Abstract
The impact of the coronavirus disease 2019 (COVID-19) pandemic has been immense, and it continues to have lasting repercussions. While the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus primarily infects the respiratory system, other organ systems are affected, including the liver. Scientific knowledge on the role of SARS-CoV-2 infection and liver injury has evolved rapidly, with recent data suggesting specific hepatotropism of SARS-CoV-2. Moreover, additional concerns have been raised in regard to long-term liver damage, related to emerging cases of post-COVID-19 cholangiopathy and chronic cholestasis. Great effort has also been focused on studying how specific subpopulations with chronic medical conditions might be disproportionately impacted by COVID-19. One such population includes individuals with chronic liver disease (CLD) and cirrhosis, with an expanding body of research indicating these patients being particularly susceptible to adverse outcomes. In this review, we provide an updated summary on the current pathogenesis and mechanism of liver injury in the setting of SARS-CoV-2 infection, the association between health outcomes and SARS-CoV-2 infection in patients with CLD, and the unique consequences of the COVID-19 pandemic on the routine care of patients with CLD.
Keywords: COVID-19, Liver, Cirrhosis, SARS-CoV-2, Tropism
INTRODUCTION
Since the first reported cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),1 the world has been shaken by the effects of the coronavirus disease 2019 (COVID-19) global pandemic. Indeed, what is known about the pathogenesis, epidemiology, and treatment/prevention strategies in regard to SARS-CoV-2 infection has expanded immensely during the pandemic. In an effort to help guide targeted public health strategies and medical interventions, an important area of research has been identifying how specific chronic medical conditions may be associated with worse outcomes from SARS-CoV-2 infection. This includes studying the impact of chronic liver disease (CLD) and SARS-CoV-2 outcomes.2,3
Individuals with CLD are of particular importance given the immense global burden of CLD,4,5 as well as concerns that immune dysfunction associated with cirrhosis might make these patients more vulnerable to severe infection.2,6 Indeed, recent research has shown significantly increased mortality from SARS-CoV-2 in patients with decompensated cirrhosis, as well as other subpopulations of CLD.2 Specifically, patients with alcohol-associated liver disease (ALD) have been identified as having a higher mortality risk from COVID-19.2,6 Given these concerns, current recommendations are that patients with CLD receive vaccination against SARS-CoV-2 infection,7 though concerns remain regarding reduced vaccine immune response in patients with cirrhosis.8 Liver injury has been identified as an extrapulmonary complication of SARS-CoV-2 infection, with some association between abnormal liver biochemistries and worse clinical outcomes.9,10 This association of liver injury and COVID-19 has led some to investigate whether SARS-CoV-2 directly infects the liver, or if liver injury is more of a bystander result of severe infection.11 In this review, we focus on the current understanding of the pathogenesis and mechanism of liver injury in the setting of SARS-CoV-2 infection, clinical outcomes of SARS-CoV-2 infection in patients with CLD, and the impact of the COVID-19 pandemic on the management and care of patients with CLD.
PATHOGENESIS AND MECHANISM OF LIVER INJURY IN SARS-COV-2 INFECTION
SARS-CoV-2 primarily infects the respiratory system, and is known to cause pneumonia as well as acute respiratory distress syndrome.1,12 A variety of extrapulmonary consequences related to SARS-CoV-2 infection have also been described, including manifestations in the gastrointestinal and hepatobiliary systems.9,13 Existing data demonstrate that elevations of liver enzymes, such as alanine aminotransferase and aspartate aminotransferase, are quite common in the setting of COVID-19.10,14,15 While the incidence of elevated liver biochemistries in patients with COVID-19 varies between studies, some have reported a frequency over 80% in hospitalized patients, with a predominantly hepatocellular pattern of elevation. The degree of elevation is usually mild, and incidence of significant elevations of alanine aminotransferase or aspartate aminotransferase (>1,000 units/L), liver synthetic dysfunction, or elevations in serum bilirubin levels are relatively rare in patients with COVID-19.10,16,17 Long-term follow-up studies have recently reported that, after SARS-CoV-2 infection, progressive cholestasis may occur in up to 20% of patients with CLD and is often severe.18
Despite the high frequency of COVID-19 associated liver injury, evidenced by abnormal liver biochemistries, the clinical significance of liver injury and need for aggressive workup has been debated.19 Over the course of the pandemic, there have been several studies that showed an association between abnormal liver tests and poor prognosis. Specifically, significant elevations of alanine aminotransferase and aspartate aminotransferase in the setting of COVID-19 are associated with a more severe infection, need for intensive care unit (ICU) admission, mechanical ventilation and even death.10,20-25 Other research has described an association between markers of liver dysfunction, including hypoalbuminemia and gamma-glutamyl transferase elevation, with worse outcomes from COVID-19.26-28
What is also concerning are the potential long-term sequelae of COVID-19 associated liver injury, including the recently described post-COVID-19 cholangiopathy.18,29,30 Secondary sclerosing cholangitis in critically ill patients is a rare disease entity that has been described before, and is generally felt to be related to ischemic injury to the bile ducts as well as formation of toxic bile leading to necrosis and cast formation.31,32 Yet, certain histologic features appear to be unique to cholangiopathy after severe SARS-CoV-2 infection and these may be more suggestive of a primary insult related to direct hepatic injury from the SARS-CoV-2 virus itself, rather than ischemic related injury.30 Moreover, when compared to similar cohorts of critically ill patients due to influenza infection, the presence of secondary sclerosing cholangitis was more common amongst critically ill patients with SARS-CoV-2.33 Hartl et al.18 recently reported that, among patients with CLD and COVID-19, development of cholestatic liver failure occurred in 23% and secondary sclerosing cholangitis occurred in 15% of cases. In addition, they demonstrated that patients with preexisting CLD were more likely to develop liver injury and progressive cholestasis after COVID-19 compared to non-COVID-19 pneumonia. Thus, given the large-scale nature of the pandemic, with many patients recovering from severe infection, post-COVID-19 cholangiopathy could lead to long-term hepatic morbidity in large numbers of patients.
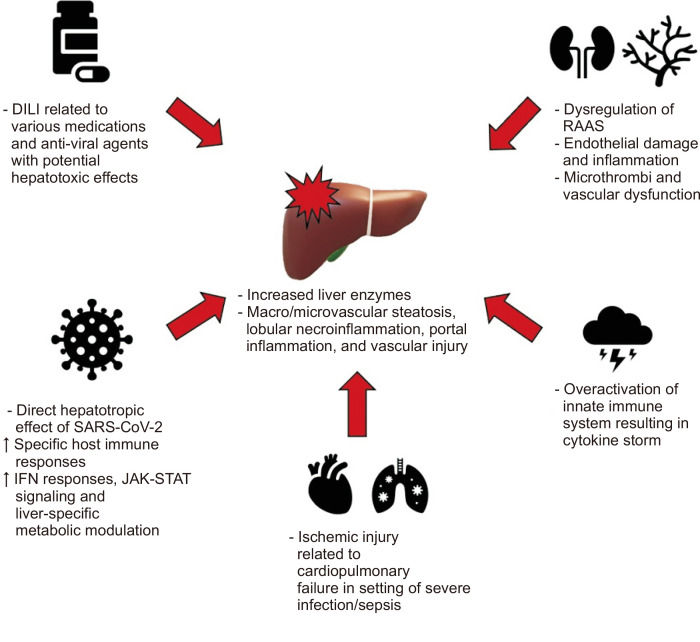
In regards to the general pathogenesis of SARS-CoV-2, current data postulates that the virus gains cell entry via interaction between its spike protein and the angiotensin converting enzyme 2 (ACE2) receptor, with priming of the spike protein by the transmembrane serine protease 2 (TMPRSS2).9,34 Upon entering host cells, the virus has several key mechanisms by which it can cause wide spread damage effecting multiple organ systems: direct viral toxicity, endothelial cell damage and inflammation leading to microthrombi deposition and microvascular dysfunction, overactivation of the innate immune system and cytokine release syndrome, and dysregulation of the renin-angiotensin-aldosterone system leading to tissue damage from vasoconstriction and vascular permeability.9,35 These mechanisms likely also play a role in regard to COVID-19 associated liver injury, though other mechanisms have also been proposed, including hepatic congestion in the setting of cardiac dysfunction and drug induced liver injury given the use of various medications and anti-viral agents with potential hepatotoxic effects.16,36,37 The diverse histologic pattern of injury in patients infected with SARS-CoV-2, characterized by steatosis (macrovascular and microvascular), lobular necroinflammation, portal inflammation, and vascular pathology, likely also supports the multifactorial nature of abnormal liver tests in setting of COVID-19 associated liver injury (Fig. 1).16,17,38-42 It is also worth acknowledging the possible contribution of non-liver-related elevations in biochemistries, including release from cardiac and skeletal muscle breakdown.17,37
Fig. 1.
Schematic of proposed mechanisms of coronavirus disease 2019 (COVID-19) related liver injury.
DILI, drug induced liver injury; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IFN, interferon; JAK-STAT, Janus kinase-signal transducer and activator of transcription; RAAS, renin-angiotensin-aldosterone system.
The question of whether SARS-CoV-2 displays specific liver tropism, causing liver injury via direct infection, has been a subject of debate. Early on in the pandemic, several discoveries were made that suggested direct infection of the liver by SARS-CoV-2, and its role as an important factor in hepatic impairment. This includes single-cell RNA sequencing analyses in healthy livers demonstrating gene expression levels for ACE2 in liver cells, with primarily expression in cholangiocytes as opposed to hepatocytes.43,44 This may lead one to expect a cholestatic pattern of hepatic injury seen in COVID-19 infection as opposed to the hepatocellular liver injury pattern more frequently reported in clinical practice. This apparent discrepancy may, in part, be explained by the fact that ACE2 is expressed at rest by cholangiocytes but can be expressed on hepatocytes in the setting of inflammation. Post-mortem autopsies provide evidence supporting direct infection of liver cells by SARS-CoV-2. Several studies report that SARS-CoV-2 was detected in a majority of post-mortem liver biopsies using polymerase chain reaction (PCR) and in situ hybridization, though evidence of direct viral invasion of hepatocytes was lacking.38,41 Some investigators were able to demonstrate specific coronavirus particles, such as spike structures, in the cytoplasm of hepatocytes in COVID-19 cases, along with associated mitochondrial swelling and apoptosis.45
Perhaps the most compelling evidence of SARS-CoV-2 liver tropism was recently published by Wanner et al.46 The authors of this study were able to show multiple levels of evidence for SARS-CoV-2 liver tropism, including SARS-CoV-2 RNA identified directly in hepatic parenchymal cells with high spatial resolution using in situ hybridization, as well as successful isolation of infectious SARS-CoV-2 from liver tissue post-mortem. Moreover, these authors identified transcriptomic and proteomic based activity profiles in hepatic samples. This confirmed the expression of known SARS-CoV-2 entry receptors and facilitators of infection including ACE2, TMPRSS2, procathepsin L, and Ras-related protein Rab-7a. Transcriptomic and proteomic analysis also exhibited strong upregulation of interferon responses, JAK-STAT signaling and liver-specific metabolic modulation, revealing a viral activity profile that has broad overlap with other hepatotropic viral infections (such as hepatitis C virus [HCV] infection).46,47 These recent revelations provide valuable insight into the pathogenesis of COVID-19 related liver injury.
CLINICAL OUTCOMES OF COVID-19 IN PATIENTS WITH CLD AND CIRRHOSIS
Patients with cirrhosis are vulnerable to infection due to dysfunction in the complement system, macrophage activation and compromised neutrophil and lymphocyte function, collectively known as cirrhosis-associated immune dysfunction. Increased susceptibility to complications from bacterial, viral and fungal infections have been demonstrated among patients with cirrhosis.61,62 This includes prior research indicating patients with cirrhosis suffering more severe infection, multiorgan failure and death due to influenza, as compared to patients without CLD.63 Data from early in the COVID-19 pandemic suggested that, while not infected with SARS-CoV-2 at higher rates, patients with cirrhosis and COVID-19 had poor outcomes (see Table 1 for summary of SARS-CoV-2 infection and CLD outcomes).50,52,64
Table 1.
Clinical Outcomes of SARS-CoV-2 Infection in Patients with CLD and Cirrhosis
| Study (year) | Description | Findings |
|---|---|---|
| Ji et al.(April 2020)48 | Data collected from 202 consecutive patients admitted to two designated COVID-19 hospitals in China. Patients had confirmed SARS-CoV-2, and information relating to NAFLD status was also obtained. | Presence of NAFLD was associated with progression of COVID-19 infection (OR, 6.4; 95% CI, 1.5–31.2). NAFLD patients had higher risk of disease progression (6.6% vs 44.7%, p<0.0001), likelihood of abnormal liver function at discharge (70.0% vs 11.1%, p<0.0001) and longer viral shedding (17.5±5.2 days vs 12.1±4.4 days, p<0.0001). |
| Zhou et al.(April 2020)49 | Investigators studied effect of MAFLD on COVID-19 severity in older (>60 years old) vs younger patients (<60 years old). 327 adult patients with SARS-CoV-2 recruited across four hospitals in China. | In younger patients (<60 years old) with MAFLD, there was >2-fold higher prevalence of severe COVID-19 (adjusted OR, 2.67; 95% CI, 1.13–6.34; p=0.03). |
| Williamson et al.(July 2020)50 | Investigators analyzed data from OpenSAFELY, a secure database that covers 40% of all patients in England, to examine factors associated with COVID-19 related death. Records of 17,278,392 adults were linked to 10,926 COVID-19-related deaths. | Liver disease was associated with increased risk of COVID-19 related death (HR adjusted for age and sex, 2.39; 95% CI, 2.06–2.77 and HR fully adjusted, 1.75; 95% CI, 1.51–2.03). |
| Marjot et al.(Oct 2020)2 | International registry study, including 29 countries, examining impact COVID-19 on patients with CLD. The study included patients positive for SARS-CoV-2 with cirrhosis (n=386), patients positive for SARS-CoV-2 with CLD but without cirrhosis (n=359), patients positive for SARS-CoV-2 without CLD (n=620). | Overall, mortality was higher amongst patients with cirrhosis as compared to those without (32% vs 8%, p<0.001). Amongst patients with CLD, mortality increased according to Child-Pugh class: Child-Pugh A (OR, 1.90; 1.03–3.52), B (OR, 4.14; 2.4–7.65), C (OR, 9.32; 4.80–18.08). Additionally, increased age (OR, 1.02; 1.01–1.04) and ALD (OR, 1.79; 1.03–3.13) were associated with higher mortality in patients with CLD. |
| Lopez-Mendez et al.(Oct 2020)51 | Retrospective study of patients hospitalized for SARS-CoV-2 in Mexico. Outcomes studied included ICU admission and mortality. 155 Patients included, with 66 having liver steatosis. | No variables showed an independent association with clinical outcomes. |
| Ioannou et al.(Nov 2020)52 | Population-based study utilizing data collected from the Veterans Affairs (VA) national health care system. Four groups analyzed included SARS-CoV-2 negative without cirrhosis (n=75,315), SARS-CoV-2 positive without cirrhosis (n=9,826), SARS-CoV-2 negative with cirrhosis (n=3,301), SARS-CoV-2 positive with cirrhosis (n=305). | Patients with cirrhosis and positive for SARS-CoV-2 were 4.1 times more likely to receive mechanical ventilation (adjusted HR, 4.12; 95% CI, 2.79–6.10) and 3.5 times more likely to die (adjusted HR, 3.54; 95% CI, 2.55–4.90). |
| Marjot et al.(Jan 2021)53 | International registry study, including 35 countries, examining outcomes of COVID-19 in patients with AIH. Data from 932 patients with CLD and SARS-CoV-2, including 70 patients with AIH. 83% of the AIH group was taking at least one immunosuppressive drug. | No statistically significant differences in outcomes between patients with AIH and non-AIH CLD, including ICU admission (29% vs 23%, p=0.240) and death (23% vs 20%, p=0.643). Among the AIH group, use of immunosuppression not associated with increased risk of death (OR, 0.79; 95% CI, 0.10–6.25; p=0.822). |
| Butt et al.(Feb 2021)54 | Data obtained using the Electronically Retrieved Cohort of HCV infected Veterans (ERCHIVES) database. Investigated associations between HCV infection and the rates of hospitalization, ICU admission and all-cause mortality. Compared patients with chronic HCV and SARS-CoV-2 (n=975) to propensity score matched non-HCV persons with SARS-CoV-2 infection (n=975). | HCV+ patients were more likely to be hospitalized compared to HCV– (24.0% vs 18.3%, p=0.002). However, no statistically significant difference was found in regard to ICU admission and mortality between the groups (6.6% vs 6.5%, p=0.9). |
| Roca-Fernández et al. (Mar 2021)55 | Prospective cohort study using data from the UK Biobank, a large-scale biomedical database with half a million UK participants. 1,043 Patients with SARS-CoV-2 were identified. | Among SARS-CoV-2 infected patients, the presence of obesity (body mass index >30 kg/m2) and fatty liver (>10% fat on imaging) was associated with 5.31 times higher risk of being hospitalized (OR, 5.31; CI, 2.01–13.11, p=0.0006). |
| Yoo et al.(May 2021)56 | Population-based study using data from Korean National Health Insurance Service, aimed to examine association between NAFLD and COVID-19 related outcomes. Cohorts divided into three groups, based on three different definitions for classifying NAFLD. | In all three groups of patients classified with NAFLD, the risk of COVID-19 infection, risk of severe COVID-19 disease and risk of COVID-19 related mortality were all higher than those without NAFLD. For example, patients with COVID-19 and NAFLD based on hepatic steatosis index >36 had 2.22 higher risk of COVID-19 related death (adjusted OR, 2.22; 95% CI, 1.18–4.00). |
| Yip et al.(May 2021)57 | Retrospective cohort study from Hong Kong. Primary outcome mortality. 5,639 SARS-CoV-2 positive patients included, 353 patients had current HBV infection, and 359 had history of past HBV infection. | Neither current nor past HBV infection was associated with increased mortality (current HBV adjusted HR, 1.29; 95% CI, 0.61–2.70; p=0.507 and past HBV adjusted HR, 0.90; 95% CI, 0.56–1.46; p=0.681). |
| Ge et al.(July 2021)58 | Investigators utilized the National COVID cohort collaborative called N3C, a harmonized U.S. database of over 6.4 million patients, to study SARS-CoV-2 outcomes in patients with CLD and cirrhosis. Primary outcome 30-day mortality. The study included 128,864 patients with no cirrhosis/SARS-CoV-2 negative, 29,446 no cirrhosis/SARS-CoV-2 positive, 53,476 cirrhosis/SARS-CoV-2 negative, and 8,941 cirrhosis/SARS-CoV-2 positive. | 30-Day mortality was higher in patients with cirrhosis with SARS-CoV-2 compared to cirrhosis without SARS-CoV-2 (3.9%; 3.7–4.0 vs 8.9%; 8.3–9.5). In multivariable analysis, SARS-CoV-2 infection with cirrhosis was associated with 2.38 times increase in 30-day mortality (adjusted HR, 2.38; 2.18–2.59). Among patients with CLD plus SARS-CoV-2, presence of cirrhosis associated with 3.31 times increase in 30-day mortality (adjusted HR, 3.31; 2.91–3.77). |
| Kim et al.(July 2021)59 | Multicenter, observational cohort study across 21 institutions in the United States, aimed at examining predictors of mortality and risk of severe COVID-19 in patients with CLD. Included 867 patients with CLD and laboratory-confirmed SARS-CoV-2. | Factors associated with higher mortality included ALD (HR, 2.42; 95% CI, 1.29–4.55), decompensated cirrhosis (HR, 2.91; 95% CI, 1.70–5.00) and hepatocellular carcinoma (HR, 3.31; 95% CI, 1.53–7.16). |
| Jeon et al.(Aug 2021)60 | Using National Health Insurance claims data from Korea, authors examined association between cirrhosis and severe complications from COVID-19. Groups included patients with SARS-CoV-2 infection only (n=333), SARS-CoV-2 infection and cirrhosis (n=67), cirrhosis only (n=332). | The rate of severe complications was higher amongst patients with SARS-CoV-2 infection and cirrhosis as compared to SARS-CoV-2 infection only (26.9% vs 18.0%, p=0.12), though the findings were not statistically significant. |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CLD, chronic liver disease; COVID-19, coronavirus disease 2019; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval; MAFLD, metabolic associated fatty liver disease; HR, hazard ratio; ALD, alcohol-associated liver disease; ICU, intensive care unit; AIH, autoimmune hepatitis; HCV, hepatitis C virus; HBV, hepatitis B virus.
The SECURE-Liver and COVID-Hep registries provided early information on lab-confirmed COVID-19 among patients with CLD and cirrhosis.2 The published analysis included 745 CLD patients (including 386 with cirrhosis) and 620 patients without CLD diagnosed with COVID-19. Among cirrhosis patients, 90% were hospitalized, 46% developed a new liver-related decompensation, and 32% died. The most common cause of death was COVID-19 related lung disease. In multivariable models, advanced age, Child-Pugh class, and ALD were all independently associated with increased mortality. In an analysis of mortality by presence and severity of CLD using propensity score matching, mortality was significantly increased among patients with Child-Pugh B or C cirrhosis.
This early work was subsequently corroborated in larger, nationally-representative samples. Ge et al.58 assessed COVID-19 outcomes in CLD using the U.S. National COVID cohort collaborative called N3C, which is a harmonized dataset of over 6.4 million patients developed to assess COVID-19 outcomes. This study included over 220 thousand patients with CLD and cirrhosis and reported that patients with cirrhosis infected by SARS-CoV-2 had a 30-day mortality of 8.9% compared to 3.9% among cirrhosis patients without SARS-CoV-2. In a multivariable analysis, having cirrhosis and COVID-19 was associated with a 2.38 times hazard of death within 30 days (95% confidence interval, 2.18 to 2.59) compared to cirrhosis without COVID-19. Furthermore, compared to CLD patients without cirrhosis who had SARS-CoV-2, cirrhosis patients with COVID-19 had a 3.31 times adjusted hazard of death at 30 days.
Conversely, in a nationwide cohort study in Korea, patients with cirrhosis with COVID-19 were not at significantly higher risk of severe complications or death compared to non-cirrhosis patients with COVID-19.60 Given that there were only 67 patients with cirrhosis and COVID-19 and six deaths among this group, this study may not have been powered to detect a clinically important difference. While not statistically significant, compared to COVID-19 positive patients without cirrhosis, those with cirrhosis and COVID-19 had numerically higher rates of severe complications (26.9% vs 18.0%) and deaths (9.0% vs 6.9%). The authors proposed that these differences could additionally be explained by the cohort’s higher proportion of non-hospitalized COVID-19 cases, younger age and increased representation of hepatitis B, as opposed to nonalcoholic fatty liver disease (NAFLD) or ALD, as the underlying etiology of cirrhosis.
While chronic hepatitis B or C infections without cirrhosis have not been associated with COVID-19 mortality,2,54,57 the impact of underlying liver disease etiology can undoubtedly influence COVID-19 outcomes. NAFLD may be associated with poor COVID-19 outcomes given the well-documented association between metabolic risk factors such as obesity, diabetes and hypertension on COVID-19 severity.65 There has been heterogeneity in findings on studies assessing COVID-19 outcomes among patients with NAFLD, potentially due to inconsistencies in NAFLD definitions or difficulties differentiating the effects of NAFLD from underlying metabolic comorbidities. Early retrospective studies reported an association between NAFLD and SARS-CoV-2 infection risk and severe COVID-19.48,49,55 Conversely, studies of inpatients with COVID-1951 and international registry data2 did not demonstrate significant associations between NAFLD etiology and COVID-19 mortality. Newly published research utilizing two-sample Mendelian randomization analysis shows no support for causal relationships between COVID-19 severity and NAFLD.66,67 Of note, a study using the Korean National Health Insurance Service database reported an association between preexisting NAFLD with SARS-CoV-2 infection and COVID-19 severity.56
ALD, on the other hand, has been more consistently identified as a risk factor for poor outcomes from COVID-19.2,58,59 Potential reasons underlying this association include alcohol-related immune dysfunction, increased susceptibility to acute respiratory distress syndrome in those with chronic alcohol consumption or a higher burden of non-liver disease comorbidities among patients with ALD.68-75
Patients with autoimmune hepatitis (AIH) represent a potentially vulnerable subpopulation given the need for treatment with immunosuppression. Reassuringly, patients with AIH appear to be at no greater risk of ICU requirement, mechanical ventilation or death compared to matched non-autoimmune liver disease patients or non-CLD patients.53 Of note, use of immunosuppression or specific type of immunosuppression was not associated with poor outcomes.
Some studies have reported that patients with cholestatic liver disease may be at lower risk of mortality from COVID-19,58 even after controlling for potential confounders including age, sex and comorbidities. There is some indication that this association could be mediated, at least in part, through a higher utilization of ursodeoxycholic acid (UDCA) in this population.76 UDCA has been shown to downregulate ACE2 levels, reduce susceptibility to SARS-CoV-2 infection in cholangiocyte organoids and ex vivo. Furthermore, use of UDCA among patients with SARS-CoV-2 infection has been associated with lower risk of hospitalization, ICU admission and death.
While patients with CLD appear a uniquely vulnerable population, SARS-CoV-2 vaccination is an effective strategy for preventing infection, reducing the likelihood of severe infection, and improving outcomes for these individuals. Currently, there are a number of approved SARS-CoV-2 vaccinations including mRNA vaccines (Pfizer-BioNTech, Moderna), viral vector vaccines (Oxford-AstraZeneca, Janssen) and protein subunit vaccines (Novavax). Given the increased mortality rates and worse outcomes for patients with decompensated cirrhosis, as described above, the Centers for Disease Control and Prevention, American Association for the Study of Liver Diseases, Korean Association for the Study of the Liver, and European Association for the Study of the Liver all recommend that patients with cirrhosis should receive vaccination.7,77-81 Prior studies have demonstrated that vaccination was overall safe in patients with CLD, though more emerging data indicates concerns over markedly reduced or rapidly waning immune response for patients with cirrhosis.8,82-84 In addition, some concern has been raised about the possibility of vaccine induced AIH. Some case reports indicate not only a presentation of acute hepatitis following mRNA vaccination similar to AIH, but also presented liver biopsy samples from the patients showing panlobular immune infiltrate with activated cytotoxic CD8 T cells.85 Though, it remains somewhat controversial as to how clinically significant this is, as an actual rise in incidence of AIH cases has not been identified, with some studies actually showing a decrease of AIH cases even after COVID-19 vaccines had been widely available.86 Along similar lines, questions remain in regard to whether administration of COVID-19 vaccines to patients with hepatocellular carcinoma (HCC) receiving immune checkpoint inhibitors (ICIs) is safe, due to concerns of increased risk of immune-related adverse events.79 Yet, prior studies demonstrated no safety concerns with other inactive vaccines, such as influenza vaccination, in patients receiving ICIs.87 Moreover, recent prospective studies investigating cytokine release syndrome response after COVID-19 vaccination in patients receiving ICIs showed that while some cytokines may be upregulated there were no clinically relevant symptoms or increase in immune-related adverse events.88 Despite concerns, real-world data demonstrates that SARS-CoV-2 vaccination among patients with cirrhosis is associated with reduced infection, hospitalization and death.89-91 Therefore, prompt vaccination of all patients with CLD is recommended. Additional research is needed to determine duration of immunogenicity and need for additional booster doses in this patient population.92
INDIRECT IMPACTS OF THE COVID-19 PANDEMIC ON LIVER DISEASE CARE
Undoubtedly COVID-19 itself has enacted a hefty toll on patients with liver disease, which has been mitigated by the rapid development of effective vaccinations. But there have also been important indirect effects of the COVID-19 pandemic resulting in delays or missed care.
Early on in the pandemic, there was a clear reduction in necessary care. Two clear examples of these pandemic-related interruptions included variceal and HCC surveillance. Timely endoscopy procedures were delayed during the pandemic, which likely had a direct impact on surveillance of esophageal varices.93 One large retrospective study reported significant delays in endoscopic procedures, including a high proportion with a listed indication of esophageal variceal screening or surveillance. At the time of their study completion, nearly half of patients had still not had their delayed procedures completed. These concerning findings were later corroborated in a study using a large national database of endoscopic procedures.94
HCC surveillance with abdominal ultrasound with or without serum alpha-fetoprotein was also disrupted during the COVID-19 pandemic. Data from the U.S. Veterans Affairs system demonstrated clear decreases in HCC surveillance in early 2020 with a median delay of approximately 8.5 months.95 These screening delays improved somewhat after April 2020 but failed to reach pre-pandemic levels through August 2020. Another study performed within the Veterans Affairs system demonstrated that patients with a high predicted risk of developing HCC (Fibrosis-4 score ≥3.25 or estimate HCC risk ≥1.5%) were not necessarily more likely to receive surveillance in the post-COVID era.96 This study highlighted the potential role of risk stratification to identify those most likely to benefit from screening outreach. Similar delays were reported elsewhere including in a large international survey reporting significant modifications in HCC surveillance and treatment practices.97 Similarly, a large survey of 14 Asia-Pacific countries demonstrated a large decline in incident HCC cases and delays in diagnosis and treatment.98 Another indirect impact of COVID-19 that may further contribute to HCC burden is the significant slowing of HCV elimination programs. One study conducted during the pandemic estimated an additional 44,800 liver cancers and 72,300 deaths from HCV globally by 2030 due to lapses of HCV elimination efforts due to COVID-19.99
The COVID-19 pandemic may have additionally influenced patient behaviors, including the patterns of alcohol use. There were many potential contributors to changes in alcohol consumption during the pandemic including disruptions to work, psychosocial stressors, social isolation and reduced access to in-person alcohol use disorder counseling. Early data suggested an increase in alcohol sales in the United States, raising concerns for a subsequent tide of deaths from ALD.100 National death certificate data from the United States have since confirmed these fears, demonstrating dramatic increases in monthly ALD-related deaths in early 2020 with the highest relative increases among females and younger adults.5 Reassuringly, in Korea, data from a large online survey did not show dramatic changes in overall alcohol consumption patterns.101 However, compared to individuals age 60 or older, individuals age 20 to 29 were significantly more likely to report increased alcohol use. Similar to the United States, these younger patients may be at higher risk of liver-related complications of high-risk alcohol use including alcohol-related hepatitis. Improved identification of alcohol use disorder and linkage to guideline-recommended care may help mitigate these downstream effects.
Expansion of telehealth services was one potentially positive innovation that arose out of necessity during the COVID-19 pandemic to address alcohol use disorder and liver disease in general. The use of telephone and video services allowed for timely delivery of care during a time when in-person clinic visits may have imperiled vulnerable patients. Early in the pandemic, telehealth was rapidly adopted in many health systems and was generally well-accepted by patients.102 Similar increases in telehealth use, particularly during COVID-19 surges, were reported in Korea.103 Increased experience with telehealth has led some to advocate for better incorporation of these services into routine care, which could help reduce costs and minimize delays in care. However, potential disparities in telehealth utilization by race, ethnicity, insurance coverage, or socioeconomic status have highlighted the potential need for tailored strategies for vulnerable populations.102-104
CONCLUSIONS
The ongoing COVID-19 pandemic has had immeasurable effects on individuals worldwide. COVID-19 has several extrapulmonary effects, including on the liver, and some data suggest this may be mediated by hepatotropism of SARS-CoV-2. Emerging cases of post-COVID-19 cholangiopathy and persistent cholestasis have raised concerns of long-term, direct liver damage of SARS-CoV-2 infection. High morbidity and mortality of COVID-19 among patients with cirrhosis, and particularly decompensated disease, has clearly demonstrated the vulnerability of these patients. Furthermore, patients with cirrhosis and CLD remain susceptible to significant health consequences borne of pandemic-related delays in care and increases in alcohol use. Undoubtedly, the rapid development and distribution of highly effective COVID-19 vaccinations and therapeutics have significantly mitigated the harmful effects of the pandemic. While much work is ongoing, the lessons of this pandemic for patients with CLD and cirrhosis will be instructive for subsequent waves of COVID-19 and future pandemics.
ACKNOWLEDGEMENTS
T.M. receives funding via a Wellcome Trust Clinical Research Training Fellowship (ref. 102176/B/13/Z) and has received registry grant funding from the European Association for the Study of the Liver (ref. 2020RG03). E.B. is funded by the Oxford NIHR Biomedical Research Centre andis an NIHR Senior Investigator. A.M.M. was supported by an Advanced/Transplant Hepatology Grant from the American Association for the Study of Liver Diseases (AASLD) Foundation. The SECURE-Liver registry was supported by the AASLD.
Footnotes
CONFLICTS OF INTEREST
A.M.M. consulted for Target RWE. A.S.B. consulted for Target RWE and Sarepta INC., served on the DSMB for Pfizer and did medical writing for Novo Nordisk. Except for that, no potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marjot T, Moon AM, Cook JA, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moon AM, Webb GJ, Aloman C, et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020;73:705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2017 Cirrhosis Collaborators, author. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutsch-Link S, Jiang Y, Peery AF, Barritt AS, Bataller R, Moon AM. Alcohol-associated liver disease mortality increased from 2017 to 2020 and accelerated during the COVID-19 pandemic. Clin Gastroenterol Hepatol. 2022;20:2142–2144.e2. doi: 10.1016/j.cgh.2022.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fix OK, Blumberg EA, Chang KM, et al. American Association for the Study of Liver Diseases expert panel consensus statement: vaccines to prevent coronavirus disease 2019 infection in patients with liver disease. Hepatology. 2021;74:1049–1064. doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willuweit K, Frey A, Passenberg M, et al. Patients with liver cirrhosis show high immunogenicity upon COVID-19 vaccination but develop premature deterioration of antibody titers. Vaccines (Basel) 2022;10:377. doi: 10.3390/vaccines10030377.13e4379f41c342a3aa1bf2b7081c383b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 10.Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal liver tests in COVID-19: a retrospective observational cohort study of 1,827 patients in a major U.S. hospital network. Hepatology. 2020;72:1169–1176. doi: 10.1002/hep.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bangash MN, Patel JM, Parekh D, et al. SARS-CoV-2: is the liver merely a bystander to severe disease? J Hepatol. 2020;73:995–996. doi: 10.1016/j.jhep.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao R, Qiu Y, He JS, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 15.Cai Q, Huang D, Yu H, et al. COVID-19: abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20–32. doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marjot T, Webb GJ, Barritt AS, 4th, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348–364. doi: 10.1038/s41575-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartl L, Haslinger K, Angerer M, et al. Progressive cholestasis and associated sclerosing cholangitis are frequent complications of COVID-19 in patients with chronic liver disease. Hepatology. 2022;76:1563–1575. doi: 10.1002/hep.32582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon AM, Barritt AS., 4th Elevated liver enzymes in patients with COVID-19: look, but not too hard. Dig Dis Sci. 2021;66:1767–1769. doi: 10.1007/s10620-020-06585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balderramo D, Mattos AZ, Mulqui V, et al. Abnormal liver tests during hospitalization predict mortality in patients with COVID-19: a multicenter study from South America. Can J Gastroenterol Hepatol. 2021;2021:1622533. doi: 10.1155/2021/1622533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yip TC, Lui GC, Wong VW, et al. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70:733–742. doi: 10.1136/gutjnl-2020-321726. [DOI] [PubMed] [Google Scholar]
- 22.Lei F, Liu YM, Zhou F, et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020;72:389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloom PP, Meyerowitz EA, Reinus Z, et al. Liver biochemistries in hospitalized patients with COVID-19. Hepatology. 2021;73:890–900. doi: 10.1002/hep.31326. [DOI] [PubMed] [Google Scholar]
- 24.Fu Y, Zhu R, Bai T, et al. Clinical features of patients infected with coronavirus disease 2019 with elevated liver biochemistries: a multicenter, retrospective study. Hepatology. 2021;73:1509–1520. doi: 10.1002/hep.31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song JE, Kang MK, Lee YR, et al. Multicenter analysis of clinical features and prognosis of COVID-19 patients with hepatic impairment. Gut Liver. 2021;15:606–615. doi: 10.5009/gnl20267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber S, Hellmuth JC, Scherer C, Muenchhoff M, Mayerle J, Gerbes AL. Liver function test abnormalities at hospital admission are associated with severe course of SARS-CoV-2 infection: a prospective cohort study. Gut. 2021;70:1925–1932. doi: 10.1136/gutjnl-2020-323800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T, Smith DA, Campbell C, et al. Longitudinal analysis of the utility of liver biochemistry as prognostic markers in hospitalized patients with corona virus disease 2019. Hepatol Commun. 2021;5:1586–1604. doi: 10.1002/hep4.1739.2afadca7b5a847cd9da8894149e8af77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faruqui S, Okoli FC, Olsen SK, et al. Cholangiopathy after severe COVID-19: clinical features and prognostic implications. Am J Gastroenterol. 2021;116:1414–1425. doi: 10.14309/ajg.0000000000001264. [DOI] [PubMed] [Google Scholar]
- 30.Roth NC, Kim A, Vitkovski T, et al. Post-COVID-19 cholangiopathy: a novel entity. Am J Gastroenterol. 2021;116:1077–1082. doi: 10.14309/ajg.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 31.Edwards K, Allison M, Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS-CoV-2 infection. BMJ Case Rep. 2020;13:e237984. doi: 10.1136/bcr-2020-237984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laurent L, Lemaitre C, Minello A, et al. Cholangiopathy in critically ill patients surviving beyond the intensive care period: a multicentre survey in liver units. Aliment Pharmacol Ther. 2017;46:1070–1076. doi: 10.1111/apt.14367. [DOI] [PubMed] [Google Scholar]
- 33.Bütikofer S, Lenggenhager D, Wendel Garcia PD, et al. Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID-19. Liver Int. 2021;41:2404–2417. doi: 10.1111/liv.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 35.Da BL, Kushner T, El Halabi M, et al. Liver injury in patients hospitalized with coronavirus disease 2019 correlates with hyperinflammatory response and elevated interleukin-6. Hepatol Commun. 2020;5:177–188. doi: 10.1002/hep4.1631.2d39791815eb4dffac41371fe4e0217f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le Louët H. Drug-induced liver injury and COVID-19 infection: the rules remain the same. Drug Saf. 2020;43:615–617. doi: 10.1007/s40264-020-00954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231–1240. doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lagana SM, Kudose S, Iuga AC, et al. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147–2155. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Díaz LA, Idalsoaga F, Cannistra M, et al. High prevalence of hepatic steatosis and vascular thrombosis in COVID-19: a systematic review and meta-analysis of autopsy data. World J Gastroenterol. 2020;26:7693–7706. doi: 10.3748/wjg.v26.i48.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohammed SA, Eid KM, Anyiam FE, et al. Liver injury with COVID-19: laboratory and histopathological outcome-systematic review and meta-analysis. Egypt Liver J. 2022;12:9. doi: 10.1186/s43066-022-00171-6.b4c4d519706a471682f5a7b1e18f5d0d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonzogni A, Previtali G, Seghezzi M, et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038–2040. doi: 10.1111/liv.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Liu S, Liu H, et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wanner N, Andrieux G, Badia-I-Mompel P, et al. Molecular consequences of SARS-CoV-2 liver tropism. Nat Metab. 2022;4:310–319. doi: 10.1038/s42255-022-00552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnes E. Infection of liver hepatocytes with SARS-CoV-2. Nat Metab. 2022;4:301–302. doi: 10.1038/s42255-022-00554-4. [DOI] [PubMed] [Google Scholar]
- 48.Ji D, Qin E, Xu J, et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou YJ, Zheng KI, Wang XB, et al. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: a multicenter preliminary analysis. J Hepatol. 2020;73:719–721. doi: 10.1016/j.jhep.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using opensafely. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez-Mendez I, Aquino-Matus J, Gall SM, et al. Association of liver steatosis and fibrosis with clinical outcomes in patients with SARS-CoV-2 infection (COVID-19) Ann Hepatol. 2021;20:100271. doi: 10.1016/j.aohep.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ioannou GN, Liang PS, Locke E, et al. Cirrhosis and severe acute respiratory syndrome coronavirus 2 infection in US veterans: risk of infection, hospitalization, ventilation, and mortality. Hepatology. 2021;74:322–335. doi: 10.1002/hep.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marjot T, Buescher G, Sebode M, et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74:1335–1343. doi: 10.1016/j.jhep.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Butt AA, Yan P, Chotani RA, Shaikh OS. Mortality is not increased in SARS-CoV-2 infected persons with hepatitis C virus infection. Liver Int. 2021;41:1824–1831. doi: 10.1111/liv.14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roca-Fernández A, Dennis A, Nicholls R, et al. Hepatic steatosis, rather than underlying obesity, increases the risk of infection and hospitalization for COVID-19. Front Med (Lausanne) 2021;8:636637. doi: 10.3389/fmed.2021.636637.d29bb3e833174b098ff1f0df9357effe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoo HW, Shin JI, Yon DK, Lee SW. COVID-19 morbidity and severity in patients with nonalcoholic fatty liver disease in South Korea: a nationwide cohort study. Clin Gastroenterol Hepatol. 2022;20:e1217–e1218. doi: 10.1016/j.cgh.2021.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yip TC, Wong VW, Lui GC, et al. Current and past infections of HBV do not increase mortality in patients with COVID-19. Hepatology. 2021;74:1750–1765. doi: 10.1002/hep.31890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ge J, Pletcher MJ, Lai JC N3C Consortium, author. Outcomes of SARS-CoV-2 infection in patients with chronic liver disease and cirrhosis: a national COVID cohort collaborative study. Gastroenterology. 2021;161:1487–1501.e5. doi: 10.1053/j.gastro.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim D, Adeniji N, Latt N, et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study. Clin Gastroenterol Hepatol. 2021;19:1469–1479.e19. doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeon D, Son M, Choi J. Impact of liver cirrhosis on the clinical outcomes of patients with COVID-19: a nationwide cohort study of Korea. Korean J Intern Med. 2021;36:1092–1101. doi: 10.3904/kjim.2020.486.1dac17e14407419bba2746a3311c42a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pose E, Coll M, Martínez-Sánchez C, et al. Programmed death ligand 1 is overexpressed in liver macrophages in chronic liver diseases, and its blockade improves the antibacterial activity against infections. Hepatology. 2021;74:296–311. doi: 10.1002/hep.31644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piano S, Brocca A, Mareso S, Angeli P. Infections complicating cirrhosis. Liver Int. 2018;38 Suppl 1:126–133. doi: 10.1111/liv.13645. [DOI] [PubMed] [Google Scholar]
- 63.Schütte A, Ciesek S, Wedemeyer H, Lange CM. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatol. 2019;70:797–799. doi: 10.1016/j.jhep.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 64.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kompaniyets L, Pennington AF, Goodman AB, et al. Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020-March 2021. Prev Chronic Dis. 2021;18:E66. doi: 10.5888/pcd18.210123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J, Tian A, Zhu H, et al. Mendelian randomization analysis reveals no causal relationship between nonalcoholic fatty liver disease and severe COVID-19. Clin Gastroenterol Hepatol. 2022;20:1553–1560.e78. doi: 10.1016/j.cgh.2022.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu D, Zhang Q, Bai P, Zhao J. Assessing causal relationships between COVID-19 and non-alcoholic fatty liver disease. J Hepatol. 2022;76:740–742. doi: 10.1016/j.jhep.2021.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szabo G, Saha B. Alcohol's effect on host defense. Alcohol Res. 2015;37:159–170. [PMC free article] [PubMed] [Google Scholar]
- 69.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simou E, Leonardi-Bee J, Britton J. The effect of alcohol consumption on the risk of ARDS: a systematic review and meta-analysis. Chest. 2018;154:58–68. doi: 10.1016/j.chest.2017.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50–54. doi: 10.1001/jama.1996.03530250054027. [DOI] [PubMed] [Google Scholar]
- 72.Moss M, Parsons PE, Steinberg KP, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31:869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 73.Boé DM, Vandivier RW, Burnham EL, Moss M. Alcohol abuse and pulmonary disease. J Leukoc Biol. 2009;86:1097–1104. doi: 10.1189/jlb.0209087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fan AZ, Russell M, Naimi T, et al. Patterns of alcohol consumption and the metabolic syndrome. J Clin Endocrinol Metab. 2008;93:3833–3838. doi: 10.1210/jc.2007-2788. [DOI] [PubMed] [Google Scholar]
- 75.Shankar A, Klein R, Klein BE. The association among smoking, heavy drinking, and chronic kidney disease. Am J Epidemiol. 2006;164:263–271. doi: 10.1093/aje/kwj173. [DOI] [PubMed] [Google Scholar]
- 76.Brevini T, Maes M, Webb GJ, et al. FXR inhibition reduces ACE2 expression, SARS-CoV-2 infection and may improve COVID-19 outcome. [cited 2022 Jul 24];bioRxiv: 2021.06.06.446781 [Preprint] 2021 doi: 10.1101/2021.06.06.446781. Available from: https://doi.org/10.1101/2021.06.06.446781 . [DOI] [Google Scholar]
- 77.Centers for Disease Control and Prevention (CDC), author Stay up to date with your COVID-19 vaccines including boosters [Internet] CDC; Atlanta: c2022. [cited 2022 Jul 24]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html#print . [Google Scholar]
- 78.National Center for Immunization and Respiratory Diseases (NCIRD), author Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. NCIRD; Atlanta: c2022. [cited 2022 Jul 24]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html . [Google Scholar]
- 79.Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cho JY, Lee YS, Kim SS, Song DS, Lee JH, Kim JH. Update on liver disease management during the pandemic of coronavirus disease 2019 (COVID-19): 2021 KASL guideline. Clin Mol Hepatol. 2021;27:515–523. doi: 10.3350/cmh.2021.0293.c2163dc131a045ceab250f4e9c82726d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cho JY, Kim SS, Lee YS, Song DS, Lee JH, Kim JH. Management of liver diseases during the pandemic of coronavirus disease-19. Clin Mol Hepatol. 2020;26:243–250. doi: 10.3350/cmh.2020.0111.37f16b05ea6343b9b69cc1110b4ac337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J, Hou Z, Liu J, et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol. 2021;75:439–441. doi: 10.1016/j.jhep.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang J, Zhang Q, Ai J, et al. Safety and immunogenicity of SARS-CoV-2 vaccines in Chinese patients with cirrhosis: a prospective multicenter study. Hepatol Int. 2022;16:691–701. doi: 10.1007/s12072-022-10332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marjot T, Murray S, Pose E, et al. Humoral and cellular immune responses to SARS-CoV-2 vaccination across multiple vaccine platforms and liver disease types: an EASL registry multicentre prospective cohort study. J Hepatol. 2022;77:S54–S55. doi: 10.1016/S0168-8278(22)00515-3. [DOI] [Google Scholar]
- 85.Boettler T, Csernalabics B, Salié H, et al. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J Hepatol. 2022;77:653–659. doi: 10.1016/j.jhep.2022.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rüther DF, Weltzsch JP, Schramm C, Sebode M, Lohse AW. Autoimmune hepatitis and COVID-19: no increased risk for AIH after vaccination but reduced care. J Hepatol. 2022;77:250–251. doi: 10.1016/j.jhep.2022.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chong CR, Park VJ, Cohen B, Postow MA, Wolchok JD, Kamboj M. Safety of inactivated influenza vaccine in cancer patients receiving immune checkpoint inhibitors. Clin Infect Dis. 2020;70:193–199. doi: 10.1093/cid/ciz202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walle T, Bajaj S, Kraske JA, et al. Cytokine release syndrome-like serum responses after COVID-19 vaccination are frequent and clinically inapparent under cancer immunotherapy. Nat Cancer. 2022;3:1039–1051. doi: 10.1038/s43018-022-00398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moon AM, Webb GJ, García-Juárez I, et al. SARS-CoV-2 infections among patients with liver disease and liver transplantation who received COVID-19 vaccination. Hepatol Commun. 2022;6:889–897. doi: 10.1002/hep4.1853.bc19c05fc26b4f4fba700019feffbd42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.John BV, Deng Y, Scheinberg A, et al. Association of BNT162b2 mRNA and mRNA-1273 vaccines with COVID-19 infection and hospitalization among patients with cirrhosis. JAMA Intern Med. 2021;181:1306–1314. doi: 10.1001/jamainternmed.2021.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.John BV, Sidney Barritt A, 4th, Moon A, et al. Effectiveness of COVID-19 Viral Vector Ad.26.COV2.S vaccine and comparison with mRNA vaccines in cirrhosis. Clin Gastroenterol Hepatol. 2022;20:2405–2408. doi: 10.1016/j.cgh.2022.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marjot T, Webb GJ, Barritt AS, et al. SARS-CoV-2 vaccination in patients with liver disease: responding to the next big question. Lancet Gastroenterol Hepatol. 2021;6:156–158. doi: 10.1016/S2468-1253(21)00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Issaka RB, Feld LD, Kao J, et al. Real-world data on the impact of COVID-19 on endoscopic procedural delays. Clin Transl Gastroenterol. 2021;12:e00365. doi: 10.14309/ctg.0000000000000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Calderwood AH, Calderwood MS, Williams JL, Dominitz JA. Impact of the COVID-19 pandemic on utilization of EGD and colonoscopy in the United States: an analysis of the GIQuIC registry. Tech Innov Gastrointest Endosc. 2021;23:313–321. doi: 10.1016/j.tige.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mahmud N, Kaplan DE, Goldberg DS, Taddei TH, Serper M. Changes in hepatocellular carcinoma surveillance and risk factors for noncompletion in the Veterans Health Administration cohort during the coronavirus disease 2019 pandemic. Gastroenterology. 2021;160:2162–2164.e3. doi: 10.1053/j.gastro.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim NJ, Rozenberg-Ben-Dror K, Jacob DA, Berry K, Ioannou GN. The COVID-19 pandemic highlights opportunities to improve hepatocellular carcinoma screening and diagnosis in a national health system. Am J Gastroenterol. 2022;117:678–684. doi: 10.14309/ajg.0000000000001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muñoz-Martínez S, Sapena V, Forner A, et al. Assessing the impact of COVID-19 on liver cancer management (CERO-19) JHEP Rep. 2021;3:100260. doi: 10.1016/j.jhepr.2021.100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gandhi M, Ling WH, Chen CH, et al. Impact of COVID-19 on hepatocellular carcinoma management: a multicountry and region study. J Hepatocell Carcinoma. 2021;8:1159–1167. doi: 10.2147/JHC.S329018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blach S, Kondili LA, Aghemo A, et al. Impact of COVID-19 on global HCV elimination efforts. J Hepatol. 2021;74:31–36. doi: 10.1016/j.jhep.2020.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moon AM, Curtis B, Mandrekar P, Singal AK, Verna EC, Fix OK. Alcohol-associated liver disease before and after COVID-19: an overview and call for ongoing investigation. Hepatol Commun. 2021;5:1616–1621. doi: 10.1002/hep4.1747.a5482d8e679f4af89ffc28c9bb555a74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang E, Lee H, Sohn JH, Yun J, Lee JY, Hong YC. Impact of the COVID-19 pandemic on the health status and behaviors of adults in Korea: national cross-sectional web-based self-report survey. JMIR Public Health Surveill. 2021;7:e31635. doi: 10.2196/31635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Serper M, Nunes F, Ahmad N, Roberts D, Metz DC, Mehta SJ. Positive early patient and clinician experience with telemedicine in an academic gastroenterology practice during the COVID-19 pandemic. Gastroenterology. 2020;159:1589–1591.e4. doi: 10.1053/j.gastro.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim HS, Kim B, Lee SG, Jang SY, Kim TH. COVID-19 case surge and telemedicine utilization in a tertiary hospital in Korea. Telemed J E Health. 2022;28:666–674. doi: 10.1089/tmj.2021.0157. [DOI] [PubMed] [Google Scholar]
- 104.Wegermann K, Wilder JM, Parish A, et al. Racial and socioeconomic disparities in utilization of telehealth in patients with liver disease during COVID-19. Dig Dis Sci. 2022;67:93–99. doi: 10.1007/s10620-021-06842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]