Abstract
Background
Antibiotics significantly increased life expectancy and decreased mortality rates due to infections. However, this trend is starting to fade with the rise of multidrug-resistant organisms (MDR); these strains are becoming a global burden on healthcare and the economy. The dramatic increase and spread of carbapenem-resistant gram-negative bacteria (CRGNB) has become a serious global public health concern. In this retrospective cross-sectional study, we aimed to estimate the rates of gram-negative bacteremia in five tertiary care hospitals in different geographical locations in Saudi Arabia for five years.
Methods
A retrospective cross-sectional study was conducted in five tertiary care hospitals in Saudi Arabia among patients with bacteremia due to CRGNB. Electronic medical records were used to retrieve data regarding patient demographics and antimicrobial susceptibility testing (AST) over five years between January 2016 and December 2020. Patients with positive blood cultures for carbapenem-resistant Escherichia (E.) coli, Klebsiella (K.) pneumonia, Pseudomonas (P.) aeruginosa, and Acinetobacter (A.) baumannii comprise the final study population.
Results
This retrospective multicentric study was conducted between 2016 and 2020 in five tertiary care hospitals across five cities in Saudi Arabia. E. coli (n=2190, 38.03%), K. pneumoniae (n=2154, 37.41%), P. aeruginosa (n = 918, 15.94%), and A. baumannii (n=496, 8.61%) constitute the 5758 gram-negative bacteria isolates. E. coli was the most frequently identified species in Riyadh, AlAhsa, Dammam, and Madinah (40%, 46.50%, 61.67%, and 43.66%, respectively), with a p-value of (p<0.001), except in Jeddah, where K. pneumoniae was the most prevalent (42%). The mean age of patients across Riyadh, AlAhsa, Dammam, and Madinah was 62.2 years (± 4.24). In contrast to Jeddah, where the majority of isolates (702; 41.8%) belonged to the adult age group. Most isolates were from male patients (3045; 52.9%), compared to 2713 (47.1%) from female patients. K. pneumoniae 1226 (40.3%) was the most prevalent isolate among male patients while E. coli (1135; 41.8%) was the most prevalent isolate among female patients.
Conclusion
Our study showed that the prevalence of carbapenem non-susceptible Gram-negative bacteria is relatively high, which therefore makes them very challenging to treat. The results show an urgent need for improved antibiotic stewardship strategies, including better surveillance and more effective infection control measures to reduce this issue. Further research into the molecular epidemiology and risk factors associated with these infections is necessary to guide public health policymakers in developing interventions to help control the spread of carbapenem-resistant Gram-negative bacteria.
Keywords: surveillance, antimicrobial resistance, saudi arabia, antimicrobial resistance surveillance, gram-negative bacteria, carbapenem resistance
Introduction
Antibiotics significantly increased life expectancy and decreased mortality rates due to infections. However, this trend is starting to fade with the rise of multidrug-resistant organisms (MDR); these strains are becoming a global burden on healthcare and the economy [1,2]. The dramatic increase and spread of carbapenem-resistant gram-negative bacteria (CRGNB) has become a serious global public health concern [3].
The transition from susceptible pathogens to multiple drug resistance (MDR) pathogens has resulted in additional healthcare and economic burdens and will cost the world approximately $100 trillion over the next 30 years [2,4]. Antimicrobial resistance has increased at an alarming rate, as it has been estimated that MDR-related mortality will increase to 10 million by 2050 [3]. Resistance to third-generation cephalosporins, fluoroquinolones, and aminoglycosides has increased due to the spread of antimicrobial-resistant Gram-negative bacteria. In particular, the dramatic increase and spread of carbapenem-resistant-Gram-negative bacteria (CRGNB) has become a serious global public health concern, including in Saudi Arabia [5-8]. The World Health Organization (WHO) has classified CRGNB as a critical pathogen, indicating that it poses significant threats to human health and necessitates immediate research and development of novel antimicrobials [9-11].
Generally, bacteria have developed different mechanisms in which they become resistant, including enzymatic inactivations, mutations in the target site, and efflux pumps. The development of carbapenem resistance can be due to either one of two mechanisms; acquired or intrinsic resistance, and in some instances, both mechanisms can occur simultaneously. The enzymatic inactivations (acquired carbapenemases) are the most common mechanism for carbapenem resistance. This resistance mechanism includes (1) the destruction of carbapenems (which are resistant to hydrolysis by plasmid AmpCs) in conjunction with ESBL enzymes, (2) ESBL genes being transferred between organisms, and (3) mutation of porin with expression modulation. On the other hand, intrinsic resistance occurs by reducing uptake (due to modified porin channels) and by reducing the permeability of the membrane for B-lactam drugs. Pseudomonas (P.) aeruginosa organisms have a certain mechanism of resistance in which efflux pump overactivation is capitalized to develop resistance toward carbapenems [12]. The aim of the current study was, therefore, to estimate the rate of CRGNB-causing bacteremia in five tertiary hospitals in Saudi Arabia over the duration from 2016 to 2020.
Materials and methods
Study setting, design, and subjects
The current study was conducted at King Abdulaziz Medical City (KAMC) and at King Abdullah International Medical Research Centre (KAIMRC), which are part of the Ministry of National Guard Health Affairs (MNGHA) in Saudi Arabia. MNGHA hospitals are tertiary healthcare hospitals that provide healthcare services to National Guard personnel and their families. Non-probability convenience sampling and a retrospective cross-sectional study were implemented, and all accessible medical records of patients with bacteremia due to carbapenem-resistant Gram-negative bacteria at five hospitals that met inclusion criteria between January 2016 and December 2020 were collected. The inclusion criteria consisted of patients of different age groups with confirmed diagnoses of bacteremia due to positive blood culture carbapenem-resistant Gram-negative bacteria (Escherichia (E.) coli, Klebsiella (K.) pneumonia, P. aeruginosa, and Acinetobacter (A.) baumannii). Data were gathered from the patient’s medical records, including demographics and microbiology laboratory findings. The total number of bacterial isolates in each age group and the rate of carbapenem resistance were calculated for 5,759 patients with CRGNB-related bacteremia.
Criteria for bacteremia diagnosis
A retrospective cross-sectional study was conducted in five tertiary care hospitals in Saudi Arabia among patients with bacteremia due to CRGNB. Electronic medical records (BestCare) were used to retrieve data regarding patient demographics and antimicrobial susceptibility testing (AST) over five years between January 2016 and December 2020. Patients with positive blood cultures for carbapenem-resistant E. coli, K. pneumonia, P. aeruginosa, and A. baumannii comprise the final study population.
Ethical considerations
The procedures followed in the current study were in accordance with the Declaration of Helsinki of 1975 (revised in 2000) and with the ethical standards of the institutional review board of KAIMRC in Riyadh, Saudi Arabia, where the study was conducted (protocol approval number: 1104/19, 7 July 2019). Permission was granted to access the patient’s medical records. As the current study was retrospective in nature, patient consent was waived.
Statistical analysis
Quantitative variables were presented as means with standard deviations while qualitative variables were presented as frequencies. The variables of the study were compared using the chi-square test. The information was analyzed using Microsoft Excel (2017) (Microsoft Corporation, Redmond, WA).
Results
Bacterial isolates and demographics
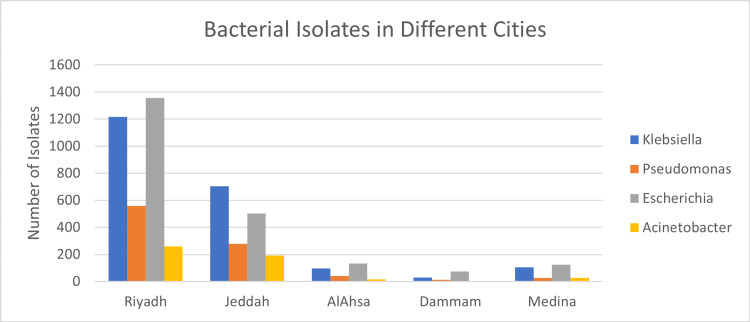
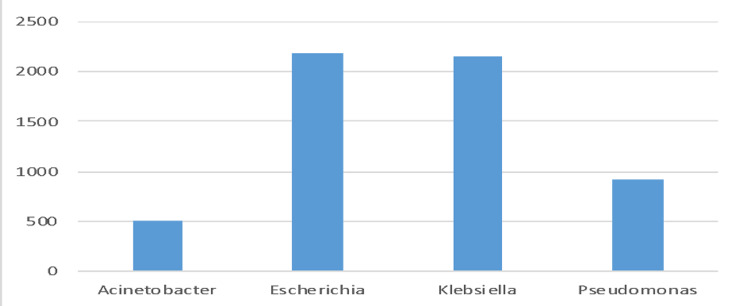
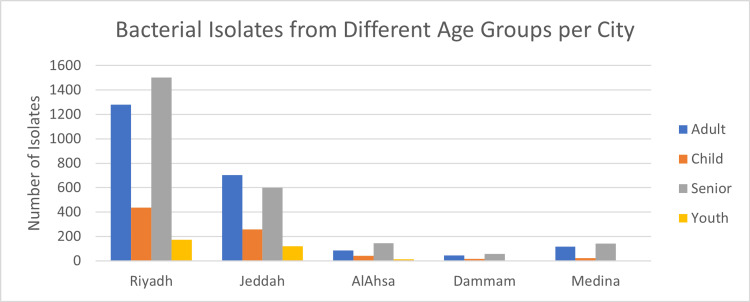
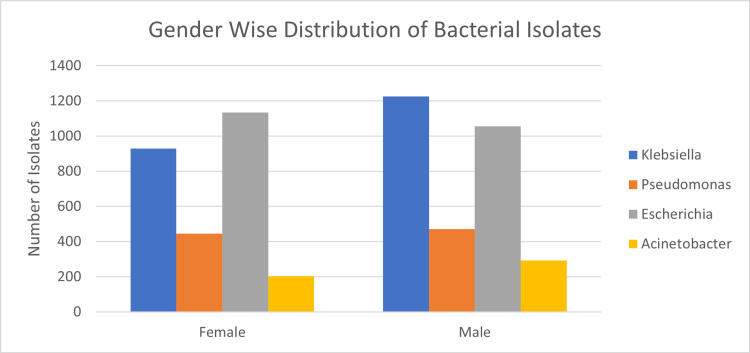
This retrospective multi-centric study was conducted between 2016 and 2020 in five tertiary care hospitals across five cities in Saudi Arabia. Riyadh (n=3390, 58.86%), Jeddah (n=1678, 29.13%), AlAhsa, (n=286, 4.96%), Madinah (n=284, 4.93%), and Dammam (n=120, 2.08%) were among the regions from which the data were recovered (Table 1 and Figure 1). E. coli (n=2190, 38.03%), K. pneumoniae (n=2154, 37.41%), P. aeruginosa (n=918, 15.94%), and A. baumannii (n=496, 8.61%) constitute the 5758 gram-negative bacteria isolates (Figure 2). E. coli was the most frequently identified species in Riyadh, AlAhsa, Dammam, and Madinah (40%, 46.50%, 61.67%, and 43.66%, respectively), except in Jeddah, where K. pneumoniae was the most prevalent (42%). The mean age of patients across Riyadh, AlAhsa, Dammam, and Madinah was 62.2 years (± 4.24), with 42.4% of patients being elderly (age over 65), 38.7% being adults (age between 25 and 64), 5.5% being youth (age between 15 and 24), and 13.4% being children (age below 15). Riyadh had 1,503 (44.3%), AlAhsa 145 (50.7%), Dammam 56 (46.7%), and Madinah 141 (49.6%) older adults among the total number of isolated individuals (Table 2). In contrast to Jeddah, where the majority of isolates, 702 (41.8%), belonged to the adult age group illustrated in Figure 3 (P < 0.001). Most isolates were from male patients, 3045 (52.9%), compared to 2713 (47.1%) from female patients (Table 2). K. pneumoniae 1226 (40.3%) was the most prevalent isolate among male patients, while E. coli 1135 (41.8%) was the most prevalent isolate among female patients, as shown in Figure 4 (P < 0.001).
Table 1. Distribution of positive blood culture among five cities and frequency of each bacteria in each city.
E.: Escherichia; K.: Klebsiella; P.: Pseudomonas; A.: Acinetobacter
| Species | City | |||||
| Riyadh (n) | Jeddah (n) | AlAhsa (n) | Dammam (n) | Medina (n) | Total (n) | |
| K. pneumoniae | 1217 | 705 | 96 | 30 | 106 | 2154 |
| E. coli | 1356 | 503 | 133 | 74 | 124 | 2190 |
| P. aeruginosa | 559 | 278 | 41 | 14 | 26 | 918 |
| A. baumannii | 258 | 192 | 16 | 2 | 28 | 496 |
| Total | 3390 | 1678 | 286 | 120 | 284 | 5758 |
Table 2. Shows the distribution of patient age and gender across the five cities.
| Age in Years | Cross all cities (Mean ± SD) | Elderly age over 65 (%) | Adult ages 25-64 (%) | Youth ages 15-24 (%) |
| 62.2±4.24 | 42.4 | 38.7 | 5.5 | |
| Gender | n (%) | |||
| Male | 3045 (52.9) | |||
| Female | 2713 (47.1) | |||
| Cities | n (%) | |||
| Riyadh | 3390 (58.86) | |||
| Jeddah | 1678 (29.13) | |||
| Madinah | 284 (4.93) | |||
| Dammam | 120 (2.08) | |||
| AlAhsa | 1678 (29.13) | |||
Figure 1. Illustration of the four gram-negative bacteria (K. pneumoniae, P. aeruginosa, A. baumannii, and E. coli) across cities.
E.: Escherichia; K.: Klebsiella; P.: Pseudomonas; A.: Acinetobacter
Figure 2. Illustrates the total number of isolates in blood cultures among the four species (n=5759).
Figure 3. Demonstrates distribution of CR gram-negative bacteremia among different age groups according to each city.
CR: carbapenem resistance
Figure 4. Illustrates CR gram-negative bacteremia among the four species according to gender.
CR: carbapenem resistance
Carbapenem resistance (CR) rate
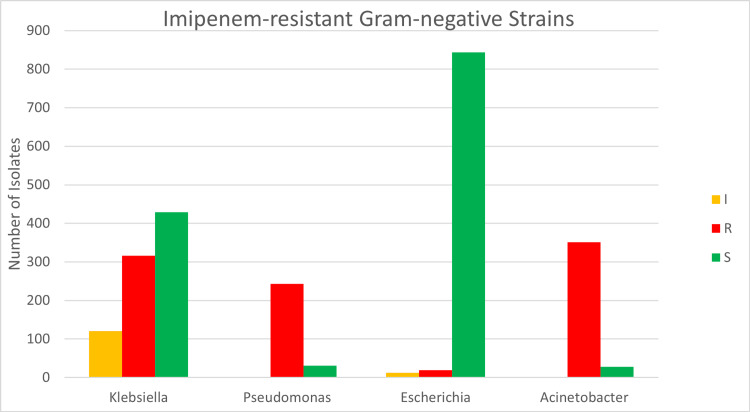
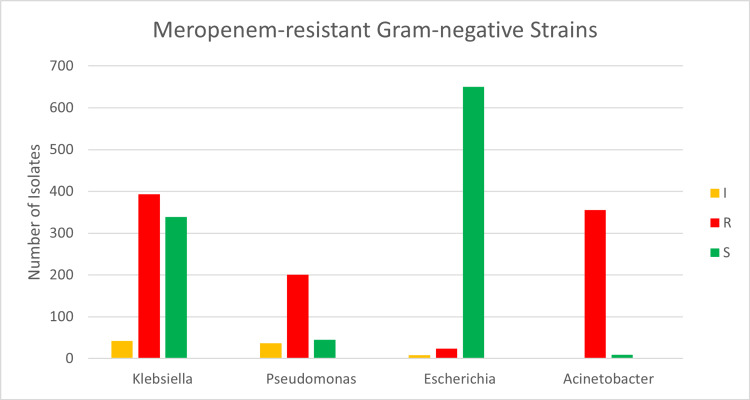
Overall, the CR rate for the four pathogens was 38% (929/2396) for imipenem and 46% (973/2104) for meropenem. A. baumannii illustrates the highest level of resistance to Imipenem; out of 381 samples, 351 (92%) were resistant, whereas 243 (88%) of 275 P. aeruginosa samples were resistant. Among 865 samples of K. pneumoniae, 316 (37%) were resistant, and 120 (14%) exhibited intermediate resistance to imipenem. E. coli had the highest number of tested samples (n=875), of which 844 (96%) were susceptible to imipenem (P<0.001) (Table 3 and Figure 5). Resistance to meropenem exhibits a similar pattern, as shown in Table 4 and Figure 6.
Table 3. Illustrates degrees of resistance to meropenem among the four species.
Intermediate (I), Resistant (R), Sensitive (S)
E.: Escherichia; K.: Klebsiella; P.: Pseudomonas; A.: Acinetobacter
| Meropenem | I | R | S | Total |
| K. pneumoniae | 42 | 393 | 339 | 774 |
| E. coli | 8 | 24 | 650 | 682 |
| P. aeruginosa | 37 | 201 | 45 | 283 |
| A. baumannii | 1 | 355 | 9 | 365 |
| Total | 88 | 973 | 1043 | 2104 |
Table 4. Illustrates degrees of resistance to imipenem among the four species.
Intermediate (I), Resistant (R), Sensitive (S)
E.: Escherichia; K.: Klebsiella; P.: Pseudomonas; A.: Acinetobacter
| Imipenem | I | R | S | Grand Total |
| K. pneumoniae | 120 | 316 | 429 | 865 |
| E. coli | 12 | 19 | 844 | 875 |
| P. aeruginosa | 1 | 243 | 31 | 275 |
| A. baumannii | 2 | 351 | 28 | 381 |
| Total | 135 | 929 | 1332 | 2396 |
Figure 5. Illustrates degrees of resistance to imipenem among the four species.
Intermediate (I), Resistant (R), Sensitive (S)
Figure 6. Illustrates degrees of resistance to meropenem among the four species.
Intermediate (I), Resistant (R), Sensitive (S)
Discussion
Our research revealed that E. coli was the most prevalent organism discovered in blood cultures. However, a different study conducted by Ayobami (2019) at MNGHA between 2006 and 2017 contradicts this result; the study showed that P. Aeruginosa was the most prevalent organism in blood cultures taken between 2006 to 2017 [9]. Moreover, our research demonstrates that the incidence of E. coli infections has risen. Similarly, a study conducted by Miranda (2020) in Mexico between 2016 and 2017 on 4382 blood isolates revealed that E. coli and K. pneumoniae are the most prevalent gram-negative species, exhibiting over 30% resistance to most commonly used antibiotics and less than 20% resistance to carbapenem [13].
The mean age of patients was 62.2, with seniors comprising 42.4% of the patient population, followed by adults comprising 38.8%. This may result from an increase in co-morbidities, hospitalizations, and interventions. Most of the isolates were obtained from male patients (52.9%), which was consistent across all Gram-negative bacteria, except for E. coli, where female isolates were more prevalent. The prevalence of urinary tract infections among females may have contributed to these results. Comparatively, males had a higher prevalence of gram-negative bacteria worldwide [2]. Except for E. coli, all isolates exhibited high resistance to carbapenem. This is somewhat concerning and may be related to the increased use of antibiotics over the past few decades. In 2010, a study conducted in a hospital in Riyadh, Saudi Arabia, revealed an overuse of antimicrobial agents in four adult intensive care units (ICUs), with meropenem being the most commonly used antibiotic (33.2 defined daily doses (DDD) per 100 bed-days) [14].
A cross-sectional study conducted by Yang (2018) in China across 153 hospitals linked CRGNB and antibiotic consumption intensity, finding that increased use of meropenem has been related to the rate of carbapenem-resistant K. pneumonia (CRKP). Carbapenemases such as New Delhi Metallo-lactamase-1 (NDM-1) And K. pneumoniae carbapenemases (KPC) are produced by increased carbapenem usage; the study linked Carbapenem resistance to increased antibiotic consumption in all remaining species [15].
In Europe, a 2017 study surveyed carbapenem resistance (CR) across three species: K. pneumonia, P. aeruginosa, and A. baumannii. The study revealed a disparity in resistance profiles between Western and Eastern Europe, which may be attributable to antibiotic use. Globally, CR for A. baumannii is typically quite high, whereas some European nations have lower resistance. It ranges from 0% in Belgium to 95% in Greece. CR rates are typically collected from a single region or hospital, so additional research is required [16]. This also emphasizes the significance of global AMR surveillance programs. Fortunately, new policies and regulations have addressed this issue head-on in recent years. Nationwide antimicrobial stewardship and surveillance programs are utilized to combat this problem.
A systemic review and meta-analysis in 2017 revealed a correlation between CR and K. pneumonia mortality rate. There were 2,167 patients across 15 studies, of which 1,148 had CSKP bacteremia and 1,019 had CRKP bacteremia. CRKP bacteremia patients had a higher mortality rate than CSKP bacteremia patients [17].
Conclusions
Our study showed that the prevalence of carbapenem non-susceptible Gram-negative bacteria is relatively high, which therefore makes them very challenging to treat. The results show an urgent need for improved antibiotic stewardship strategies, including better surveillance and more effective infection control measures to reduce this issue. Antimicrobial resistance (AMR) trends must be identified on a national and international scale, necessitating routine surveillance studies to provide crucial data. Further research into the molecular epidemiology and risk factors associated with these infections is necessary to guide public health policymakers in developing interventions to help control the spread of carbapenem-resistant Gram-negative bacteria. Further research is recommended to establish a connection between CR and clinical outcomes and mortality rate.
Acknowledgments
We sincerely thank the data management staff at King Abdullah International Medical Research Centre for their help in the process of data collection.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Institutional review board of KAIMRC, Riyadh, Saudi Arabia issued approval 1104/19, 7 July 2019
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Molecular mechanisms of antibiotic resistance. Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Nat Rev Microbiol. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 2.Mechanisms of antibiotic resistance. Munita JM, Arias CA. Microbiol Spectr. 2016;4 doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Review on antimicrobial resistance. Antimicrobial resistance: tackling a crisis for the health and wealth of Nations. [ Jul; 2022 ]. 2014. https://wellcomecollection.org/works/rdpck35v https://wellcomecollection.org/works/rdpck35v
- 4.Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Antimicrobial Resistance Collaborators. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molecular mechanisms and epidemiology of carbapenem-resistant Escherichia coli isolated from Chinese patients during 2002-2017. Tian X, Zheng X, Sun Y, et al. Infect Drug Resist. 2020;13:501–512. doi: 10.2147/IDR.S232010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbapenem-resistant Enterobacteriaceae: an update narrative review from Saudi Arabia. Alotaibi F. J Infect Public Health. 2019;12:465–471. doi: 10.1016/j.jiph.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Genomic characterization of carbapenem-non-susceptible Pseudomonas aeruginosa clinical isolates from Saudi Arabia revealed a global dissemination of GES-5-producing ST235 and VIM-2-producing ST233 sub-lineages. Doumith M, Alhassinah S, Alswaji A, et al. Front Microbiol. 2021;12:765113. doi: 10.3389/fmicb.2021.765113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevalence of carbapenem-resistant Gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Cai B, Echols R, Magee G, et al. https://doi.org/10.1093/ofid/ofx176. Open Forum Infect Dis. 2017;4:0. doi: 10.1093/ofid/ofx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The incidence and prevalence of hospital-acquired (carbapenem-resistant) Acinetobacter baumannii in Europe, Eastern Mediterranean and Africa: a systematic review and meta-analysis. Ayobami O, Willrich N, Harder T, Okeke IN, Eckmanns T, Markwart R. Emerg Microbes Infect. 2019;8:1747–1759. doi: 10.1080/22221751.2019.1698273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ten-year resistance trends in pathogens causing healthcare-associated infections; reflection of infection control interventions at a multi-hospital healthcare system in Saudi Arabia, 2007-2016. Balkhy HH, El-Saed A, Alshamrani MM, et al. Antimicrob Resist Infect Control. 2020;9:21. doi: 10.1186/s13756-020-0678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO publishes list of bacteria for which new antibiotics are urgently needed. World health organization. 2017. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed
- 12.Aslam B, Rasool M, Muzammil S, et al. Pathogenic Bacteria. London, UK: IntechOpen; 2020. Carbapenem Resistance: Mechanisms and Drivers of Global Menace. [Google Scholar]
- 13.Antimicrobial resistance and antibiotic consumption in Mexican hospitals. Miranda-Novales MG, Flores-Moreno K, López-Vidal Y, Rodríguez-Álvarez M, Solórzano-Santos F, Soto-Hernández JL, Ponce de León-Rosales S. Salud Publica Mex. 2020;62:42–49. doi: 10.21149/10543. [DOI] [PubMed] [Google Scholar]
- 14.Antimicrobial resistance in Saudi Arabia. An urgent call for an immediate action. Zowawi HM. Saudi Med J. 2016;37:935–940. doi: 10.15537/smj.2016.9.16139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Association between antibiotic consumption and the rate of carbapenem-resistant Gram-negative bacteria from China based on 153 tertiary hospitals data in 2014. Yang P, Chen Y, Jiang S, Shen P, Lu X, Xiao Y. Antimicrob Resist Infect Control. 2018;7:137. doi: 10.1186/s13756-018-0430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Global antimicrobial resistance in Gram-negative pathogens and clinical need. Theuretzbacher U. Curr Opin Microbiol. 2017;39:106–112. doi: 10.1016/j.mib.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 17.Carbapenem resistance, initial antibiotic therapy, and mortality in Klebsiella pneumoniae bacteremia: a systematic review and meta-analysis. Kohler PP, Volling C, Green K, Uleryk EM, Shah PS, McGeer A. Infect Control Hosp Epidemiol. 2017;38:1319–1328. doi: 10.1017/ice.2017.197. [DOI] [PubMed] [Google Scholar]