Abstract
Green chemistry has been a growing multidisciplinary field in recent years showing great promise in biomedical applications, especially for cancer therapy. Chitosan (CS) is an abundant biopolymer derived from chitin and is present in insects and fungi. This polysaccharide has favorable characteristics, including biocompatibility, biodegradability, and ease of modification by enzymes and chemicals. CS‐based nanoparticles (CS‐NPs) have shown potential in the treatment of cancer and other diseases, affording targeted delivery and overcoming drug resistance. The current review emphasizes on the application of CS‐NPs for the delivery of a chemotherapeutic agent, doxorubicin (DOX), in cancer therapy as they promote internalization of DOX in cancer cells and prevent the activity of P‐glycoprotein (P‐gp) to reverse drug resistance. These nanoarchitectures can provide co‐delivery of DOX with antitumor agents such as curcumin and cisplatin to induce synergistic cancer therapy. Furthermore, co‐loading of DOX with siRNA, shRNA, and miRNA can suppress tumor progression and provide chemosensitivity. Various nanostructures, including lipid‐, carbon‐, polymeric‐ and metal‐based nanoparticles, are modifiable with CS for DOX delivery, while functionalization of CS‐NPs with ligands such as hyaluronic acid promotes selectivity toward tumor cells and prevents DOX resistance. The CS‐NPs demonstrate high encapsulation efficiency and due to protonation of amine groups of CS, pH‐sensitive release of DOX can occur. Furthermore, redox‐ and light‐responsive CS‐NPs have been prepared for DOX delivery in cancer treatment. Leveraging these characteristics and in view of the biocompatibility of CS‐NPs, we expect to soon see significant progress towards clinical translation.
Keywords: chitosan, drug resistance, gene therapy, stimuli‐responsive nanocarriers, synergistic therapy
Abbreviations
- AA
acrylic acid
- ABC
ATP‐binding cassette
- AGO
amine‐functionalized GO
- BSA
bovine serum albumin
- CHOL
cholesterol
- CMC
carboxymethyl CS
- CSO
CS oligosaccharide
- CXB
celecoxib
- DCA
deoxycholic acid
- DOX
doxorubicin
- FA
folic acid
- GA
glycyrrhetinic acid
- GO
graphene oxide
- GSH
glutathione
- HA
hyaluronic acid
- HSPC
hydrogenated soy phosphatidyl choline
- IA
itaconic acid
- miRNA
microRNA
- M‐MSNs
magnetic mesoporous silica nanoparticles
- MOFs
metal organic frameworks
- OA
oleanolic acid
- P‐gp
P‐glycoprotein
- PHA
pheophorbide A
- PTX
paclitaxel
- RAPA
rapamycin
- ROS
reactive oxygen species
- SA
stearic acid
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- SOC
N‐succinyl‐N′‐octyl chitosan
1. INTRODUCTION
Cancer treatment requires development of therapeutic strategies for minimizing growth and migration of tumor cells to improve overall survival of patients. For exerting such activities, antitumor compounds should be effectively internalized by cancer cells and induce a therapeutic effect at the cellular level by affecting the molecular pathways and mechanisms responsible for organelle organization and function, such as mitochondria and endoplasmic reticulum. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 Due to advancement in the fields of medicinal chemistry, various antitumor compounds, namely cisplatin, paclitaxel, docetaxel, and doxorubicin (DOX) among others, have been developed in cancer therapy. 9 , 10 Chemotherapy is currently a first‐line option for the treatment of cancer patients to eradicate tumor progression and improve prognosis. Furthermore, chemotherapy is preferred to surgery, as it is a noninvasive strategy in cancer treatment. However, some of the cancers are inherently resistant to chemotherapy, or attained drug resistance during the treatment affecting antitumor agent or its target. As chemoresistance threatens the life of many people around the world, there have been incessant efforts in understanding underlying factors in this process. The drug resistance is a multifactorial condition and each factor can independently participate in decreasing cytotoxicity of antitumor agent. The enhanced drug efflux, diminution in drug uptake, mutation, drug inactivation, apoptosis machinery impairment, signaling networks (upregulation of tumor‐promoting factors and downregulation of tumor‐suppressor), and phenotype switching are the mechanisms that can lead to cancer drug resistance. 11 , 12 , 13 , 14 Given the importance of drug resistance in chemotherapy failure, scientists have followed some strategies for overcoming this condition by applying nanostructures that improve drug delivery potential, enhance intracellular accumulation, and provide targeted delivery and co‐delivery with other antitumor agents or nucleic acid therapeutics. 15 , 16
The aim of present review is to discuss the role of chitosan (CS) for the delivery of DOX as one of the most well‐known chemotherapeutic agents in cancer therapy and introducing CS chemistry, structure, and potential applications in medicine. The function of DOX in cancer suppression, factors responsible for its resistance and role of nanoparticles in reversing DOX resistance are discussed with emphasis on CS‐based nanostructures for its delivery; pH‐ and redox‐sensitive assorted CS nanoparticles are highlighted including their use for co‐delivery of DOX with antitumor agents and nucleic acid therapeutics. Finally, the modification of various nanoparticles and appropriate solutions for their clinical applications are described to shed a light on the deployment of these nanostructures for cancer chemotherapy.
2. CHITOSAN: CHEMISTRY AND BIOMEDICAL APPLICATION
The green technology is one of the newest and most recent approaches for the development of nanopharmaceuticals in treatment of diseases. The green chemistry approach utilizes compounds and agents derived from nature for synthesis and modification of nanocarriers to improve their characteristics and make them better options for disease treatment. In this strategy, the hazardous material application is avoided and in turn, safe, biorenewable and biocompatible agents isolated from nature are utilized to develop nanoparticles. Since green‐based nanocarriers demonstrate good safety profile and biocompatibility, the way for their clinical application is paved. The delivery of drugs and nucleic acid therapeutics is essential in cancer therapy due to their low accumulation at tumor site and emergence of drug resistance; hence, green synthesis or green modification of nanoparticles can be beneficial in this case for improving efficacy in cancer therapy. 17 , 18 , 19 In the present review, our aim is to highlight greener modifications of nanoparticles with CS as a natural compound to show its potential for cancer chemotherapy and possible clinical applications in the near future.
After cellulose, chitin is the most abundant natural polymer 20 and CS is derived from chitin (Figure 1), 22 an essential component comprising shells of insects, crustaceans, and cell walls of fungi. 23 , 24 , 25 The annual production of chitin is estimated to be 10–100 Gt and the commercialized chitin/CS can result from seafood waste in which α‐ and β‐chitins are derived from shells of crab and shrimp, while CS is prepared by deacetylation of chitin. 23 , 24 , 26 , 27 , 28 The amount of deacetylation seems to be more than 60% in commercialized CS where Japan is considered as the major producer of CS. Based on the estimates, the value of CS market has been ~6.8 B$ in 2019. 20 The unique chemical structure of CS has made it a suitable option for biomedical and engineering applications. The most important feature of CS is its great solubility in aqueous solution due to the presence of amino groups at C2 position. In aqueous acidic solvents, CS undergoes protonation to generate NH3 + that is beneficial in the design of nanoarchitectures and their synthesis via bottom‐up approach. Importantly, amino and acetylamino groups in CS are main sources of nitrogen for generating fertilizers and N‐doped carbon materials for deployment as catalyst. 20 , 29
FIGURE 1.
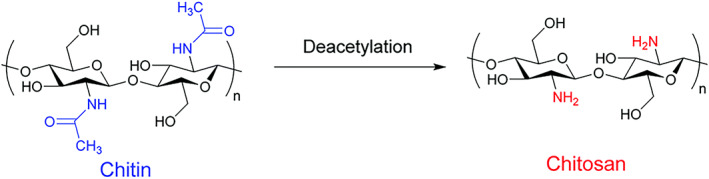
Chemical structures of chitin and CS via deacetylation.Source: Reprinted with permission from Ref 21
The application of CS in industry has demonstrated potential in reducing environmental pollution as a biodegradable and renewable abundant material that should not be discarded in to scarce landfills. The aim of green chemistry is to limit industrial production of hazardous compounds and prevent destructive impacts, both short‐term and long‐term, on ecosystem. 30 , 31 , 32 Besides, green chemistry is beneficial in decreasing energy consumption and substituting conventional solvents with newer options that are renewable and demonstrate low destructive impact on environment. 33 The precursor of CS, chitin is made of up to 3000 repeating units comprising N‐acetyl‐d‐glucosamine 34 that are interconnected via β(1 → 4) glycosidic bonds. The chitin exhibits high similarity to cellulose in terms of chemical structure with the difference that hydroxyl group at position C2 is substituted by acetamido group. 35 There are various kinds of chitin including α, β, and γ, which show variation in hydration, size, and number of chains 36 and are present in various structures and sources. For instance, α‐chitin is found in shells and cell walls, β‐chitin is present in endocycleton of squid pens 36 , 37 and γ‐chitin is observed in stomach lining of squid and cuttlefish. 36
There are two saccharides, namely, N‐acetyl‐d‐glucosamine and β 1–4 d‐glucosamine in the CS structure and during the deacetylation of chitin, N‐acetyl‐d‐glucosamine monomers are transformed into d‐glucosamine to generate CS. The LD50 of CS is 16 g/kg body weight and it shows a great safety profile. The various kinds of CS are categorized based on molecular weight and deacetylation degree 38 ; CS is a polycation and its charge density is determined by pH and deacetylation degree including the solubility aspects. The CS oligomers display solubility in acidic and basic media but with increase in its molecular weight, it is only soluble in acidic media even with a higher deacetylation degree. Consequently, significant efforts have been made in synthesizing CS derivatives that are soluble under neutral and basic pH conditions by altering acetylation, polymerization, and quaternization 39 ; pKa value is suggested to be 6.5 and protonation of —NH2 groups provides CS solubility in acidic media 40 as has been affirmed that protonation of 50% of amine groups leads to CS solubility. 41 The molecular weight and deacetylation degree determine the viscosity of CS and reduction in molecular weight significantly diminishes CS's viscosity. 41 , 42 , 43 The biocompatibility and biodegradability are other beneficial characteristics of CS. 44 , 45 , 46
The biomedical application of CS nanostructures 47 has garnered much attention in recent years, especially in cancer therapy, which has been investigated in detail; CS nanoparticles (CS NPs) can mediate drug and nucleic acid therapeutic delivery, 48 chemotherapy, 49 phototherapy, 50 and imaging in cancer treatment. 51 The redox‐sensitive micelles with carboxymethyl CS decoration can be utilized for NIR imaging of liver cancer cells and simultaneously, photo‐ and chemo‐therapy. 49 , 52 Another study evaluated the potential of gold (Au)‐embedded CS nanostructures for delivery of drugs in a pH‐sensitive manner and providing fluorescence imaging. 53 Notably, the surface modification of CS nanostructures is of importance for nucleic acid therapeutic delivery and synthesizing vectors that can form stable complexes with genetic tools. In a recent study, CS‐Au nanostructures have been applied in photoacoustic imaging‐guided nucleic acid therapeutic delivery and photothermal therapy to exert a synergistic impact for cancer suppression. 54 The CS can be utilized for the development of pulmonary drug delivery systems. Notably, CS‐based nano‐scale delivery systems for pulmonary delivery are of importance in treatment of related cancers such as lung tumor. Furthermore, it can be used in treatment of infectious diseases such as COVID‐19 that is inflicting nowadays. 55 , 56 , 57 , 58 , 59 , 60 The CS‐based nanostructures have demonstrated great efficacy in prolonged delivery of drugs 61 , 62 that is of utmost importance in cancer therapy. Due to CS's positive charge, it can easily form complexes with negatively charged nucleic acids. A recent study has exploited CS‐hyaluronate‐SPION nanoparticles for the delivery of siRNA and EP4 antagonist in cancer therapy; these nanoparticles effectively suppress HIF‐1α/EP4 axis in impairing growth and invasion of tumor cells. 63 Furthermore, CS NPs can mediate pulmonary delivery of CRISPR/Cas9 system, as a new emerging genetic tool. 56 Besides cancer therapy, CS has a therapeutic impact in alleviating osteoarthritis 64 and, recently, a hydrogel system based on lactate‐modified CS has been prepared for osteoarthritis treatment due to its antioxidant activity, where it exhibits high biocompatibility. 65 Furthermore, CS‐based complexes can ameliorate osteoarthritis by enhancing proliferation rate of chondrocytes and preventing apoptosis. 66 , 67 In another study, CS NPs were used for the delivery of rosuvastatin to decrease cholesterol levels upon atherosclerosis treatment and to prevent the development of cardiovascular diseases. 68 Further, CS NPs can be utilized in anti‐inflammatory formulations by delivery of drugs such as diclofenac sodium 69 and dexamethasone, 70 among others. Overall, studies highlight the fact that CS‐based NPs demonstrate good biomedical applications. 71 , 72 , 73 , 74 , 75 , 76 The following sections explore the role of CS NPs in the delivery of anticancer drug—doxorubicin (DOX).
3. DOXORUBICIN: MECHANISM OF ACTION AND RESISTANCE
The DOX is an anthracycline antibiotic derived from Streptomyces peucetius caesius with high antitumor activity 77 , 78 , 79 as it displays efficacy even at low doses in suppressing different neoplasms. 80 The animal experiments evaluating anticancer activity of DOX have affirmed its potential in minimizing tumor progression and improving survival of animal models. 81 Antisuppressive activity of DOX has been successfully demonstrated in preclinical models and clinical trials on various cancers, including leukemia, lymphoma, sarcoma, and urogenital cancers, among others. 80 , 82 DOX mainly targets genetic components in nucleus and mitochondria inhibiting the cell growth and division. However, DOX alone is not cell‐selective and affects also the function of healthy cells. This antitumor agent is capable of intercalating with DNA to prevent DNA replication and protein synthesis. Furthermore, DOX stimulates DNA damage in tumor cells by preventing the activity of topoisomerase II enzymes. Additional investigations revealed that DOX enhances the production of reactive oxygen species (ROS) to induce DNA damage and destroy cell membrane via direct interaction. 83 , 84 , 85 , 86
However, DOX resistance evolution in tumor cells is considered a major challenge as various underlying molecular pathways and mechanisms are responsible for the development of DOX resistance. 87 , 88 , 89 The breast tumor is a heterogeneous cancer with different subtypes and its incidence rate is various based on geographical differences. Although chemotherapy is used for breast cancer treatment, its therapy is still a challenge. 90 The lncRNA H19 is involved in triggering DOX resistance in breast tumor (in vitro and in vivo) via PARP1 downregulation. 91 The lncRNA TUG1 decreases miRNA‐9 expression via sponging to induce DOX resistance in breast cancer. 92 The circRNA‐0002060 mediates the DOX resistance in osteosarcoma via miRNA‐198 downregulation and subsequent increase in the expression level of ABCB1. 93 The TCF4 and EIF5A2 are other molecular pathways that are affected in cancer cells to regulate DOX chemotherapy response. 93 , 94 However, antitumor agents, such as trabectedin and resveratrol among others, have shown potential in reversing DOX resistance. 95 , 96
Different studies provide novel insights and pathways for development of DOX resistance. The DOX resistance in osteosarcoma can be mediated by TCF4 overexpression. In this case, circ‐0001721 promotes TCF4 expression via miRNA‐758 downregulation to induce DOX resistance. 94 Another experiment reveals that circATXN7 increases HOXA11 expression via miRNA‐149‐5p downregulation to increase breast cancer progression and to inhibit DOX resistance. 97 Furthermore, EMT is responsible for cancer metastasis 98 and its induction can lead to DOX resistance in tumor cells. Therefore, targeting these pathways can effectively suppress DOX resistance in cancer. For instance, silencing RNF8, 99 CIP2A, 100 and miRNA‐21 101 can inhibit DOX resistance in various tumors and impairs their proliferation. A more advanced strategy in reversing DOX resistance is the application of nanoparticles for targeted delivery of DOX at the tumor site and co‐delivery of DOX with other antitumor agents or nucleic acid therapeutics. 88 , 102 , 103 , 104
4. NANOSTRUCTURES OF CHITOSAN‐DOXORUBICIN
4.1. Stimuli‐responsive nanocarriers
4.1.1. pH‐responsive
The tumor microenvironment has a mild acidic pH (approximately 6.5) that is lower than physiological condition and such difference in pH level can be exploited for drug release at tumor site; functionalization of nanomaterials and their drug conjugates can lead to release at this mild acidic pH. Based on this system, the bond between the material and the drug degrades under acidic pH of tumor site to mediate drug release. 105 Various kinds of bonds including imine, hydrazone, oxime, amide, acetals, and orthoester can be deployed for synthesizing pH‐sensitive nanocarriers and drug release at tumor site 106 as has been discussed in this section. Figure 2 provides a schematic representation of stimuli‐responsive CS‐based nano‐scale delivery systems in cancer therapy.
FIGURE 2.
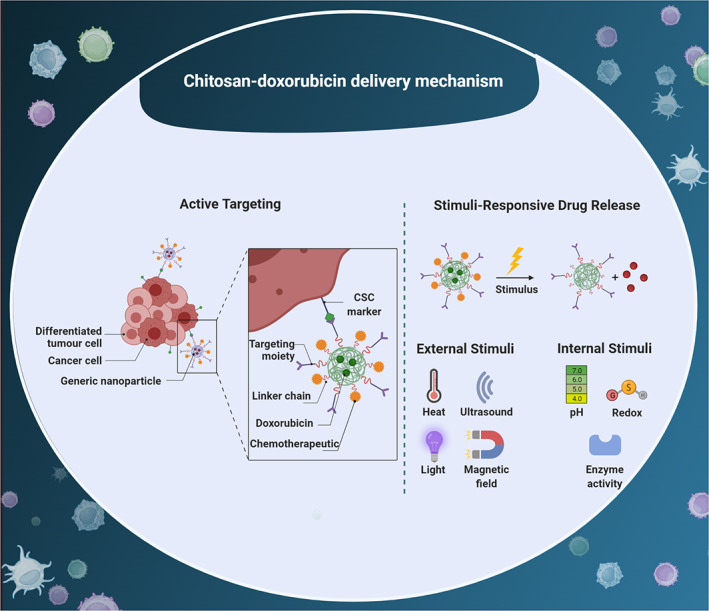
Active targeting and stimuli‐responsive drug release of CS‐doxorubicin nanocarrier. The surface modification of CS‐based nanostructures with ligands can increase their internalization in tumor cells. Besides, External and internal stimuli can be utilized for developing smart nanocarriers in cancer therapy. CSC, cancer stem cell
Recently, a CS‐based polymeric prodrug, that is pH sensitive and can provide a platform for co‐delivery of DOX and siRNA, has been synthesized. The DOX and Bcl‐2‐siRNA can be conjugated to CS‐modified polymeric nanoparticles and then, synergistic cancer therapy is provided. These nanostructures are internalized by hepatoma cells via glycyrrhetinic acid receptor‐mediated endocytosis. After 10 h, nanostructures can release siRNA and DOX as much as 90.2% and 81.3%, respectively. This CS‐based polymeric prodrug efficiently suppresses tumor progression (HepG2 cells) by 88% via mediating both chemo‐ and nucleic acid‐therapy. 107
The CS is a pH‐sensitive agent due to the presence of amine groups (—NH2) that undergoes protonation in acidic pH 108 , 109 ; higher pH significantly decreases the solubility of CS. 109 On the other hand, polyvinylpyrrolidone (PVP) is often utilized for the synthesis of nanoparticles, but it significantly decreases the initial burst release. 110 For overcoming such issues, the combination of PVP and CS has been suggested to improve the solubility of CS at high pH levels and mechanical characteristic of PVP, simultaneously. 109 , 110 , 111 A recent study demonstrated that CS/PVP/α‐Fe2O3 nanocomposites for the delivery of DOX, where nanoparticles had a spherical structure, and they could load Fe2O3 in CS/PVP. The nanostructures showed a particle size of 247 nm and due to conjugation of α‐Fe2O3, they demonstrated prolonged release and increased retention of DOX at tumor site. The CS/PVP‐based nanocomposites release DOX in a pH‐sensitive manner, mimicking pH level of tumor microenvironment and they induced apoptosis to significantly decrease the viability of breast cancer (MCF‐7 cells). 112
There are several reasons for using pH‐sensitive nanocarriers for cancer therapy and also delivery of chemotherapeutic agents. The nonspecificity of systemic chemotherapy can negatively affect normal and healthy cells after intravenous injection. 113 , 114 Besides, repeating injections can lead to pain, infection, and hospitalization. Therefore, the application of nanoparticles for sustained delivery of chemotherapeutic agents eliminates the need for repeated injections and can improve the chance in the fight against cancer. 114 , 115 , 116 Due to glycolysis phenomenon in the tumor microenvironment and conversion of glucose to lactate, pH level is significantly diminished, which is beneficial for cancer progression. 117 , 118 A recent experiment has advanced graphene‐CS nanocomposites for DOX delivery, which were stabilized with bovine serum albumin (BSA); the presence of BSA is beneficial in preventing burst release of drug from CS nanocomposites. They show uniform release over 24 h and can release drug for up to 28 days (84% of drug). Such prolonged release of DOX from CS‐decorated nanocomposites that is pH‐sensitive can improve cytotoxicity against cancer cells. 119 In another study, a composite structure consists of halloysite nanotubes as a natural aluminosilicate ceramic and CS was designed for pH‐responsive release of DOX for breast cancer therapy. The drug‐loaded carrier showed a sustained release in cell lysate and the mechanism of action was to penetrate into mitochondria followed by inducing damage. Moreover, the IC50 of nanocarrier against MCF‐7 cells was found 1.17 μg ml−1 lower than that of free DOX (2.43 μg ml−1). The in vivo studies revealed that the tumor inhibition ratio of the nanocarrier was 83.5%, whereas the free DOX showed 46.1%. Notably, the treated mice with the DOX‐loaded carrier survived over 60 days without a significant systemic cytotoxicity (Figure 3). 120
FIGURE 3.
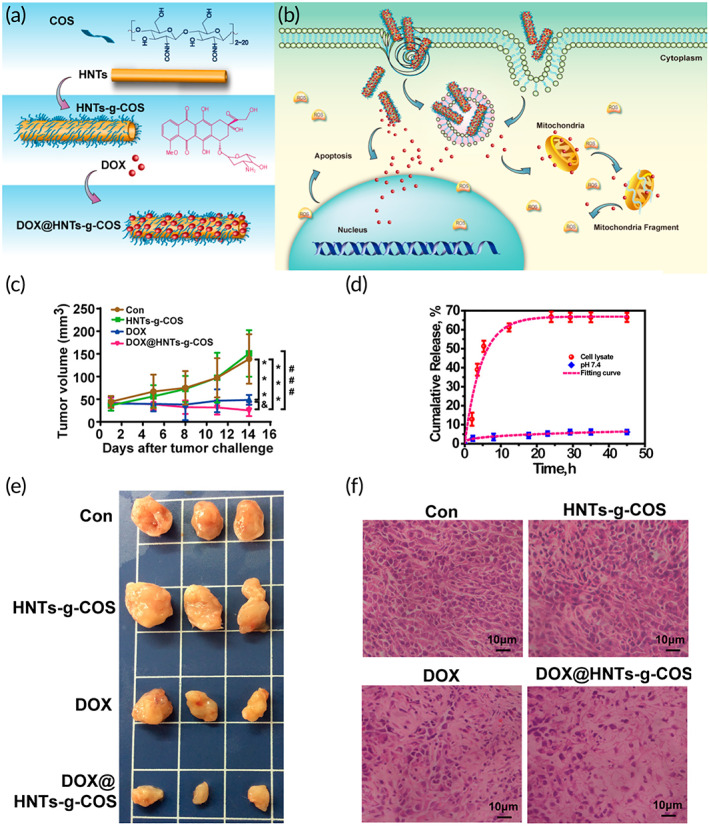
A pH‐responsive CS carrier in combination with halloysite nanotubes for breast cancer therapy. (a,b) The synthesis procedure followed by loading of DOX molecules on the chitosan (COS)‐halloysite nanotubes (HNTs) and the uptake process by which the drug‐loaded carrier induce cytotoxicity toward cancerous cells. (c) Tumor volume changes after being treated with different samples including control (CON), free DOX, unloaded carrier, and DOX‐loaded carrier; significant difference with the control group ***P < 0.001, unloaded carrier at ###P < 0.001, and DOX at &P < 0.01. (d) The drug release from the carrier at different pH. (e) The excised tumors removed at the end of treatment after being treated with different samples. (f) The histology that was accomplished through H&E staining on the 14th day of treatment. Source: Reprinted from Ref 120 with permission from ACS
The UV‐triggered injectable CS hydrogels are extensively applied in biomedicine. 121 , 122 , 123 , 124 Different UV‐crosslinkable CS derivatives have been designed via covalent attachment of UV‐responsive components 125 , 126 , 127 , 128 to improve their solubility. The “thiol‐ene” click chemistry can be utilized for the development of pH‐responsive UV crosslinkable CS hydrogel. The UV crosslinking ability and pH‐sensitive capacity of CS ensue from allyl groups on C6 site and amine groups on C2 site. At various pH levels, CS‐based hydrogels show different behaviors, and their swelling and shrinkage can lead to the release of DOX in a pH‐sensitive manner. 129 The purpose of using CS in the modification of nanoparticles is its capacity in functionalizing or loading various antitumor drugs (DOX), targeting ligands (aptamer), coating polymers and imaging probes. 130 For this purpose, Au nanoparticles were modified with CS and DNA aptamer to mediate selective targeting to glioblastoma cells. Obtained nanostructures were utilized for delivery of 5‐flourouracil (5‐FU) and DOX in glioblastoma suppression. The prepared CS‐Au NPs had a particle size of 196.2 nm with zeta ζ‐potential of 16.26 mV and they significantly enhanced the cytotoxicity of DOX and 5‐FU against glioblastoma cells and induced cell death and G0/G1 cell cycle arrest. 131 Based on these results, it can be concluded that CS is a promising agent for the synthesis of pH‐responsive nanocarriers for DOX delivery and cancer suppression 132 and can be easily functionalized by other ligands to improve the selectivity of NPs toward cancer cells, while simultaneously providing co‐delivery of DOX and other antitumor agents such as 5‐FU. By providing pH‐sensitive feature, these nanocarriers release DOX at the tumor site and also mediate sustained delivery, which are both beneficial in cancer suppression.
4.1.2. Redox responsive
The presence of redox imbalance is another unique feature of tumor microenvironment that is responsible for enhancing tumor proliferation rate. The production of ROS, initiated by inflammatory cells, endothelial cells, and cancer‐associated fibroblasts, induces an aerobic glycolysis and significantly increases tumor progression. On the other hand, glutathione (GSH) is an enzyme that regulates oxidative stress and diminishes ROS levels. In the pharmaceutical industry, there have been efforts in synthesizing redox‐sensitive nanomaterials. Preparation of such nanostructures usually involves incorporation of disulfide as it undergoes degradation by GSH. 133 , 134 This section focuses on redox‐sensitive CS‐NPs and disulfide bond decomposition by GSH at the tumor site for DOX release.
As has been discussed in the previous sections, a combination of CS with other agents is applied to synthesize NPs and improve features that are important for cargo delivery. The CS oligosaccharide (CSO) and stearic acid (SA) can be utilized for the preparation of glycolipid‐like copolymer 135 and ensued CSO‐SA copolymer has high stability that can be further exploited for drug delivery applications 136 , 137 , 138 , 139 as it exhibits high internalization and cellular uptake. 136 However, micelles prepared from CSO‐SA have a major challenge to efficiently release the drug in vitro due to slow degradation kinetics of amide bond. To improve drug release profile from CSO‐SA‐based NPs, DOX can be conjugated with CSO‐SA via disulfide bond. This approach is advantageous in synthesizing CSO‐SA‐based nanoparticles that are redox sensitive and due to higher GSH levels in cells, they release DOX to suppress breast cancer progression (MCF‐7 cells). 140 In another study, carboxymethyl CS (CMC)‐based micelles for delivery of DOX in cancer therapy were prepared. The poly‐ε‐caprolactone (PCL)‐SS‐CMC self‐assembled into micelles for improvement of their selectivity against cancer cells (liver and cervical cancers) and were modified with glycyrrhetinic acid (GA). Then, DOX and another antitumor agent known as pheophorbide A (PHA) were loaded on these NPs, which showed a release profile up to 86.3% and 92.1% of DOX and PHA, respectively, after 48 h. This approach is beneficial in enhancing intracellular accumulation of DOX and PHA by providing GA receptor‐mediated endocytosis and redox‐sensitive system. 49 Although a few experiments have evaluated the potential of redox‐sensitive CS‐based NPs for delivery of DOX, 140 they highlight the fact that disulfide bond between CS and DOX can be easily degraded in the presence of GSH. This system is biocompatible and its modification with ligands can be performed to promote its potential in DOX delivery.
4.1.3. Light responsive
A few experiments have exploited the role of CS‐based nanostructures for light‐mediated release of DOX, which deployed nanobubbles. 141 The nanobubbles have spherical core–shell structure and their surface can be conjugated with functional groups. The nanobubbles possess enhanced permeability and retention (EPR) effect that is of importance for crossing over endothelial barrier. The nanobubbles can be administered via intravenous route and in order to improve their biocompatibility and biodegradability, surface modification of nanoparticles with PLGA and PCL can be performed. 142 In an experiment, modification of nanobubbles with CS has been conducted. The CS nanobubbles can release DOX in vitro upon irradiation and significantly enhance uptake of DOX in mammalian cells. These biocompatible nanobubbles can be used for inhibiting breast tumor suppression (MCF‐7) via light‐mediated DOX delivery. 141 A light‐responsive theranostic platform composed of DOX‐loaded gold nanoparticles and CS was reported for breast cancer therapy. Applying NIR irradiation not only facilitated the payload release in the cancer cells but also caused the gold nanoparticles to elevate the inner temperature up to level, which leads apoptosis. Besides photothermal therapy, it was observed that the liberated DOX caused an oxidative stress through generation of ROS. 143 Through Figure 4, the preparation and the mechanism of action of the light‐responsive platform are indicated.
FIGURE 4.
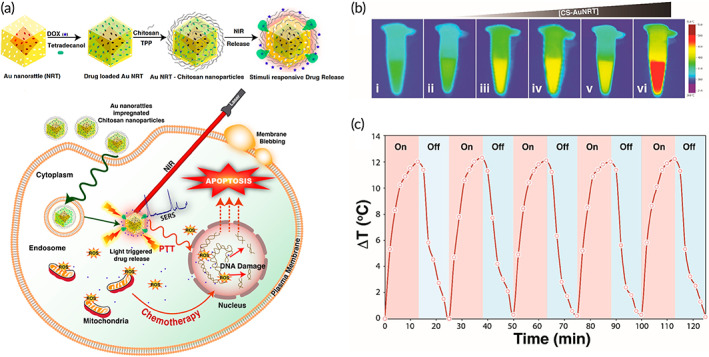
The light‐responsive DOX‐loaded gold‐chitosan nanocomposite. (a) The preparation of CS‐gold nanorattles (AuNRT), DOX loading and the effect of NIR irradiation on the payload release. (b) The thermal images related to the nanocomposite after being irradiated with 785 nm laser for 15 min with various concentrations including (i) water as the control, (ii) 75, (iii) 150, (iv) 200, (v) 500, and (vi) 800 μg ml−1. (c) Measurements of temperature increase after applying on/off cycles at a power density of 5 W/cm2. Source: Reprinted from Ref 143 with permission from ACS
4.1.4. Thermosensitive
The thermosensitive hydrogels have garnered much attention in recent years as an implantable delivery system. Thermosensitive hydrogels are injected into tumor site in a liquid state and undergo conversion into a solid gel at body temperature. Besides, hydrogels can provide the prolonged release of antitumor drugs at the tumor site. 144 , 145 , 146 A study describes the synthesis of thermosensitive CS hydrogel to encapsulate liposomal DOX with a particle size of 94.2 nm and encapsulation efficiency as much as 98%. This thermosensitive hydrogel changes to a solid gel at body temperature and provides sustained release of DOX. These hydrogels demonstrated a high safety profile and biocompatibility, and simultaneously, they showed capacity in suppressing tumor progression (H22 hepatoma cells and tumor‐bearing mice). 147 The application of CS prevents the burst release of DOX from hydrogels. Notably, CS‐DOX conjugate in hydrogels does not affect the antitumor activity of DOX and it is comparable to free DOX. Both in vitro and in vivo experiments have shown the potential of hydrogels containing CS‐DOX conjugate for cancer suppression (A549 lung cancer cells and nude mice). 148 Furthermore, loading liposomal DOX in hydrogel does not negatively affect the entrapment efficiency of liposomes. For instance, an experiment synthesized CS‐based thermosensitive hydrogel containing liposomal DOX for topical cancer treatment (hepatoma). The entrapment efficiency was 90% and after loading liposomal DOX in the hydrogel, its entrapment efficiency did not change. 149 Notably, carbon nanotube (CNT)‐CS can be loaded in thermosensitive hydrogels for controlling DOX release. Their exposure to irradiation provides a photothermal effect of CNTs that is beneficial in destroying hydrogel structure and mediating DOX delivery. 150 Therefore, a thermosensitive hydrogel can be synthesized first for conversion to solid gel at body temperature and in the next step, CS‐carbon nanotubes are loaded into the hydrogel for regulating DOX release upon irradiation. 150 The succeeding section focuses on multisensitive CS‐based nanocarriers for DOX delivery.
4.1.5. Multiresponsive
There have been many efforts in developing multifunctional CS‐based NPs for DOX delivery in cancer treatment including nanocomposites that are triple sensitive. For preparing such systems, CS is utilized as a pH‐sensitive agent, g‐poly(N‐vinylcaprolactam) (PNVCL) as a thermosensitive agent and H6R6 as a cell‐penetrating peptide. Then, DOX and oleanolic acid (OA) are loaded on nanocomposites with a particle size of 190 nm, the loading efficiency being 13.2% and 7.3% for DOX and OA, respectively. These nanocomposites are accumulated in the tumor microenvironment and the drug is released at the tumor site. The in vitro and in vivo experiments demonstrated the potential of DOX and OA‐loaded CS/PNVCL/H6R6 nanocomposites in suppressing cancer progression and apoptosis induction (SKOV3 ovarian cancer cells and nude mice). 151 Another strategy for improving the physicochemical properties of CS exploited its conjugation with PEG, which significantly enhances solubility and biocompatibility. The hollow mesoporous silica NPs have been modified with CS and PEG with loading efficiency of 32.8%. The DOX is loaded in PEF‐CS‐silica nanoparticles and there is no release of DOX at low levels of GSH and pH 7.4. However, a mild acidic pH or higher levels of GSH can induce DOX release in breast cancer suppression. 152 Another study prepared alginate/CS‐based NPs for delivery of DOX that are pH‐ and light‐responsive where alginate improved stability of CS‐based NPs and could release DOX in pH‐ and light‐sensitive manner after irradiation and in mildly acidic pH (Figure 5). 153 Based on these studies, CS and its combination with other agents can be beneficial in the development of multifunctional nanostructures for DOX delivery (Table 1). 159 , 160 , 161
FIGURE 5.
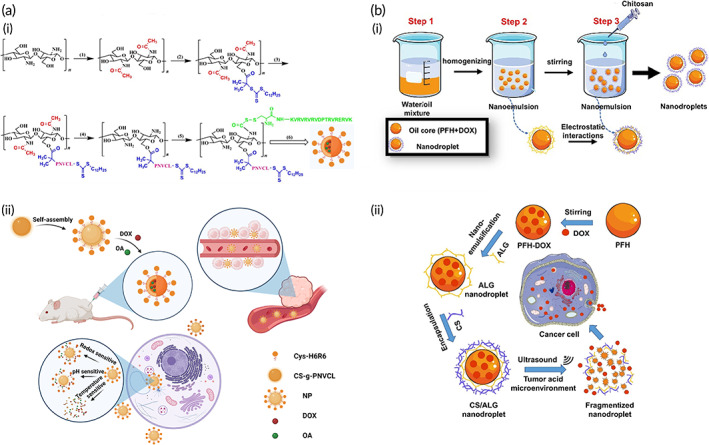
Multiresponsive CS‐NPs for DOX delivery. (a) (i) Step‐by‐step synthesis of multiresponsive (pH, thermo, and redox responsive) DOX/(oleanolic acid [OA[)@functionalized cell‐penetrating peptide (H6R6)‐chitosan (CS)‐g‐poly(N‐vinylcaprolactam) (PNVCL) NPs. (ii) The applicability of nanoparticles for anticancer therapy; the improved permeation and retention leads the NPs to remain in the tumor environment followed by triggering triple sensitivity to release doxorubicin (DOX) and OA. Source: Reprinted from Ref 151 with permission from Elsevier. (b) (i) Synthesis of DOX‐loaded alginate (ALG)/chitosan (CS) stabilized perfluorohexane (PFH) NPs through nano‐emulsion technique. (ii) Double sensitivity (ultrasound and pH) of the DOX‐loaded nanodroplets against cancer cells. Source: Reprinted from Ref 153 with permission from Elsevier
TABLE 1.
The application of stimuli‐responsive CS‐based nanocarriers for DOX delivery.
| Nanovehicle | Stimulus response | Particle size (nm); zeta potential (mV); encapsulation or loading efficiency (%) | Antitumor agent | Remarks | References |
|---|---|---|---|---|---|
| Polymeric nanoparticles | pH‐ and thermo‐sensitive | 190 nm; 13.2% for DOX and 7.3% for oleanolic acid | Doxorubicin; oleanolic acid |
CS provides pH‐sensitive feature and PNVCL leads to thermosensitive feature Accumulation at the tumor site and suppressing ovarian cancer progression Synergistic impact between antitumor compounds |
151 |
| PEG‐ and CS‐conjugated hollow mesoporous silica nanoparticles | Redox‐ and pH‐sensitive | 230 nm; −11.5 mV | Doxorubicin |
Addition of 10 mM of GSH induces DOX release from nanostructures DOX release at mild acidic pH High stability and biocompatibility |
152 |
| Alginate/CS‐stabilized nanoparticles | Ultrasound‐ and pH‐responsive | 73.3–132.7 nm; 6.41 mV (Step 1), −62.13 mV (Step 2) an d −26.83 mV (Step 3) | Doxorubicin |
High biocompatibility Decreasing survival of tumor cells, showing their cytotoxicity |
153 |
| GA‐functionalized CS‐based micelles | Redox sensitive | 122.4 nm | Doxorubicin; pheophorbide A |
Increased cellular uptake by HepG2 cells due to GA modification Providing both photo‐ and chemo‐therapy in cancer suppression |
49 |
| CS‐modified Fe3O4/rGO nanocomposites | pH‐sensitive | ‐ | Doxorubicin | Promising drug delivery systems for DOX and their modification with folic acid remarkably elevates the accumulation of DOX in cancer cells | 154 |
| Aptamer‐functionalized CS‐gold nanoparticles | pH sensitive | 196.2 nm; 16.26 mV; up to 96% | Doxorubicin; 5‐fluorouracil |
Inducing cell cycle arrest at G0/G1 phase Cell death induction Impairing progression of glioblastoma pH‐sensitive release of DOX and 5‐FU in synergistic tumor ablation |
131 |
| CS‐mesoporous silica nanoparticle | pH‐sensitive | 155 nm;−1.66 mV | Doxorubicin |
CS functions as a gatekeeper and covers surface of MSN DOX release at the tumor site Suppressing cancer progression in vitro and in vivo Apoptosis induction and decreasing proliferation rate |
155 |
| Nanomicelle | pH‐sensitive | 133.52 nm; 13.5 mV; more than 80% | Doxorubicin; quercetin |
Providing lysosomal escape by CS‐based micelles DOX and quercetin release at the cytoplasm of breast cancer cells |
156 |
| CS‐tripolyphosphate nanostructures | pH‐sensitive | ‐ | Doxorubicin |
Apoptosis induction Cell cycle arrest at G2/M or S phase Increasing DOX uptake by cervical cancer cells |
157 |
| CS‐poly (N‐isopropylacrylamide)‐coated mesoporous silica nanoparticles | pH‐ and thermo‐sensitive | 70 nm | Doxorubicin |
High biocompatibility and stability Suppressing progression of HeLa cells |
158 |
| CS nanobubbles | Ultrasound‐responsive | 641 nm; +67.12 mV; 54.18% | Doxorubicin | Ultrasound induces the release of DOX from CS‐modified nanobubbles to suppress breast cancer progression | 141 |
Abbreviations: CS, chitosan; DOX, doxorubicin; 5‐FU, 5‐fluorouracil; GSH, glutathione; GA, glycyrrhetinic acid; GO, graphene oxide; MSN, mesoporous silica nanoparticle; PNVCL, poly(N‐vinylcaprolactam).
Besides internal‐responsive CS‐NPs for DOX delivery, there is another category termed hyperthermia‐based external‐stimuli drug delivery systems. These systems are responsive to an external stimulus, like magnetic field and light capable of increasing the temperature of the tumor microenvironment followed by killing the cancerous cells. 162 Moreover, after being triggered by the mentioned stimuli agents, the anticancer drug release rate undergoes a significant increase thus improving the efficiency of the delivery system. 163 This strategy has been exemplified by a multifunctional chemo‐phototherapeutic delivery system where black phosphorus nanosheets have been adopted as an inorganic light‐responsive material; the DOX has been first loaded onto the nanosheets followed by being surface modified with CS‐PEG and folic acid to form the final platform (BP‐DcF). Next, small interfering RNA and programmed death‐ligand 1 (PL) are encapsulated into the delivery system. Upon irradiation of near‐infrared, a hyperthermia effect and burst release of DOX could be observed and the combination of both chemo and phototherapies culminated in an effective tumor cells apoptosis (Figure 6). 164
FIGURE 6.
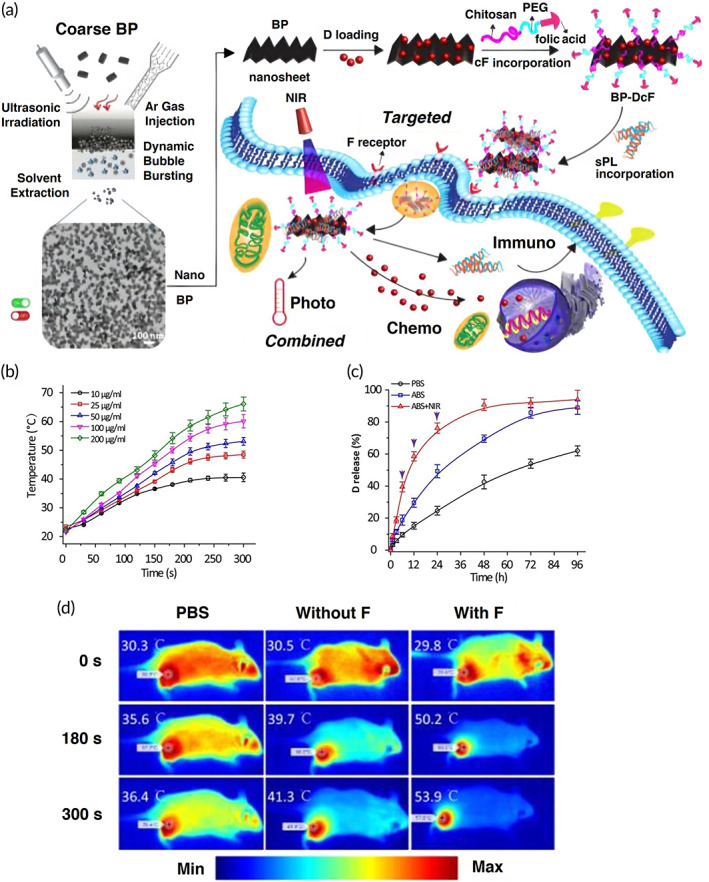
Combination of chemo and photothermal therapies for cancer therapy. (a) An illustration of synthesis of black phosphorus (BP) nanosheets followed by being modified with doxorubicin (D)‐chitosan‐PEG‐folic acid (cF) and small interfering RNA and programmed death ligand 1 (sPL) to form a multifunctional platform called BP‐DcF for cancer therapy. (b) Assessment of temperature increase when the NPs were being exposed to near‐infrared irradiation (808 nm, 1.5 W/cm2) for 5 min in a concentration‐dependent manner. (c) The D release profiles from the NPs in different media including acetate‐buffered saline (ABS) pH 5.0 and phosphate‐buffered saline (PBS) pH 7.4. In the case of ABS + NIR, there are some black arrows representing the applied infrared in those time intervals. (d) The photothermal therapy (808 nm, 1.5 W/cm2, 5 min) applied in the tumor's sites treated with two samples including without F (Cy5.5‐labeled BP‐Dc@s) and with F (BP‐DcF@s) plus the control group (PBS). Source: Reprinted from Ref 164 with permission from ACS
Overall, some technical conclusions about CS‐based nanocarriers for DOX delivery can be provided. Due to protonation of amine groups of CS at acidic pH that is similar to tumor microenvironment pH, the pH‐sensitive release of DOX occurs upon CS modification of nanocarriers. Notably, by loading some kinds of nanoparticles such as Fe3O4 in CS‐based nanocarriers, multifunctional nanoarchitectures are designed that can provide stimulus‐responsive release of DOX and simultaneous imaging.
4.2. Reversing drug resistance
There are several reasons responsible for role of NPs in reversing chemoresistance. Frequent application and high doses of chemotherapeutic agents can result in drug resistance development and in targeted delivery, a low amount of anticancer agent is loaded that reduces chance of drug resistance, while it maintains tumor‐suppressor activity. 165 , 166 , 167 Additionally, increasing accumulation of chemotherapeutic agents in tumor cells prevents drug resistance and NPs are promising in promoting cellular uptake of anticancer agents. There have been some efforts in application of CS‐based nanostructures for suppressing DOX resistance; diverse applied strategies are reviewed in this section.
Preventing drug efflux can be beneficial in reversing drug resistance. One of the factors involved in mediating drug efflux is P‐glycoprotein (P‐gp), as a transmembrane transporter belonging to the family of ATP‐binding cassette (ABC) transporters. The enhanced activity and expression of P‐gp are responsible for inducing the drug resistance. 13 , 167 In this case, CS‐g‐d‐α‐tocopheryl polyethylene glycol 1000 (TPGS) nanostructures have been developed for DOX delivery. The nanoparticles are synthesized using a modified solvent extraction/evaporation method mixed with ionic cross‐linking. The ensuing nanostructures showed a particle size of 140–180 nm with loading efficiency of 40% for DOX and being pH‐sensitive, they could release DOX at the tumor site in response to mild acidic pH of the tumor microenvironment. The reason for using TPGS is its potential to inhibit P‐gp activity. Therefore, CS‐g‐TPGS NPs suppress P‐gp activity and significantly decrease the ATP levels that are beneficial in preventing DOX efflux. Furthermore, they provide an increase in internalization of DOX for apoptosis induction and decrease the survival of tumor cells (liver and breast cancers). 168 These mechanisms participate in suppressing DOX resistance in cancer cells.
To investigate efficacy of CS NPs in preventing the progression of DOX‐resistant cancer cells, a study developed CS‐dextran sulfate‐coated PLGA‐PVA NPs for DOX delivery and inhibiting the progression of DOX‐resistant breast tumor cells; spherical NPs showed high stability with ζ‐potential of +2.89 mV. The double coating of PLGA‐PVA nanostructures with CS and dextran sulfate is beneficial in inducing cytotoxicity against breast cancer cells (MCF‐7 cells); these ensued NPs significantly enhanced DOX uptake in MCF‐7 cells. Furthermore, they induced apoptosis, DNA damage and S cell cycle arrest, while they reduced invasion of parental cells that are beneficial in suppressing DOX resistance. The upregulation of Bax, PARP1, p21, and p23 has been observed upon using DOX‐loaded CS‐dextran sulfate‐coated PLGA‐PVA nanoparticles. 169 The deployment of CS promotes the stability of NPs and pH‐sensitive capacity that are both vital for overcoming DOX resistance. 170 Therefore, CS‐NPs can effectively suppress DOX resistance, 171 , 172 , 173 and based on the studies, apoptosis induction, cell cycle arrest, inhibiting migration, promoting cellular uptake, preventing drug efflux via P‐gp down‐regulation and affecting tumor‐promoting and tumor‐suppressor pathways can be followed.
4.3. Doxorubicin and gene delivery
Gene therapy is a new emerging field for disease management and it can be considered as an option for treatment of diseases that are incurable with conventional medicines. 174 , 175 Various nucleic acid drugs have been developed for purpose of gene therapy and have shown promising results. 176 However, gene therapy has its own drawbacks and naked genetic tools show limited efficacy in disease treatment, especially cancer. Thus, there is a need for developing nanoscale delivery systems for the delivery of genes. Furthermore, nanoarchitectures can provide co‐delivery of genes and drugs for synergistic disease therapy that is common in cancer treatment. 177 , 178 , 179 , 180 , 181 This section focuses on CS‐based NPs for co‐delivery of DOX and genes in cancer suppression (Table 2 and Figure 7).
TABLE 2.
CS‐based nanocarriers for co‐delivery of DOX and genes in cancer suppression.
| Nanovehicle | Gene | Antitumor agent | Particle size (nm); zeta potential (mV); encapsulation efficiency (%) | Remarks | Refernces |
|---|---|---|---|---|---|
| PAMAM‐CS‐DCA nanoparticles | pDNA | Doxorubicin | 140–220 nm |
Encapsulating DOX at core Loading pDNA on PAMAM shell with positive charge Transfection efficiency as much as 74% Exerting synergistic activity and suppressing cancer progression |
182 |
| CS‐based nanostructures | IL17RB‐siRNA | Doxorubicin | 114 nm; 10.1 mV |
Inducing apoptosis in breast cancer cells and suppressing their migration and invasion siRNA promotes cytotoxicity of DOX against tumor cells Targeted delivery by nanocarriers Decreasing expression levels of Bcl‐2 and NF‐κB |
183 |
| Micellar polyplexes | MDR‐1‐siRNA | Doxorubicin | 92 nm; 7–10 mV |
Impairing progression of breast cancer cells (4 T1 cells) Enhancing survival of animal model Down‐regulating MDR1 expression and promoting DOX sensitivity of tumor cells |
184 |
| CS nanostructures | HMGA2‐siRNA | Doxorubicin | 110–174 nm; 11.6–13.2 mV; up to 78% |
Stability against serum and heparin Inducing cell death Decreasing expression level of HMGA2, vimentin and MMP‐9, while promoting E‐cadherin levels in impairing progression of colorectal tumor cells Exerting synergistic impact between DOX and siRNA |
185 |
| LDL‐CS nanoparticles | MDR‐1‐siRNA | Doxorubicin | 23.4 nm; −1.65 mV; 71.06% |
Protecting siRNA against macrophage phagocytosis Decreasing MDR1 expression Accumulating at tumor site Suppressing liver cancer progression |
186 |
| CS‐gold nanoparticles | MDR‐1‐siRNA | ‐ | 94.5 nm | Reducing expression level of MDR‐1 and P‐gp to significantly enhance DOX internalization in breast cancer cells | 187 |
| CS nanoparticles | IGF‐1‐siRNA | Doxorubicin | 176 nm; 11 mV | Apoptosis induction due to synergistic impact between siRNA and DOX and subsequent impairment of A549 progression | 188 |
| Glycol CS nanoparticles | Bcl‐2‐siRNA | Doxorubicin | 290 nm; 0.86 mV; up to 93% |
Release of cargo in a pH‐sensitive manner Reducing cancer cell survival via Bcl‐2 down‐regulation and enhancing DOX sensitivity |
189 |
| CS‐based nanostructures | HMGA2‐siRNA | Doxorubicin | 207 nm; 16.3 mV |
Apoptosis induction Impairing migration and invasion of cancer cells Decreasing HMGA2, vimentin and MMP‐9 levels Enhancing E‐cadherin levels |
190 |
| Trimethyl CS nanostructures | HMGA2 | Doxorubicin | 120–205 nm; 13.5–17.3 mV; up to 80.2% |
Impairing migratory ability of breast cancer cells via co‐delivery of siRNA and DOX Suppressing breast tumor proliferation |
191 |
Abbreviations: CS, chitosan; DOX, doxorubicin; DCA, deoxycholic acid; HMGA2, high mobility group A2; MDR1, multidrug resistance 1; MMP, matrix metalloproteinase; NF‐κB; nuclear factor‐kappa B; PAMAM, poly(amidoamine); P‐gp, P‐glycoprotein.
FIGURE 7.
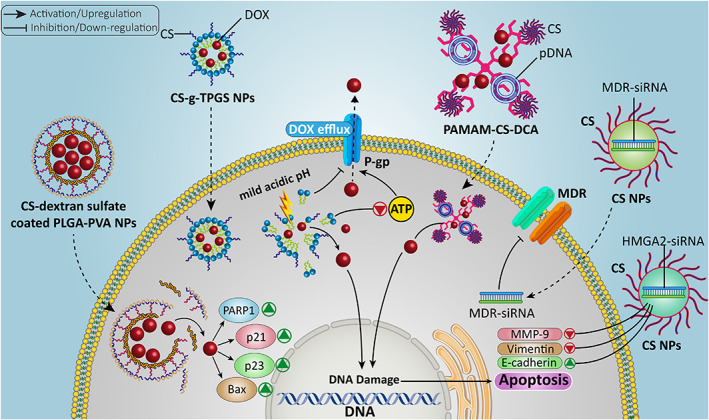
The CS‐based nano‐scale delivery systems for the purpose of gene and DOX delivery in cancer. The nucleic acids lead to apoptosis, DNA damage, and alterations in expression of genes to sensitize tumor cells to chemotherapy. Besides, efflux of chemotherapeutic agents is prevented via reducing activity and expression of P‐gp. Then, CS‐based nano‐scale delivery systems increase accumulation of anticancer drugs in tumor cells and promote their capacity in decreasing tumor progression
Although CS has a positive charge and can form stable complexes with genes possessing a negative charge, it has a hydrophilic segment that reduces its affinity toward genes and shows poor solubility. 192 To overcome these drawbacks, a dendronized CS derivative (poly(admidoamine) [PAMAM]‐CS) and PAMAM have been grafted into CS. 193 A recent study prepared PAMAM‐CS and then embedded hydrophobic deoxycholic acid (DCA) into CS backbone. The final PAMAM‐CS‐DCA has an amphiphilic nature and can self‐assemble into cationic nanostructures. The resulting nanocarriers demonstrated a particle size of 140–220 nm. The DOX as a hydrophobic drug is loaded in the core of NPs, while plasmid DNA (pDNA) forms a stable complex with a positively charged PAMAM shell. These NPs exhibited high transfection efficiency (74%) in 293 T cells and they may be considered promising for delivery applications. 182 Another important strategy for co‐delivery of DOX and gene is loading CS‐gene conjugate and DOX in other vectors. For this purpose, double‐walled microspheres can be prepared and then, DOX and CS‐DNA nanostructures containing p53 gene can be loaded to provide simultaneous chemotherapy and gene therapy of tumor cells. 194 , 195
The CS has been considered as a promising agent for modification of NPs for the delivery of small interfering RNA (siRNA) due to its positive charge, capacity in generating stable complex, high biocompatibility, and effective suppression of tumor cells. 177 The interest for the delivery of siRNA is due to circumventing a number of challenges such as poor internalization in tumor cells, prolonging blood circulation and preventing siRNA degradation by RNase enzymes. 13 , 196 , 197 , 198 The CS‐based nanostructures can provide a platform for the co‐delivery of DOX and siRNA in cancer chemo‐ and gene therapy. A study described the preparation of DOX‐loaded mixed micelles as core via thin‐film hydration method and then used CS as a coating agent and finally, complexation with multidrug resistance (MDR)‐siRNA has been made. To improve the selectivity toward cancer cells, modification of nanostructures with folic acid was accomplished. Due to the presence of CS, these nanoparticles are pH‐sensitive and can release MDR‐siRNA at the tumor site and significantly promote its internalization. Furthermore, the combination of DOX and MDR‐siRNA exerts a synergistic impact and suppresses tumor progression in vitro and in vivo (4 T1 breast tumor cells and Balb/c mice). 184 Although CS is an ideal agent in the synthesis of NPs, it has also some problems that need to be addressed. The CS nanostructures have high complexity, high degree of mutability, and complicated extraction process. 199 , 200 Therefore, carboxymethyl dextran (CMD) can be used to improve the features of CS NPs in drug and nucleic acid therapeutic delivery. An experiment has prepared CMD‐CS nanostructures for colorectal tumor suppression via siRNA and DOX co‐delivery and the nanosystem exhibited high encapsulation efficiency for both high mobility group A2 (HMGA2)‐siRNA (78%) and DOX (75%) and can induce cell death. Furthermore, HMGA2‐siRNA delivery by CMD‐CS nanoparticles suppresses metastasis of colorectal tumor cells via down‐regulating MMP‐9 and vimentin, and upregulating E‐cadherin that are beneficial in enhancing DOX cytotoxicity. 185
In addition to siRNA, short hairpin RNA (shRNA) has been utilized for regulating gene expression. Overall, shRNA has similar function to siRNA and is capable of reducing the expression level of target gene in cancer therapy. 201 , 202 , 203 , 204 A study synthesized magnetic mesoporous silica nanoparticles (M‐MSNs) and then conjugated them to DOX and chlorin e6 as a photosensitizer. Subsequently, this nanosystem was conjugated to CS and alginate to provide a pH‐sensitive platform and to improve adsorption of P‐gp‐shRNA. This nanosystem with a particle size of 280 nm can offer simultaneous chemotherapy, gene therapy and phototherapy; the release of drug at the tumor site (pH sensitive) and exposure to irradiation significantly enhances the production of singlet oxygen. The high cellular uptake after intravenous injection in tumor‐bearing mice with the synergistic impact between P‐gp‐shRNA, DOX and phototherapy in breast cancer therapy has been illustrated. 205
Another nucleic acid therapeutic approach is to affect the expression level of microRNAs (miRNAs), as their aberrant expression mediates initiation, development, and progression of various cancers. 206 , 207 , 208 , 209 , 210 , 211 , 212 The downregulation of miRNA‐34a is observed in breast tumor and promoting its expression could be beneficial in impairing tumor progression. CS‐based nanocarriers can provide co‐delivery of DOX and miRNA‐34a in breast tumor suppression. This combination therapy significantly reduces the Bcl‐2 expression to induce apoptosis. Furthermore, surface modification of CS nanoparticles by hyaluronic acid selectively targets breast cancer cells overexpressing CD44 receptor. 213 Therefore, CS nanostructures can be utilized in nucleic acid therapeutic delivery in enhancing cytotoxicity of DOX against cancer cells. 205 , 214 , 215 , 216 Notably, there has been no instance of the co‐delivery of DOX and CRISPR/Cas9 in cancer chemotherapy and future studies can focus on this aspect.
The co‐delivery of DOX and nucleic acids appears to be promising for purpose of effective cancer chemotherapy. There are some underlying reasons for this combination therapy. There are a number of tumor‐promoting factors in tumor cells such as IL‐17RB, IGF‐1R, MDR1, Bcl‐2, and HMGA2, among others, that can mediate resistance of cancer cells to DOX chemotherapy. Therefore, co‐application of nucleic acids for downregulation of these oncogenic factors can sensitize tumor cells to DOX chemotherapy. The surface modification of nanocarriers with positively charged CS leads to proper interaction with negatively charged nucleic acids and forms stable complexes. Furthermore, CS provides a site for conjugation to ligands to selectively target receptors overexpressed on tumor cells. 183 , 186 , 187 , 188 , 189 , 190 These benefits advocate application of CS‐based nano‐scale delivery systems for DOX chemotherapy.
4.4. Doxorubicin and antitumor drug delivery
In addition to gene therapy as a promising strategy in promoting cytotoxicity of DOX against tumor cells, there have been attempts to recognize drugs that can exert synergistic impact with DOX in tumor suppression. The most well‐known mechanism is that a certain antitumor agent induces DNA damage and apoptosis in cancer cells and then, the pathway is paved for DOX to inhibit tumor cell growth and invasion. 217 , 218 This section focuses on DOX and drug co‐delivery by CS NPs in cancer therapy.
Lung cancer is a leading cause of death worldwide and based on new estimates, it is the most common cancer in both males and females. 219 These malignant cells can attain resistance to various chemotherapeutic agents and different underlying pathways and mechanisms are responsible for drug resistance in lung cancer. 8 , 220 , 221 , 222 A recent experiment has described the preparation of glycol CS NPs for the co‐delivery of DOX and celecoxib (CXB) in lung cancer suppression. For selective targeting of lung cancer cells, DOX‐ and CXB‐loaded CS NPs are modified by hyaluronic acid (HA) to target CD44 receptor; ensued CS NPs have a uniform spherical shape with a particle size of 150 nm. Further, this nanosystem is pH‐sensitive and can release DOX and CXB in response to mild acidic pH of the tumor microenvironment (pH 4–6). This combination therapy exerted synergistic impact and suppressed proliferation, migration, and inflammation in lung cancer. 223 Another study has synthesized UiO‐66 metal organic framework (MOF) for co‐loading DOX and folic acid and then loaded them in CMD/poly ethylene oxide (PEO)/polyurethane core–shell nanofibers for regulated release of drugs. This nanosystem induced cell death in breast cancer (MCF‐7) cells and significantly impaired cancer progression. 224
The interesting theme is the potential of plant derived‐natural compounds in reversing the DOX resistance in cancer suppression. The curcumin is derived from Curcuma longa and can induce apoptosis in tumor cells and it inhibits migration and proliferation that are beneficial in promoting chemosensitivity of tumor cells. 8 , 222 , 225 , 226 , 227 The inhalable bioresponsive CS microspheres have been developed for the co‐delivery of soluble curcumin and DOX in lung cancer therapy. These NPs bear elastin as a stimuli‐responsive agent. At pH 5.5, CS microspheres release DOX due to existence of elastase enzyme. Notably, curcumin is also released from CS microspheres by the function of elastase enzyme and independent of pH level. These nanoparticles can provide co‐delivery of curcumin and DOX that is beneficial in exerting synergistic therapy and inducing apoptosis in A549 cells. Furthermore, this combination and targeted delivery can reduce IC50 to 3.4 μM compared to 6.5 μM of NPs lacking elastin. 228 Another experiment has prepared CS liposomal nanocarriers for co‐delivery of DOX and rapamycin (RAPA). At the first step, a conjugation of glycol CS and DOX is formed and then, electrostatic interaction mediates complexation between glycol CS‐DOX and docosahexaenoic acid RAPA‐liposomes. The resulting nanoparticles had a particle size of 131.3 nm with ζ‐potential of −14.5 mV, loading efficiency being 4.1% and 6.2% for DOX and RAPA, respectively. These glycol CS liposomes are stable and exhibit pH‐sensitive behavior, capable of releasing drugs at the tumor site and exerting synergistic breast cancer therapy. 229 Based on these experiments, CS NPs can mediate co‐delivery of DOX with other antitumor agents. Although a few studies have been performed, more efforts should be made in co‐delivery of DOX with other well‐known antitumor agents such as cisplatin, docetaxel, paclitaxel, and resveratrol among others. Furthermore, other kinds of nanocarriers such as CS‐based micelles and carbon nanomaterials should be evaluated in terms of co‐delivery of DOX and other drugs.
4.5. Lipid nanoparticle modification
Liposomes are synthetic lipid NPs that have been first discovered in the 1960s and comprise a lipid bilayer with an aqueous core. 230 The liposomal nanocarriers can be exploited for the delivery of both hydrophilic and hydrophobic drugs. The hydrophilic drugs can be loaded in the core, while hydrophobic drugs can be loaded in lipid bilayer. The drugs loaded in liposomes are protected against inactivation in blood circulation, dilution, and degradation. The clinical application of liposomes has some impediments such as their rapid clearance, immune system activation, and accumulation in other organs. 231 Recent experiments have exploited liposomes for delivery of DOX or its co‐delivery with other agents such as hispolon, curcumin, and linalool in combination cancer therapy. 232 , 233 , 234 , 235 Therefore, liposomal nanocarriers can provide delivery of DOX at the tumor site and this section focuses on CS‐modified liposomes in DOX delivery.
An experiment has conjugated DOX to amphiphilic stearolylspermine anchor to produce a prodrug. Then, this prodrug has been loaded into liposomal nanocarriers for colorectal cancer therapy. For improving the stability of DOX‐loaded liposomes, their modification with CS and PEG has been made. Furthermore, stearoyl chains promote local microfluidity of liposomes and spermine via amine groups interacts with phosphate groups of lipids in improving liposome stability. In addition to stability, CS‐PEG modification of liposomes prevents aggregation and this coating mediates charge neutralization. These neutral liposomal nanocarriers demonstrated cytotoxicity against A549 (lung cancer) and Caco‐2 cells (colorectal tumor) and they showed high stability. 236 Another study described the preparation of liposomes using hydrogenated soy phosphatidylcholine (HSPC) and cholesterol (CHOL) and glycol CS conjugation has been performed during film preparation. Then, DOX was embedded in preformed liposomes via transmembrane pH gradient loading strategy. These CS‐based liposomes enhance internalization of DOX in tumor cells (HT1080 cells) and were successful in enhancing the therapeutic efficacy of DOX in vitro and in vivo. This system was pH‐sensitive and could be exploited for the treatment of other cancer types. 237
Another kind of lipid‐based nanoparticles that can be utilized for cancer therapy are micelles as they are considered as promising vectors in drug delivery and cancer treatment due to their ease of synthesis and chemical modification. Furthermore, the size of micellar NPs is tunable and they can enhance drug solubility in water and significantly promote blood circulation of drugs. The increased bioavailability of the drug, lowering adverse impacts and high accumulation at tumor site are other benefits of using micelles for drug delivery. The micelles can provide pH‐responsive release of DOX at the tumor site and provide co‐delivery of DOX with other antitumor agents such as cisplatin for synergistic cancer therapy. Furthermore, surface modification of micelles, for instance, with phenyboronic acid promotes selectivity toward tumor cells and enhances the tumor‐suppressor activity of DOX. 238 , 239 , 240 , 241 , 242
The DOX‐loaded micelles can be prepared using alginate and CS in a water‐in‐oil emulsion method with a spherical particle size of 80 nm. This is an interesting method for loading DOX in nanocarriers and uses an aqueous phase dispersed in a cyclohexane/dodecylamine organic phase. These nanocarriers showed high cellular uptake by breast cancer cells and can suppress proliferation of 4 T1 cells. 243 The CS‐modified micelles can also provide co‐delivery of DOX and curcumin in liver cancer therapy. The CS‐cystamine‐poly(ε‐caprolactone) copolymer micelles have been prepared for curcumin and DOX co‐delivery and then, modification with GA has been performed in enhancing their cellular uptake. They showed drug loading efficiency of 19.8% and 8.9% for DOX and curcumin, respectively. They had a spherical shape with a particle size of 110 nm. The GA modification enhanced its internalization in cells via endocytosis and exposure to the tumor microenvironment induced changes in charge of nanocarriers from negative to positive. These CS‐modified micelles are pH‐ and redox sensitive and 10 mM of GSH induces the release of DOX (80.6%) and curcumin (67.2%). This combination therapy exerts a synergistic impact and is beneficial in suppressing the progression of hepatoma cells. 244 Overall, CS derivatives that can self‐assemble into micelles, are able to provide nanocarriers that are biocompatible, have low immunogenicity, and provide nanoplatforms for drug delivery. 245 , 246 , 247 An experiment has prepared N‐succinyl‐N′‐octyl chitosan (SOC)‐based micelles for DOX delivery and increasing the ocetyl chain amount, promotes capacity of these micelles in DOX loading. Drug loading and ocetyl chain number determine the size of micelles and they have a particle size of 100–200 nm. They showed high antitumor activity against various cancer types including HepG2, A549, BGC, and K562 cells. 248 Hence, similar to liposomes, micellar nanoparticles are potential vectors for DOX delivery and cancer suppression as well as preventing drug resistance development. 249 , 250
4.6. Metal nanoparticle modification
Metal–organic frameworks (MOFs) can be considered as ideal options for drug delivery due to their nanoscale size, high surface area, and porosity as well as adjustable size. 251 , 252 To improve the property of MOFs in drug release, their modification with polymers has been performed; CS modification of MOFs renders them pH‐sensitive feature and provides a condition for sustained release of DOX in cancer chemotherapy. Furthermore, CS can be functionalized by folic acid (FA) for selective targeting of tumor cells overexpressing folate receptor. Then, DOX can be loaded in CS‐modified MOFs with a high drug loading capacity (1.63 g). Notably, MOFs can encapsulate carbon dots for providing imaging. These CS‐based metal NPs provide simultaneous chemotherapy and bioimaging that are beneficial in cervical cancer treatment. 253 When exposed to mild acidic pH of the tumor microenvironment, CS layers located on the surface of MOFs would collapse and swell, leading to the release of DOX at the tumor site and subsequent breast cancer suppression. 254
The interesting note is the modification of Fe3O4 nanoparticles with CS that provides a drug delivery system that can mediate bioimaging. The surface modification of Fe3O4 with CS is of importance for loading DOX. The CS can form a stable complex with DOX and produces NH2‐Zn(II)‐DOX structure. Exposing to certain pH destroys the bond between DOX and CS and leads to the pH‐sensitive release of DOX and Fe3O4 nanoparticles can provide magnetic resonance imaging. 255 As has been mentioned in the Introduction section, CS can undergo changes by chemicals and enzymes. There have been efforts in the chemical modification of CS and improving its properties. It has been reported that CS modification with guanidine moieties significantly elevates its intracellular accumulation; guadinylated CS can form chelates with copper via copper‐nitrogen coordination. The CS‐copper complexes enhance the production of ROS in impairing lung cancer progression and exert synergistic cancer therapy with DOX. 256 Based on these experiments, modification of metal‐based nanostructures with CS improves their biocompatibility and loading of DOX. Notably, further progress can be made by the modification of CS‐metal NPs. A recent experiment has synthesized CS‐Au nanostructures and for enhancing their selectivity toward lung (A549) and breast (4 T1) cancer cells, their modification with nucleolin aptamer (AS1411) was conducted. These nanocomplexes targeted the tumor cells and effectively penetrated into them, leading to DOX accumulation and subsequent cancer progression suppression. 257 Therefore, CS is used as a reducing and stabilizing agent for loading DOX on Au NPs and further modification of CS‐Au NPs with polyethylene glycol promoters their blood circulation that is of importance for elevating cytotoxicity of DOX against lung cancer cells. 258 Taking everything together, efficacy of metal‐based NPs for DOX delivery and cancer suppression can significantly enhance by CS modification. 259 , 260 , 261
4.7. Carbon‐based nanoparticle modification
A recent experiment has provided novel insights about the surface modification of graphene composites by CS and its impact on DOX conjugation and release. The concentration or pH of the solution (level of CS protonation) affects the aggregation and dispersion of CS on the surface of graphene composites. At low concentration levels of CS and DOX, the bare surface of graphene composites enhances. Increasing the numbers of —NH2 CS and DOX molecules promotes the adsorption of DOX on bare surface areas of graphene and mediates encapsulation of DOX by CS clusters on the surface or results in conjugation with CS chains. On the other hand, when levels of —NH3 CS and DOX increase, there will be higher positive charges and lower bare surface area of graphene that can provide conditions for the release of DOX. Therefore, at mild acidic pH of the tumor microenvironment, protonation of CS occurs and leads to the release of DOX from nanocarriers. 262 Graphene oxide (GO) is a derivative of graphite or graphene and has a two‐dimensional plate‐like structure. The GO sheets have both sides and due to their large surface area, they are considered as promising structures for the delivery of antitumor drugs. Furthermore, aromatic molecules and nucleobases can be loaded on GO composites via π–π stacking and hydrogen bonding interactions. 263 , 264 For improving the biodegradability of CS and its solubility, conjugation of CS with other agents such as acrylic acid (AA) and itaconic acid (IA) monomers has been performed to improve its functionality via COOH group. Furthermore, modification by folic acid (FA) selectively targets tumor cells overexpressing folate receptor. A recent experiment has first converted GO sheets to amine‐functionalized GO (AGO) to provide a function of a cationic polyelectrolyte. Next, CS and FA conjugation via N, N′‐dicyclohexylcarbodiimide was performed. Then, CS was chemically modified by AA and IA monomers by binding to COOH group via ethyleneglycol dimethacrylate as cross‐linker and potassium peroxydisulfate as an initiator. Subsequently, DOX was embedded into FA‐chemically modified chitosan (CMCS)/AGO nanocomposites via π–π stacking interactions. The prepared nanocomposites demonstrated drug loading capacity as much as 95% and they released DOX at a pH‐sensitive manner (release of DOX at pH 5.3 compared to pH 7.4). These DOX‐loaded nanoparticles were able to significantly reduce the viability of tumor cells including HeLa and MCF‐7 cells. 265
Another experiment has prepared injectable hydrogels for the controlled release of DOX. This hydrogel is based on cross‐linking of graphene, CS, and cellulose nanowhiskers where Schiff base reaction by a synthetic dialdehyde has been used. This hydrogel is responsive to pH and other stimuli by adding benzaldehyde and amino acid cysteine and can be administered subcutaneously to deliver both DOX and curcumin in synergistic cancer therapy. 266 In addition, CNTs can be modified with CS to provide pH‐sensitive release of DOX in cancer therapy; CNTs can be noncovalently wrapped with CS and then loaded with DOX. Due to the deprotonation form of CS, DOX release does not occur at physiological pH, whereas protonation of CS at pH 5–6.5 leads to DOX release due to charge–charge repulsion between CS and DOX, resulting in controlled drug release 267 that is beneficial for cancer therapy. Another experiment prepared graphene and CNTs for co‐delivery of DOX and paclitaxel (PTX). For improving loading efficiency and release features, further functionalization by CS has been performed. Furthermore, CS provides pH‐sensitive manner release of DOX and PTX and slow release of these antitumor drugs promotes tumor‐suppressor activity. 268 Based on these experiments, carbon‐based NPs can provide delivery of DOX in cancer therapy and CS modification improves their beneficial features. 99 , 269 , 270 , 271 , 272 , 273 , 274 , 275 Figure 7 demonstrates modification of carbon‐based nanomaterials with CS and then, conjugation of folic acid as ligand on nanocarriers to mediate their internalization in cervical cancer cells via endocytosis, resulting in a significant increase in accumulation of DOX in tumor cells. 253 , 276 , 277 Table 3 provides a summary of various nanoparticles modified by CS for purpose of DOX delivery in cancer suppression.
TABLE 3.
A summary of CS‐based nanostructures for DOX delivery in cancer suppression
| Nanostructure | Particle size (nm); zeta potential (mV); encapsulation efficiency or drug loading (%) | Cancer type | In vitro/in vivo | Cell line/animal model | Remarks | Referencs |
|---|---|---|---|---|---|---|
| Estrogen‐functionalized CS nanoparticles | 198.2 and 206.4 nm; 28.3 and 30.6 mV; up to 66.33% | Breast cancer | In vitro | MCF‐7 cells | High biocompatibility and antineoplastic activity | 278 |
| CS‐raloxifene nanoparticles | 26.85 and 34.75 nm; 0.17 and −0.49 mV; up to 98% | Breast cancer | In vitro | MCF‐7 cells |
Decreasing proliferation rate by 60% Nanoparticles inhibit cancer progression via suppressing estrogen receptor |
279 |
| DOX‐loaded LGCC NPs | 200 nm; 20–35 mV; up to 86.4% | Breast and liver cancers | In vitro; in vivo |
QGY‐7703 and 4 T1 cells H22 hepatocarcinoma model |
Penetrating directly via cell membrane and circumventing endocytic vesicles Cargo release under high GSH levels Endosomal and lysosomal escape High nuclear distribution |
262 |
| Catechol‐modified CS‐hyaluronic acid nanoparticles | 160 nm; −19.8 mV | Oral cancer | In vitro | HN22 cells |
Negative charge and spherical shape High mucoadhesive ability Prolonged release of DOX Reducing cancer proliferation |
280 |
| Ethyl cellulose/CS/g‐C3N4/MoS2 core–shell nanofibers | 285–370 nm | Breast and cervical cancers | In vitro | MCF‐7 and HeLa cells |
Sustained delivery of DOX Inducing cell death up to 89% and 85% in MCF‐7 and HeLa cells, respectively in 7 days |
281 |
| Aptamer‐functionalized CS‐bases silica nanostructures | 87 nm; 35.9 to −32.3 mV | Breast cancer | In vitro; in vivo |
MCF‐7 and 4 T1 cells C26 tumor‐bearing mice |
Enhanced cellular uptake Targeted delivery of DOX and anti‐miRNA‐21 in cancer suppression |
282 |
| PEGylated CS nanoparticles | 169–192 nm; up to 43 mV | Breast cancer | In vitro | MCF‐7 cells |
Functionalization of CS nanoparticles with anti‐hMAM and anti‐HER2 promotes selectivity toward cancer cells Exerting dose‐dependent toxicity against cancer cells |
283 |
| CMC/PCL nanofibers | 300 nm; higher than −30 mV; 90% | Breast cancer | In vitro | MCF‐7 cells |
Lack of initial burst release Sustained release for 7 and 25 days Cytotoxicity against tumor cells up to 85% |
284 |
| HMSN grafted with CS‐copper sulfide composites | 150 nm; −19.6 mV; 46.1% | Breast cancer | In vitro; in vivo |
MDA‐MB‐231 cells Mouse model of breast cancer |
High biocompatibility Increased cellular uptake by cancer cells Apoptosis induction Increasing survival of mice |
285 |
| CS‐, PEG‐ and PVA‐modified MgFe2O4 ferrite magnetic nanoparticles | 78–140 nm; below −21 mV | Breast and colorectal cancers | In vitro | Caco‐2 and SKBR‐3 cells |
Decreasing cancer cell viability in a concentration‐dependent manner pH‐sensitive release of DOX 85.86% release of DOX after 72 h |
286 |
| CS hydrogel beads | 13.5 mV | Breast cancer | In vitro | MCF‐7 cells |
High swelling rate (426%) and drug release (81.33% in 144 h) at pH of 5.8 High biocompatibility Decreasing proliferation rate of MCF‐7 cells |
287 |
| CMCS/MAGG hydrogel | ‐ | Breast cancer | In vitro | MCF‐7 cells |
pH‐responsive swelling of hydrogels 67.06% release of DOX after 5 days in pH of 5.5 32.13% release of DOX at pH of 7.4 High biocompatibility Cytotoxicity against MCF‐7 cells |
287 |
Abbreviations: CMC, N‐carboxymethyl chitosan; CS, chitosan; DOX, doxorubicin; GSH, glutathione; HMSN, hollow mesoporous silica nanoparticle; NPs, nanoparticles; PCL, poly(ε‐caprolactone); PVA, polyvinyl alcohol, PEG, polyethylene glycol.
Taking everything together, these studies highlight the fact that various kinds of lipid‐, carbon‐ and metal‐based nanostructures can be modified with CS in improving their characteristics. The nanoparticles demonstrate low particle size, sustained drug release, and high encapsulation efficiency. Furthermore, CS modification may significantly enhance biocompatibility and stability of nanocarriers. Modification of CS‐based nano‐scale delivery systems with ligands such as HA and folic acid increases their selectivity toward tumor cells for specific accumulation of DOX. The CS modification appears to be vital for metal‐ and carbon‐based nanocarriers, as they may show toxicity toward normal cells and such modification improves their biocompatibility (Figure 8). 278 , 279 , 280 , 281 , 282 , 283 , 284 , 285 , 286 , 287
FIGURE 8.
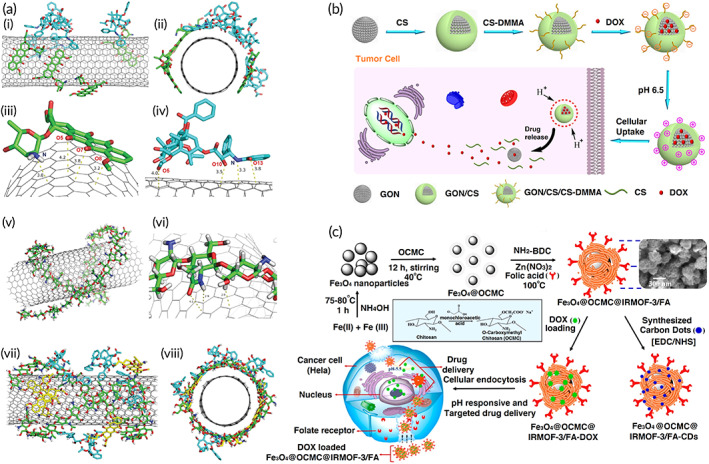
Modification of various kinds of carbon‐based nanostructures with CS for DOX delivery and cancer suppression. (a) Surface modification of single‐walled carbon nanotubes (SWCNs) with CS followed by loading doxorubicin (DOX) and paclitaxel (PTX); (i and ii) DOX and PTX loading on the bare SWCNs from front and side views; a close view of DOX (iii) and PTX (iv) orientation on the side of SWCNs; CS modification on the surface of SWCNs (v and vi); the final drug‐loaded CS‐coated SWCNs from front and side views (vii and viii). Source: Reprinted from Ref 277 with permission from RSC. (b) A schematic on the synthesis and applicability of ternary DOX‐loaded graphene oxide nanoparticles (GON)‐CS‐dimethylmaleic anhydride (DMMA) for cancer therapy. Source: Reprinted from Ref 276 with permission from ACS publication. (c) A schematic on the synthesis of folic acid (FA)‐anchored O‐carboxymethyl CS (OCMC)‐Fe3O4 modified with carbon dots (CDs) for DOX delivery to the cancer cells. Source: Reprinted from Ref 253 with permission from ACS publication. NH2‐H2BDC, 2‐amino terepthalic acid; IRMOF‐3, metal organic framework
4.8. Directions for clinical application
The current review article demonstrated that rational integration of engineering and biology appears to be promising in treatment of cancer. The gold standard for improving prognosis and survival of cancer patients is chemotherapy. However, chemotherapy failure is a common outcome in cancer patients due to drug resistance. Therefore, there is an urgent need toward development of targeted delivery systems for chemotherapeutic agents. Since DOX is frequently used in clinic for treatment of cancer patients, resistance to its anticancer activities is common. Although problem (drug resistance) is obvious and one of solutions is application of nanocarriers, there are still a number of challenges for use of DOX‐loaded nanostructures in clinical course. The first problem is related to biocompatibility of nanoparticles for DOX delivery in cancer patients. The second problem is affordability and final difficulty is related to large‐scale production of nanocarriers. All of these problems can be solved using greener modification of nanoparticles. Throughout this review article, it was shown that surface modification of various nanocarriers by CS promotes their biocompatibility, safety profile and increases their stability. Furthermore, CS is a natural product that is affordable and can be used for synthesis on larger scale. A number of clinical studies (clinicaltrials.gov) have been conducted on CS application in patients; however, there is no experiment pertaining to the use of CS‐based nanocarriers for DOX delivery in treatment of cancer patients that exploits the afore mentioned benefits of CS. Hopefully, it will occur in the near future.
5. CONCLUSION AND REMARKS
The CS is among the most abundant polysaccharides that have received much attention in recent years and a large number of experiments have been performed in revealing therapeutic potentials of CS and its efficacy in NPs synthesis and their modification. Furthermore, as CS is a nature‐derived agent, it is affordable and can be utilized in both preclinical and clinical studies. Regardless of the protective impacts of CS in diabetes, anti‐inflammatory diseases and cardiovascular diseases among others, CS appliance in cancer has undergone a surge due to its cytotoxicity against tumor cells and ability in nanostructure preparation with high biocompatibility. On the other hand, cancer treatment depends on overcoming drug resistance to improve chemotherapy efficacy in tumor cell suppression. Among various chemotherapeutic agents, DOX is a well‐known compound and that is why DOX it has been discussed in this review article. Frequent application of DOX leads to drug resistance and as DOX is an FDA‐approved agent and is utilized in the clinic for the treatment of cancer patients, efforts should be made in reversing this resistance. The present review focused on CS‐NPs for DOX delivery in cancer treatment. Different kinds of stimuli‐responsive CS‐based nanostructures have been developed for DOX delivery including pH‐, redox‐, thermo‐ and multi‐sensitive nanocarriers. The pH of the tumor microenvironment tends to be acidic and is lower than physiological pH. Therefore, pH‐sensitive CS‐based NPs can deliver DOX at tumor site. Due to glycolysis and high proliferation rate of tumor cells, redox‐sensitive NPs are also of importance and GSH levels can induce DOX release from CS nanostructures. The thermosensitive CS‐based hydrogels have been also developed that can be transformed to solid gel at body temperature and provide sustained release of DOX that is of importance for enhancing cytotoxicity against cancer cells and preventing the drug resistance development.
Based on the documented experiments, CS has antitumor activity and it can exert a synergistic impact with DOX. Furthermore, CS‐based NPs promote DOX internalization in cancer cells. The enhanced cellular uptake is vital for promoting the sensitivity of cancer cells to DOX chemotherapy. Furthermore, using other agents such as TPGS with CS can prevent P‐gp activity in inhibiting DOX efflux from tumor cells. Hence, CS‐based NPs can prevent the development of DOX resistance. Notably, CS‐based NPs can provide co‐delivery of DOX with antitumor drugs and nucleic acid therapeutics. This strategy can induce apoptosis in tumor cells in providing DOX sensitivity. Furthermore, the proliferation rate and the invasion of tumor cells undergo a decrease, and the pathway is paved for DOX to induce its tumor‐suppressor activity. Besides, various kinds of NPs including carbon‐, lipid‐, polymer‐ and metal‐based nanostructures can be modified with CS to improve their stability and biocompatibility and provide conditions for DOX complex formation. A search at clinicaltrials.gov shows that CS is currently applied in the clinical course for the treatment of various cancer such as prostate and breast cancers (NCT03202446; NCT03712371). Therefore, future experiments can be directed toward using DOX‐loaded CS‐based nanoarchitectures in the clinic. The application of some of the CS‐modified nanoparticles in clinical course still needs extensive investigation. For instance, CS‐modified metal nanostructures still require examination in terms of biocompatibility, as metal nanoparticles are toxic toward normal cells. The same explanation can be provided for carbon‐based nanomaterials. However, lipid‐based nanoparticles have demonstrated long‐term biocompatibility, especially liposomes and their modification with CS not only increases their biocompatibility but also improves drug release profile and their stability, paving the way for clinical application.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/btm2.10325.
ACKNOWLEDGMENT
Gorka Orive wishes to thank the Spanish Ministry of Economy, Industry, and Competitiveness (PID2019‐106094RB‐I00/AEI/10.13039/501100011033) and technical assistance from the ICTS NANBIOSIS (Drug Formulation Unit, U10) at the University of the Basque Country. The authors also appreciate the support from the Basque Country Government (Grupos Consolidados, No ref: IT907‐16). Ebrahim Mostafavi would like to acknowledge the support from the National Institute of Biomedical Imaging and Bioengineering (5T32EB009035). Alan Prem Kumar was supported by a grant from the Singapore Ministry of Education (MOE‐T2EP30120‐0016).
Ashrafizadeh M, Hushmandi K, Mirzaei S, et al. Chitosan‐based nanoscale systems for doxorubicin delivery: Exploring biomedical application in cancer therapy. Bioeng Transl Med. 2023;8(1):e10325. doi: 10.1002/btm2.10325
Funding information National Institute of Biomedical Imaging and Bioengineering, Grant/Award Number: 5T32EB009035; University of the Basque Country; Ministry of Economy, Grant/Award Number: PID2019‐106094RB‐I00/AEI
Contributor Information
Pooyan Makvandi, Email: pooyan.makvandi@iit.it.
Nicolas H. Voelcker, Email: nicolas.voelcker@monash.edu.
Gorka Orive, Email: gorka.orive@ehu.eus.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed.
REFERENCES
- 1. Kumar KSS, Girish YR, Ashrafizadeh M, et al. AIE‐featured tetraphenylethylene nanoarchitectures in biomedical application: bioimaging, drug delivery and disease treatment. Coord Chem Rev. 2021;447:214135. [Google Scholar]
- 2. Makvandi P, Chen M, Sartorius R, et al. Endocytosis of abiotic nanomaterials and nanobiovectors: inhibition of membrane trafficking. Nano Today. 2021;40:101279. doi: 10.1016/j.nantod.2021.101279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mirzaei S, Mohammadi AT, Gholami MH, et al. Nrf2 signaling pathway in cisplatin chemotherapy: potential involvement in organ protection and chemoresistance. Pharmacol Res. 2021;167:105575. [DOI] [PubMed] [Google Scholar]
- 4. Ashrafizaveh S, Ashrafizadeh M, Zarrabi A, et al. Long non‐coding RNAs in the doxorubicin resistance of cancer cells. Cancer Lett. 2021;508:104‐114. [DOI] [PubMed] [Google Scholar]
- 5. Mirzaei S, Gholami MH, Hushmandi K, et al. The long and short non‐coding RNAs modulating EZH2 signaling in cancer. J Heamto Oncol. 2022;15(1):1‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ashrafizadeh M, Mirzaei S, Hashemi F, et al. New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed Pharmacother. 2021;141:111824. [DOI] [PubMed] [Google Scholar]
- 7. Mirzaei S, Gholami MH, Zabolian A, et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: new hope in the fight against cancer. Pharmacol Res. 2021;171:105759. doi: 10.1016/j.phrs.2021.105759 [DOI] [PubMed] [Google Scholar]
- 8. Ashrafizadeh M, Zarrabi A, Hashemipour M, et al. Sensing the scent of death: modulation of microRNAs by curcumin in gastrointestinal cancers. Pharmacol Res. 2020;160:105199. doi: 10.1016/j.phrs.2020.105199 [DOI] [PubMed] [Google Scholar]
- 9. Akbari E, Mousazadeh H, Hanifehpour Y, et al. Co‐loading of cisplatin and methotrexate in nanoparticle‐based PCL‐PEG system enhances lung cancer chemotherapy effects. J Cluster Sci. 2021;1‐12. doi: 10.1007/s10876-021-02101-9 [DOI] [Google Scholar]
- 10. Rabiee N, Bagherzadeh M, Ghadiri AM, et al. Calcium‐based nanomaterials and their interrelation with chitosan: optimization for pCRISPR delivery. J Nanostructure Chem. 2021;1‐14. doi: 10.1007/s40097-021-00446-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ward RA, Fawell S, Floc'h N, et al. Challenges and opportunities in cancer drug resistance. Chem Rev. 2020;121(6):3297‐3351. [DOI] [PubMed] [Google Scholar]
- 12. Kirtonia A, Ashrafizadeh M, Zarrabi A, et al. Long noncoding RNAs: a novel insight in the leukemogenesis and drug resistance in acute myeloid leukemia. J Cell Physiol. 2021;237(1):450‐65. [DOI] [PubMed] [Google Scholar]
- 13. Mirzaei S, Zarrabi A, Hashemi F, et al. Regulation of nuclear factor‐KappaB (NF‐κB) signaling pathway by non‐coding RNAs in cancer: inhibiting or promoting carcinogenesis? Cancer Lett. 2021;509:63‐80. [DOI] [PubMed] [Google Scholar]
- 14. Ashrafizadeh M, Zarrabi A, Hushmandi K, et al. Association of the epithelial–mesenchymal transition (EMT) with cisplatin resistance. Int J Mol Sci. 2020;21(11):4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mostafavi E, Soltantabar P, Webster TJ. Nanotechnology and picotechnology: a new arena for translational medicine. Biomaterials in Translational Medicine. Elsevier; 2019:191‐212. [Google Scholar]
- 16. Dehshahri A, Ashrafizadeh M, Ghasemipour Afshar E, et al. Topoisomerase inhibitors: pharmacology and emerging nanoscale delivery systems. Pharmacol Res. 2020;151:104551. doi: 10.1016/j.phrs.2019.104551 [DOI] [PubMed] [Google Scholar]
- 17. Jahangirian H, Ghasemian lemraski E, Webster TJ, Rafiee‐Moghaddam R, Abdollahi Y. A review of drug delivery systems based on nanotechnology and green chemistry: green nanomedicine. Int J Nanomed. 2017;12:2957‐2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Makvandi P, Ghomi M, Ashrafizadeh M, et al. A review on advances in graphene‐derivative/polysaccharide bionanocomposites: therapeutics, pharmacogenomics and toxicity. Carbohydr Polym. 2020;250:116952. doi: 10.1016/j.carbpol.2020.116952 [DOI] [PubMed] [Google Scholar]
- 19. Ashrafizadeh M, Ahmadi Z, Mohamadi N, et al. Chitosan‐based advanced materials for docetaxel and paclitaxel delivery: recent advances and future directions in cancer theranostics. Int J Biol Macromol. 2020;145:282‐300. doi: 10.1016/j.ijbiomac.2019.12.145 [DOI] [PubMed] [Google Scholar]
- 20. Takeshita S, Zhao S, Malfait WJ, Koebel MM. Chemistry of chitosan aerogels: three‐dimensional pore control for tailored applications. Angew Chem. 2021;60(18):9828‐9851. [DOI] [PubMed] [Google Scholar]
- 21. Jin T, Liu T, Lam E, Moores A. Chitin and chitosan on the nanoscale. Nanoscale Horizon. 2021;6(7):505‐542. [DOI] [PubMed] [Google Scholar]
- 22. Sharifi E, Chehelgerdi M, Fatahian‐Kelishadrokhi A, Yazdani‐Nafchi F, Ashrafi‐Dehkordi K. Comparison of therapeutic effects of encapsulated mesenchymal stem cells in Aloe vera gel and chitosan‐based gel in healing of grade‐II burn injuries. Regen Ther. 2021;18:30‐37. doi: 10.1016/j.reth.2021.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumar MR, Muzzarelli RAA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. ACS Publ. 2004;104(12):6017‐6084. [DOI] [PubMed] [Google Scholar]
- 24. Ifuku SJM. Chitin and chitosan nanofibers: preparation and chemical modifications. Molecules. 2014;19(11):18367‐18380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zafari M, Boroujeni MM, Omidghaemi S, et al. Physical and biological properties of blend‐electrospun polycaprolactone/chitosan‐based wound dressings loaded with N‐decyl‐N, N‐dimethyl‐1‐decanaminium chloride: an in vitro and in vivo study. J Biomed Mater Res. 2020;108(8):3084‐3098. [DOI] [PubMed] [Google Scholar]
- 26. Yan N, Chen XJNN. Sustainability: don't waste seafood waste. Nature. 2015;524(7564):155. [DOI] [PubMed] [Google Scholar]
- 27. Prashanth KVH, Tharanathan RN. Chitin/chitosan: modifications and their unlimited application potential ‐ an overview. Trends Food Sci Technol. 2007;18(3):117‐131. doi: 10.1016/j.tifs.2006.10.022 [DOI] [Google Scholar]
- 28. Bellich B, D'Agostino I, Semeraro S, Gamini A, Cesàro A. “The good, the bad and the ugly” of chitosans. Mar Drugs. 2016;14(5):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verma S, Nadagouda MN, Varma RS. Porous nitrogen‐enriched carbonaceous material from marine waste: chitosan‐derived carbon nitride catalyst for aerial oxidation of 5‐hydroxymethylfurfural (HMF) to 2,5‐furandicarboxylic acid. Sci Rep. 2017;7(1):13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Varma RS. Biomass‐derived renewable carbonaceous materials for sustainable chemical and environmental applications. ACS Sustain Chem Eng. 2019;7(7):6458‐6470. doi: 10.1021/acssuschemeng.8b06550 [DOI] [Google Scholar]
- 31. Nik AB, Zare H, Razavi S, et al. Smart drug delivery: capping strategies for mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2020;299:110115. [Google Scholar]
- 32. Maghsoudi S, Shahraki BT, Rabiee N, et al. Burgeoning polymer nano blends for improved controlled drug release: a review. Int J Nanomed. 2020;15:4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Özel N, Elibol MJCP. A review on the potential uses of deep eutectic solvents in chitin and chitosan related processes. 2021;262:117942. doi: 10.1016/j.carbpol.2021.117942 [DOI] [PubMed] [Google Scholar]
- 34. Kadokawa J‐I. Dissolution, derivatization, and functionalization of chitin in ionic liquid. Int J Biol Macromol. 2019;123:732‐737. [DOI] [PubMed] [Google Scholar]
- 35. Shahidi F, Abuzaytoun R. Chitin, chitosan, and co‐products: chemistry, production, applications, and health effects. Advances in Food and Nutrition Research. Academic Press; 2005:93‐135. [DOI] [PubMed] [Google Scholar]
- 36. Kim S‐K. Chitin, Chitosan, Oligosaccharides and their Derivatives: Biological Activities and Applications. CRC Press; 2010. [Google Scholar]
- 37. Reys L, Silva SS, Oliveira JM, et al. Revealing the potential of squid chitosan‐based structures for biomedical applications. Biomed Mater. 2013;8(4):045002. [DOI] [PubMed] [Google Scholar]
- 38. Ferreira LM, Dos Santos AM, Boni FI, et al. Design of chitosan‐based particle systems: a review of the physicochemical foundations for tailored properties. Carbohydr Polym. 2020;250:116968. doi: 10.1016/j.carbpol.2020.116968 [DOI] [PubMed] [Google Scholar]
- 39. Aranaz I, Harris R, Heras A. Chitosan amphiphilic derivatives. Chemistry and applications. Curr Organ Chem. 2010;14(3):308‐330. [Google Scholar]
- 40. Domard A. pH and c.d. measurements on a fully deacetylated chitosan: application to CuII—polymer interactions. Int J Biol Macromol. 1987;9(2):98‐104. [Google Scholar]
- 41. Rinaudc M, Pavlov G, Desbrières J. Solubilization of chitosan in strong acid medium. Int J Polymer Anal Charact. 1999;5(3):267‐276. [Google Scholar]
- 42. Chattopadhyay D, Inamdar MS. Aqueous behaviour of chitosan. Int J Polym Sci. 2010. doi: 10.1155/2010/939536 [DOI] [Google Scholar]
- 43. Wang W, Xu D. Viscosity and flow properties of concentrated solutions of chitosan with different degrees of deacetylation. Int J Biol Macromol. 1994;16(3):149‐152. [DOI] [PubMed] [Google Scholar]
- 44. Bagheri M, Validi M, Gholipour A, Makvandi P, Sharifi E. Chitosan nanofiber biocomposites for potential wound healing applications: antioxidant activity with synergic antibacterial effect. Bioeng Transl Med. 2022;7(1):e10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abadehie FS, Dehkordib AH, Zafaric M, et al. Lawsone‐encapsulated chitosan/polyethylene oxide nanofibrous mat as a potential antibacterial biobased wound dressing. Eng Reg. 2021;2:219‐226. [Google Scholar]
- 46. Nikbakht M, Karbasi S, Rezayat SM, Tavakol S, Sharifi E. Evaluation of the effects of hyaluronic acid on poly (3‐hydroxybutyrate)/chitosan/carbon nanotubes electrospun scaffold: structure and mechanical properties. Polym‐Plast. Technol Mater. 2019;58(18):2031‐2040. [Google Scholar]
- 47. Jhaveri J, Raichura Z, Khan T, Momin M, Omri A. Chitosan nanoparticles‐insight into properties, functionalization and applications in drug delivery and theranostics. Molecules. 2021;26(2):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hajebi S, Hajebi S, Bagherzadeh M, et al. Stimulus‐responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019;92:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rabiee N, Bagherzadeh M, Ghadiri AM, et al. Turning toxic nanomaterials into a safe and bioactive nanocarrier for co‐delivery of DOX/pCRISPR. ACS Appl Bio Mater. 2021;4:5336‐5351. doi: 10.1021/acsabm.1c00447 [DOI] [PubMed] [Google Scholar]
- 50. Nasseri B, Kosemehmetoglu K, Kaya M, Piskin E, Rabiee N, Webster TJ. The pimpled gold nanosphere: a superior candidate for plasmonic photothermal therapy. Int J Nanomed. 2020;15:2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rabiee N, Yaraki MT, Garakani SM, et al. Recent advances in porphyrin‐based nanocomposites for effective targeted imaging and therapy. Biomaterials. 2020;232:119707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee S, Jo G, Jung JS, Yang DH, Hyun H. Near‐infra‐red fluorescent chitosan oligosaccharide lactate for targeted cancer imaging and photothermal therapy. Artif Cells Nanomed Biotechnol. 2020;48(1):1144‐1152. doi: 10.1080/21691401.2020.1817054 [DOI] [PubMed] [Google Scholar]
- 53. Ma K, Cheng Y, Wei X, Chen D, Zhao X, Jia P. Gold embedded chitosan nanoparticles with cell membrane mimetic polymer coating for pH‐sensitive controlled drug release and cellular fluorescence imaging. J Biomater Appl. 2021;35(7):857‐868. doi: 10.1177/0885328220952594 [DOI] [PubMed] [Google Scholar]
- 54. Dai X, Zhao X, Liu Y, et al. Controlled synthesis and surface engineering of Janus chitosan‐gold nanoparticles for photoacoustic imaging‐guided synergistic gene/photothermal therapy. Small. 2021;17(11):e2006004. doi: 10.1002/smll.202006004 [DOI] [PubMed] [Google Scholar]
- 55. Rasul RM, Tamilarasi Muniandy M, Zakaria Z, et al. A review on chitosan and its development as pulmonary particulate anti‐infective and anti‐cancer drug carriers. Carbohydr Polym. 2020;250:116800. doi: 10.1016/j.carbpol.2020.116800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miao YQ, Chen MS, Zhou X, et al. Chitosan oligosaccharide modified liposomes enhance lung cancer delivery of paclitaxel. Acta Pharmacol Sin. 2021;42(10):1714‐1722. doi: 10.1038/s41401-020-00594-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vikas, Viswanadh MK, Mehata AK, et al. Bioadhesive chitosan nanoparticles: dual targeting and pharmacokinetic aspects for advanced lung cancer treatment. Carbohydr Polym. 2021;274:118617. [DOI] [PubMed] [Google Scholar]
- 58. Fernández‐Álvarez F, García‐García G, Arias JL. A tri‐stimuli responsive (maghemite/PLGA)/chitosan nanostructure with promising applications in lung cancer. Pharmaceutics. 2021;13(8):1232. doi: 10.3390/pharmaceutics13081232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Safarzadeh M, Sadeghi S, Azizi M, Rastegari‐Pouyani M, Pouriran R, Haji Molla Hoseini M. Chitin and chitosan as tools to combat COVID‐19: a triple approach. Int J Biol Macromol. 2021;183:235‐244. doi: 10.1016/j.ijbiomac.2021.04.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jaber N, al‐Remawi M, al‐Akayleh F, al‐Muhtaseb N, al‐Adham ISI, Collier PJ. A review of the antiviral activity of chitosan, including patented applications and its potential use against COVID‐19. J Appl Microbiol. 2022;132(1):41‐58. doi: 10.1111/jam.15202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lotfy VF, Basta AH. Optimizing the chitosan‐cellulose based drug delivery system for controlling the ciprofloxacin release versus organic/inorganic crosslinker, characterization and kinetic study. Int J Biol Macromol. 2020;165:1496‐1506. [DOI] [PubMed] [Google Scholar]
- 62. Zhang X, Pan Y, Li S, et al. Doubly crosslinked biodegradable hydrogels based on gellan gum and chitosan for drug delivery and wound dressing. Int J Biol Macromol. 2020;164:2204‐2214. doi: 10.1016/j.ijbiomac.2020.08.093 [DOI] [PubMed] [Google Scholar]
- 63. Karpisheh V, Fakkari Afjadi J, Nabi Afjadi M, et al. Inhibition of HIF‐1α/EP4 axis by hyaluronate‐trimethyl chitosan‐SPION nanoparticles markedly suppresses the growth and development of cancer cells. Int J Biol Macromol. 2021;167:1006‐1019. doi: 10.1016/j.ijbiomac.2020.11.056 [DOI] [PubMed] [Google Scholar]
- 64. Hamza RZ, Al‐Salmi FA, El‐Shenawy NS. Chitosan and lecithin ameliorate osteoarthritis symptoms induced by monoiodoacetate in a rat model. Molecules. 2020;25(23):5738. doi: 10.3390/molecules25235738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Scognamiglio F, Travan A, Donati I, Borgogna M, Marsich E. A hydrogel system based on a lactose‐modified chitosan for viscosupplementation in osteoarthritis. Carbohydr Polym. 2020;248:116787. doi: 10.1016/j.carbpol.2020.116787 [DOI] [PubMed] [Google Scholar]
- 66. Liu Z, Mo X, Ma F, et al. Synthesis of carboxymethyl chitosan‐strontium complex and its therapeutic effects on relieving osteoarthritis. Carbohydr Polym. 2021;261:117869. doi: 10.1016/j.carbpol.2021.117869 [DOI] [PubMed] [Google Scholar]
- 67. Medina‐Cruz D, Mostafavi E, Vernet‐Crua A, et al. Green nanotechnology‐based drug delivery systems for osteogenic disorders. Expert Opin Drug Deliv. 2020;17(3):341‐356. [DOI] [PubMed] [Google Scholar]
- 68. Chen L, Wang C, Wu Y. Cholesterol (blood lipid) lowering potential of Rosuvastatin chitosan nanoparticles for atherosclerosis: preclinical study in rabbit model. Acta Biochim pol. 2020;67(4):495‐499. [DOI] [PubMed] [Google Scholar]
- 69. Gull N, Khan SM, Butt OM, et al. Inflammation targeted chitosan‐based hydrogel for controlled release of diclofenac sodium. Int J Biol Macromol. 2020;162:175‐187. doi: 10.1016/j.ijbiomac.2020.06.133 [DOI] [PubMed] [Google Scholar]
- 70. Shen K, Tang Q, Fang X, et al. The sustained release of dexamethasone from TiO(2) nanotubes reinforced by chitosan to enhance osteoblast function and anti‐inflammation activity. Mater Sci Eng C Mater Biol Appl. 2020;116:111241. doi: 10.1016/j.msec.2020.111241 [DOI] [PubMed] [Google Scholar]
- 71. Wang X, Almoallim HS, Cui Q, Alharbi SA, Yang H. In situ decorated au NPs on chitosan‐encapsulated Fe(3)O(4)‐NH(2) NPs as magnetic nanocomposite: investigation of its anti‐colon carcinoma, anti‐gastric cancer and anti‐pancreatic cancer. Int J Biol Macromol. 2021;171:198‐207. doi: 10.1016/j.ijbiomac.2020.12.037 [DOI] [PubMed] [Google Scholar]
- 72. Sarkar S, Das D, Dutta P, Kalita J, Wann SB, Manna P. Chitosan: a promising therapeutic agent and effective drug delivery system in managing diabetes mellitus. Carbohydr Polym. 2020;247:116594. doi: 10.1016/j.carbpol.2020.116594 [DOI] [PubMed] [Google Scholar]
- 73. Othman SI, Alturki AM, Abu‐Taweel GM, Altoom NG, Allam AA, Abdelmonem R. Chitosan for biomedical applications, promising antidiabetic drug delivery system, and new diabetes mellitus treatment based on stem cell. Int J Biol Macromol. 2021;190:417‐432. doi: 10.1016/j.ijbiomac.2021.08.154 [DOI] [PubMed] [Google Scholar]
- 74. Arnaldi P, Pastorino L, Monticelli O. On an effective approach to improve the properties and the drug release of chitosan‐based microparticles. Int J Biol Macromol. 2020;163:393‐401. doi: 10.1016/j.ijbiomac.2020.07.016 [DOI] [PubMed] [Google Scholar]
- 75. Shi W, Ching YC, Chuah CH. Preparation of aerogel beads and microspheres based on chitosan and cellulose for drug delivery: a review. Int J Biol Macromol. 2021;170:751‐767. doi: 10.1016/j.ijbiomac.2020.12.214 [DOI] [PubMed] [Google Scholar]
- 76. Silva TND, Reynaud F, Picciani PHS, de Holanda e Silva KG, Barradas TN. Chitosan‐based films containing nanoemulsions of methyl salicylate: formulation development, physical‐chemical and in vitro drug release characterization. Int J Biol Macromol. 2020;164:2558‐2568. doi: 10.1016/j.ijbiomac.2020.08.117 [DOI] [PubMed] [Google Scholar]
- 77. Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ. Doxorubicin‐induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev. 2014;34(1):106‐135. [DOI] [PubMed] [Google Scholar]
- 78. Soltantabar P, Calubaquib EL, Mostafavi E, Biewer MC, Stefan MC. Enhancement of loading efficiency by coloading of doxorubicin and quercetin in thermoresponsive polymeric micelles. Biomacromolecules. 2020;21(4):1427‐1436. [DOI] [PubMed] [Google Scholar]
- 79. Rabiee N, Haris MH, Ghadiri AM, Moghaddam FM, Fatahi Y. Polymer‐coated NH2‐UiO‐66 for the codelivery of DOX/pCRISPR. ACS Appl Mater Interfaces. 2021;13(9):10796‐10811. [DOI] [PubMed] [Google Scholar]
- 80. Bonadonna G, Monfardini S, De Lena M, Fossati‐Bellani F, Beretta G. Phase I and preliminary phase II evaluation of adriamycin (NSC 123127). Cancer Res. 1970;30(10):2572‐2582. [PubMed] [Google Scholar]
- 81. Bonadonna G, Monfardini S, de Lena M, Fossati‐Bellani F. Clinical evaluation of adriamycin, a new antitumour antibiotic. Br Med J. 1969;3(5669):503‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Carvalho C, Santos RX, Cardoso S, et al. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16(25):3267‐3285. [DOI] [PubMed] [Google Scholar]
- 83. Hortobagyi GJ. Anthracyclines in the treatment of cancer. Drugs. 1997;54(4):1‐7. [DOI] [PubMed] [Google Scholar]
- 84. Aubel‐Sadron G, Londos‐Gagliardi DJB. Daunorubicin and doxorubicin, anthracycline antibiotics, a physicochemical and biological review. Biochimie. 1984;66(5):333‐352. [DOI] [PubMed] [Google Scholar]
- 85. Ashrafizaveh S, Ashrafizadeh M, Zarrabi A, et al. Long non‐coding RNA in the doxorubicin resistance of cancer cells. Cancer Lett. 2021;508:104‐114. doi: 10.1016/j.canlet.2021.03.018 [DOI] [PubMed] [Google Scholar]
- 86. Maghsoudi S, Shahraki BT, Rabiee N, et al. Recent advancements in aptamer‐bioconjugates: sharpening stones for breast and prostate cancers targeting. J Drug Deliv Sci Technol. 2019;53:101146. [Google Scholar]
- 87. Mirzaei S et al. The involvement of epithelial‐to‐mesenchymal transition in doxorubicin resistance: possible molecular targets. Eur J Pharmacol. 2021;908:174344. [DOI] [PubMed] [Google Scholar]
- 88. Mirzaei S, Zarrabi A, Hashemi F, et al. Nrf2 signaling pathway in chemoprotection and doxorubicin resistance: potential application. Drug Discov. 2021;10(3):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ashrafizadeh M, Zarrabi A, Hashemi F, et al. Polychemotherapy with curcumin and doxorubicin via biological nanoplatforms: enhancing antitumor activity. Pharmaceutics. 2020;12(11):1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang C, Kar S, Lai X, et al. Triple negative breast cancer in Asia: an insider's view. Cancer Treat Rev. 2018;62:29‐38. doi: 10.1016/j.ctrv.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 91. Wang Y, Zhou P, Li P, Yang F, Gao XQ. Long non‐coding RNA H19 regulates proliferation and doxorubicin resistance in MCF‐7 cells by targeting PARP1. Bioengineered. 2020;11(1):536‐546. doi: 10.1080/21655979.2020.1761512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang S, Cheng M, Zheng X, et al. Interactions between lncRNA TUG1 and miR‐9‐5p modulate the resistance of breast cancer cells to doxorubicin by regulating eIF5A2. Onco Targets Ther. 2020;13:13159‐13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ji Y, Liu J, Zhu W, Ji J. circ_0002060 enhances doxorubicin resistance in osteosarcoma by regulating the miR‐198/ABCB1 Axis. Cancer Biother Radiopharm. 2020. doi: 10.1089/cbr.2020.4240 [DOI] [PubMed] [Google Scholar]
- 94. Guan H, Xu H, Chen J, et al. Circ_0001721 enhances doxorubicin resistance and promotes tumorigenesis in osteosarcoma through miR‐758/TCF4 axis. Cancer Cell Int. 2021;21(1):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Xiong L, Lin XM, Nie JH, Ye HS, Liu J. Resveratrol and its nanoparticle suppress doxorubicin/docetaxel‐resistant anaplastic thyroid cancer cells in vitro and in vivo. Nanotheranostics. 2021;5(2):143‐154. doi: 10.7150/ntno.53844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Casagrande N, Borghese C, Favero A, Vicenzetto C, Aldinucci D. Trabectedin overcomes doxorubicin‐resistance, counteracts tumor‐immunosuppressive reprogramming of monocytes and decreases xenograft growth in Hodgkin lymphoma. Cancer Lett. 2021;500:182‐193. doi: 10.1016/j.canlet.2020.12.015 [DOI] [PubMed] [Google Scholar]
- 97. Wang H, Shan S, Wang H, Wang X. CircATXN7 contributes to the progression and doxorubicin resistance of breast cancer via modulating miR‐149‐5p/HOXA11 pathway. Anticancer Drugs. 2022;33(1):e700‐e710. doi: 10.1097/CAD.0000000000001243 [DOI] [PubMed] [Google Scholar]
- 98. Cheng J‐T, Wang L, Wang H, et al. Insights into biological role of LncRNAs in epithelial‐mesenchymal transition. Cell. 2019;8(10):1178. doi: 10.3390/cells8101178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Xie M, Li J, Deng T, Yang N, Yang M. Modification of magnetic molybdenum disulfide by chitosan/carboxymethylcellulose with enhanced dispersibility for targeted photothermal−/chemotherapy of cancer. J Mater Chem B. 2021;9(7):1833‐1845. doi: 10.1039/D0TB01664K [DOI] [PubMed] [Google Scholar]
- 100. Zhu Z, Wei Z. CIP2A silencing alleviates doxorubicin resistance in MCF7/ADR cells through activating PP2A and autophagy. Clin Transl Oncol. 2021;23(8):1542‐1548. doi: 10.1007/s12094-021-02616-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhao W, Ning L, Wang L, et al. miR‐21 inhibition reverses doxorubicin‐resistance and inhibits PC3 human prostate cancer cells proliferation. Andrologia. 2021;53(5):e14016. doi: 10.1111/and.14016 [DOI] [PubMed] [Google Scholar]
- 102. Ashrafizadeh M, Mirzaei S, Gholami MH, et al. Hyaluronic acid‐based nanoplatforms for doxorubicin: a review of stimuli‐responsive carriers, co‐delivery and resistance suppression. Carbohydr Polym. 2021;272:118491. doi: 10.1016/j.carbpol.2021.118491 [DOI] [PubMed] [Google Scholar]
- 103. Paskeh MDA, Saebfar H, Mahabady MK, et al. Overcoming doxorubicin resistance in cancer: siRNA‐loaded nanoarchitectures for cancer gene therapy. Life Sci. 2022;298:120463. doi: 10.1016/j.lfs.2022.120463 [DOI] [PubMed] [Google Scholar]
- 104. Ashrafizadeh M, Saebfar H, Gholami MH, et al. Doxorubicin‐loaded graphene oxide nanocomposites in cancer medicine: stimuli‐responsive carriers, co‐delivery and suppressing resistance. Expert Opin Drug Deliv. 2022;1‐28. doi: 10.1080/17425247.2022.2041598 [DOI] [PubMed] [Google Scholar]
- 105. Makvandi P, Jamaledin R, Chen G, et al. Stimuli‐responsive transdermal microneedle patches. Mater Today. 2021;47:206‐222. doi: 10.1016/j.mattod.2021.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhuo S, Zhang F, Yu J, Zhang X, Yang G, Liu X. pH‐sensitive biomaterials for drug delivery. Molecules (Basel, Switzerland). 2020;25(23):5649. doi: 10.3390/molecules25235649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yan T, Zhu S, Hui W, He J, Liu Z, Cheng J. Chitosan based pH‐responsive polymeric prodrug vector for enhanced tumor targeted co‐delivery of doxorubicin and siRNA. Carbohydr Polym. 2020;250:116781. doi: 10.1016/j.carbpol.2020.116781 [DOI] [PubMed] [Google Scholar]
- 108. Wang R, Shou D, Lv O, Kong Y, Deng L, Shen J. pH‐controlled drug delivery with hybrid aerogel of chitosan, carboxymethyl cellulose and graphene oxide as the carrier. Int J Biol Macromol. 2017;103:248‐253. [DOI] [PubMed] [Google Scholar]
- 109. Marsano E, Bianchi E, Vicini S, et al. Stimuli responsive gels based on interpenetrating network of chitosan and poly (vinylpyrrolidone). Polymer. 2005;46(5):1595‐1600. [Google Scholar]
- 110. Hasan A, Waibhaw G, Tiwari S, Dharmalingam K, Shukla I, Pandey LM. Fabrication and characterization of chitosan, polyvinylpyrrolidone, and cellulose nanowhiskers nanocomposite films for wound healing drug delivery application. J Biomed Mater Res A. 2017;105(9):2391‐2404. [DOI] [PubMed] [Google Scholar]
- 111. Risbud MV, Hardikar AA, Bhata SV, Bhonde RR. pH‐sensitive freeze‐dried chitosan–polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J Controlled Release. 2000;68(1):23‐30. [DOI] [PubMed] [Google Scholar]
- 112. Gerami SE, Pourmadadi M, Fatoorehchi H, Yazdian F, Rashedi H, Nigjeh MN. Preparation of pH‐sensitive chitosan/polyvinylpyrrolidone/α‐Fe(2)O(3) nanocomposite for drug delivery application: emphasis on ameliorating restrictions. Int J Biol Macromol. 2021;173:409‐420. doi: 10.1016/j.ijbiomac.2021.01.067 [DOI] [PubMed] [Google Scholar]
- 113. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7‐30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 114. Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153(3):198‐205. doi: 10.1016/j.jconrel.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Shi Y, Li LC. Current advances in sustained‐release systems for parenteral drug delivery. Expert Opin Drug Deliv. 2005;2(6):1039‐1058. doi: 10.1517/17425247.2.6.1039 [DOI] [PubMed] [Google Scholar]
- 116. Sartipzadeh O, Naghib SM, Shokati F, et al. Microfluidic‐assisted synthesis and modelling of monodispersed magnetic nanocomposites for biomedical applications. J Nanotechnol Rev. 2020;9(1):1397‐1407. [Google Scholar]
- 117. Yin Q, Shen J, Zhang Z, Yu H, Li Y. Reversal of multidrug resistance by stimuli‐responsive drug delivery systems for therapy of tumor. Adv Drug Deliv Rev. 2013;65(13–14):1699‐1715. doi: 10.1016/j.addr.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 118. Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441(7092):437‐443. doi: 10.1038/nature04871 [DOI] [PubMed] [Google Scholar]
- 119. Gooneh‐Farahani S, Naghib SM, Naimi‐Jamal MR, Seyfoori A. A pH‐sensitive nanocarrier based on BSA‐stabilized graphene‐chitosan nanocomposite for sustained and prolonged release of anticancer agents. Sci Rep. 2021;11(1):17404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yang J, Naghib SM, Shokati F, et al. Enhanced therapeutic efficacy of doxorubicin for breast cancer using chitosan oligosaccharide‐modified halloysite nanotubes. Nanotechnol Rev. 2016;8(40):26578‐26590. [DOI] [PubMed] [Google Scholar]
- 121. Gao X, Zhou Y, Ma G, et al. A water‐soluble photocrosslinkable chitosan derivative prepared by Michael‐addition reaction as a precursor for injectable hydrogel. Carbohydr Polym. 2010;79(3):507‐512. [Google Scholar]
- 122. Kufelt O, El‐Tamer A, Sehring C, Meißner M, Schlie‐Wolter S, Chichkov BN. Water‐soluble photopolymerizable chitosan hydrogels for biofabrication via two‐photon polymerization. Acta Biomaterialia. 2015;18:186‐195. [DOI] [PubMed] [Google Scholar]
- 123. Li B, Wang L, Xu F, et al. Hydrosoluble, UV‐crosslinkable and injectable chitosan for patterned cell‐laden microgel and rapid transdermal curing hydrogel in vivo. Acta Biomater. 2015;22:59‐69. [DOI] [PubMed] [Google Scholar]
- 124. Zhou Y, Ma G, Shi S, Yang D, Nie J. Photopolymerized water‐soluble chitosan‐based hydrogel as potential use in tissue engineering. Int J Biol Macromol. 2011;48(3):408‐413. [DOI] [PubMed] [Google Scholar]
- 125. Carvalho IC, Mansur HS. Engineered 3D‐scaffolds of photocrosslinked chitosan‐gelatin hydrogel hybrids for chronic wound dressings and regeneration. Mater Sci Eng C. 2017;78:690‐705. [DOI] [PubMed] [Google Scholar]
- 126. Cho IS, Cho MO, Li Z, et al. Synthesis and characterization of a new photo‐crosslinkable glycol chitosan thermogel for biomedical applications. Carbohydr Polym. 2016;144:59‐67. [DOI] [PubMed] [Google Scholar]
- 127. He M, Jiang Z, Yang Y, Peng Y, Liu W. Synthesis of a chitosan‐based photo‐sensitive hydrogel and its biocompatibility and biodegradability. Carbohydr Polym. 2017;166:228‐235. [DOI] [PubMed] [Google Scholar]
- 128. Nada AA, Ali EA, Solimanc AAF. Biocompatible chitosan‐based hydrogel with tunable mechanical and physical properties formed at body temperature. Int J Biol Macromol. 2019;131:624‐632. [DOI] [PubMed] [Google Scholar]
- 129. Ding H, Li B, Jiang Y, et al. pH‐responsive UV crosslinkable chitosan hydrogel via "thiol‐ene" click chemistry for active modulating opposite drug release behaviors. Carbohydr Polym. 2021;251:117101. doi: 10.1016/j.carbpol.2020.117101 [DOI] [PubMed] [Google Scholar]
- 130. Fathi M, Majidi S, Zangabad PS, Barar J, Erfan‐Niya H, Omidi Y. Chitosan‐based multifunctional nanomedicines and theranostics for targeted therapy of cancer. Med Res Rev. 2018;38(6):2110‐2136. [DOI] [PubMed] [Google Scholar]
- 131. Sathiyaseelan A, Saravanakumar K, Mariadoss AVA, Wang MH. pH‐controlled nucleolin targeted release of dual drug from chitosan‐gold based aptamer functionalized nano drug delivery system for improved glioblastoma treatment. Carbohydr Polym. 2021;262:117907. doi: 10.1016/j.carbpol.2021.117907 [DOI] [PubMed] [Google Scholar]
- 132. Raja MA, Arif M, Feng C, Zeenat S, Liu CG. Synthesis and evaluation of pH‐sensitive, self‐assembled chitosan‐based nanoparticles as efficient doxorubicin carriers. J Biomater Appl. 2017;31(8):1182‐1195. doi: 10.1177/0885328216681184 [DOI] [PubMed] [Google Scholar]
- 133. Mirhadi E, Mashreghi M, Faal Maleki M, et al. Redox‐sensitive nanoscale drug delivery systems for cancer treatment. Int J Pharm. 2020;589:119882. doi: 10.1016/j.ijpharm.2020.119882 [DOI] [PubMed] [Google Scholar]
- 134. Ahmadi S, Rabieec N, Bagherzadeh M, et al. Stimulus‐responsive sequential release systems for drug and gene delivery. Nano Today. 2020;34:100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hu F‐Q, Zhao M‐d, Yuan H, You J, Du Y, Zeng S. A novel chitosan oligosaccharide–stearic acid micelles for gene delivery: properties and in vitro transfection studies. Int J Pharm. 2006;315(1–2):158‐166. [DOI] [PubMed] [Google Scholar]
- 136. Li J, Huo M, Wang J, et al. Redox‐sensitive micelles self‐assembled from amphiphilic hyaluronic acid‐deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials. 2012;33(7):2310‐2320. [DOI] [PubMed] [Google Scholar]
- 137. Yan J, Du Y‐Z, Chen F‐Y, You J, Yuan H, Hu F‐Q. Effect of proteins with different isoelectric points on the gene transfection efficiency mediated by stearic acid grafted chitosan oligosaccharide micelles. Mol Pharm. 2013;10(7):2568‐2577. [DOI] [PubMed] [Google Scholar]
- 138. Zhao M‐D, Hu F‐Q, Du Y‐Z, et al. Coadministration of glycolipid‐like micelles loading cytotoxic drug with different action site for efficient cancer chemotherapy. Nanotechnology. 2009;20(5):055102. [DOI] [PubMed] [Google Scholar]
- 139. You J, Wang Z, Du Y, et al. Specific tumor delivery of paclitaxel using glycolipid‐like polymer micelles containing gold nanospheres. Biomaterials. 2013;34(18):4510‐4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Su Y, Hu Y, du Y, et al. Redox‐responsive polymer‐drug conjugates based on doxorubicin and chitosan oligosaccharide‐g‐stearic acid for cancer therapy. Mol Pharm. 2015;12(4):1193‐1202. doi: 10.1021/mp500710x [DOI] [PubMed] [Google Scholar]
- 141. Zhou X, Guo L, Shi D, Duan S, Li J. Biocompatible chitosan Nanobubbles for ultrasound‐mediated targeted delivery of doxorubicin. Nanoscale Res Lett. 2019;14(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Xu RX. Multifunctional microbubbles and nanobubbles for photoacoustic imaging. Contrast Media Mol Imaging. 2011;6(5):401‐411. [DOI] [PubMed] [Google Scholar]
- 143. Sarkar A, Roy S, Sanpui P, Jaiswal A. Plasmonic gold nanorattle impregnated chitosan nanocarrier for stimulus responsive theranostics. ACS Appl Bio Mater. 2019;2(11):4812‐4825. doi: 10.1021/acsabm.9b00568 [DOI] [PubMed] [Google Scholar]
- 144. Ruel‐Gariepy E, Leroux J‐C. In situ‐forming hydrogels—review of temperature‐sensitive systems. Eur J Pharm Biopharm. 2004;58(2):409‐426. [DOI] [PubMed] [Google Scholar]
- 145. Ruel‐Gariepy E, Chenite A, Chaput C, Guirguis S, Leroux J. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int J Pharm. 2000;203(1–2):89‐98. [DOI] [PubMed] [Google Scholar]
- 146. Lalloo A, Chao P, Hu P, Stein S, Sinko PJ. Pharmacokinetic and pharmacodynamic evaluation of a novel in situ forming poly (ethylene glycol)‐based hydrogel for the controlled delivery of the camptothecins. J Control Release. 2006;112(3):333‐342. [DOI] [PubMed] [Google Scholar]
- 147. Ren S, Dai Y, Li C, et al. Pharmacokinetics and pharmacodynamics evaluation of a thermosensitive chitosan based hydrogel containing liposomal doxorubicin. Eur J Pharm Sci. 2016;92:137‐145. doi: 10.1016/j.ejps.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 148. Cho YI, Park S, Jeong SY, Yoo HS. In vivo and in vitro anti‐cancer activity of thermo‐sensitive and photo‐crosslinkable doxorubicin hydrogels composed of chitosan‐doxorubicin conjugates. Eur J Pharm Biopharm. 2009;73(1):59‐65. doi: 10.1016/j.ejpb.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 149. Wang W, Zhang P, Shan W, Gao J, Liang W. A novel chitosan‐based thermosensitive hydrogel containing doxorubicin liposomes for topical cancer therapy. J Biomater Sci Polym Ed. 2013;24(14):1649‐1659. doi: 10.1080/09205063.2013.789357 [DOI] [PubMed] [Google Scholar]
- 150. Dong X, Wei C, Liang J, Liu T, Kong D, Lv F. Thermosensitive hydrogel loaded with chitosan‐carbon nanotubes for near infrared light triggered drug delivery. Colloids Surf B Biointerfaces. 2017;154:253‐262. doi: 10.1016/j.colsurfb.2017.03.036 [DOI] [PubMed] [Google Scholar]
- 151. Chen X, Niu S, Bremner DH, et al. Co‐delivery of doxorubicin and oleanolic acid by triple‐sensitive nanocomposite based on chitosan for effective promoting tumor apoptosis. Carbohydr Polym. 2020;247:116672. doi: 10.1016/j.carbpol.2020.116672 [DOI] [PubMed] [Google Scholar]
- 152. Jiao J, Li X, Zhang S, et al. Redox and pH dual‐responsive PEG and chitosan‐conjugated hollow mesoporous silica for controlled drug release. Mater Sci Eng C Mater Biol Appl. 2016;67:26‐33. doi: 10.1016/j.msec.2016.04.091 [DOI] [PubMed] [Google Scholar]
- 153. Gao Y, Ma Q, Cao J, et al. Bifunctional alginate/chitosan stabilized perfluorohexane nanodroplets as smart vehicles for ultrasound and pH responsive delivery of anticancer agents. Int J Biol Macromol. 2021;191:1068‐1078. doi: 10.1016/j.ijbiomac.2021.09.166 [DOI] [PubMed] [Google Scholar]
- 154. Karthika V, AlSalhi MS, Devanesan S, Gopinath K, Arumugam A, Govindarajan M. Chitosan overlaid Fe(3)O(4)/rGO nanocomposite for targeted drug delivery, imaging, and biomedical applications. Sci Rep. 2020;10(1):18912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Liao T, Liu C, Ren J, et al. A chitosan/mesoporous silica nanoparticle‐based anticancer drug delivery system with a "tumor‐triggered targeting" property. Int J Biol Macromol. 2021;183:2017‐2029. doi: 10.1016/j.ijbiomac.2021.06.004 [DOI] [PubMed] [Google Scholar]
- 156. Mu Y, Wu G, Su C, et al. pH‐sensitive amphiphilic chitosan‐quercetin conjugate for intracellular delivery of doxorubicin enhancement. Carbohydr Polym. 2019;223:115072. doi: 10.1016/j.carbpol.2019.115072 [DOI] [PubMed] [Google Scholar]
- 157. Nogueira‐Librelotto DR, Scheeren LE, Macedo LB, Vinardell MP, Rolim CMB. pH‐sensitive chitosan‐tripolyphosphate nanoparticles increase doxorubicin‐induced growth inhibition of cervical HeLa tumor cells by apoptosis and cell cycle modulation. Colloids Surf B Biointerfaces. 2020;190:110897. doi: 10.1016/j.colsurfb.2020.110897 [DOI] [PubMed] [Google Scholar]
- 158. Huang N, Wang J, Cheng X, Xu Y, Li W. Fabrication of PNIPAM‐chitosan/decatungstoeuropate/silica nanocomposite for thermo/pH dual‐stimuli‐responsive and luminescent drug delivery system. J Inorg Biochem. 2020;211:111216. doi: 10.1016/j.jinorgbio.2020.111216 [DOI] [PubMed] [Google Scholar]
- 159. Verma NK, Purohit MP, Equbal D, et al. Targeted smart pH and thermoresponsive N,O‐carboxymethyl chitosan conjugated Nnanogels for enhanced therapeutic efficacy of doxorubicin in MCF‐7 breast cancer cells. Bioconjug Chem. 2016;27(11):2605‐2619. doi: 10.1021/acs.bioconjchem.6b00366 [DOI] [PubMed] [Google Scholar]
- 160. Xia B, Zhang W, Tong H, Li J, Chen Z, Shi J. Multifunctional chitosan/porous silicon@au nanocomposite hydrogels for long‐term and repeatedly localized combinatorial therapy of cancer via a single injection. ACS Biomater Sci Eng. 2019;5(4):1857‐1867. doi: 10.1021/acsbiomaterials.8b01533 [DOI] [PubMed] [Google Scholar]
- 161. Kong M, Zuo Y, Wang M, Bai X, Feng C, Chen X. Simply constructed chitosan nanocarriers with precise spatiotemporal control for efficient intracellular drug delivery. Carbohydr Polym. 2017;169:341‐350. doi: 10.1016/j.carbpol.2017.03.090 [DOI] [PubMed] [Google Scholar]
- 162. Bigham A, Foroughi F, Rezvani Ghomi E, Rafienia M, Neisiany RE, Ramakrishna S. The journey of multifunctional bone scaffolds fabricated from traditional toward modern techniques. Bio‐Design Manufact. 2020;3(4):281‐306. doi: 10.1007/s42242-020-00094-4 [DOI] [Google Scholar]
- 163. Bigham A, Aghajanian AH, Saudi A, Rafienia M. Hierarchical porous Mg2SiO4‐CoFe2O4 nanomagnetic scaffold for bone cancer therapy and regeneration: surface modification and in vitro studies. Mater Sci Eng C. 2020;109:110579. [DOI] [PubMed] [Google Scholar]
- 164. Ou W, Byeon JH, Thapa RK, Ku SK, Yong CS, Kim JO. Plug‐and‐play Nanorization of coarse black phosphorus for targeted chemo‐photoimmunotherapy of colorectal cancer. ACS Nano. 2018;12(10):10061‐10074. doi: 10.1021/acsnano.8b04658 [DOI] [PubMed] [Google Scholar]
- 165. Mirzaei S, Gholami MH, Hashemi F, et al. Employing siRNA tool and its delivery platforms in suppressing cisplatin resistance: approaching to a new era of cancer chemotherapy. Life Sci. 2021;277:119430. [DOI] [PubMed] [Google Scholar]
- 166. Delfi M, Sartorius R, Ashrafizadeh M, et al. Self‐assembled peptide and protein nanostructures for anti‐cancer therapy: targeted delivery, stimuli‐responsive devices and immunotherapy. Nano Today. 2021;38:101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Mirzaei S, Hushmandi K, Zabolian A, et al. Elucidating role of reactive oxygen species (ROS) in cisplatin chemotherapy: a focus on molecular pathways and possible therapeutic strategies. Molecules. 2021;26(8):2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Guo Y, Chu M, Tan S, et al. Chitosan‐g‐TPGS nanoparticles for anticancer drug delivery and overcoming multidrug resistance. Mol Pharm. 2014;11(1):59‐70. doi: 10.1021/mp400514t [DOI] [PubMed] [Google Scholar]
- 169. Siddharth S, Nayak A, Nayak D, Bindhani BK, Kundu CN. Chitosan‐dextran sulfate coated doxorubicin loaded PLGA‐PVA‐nanoparticles caused apoptosis in doxorubicin resistance breast cancer cells through induction of DNA damage. Sci Rep. 2017;7(1):2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Luesakul U, Puthong S, Neamati N, Muangsin N. pH‐responsive selenium nanoparticles stabilized by folate‐chitosan delivering doxorubicin for overcoming drug‐resistant cancer cells. Carbohydr Polym. 2018;181:841‐850. doi: 10.1016/j.carbpol.2017.11.068 [DOI] [PubMed] [Google Scholar]
- 171. Meng T, Qiu G, Hong Y, et al. Effect of chitosan based glycolipid‐like nanocarrier in prevention of developing acquired drug resistance in tri‐cycle treatment of breast cancer. Int J Pharm. 2019;555:303‐313. doi: 10.1016/j.ijpharm.2018.11.056 [DOI] [PubMed] [Google Scholar]
- 172. Giarra S, Zappavigna S, Campani V, et al. Chitosan‐based polyelectrolyte complexes for doxorubicin and Zoledronic acid combined therapy to overcome multidrug resistance. Pharmaceutics. 2018;10(4):180. doi: 10.3390/pharmaceutics10040180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Duan J, Mansour HM, Zhang Y, et al. Reversion of multidrug resistance by co‐encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticles. Int J Pharm. 2012;426(1–2):193‐201. doi: 10.1016/j.ijpharm.2012.01.020 [DOI] [PubMed] [Google Scholar]
- 174. Ahmadi S, Rabiee N, Fatahi Y, et al. Controlled gene delivery systems: nanomaterials and chemical approaches. J Biomed Nanotechnol. 2020;16(5):553‐582. [DOI] [PubMed] [Google Scholar]
- 175. Rabiee N et al. Carbosilane dendrimers: drug and gene delivery applications. J Drug Deliv Sci Technol. 2020:101879. [Google Scholar]
- 176. Rabiee N, Ahmadi S, Arab Z, et al. Aptamer hybrid nanocomplexes as targeting components for antibiotic/gene delivery systems and diagnostics: a review. Int J Nanomed. 2020;15:4237‐4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ashrafizade M, Delfi M, Hashemi F, et al. Biomedical application of chitosan‐based nanoscale delivery systems: potential usefulness in siRNA delivery for cancer therapy. Carbohydr Polym. 2021;260:117809. doi: 10.1016/j.carbpol.2021.117809 [DOI] [PubMed] [Google Scholar]
- 178. Makvandi P, Josic U, Delfi M, et al. Drug delivery (nano) platforms for oral and dental applications: tissue regeneration, infection control, and cancer management. Adv Sci. 2021;8(8):2004014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Gantenbein B, Tang S, Guerrero J, et al. Non‐viral gene delivery methods for bone and joints. Front Bioeng Biotechnol. 2020;8:1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Duan L, Xu L, Xu X, et al. Exosome‐mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13(3):1387‐1397. [DOI] [PubMed] [Google Scholar]
- 181. Kimura S, Harashima HJP. Current status and challenges associated with CNS‐targeted gene delivery across the BBB. Pharmaceutics. 2020;12(12):1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Chen S, Deng J, Zhang LM. Cationic nanoparticles self‐assembled from amphiphilic chitosan derivatives containing poly(amidoamine) dendrons and deoxycholic acid as a vector for co‐delivery of doxorubicin and gene. Carbohydr Polym. 2021;258:117706. doi: 10.1016/j.carbpol.2021.117706 [DOI] [PubMed] [Google Scholar]
- 183. Alinejad V, Hossein Somi M, Baradaran B, et al. Co‐delivery of IL17RB siRNA and doxorubicin by chitosan‐based nanoparticles for enhanced anticancer efficacy in breast cancer cells. Biomed Pharmacother. 2016;83:229‐240. doi: 10.1016/j.biopha.2016.06.037 [DOI] [PubMed] [Google Scholar]
- 184. Butt AM, Amin MCIM, Katas H, Abdul Murad NA, Jamal R, Kesharwani P. Doxorubicin and siRNA codelivery via chitosan‐coated pH‐responsive mixed micellar Polyplexes for enhanced cancer therapy in multidrug‐resistant tumors. Mol Pharm. 2016;13(12):4179‐4190. doi: 10.1021/acs.molpharmaceut.6b00776 [DOI] [PubMed] [Google Scholar]
- 185. Siahmansouri H, Somi MH, Babaloo Z, et al. Effects of HMGA2 siRNA and doxorubicin dual delivery by chitosan nanoparticles on cytotoxicity and gene expression of HT‐29 colorectal cancer cell line. J Pharm Pharmacol. 2016;68(9):1119‐1130. doi: 10.1111/jphp.12593 [DOI] [PubMed] [Google Scholar]
- 186. Zhu QL, Zhou Y, Guan M, et al. Low‐density lipoprotein‐coupled N‐succinyl chitosan nanoparticles co‐delivering siRNA and doxorubicin for hepatocyte‐targeted therapy. Biomaterials. 2014;35(22):5965‐5976. doi: 10.1016/j.biomaterials.2014.03.088 [DOI] [PubMed] [Google Scholar]
- 187. Han L, Zhao J, Zhang X, et al. Enhanced siRNA delivery and silencing gold‐chitosan nanosystem with surface charge‐reversal polymer assembly and good biocompatibility. ACS Nano. 2012;6(8):7340‐7351. doi: 10.1021/nn3024688 [DOI] [PubMed] [Google Scholar]
- 188. Shali H, Shabani M, Pourgholi F, et al. Co‐delivery of insulin‐like growth factor 1 receptor specific siRNA and doxorubicin using chitosan‐based nanoparticles enhanced anticancer efficacy in A549 lung cancer cell line. Artif Cells Nanomed Biotechnol. 2018;46(2):293‐302. doi: 10.1080/21691401.2017.1307212 [DOI] [PubMed] [Google Scholar]
- 189. Yoon HY, Son S, Lee SJ, et al. Glycol chitosan nanoparticles as specialized cancer therapeutic vehicles: sequential delivery of doxorubicin and Bcl‐2 siRNA. Sci Rep. 2014;4:6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Seifi‐Najmi M, Hajivalili M, Safaralizadeh R, et al. SiRNA/DOX lodeded chitosan based nanoparticles: development, characterization and in vitro evaluation on A549 lung cancer cell line. Cell Mol Biol (Noisy‐le‐Grand). 2016;62(11):87‐94. [PubMed] [Google Scholar]
- 191. Eivazy P, Atyabi F, Jadidi‐Niaragh F, et al. The impact of the codelivery of drug‐siRNA by trimethyl chitosan nanoparticles on the efficacy of chemotherapy for metastatic breast cancer cell line (MDA‐MB‐231). Artif Cells Nanomed Biotechnol. 2017;45(5):889‐896. [DOI] [PubMed] [Google Scholar]
- 192. Choi C et al. Application of chitosan and chitosan derivatives as biomaterials. 2016;33:1‐10. [Google Scholar]
- 193. Deng J, Nam J‐P, Nah J‐W. Dendronized chitosan derivative as a biocompatible gene delivery carrier. J Indus Eng Chem. 2011;12(3):642‐649. [DOI] [PubMed] [Google Scholar]
- 194. Xu Q, Leong J, Chua QY, et al. Combined modality doxorubicin‐based chemotherapy and chitosan‐mediated p53 gene therapy using double‐walled microspheres for treatment of human hepatocellular carcinoma. Biomaterials. 2013;34(21):5149‐5162. doi: 10.1016/j.biomaterials.2013.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Xu Q, Xia Y, Wang CH, Pack DW. Monodisperse double‐walled microspheres loaded with chitosan‐p53 nanoparticles and doxorubicin for combined gene therapy and chemotherapy. J Control Release. 2012;163(2):130‐135. doi: 10.1016/j.jconrel.2012.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Mirzaei S, Mahabady MK, Zabolian A, et al. Small interfering RNA (siRNA) to target genes and molecular pathways in glioblastoma therapy: current status with an emphasis on delivery systems. Life Sci. 2021;275:119368. doi: 10.1016/j.lfs.2021.119368 [DOI] [PubMed] [Google Scholar]
- 197. Ashrafizadeh M, Zarrabi A, Hushmandi K, et al. Progress in natural compounds/siRNA co‐delivery employing nanovehicles for cancer therapy. ACS Comb Sci. 2020;22(12):669‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ashrafizadeh M, Hushmandi K, Moghadam ER, et al. Progress in delivery of siRNA‐based therapeutics employing nano‐vehicles for treatment of prostate cancer. Bioengineering (Basel). 2020;7(3):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75(1):1‐18. [DOI] [PubMed] [Google Scholar]
- 200. Muhamad II, Selvakumaran S, Lazim NAMJN. Designing polymeric nanoparticles for targeted drug delivery system. Nanomedicine. 2014;287:287. [Google Scholar]
- 201. Cai W, Cai W, Jiang H, Weizmann Y, Wang X. Intelligent bio‐responsive fluorescent Au‐shRNA complexes for regulated autophagy and effective cancer bioimaging and therapeutics. Biosensors (Basel). 2021;11(11):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Zhang X, Taoka R, Liu D, et al. Knockdown of RRM1 with adenoviral shRNA vectors to inhibit tumor cell viability and increase chemotherapeutic sensitivity to gemcitabine in bladder cancer cells. Int J Mol Sci. 2021;22(8):4102. doi: 10.3390/ijms22084102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Wang J, Wang K, Liang J, Jin J, Wang X, Yan S. Chitosan‐tripolyphosphate nanoparticles‐mediated co‐delivery of MTHFD1L shRNA and 5‐aminolevulinic acid for combination photodynamic‐gene therapy in oral cancer. Photodiagnosis Photodyn Ther. 2021;36:102581. doi: 10.1016/j.pdpdt.2021.102581 [DOI] [PubMed] [Google Scholar]
- 204. Lu M, Xu Z, Wei F, et al. Oncolytic adenovirus carrying SPAG9‐shRNA enhanced the efficacy of docetaxel for advanced prostate cancer. Anticancer Drugs. 2022;33(2):142‐148. doi: 10.1097/CAD.0000000000001251 [DOI] [PubMed] [Google Scholar]
- 205. Yang H, Chen Y, Chen Z, et al. Chemo‐photodynamic combined gene therapy and dual‐modal cancer imaging achieved by pH‐responsive alginate/chitosan multilayer‐modified magnetic mesoporous silica nanocomposites. Biomater Sci. 2017;5(5):1001‐1013. 10.1039/C7BM00043J [DOI] [PubMed] [Google Scholar]
- 206. Paskeh MDA, Mirzaeic S, Oroueid S, et al. Revealing the role of miRNA‐489 as a new onco‐suppressor factor in different cancers based on pre‐clinical and clinical evidence. Int J Biol Macromol. 2021;191:727‐737. doi: 10.1016/j.ijbiomac.2021.09.089 [DOI] [PubMed] [Google Scholar]
- 207. Ashrafizadeh M, Zarrabi A, Orouei S, et al. Interplay between SOX9 transcription factor and microRNAs in cancer. Int J Biol Macromol. 2021;183:681‐694. doi: 10.1016/j.ijbiomac.2021.04.185 [DOI] [PubMed] [Google Scholar]
- 208. Mirzaei S, Zarrabi A, Asnaf SE, et al. The role of microRNA‐338‐3p in cancer: growth, invasion, chemoresistance and mediators. Life Sci. 2021;268:119005. [DOI] [PubMed] [Google Scholar]
- 209. Mirzaei S, Saebfar H, Gholami MH, et al. MicroRNAs regulating SOX2 in cancer progression and therapy response. Expert Rev Mol Med. 2021;23. [DOI] [PubMed] [Google Scholar]
- 210. Meijuan W, Mao X, Wang S. Clinical significance of miR‐139‐5p and FGF2 in ovarian cancer. J BUON. 2021;26(3):663‐669. [PubMed] [Google Scholar]
- 211. Hang J, Wei F, Yan Z, Zhang X, Xu K, Zhu Y. The value of miR‐510 in the prognosis and development of colon cancer. Open Med (Wars). 2021;16(1):795‐804. doi: 10.1515/med-2021-0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Wang J. Tripterine and miR‐184 show synergy to suppress breast cancer progression. Biochem Biophys Res Commun. 2021;561:19‐25. doi: 10.1016/j.bbrc.2021.04.108 [DOI] [PubMed] [Google Scholar]
- 213. Deng X, Cao M, Zhang J, et al. Hyaluronic acid‐chitosan nanoparticles for co‐delivery of MiR‐34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials. 2014;35(14):4333‐4344. doi: 10.1016/j.biomaterials.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 214. Unsoy G, Gunduz U. Targeted silencing of Survivin in cancer cells by siRNA loaded chitosan magnetic nanoparticles. Expert Rev Anticancer Ther. 2016;16(7):789‐797. doi: 10.1080/14737140.2016.1184981 [DOI] [PubMed] [Google Scholar]
- 215. Zhao Z, Ji M, Wang Q, He N, Li Y. Ca(2+) signaling modulation using cancer cell membrane coated chitosan nanoparticles to combat multidrug resistance of cancer. Carbohydr Polym. 2020;238:116073. doi: 10.1016/j.carbpol.2020.116073 [DOI] [PubMed] [Google Scholar]
- 216. Wang C, Ravi S, Garapati US, et al. Multifunctional chitosan magnetic‐graphene (CMG) nanoparticles: a Theranostic platform for tumor‐targeted co‐delivery of drugs, genes and MRI contrast agents. J Mater Chem B. 2013;1(35):4396‐4405. doi: 10.1039/c3tb20452a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Abadi AJ, Mirzaei S, Mahabady MK, et al. Curcumin and its derivatives in cancer therapy: potentiating antitumor activity of cisplatin and reducing side effects. Phytother Res. 2022;36(1):189‐213. [DOI] [PubMed] [Google Scholar]
- 218. Ashrafizadeh M, Zarrabi A, Mirzaei S, et al. Gallic acid for cancer therapy: molecular mechanisms and boosting efficacy by nanoscopical delivery. Food Chem Toxicol. 2021;157:112576. [DOI] [PubMed] [Google Scholar]
- 219. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7‐33. [DOI] [PubMed] [Google Scholar]
- 220. Ashrafizadeh M, Mirzaei S, Hushmandi K, et al. Therapeutic potential of AMPK signaling targeting in lung cancer: advances, challenges and future prospects. Life Sci. 2021;278:119649. [DOI] [PubMed] [Google Scholar]
- 221. Abadi AJ, Zarrabi A, Gholami MH, et al. Small in size, but large in action: microRNAs as potential modulators of PTEN in breast and lung cancers. Biomolecules. 2021;11(2):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Ashrafizadeh M, Zarrabi A, Hushmandi K, et al. Toward regulatory effects of curcumin on transforming growth factor‐beta across different diseases: a review. Front Pharmacol. 2020;11:1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Lee R, Choi YJ, Jeong MS, et al. Hyaluronic acid‐decorated glycol chitosan nanoparticles for pH‐sensitive controlled release of doxorubicin and celecoxib in nonsmall cell lung cancer. Bioconjug Chem. 2020;31(3):923‐932. doi: 10.1021/acs.bioconjchem.0c00048 [DOI] [PubMed] [Google Scholar]
- 224. Farboudi A, Mahboobnia K, Chogan F, et al. UiO‐66 metal organic framework nanoparticles loaded carboxymethyl chitosan/poly ethylene oxide/polyurethane core‐shell nanofibers for controlled release of doxorubicin and folic acid. Int J Biol Macromol. 2020;150:178‐188. doi: 10.1016/j.ijbiomac.2020.02.067 [DOI] [PubMed] [Google Scholar]
- 225. Ashrafizadeh M, Ahmadi Z, Mohamamdinejad R, et al. Curcumin therapeutic modulation of the Wnt signaling pathway. Curr Pharm Biotechnol. 2020;21(11):1006‐1015. [DOI] [PubMed] [Google Scholar]
- 226. Ashrafizadeh M, Yaribeygi H, Sahebkar A. Therapeutic effects of curcumin against bladder cancer: a review of possible molecular pathways. Anticancer Agents Med Chem. 2020;20(6):667‐677. [DOI] [PubMed] [Google Scholar]
- 227. Azarian M, Azarian M, Hossein HHS, et al. Folic acid‐adorned curcumin‐loaded iron oxide nanoparticles for cervical cancer. ACS Appl Bio Mater. 2022;5(3):1305‐1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Jyoti K, Pandey RS, Kush P, Kaushik D, Jain UK, Madan J. Inhalable bioresponsive chitosan microspheres of doxorubicin and soluble curcumin augmented drug delivery in lung cancer cells. Int J Biol Macromol. 2017;98:50‐58. doi: 10.1016/j.ijbiomac.2017.01.109 [DOI] [PubMed] [Google Scholar]
- 229. Kim MW, Niidome T, Lee R. Glycol chitosan‐docosahexaenoic acid liposomes for drug delivery: synergistic effect of doxorubicin‐rapamycin in drug‐resistant breast cancer. Mar Drugs. 2019;17(10) :581. doi: 10.3390/md17100581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. van der Koog L, Gandek TB, Nagelkerke A. Liposomes and extracellular vesicles as drug delivery systems: a comparison of composition, pharmacokinetics, and functionalization. Adv Healthc Mater. 2022;11:e2100639. doi: 10.1002/adhm.202100639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Al Saqr A et al. Co‐delivery of Hispolon and doxorubicin liposomes improves efficacy against melanoma cells. AAPS PharmSciTech. 2020;21(8):304. [DOI] [PubMed] [Google Scholar]
- 233. Murugesan K, Srinivasan P, Mahadeva R, Gupta CM, Haq W. Tuftsin‐bearing liposomes co‐encapsulated with doxorubicin and curcumin efficiently inhibit EAC tumor growth in mice. Int J Nanomedicine. 2020;15:10547‐10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Wi TI, Won JE, Lee CM, et al. Efficacy of combination therapy with linalool and doxorubicin encapsulated by liposomes as a two‐in‐one hybrid carrier system for epithelial ovarian carcinoma. Int J Nanomedicine. 2020;15:8427‐8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Lian B, Wei H, Pan R, et al. Galactose modified liposomes for effective co‐delivery of doxorubicin and Combretastatin A4. Int J Nanomedicine. 2021;16:457‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Deygen IM, Seidl C, Kölmel DK, et al. Novel prodrug of doxorubicin modified by stearoylspermine encapsulated into PEG‐chitosan‐stabilized liposomes. Langmuir. 2016;32(42):10861‐10869. doi: 10.1021/acs.langmuir.6b01023 [DOI] [PubMed] [Google Scholar]
- 237. Yan L, Crayton SH, Thawani JP, Amirshaghaghi A, Tsourkas A, Cheng Z. A pH‐responsive drug‐delivery platform based on glycol chitosan‐coated liposomes. Small. 2015;11(37):4870‐4874. doi: 10.1002/smll.201501412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Cavalcante CH, Fernandes RS, de Oliveira Silva J, et al. Doxorubicin‐loaded pH‐sensitive micelles: a promising alternative to enhance antitumor activity and reduce toxicity. Biomed Pharmacother. 2021;134:111076. doi: 10.1016/j.biopha.2020.111076 [DOI] [PubMed] [Google Scholar]
- 239. Feng R, Wang W, Zhu L, Xu H, Chen S, Song Z. Phenylboronic acid‐functionalized F127‐oligochitosan conjugate micelles for doxorubicin encapsulation. J Biomed Mater Res B Appl Biomater. 2020;108(8):3345‐3355. doi: 10.1002/jbm.b.34670 [DOI] [PubMed] [Google Scholar]
- 240. Wang C, Qi P, Lu Y, et al. Bicomponent polymeric micelles for pH‐controlled delivery of doxorubicin. Drug Deliv. 2020;27(1):344‐357. doi: 10.1080/10717544.2020.1726526 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 241. Gao X, Lu Y, Wu B, et al. Interface cisplatin‐crosslinked doxorubicin‐loaded triblock copolymer micelles for synergistic cancer therapy. Colloids Surf B Biointerfaces. 2020;196:111334. doi: 10.1016/j.colsurfb.2020.111334 [DOI] [PubMed] [Google Scholar]
- 242. Xie L, Liu R, Chen X, He M, Zhang Y, Chen S. Micelles based on lysine, histidine, or arginine: designing structures for enhanced drug delivery. Front Bioeng Biotechnol. 2021;9:744657. doi: 10.3389/fbioe.2021.744657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Rosch JG, Winter H, DuRoss AN, Sahay G, Sun C. Inverse‐micelle synthesis of doxorubicin‐loaded alginate/chitosan nanoparticles and in vitro assessment of breast cancer cytotoxicity. Colloid Interface Sci Commun. 2019;28:69‐74. doi: 10.1016/j.colcom.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Yan T, Li D, Li J, et al. Effective co‐delivery of doxorubicin and curcumin using a glycyrrhetinic acid‐modified chitosan‐cystamine‐poly(ε‐caprolactone) copolymer micelle for combination cancer chemotherapy. Colloids Surf B Biointerfaces. 2016;145:526‐538. doi: 10.1016/j.colsurfb.2016.05.070 [DOI] [PubMed] [Google Scholar]
- 245. Kittur F, Kittura KV, Prashantha H, Sankarb KU, Tharanathana RN. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr Polym. 2002;49(2):185‐193. [Google Scholar]
- 246. Win PP, Shin‐ya Y, Kyung‐JinHong TK. Formulation and characterization of pH sensitive drug carrier based on phosphorylated chitosan. Carbohydr Polym. 2003;53(3):305‐310. [Google Scholar]
- 247. Sarasam AR, Krishnaswamy RK, Madihally SVJB. Blending chitosan with polycaprolactone: effects on physicochemical and antibacterial properties. Biomacromolecules. 2006;7(4):1131‐1138. [DOI] [PubMed] [Google Scholar]
- 248. Xiangyang X, Ling L, Jianping Z, et al. Preparation and characterization of N‐succinyl‐N'‐octyl chitosan micelles as doxorubicin carriers for effective anti‐tumor activity. Colloids Surf B Biointerfaces. 2007;55(2):222‐228. doi: 10.1016/j.colsurfb.2006.12.006 [DOI] [PubMed] [Google Scholar]
- 249. Su CW, Chiang MY, Lin YL, et al. Sodium dodecyl sulfate‐modified doxorubicin‐loaded chitosan‐lipid Nanocarrier with multi polysaccharide‐lecithin Nanoarchitecture for augmented bioavailability and stability of Oral administration in vitro and in vivo. J Biomed Nanotechnol. 2016;12(5):962‐972. doi: 10.1166/jbn.2016.2227 [DOI] [PubMed] [Google Scholar]
- 250. Hu FQ, Liu LN, du YZ, Yuan H. Synthesis and antitumor activity of doxorubicin conjugated stearic acid‐g‐chitosan oligosaccharide polymeric micelles. Biomaterials. 2009;30(36):6955‐6963. doi: 10.1016/j.biomaterials.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 251. Saeb MR, Rabiee N, Mozafari M, Mostafavi E. Metal‐organic frameworks (MOFs)‐based nanomaterials for drug delivery. Materials. 2021;14(13):3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Rabiee N, Rabiee M, Sojdeh S, et al. Porphyrin molecules decorated on metal–organic frameworks for multi‐functional biomedical applications. Biomolecules. 2021;11(11):1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Chowdhuri AR, Singh T, Ghosh SK, Sahu SK. Carbon dots embedded magnetic nanoparticles @chitosan @metal organic framework as a Nanoprobe for pH sensitive targeted anticancer drug delivery. ACS Appl Mater Interfaces. 2016;8(26):16573‐16583. doi: 10.1021/acsami.6b03988 [DOI] [PubMed] [Google Scholar]
- 254. Abazari R, Mahjoub AR, Ataei F, et al. Chitosan immobilization on bio‐MOF nanostructures: a biocompatible pH‐responsive Nanocarrier for doxorubicin release on MCF‐7 cell lines of human breast cancer. Inorg Chem. 2018;57(21):13364‐13379. doi: 10.1021/acs.inorgchem.8b01955 [DOI] [PubMed] [Google Scholar]
- 255. Zhao G, Wang J, Peng X, Li Y, Yuan X, Ma Y. Facile solvothermal synthesis of mesostructured Fe3O4/chitosan nanoparticles as delivery vehicles for pH‐responsive drug delivery and magnetic resonance imaging contrast agents. Chem Asian J. 2014;9(2):546‐553. doi: 10.1002/asia.201301072 [DOI] [PubMed] [Google Scholar]
- 256. Pandit A, Khare L, Jahagirdar D, Srivastav A, Jain R, Dandekar P. Probing synergistic interplay between bio‐inspired peptidomimetic chitosan‐copper complexes and doxorubicin. Int J Biol Macromol. 2020;161:1475‐1483. doi: 10.1016/j.ijbiomac.2020.07.241 [DOI] [PubMed] [Google Scholar]
- 257. Khademi Z, Lavaee P, Ramezani M, Alibolandi M, Abnous K, Taghdisi SM. Co‐delivery of doxorubicin and aptamer against Forkhead box M1 using chitosan‐gold nanoparticles coated with nucleolin aptamer for synergistic treatment of cancer cells. Carbohydr Polym. 2020;248:116735. doi: 10.1016/j.carbpol.2020.116735 [DOI] [PubMed] [Google Scholar]
- 258. Kang JW, Cho HJ, Lee HJ, Jin HE, Maeng HJ. Polyethylene glycol‐decorated doxorubicin/carboxymethyl chitosan/gold nanocomplex for reducing drug efflux in cancer cells and extending circulation in blood stream. Int J Biol Macromol. 2019;125:61‐71. doi: 10.1016/j.ijbiomac.2018.12.028 [DOI] [PubMed] [Google Scholar]
- 259. Al‐Musawi S, Albukhaty S, Al‐Karagoly H, Almalki F. Design and synthesis of multi‐functional superparamagnetic core‐gold shell coated with chitosan and folate nanoparticles for targeted antitumor therapy. Nanomaterials (Basel). 2020;11(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Horo H, Bhattacharyya S, Mandal B, Kundu LM. Synthesis of functionalized silk‐coated chitosan‐gold nanoparticles and microparticles for target‐directed delivery of antitumor agents. Carbohydr Polym. 2021;258:117659. doi: 10.1016/j.carbpol.2021.117659 [DOI] [PubMed] [Google Scholar]
- 261. Rasoulzadehzali M, Namazi H. Facile preparation of antibacterial chitosan/graphene oxide‐ag bio‐nanocomposite hydrogel beads for controlled release of doxorubicin. Int J Biol Macromol. 2018;116:54‐63. doi: 10.1016/j.ijbiomac.2018.04.140 [DOI] [PubMed] [Google Scholar]
- 262. Shen JW, Li J, Dai J, et al. Molecular dynamics study on the adsorption and release of doxorubicin by chitosan‐decorated graphene. Carbohydr Polym. 2020;248:116809. doi: 10.1016/j.carbpol.2020.116809 [DOI] [PubMed] [Google Scholar]
- 263. Anirudhan T, Deepa JR. Nano‐zinc oxide incorporated graphene oxide/nanocellulose composite for the adsorption and photo catalytic degradation of ciprofloxacin hydrochloride from aqueous solutions. J Colloid Interface Sci. 2017;490:343‐356. [DOI] [PubMed] [Google Scholar]
- 264. Fiorica C, Mauro N, Pitarresi G, Scialabba C, Palumbo FS, Giammona G. Double‐network‐structured graphene oxide‐containing nanogels as photothermal agents for the treatment of colorectal cancer. Biomacromolecules. 2017;18(3):1010‐1018. [DOI] [PubMed] [Google Scholar]
- 265. Anirudhan TS, Chithra Sekhar V, Athira VS. Graphene oxide based functionalized chitosan polyelectrolyte nanocomposite for targeted and pH responsive drug delivery. Int J Biol Macromol. 2020;150:468‐479. doi: 10.1016/j.ijbiomac.2020.02.053 [DOI] [PubMed] [Google Scholar]
- 266. Omidi S, Pirhayati M, Kakanejadifard A. Co‐delivery of doxorubicin and curcumin by a pH‐sensitive, injectable, and in situ hydrogel composed of chitosan, graphene, and cellulose nanowhisker. Carbohydr Polym. 2020;231:115745. doi: 10.1016/j.carbpol.2019.115745 [DOI] [PubMed] [Google Scholar]
- 267. Rungnim C, Rungrotmongkol T, Poo‐Arporn RP. pH‐controlled doxorubicin anticancer loading and release from carbon nanotube noncovalently modified by chitosan: MD simulations. J Mol Graph Model. 2016;70:70‐76. doi: 10.1016/j.jmgm.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 268. Khoshoei A, Ghasemy E, Poustchi F, Shahbazi MA, Maleki R. Engineering the pH‐sensitivity of the graphene and carbon nanotube based nanomedicines in smart cancer therapy by grafting trimetyl chitosan. Pharm Res. 2020;37(8):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Wang H, Mukherjee S, Yi J, Banerjee P, Chen Q, Zhou S. Biocompatible chitosan‐carbon dot hybrid Nanogels for NIR‐imaging‐guided synergistic Photothermal‐chemo therapy. ACS Appl Mater Interfaces. 2017;9(22):18639‐18649. doi: 10.1021/acsami.7b06062 [DOI] [PubMed] [Google Scholar]
- 270. Wang C, Zhang Z, Chen B, Gu L, Li Y, Yu S. Design and evaluation of galactosylated chitosan/graphene oxide nanoparticles as a drug delivery system. J Colloid Interface Sci. 2018;516:332‐341. doi: 10.1016/j.jcis.2018.01.073 [DOI] [PubMed] [Google Scholar]
- 271. Arjmand O, Ardjmand M, Amani AM, Eikani MH. Development of a novel system based on green magnetic/graphene oxide/chitosan/allium sativum/quercus/nanocomposite for targeted release of doxorubicin anti‐cancer drug. Anticancer Agents Med Chem. 2020;20(9):1094‐1104. [DOI] [PubMed] [Google Scholar]
- 272. Zaharie‐Butucel D, Potara M, Suarasan S, Licarete E, Astilean S. Efficient combined near‐infrared‐triggered therapy: phototherapy over chemotherapy in chitosan‐reduced graphene oxide‐IR820 dye‐doxorubicin nanoplatforms. J Colloid Interface Sci. 2019;552:218‐229. doi: 10.1016/j.jcis.2019.05.050 [DOI] [PubMed] [Google Scholar]
- 273. Bae Y, Joo C, Park KH, Kang SW, Huh KM, Choi JS. Preparation and characterization of 3D human glioblastoma spheroids using an N‐octanoyl glycol chitosan hydrogel. Int J Biol Macromol. 2021;185:87‐97. doi: 10.1016/j.ijbiomac.2021.06.083 [DOI] [PubMed] [Google Scholar]
- 274. Sun R, Fang L, Lv X, et al. In vitro and in vivo evaluation of self‐assembled chitosan nanoparticles selectively overcoming hepatocellular carcinoma via asialoglycoprotein receptor. Drug Deliv. 2021;28(1):2071‐2084. doi: 10.1080/10717544.2021.1983077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Xie P, du P, Li J, Liu P. Stimuli‐responsive hybrid cluster bombs of PEGylated chitosan encapsulated DOX‐loaded superparamagnetic nanoparticles enabling tumor‐specific disassembly for on‐demand drug delivery and enhanced MR imaging. Carbohydr Polym. 2019;205:377‐384. doi: 10.1016/j.carbpol.2018.10.076 [DOI] [PubMed] [Google Scholar]
- 276. Zhao X, Wei Z, Zhao Z, et al. Design and development of graphene oxide nanoparticle/chitosan hybrids showing pH‐sensitive surface charge‐reversible ability for efficient intracellular doxorubicin delivery. ACS Appl Mater Interfaces. 2018;10(7):6608‐6617. doi: 10.1021/acsami.7b16910 [DOI] [PubMed] [Google Scholar]
- 277. Karnati KR, Wang Y. Understanding the co‐loading and releasing of doxorubicin and paclitaxel using chitosan functionalized single‐walled carbon nanotubes by molecular dynamics simulations. Phys Chem Chem Phys. 2018;20(14):9389‐9400. doi: 10.1039/C8CP00124C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Kurmi BD, Paliwal R, Paliwal SR. Dual cancer targeting using estrogen functionalized chitosan nanoparticles loaded with doxorubicin‐estrone conjugate: a quality by design approach. Int J Biol Macromol. 2020;164:2881‐2894. doi: 10.1016/j.ijbiomac.2020.08.172 [DOI] [PubMed] [Google Scholar]
- 279. Mohammadi Z, Samadi FY, Rahmani S, Mohammadi Z. Chitosan‐Raloxifene nanoparticle containing doxorubicin as a new double‐effect targeting vehicle for breast cancer therapy. Daru. 2020;28(2):433‐442. doi: 10.1007/s40199-020-00338-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Pornpitchanarong C, Rojanarata T, Opanasopit P, Ngawhirunpat T, Patrojanasophon P. Catechol‐modified chitosan/hyaluronic acid nanoparticles as a new avenue for local delivery of doxorubicin to oral cancer cells. Colloids Surf B Biointerfaces. 2020;196:111279. doi: 10.1016/j.colsurfb.2020.111279 [DOI] [PubMed] [Google Scholar]
- 281. Nouri A, Faraji Dizaji B, Kianinejad N, et al. Simultaneous linear release of folic acid and doxorubicin from ethyl cellulose/chitosan/g‐C(3) N(4) /MoS(2) core‐shell nanofibers and its anticancer properties. J Biomed Mater Res A. 2021;109(6):903‐914. doi: 10.1002/jbm.a.37081 [DOI] [PubMed] [Google Scholar]
- 282. Khatami F, Matin MM, Danesh NM, Bahrami AR, Abnous K, Taghdisi SM. Targeted delivery system using silica nanoparticles coated with chitosan and AS1411 for combination therapy of doxorubicin and antimiR‐21. Carbohydr Polym. 2021;266:118111. doi: 10.1016/j.carbpol.2021.118111 [DOI] [PubMed] [Google Scholar]
- 283. Helmi O, Elshishiny F, Mamdouh W. Targeted doxorubicin delivery and release within breast cancer environment using PEGylated chitosan nanoparticles labeled with monoclonal antibodies. Int J Biol Macromol. 2021;184:325‐338. doi: 10.1016/j.ijbiomac.2021.06.014 [DOI] [PubMed] [Google Scholar]
- 284. Abasalta M, Asefnejad A, Khorasani MT, Saadatabadi AR. Fabrication of carboxymethyl chitosan/poly(ε‐caprolactone)/doxorubicin/nickel ferrite core‐shell fibers for controlled release of doxorubicin against breast cancer. Carbohydr Polym. 2021;257:117631. doi: 10.1016/j.carbpol.2021.117631 [DOI] [PubMed] [Google Scholar]
- 285. Niu S, Zhang X, Williams GR, et al. Hollow mesoporous silica nanoparticles gated by chitosan‐copper sulfide composites as theranostic agents for the treatment of breast cancer. Acta Biomater. 2021;126:408‐420. doi: 10.1016/j.actbio.2021.03.024 [DOI] [PubMed] [Google Scholar]
- 286. Ramnandan D, Mokhosi S, Daniels A, Singh M, et al. Chitosan, polyethylene glycol and polyvinyl alcohol modified MgFe(2)O(4) ferrite magnetic nanoparticles in doxorubicin delivery: a comparative study in vitro. Molecules. 2021;26(13):877. doi: 10.3390/molecules26133893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Pandit AH, Nisar S, Imtiyaz K, et al. Injectable, self‐healing, and biocompatible N,O‐carboxymethyl chitosan/multialdehyde guar gum hydrogels for sustained anticancer drug delivery. Biomacromolecules. 2021;22(9):3731‐3745. doi: 10.1021/acs.biomac.1c00537 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed.


