Abstract
Background
The efficacy of selective serotonin reuptake inhibitors (SSRIs) in the treatment of acute COVID-19 is still under investigation, with conflicting results reported from randomized controlled trials (RCTs). Different dosing regimens may have contributed to the contradictory findings.
Objectives
To evaluate the efficacy and safety of SSRIs and the effect of different dosing regimens on the treatment of acute COVID-19.
Data sources
Seven databases were searched from January 2020 to December 2022. Trial registries, previous reviews, and preprint servers were hand-searched.
Study eligibility criteria
RCTs and observational studies with no language restrictions.
Participants
COVID-19 inpatients/outpatients.
Interventions
SSRIs prescribed after diagnosis were compared against a placebo or standard of care.
Assessment of risk of bias
Risk of bias was rated using the revised Cochrane Risk of Bias Tool for Randomized Trials version 2.0 and Risk of Bias in Non-Randomized Studies of Interventions.
Methods of data synthesis
Outcomes were mortality, hospitalization, composite of hospitalization/emergency room visits, hypoxemia, requirement for supplemental oxygen, ventilator support, and serious adverse events. RCT data were pooled in random-effects meta-analyses. Observational findings were narratively described. Subgroup analyses were performed on the basis of SSRI dose, and sensitivity analyses were performed excluding studies with a high risk of bias. The Grading of Recommendations, Assessment, Development and Evaluations framework was used to assess the quality of evidence.
Results
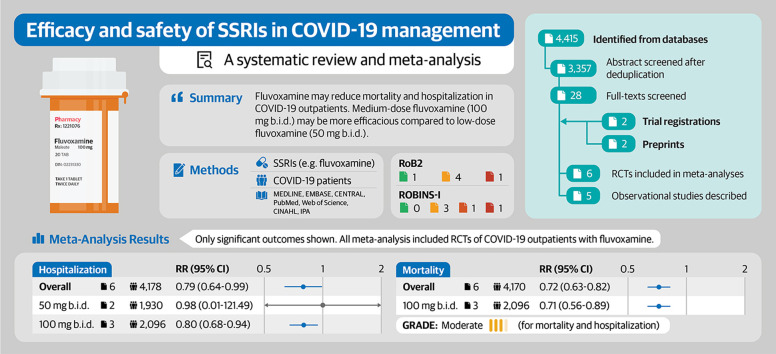
Six RCTs (N = 4197) and five observational studies (N = 1156) were included. Meta-analyses associated fluvoxamine with reduced mortality (risk ratio, 0.72; 95% CI, 0.63–0.82) and hospitalization (risk ratio, 0.79; 95% CI, 0.64–0.99) on the basis of moderate quality of evidence. Medium-dose fluvoxamine (100 mg twice a day) was associated with reduced mortality, hospitalization, and composite of hospitalization/emergency room visits, but low-dose fluvoxamine (50 mg twice a day) was not. Fluvoxamine was not associated with increased serious adverse events. Observational studies support the use of fluvoxamine and highlight fluoxetine as a possible alternative to SSRIs for the treatment of COVID-19.
Discussion
Fluvoxamine remains a candidate pharmacotherapy for treating COVID-19 in outpatients. Medium-dose fluvoxamine may be preferable over low-dose fluvoxamine.
Keywords: COVID-19, Fluvoxamine, Meta-analysis, SARS-CoV-2, Selective serotonin reuptake inhibitor
Graphical abstract
Introduction
Despite the introduction of COVID-19 vaccines and effective anti-COVID therapies, such as nirmatrelvir/ritonavir, constraints such as cost, availability, and vaccine reluctance hamper their adoption and distribution [1,2]. Thus, there remains a need for identifying effective, accessible, and affordable therapies against COVID-19. In particular, recent studies have established selective serotonin reuptake inhibitors (SSRIs) as a new category of repurposed pharmacotherapies with potential efficacy against COVID-19 [[3], [4], [5]].
It has been hypothesized that SSRIs exert their anti-COVID effects by binding to serotonin transporters and σ-1 receptors to attenuate inflammation [6,7]. SSRIs may also interact with acid sphingomyelinase and σ-1 receptors to disrupt viral entry and virion assembly [6,7]. Among the SSRIs under investigation, fluvoxamine has garnered considerable research interest owing to its high binding affinity to σ-1 receptors compared with other SSRIs [8,9].
However, findings regarding the efficacy of fluvoxamine from randomized controlled trials (RCTs) have been contradictory. Although early outpatient RCTs, such as TOGETHER and STOP COVID, have found significant benefits associated with fluvoxamine in terms of reduced deterioration and hospitalization [10,11], more recent trials have found no such effects [12,13]. A commentary in response to the latest COVID-OUT trial had even concluded that fluvoxamine should not be prescribed due to a lack of benefits [14]. It is possible that different dosing regimens used in the RCTs are implicated in this controversy, with earlier RCTs using higher doses of fluvoxamine than those used by newer RCTs [12].
Previously, two systematic reviews assessing the efficacy of fluvoxamine have been published [15,16]. However, they included the same set of RCTs [10,11,17] with low statistical power. Hence, they could not associate fluvoxamine with reduced hospitalization and could only assess investigator-defined primary outcomes. Additionally, they could not explore the effect of SSRI dosing regimens on efficacy and safety. Thus, we conducted this systematic review and meta-analysis to provide an updated assessment of SSRIs' efficacy and safety for the management of patients with COVID-19 with regards to mortality, hospitalization, hypoxemia, oxygen requirements, and serious adverse events (SAEs) compared with those of standard of care or placebo. We also assessed the effect of SSRI dosing levels on clinical outcomes in patients with COVID-19.
Methods
We conducted this systematic review and meta-analysis with guidance from Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statements [18] and the Cochrane Handbook [19]. The completed PRISMA checklist is included as Table S1. This review was prospectively registered on PROSPERO (CRD42021275303) [20].
Study identification
A database search was performed in MEDLINE, Embase, CENTRAL, PubMed Clinical Queries, Web of Science (Core Collection), CINAHL, and International Pharmaceutical Abstracts from 1 January 2020 to 17 December 2022. The complete strategies are tabulated in Tables S2–S8. We also hand-searched the reference sections of previous reviews, clinical trial registries (ClinicalTrials.gov and the WHO International Clinical Trials Registry), and preprint servers (medRxiv and Research Square) for additional records.
Eligibility criteria
We included RCTs and prospective or retrospective observational studies that included inpatients and/or outpatients of any age with COVID-19 and compared the use of SSRIs to the use of the standard of care or placebo. Studies assessing patients with past or ongoing SSRI prescriptions at the time of diagnosis were specifically excluded. We did not impose any language restrictions.
Outcome measures
Efficacy was assessed through the following outcomes: 1) mortality, 2) hospitalization, 3) composite outcome of emergency room visits or hospitalization, 4) hypoxemia, 5) requirement for supplemental oxygen, and 6) requirement for ventilator support. Safety was assessed through the incidence of SAEs.
Study selection
Eight investigators (J.D., D.R., H.B.R., U.A., E.H., K.H., F.Z., and Y.-J.P.) performed title and abstract screening independently and in-duplicate using Rayyan [21]. Records deemed eligible for inclusion by two investigators were subsequently retrieved and entered into an in-duplicate full-text screening process. Disagreements were resolved by recruiting a third author (J.D. or C.G.) to attain consensus.
Data extraction
Data extraction was performed independently and in-duplicate by three reviewers (J.D., E.H., and K.H.) using standardized extraction sheets developed a priori. Disagreements in the extracted data were identified and resolved by a third author (C.G. or M.M.) who reviewed the conflicts and attained consensus. In the case of missing data, we made attempts to contact the corresponding authors for unpublished information.
Risk-of-bias assessment
We assessed the risk of bias of RCTs using the revised Cochrane Risk of Bias Tool for Randomized Trials version 2.0 [22]. The risk of bias in non-randomized studies was assessed using the Risk of Bias in Non-Randomized Studies of Interventions tool [23]. Risk-of-bias assessments were completed in duplicate by three reviewers (K.H., F.Z., and D.R.). Disagreements were resolved by recruiting a third author (J.D.) to attain consensus.
Statistical analysis
RCT results were pooled in random-effects meta-analyses using the meta 6.0 package in R [24]. Dichotomous outcome data were expressed as risk ratios (RRs) and 95% CIs. For studies reporting zero events in one or both of its treatment arms, we applied treatment-arm continuity correction to complete the meta-analyses [25]. Heterogeneity was examined using Cochran's Q test with a significance level of <0.10 (pQ < 0.10) and further quantified using I 2 statistics. We interpreted an I 2 between 30% and 75% as moderate heterogeneity and an I 2 of ≥75% as serious heterogeneity [19]. The results of the meta-analyses were illustrated in forest plots.
We did not include observational studies in the data synthesis owing to high heterogeneity in participant characteristics and intervention medications and biases inherent to observational study designs. Findings from observational studies were described narratively.
Subgroup and sensitivity analyses
Subgroup analyses were performed on the basis of SSRI dose (excluding loading or taper doses). SSRI dose was prospectively categorized as low, medium, and high daily doses according to classifications established by previous reviews [26,27]. In the context of this review, a low dose of fluvoxamine was defined as 50 mg twice a day, a moderate dose of fluvoxamine was defined as 100 mg twice a day, and a high dose of fluvoxamine was defined as 100 mg three times a day.
Additionally, post hoc sensitivity analyses were performed, excluding studies with a high risk of bias to assess the effect of the methodological quality of RCTs on the pooled treatment effects.
Quality of evidence
The quality of evidence generated from meta-analyses and subgroup analyses of RCTs was assessed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework [28]. A summary of study findings and the associated GRADE ratings are presented in a GRADE summary of findings table generated using GRADEpro GDT (https://gradepro.org/) [29].
Additional methods
Additional methods and deviations from the original study protocol are reported in Method S1.
Results
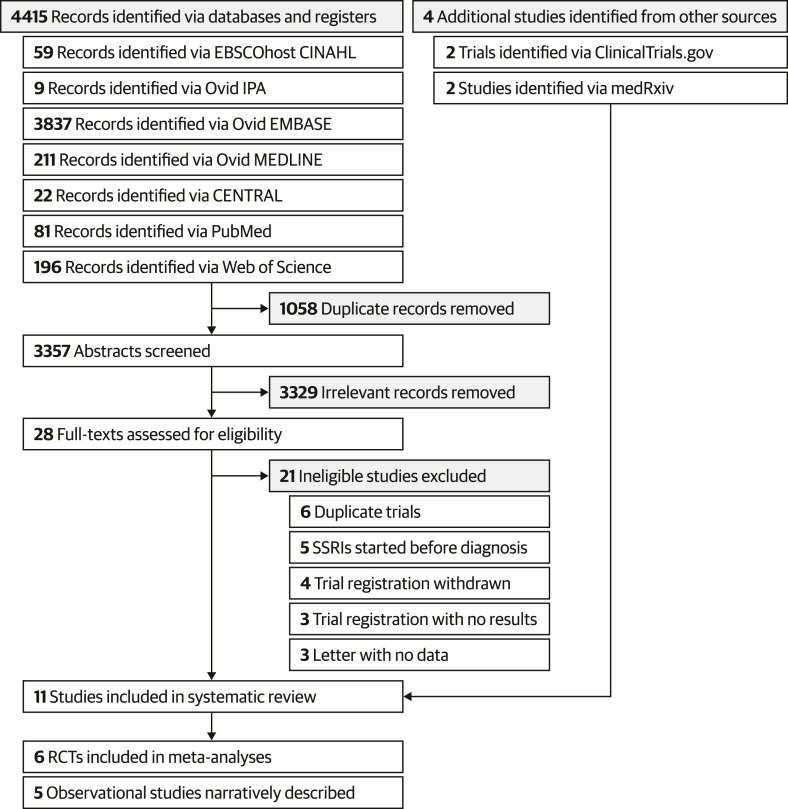
The database search yielded 4415 records, of which 3357 were screened after de-duplication. Twenty-eight full texts were retrieved for further screening, and seven studies were included. A list of studies excluded during the full-text screening process can be found in Result S1. In addition, two relevant studies were retrieved from medRxiv and the results of two trials were retrieved from ClinicalTrials.gov. In total, six RCTs (N = 4197) [[10], [11], [12], [13],17,30] and five comparative observational studies (N = 1156) [[31], [32], [33], [34], [35]] were included in this review. The PRISMA flowchart for the study selection process is illustrated in Fig. 1 , and study characteristics are tabulated in Table S9.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 flowchart for the identification and selection of relevant studies. RCT, randomized controlled trial; SSRI, selective serotonin reuptake inhibitor.
Risk of bias
Based on the revised Cochrane Risk of Bias Tool for Randomized Trials version 2.0 ratings, four RCTs [10,11,13,17] had some concerns in regards to risk of bias, one RCT [30] had a high risk of bias, and one RCT [12] had a low risk of bias. Specifically, three RCTs [13,17,30] in which allocation concealment was not reported and could not be reasonably deduced had some concerns regarding randomization. Additionally, three RCTs [10,13,17] had some concerns about deviation from the intended intervention due to the presence of significant deviation or a lack of reporting while using intention-to-treat analysis. One RCT [30] failed to report deviations and did not statistically correct for the effects of potential deviation, leading to a high risk-of-bias rating. Furthermore, one RCT [11] had some concerns regarding missing data owing to censoring without valid reasons. All RCTs were rated as having a low risk of bias on outcome measurement and selective reporting.
Based on the Risk of Bias in Non-Randomized Studies of Interventions ratings, three studies [32,34,35] had a serious risk of bias due to insufficient adjustments for potential confounders and a lack of a priori protocols. The study by Calusic et al. [31] was rated as having a moderate risk of bias because it used a control group that was matched for age, sex, vaccination, disease severity, and comorbidities, thus decreasing bias owing to confounding. Lastly, the study by Németh et al. [33] was rated as having a critical risk of bias owing to both insufficient confounder adjustments and the use of a case-control design, which selected participants on the basis of outcomes.
The full per-study risk-of-bias ratings are tabulated in Tables S10 and S11.
Meta-analysis of RCTs
All RCTs assessed the efficacy and safety of fluvoxamine in non-hospitalized patients with COVID-19.
Mortality
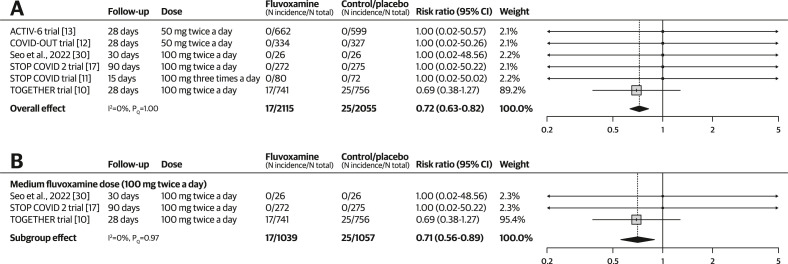
Based on pooled results from six RCTs (N = 4170) [[10], [11], [12], [13],17,30], treatment with fluvoxamine reduced mortality with a RR of 0.72 (95% CI, 0.63–0.82; I 2 = 0%, pQ 1.00) among outpatients with COVID-19 (see Fig. 2 a). The quality-of-evidence rating was moderate, with a downgrade due to concerns regarding the risk of bias.
Fig. 2.
Forest plots illustrating the individual and pooled randomized controlled trial effects for the outcome of mortality. A risk ratio of <1 indicates beneficial treatment effects associated with fluvoxamine. (a) Meta-analysis for mortality. (b) Subgroup analysis by dose for mortality.
There was no change in the treatment effect when the analysis was limited to a subgroup of studies using a medium-dose of fluvoxamine (RR, 0.71; 95% CI, 0.56–0.89; I 2 = 0%; pQ 0.97). Subgroup analyses for other dosing levels were not conducted because no events were reported in those subgroups (Fig. 2b). There was no change in the treatment effect when one study with a high risk of bias was excluded (RR, 0.72; 95% CI, 0.62–0.83; I 2 = 0%; pQ 1.00; see Fig. S1).
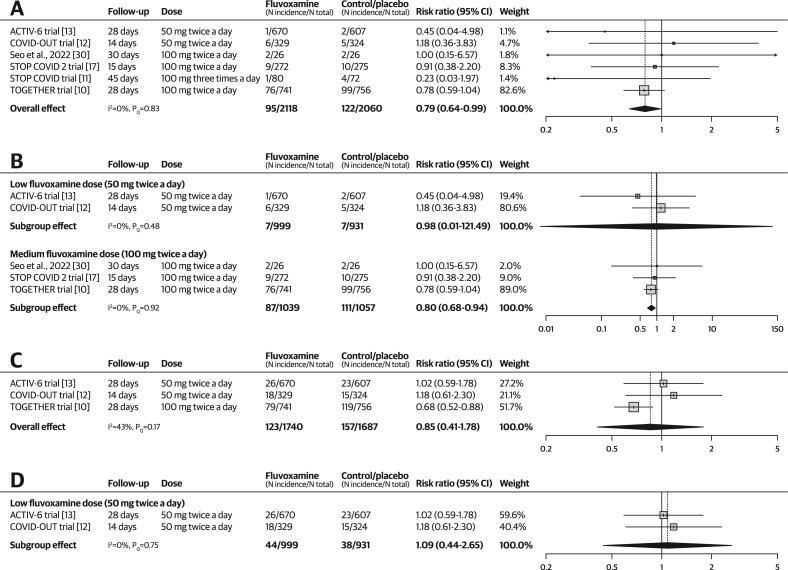
Hospitalization and emergency room visits
The pooling of six RCTs (N = 4178) [[10], [11], [12], [13],17,30] showed that fluvoxamine reduced hospitalization (RR, 0.79; 95% CI, 0.64–0.99; I 2 = 0%; pQ 0.83) among outpatients with COVID-19 (see Fig. 3 a). The quality-of-evidence rating was moderate, with a downgrade due to concerns regarding the risk of bias. In the subgroup analysis by dose, medium-dose fluvoxamine was associated with reduced hospitalization (RR, 0.80; 95% CI, 0.68–0.94; I 2 = 0%; pQ 0.92) but low-dose fluvoxamine was not (RR, 0.98; 95% CI, 0.01–121.49; I 2 = 0%; PQ 0.48; see Fig. 3b). Only one trial [11] reported data on high-dose fluvoxamine, which did not associate its use with reduced hospitalization (RR, 0.23; 95% CI, 0.03–1.97). Excluding one study with a high risk of bias caused the 95% CI to widen, resulting in a non-significant treatment effect (RR, 0.79; 95% CI, 0.61–1.03; I 2 = 0%; pQ 0.73; see Fig. S2).
Fig. 3.
Forest plots illustrating the individual and pooled randomized controlled trial effects for hospitalization-related outcomes. A risk ratio of <1 indicates beneficial treatment effects associated with fluvoxamine. (a) Meta-analysis for hospitalization. (b) Subgroup analysis by dose for hospitalization. (c) Meta-analysis for the composite outcome. (d) Subgroup analysis by dose for the composite outcome.
In terms of the composite outcome including both hospitalization and emergency room visits, pooled results from three RCTs (N = 3427) [10,12,13] showed that fluvoxamine was not associated with reduced incidence of the composite outcome (RR, 0.85; 95% CI, 0.41–1.78; I 2 = 43%; pQ 0.17; see Fig. 3c). The quality-of-evidence rating was very low, with downgrades due to the risk of bias, imprecision, and inconsistency. In the subgroup analysis, low-dose fluvoxamine was not associated with reduced incidence of the composite outcome (RR, 1.09; 95% CI, 0.44–2.65; I 2 = 0%; pQ 0.75; see Fig. 3d). The TOGETHER [10] trial reported data on medium-dose fluvoxamine, which showed a significant reduction in the incidence of the composite outcome (RR, 0.68; 95% CI, 0.52–0.88).
Hypoxemia, requirement for supplemental oxygen, and ventilator support
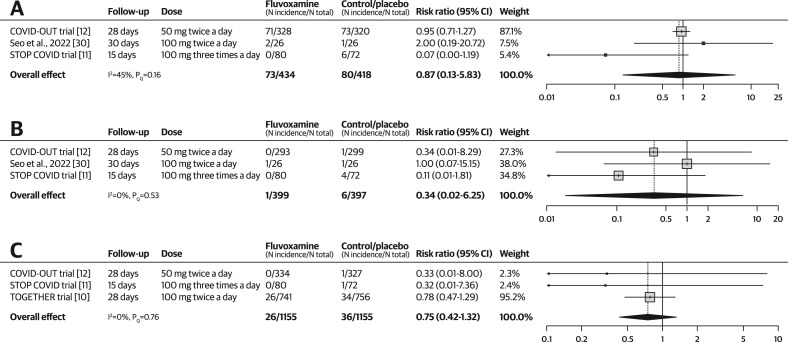
The use of fluvoxamine was not associated with significantly reduced incidence of hypoxemia, requirement for supplemental oxygen, or ventilator support (see Fig. 4 ). Three RCTs were included in the meta-analysis of each outcome. The quality-of-evidence rating was very low for all three outcomes because of the risk of bias and imprecision. Hypoxemia was also downgraded because of inconsistency. No subgroup pooling by dose was performed for any of the three outcomes because only one RCT from each dosing level was included in the meta-analyses. Excluding one study with a high risk of bias did not change the significance of the treatment effects in the aforementioned outcomes (see Figs S3 and S4).
Fig. 4.
Forest plots illustrating the individual and pooled randomized controlled trial effects for the outcome of hypoxemia, requirement for supplemental oxygen, and requirement for ventilator support. A risk ratio of <1 indicates beneficial treatment effects associated with fluvoxamine. (a) Meta-analysis for hypoxemia. (b) Meta-analysis for supplemental oxygen requirement. (c) Meta-analysis for ventilator requirement.
Serious adverse events
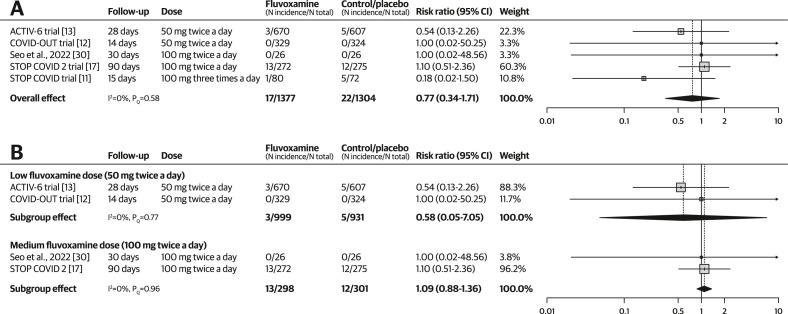
Based on pooled results from five RCTs (N = 2641) [[11], [12], [13],17,30], the use of fluvoxamine was not associated with significantly increased incidence of SAEs (RR, 0.77; 95% CI, 0.34–1.71; I 2 = 0%; pQ 0.58; see Fig. 5 a). The quality-of-evidence rating was very low because of the risk of bias and concerns regarding imprecision.
Fig. 5.
Forest plots illustrating the individual and pooled randomized controlled trial effects for the outcome of serious adverse events (SAEs). A risk ratio of <1 indicates beneficial treatment effects associated with fluvoxamine. (a) Meta-analysis for SAE. (b) Subgroup analysis by dose for SAE.
In the subgroup analysis by dosing levels, both low-dose fluvoxamine (RR, 0.58; 95% CI, 0.05–7.05; I 2 = 0%; pQ 0.77) and medium-dose fluvoxamine (RR, 1.09; 95% CI, 0.88–1.36; I 2 = 0%; pQ 0.96) were not associated with significantly increased incidence of SAEs (see Fig. 5b). The STOP COVID [11] trial reported results for high-dose fluvoxamine that did not show significantly increased SAEs (RR, 0.18; 95% CI, 0.02–1.50). Excluding one study with a high risk of bias did not change the significance of the treatment effect (RR, 0.73; 95% CI, 0.24–2.23; I 2 = 0%; pQ 0.42; see Fig. S5).
GRADE summary of findings table
A summary of the meta-analysis results and quality-of-evidence ratings is tabulated in Table 1 .
Table 1.
Summary of randomized controlled trial findings, fluvoxamine compared with placebo for the management of COVID-19 outpatients
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects (95% CI)a |
No. of patients (No. of studies) | Quality of evidence (GRADE) | ||
|---|---|---|---|---|---|---|
| Risk without fluvoxamine | Risk with fluvoxamine | Risk difference (95% CI) | ||||
|
Mortality (Median follow-up: 28 d) |
RR, 0.72 (0.63–0.82) | 12 per 1000 | 9 per 1000 (7–10) | 3 fewer per 1000 (5 fewer to 2 fewer) | 4170 (6 RCTs) | ⨁⨁⨁◯ Moderateb |
|
Hospitalization (Median follow-up: 28 d) |
RR, 0.79 (0.64–0.99) | 59 per 1000 | 47 per 1000 (38–58) | 12 fewer per 1000 (21 fewer to 1 fewer) | 4178 (6 RCTs) | ⨁⨁⨁◯ Moderateb |
|
Hospitalization or emergency room visits (Median follow-up: 28 d) |
RR, 0.85 (0.41–1.78) | 93 per 1000 | 79 per 1000 (38–166) | 14 fewer per 1000 (55 fewer to 73 more) | 3427 (3 RCTs) | ⨁◯◯◯ Very lowb,c,d |
|
Hypoxemia (Median follow-up: 28 d) |
RR, 0.87 (0.13–5.83) | 191 per 1000 | 166 per 1000 (24–1000) | 25 fewer per 1000 (167 fewer to 924 more) | 852 (3 RCTs) | ⨁◯◯◯ Very lowb,c,d |
|
Requirement for supplemental oxygen (Median follow-up: 28 d) |
RR, 0.34 (0.02–6.25) | 15 per 1000 | 5 per 1000 (0–94) | 10 fewer per 1000 (15 fewer to 79 more) | 796 (3 RCTs) | ⨁◯◯◯ Very lowb,c |
|
Requirement for ventilator support (Median follow-up: 28 d) |
RR, 0.75 (0.42–1.32) | 31 per 1000 | 23 per 1000 (13–41) | 8 fewer per 1000 (18 fewer to 10 more) | 2310 (3 RCTs) | ⨁◯◯◯ Very lowb,c |
|
Serious adverse events (Median follow-up: 28 d) |
RR, 0.77 (0.34–1.71) | 170 per 1000 | 166 per 1000 (159–182) | 4 fewer per 1000 (11 fewer to 12 more) | 2681 (5 RCTs) | ⨁◯◯◯ Very lowb,c |
GRADE Working Group quality-of-evidence rating [28].
High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of the effect.
CI: Confidence Interval GRADE, Grading of Recommendations, Assessment, Development and Evaluations; RCT, randomized controlled trial; RR, risk ratio.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Downgraded by one level due to risk of bias. A majority of included studies were rated as having ‘some concerns’ on the revised Cochrane Risk of Bias Tool for Randomized Trials version 2.0.
Downgraded by two levels owing to imprecision. Using the Optimal Information Size method, the ratio between the upper and lower bounds of the CI is > 3 for risk ratio.
Downgraded by one level owing to inconsistency. I2 statistics of the meta-analysis indicated the presence of moderate heterogeneity.
Results from observational studies
In a prospective cohort study, Pineda et al. [34] assessed fluvoxamine 100 mg twice a day or three times a day in 657 non-hospitalized patients with COVID-19 (fluvoxamine, N = 63; control, N = 594). After adjusting for hypertension, diabetes, age, vaccination status, or premedication with steroids at baseline, the control group had significantly increased odds of mortality (adjusted OR, 24; 95% CI, 2.6–233.5), hospitalization (adjusted OR, 2.38; 95% CI, 1.04–5.47), and oxygen requirement (adjusted OR, 5.08; 95% CI, 2.18–11.81) compared with those in the fluvoxamine group.
In a prospective cohort study, Seftel et al. [35] assessed fluvoxamine 50 mg twice a day in 113 non-hospitalized patients with COVID-19 (fluvoxamine, N = 65; control, N = 48). No patients required hospitalization in the fluvoxamine group, whereas six control patients were hospitalized (OR, 0.06; 95% CI, 0.00–0.88). Two of these 6 control patients progressed to intensive care unit (ICU) stay with mechanical ventilation (OR, 0.17; 95% CI, 0.01–3.05), and one of these 2 patients died (OR, 0.29; 95% CI, 0.01–6.38). Fluvoxamine was well tolerated, with no SAEs and no adverse events leading to discontinuation.
Calusic et al. [31] conducted a prospective cohort study assessing the efficacy of fluvoxamine 100 mg three times a day in 102 patients hospitalized in the ICU against a matched control group on the basis of age, sex, vaccination against COVID-19, disease severity, and comorbidities (fluvoxamine, N = 51; control, N = 51). Fluvoxamine was associated with reduced mortality (hazard ratio, 0.58; 95% CI, 0.36–0.94) but not reduced length of stay, duration of ICU stay, or duration of ventilator support compared with those in controls.
Németh et al. [33] conducted a retrospective case-control study assessing the use of fluoxetine 20 mg daily in 205 living and 64 deceased patients (fluoxetine, N = 110; control, N = 159). Mortality in 2–28 days was significantly higher among control patients than among patients using fluoxetine (30.8% vs. 13.6%; p 0.001).
Lastly, a small, unpublished prospective cohort study on a mix of inpatients and outpatients (NCT04377308) [32] found that none of the 11 patients in the fluoxetine (20–60 mg daily) group required intubation or died compared with one of the four patients in the control group who required intubation and one of the four patients in the control group who died of the nine outpatients in the fluoxetine group, none required hospitalization.
Discussion
This systematic review and meta-analysis found that COVID-19 treatment with fluvoxamine was associated with a 28% reduction in the risk of mortality and a 21% reduction in the risk of hospitalization compared with those with placebo on the basis of moderate quality of evidence. Fluvoxamine was not associated with improvements in other efficacy outcomes or increased SAEs compared with placebo; however, the quality of evidence was very low. Medium-dose fluvoxamine (100 mg twice a day) was associated with reduced risk of mortality, hospitalization, and composite of hospitalization or emergency room visits but not with low-dose fluvoxamine (50 mg twice a day). These findings, combined with observational findings, demonstrate that fluvoxamine may be effective for treating outpatients with COVID-19, with medium-to high-dose fluvoxamine being preferable to low-dose fluvoxamine. The difference in effects between dosing regimens may be attributable to insufficient effector receptor occupancy associated with low-dose fluvoxamine [36].
In addition to dosing considerations, other factors, such as differences in patient characteristics between early and recent fluvoxamine RCTs, should also be taken into account. For instance, the TOGETHER trial included patients with risk factors for disease progression, whereas the ACTIV-6 trial did not impose such a restriction. Vaccination rates in the ACTIV-6 and COVID-OUT trials were also higher than those in the TOGETHER trial [10,12,13]. As COVID-19 transitions into an endemic, it may be more difficult to observe clinical events and treatment effects in outpatient trials. For example, the STOP COVID 2 trial was prematurely terminated owing to a lack of recruitment and clinical events. Therefore, future COVID-19 trials should be limited to patients at risk of deterioration who are more likely to experience clinical events and benefit from SSRIs.
Results from observational studies also highlighted fluoxetine as an alternative SSRI therapy for COVID-19 management. However, given that fluoxetine has less binding affinity for σ-1 receptors than fluvoxamine [9], further investigations are needed to elucidate its efficacy, safety, and mechanism of action.
Clinical considerations
Currently, guidelines from the Infectious Diseases Society of America, the National Institutes of Health, and WHO do not recommend the use of fluvoxamine for the treatment of COVID-19 outside the context of clinical trials [[37], [38], [39]]. However, given its wide availability and low cost (as little as $1 per day) [15], fluvoxamine may play a crucial role in managing high-risk outpatients who do not have access to effective antivirals, vaccines, or monoclonal antibodies. Although we found that fluvoxamine was not associated with SAEs, clinicians should remain vigilant of contraindications and drug-drug interactions associated with SSRIs, such as interactions with monoamine oxidase inhibitors, QT-prolonging medications, and drugs that rely on cytochrome P450 for metabolism [40].
It is notable that previous research has shown reductions in SSRI efficacy among patients living with depression and obesity or a high body mass index possibly due to pharmacokinetic changes caused by adipose tissue, comorbid conditions, and genetic causes, among other factors [41,42]. Given that obesity is a well-recognized risk factor for severe COVID-19 [43], further research is needed to determine whether a higher or weight-adjusted SSRI regimen would be beneficial in this population for the management of COVID-19.
Review limitations
There are several limitations associated with the review. First of all, continuity correction was used extensively for the outcome of mortality because of a low number of reported events. Although continuity correction helps to incorporate precision from zero-event studies that would have been otherwise excluded [44], its use may also pull the treatment effect towards null [45]. Although we used treatment-arm continuity correction, which performs better than a constant continuity correction factor [25], our findings regarding mortality should be interpreted with caution. An additional weakness is that most outcomes were rated as having very low quality of evidence. Findings from low quality outcomes need to be further explored and confirmed in future studies.
Lastly, we found that fluvoxamine was not associated with reduced hospitalization once we excluded one study with a high risk-of-bias rating. This was likely due to loss of precision from a reduced sample size given the small number of studies and patients included in this review. The incorporation of larger, better-designed RCTs in future meta-analyses can help confirm our findings relating to hospitalization.
In conclusion, this systematic review and meta-analysis of six RCTs and five observational studies found that fluvoxamine may reduce mortality and hospitalization on the basis of moderate quality of evidence. Medium-dose fluvoxamine (100 mg twice a day) was associated with reduced mortality, hospitalization, and composite of hospitalization and emergency room visits but low-dose fluvoxamine (50 mg twice a day) was not. These findings support further assessment of medium-dose fluvoxamine for the treatment of COVID-19 in clinical trials. Apart from fluvoxamine, observational evidence highlighted fluoxetine as an alternative SSRI for the treatment of COVID-19; however, its efficacy, safety, and mechanism of action need to be investigated in future trials.
Author contributions
JD, KH, and FZ conceptualized the study. JD, DR, and FZ developed the methodology. JD was responsible for the software, data duration, visualization, and supervision. JD, DR, and FZ performed the validation. JD and DR were responsible for the resources. JD and FZ performed the formal analysis. JD, DR, HBR, UA, CG, KH, FZ, EH, Y-JP, and MM performed the investigation. JD and FZ were responsible for project administration. JD and FZ wrote the original draft of the manuscript. JD, DR, HBR, UA, CG, KH, FZ, EH, Y-JP, and MM reviewed and edited the manuscript.
Data sharing
All relevant data are disclosed in the manuscript, its associated figures, and the supplementary materials.
Transparency declaration
The authors declare that they have no conflicts of interest. This review and its authors received no funding support from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgements
We would like to thank Dr. Hyeonji Seo, who is affiliated with the Division of Infectious Diseases at the Hallym University Dongtan Sacred Heart Hospital, for providing us with unpublished mortality data needed to complete our analyses.
Editor: A. Huttner
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2023.01.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Pepperrell T., Ellis L., Wang J., Hill A. Barriers to worldwide access for Paxlovid, a new treatment for COVID-19. Open Forum Infect Dis. 2022;9:ofac174. doi: 10.1093/ofid/ofac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nawas GT, Zeidan RS, Edwards CA, El-Desoky RH. Barriers to COVID-19 vaccines and strategies to improve acceptability and uptake. J Pharm Pract. In press. 10.1177/08971900221081621. [DOI] [PMC free article] [PubMed]
- 3.Oskotsky T., Maric I., Tang A., Oskotsky B., Wong R.J., Aghaeepour N., et al. Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.33090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoertel N., Sánchez-Rico M., Kornhuber J., Gulbins E., Reiersen A.M., Lenze E.J., et al. Antidepressant use and its association with 28-day mortality in inpatients with SARS-CoV-2: support for the FIASMA model against COVID-19. J Clin Med Res. 2022;11:5882. doi: 10.3390/jcm11195882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Min K.H., Kim T.H., Oh S.J., Kim W., Lee K.E. COVID-19 prognosis in association with antidepressant use. Pharmacopsychiatry. 2022;55:220–227. doi: 10.1055/a-1842-7859. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto Y., Suzuki T., Hashimoto K. Mechanisms of action of fluvoxamine for COVID-19: a historical review. Mol Psychiatry. 2022;27:1898–1907. doi: 10.1038/s41380-021-01432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto K. Repurposing of CNS drugs to treat COVID-19 infection: targeting the sigma-1 receptor. Eur Arch Psychiatry Clin Neurosci. 2021;271:249–258. doi: 10.1007/s00406-020-01231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vela J.M. Repurposing sigma-1 receptor ligands for COVID-19 therapy? Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.582310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto K. Activation of sigma-1 receptor chaperone in the treatment of neuropsychiatric diseases and its clinical implication. J Pharmacol Sci. 2015;127:6–9. doi: 10.1016/j.jphs.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Reis G., Dos Santos Moreira-Silva E.A., Silva D.C.M., Thabane L., Milagres A.C., Ferreira T.S., et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10:e42–e51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenze E.J., Mattar C., Zorumski C.F., Stevens A., Schweiger J., Nicol G.E., et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324:2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bramante C.T., Huling J.D., Tignanelli C.J., Buse J.B., Liebovitz D.M., Nicklas J.M., et al. Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19. N Engl J Med. 2022;387:599–610. doi: 10.1056/NEJMoa2201662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy M.W., Naggie S., Boulware D.R., Lindsell C.J., Stewart T.G., Felker G.M., et al. Fluvoxamine for outpatient treatment of COVID-19: a decentralized, placebo-controlled, randomized, platform clinical trial. medRxiv. 2022 doi: 10.1101/2022.10.17.22281178v2. [DOI] [Google Scholar]
- 14.Abdool Karim S.S., Devnarain N. Time to stop using ineffective Covid-19 drugs. N Engl J Med. 2022;387:654–655. doi: 10.1056/NEJMe2209017. [DOI] [PubMed] [Google Scholar]
- 15.Lee T.C., Vigod S., Bortolussi-Courval É., Hanula R., Boulware D.R., Lenze E.J., et al. Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo C.M., Harari O., Chernecki C., Thorlund K., Forrest J.I. Fluvoxamine for the early treatment of COVID-19: a meta-analysis of randomized clinical trials. Am J Trop Med Hyg. 2022;106:1315–1320. doi: 10.4269/ajtmh.21-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ClinicalTrialsgov Fluvoxamine for early treatment of Covid-19 (stop Covid 2) https://clinicaltrials.gov/ct2/show/NCT04668950 [Internet]. 2022 [cited 7 November 2022]. Available from:
- 18.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J.P.T., Green S. Wiley; New Jersey: 2008. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 20.Schiavo J.H. PROSPERO: an international register of systematic review protocols. Med Ref Serv Q. 2019;38:171–180. doi: 10.1080/02763869.2019.1588072. [DOI] [PubMed] [Google Scholar]
- 21.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 23.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarzer G., Carpenter J.R., Rucker G. 1st ed. Springer International Publishing; Cham, Switzerland: 2015. Meta-analysis with R. [DOI] [Google Scholar]
- 25.Sweeting M.J., Sutton A.J., Lambert P.C. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 26.Bloch M.H., McGuire J., Landeros-Weisenberger A., Leckman J.F., Pittenger C. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15:850–855. doi: 10.1038/mp.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bollini P., Pampallona S., Tibaldi G., Kupelnick B., Munizza C. Effectiveness of antidepressants. Meta-analysis of dose-effect relationships in randomised clinical trials. Br J Psychiatry. 1999;174:297–303. doi: 10.1192/bjp.174.4.297. [DOI] [PubMed] [Google Scholar]
- 28.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyatt G.H., Oxman A.D., Santesso N., Helfand M., Vist G., Kunz R., et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013;66:158–172. doi: 10.1016/j.jclinepi.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Seo H., Kim H., Bae S., Park S., Chung H., Sung H.S., et al. Fluvoxamine treatment of patients with symptomatic COVID-19 in a community treatment center: a preliminary result of randomized controlled trial. Infect Chemother. 2022;54:102–113. doi: 10.3947/ic.2021.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calusic M., Marcec R., Luksa L., Jurkovic I., Kovac N., Mihaljevic S., et al. Safety and efficacy of fluvoxamine in COVID-19 ICU patients: an open label, prospective cohort trial with matched controls. Br J Clin Pharmacol. 2022;88:2065–2073. doi: 10.1111/bcp.15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04377308 Fluoxetine to reduce intubation and death after COVID19 infection [Internet]. 2022 [cited 7 November 2022]. Available from:
- 33.Németh Z.K., Szûcs A., Vitrai J., Juhász D., Németh J.P., Holló A. Fluoxetine use is associated with improved survival of patients with COVID-19 pneumonia: a retrospective case-control study. Ideggyogy Sz. 2021;74:389–396. doi: 10.18071/isz.74.0389. [DOI] [PubMed] [Google Scholar]
- 34.Pineda E., Singh J., Pineda M.V., Umanzor J.G., Baires F., Benitez L.G., et al. Impact of fluvoxamine on outpatient treatment of COVID-19 in Honduras. medRxiv. 2022 doi: 10.1101/2022.09.27.22280428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seftel D., Boulware D.R. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab050. ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishikawa M., Ishiwata K., Ishii K., Kimura Y., Sakata M., Naganawa M., et al. High occupancy of sigma-1 receptors in the human brain after single oral administration of fluvoxamine: a positron emission tomography study using [11C]SA4503. Biol Psychiatry. 2007;62:878–883. doi: 10.1016/j.biopsych.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 37.National Institutes of Health https://www.covid19treatmentguidelines.nih.gov COVID-19 treatment guidelines [Internet]. 2023 [cited 21 December 2022]. Available from:
- 38.Bhimraj A., Morgan R.L., Shumaker A.H., Baden L., Cheng V.C., Edwards K.M., et al. Infectious Disease Society of America. IDSA guidelines on the treatment and management of patients with COVID-19 [Internet] https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ 2022 [cited 21 December 2022]. Available from:
- 39.World Health Organization Living guidance for clinical management of COVID-19 [Internet] https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 2021 [cited 21 December 2022]. Available from:
- 40.Marzolini C., Marra F., Boyle A., Khoo S., Back D.J. Fluvoxamine for the treatment of COVID-19. Lancet Glob Health. 2022;10:e331. doi: 10.1016/S2214-109X(21)00592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puzhko S., Aboushawareb S.A.E., Kudrina I., Schuster T., Barnett T.A., Renoux C., et al. Excess body weight as a predictor of response to treatment with antidepressants in patients with depressive disorder. J Affect Disord. 2020;267:153–170. doi: 10.1016/j.jad.2020.01.113. [DOI] [PubMed] [Google Scholar]
- 42.Khan A., Schwartz K.A., Kolts R.L., Brown W.A. BMI, sex, and antidepressant response. J Affect Disord. 2007;99:101–106. doi: 10.1016/j.jad.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 43.Sattar N., Valabhji J. Obesity as a risk factor for severe COVID-19: summary of the best evidence and implications for health care. Curr Obes Rep. 2021;10:282–289. doi: 10.1007/s13679-021-00448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng J., Pullenayegum E., Marshall J.K., Iorio A., Thabane L. Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: a simulation study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren Y., Lin L., Lian Q., Zou H., Chu H. Real-world performance of meta-analysis methods for double-zero-event studies with dichotomous outcomes using the Cochrane database of systematic reviews. J Gen Intern Med. 2019;34:960–968. doi: 10.1007/s11606-019-04925-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.