Abstract
Background and Objectives
The menopause transition is increasingly recognized as a time of importance for women's brain health. A growing body of work indicates that the classic menopausal symptom, vasomotor symptom (VMS), may be associated with poorer cardiovascular health. Other work links VMS to poorer cognition. We investigate whether VMS, when rigorously assessed using physiologic measures, are associated with greater white matter hyperintensity volume (WMHV) among midlife women. We consider a range of potential explanatory factors in these associations and explore whether VMS are associated with the spatial distribution of WMHV.
Methods
Women aged 45–67 years and free of hormone therapy underwent 24 hours of physiologic VMS monitoring (sternal skin conductance), actigraphy assessment of sleep, physical measures, phlebotomy, and 3 Tesla neuroimaging. Associations between VMS (24-hour, wake, and sleep VMS, with wake and sleep intervals defined by actigraphy) and whole brain WMHV were considered in linear regression models adjusted for age, race, education, smoking, body mass index, blood pressure, insulin resistance, and lipids. Secondary models considered WMHV in specific brain regions (deep, periventricular, frontal, temporal, parietal, and occipital) and additional covariates including sleep.
Results
The study sample included 226 women. Physiologically assessed VMS were associated with greater whole brain WMHV in multivariable models, with the strongest associations observed for sleep VMS (24-hour VMS, B[SE] = 0.095 [0.045], p = 0.032; Wake VMS, B[SE] = 0.078 [0.046], p = 0.089, Sleep VMS, B[SE] = 0.173 [0.060], p = 0.004). Associations were not accounted for by additional covariates including actigraphy-assessed sleep (wake after sleep onset). When considering the spatial distribution of WMHV, sleep VMS were associated with both deep WMHV, periventricular WMHV, and frontal lobe WMHV.
Discussion
VMS, particularly VMS occurring during sleep, were associated with greater WMHV. Identification of female-specific midlife markers of poor brain health later in life is critical to identify women who warrant early intervention and prevention. VMS have the potential to serve as female-specific midlife markers of brain health in women.
The menopause is an important reproductive transition for many midlife women. The menopause is characterized by irregularity and ultimately cessation of menses, changes in sex steroid hormones and characteristic symptoms. The menopause transition is increasingly appreciated to be important to brain health and is accompanied by changes in cognitive performance1-3 and possibly brain structure and metabolism.4 Although prior work on the relationship of menopause to brain health has largely focused on menopause-related changes in sex hormones such as estradiol (E2),4,5 emerging data underscore the importance of other menopause-related factors.6
Hot flashes and night sweats, collectively known as vasomotor symptoms (VMS), are the classic menopause symptoms that are experienced by over 70% of women.7 For a third of women, VMS are frequent or severe.7,8 Although VMS were once believed to last for only a few years, new data indicate that frequent or moderate-severe VMS last on average 7–10 years and for many women, much longer.9,10 VMS have long been regarded as midlife symptoms important to quality of life. However, a burgeoning body of data links them to physical health. For example, multiple studies have found more frequent VMS associated with an adverse cardiovascular disease (CVD) risk factor profile, greater subclinical CVD, and increased risk of clinical CVD events.11-13 These associations are typically not explained by endogenous sex steroid hormones such as estradiol (E2).
Some limited data suggest that VMS may also be linked to markers of cerebrovascular health and specifically the volume of brain white matter hyperintensities (WMHVs). Lesions in the white matter appear as WMHs on T2-weighted MRI. WMHs are a marker of cerebral small vessel damage believed to develop because of small vessel disease and linked to later cognitive decline, dementia, and mortality.14 Because they can be detected decades before the onset of these brain disorders,15,16 they can serve as early markers for risk of development of these brain disorders. We previously reported the results of a pilot study of 20 women showing that VMS, particularly physiologically assessed nocturnal VMS, are associated with greater WMHV.17 However, because this was a small pilot study, further investigation of VMS-WMH associations in an adequately powered study with rigorous consideration of confounders and mechanisms is warranted.
Thus, this study tested the primary research hypothesis that more frequent VMS, particularly physiologically assessed VMS during sleep, would be associated with greater WMHV. We tested this hypothesis among 226 midlife women participating in the MsBrain study. We used physiologic assessments of VMS given prior findings demonstrating that links between VMS and neurocognitive health were specific to physiologically assessed VMS,18,19 particularly VMS during sleep.20 We tested whether associations between VMS and WMHV persisted when adjusting for a range of potential factors including CVD risk factors. Furthermore, because prior data have suggested the potential for a differential etiology or clinical implications of WMHs depending on their spatial location,21 we explored associations between VMS and WMHV in specific brain regions. In additional models, we considered other potential explanatory factors such as actigraphy-assessed sleep, endogenous E2, depressive symptoms, and inflammation.
Methods
Sample
Women assigned female at birth and aged 45–67 years were enrolled in the MsBrain Study, a study of menopause and brain aging. Women were recruited between 2017 and 2020 from the Allegheny County, PA community through advertisements in local registries, fliers and brochures placed in community locations, listservs and electronic mailing lists, and from the MsHeart study, a study of nonsmoking women free of clinical CVD who were previously studied for menopause and cardiovascular health.22 Inclusion criteria were being aged 45–67 years, having a uterus and at least 1 ovary, and being late perimenopausal or postmenopausal status (no menstrual periods in the past 2 months).23 Exclusion criteria included a reported history of stroke or cerebrovascular accident, dementia, seizure disorder, brain tumor, or Parkinson disease; a history of head trauma with loss of consciousness for >60 minutes; contraindications to MRI; active substance use (established through urine toxicology screen and interview); current chemotherapy; pregnancy; a history of hysterectomy and/or bilateral oophorectomy or other procedure that resulted in permanent end of menses; current intrauterine device; and current use of select medications affecting VMS, including hormone therapy (oral or transdermal estrogen and/or progesterone), selective estrogen receptor modulators, aromatase inhibitors, selective serotonin reuptake inhibitors (SSRIs), or serotonin norepinephrine reuptake inhibitors (SNRIs).
Standard Protocol Approvals, Registrations, and Patient Consents
All study procedures were reviewed and approved by the University of Pittsburgh Human Research Protection Office. All participants provided written informed consent.
Participant Assessments
Screening, Physical Measures, and Questionnaires
Women responding to study advertisements or a recruitment letter contacted this study and underwent telephone screening procedures with a trained staff member. Women who met initial eligibility criteria through telephone subsequently came to the study laboratory for further orientation and in-person screening and consenting procedures. Conducted by a trained study staff member, in-person screening procedures included a medical history interview (medical, reproductive, and psychiatric history, current medication use) and a urine toxicology screen. Eligible participants subsequently completed physical measures. Height was measured using a fixed stadiometer and weight using a balance beam scale. Body mass index (BMI) was calculated [weight (kg)/height2 (m)]. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were the average of 3 seated measurements taken using a Dinamap v100. Women also completed written questionnaires assessing demographics and health behaviors, including smoking, alcohol use, and current and prior substance use. Race/ethnicity was self-reported. Educational attainment was assessed as years of completed education (classified as high school/vocational, college graduate, and >college). Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale.24
Ambulatory VMS Monitoring
Participants were equipped with a VMS monitor and VMS diary to undergo 3 days of ambulatory VMS monitoring, the first 24 hours of which included physiologic VMS monitoring. The wearable VMS monitor (VU-AMS 5 fs, VU University Amsterdam, the Netherlands) quantifies VMS through sternal skin conductance, a validated physiologic measure of VMS.25 Participants wore the VU-AMS monitor for 24 hours, after which time they removed it and stored it in a carrying case. Women reported VMS on the monitor by completing an electronic diary and pressing event mark buttons on the VU-AMS monitor. For the remaining 2 days, women completed the VMS diary (Moto G5S Plus 4 G LTE, Motorola, Chicago, IL; MovisensXS software, movisens GmbH, Karlsruhe, Germany), which provided a date-stamped and time-stamped VMS report. After monitoring, VMS data were downloaded and scored using UFI software (DPS; Morro Bay, CA) according to validated methods that have demonstrated reliability, including in the present laboratory and for this study (ĸ = 0.89). Only physiologic VMS files with ≥70% of usable data were included. VMS during wake and sleep were categorized based on actigraphy-defined wake and sleep times. VMS rates were calculated as the number of VMS/monitoring time and standardized to a 24-hour day (17 hours wake, 7 hours sleep) to account for variations in monitoring durations across women.
Actigraphy
For the 3 days of monitoring, women also wore a Actiwatch 2 wrist actigraph unit on the wrist of the nondominant hand (Respironics, Inc., Murrysville, PA)26 and completed a sleep diary.27 Actigraphy data were collected in 1-minute epochs and analyzed with Philips Actiware v6.0.0 software, with a wake threshold of 40 and the number of epochs of sleep/wake for sleep onset/offset of 10. Wake after sleep onset (WASO; minutes of wakefulness between actigraphy-defined sleep onset time and actigraphy-defined final wake time) was our primary sleep measure given its relationship to WMHV in this sample.28
Phlebotomy
After the ambulatory monitoring period, participants returned to the laboratory in the morning (approximately 6:00-10:00 am) after an overnight fast and underwent phlebotomy. Glucose, insulin, and lipids (high-density lipoprotein-cholesterol [HDL-C], triglycerides) were assessed using an enzymatic assay or immunoturbidimetric assay. Low-density lipoprotein was calculated using the Friedewald equation.29 Homeostatic model assessment (HOMA) for insulin resistance was calculated as [(fasting insulin × fasting glucose)/22.5].30 High-sensitivity C-reactive protein (hsCRP) was assessed using ELISA (R&D Systems, DCRP00), with a sensitivity of 0.10 pg/mL, interassay CV of 6.0%–7.0%, and intra-assay CV of 3.8%–8.3%. E1 and E2 were measured at the University of Pittsburgh's Small Molecule Biomarker Core using ultra-performance liquid chromatography-tandem mass spectrometry, the reference method for the measurement of estrogens in postmenopausal women.31,32 The E1 and E2 assays had a lower limit of quantitation of 1.0 pg/mL; and for E2, intraday errors <8.1% and relative SDs (RSDs) < 10.4% and interday errors <5.0% and RSD <7.4%; and for E1, intraday errors <15.0% and RSD < 11.2% and interday errors <11.0% and RSD <10.9%. FSH was assessed using a commercially available ELISA from Cayman Chemical Co. (#500710; interassay CVs of 4.5%–8.9% and intra-assay CVs: 1.0%–4.5%). Genotypes for 2 APOE polymorphisms, rs429358 (APOE*4) and rs7412 (APOE*2), were determined using TaqMan genotyping assays.33 Because of the strong linkage disequilibrium between the 2 sites, this is also treated as a three-allele APOE polymorphism: APOE*2, APOE*3, and APOE*4, resulting in 6 genotypes (2/2, 2/3, 2/4, 3/3, 3/4, 4/4). Participants were classified as either APOE*4 positive (2/4, 3/4, 4/4) or negative (2/2, 2/3, 3/3) for analysis; we also considered this categorization excluding women who were APOE*2 positive. As findings were comparable, findings based on the full sample are reported here.
Carotid Ultrasound
Participants also underwent a carotid ultrasound for assessment of intima-media thickness (IMT). Sonographers at the University of Pittsburgh's Ultrasound Research Laboratory obtained bilateral carotid images through B-mode ultrasound using a Sonoline Antares (Siemens, Malvern, PA) high-resolution duplex scanner equipped with a VF10-5 transducer.34,35 Digitized images were obtained at end-diastole from 8 locations (4 locations each from the left and right carotid arteries): the near and far walls of the distal common carotid artery, the far walls of the carotid bulb, and the internal carotid artery. Images were read using semiautomated reading software.36 IMT was the mean of the readings across the 8 locations. Reproducibility was excellent (intraclass correlation coefficient between sonographers ≥0.87, between readers = 0.92).
MRI
Participants completed MRI scanning, which was performed at the MR Research Center of the University of Pittsburgh at an average of 11.37 days (SD = 7.32) from the VMS measurements. A 3 T Siemens Tim Trio MR scanner was used, with a Siemens 64-channel head coil. Two series of MR images were analyzed for this study: a magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted sequence and T2-weighted (T2w) fluid-attenuated inversion recovery (FLAIR) sequence. MPRAGE images were acquired in the axial plane using the following parameters: repetition time (TR) = 2,400 ms, echo time (TE) = 2.22 ms, inversion time (TI) = 1,000 ms, flip angle = 8°, field of view (FOV) = 256 × 240 mm, slice thickness = 0.8 mm, voxel size = 0.8 × 0.8 mm, matrix size = 320 × 300, and number of slices = 208. FLAIR images were acquired in the axial plane using the following parameters: TR = 9,690 or 10000 ms, TE = 91 ms, TI = 2,500 ms, flip angle = 135°, FOV = 256 × 256 mm, matrix = 320 × 320, slice thickness = 1.6 mm, voxel size = 0.8 × 0.8 mm, and number of slices = 104. The small change in TR from 9,690 to 10,000 was performed 1 year into this study to meet the Specific Absorption Rate human safety guidelines for participants with a higher BMI. This change slightly increased the time of acquisition but had minimal effect on image contrast.
An automated pipeline was used to segment WMHs on the T2w FLAIR images using methods developed previously.37,38 For each participant, cerebral and cerebellar white matter were segmented on the T1w image and mapped into the T2w FLAIR image space using SPM12 (fil.ion.ucl.ac.uk/spm/) and FreeSurfer (version 7.1.1, surfer.nmr.mgh.harvard.edu/). Because there were very few lesions in the cerebellum in our participants, cerebellar whiter matter represented normal appearing white matter, and its intensity mean and standard deviation were used for Z-transform of the T2w FLAIR image. Manual inspection of each image was performed to rule out cerebellar WMHs. A threshold of 2 was then applied on Z-transformed FLAIR images. This method uses individual mean and standard deviation from normal cerebellar white matter to standardize individual FLAIR images, which avoids systematic bias in seed selection between participants with significant cerebral WMHV vs those with few WMHV. Z-transformation also reduces intensity variations across individual FLAIR images. In FreeSurfer, white matter was also parcellated according to its nearest cortex with the Desikan/Killiany atlas, which was used to generate the cortical white matter masks for the frontal, temporal, parietal, and occipital lobes for the localization of WMHV. Specifically, white matter parcellations corresponding to frontal cortex regions in the Desikan/Killiany atlas were combined to create a frontal cortical white matter mask to localize frontal WMH. Similarly, cortical white matter masks were generated for temporal, parietal, and occipital lobes. These lobular cortical white matter masks did not overlap each other and were combined to create an overall cortical/deep white matter mask. White matter surrounding the ventricles that is not part of the cortical/deep white matter mask comprised the periventricular white matter mask. These lobular cortical and periventricular white matter masks allow us to investigate additional models of regional WMHV. The total and regional WMHV (in cubic centimeters) were normalized by intracranial volumes and log transformed for analysis.
Statistical Analysis
Variables were examined for distributions, outliers, and cell sizes. WMHV, BMI, WASO, E1, and E2 were log transformed to conform to model assumptions of normality. Bivariate relations between study variables and WMHV were examined using Pearson and Spearman correlation coefficients. We tested the relation of VMS (total, sleep, and wake) to whole brain WMHV separately in linear regression models. We next considered VMS in relation to WMHV in specific brain regions (periventricular, deep, and frontal, temporal, parietal, and occipital lobes). Select covariates (age, race, and education) were a priori selected for inclusion in models, and all other covariates were included based on their relationship with the outcome at p < 0.10. Covariates included CVD risk factors (smoking, BMI, SBP, antihypertensive medications, triglycerides, HDL-C, and HOMA); only 1 blood pressure variable was included due to the high correlation between SBP and DBP. In secondary models, we also considered WASO, hormones (E1, E2, FSH), depressive symptoms, hsCRP, and carotid IMT as additional covariates and mean arterial pressure (MAP) as an alternate covariate to BP. Effect modification of VMS-WMH relationships by APOE*4 status was tested. All tests were 2-tailed with an alpha set to 0.05. Analyses were conducted using SAS v9.4 (SAS Institute, Cary, NC).
Data Availability
The data and associated materials that support the findings of this study are available from the corresponding author on reasonable request.
Results
Of the 664 who underwent initial telephone screening, 274 women met eligibility criteria, were enrolled, and underwent study procedures and 239 of these women also underwent neuroimaging. The 239 women who underwent neuroimaging, relative to the 35 women who did not, had higher education (16 years vs 14 years, p = 0.03) and a lower BMI (27.12 vs 30.80, p = 0.04). Of these 239 women, 8 women were excluded because of detection of brain tumor, stroke, or seizure disorder and 5 because of a history of chemotherapy. Thus, the final sample for analysis was N = 226. Furthermore, 2 additional women missing phlebotomy data resulted in N = 224 for models incorporating blood-based biomarkers.
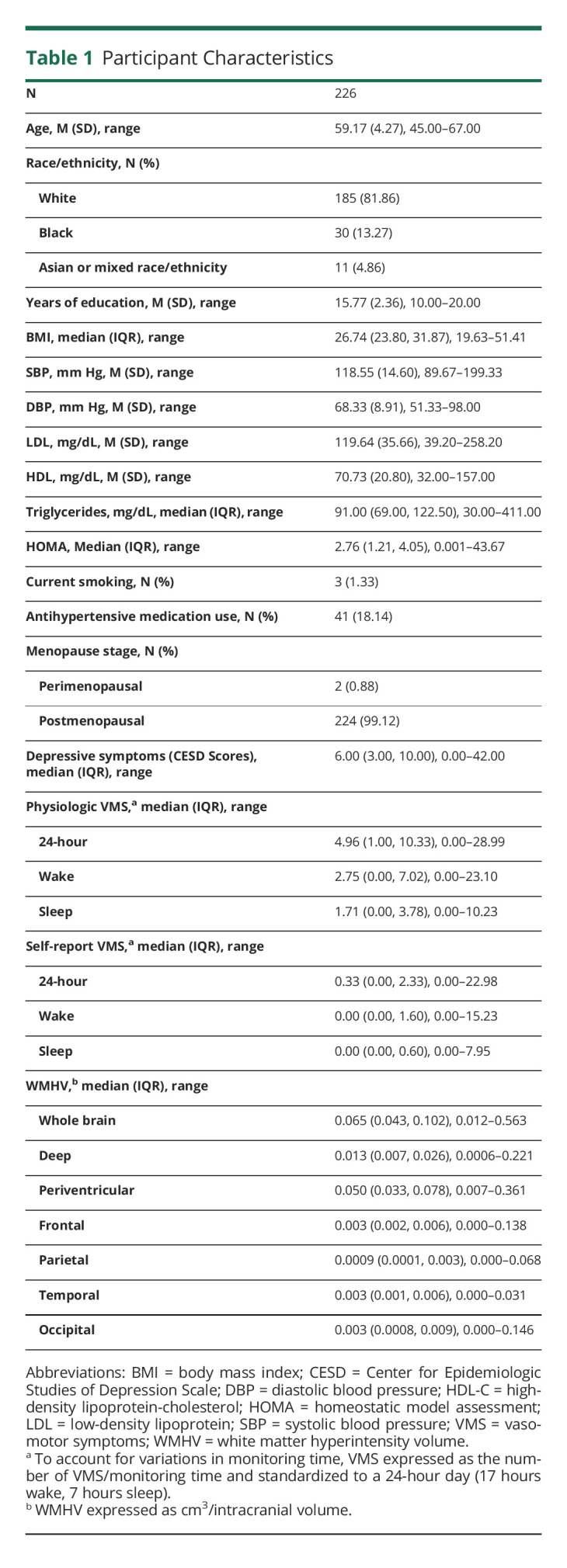
Participants were on average age 59 years, overweight, and normotensive (Table 1). The women had on average approximately 5 physiologically detected VMS over a 24-hour period (approximately 3 during wake and 2 during sleep).
Table 1.
Participant Characteristics
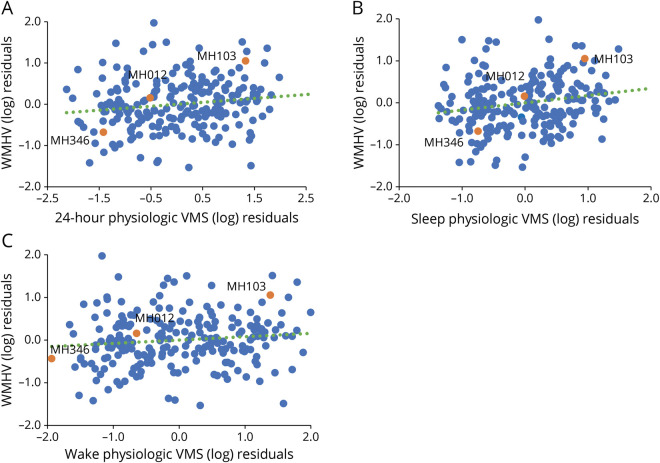
More frequent physiologic VMS were associated with greater whole brain WMHV (Table 2, Figures 1–2). When wake and sleep VMS were considered separately, associations were most pronounced for sleep VMS. Associations persisted after adjustment for a range of covariates, including demographic factors and CVD risk factors.
Table 2.
Associations of Physiologically Assessed VMS to Whole Brain WMHV
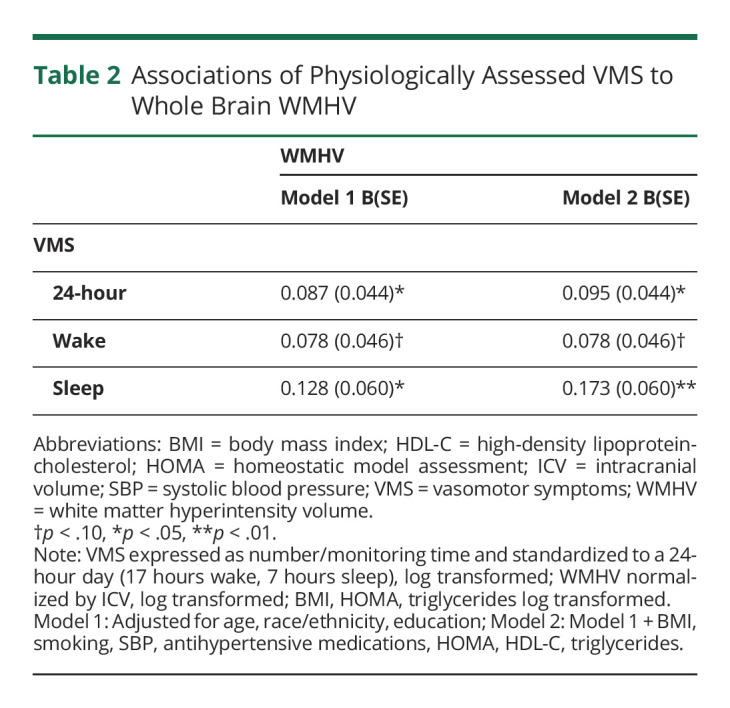
Figure 1. Scatterplots of Residuals for Associations Between 24-Hour Vasomotor Symptoms (VMS) (A), Wake VMS (B), and Sleep VMS (C) and White Matter Hyperintensity Volume (WMHV).
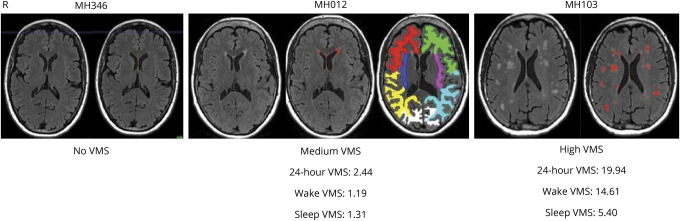
Figure 2. MRI Scans of White Matter (WM) Hyperintensities in Representative Women With No, Medium, and High-Frequency Vasomotor Symptoms (VMS).
Lobular cortical WM masks of MH012 are depicted: red/green—right/left frontal WM, blue/pink—right/left temporal WM, yellow/cyan—right/left parietal WM, and white occipital WM.
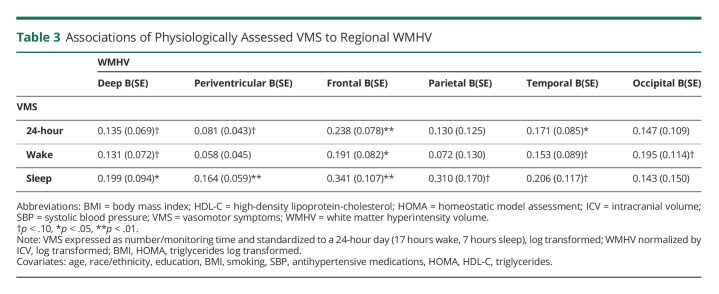
We next considered associations between physiologic VMS and regional WMHV. Physiologically detected VMS, particularly sleep VMS, were associated with both greater deep WMHV and greater periventricular WMHV (Table 3). When considering WMHV in specific brain regions, VMS were most consistently associated with frontal lobe WMHV; this pattern was observed across 24-hour, wake, and sleep VMS.
Table 3.
Associations of Physiologically Assessed VMS to Regional WMHV
In additional models, we carefully considered WASO as a covariate given the pronounced associations between sleep VMS and WMHV here and prior findings of links between WASO and WMHV in this cohort.28 However, when adjusting for WASO, the associations between sleep VMS and WMHV persisted (e.g., sleep VMS and whole brain WMHV, B[SE] = 0.174 [0.060], p = 0.004, controlling for age, race, education, CVD risk factors, and WASO). We also considered additional covariates endogenous E2, E1, FSH, depressive symptoms, hsCRP, or carotid IMT in multivariable models, and associations between VMS and WMHV persisted (data not shown). Furthermore, we considered MAP instead of SBP as a covariate in multivariable models, and findings were similar (data not shown). Moreover, 21.2% (N = 48) of participants were APOE*4 allele carriers, and we considered modification of associations between VMS and WMHV (whole brain and regional) by APOE*4 status. No significant effect modification was observed (VMS and APOE*4 interaction p-values >0.21). Finally, we examined whether self-reported VMS were associated with WMHV. Self-reported VMS were not significantly associated with WMHV in multivariable models (e.g., 24-hour VMS, B[SE] = 0.035 [0.058], p = 0.55; wake VMS, B[SE] = 0.054 [0.066], p = 0.41; sleep VMS, B[SE] = -0.020 [0.104], p = 0.85, adjusted for age, race/ethnicity, education, CVD risk factors).
Discussion
In this study of over 200 midlife women, those with more physiologically detected VMS, particularly VMS occurring during sleep, had greater brain WMHV. These relationships were not explained by CVD risk factors or by sleep (WASO) nor by endogenous E2 levels or depressive symptoms. Associations were observed for both deep and periventricular WMHV yet were most pronounced for WMHV in the frontal lobe. These findings underscore the potential importance of VMS, particularly nocturnal VMS, for women's brain health.
Our findings are consistent with a growing body of literature underscoring the potential significance of VMS to women's cardiovascular and brain health. A burgeoning literature underscores the associations between VMS and CVD risk factors as well as clinical CVD.11-13 Emerging data also show VMS, particularly physiologically assessed VMS19 during sleep,20 related to poorer cognitive performance. Finally, our pilot work links sleep physiologic VMS to WMHV.17 Thus, our findings importantly add to this literature with the most definitive study to date linking VMS to WMHV, with a large sample size, state-of-the-art assessments of VMS, and rigorous assessment of covariates and potential mechanisms.
Associations between VMS and WMHV were observed for physiologically detected VMS but not self-reported VMS. Subjective reports of VMS are critical to understanding their implications for quality of life. However, the importance of assessing VMS physiologically when considering their implications for brain health has become increasingly clear. Prior work has underscored the specificity of links between physiologically assessed VMS, but not self-reported VMS and indicators of brain health.6 The reasons for this pattern of findings are likely multiple. It is well established that VMS are underreported relative to physiologic assessments.22,39 The accuracy of VMS reports during sleep is a particular challenge, with these reports influenced by the quality of the sleep itself.39,40 Furthermore, self-reported VMS are subject to multiple influences; for example, negative mood can increase VMS reports41 and poorer memory and nonadherence to VMS reporting masquerade as the absence of subjective VMS.39 Therefore, self-reported VMS are important to understanding the subjective experience of VMS, but physiologic assessments of VMS are vital to understanding their potential mechanistic links to health.
We also considered whether VMS were associated with a specific spatial distribution of WMHs. WMHs are heterogenous in origin, and it has been postulated that their location may be relevant to their etiology and clinical implications.21 Associations between VMS and WMHV were observed for both periventricular WMHV and deep WMHV. In this study, VMS were most associated with frontal WMHV. Some prior data indicate that WMHs may emerge earlier in life (i.e., the fifth decade of life) within the frontal region compared with other locations.21 However, in this study, the distributions of frontal lobe WMHV were not strikingly different than those in other brain regions. An increasing body of research links greater frontal lobe WMHV to adverse CVD risk factors,42 particularly hypertension.21 Notably, we did observe that associations between VMS and WMHV (whole brain or frontal) persisted after controlling for CVD risk factors, suggesting that these risk factors, at least when assessed in midlife, do not fully explain associations between VMS and frontal WMHV. However, the present findings are overall consistent with prior literature linking VMS to cardiovascular health and expand these associations to indicators of cerebrovascular health.
Several mechanisms may link VMS to WMHs. We considered both the standard and novel CVD risk factors such as blood pressure, lipids, insulin resistance, hsCRP, and carotid atherosclerosis, which have been linked to VMS12 and WMHV43-45; however, these factors did not explain associations between VMS and WMH. We further considered endogenous E2 with rigorous assessments of E2 sensitive to the low levels of E2 observed in postmenopausal women. We also considered E1, an estrogen largely produced by body fat, and the gonadotropin FSH. However, neither E2, E1, nor FSH explained VMS-WMHV associations. We also considered depressive symptoms, yet depressive symptoms played little role in associations. Finally, we carefully considered sleep for several reasons. The most pronounced associations between VMS and WMHV observed here and in prior work were for sleep VMS. In this cohort, nocturnal VMS have been associated with wakening46 and WASO has been linked to greater WMHV.28 However, in the present analysis, objective measures of sleep, particularly WASO, did not explain associations between VMS and WMHV. Taken together, these findings point to the potential of other aspects of physiology, particularly nocturnal physiology, as potential mechanisms linking VMS and WMHV.
This work had several limitations. Although the racial/ethnic composition of the sample was reflective of the region in which this study was conducted, future work should include more racially/ethnically diverse samples, particularly greater representation of Black, Asian, and Hispanic women. This study was conducted largely among postmenopausal women; future work should consider a larger number of perimenopausal women who tend to be among the most highly symptomatic with respect to VMS. Furthermore, this study enrolled cisgender women; future research should consider sex diverse cohorts. Women taking SSRIs/SNRIs were excluded because of the impact of these agents on VMS, and thus, women with certain mental health conditions were likely underrepresented here and should be considered in future work. Furthermore, we considered sex hormones relevant to the menopause transition and VMS, including E2, E1, and FSH, but other hormones such as progesterone, luteinizing hormone, or testosterone may also warrant consideration in future studies. Finally, because this was an observational study, it is unknown whether sleep VMS play a causal role in WMHV.
This work has several strengths. This work was conducted in a large sample of well-characterized women. VMS were assessed through state-of-the-art physiologic measures. Total and regional WMHV were considered, lending insight into any region-specific links with VMS. A range of potential mechanisms and confounders were considered, including standard and novel CVD risk factors, objectively assessed sleep, and rigorously assessed estrogens.
In summary, we found that physiologic VMS, particularly occurring during sleep, were associated with greater brain WMHV. Findings were not explained by a range of potential confounders or explanatory factors. These study results call into question the perception of VMS as a benign midlife symptom of limited clinical significance and underscore the potential links of VMS to brain health. Women are at particular risk for poor brain health as they age, including for stroke and dementia. Although further work is needed to elucidate whether intervention on VMS improves brain health, identification of midlife female-specific markers of brain health hold promise in identifying targets for early intervention and prevention. These findings indicate that VMS have the potential to serve as a female-specific midlife marker of later risk for brain health.
Glossary
- BMI
body mass index
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- FLAIR
fluid-attenuated inversion recovery
- FOV
field of view
- HDL
high-density lipoprotein-cholesterol
- hsCRP
high sensitivity C-reactive protein
- HOMA
homeostatic model assessment
- IMT
intima-media thickness
- MPRAGE
magnetization-prepared rapid gradient echo
- RSD
relative SD
- SBP
systolic blood pressure
- SNRI
serotonin norepinephrine reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
- TE
echo time
- TI
inversion time
- TR
repetition time
- VMS
vasomotor symptom
- WASO
wake after sleep onset
- WMHV
white matter hyperintensities
Appendix. Authors
Study Funding
This research was supported by the NIH (NIH), National Institute on Aging (RF1AG053504 and R01AG053504 to Thurston & Maki), and the NIH Heart Lung and Blood Institute (R01HL105647 and 2K24HL123565 to Thurston). This work was also supported by the University of Pittsburgh Clinical and Translational Science Institute (NIH Grant UL1TR000005) and the University of Pittsburgh Small Molecule Biomarker Core (NIH Grant S10RR023461 to Poloyac).
Disclosure
R.C. Thurston is a consultant for Astellas, Bayer, Happify Health, Hello Therapeutics, and Vira Health. H.J. Aizenstein is an advisor to Eisai. P. Maki is a consultant for AbbVie, Balchem, and Pfizer and Advisor to Astellas, Bayer, and Johnson&Johnson. M. Wu, Y. Chang, C.A. Derby, and E.A. Barinas-Mitchell have no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Epperson CN, Sammel MD, Freeman EW. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metab. 2013;98(9):3829-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greendale GA, Karlamangla AS, Maki PM. The menopause transition and cognition. JAMA. 2020;323(15):1495-1496. [DOI] [PubMed] [Google Scholar]
- 3.Taylor CM, Pritschet L, Yu S, Jacobs EG. Applying a women's health lens to the study of the aging brain. Front Hum Neurosci. 2019;13:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosconi L, Berti V, Dyke J, et al. . Menopause impacts human brain structure, connectivity, energy metabolism, and amyloid-beta deposition. Sci Rep. 2021;11(1):10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schelbaum E, Loughlin L, Jett S, et al. . Association of reproductive history with brain MRI biomarkers of dementia risk in midlife. Neurology. 2021;97(23):e2328-e2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maki PM, Thurston RC. Menopause and brain health: hormonal changes are only a part of the story. Front Neurol. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold E, Colvin A, Avis N, et al. . Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: study of Women's Health Across the Nation (SWAN). Am J Public Health. 2006;96(7):1226-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams RE, Kalilani L, DiBenedetti DB, et al. . Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric. 2008;11(1):32-43. [DOI] [PubMed] [Google Scholar]
- 9.Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avis NE, Crawford SL, Greendale G, et al. . Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thurston RC, Aslanidou Vlachos HE, Derby CA, et al. . Menopausal vasomotor symptoms and risk of incident cardiovascular disease events in SWAN. JAMA. 2021:e017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thurston RC. Vasomotor symptoms: natural history, physiology, and links with cardiovascular health. Climacteric. 2018;21(2):96-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu D, Chung HF, Dobson AJ, et al. . Vasomotor menopausal symptoms and risk of cardiovascular disease: a pooled analysis of six prospective studies. Am J Obstet Gynecol. 2020;223(6):898.e891-898.e816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamin EJ, Muntner P, Alonso A, et al. . Heart disease and stroke statistics-2019 update: a report from the American Heart association. Circulation. 2019;139(10):e56-e528. [DOI] [PubMed] [Google Scholar]
- 16.Lambert MA, Bickel H, Prince M, et al. . Estimating the burden of early onset dementia; systematic review of disease prevalence. Eur J Neurol. 2014;21(4):563-569. [DOI] [PubMed] [Google Scholar]
- 17.Thurston RC, Aizenstein HJ, Derby CA, Sejdic E, Maki PM. Menopausal hot flashes and white matter hyperintensities. Menopause. 2016;23(1):27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maki PM, Rubin LH, Savarese A, et al. . Stellate ganglion blockade and verbal memory in midlife women: evidence from a randomized trial. Maturitas. 2016;92:123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fogel J, Rubin LH, Kilic E, Walega DR, Maki PM. Physiologic vasomotor symptoms are associated with verbal memory dysfunction in breast cancer survivors. Menopause. 2020;27(11):1209-1219. [DOI] [PubMed] [Google Scholar]
- 20.Maki PM, Drogos LL, Rubin LH, Banuvar S, Shulman LP, Geller SE. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15(5):848-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habes M, Sotiras A, Erus G, et al. . White matter lesions: spatial heterogeneity, links to risk factors, cognition, genetics, and atrophy. Neurology. 2018;91(10):e964-e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thurston RC, Chang Y, Barinas-Mitchell E, et al. . Menopausal hot flashes and carotid intima media thickness among midlife women. Stroke. 2016;47(12):2910-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harlow SD, Gass M, Hall JE, et al. . Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401. [Google Scholar]
- 25.Carpenter JS, Andrykowski MA, Freedman RR, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6(3):209-215. [DOI] [PubMed] [Google Scholar]
- 26.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342-392. [DOI] [PubMed] [Google Scholar]
- 27.Monk TH, Reynolds CF, Kupfer DJ, et al. . The Pittsburgh sleep diary. J Sleep Res. 1994;3(2):111-120. [PubMed] [Google Scholar]
- 28.Thurston R, Wu M, Aizenstein H, et al. . Sleep characteristics and white matter hyperintensities among midlife women. Sleep. 2020; 43(6):zsz298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502. [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Teacher DF, Turner RC. Homeostasis model assessment: insulin resistance and B cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 31.Demers LM. Testosterone and estradiol assays: current and future trends. Steroids. 2008;73(13):1333-1338. [DOI] [PubMed] [Google Scholar]
- 32.Nelson RE, Grebe SK, O'Kane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50(2):373-384. [DOI] [PubMed] [Google Scholar]
- 33.Kamboh MI. Genomics and functional genomics of Alzheimer's disease. Neurotherapeutics. 2022;19(1):152-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thurston RC, Fritz MM, Chang Y, Barinas Mitchell E, Maki PM. Self-compassion and subclinical cardiovascular disease among midlife women. Health Psychol. 2021;40(11):747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton-Tyrrell K, Wolfson SK, Jr., Thompson T, Kelsey SF. Measurement variability in duplex scan assessment of carotid atherosclerosis. Stroke. 1992;23(2):215-220. [DOI] [PubMed] [Google Scholar]
- 36.Wendelhag I, Gustavsson T, Suurkula M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clin Physiol. 1991;11(6):565-577. [DOI] [PubMed] [Google Scholar]
- 37.Wu M, Fatukasi O, Yang S, et al. . HIV disease and diabetes interact to affect brain white matter hyperintensities and cognition. AIDS. 2018;32(13):1803-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu M, Rosano C, Butters M, et al. . A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148(2-3):133-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter JS, Monahan PO, Azzouz F. Accuracy of subjective hot flush reports compared with continuous sternal skin conductance monitoring. Obstet Gynecol. 2004;104(6):1322-1326. [DOI] [PubMed] [Google Scholar]
- 40.Fu PB, Matthews KA, Thurston RC. How well do different measurement modalities estimate the number of vasomotor symptoms? Findings from the Study of Women's Health Across the Nation FLASHES Study. Menopause. 2014;21(2):124-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Emotional antecedents of hot flashes during daily life. Psychosom Med. 2005;67(1):137-146. [DOI] [PubMed] [Google Scholar]
- 42.Palhaugen L, Sudre CH, Tecelao S, et al. . Brain amyloid and vascular risk are related to distinct white matter hyperintensity patterns. J Cereb Blood Flow Metab. 2021;41(5):1162-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: a clinical review. Neurology. 2019;92(24):1146-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Della-Morte D, Dong C, Markert MS, et al. . Carotid intima-media thickness is associated with white matter hyperintensities: the Northern Manhattan Study. Stroke. 2018;49(2):304-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology. 2012;78(10):720-727. [DOI] [PubMed] [Google Scholar]
- 46.Thurston RC, Chang Y, Buysse DJ, Hall MH, Matthews KA. Hot flashes and awakenings among midlife women. Sleep. 2019; 42(9):zsz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and associated materials that support the findings of this study are available from the corresponding author on reasonable request.