell Rep 2016; 15:574–87. [DOI] [PubMed] [Google Scholar]
- 15.Mavrakis KJ, McDonald ER 3rd, Schlabach MR, Billy E, Hoffman GR, deWeck A, et al. Disordered methionine metabolism in MTAP/CDKN2A-deleted cancers leads to dependence on PRMT5. Science 2016; 351:1208–13. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Chang WH, Fong LWR, Weiss RH, Yu SL, Chen CH. Targeting the insulin-like growth factor-1 receptor in MTAP-deficient renal cell carcinoma. Signal Transduct Target Ther 2019; 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang WH, Chen YJ, Hsiao YJ, Chiang CC, Wang CY, Chang YL, et al. Reduced symmetric dimethylation stabilizes vimentin and promotes metastasis in MTAP-deficient lung cancer. EMBO Rep 2022:e54265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahrmann JF, Grapov DD, Wanichthanarak K, DeFelice BC, Salemi MR, Rom WN, et al. Integrated Metabolomics and Proteomics Highlight Altered Nicotinamide- and Polyamine Pathways in Lung Adenocarcinoma. Carcinogenesis 2017; 38:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan Z, Kong X, Xia J, Wu X, Li H, Xu H, et al. The arginine methyltransferase PRMT5 regulates CIITA-dependent MHC II transcription. Biochim Biophys Acta 2016; 1859:687–96. [DOI] [PubMed] [Google Scholar]
- 20.Henrich FC, Singer K, Poller K, Bernhardt L, Strobl CD, Limm K, et al. Suppressive effects of tumor cell-derived 5'-deoxy-5'-methylthioadenosine on human T cells. Oncoimmunology 2016; 5:e1184802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs B, Schlogl S, Strobl CD, Volkl S, Stoll A, Mougiakakos D, et al. The Oncometabolite 5'-Deoxy-5'-Methylthioadenosine Blocks Multiple Signaling Pathways of NK Cell Activation. Front Immunol 2020; 11:2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Li A, Lei Q, Zhang Y. Tumor-intrinsic signaling pathways: key roles in the regulation of the immunosuppressive tumor microenvironment. J Hematol Oncol 2019; 12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev 2002; 13:155–68. [DOI] [PubMed] [Google Scholar]
- 24.O'Neill RE, Cao X. Co-stimulatory and co-inhibitory pathways in cancer immunotherapy. Adv Cancer Res 2019; 143:145–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tugues S, Burkhard SH, Ohs I, Vrohlings M, Nussbaum K, Vom Berg J, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ 2015; 22:237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ullrich KA, Schulze LL, Paap EM, Muller TM, Neurath MF, Zundler S. Immunology of IL-12: An update on functional activities and implications for disease. EXCLI J 2020; 19:1563–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirlekar B, Pylayeva-Gupta Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers (Basel) 2021; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorgovanovic D, Song M, Wang L, Zhang Y. Roles of IFN-gamma in tumor progression and regression: a review. Biomark Res 2020; 8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol 2010; 108:111–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 2020; 20:7–24. [DOI] [PubMed] [Google Scholar]
- 31.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 1996; 2:1096–103. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton JA, Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol 2013; 34:81–9. [DOI] [PubMed] [Google Scholar]
- 33.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 2014; 26:623–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang M, Diao J, Gu H, Khatri I, Zhao J, Cattral MS. Toll-like Receptor 2 Activation Promotes Tumor Dendritic Cell Dysfunction by Regulating IL-6 and IL-10 Receptor Signaling. Cell Rep 2015; 13:2851–64. [DOI] [PubMed] [Google Scholar]
- 35.MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med 2003; 198:615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, et al. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 2016; 30:324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villablanca EJ, Raccosta L, Zhou D, Fontana R, Maggioni D, Negro A, et al. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med 2010; 16:98–105. [DOI] [PubMed] [Google Scholar]
- 38.Ness S, Lin S, Gordon JR. Regulatory Dendritic Cells, T Cell Tolerance, and Dendritic Cell Therapy for Immunologic Disease. Front Immunol 2021; 12:633436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res 2019; 79:4557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hung MH, Lee JS, Ma C, Diggs LP, Heinrich S, Chang CW, et al. Tumor methionine metabolism drives T-cell exhaustion in hepatocellular carcinoma. Nat Commun 2021; 12:1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bian Y, Li W, Kremer DM, Sajjakulnukit P, Li S, Crespo J, et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature 2020; 585:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vigano S, Alatzoglou D, Irving M, Menetrier-Caux C, Caux C, Romero P, et al. Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function. Front Immunol 2019; 10:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA sequencing data in this study are publicly available in the NCBI SRA database (PRJNA720919).


