Abstract
Pyroptosis could be responsible for the bone loss from bone metabolic diseases, leading to the negative impact on people's health and life. It has been shown that osteoclasts, osteoblasts, macrophages, chondrocytes, periodontal and gingival cells may be involved in bone loss linked with pyroptosis. So far, the involved mechanisms have not been fully elucidated. In this review, we introduced the related cells involved in the pyroptosis associated with bone loss and summarized the role of these cells in the bone metabolism during the process of pyroptosis. We also discuss the clinical potential of targeting mechanisms in the osteoclasts, osteoblasts, macrophages, chondrocytes, periodontal and gingival cells touched upon pyroptosis to treat bone loss from bone metabolic diseases as well as the challenges of avoiding potential side effects and producing efficient treatment methods.
Keywords: Pyroptosis, NLRP3, Inflammasome, Bone loss, Osteoclast
Introduction
Over 200 million people worldwide are suffering from bone metabolic diseases that result in bone loss, including osteoporosis, rheumatoid arthritis, psoriatic arthritis, and periodontitis [1, 2], which represents increasing medical and socio-economic challenges [3]. The primary cause of bone loss is an imbalance between osteoclastic bone resorption and osteoblastic bone formation [4]: the increased osteoclast activities and dysregulated osteoblast activities contributes to excessive bone loss [5]. Actually, in bone metabolic diseases, it is clear that multiple inflammatory factors and inflammatory signaling pathways are fundamentally linked to osteoclast and osteoblast activities in the process of bone loss [6, 7]. Moreover, the process of bone resorption and formation can be mediated by the inflammatory response, leading to bone loss caused by a great number of autoinflammatory diseases [8, 9]. For instance, researchers have shown that particular stimulation of the body contributed to an increase in the innate immune function, thus upregulating the tumor necrosis factor α (TNF-α) from activated T cells as an inflammatory factor and facilitating bone loss [10]. The immune system and bone loss are mutually also influential: an imbalance in the immune system, resulting in inflammatory stimuli may induce an imbalance in bone turnover via induction of osteoclast differentiation and inhibition of osteoblast differentiation, leading to various bone metabolic diseases [11, 12]; meanwhile, bone cells, including osteoblasts, osteoclasts, osteocytes, periodontal and gingival cells influence the cellular functions of immune cells and contribute to immune regulation by modulating immune cell differentiation and/or function through the maintenance of the bone marrow microenvironment [13].
Pyroptosis, a form of programmed cell death accompanied by inflammation and immune response [14], is described as a critical area of interest within the field of the immune system and the accompanying bone loss, which gradually attracts higher attention in the near future [15]. Canonically, pyroptosis launches after pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) were recognized by pattern recognition receptors (PRRs) [16, 17]. Then, PRRs interact and activate the apoptosis-associated speck-like protein (ASC) that then oligomerizes and uses its caspase activation and recruitment domain (CARD) to bind to the CARD of pro-caspase-1 [18]. One common inflammasome consists of nucleotide-binding oligomerization domain (NOD)-like receptor family pyrin domain containing 3 (NLRP3), ASC and pro-caspase-1 [19, 20]. The assembly of inflammasome triggers the activation of caspase-1 to cleave gasdermin D (GSDMD) and pro-IL-1β/IL-18 into their mature forms [21]. Then, GSDMD interacts preferentially with membrane lipids to form transmembrane pores and induces cells to swell until the cell membrane ruptures, allowing the release of cellular contents (e.g. IL-1β and IL-18), triggering a strong inflammatory response [6, 7]. Additionally, the canonical pathway also exploits toll-like receptors (TLRs) and tumor necrosis factor receptor (TNFR) that are stimulated via aging, infection, estrogen deficiency or inflammatory condition for priming particular PRRs to induce Nuclear factor kappa B (NF-kB) signaling [8, 9], resulting in increased expression of NLRP3, pro-IL-1β, and pro-IL-18 [10], and subsequently NLRP3 is activated, leading to the assembly of inflammasome assembly and the maturation of caspase-1 [22]. Differently, in the noncanonical pathway, intracellular lipopolysaccharides (LPS) of Gram-negative bacteria can be recognized [23], and LPS directly binds to CARD of caspase-11 in rodents or caspase 4/5 in humans (instead of caspase 1), resulting in the activation of caspase-4/5/11 [24, 25]. The caspase-4/5/11 cleaves GSDMD that is oligomerized and transferred to the cell membrane to form plasma membrane pores, leading to the development of pyroptosis [26, 27]. However, caspase-4/5/11 cannot directly cleave pro-IL-1β/pro-IL-18 [16, 26], but they can regulate the maturation and secretion of IL-1β/IL-18 via the NLRP3/caspase-1 pathway.
Appropriate pyroptosis induces strong inflammatory reactions and immune response to aid in host defenses infection, while excessive pyroptosis leads to numerous inflammatory diseases, including bone metabolic disorders [5, 16]. Sartoretto et al. have reported that inflammasome activities including pyroptosis could not only negatively affect host immune defense but also influence osteoclastic bone resorption and osteoblastic bone formation [28]. For instance, during the process of pyroptosis, PAMPs and DAMPs can activate the inflammasome in osteoclasts, promote the production of IL-1β and TNF-α to induce excessive inflammasome activities [29], and stimulate osteoclast differentiation, thus resulting in bone loss [30, 31]. Moreover, the pyroptosis of osteoblasts may induce the release of IL-1β and RANKL, further leading to bone loss [32]. In fact, osteoclasts, osteoblasts, macrophages, chondrocytes, periodontal and gingival cells are all involved in the process of pyroptosis, which eventually leads to bone loss and causes bone metabolic diseases [5, 16, 33].
In this review, we introduced recent advancements and insights into the potential specific mechanisms of pyroptosis in bone loss and summarized the various role of different cells as crucial parts during the process of pyroptosis in osteoclastic bone resorption and osteoblastic bone formation. Furthermore, we discussed the promising potential and underlying challenges of therapeutic strategies for pyroptosis in bone loss and proposed the direction for the development of the identification of therapeutic targets and the development of novel anti-inflammatory drugs.
Pyroptosis
Pyroptosis is defined as a programmed death, which causes cells to swell until the cell membrane ruptures, resulting in the release of cell contents and activating a strong inflammatory response [34]. The process of pyroptosis is classified into the canonical pathway that depends on caspase-1 and the noncanonical pathway that depends on caspase-4/5/11 [16]. As pathogen invasion and tissue damage activate innate immunity, the canonical pathway subsequently commences with the assembly of inflammasomes (an intracellular supramolecular protein complex) that occurs in response to PAMPs and DAMPs(such as toxins, pathogens, metabolites and nucleic acids) [35]. The canonical inflammasome sensors (one component of canonical inflammasome) can be divided into NOD-like receptors (NLRs) [36], melanoma 2 (AIM2)-like receptors (ALRs) [37], and tripartite motif family (TRIM) [38]. Among them, NLRP3 is the well-studied inflammasome sensor in the NLR family and displays an essential role in the innate immune as an important factor of pyroptosis [39], requiring signals to activate: the NF-κB-dependent pathway (an critical pathway in osteoclastic activities) [32] and varieties of stimulus (e.g. PAMPs and DAMPs) [40]. The TLR and TNFR stimulated by aging, infection, estrogen deficiency or inflammatory condition can also prompt NF-kB signaling, leading to the upregulated expression of NLRP3, pro-IL-1β, and pro-IL-18 [41, 42]. Upon stimulation, NLRP3 recruits the adaptor ASC through PYD-PYD domain association [43]. With NLRP3, ASC further recruits pro-caspase-1 through CARD-CARD domain interaction, forming the signaling ternary complex known as the inflammasome [44]. Activated caspase-1 cleaves GSDMD into a hydrophilic GSDMD-C-terminal domain and a lipophilic GSDMD-N-terminal domain [45] that is anchored on the cell membrane and polymerizes to form a hollow diameter cyclic oligomer (GSDMD pores). Subsequently, the regular permeability barrier of the plasma membrane is disrupted by breaking the concentration gradients of sodium and potassium, resulting in osmotic pressure changes [46], meanwhile, with more pores forming, eventful swelling leads to membrane rupture [47]. Moreover, in this process, the IL-1β and IL-18 precursors are also cleaved into the mature form by caspase-1, and then the mature IL-1β and IL-18 released via the GSDMD pores or later during membrane rupture [48]. It has been reported that IL-1β can stimulate the level of RANKL in osteoblasts or bone marrow mesenchymal stem cells as well as the osteoclasts activities [49]. Furthermore, IL-1β can bind with its receptors on macrophages to induce the expression of RANKL that binds with RANK on the osteoclast precursors to promote osteoclast differentiation [50]. IL-18 are closely concerned with IL-1β since they belong to the same family, and IL-18 also facilitates osteoclast differentiation via various pathways [51]. For example, the increased IL-18 in ovariectomized (OVX) mice promoted the differentiation from peripheral monocytes to osteoclasts and activated the molecules related to NLRP3 inflammasome, as well as suppressed osteoblast activities [52, 53]. Notably, NLRP3 is one of the most prevalent inflammatory bodies linked to numerous disorders, such as cardiovascular disease, diabetes, multiple sclerosis, and cancer [8]. Obviously, IL-1β and IL-18 as well as NLRP3 are critical factors contributing to the release of caspases and resulting in the programmed cell death caused by pyroptosis [21, 32].
In the noncanonical pathways, stimulated by direct interactions between lipid A of cytoplasmic LPS from Gram-negative bacteria and CARD of caspase [54], the caspase-11 in rodents (caspase-4/-5 in human) activated and oligomerized, [55] thus cleaving GSDMD to release its N-terminus, forming pore and resulting in cell rupture [26, 56]. Meanwhile, although caspase-4/5/11 cannot directly cleave pro-IL-1β/pro-IL-18 [16, 26], the enhanced cellular stress induced by noncanonical inflammasome activation may contribute to the activation of the canonical NLRP3/caspase-1 pathway, thereby increasing the secretion of IL-1β and IL-18 [57] (Fig. 1).
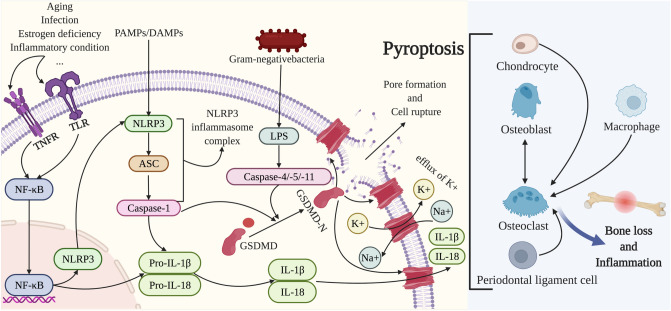
Fig.1.
Pyroptosis modulates the activities of osteoclasts, osteoblasts, macrophages, chondrocytes, and periodontal ligament cells to play a non-negligible role in bone loss. In the canonical pathway, after exposure to PAMP and DAMP, NLRP3 inflammasome is activated and followed by the recruitment of ASC and pro-caspase-1, subsequently producing the pro-inflammatory cytokines IL-1β and IL-18. The activation mediated by aging, infection, estrogen deficiency or inflammatory condition of TLR or TNFR prompts NF-kB signaling, causing increased expression of NLRP3, pro-IL-1β, and pro-IL-18. In the noncanonical pathway, caspase-4/-5/-11 is activated upon binding to LPS of Gram-negative bacteria. Caspase-1 and caspase-4/-5/-11 cleave GSDMD into a GSDMD-N-terminal domain which anchors in the cell membrane and results in cell rupture as well as the efflux of K + . In the process of pyroptosis, inflammasomes facilitate osteoclast activities and thus improve the bone resorption ability of osteoclasts. Osteoblasts, chondrocytes, periodontal ligament cells, and macrophages can elevate osteoclast activity in the context of inflammasome activation, meanwhile, the decreased osteoblast activity and increased pyroptosis of osteoblasts and periodontal ligament cells can directly upregulate bone loss and inflammation
Pyroptosis in bone loss
The delicate balance between bone resorption and formation is a complex process involving the regulation of pyroptosis that could be effectively modulated via the activities of osteoclasts, osteoblasts, macrophages, chondrocytes, periodontal and gingival cells. Compelling evidence has demonstrated that canonical inflammasomes are composed to activate caspase-1, produce mature IL-1β and IL-18, and bring about pyroptosis in response to PAMPs and DAMPs, meanwhile noncanonical caspases bind to stimuli including LPS, and contribute to pyroptosis. The improper activation of inflammasomes may lead to a pro-inflammatory microenvironment and excessive cell rupture, thus leading to the involved bone loss in the bone metabolic diseases [39]. Here we summarize various cells that have specific influence on bone loss under the regulation of pyroptosis, and how the mechanisms are engaged in the bone loss related to the pyroptosis.
Pyroptosis in osteoclasts
Osteoclasts are the main functional cells mediating bone resorption [58, 59]. A variety of immune cytokines and receptors participate in bone metabolism by regulating the function of osteoclasts. On the other hand, recent studies point out that certain signals in osteoclast formation (e.g., NFATc1 and NF-κB) also play roles in normal and aberrant immune responses, lymph node formation, and inflammatory arthritis, opening up new frontiers in osteoimmunology [60]. In detail, osteoclasts and osteoclast precursors (OCPs) regulate bone immune responses by means of direct cell–cell contacts through ligands and receptors, such as semaphorins (Semas) and plexins, and Ephs and ephrins [60]. For instance, osteoblast-expressed Sema6d signal through binding to receptor plexin to enhance OC formation by activating NFATc1 and promote OC activation by producing podosome [61]. Moreover, ephrin B2 on the surface of OCPs binding to receptor Eph4 on osteoblastic cells reduces the expression of c-Fos and NFATc1, inhibiting OC formation [62].
According to their immune function of osteoclasts, during inflammatory processes, osteoclasts could serve as major participants and regulators of the pyroptosis to affect bone immune status [63]. PAMPs and DAMPs can activate inflammasomes in osteoclasts and OCPs, prompting hyper multinucleation and IL-1β production [31]. Some essential factors associated with pyroptosis activator of nuclear factor-κB ligand (RANKL), macrophage colony-stimulating factor (M-CSF), TNF-α, and IL family, stimulate further differentiation and fusion of OCPs into multinucleated osteoclasts, leading to bone loss [64, 65] (Table 1).
Table 1.
The regulation of pyroptosis on the osteoclast in the process of bone loss
| Target of action | Intervention | Mechanism | Experimental models | Effect | Diseases | References |
|---|---|---|---|---|---|---|
| NLRP3 | Signals originating from bone matrix | NLRP3/IL-1β | Mice | Promoted bone loss | Not given | [66] |
| NLRP3 | MCC950 | NLRP3/IL-1β | Mice | Inhibited bone loss | Ligature-induced periodontitis | [67] |
| NLRP3 | MCC950 | NLRP3/IL-1β | Aged mice | Inhibited bone loss | Periodontitis | [68] |
| NLRP3 |
High glucose Induced ROS |
ROS/MAPKs/NF-κB/NLRP3 | BMMs | Promoted bone loss | Osteoporosis | [69] |
| NLRP3 | Exosomes from derived MSCs | NLRP3/IL-1β | Diabetic osteoporosis rats | Inhibited bone loss | Osteoporosis | [70] |
| NLRP3 | Glyburide | NLRP3/IL-1β | Mice | Inhibited bone loss | Diabetic-induced fracture | [71] |
| NLRP3 | LncRNA ORLNC1 | microRNA-200b-3p/FoxO3/ CML | BMSCs | Inhibited bone loss | Osteoporosis | [72] |
| NLRP3 | Not given | NLRP3/IL-1β | BMMs | Inhibited bone loss | NOMID | [73] |
| NLRP3 | Not given | NLRP3/IL-1β | Mice | Inhibited bone loss | NOMID | [31] |
| Caspase-1 | Aa | LPS/NLRP3/ caspase-1 | Mice | Promoted bone loss | Periodontitis | [74] |
| RANKL | UA | NF-κB/NLRP3 | OVX mice | Inhibited bone loss | Postmenopausal osteoporosis | [75] |
| IL-1β | Auranofin | IL-1β/RANKL | OVX mice | Inhibited bone loss | Osteoporosis | [76] |
| Smad3 | Alendronate | Smad3/NLRP3/ASC |
Murine OCPs |
Promoted bone loss | Not given | [77] |
| RIPK1 / RIPK3 | NEC-1 and GSK-872 | MAPKs/NF-κB/NLRP3 | OVX mice | Inhibited bone loss | Osteoporosis | [78] |
As a crucial participant in pyroptosis, NLRP3 can regulate the immune status of bone, ultimately leading to bone resorption, and a hyperactive NLRP3 inflammasome can also enhance osteoclast bone resorption ability by reorganizing the actin cytoskeleton [73]. Recent studies have shown that the intervention on NLRP3 can affect osteoclast pyroptosis and thereby modulate bone loss. The study by Chen et al. showed that NLRP3 could regulate the bone loss in ligature‐induced periodontitis by promoting osteoclastic differentiation, while MCC950, a potent inhibitor of the NLRP3 inflammasome, could inhibit bone loss with decreased IL‐1β activation and osteoclast differentiation in ligature‐induced periodontitis [67]. Moreover, the interference effect of MCC950 on NLRP3 seems to be affected by age. In the age-related alveolar bone loss from periodontitis, Zang et al. found that the treatment with MCC950 significantly suppressed alveolar bone loss with reduced caspase-1 activation in aged mice but not in young mice [68]. However, in the Aggregatibacter actinomycetemcomitans (Aa) induced periodontal disease, results from Rocha et al. suggested that NLRP3 inflammasome did not play a significant role in inflammation and bone resorption in vivo but caspase-1 had an affirmative role in alveolar bone resorption [74].
In addition, it has been demonstrated that diabetes increases fracture risk and impairs bone repair [79]. Numerous studies have shown a strong link between diabetes and inflammation: diabetes generates higher inflammatory cytokines, which stimulate the development of osteoclasts and suppress fracture healing [80–82]. The inflammatory cascade accelerates the synthesis of advanced glycosylation end products (AGEs) and increases the production of reactive oxidants, causing diabetic patients to experience increased bone resorption and delayed fracture repair [83]. Notably, a high-glucose environment can upregulate the activity of NLRP3, which accordingly promotes bone loss and meanwhile, under diabetic conditions, overexpression of NLRP3 has been demonstrated to cause stronger inflammatory cytokines and subsequently promote diabetic-induced impaired fracture healing [84, 85]. It has been reported that the exposure to high glucose concentrations of mouse bone marrow cells (BMMs) upregulated NLRP3 inflammasome expression via reactive oxygen species (ROS)/MAPKs/NF-kB pathway, while NF-κB inhibitors significantly reduce the expression level of NLRP3 inflammasome and alleviate bone resorption [69]. In a similar pathway, a study by Alippe et al. demonstrated that the NLRP3 inflammasome is also activated when exposed to the signals originating from bone matrix under multiple bone turnover states (e.g., estrogen deficiency and persistent parathyroid hormone exposure), resulting in increased NF-κB and MAPK phosphorylation [66]. In osteoclasts, the effect of exposure to high concentrations of glucose could be reversed by exosomes originating from mesenchymal stem cells (MSCs) that inhibited the overactivated NLRP3 inflammasome [70]. Furthermore, glyburide is one of the most commonly prescribed medications for the treatment of diabetes [86], and it has been shown to accelerate the healing of diabetes-induced fractures while inhibiting NLRP3 activation in mouse and human macrophages [87]. Yang et al. proved that treatment of NLRP3 inflammasome inhibitor glyburide significantly decreased the expressions of TNF-α, and IL-6 in the fracture calluses in the diabetic-induced fracture model, as well as increased bone callus volume and bone volume fraction, contributing to diabetic-induced fracture healing [71].
Except for NLRP3, RANKL, IL-1β, and Smad3 are also the target of action to be regulated, affecting bone loss involved in pyroptosis. Tao et al. found that Urolithin A (UA) inhibited RANKL-triggered osteoclastogenesis in a concentration-dependent manner, which finally contributed to downregulated cytoplasmic secretion of IL-1β and IL-18 and reduced pyroptosis to inhibit bone loss [75]. Besides, Kim et al. have demonstrated that the expression of IL-1βmodulated by inflammasomes could be suppressed by auranofin to inhibit osteoclastogenesis induced by RANKL, critically decreasing the bone loss related to pyroptosis [76]. Moreover, Tamai et al. reported that alendronate could directly regulate Smad3 to accelerate IL-1β production and NLRP3-dependent pyroptosis, preventing OCPs from differentiation into osteoclasts and thus inhibiting bone loss [77] (Fig. 2).
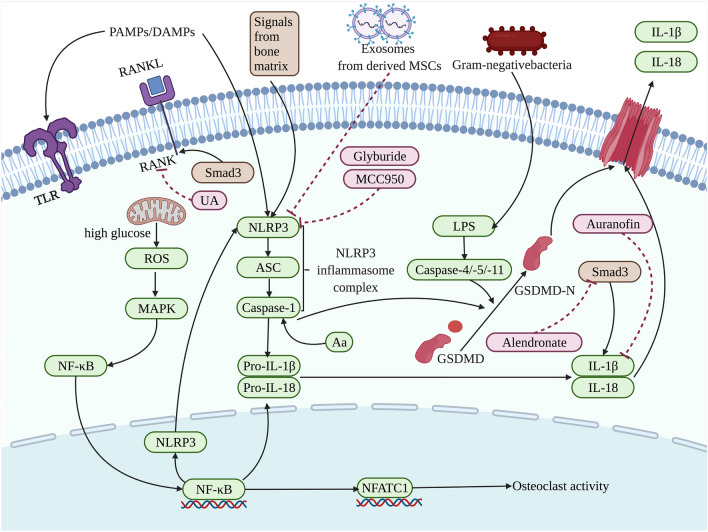
Fig. 2.
The pyroptosis related to the osteoclasts in the process of bone loss is affected by the regulation on different targets of actions. Exposure to PAMPs or DAMPs and then influenced by NF-κB, the NLRP3 inflammasome complex assemblies and activates caspase-1 and subsequently results in the cleavage of the proinflammatory cytokines IL-1β and IL-18. Caspase-1 and caspase-4/-5/-11 cleave GSDMD into a GSDMD-N-terminal domain that anchors in the cell membrane, leading to the cell rupture. Signals from bone matrix [66], exosomes from derived MSCs [67], glyburide [71], and MCC950 [67, 68] have been developed in the presented pathway targeting NLRP3. Meanwhile, RANKL, IL-1β, and Smad3 are also the target of action to be regulated to affect bone loss involved in pyroptosis, which can be modulated by Aa [74], UA [75], Auranofin [76] or Alendronate [77], respectively. The factors that promote pyroptosis are indicated in green and pyroptosis-suppressor factors are indicated in pink as well as factors that can have both effects are indicated in brown
Pyroptosis in osteoblasts
Osteoblasts, the bone-forming cells, are indispensable for bone metabolism, and the proliferation and differentiation of osteoblasts are essential elements in the bone loss from bone metabolic diseases [88]. Osteoblasts can differentiate from bone marrow mesenchymal stem cells (BMSCs), and the expression of NLRP3, ASC, caspase-1, IL-1β, and TNF-α can be also upregulated in response to LPS, thereby affecting the potential for osteogenic differentiation [89]. During the progression of bone loss, BMSCs exhibit inhibited osteogenic capacity, resulting in reduced bone formation [90], and it has been demonstrated that the NLRP3 inflammasome is involved in this process by affecting the differentiation of BMSCs via certain active molecules [91]. Besides, NLRP3 also directly inhibits osteogenesis by influencing osteoblasts and plays a core role in osteoporosis induced by inflammation. In estrogen-deficient osteoporosis, direct bacterial infection was not observed but NLRP3 levels still rose, while the viability of osteoblasts was significantly increased and the bone loss was also relieved after NLRP3 was inhibited or inactivated [92]. McCall SH et al. also proved that osteoblasts highly expressed NLRP3 that mediated bacterially induced cell death and participated in bone loss during inflammation [93]. Actually, when NLRP3 inflammasomes increase in osteoblasts, the caspase-1 pathway is activated and the expression levels of IL-1 and IL-18 are upregulated, resulting in the death of osteoblasts [94, 95]. Moreover, osteoblasts undergoing pyroptosis will facilitate osteoclastogenesis and the activation of osteoclasts through increased production of cytokines and chemokines, such as RANKL, or decreased OPG levels, leading to increased bone loss [32] (Table 2).
Table 2.
The regulation of pyroptosis on the osteoblast in the process of bone loss
| Target of action | Intervention | Mechanism | Experimental models | Effect | Diseases | References |
|---|---|---|---|---|---|---|
| NLRP3 | NLRP3 knock-out | NFκB/NLRP3 | Osteoblasts | Promoted bone loss | Not given | [96] |
| NLRP3 | IL-17 | IL-17/NLRP3 | Osteoblasts | Promoted bone loss | Not given | [97] |
| NLRP3 | Wnt/β-catenin signaling | melatonin/ Wnt/β-catenin signaling/ NLRP3 | OVX mice | Inhibited bone loss | Osteoporosis | [98] |
| NLRP3 | ROS produced by LPS | LPS/NLRP3 | MG63 cells | Promoted bone loss | Periodontitis | [99] |
| NLRP3 | TXNIP | TXNIP/NLRP3/IL-1β | BMSCs | Inhibited bone loss | Not given | [89] |
| NLRP3 | shRNA | shRNA/NLRP3/caspase-1/IL-1β | Rats | Inhibited bone loss | Diabetes | [85] |
| NLRP3/ caspase-1 | LPS/PA | NLRP3/caspase-1/ | MSCs | Promoted bone loss | Osteoporosis | [100] |
| NLRP3/ caspase-1 | IL-18BP | NLRP3/caspase-1/ IL18 | Osteoblasts | Promoted bone loss | Postmenopausal osteoporosis | [52] |
| caspase-1 | High glucose | caspase-1/GSDMD/IL-1β | ME3T3-E1 cells | Promoted bone loss | Alveolar bone disease | [101] |
| LPS | Necrosulfonamide | NLRP3/caspase-1/GSDMD | Osteoblasts | Promoted bone loss | Bone fracture | [102] |
| NF-κB | PKR | PKR/NF-κB/NLRP3 | Osteoblasts | Promoted bone loss | Periodontal disease | [103] |
In the process of pyroptosis, with the increase of NLRP3 inflammasome, the caspase-1 pathway is activated, and IL-1 and IL-18 are facilitated, contributing to the programmed death of osteoblasts [94, 95]. It has been demonstrated that in the auto-immune diseases including some bone metabolic diseases, NLRP3 inflammasome not only results in the severe bone loss caused by the activation of osteoclasts, but also mediates the osteoblast activity to prevent bone loss from the pyroptosis associated with NLRP3 inflammasome [96]. The results from Wang et al. have proved that NLRP3 inflammasome cascade activation was related to suppressed osteogenesis of MSCs (A type of cell that can differentiate into osteoblasts), while the downregulated capsase-1 induced osteogenic differentiation of MSCs, avoiding bone loss [100]. Recently, Lei et al. have discovered that IL-17 could prompt pyroptosis of osteoblasts involved in NLRP3 inflammasome pathway, which may promote the release of IL-1β as well as RANKL and subsequently further conduce to bone loss [97]. Coincidentally, Mansoori et al. have reported that IL-18BP (a natural antagonist of pro-inflammatory IL-18 cytokine related to autoimmune disorders) that displayed the different role from IL-17 could suppress NLRP3 inflammasome and caspase-1, which promoted osteoblast differentiation, reduced osteoclastogenesis, and thus restored trabecular microarchitecture [52]. Beyond IL-18BP, the results from Xu et al. showed that Wnt/β-catenin signaling (participates in osteoblast differentiation) activated by melatonin could also inhibit NLRP3 inflammasome activity, weakening osteogenic differentiation and attenuating the bone loss [98]. Furthermore, it is noteworthy that hyperglycemia-induced exaggerated inflammatory response caused by elevated NLRP3 inflammasome activation impairs diabetic-induced alveolar bone defect healing [85, 104], it has been reported that NLRP3 and the released IL 1β lead to diabetes-associated persistent inflammatory responses that may lead to impaired bone healing of alveolar socket wounds [105]. The results in diabetic rats have proved that a lentiviral short hairpin RNA (shRNA) could negatively target NLRP3 and simultaneously induced the expression of osteogenic markers Runt-related transcription factor 2 and osteocalcin, suggesting that the inhibition of NLRP3 inflammasome could increase alveolar bone defect healing [85].
In the process of osteoblast-related pyroptosis, induced LPS can lead to increased expression of NLRP3, caspase-1, and so on, which promotes pyroptosis and cell migration injury of osteoblasts, contributing to concomitant bone damage. Liu et al. demonstrated that ROS produced by LPS in osteoblasts could lead to the pyroptosis of osteoblasts mediated by NLRP3 inflammasome as well as decreased cell migration, while MCC950, a potent inhibitor of the NLRP3 inflammasome, could enhance osteoblast migration and repair the expression of osteogenic differentiation-related proteins. And the inhibition of ROS with N-acetyl-L-cysteine could also weaken oxidative stress-mediated pyroptosis and improved migration injury in osteoblasts treated with LPS [99]. Moreover, it has been found that necrosulfonamide reversed the influence of LPS on cell viability and pyroptosis, as well as on the mRNA and protein expression of pyroptosis-related genes in osteoblasts, inhibiting the secretion of IL-6, TNF-α and IL-1β [102].
Apart from NLRP3, intervention on the caspase-1 and NF-κB signaling also affects osteoblasts involved in the process of pyroptosis. Yang et al. found that high glucose could produce the pyroptosis through the caspase-1/GSDMD/IL-1β pathway to inhibit the proliferation and differentiation of osteoblast in alveolar bone, resulting in the bone loss, while caspase-1 inhibitor could reverse the negative role of high glucose on the osteoblasts [101]. Additionally, the findings from Yoshida et al. suggested that in the Porphyromonas gingivalis-infected osteoblasts, the NF-κB signaling could be induced by double-stranded RNA-dependent kinase (PKR) to facilitate NLRP3, influencing the process of pyroptosis related to bone loss [103] (Fig. 3).
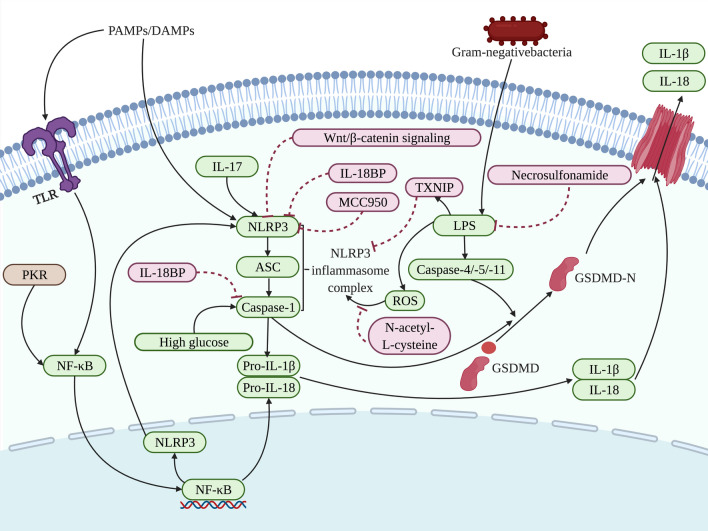
Fig.3.
The pyroptosis related to the osteoblasts in the process of bone loss is affected by the regulation on different targets of actions. Exposure to PAMPs or DAMPs and then influenced by NF-κB, the NLRP3 inflammasome complex assemblies and activates caspase-1 and subsequently results in the cleavage of the proinflammatory cytokines IL-1β and IL-18. Caspase-1 and caspase-4/-5/-11 cleave GSDMD into a GSDMD-N-terminal domain that anchors in the cell membrane, leading to the cell rupture. Wnt/β-catenin signaling [98], IL-17 [97], IL-18BP [52], MCC950 [99], TXNIP [89], shRNA [85] and ROS that could be inhibited by N-acetyl-L-cysteine [99], have been developed in the presented pathway targeting NLRP3. Meanwhile, NF-κB, LPS and caspase-1 are also the target of action to be regulated by PKR [103], Necrosulfonamide [102], high glucose [101] or IL-18BP [52], affecting bone loss involved in pyroptosis. The factors that promote pyroptosis are indicated in green and pyroptosis-suppressor factors are indicated in pink as well as factors that can have both effects are indicated in brown
Pyroptosis in macrophages
Macrophages, the essential inflammatory cells involved in the bone metabolic diseases [106], possess the ability to differentiate into osteoclasts and function as a part of the innate immune system [107]. Activated macrophages can polarize into pro-inflammatory M1-like macrophages or anti-inflammatory M2-like macrophages, both of which are characterized depending on cytokine release patterns and metabolic signatures [33, 108]. Macrophages can not only mediate bone loss in bone metabolism via their communication with osteoclasts during the pyroptosis, but also participate in the process of inflammasome activation in macrophages to facilitate bone destruction [109, 110]. Exposed to PAMPs/DAMPs or bacteria [111], the M1-like polarization of macrophages can be dependent on inflammasome activation which upregulates the levels of IL-1β and IL-18, causing the pyroptosis in pro-inflammatory microenvironment and thus leading to inflammatory bone loss [112, 113] (Table 3).
Table 3.
The regulation of pyroptosis on the macrophages in the process of bone loss
| Target of action | Intervention | Mechanism | Experimental models | Effect | Diseases | References |
|---|---|---|---|---|---|---|
| NLRP3 | LPS | NLRP3/caspase-1/IL-1β and IL-18 | Mice | Promoted bone loss | Osteoporosis | [114] |
| NLRP3 | HPDLCs stimulated by force | NLRP3/ IL-1β | Periodontal tissues and rats | Promoted bone loss | Root resorption | [115] |
| NLRP3 | Porphyromonas gingivalis | Porphyromonas gingivalis/NLRP3/caspase-1/IL-1β and IL-18 | Mice | Promoted bone loss | Periodontal disease | [116] |
| NLRP3 | MARK4 | MARK4/NLRP3/caspase-1/IL-1β and IL-18 | Healthy and inflamed human gingival tissues | Promoted bone loss | Periodontitis | [117] |
| NLRP3 | Mycoplasma salivarium | NLRP3/caspase-1/IL-1β | BMMs | Promoted bone loss | Periodontal disease | [118] |
| NLRP3 | Staphylococcus aureus | NLRP3/caspase-1/GSDMD | Murine and human infectious bone fragments | Promoted bone loss | Osteomyelitis | [119] |
| NLRP3 | glyburide | glyburide/NLRP3/caspase-1/IL-1β | Human monocyte cells | Inhibited bone loss | Periodontitis | [120] |
| NLRP3 | dioscin | dioscin/NLRP3/caspase-1/IL-1β | BMMs and MC3T3-E1 cells | Inhibited bone loss | Apical periodontitis | [91] |
| NLRP3 |
lncRNA_1810058I24Rik lncRNA_Gm12474 lncRNA_Gm41514 Metformin |
NLRP3/caspase-1/IL-1β and IL-18 | Mice | Inhibited bone loss | Diabetes-associated periodontitis | [121] |
| NLRP3 | resveratrol | Resveratrol/Pink1/Parkin/NLRP3/caspase-1/IL-1β and IL-18 | Arthritis rats | Inhibited bone loss | Gouty arthritis | [122] |
| TLR4 | MDP | TLR4/NLRP3/GSDMD | Macrophage of adjuvant arthritis rats | Inhibited bone loss | Adjuvant arthritis | [123] |
| caspase-1 | cyclic stretch | cyclic stretch/AMPK/caspase-1/IL-1β | BMMs | Inhibited bone loss | Not given | [124] |
| caspase-1 | cyclic stretch | cyclic stretch/AMPK/caspase-1/IL-1β | BMMs | Inhibited bone loss | Not given | [125] |
| caspase-1 | DEX | HMGB1/DEX/caspase-1/IL-1β and IL-18 | BMMs | Inhibited bone loss | infection and trauma-derived inflammation | [126] |
| caspase-1 | cranberry PACs | cranberry PACs/caspase-1/IL-1β and IL-18 | BMMs | Promoted bone loss | Periodontitis | [127] |
| NF-κB | PUN | PUN/NF-κB/ NLRP3/caspase-1/IL-1β and IL-18 | BMMs | Inhibited bone loss | rheumatoid arthritis | [128] |
| NF-κB | cyclic stretch | cyclic stretch/NF-κB/exosomes from HPDLCs/IL-1β | Human macrophages | Inhibited bone loss | Periodontitis | [129] |
| GSDMD | deficiency | GSDMD deficiency/caspase-1/IL-1β and IL-18 | Mice | Inhibited bone loss | PTOA | [130] |
| AIM2 | GSDMD | GSDMD/AIM2/caspase-1/IL-1β and IL-18 | BMMs | Promoted bone loss | Francisella novicida infection | [131] |
In orchestrated innate immune reactions in macrophages, the NLRP3 inflammasome could activate caspase-1 and promote the secretion of IL-1β as well as IL-18, meanwhile the inflammasome activity results in the progress of pyroptosis and the disorders of bone metabolism [114]. Lately, it has been demonstrated that the activation of NLRP3 inflammasome in M1 macrophages could prompt the levels of IL-1β and the development of inflammation and pyroptosis, thereby triggering bone loss [115]. NLRP3 could also be upregulated in macrophages by microorganisms (e.g., porphyromonas gingivalis, mycoplasma salivarium and staphylococcus aureus) to positively mediate the process of pyroptosis, inducing concomitant bone loss [116–119]. In the macrophages from wild-type and NLRP3-deficient mice, Yamaguchi et al. have found that porphyromonas gingivalis could activate innate immune cells through activating NLRP3 inflammasome, inducing proinflammatory cytokines during pyroptosis and subsequently enhancing alveolar bone loss [116]. Moreover, in the macrophages infected by porphyromonas gingivalis, overexpressed microtubule affinity regulating kinase 4 (MARK4) could further increased inflammasome activation and the pyroptosis, promoting alveolar bone loss [117]. Mycoplasma salivarium had the similar role with porphyromonas gingivalis, which could activate NLRP3 to induce IL-1β and promote periodontal disease accompanying bone loss [118]. Additionally, in the osteomyelitis, staphylococcus aureus displayed a key role on upregulating the expressions of NLRP3, caspase-1 as well as GSDMD to enhance pyroptosis and bone disruption, [119].
However, NLRP3 could also be inhibited by glyburide, dioscin, metformin and resveratrol, preventing the bone loss from the progress of pyroptosis. In pyroptosis and inflammation caused by periodontopathic bacteria-infected macrophages, sulfonylureas (such as glyburide) could directly downregulate the expression of NLRP3 and reduce the release of inflammatory cytokines, inhibiting inflammatory bone resorption [120]. Besides, Yin et al. have reported that in the macrophages, dioscin, a new NLRP3 inflammasome inhibitor, could not only suppress inflammatory cell infiltration but also prompt the expression of osteogenic-related factors in osteoblast, which enhanced osteogenesis as well as reduced osteoclastogenesis [91]. Furthermore, in macrophages, NLRP3 leads to a persistent inflammatory response and the release of inflammatory factors including IL-1β and IL-18, which also results in impaired healing of bone wounds induced by diabetes [132]. When it comes to diabetes-associated periodontitis and concomitant bone destruction, it has been proved that metformin could reverse NLRP3-induced pyroptosis and alveolar bone loss in BMMs from diabetes-associated periodontitis mice, and the role of both NLRP3 and GSDMD may also be modulated by lncRNA_1810058I24Rik, lncRNA_Gm12474 and lncRNA_Gm41514 [121]. Differently, resveratrol, a non-flavonoid phenolic substance, inhibited NLRP3 through launching the Pink1/Parkin pathway to induce mitophagy, attenuating bone loss [122]. Moreover, it has been investigated that monomer derivative of paeoniflorin (MDP) could restrain TLR4/NLRP3/GSDMD signaling pathway and decrease the expressions of TLR4, NLRP3, saspase-1, ASC, and GSDMD-N in vivo and in vitro, thereby reducing the ratio of macrophage-related pyroptosis and subsequently mitigating bone loss [123].
Furthermore, different targets of action could directly regulate the caspase-1, affecting the pyroptos related to the macrophages in the process of bone loss. Notably, in pyroptosis associated with macrophages, cyclic stretch could suppress NLRP3 inflammasome-dependent IL-1β secretion in BMMs via the inhibition of caspase-1 activity attributed to weakening the AMPK pathway, attenuating inflammation and concomitant bone loss [124, 125]. Moreover, it has been reported that dexmedetomidine (DEX) could also restrain the expression of caspase-1 and the process of pyroptosis, improving cellular injury in potential bone destruction due to inflammation [126]. However, Lagha et al. have revealed different results in suppressing pyroptosis by the repression of caspase-1: cranberry proanthocyanidins (PACs) could also repress the activation of caspase-1 in BMMs and consequently restrain the secretion of both IL-1β and IL-18, inhibiting pyroptosis, but lead to the development of periodontitis and the differentiation of osteoclasts through cell migration [127].
In addition to NLRP3 and caspase-1, the inhibition of NF-κB signaling by punicalagin (PUN) and cyclic stretch also reduces macrophage-related pyroptosis, ameliorating bone disruption [128, 129]. Gao et al. have found that the treatment of PUN could inhibit NF-κB signaling, and subsequently downregulates NLRP3 as well as caspase-1 to attenuate pyroptosis, thereby mitigating inflammatory cell death caused by the secretion of IL-1β and IL-18, while promoting macrophages to switch from an M1 pro-inflammatory phenotype to an M2 anti-inflammatory phenotype, ultimately repressing bone loss [128]. Moreover, NF-κB signaling in LPS-stimulated macrophages could be also retrained by cyclic stretch-induced exosomes from human periodontal ligament cells (PDLCs), which subsequently leads to the repression of IL-1β as well as inhibition of pyroptosis and concomitant alveolar bone loss [129] (Fig. 4).
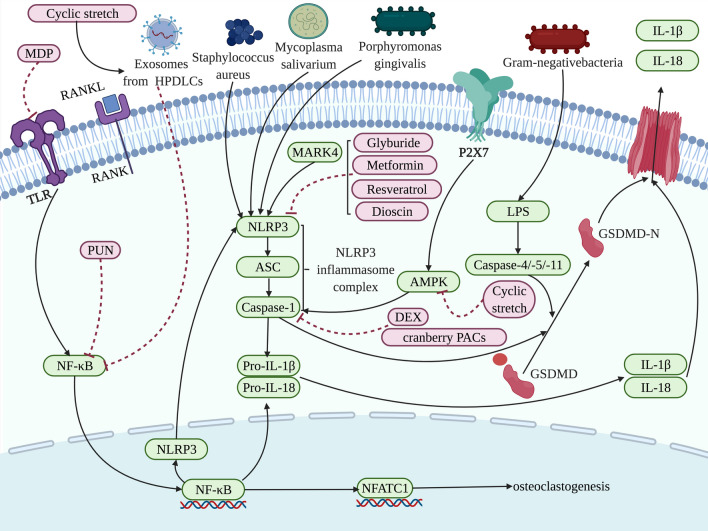
Fig.4.
Different targets of action are regulated to affect the pyroptosis related to the macrophages in the process of bone loss. Influenced by NF-κB, the NLRP3 inflammasome complex assemblies and activates caspase-1 and subsequently results in the cleavage of the proinflammatory cytokines IL-1β and IL-18. Caspase-1 and caspase-4/-5/-11 cleave GSDMD into a GSDMD-N-terminal domain that anchors in the cell membrane, leading to the cell rupture. MARK4 [117], mycoplasma salivarium [118], staphylococcus aureus [119], and porphyromonas gingivalis [116] have been found in the presented pathway positively targeting NLRP3 to facilitate pyroptosis, yet glyburide [120], dioscin [91], metformin [121], resveratrol [122] target to suppress NLRP3. Meanwhile, caspase-1 is also the target of action to be modulated by P2X7/AMPK/cyclic stretch [124, 125] and DEX [126], cranberry PACs [127], affecting bone loss involved in pyroptosis. And NF-κB signaling is repressed by PUN [128] and cyclic stretch induced exosomes from human PDLCs [129]. The factors that promote pyroptosis are indicated in green and pyroptosis-suppressor factors are indicated in pink
Pyroptosis in chondrocytes
The immune and bone systems retain homeostasis through interplaying intently with each other, and arthritis is a pathological consequence of their interaction [133], which may be attributed to the immune responses from chondrocytes, resulting in bone loss [134]. In the chondrocytes in osteoarthritis, the expression of genes that encode proteins related to the inflammatory and catabolic responses is increased primarily by signal transduction pathways involving NF-κB and other factors activated via inflammation [135]. Meanwhile, with IL-1β and IL-18 into the synovial fluid following the activation of caspase-1 [136], the process of pyroptosis in chondrocytes and other cells induces proinflammatory cytokines and inflammatory response cascade in chondrocytes and other cells, ultimately contributing to bone loss [137] (Table 4).
Table 4.
The regulation of pyroptosis on the chondrocytes in the process of bone loss
| Target of action | Intervention | Mechanism | Experimental models | Effect | Diseases | References |
|---|---|---|---|---|---|---|
| NF-κB signaling | Loganin | Loganin/NF-κB/NLRP3/caspase-1 | Mice | Inhibited bone loss | Osteoarthritis | [138] |
| NF-κB signaling | Morroniside | morroniside /NF-κB/NLRP3/caspase-1 | Mice | Inhibited bone loss | Osteoarthritis | [139] |
| NF-κB signaling | Nrf2/HO-1 | Licochalcone A /Nrf2/HO-1/NF-κB/LPS/NLRP3 | Mice and osteoarthritis chondrocytes | Inhibited bone loss | Osteoarthritis | [140] |
| NF-κB signaling | MicroRNA-326 delivered by BMSC-derived exosomes | microRNA-326 NF-κB/NLRP3 | Osteoarthritis chondrocytes | Inhibited bone loss | Osteoarthritis | [141] |
| NF-κB/NLRP3 crosstalk | P2X7 | P2X7/NF-κB/NLRP3 crosstalk | Osteoarthritis chondrocytes | Promoted bone loss | Osteoarthritis | [142] |
| NLRP3 | Ca 2 + | ASIC1a/ Ca 2 + /NLRP3 | Adjuvant arthritis rats | Promoted bone loss | Rheumatoid arthritis | [143] |
| NLRP3 | Icariin | Icariin/NLRP3/caspase-1 | Chondrocytes and mice | Inhibited bone loss | Osteoarthritis | [144] |
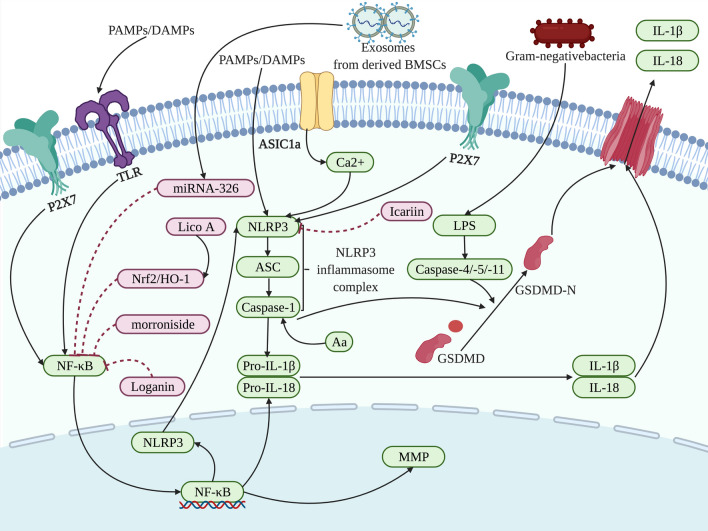
In chondrocytes, the NF-κB signaling pathway induces the expression of proteins related to inflammation and metabolic responses, and promotes the role of inflammatory cytokines during pyroptosis, facilitating the development of pyroptosis and the concomitant bone loss [135], while it has been demonstrated that loganin and morroniside that both are the iridoid glycosides could suppress NF-κB signaling to inhibit pyroptosis, ameliorating bone loss [138, 139]. The results from mice have shown that loganin and morroniside could directly restrain NF-κB signaling, reducing the expression of MMP, NLRP3 and caspase-1 in chondrocytes, which suppressed the progression of inflammation and attenuated the loss of cartilage [138, 139]. In addition, NF-κB signaling could also be repressed by Licochalcone A through nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling and then NLRP3, ASC, GSDMD, caspase-1, IL-1β and IL-18 was decreased in the LPS-induced chondrocytes, reducing pyroptosis and the degeneration of bone [140]. Moreover, exosomes from BMSCs also target NF-κB signaling via delivering microRNA-326 to inhibit the levels of inflammatory cytokines and pyroptosis-related proteins, participating in the inhibition of chondrocyte-related pyroptosis and reducing bone loss [141]. Differently, the activated P2X7 directly targeted the crosstalk between NF-κB and NLRP3 to prompt pyroptosis, thereby aggravating the secretion of proinflammatory cytokines and the degradation of bone [142].
Beyond NF-κB signaling, NLRP3 can also be mediated by chemical compounds and ions to affect the regulation of pyroptosis on the chondrocytes in the process of bone loss [143, 144]. Wu et al. have found that the expression of the NLRP3 inflammasome could be enhanced by Ca2+ in acid-sensing ion channel 1a, inducing the pyroptosis in chondrocytes and causing accompanying bone loss [143]. However, icariin could alleviate the pyroptosis in chondrocytes by suppressing NLRP3 signaling and caspase-1 in vitro and in vivo, suggesting the potential of refraining bone loss [144] (Fig. 5).
Fig.5.
Different targets of action are regulated to affect the pyroptosis related to the chondrocytes in the process of bone loss. Exposure to PAMPs or DAMPs and then influenced by NF-κB, the NLRP3 inflammasome complex assemblies and activates caspase-1 and subsequently results in the cleavage of the proinflammatory cytokines IL-1β and IL-18. Caspase-1 and caspase-4/-5/-11 cleave GSDMD into a GSDMD-N-terminal domain that anchors in the cell membrane, leading to the cell rupture. Loganin [138], morroniside [139], Nrf2/HO-1 induced by Lico A (Licochalcone A) [140], and microRNA-326 delivered by BMSC-derived exosomes [141], have been developed in the presented pathway targeting NF-κB signaling, and P2X7 directly targeted the crosstalk between NF-κB and NLRP3 [142]. Meanwhile, NLRP3 are also the target of action to be regulated by Ca2+ [143] and icariin [144], affecting bone loss involved in pyroptosis. The factors that promote pyroptosis are indicated in green and pyroptosis-suppressor factors are indicated in pink
Pyroptosis in periodontal and gingival cells
Periodontal and gingival cells are critically associated with alveolar bone diseases involving apical periodontitis and periodontitis [145]. In the periodontal and gingival cells, the activation of inflammasome can induce inflammatory cytokines that can modulate the process of pyroptosis related to bone loss [33, 146]. The activitated IL-1β that can be produced via caspase-1 induce RANKL production and improve osteoclast activity [147]. Meanwhile, IL-18 can promote protein levels of MMP in PDLCs, increasing osteoclastogenesis [148]. Furthermore, pyroptosis can directly cause the damage of periodontal and gingival cells to lead to the inflammation, ultimately facilitating bone loss [149, 150] (Table 5).
Table 5.
The regulation of pyroptosis on the periodontal and gingival cells in the process of bone loss
| Target of action | Intervention | Mechanism | Experimental models | Effect | Diseases | References |
|---|---|---|---|---|---|---|
| LPS | Periodontal bacteria | LPS/caspase-4/GSDMD | GCF and periodontium | Promoted bone loss | Periodontitis | [39] |
| LPS | Porphyromonas gingivalis | LPS/caspase-4/GSDMD | HGFs and gingival tissues | Promoted bone loss | Periodontitis | [13] |
| LPS | ED-71 | Nrf2/HO-1/ LPS/caspase-4/GSDMD | Gingival fibroblasts | Inhibited bone loss | Periodontitis | [151] |
| NLRP3 | LPS | LPS/NLRP3/caspase-1/IL-1β | HPDLFs | Promoted bone loss | Apical periodontitis | [150] |
| NLRP3 | Cyclic stretch | cyclic stretch/NLRP3 /caspase-1/IL-1β and IL-18 | PDL tissues from teenagers | Promoted bone loss | Periodontitis | [152] |
| GSDMD | Cyclic stretch | caspase-1/GSDMD/IL-1β and IL-18 | PDL tissues from teenagers | Promoted bone loss | Periodontitis | [153] |
| Caspase-1 and IL-1β | VX765 | caspase-1/GSDMD/IL-1β and IL-18 | HPDLFs | Inhibited bone loss | Apical periodontitis | [154] |
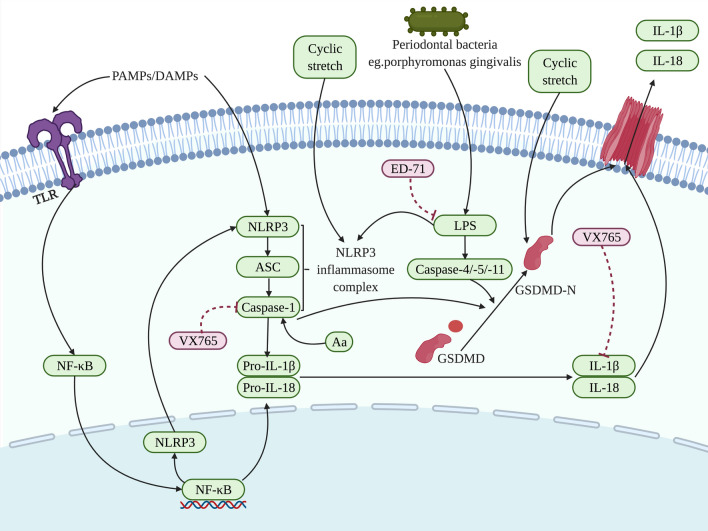
In the periodontal and gingival cells, pyroptosis is dominantly triggered by a non-canonical pathway, which usually involves the activation of LPS. It has been demonstrated that LPS can activate the NLRP3 inflammasome and subsequently induce IL-1β secretion, thereby inducing tissue inflammation, which may lead to bone loss [150]. Moreover, the results of gingival crevicular fluid and periodontium from periodontitis patients and healthy patients have proved that the pyroptosis modulated by the caspase-4/GSDMD lead to the death of periodontal ligament stem cells, which was attributed to the periodontal bacteria and cytoplasmic LPS, and subsequently, IL-1β was released into the tissue microenvironment to enhance inflammation and induce osteoclastogenesis [39]. Li et al. have found that LPS released by porphyromonas gingivalis could promote the pyroptosis of gingival fibroblasts with increasing inflammatory cytokines associated with pyroptosis, may inducing alveolar bone loss [13]. The regulation of LPS on pyroptosis could be inhibited via eldecalcitol (ED-71), in which upregulated Nrf2/HO-1 signaling displayed the role on suppressing pyroptosis, as well as the levels of NLRP3, caspase-1 and IL-1β decreased [151].
Except for LPS, NLRP3 inflammasome and GSDMD can be activated by cyclic stretch to prompt the process of pyroptosis. Zhao et al. have investigated that in the human PDLCs, cyclic stretch could positively stimulate the NLRP3 inflammasome and then induce the secretion of IL-1β, thereby enhancing pyroptosis and bone loss [152]. Besides, GSDMD could also be activated via cyclic stretch, and subsequently IL-1β as well IL-18 were liberated, leading to bone loss related to pyroptosis [153]. Additionally, it has been reported that the role of caspase-1 and IL-1β in the process of pyroptosis could be directly restrained by VX765, suppressing the development of apical periodontitis and the concomitant bone resorption [154] (Fig. 6).
Fig.6.
Different targets of action are regulated to affect the pyroptosis related to the periodontal and gingival cells in the process of bone loss. Exposure to PAMPs or DAMPs and then influenced by NF-κB, the NLRP3 inflammasome complex assemblies and activates caspase-1 and subsequently results in the cleavage of the proinflammatory cytokines IL-1β and IL-18. Caspase-1 and caspase-4/-5/-11 cleave GSDMD into a GSDMD-N-terminal domain that anchors in the cell membrane, leading to the cell rupture. Periodontal bacteria [39] including porphyromonas gingivalis [13] and ED-71 [151] have been found in the presented pathway targeting LPS. Meanwhile, NLRP3 and GSDMD are also the target of action to be regulated through cyclic stretch [152, 153], and Caspase-1 as well as IL-1β are negatively targeted by VX765 [154], affecting bone loss involved in pyroptosis. The factors that promote pyroptosis are indicated in green and pyroptosis-suppressor factors are indicated in pink
Pyroptosis in other cells
Apart from osteoclasts, osteoblasts, macrophages, chondrocytes, periodontal and gingival cells, in the process of pyroptosis associated with bone loss, osteocytes, fibroblast-like synoviocytes as well as cholesteatoma keratinocytes are also included. Zhang et al. reported that the pyroptotic death of osteocytes could be attributed to Bisphenol A which launched the pyroptosis through ROS/NLRP3/caspase-1 pathway, promoting bone erosion [155]. Besides, in the fibroblast-like synoviocytes, pyroptosis could be triggered via NLRP3/caspase-1/GSDMD signaling pathways, which were mediated by LPS as well as the NF-κB signaling that induced the activation of inflammasomes, promoting inflammation and accompanying bone loss [156]. While the activated inflammasome is distinct from NLRP3, it has been reported that the pyroptosis of cholesteatoma keratinocytes could be also induced by AIM2, which facilitated the development of cholesteatoma ( a kind of the non-permanent bone lesion) as well as bone loss [157] (Table 6).
Table 6.
The regulation of pyroptosis on other cells in the process of bone loss
| Target of action | Intervention | Mechanism | Experimental models | Effect | Diseases | References |
|---|---|---|---|---|---|---|
| ROS | Bisphenol A | ROS/NLRP3/caspase-1 | MLO-Y4 cells | Promoted bone loss | Not given | [155] |
| NLRP3 and caspase3 | LPS the NF-κB signaling | NLRP3/caspase-1/GSDMD and caspase-3/gasdermin E | fibroblast-like synoviocytes | Promoted bone loss | Rheumatoid arthritis | [156] |
| AIM2 | IFN-γ | IFN-γ/AIM2/caspase-1/ IL-1β and IL-18 | Cholesteatoma tissue | Promoted bone loss | Cholesteatoma | [157] |
Perspectives and conclusion
This review summarizes recent shreds of evidences that the pyroptosis in bone loss is associated with osteoclasts, osteoblasts, macrophages, chondrocytes, periodontal and gingival cells. The targets in the process of pyroptosis related to these cells have the potential to become an effective approach for future interventions in bone metabolic diseases, with implications for the prevention and treatment of bone metabolic diseases caused by pyroptosis.
However, some obstacles significantly hamper the application of the targets at this phase, mainly concerning the far from enough understanding of the clear association between pyroptosis and the bone loss, as well as lacking the further research of involved underlying mechanisms. For instance, a breakthrough of the restrictive assembly, activation, or other response mechanisms is required to interfere with pyroptosis by inhibiting the inflammasome, as well as the possible other roles of inflammasome-mediated pyroptosis in bone metabolic function in addition to the modulation on maturation of IL-1β, IL-18 and GSDMD. Meanwhile, for the prevention of bone metabolic diseases related to pyroptosis, it is of a notable necessity that clinical studies should be deeply investigated, which involve exploring effective strategies for integrating the Wnt/β-catenin pathway, MAPK pathway as well as other signaling pathways to block the NLRP3 inflammasome and repressing pyroptosis [158]. Furthermore, despite the fact that the combination of numerous treatments as well as various drugs developed on the basis of NLRP3, caspase, or the GSDMD may contribute to more hope for healing bone loss in bone metabolic diseases, it is still significant to evaluate the potential negative interaction mechanisms of these treatments that could trigger the disturbing effects on pyroptosis and thus reducing the therapeutic effect. In particular, the prospective association of versatile medication strategies should be balanced with the advantageous dedication of pyroptosis activation in host defense, preventing from mutually antagonistic intervention.
In terms of the current treatment targeting pyroptosis in bone metabolic diseases, developing direct inhibitors that directly target the NLRP3 protein and other indirect inhibitors that target components of the NLRP3 inflammasome, such as caspase-1, IL-1β, and IL-18, are currently reported to be more commonly therapies targeted against pyroptosis [158]. New drugs and related inhibitor molecules that directly target NLRP3 include MCC950 [68], glyburide [71], irisin [159], melatonin [98], dioscin [91], etc., which are effective in osteoporosis, impaired fracture healing caused by diabetes, osteoarthritis, osteomyelitis, and so on, displaying certain clinical therapeutic potential. In addition, VX765 targets Caspase-1 [154], and IL-18BP targets IL-18 [52], both of which have been found to partially reduce bone resorption, while the oral drug auranofin effectively inhibits IL-1β to suppress the progression of osteoclasts differentiation [76]. Some studies have focused on the post-transcriptional control of microRNA based on NLRP3, in which NLRP3 targeting miRNA is a relatively successful treatment method for rheumatoid arthritis, etc. [160], but it is not yet widely used in some specific bone metabolic diseases including osteoporosis [161]. Moreover, attenuating inflammasome activity through other indirect means has also shown some clinical therapeutic effects: using E and D series resolvins decreases NF-κB activity to suppress inflammasome activation [162]; using antioxidant drugs that target intracellular ROS regulates upstream signaling linked to inflammasome oligomerization and activation [163].
When it comes to the development direction of targets on pyroptosis therapy into clinics in the future, it is progressive for bone metabolic diseases to obtain suitable drugs which should be decreased the possibility of side effects that include the osteolytic inflammatory responses contemporaneous with targeted regulation of the NLRP3 inflammasome. Additionally, since it has been found that the activation of inflammasome in infected macrophages could trigger the release of IL-1 and IL-18 and the process of pyroptosis, driving the inflammatory process in covid-19, the concomitant effects on pulmonary inflammatory pathology can be referred in further application on the production and development of therapeutic strategies targeting pyroptosis [164]. Moreover, since current drugs are administered orally or subcutaneously [146], drug delivery methods that comply with the stability, specificity and efficacy of targeting are needed to develop. For example, in terms of drug delivery carriers, appropriate materials with excellent drug emancipate dynamics is advocated to devise and produce to prompt local delivery of potential anti-inflammatory drugs [5], exquisite candidates of which may include hydrogels. Carriers such as hydrogels are supplied with modifiable tunable physicochemical characteristics, effectively dominating drug release kinetics as well as probably solving the conundrum of systemic side effects [165, 166]. Besides, it is critical to design adequate and effective regulatory inhibitors to restrain pyroptosis and accompanying bone loss, which could be achieved through the regulation of inflammasome components, inflammasome initiation, inflammasome oligomerization as well as activation, proinflammatory influence on inflammasome-dependent cytokines. As described above, for instance, utilizing VX765 to target caspase-1 and utilizing MCC950 to target NLRP3, nevertheless, more potent inhibitors against different targets need to be developed to alleviate bone metabolic diseases related to pyroptosis in the future. Since it has been demonstrated that classical pathway and nonclassical pathway of pyroptosis will interact as well as activate each other, and subsequently their interdependent activation could enhance the expression of the NLRP3 inflammasome, suggesting that the development of vaccine needs to target both pathways to achieve superior consequent in treatment [167].
Acknowledgements
The figures were created with BioRender.
Abbreviations
- TLR
Toll-like receptors
- OVX
Ovariectomy
- BMMs
Mouse bone marrow cells
- MSCs
Mesenchymal stem cells
- BMSCs
Bone marrow stromal cells
- NOMID
Neonatal onset multisystem inflammatory disease
- HMGB1
High mobility group box 1
- PTOA
Post-traumatic osteoarthritis
- Lico A
Licochalcone A
- ASIC1a
Acid-sensing ion channel 1a
- TXNIP
Thioredoxin-interacting protein
- HPDLCs
Human periodontal ligament cells
- HPDLFs
Human periodontal ligament fibroblasts
- PDL
Human periodontal ligament
- GCF
Gingival crevicular fluid
- HGFs
Human gingival fibroblasts
Author contributions
XYL and LJ wrote the manuscript with feedback from all authors. XRM, XYC and MHZ polished the manuscript. SSH and SC gave their comments and suggestions to the manuscript. All authors read and approved the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (Grant Number 81671021), Science and Technology Foundation of Sichuan Province, China (Grant Number 2022YFS0127).
Data availability
All data relevant to this review is included in the text, references, and figures.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors approve the submission of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xinyi Li and Ling Ji have contributed equally to this work.
Contributor Information
Shushu He, Email: heshushu-03@163.com.
Song Chen, Email: songchen882002@hotmail.com.
References
- 1.Inoue K, Nakano S, Zhao B. Osteoclastic microRNAs and their translational potential in skeletal diseases. Semin Immunopathol. 2019;41:573–582. doi: 10.1007/s00281-019-00761-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldring SR, et al. Bone remodelling in inflammatory arthritis. Ann Rheum Dis. 2013;72(Suppl 2):ii52–ii55. doi: 10.1136/annrheumdis-2012-202199. [DOI] [PubMed] [Google Scholar]
- 3.Sozen T, Ozisik L, Basaran NC. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4:46–56. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 5.Yu C, Zhang C, Kuang Z, Zheng Q. The role of NLRP3 inflammasome activities in bone diseases and vascular calcification. Inflammation. 2021;44:434–449. doi: 10.1007/s10753-020-01357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liston A, Masters SL. Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nat Rev Immunol. 2017;17:208–214. doi: 10.1038/nri.2016.151. [DOI] [PubMed] [Google Scholar]
- 7.He WT, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carty M et al (2019) Cell survival and cytokine release after inflammasome activation is regulated by the Toll-IL-1R protein SARM. Immunity 50:1412–1424 e1416 (2019). 10.1016/j.immuni.2019.04.005 [DOI] [PubMed]
- 11.Tanaka Y. Clinical immunity in bone and joints. J Bone Miner Metab. 2019;37:2–8. doi: 10.1007/s00774-018-0965-5. [DOI] [PubMed] [Google Scholar]
- 12.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 13.Yang N, Liu Y. The role of the immune microenvironment in bone regeneration. Int J Med Sci. 2021;18:3697–3707. doi: 10.7150/ijms.61080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu P, et al. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou J, Hsu JM, Hung MC. Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol Cell. 2021;81:4579–4590. doi: 10.1016/j.molcel.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burdette BE, Esparza AN, Zhu H, Wang S. Gasdermin D in pyroptosis. Acta Pharm Sin B. 2021;11:2768–2782. doi: 10.1016/j.apsb.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tummers B, Green DR. The evolution of regulated cell death pathways in animals and their evasion by pathogens. Physiol Rev. 2022;102:411–454. doi: 10.1152/physrev.00002.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verdonck S, Nemegeer J, Vandenabeele P, Maelfait J. Viral manipulation of host cell necroptosis and pyroptosis. Trends Microbiol. 2021 doi: 10.1016/j.tim.2021.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Pollard KM, Kono DH. Requirements for innate immune pathways in environmentally induced autoimmunity. BMC Med. 2013;11:100. doi: 10.1186/1741-7015-11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redondo-Castro E, et al. Development of a characterised tool kit for the interrogation of NLRP3 inflammasome-dependent responses. Sci Rep. 2018;8:5667. doi: 10.1038/s41598-018-24029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kesavardhana S, Malireddi RKS, Kanneganti TD. Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol. 2020;38:567–595. doi: 10.1146/annurev-immunol-073119-095439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 23.Galluzzi L, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park YH, Wood G, Kastner DL, Chae JJ. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol. 2016;17:914–921. doi: 10.1038/ni.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rathinam VAK, Zhao Y, Shao F. Innate immunity to intracellular LPS. Nat Immunol. 2019;20:527–533. doi: 10.1038/s41590-019-0368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayagaki N, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 27.Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Sartoretto S, et al. Apoptosis-associated speck-like protein containing a caspase-1 recruitment domain (ASC) contributes to osteoblast differentiation and osteogenesis. J Cell Physiol. 2019;234:4140–4153. doi: 10.1002/jcp.27226. [DOI] [PubMed] [Google Scholar]
- 29.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Kolly L, et al. Expression and function of the NALP3 inflammasome in rheumatoid synovium. Immunology. 2010;129:178–185. doi: 10.1111/j.1365-2567.2009.03174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonar SL et al (2012) Constitutively activated NLRP3 inflammasome causes inflammation and abnormal skeletal development in mice. PLoS One 7:e35979. 10.1371/journal.pone.0035979 [DOI] [PMC free article] [PubMed]
- 32.Tao Z et al (2020) Pyroptosis in osteoblasts: a novel hypothesis underlying the pathogenesis of osteoporosis. Front Endocrinol (Lausanne) 11:548812 [DOI] [PMC free article] [PubMed]
- 33.Li Y, Ling J, Jiang Q. Inflammasomes in alveolar bone loss. Front Immunol. 2021;12:691013. doi: 10.3389/fimmu.2021.691013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Chen X, Gueydan C, Han J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018;28:9–21. doi: 10.1038/cr.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection. Nat Rev Immunol. 2017;17:151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegel RM. Caspases at the crossroads of immune-cell life and death. Nat Rev Immunol. 2006;6:308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- 37.Brunette RL, et al. Extensive evolutionary and functional diversity among mammalian AIM2-like receptors. J Exp Med. 2012;209:1969–1983. doi: 10.1084/jem.20121960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chae JJ, et al. Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity. 2011;34:755–768. doi: 10.1016/j.immuni.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Q et al (2021) Periodontal inflammation-triggered by periodontal ligament stem cell pyroptosis exacerbates periodontitis. Front Cell Dev Biol 9:663037 (2021). 10.3389/fcell.2021.663037 [DOI] [PMC free article] [PubMed]
- 40.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13:148–159. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rathinam VA, Fitzgerald KA. Inflammasome complexes: emerging mechanisms and effector functions. Cell. 2016;165:792–800. doi: 10.1016/j.cell.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown J, Wang H, Hajishengallis GN, Martin M. TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2011;90:417–427. doi: 10.1177/0022034510381264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao Y, et al. Antigen-specific CD8(+) T cell feedback activates NLRP3 inflammasome in antigen-presenting cells through perforin. Nat Commun. 2017;8:15402. doi: 10.1038/ncomms15402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 46.Ding J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 47.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265:130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X, Xia S, Zhang Z, Wu H, Lieberman J. Channelling inflammation: gasdermins in physiology and disease. Nat Rev Drug Discov. 2021;20:384–405. doi: 10.1038/s41573-021-00154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pietschmann P, Mechtcheriakova D, Meshcheryakova A, Foger-Samwald U, Ellinger I. Immunology of osteoporosis: a mini-review. Gerontology. 2016;62:128–137. doi: 10.1159/000431091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruscitti P et al (2015) The role of IL-1beta in the bone loss during rheumatic diseases. Mediat Inflamm. 10.1155/2015/782382 [DOI] [PMC free article] [PubMed]
- 51.Ge Y, Huang M, Yao YM. Recent advances in the biology of IL-1 family cytokines and their potential roles in development of sepsis. Cytokine Growth Factor Rev. 2019;45:24–34. doi: 10.1016/j.cytogfr.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Mansoori MN, et al. IL-18BP is decreased in osteoporotic women: prevents inflammasome mediated IL-18 activation and reduces Th17 differentiation. Sci Rep. 2016;6:33680. doi: 10.1038/srep33680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kristo C, et al. Raised serum levels of interleukin-8 and interleukin-18 in relation to bone metabolism in endogenous Cushing's syndrome. Eur J Endocrinol. 2002;146:389–395. doi: 10.1530/eje.0.1460389. [DOI] [PubMed] [Google Scholar]
- 54.Shahbaz SK, et al. Inflammasomes and colorectal cancer. Cells. 2021 doi: 10.3390/cells10092172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yi YS. Caspase-11 non-canonical inflammasome: emerging activator and regulator of infection-mediated inflammatory responses. Int J Mol Sci. 2020 doi: 10.3390/ijms21082736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wein T, Sorek R. Bacterial origins of human cell-autonomous innate immune mechanisms. Nat Rev Immunol. 2022 doi: 10.1038/s41577-022-00705-4. [DOI] [PubMed] [Google Scholar]
- 57.Schmid-Burgk JL, et al. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur J Immunol. 2015;45:2911–2917. doi: 10.1002/eji.201545523. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Grainger DW. RNA therapeutics targeting osteoclast-mediated excessive bone resorption. Adv Drug Deliv Rev. 2012;64:1341–1357. doi: 10.1016/j.addr.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Y, Humphrey MB, Nakamura MC. Osteoclasts - the innate immune cells of the bone. Autoimmunity. 2008;41:183–194. doi: 10.1080/08916930701693180. [DOI] [PubMed] [Google Scholar]
- 60.Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92:860–867. doi: 10.1177/0022034513500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang S, Kumanogoh A. Semaphorins in bone development, homeostasis, and disease. Semin Cell Dev Biol. 2013;24:163–171. doi: 10.1016/j.semcdb.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 62.Zhao C, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 63.Madel MB, et al. Immune function and diversity of osteoclasts in normal and pathological conditions. Front Immunol. 2019;10:1408. doi: 10.3389/fimmu.2019.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakashima T, Takayanagi H. New regulation mechanisms of osteoclast differentiation. Ann N Y Acad Sci. 2011;1240:E13–18. doi: 10.1111/j.1749-6632.2011.06373.x. [DOI] [PubMed] [Google Scholar]
- 65.Nakashima T, Hayashi M, Takayanagi H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol Metab. 2012;23:582–590. doi: 10.1016/j.tem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 66.Alippe Y, et al. Bone matrix components activate the NLRP3 inflammasome and promote osteoclast differentiation. Sci Rep. 2017;7:6630. doi: 10.1038/s41598-017-07014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y, et al. NLRP3 regulates alveolar bone loss in ligature-induced periodontitis by promoting osteoclastic differentiation. Cell Prolif. 2021;54:e12973. doi: 10.1111/cpr.12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zang Y, et al. Targeting NLRP3 inflammasome reduces age-related experimental alveolar bone loss. J Dent Res. 2020;99:1287–1295. doi: 10.1177/0022034520933533. [DOI] [PubMed] [Google Scholar]
- 69.An Y, et al. Activation of ROS/MAPKs/NF-kappaB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. 2019;33:12515–12527. doi: 10.1096/fj.201802805RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L, Wang Q, Su H, Cheng J. Exosomes from adipose derived mesenchymal stem cells alleviate diabetic osteoporosis in rats through suppressing NLRP3 inflammasome activation in osteoclasts. J Biosci Bioeng. 2021;131:671–678. doi: 10.1016/j.jbiosc.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 71.Yang X, Qu C, Jia J, Zhan Y. NLRP3 inflammasome inhibitor glyburide expedites diabetic-induced impaired fracture healing. Immunobiology. 2019;224:786–791. doi: 10.1016/j.imbio.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 72.Zhang L, Li S, Li J, Li Y. LncRNA ORLNC1 promotes bone marrow mesenchyml stem cell pyroptosis induced by advanced glycation end production by targeting miR-200b-3p/Foxo3 pathway. Stem Cell Rev Rep. 2021;17:2262–2275. doi: 10.1007/s12015-021-10247-2. [DOI] [PubMed] [Google Scholar]
- 73.Qu C, et al. NLRP3 mediates osteolysis through inflammation-dependent and -independent mechanisms. FASEB J. 2015;29:1269–1279. doi: 10.1096/fj.14-264804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rocha FRG, et al. Relevance of caspase-1 and Nlrp3 inflammasome on inflammatory bone resorption in a murine model of periodontitis. Sci Rep. 2020;10:7823. doi: 10.1038/s41598-020-64685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tao H et al (2021) Urolithin A suppresses RANKL-induced osteoclastogenesis and postmenopausal osteoporosis by, suppresses inflammation and downstream NF-kappaB activated pyroptosis pathways. Pharmacol Res 174 [DOI] [PubMed]
- 76.Kim HY, et al. Auranofin inhibits RANKL-induced osteoclastogenesis by suppressing inhibitors of kappab kinase and inflammasome-mediated interleukin-1beta secretion. Oxid Med Cell Longev. 2019;2019:3503912. doi: 10.1155/2019/3503912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tamai R, Kiyoura Y. Alendronate augments lipid A-induced IL-1beta release and Smad3/NLRP3/ASC-dependent cell death. Life Sci. 2018;198:8–17. doi: 10.1016/j.lfs.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 78.Liang S, Nian Z, Shi K. Inhibition of RIPK1/RIPK3 ameliorates osteoclastogenesis through regulating NLRP3-dependent NF-kappaB and MAPKs signaling pathways. Biochem Biophys Res Commun. 2020;526:1028–1035. doi: 10.1016/j.bbrc.2020.03.177. [DOI] [PubMed] [Google Scholar]
- 79.Cortet B, et al. Bone disorders associated with diabetes mellitus and its treatments. Joint Bone Spine. 2019;86:315–320. doi: 10.1016/j.jbspin.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 80.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 82.Kanazawa I, Sugimoto T. Diabetes mellitus-induced bone fragility. Intern Med. 2018;57:2773–2785. doi: 10.2169/internalmedicine.0905-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiao H, Xiao E, Graves DT. Diabetes and its effect on bone and fracture healing. Curr Osteoporos Rep. 2015;13:327–335. doi: 10.1007/s11914-015-0286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garcia-Hernandez AL, et al. Upregulation of proteins of the NLRP3 inflammasome in patients with periodontitis and uncontrolled type 2 diabetes. Oral Dis. 2019;25:596–608. doi: 10.1111/odi.13003. [DOI] [PubMed] [Google Scholar]
- 85.Li H, Zhong X, Chen Z, Li W. Suppression of NLRP3 inflammasome improves alveolar bone defect healing in diabetic rats. J Orthop Surg Res. 2019;14:167. doi: 10.1186/s13018-019-1215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riddle MC. Editorial: sulfonylureas differ in effects on ischemic preconditioning–is it time to retire glyburide? J Clin Endocrinol Metab. 2003;88:528–530. doi: 10.1210/jc.2002-021971. [DOI] [PubMed] [Google Scholar]
- 87.Lamkanfi M, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee WC, Guntur AR, Long F, Rosen CJ. Energy metabolism of the osteoblast: implications for osteoporosis. Endocr Rev. 2017;38:255–266. doi: 10.1210/er.2017-00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang X, et al. Nacetyl cysteine inhibits the lipopolysaccharideinduced inflammatory response in bone marrow mesenchymal stem cells by suppressing the TXNIP/NLRP3/IL1beta signaling pathway. Mol Med Rep. 2020;22:3299–3306. doi: 10.3892/mmr.2020.11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun W, et al. Overexpression of Sirt1 in mesenchymal stem cells protects against bone loss in mice by FOXO3a deacetylation and oxidative stress inhibition. Metabolism. 2018;88:61–71. doi: 10.1016/j.metabol.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 91.Yin W, et al. A new NLRP3 inflammasome inhibitor, dioscin promotes osteogenesis. Small. 2020;16:e1905977. doi: 10.1002/smll.201905977. [DOI] [PubMed] [Google Scholar]
- 92.Greene E, et al. Double-stranded RNA is a novel molecular target in osteomyelitis pathogenesis: a translational avian model for human bacterial chondronecrosis with osteomyelitis. Am J Pathol. 2019;189:2077–2089. doi: 10.1016/j.ajpath.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 93.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shi J, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 95.Rathinam VA, et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Detzen L, et al. NLRP3 is involved in long bone edification and the maturation of osteogenic cells. J Cell Physiol. 2021;236:4455–4469. doi: 10.1002/jcp.30162. [DOI] [PubMed] [Google Scholar]
- 97.Lei L, et al. Interleukin-17 induces pyroptosis in osteoblasts through the NLRP3 inflammasome pathway in vitro. Int Immunopharmacol. 2021;96:107781. doi: 10.1016/j.intimp.2021.107781. [DOI] [PubMed] [Google Scholar]
- 98.Xu L, et al. Melatonin suppresses estrogen deficiency-induced osteoporosis and promotes osteoblastogenesis by inactivating the NLRP3 inflammasome. Calcif Tissue Int. 2018;103:400–410. doi: 10.1007/s00223-018-0428-y. [DOI] [PubMed] [Google Scholar]
- 99.Liu S, et al. Oxidative stress induced pyroptosis leads to osteogenic dysfunction of MG63 cells. J Mol Histol. 2020;51:221–232. doi: 10.1007/s10735-020-09874-9. [DOI] [PubMed] [Google Scholar]
- 100.Wang L, et al. NLRP3 inflammasome activation in mesenchymal stem cells inhibits osteogenic differentiation and enhances adipogenic differentiation. Biochem Biophys Res Commun. 2017;484:871–877. doi: 10.1016/j.bbrc.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 101.Yang L, Liu J, Shan Q, Geng G, Shao P. High glucose inhibits proliferation and differentiation of osteoblast in alveolar bone by inducing pyroptosis. Biochem Biophys Res Commun. 2020;522:471–478. doi: 10.1016/j.bbrc.2019.11.080. [DOI] [PubMed] [Google Scholar]
- 102.Zhang J, Wei K. Necrosulfonamide reverses pyroptosis-induced inhibition of proliferation and differentiation of osteoblasts through the NLRP3/caspase-1/GSDMD pathway. Exp Cell Res. 2021;405:112648. doi: 10.1016/j.yexcr.2021.112648. [DOI] [PubMed] [Google Scholar]
- 103.Yoshida K, et al. PKR induces the expression of NLRP3 by regulating the NF-kappaB pathway in Porphyromonas gingivalis-infected osteoblasts. Exp Cell Res. 2017;354:57–64. doi: 10.1016/j.yexcr.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 104.Colombo JS, et al. Delayed osteoblast differentiation and altered inflammatory response around implants placed in incisor sockets of type 2 diabetic rats. Clin Oral Implants Res. 2011;22:578–586. doi: 10.1111/j.1600-0501.2010.01992.x. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Q, et al. Bisphosphonate induces osteonecrosis of the jaw in diabetic mice via NLRP3/Caspase-1-dependent IL-1beta mechanism. J Bone Miner Res. 2015;30:2300–2312. doi: 10.1002/jbmr.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alexander KA, et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res. 2011;26:1517–1532. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- 107.Gierut A, Perlman H, Pope RM. Innate immunity and rheumatoid arthritis. Rheum Dis Clin North Am. 2010;36:271–296. doi: 10.1016/j.rdc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guilliams M, Thierry GR, Bonnardel J, Bajenoff M. Establishment and maintenance of the macrophage niche. Immunity. 2020;52:434–451. doi: 10.1016/j.immuni.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 109.Chen K, et al. Communications between bone marrow macrophages and bone cells in bone remodeling. Front Cell Dev Biol. 2020;8:598263. doi: 10.3389/fcell.2020.598263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–396. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- 111.de Andrade KQ, Almeida-da-Silva CLC, Coutinho-Silva R. Immunological pathways triggered by Porphyromonas gingivalis and fusobacterium nucleatum: therapeutic possibilities? Mediators Inflamm. 2019;2019:7241312. doi: 10.1155/2019/7241312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ip WK, Medzhitov R. Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation. Nat Commun. 2015;6:6931. doi: 10.1038/ncomms7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Munoz J, Akhavan NS, Mullins AP, Arjmandi BH. Macrophage polarization and osteoporosis: a review. Nutrients. 2020 doi: 10.3390/nu12102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Snouwaert JN, et al. An NLRP3 mutation causes arthropathy and osteoporosis in humanized mice. Cell Rep. 2016;17:3077–3088. doi: 10.1016/j.celrep.2016.11.052. [DOI] [PubMed] [Google Scholar]
- 115.Zhang J, et al. NLRP3 inflammasome mediates M1 macrophage polarization and IL-1beta production in inflammatory root resorption. J Clin Periodontol. 2020;47:451–460. doi: 10.1111/jcpe.13258. [DOI] [PubMed] [Google Scholar]
- 116.Yamaguchi Y, Kurita-Ochiai T, Kobayashi R, Suzuki T, Ando T. Regulation of the NLRP3 inflammasome in Porphyromonas gingivalis-accelerated periodontal disease. Inflamm Res. 2017;66:59–65. doi: 10.1007/s00011-016-0992-4. [DOI] [PubMed] [Google Scholar]
- 117.Wang L, Pu W, Wang C, Lei L, Li H. Microtubule affinity regulating kinase 4 promoted activation of the NLRP3 inflammasome-mediated pyroptosis in periodontitis. J Oral Microbiol. 2022;14:2015130. doi: 10.1080/20002297.2021.2015130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sugiyama M, et al. Activation of inflammasomes in dendritic cells and macrophages by Mycoplasma salivarium. Mol Oral Microbiol. 2016;31:259–269. doi: 10.1111/omi.12117. [DOI] [PubMed] [Google Scholar]
- 119.Zhu X et al (2019) Inhibition of pyroptosis attenuates Staphylococcus aureus-induced bone injury in traumatic osteomyelitis. Ann Transl Med 7:170. 10.21037/atm.2019.03.40 [DOI] [PMC free article] [PubMed]
- 120.Kawahara Y, et al. Effects of sulfonylureas on periodontopathic bacteria-induced inflammation. J Dent Res. 2020;99:830–838. doi: 10.1177/0022034520913250. [DOI] [PubMed] [Google Scholar]
- 121.Zhou X, et al. Metformin ameliorates the NLPP3 inflammasome mediated pyroptosis by inhibiting the expression of NEK7 in diabetic periodontitis. Arch Oral Biol. 2020;116:104763. doi: 10.1016/j.archoralbio.2020.104763. [DOI] [PubMed] [Google Scholar]
- 122.Fan W, Chen S, Wu X, Zhu J, Li J. Resveratrol relieves gouty arthritis by promoting mitophagy to inhibit activation of NLRP3 inflammasomes. J Inflamm Res. 2021;14:3523–3536. doi: 10.2147/JIR.S320912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu L, et al. The monomer derivative of paeoniflorin inhibits macrophage pyroptosis via regulating TLR4/ NLRP3/ GSDMD signaling pathway in adjuvant arthritis rats. Int Immunopharmacol. 2021;101:108169. doi: 10.1016/j.intimp.2021.108169. [DOI] [PubMed] [Google Scholar]
- 124.Maruyama K, Nemoto E, Yamada S. Mechanical regulation of macrophage function - cyclic tensile force inhibits NLRP3 inflammasome-dependent IL-1beta secretion in murine macrophages. Inflamm Regen. 2019;39:3. doi: 10.1186/s41232-019-0092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maruyama K, et al. Cyclic stretch negatively regulates IL-1beta secretion through the inhibition of NLRP3 inflammasome activation by attenuating the AMP kinase pathway. Front Physiol. 2018;9:802. doi: 10.3389/fphys.2018.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ji X, et al. Dexmedetomidine protects against high mobility group box 1-induced cellular injury by inhibiting pyroptosis. Cell Biol Int. 2019;43:651–657. doi: 10.1002/cbin.11140. [DOI] [PubMed] [Google Scholar]
- 127.Ben Lagha A, Howell A, Grenier D (2019) Cranberry proanthocyanidins neutralize the effects of Aggregatibacter actinomycetemcomitans Leukotoxin. Toxins (Basel). 10.3390/toxins11110662 [DOI] [PMC free article] [PubMed]
- 128.Ge G, et al. Punicalagin ameliorates collagen-induced arthritis by downregulating M1 macrophage and pyroptosis via NF-kappaB signaling pathway. Sci China Life Sci. 2022;65:588–603. doi: 10.1007/s11427-020-1939-1. [DOI] [PubMed] [Google Scholar]
- 129.Wang Z, et al. Cyclic stretch force induces periodontal ligament cells to secrete exosomes that suppress IL-1beta production through the inhibition of the NF-kappaB signaling pathway in macrophages. Front Immunol. 2019;10:1310. doi: 10.3389/fimmu.2019.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang T, et al. Gasdermin D deficiency attenuates arthritis induced by traumatic injury but not autoantibody-assembled immune complexes. Arthritis Res Ther. 2021;23:286. doi: 10.1186/s13075-021-02668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu Q, Zheng M, Balakrishnan A, Karki R, Kanneganti TD. Gasdermin D promotes AIM2 inflammasome activation and is required for host protection against Francisella novicida. J Immunol. 2018;201:3662–3668. doi: 10.4049/jimmunol.1800788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. 2014;63:1103–1114. doi: 10.2337/db13-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Komatsu N, Takayanagi H. Immune-bone interplay in the structural damage in rheumatoid arthritis. Clin Exp Immunol. 2018;194:1–8. doi: 10.1111/cei.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weber A, Chan PMB, Wen C. Do immune cells lead the way in subchondral bone disturbance in osteoarthritis? Prog Biophys Mol Biol. 2019;148:21–31. doi: 10.1016/j.pbiomolbio.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 135.Fosang AJ, Beier F. Emerging frontiers in cartilage and chondrocyte biology. Best Pract Res Clin Rheumatol. 2011;25:751–766. doi: 10.1016/j.berh.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 136.Wang M, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15:R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.An S, Hu H, Li Y, Hu Y (2020) Pyroptosis plays a role in osteoarthritis. Aging Dis 11:1146–1157. 10.14336/AD.2019.1127 [DOI] [PMC free article] [PubMed]
- 138.Hu J, et al. Loganin ameliorates cartilage degeneration and osteoarthritis development in an osteoarthritis mouse model through inhibition of NF-kappaB activity and pyroptosis in chondrocytes. J Ethnopharmacol. 2020;247:112261. doi: 10.1016/j.jep.2019.112261. [DOI] [PubMed] [Google Scholar]
- 139.Yu H, et al. Morroniside attenuates apoptosis and pyroptosis of chondrocytes and ameliorates osteoarthritic development by inhibiting NF-kappaB signaling. J Ethnopharmacol. 2021;266:113447. doi: 10.1016/j.jep.2020.113447. [DOI] [PubMed] [Google Scholar]
- 140.Yan Z, et al. Activating Nrf2 signalling alleviates osteoarthritis development by inhibiting inflammasome activation. J Cell Mol Med. 2020;24:13046–13057. doi: 10.1111/jcmm.15905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu H, Xu B. BMSC-derived exosomes ameliorate osteoarthritis by inhibiting pyroptosis of cartilage via delivering miR-326 targeting HDAC3 and STAT1//NF-kappaB p65 to chondrocytes. Mediat Inflamm. 2021;2021:9972805. doi: 10.1155/2021/9972805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li Z, et al. P2X7 receptor induces pyroptotic inflammation and cartilage degradation in osteoarthritis via NF-kappaB/NLRP3 crosstalk. Oxid Med Cell Longev. 2021;2021:8868361. doi: 10.1155/2021/8868361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu X, Ren G, Zhou R, Ge J, Chen FH. The role of Ca(2+) in acid-sensing ion channel 1a-mediated chondrocyte pyroptosis in rat adjuvant arthritis. Lab Invest. 2019;99:499–513. doi: 10.1038/s41374-018-0135-3. [DOI] [PubMed] [Google Scholar]
- 144.Zu Y, Mu Y, Li Q, Zhang ST, Yan HJ. Icariin alleviates osteoarthritis by inhibiting NLRP3-mediated pyroptosis. J Orthop Surg Res. 2019;14:307. doi: 10.1186/s13018-019-1307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiang N, et al. Periodontal ligament and alveolar bone in health and adaptation: tooth movement. Front Oral Biol. 2016;18:1–8. doi: 10.1159/000351894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marchesan JT, et al. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontol. 2020;2000(82):93–114. doi: 10.1111/prd.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murayama R, Kobayashi M, Takeshita A, Yasui T, Yamamoto M. MAPKs, activator protein-1 and nuclear factor-kappaB mediate production of interleukin-1beta-stimulated cytokines, prostaglandin E(2) and MMP-1 in human periodontal ligament cells. J Periodontal Res. 2011;46:568–575. doi: 10.1111/j.1600-0765.2011.01374.x. [DOI] [PubMed] [Google Scholar]
- 148.Wang F, Guan M, Wei L, Yan H. IL18 promotes the secretion of matrix metalloproteinases in human periodontal ligament fibroblasts by activating NFkappaB signaling. Mol Med Rep. 2019;19:703–710. doi: 10.3892/mmr.2018.9697. [DOI] [PubMed] [Google Scholar]
- 149.Li YY, et al. The effect of Porphyromonas gingivalis lipopolysaccharide on the pyroptosis of gingival fibroblasts. Inflammation. 2021;44:846–858. doi: 10.1007/s10753-020-01379-7. [DOI] [PubMed] [Google Scholar]
- 150.Lu WL, et al. NLRP3 inflammasome may regulate inflammatory response of human periodontal ligament fibroblasts in an apoptosis-associated speck-like protein containing a CARD (ASC)-dependent manner. Int Endod J. 2017;50:967–975. doi: 10.1111/iej.12722. [DOI] [PubMed] [Google Scholar]
- 151.Huang C, et al. Eldecalcitol inhibits LPS-induced NLRP3 inflammasome-dependent pyroptosis in human gingival fibroblasts by activating the Nrf2/HO-1 signaling pathway. Drug Des Devel Ther. 2020;14:4901–4913. doi: 10.2147/DDDT.S269223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhao D, Wu Y, Zhuang J, Xu C, Zhang F. Activation of NLRP1 and NLRP3 inflammasomes contributed to cyclic stretch-induced pyroptosis and release of IL-1beta in human periodontal ligament cells. Oncotarget. 2016;7:68292–68302. doi: 10.18632/oncotarget.11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhuang J, et al. Gasdermin-d played a critical role in the cyclic stretch-induced inflammatory reaction in human periodontal ligament cells. Inflammation. 2019;42:548–558. doi: 10.1007/s10753-018-0912-6. [DOI] [PubMed] [Google Scholar]
- 154.Cheng R, et al. The extent of pyroptosis varies in different stages of apical periodontitis. Biochim Biophys Acta Mol Basis Dis. 2018;1864:226–237. doi: 10.1016/j.bbadis.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 155.Zhang Y, et al. Bisphenol A induces pyroptotic cell death via ROS/NLRP3/Caspase-1 pathway in osteocytes MLO-Y4. Food Chem Toxicol. 2022;159:112772. doi: 10.1016/j.fct.2021.112772. [DOI] [PubMed] [Google Scholar]
- 156.Yang P, et al. LPS induces fibroblast-like synoviocytes RSC-364 cells to pyroptosis through NF-kappaB mediated dual signalling pathway. J Mol Histol. 2021;52:661–669. doi: 10.1007/s10735-021-09988-8. [DOI] [PubMed] [Google Scholar]
- 157.Zhang C, Chen M, Chi Z. Cytokine secretion and pyroptosis of cholesteatoma keratinocytes mediated by AIM2 inflammasomes in response to cytoplasmic DNA. Mol Med Rep. 2021 doi: 10.3892/mmr.2021.11983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jiang N, et al. NLRP3 inflammasome: a new target for prevention and control of osteoporosis? Front Endocrinol (Lausanne) 2021;12:752546. doi: 10.3389/fendo.2021.752546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu L, et al. Effects of Irisin on osteoblast apoptosis and osteoporosis in postmenopausal osteoporosis rats through upregulating Nrf2 and inhibiting NLRP3 inflammasome. Exp Ther Med. 2020;19:1084–1090. doi: 10.3892/etm.2019.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tezcan G, et al. MicroRNA post-transcriptional regulation of the NLRP3 inflammasome in immunopathologies. Front Pharmacol. 2019;10:451. doi: 10.3389/fphar.2019.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xie Q, et al. MicroRNA-33 regulates the NLRP3 inflammasome signaling pathway in macrophages. Mol Med Rep. 2018;17:3318–3327. doi: 10.3892/mmr.2017.8224. [DOI] [PubMed] [Google Scholar]
- 162.Van Dyke TE. Pro-resolving mediators in the regulation of periodontal disease. Mol Aspects Med. 2017;58:21–36. doi: 10.1016/j.mam.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jiang Q, et al. Mitochondria-targeted antioxidants: a step towards disease treatment. Oxid Med Cell Longev. 2020;2020:8837893. doi: 10.1155/2020/8837893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sefik E, et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature. 2022 doi: 10.1038/s41586-022-04802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fernandez-Yague MA, et al. Analyzing immune response to engineered hydrogels by hierarchical clustering of inflammatory cell subsets. Sci Adv. 2022;8:eabd8056. doi: 10.1126/sciadv.abd8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vigata M, Meinert C, Pahoff S, Bock N, Hutmacher DW. Gelatin methacryloyl hydrogels control the localized delivery of albumin-bound paclitaxel. Polymers (Basel) 2020 doi: 10.3390/polym12020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moretti J, et al. Caspase-11 interaction with NLRP3 potentiates the noncanonical activation of the NLRP3 inflammasome. Nat Immunol. 2022 doi: 10.1038/s41590-022-01192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to this review is included in the text, references, and figures.