Abstract
The objective of the following study was to investigate the effects of naturally oxidized corn oil on the antioxidant capacity and lipid metabolism of broilers. A total of 450, 1-day-old Arbor Acres male broilers were randomly divided into 5 treatments with 6 replicate cages and 15 birds/cage. The dietary treatment array consisted of ratios of naturally oxidized corn oil to non-oxidized corn oil from 0:100, 25:75, 50:50, 75:25, and 100:0, respectively. Serum, liver, and abdominal fat samples were taken at 42 d. The results showed that the liver organ index, liver catalase (CAT) activity, malondialdehyde (MDA) content, and the serum aspartate aminotransferase (AST) content had significant quadratic relationships with the ratio of naturally oxidized corn oil (P < 0.05). Inflammatory infiltrating cells appeared in the liver of the 50% and 75% oxidized corn oil group. The percentage of abdominal fat, and serum free fatty acids (FFA) content increased linearly with the increased proportion of oxidized corn oil (P < 0.05). The mRNA expression of NADH quinone oxidoreductase 1 (NQO-1), nuclear factor kappa B (NF-κB), toll-like receptor-4 (TLR-4), peroxisome proliferators activate receptor-α (PPARα), carnitine acyltransferase (CPT1), and acyl-coenzyme oxidase (ACO) of the liver increased linearly while oxidized corn oil increased in the diet (P < 0.05). Diets containing 100% oxidized corn oil significantly changed the mRNA expression of liver Caveolin compared with other treatment groups (P < 0.05). Taken together, this study demonstrated that naturally oxidized corn oil could change liver lipid metabolism and accelerate lipid deposition of broilers by upregulating PPARα.
Key words: oxidized oil, liver, antioxidation, lipid metabolism, broiler chicken
INTRODUCTION
Oil is typically used in the diet to partially fulfill the energy requirements of poultry. However, vegetable oil is rich in polyunsaturated fatty acids, which are easily oxidized and broken-down into small molecular acids, aldehydes, and ketones during feed storage (Grotto et al., 2009; Vieira et al., 2017); however, this process might be altered differently from the effects of artificial oxidation (Taghvaei and Jafari, 2015). In addition, aged corn is popularly used as an animal feed ingredient in China and other areas of the world. The issues with aged corn could be related to the natural oxidation of corn oil (Winkler-Moser and Breyer, 2011). These oxidation products are potentially toxic and can promote the production of many free radicals in poultry (Ehr et al., 2015), disrupt the normal metabolic processes, and impair the physiological and biochemical functions of cells (Liang et al., 2015; Dong et al., 2020).
As the center of metabolism and detoxification of various nutrients, the liver plays an important role in lipid metabolism, and is considered the main site of fat synthesis in poultry (Nguyen et al., 2008). However, oxidized oil generates high free fatty acids in serum, which increases the de novo synthesis of liver fatty acids and leads to abnormal accumulation of liver fat, thus exacerbating liver lipid metabolism (Yue et al., 2011). Meanwhile, the abdominal adipose tissue of poultry mainly comes from the production and metabolism of fatty acids in the liver (Wang et al., 2017). Lipid oxidation also produces a large number of reactive oxygen species (ROS), and excessive ROS can severely damage the antioxidant systems in the liver (Ju et al., 2019). Moreover, malondialdehyde (MDA) is a major product of lipid peroxidation produced by free radicals, and attacks the cell membrane and interacts with the peroxisome proliferators activate receptor-α (PPARα) pathway of lipid metabolism (Lv et al., 2018). PPARα mainly regulates the expression of fatty acid oxidation-related genes in the liver and is the primary regulator of fatty acid oxidation (Ament et al., 2016). Importantly, the NF-κB pathway of the inflammatory response and the Nrf2-Keap1 pathway of antioxidants also interact with the PPARα pathway (Agca et al., 2014).
Many publish papers have studied the effects of oxidized vegetable oil on animals by artificial oxidation using high temperatures, however, vegetable oil is easily oxidized during transport and storage. This process could be better characterized as natural oxidation, and its products may have different effects on animals relative to those of artificially oxidized oil products. However, the effects of naturally oxidized oil on liver lipid metabolism are unknown. Therefore, naturally oxidized corn oil was used in this experiment to investigate the antioxidant capacity and liver lipid metabolism of broilers, providing insight into the potential usage of oxidized oil in poultry production.
MATERIALS AND METHODS
Ethics Statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of China Agricultural University and approved by the Animal Ethics Committee of China Agricultural University (approval number: AW04129102-1-1), and all experiments followed institutional guidelines.
Preparation of Oxidized Oil
Fresh corn oil was purchased from a crude oil manufacturing facility. Part of the corn oil was stored in an open container outdoors for 60 d to create the naturally oxidized oil. The temperature during this period was 30°C to 40°C, and the average light intensity was approximately 1,000 Lux. The other was stored in a closed container indoors at a temperature 20°C to 30°C. The acid values of the non-oxidized oil and oxidized oil were 3.02 mg/g and 5.74 mg/g, respectively. The acid value was determined as FFA amount in lipids that depended on the titration with a methanolic potassium hydroxide solution versus phenolphthalein as an endpoint indicator (AOCS, 2004).
Birds, Diets, and Experimental Design
A total of 450, 1-day-old male Arbor Acres broilers were randomly assigned to 5 dietary treatments (6 replicate cages with 15 birds each). The basal diet was formulated to meet or exceed the nutrient requirement for broilers recommended by NRC (1994). The ingredient and nutrient compositions of the basal diets are shown in Table 1. The treatment groups were 0%, 25%, 50%, 75%, and 100% naturally oxidized oil in place of non-oxidized oil, respectively. The experimental period was 42 d. The starter diet was pelleted and crumbled, whereas the finisher diet was fed in pelleted form. Water was provided ad libitum using a nipple-type drinker. The room temperature was maintained at 33-35°C during the first 3 d, followed by a reduction to 28°C to 30°C during the next 2 wk and 22°C to 25°C for the remainder of the trial. A standard lighting regime was followed: 23 h of light and 1 h of darkness for the first 8 d, followed by 20 h of light and 4 h of darkness from 9 d of age until the end of the trial.
Table 1.
Ingredients and composition of the basal experimental diets.
| Ingredients, % | Starter diet | Finisher diet |
|---|---|---|
| Corn | 51.50 | 56.60 |
| Soybean meal | 30.58 | 26.10 |
| Corn DDGS | 5.00 | 5.00 |
| Corn gluten meal | 4.00 | 4.00 |
| Corn oil1 | 2.70 | 4.70 |
| Wheat flour | 2.00 | 0.00 |
| Dicalcium phosphate | 1.64 | 1.20 |
| Limestone | 1.27 | 1.20 |
| Salt | 0.35 | 0.35 |
| Trace mineral premix2 | 0.20 | 0.2 |
| Vitamin premix3 | 0.03 | 0.02 |
| Choline chloride (50%) | 0.20 | 0.20 |
| DL-Methionine | 0.24 | 0.20 |
| L-Lysine sulfate | 0.26 | 0.20 |
| Antioxidant | 0.02 | 0.02 |
| Phytase | 0.01 | 0.01 |
| Nutrient composition, %4 | ||
| ME (kcal/kg) | 2950 | 3146 |
| CP, % | 22.50 | 20.50 |
| Lysine, % | 1.30 | 1.12 |
| Methionine, % | 0.59 | 0.52 |
| Methionine+Cysteine, % | 0.94 | 0.85 |
| Calcium, % | 1.00 | 0.80 |
| NPP, % | 0.45 | 0.32 |
The same percentage of corn oil was used in different treatment groups. The treatment groups were as follows: the ratio of non-oxidized corn oil to naturally oxidized oil was 0:100, 25:75, 50:50, 75:25, 100:0, respectively.
The trace mineral premix provided the following per kg of diets: Cu,16 mg (as CuSO4·5H2O); Zn, 110 mg (as ZnSO4); Fe, 80 mg (as FeSO4·H2O); Mn, 120 mg (as MnO); Se, 0.3 mg (as Na2SeO3); I, 1.5 mg(as KI); Co, 0.5 mg.
The vitamin premix provided the following per kg of diets: vitamin A, 10 000 IU; vitamin D3, 2400 IU; vitamin E, 20 mg; vitamin K3, 2 mg; vitamin B1, 2 mg; vitamin B2, 6.4 mg; VB6, 3 mg;VB12, 0.02 mg; biotin, 0.1 mg; folic acid, 1 mg; pantothenic acid,10 mg; nicotinamide, 30 mg.
All the nutrient levels are calculated values.
Sample Collection
On day 42, 6 birds (one bird per cage) of each treatment were randomly selected and weighed. A 4-mL blood sample was collected from the wing vein of the broiler and was placed in a coagulation tube. After centrifugation at 3,000 × g at 4°C for 10 min, serum was separated and stored at −20°C.
Chickens were killed by intracardial administration of sodium pentobarbital (30 mg/kg of body weight). The liver and abdominal fat were collected and weighed. Then, 1g liver was collected snap-frozen in liquid nitrogen and stored at -80°C for subsequent analysis. 1cm2 of liver from the liver tip was removed and fixed in a 4% paraformaldehyde solution. After tissue sections were prepared and stained with eosin–hematoxylin (HE), the inflammatory cell infiltration was observed by the OLYMPUS BX-41TF microscope at 400×.
Antioxidant Capacity of the Liver
Tissue samples from the liver (0.1g) were added into 0.9% normal saline based on the ratio of 1:9 (mass g/volume mL). The homogenate was centrifuged at 3,000 × g for 10 min at 4°C, and the supernatant was stored at −20°C for further analysis. The total protein content was determined by the BCA method. The activities of glutathione peroxidase (GSH-Px, cat# A005-1), superoxide dismutase (SOD, cat# A001-3), catalase (CAT, cat# A007-1), and MDA (cat# A003-1) in the liver were determined using commercial analytical kits according to the manufacturer's recommendations (JianCheng Bioengineering Institute, Nanjing, China).
Serum Lipid Metabolism Index
Aspartate aminotransferase (AST, cat# JH-00020), alanine aminotransferase (ALT, cat# JH-00019), total cholesterol (TC, cat# JH-00028), triglyceride (TG, cat# JH-00029), high-density lipoprotein (HDL, cat# JH-00030), low-density lipoprotein (LDL, cat# JH-00031) and free fatty acid (FFA, cat# JH-00116) contents were determined using commercial analytical kits according to the manufacturer's recommendations (Jinhai Keyu Biotechnology Co., Ltd., Beijing, China).
RNA Isolation and Quantitative Reverse Transcription Polymerase Chain Reaction
The RNA was extracted from the liver using the Eastep Super Total RNA Extraction Kit (Promaga Co., Shanghai, China). RNA quantity was measured using the Nanodrop (Thermo Fisher, Waltham, MA). Then, total RNA was reversed transcribed to cDNA using the PrimeScrip RT reagent Kit with gDNA Eraser Perfect Real-Time (Takara Biomedical Technology, Beijing, China), and the gene expression levels were determined using the SYBR Premix Ex Taq Tli RNaseH Plus (Takara Biomedical Technology, Beijing, China) according to the product protocols. Primer sequences of the β-actin, catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), heme oxygenase-1 (HO-1), NADH quinone oxidoreductase 1 (NQO-1), nuclear factor erythroid related factor 2 (Nrf2), kelch-like epichlorohydrin associated protein-1 (Keap-1), nuclear factor kappa B (NF-κB), toll-like receptor-4 (TLR-4), PPARα, acyl coenzyme oxidase (ACO), carnitine acyltransferase (CPT1), fatty acid transporter (FATP), fatty acid-binding protein (FABP), fatty acid transferase (CD36), and caveolin in the liver are listed in Table 2. All the gene sequences were quoted from NCBI. All the measurements were carried out in triplicate (n = 6, the cage was used as the experimental unit), and the average values were calculated. Relative expression levels of different genes were normalized to the expression of β-actin using the 2 −∆∆CT method.
Table 2.
Primers used in real-time quantitative PCR.
| Gene | Gene bank ID | Primer sequence (5’-3′) |
|---|---|---|
| β-actin | NM_205518.1 | F: CCACCGCAAATGCTTCTAAAC R: AAGACTGCTGCTGACACCTTC |
| CAT | NM_001031215.2 | F: TCAGAGGGACGGGCCAATGTG R: CTGAAACGGACATGCGGCTCTC |
| SOD | NM_205064.1 | F: GGCAAGCAGCACGGTGGACR: CTTCTGCCACTCCTCCCTTTGC |
| GSH-Px | NM_001277853.1 | F: GACCAACCCGCAGTACATCA R: GAGGTGCGGGCTTTCCTTTA |
| HO-1 | NM_205344.1 | F: GGCAGAGCTTCGCACAAGGAG R: CCACCGCACCAGGGGAGAG |
| NQO-1 | NM_001277619.1 | F: AACCTCTTTCAACCACGCCA R: TTCTTGAGGGGTCCGGTGAT |
| Nrf2 | NM_001007858.1 | F: GAGCCCATGGCCTTTCCTAT R: CACAGAGGCCCTGACTCAAA |
| Keap-1 | KU321503.1 | F: GGTCGCAGTGCTGAACATACT R: CCATGGCGTAGATGCAGTTG |
| NF-κB | NM_205129.1 | F: ACCCCTTCAATGTGCCAATG R: TCAGCCCAGAAACGAACCTC |
| TLR-4 | NM_001030693.1 | F: GGATCTTTCAAGGTGCCACA R: CAAGTGTCCGATGGGTAGGT |
| PPARα | NM_001001464.1 | F: ACCTTGTGCATGGCAGAGAA R: TACACTGGCAGCAGTGGAAG |
| ACO | XM_015295164.2 | F: GATTTTTTGCAGGCGGGT R: CACACGCTGGTTCACCTGAGT |
| CPT1 | XM_025150696.1 | F: TGGCTGATGATGGTTACGGTG R: TTCCAAAGCGATGAGAATCCGTT |
| FATP | XM_015279553.2 | F: GCTTCCATCTGGCGAGAGTT R: CCACAGGACCCGACGTTATT |
| FABP | NM_001007923.1 | F: TGGAAGCAATGGGCGTGAAT R: TCGATGTCGATGGTACGGAA |
| CD36 | XM_025147449.1 | F: AAGAAGGGAGACTGAGCTTCTC R: TCCTTTGACAGGGTGCAGAC |
| Caveolin | NM_001105664.2 | F: GACGACGTGGTGAAGATTGA R: AACTGGCCTTCCAAATCCCAT |
Abbreviations: F, forward; R, reverse.
Statistical Analysis
Data were statistically analyzed by one-way ANOVA analysis using SPSS 20.0 for Windows (SPSS Inc., Chicago, IL). All data were tested for homogeneity of variances using Levene's test. Different proportions of naturally oxidized corn oil in the basal diet were analyzed to test for linear or quadratic responses. The significance among the groups was identified using the Duncan test for multiple comparisons. Results were presented as mean values with pooled standard errors. A P value of < 0.05 was considered to be statistically significant. The regression equation was calculated by Microsoft Excel (2016).
RESULTS
Effects of Different Proportions of Naturally Oxidized Corn Oil on Liver Organ Index, Histomorphology, and Abdominal Fat Rate of Broilers
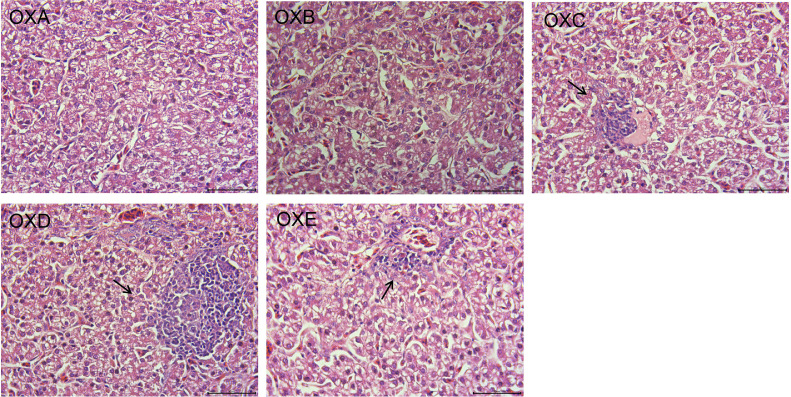
The liver organ index (y = −13.72x2 + 15.04x + 17.78, R2 = 0.859, P < 0.05) showed a significant quadratic relationship with the increasing ratio of oxidized corn oil (Table 3). According to the fitting equation, the liver organ index reached the maximum value when 50% oxidized corn oil was added. Inflammatory infiltrating cells appeared in the liver of the 50% and 75% oxidized corn oil group, and the number of inflammatory infiltrating cells in the liver of the 100% oxidized corn oil group was alleviated (Figure 1). The abdominal fat percentage was linearly increased with the increasing proportion of oxidized corn oil (P < 0.05).
Table 3.
Effects of different proportions of naturally oxidized corn oil on liver organ index and abdominal fat rate of broilers.
| Oxidized corn oil: non-oxidized corn oil |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 0:100 | 25:75 | 50:50 | 75:25 | 100:0 | SEM | P-value | P-linear | P-quadratic |
| Liver organ index (g/kg) | 18.18c | 19.63bc | 22.63a | 21.38ab | 18.95c | 0.05 | 0.003 | 0.686 | 0.006 |
| Abdominal fat rate (%) | 1.02bc | 0.87c | 1.44a | 1.06bc | 1.31ab | 0.05 | 0.007 | 0.026 | 0.849 |
Different letters in the same row indicate significant differences (P < 0.05), and the same letter means no significant difference (P > 0.05).
Figure 1.
Effects of different proportions of naturally oxidized oil on the histomorphology of liver of broilers. At the end of day 42, 1 cm2 of liver from the liver tip was removed and fixed in a 4% paraformaldehyde solution. After tissue sections were prepared and stained with eosin–hematoxylin (HE), the inflammatory cell infiltration was observed by the OLYMPUS BX-41TF microscope at 400×. Abbreviations: OXA, basal diet with 0% naturally oxidized oil; OXB, basal diet with 25% naturally oxidized oil; OXC, basal diet with 50% naturally oxidized oil; OXD, basal diet with 75% naturally oxidized oil; OXE, basal diet with 100% naturally oxidized oil.
Effects of Different Proportions of Naturally Oxidized Corn Oil on Antioxidant Capacity of the Liver
The liver GSH-Px activity was linearly increased with the increasing proportion of oxidized corn oil (P < 0.05) (Table 4). There was a significant quadratic relationship between CAT activity and MDA content in the liver with the rise of the proportion of oxidized corn oil, and CAT activity in the liver reached the highest value when 50% oxidized corn oil was added. The content of MDA in the liver began to increase significantly after adding 50% oxidized corn oil and reached the maximum value after adding 75% oxidized corn oil (P < 0.05).
Table 4.
Effects of different proportions of naturally oxidized corn oil on antioxidant capacity of liver.
| Oxidized corn oil: non-oxidized corn oil |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 0:100 | 25:75 | 50:50 | 75:25 | 100:0 | SEM | P-value | P-linear | P-quadratic |
| GSH-Px (U/mgprot) | 28.76b | 28.28b | 30.11ab | 31.70ab | 33.11a | 0.79 | 0.008 | 0.002 | 0.162 |
| SOD (U/mgprot) | 253.34 | 266.20 | 253.18 | 242.16 | 256.01 | 8.12 | 0.186 | 0.084 | 0.086 |
| CAT (U/mgprot) | 16.86b | 17.11b | 19.84a | 18.67ab | 17.87ab | 0.33 | 0.043 | 0.858 | 0.027 |
| MDA (nmol/mgprot) | 2.05c | 2.20bc | 2.69b | 3.59a | 2.73b | 0.03 | <0.001 | 0.112 | 0.041 |
Abbreviations: CAT, catalase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase.
Different letters in the same row indicate significant differences (P < 0.05), and the same letter means no significant difference (P > 0.05).
Effects of Different Proportions of Naturally Oxidized Corn Oil on Relative mRNA Expression of Liver Antioxidants
The expression of CAT mRNA in the liver increased firstly and then decreased with the increasing proportion of oxidized corn oil, and the expression of CAT mRNA in the liver began to increase significantly after adding 50% oxidized corn oil and reached the highest value after adding 75% oxidized corn oil (Table 5). The mRNA expressions of NQO-1, NF-κB, and TLR-4 were linearly increased with the rise of the proportion of oxidized corn oil (P < 0.05).
Table 5.
Effects of different proportions of naturally oxidized corn oil on relative mRNA expression of liver antioxidant of broilers.
| Oxidized corn oil: non-oxidized corn oil |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter1 | 0:100 | 25:75 | 50:50 | 75:25 | 100:0 | SEM | P-value | P-linear | P-quadratic |
| CAT | 1.00b | 1.17b | 2.86a | 3.64a | 1.95b | 0.09 | <0.001 | 0.004 | 0.002 |
| SOD | 1.00 | 2.49 | 1.09 | 2.23 | 2.09 | 0.56 | 0.125 | 0.303 | 0.542 |
| GSH-Px | 1.00 | 1.15 | 1.15 | 1.54 | 1.51 | 0.19 | 0.071 | 0.781 | 0.511 |
| HO-1 | 1.00 | 3.24 | 1.19 | 3.10 | 1.51 | 0.13 | 0.063 | 0.679 | 0.153 |
| iNOS | 1.00 | 1.72 | 1.38 | 2.88 | 1.62 | 0.14 | 0.077 | 0.109 | 0.222 |
| NQO-1 | 1.00b | 0.78b | 1.34b | 3.05a | 2.86a | 0.08 | 0.009 | 0.001 | 0.544 |
| Nrf2 | 1.00 | 0.05 | 0.15 | 0.33 | 1.13 | 0.05 | 0.072 | 0.596 | 0.006 |
| Keap-1 | 1.00 | 0.26 | 0.17 | 0.65 | 0.18 | 0.11 | 0.055 | 0.089 | 0.194 |
| NF-κB | 1.00b | 1.34b | 2.74b | 3.65ab | 4.23a | 0.08 | 0.003 | 0.003 | 0.566 |
| TLR-4 | 1.00b | 1.39b | 2.84b | 3.67ab | 4.50a | 0.07 | 0.001 | 0.003 | 0.304 |
Abbreviations: CAT, catalase; GSH-Px, glutathione peroxidase; HO-1, heme oxygenase-1; iNOS, inductible nitric oxide synthase; Keap-1, kelch-like epichlorohydrin associated protein-1; NF-κB, nuclear factor kappa B; NQO-1, NADH quinone oxidoreductase 1; Nrf2, nuclear factor erythroid related factor 2; SOD, superoxide dismutase; TLR-4, toll like receptor 4.
Different letters in the same row indicate significant differences (P < 0.05), and the same letter means no significant difference (P > 0.05).
Relative gene expression in different treatments of broilers (N = 6).
Effects of Different Proportions of Naturally Oxidized Corn Oil on Serum Lipid Metabolism of Broilers
The serum AST content had a quadratic relationship with the increasing proportion of oxidized corn oil (P < 0.05), and the serum AST content reached the highest value when 50% oxidized corn oil was added. The serum TG content was significantly changed with the increasing proportion of oxidized corn oil, and when the oxidized corn oil proportion was more than 50%, the serum TG content was significantly decreased (P < 0.05) (Table 6). Serum FFA content was linearly increased, with the increased proportion of oxidized corn oil (P<0.05). This indicated that oxidized corn oil had adverse effects on liver lipid metabolism.
Table 6.
Effects of different proportions of naturally oxidized corn oil on serum lipid metabolism of broilers.
| Oxidized corn oil: non-oxidized corn oil |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 0:100 | 25:75 | 50:50 | 75:25 | 100:0 | SEM | P-value | P-linear | P-quadratic |
| AST (U/L) | 117.04d | 115.24d | 197.96a | 157.86b | 136.10c | 5.89 | <0.001 | <0.001 | <0.001 |
| ALT (U/L) | 30.62 | 30.26 | 31.01 | 30.64 | 28.64 | 0.68 | 0.849 | 0.484 | 0.468 |
| TC (mmol/L) | 2.65 | 2.70 | 2.85 | 2.69 | 2.76 | 0.03 | 0.400 | 0.368 | 0.342 |
| TG (mmol/L) | 1.60a | 1.41ab | 0.85c | 1.20b | 1.22b | 0.06 | 0.001 | 0.237 | 0.063 |
| HDL (mmol/L) | 1.20 | 1.18 | 1.25 | 1.20 | 1.23 | 0.01 | 0.539 | 0.489 | 0.837 |
| LDL (mmol/L) | 1.21 | 1.30 | 1.28 | 1.25 | 1.26 | 0.01 | 0.353 | 0.729 | 0.141 |
| FFA (umol/L) | 298.17c | 322.59bc | 366.78ab | 393.96a | 375.24ab | 11.38 | 0.032 | 0.004 | 0.229 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; FFA, free fatty acid; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglyceride.
Different letters in the same row indicate significant differences (P < 0.05), and the same letter means no significant difference (P > 0.05).
Effects of Different Proportions of Naturally Oxidized Corn Oil on Relative mRNA Expression of Liver Lipid Metabolism of Broilers
With the proportion of oxidized corn oil increasing, the mRNA expressions of PPARα, CPT1, and ACO in the liver were linearly increased (P < 0.05) (Table 7). The expression of Caveolin mRNA in the liver changed significantly with the increasing proportion of oxidized corn oil, and the expression of Caveolin mRNA in the liver in the 100% oxidized corn oil group was significantly higher than that in other treatment groups (P < 0.05).
Table 7.
Effects of different proportions of naturally oxidized corn oil on relative mRNA expression of liver lipid metabolism of broilers.
| Oxidized corn oil: non-oxidized corn oil |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter1 | 0:100 | 25:75 | 50:50 | 75:25 | 100:0 | SEM | P-value | P-linear | P-quadratic |
| PPARα | 1.00b | 1.87b | 3.83ab | 3.85ab | 6.88a | 0.09 | 0.002 | 0.034 | 0.158 |
| CPT1 | 1.00b | 0.87b | 3.52b | 6.69a | 5.23ab | 0.08 | 0.001 | 0.006 | 0.219 |
| ACO | 1.00b | 0.81b | 1.83ab | 3.28a | 2.81a | 0.04 | 0.016 | 0.002 | 0.949 |
| FATP | 1.00 | 0.92 | 1.20 | 0.89 | 1.27 | 0.10 | 0.382 | 0.331 | 0.592 |
| FABP | 1.00 | 0.84 | 1.34 | 0.85 | 1.10 | 0.08 | 0.558 | 0.773 | 0.844 |
| CD36 | 1.00 | 1.73 | 1.23 | 0.79 | 1.11 | 0.06 | 0.183 | 0.403 | 0.461 |
| Caveolin | 1.00b | 0.65b | 0.63b | 0.62b | 2.06a | 0.08 | 0.023 | 0.148 | 0.351 |
Abbreviations: ACO, acyl coenzyme oxidase; CD36, fatty acid transferase; CPT1, carnitine acyltransferase; FABP, fatty acid-binding protein; FATP, fatty acid transporter; PPARα, peroxisome proliferators activate receptor-α.
Different letters in the same row indicate significant differences (P < 0.05), and the same letter means no significant difference (P > 0.05).
Relative gene expression in different treatments of broilers (N = 6).
DISCUSSION
Lipid peroxidation inevitably occurs during the storage of vegetable oil and feed preparation, which would promote the production of ROS (Andarwulan et al., 2014), and cause animal oxidative stress (BV et al., 2011). Broilers are easily exposed to accumulated oxidative by-products through the ingestion of oxidized oil, which causes an imbalance of redox status, especially in the liver (Dong et al., 2020). In the current study, we found that the liver organ index and serum AST content reached the maximum value when 50% oxidized corn oil was added. During metabolism, liver injury was usually accompanied by increased ALT and AST activities in serum (Giannini et al., 1999). A high level of AST indicated that oxidized oil could cause liver damage in broilers and increase the liver index. Meanwhile, inflammatory infiltrating cells were observed in the liver when 50% oxidized corn oil was added. However, with the increase of the proportion of oxidized corn oil, the liver index and the number of liver inflammatory infiltrating cells decreased. In this study, the mRNA expressions of NF-κB and TLR-4 in the liver increased linearly with the increasing proportion of oxidized corn oil. The TLR4-mediated NF-κB signaling pathway can activate hepatic Kupffer's cells under oxidative stress (Yang et al., 2014), leading to excessive production of inflammatory cytokines, which induces hepatocyte injury (Cheng et al., 2017). Therefore, it could be speculated that the inflammatory infiltrating cells in the liver after the addition of 50% oxidized corn oil might be due to the activation of the NF-κB signaling pathway by excessive oxidized corn oil, which prompted the body's immune homeostasis to shift towards the anti-inflammatory direction.
ROS can regulate the body's natural redox system through endogenous free radical scavengers such as SOD, CAT, and GSH-Px (Hu et al., 2019). In addition, MDA is a reliable marker of oxidative stress-mediated lipid peroxidation, and its level can be used to evaluate oxidative stress in liver injury (Grotto et al., 2009). Tan et al. (2018) found that oxidized oil increased liver MDA level, and decreased total antioxidant capacity (T-AOC) and SOD of broilers. On the contrary, the current study showed that naturally oxidized corn oil increased the GSH-Px and CAT in the liver, which might reflect a compensatory impact or a precursor of oxidative tissue stress, and improved the ability of the tissue to clear free radicals (Tan et al., 2019). The increase of antioxidant enzyme activity could clear MDA, the marker of oxidative stress in liver injury, which explained the quadratic curve change of MDA content which first increased and then decreased. The decrease of MDA in the 100:0 group suggested that excess oxidized oil might induce the body to adapt to the mild oxidation reaction. Due to the above results, the decrease of MDA content and the relief of damage in the liver may be related to the activation of antioxidant capacity by a higher dose of oxidized oil. It should be pointed out that Nrf2 is a critical molecule that regulates endogenous antioxidant system responses which can protect cells from ROS and toxic compounds (Li et al., 2020). In the current study, the mRNA expression of both NQO-1 and CAT in the liver increased with the increase of the proportion of oxidized corn oil. This suggests that a high dose of naturally oxidized corn oil may activate the downstream gene of the Nrf2 antioxidant signaling pathway, which in turn enhances the activities of antioxidant enzymes such as GSH-Px and CAT, thereby playing a protective role. The results of oxidized corn oil on antioxidant enzymes were consistent with the growth performance of broilers. Our previous study found that 100% oxidized oil had no difference compared to 0% oxidized oil in growth performance when the oxidized oil is excessive, which indicated that the oxidative stress level did not exceed the adaptability of broilers and would not affect growth performance (Zhang et al., 2022). The results further demonstrated that high-dose oxidized oil may activate the antioxidant defense system of broilers, which could enhance the resistance to oxidative stress, but its mechanism needed to be further explored.
The liver lipid metabolism disorder caused by oxidized vegetable oil will affect the abdominal fat rate of broilers, which could accelerate abnormal lipid metabolites in serum and promote the formation of adipose tissue (Fouad and El-Senousey, 2014). We observed that the excessive oxidized corn oil significantly increased FFA content in the serum of broilers and significantly decreased TG concentrations. Based on the previous findings, it should be noted that the concentration of TG and cholesterol in the liver and serum of animals decreased after feeding oxidized oil (Yue et al., 2011). However, decreased serum TG and cholesterol concentrations were accompanied by increased serum FFA, which are the major source of lipids in the body (Crespo and Esteve-Garcia, 2001). Increased levels of serum FFA would accelerate the absorption of fatty acids, and thus, could increase the abdominal fat percentage (Wu et al., 2011). In the previous study, we found that there was no significant difference in body weight gain between broilers fed oxidized corn oil and non-oxidized corn oil (Zhang et al., 2022). However, the abdominal fat rate was significantly increased, suggesting that excessive oxidized corn oil may change nutrient absorption and utilization, or lipid metabolism of broilers.
A previous study found that the decrease of serum TG content might be related to the activation of PPARα and the accelerated production of O2− in the mitochondrial electron transport chain and this accumulation of free radicals could further trigger oxidative stress (Fernández-Sánchez et al., 2011). Meanwhile, oxidized oil was found to be an effective activator of PPARα, and feeding thermally oxidized oil to broilers showed that it could accelerate the activation of PPARα in the liver (Ali et al., 2020). Therefore, we further examined the genes related to the liver PPARα pathway. In the present study, the mRNA expressions of PPARα, CPT1, and ACO in the liver increased linearly with the rise of the proportion of oxidized corn oil. PPARα is a ligand-activated transcription factor, which is highly expressed in the liver (Nguyen et al., 2008). Moreover, PPARα can control lipid content in the liver by regulating fatty acid transport and β-oxidation (Perez et al., 2020). Meanwhile, PPARα regulates fatty acid oxidation mainly by upregulating target genes CPT-1 and ACO, which are vital rate-limiting enzymes controlling lipid metabolism (Begriche et al., 2006). Based on the results of this study, it should be noted that excessive oxidized corn oil could activate the PPARα pathway, which may facilitate liver fatty acid β-oxidation, high energy consumption, and increases in serum FFA production. In addition, Caveolin is a cellular membrane vesicle structural protein that helps liver cells absorb free fatty acids to synthesize TG and participates in the regulation of cellular cholesterol (Martin, 2013). In this study, excessive oxidized corn oil significantly upregulated the expression of Caveolin mRNA in the liver of broilers, suggesting that naturally oxidized corn oil could promote lipid synthesis in broilers, which potentially explains the increase in the liver index. Based on these results, the adverse effects of high-dose oxidized corn oil on liver lipid metabolism are clearly illustrated, which led to the change of abdominal fat of broilers. Therefore, we suggested that naturally oxidized corn oil should be avoided to be added in poultry feed.
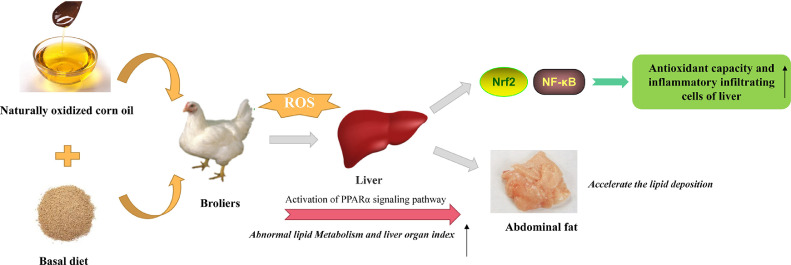
The results clearly showed that naturally oxidized corn oil could damage liver lipid metabolism and accelerate lipid deposition of broilers by upregulating PPARα. Additionally, naturally oxidized corn oil could cause liver inflammation and damage the liver by activating the NF-κB signaling pathway. In this case, the body could improve antioxidant capacity by activating Nrf2 signaling pathway and resist the negative effects caused by the addition of oxidized oil to a certain extent, but its mechanism needs to be further explored (Figure 2).
Figure 2.
Oxidized corn oil changes the liver lipid metabolism of broilers by upregulating PPARα. The results clearly showed that naturally oxidized corn oil could damage liver lipid metabolism and accelerate lipid deposition of broilers by upregulating PPARα. Additionally, naturally oxidized corn oil could cause liver inflammation and damage the liver by activating the NF-κB signaling pathway. In this case, the body could improve antioxidant capacity by activating Nrf2 signaling pathway and resist the negative effects caused by the addition of oxidized oil to a certain extent, but its mechanism needs to be further explored. Abbreviations: NF-κB, nuclear factor kappa B; Nrf2, nuclear factor erythroid related factor 2; PPARα, peroxisome proliferators activate receptor-α; ROS, reactive oxygen species.
CONCLUSION
In poultry production, naturally oxidized corn oil could damage liver lipid metabolism and accelerate lipid deposition of broilers by upregulating PPARα. Therefore, oxidized corn oil should be avoided to use in broiler diet.
ACKNOWLEDGMENTS
We acknowledge the System for Poultry Production Technology, Beijing Agriculture Innovation Consortium (Project Number: BAIC04-2021) for supporting this research.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Agca C.A., Tuzcu M., Hayirli A., Sahin K. Taurine ameliorates neuropathy via regulating NF-κB and Nrf2/HO-1 signaling cascades in diabetic rats. Food Chem. Toxicol. 2014;71:116–121. doi: 10.1016/j.fct.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Ali S.A.-F., Ismail A., Abdel-Hafez S.A., El-Genaidy H.M.A. Influence of thermally oxidized palm oil on growth performance and PPAR-α gene expression in broiler chickens. Physiol. Mol. Biol. 2020;12:23–37. [Google Scholar]
- Ament Z., West J.A., Stanley E., Ashmore T., Roberts L.D., Wright J., Nicholls A.W., Griffin J.L. PPAR-pan activation induces hepatic oxidative stress and lipidomic remodelling. Free Radic. Biol. Med. 2016;95:357–368. doi: 10.1016/j.freeradbiomed.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andarwulan N., Gitapratiwi D., Laillou A., Fitriani D., Hariyadi P., Moench-Pfanner R., Martianto D. Quality of vegetable oil prior to fortification is an important criteria to achieve a health impact. Nutrients. 2014;6:5051–5060. doi: 10.3390/nu6115051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begriche K., Igoudjil A., Pessayre D., Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- BV S.K., Ajeet K., Meena K. Effect of heat stress in tropical livestock and different strategies for its amelioration. J Physiol. Biochem. 2011;7:45–54. [Google Scholar]
- Cheng P., Wang T., Li W., Muhammad I., Wang H., Sun X., Yang Y., Li J., Xiao T., Zhang X. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-κB pathway. Front. Pharmacol. 2017;8:547. doi: 10.3389/fphar.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo N., Esteve-Garcia E. Dietary fatty acid profile modifies abdominal fat deposition in broiler chickens. Poult. Sci. 2001;80:71–78. doi: 10.1093/ps/80.1.71. [DOI] [PubMed] [Google Scholar]
- Dong Y., Lei J., Zhang B. Effects of dietary quercetin on the antioxidative status and cecal microbiota in broiler chickens fed with oxidized oil. Poult. Sci. 2020;99:4892–4903. doi: 10.1016/j.psj.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehr I., Kerr B., Persia M. Effects of peroxidized corn oil on performance, AMEn, and abdominal fat pad weight in broiler chicks. Poult. Sci. 2015;94:1629–1634. doi: 10.3382/ps/pev131. [DOI] [PubMed] [Google Scholar]
- Fernández-Sánchez A., Madrigal-Santillán E., Bautista M., Esquivel-Soto J., Morales-González Á., Esquivel-Chirino C., Durante-Montiel I., Sánchez-Rivera G., Valadez-Vega C., Morales-González J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011;12:3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad A., El-Senousey H. Nutritional factors affecting abdominal fat deposition in poultry: a review. Asian-Australas. J. Anim. Sci. 2014;27:1057. doi: 10.5713/ajas.2013.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini E., Botta F., Fasoli A., Ceppa P., Risso D., Lantieri P.B., Celle G., Testa R. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig. Dis. Sci. 1999;44:1249–1253. doi: 10.1023/a:1026609231094. [DOI] [PubMed] [Google Scholar]
- Grotto D., Maria L.S., Valentini J., Paniz C., Schmitt G., Garcia S.C., Pomblum V.J., Rocha J.B.T., Farina M. Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quim Nova. 2009;32:169–174. [Google Scholar]
- Hu R., He Y., Arowolo M.A., Wu S., He J. Polyphenols as potential attenuators of heat stress in poultry production. Antioxidants. 2019;8:67. doi: 10.3390/antiox8030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J., Zheng Z., Xu Y.-j., Cao P., Li J., Li Q., Liu Y. Influence of total polar compounds on lipid metabolism, oxidative stress and cytotoxicity in HepG2 cells. Lipids Health Dis. 2019;18:1–13. doi: 10.1186/s12944-019-0980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Li K., Zou C., Tong C., Sun L., Cao Z., Yang S., Lyu Q. Selenium yeast alleviates ochratoxin A-induced hepatotoxicity via modulation of the PI3K/AKT and Nrf2/Keap1 signaling pathways in chickens. Toxins. 2020;12:143. doi: 10.3390/toxins12030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F., Jiang S., Mo Y., Zhou G., Yang L. Consumption of oxidized soybean oil increased intestinal oxidative stress and affected intestinal immune variables in yellow-feathered broilers. Asian-Australas. J. Anim. Sci. 2015;28:1194. doi: 10.5713/ajas.14.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z., Xing K., Li G., Liu D., Guo Y. Dietary genistein alleviates lipid metabolism disorder and inflammatory response in laying hens with fatty liver syndrome. Front. Physiol. 2018;9:1493. doi: 10.3389/fphys.2018.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. Caveolae, lipid droplets, and adipose tissue biology: pathophysiological aspects. Horm. Mol. Biol. Clin. Investig. 2013;15:11–18. doi: 10.1515/hmbci-2013-0035. [DOI] [PubMed] [Google Scholar]
- Nguyen P., Leray V., Diez M., Serisier S., Bloc'h J.L., Siliart B., Dumon H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008;92:272–283. doi: 10.1111/j.1439-0396.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- Perez V.M., Gabell J., Behrens M., Wase N., DiRusso C.C., Black P.N. Deletion of fatty acid transport protein 2 (FATP2) in the mouse liver changes the metabolic landscape by increasing the expression of PPARα-regulated genes: FATP2 expression is linked to PPARα-regulated genes in liver. J. Biol. Chem. 2020;295:5737–5750. doi: 10.1074/jbc.RA120.012730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Society A.O.C. Official methods and recommended practices of the American Oil Chemists’ Society. J Am. Oil Chem. Soc. 2004 [Google Scholar]
- Taghvaei M., Jafari S.M. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J. Food Sci. Tech. 2015;52:1272–1282. doi: 10.1007/s13197-013-1080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Rong D., Yang Y., Zhang B. Effect of oxidized soybean oils on oxidative status and intestinal barrier function in broiler chickens. Braz. J. Poult. Sci. 2018;20:333–342. [Google Scholar]
- Tan L., Rong D., Yang Y., Zhang B. The effect of oxidized fish oils on growth performance, oxidative status, and intestinal barrier function in broiler chickens. J. Appl. Poult. Res. 2019;28:31–41. [Google Scholar]
- Vieira S.A., Zhang G., Decker E.A. Biological implications of lipid oxidation products. J. Am. Oil Chem. Soc. 2017;94:339–351. [Google Scholar]
- Wang G., Kim W.K., Cline M.A., Gilbert E.R. Factors affecting adipose tissue development in chickens: a review. Poult. Sci. 2017;96:3687–3699. doi: 10.3382/ps/pex184. [DOI] [PubMed] [Google Scholar]
- Winkler-Moser J.K., Breyer L. Composition and oxidative stability of crude oil extracts of corn germ and distillers grains. Ind. Crop Prod. 2011;33:572–578. [Google Scholar]
- Wu H., Gong L., Guo L., Zhang L., Li J. Effects of the free fatty acid content in yellow grease on performance, carcass characteristics, and serum lipids in broilers. Poult. Sci. 2011;90:1992–1998. doi: 10.3382/ps.2010-01298. [DOI] [PubMed] [Google Scholar]
- Yang L., Roh Y.S., Song J., Zhang B., Liu C., Loomba R., Seki E. Transforming growth factor beta signaling in hepatocytes participates in steatohepatitis through regulation of cell death and lipid metabolism in mice. Hepatology. 2014;59:483–495. doi: 10.1002/hep.26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue H., Wang J., Qi X., Ji F., Liu M., Wu S., Zhang H., Qi G. Effects of dietary oxidized oil on laying performance, lipid metabolism, and apolipoprotein gene expression in laying hens. Poult. Sci. 2011;90:1728–1736. doi: 10.3382/ps.2011-01354. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Mahmood T., Tang Z., Wu Y., Yuan J. Effects of naturally oxidized corn oil on inflammatory reaction and intestinal health of broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101541. [DOI] [PMC free article] [PubMed] [Google Scholar]