Abstract
Hydrogen sulfide (H2S) is an important signaling molecule in colorectal cancer (CRC). It is produced in the colon by the catalytic synthesis of the colonocytes' enzymatic systems and the release of intestinal microbes, and is oxidatively metabolized in the colonocytes' mitochondria. Both endogenous H2S in colonic epithelial cells and exogenous H2S in intestinal lumen contribute to the onset and progression of CRC. The up-regulation of endogenous synthetases is thought to be the cause of the elevated H2S levels in CRC cells. Different diagnostic probes and combination therapies, as well as tumor treatment approaches through H2S modulation, have been developed in recent years and have become active area of investigation for the diagnosis and treatment of CRC. In this review, we focus on the specific mechanisms of H2S production and oxidative metabolism as well as the function of H2S in the occurrence, progression, diagnosis, and treatment of CRC. We also discuss the present challenges and provide insights into the future research of this burgeoning field.
Keywords: Hydrogen sulfide, Colorectal cancer, Sulfur metabolism, Colorectal carcinogenesis, Diagnosis, Cancer therapy
Graphical abstract
Highlights
-
•
H2S in the colon can be produced by endogenous synthetases and gut bacteria.
-
•
Both endogenous H2S and exogenous H2S contribute to the onset and progression of CRC.
-
•
Probes and therapies are developed in response to high levels of H2S in CRC cells.
-
•
H2S modulation strategies are applied to CRC treatment.
List of abbreviations
- ACLY
adenosine triphosphate citrate lyase
- ADT
anethole dithione
- AIE
aggregation-induced emission
- AOAA
aminooxyacetic acid
- Apr
adenosine-5′-phosphosulfate reductase
- Asr
anaerobic sulfite reductase
- AzMC
7-azido-4-methylcoumarin
- CAC
colitis-associated cancer
- CAT
cysteine aminotransferase
- CBS
cystathionine β-synthase
- CO
carbon monoxide
- CPT
camptothecin
- CRC
colorectal cancer
- CSE
cystathionine γ-lyase
- CT
computed tomography
- Cur
curcumin
- CyR61
cysteine-rich angiogenic inducer 61
- Cys
cysteine
- 2D
two-dimensional
- 3D
three-dimensional
- DADS
diallyl disulfide
- DAO
d-amino acid oxidase
- DAS
diallyl sulfide
- DATS
diallyl trisulfide
- DHLA
dihydrolipoic acid
- DHPS
2,3-dihydroxypropane-1-sulfonate
- DOX
doxorubicin
- Dsr
dissimilatory sulfite reductase
- DTTs
dithiothiones
- EGCG
epigallocatechin gallate
- ETHE1
ethylmalonic encephalopathy protein 1
- FR
far-red
- 5-FU
5-fluorouracil
- GRE
glycyl radical enzyme
- GSH
glutathione
- GSSH
glutathione persulfide
- GT
gemcitabine
- HAS
human serum albumin
- HMPB
hollow mesoporous Prussian blue
- H2O2
hydrogen peroxide
- H2S
hydrogen sulfide
- HSA
human serum albumin
- HS-NSAIDs
Hydrogen sulfide-releasing non-steroidal anti-inflammatory drugs
- ICG
indocyanine green
- IslA
isethionate sulfite-lyase
- IslB
isethionate sulfite-lyase's cognate activating enzyme
- LA
lauric acid
- LP
liposome
- MET
mesenchymal-epithelial transition
- 3-MP
3-mercaptopyruvate
- MRI
magnetic resonance imaging
- MSOT
multispectral optoacoustic tomography
- 3-MST
3-mercaptopyruvate sulfurtransferase
- NAD(P)H
nicotinamide adenine dinucleotide (phosphate)
- NBD
7-nitro-1,2,3-benzoxadiazole
- NIR-I/II
Near-infrared-I/II
- NO
nitric oxide
- NPs
nanoparticles
- OH
hydroxyl radical
- PA
photoacoustic
- PAG
dl-propargylglycine
- PAI
photoacoustic imaging
- PDA
polydopamine
- PDO
persulfide dioxygenase
- PDT
photodynamic therapy
- PLP
pyridoxal-5′-phosphate
- PMSN
polydopamine decorated mesoporous silica nanoparticles
- PTT
photothermal therapy
- PW
prussian white
- Rhd
rhodanese
- ROS
reactive oxygen species
- SAM
S-adenosyl-l-methionine
- SarD
sulfoacetaldehyde reductase
- Sat
sulfate adenylyltransferase
- SATO
S-aroylthiooxime
- SCM
surface cross-linked micelles
- SL
sulfolactate
- SOU
sulfide oxidation unit
- SPECT
single-photon emission computed tomography
- SQ
sulfoquinovose
- SQDG
sulfolipid sulfoquinovosyl diacylglycerol
- SQR
sulfide: quinone oxidoreductase
- SRB
sulfate-reducing bacteria
- SUOX
sulfite oxidase
- THBH
2,3,4-trihydroxybenzylhydrazine
- TME
tumor microenvironment
- Toa
taurine:2-oxoglutarate aminotransferase
- Trx
thioredoxin
- TST
thiosulfate thiotransferase
- UCL
upconversion luminescence
- UCNPs
upconversion nanophosphors
- Xsc
sulfoacetaldehyde acetyltransferase
1. Introduction
Hydrogen sulfide (H2S) is an irritating gas that smells like rotten eggs. As the third identified gasotransmitter after nitric oxide (NO) and carbon monoxide (CO), H2S has been recognized to have a variety of biological effects on human health and diseases, covering the nervous system, cardiovascular system, immune system, and digestive system [ [[1], [2], [3], [4], [5]]]. Moreover, H2S is widely accepted as a key signaling molecule in cancer biology because of its unique chemical properties, reaction mechanisms, ability to alter proteins, and active participation in many metal redox processes [6].
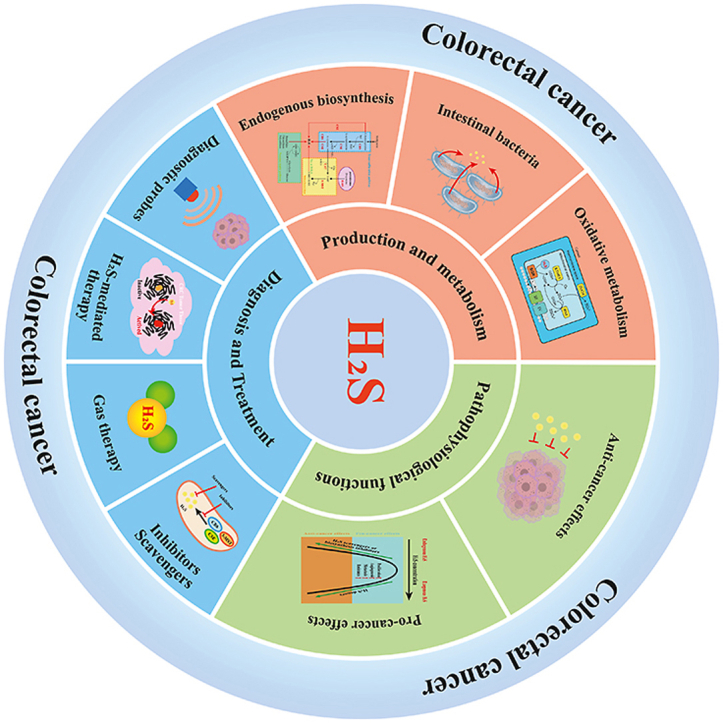
Colorectal cancer (CRC) is one of the most frequently diagnosed cancers and one of the leading causes of cancer-related deaths worldwide endogenous or exogenous [7]. A number of studies have found that H2S plays an important role in CRC [ [[8], [9], [10], [11], [12], [13]]]. This review summarizes recent studies on H2S in the field of CRC. We first discuss the production of H2S in the colon, the mechanism of oxidative metabolism, and the importance of H2S in the development of CRC. Following that, we review the strategies for using H2S in the diagnosis and treatment of CRC, and sort and discuss the currently developed probes, donors, therapeutics, and so on according to the diagnosis and treatment strategies.
2. Production and metabolism of H2S in the colon
2.1. Luminal H2S produced by intestinal bacteria
The release of luminal H2S from the intestinal bacterial metabolism is the dominant production mode [13]. Previous studies on microbial sulfidogenesis in the human gut mainly focused on the bacterial inorganic sulfur metabolism. However, increasing evidence suggests that organic sulfur metabolism by intestinal microbiota may be a critical mechanism linking diet and CRC [11]. In this section, we will look at how gut bacteria utilize inorganic sulfur salts or organic substrates (taurine/sulfoquinovose/cysteine/methionine) to produce H2S.
2.1.1. Metabolism of inorganic sulfur salts
Sulfate-reducing bacteria (SRB) are a very old class of anaerobic bacteria that rely on inorganic sulfates as electron acceptors for energy production [14]. As an important component of the intestinal flora, SRB can colonize the gut of a variety of animals. Among these SRB, Desulfovibrio, Desulfomicrobium, and Bilophila are the main members in the human intestinal tract [15].
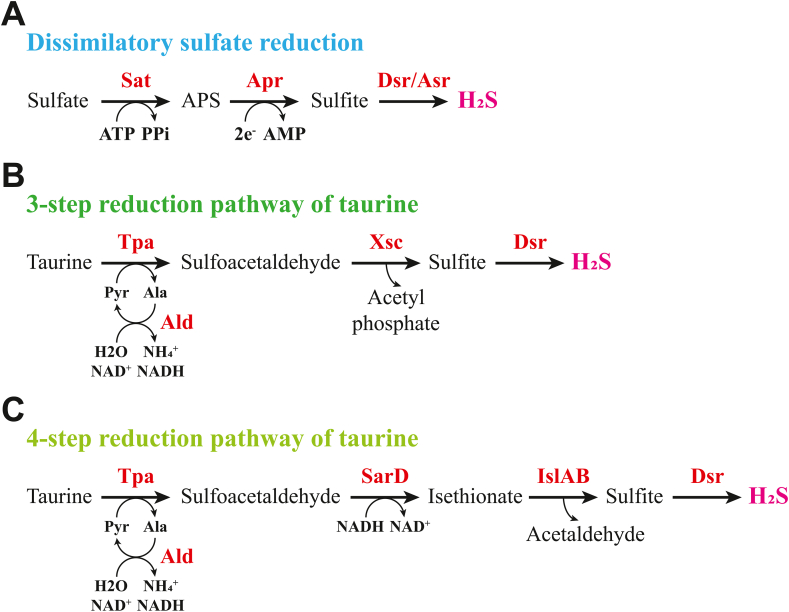
SRB produce H2S in the intestine by reducing sulfur oxidation products like sulfate, sulfite, and thiosulfate, while obtaining energy for their own growth through these biochemical reactions. The main method of producing H2S from inorganic sulfur salts released by bacteria in the intestinal lumen is dissimilatory sulfate reduction of SRB [16]. This process involves sulfate adenylyltransferase (Sat; an ATP sulfurylase), adenosine phosphosulfate reductase (Apr), dissimilatory sulfite reductase (Dsr), and anaerobic sulfite reductase (Asr) (Fig. 1A) [ [[17], [18], [19]]]. Dsr and Asr catalyze the six-electron reduction of sulfite to H2S in the final step of the process. Previous studies of SRB concentrate dissimilatory sulfate reduction on bacteria containing Dsr enzyme. Nevertheless, the latest research indicates that the Asr gene is more prevalent in human intestinal bacteria than the Dsr gene, implying that Asr may be a more important contributor to sulfate reduction in the gut than Dsr [11].
Fig. 1.
Metabolic pathways of inorganic sulfur salts and taurine in bacteria. A) Dissimilatory sulfate reduction pathway of SRB. B) Previously thought three-step reduction pathway for taurine in Bilophila wadsworthia. C) Four-step reduction pathway for taurine in Bilophila wadsworthia. Ala, alanine; Ald, alanine dehydrogenase; AMP, adenosine monophosphate; Apr, adenosine phosphosulfate reductase; APS, adenosine phosphosulfate; Asr, anaerobic sulfite reductase; ATP, adenosine triphosphate; Dsr, dissimilatory sulfite reductase; IslAB, isethionate sulfite-lyase (IslA) and its cognate activating enzyme (IslB); NAD(P)H, nicotinamide adenine dinucleotide (phosphate); PPi, inorganic pyrophosphate; Pyr, pyruvate; SarD, sulfoacetaldehyde reductase; Sat, sulfate adenylyltransferase; Tpa, taurine: pyruvate aminotransferase; Xsc, sulfoacetaldehyde acetyltransferases.
2.1.2. Metabolism of taurine
Taurine is the second most abundant free amino acid in colon tissue [20]. It is a substrate for the human gut microbiota that is obtained directly from the diet or from taurine-conjugated bile acids hydrolyzed by bile salt hydrolase [21]. Microbial taurine metabolism has received considerable critical attention after being identified as a potential mechanism by which dietary differences affect the development of colitis and CRC [22,23].
For a long time, Bilophila wadsworthia was the only known taurine-reducing bacterium in the human gut. This bacterium is thought to metabolize taurine via a three-step pathway that involves sulfoacetaldehyde acetyltransferase (Xsc) (Fig. 1B) [24]. Surprisingly, recent studies have revealed that Bilophila wadsworthia does not have the above pathway, but instead metabolizes taurine via a four-step reduction pathway involving sulfoacetaldehyde reductase (SarD) and glycyl radical enzyme (GRE)-isethionate sulfite-lyase (IslA) and its cognate activating enzyme (IslB) to produce H2S (Fig. 1C) [11,25].
Given the colon's taurine-rich environment, it is likely that there are other bacteria capable of performing this metabolic process that have yet to be discovered. One study found taurine:2-oxoglutarate aminotransferase (Toa) in Bifidobacterium kashiwanohense, which can catalyze the deamination in the first step of taurine reduction [26]. Another recent study looked at the microbiome of sulfur metabolism genes in human gut and identified twelve potential pathways for gut microbes to reduce taurine, as well as new bacterial genera that contain these pathways [11]. Their findings point to new microbial targets for novel enzyme discovery or taurine metabolism research.
2.1.3. Metabolism of sulfoquinovose
Sulfoquinovose (SQ; 6-deoxy-6-sulfoglucose) is the polar head group of sulfolipid sulfoquinovosyl diacylglycerol (SQDG), which is the main organic sulfur reservoir in photosynthetic organisms and a source of carbon and sulfur for various microbial communities [27]. Because vegetarian diets typically contain SQDG and some gut bacteria have the ability to catabolize SQ to H2S, it is hypothesized that SQ could be a long-neglected source of H2S in the intestine derived from green diets [28].
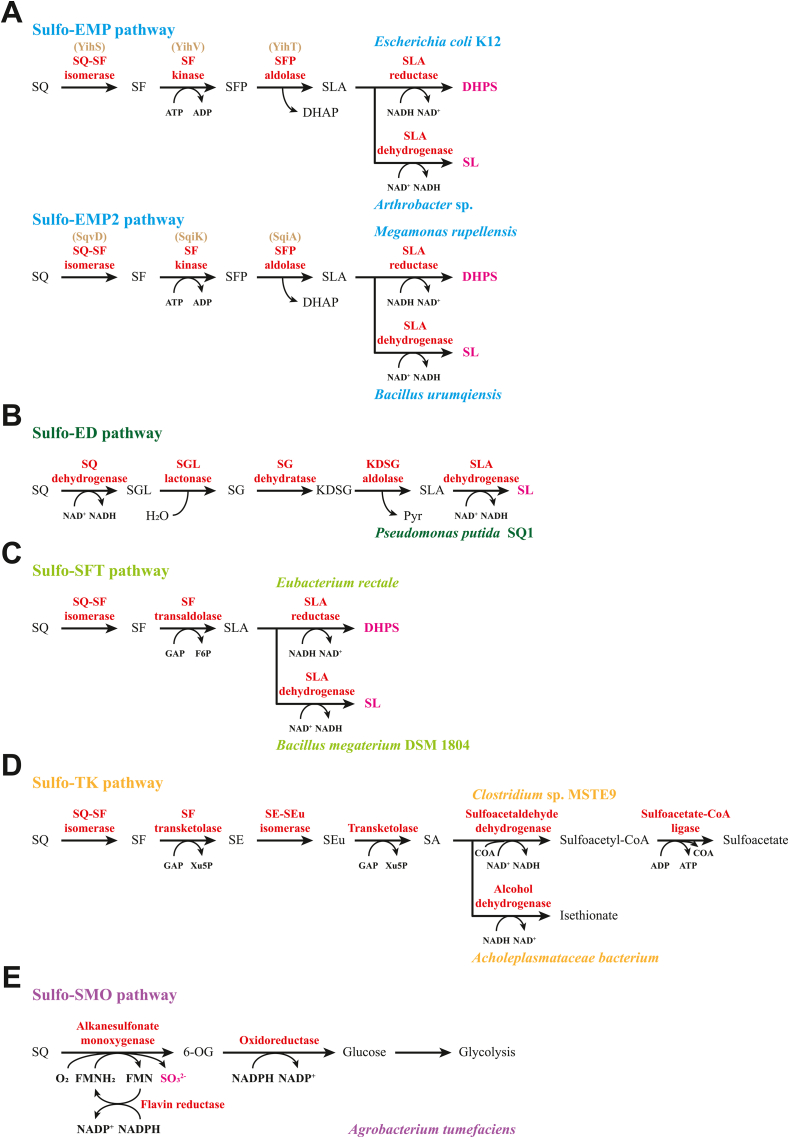
In recent years, some advances have been made in the SQ metabolism (described as sulfoglycolysis) of microorganisms. A total of five pathways have been identified, including the sulfo-Embden-Meyerhof-Parnas (sulfo-EMP) pathway [ [[29], [30], [31], [32], [33]]], the sulfo-Entner-Doudoroff (sulfo-ED) pathway [34,35], the sulfo-sulfofructose transaldolase (sulfo-SFT; or named sulfo-transaldolase, sulfo-TAL) pathway [36,37], the sulfo-transketolase (sulfo-TK) pathway [33], and the sulfo-sulfoquinovose monooxygenase (sulfo-SMO; or named sulfo-alkanesulfonate monooxygenase, sulfo-ASMO) pathway [33,38]. Among them, the sulfo-SMO pathway allows for complete SQ metabolism and sulfite release, while the other pathways utilize only part of the carbons within the SQ skeleton (Fig. 2). These pathways that incompletely metabolize SQ (with the exception of the sulfo-TK pathway) produce 2,3-dihydroxypropane-1-sulfonate (DHPS) and/or sulfolactate (SL), which are biomineralized to sulfite/sulfate by other members of the microbial community.
Fig. 2.
Sulfoglycolytic pathways in bacteria. A) The sulfo-Embden-Meyerhof-Parnas (sulfo-EMP) and sulfo-EMP2 pathways. B) The sulfo-Entner-Doudoroff (sulfo-ED) pathway. C) The sulfo-sulfofructose transaldolase (sulfo-SFT) pathway. D) The sulfo-transketolase (sulfo-TK) pathway. E) The sulfo-sulfoquinovose monooxygenase (sulfo-SMO) pathway. ADP, adenosine diphosphate; ATP, adenosine triphosphate; CoA, coenzyme A; DHAP, dihydroxyacetone phosphate; DHPS, 2,3-dihydroxypropanesulfonate; FMN, flavin mononucleotide; F6P, fructose-6-phosphate; GAP, glyceraldehyde-3-phosphate; KDSG, 2-keto-3,6-dideoxy-6-sulfogluconate; NAD(P)H, nicotinamide adenine dinucleotide (phosphate); 6-OG, 6-oxo-glucose; Pyr, pyruvate; SA, sulfoacetaldehyde; SE, 4-deoxy-4-sulfoerythrose; SEu, 4-deoxy-4-sulfoerythrulose; SF, sulfofructose; SFP, sulfofructose-1-phosphate; SG, 6-deoxy-6-sulfogluconate; SGL, 6-deoxy-6-sulfogluconolactone; SL, sulfolactate; SLA, sulfolactaldehyde; SO32−, sulfite; SQ, sulfoquinovose; Xu5P; xylulose-5-phosphate.
SRB can degrade DHPS in the digestive tracts of terrestrial animals, resulting in the conversion of sulfonate sulfur to H2S. For example, Desulfovibrio sp. Strain DF1 can ferment DHPS and SL under anaerobic conditions to produce acetate and H2S via the DHPS/SL desulfurization pathway (related genes also exist in the SRB of the human gut microbiota) [39]. Furthermore, two oxygen-sensitive GREs in anaerobic bacteria were reported for DHPS degradation through different mechanisms. DHPS sulfolyase (HpsG) in SRB catalyzes C–S cleavage to release sulfite, which acts as a terminal electron acceptor in respiration, resulting in production of H2S [40]. On this basis, a study found that Eubacterium rectale (via the sulfo-SFT pathway) and Bilophila wadsworthia (via the HpsG pathway) were involved in the interspecies DHPS transfer of human gut microbiota to jointly degrade plant-diet-derived SQ to H2S. Meanwhile, the impact of SQ on human health and disease was also highlighted as a unique microbial nutrient and an additional source of intestinal H2S [28].
2.1.4. Metabolism of cysteine/methionine
Some bacteria in the colon can also produce H2S through cysteine desulfurase-catalyzed degradation of the substrate cysteine. The first L- or d-cysteine desulfurases were discovered in the 1950s in the Escherichia coli, which is a well-known resident of the human gut [41,42]. Since then, cysteine desulfurase-mediated desulfurization reactions have been described in a variety of common colonic bacteria, including Clostridium, Enterobacter, Klebsiella, Streptococcus, and Desulfovibrio of the SRB genus [17].
Notably, a high proportion of bacterial genomes contain mammalian homologous CBS, CSE, and 3-MST-encoding genes that are involved in endogenous H2S production in cells. Pathogenic strains, such as Escherichia coli and Staphylococcus aureus, can convert cysteine to H2S through the activity of one of these enzymes [43]. According to the recent studies, genes involved in microbial cysteine and methionine metabolism are more widely and diversely distributed in the human intestinal microbiome than previously thought [11]. These findings suggest that the sulfur-containing amino acids cysteine and methionine may be understudied and abundant sources of H2S derived from microbial organosulfur metabolism in the human gut.
2.2. Endogenous production of H2S
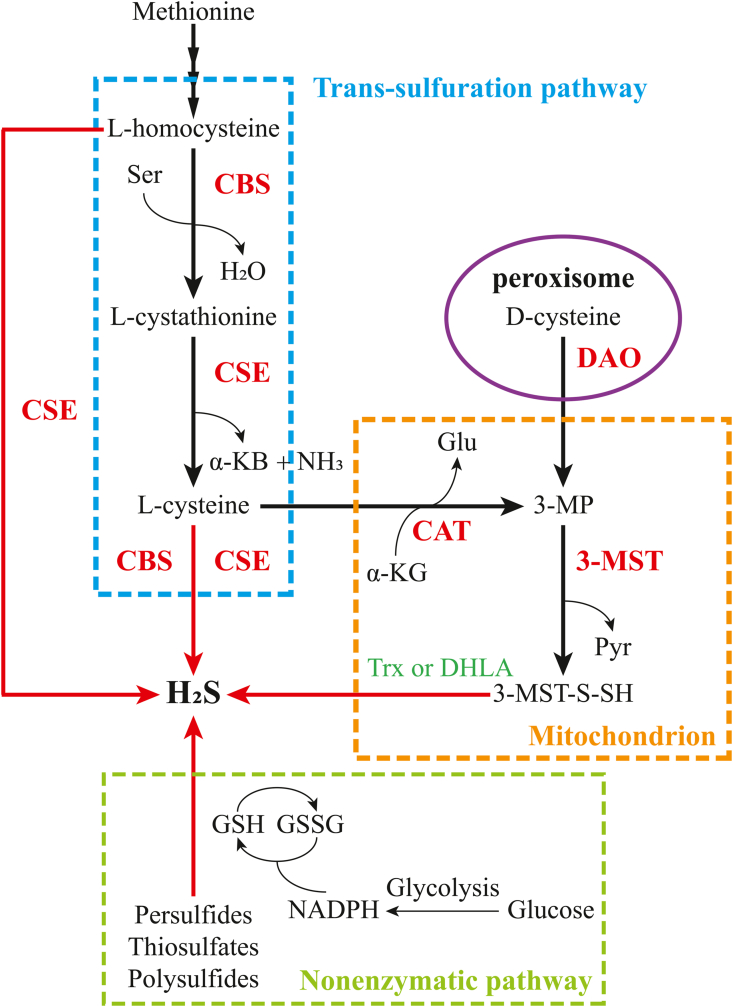
Endogenous H2S can be produced in mammals via enzymatic reactions as well as non-enzymatic chemical reduction methods. Three enzymes involved in the metabolization of cysteine (Cys) catalyze the production of H2S: the pyridoxal-5′-phosphate (PLP)-dependent enzymes cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), and the PLP-independent enzyme 3-mercaptopyruvate sulfurtransferase (3-MST) (Fig. 3) [1,3,44]. The important substrate cysteine can be derived from dietary absorption or release from endogenous proteins, or it can be synthesized from methionine through the trans-sulfuration pathway [45,46].
Fig. 3.
Enzymatic and non-enzymatic pathways for endogenous H2S production in mammalian cells. Endogenous H2S from mammalian cells can be produced by enzymatic reactions with the help of CBS, CSE, 3MST coupled to CAT. As an important substrate in the enzymatic pathway, l-cysteine can be converted from methionine via the trans-sulfur pathway (blue dashed rectangle). l-cysteine is catalyzed by CAT to produce 3-MP, which enters the mitochondria for further conversion to H2S via 3-MST (orange dashed rectangle). In addition, 3-MP can be generated from d-cysteine in brain and kidney, catalyzed by DAO located in the peroxisome (purple circles). A portion of endogenous H2S can also be obtained by non-enzymatic chemical reduction (green dashed rectangle). The active sulfur species in persulfides, thiosulfates and polysulfides release H2S, which can be converted from GSSG via NADPH in the presence of thiols such as GSH. α-KB, α-ketobutyrate; α-KG, α-ketoglutarate; CAT, cysteine aminotransferase; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; DAO, d-amino acid oxidase; DHLA, dihydrolipoic acid; Glu, glutamate; GSH, glutathione; GSSG, oxidized glutathione; H2S, hydrogen sulfide; 3-MP, 3-mercaptopyruvate; 3-MST, 3-mercaptopyruvate sulfurtransferase; 3-MST-S-SH, 3-MST-bound persulfide; NAD(P)H, nicotinamide adenine dinucleotide (phosphate); NH3, ammonia; Pyr, pyruvate; Ser, serine; Trx, thioredoxin. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
As the first (and rate-limiting) enzyme in the mammalian trans-sulfuration pathway, CBS catalyzes the typical β-replacement reaction for condensation of l-serine and l-homocysteine to form water and l-cystathionine. The latter is the physiological substrate for CSE, which catalyzes its conversion to l-cysteine, α-ketobutyrate and ammonia [47]. CBS can also catalyze multiple H2S production reactions, such as the reaction of cysteine with l-homocysteine, l-cysteine, and water to generate H2S. In contrast, CSE is a more promiscuous enzyme than CBS in the trans-sulfuration pathway. CSE can generate H2S from one or 2 mol of homocysteine in addition to common reactions with CBS in H2S production [1,47,48]. Notably, an intact trans-sulfuration pathway can support cell growth in the presence of extracellular cysteine limitation. When the catalytic activity of CBS and CSE is inhibited, cells are unable to synthesize cysteine, which may have deleterious effects on the organism at low cysteine availability [ [[49], [50], [51]]].
The 3-MST catalyzed H2S-producing reactions require the assistance of cysteine aminotransferase (CAT). l-cysteine and α-ketoglutarate are converted to glutamate and 3-mercaptopyruvate (3-MP) through the CAT-based catalysis. The latter is catalyzed by 3-MST to generate pyruvate and MST-bound persulfide, which can produce H2S in the presence of thioredoxin (Trx) or dihydrolipoic acid (DHLA) [1,52]. Moreover, 3-MP can be synthesized from d-cysteine in the kidneys or the brain by d-amino acid oxidase (DAO) to participate in the subsequent H2S synthesis [53].
Endogenous H2S can also be produced by non-enzymatic pathways in addition to enzymatic reactions. Persulfides, thiosulfates, and polysulfides can be converted to H2S and other metabolites in the presence of reducing equivalents, such as nicotinamide adenine dinucleotide (NADH) and nicotinamide adenine dinucleotide phosphate (NADPH) [45,46,54].
2.3. The catabolism of H2S
Sulfide can exist in three forms in the intestinal lumen: H2S gas partially dissolved in the aqueous phase, hydrosulfide anion HS− and sulfide ion S2− (generally negligible under physiological conditions) [13]. Because of its high membrane permeability, luminal H2S easily penetrates biofilms and enters colon cells [55,56].
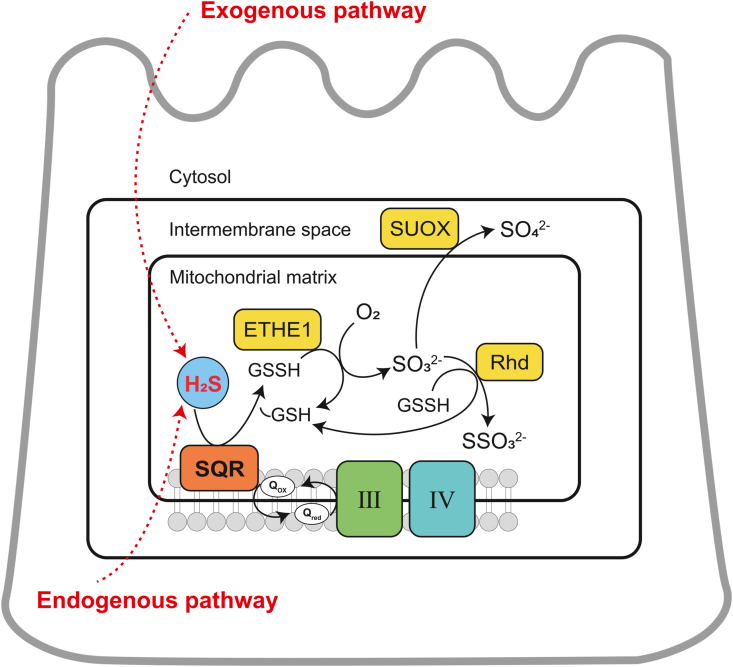
The sulfide oxidation unit (SOU) is a mitochondrial multi-enzyme system that is responsible for the intracellular oxidative metabolism of H2S in colonic epithelial cells (Fig. 4). This sulfide metabolic process generates electrons that enter the electron transport chain at complex III (from sulfide: quinone oxidoreductase (SQR)) and cytochrome c/complex IV (from sulfite oxidase (SUOX)), respectively, driving ATP synthesis [57,58].
Fig. 4.
Oxidation pathway for H2S in colonic epithelial cells. ETHE1, ethylmalonic encephalopathy protein 1; H2S, hydrogen sulfide; GSH, glutathione; GSSH, glutathione persulfide; O2, oxygen; Rhd, rhodanese; SO32−, sulfite; SO42−, sulfate; SUOX, sulfite oxidase; SSO32−, thiosulfate; SQR, sulfide: quinone oxidoreductase; III and IV, complex III and IV.
It is generally accepted that H2S binds to the SQR enzyme and is oxidized to sulfane sulfur in the first step of the oxidation pathway. However, there are different perspectives on what the physiological receptor of sulfane sulfur is. One view is that the sulfane sulfur generated by the reaction is directly transferred to glutathione (GSH) to form glutathione persulfide (GSSH) [59]. Subsequently, GSSH is oxidized by iron-dependent persulfide dioxygenase (PDO), also known as ethylmalonic encephalopathy protein 1 (ETHE1), regenerating GSH and producing sulfite [60,61], which is oxidized to sulfate by SUOX [62], or reacts with GSSH to generate GSH and thiosulfate under the catalysis of rhodanese (Rhd), or thiosulfate thiotransferase (TST) [59,63]. Another view is that sulfane sulfur uses sulfite as an acceptor to produce thiosulfate, which is then transferred to GSH to form GSSH [64,65]. Nevertheless, this view has been challenged by other studies. First, this indirect pathway was proposed based on in vitro kinetic data, and its research model was inconsistent with physiologically low intracellular sulfite concentrations [66]. Second, the rhodanese-catalyzed sulfur transfer reaction of thiosulfate to GSSH is kinetically unfavorable [59]. Finally, since sulfite itself is an oxidation product of sulfide and is inherently toxic, it is unlikely to be used as a substrate for sulfide oxidation [58]. Overall, the now widely accepted view is that GSH is the primary sulfane sulfur receptor for the SQR reaction, which directly generates GSSH as a product. However, it is worth noting that the importance of sulfite as a receptor in the SQR response may increase under pathological conditions that lead to elevated concentrations [58].
Colonic epithelium express higher levels of SQR compared with other tissues, which may be an adaptation to the colon microenvironment with high sulfide load [67]. The SQR reaction of intestinal epithelial cells prevents H2S accumulation and produces highly reactive persulfides, which may play an important role in H2S/polysulfide-mediated protein persulfidation, also known as S-sulfuration or S-sulfhydration [1,58,[68], [69], [70]]. In addition, H2S can be considered as an inorganic energy substrate for colonocytes [71]. Electrons extracted from H2S are injected into the mitochondrial respiratory chain via SQR during oxidative metabolism. At excessive concentration, H2S inhibits the catalytic activity of cytochrome c oxidase, leading to inhibition of colonocyte respiration. Whereas micromolar concentration of H2S is able to increase colonocyte respiration and to stimulate mitochondria allowing these cells to detoxify and to recover energy from luminal sulfide [ [[71], [72], [73], [74], [75]]]. Furthermore, the metabolic utilization of H2S by intestinal epithelial cells may play an important role in the regulation of overall H2S homeostasis in animals. The researchers found that plasma levels of free H2S and bound sulfane sulfur were significantly lower in germ-free mice compared to conventional mice, suggesting that microbial-derived intestinal H2S is closely related to the total circulating H2S in animals [76].
Notably, colonic epithelial cells can synthesize H2S via endogenous synthetic pathways (as described in Section 2.1) in addition to receiving exogenous H2S from their luminal side. Thus, the intracellular H2S concentration in colonocytes is determined by the diffusion of luminal H2S across the cell membrane, cysteine-based intracellular synthesis, and the cellular capacity to metabolize H2S.
3. The role of H2S in the pathophysiology of colorectal cancer
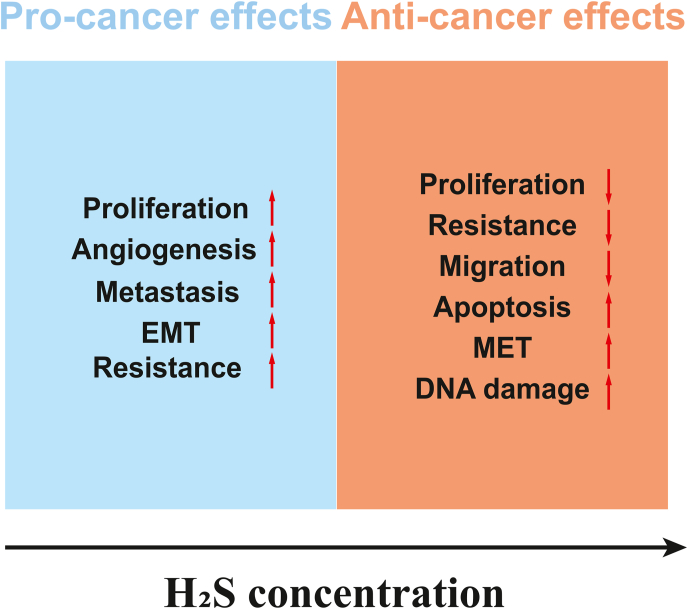
The biological effects of H2S show a typical bell-shaped concentration-response in cancer. Appropriate concentrations of H2S can promote cancer cell growth, stimulate cellular bioenergetics, enhance angiogenesis, induce dedifferentiation, invasion, and metastasis, and confer chemotherapeutic resistance. When the concentration of H2S exceeds a certain threshold, it exerts tumor suppressor effects such as reducing cancer cell proliferation, migration and bioenergetics, inducing cancer cells apoptosis, sensitizing cancer cells to chemotherapeutics, and inducing mesenchymal-to-epithelial transition (Fig. 5) [6,68,77].
Fig. 5.
The schematic diagram of bell-shaped effects of H2S on cancer. H2S promotes cancer growth within an appropriate concentration range while acting as a tumor suppressor above the concentration threshold. The models suggest that exogenous delivery of high doses of H2S by donors and reduction of endogenous H2S by scavengers or biosynthesis inhibitors represent two strategies to treat cancer.
H2S-mediated persulfidation (a post-translational modification of proteins) plays a role in regulating tumor growth and metastasis, such as mediating MEK1 persulfidation to regulate ERK1/2 activity and thus affect tumor growth [78,79], and mediating NF-κB persulfidation to induce metastasis-promoting gene expression [80,81]. H2S acts as a pro-angiogenic factor in vitro and in vivo under different physiological and disease conditions, including cancer. One possibility is H2S may mediate angiogenesis through persulfidation of Kir 6.1 subunit of KATP channel since inhibition of KATP channel attenuates VEGF mediated endothelial cell migration. The other possibility is the activation of NF-κB/IL-1β signaling through H2S-mediated NF-κB persulfidation [80,81], since IL-1β is a known pro-angiogenic cytokine through induction of VEGF during cancer progression [82]. Inhibition of mitochondrial complex IV is usually considered as the main mode of cytotoxic action of H2S, which leads to blocked mitochondrial electron transport and inhibits aerobic ATP production [83]. However, at lower concentrations, H2S can also act as a metabolic substrate to stimulate the mitochondrial electron transport chain. Oxidation of H2S by SQR leads to electron transfer to coenzyme Q, which promotes aerobic respiratory ATP synthesis [83,84]. In addition, H2S increases the catalytic activity of the mitochondrial ATP synthase through persulfidation [85]. In addition to stimulating mitochondrial ATP production, the role of endogenous H2S in cancer cells includes maintaining mitochondrial organization (preventing mitochondrial fission) and maintaining mitochondrial DNA repair (by stimulating the assembly of mitochondrial DNA repair complexes) [83]. In addition, H2S regulates the Wnt/β-catenin pathway, which is associated with differentiation/dedifferentiation of tumor cells, by inducing Sp3 transcription factors to activate the promoter of the ATP citrate lyase (ACLY) gene [86,87]. Notably, both endogenous H2S synthesis in colonic epithelial cells and exogenous H2S in the lumen are implicated in tumorigenesis in CRC [13,88].
3.1. Exogenous H2S and CRC
Exogenous H2S in the lumen is primarily derived from the gut microbiota, which is associated with the occurrence of CRC [13,89,90]. It has been shown that gastrointestinal exhaust samples recovered from CRC patients contained higher levels of sulfur-containing compounds compared to samples recovered from healthy subjects [91]. Moreover, the colons of CRC patients had a reduced ability to detoxify H2S [92]. Another study found that fecal H2S levels were significantly higher in patients with colon tumors and sigmoid surgery compared to the healthy population [93]. However, owing to the good volatility of H2S, direct measurement of fecal H2S concentrations does not accurately reflect intestinal H2S levels. Hale et al. indirectly assessed H2S production by intestinal bacteria in CRC by quantifying the amino acids produced with H2S. The experimental results showed that the production of H2S originated from intestinal microbes was increased in CRC samples, compared to non-cancerous samples [94]. Unfortunately, the combination of only four amino acids (serine, homoserine, lanthionine, and cystathionine) does not fully respond to the intestinal H2S production. The available evidence suggests that H2S production by gut microbes is closely related to CRC. Nevertheless, studies directly assessing the role of intestinal bacterial sources of H2S on CRC are lacking. The precise quantification of the level of H2S production by intestinal bacteria remains a challenge.
In contrast, quantifying the relative abundance of H2S-producing bacteria is much less challenging [94]. Multiple studies investigating changes in the gut microbiota of colonic adenomas and/or CRC found that some major groups of sulfur-producing bacteria (eg, Bilophila, Desulfovibrio, Bilophila wadsworthia, Fusobacterium) levels increased in those with adenomatous polyps (the most common precursor of CRC) [11,23,[95], [96], [97], [98], [99], [100]]. Among them, Bilophila, Desulfovibrio, and Bilophila wadsworthia are important H2S-producing bacteria (their mechanisms of H2S production have been discussed in Section 2.2). Fusobacterium is a periodontal disease-related H2S producer, which has attracted wide attention in recent years due to its close relationship with CRC promotion [ [99,101,102]]. These gut bacteria involved in sulfur metabolism use dietary and endogenous organic and inorganic compounds to produce H2S, which may be the key linking diet, microbiota, and CRC [11,12,97]. In fact, dietary habits, as mediated by intestinal sulfur metabolizing bacteria, have been linked to an increased risk of early-onset adenomas and CRC [12,23]. Long-term adherence to a sulfur microbial diet (characterized by high intakes of low-calorie beverages, red and processed meats, and low intakes of fruits, whole grains, and vegetables, as well as relative abundance of metabolic gut bacteria) is associated with an increased risk of distal CRC and rectal cancer [97]. Another study, which included men and women and used larger, more diverse data as well as an analysis method that accommodated multiple cohorts, found that higher adherence to a sulfuric microbial diet was associated with higher risk of developing distal CRC [103]. Follow-up studies are needed to determine the specific mechanism of action of H2S as an associated factor in linking dietary pattern-gut microbiota-CRC.
Donors that release H2S rapidly or slowly can be used to mimic the effect of exogenous H2S on CRC cell lines. A study reported that treating HCT116 cells with low concentrations (0.3 mM) of GYY4137, a slow-release H2S donor, enhanced mitochondrial function and glycolysis while also encouraging cell proliferation [104]. Furthermore, H2S donor stimulated cell proliferation using 0.2 mM NaHS on the same cell line by increasing Akt and ERK activation while inhibiting p21 (Waf1/Cip 1) [105]. Likewise, treatment of colon tumor cells with exogenous H2S at a concentration of 1 mM using NaHS as a donor inhibited the chemo-preventive PEITC-induced apoptosis [106]. The effects of H2S are biphasic, concentration- and time-dependent. Exogenous H2S stimulation at optimum quantities can enhance the proliferation of CRC cells, while high concentration and sustained H2S therapy can decrease the proliferative vitality of tumor cells. Szabo et al. evaluated the effect of different concentrations of NaHS (fast-release) or GYY4137 (slow-release) H2S donors on the proliferation of the human colon cancer cell line HCT116. The results showed that low concentrations (0.03–0.3 mM) of NaHS stimulated tumor cell proliferation, while higher concentrations (1–3 mM) inhibited tumor cell growth. In the case of pretreatment with the CBS inhibitor Aminooxyacetic acid (AOAA), not only the basal proliferation of HCT116 cells was attenuated, but also the toxic effect of higher concentrations of NaHS on the cells was diminished. In addition, without AOAA pretreatment, 0.03–3 mM of GYY4137 stimulated the proliferation of HCT116 cells. In contrast, only 3 mM of GYY4137 stimulated HCT116 cell proliferation beyond the basal growth rate after using AOAA [107]. These studies on the mechanism of action of various H2S donors in CRC and their therapeutic applications will be described in detail later.
3.2. H2S endogenous synthesis pathway and CRC
Several experimental and clinical studies have shown that the expression of H2S endogenous synthase CBS is higher in colon tumor tissues than in normal surrounding tissues [ [82,[108], [109], [110]]]. Moreover, CBS levels are closely related to CRC severity/tumor stage as well as patient survival [47,82]. These findings suggest that the CBS/H2S system is essential in the occurrence and progression of CRC. CBS-derived H2S has been shown in studies to be involved in maintaining the bioenergetics of CRC cells to support tumor growth and proliferation, as well as promoting angiogenesis and vasodilation to provide blood and nutrients to tumors [82]. In addition, some studies have pointed out that the CBS-H2S axis may regulate CRC angiogenesis and liver metastasis via positive feedback between the CBS-H2S axis and VEGF [111]. Considering the bell-shaped effect of H2S, colon cancer cell proliferation can be suppressed by up-regulating CBS expression or delivering H2S exogenously in a state where the CBS-H2S axis is already highly activated. The underlying mechanism could be that H2S inhibits the functional activity of transcription factor SP-1, thereby negatively regulating CD44 expression [112]. Similar bell-shaped functional responses were observed in HCT116 cells treated with the allosteric activator of CBS, S-adenosyl-l-methionine (SAM): lower concentrations/shorter incubation times stimulated HCT116 cell proliferation and bioenergetics, while higher concentrations and longer exposure to SMA inhibited cell viability and exerted cytotoxic effects [113]. Of course, whether the SAM's anti-proliferative mechanism is related to the “overstimulation” of the CBS-H2S axis is still controversial, and the current consensus is that it is more likely to be an independent mechanism involving CBS. According to a recent study on the oncogenic role of CBS, protein expression of CBS is low in healthy colonic mucosa, but gradually increases with epithelial cell transformation into polyps, hyperplastic polyps, tubular adenomas (dysplasia), and adenocarcinomas (in situ). Furthermore, up-regulation of CBS leads to metabolic reprogramming and the induction of an aggressive tumorigenic phenotype in precancerous colon epithelial cell lines, suggesting that activation of the CBS/H2S axis may promote colon carcinogenesis [114].
CSE, another key endogenous H2S synthase, is also expressed in colon tumor cell lines [82,105,115]. The role of CSE/H2S is currently less known. Activation of the Wnt/β-catenin pathway has been shown to up-regulate CSE expression and stimulate the proliferation of SW480 cells, a CRC cell line, in vitro migration and tumor xenograft growth in vivo [115]. Another study found that stable silencing of CSE via lentiviral transduction (shCSE) or the small molecule inhibitor dl-propargylglycine (PAG) had no effect on H2S production or cell proliferation in HCT116 cells [82]. Thanki et al. found that loss or inhibition of CSE function promotes excessive inflammation, leading to the development of colitis-associated cancer (CAC). Meanwhile, loss of CSE expression in bone marrow-derived cells alters the balance of mucosal IL-6 and IL-10 expression and accelerates the development and progression of CAC. These results suggest that CAC may result from a failure of normal physiological functions regulating intestinal immune homeostasis and tissue repair via CSE and H2S [116].
3-MST expression and/or catalytic activity has been demonstrated in several CRC cell lines (HCT116, LoVo, HT29, CT26) [6,117]. The recent discovery of the novel pharmacological inhibitor HMPSNE [118] has changed the lack of direct studies on the functional role of 3-MST. Szabo's group discovered that reduction of H2S biosynthesis by inhibiting the catalytic activity of 3-MST could promote mesenchymal-epithelial transition (MET) in HCT116 cells [87]. The MET regulation mechanism could be related to the involvement of endogenous H2S in the regulation of ACLY protein expression. ACLY regulates the Wnt/β-catenin pathway, which is an important regulator of EMT/MET balance. In addition, the 3-MST/H2S system maintains tumor cell proliferation, migration, and cellular bioenergetics in murine CRC cell CT26 [117].
3.3. H2S oxidation pathway and CRC
In normal colonic epithelium, the sulfide oxidation pathway is mainly located apically at the host-microbiota interface, i.e., the human colonic crypt [13,67]. In contrast, the expression of three enzymes involved in oxidative metabolism (SQR, ETHE1, and TST) was elevated in CRC tissues and showed a diffuse distribution. Moreover, elevated expression of SQR and ETHE1 was observed in all six CRC cell lines (HT29, LoVo, Caco-2, RKO, DLD-1, and HCT116), compared to the non-cancerous colon cell line HCEC [1]. It has been noted that ETHE1 expression is significantly increased in CRC and increases with CRC tumor grade compared to benign colonic epithelium [119]. It has also been found that human colon cancer cells HT-29 Glc (−/+) have an enhanced ability to oxidize sulfide when differentiated spontaneously or induced by butyrate [75]. These results suggest that enhanced SOU function is associated with the development of CRC. However, a different perspective was proposed by Piran et al. They found through meta-analysis that the expression of SQR, ETHE1, and TST genes tended to decrease during the process of colon tumors from normal to primary and then to liver and lung metastases. They speculated that H2S is a supplementary energy source in the hypoxic environment of colon tumors. Unlike normal colon cells exposed to tubular H2S, CRC cells may need to avoid excessive consumption of H2S by reducing sulfide metabolic function during tumorigenesis and metastasis, which helps CRC cells survive from a hypoxic environment [120]. Szabo's group found that in human intestinal epithelial-like organs with harboring early mutations of the Vogelstein sequence, upregulation of H2S synthase and metabolizing enzymes occurred simultaneously, and live cell imaging did not detect a significant increase in cellular H2S levels. But, in organoids with late mutations, the expression of H2S synthase was further increased, while the expression of certain H2S metabolizing enzymes (e.g., SQR) was reduced and cellular H2S levels were elevated. This consecutive mutations leads to effects that coincide with the upregulation of cellular bioenergetics (mitochondrial respiration and/or glycolysis) and the upregulation of the Wnt/β-catenin pathway, a key effector of EMT [121]. This study contributes to the proper understanding of the important role of H2S synthesis and metabolism in the pathogenesis of CRC.
In conclusion, the current study suggests that the oxidative metabolic pathway of H2S is closely related to the development of CRC. However, direct experiments to validate the role of oxidative pathway-related enzyme systems on CRC, such as observation of the effect of SQR catalytic activity inhibition on CRC by suitable SQR inhibitors, are lacking. Several recent studies have pointed to selective inhibitors of SQR as an attractive target for drug development and have provided some SQR inhibitor candidates, such as STI1, which can bind highly selectively to the coenzyme Q-binding pocket in human SQR [[122], [123], [124]]. Unfortunately, there are no reports of these inhibitors being used in CRC-related biological experiments. It is also important to note that inhibition of the function of these enzymes may to lead to some undesired effects, for example, the restricted function of ETHE1 may lead to the accumulation of H2S in the colonic mucosa, liver, muscle, and brain [125,126].
3.4. Persulfidation, persulfides, polysulfides and CRC
Persulfidation (S-sulfuration) is a post-translational modification of proteins characterized by the addition of sulfur atoms to specific cysteine residues via H2S or polysulfides (mainly polysulfides), resulting in peroxisulfide adducts on small molecules and proteins [1,[127], [128], [129]]. This process is considered to be a key step in the biological function of reactive sulfur species [69,70,130,131]. Some of the enzymes involved in H2S anabolism (mainly 3-MST, SQR), as well as some other proteins (haemoglobin, neuroglobin) and enzymes (catalase, peroxidases, super oxide dismutase, and cysteine tRNA synthetase), have been identified to produce polysulfides, GSSH, and other S-sulfurated molecules (S-sulfated molecules) [66,132,133].
These generated polysulfides, persulfides, have recently been recognized to play an important role in cancer regulation [68,70,131]. Cysteine-rich angiogenic inducer 61 (CyR61) is a matricellular protein encoded by the CYR61 gene, which promotes colon cancer cell migration, invasion and metastasis, and its high expression is associated with poor CRC prognosis [ [[134], [135], [136], [137]]]. Recent studies suggest that H2S and polysulfides activate the CyR61 promoter in colon cancer cells. Endogenous H2S/polysulfide biosynthesis of 3-MST in colon cancer cells facilitates the induction of CyR61 mRNA, most likely through the persulfidation of Sp1, and through the activation of S1PR, ATF1 and CREB [137]. It has also been shown that in colon cancer cells, the trans-sulfuration pathway –H2S axis stabilizes xCT (the functional subunit of system Xc-) through the persulfidation of OTUB1, which imports cysteine and converts it to cysteine, providing a substrate for endogenous H2S biosynthesis. Increasing xCT and the transsulfuration pathway are associated with the metabolic reprogramming of CRC [138]. Furthermore, it was revealed that the stimulatory effect of low concentrations of exogenous H2S on glycolysis may be due to H2S/polysulfide-mediated persulfidation of lactate dehydrogenase, which enhances its catalytic activity [104]. N-acetylcysteine (NAC) is a commonly used antioxidant in biological experiments as well as in clinical studies [139,140]. NAC treatment was found to promote the expression activity of SW480 cell line 3-MST and SQR, enzymes involved in the production and consumption of H2S and thiane sulfur. Moreover, NAC can act as a direct thiane sulfur accepting co-substrate for 3-MST and undergo persulfidation. The NAC persulfide formed is hypothesized to promote resistance to oxidative stress and increase drug resistance in colon cancer cells, given the specific novelty of the reaction of the persulfide with electrophilic reagents and the reference to the already reported cysteine persulfide [141]. Thus, further investigations are necessary to validate this hypothesis and whether the effect of NAC on colon cancer cells is mediated through H2S and related active substances.
Many natural polysulfides have antitumor effects. Studies have pointed out that in CRC, diallyl polysulfides (garlic-derived organosulfur compounds) and their derivatives can exert anticancer activity by inducing cell growth arrest and apoptosis [[142], [143], [144]]. Moreover, diallyl sulfide (DAS), diallyl disulfide (DADS) and diallyl trisulfide (DATS) could promote the expression of multidrug-resistant genes in colo 205 human colon cancer cells [145]. In addition, dibenzyl tetrasulfide, a derivative of diallyl tetrasulfide, was also identified as a mitogenic inhibitor and apoptosis inducer in colon cancer cells and induced cell type-dependent autophagic damage involving p62 [146].
4. H2S diagnosis and treatment strategies for colon cancer
Existing studies have shown that the moderate supply of exogenous H2S and the elevated endogenous concentrations of H2S may be involved in promoting the occurrence of CRC. Increased H2S production in colon tumor cells is ascribed to increased activity of endogenous H2S synthases (mainly CBS). These provide new ideas for the diagnosis and treatment of CRC: strategies for H2S modulation (exogenous delivery of high doses of H2S or reduced expression of endogenous H2S) in colon tumor treatment, and using the high level of H2S in CRC tissue cells to develop diagnostic probes and combination therapies that respond to endogenous H2S.
4.1. Inhibition and clearance of endogenous H2S
The reduction of endogenous H2S is currently receiving wide attention as a method of applying H2S regulation to tumor therapy [147]. In related studies, a common and well-established approach is to inhibit the endogenous biosynthesis of H2S. To this end, a series of small molecule inhibitors were screened and synthesized to inhibit three key enzymes involved in endogenous H2S synthesis, CBS, CSE, and 3-MST. Hence, another more desirable approach is the development of endogenous H2S scavengers. Targeted H2S scavenging is achieved by specific, rapid-response, targeted endogenous scavengers. In this section, we summarize and discuss reports on the application of inhibition of H2S production in the field of CRC, focusing on H2S-producing enzyme inhibitors and H2S scavengers (Table 1).
Table 1.
Summary of recent reported CRC treatment strategies via H2S modulation.
| Therapeutic agent | Therapeutic strategy | Anticancer mechanism |
|---|---|---|
| AOAA | Endogenous H2S biosynthesis inhibitors | Anti-proliferative activity; Enhanced sensitivity to oxaliplatin via exaggerating apoptosis induced by ROS. |
| AOAA derivatives | Endogenous H2S biosynthesis inhibitors | Anti-proliferative activity. |
| Benserazide and its metabolite, THBH | Endogenous H2S biosynthesis inhibitors | Anti-proliferative activity; Therapeutic effects on cancer cells with a multidrug-resistant phenotype. |
| EGCG | Endogenous H2S biosynthesis inhibitors | Anti-proliferative activity; CBS specific inhibitor. |
| PAG | Endogenous H2S biosynthesis inhibitors | Anti-proliferative activity. |
| HMPSNE | Endogenous H2S biosynthesis inhibitors | HMPSNE exhibited anti-proliferative activity and induced MET through the inhibition of 3-MST, CBS and key enzymes involved in H2S degradation ETHE1 and TST. |
| HMPSNE derivatives | Endogenous H2S biosynthesis inhibitors | Anti-proliferative activity. |
| CuDDC | Endogenous H2S biosynthesis inhibitors; H2S scavenger | Anti-proliferative activity. |
| VZnO | H2S scavenger | Anti-proliferative activity. |
| EA-Fe@BSA | H2S scavenger | Anti-proliferative activity; CDT/PTT; MRI imaging. |
| PMSN-Fe-LA-BSA | H2S scavenger | Anti-proliferative activity; CDT/PTT. |
| HKUST-1 | H2S scavenger | Anti-proliferative activity; CDT/PTT. |
| 2D Cu-MOF | H2S scavenger | Anti-proliferative activity; CDT/PTT; PA imaging. |
| ADT-OH conjugate | H2S donor | Anti-proliferative activity. |
| SATO | H2S donor | Anti-proliferative activity. |
| SATO-functionalized micelles | H2S donor | Anti-proliferative activity. |
| Natural polysulfides | H2S donor | Anti-proliferative activity. |
| HS-NSAIDs | H2S donor | Induced cell proliferation inhibition, apoptosis and G0/G1 phase cell cycle arrest; Phase II metabolic enzyme inducers; Synergistic treatment with NO. |
| NiNPs | Carrier | H2S promoted the anticancer efficiency of 5-FU in the presence of NiNPs. |
4.1.1. CBS inhibitors
AOAA is one of the best-known CBS inhibitors. In aqueous solution, AOAA is able to react with the aldehyde group of vitamin B6 (pyridoxal form) and generate a stable oxime [148]. As the metabolically active form of vitamin B6, PLP is involved in a series of enzymatic reactions in biological systems and is a cofactor of enzymes such as CBS and CSE. Therefore, in the presence of AOAA, oxime is produced irreversibly, inhibiting the CBS-catalyzed H2S production. In the field of colon tumor research, Szabo et al. reported that the use of AOAA could inhibit CRC cell proliferation in vitro and reduce tumor growth in vivo [82]. Zhao et al. treated HCT116 cells with AOAA and examined the ability of cancer cells to target H2S probes through the negative control [149]. Yue et al. found that AOAA sensitizes CRC cells to oxaliplatin via exaggerating apoptosis induced by ROS both in vitro and in vivo [150]. But it is worth noting that AOAA is not a specific inhibitor of CBS, and it inhibits a variety of PLP-dependent enzymes, including CSE. Moreover, AOAA exhibited higher inhibitory potency to CSE than CBS (CSE's IC50 is 1 μM in the same report where CBS's IC50 is 8 μM) [151]. In addition, AOAA can also indirectly inhibit H2S formation by the 3-MST system via inhibiting the enzymatic generation of the 3-MST substrate, 3-MP. Furthermore, AOAA can even inhibit some non-enzymatic pathways of H2S generation [46]. In addition, the poor lipophilicity of AOAA (water/octanol coefficient: 0.0019) makes its action in cells inefficient. In HCT116 cells, up to 100 μM AOAA was required to significantly inhibit H2S production, which may be attributable to the low cellular uptake [147].
To improve its lipophilicity, Szabo's group designed and synthesized a series of AOAA-based prodrugs that release AOAA after penetrating the cell membrane and hydrolyze the ester bond on the prodrug by cellular esterases [110,152]. Among them, YD0171, a methyl ester derivative of AOAA, possesses stronger lipophilicity and higher inhibition efficiency compared with AOAA. In the treatment of HCT116 cells, CBS-induced inhibition of H2S production is inhibited with 30 μM YD0171, whereas a similar effect required 100 μM AOAA. The in vivo efficacy is nine times higher than that of AOAA. At the same time, YD0171 administration has low systemic toxicity and can target the metabolism of colon tumors [152]. YD0251, an isopropyl ester derivative of AOAA, inhibits HCT116 tumor growth in vivo 18 times more potently than AOAA and exerts anticancer effects at doses that do not induce severe organ damage. It also inhibited the growth of patient-derived tumor xenografts and exerted anti-proliferative effects on CRC cells with a multidrug-resistant phenotype. Furthemore, they proposed that matching CBS inhibitor therapy to patients with high CBS expression could be a useful application for future CRC therapeutic diagnosis. Likewise, they identified lanthionine, a metabolic intermediate of CBS-mediated H2S biosynthesis, as a suitable biomarker for identifying CRC target populations with high CBS expression [110].
Some hydrazine derivatives can antagonize the action of vitamin B6. Among them, benserazide was found to inhibit CBS activity and suppress colon cancer cell proliferation and bioenergetics in vitro, as well as tumor growth in vivo [153,154]. It can bind to the active site of the enzyme and inhibit CBS by forming a reversible but kinetically stable Schiff base-like adduct with the formyl portion of pyridoxal, and reacting with PLP cofactors. Moreover, its main metabolite, 2,3,4-trihydroxybenzylhydrazine (THBH), can act as a CBS inhibitor and an anti-proliferative agent for CRC cells. In buffer, the inhibitory effect of benserazide on CBS (IC50: ∼30 μM) was much lower than that of AOAA (IC50: ∼3 μM). However, it inhibited the proliferation of HCT116 colon cancer cells (IC50: ∼20 μM) with greater potency than AOAA (IC50: ∼300 μM) in cytological assays, probably owing to the good cellular uptake. Meanwhile, benserazide showed some selectivity for CBS, with the activity inhibition of CSE and 3-MST being 16% and 35%, respectively, after 2 h of 100 μM benserazide treatment, while the activity inhibition of CBS was as high as 66%. It is noted that this conclusion was drawn from the comparison under different substrate concentration conditions, and the inhibition of benserazide is related to the substrate concentration, which remains to be further verified [147]. The efficacy of many anticancer drugs is reduced when cancer cells have a multidrug-resistant phenotype. Szabo's group evaluated the role of AOAA and benserazide in multidrug-resistant phenotypic tumor cells by constructing a 5-FU resistant HCT116 cell line that also exhibited partial resistance to the unrelated chemical oxaliplatin. They found that 5-FU resistance in HCT116 cells was associated with up-regulation of drug-metabolizing enzymes and enhanced endogenous H2S production. Moreover, 5-FU-resistant cells showed reduced sensitivity to AOAA, but remained sensitive to the anti-proliferative effect of benserazide. This contributes to the development of new strategies for the treatment of advanced CRC [155].
Epigallocatechin gallate (EGCG) is a major bioactive component of green tea with antioxidant, anti-inflammatory, anticancer, and anti-neurodegenerative effects [ [[156], [157], [158], [159]]]. Moreover, the antiproliferative effect of EGCG is known to be more effective than a chemotherapeutic drug, 5-FU, on CRC [160]. Many of the biological effects of EGCG have been attributed to binding to or sterically interfering with certain enzymes or other regulatory proteins, because they can form covalent adducts with protein and non-protein thiols [161]. In the past, many polyphenolic compounds similar to EGCG were selected in the screening of CBS inhibitors, but most of them were not as effective as the most commonly used AOAA for inhibition. In contrast, EGCG inhibits CBS-catalyzed H2S production at sub-micromolar concentrations (IC50: 0.12 μM) and has been shown to significantly inhibit H2S production in CRC or Down syndrome cell models in vitro [162]. Remarkably, unlike AOAA and its derivatives or hydrazine CBS inhibitors, the inhibition of CBS by EGCG does not target PLP or response mechanisms, but exploits certain structural or conformational features unique to CBS, which makes it a CBS-specific inhibitor. In addition, it has been proposed that EGCG can oxidize H2S produced by CBS to form polysulfides [161], indicating that EGCG may act as a scavenger of H2S. Nonetheless, a study by Szabo's group discovered that EGCG exhibited some H2S scavenging activity only at higher concentrations and also inhibited non–H2S–generating HsCBS, indicating that it is a true CBS inhibitor [162]. Although EGCG and other polyphenolic bioflavonoids usually have a variety of pharmacological targets and actions, their therapeutic effects on CRC may not be attributed to CBS inhibition alone. Putting aside the PLP, the studies on EGCG still provide a new idea for the development of CBS inhibitors by targeting specific sites on CBS, resulting in the development of new potent CBS-specific inhibitors.
There are many challenges in translational application of EGCG, including the low systemic bioavailability, less stability in alkaline media, low membrane permeability, high oxidative degradation, and metabolic transformations [156]. A clinical trial of EGCG showed that all the patients treated with EGCG could not respond to it as this unstable EGCG exhibited alterable bioavailability [163]. Therefore, how to improve the stability and bioavailability of EGCG is the focus of further research.
4.1.2. CSE inhibitors
There are few reports on the application of CSE inhibitors in the treatment of CRC, due to the lack of direct evidence to prove that CSE plays a significant role in CRC. dl-propargylglycine (PAG) is an early discovered irreversible CSE inhibitor [164]. Unlike most PLP-dependent enzyme inhibitors, PAG irreversibly changes the CSE active center, thereby inactivating enzyme function. The whole inhibition process is closely related to several key amino acid residues of CSE, including Arg 62, Lys 212 and Tyr114 [147]. Fan et al. reported that treatment of SW480 cells with CSE inhibitors PAG or shCSE effectively reduced tumor cell proliferation, migration, and in vivo tumor xenograft growth [115]. However, this PAG or shCSE-mediated CSE silencing was ineffective in inhibiting H2S production or cell proliferation in HCT116 cells [82]. The role of CSE inhibitors in the treatment of CRC remains to be explored.
4.1.3. 3-MST inhibitors
High throughput screening has been employed to find suitable endogenous H2S synthase inhibitors. Hanaoka et al. identified four novel 3-MST inhibitors with IC50 values in the micromolar range by high-throughput screening of a library of 174,118 compounds using the H2S-selective fluorescent probe HSip-1. Among them, (2-[(4-hydroxy-6-methylpyrimidin-2-yl)sulfanyl]-1-(naphthalen-1-yl)ethan-1-one) or HMPSNE is considered to be the most effective and selective [118]. Inhibition of H2S biosynthesis in CRC cell lines by HMPSNE inhibits tumor cell proliferation, migration, and induces MET [87,117]. Particularly, when treated with 300 μM HMPSNE, expression inhibition of 3-MST, CBS and key enzymes involved in H2S degradation ETHE1 and TST was observed in HCT116 cells. The effect of HMPSNE on HCT116 cells at this concentration must be explained by the direct inhibition of 3-MST catalytic activity and the combined effect of CBS down-regulation [87]. Bantzi et al. synthesized a library of 63 compounds using the central core of HMPSNE (renamed 1a) to independently modify the pyrimidinone and aryl ketone sides, and then evaluated in vitro the efficacy of these new derivatives as 3-MST inhibitors and their effects on CRC cell proliferation and viability [165]. Six novel 3-MST inhibitors were found to be effective in inhibiting the proliferation of two mouse CRC cells (MC38 and CT26). Although the inhibitory effect of compound (1 b) on recombinant 3-MST was not as good as that of starting compound 1a, it was more effective than 1a in inhibiting tumor growth in tumor-bearing mice in vivo.
4.1.4. H2S scavengers
The ideal scavenger should have high reactivity and high selectivity to H2S, and the product after the reaction should have the lowest biological activity [147]. Researchers have developed some small molecule endogenous H2S scavengers based on sulfonyl azides [166] or NBD [167], because these properties are very similar to those of H2S sensors. Szabo ‘s group found that while the clinical drug disulfiram did not directly inhibit H2S production in CRC cells, its metabolite bis(N, N-diethyldithiocarbamate)-copper (II) complex (CuDDC) could be used as an effective inhibitor of CBS and CSE as well as an H2S scavenger to inhibit CRC cell proliferation [168].
Thanks to the development of modern nanotechnology, researchers have begun to explore the direct elimination of endogenous H2S by appropriate nanomaterials. Zinc oxide has more prominent desulfurization performance than other metal oxide desulfurizers [169], and has better biocompatibility and low toxicity when made into nanoparticles [170]. Based on these characteristics, Pan et al. developed a ZnO-coated virus-like silica (VZnO) nanoparticle H2S-responsive nanoplatform for CRC treatment. After entering the cells, VZnO can reduce the level of endogenous H2S, lead to a significant decrease in intracellular GSH levels, and ultimately lead to ferroptosis in CRC cells. They injected fluorescently labeled VZnO@FITC intravenously into a mouse model of CRC to evaluate the tumor selectivity of this scavenger. The results showed that the tumor tissues had the highest uptake of VZnO@FITC compared with most normal tissues, except the liver. Moreover, the scavenger has no therapeutic effect on the 4T1 breast cancer model (non H2S rich cancer), suggesting that it may be selective for cells with high H2S levels [171].
Chemodynamic therapy (CDT) is a highly tumor-specific and minimally invasive treatment modality. It uses Fenton or Fenton-like drugs to degrade the highly expressed hydrogen peroxide (H2O2) in tumors and generate highly toxic hydroxyl radicals (•OH) to kill cancer cells [ [[172], [173], [174]]]. However, its therapeutic efficacy is limited by the low efficiency of •OH production. In particular, in CRC, the high expression of H2S with strong reducibility leads to the depletion of the generated •OH, which further weakens the efficacy of CDT [175]. Therefore, a therapeutic strategy combining endogenous H2S scavengers, CDT, and photothermal therapy (PTT) has been proposed for CRC treatment. The key to this effective strategy is that the heating effect of activated PTT can accelerate •OH production while depleting endogenous H2S, thus improving CDT efficiency and providing better synergistic therapeutic effects.
The classical CDT based on Fe(II)-mediated Fenton reaction is strictly limited by the catalytic efficiency of Fe(II). To this end, Yang ‘s group assembled EA-Fe@BSA nanoparticles (NPs) using natural polyphenols, Fe(III), and albumin [176]. These NPs have excellent T1-weighted MRI performance as well as enhanced CDT effects through the combined effect of the strong reducing properties of high concentrations of H2S in CRC, which accelerates Fe(III)/Fe(II) conversion, and the thermal effects of PTT. This holds great potential for effective colon cancer theranostics. In addition, Wang et al. designed an iron-triggered tumor microenvironment (TME)-responsive PMSN-Fe-LA-BSA (PMFLB) nanotherapeutic agent combined with CDT/PTT for colon cancer [177]. It is loaded with Fe3+ by polydopamine (PDA) decorated mesoporous silica nanoparticles (PMSN), and uses the phase change ligand lauric acid (LA) to prevent Fe3+ leakage. Under NIR laser irradiation, PDA generates a large amount of heat to kill colon tumor cells by hyperthermia, and induces the phase transition of LA to release Fe3+. Fe3+ further reacts with endogenous H2S in TME to form Fe2+, which reacts with H2O2 in TME to form •OH and is then converted back to Fe3+. This repeated reaction with endogenous H2S produces more •OH, thus enhancing CDT.
Cu(II)/Cu(I) pair has lower redox potential (−1.6 eV) and higher catalytic activity than Fe(II)-based Fenton reagents [178,179]. Li et al. developed a non-photoactive copper-based metal-organic framework named HKUST-1, which can be activated by endogenous H2S in colon tumors to produce photoactive CuS for thermal imaging and PTT [180]. At the same time, HKUST-1 exhibits horseradish peroxidase (HRP) mimetic activity that effectively converts overexpressed H2O2 in CRC cells into a more toxic •OH, as well as synergistic therapy of endogenous H2S scavenging, PTT and CDT. Wang et al. also designed a two-dimensional (2D) Cu-bipyridine [Cu(bpy)2(OTf)2] metal-organic framework (2D Cu-MOF) nanosheet for combination therapy [175]. This nanotherapeutics can significantly improve the applicability of CDT in CRC treatment by inhibiting the consumption of the generated •OH through rapid depletion of H2S via 2D Cu-MOF, promoting the Fenton-like reaction by ultra-small CuS produced by in situ vulcanization, and generating CuS with good photothermal properties because of its strong NIR-II absorption.
4.2. H2S donor therapy
H2S donor therapy has gained widespread attention in recent years as an important gas chemical transmitter. H2S donors, i.e., exogenous compounds that can release H2S, are the key to H2S gas treatment. Considering the intricate mechanism of the H2S dose response, its effects are dual in nature and are influenced by many factors such as in vivo concentration and biodistribution [181]. Targeted delivery and controlled release of H2S is thus critical for efficient and safe precision gas therapy. To this end, scientists have developed a number of small molecules or macromolecular H2S donors based on biocompatible polymeric materials that can respond to various stimuli, such as pH, thiol-containing compounds, enzymes, and other pathological microenvironments, as well as external stimuli, such as light and ultrasound, resulting in a controlled release of H2S gas [182]. In this section, we review the application of small and large molecule H2S donors with different activation mechanisms in the field of CRC research (Table 1) and discuss some new strategies for H2S donor therapy in CRC.
Inorganic sulfide salts, e.g., Na2S and NaHS, are the simplest and most common class of donors in H2S-related biological studies. Although commonly categorized as H2S donors, these sulfide salts are a direct source of H2S once dissolved in water. They immediately hydrolyze and establish an equilibrium between H2S, HS−, and S2−, followed by rapid volatilization of H2S [183]. These gaseous H2S substitutes have now been widely used to assess the therapeutic efficacy of exogenous H2S in a variety of cancer studies, including CRC [184]. However, simple sulfide salts are flawed as chemical tools for studying the biology of H2S and as potential therapeutics. Due to their direct and rapid gas release mode, they are difficult to use to mimic the biological effects of endogenous H2S, and they usually fail to maintain the therapeutic effect and may cause side effects. Furthermore, because sulfide salts lack targeting ability, they are only useful for systemic delivery [185].
Morpholin-4-ium 4 methoxyphenyl (morpholino) phosphinodithioate (GYY4137) is a class of Lawson's reagent-derived hydrolysis-activated H2S donors with slow-release properties that allow for the slow release of H2S in aqueous solution [186]. It showed in vitro killing effects on several types of cancer cells, including HCT116 [187]. GYY4137 also exhibited anti-proliferative activity against Caco-2 cells, possibly through the production of H2S-induced G2/M phase cell cycle arrest, apoptosis, and necrosis [188,189]. In addition, NHE1 internalization in colorectal cancer DLD1 cells and uncoupling of the NCX1/NHE1 complex after treatment with GYY4137 resulted in intracellular acidification and apoptosis [190]. Researchers have also proposed a new combination therapy strategy based on the mechanism of action of GYY4137 in CRC. Kajsik et al. reported that GYY4137 could be used to increase the sensitivity of CRC cells to the potent chemotherapeutic agent paclitaxel, which is commonly used in the treatment of a variety of cancers but has low efficacy in CRC [191].
Dithiothiones (DTTs), represented by anethole dithione (ADT), are generally considered as hydrolysis-activated H2S donors. However, recent studies have shown that H2S release from ADTs may occur through an enzymatic process in the presence of hepatic microsomes and reduced NADPH [182]. Li et al. designed and synthesized three series of the H2S-releasing oridonin derivatives using its demethylated analogue (ADT-OH). Among them, compound 12 b showed strong anti-proliferative effect in Caco-2 or HCT116 cells and low toxicity to two human normal cells, making it a potential anticancer candidate [192]. In addition, Hu et al. proposed a new strategy to design H2S donor-based antitumor drugs by combining a potent natural compound evodiamine with the H2S donor ADT-OH or α-thioctic acid. They tested the anti-proliferative activity of all synthesized new compounds in five human cancer cell lines and normal cells, and found that the most effective ADT-OH conjugate 12c exhibited significant cytotoxicity against both Caco-2 and HL-60 cell lines with IC50 values of 2.02 and 0.58 μM, respectively. And its enhanced anti-proliferative efficacy accompanied by increased selectivity is a class of promising antitumor candidates [193].
Some H2S donors can be reduced by thiol-containing bioactive compounds lke Cys or GSH to release H2S, resulting in a smart polymeric H2S delivery system for therapeutic applications. For example, S-aroylthiooxime (SATO) can react with Cys-containing compounds to release H2S [194] and reduce the proliferation viability of HCT116 cells [195]. In addition, natural polysulfides, such as diallyl trisulfide (DATS), are thiol-activated H2S donors [196], which are cytotoxic to cancer cell lines like HCT116 [ [[197], [198], [199]]].
Hydrogen sulfide-releasing non-steroidal anti-inflammatory drugs (HS-NSAIDs) are a special class of anticancer drugs that covalently link traditional non-steroidal anti-inflammatory drugs with hydrogen sulfide-releasing parts. Kashfi's group reported that H2S-donating aspirin derivatives (HS-ASA) can act as phase II metabolic enzyme inducers for HT29 cells, with great potential for CRC prevention [200]. They also investigated the effects of four different HS-NSAIDs (HS-aspirin, -sulindac, -iburofen, -naproxen) on the growth characteristics of different human cancer cell lines. The results showed that HS-NSAIDs inhibited cell proliferation, induced apoptosis, and caused G0/G1 cell cycle arrest in a variety of cancer cells [201]. Moreover, it has higher anti-inflammatory activity, tumor growth inhibitory potency, and chemo-preventive effects than conventional counterparts [ [[201], [202], [203], [204]]]. Based on NO-releasing NSAIDs (NO-NSAIDs) and HS-NSAIDs, a new strategy for the synergistic cancer treatment with NO and H2S was proposed. Kashfi's group designed and synthesized a hybrid of NOSH-NSAIDs that can simultaneously release NO and H2S, including NOSH-aspirin, NOSH-sulindac, and NOSH-naproxen for anti-inflammatory and antitumor studies [205,206]. After a series of in vivo and in vitro studies, these NOSH-NSAID drugs showed excellent performance in inhibiting the growth of CRC cells and are a class of drugs with considerable potential in the prevention and treatment of CRC [ [[205], [206], [207], [208], [209], [210], [211], [212]]].
Despite the great progress in the development of small molecule H2S donors, they usually do not meet the requirements for in vivo applications considering the stability, water solubility, trigger specificity, toxicity, and by-products generated upon H2S release of the donors themselves. Therefore, macromolecular/supramolecular H2S donor systems attracted a lot of interest from researchers [213]. For direct release of H2S from Na2S and NaHS, polymeric carrier encapsulated delivery is an effective strategy for achieving controlled H2S delivery. Other sulfide salts, such as MnS, FeS, and ZnS, are less water soluble but slowly hydrolyze at acidic pH to generate H2S and metal ions, and have been used to develop pH-responsive nanotherapeutic release [182]. Foster et al. prepared an H2S-releasing micelle from SATO-functionalized polymeric amphiphiles as an in vitro or potential in vivo targeting delivery tool. The micelles were selectively toxic to cancer cells and reduced the survival of CRC cells (HCT116) more significantly than other common H2S donors including Na2S, the small molecules SATO and GYY4137 [195]. Furthermore, Housein et al. evaluated the effects of different combinations of nickel nanoparticles (NiNPs) with 5-FU, H2S, and NO donors on CRC cells. They found that H2S promoted the anticancer efficiency of 5-FU in the presence of NiNPs, while NO had anti-apoptotic activity on CRC cell lines [214]. This provides a new idea for H2S donor therapy in combination with chemotherapy. In the future, a new combination therapeutic nanoagent that delivers both H2S and chemotherapeutics can be developed for colon tumors by loading NiNPs with H2S donors and 5-FU.
Overall, all donors mentioned in this section failed to achieve targeted delivery and controlled release of H2S in vivo, even with SATO-functionalized micelles, which were only evaluated in vitro on the selectivity for HCT116 and mouse embryonic fibroblasts NIH/3T3. Indeed, targeted and controlled delivery of H2S by nanotechnology is feasible. For example, Li et al. provided a strategy combining H2S donor therapy, PTT, and imaging technology applied to breast cancer treatment to improve the targeting of nanotubes to tumors by responding to GSH enriched in tumor cells [215]. Therefore, an effective strategy to achieve targeted delivery and controlled, on-demand release of H2S is that combines imaging technology (dynamic monitoring of H2S levels) and delivery vehicles that respond to tumor microenvironmental factors (e.g., pH, thiols) or external stimuli (e.g., acoustic signals, light signals). In addition, the development of prodrugs through surface modification, or the use of cancer cell-selective or semi-selective enzymes may be an effective way to improve tumor targeting.
4.3. H2S-mediated colon cancer therapy
Targeted therapy allows for better therapeutic outcomes and reduced side effects compared to conventional CRC treatment. H2S is a TME factor that can be exploited for targeted therapy in CRC, due to elevated levels of endogenous H2S in CRC cells [114,127,216]. In this section, we focus on therapeutic agents activated by H2S designed to target the high levels of endogenous H2S in CRC, including H2S-activating chemotherapy, photodynamic therapy, photothermal therapy, chemodynamic therapy, and some combination therapy strategies (Table 2). These promising CRC-targeted therapeutics are currently in development and have not yet been translated into clinical trials.
Table 2.
Summary of recent reported H2S-mediated CRC therapy.
| Therapeutic agent | Therapeutic strategy | Imaging method | Stimulus | Colon cancer cells |
|---|---|---|---|---|
| MSNP-N3-FA/DOX | Chemotherapy | – | H2S | HCT116; |
| HT-29 | ||||
| TP-HS | Chemotherapy | Fluorescence imaging | H2S | HCT116 |
| Ru-NBD | PDT | Fluorescence imaging | H2S; | HCT116 |
| Light | ||||
| MOF NPs | PDT | Fluorescence imaging | H2S; | HCT116; |
| Light | LoVo | |||
| TNP-SO | PDT | NIR imaging | H2S; | HCT116 |
| Light | ||||
| 12+-PSs-FA | PDT | NIR imaging | H2S; | HCT116; |
| NIR light | HT-29 | |||
| ZNNPs@FA | PDT | PA imaging | H2S; | HCT116 |
| Light | ||||
| Nano-PT | PTT | NIR-II imaging | H2S; | HCT116 |
| Ligh | ||||
| t | ||||
| Cu2O NPs | PTT | PA imaging | H2S; | HCT116 |
| NIR light | ||||
| Au@Cu2O | PTT | PA imaging | H2S; | HCT116 |
| NIR light | ||||
| Bi:Cu2O@HA | PTT | CT imaging | H2S; | CT26 |
| NIR light | ||||
| FeOOH NSs | PTT/Ferroptosis/Scavenging endogenous H2S | MRI imaging | H2S; | CT26 |
| NIR light | ||||
| PL-Cu | Chemotherapy/PTT | – | H2S; | HCT116 |
| NIR light | ||||
| N3-GT-CPT/ICG | Chemotherapy/PTT | NIR imaging | H2S; | CT26 |
| NIR light | ||||
| NPs@BOD/CPT | Chemotherapy/PTT | NIR-II imaging | H2S; | HCT116 |
| NIR light | ||||
| CatCry-AgNP-DOX | Chemotherapy/PTT | NIR-II imaging | H2S; | HCT116 |
| NIR light | ||||
| Cu-DhaTph COF | PDT/PTT | – | H2S; | HCT116 |
| NIR light | ||||
| FR-H2S | PDT/PTT | Fluorescence imaging | H2S; | HCT116 |
| Far-red light | ||||
| NP-Cu | PDT/PTT/CDT | – | H2S; | HCT116 |
| NIR light; | ||||
| Cu2O@CaCO3@HA | PDT/PTT/CDT/Calcium overload | – | H2S; | CT26 |
| NIR-II light; | ||||
| pH | ||||
| 5-Fu/Cur-P@HMPB | Chemotherapy/CDT/Autophagy | – | H2S | HCT116 |
4.3.1. H2S in chemotherapy
Chemotherapy is the most commonly used treatment strategy in the clinical practice. Chemotherapeutics that are commonly used include doxorubicin (DOX), camptothecin (CPT), 5-FU, and curcumin (Cur), which are employed in the treatment of many cancers, including CRC [217]. However, chemotherapeutics have a number of limitations in their use, including rapid clearance after administration, non-specific distribution, and non-discriminatory damage to normal tissues [218]. As a result, scientists have been working for a long time to develop a drug carrier that can precisely control the on-demand release of chemotherapeutics in order to increase targeting to tumor tissues and improve therapeutic efficacy.
Recently, considering the high endogenous H2S levels in some tumor cells (e.g., colon cancer, ovarian cancer [219]), some carriers using H2S as a specific environmental stimulus to control the release of chemotherapeutics have been developed. Thirumalaivasan et al. designed H2S-activated folate-modified azide functionalized biocompatible mesoporous silica nanoparticles (MSNPs) capable of targeting tumor cells via folate receptor [220]. In the presence of H2S, the ester bond in DOX-loaded MSNP-N3-FA breaks and leads to the release of the drug from the MSNP nanocarriers. At the same time, the therapeutic effect of DOX delivery via MSNP-N3-FA was verified in HT29 (human colon cancer cells) tumor-bearing mice.
In addition, Bobba et al. developed a therapeutic diagnostic molecular prodrug (TP-HS) in combination with fluorescence imaging technology [221]. It is capable of synergistically releasing rhodol and the active therapeutic component SN-38 under the stimulation of endogenous H2S, which can be monitored dynamically and quantitatively by fluorescence imaging. Based on the experimental results in colon cancer (HCT116 cells) and lung cancer (A549 cells), it was shown that TP-HS is an effective therapeutic diagnostic delivery system that can be activated in tumor-specific regions and allows the assessment of free drug content by fluorescence modulation.
It is important to note that although these H2S-activated nanodrug carriers exhibit excellent delivery of chemotherapeutic drugs in cellular or tumor-bearing mouse models, it is difficult to achieve experimental drug delivery in translational applications in organisms due to complex biological barriers.
4.3.2. H2S in photodynamic therapy
Photodynamic therapy (PDT) is primarily based on the accumulation of non-toxic photosensitizers, oxygen and light to produce reactive oxygen species (ROS), particularly singlet oxygen (1O2), which selectively induces apoptosis and necrosis in cancer cells [222,223]. It is a promising approach for cancer therapy due to its high selectivity, minimal invasiveness, significant efficacy, low impact on the host system, and many other advantages. Monitoring the phototherapy process by detecting changes in H2S-activated optical signals to guide the on-demand treatment of tumors is another effective strategy that maximizes the effect of PDT. Based on this idea, Yuan et al. developed a multifunctional H2S probe, Ru-NBD, which can act not only as an effective PDT reagent for H2S activation, but also for real-time and in situ monitoring of therapeutic effects on tumors through restored luminescence during PDT [224]. Similarly, Ma et al. reported a novel metal-organic framework nanoparticle (MOF NP) for photodynamic therapy of colon cancer via controlled release of H2S-activated 1O2 in TME [225]. They used zinc-metallized 5,10,15,20-tetra(4-methoxycarbonylphenyl)porphyrin (ZnTCPP) as a photosensitive bridging ligand to construct a new one-component MOF NP PS. Cu2+ ions as metal nodes of the network quench ligand-based fluorescence and significantly reduce the release of 1O2. When H2S appears, Cu2+ ions are taken out from the MOF nodes, thereby simultaneously obtaining the luminophore photosensitive ligand. Since Cu2+ ions are detached from the MOF network after exposure to H2S, the fluorescence of the ligand is turned on and the production of 1O2 is enhanced. Through controlled release of photosensitive ligands, this turn-on MOF nanophotosensitizer achieves effective photodynamic therapy for cancer.
To combine NIR imaging with PDT, Wang et al. validated a designed therapeutic diagnostic prodrug, TNP-SO, in human colon cancer cells as well as in tumor-bearing mouse models. It consists of an H2S -activated imaging probe, NIR-BSO, and a photosensitive drug, 3I-BOD, with both H2S-activated NIR-emitting light and efficient 1O2 generation [226]. The experimental results showed that the TNP-SO platform accurately guided where light is applied to generate cytotoxic ROS for on-demand cancer treatment through cancer imaging. In addition, Wu et al. doped an electrochromic material based on an organic π-electron structure (bicationic 1,1,4,4-tetraarylbutadiene, 12+) into semiconductor polymer nanoparticles (SPNs) and constructed tumor-targeted fluorescent probes (12+-SNP830-FA) by folic acid modification for H2S-related tumor imaging [227]. On this basis, they further developed a new tumor-targeted photosensitizer (12+-PSs-FA) by replacing SNP with organic photosensitizer, which generates ROS under 808 nm laser irradiation after H2S-specific activation. The reagent exhibited a significant photodynamic therapeutic effect on tumor tissues while showing negligible phototoxicity to normal tissues.
Based on PA imaging technology, Zhang et al. developed a H2S-responsive and consumable nanoplatform ZNNPs for early diagnosis and treatment of H2S-related diseases [10]. In the presence of H2S, ZNNPs exhibited NIR conversion (F1070→F720) and ratiometric PA (PA680/PA900) signals, thereby sensitively and visually detecting endogenous H2S levels in acute hepatotoxicity, brain hemorrhage models, and colorectal tumors in living mice. In addition, the designed ZNNPs@FA can deplete mitochondrial H2S in tumors to cause significant ATP reduction and severe mitochondrial damage, and simultaneously activate photodynamic effects for effective inhibition of colorectal tumors in vivo.
Notably, the therapeutic efficacy of PDT is influenced by factors such as penetration depth, photosensitizer distribution and permeability, and especially oxygen supply (the hypoxic environment of most solid tumors results in limited PDT efficacy), which need to be considered when designing PDT drugs.
4.3.3. H2S in photothermal therapy
PTT is a simple, safe and non-invasive treatment method. It uses the heat generated by photothermal agents under near-infrared light irradiation to achieve local hyperthermia to kill tumor cells [ [[228], [229], [230]]]. However, conventional photothermal agents have limitations such as non-specificity and treatment-related side effects. To address these issues, photothermal agents with intelligent responses need to be developed to turn on the photothermal effect at the tumor site through specific stimuli while remaining silent in normal tissues. Furthermore, scientists are interested in combining imaging technology and photothermal therapy to improve photothermal conversion performance and photothermal agent targeting in order to improve the therapeutic effect of PTT on tumors. Taking advantage of the high endogenous H2S in colon cancer TME, Shi et al. developed an H2S-activatable nanostructured photothermal agent (Nano-PT) [231]. Through in vivo studies, they found that the Nano-PT probe was specifically activated in H2S-rich CRC tissues, emitting NIR-II fluorescence and generating high NIR absorption, thereby achieving efficient photothermal conversion under NIR laser irradiation, while remaining inactivated in normal tissues. Based on these characteristics, Nano-PT was able to achieve effective photothermal ablation of CRC tumors using NIR-II imaging. Yang's group designed a switchable diagnostic and therapeutic probe with combined diagnostic and therapeutic functions using the PA signal activated by the in situ reaction between endogenous H2S and Cu2O at the colon tumor site and PTT [232]. On this basis, they developed a plasmonic hybrid Au@Cu2O with intelligent response to H2S [233]. The photothermal conversion efficiency of Au@Cu2O in the presence of H2S is much higher than that of pure Cu2O due to the localized surface plasmon resonance coupling effect between noble metals and plasmonic semiconductors. Au@Cu2O can be used for CRC diagnosis and treatment due to its improved PAI and PTT. Bismuth (Bi)-based nanoparticles are widely used as computed tomography (CT) reagents [ [[234], [235], [236]]]. Bi doping can improve the NIR absorption of Cu2O triggered by endogenous H2S [237]. Based on these features, Cheng et al. developed a smart H2S-responsive nanoplatform, Bi:Cu2O@HA NPs, combining tumor-targeted delivery, high-performance CT imaging, and enhanced PTT into one system for colon cancer treatment [238].
Furthermore, MRI can be used to guide photothermal treatment of CRC. Li et al. developed a FeOOH NSs nanotherapeutic drug for MRI, ferroptosis, and H2S-based cascade-enhanced combination CRC therapy [239]. FeOOH NSs can effectively scavenge endogenous H2S through reduction reactions and generate H2S-driven cascades of FeS with photothermal therapeutic ability and Fe2+-mediated ferroptosis. Surprisingly, in vivo experiments revealed that FeOOH NSs do not have curative effects on other cancer types and can be used as a specific therapeutic agent for CRC.
It is important to note that PTT has side effects such as damage to normal tissues around the tumor, inflammatory reaction after treatment, and short-term massive release of tumor cell contents during thermal ablation. For this purpose, mild-temperature photothermal therapy was introduced, which solved some side effects, but some new and other problems emerged, such as decreased therapeutic effect (due to the heat resistance of cancer cells). Combining other therapies, such as chemotherapy and PDT, to synergistically assist the therapeutic effect of PTT is a feasible strategy.
4.3.4. H2S in combination therapy of chemotherapy and PTT
The combination of nanotechnology-based targeted chemotherapy and PTT can synergistically enhance the efficiency of cancer treatment, thus reducing the systemic toxic effects of the drugs on patients because of their high targeting and lower concentrations of use [240,241]. Taking advantage of the rapid development of nanotechnology, new advancements has been made in the design of a nanotherapeutic platform that integrates PTT and targeted delivery of chemotherapeutics.
Sun et al. reported a copper complex modified drug-loaded liposome nanoplatform (PL-Cu) [242]. The synergistic ablation of colon tumors at mild apparent temperature achieved successfully performed in a mouse model using photothermal inactivation of tumor cells mediated by tumor endogenous H2S stimulation and drug release induced by thermal effects. To better guide and control drug release, Hou et al. developed a multifunctional therapeutic nanosystem using a small molecule prodrug N3-GT-CPT designed to link two chemotherapeutic drugs, CPT and gemcitabine (GT), to easily co-precipitate with the photothermal agent indocyanine green (ICG) [243]. The N3-GT-CPT/ICG nanosystem showed selective H2S-triggered drug release behavior by a lock GT strategy using a H2S-responsive azide group. At the same time, a synergistic chemotherapy, PTT and NIR imaging for tumors was achieved by combining N3-GT-CPT with ICG. In addition, Shi et al. developed a photoresponsive drug delivery system NPs@BOD/CPT combined with NIR-II imaging [244]. NPs@BOD/CPT was developed by co-encapsulating a rationally designed borodipyrrole methylene (InTBOD-Cl) dye (used as an H2S-activatable NIR photothermal agent) and the clinical drug camptothecin-11 (CPT-11). Under NIR laser irradiation, H2S induces an increase in local temperature and conformational changes in the thermosensitive matrix, resulting in the instantaneous release of encapsulated CPT to inhibit tumor growth in vivo in a tumor-bearing mouse model of HCT116 (human colon cancer cells).
It is worth noting that the tedious preparation steps, the introduction of toxic reagents, and uncertain long-term toxicity may cause unpredictable limitations to the clinical application of nanocarriers [245]. Zhou et al. introduced catalase nanocrystals (CatCry) as a TME-activated nanoplatform into the CRC treatment and prepared a nanoformulation (CatCry-AgNP-DOX) by a one-step co-crystallization method using catalase, AgNO3, and DOX as substrates [246]. The CatCry-AgNP-DOX nanoformulation reacted in situ to form Ag2S nanoparticles under the action of large amounts of H2S in the tumor, which provided NIR-I-activated photothermal effects and NIR-II imaging capabilities while triggering the release of loaded DOX chemotherapeutics. Moreover, CatCry catalyzes the conversion of abundant H2O2 in TME to O2, thereby relieving tumor hypoxia and correspondingly enhancing DOX-induced chemotherapy.
4.3.5. H2S in the combination therapy of PDT and PTT
At the moment, the combination of PDT and PTT therapy is still the research focus, because it not only improves the treatment efficiency, but also avoids unnecessary damage to normal tissues caused by the high power and prolonged irradiation generated by single-peak phototherapy [247]. However, how to integrate the photosensitizer responsible for ROS production during light irradiation and the photothermal agent responsible for local warming into a single nanosystem to construct a multifunctional therapeutic nanoagent remains a major challenge.
Feng et al. designed a Cu(II)-porphyrin-derived nanoscale covalent organic framework (Cu-DhaTph COF) [248]. Upon arrival of the Cu-DhaTph COF at the colon tumor site, Cu(II) is captured by endogenous H2S to form the photothermal agent CuS, allowing the recovery of porphyrin-derived fluorescence. Moreover, the synchronously released photosensitizer DhaTph produces 1O2 in photodynamic mode under light irradiation to achieve in situ activated PDT/PTT combination therapy. In addition, Zhu et al. constructed a far-red (FR) absorbing H2S -responsive nanosystem (FR- H2S) based on the prodrug dinitrophenyl BODIPY (DNP-BDP) [249]. DNP-BDP has fluorescence turn-on properties after endogenous H2S-specific thiolation in tumors, as well as the ability to generate abundant 1O2 and heat for imaging-guided CRC phototherapy.
4.3.6. Other combination therapy
Recently, researchers have provided a three-mode cascade treatment strategy for endogenous H2S-responsive PDT, PTT, and CDT in CRC treatment. Yang et al. developed an intelligent multifunctional nanoplatform (NP–Cu) by clickable assembly of dibenzocyclooctayne (DIBO) functionalized lysine (D-K), the photosensitizer chlorin e6 (Ce6)-responsive prodrug oxyanthraquinone (AQ4N), and cyclen-Cu2+ complex [9]. In NP-Cu, Cu2+ quenched the luminescence of Ce6 in the inactivated state, resulting in PDT in the “turn off” state, and in situ reaction with endogenous H2S led to the “turn on” state of PDT. In particular, the simultaneous CuS production not only provides additional PTT but also amplifies intracellular hypoxic stress to trigger AQ4N-associated CDT. Chang et al. synthesized a nanoformulation Cu2O@CaCO3@HA that can respond to colonic TME by in situ mineralization of hollow mesoporous Cu2O coated with CaCO3 shells and modified with HA [250]. The protective CaCO3 shell decomposes and releases calcium in the acidic colonic TME, allowing the Cu2O core sulfidated by H2S to form metabolizable Cu31S16 nanocrystals, which exhibit excellent PDT/PTT/CDT effects under 1064 nm laser irradiation. After HA modification, the nanocomposite can achieve synergistic CRC targeting and TME-triggered PDT/PTT/CDT/calcium overload-mediated combination therapy.
In addition, Chen et al. developed a novel 5-Fu/Cur-P@HMPB nanomedicine by co-encapsulating Cur and 5-Fu into a hollow mesoporous Prussian blue (HMPB), combining enhanced chemotherapy and CDT and amplifying autophagy [8]. 5-Fu/Cur-P@HMPB enter CRC's H2S-rich TME, PB with low Fenton catalytic activity is responsively converted to Prussian white (PW) with high catalytic activity, which in situ produces high levels of •OH to activate CDT while triggering autophagy. The natural anticancer drug Cur can amplify autophagy to induce autophagic cell death, and can also be used as a clinical chemotherapy drug 5-Fu specific chemical sensitizer, with a good synergistic antitumor effect.
It is important to note that combination therapeutic strategies, including sections 4.2.4 and 4.2.5, and the approaches described in this section, require a balance of the characteristics of each system, which increases the difficulty of design development. Moreover, the complexity of the systems may have unknown implications in practical applications and represent a significant challenge for the clinical translation of the drugs.
4.4. H2S-activated probes
Abnormal H2S levels in organisms have been associated with the development of many diseases, including tumors, Alzheimer's disease, and cardiovascular disease [127]. Therefore, highly sensitive probes targeting H2S concentrations in animals are very important. They can help us to understand the effects of H2S on various physiological and pathological processes and serve as potential diagnostic tools for relevant diseases. Optical imaging techniques are widely used for the development of H2S probes because of their high sensitivity, short response time, non-invasiveness, and real-time monitoring capability. For example, the H2S fluorescent probe SF7-AM based on visible light imaging allows for direct, real-time visualization of endogenous H2S produced in live human umbilical vein endothelial cells stimulated by VEGF [251]. Nanoprobe 1-PEI-DCNPs based on near-infrared imaging can monitor endogenous H2S in lipopolysaccharide-induced liver inflammation in animal models [ [252]]. Some other imaging modalities, such as photoacoustic imaging (PAI), magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), and combined imaging, have also been explored for potential use in H2S detection and diagnosis. Recently, due to the increased levels of H2S in CRC, many probes and H2S-based in vivo diagnosis have been developed to detect intracellular levels of H2S in CRC cells [ [181,253,254]]. In this section, we summarize the H2S-activatable probes relevant in the field of CRC research (Table 3) and described in detail below.
Table 3.
Summary of recent reported H2S-activated probes in CRC.
| H2S probe | Imaging method | Signal detection | Detection limit | In vivo Experimental subject |
|---|---|---|---|---|
| AzMC | Visible light | 450 nm | 200 nM | – |
| AM-BODIPY-NBD | Visible light | 520 nm | 15.7 nM | – |
| TPANF | Visible light | 468 nm | 170 nM | HCT116 tumor mice |
| Probe 1 | NIR-I | 796 nm | 39.6 nM | HCT116, HT29 tumor mice |
| Ag-CEW | NIR-II | 1090 nm | 35 nM | HCT116 tumor mice |
| NanoBOD-SCM | NIR-I | 710/610 nm | 198 nM | – |
| NIR-II@Si | NIR-II | 700/900 nm | 37 nM | HCT116 tumor mice |
| DCNP@HSA-Ag+ | NIR-II | 1050/1550 nm | 210 μM | MC38-luc tumor mice |
| F12+-ANP | Afterglow luminescent | 790 nm | 100 nM | – |
| TPAMC-UCNPs@PEG | UCL | 540/800 nm | 220 nM | HCT116 tumor mice |
| Si@BODPA | PA | 780 nm | 53 nM | HCT116 tumor mice |
| AzHD-LP | PA | 680 nm | 500 nM | HCT116 tumor mice |
| NR-NO2 | PA | 710 nm | 40 nM | HCT116 tumor mice |
| [99mTc]Tc-Gluconate | SPECT | gamma-ray | – | – |
| Fe3O4@Cu2O-lipid-mPEG | PA; MRI | 770–1100 nm; MRI signals | 110/4.10 μM; 214/2.15 μM (wide/narrow concentration ranges) | HCT116 tumor mice |
| Amphiphilic probes 1 and 2 | PA; NIR-I | 718 nm | 60 nM | HCT116 tumor mice |
| SiO2@Ag | PA; NIR-II | 1000–1400 nm | 0.92 nM | HCT116 tumor mice |
4.4.1. Visible light imaging probes
In living systems, selective detection and differentiation of endogenous H2S are challenging considering the interference of other active substances such as intracellular GSH [255]. For H2S fluorescent probes, the selectivity of the sensing reaction should be considered as one of the most important properties. Probes based on H2S-mediated azide reduction are a widely adopted design strategy for H2S fluorescent probes [ [[256], [257], [258]]]. Upon reduction by H2S, the electron-withdrawing azido group will be converted into an electron-donating amino group and turn on the fluorescent signal of the fluorophore. 7-Azido-4-methylcoumarin (AzMC) is a commercial fluorescent probe which has been applied in CRC-related biological studies, including screening potential CRC therapeutic drugs (CBS inhibitors) and evaluating the role of 3-MST in CRC, because AzMC is easy to synthesize and can selectively and sensitively detect H2S levels in colon tumor cells [ [154] [117,153,168]]. Compared with H2S-mediated azide reduction, H2S-mediated thiolysis of 7-nitro-1,2,3-benzoxadiazole (NBD) amines possess better selectivity for H2S even in the presence of millimolar GSH [ [[259], [260], [261]]]. Taking advantage of NBD amines, Ye et al. developed a cell-capable probe (AM-BODIPY-NBD) for highly selective and ultrasensitive imaging of intracellular H2S to reveal a positive correlation between endogenous H2S and the metastatic potential of CRC and breast cancer cells [259].
Most of the traditional fluorescent probes still suffered from the aggregation-caused quenching effect which adversely impacts the performance of the probes. In contrast, molecules with aggregation-induced emission (AIE) characteristics usually exhibit weak emission when dissolved in solvents but intense fluorescence in the aggregated state [262,263]. Based on these characteristics, Xu et al. developed a fluorescent probe based on a triphenylamine benzopyridine platform (TPANF) with AIE characteristics to identify changes in H2S concentration in living cells and to image endogenous H2S in HCT116 (human colon cancer cells) xenograft tumor tissue [264]. This probe has the advantages of high stability to photobleaching and low background fluorescence interference compared with traditional fluorescent probes. Notably, AzMC and AM-BODIPY-NBD are usually only used to detect H2S levels in cells, while TPANF with high photostability can be applied to in vivo animal imaging, but it is limited by the low tissue penetration and poor spatial resolution of visible light imaging mode.
4.4.2. Near-infrared-I/II (NIR-I/II) imaging probes
Because of the numerous problems that arise during the transition from solution, cells, and tissues to whole organisms, NIR fluorescent probes with good penetration and low phototoxicity are preferable to fluorescent probes emitting in the visible region for H2S monitoring and imaging in living animal tissues [265]. Zhang and co-workers developed a cyanine-based NIR-I probe (Probe 1) for rapid and highly selective NIR-I imaging of endogenous H2S in live cells, tissues and mouse models via H2S-mediated thiolysis of NBD amines [266]. Compared to conventional visible light (450–750 nm) and NIR-I (750–900 nm) imaging, fluorescence imaging in the second near-infrared window (NIR-II, 1000–1700 nm) has higher in vivo spatial resolution compared to conventional visible (450–750 nm) and NIR-I (750–900 nm) imaging [267]. On this basis, Deng et al. developed an in situ H2S-activatable NIR–II–emitting nanoprobe for illumination of colorectal cancer in vivo [268]. This probe is based on an Ag-chicken egg white (Ag-CEW) complex, which forms Ag2S quantum dots via endogenous H2S-induced in situ chemistry with effective NIR-II emission at 1000–1400 nm, enabling specific visualization of colon cancer guided by NIR-II imaging as well as precise localization.
Experiment results for most single-window response fluorescent probes tend to fluctuate with changes in experimental conditions [269]. In contrast, ratiometric fluorescent probes with two or more emissions construct a self-calibrating system that minimizes the interference caused by experimental conditions [270]. Liu and co-workers prepared nanoprobes (NanoBOD-SCM) by trapping a small molecule probe BODVA-Cl inside the hydrophobic interior of organic nanoparticles made from surface cross-linked micelles (SCM) [271]. This probe provided H2S-specific ratiometric fluorescence patterns (I710/I610) and was successfully used to image H2S-rich colon cancer cells in a dual-color imaging modality. To visualize colorectal cancer in vivo, Xu et al. designed an activatable NIR-II nanoprobe (NIR-II@Si) consisting of two dyes, boron-dipyrromethene (ZX-NIR) and aza-BODIPY (aza-BOD) [267]. This targeted probe exhibits high selectivity and proportional fluorescence response to H2S, allowing deep tissue imaging and identification of colon tumors in animal models. In addition, Wang and co-workers bound human serum albumin (HSA) to Ag+ on the surface of DCNP to form the DCNP@HSA-Ag+ nanoprobe, which generate H2S concentration-dependent ratiometric F1050Em, 808Ex/F1550Em, 980Ex signals based on H2S-induced in situ reduction reaction to precisely localize CRC in vivo [255].
In conclusion, H2S-activated NIR imaging probes have the following features: NIR-II has higher spatial resolution compared to NIR-I imaging probes; ratiometric probes reduce interference caused by experimental conditions; and probes based on H2S-mediated in situ reactions (Ag-CEW and DCNP@HSA-Ag+) allow precise localization of CRC in vivo.
4.4.3. Afterglow luminescent probes
Afterglow luminescence probes, also known as persistent luminescence probes, which capture excitation energy in defects and slowly release photons after the cessation of photoexcitation, have recently emerged as promising tools to overcome the limitations of fluorescent probes in biosensing and molecular imaging [ [[272], [273], [274]]]. Wu et al. designed and developed H2S-activatable NIR afterglow luminescence probes (F12+-ANP) by doping EM F12+ (an organic electrochromic material) and NIR775 (a NIR photosensitizer) into MEH-PPV-based organic afterglow nanoparticles [275]. This probe exhibited a fast response rate (1563 ± 141 M−1 s−1) and large afterglow turn-on ratio (∼122-fold) toward H2S, which can rapidly quantify H2S concentrations in blood samples from healthy individuals and patients diagnosed with CRC or hepatocellular carcinoma. The afterglow signal can persist for more than 40 min after the stop of the pre-irradiated laser with a half-life of ∼6.6 min, and can be charged by multiple irradiations with the 808 nm laser for long-term imaging (validated with 15 cycles over 3 days).
It is noted that there are no afterglow imaging probes for the in vivo detection of colonic tumors. It might be possible to obtain these probes through surface modifications. For example, Wu et al. prepared F12+-ANP-Gal by introducing β-Gal (targeting the β-Gal receptor on HepG2 cells) to the F1 surface of the abovementioned probe for detecting subcutaneous and in situ tumors in living mice, as well as for depicting tumor lesions in clinical liver resection specimens [275].
4.4.4. Upconversion luminescence (UCL) probes
Upconversion nanophosphors (UCNPs) can absorb NIR light and convert it to high-energy light in the wavelength range from UV to NIR [ [[276], [277], [278]]]. Based on the UCL system, Li et al. developed a lysosomal-assisted mitochondrial targeting probe (TPAMC-UCNPs@PEG) consisting of a pH-sensitive PEG shell and a sensing core of merocyanine triphenylamine-merocyanine (TPAMC)-modified UCNPs [270]. TPAMC, which acts as an H2S -responsive site, is initially hidden under the PEG shell and upon entering the cell via endocytosis the mitochondria-targeting site is exposed due to the acidic environment in the lysosome enabling further attachment to the mitochondria. The strong Förster resonance energy transfer effect between UCNPs and TPAMC allows sensitive detection of H2S by using ratiometric UCL signals. The group revealed the acid-activated mitochondrial targeting processes via lysosomal delivery of this probe, and demonstrated that this probe can be used to monitor mitochondrial H2S levels in living cells and a mouse model of CRC by ratiometric UCL imaging.
Compared to conventional two-photon materials, they have higher conversion efficiency due to the presence of stable intermediate states, which also allow continuous-wave excitation at lower power densities [259,260,279,280]. Considering that mitochondria are the main metabolic site of H2S in living organisms, UCL nanoprobes are expected to be an effective and promising tool for studying the biological and pathological roles of mitochondrial H2S.
4.4.5. Photoacoustic (PA) imaging probes
Researchers have developed a probe with a new imaging modality, PA imaging, which relies on the translation of excitation light into ultrasonic waves based on the PA effect. This imaging method combines the advantages of high resolution of optical imaging and high penetration depth of ultrasound imaging for deep tissues and whole animals imaging [254,281].
Efforts have been made in recent years to develop PA probes for the detection of H2S in vivo. Shi et al. developed an H2S-activated Si@BODPA PA probe by encapsulating a hemicyanine-BODIPY hybrid dye (BODPA) inside a silica nanocomposite [282]. The probe produces a strong PA signal output in the NIR region in the presence of H2S, converting BODPA within nanoparticles to BOD-HS via aromatic nucleophilic substitution. The probe shows an extremely fast response and can detect transient changes in H2S, which allows direct PA tracking of endogenous H2S production in a CRC cell-bearing mouse model. To reduce the interference of experimental results by factors such as instrumentation, probe concentration and external environment, Ma et al. developed an AzHD-LP ratiometric PA nanoprobe consisting of a liposome (LP) encapsulating an H2S-responsive NIR dye (AzHD) for in vivo detection of H2S [283]. Upon H2S-mediated reduction of the probe's azide group to amine, the absorption of AzHD-LP at 600 nm decreased while the absorption at 700 nm increased, producing a proportional PA signal. By combining AzHD-LP with the tumor-targeting peptide c (RGDyK), this probe enabled the detection of intra-tumor H2S production in a mouse model of CRC with ratiometric PA signal.
Multispectral optoacoustic tomography (MSOT) is an emerging technology combining optoacoustic tomography and multiwavelength illumination, which allows each light absorber to be visualized individually in the target tissue and allows the generation of three-dimensional (3D) MSOT images [ [[284], [285], [286]]]. Sun et al. proposed a new strategy for the effective assessment of drug side effects by tracking their metabolism-related products through H2S turn-on optoacoustic probes (NR-NO2) [287]. This probe was first developed for monitoring liver injury induced by metformin, which induces excessive H2S expression, through detecting hepatic H2S. It can successfully identify and precisely localize liver injury with the help of MSOT imaging method, while visualizing the volume of liver injury. It has also achieved desirable results in the evaluation of the effects of AOAA (a CBS inhibitor) used in a mouse model of colon tumors [287].
In general, compared with conventional optical imaging, PA imaging overcomes the challenges of tissue penetration and in vivo spatial resolution, and the reconstruction of 3D photoacoustic images by MSOT has promising applications in visual cancer diagnosis, drug efficacy assessment, and other fields. It is to be noted that the spectral coloring effect and the spectral crosstalk are the two main challenges of multispectral PA imaging, and further research is needed to deal with them.
4.4.6. SPECT imaging
SPECT is an in vivo imaging modality that uses the SPECT camera to measures gamma rays emitted by radiotracers injected into the body [ [[288], [289], [290]]]. Technetium-99 m (99mTc) is one of the most commonly used isotopes for diagnostic purposes, which has a short half-life of 6 h and is cheap and can be easily purchased from hospitals or obtained from 99Mo/99mTc generators [291,292]. Jeong's group labeled various α-hydroxy acids with 99mTc and developed 99mTc-labeled reagents for in vitro and in vivo H2S quantification [293]. These 99mTc-labeled reagents, particularly [99mTc] Tc-gluconate, can form insoluble complexes in the presence of H2S, thus enabling imaging of endogenously produced H2S. In addition, after evaluating a mouse CRC cell model under hypoxic conditions and a mouse model of acute hindlimb ischemia-reperfusion, they found a significant increase in the uptake of [99mTc] Tc-gluconate under hypoxic conditions, implying that this imaging agent could be used to detect endogenous H2S produced in hypoxic tissues [294]. Considering that SPECT is a commonly utilized imaging technique in clinical practice [295,296], assays based on SPECT imaging principles, such as [99mTc]Tc-Gluconate, have great promise for translational applications.
4.4.7. Dual-mode imaging probe
The limitations of single-modal probes in practical applications, such as poor tissue penetration and photostability of conventional optical imaging probes, are exacerbated in complex physiological settings. As a result, it is critical to develop novel dual-modality probes that are sensitive to H2S. Moreover, single-modal imaging rarely provides enough information to make an accurate cancer diagnosis. Hence, it is important to develop novel dual-mode probes that are sensitive to H2S [297].
Magnetic probes are not affected by fluorescence quenching or problems associated with tissue penetration depth and have been widely used for ultrasensitive detection of bacteria, proteins, viruses, and nucleic acids [ [[298], [299], [300], [301]]]. However, the resolution of magnetic probes is insufficient to detect H2S in vivo. In contrast, PA probes have recently been used to detect H2S in vivo because of their high resolution and the absence of ambiguous photoluminescence signal caused by tissue-induced optical extinction and sporadic autofluorescence in the visible range when using conventional optical imaging techniques. Thus, combining the two modes into a single system can provide complementary benefits and is expected to greatly improve the accuracy of H2S detection in vivo. Yan and co-workers constructed core-shell Fe3O4@Cu2O nanoparticles as a magnetic-optoacoustic dual-mode probe for CRC diagnosis based on the in situ response of Cu2O to endogenous H2S in colon tumors [297]. The obtained Cu2O with weak NIR absorption was sulfated in the presence of H2S to generate Cu9S8 with strong NIR absorption and activated PA signal. Meanwhile, the in situ response of Cu2O formed a layer of cavities between the core and the initial coating, leading to enhanced r1-and T1-weighted MRI.
In addition, other researchers have combined NIR-I/II probes with PA probes to form complementary multimodal probes that provide ultra-spatial resolution and sensitivity for tumor imaging and monitoring. Wang et al. designed and synthesized two small molecule probes using hydrophilic N-methylpyridinium to act as an electron-withdrawing group and a hydrophobic monochlorinated BODIPY core to act as an H2S-responsive unit [149]. The designed amphiphilic probes 1 and 2 are able to spontaneously self-assemble into nanoprobes with well-defined nanostructures and show an unprecedented aggregation-enhanced responsiveness to H2S. This probe in the aggregated state rather than the molecularly soluble state emits NIR fluorescence as well as PA signals upon specific activation by H2S, thus allowing in vivo visualization and differentiation of CRC based on differences in H2S content. In addition, Bi and co-workers developed a NIR-II/PA dual-mode nanoprobe (SiO2@Ag) based on the H2S-mediated in situ sulfation reaction for specific monitoring of colorectal tumors [302]. Upon activation by endogenous H2S, this nanoprobe present turned-on NIR-II fluorescence from 1000 to 1400 nm as well as an excellent PA signal, showing high sensitivity and specificity in the diagnosis of colon cancer in vivo.
The development of H2S-activated dual-mode imaging probes and even multimode imaging probes is currently an emerging field. It remains a challenge to rationally integrate multimodal imaging modalities into a single probe while responding to endogenous H2S.
5. Conclusion and outlook
H2S has been identified as an important regulator of CRC biology among numerous compounds released by the colon and colonic microbiota. CBS, CSE, and 3-MST are the three major H2S-generating enzymes that catalyze the production of endogenous H2S in colon cells to varying degrees. In addition, H2S produced by gut microbes using inorganic or organic substrates can easily penetrate the cell membrane and enter colon cells. These intracellular H2S molecules are oxidatively metabolized in mitochondria to generate energy or used in other biological reactions. The occurrence and progression of CRC are influenced by both endogenous synthesis of colonic epithelial cells and exogenous delivery of H2S from the luminal side. The effect of H2S on CRC showed duality, promoting energy metabolism, proliferation, and migration of tumor cells within an appropriate concentration range while acting as a tumor suppressor above the concentration threshold. As a result, determining H2S release kinetics, concentration-dependent effect curves, and optimal therapeutic doses is critical when using it in therapy. Furthermore, considering the close relationship between intestinal H2S, microbes, and diet, H2S is regarded as an important factor linking dietary patterns, gut microbiota, and CRC. Modifying dietary patterns to reduce S-containing substrates for H2S production and/or limiting the number of H2S-producing gut microbes may be a promising approach to CRC prevention.
In terms of H2S synthase inhibitors, the current focus needs to be on finding effective, selective, and non-toxic inhibitors that can be translated. Traditional small molecule inhibitors such as AOAA and EGCG were once of great interest, however, they were not finally used in clinical treatment because of their respective shortcomings. Unlike AOAA, the inhibition of CBS by EGCG does not target PLP, but exploits certain structural or conformational features unique to CBS, which makes it a CBS-specific inhibitor. According to this idea, the development of new potent specific inhibitors targeting specific sites on the synthase is a feasible strategy. In addition, the search for suitable inhibitors from compound libraries by high-throughput screening techniques also deserves researchers’ attention. In terms of scavengers, the H2S scavenger options available in CRC therapy are still restricted. Moreover, H2S scavengers require higher doses to deal with the continuous generation of H2S and its byproducts [147], which is still under investigation. It is worth mentioning that H2S enzymes are prevalent in the body and perform important biological functions, and that endogenous H2S is an important signaling molecule in physiological or pathological states. Inhibitors or scavengers are likely to have undesired consequences during practical use. For example, CBS and CSE are key enzymes in the cellular synthesis of cysteine and H2S plays an important role in the maintenance of vascular tone and angiogenesis. Inhibition of these effects inevitably causes damage to the body. Therefore, a comprehensive assessment must be undertaken during drug development and translation to target and eliminate or attenuate possible side effects.
Although conventional small molecule H2S donors offer numerous advantages over sulfide salts in H2S donor therapy, they are intrinsically unstable molecules. To achieve improved therapeutic effects, stimulus-responsive delivery as well as gradual and prolonged release must be established. One of the tactics that has received a lot of attention and is effective in managing the transport and release of H2S gas is the use of macromolecular/supramolecular polymer carriers in the development of delivery vehicles. Delivery systems that combine carriers and detection imaging technologies that respond to the TME as well as to external stimuli are one way to improve the therapeutic targeting of H2S donors and to achieve controlled and on-demand gas release.
The development of tumor-targeted intelligent diagnostic and therapeutic drugs based on TME characteristics is currently a popular cancer treatment strategy [218,[303], [304], [305], [306]]. The high level of H2S in CRC cells makes it a promising endogenous microenvironment molecule for the design and synthesis of H2S-responsive diagnostic therapeutics targeting CRC. Currently, H2S-activated therapeutics developed in combination with nanotechnology are being investigated in CRC mainly in the fields of chemotherapy, PDT, PTT, and CDT. Combining these therapeutic strategies may lead to better therapeutic outcomes, such as the combination of CDT and PTT (thermal effects can accelerate •OH production), the combination of chemotherapy and PTT (controlled drug delivery through thermal effects), and the combination of PDT and PTT (many photosensitizers have photothermal effects themselves). The combination of H2S-activated therapeutic agents with the combination of other emerging therapeutic modalities, such as immunotherapy, sonodynamic therapy, and electrotherapy, remains to be further explored.
It is to be noted that these nanotherapeutics, including nanocarriers introduced for controlled gas delivery and H2S-activated targeted nanodrugs, can be used in a tumor-bearing mouse model to achieve good tumor suppression by direct injection of the therapeutic agent at the tumor site, but the actual application in the organism often does not yield as effective therapeutic results as in vitro experiments. A complex set of biological barriers limit the bioavailability of drugs at specific sites and prevent good therapeutic outcomes. These barriers include mononuclear phagocyte systems, nonspecific distribution, blood rheology/vascular flow restrictions, pressure gradients, cellular internalization, escape from endosomal and lysosomal compartments, and drug efflux pumps [216,307]. In addition, nanomedicines, prior to clinical translation, must undergo long-term pharmacodynamic and toxicological studies to fully assess the effects on the human body, due to the frequently complex and inconsistent biological profiles of nanomaterials. In the future, these barriers should be addressed in a targeted manner while rationally incorporating innovative designs in the development of new generations of CRC tumor-targeting nanotherapeutics to achieve optimal therapeutic outcomes.
In the last twenty years, H2S detection methods have evolved from initial methods that require handling and/or destruction of tissues or cells, including colorimetric method, electrochemical method, gas chromatography and sulfide precipitation method, to visible light imaging fluorescent probes that can be used on living cells, to optical imaging modalities, e.g., NIR-I/II imaging, UCL imaging and afterglow imaging, and other non-optical imaging modalities, e.g., PA imaging, SPECT imaging, MRI imaging. In response to H2S in the tumor environment, NBD amines have a higher selectivity for H2S compared to azides. The probes developed based on the H2S-mediated in situ response are able to illuminate tumor sites in situ when applied in vivo. Conventional visible light imaging modalities have many limitations such as low tissue penetration, poor spatial resolution of deep tissue, interference from autofluorescence, and poor photostability when applied in vivo to animals. In contrast, novel optical imaging modalities such as NIR-I/II imaging has higher spatial resolution, UCL imaging has high tissue penetration and strong photostability, afterglow imaging does not require real-time external light excitation, is not subject to autofluorescence interference, and has the potential for long-term imaging. Compared to optical imaging modalities, PA imaging combines the features of optical imaging and ultrasound imaging and allows the reconstruction of 3D photoacoustic images by MSOT. Remarkably, PA imaging has been clinically translated in the field of gynecology with high-resolution imaging (down to capillary size resolution) of the cervical microvascular system by transvaginal fast-scan photoacoustic endoscopy [308]. Likewise, there have been several studies on handheld photoacoustic imaging devices and platforms that have greatly increased the potential of this modality for commercial as well as clinical applications [309]. Similar to the vagina, researchers can develop high-performance PA imaging probes through CRC tumor microenvironmental factors, e.g., H2S, or other specific targets to detect PA signals transintestinally or directly in vitro for accurate diagnosis of CRC. Other non-optical imaging modalities, such as SPECT imaging and MRI imaging, are already widely used imaging techniques in clinical practice, and the development of corresponding CRC diagnostic probes based on this technology may facilitate clinical translation.
Overall, H2S-related researches are fruitful in the areas of mechanistic studies of colon cancer development, diagnosis, and treatment. The current focus is on how to translate the existing researches into clinical applications. In addition, researchers need to further explore new H2S-mediated therapeutic modalities, such as H2S-mediated anticancer immunotherapy or H2S-mediated antibacterial therapy in CRC, whose mechanisms or therapeutic effects are still not fully known.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Key Research and Development Program of China [2021YFC2500403]; the Shanghai International Science and Technology Collaboration Program [214907137000]; and the Program of Shanghai Academic Research Leader [20XD1400900].
Contributor Information
Dongqin Yang, Email: kobesakura@fudan.edu.cn.
Ning Ren, Email: ren.ning@zs-hospital.sh.cn.
Zhiling Zhu, Email: zlzhu@qust.edu.cn.
Qiongzhu Dong, Email: qzhdong@fudan.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Cirino G., Szabo C., Papapetropoulos A. Physiological roles of hydrogen sulfide in mammalian cells, tissues, and organs. Physiol. Rev. 2023;103(1):31–276. doi: 10.1152/physrev.00028.2021. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R., Sahu M., Tripathi R., Ambasta R.K., Kumar P. Protein S-sulfhydration: unraveling the prospective of hydrogen sulfide in the brain, vasculature and neurological manifestations. Ageing Res. Rev. 2022;76 doi: 10.1016/j.arr.2022.101579. [DOI] [PubMed] [Google Scholar]
- 3.Kolluru G.K., Shackelford R.E., Shen X., Dominic P., Kevil C.G. Sulfide regulation of cardiovascular function in health and disease. Nat. Rev. Cardiol. 2022:1–17. doi: 10.1038/s41569-022-00741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dilek N., Papapetropoulos A., Toliver-Kinsky T., Szabo C. Hydrogen sulfide: an endogenous regulator of the immune system. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105119. [DOI] [PubMed] [Google Scholar]
- 5.Głowacka U., Brzozowski T., Magierowski M. Synergisms, discrepancies and interactions between hydrogen sulfide and carbon monoxide in the gastrointestinal and digestive system physiology, pathophysiology and pharmacology. Biomolecules. 2020;10(3):445. doi: 10.3390/biom10030445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khattak S., Rauf M.A., Khan N.H., Zhang Q.Q., Chen H.J., Muhammad P., et al. Hydrogen sulfide biology and its role in cancer. Molecules. 2022;27(11):3389. doi: 10.3390/molecules27113389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dekker E., Rex D.K. Advances in CRC prevention: screening and surveillance. Gastroenterology. 2018;154(7):1970–1984. doi: 10.1053/j.gastro.2018.01.069. [DOI] [PubMed] [Google Scholar]
- 8.Chen J., Xue F., Du W., Yu H., Yang Z., Du Q., et al. An endogenous H2S-activated nanoplatform for triple synergistic therapy of colorectal cancer. Nano Lett. 2022;22(15):6156–6165. doi: 10.1021/acs.nanolett.2c01346. [DOI] [PubMed] [Google Scholar]
- 9.Yang L., Zhu Y., Liang L., Wang C., Ning X., Feng X. Self-assembly of intelligent nanoplatform for endogenous H2S-triggered multimodal cascade therapy of colon cancer. Nano Lett. 2022;22(10):4207–4214. doi: 10.1021/acs.nanolett.2c01131. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Fang J., Ye S., Zhao Y., Wang A., Mao Q., et al. A hydrogen sulphide-responsive and depleting nanoplatform for cancer photodynamic therapy. Nat. Commun. 2022;13(1):1685. doi: 10.1038/s41467-022-29284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf P.G., Cowley E.S., Breister A., Matatov S., Lucio L., Polak P., et al. Diversity and distribution of sulfur metabolic genes in the human gut microbiome and their association with colorectal cancer. Microbiome. 2022;10(1):64. doi: 10.1186/s40168-022-01242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen L.H., Cao Y., Hur J., Mehta R.S., Sikavi D.R., Wang Y., et al. The sulfur microbial diet is associated with increased risk of early-onset colorectal cancer precursors. Gastroenterology. 2021;161(5):1423–1432. doi: 10.1053/j.gastro.2021.07.008. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blachier F., Andriamihaja M., Larraufie P., Ahn E., Lan A., Kim E. Production of hydrogen sulfide by the intestinal microbiota and epithelial cells and consequences for the colonic and rectal mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2021;320(2) doi: 10.1152/ajpgi.00261.2020. G125-g35. [DOI] [PubMed] [Google Scholar]
- 14.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 2012;92(2):791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 15.Kushkevych I., Dordević D., Vítězová M. Possible synergy effect of hydrogen sulfide and acetate produced by sulfate-reducing bacteria on inflammatory bowel disease development. J. Adv. Res. 2021;27:71–78. doi: 10.1016/j.jare.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kushkevych I., Cejnar J., Treml J., Dordević D., Kollar P., Vítězová M. Recent advances in metabolic pathways of sulfate reduction in intestinal bacteria. Cells. 2020;9(3):698. doi: 10.3390/cells9030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carbonero F., Benefiel A.C., Alizadeh-Ghamsari A.H., Gaskins H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012;3:448. doi: 10.3389/fphys.2012.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller A.L., Kjeldsen K.U., Rattei T., Pester M., Loy A. Phylogenetic and environmental diversity of DsrAB-type dissimilatory (bi)sulfite reductases. ISME J. 2015;9(5):1152–1165. doi: 10.1038/ismej.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anantharaman K., Hausmann B., Jungbluth S.P., Kantor R.S., Lavy A., Warren L.A., et al. Expanded diversity of microbial groups that shape the dissimilatory sulfur cycle. ISME J. 2018;12(7):1715–1728. doi: 10.1038/s41396-018-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahlman B., Leijonmarck C.E., Lind C., Vinnars E., Wernerman J. Free amino acids in biopsy specimens from the human colonic mucosa. J. Surg. Res. 1993;55(6):647–653. doi: 10.1006/jsre.1993.1198. [DOI] [PubMed] [Google Scholar]
- 21.Ridlon J.M., Wolf P.G., Gaskins H.R. Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microb. 2016;7(3):201–215. doi: 10.1080/19490976.2016.1150414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devkota S., Wang Y., Musch M.W., Leone V., Fehlner-Peach H., Nadimpalli A., et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487(7405):104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yazici C., Wolf P.G., Kim H., Cross T.L., Vermillion K., Carroll T., et al. Race-dependent association of sulfidogenic bacteria with colorectal cancer. Gut. 2017;66(11):1983–1994. doi: 10.1136/gutjnl-2016-313321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei Y., Zhang Y. Glycyl radical enzymes and sulfonate metabolism in the microbiome. Annu. Rev. Biochem. 2021;90:817–846. doi: 10.1146/annurev-biochem-080120-024103. [DOI] [PubMed] [Google Scholar]
- 25.Peck S.C., Denger K., Burrichter A., Irwin S.M., Balskus E.P., Schleheck D. A glycyl radical enzyme enables hydrogen sulfide production by the human intestinal bacterium Bilophila wadsworthia. Proc. Natl. Acad. Sci. U. S. A. 2019;116(8):3171–3176. doi: 10.1073/pnas.1815661116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M., Wei Y., Yin J., Lin L., Zhou Y., Hua G., et al. Biochemical and structural investigation of taurine:2-oxoglutarate aminotransferase from Bifidobacterium kashiwanohense. Biochem. J. 2019;476(11):1605–1619. doi: 10.1042/BCJ20190206. [DOI] [PubMed] [Google Scholar]
- 27.Snow A.J.D., Burchill L., Sharma M., Davies G.J., Williams S.J. Sulfoglycolysis: catabolic pathways for metabolism of sulfoquinovose. Chem. Soc. Rev. 2021;50(24):13628–13645. doi: 10.1039/d1cs00846c. [DOI] [PubMed] [Google Scholar]
- 28.Hanson B.T., Dimitri Kits K., Löffler J., Burrichter A.G., Fiedler A., Denger K., et al. Sulfoquinovose is a select nutrient of prominent bacteria and a source of hydrogen sulfide in the human gut. ISME J. 2021;15(9):2779–2791. doi: 10.1038/s41396-021-00968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denger K., Weiss M., Felux A.K., Schneider A., Mayer C., Spiteller D., et al. Sulphoglycolysis in Escherichia coli K-12 closes a gap in the biogeochemical sulphur cycle. Nature. 2014;507(7490):114–117. doi: 10.1038/nature12947. [DOI] [PubMed] [Google Scholar]
- 30.Sharma M., Abayakoon P., Epa R., Jin Y., Lingford J.P., Shimada T., et al. Molecular basis of sulfosugar selectivity in sulfoglycolysis. ACS Cent. Sci. 2021;7(3):476–487. doi: 10.1021/acscentsci.0c01285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma M., Abayakoon P., Lingford J., Epa R., John A., Jin Y., et al. Dynamic structural changes accompany the production of dihydroxypropanesulfonate by sulfolactaldehyde reductase. ACS Catal. 2020;10(4):2826–2836. [Google Scholar]
- 32.Kaur A., van der Peet P.L., Mui J.W., Herisse M., Pidot S., Williams S.J. Genome sequences of Arthrobacter spp. that use a modified sulfoglycolytic Embden-Meyerhof-Parnas pathway. Arch. Microbiol. 2022;204(3):193. doi: 10.1007/s00203-022-02803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J., Wei Y., Ma K., An J., Liu X., Liu Y., et al. Mechanistically diverse pathways for sulfoquinovose degradation in bacteria. ACS Catal. 2021;11(24):14740–14750. [Google Scholar]
- 34.Felux A.K., Spiteller D., Klebensberger J., Schleheck D. Entner-Doudoroff pathway for sulfoquinovose degradation in Pseudomonas putida SQ1. Proc. Natl. Acad. Sci. U. S. A. 2015;112(31):E4298–E4305. doi: 10.1073/pnas.1507049112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J., Epa R., Scott N.E., Skoneczny D., Sharma M., Snow A.J.D., et al. A sulfoglycolytic entner-doudoroff pathway in rhizobium leguminosarum bv. trifolii SRDI565. Appl. Environ. Microbiol. 2020;86(15) doi: 10.1128/AEM.00750-20. e00750-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frommeyer B., Fiedler A.W., Oehler S.R., Hanson B.T., Loy A., Franchini P., et al. Environmental and intestinal phylum firmicutes bacteria metabolize the plant sugar sulfoquinovose via a 6-Deoxy-6-sulfofructose transaldolase pathway. iScience. 2020;23(9) doi: 10.1016/j.isci.2020.101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y., Wei Y., Zhou Y., Ang E.L., Zhao H., Zhang Y. A transaldolase-dependent sulfoglycolysis pathway in Bacillus megaterium DSM 1804. Biochem. Biophys. Res. Commun. 2020;533(4):1109–1114. doi: 10.1016/j.bbrc.2020.09.124. [DOI] [PubMed] [Google Scholar]
- 38.Sharma M., Lingford J.P., Petricevic M., Snow A.J.D., Zhang Y., Järvå M.A., et al. Oxidative desulfurization pathway for complete catabolism of sulfoquinovose by bacteria. Proc. Natl. Acad. Sci. U. S. A. 2022;119(4) doi: 10.1073/pnas.2116022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burrichter A., Denger K., Franchini P., Huhn T., Müller N., Spiteller D., et al. Anaerobic degradation of the plant sugar sulfoquinovose concomitant with H2S production: Escherichia coli K-12 and Desulfovibrio sp. strain DF1 as Co-culture model. Front. Microbiol. 2018;9:2792. doi: 10.3389/fmicb.2018.02792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J., Wei Y., Lin L., Teng L., Yin J., Lu Q., et al. Two radical-dependent mechanisms for anaerobic degradation of the globally abundant organosulfur compound dihydroxypropanesulfonate. Proc. Natl. Acad. Sci. U. S. A. 2020;117(27):15599–15608. doi: 10.1073/pnas.2003434117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metaxas M.A., Delwiche E.A. The L-cysteine desulfhydrase of Escherichia coli. J. Bacteriol. 1955;70(6):735–737. doi: 10.1128/jb.70.6.735-737.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saz A., Brownell L.W. D-Cysteine desulfhydrase in Escherichia coli. Arch. Biochem. Biophys. 1954;52(1):291–293. doi: 10.1016/0003-9861(54)90117-4. [DOI] [PubMed] [Google Scholar]
- 43.Shatalin K., Shatalina E., Mironov A., Nudler E. H2S: a universal defense against antibiotics in bacteria. Science. 2011;334(6058):986–990. doi: 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- 44.Sun H.J., Wu Z.Y., Nie X.W., Wang X.Y., Bian J.S. Implications of hydrogen sulfide in liver pathophysiology: mechanistic insights and therapeutic potential. J. Adv. Res. 2021;27:127–135. doi: 10.1016/j.jare.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szabó C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6(11):917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 46.Zuhra K., Augsburger F., Majtan T., Szabo C. Cystathionine-β-Synthase: molecular regulation and pharmacological inhibition. Biomolecules. 2020;10(5):697. doi: 10.3390/biom10050697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ascenção K., Szabo C. Emerging roles of cystathionine β-synthase in various forms of cancer. Redox Biol. 2022;53 doi: 10.1016/j.redox.2022.102331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banerjee R. Catalytic promiscuity and heme-dependent redox regulation of H2S synthesis. Curr. Opin. Chem. Biol. 2017;37:115–121. doi: 10.1016/j.cbpa.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu J., Berisa M., Schwörer S., Qin W., Cross J.R., Thompson C.B. Transsulfuration activity can support cell growth upon extracellular cysteine limitation. Cell Metabol. 2019;30(5):865–876. doi: 10.1016/j.cmet.2019.09.009. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H.F., Klein Geltink R.I., Parker S.J., Sorensen P.H. Transsulfuration, minor player or crucial for cysteine homeostasis in cancer. Trends Cell Biol. 2022;32(9):800–814. doi: 10.1016/j.tcb.2022.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sbodio J.I., Snyder S.H., Paul B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019;176(4):583–593. doi: 10.1111/bph.14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Augsburger F., Szabo C. Potential role of the 3-mercaptopyruvate sulfurtransferase (3-MST)-hydrogen sulfide (H2S) pathway in cancer cells. Pharmacol. Res. 2020;154 doi: 10.1016/j.phrs.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 53.Shibuya N., Koike S., Tanaka M., Ishigami-Yuasa M., Kimura Y., Ogasawara Y., et al. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013;4:1366. doi: 10.1038/ncomms2371. [DOI] [PubMed] [Google Scholar]
- 54.Olson K.R., Deleon E.R., Gao Y., Hurley K., Sadauskas V., Batz C., et al. Thiosulfate: a readily accessible source of hydrogen sulfide in oxygen sensing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305(6):R592–R603. doi: 10.1152/ajpregu.00421.2012. [DOI] [PubMed] [Google Scholar]
- 55.Mathai J.C., Missner A., Kügler P., Saparov S.M., Zeidel M.L., Lee J.K., et al. No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl. Acad. Sci. U. S. A. 2009;106(39):16633–16638. doi: 10.1073/pnas.0902952106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hemelrijk S.D., Dirkes M.C., van Velzen M.H.N., Bezemer R., van Gulik T.M., Heger M. Exogenous hydrogen sulfide gas does not induce hypothermia in normoxic mice. Sci. Rep. 2018;8(1):3855. doi: 10.1038/s41598-018-21729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olson K.R., Straub K.D. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology. 2016;31(1):60–72. doi: 10.1152/physiol.00024.2015. [DOI] [PubMed] [Google Scholar]
- 58.Landry A.P., Ballou D.P., Banerjee R. Hydrogen sulfide oxidation by sulfide quinone oxidoreductase. Chembiochem. 2021;22(6):949–960. doi: 10.1002/cbic.202000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Libiad M., Yadav P.K., Vitvitsky V., Martinov M., Banerjee R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J. Biol. Chem. 2014;289(45):30901–30910. doi: 10.1074/jbc.M114.602664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiranti V., Viscomi C., Hildebrandt T., Di Meo I., Mineri R., Tiveron C., et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med. 2009;15(2):200–205. doi: 10.1038/nm.1907. [DOI] [PubMed] [Google Scholar]
- 61.Kabil O., Banerjee R. Characterization of patient mutations in human persulfide dioxygenase (ETHE1) involved in H2S catabolism. J. Biol. Chem. 2012;287(53):44561–44567. doi: 10.1074/jbc.M112.407411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maiti B.K. Cross-talk between (Hydrogen)Sulfite and metalloproteins: impact on human health. Chemistry. 2022;28(23) doi: 10.1002/chem.202104342. [DOI] [PubMed] [Google Scholar]
- 63.Libiad M., Sriraman A., Banerjee R. Polymorphic variants of human rhodanese exhibit differences in thermal stability and sulfur transfer kinetics. J. Biol. Chem. 2015;290(39):23579–23588. doi: 10.1074/jbc.M115.675694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jackson M.R., Melideo S.L., Jorns M.S. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry. 2012;51(34):6804–6815. doi: 10.1021/bi300778t. [DOI] [PubMed] [Google Scholar]
- 65.Melideo S.L., Jackson M.R., Jorns M.S. Biosynthesis of a central intermediate in hydrogen sulfide metabolism by a novel human sulfurtransferase and its yeast ortholog. Biochemistry. 2014;53(28):4739–4753. doi: 10.1021/bi500650h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Landry A.P., Ballou D.P., Banerjee R. H2S oxidation by nanodisc-embedded human sulfide quinone oxidoreductase. J. Biol. Chem. 2017;292(28):11641–11649. doi: 10.1074/jbc.M117.788547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Libiad M., Vitvitsky V., Bostelaar T., Bak D.W., Lee H.J., Sakamoto N., et al. Hydrogen sulfide perturbs mitochondrial bioenergetics and triggers metabolic reprogramming in colon cells. J. Biol. Chem. 2019;294(32):12077–12090. doi: 10.1074/jbc.RA119.009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang R.H., Chu Y.H., Lin K.T. The hidden role of hydrogen sulfide metabolism in cancer. Int. J. Mol. Sci. 2021;22(12) doi: 10.3390/ijms22126562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iciek M., Bilska-Wilkosz A., Kozdrowicki M., Górny M. Reactive sulfur species and their significance in health and disease. Biosci. Rep. 2022;42(9) doi: 10.1042/BSR20221006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zuhra K., Tomé C.S., Forte E., Vicente J.B., Giuffrè A. The multifaceted roles of sulfane sulfur species in cancer-associated processes. Biochim. Biophys. Acta Bioenerg. 2021;1862(2) doi: 10.1016/j.bbabio.2020.148338. [DOI] [PubMed] [Google Scholar]
- 71.Goubern M., Andriamihaja M., Nübel T., Blachier F., Bouillaud F. Sulfide, the first inorganic substrate for human cells. Faseb. J. 2007;21(8):1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- 72.Blachier F., Davila A.M., Mimoun S., Benetti P.H., Atanasiu C., Andriamihaja M., et al. Luminal sulfide and large intestine mucosa: friend or foe? Amino Acids. 2010;39(2):335–347. doi: 10.1007/s00726-009-0445-2. [DOI] [PubMed] [Google Scholar]
- 73.Abou-Hamdan A., Guedouari-Bounihi H., Lenoir V., Andriamihaja M., Blachier F., Bouillaud F. Oxidation of H2S in mammalian cells and mitochondria. Methods Enzymol. 2015;554:201–228. doi: 10.1016/bs.mie.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 74.Beaumont M., Andriamihaja M., Lan A., Khodorova N., Audebert M., Blouin J.M., et al. Detrimental effects for colonocytes of an increased exposure to luminal hydrogen sulfide: the adaptive response. Free Radic. Biol. Med. 2016;93:155–164. doi: 10.1016/j.freeradbiomed.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 75.Mimoun S., Andriamihaja M., Chaumontet C., Atanasiu C., Benamouzig R., Blouin J.M., et al. Detoxification of H2S by differentiated colonic epithelial cells: implication of the sulfide oxidizing unit and of the cell respiratory capacity. Antioxidants Redox Signal. 2012;17(1):1–10. doi: 10.1089/ars.2011.4186. [DOI] [PubMed] [Google Scholar]
- 76.Shen X., Carlström M., Borniquel S., Jädert C., Kevil C.G., Lundberg J.O. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic. Biol. Med. 2013;60:195–200. doi: 10.1016/j.freeradbiomed.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shackelford R.E., Mohammad I.Z., Meram A.T., Kim D., Alotaibi F., Patel S., et al. Molecular functions of hydrogen sulfide in cancer. Pathophysiology. 2021;28(3):437–456. doi: 10.3390/pathophysiology28030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao K., Ju Y., Li S., Altaany Z., Wang R., Yang G. S-sulfhydration of MEK1 leads to PARP-1 activation and DNA damage repair. EMBO Rep. 2014;15(7):792–800. doi: 10.1002/embr.201338213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu Z., Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58(11):621–631. doi: 10.1080/15216540600957438. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y.H., Huang J.T., Chen W.L., Wang R.H., Kao M.C., Pan Y.R., et al. Dysregulation of cystathionine γ-lyase promotes prostate cancer progression and metastasis. EMBO Rep. 2019;20(10) doi: 10.15252/embr.201845986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoesel B., Schmid J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer. 2013;12(1):86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Szabo C., Coletta C., Chao C., Módis K., Szczesny B., Papapetropoulos A., et al. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. U. S. A. 2013;110(30):12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szabo C. Hydrogen sulfide, an endogenous stimulator of mitochondrial function in cancer cells. Cells. 2021;10(2):220. doi: 10.3390/cells10020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paul B.D., Snyder S.H., Kashfi K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 2021;38 doi: 10.1016/j.redox.2020.101772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Módis K., Ju Y., Ahmad A., Untereiner A.A., Altaany Z., Wu L., et al. S-Sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol. Res. 2016;113:116–124. doi: 10.1016/j.phrs.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wen J., Min X., Shen M., Hua Q., Han Y., Zhao L., et al. ACLY facilitates colon cancer cell metastasis by CTNNB1. J. Exp. Clin. Cancer Res. 2019;38(1):401. doi: 10.1186/s13046-019-1391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ascenção K., Dilek N., Augsburger F., Panagaki T., Zuhra K., Szabo C. Pharmacological induction of mesenchymal-epithelial transition via inhibition of H2S biosynthesis and consequent suppression of ACLY activity in colon cancer cells. Pharmacol. Res. 2021;165 doi: 10.1016/j.phrs.2020.105393. [DOI] [PubMed] [Google Scholar]
- 88.Guo F.F., Yu T.C., Hong J., Fang J.Y. Emerging roles of hydrogen sulfide in inflammatory and neoplastic colonic diseases. Front. Physiol. 2016;7:156. doi: 10.3389/fphys.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fang Y., Yan C., Zhao Q., Xu J., Liu Z., Gao J., et al. The roles of microbial products in the development of colorectal cancer: a review. Bioengineered. 2021;12(1):720–735. doi: 10.1080/21655979.2021.1889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dalal N., Jalandra R., Bayal N., Yadav A.K., Harshulika, Sharma M., et al. Gut microbiota-derived metabolites in CRC progression and causation. J. Cancer Res. Clin. Oncol. 2021;147(11):3141–3155. doi: 10.1007/s00432-021-03729-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamagishi K., Onuma K., Chiba Y., Yagi S., Aoki S., Sato T., et al. Generation of gaseous sulfur-containing compounds in tumour tissue and suppression of gas diffusion as an antitumour treatment. Gut. 2012;61(4):554–561. doi: 10.1136/gutjnl-2011-300721. [DOI] [PubMed] [Google Scholar]
- 92.Ramasamy S., Singh S., Taniere P., Langman M.J.S., Eggo M.C. Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291(2):G288–G296. doi: 10.1152/ajpgi.00324.2005. [DOI] [PubMed] [Google Scholar]
- 93.Kanazawa K., Konishi F., Mitsuoka T., Terada A., Itoh K., Narushima S., et al. Factors influencing the development of sigmoid colon cancer: bacteriologic and biochemical studies. Cancer. 1996;77(8):1701–1706. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1701::AID-CNCR42>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 94.Linden D.R., Levitt M.D., Farrugia G., et al. Endogenous production of H2S in the gastrointestinal tract: still in search of a physiologic function. Antioxidants Redox Signal. 2010;12(9):1135–1146. doi: 10.1089/ars.2009.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feng Q., Liang S., Jia H., Stadlmayr A., Tang L., Lan Z., et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat. Commun. 2015;6:6528. doi: 10.1038/ncomms7528. [DOI] [PubMed] [Google Scholar]
- 96.Hale V.L., Chen J., Johnson S., Harrington S.C., Yab T.C., Smyrk T.C., et al. Shifts in the fecal microbiota associated with adenomatous polyps. Cancer Epidemiol. Biomarkers Prev. 2017;26(1):85–94. doi: 10.1158/1055-9965.EPI-16-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nguyen L.H., Ma W., Wang D.D., Cao Y., Mallick H., Gerbaba T.K., et al. Association between sulfur-metabolizing bacterial communities in stool and risk of distal colorectal cancer in men. Gastroenterology. 2020;158(5):1313–1325. doi: 10.1053/j.gastro.2019.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hale V.L., Jeraldo P., Mundy M., Yao J., Keeney G., Scott N., et al. Synthesis of multi-omic data and community metabolic models reveals insights into the role of hydrogen sulfide in colon cancer. Methods. 2018;149:59–68. doi: 10.1016/j.ymeth.2018.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yachida S., Mizutani S., Shiroma H., Shiba S., Nakajima T., Sakamoto T., et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. 2019;25(6):968–976. doi: 10.1038/s41591-019-0458-7. [DOI] [PubMed] [Google Scholar]
- 100.Romanov V.A., Karasev I.A., Klimenko N.S., Koshechkin S.I., Tyakht A.V., Malikhova O.A. Luminal and tumor-associated gut microbiome features linked to precancerous lesions malignancy risk: a compositional approach. Cancers. 2022;14(21):5207. doi: 10.3390/cancers14215207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang N., Fang J.Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2022;14(23):5813. doi: 10.1016/j.tim.2022.08.010. [DOI] [PubMed] [Google Scholar]
- 102.Bullman S., Pedamallu C.S., Sicinska E., Clancy T.E., Zhang X., Cai D., et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358(6369):1443–1448. doi: 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y., Nguyen L.H., Mehta R.S., Song M., Huttenhower C., Chan A.T. Association between the sulfur microbial diet and risk of colorectal cancer. JAMA Netw. Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.34308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Untereiner A.A., Oláh G., Módis K., Hellmich M.R., Szabo C. H2S-induced S-sulfhydration of lactate dehydrogenase a (LDHA) stimulates cellular bioenergetics in HCT116 colon cancer cells. Biochem. Pharmacol. 2017;136:86–98. doi: 10.1016/j.bcp.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai W.J., Wang M.J., Ju L.H., Wang C., Zhu Y.C. Hydrogen sulfide induces human colon cancer cell proliferation: role of Akt, ERK and p21. Cell Biol. Int. 2010;34(6):565–572. doi: 10.1042/CBI20090368. [DOI] [PubMed] [Google Scholar]
- 106.Rose P., Moore P.K., Ming S.H., Nam O.C., Armstrong J.S., Whiteman M. Hydrogen sulfide protects colon cancer cells from chemopreventative agent beta-phenylethyl isothiocyanate induced apoptosis. World J. Gastroenterol. 2005;11(26):3990–3997. doi: 10.3748/wjg.v11.i26.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hellmich M.R., Coletta C., Chao C., et al. The therapeutic potential of cystathionine β-synthetase/hydrogen sulfide inhibition in cancer. Antioxidants Redox Signal. 2015;22(5):424–448. doi: 10.1089/ars.2014.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oláh G., Módis K., Törö G., Hellmich M.R., Szczesny B., Szabo C. Role of endogenous and exogenous nitric oxide, carbon monoxide and hydrogen sulfide in HCT116 colon cancer cell proliferation. Biochem. Pharmacol. 2018;149:186–204. doi: 10.1016/j.bcp.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen S., Yue T., Huang Z., Zhu J., Bu D., Wang X., et al. Inhibition of hydrogen sulfide synthesis reverses acquired resistance to 5-FU through miR-215-5p-EREG/TYMS axis in colon cancer cells. Cancer Lett. 2019;466:49–60. doi: 10.1016/j.canlet.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 110.Hellmich M.R., Chao C., Módis K., Ding Y., Zatarain J.R., Thanki K., et al. Efficacy of novel aminooxyacetic acid prodrugs in colon cancer models: towards clinical translation of the cystathionine β-synthase inhibition concept. Biomolecules. 2021;11(8) doi: 10.3390/biom11081073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guo S., Li J., Huang Z., Yue T., Zhu J., Wang X., et al. The CBS-H2S axis promotes liver metastasis of colon cancer by upregulating VEGF through AP-1 activation. Br. J. Cancer. 2022;126(7):1055–1066. doi: 10.1038/s41416-021-01681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Y., Chen S., Zhu J., Guo S., Yue T., Xu H., et al. Overexpression of CBS/H2S inhibits proliferation and metastasis of colon cancer cells through downregulation of CD44. Cancer Cell Int. 2022;22(1):85. doi: 10.1186/s12935-022-02512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Módis K., Coletta C., Asimakopoulou A., Szczesny B., Chao C., Papapetropoulos A., et al. Effect of S-adenosyl-L-methionine (SAM), an allosteric activator of cystathionine-β-synthase (CBS) on colorectal cancer cell proliferation and bioenergetics in vitro. Nitric Oxide. 2014;41:146–156. doi: 10.1016/j.niox.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Phillips C.M., Zatarain J.R., Nicholls M.E., Porter C., Widen S.G., Thanki K., et al. Upregulation of cystathionine-β-synthase in colonic epithelia reprograms metabolism and promotes carcinogenesis. Cancer Res. 2017;77(21):5741–5754. doi: 10.1158/0008-5472.CAN-16-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fan K., Li N., Qi J., Yin P., Zhao C., Wang L., et al. Wnt/β-catenin signaling induces the transcription of cystathionine-γ-lyase, a stimulator of tumor in colon cancer. Cell. Signal. 2014;26(12):2801–2808. doi: 10.1016/j.cellsig.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 116.Thanki K.K., Johnson P., Higgins E.J., Maskey M., Phillips C., Dash S., et al. Deletion of cystathionine-γ-lyase in bone marrow-derived cells promotes colitis-associated carcinogenesis. Redox Biol. 2022;55 doi: 10.1016/j.redox.2022.102417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Augsburger F., Randi E.B., Jendly M., Ascencao K., Dilek N., Szabo C. Role of 3-mercaptopyruvate sulfurtransferase in the regulation of proliferation, migration, and bioenergetics in murine colon cancer cells. Biomolecules. 2020;10(3):447. doi: 10.3390/biom10030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hanaoka K., Sasakura K., Suwanai Y., Toma-Fukai S., Shimamoto K., Takano Y., et al. Discovery and mechanistic characterization of selective inhibitors of H2S-producing enzyme: 3-mercaptopyruvate sulfurtransferase (3MST) targeting active-site cysteine persulfide. Sci. Rep. 2017;7 doi: 10.1038/srep40227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ozluk E., Coppola D., Mohammad I.Z., Islam T., Ghali G., Kevil C.G., et al. Ethylmalonic encephalopathy 1 protein is increased in colorectal adenocarcinoma. Anticancer Res. 2021;41(10):4719–4723. doi: 10.21873/anticanres.15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Piran M., Sepahi N., Moattari A., Rahimi A., Ghanbariasad A. Systems biomedicine of primary and metastatic colorectal cancer reveals potential therapeutic targets. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.597536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ascenção K., Dilek N., Zuhra K., Módis K., Sato T., Szabo C. Sequential accumulation of 'driver' pathway mutations induces the upregulation of hydrogen-sulfide-producing enzymes in human colonic epithelial cell organoids. Antioxidants. 2022;11(9):1823. doi: 10.3390/antiox11091823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jackson M.R., Cox K.D., Baugh S.D.P., Wakeen L., Rashad A.A., Lam P.Y.S., et al. Discovery of a first-in-class inhibitor of sulfide:quinone oxidoreductase that protects against adverse cardiac remodelling and heart failure. Cardiovasc. Res. 2021;118(7):1771–1784. doi: 10.1093/cvr/cvab206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baugh S.D.P., Jackson M.R., Rashad A.A., Reitz A.B., Lam P.Y.S., Jorns M.S. Synthesis and evaluation of potent novel inhibitors of human sulfide:quinone oxidoreductase. Bioorg. Med. Chem. Lett. 2021;54 doi: 10.1016/j.bmcl.2021.128443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Linden D., Furne J., Stoltz G., Abdel-Rehim M., Levitt M., Szurszewski J. Sulphide quinone reductase contributes to hydrogen sulphide metabolism in murine peripheral tissues but not in the CNS. Br. J. Pharmacol. 2012;165(7):2178–2190. doi: 10.1111/j.1476-5381.2011.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Di Meo I., Lamperti C., Tiranti V. Mitochondrial diseases caused by toxic compound accumulation: from etiopathology to therapeutic approaches. EMBO Mol. Med. 2015;7(10):1257–1266. doi: 10.15252/emmm.201505040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grings M., Wajner M., Leipnitz G. Mitochondrial dysfunction and redox homeostasis impairment as pathomechanisms of brain damage in ethylmalonic encephalopathy: insights from animal and human studies. Cell. Mol. Neurobiol. 2022;42(3):565–575. doi: 10.1007/s10571-020-00976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Filipovic M.R., Zivanovic J., Alvarez B., Banerjee R. Chemical biology of H2S signaling through persulfidation. Chem. Rev. 2018;118(3):1253–1337. doi: 10.1021/acs.chemrev.7b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mustafa A.K., Gadalla M.M., Sen N., Kim S., Mu W., Gazi S.K., et al. H2S signals through protein S-sulfhydration. Sci. Signal. 2009;2(96):ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun H.J., Wu Z.Y., Nie X.W., Bian J.S. Role of hydrogen sulfide and polysulfides in neurological diseases: focus on protein S-persulfidation. Curr. Neuropharmacol. 2021;19(6):868–884. doi: 10.2174/1570159X18666200905143550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kimura H. Signalling by hydrogen sulfide and polysulfides via protein S-sulfuration. Br. J. Pharmacol. 2020;177(4):720–733. doi: 10.1111/bph.14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang C.T., Devarie-Baez N.O., Hamsath A., Fu X.D., Xian M., S-Persulfidation Chemistry, chemical biology, and significance in health and disease. Antioxidants Redox Signal. 2020;33(15):1092–1114. doi: 10.1089/ars.2019.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kimura Y., Koike S., Shibuya N., Lefer D., Ogasawara Y., Kimura H. 3-Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine- and glutathione-persulfide (Cys-SSH and GSSH) together with signaling molecules H2S2, H2S3 and H2S. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-11004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shibuya N., Tanaka M., Yoshida M., Ogasawara Y., Togawa T., Ishii K., et al. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxidants Redox Signal. 2009;11(4):703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 134.Huang X., Xiang L., Li Y., Zhao Y., Zhu H., Xiao Y., et al. Snail/FOXK1/Cyr61 signaling Axis regulates the epithelial-mesenchymal transition and metastasis in colorectal cancer. Cell. Physiol. Biochem. 2018;47(2):590–603. doi: 10.1159/000490015. [DOI] [PubMed] [Google Scholar]
- 135.Monnier Y., Farmer P., Bieler G., Imaizumi N., Sengstag T., Alghisi G.C., et al. CYR61 and alphaVbeta5 integrin cooperate to promote invasion and metastasis of tumors growing in preirradiated stroma. Cancer Res. 2008;68(18):7323–7331. doi: 10.1158/0008-5472.CAN-08-0841. [DOI] [PubMed] [Google Scholar]
- 136.Baek M., Bae S., Jeong D. Relationship of pro-angiogenic factor Cyr61 to colorectal cancer development and prognosis. J. Clin. Oncol. 2011;29(4_suppl):446. [Google Scholar]
- 137.Ascenção K., Lheimeur B., Szabo C. Regulation of CyR61 expression and release by 3-mercaptopyruvate sulfurtransferase in colon cancer cells. Redox Biol. 2022;56 doi: 10.1016/j.redox.2022.102466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen S., Bu D., Zhu J., Yue T., Guo S., Wang X., et al. Endogenous hydrogen sulfide regulates xCT stability through persulfidation of OTUB1 at cysteine 91 in colon cancer cells. Neoplasia. 2021;23(5):461–472. doi: 10.1016/j.neo.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pedre B., Barayeu U., Ezeriņa D., Dick T.P. The mechanism of action of N-acetylcysteine (NAC): the emerging role of H2S and sulfane sulfur species. Pharmacol. Ther. 2021;228 doi: 10.1016/j.pharmthera.2021.107916. [DOI] [PubMed] [Google Scholar]
- 140.Kalyanaraman B. NAC, NAC, Knockin’ on Heaven's door: interpreting the mechanism of action of N-acetylcysteine in tumor and immune cells. Redox Biol. 2022;57 doi: 10.1016/j.redox.2022.102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zuhra K., Tomé C.S., Masi L., Giardina G., Paulini G., Malagrinò F., et al. N-acetylcysteine serves as substrate of 3-mercaptopyruvate sulfurtransferase and stimulates sulfide metabolism in colon cancer cells. Cells. 2019;8(8):828. doi: 10.3390/cells8080828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Busch C., Jacob C., Anwar A., Burkholz T., Aicha Ba L., Cerella C., et al. Diallylpolysulfides induce growth arrest and apoptosis. Int. J. Oncol. 2010;36(3):743–749. doi: 10.3892/ijo_00000550. [DOI] [PubMed] [Google Scholar]
- 143.Saidu N.E.B., Valente S., Bana E., Kirsch G., Bagrel D., Montenarh M. Coumarin polysulfides inhibit cell growth and induce apoptosis in HCT116 colon cancer cells. Bioorg. Med. Chem. 2012;20(4):1584–1593. doi: 10.1016/j.bmc.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 144.Siegers C.P., Steffen B., Röbke A., Pentz R. The effects of garlic preparations against human tumor cell proliferation. Phytomedicine. 1999;6(1):7–11. doi: 10.1016/S0944-7113(99)80028-2. [DOI] [PubMed] [Google Scholar]
- 145.Lai K.C., Kuo C.L., Ho H.C., Yang J.S., Ma C.Y., Lu H.F., et al. Diallyl sulfide, diallyl disulfide and diallyl trisulfide affect drug resistant gene expression in colo 205 human colon cancer cells in vitro and in vivo. Phytomedicine. 2012;19(7):625–630. doi: 10.1016/j.phymed.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 146.Yagdi Efe E., Mazumder A., Lee J.Y., Gaigneaux A., Radogna F., Nasim M.J., et al. Tubulin-binding anticancer polysulfides induce cell death via mitotic arrest and autophagic interference in colorectal cancer. Cancer Lett. 2017;410:139–157. doi: 10.1016/j.canlet.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 147.Wang Y., Ni X., Chadha R., McCartney C., Lam Y., Brummett B., et al. Methods for suppressing hydrogen sulfide in biological systems. Antioxidants Redox Signal. 2022;36(4–6):294–308. doi: 10.1089/ars.2021.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McCormick D.B., Snell E.E. Pyridoxal phosphokinases. II. Effects of inhibitors. J. Biol. Chem. 1961;236:2085–2088. [PubMed] [Google Scholar]
- 149.Wang R., Gu X., Li Q., Gao J., Shi B., Xu G., et al. Aggregation enhanced responsiveness of rationally designed probes to hydrogen sulfide for targeted cancer imaging. J. Am. Chem. Soc. 2020;142(35):15084–15090. doi: 10.1021/jacs.0c06533. [DOI] [PubMed] [Google Scholar]
- 150.Yue T., Zuo S., Bu D., Zhu J., Chen S., Ma Y., et al. Aminooxyacetic acid (AOAA) sensitizes colon cancer cells to oxaliplatin via exaggerating apoptosis induced by ROS. J. Cancer. 2020;11(7):1828–1838. doi: 10.7150/jca.35375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Asimakopoulou A., Panopoulos P., Chasapis C.T., Coletta C., Zhou Z., Cirino G., et al. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE) Br. J. Pharmacol. 2013;169(4):922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chao C., Zatarain J.R., Ding Y., Coletta C., Mrazek A.A., Druzhyna N., et al. Cystathionine-beta-synthase inhibition for colon cancer: enhancement of the efficacy of aminooxyacetic acid via the prodrug approach. Mol. Med. 2016;22:361–379. doi: 10.2119/molmed.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Druzhyna N., Szczesny B., Olah G., Módis K., Asimakopoulou A., Pavlidou A., et al. Screening of a composite library of clinically used drugs and well-characterized pharmacological compounds for cystathionine β-synthase inhibition identifies benserazide as a drug potentially suitable for repurposing for the experimental therapy of colon cancer. Pharmacol. Res. 2016;113(Pt A):18–37. doi: 10.1016/j.phrs.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Thorson M.K., Majtan T., Kraus J.P., Barrios A.M. Identification of cystathionine β-synthase inhibitors using a hydrogen sulfide selective probe. Angew Chem. Int. Ed. Engl. 2013;52(17):4641–4644. doi: 10.1002/anie.201300841. [DOI] [PubMed] [Google Scholar]
- 155.Untereiner A.A., Pavlidou A., Druzhyna N., Papapetropoulos A., Hellmich M.R., Szabo C. Drug resistance induces the upregulation of H2S-producing enzymes in HCT116 colon cancer cells. Biochem. Pharmacol. 2018;149:174–185. doi: 10.1016/j.bcp.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mehmood S., Maqsood M., Mahtab N., Khan M.I., Sahar A., Zaib S., et al. Epigallocatechin gallate: phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022;46(8) doi: 10.1111/jfbc.14189. [DOI] [PubMed] [Google Scholar]
- 157.Kim J.M., Heo H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022;31(8):957–970. doi: 10.1007/s10068-022-01069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alam M., Ali S., Ashraf G.M., Bilgrami A.L., Yadav D.K., Hassan M.I. Epigallocatechin 3-gallate: from green tea to cancer therapeutics. Food Chem. 2022;379 doi: 10.1016/j.foodchem.2022.132135. [DOI] [PubMed] [Google Scholar]
- 159.Payne A., Nahashon S., Taka E., Adinew G.M., Soliman K.F.A. Epigallocatechin-3-Gallate (EGCG): new therapeutic perspectives for neuroprotection, aging, and neuroinflammation for the modern age. Biomolecules. 2022;12(3):371. doi: 10.3390/biom12030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Du G.J., Zhang Z., Wen X.D., Yu C., Calway T., Yuan C.S., et al. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients. 2012;4(11):1679–1691. doi: 10.3390/nu4111679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Olson K.R., Briggs A., Devireddy M., Iovino N.A., Skora N.C., Whelan J., et al. Green tea polyphenolic antioxidants oxidize hydrogen sulfide to thiosulfate and polysulfides: a possible new mechanism underpinning their biological action. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zuhra K., Petrosino M., Gupta B., Panagaki T., Cecconi M., Myrianthopoulos V., et al. Epigallocatechin gallate is a potent inhibitor of cystathionine beta-synthase: structure-activity relationship and mechanism of action. Nitric Oxide. 2022;128:12–24. doi: 10.1016/j.niox.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 163.Mereles D., Hunstein W. Epigallocatechin-3-gallate (EGCG) for clinical trials: more pitfalls than promises? Int. J. Mol. Sci. 2011;12(9):5592–5603. doi: 10.3390/ijms12095592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Abeles R.H., Walsh C.T. Acetylenic enzyme inactivators. Inactivation of gamma-cystathionase, in vitro and in vivo, by propargylglycine. J. Am. Chem. Soc. 1973;95(18):6124–6125. doi: 10.1021/ja00799a053. [DOI] [PubMed] [Google Scholar]
- 165.Bantzi M., Augsburger F., Loup J., Berset Y., Vasilakaki S., Myrianthopoulos V., et al. Novel aryl-substituted pyrimidones as inhibitors of 3-mercaptopyruvate sulfurtransferase with antiproliferative efficacy in colon cancer. J. Med. Chem. 2021;64(9):6221–6240. doi: 10.1021/acs.jmedchem.1c00260. [DOI] [PubMed] [Google Scholar]
- 166.Yang C.T., Wang Y., Marutani E., Ida T., Ni X., Xu S., et al. Data-driven identification of hydrogen sulfide scavengers. Angew Chem. Int. Ed. Engl. 2019;58(32):10898–10902. doi: 10.1002/anie.201905580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ismail I., Chen Z., Sun L., Ji X., Ye H., Kang X., et al. Highly efficient H2S scavengers via thiolysis of positively-charged NBD amines. Chem. Sci. 2020;11(30):7823–7828. doi: 10.1039/d0sc01518k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zuhra K., Panagaki T., Randi E.B., Augsburger F., Blondel M., Friocourt G., et al. Mechanism of cystathionine-β-synthase inhibition by disulfiram: the role of bis(N,N-diethyldithiocarbamate)-copper(II) Biochem. Pharmacol. 2020;182 doi: 10.1016/j.bcp.2020.114267. [DOI] [PubMed] [Google Scholar]
- 169.Liu D., Li B., Wu J., Liu Y. Sorbents for hydrogen sulfide capture from biogas at low temperature: a review. Environ. Chem. Lett. 2020;18(1):113–128. [Google Scholar]
- 170.Aksoy B., Atakan N., Aksoy H.M., Tezel G.G., Renda N., Ozkara H.A., et al. Effectiveness of topical zinc oxide application on hypertrophic scar development in rabbits. Burns. 2010;36(7):1027–1035. doi: 10.1016/j.burns.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 171.Pan X., Qi Y., Du Z., He J., Yao S., Lu W., et al. Zinc oxide nanosphere for hydrogen sulfide scavenging and ferroptosis of colorectal cancer. J. Nanobiotechnol. 2021;19(1):392. doi: 10.1186/s12951-021-01069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jia C., Guo Y., Wu F.G. Chemodynamic therapy via Fenton and fenton-like nanomaterials: strategies and recent advances. Small. 2022;18(6) doi: 10.1002/smll.202103868. [DOI] [PubMed] [Google Scholar]
- 173.Zhou Y., Fan S., Feng L., Huang X., Chen X. Manipulating intratumoral Fenton chemistry for enhanced chemodynamic and chemodynamic-synergized multimodal therapy. Adv. Mater. 2021;33(48) doi: 10.1002/adma.202104223. [DOI] [PubMed] [Google Scholar]
- 174.Tang Z., Liu Y., He M., Bu W. Chemodynamic therapy: tumour microenvironment-mediated Fenton and fenton-like reactions. Angew Chem. Int. Ed. Engl. 2019;58(4):946–956. doi: 10.1002/anie.201805664. [DOI] [PubMed] [Google Scholar]
- 175.Wang C., Xue F., Wang M., An L., Wu D., Tian Q. 2D Cu-bipyridine MOF nanosheet as an agent for colon cancer therapy: a three-in-one approach for enhancing chemodynamic therapy. ACS Appl. Mater. Interfaces. 2022;14(34):38604–38616. doi: 10.1021/acsami.2c11999. [DOI] [PubMed] [Google Scholar]
- 176.Tian Q., An L., Tian Q., Lin J., Yang S. Ellagic acid-Fe@BSA nanoparticles for endogenous H2S accelerated Fe(III)/Fe(II) conversion and photothermal synergistically enhanced chemodynamic therapy. Theranostics. 2020;10(9):4101–4115. doi: 10.7150/thno.41882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang S., Yang Y., Wu H., Li J., Xie P., Xu F., et al. Thermosensitive and tum or microenvironment activated nanotheranostics for the chemodynamic/photothermal therapy of colorectal tumor. J. Colloid Interface Sci. 2022;612:223–234. doi: 10.1016/j.jcis.2021.12.126. [DOI] [PubMed] [Google Scholar]
- 178.Ma B., Wang S., Liu F., Zhang S., Duan J., Li Z., et al. Self-assembled copper-amino acid nanoparticles for in situ glutathione "AND" H2O2 sequentially triggered chemodynamic therapy. J. Am. Chem. Soc. 2019;141(2):849–857. doi: 10.1021/jacs.8b08714. [DOI] [PubMed] [Google Scholar]
- 179.Wu W., Yu L., Jiang Q., Huo M., Lin H., Wang L., et al. Enhanced tumor-specific disulfiram chemotherapy by in situ Cu2+ chelation-initiated nontoxicity-to-toxicity transition. J. Am. Chem. Soc. 2019;141(29):11531–11539. doi: 10.1021/jacs.9b03503. [DOI] [PubMed] [Google Scholar]
- 180.Li Y., Zhou J., Wang L., Xie Z. Endogenous hydrogen sulfide-triggered MOF-based nanoenzyme for synergic cancer therapy. ACS Appl. Mater. Interfaces. 2020;12(27):30213–30220. doi: 10.1021/acsami.0c08659. [DOI] [PubMed] [Google Scholar]
- 181.Ding H., Chang J., He F., Gai S., Yang P. Hydrogen sulfide: an emerging precision strategy for gas therapy. Adv Healthc Mater. 2022;11(4) doi: 10.1002/adhm.202101984. [DOI] [PubMed] [Google Scholar]
- 182.Rong F., Wang T., Zhou Q., Peng H., Yang J., Fan Q., et al. Intelligent polymeric hydrogen sulfide delivery systems for therapeutic applications. Bioact. Mater. 2023;19:198–216. doi: 10.1016/j.bioactmat.2022.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wu D., Hu Q., Zhu Y. Therapeutic application of hydrogen sulfide donors: the potential and challenges. Front. Med. 2016;10(1):18–27. doi: 10.1007/s11684-015-0427-6. [DOI] [PubMed] [Google Scholar]
- 184.Ngowi E.E., Afzal A., Sarfraz M., Khattak S., Zaman S.U., Khan N.H., et al. Role of hydrogen sulfide donors in cancer development and progression. Int. J. Biol. Sci. 2021;17(1):73–88. doi: 10.7150/ijbs.47850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Powell C.R., Dillon K.M., Matson J.B. A review of hydrogen sulfide (H2S) donors: chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018;149:110–123. doi: 10.1016/j.bcp.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li L., Whiteman M., Guan Y.Y., Neo K.L., Cheng Y., Lee S.W., et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117(18):2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 187.Lee Z.W., Zhou J., Chen C.S., Zhao Y., Tan C.H., Li L., et al. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sakuma S., Minamino S., Takase M., Ishiyama Y., Hosokura H., Kohda T., et al. Hydrogen sulfide donor GYY4137 suppresses proliferation of human colorectal cancer Caco-2 cells by inducing both cell cycle arrest and cell death. Heliyon. 2019;5(8) doi: 10.1016/j.heliyon.2019.e02244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xu W., Watanabe K., Mizukami Y., Yamamoto Y., Suzuki T. Hydrogen sulfide suppresses the proliferation of intestinal epithelial cells through cell cycle arrest. Arch. Biochem. Biophys. 2021;712 doi: 10.1016/j.abb.2021.109044. [DOI] [PubMed] [Google Scholar]
- 190.Szadvari I., Hudecova S., Chovancova B., Matuskova M., Cholujova D., Lencesova L., et al. Sodium/calcium exchanger is involved in apoptosis induced by H2S in tumor cells through decreased levels of intracellular pH. Nitric Oxide. 2019;87:1–9. doi: 10.1016/j.niox.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 191.Kajsik M., Chovancova B., Liskova V., Babula P., Krizanova O. Slow sulfide donor GYY4137 potentiates effect of paclitaxel on colorectal carcinoma cells. Eur. J. Pharmacol. 2022;922 doi: 10.1016/j.ejphar.2022.174875. [DOI] [PubMed] [Google Scholar]
- 192.Li H., Mu J., Sun J., Xu S., Liu W., Xu F., et al. Hydrogen sulfide releasing oridonin derivatives induce apoptosis through extrinsic and intrinsic pathways. Eur. J. Med. Chem. 2020;187 doi: 10.1016/j.ejmech.2019.111978. [DOI] [PubMed] [Google Scholar]
- 193.Hu X., Jiao R., Li H., Wang X., Lyu H., Gao X., et al. Antiproliferative hydrogen sulfide releasing evodiamine derivatives and their apoptosis inducing properties. Eur. J. Med. Chem. 2018;151:376–388. doi: 10.1016/j.ejmech.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 194.Foster J.C., Powell C.R., Radzinski S.C., Matson J.B., S-aroylthiooximes A facile route to hydrogen sulfide releasing compounds with structure-dependent release kinetics. Org. Lett. 2014;16(6):1558–1561. doi: 10.1021/ol500385a. [DOI] [PubMed] [Google Scholar]
- 195.Foster J.C., Radzinski S.C., Zou X., Finkielstein C.V., Matson J.B. H2S-Releasing polymer micelles for studying selective cell toxicity. Mol. Pharm. 2017;14(4):1300–1306. doi: 10.1021/acs.molpharmaceut.6b01117. [DOI] [PubMed] [Google Scholar]
- 196.Piragine E., Flori L., Di Cesare Mannelli L., Ghelardini C., Pagnotta E., Matteo R., et al. Eruca sativa Mill. seed extract promotes anti-obesity and hypoglycemic effects in mice fed with a high-fat diet. Phytother Res. 2021;35(4):1983–1990. doi: 10.1002/ptr.6941. [DOI] [PubMed] [Google Scholar]
- 197.Bhattacherjee D., Basu C., Bhardwaj Q., Mal S., Sahu S., Sur R., et al. Design, synthesis and anti‐cancer activities of benzyl analogues of garlic‐derived diallyl disulfide (DADS) and the corresponding diselenides. ChemistrySelect. 2017;2(24):7399–7406. [Google Scholar]
- 198.Gojon G., Morales G.A. SG1002 and catenated divalent organic sulfur compounds as promising hydrogen sulfide prodrugs. Antioxidants Redox Signal. 2020;33(14):1010–1045. doi: 10.1089/ars.2020.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yagdi E., Cerella C., Dicato M., Diederich M. Garlic-derived natural polysulfanes as hydrogen sulfide donors: friend or foe? Food Chem. Toxicol. 2016;95:219–233. doi: 10.1016/j.fct.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 200.Chattopadhyay M., Kodela R., Nath N., Street C.R., Velázquez-Martínez C.A., Boring D., et al. Hydrogen sulfide-releasing aspirin modulates xenobiotic metabolizing enzymes in vitro and in vivo. Biochem. Pharmacol. 2012;83(6):733–740. doi: 10.1016/j.bcp.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 201.Chattopadhyay M., Kodela R., Nath N., Dastagirzada Y.M., Velázquez-Martínez C.A., Boring D., et al. Hydrogen sulfide-releasing NSAIDs inhibit the growth of human cancer cells: a general property and evidence of a tissue type-independent effect. Biochem. Pharmacol. 2012;83(6):715–722. doi: 10.1016/j.bcp.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 202.Elsheikh W., Blackler R.W., Flannigan K.L., Wallace J.L. Enhanced chemopreventive effects of a hydrogen sulfide-releasing anti-inflammatory drug (ATB-346) in experimental colorectal cancer. Nitric Oxide. 2014;41:131–137. doi: 10.1016/j.niox.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 203.Kodela R., Nath N., Chattopadhyay M., Nesbitt D.E., Velázquez-Martínez C.A., Kashfi K. Hydrogen sulfide-releasing naproxen suppresses colon cancer cell growth and inhibits NF-κB signaling. Drug Des. Dev. Ther. 2015;9:4873–4882. doi: 10.2147/DDDT.S91116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Paul-Clark M., Elsheikh W., Kirkby N., Chan M., Devchand P., Agbor T.A., et al. Profound chemopreventative effects of a hydrogen sulfide-releasing NSAID in the APCMin/+ mouse model of intestinal tumorigenesis. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0147289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kodela R., Chattopadhyay M., Kashfi K. NOSH-aspirin: a novel nitric oxide-hydrogen sulfide-releasing hybrid: a new class of anti-inflammatory pharmaceuticals. ACS Med. Chem. Lett. 2012;3(3):257–262. doi: 10.1021/ml300002m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kodela R., Chattopadhyay M., Kashfi K. Synthesis and biological activity of NOSH-naproxen (AVT-219) and NOSH-sulindac (AVT-18A) as potent anti-inflammatory agents with chemotherapeutic potential. Medchemcomm. 2013;4(11):1472–1481. doi: 10.1039/C3MD00185G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chattopadhyay M., Kodela R., Olson K.R., Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model. Biochem. Biophys. Res. Commun. 2012;419(3):523–528. doi: 10.1016/j.bbrc.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 208.Vannini F., Kodela R., Chattopadhyay M., Kashfi K. NOSH-aspirin inhibits colon cancer cell growth: effects of positional isomerism. Redox Biol. 2015;5:421. doi: 10.1016/j.redox.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 209.Vannini F., MacKessack-Leitch A.C., Eschbach E.K., Chattopadhyay M., Kodela R., Kashfi K. Synthesis and anti-cancer potential of the positional isomers of NOSH-aspirin (NBS-1120) a dual nitric oxide and hydrogen sulfide releasing hybrid. Bioorg. Med. Chem. Lett. 2015;25(20):4677–4682. doi: 10.1016/j.bmcl.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vannini F., Chattopadhyay M., Kodela R., Rao P.P.N., Kashfi K. Positional isomerism markedly affects the growth inhibition of colon cancer cells by NOSH-aspirin: COX inhibition and modeling. Redox Biol. 2015;6:318–325. doi: 10.1016/j.redox.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chattopadhyay M., Kodela R., Duvalsaint P.L., Kashfi K. Gastrointestinal safety, chemotherapeutic potential, and classic pharmacological profile of NOSH-naproxen (AVT-219) a dual NO- and H2S-releasing hybrid. Pharmacol Res Perspect. 2016;4(2) doi: 10.1002/prp2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kashfi K., Chattopadhyay M., Kodela R. NOSH-sulindac (AVT-18A) is a novel nitric oxide- and hydrogen sulfide-releasing hybrid that is gastrointestinal safe and has potent anti-inflammatory, analgesic, antipyretic, anti-platelet, and anti-cancer properties. Redox Biol. 2015;6:287–296. doi: 10.1016/j.redox.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kaur K., Carrazzone R.J., Matson J.B. The benefits of macromolecular/supramolecular approaches in hydrogen sulfide delivery: a review of polymeric and self-assembled hydrogen sulfide donors. Antioxidants Redox Signal. 2020;32(2):79–95. doi: 10.1089/ars.2019.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Housein Z., Kareem T.S., Salihi A. In vitro anticancer activity of hydrogen sulfide and nitric oxide alongside nickel nanoparticle and novel mutations in their genes in CRC patients. Sci. Rep. 2021;11(1):2536. doi: 10.1038/s41598-021-82244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li J., Xie L., Li B., Yin C., Wang G., Sang W., et al. Engineering a hydrogen-sulfide-based nanomodulator to normalize hyperactive photothermal immunogenicity for combination cancer therapy. Adv. Mater. 2021;33(22) doi: 10.1002/adma.202008481. [DOI] [PubMed] [Google Scholar]
- 216.Peng S., Xiao F., Chen M., Gao H. Tumor-microenvironment-responsive nanomedicine for enhanced cancer immunotherapy. Adv. Sci. 2022;9(1) doi: 10.1002/advs.202103836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li B., Shao H., Gao L., Li H., Sheng H., Zhu L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: a review. Drug Deliv. 2022;29(1):2130–2161. doi: 10.1080/10717544.2022.2094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Singh N., Kim J., Kim J., Lee K., Zunbul Z., Lee I., et al. Covalent organic framework nanomedicines: biocompatibility for advanced nanocarriers and cancer theranostics applications. Bioact. Mater. 2023;21:358–380. doi: 10.1016/j.bioactmat.2022.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nunes S.C., Ramos C., Lopes-Coelho F., Sequeira C.O., Silva F., Gouveia-Fernandes S., et al. Cysteine allows ovarian cancer cells to adapt to hypoxia and to escape from carboplatin cytotoxicity. Sci. Rep. 2018;8(1):9513. doi: 10.1038/s41598-018-27753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Thirumalaivasan N., Venkatesan P., Lai P.S., Wu S.P. In vitro and in vivo approach of hydrogen-sulfide-responsive drug release driven by azide-functionalized mesoporous silica nanoparticles. ACS Appl. Bio Mater. 2019;2(9):3886–3896. doi: 10.1021/acsabm.9b00481. [DOI] [PubMed] [Google Scholar]
- 221.Bobba K.N., Saranya G., Sujai P.T., Joseph M.M., Velusamy N., Podder A., et al. Endogenous H2S-assisted cancer-cell-specific activation of theranostics with emission readout. ACS Appl. Bio Mater. 2019;2(3):1322–1330. doi: 10.1021/acsabm.9b00019. [DOI] [PubMed] [Google Scholar]
- 222.Choi J., Sun I.C., Sook Hwang H., Yeol Yoon H., Kim K. Light-triggered photodynamic nanomedicines for overcoming localized therapeutic efficacy in cancer treatment. Adv. Drug Deliv. Rev. 2022;186 doi: 10.1016/j.addr.2022.114344. [DOI] [PubMed] [Google Scholar]
- 223.Carigga Gutierrez N.M., Pujol-Solé N., Arifi Q., Coll J.L., le Clainche T., Broekgaarden M. Increasing cancer permeability by photodynamic priming: from microenvironment to mechanotransduction signaling. Cancer Metastasis Rev. 2022:1–36. doi: 10.1007/s10555-022-10064-0. [DOI] [PubMed] [Google Scholar]
- 224.Yuan J., Ren T.B., Xu S., Wang C.J., Zhang X.B., Yuan L. A unique multifunctional luminescent probe for self-monitoring photodynamic therapy by detecting H2S in cancer cells. ACS Appl. Bio Mater. 2021;4(8):6016–6022. doi: 10.1021/acsabm.1c00273. [DOI] [PubMed] [Google Scholar]
- 225.Ma Y., Li X., Li A., Yang P., Zhang C., Tang B. H2S-Activable MOF nanoparticle photosensitizer for effective photodynamic therapy against cancer with controllable singlet-oxygen release. Angew Chem. Int. Ed. Engl. 2017;56(44):13752–13756. doi: 10.1002/anie.201708005. [DOI] [PubMed] [Google Scholar]
- 226.Wang R., Dong K., Xu G., Shi B., Zhu T., Shi P., et al. Activatable near-infrared emission-guided on-demand administration of photodynamic anticancer therapy with a theranostic nanoprobe. Chem. Sci. 2019;10(9):2785–2790. doi: 10.1039/c8sc04854a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wu L., Sun Y., Sugimoto K., Luo Z., Ishigaki Y., Pu K., et al. Engineering of electrochromic materials as activatable probes for molecular imaging and photodynamic therapy. J. Am. Chem. Soc. 2018;140(47):16340–16352. doi: 10.1021/jacs.8b10176. [DOI] [PubMed] [Google Scholar]
- 228.Chang M., Hou Z., Wang M., Li C., Lin J. Recent advances in hyperthermia therapy-based synergistic immunotherapy. Adv. Mater. 2021;33(4) doi: 10.1002/adma.202004788. [DOI] [PubMed] [Google Scholar]
- 229.Xu C., Pu K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2021;50(2):1111–1137. doi: 10.1039/d0cs00664e. [DOI] [PubMed] [Google Scholar]
- 230.Liu S., Pan X., Liu H. Two-dimensional nanomaterials for photothermal therapy. Angew Chem. Int. Ed. Engl. 2020;59(15):5890–5900. doi: 10.1002/anie.201911477. [DOI] [PubMed] [Google Scholar]
- 231.Shi B., Yan Q., Tang J., Xin K., Zhang J., Zhu Y., et al. Hydrogen sulfide-activatable second near-infrared fluorescent nanoassemblies for targeted photothermal cancer therapy. Nano Lett. 2018;18(10):6411–6416. doi: 10.1021/acs.nanolett.8b02767. [DOI] [PubMed] [Google Scholar]
- 232.An L., Wang X., Rui X., Lin J., Yang H., Tian Q., et al. The in situ sulfidation of Cu2O by endogenous H2S for colon cancer theranostics. Angew Chem. Int. Ed. Engl. 2018;57(48):15782–15786. doi: 10.1002/anie.201810082. [DOI] [PubMed] [Google Scholar]
- 233.Tao C., An L., Lin J., Tian Q., Yang S. Surface plasmon resonance–enhanced photoacoustic imaging and photothermal therapy of endogenous H2S‐triggered Au@Cu2O. Small. 2019;15(44) doi: 10.1002/smll.201903473. [DOI] [PubMed] [Google Scholar]
- 234.Rabin O., Manuel Perez J., Grimm J., Wojtkiewicz G., Weissleder R. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat. Mater. 2006;5(2):118–122. doi: 10.1038/nmat1571. [DOI] [PubMed] [Google Scholar]
- 235.Ai K., Liu Y., Liu J., Yuan Q., He Y., Lu L. Large-scale synthesis of Bi2S3 nanodots as a contrast agent for in vivo X-ray computed tomography imaging. Adv. Mater. 2011;23(42):4886–4891. doi: 10.1002/adma.201103289. [DOI] [PubMed] [Google Scholar]
- 236.Yao M.H., Ma M., Chen Y., Jia X.Q., Xu G., Xu H.X., et al. Multifunctional Bi2S3/PLGA nanocapsule for combined HIFU/radiation therapy. Biomaterials. 2014;35(28):8197–8205. doi: 10.1016/j.biomaterials.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 237.Zhang R., Li Y., Zhang W., Sheng Y., Wang M., Liu J., et al. Fabrication of Cu2O/Bi2S3 heterojunction photocatalysts with enhanced visible light photocatalytic mechanism and degradation pathways of tetracycline. J. Mol. Struct. 2021;1229 [Google Scholar]
- 238.Cheng Y., Bo H., Qin R., Chen F., Xue F., An L., et al. Hyaluronic acid-coated Bi:Cu2O: an H2S-responsive agent for colon cancer with targeted delivery and enhanced photothermal performance. J. Nanobiotechnol. 2022;20(1):346. doi: 10.1186/s12951-022-01555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li Y., Chen W., Qi Y., Wang S., Li L., Li W., et al. H2S-Scavenged and activated iron oxide-hydroxide nanospindles for MRI-guided photothermal therapy and ferroptosis in colon cancer. Small. 2020;16(37) doi: 10.1002/smll.202001356. [DOI] [PubMed] [Google Scholar]
- 240.Pu X.Q., Ju X.J., Zhang L., Cai Q.W., Liu Y.Q., Peng H.Y., et al. Novel multifunctional stimuli-responsive nanoparticles for synergetic chemo-photothermal therapy of tumors. ACS Appl. Mater. Interfaces. 2021;13(24):28802–28817. doi: 10.1021/acsami.1c05330. [DOI] [PubMed] [Google Scholar]
- 241.Liu B., Wang W., Fan J., Long Y., Xiao F., Daniyal M., et al. RBC membrane camouflaged prussian blue nanoparticles for gamabutolin loading and combined chemo/photothermal therapy of breast cancer. Biomaterials. 2019;217 doi: 10.1016/j.biomaterials.2019.119301. [DOI] [PubMed] [Google Scholar]
- 242.Sun J., Liu Y., Zhu X., Liao X., Wang L., Yuan J., et al. Endogenous H2S-activable liposomal nanoplatform for synergistic colorectal tumor ablation at mild apparent temperature. ACS Appl. Bio Mater. 2020;3(10):6680–6687. doi: 10.1021/acsabm.0c00535. [DOI] [PubMed] [Google Scholar]
- 243.Hou M., Ye M., Liu L., Xu M., Liu H., Zhang H., et al. Azide-locked prodrug Co-assembly into nanoparticles with indocyanine green for chemophotothermal therapy. Mol. Pharm. 2022;19(9):3279–3287. doi: 10.1021/acs.molpharmaceut.2c00452. [DOI] [PubMed] [Google Scholar]
- 244.Shi B., Ren N., Gu L., Xu G., Wang R., Zhu T., et al. Theranostic nanoplatform with hydrogen sulfide activatable NIR responsiveness for imaging-guided on-demand drug release. Angew Chem. Int. Ed. Engl. 2019;58(47):16826–16830. doi: 10.1002/anie.201909883. [DOI] [PubMed] [Google Scholar]
- 245.Bhanushali S., Tanna V., Nimbalkar Y., Ravikumar P., Sawarkar S.P. Safety of nanobiomaterials for cancer nanotheranostics. Cancer Nanotheranostics. 2021:333–368. [Google Scholar]
- 246.Zhou R., Ohulchanskyy T.Y., Xu Y., Ziniuk R., Xu H., Liu L., et al. Tumor-microenvironment-activated NIR-II nanotheranostic platform for precise diagnosis and treatment of colon cancer. ACS Appl. Mater. Interfaces. 2022;14(20):23206–23218. doi: 10.1021/acsami.2c04242. [DOI] [PubMed] [Google Scholar]
- 247.Cheng L., Wang C., Feng L., Yang K., Liu Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014;114(21):10869–10939. doi: 10.1021/cr400532z. [DOI] [PubMed] [Google Scholar]
- 248.Feng J., Ren W.X., Kong F., Dong Y.B. A covalent organic framework-based nanoagent for H2S-activable phototherapy against colon cancer. Chem. Commun. 2021;57(59):7240–7243. doi: 10.1039/d1cc02258j. [DOI] [PubMed] [Google Scholar]
- 249.Zhu Y., Chen C., Wu Q., Yang G., Liu Z., Hao E., et al. Single-wavelength phototheranostics for colon cancer via the thiolytic reaction. Nanoscale. 2020;12(22):12165–12171. doi: 10.1039/d0nr02393k. [DOI] [PubMed] [Google Scholar]
- 250.Chang M., Hou Z., Jin D., Zhou J., Wang M., Wang M., et al. Colorectal tumor microenvironment-activated bio-decomposable and metabolizable Cu2O@CaCO3 nanocomposites for synergistic oncotherapy. Adv. Mater. 2020;32(43) doi: 10.1002/adma.202004647. [DOI] [PubMed] [Google Scholar]
- 251.Lin V.S., Lippert A.R., Chang C.J. Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc. Natl. Acad. Sci. U. S. A. 2013;110(18):7131–7135. doi: 10.1073/pnas.1302193110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Liu Q., Zhong Y., Su Y., Zhao L., Peng J. Real-time imaging of hepatic inflammation using hydrogen sulfide-activatable second near-infrared luminescent nanoprobes. Nano Lett. 2021;21(11):4606–4614. doi: 10.1021/acs.nanolett.1c00548. [DOI] [PubMed] [Google Scholar]
- 253.Wang X., An L., Tian Q., Cui K. Recent progress in H2S activated diagnosis and treatment agents. RSC Adv. 2019;9(58):33578–33588. doi: 10.1039/c9ra06698e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kong L., Lu W., Cao X., Wei Y., Sun J., Wang Y. The design strategies and biological applications of probes for the gaseous signaling molecule hydrogen sulfide. J. Mater. Chem. B. 2022;10(39):7924–7954. doi: 10.1039/d2tb01210c. [DOI] [PubMed] [Google Scholar]
- 255.Wang C., Niu M., Wang W., Su L., Feng H., Lin H., et al. In situ activatable ratiometric NIR-II fluorescence nanoprobe for quantitative detection of H2S in colon cancer. Anal. Chem. 2021;93(27):9356–9363. doi: 10.1021/acs.analchem.1c00427. [DOI] [PubMed] [Google Scholar]
- 256.Wu L., Sedgwick A.C., Sun X., Bull S.D., He X.P., James T.D. Reaction-based fluorescent probes for the detection and imaging of reactive oxygen, nitrogen, and sulfur species. Acc. Chem. Res. 2019;52(9):2582–2597. doi: 10.1021/acs.accounts.9b00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yu F., Han X., Chen L. Fluorescent probes for hydrogen sulfide detection and bioimaging. Chem. Commun. 2014;50(82):12234–12249. doi: 10.1039/c4cc03312d. [DOI] [PubMed] [Google Scholar]
- 258.Kang X., Cai X., Yi L., Xi Z. Multifluorinated aryl azides for the development of improved H2S probes, and fast strain-promoted azide-alkyne cycloaddition and staudinger reactions. Chem. Asian J. 2020;15(9):1420–1429. doi: 10.1002/asia.202000005. [DOI] [PubMed] [Google Scholar]
- 259.Ye H., Sun L., Pang Z., Ji X., Jiao Y., Tu X., et al. Cell-Trappable BODIPY-NBD dyad for imaging of basal and stress-induced H2S in live biosystems. Anal. Chem. 2022;94(3):1733–1741. doi: 10.1021/acs.analchem.1c04324. [DOI] [PubMed] [Google Scholar]
- 260.Pang Z., Ye H., Ma D., Tu X., Yi L., Xi Z. A H2S-specific ultrasensitive fluorogenic probe reveals TMV-induced H2S production to limit virus replication. Chembiochem. 2021;22(13):2292–2299. doi: 10.1002/cbic.202100138. [DOI] [PubMed] [Google Scholar]
- 261.Henthorn H.A., Pluth M.D. Mechanistic insights into the H2S-mediated reduction of aryl azides commonly used in H2S detection. J. Am. Chem. Soc. 2015;137(48):15330–15336. doi: 10.1021/jacs.5b10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wang H., Zhao E., Lam J.W., Tang B.Z. AIE luminogens: emission brightened by aggregation. Mater. Today. 2015;18(7):365–377. [Google Scholar]
- 263.Wu Y., Chen J., Sun L., Zeng F., Wu S. A nanoprobe for diagnosing and mapping lymphatic metastasis of tumor using 3D multispectral optoacoustic tomography owing to aggregation/deaggregation induced spectral change. Adv. Funct. Mater. 2019;29(8) [Google Scholar]
- 264.Xu L., Ni L., Sun L., Zeng F., Wu S. A fluorescent probe based on aggregation-induced emission for hydrogen sulfide-specific assaying in food and biological systems. Analyst. 2019;144(22):6570–6577. doi: 10.1039/c9an01582e. [DOI] [PubMed] [Google Scholar]
- 265.Li H.D., Yao Q.C., Fan J.L., Jiang N., Wang J.Y., Xia J., et al. A fluorescent probe for H2S in vivo with fast response and high sensitivity. Chem. Commun. 2015;51(90):16225–16228. doi: 10.1039/c5cc06612c. [DOI] [PubMed] [Google Scholar]
- 266.Zhang K., Zhang J., Xi Z., Li L.Y., Gu X., Zhang Q.Z., et al. A new H2S-specific near-infrared fluorescence-enhanced probe that can visualize the H2S level in colorectal cancer cells in mice. Chem. Sci. 2017;8(4):2776–2781. doi: 10.1039/c6sc05646f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Xu G., Yan Q., Lv X., Zhu Y., Xin K., Shi B., et al. Imaging of colorectal cancers using activatable nanoprobes with second near-infrared window emission. Angew Chem. Int. Ed. Engl. 2018;57(14):3626–3630. doi: 10.1002/anie.201712528. [DOI] [PubMed] [Google Scholar]
- 268.Deng Z., Jiang M., Li Y., Liu H., Zeng S., Hao J. Endogenous H2S-triggered in situ synthesis of NIR-II-emitting nanoprobe for in vivo intelligently lighting up colorectal cancer. iScience. 2019;17:217–224. doi: 10.1016/j.isci.2019.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Chen Y., Zhu C., Yang Z., Chen J., He Y., Jiao Y., et al. A ratiometric fluorescent probe for rapid detection of hydrogen sulfide in mitochondria. Angew Chem. Int. Ed. Engl. 2013;52(6):1688–1691. doi: 10.1002/anie.201207701. [DOI] [PubMed] [Google Scholar]
- 270.Li X., Zhao H., Ji Y., Yin C., Li J., Yang Z., et al. Lysosome-assisted mitochondrial targeting nanoprobe based on dye-modified upconversion nanophosphors for ratiometric imaging of mitochondrial hydrogen sulfide. ACS Appl. Mater. Interfaces. 2018;10(46):39544–39556. doi: 10.1021/acsami.8b16818. [DOI] [PubMed] [Google Scholar]
- 271.Liu H., Xu G., Zhu T., Wang R., Tan J., Zhao C., et al. Rational design of water-dispersible and biocompatible nanoprobes with H2S-triggered NIR emission for cancer cell imaging. J. Mater. Chem. B. 2020;8(28):6013–6016. doi: 10.1039/d0tb00173b. [DOI] [PubMed] [Google Scholar]
- 272.Maldiney T., Lecointre A., Viana B., Bessière A., Bessodes M., Gourier D., et al. Controlling electron trap depth to enhance optical properties of persistent luminescence nanoparticles for in vivo imaging. J. Am. Chem. Soc. 2011;133(30):11810–11815. doi: 10.1021/ja204504w. [DOI] [PubMed] [Google Scholar]
- 273.Abdukayum A., Chen J.T., Zhao Q., Yan X.P. Functional near infrared-emitting Cr3+/Pr3+ co-doped zinc gallogermanate persistent luminescent nanoparticles with superlong afterglow for in vivo targeted bioimaging. J. Am. Chem. Soc. 2013;135(38):14125–14133. doi: 10.1021/ja404243v. [DOI] [PubMed] [Google Scholar]
- 274.Jiang Y., Huang J., Zhen X., Zeng Z., Li J., Xie C., et al. A generic approach towards afterglow luminescent nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 2019;10(1):2064. doi: 10.1038/s41467-019-10119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wu L., Ishigaki Y., Hu Y., Sugimoto K., Zeng W., Harimoto T., et al. H2S-activatable near-infrared afterglow luminescent probes for sensitive molecular imaging in vivo. Nat. Commun. 2020;11(1):446. doi: 10.1038/s41467-020-14307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Auzel F. Upconversion and anti-Stokes processes with f and d ions in solids. Chem. Rev. 2004;104(1):139–173. doi: 10.1021/cr020357g. [DOI] [PubMed] [Google Scholar]
- 277.Feng W., Han C., Li F. Upconversion-nanophosphor-based functional nanocomposites. Adv. Mater. 2013;25(37):5287–5303. doi: 10.1002/adma.201301946. [DOI] [PubMed] [Google Scholar]
- 278.Zhou J., Liu Z., Li F. Upconversion nanophosphors for small-animal imaging. Chem. Soc. Rev. 2012;41(3):1323–1349. doi: 10.1039/c1cs15187h. [DOI] [PubMed] [Google Scholar]
- 279.Ma C., Bian T., Yang S., Liu C., Zhang T., Yang J., et al. Fabrication of versatile cyclodextrin-functionalized upconversion luminescence nanoplatform for biomedical imaging. Anal. Chem. 2014;86(13):6508–6515. doi: 10.1021/ac5010103. [DOI] [PubMed] [Google Scholar]
- 280.Park Y.I., Lee K.T., Suh Y.D., Hyeon T. Upconverting nanoparticles: a versatile platform for wide-field two-photon microscopy and multi-modal in vivo imaging. Chem. Soc. Rev. 2015;44(6):1302–1317. doi: 10.1039/c4cs00173g. [DOI] [PubMed] [Google Scholar]
- 281.Pu K., Shuhendler A.J., Jokerst J.V., Mei J., Gambhir S.S., Bao Z., et al. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat. Nanotechnol. 2014;9(3):233–239. doi: 10.1038/nnano.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Shi B., Gu X., Fei Q., Zhao C. Photoacoustic probes for real-time tracking of endogenous H2S in living mice. Chem. Sci. 2017;8(3):2150–2155. doi: 10.1039/c6sc04703c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Ma T., Zheng J., Zhang T., Xing D. Ratiometric photoacoustic nanoprobes for monitoring and imaging of hydrogen sulfide in vivo. Nanoscale. 2018;10(28):13462–13470. doi: 10.1039/c8nr03445a. [DOI] [PubMed] [Google Scholar]
- 284.Ntziachristos V., Razansky D. Molecular imaging by means of multispectral optoacoustic tomography (MSOT) Chem. Rev. 2010;110(5):2783–2794. doi: 10.1021/cr9002566. [DOI] [PubMed] [Google Scholar]
- 285.Taruttis A., Ntziachristos V. Advances in real-time multispectral optoacoustic imaging and its applications. Nat. Photonics. 2015;9(4):219–227. [Google Scholar]
- 286.Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat. Methods. 2010;7(8):603–614. doi: 10.1038/nmeth.1483. [DOI] [PubMed] [Google Scholar]
- 287.Sun L., Wu Y., Chen J., Zhong J., Zeng F., Wu S. A turn-on optoacoustic probe for imaging metformin-induced upregulation of hepatic hydrogen sulfide and subsequent liver injury. Theranostics. 2019;9(1):77–89. doi: 10.7150/thno.30080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Broens B., Duitman J.W., Zwezerijnen G.J.C., Nossent E.J., van der Laken C.J., Voskuyl A.E. Novel tracers for molecular imaging of interstitial lung disease: a state of the art review. Autoimmun. Rev. 2022;21(12) doi: 10.1016/j.autrev.2022.103202. [DOI] [PubMed] [Google Scholar]
- 289.Younis M.H., Tang Z., Cai W. Multimodality imaging of nanoparticle-based vaccines: shedding light on immunology. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;14(5) doi: 10.1002/wnan.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Boswell C.A., Brechbiel M.W. Development of radioimmunotherapeutic and diagnostic antibodies: an inside-out view. Nucl. Med. Biol. 2007;34(7):757–778. doi: 10.1016/j.nucmedbio.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Urbano N., Scimeca M., Tancredi V., Bonanno E., Schillaci O. (99)mTC-sestamibi breast imaging: current status, new ideas and future perspectives. Semin. Cancer Biol. 2022;84:302–309. doi: 10.1016/j.semcancer.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 292.Roussel E., Capitanio U., Kutikov A., Oosterwijk E., Pedrosa I., Rowe S.P., et al. Novel imaging methods for renal mass characterization: a collaborative review. Eur. Urol. 2022;81(5):476–488. doi: 10.1016/j.eururo.2022.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Park J.Y., Kim Y.J., Lee J.Y., Lee Y.S., Jeong J.M. Imaging of the third gasotransmitter hydrogen sulfide using (99m)Tc-labeled alpha-hydroxy acids. Nucl. Med. Biol. 2019;76–77:28–35. doi: 10.1016/j.nucmedbio.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 294.Kweon Y., Park J.Y., Kim Y.J., Lee Y.S., Jeong J.M. Imaging hydrogen sulfide in hypoxic tissue with [(99m)Tc]Tc-gluconate. Molecules. 2020;26(1):96. doi: 10.3390/molecules26010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Qin X., Jiang H., Liu Y., Zhang H., Tian M. Radionuclide imaging of apoptosis for clinical application. Eur. J. Nucl. Med. Mol. Imag. 2021:1–15. doi: 10.1007/s00259-021-05641-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Pellico J., Gawne P.J., de Rosales R.T. Radiolabelling of nanomaterials for medical imaging and therapy. Chem. Soc. Rev. 2021;50(5):3355–3423. doi: 10.1039/d0cs00384k. [DOI] [PubMed] [Google Scholar]
- 297.Yan C., Liu D., An L., Wang Y., Tian Q., Lin J., et al. Magnetic-photoacoustic dual-mode probe for the visualization of H2S in colorectal cancer. Anal. Chem. 2020;92(12):8254–8261. doi: 10.1021/acs.analchem.0c00504. [DOI] [PubMed] [Google Scholar]
- 298.Ni D., Bu W., Ehlerding E.B., Cai W., Shi J. Engineering of inorganic nanoparticles as magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2017;46(23):7438–7468. doi: 10.1039/c7cs00316a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Li H., Meade T.J. Molecular magnetic resonance imaging with Gd(III)-Based contrast agents: challenges and key advances. J. Am. Chem. Soc. 2019;141(43):17025–17041. doi: 10.1021/jacs.9b09149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Angelovski G. Heading toward macromolecular and nanosized bioresponsive MRI probes for successful functional imaging. Acc. Chem. Res. 2017;50(9):2215–2224. doi: 10.1021/acs.accounts.7b00203. [DOI] [PubMed] [Google Scholar]
- 301.Angelovski G. What we can really do with bioresponsive MRI contrast agents. Angew Chem. Int. Ed. Engl. 2016;55(25):7038–7046. doi: 10.1002/anie.201510956. [DOI] [PubMed] [Google Scholar]
- 302.Bi S., Deng Z., Jiang Q., Jiang M., Zeng S. A H2S-triggered dual-modal second near-infrared/photoacoustic intelligent nanoprobe for highly specific imaging of colorectal cancer. Anal. Chem. 2021;93(39):13212–13218. doi: 10.1021/acs.analchem.1c02200. [DOI] [PubMed] [Google Scholar]
- 303.Wang X.J., Cheng J., Zhang L.Y., Zhang J.G. Self-assembling peptides-based nano-cargos for targeted chemotherapy and immunotherapy of tumors: recent developments, challenges, and future perspectives. Drug Deliv. 2022;29(1):1184–1200. doi: 10.1080/10717544.2022.2058647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yagublu V., Karimova A., Hajibabazadeh J., Reissfelder C., Muradov M., Bellucci S., et al. Overview of physicochemical properties of nanoparticles as drug carriers for targeted cancer therapy. J. Funct. Biomater. 2022;13(4):196. doi: 10.3390/jfb13040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Das C.G.A., Kumar V.G., Dhas T.S., Karthick V., Kumar C.M.V. Nanomaterials in anticancer applications and their mechanism of action - a review. Nanomedicine. 2022;47 doi: 10.1016/j.nano.2022.102613. [DOI] [PubMed] [Google Scholar]
- 306.Zhang X., Chen Y., He X., Zhang Y., Zhou M., Peng C., et al. Smart nanogatekeepers for tumor theranostics. Small. 2021;17(47) doi: 10.1002/smll.202103712. [DOI] [PubMed] [Google Scholar]
- 307.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33(9):941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Qu Y., Li C., Shi J., Chen R., Xu S., Rafsanjani H., et al. Transvaginal fast-scanning optical-resolution photoacoustic endoscopy. J. Biomed. Opt. 2018;23(12):1–4. doi: 10.1117/1.JBO.23.12.121617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Schellenberg M.W., Hunt H.K. Hand-held optoacoustic imaging: a review. Photoacoustics. 2018;11:14–27. doi: 10.1016/j.pacs.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.