Abstract
Biofilms are self-organized communities of microorganisms that are encased in an extracellular polymeric matrix and often found attached to surfaces. Biofilms are widely present on Earth, often found in diverse and sometimes extreme environments. These microbial communities have been described as recalcitrant or protective when facing adversity and environmental exposures. On the International Space Station, biofilms were found in human-inhabited environments on a multitude of hardware surfaces. Moreover, studies have identified phenotypic and genetic changes in the microorganisms under microgravity conditions including changes in microbe surface colonization and pathogenicity traits. Lack of consistent research in microgravity-grown biofilms can lead to deficient understanding of altered microbial behavior in space. This could subsequently create problems in engineered systems or negatively impact human health on crewed spaceflights. It is especially relevant to long-term and remote space missions that will lack resupply and service. Conversely, biofilms are also known to benefit plant growth and are essential for human health (i.e., gut microbiome). Eventually, biofilms may be used to supply metabolic pathways that produce organic and inorganic components useful to sustaining life on celestial bodies beyond Earth. This article will explore what is currently known about biofilms in space and will identify gaps in the aerospace industry's knowledge that should be filled in order to mitigate or to leverage biofilms to the advantage of spaceflight.
1. Introduction
Multiple biofilm studies in the spaceflight environment have been reported in the past decade [1]. In 2010 and 2011, Space Shuttle Atlantis flew biofilm studies of Pseudomonas and Staphylococcus spp via the Micro-2 experiment, and the follow-on study Micro-2A [2] for the purpose of characterizing biofilm and determining phenotypical microgravitational effects. This study resulted in the description of column-and-canopy microgravity biofilms, more than 10 years after the first scientific study on biofilms under microgravity that flew on Space Shuttle Discovery's 25th flight [3]. Most recently, biology spaceflight experiments have chosen to study transcriptomic changes, such as the study by the University of Colorado Boulder [4], which focused on the gene expression changes caused by spaceflight in Escherichia coli. Space agencies other than the National Aeronautics and Space Administration (NASA), such as the European Space Agency (ESA), have also shown interest in spaceflight biofilms. Surface materials, such as laser-patterned copper, steel, and brass, are to be studied via the experiment BIOFILMS (Siems et al., 2022) at the International Space Station (ISS). This study will focus on different material's resistance to biofilm under microgravity. Materials, biofilm phenotype, and transcriptomic studies all take place under spaceflight conditions to inform researchers and agencies on the best paths to mitigation, and on the conditions that may affect biofilm development. The diversity of experimental designs, including a range of microorganisms and transcriptomic targets, results in data gathering and analysis challenges. The broad scope and directions in these studies—including different microbial metabolisms, nutrient media, species, and methods—provide large data sets, albeit with sparse information. These studies provide insight into the impact of space environments on microorganisms, but we are still challenged for a full understanding of the importance of biofilm in space.
Microbial biofilms can exhibit resistance to harsh environments such as UV radiation, varied pH levels, extreme temperatures, nutrient limitation, starvation, high salinity, and pressurized environments (Yin et at., 2019). Such flexibility in environmental tolerance represents an issue in treating biofilm and building habitats that are less propense to such biocontamination. On the other hand, some biofilms may be optimal for processing and engineering, as seen in sewage systems on Earth containing biofilms that take part in biodegradation of organic matter. However, these biofilms can also contain and transport antimicrobial resistance genes that may affect microbial processes as part of wastewater treatments [5]. Beneficial biofilms can also be engineered and applied in microbial fuel cells, bioremediation, and certain food product bioprocesses [[6], [7], [8]].
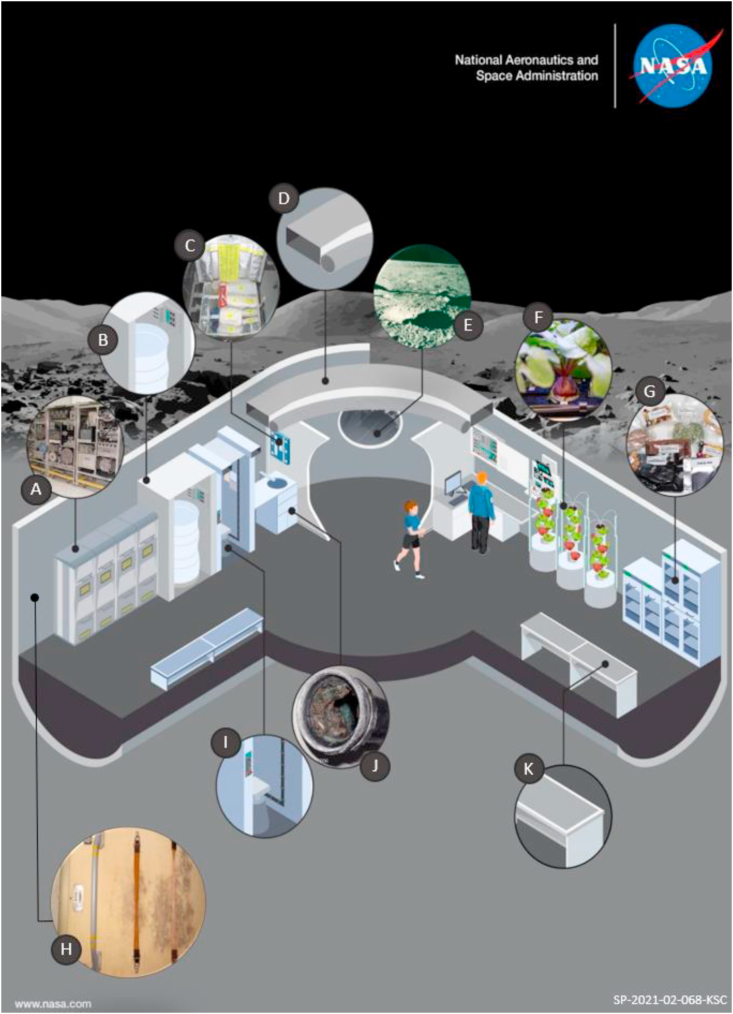
Biofilm presence on surfaces can affect hardware and other mission-critical materials, potentially leading to failures in industrial and clinical systems [9,10]. In space systems, surfaces include water recycling hardware, hatch locks, control panels, electrical connectors, oxygen electrolysis blocks, thermal control system radiators, extravehicular activity (EVA) suit headphones, and the navigation window [11,12]. These components are vital for supporting life in space due to their ability to supply water, air, and crucial mission controls (see Fig. 1). Due to the risk that biofilms represent to system performance, it is critical to continue studies aimed at understanding the biofilm life cycle, physiology, and effects on surfaces in addition to developing potential mitigation practices.
Fig. 1.
Schematic of areas that can come into contact or sustain biofilm growth during space habitation. (A) The Water Processor Assembly is an important part in the production of potable water and urine recycling for sustaining astronaut life. (B) Showers/cleaning devices are used to support astronaut hygiene. (C) Medical kits are carried on missions and medicine/medical attention is an important operations and mission planning. (D) Air conduits and collection of humidity condensate are a part of water and air system for life sustainability. (E) In-situ resource utilization (ISRU) will sustain production of needed materials from resources found where the habitat is situated. (F) Crop production in space encompasses the challenges of gathering the necessary compounds and conditions to grow nutritious food in space. Some plants benefit from biofilm although some microorganisms may be considered an infectious plant threat. (G) Food production and shipment, similarly to food crops, require safety measures and may benefit from in-situ production of specific nutrients. (H) Walls in the ISS have shown fungal growth before [13] and considering interior/exterior walls that may contain proliferating organisms is of importance for maintenance of a habitat (I) Urinals and full toilets are part of mission planning and contain surfaces that get dirty but also provide matter that can be reused (J) Part of urinals, air, and water systems are the individual components of Environmental Control and Life Support Systems (ECLSS) which contain hosing, heat exchangers, filters, and entire units with wet surfaces were biofilms may thrive (K) Other dry or humid surfaces may exist in the interior, especially science cabinets and technological equipment.
The pathogenesis of biofilms in space may directly affect astronaut health and wellbeing. However, the effects of biofilms on health in differing gravities are only partially understood due to the limitations of microgravitational studies as well as the complexity of working with biofilms composed of different species and describing their similarities under varying conditions [14,15]. In contrast to system and health risks, enhancing biofilms for food production (i.e., nutrient production/extraction and plant microbiology) may enable more autonomous and longer lasting space exploration [[16], [17], [18]]. With the proposed exploration of the Moon and Mars, the Artemis missions, and potential new space habitats, microbial monitoring and controls are sought for sustainable space operations [19]. This article will explore the current research about biofilms on wet and dry surfaces, the impact of microgravity on virulence, and how this impacts astronaut health (see Fig. 1). Furthermore, we will discuss the opportunity to use biofilms as a potential strategy in in-situ resource utilization and will explore if the fundamental aspects that define biofilms on Earth are translatable to how biofilms form and function in space.
2. Wet surfaces
Spaceflight hardware used during missions may have surfaces in direct contact with liquids such as crew water and fuels, which put surfaces at risk for microbial contamination, propagation and biofilm formation. The study of this contamination is also a part of Planetary Protection risk mitigation activities [20]. In other industries, materials such as stainless steel and aluminum alloys, common fuel tank materials, have been studied for susceptibility to abiotic and biotic corrosion [21,22]. However, these conditions are not well understood in microgravity or partial gravity. Constant wet surfaces that can be considered in crewed missions are those related to the Water Recovery and Management System in the ISS. This structure contains a Water Recovery System (WRS) and produces potable water from urine that can be used for drinking, cleaning, oxygen generation or payloads as required [23]. In recent times, parts related to the WRS have been grounded due to biofilm obstructions [11]. Such was the case of the filter for the External Filter Assembly in 2011 (Weir et al., 2012) and the obstruction of a solenoid valve in the Mostly Liquid Separator in 2009 [11]. Grounding equipment for refurbishment entails receiving the equipment on Earth and making sure that a replacement can be sent back to the space system if needed. Such services can cause delays, increase mission costs, in some cases risk crew safety and furthermore lack feasibility as missions increase in duration and distance from Earth. In addition to water systems, other liquid systems are challenged by biofilm. Biofilm contamination has been observed in aircraft fuel tanks [24] and microbial contamination of propellants has been studied by NASA [20].
In the case of future space missions, especially those that cannot be serviced due to mission distances, systems must be able to resist or limit an influx of microorganisms and continue their intended function for uninterrupted periods of time under varying conditions in space. Multiple efforts have been made to mitigate and treat biofilms in WPA systems such as UV LED treatment in the wastewater tank and antimicrobial coatings [[25], [26], [27]]. However, research in space systems is challenged by a changing microbiome caused by rotating crew members [28] and due to fluctuations of nutrients and inhibitory compounds in wastewater that can influence survival of individual microbial species [29]. Biofilm-related studies can contribute to the identification of knowledge gaps in the way that biofilms are tested, the role of microgravity in microbial behavioral, and how different species affect the outcome of such results. Nevertheless, standardized methods of validating water biofilm technologies are needed to bridge gaps in varied testing control groups. Robust contamination control and system assembly requirements can be part of the strategies for mitigating the water system bioburden which is conducive to biofilm formation.
2.1. Knowledge gaps
-
a.
Gravitational effects on biofilm formation. Life support systems will be used in Moon and Mars habitats during deep space exploration under varying gravity regimes. Therefore, multiple modes of biofilm gravity systems and the impact on biofouling in wetted systems must be understood.
-
b.
Microbial growth during system dormancy. Liquid systems in future missions may not be in constant use and wetted surfaces may stand stagnant or empty during system shutdown. Biofilm and control practices in systems prior to, during, and following system dormancy have not been fully studied [12] and is a current area of active research.
-
c.
Contamination control and engineering requirements for biofilm prevention. Space systems undergo manufacturing and assembly procedures that can be modified to include contamination control and human factor requirements. Such requirements for spacecraft systems are not uncommon, but requirements for hardware in water systems do not yet exist or have not been published.
-
d.
Sensors, astronaut interface, and potential machine learning (ML) for biofilm detection. If there are ongoing biofilm requirements that span the entirety of a mission, biofilm monitoring could enhance abilities to detect problems prior to system failure. As monitoring is not yet autonomous, such systems would need an interface for the astronauts to use. In the future, potential ML features could help anticipate issues and alarm of any operational risks or enable a more straightforward decision pipeline in case of failures or failure anticipation.
3. Dry surfaces
Because biofilms generally thrive in continuously wet or moist conditions, dry surfaces tend to represent an unfavorable environment for biofilm formation. However, dry, hard, and nonporous surfaces on the ISS can be intermittently damp due to fluctuating humidity levels and frequent human contact during normal usage [30]. Currently on the ISS, surface swabs are used to quantify surface-associated microorganisms [31]. reported the top three most frequently isolated genera of bacteria recovered were Micrococcus, Bacillus, and Staphylococcus. The top three fungal genera recovered were Hyphomycetes, Aspergillus, and Penicillium [31]. In other recent studies, different microorganisms seem to predominate but overall, the community has been shown to consist of human-associated microorganisms, which can be transient or enduring and consist of various types of bacteria and fungi [[32], [33], [34], [35]]; UC Boulder Space Biofilms, 2019, [36,37]. Furthermore, the community make up can fluctuate with the crew make up [28]. As determined by a consensus of NASA experts, the acceptable limit for surface-associated bacteria is set at 10,000 CFU/100 cm2, and surface-associated fungi is set at 100 CFU/100 cm2 [30,31]. For the purposes of this review, we focused on dry biofilms formed on surfaces in the ISS, including ceilings, walls, and other high-touch surfaces. Several studies have been conducted or are currently underway that aim at understanding the characteristics and implications of these biofilms [31,34,35].
While there are studies ongoing to understand the inherent science of the biofilms aboard the ISS, it is critical that we understand how to control them. This includes understanding how microorganisms become deposited on surfaces, which is likely through means of direct contact and air circulation since aerosol settling is not a factor in microgravity [38]. There are also questions regarding the importance of the surface material in the development and morphology of the dry biofilm [11]; UC Boulder Space Biofilms, 2019; [39]. Once deposited, it needs to be assessed how the ‘sticky’ matrix can hold the microorganisms in place. Furthermore, evaluation of how the biofilms can collect organic and other materials as well as nutrients is important [11]. Possible detrimental impacts of biofilm growth have been identified, including (1) microbially induced corrosion (MIC) or blockage of mechanical components, (2) detriment to human health through infection or allergy response, (3) potential to harbor pathogens to both humans and plants, and (4) the development of antimicrobial resistance [[39], [40], [41]].
Given these risks of dry biofilms, measures are taken on the ISS to manage biofilm growth. The ISS makes use of humidity and condensation controls, high efficiency particulate air (HEPA) filters, and cleanable surfaces as well as precleaning materials sent to the ISS [30,34,42,43]. Lastly, measures are taken to frequently clean the ISS using a variety of methods [31] that in short include weekly or daily cleaning with disinfecting wipes and vacuuming for larger debris [44]. In the context of a biofilm, it is not always possible to distinguish between sanitization (i.e., killing or inactivating cells) and surface cleaning (i.e., removing the dried biofilm). Some chemistries and procedures kill viable cells but do not remove the matrix (e.g., quaternary substances, most antimicrobial surfaces), some remove the matrix but do not kill the cells (e.g., scrubbing with a microfiber cloth), and some do both (e.g., bleach). Given that wipes are frequently employed on the ISS, it is presumed that this provides mechanical removal of the dried biofilm, and thus is beneficial in achieving both aims. Finally, precaution must always be taken to consider the toxicity of the compounds used for cleaning, especially when cleaning with volatiles within a closed system like the ISS [39].
While antimicrobial surfaces have been extensively explored as an approach to control microbial contamination on hard, non-porous, and high-touch surfaces in a hospital environment, the use of antimicrobial surfaces has not yet been implemented on the ISS [39,45]. However, studies by the European Space Agency (ESA) and Boeing are ongoing aboard the ISS to assess the antimicrobial nature of materials for use in future applications [46,47].
Given that we cannot assume that what is known about dried biofilms on Earth translates to dried biofilms on the ISS, the following assumptions on the knowledge gaps are made.
3.1. Knowledge gaps
-
a.
Microbial surfaces. There is no data to answer how the unique environment on the ISS impacts the development or prevalence of dried biofilms. Additional studies are required to address how microgravity impacts surface colonization and the matrix of dried biofilms as well as biofilm responses to antimicrobial surfaces.
-
b.
Long-term effects. Research is needed to determine how surface communities change over time and if their survival mechanism is through community structure and composition, lateral gene transfer, or mutations. It is also of critical importance to understand evolutionary biology in terms of partial gravity, increased radiation, and a possibly altered chemical environment beyond low Earth orbit (LEO) impact biofilm growth.
-
c.
Medical surfaces. On-board sterilization protocols need to be developed in the event that a surgical procedure is needed for a crew member. This becomes a significant consideration in the future for space travel beyond LEO. Surfaces for surgery and surfaces of implants or of tools must also be considered with regards to cleaning reusable medical devices.
4. Impact to astronaut health and other considerations
Biofilms are ubiquitous and play various roles in natural and human-engineered environments. While not all biofilms raise concerns for human health on Earth, they are generally known to have enhanced chemical/drug resistance, persistence, and virulence [48]. In particular, biofilms are often considered to be a serious threat for chronic infections associated with drug resistance [[49], [50], [51], [52], [53], [54], [55]]. Biofilm matrices are composed of biomolecules such as extracellular polysaccharides, nucleic acids, secreted proteins, and lipoproteins [56] and provide protection for the biofilm communities from environmental stress (pH, osmolality, fluid shear, antibiotics, etc. [57] or host immune cells [58]. It allows reduced drug susceptibility and microbial persistence that can contribute to long-term microbial adaptation and genomic modification over generations [54,59,60]. Numerous studies showed changes in microbial properties with biofilm formation, which includes consistent expression of efflux pumps, entering dormant states, shifting to alternative metabolism, and stress responses that are directly or indirectly associated with microbial virulence [53,55,57,61,62]. Thus, biofilms increase infection risk and biofilm formation in human living environments can negatively impact human health and performance, both in Earth & non-Earth environments [49,50,54,63]. In addition, biofilms can clog filtration systems or corrode system materials [[64], [65], [66]]. While mechanical removal is an effective way to control biofilm formation on easy-to-reach surfaces, it is not easy to apply to an isolated, semi-closed or closed-loop environment such as spaceflights or human habitats beyond Earth. Biofilms clogging filtration systems, corroding system materials, or causing infection can be life-threatening for astronauts relying on the life support systems for their survival [67,68].
Such adverse effects, particularly biofilms involving pathogens, are a serious concern during a long-term space mission, and it is critical to find effective ways to control the microbial load and biofilm formation in the space environment for the success of long-term space missions far from the Earth. Enhancing our understanding of biofilm formation, survival, and changes adapting to non-Earth environments will improve risk assessments of biofilms and mitigation strategies, leading to success of the currently planned and future long-term missions. Here, we list several aspects of biofilm formation that raise concerns for sustainability of human health and life support systems: 1) increase in both tolerance and resistance to some physical, chemical and biological treatments, or to host immune response [55,60,61], 2) higher densities and decreased cell-to-cell distance within biofilms can enhance horizontal gene transfer (HGT) which in turn could lead to increased virulence and drug resistance, 3) long-term microbial interactions within the biofilm community could promote evolutionary fitness within the population in the given closed system [60].
Current studies show alterations in virulence-associated microbial properties, gene expression, or biofilm formation in the spaceflight or spaceflight analogue environment [[69], [70], [71], [72]]. For example, Salmonella spp. grown in the ISS showed upregulated gene expression associated with biofilm formation such as wca/wza, ompA, fim genes and subsequent biofilm formation, as compared to synchronous ground control [71]. Candida albicans, a commensal yeast and common causative agent of fungal infection that was flown to the ISS showed enhanced cell aggregation, an increase in cell growth, and resistance to the antifungal Amphotericin B, as compared to terrestrial controls [73,74]. Moreover, studies utilizing spaceflight analogue cell culture devices such as the Rotating Wall vessel showed that Escherichia coli, Listeria monocytogenes, Enterococcus faecalis, and Staphylococcus aureus strains altered pathogenesis or virulence potential after being cultured in simulated spaceflight conditions [71,75,76].
However, some studies have not found definitive evidence suggesting significant alterations in microbial virulence or antimicrobial characteristics relevant to human health under space station conditions [77,78] or found a reduced virulence potential of Yersinia pestis or S. aureus rather than enhanced [79]; Castro et al., 2011). For example, Mora and co-workers used metagenomic analysis and several physiological tests including antibiotics susceptibility and heat-shock resistance test and showed that the ISS microbial communities are highly similar to those present in the ground-based environment [77]. The authors found that microbial diversity, distribution, microbial genetic characteristics, extreme tolerance, and antibiotics-resistance were not significantly different as compared with ground controls, thus suggesting that the genomic and physiological features of the ISS microbes may not impact human health in ways different from those on Earth [77]. Also, some studies on US space shuttle missions [80] reported a shorter lag phase and longer exponential phase in E. coli compared to ground control, while other studies found no difference in both phases in E. coli affected in spaceflight [81,82]. Nevertheless, there is a general consensus that microgravity condition presents an intrinsic risk of biofouling and biofilm formation due to reduced fluid dynamics in reduced gravity conditions [83], thus impacting system material integrity and human health and posing a potential risk to mission success [77,84,85]. Although Mora et al. did not find a direct different genetic or functional capacity between the ISS microbes and Earth microbes, the authors agreed with microbial adaptations toward biofilm formation.
Biofilms in nature do not commonly exist as pure cultures, but rather, as heterogeneous polymicrobial populations. Inter-kingdom biofilms also exist, where bacteria, archaea, fungi, phages and other viruses co-exist [86]. Interactions of mixed communities of microorganisms have been shown to affect biofilm functional characteristics, including drug resistance and morphology [87]; Burmølle et al., 2016; [85,88]. For example, a recent study conducted dual- or multispecies biofilm formation assays using six ISS potable water bacteria and showed that robustness of community biofilm formation was influenced by synergistic biofilm formation with different species such as Cupriavidus, Chryseobacterium, and Ralstonia spp [89]. Interestingly, this work found that predation by phage or predatory bacteria did not selectively remove specific bacterial community members, suggesting emerging properties of the multispecies biofilm community [89]. Indeed, Burmølle et al. documented that multispecies biofilm led to emergent properties that are triggered by bacterial social interactions and do so with the existence of a plethora of different bacterial species together [[90], [91], [92], [93], [94]]. For example, a study reported Pseudomonas aeruginosa-dependent induction of the S. aureus virulence factors Panton-Valentine leukocidin and α-hemolysin [95], and C. albicans-induced downregulation of CodY resulted in enhanced biofilm formation and virulence by S. aureus [96]. It is clear that the function and behavior of polymicrobial biofilms is highly complex and the characteristics of multispecies microbial community are not necessarily additive of those of the individual members. Therefore, in order to effectively control polymicrobial biofilms, it will be important to have mechanistic understanding of polymicrobial interactions, properties of complex communities, and long-term adaptation and evolution within the mixed-species community, [97]; Limoli et al., 2017; Botelho, Grosso & Peixe, 2019; Frydenlund, 2016; Damkiaer et al., 2013).
Conjugation, as well, can affect bacterial chemical/antimicrobial sensitivities and growth [98,99]. De Boever and others have documented that plasmid conjugation can occur in microgravitational environments [100]. This was initially documented during a single in-flight experiment in which statistical data could not be analyzed due to the ground control's lack of transconjugant growth. Therefore, it remains unclear whether the conjugation efficiency was altered compared to efficiency of conjugation in ground controls. This prior study, as well as metagenomic analysis of microbial communities on the ISS, showed that plasmids were present as expected in other microbial communities in a closed environment [84]; Venkateswaran et al., 2014). Moreover, even plasmids present as free deoxyribonucleic acid (DNA) were shown to be stable in launch conditions (Thiel, 2014). An extensive map of the microbial composition of the ISS surfaces and some crewmembers' microbiomes has been compiled from various studies from 2014 to 2021 [40]; Danko et al., 2021; Morrison et al., 2021 [84]; Venkateswaran et al., 2014). These compositional studies and characterization of genomes in ISS-derived microbial isolates indicate the presence of drug-resistant, potentially pathogenic microorganisms. It also identifies the locations of biofilms that can harbor those microorganisms such as hard surfaces and air samples.
NASA has also identified increased immune dysregulation as a threat to crew safety and mission success, and, as biofilms increase infection risks and microgravity condition may alter microbial characteristics, gene expressions, and biofilm formation [85]; Yi et al., 2016), biofilm may represent a risk to astronaut health. Therefore, as NASA shifts the focus towards long-duration missions beyond LEO, it is imperative that the altered host-microbe interactions brought on by spaceflight-associated environmental factors are understood for mission risk evaluation. Still under study, there are other factors such as how reduced gravity affects biofilm structures, biomass, and properties associated with drug resistance and virulence. Terrestrial-based simulations of microgravity have been a case of debate as platform performance of weightlessness is still a base for testing in itself [101]. Consequently, this could point to experiments with incomplete models of the environmental stresses that bacteria, archaea, viruses, and fungi experience during spaceflight. Additionally, the logistics of performing experiments in space are challenging and may introduce confounding variables, making results more difficult to interpret.
This combination of potentially altered biofilm formation, accumulation of virulence and resistance traits, and decreased human immune effectiveness during spaceflight is a concern for crew health on long-duration missions. To mitigate this, further studies must be performed to evaluate if the results and trends observed are reflective of the effects of spaceflight conditions. This added level of knowledge will allow NASA to assess and address risk more accurately in future missions and programs.
4.1. Knowledge gaps
-
a.
Increased virulence of pathogens in microgravity environment. Metabolism and behavior of microorganisms is altered in response to microgravity environments. However, the effects of a microgravity on virulence and drug resistance warrant further investigation (Simões and Andre, 2021) and future studies should assess a diverse range of pathogens to account for the variable strategies that different microorganisms employ to adapt to different gravitational conditions.
-
b.
Effect of polymicrobial biofilms. Biofilms in nature most commonly exist as complex microbial populations that interact with neighboring cells. Ecological success within the polymicrobial community can change the community characteristics thus impacting human health and space systems operation. An improved understanding of polymicrobial biofilms and how biofilm properties are affected by the surrounding environment and community members is needed.
-
c.
Importance of horizontal gene transfer (HGT). While the mechanisms and regulation of HGT have been explored under normal gravity, the effects of reduced gravity on such transfers are virtually unknown. All major types of horizontal gene transfer are tightly regulated (Frost, 2010; Lopatkin, 2016; Sysoeva, 2020) and are affected by the metabolic state of the cells. It was shown previously that bacterial physiology is altered upon gravity changes [4,102,103]. Therefore, the efficiency of HGT might be strongly affected, suggesting that commensal and pathogenic bacteria might have an increased capacity to obtain drug- and metal-resistance genes. Moreover, HGT in a biofilm environment is an actively developing area of research. Thus far, it has been revealed that, even in Earth's gravity, numerous factors contribute to the apparent efficiency of HGT in biofilms. It is unknown how these factors interplay in the absence or reduction of gravity.
-
d.
Effect of long-duration studies. While microbial interactions may appear stable in short-term studies, their relationship may be more dynamic in longer-term setting [97]; Limoli et al., 2017; Bithelo et al., 2019). For example, a recent longitudinal study using P. aeruginosa clinical isolates from cystic fibrosis patients suggested that the long-term development of metabolic divergence contributed to cooperative interspecies interactions that evolved over decades (Frydenlund et al., 2016; Damkiaer, Molin & Jelsbak, 2013). Such microbial dynamics and changes in the relationship among the members within a biofilm community are critical to better understand the functions and long-term effects of microbial community in human habitats, both on Earth and in space. The ISS is a good study platform for longitudinal experiments.
5. In-situ resource utilization (ISRU)
Although biofilm formation is often seen as a negative occurrence, biofilms could potentially play a positive role in space travel through their use in various in-situ resource utilization (ISRU) procedures. ‘Biomining’ is the blanket term for the processes by which a biological system (such as biofilm) extracts and recovers desired metals or other resources from the environment including rock ores [104]. This process can be divided into the more specific methods of bioleaching and bio-oxidation, which are both currently in use on Earth [105]. When it comes to extracting useful metals in space, biomining might be more advantageous than traditional mining methods because it has lower energy demands, less toxicity, and takes up less equipment area [106]. Bioleaching removes the target compound by oxidation or reduction processes. ESA has conducted biomining studies aboard the ISS, finding that gravitational conditions did not prevent effective bioleaching of vanadium (an element of interest due to its strength and resistance to corrosion) from basalt rock [107]. Two of the bacterial strains that were used in the biomining bioprocess increased vanadium leaching up to 283% [108]. Data from this project, called BioRock, shows that biomining “may be possible on a large scale in space,” which could enable the extraction of the elements necessary to human survival outside of the Earth [106].
Aside from biomining, biofilms can be utilized in space exploration and ISRU through bioregenerative life-support systems [12,109]. For example, some bacteria are involved in the production of methane, at the same time, methane can serve as fuel for some propulsion systems, as such cyanobacterial biofilms have been considered suitable candidates for methane production and subsequent use as fuel (Keller et al., 2021). In another example, Mars dust has been seen as a risk to crewed Mars missions. Therefore, in a related study, an area of the Mars-like Mongolian desert sand was seeded with cyanobacteria. Within fifteen days, a stable and wind-resistant crust that prevented the release of dust particles was produced [110]. Racks of these crusts could be used to filter air by removing dust from the atmosphere as it passes through. Microbial crusts and biofilms could also aid in extraterrestrial plant growth and regolith-to-soil processes, aiding in both morale and survival for astronauts [111]. The employment of microorganisms, often as biofilms, has been proposed for the production and recovery of resources such as oxygen, food, energy, and building materials, but also in biomining, wastewater recycling and even terraforming [16,[112], [113], [114], [115]]. For extraterrestrial food production, photosynthetic bacteria such as Arthrospira platensis and Arthrospira maxima (together forming the nutrient-rich supplement, spirulina), have been identified as a complimentary option to plant-based space farming [116]; Way et al., 2011).
Production of medicines on Mars to avoid degradation by radiation and temperature variations will be imperative to preserving astronaut health and safety [117]. Many pharmaceutical molecules can be produced efficiently on-demand using compact microbial bioreactors [[118], [119], [120]]. The methylotrophic yeast Pichia pastoris could potentially be a production host for medicines, metabolites, and materials on Mars due to its metabolic versatility [113]. P. pastoris can grow on methanol, derived from methane, which is a possible ISRU product on Mars ([112,121]. Biomining and bioremediation for extraction of rare Earth elements, extraction of precious metals, and removal of perchlorate are also important strategies for bio-ISRU. Several microorganisms have been utilized in proof-of-concept experiments for biomining on Earth as well as the proving-ground of the ISS [[105], [106], [107], [108],[122], [123], [124], [125], [126]]. In particular, Acidithiobacillus ferroxidans, Cupriavidus metallidurans, Shewanella oneidensis, and Sphingomonas desiccabilis are promising; most of these species perform chemolithotrophic leaching. Due to the approaches mentioned here, as well as many other potential applications, biofilms and microorganisms in general have potential value in space ISRU.
5.1. Knowledge gaps
-
a.
Impact of external materials on Earth originating microorganisms. As humans aim to return to the Moon and eventually travel to Mars, it is inevitable that microorganisms will be delivered to these surfaces. Thus, microbial associations and interactions with external materials such as regolith, rocks, and minerals that are not terrestrial should be explored to understand their effects on microbial growth. Furthermore, it is unknown which influence microgravity and partial gravity (present at the Moon or Mars) along with radiation beyond LEO will have on microbial growth [38,125,[127], [128], [129]].
-
b.
Mars In-situ Resource Utilization (ISRU). ISRU systems are currently being developed to convert organic Martian resources into chemical products useful to contemporary technologies. The Martian atmosphere contains inorganic carbon and molecular nitrogen, but the surface of the planet does not contain the same organic molecules as Earth, which has presented a challenging problem [130]. With this knowledge, the manufactured bioprocesses would need to perform under the harsh conditions on Mars while also fitting the strict requirements of space travel [130]. In addition, available materials, efficiency, and quality are important factors when evaluating the process and choice of microbial cell processes [130]. While some bacteria have proven partially effective, it is still unknown what combination of microorganisms would be best suited to the atmospheric and surface conditions of Mars.
6. Biosensors
A recent review of biofilm control strategies for extended spaceflight missions identified the need for biofilm detection and monitoring strategies within critical life-support systems such as water recovery, environmental control, and life support [12]. Several questions were posed, such as if planktonic and biofilm growth can be differentiated to better inform control strategies and assess potential problems prior to system failure.
Ideally, sensors for monitoring biofilms in spaceflight missions would be real-time, non-invasive, robust, miniaturized, autonomous, able to withstand extended missions, able to withstand dormancy, and have low power needs [131]. reviewed the latest technology in 2020 for detecting, imaging, and sensing of biofilms. They divided sensor systems into three broad categories: optical, electrochemical, and mechanical [131].
-
1.
The optical systems included modified reflectance confocal microscopy as well as a variety of spectroscopy systems including Raman, Brillouin, fiber optic evanescent wave, and synchrotron radiation Fourier transfer infrared spectroscopy methods (Yawata et al., 2010; Mattana et al., 2017; Zhong et al., 2016; Keirsse et al., 2003; Holman et al., 2009). While informative on molecular identity and quantification, these methods are not suitable for spaceflight missions because of the need for manual operation, size of the equipment (bulky), extensive data collection (increased computer memory requirements), and extensive need for data processing.
-
2.
Electrochemical sensors measure the change in electric potential due to chemical reactions, coupling a chemically selective layer to an electrode, which acts as a transducer. For biofilm research, three main electrochemical methods are often employed: a) electrochemical impedimetric systems that measure changes to the opposition of an electrochemical system to an alternating electrical stimulus; b) potentiometric systems measure the electrochemical potential between a reference electrode (defined potential under any environmental conditions) and a working electrode, where the potential depends on the growing biofilm under static conditions; c) amperometric systems measure the oxidization and reduction processes of electroactive analytes such as biofilms (van Duuren et al., 2017; Schott et al., 2019; Poma et al., 2020; Bruchmann et al., 2015). These sensors have several advantages in that they are robust, non-invasive, offer high sensitivity, have high selectivity, provide a rapid response, have low power requirements, and may be miniaturized using a micro-fabrication process.
-
3.
Mechanical biofilm detection systems are based on microgravimetry, measuring the amount of biofilm mass formed on piezoelectric films. Several techniques have been employed in biofilm monitoring, including the use of quartz crystal microbalances, quartz tuning forks, and surface acoustic wave sensors (Ripa et al., 2020; Gula et al., 2012; [132]. These sensors are low cost and offer real-time data. However, their integration into spaceflight systems is challenging due to required crew interaction and low vibrational tolerance. This could also be the case with non-impedance-based sensors.
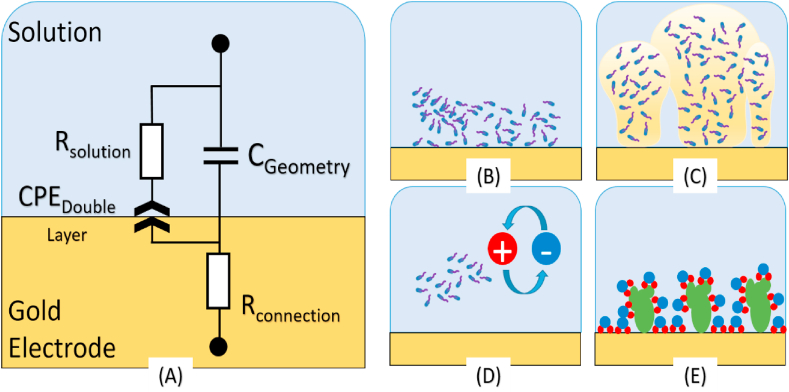
Electrochemical impedance spectroscopy sensors offer great promise within the constraints of spaceflight, and they have been commonly used for biofilm detection [131,133,134]. Impedance sensors can be microfabricated using matured semiconductor technologies, resulting in the precise manufacture of small (∼ nm to μm feature sized), lightweight, highly sensitive, minimally invasive sensors that require low power (∼<50 mW) to operate. The use of electrochemical impedance spectroscopy takes advantage of the complex behavior of biofilm growth and attachment. It can also differentiate between free-floating (planktonic) bacteria and biofilm growth, modeled as electrochemical phenomena (see Fig. 2).
Fig. 2.
(A) Equivalent complex circuit model of an electrode-electrolyte electrochemical impedimetric cell containing microorganisms and biofilm. Bacteria contribute to different mechanisms at the interface and bulk solution in an electrochemical cell. (B) bacteria in close contact with the surface contributing to the double-layer capacitance. (C) extracellular polymeric substance (EPS) production contributing to the double-layer capacitance. (D) planktonic growth contributing to the bulk solution resistance. (E) proteins and macromolecules adsorption onto the electrode surface contribute to changes in the double-layer capacitance (Image Credit: Matt McGlennen, CBE).
Furthermore, commercial impedance spectroscopy devices are available such as xCELLigence RTCA (Agilent Technologies, Inc.), DropSens (Metrohm DropSens, S. I.), and Palmsens (Palmsens BV). However, these have not yet been applied to spaceflight systems. Electrochemical impedance sensor materials and geometries can be fabricated with on-demand additive technologies such as 3D or inkjet printing. This would allow crew members to fabricate new sensors on-demand, requiring only the raw materials to be transported to space.
6.1. Knowledge gaps
-
a.
Microgravity and radiation effects. Several challenges must be overcome prior to the deployment of impedance-based biofilm sensors in critical water recovery and life-support systems. It is unknown how these systems function in microgravity. Since electrochemical impedance measures biofilm surface interactions, the effect of microgravity may not be an issue. Proposed timescales for spaceflight operations vary from a few days to a few years, thus landers, gateway stations, and spacecraft may be dormant for extended time periods [135]. Therefore, the sensors must be robust or exchanged at the end of their functional lifespan to remain operational. On the other hand, effect of ultraviolet or gamma radiation as a biofilm control strategy within water systems could affect the sensors [136]. The systems should be investigated for any photochemically-generated residual effects on measurements. Integration and material compatibility of spaceflight modules must be addressed to ensure stable, long-term sensor operation. If biocides or coatings are to be used, these must be tested with the sensors to determine their potential impacts on resistance or capacitance of the system in addition to corrosion and sensor degradation.
-
b.
Crew interface. To minimize crew time, the sensors could be included in a platform that supports autonomous monitoring. This system would detect when a biofilm development threshold is reached, notify the crew, deliver biocide, and exchange sensors.
-
c.
Biosensors against microbial consortia. Most studies using electrochemical impedance-based biofilm sensors have focused on pure cultures of microorganisms. More environmentally relevant mixed-consortia studies are needed to identify microbial interactions and effects on impedance measurements. System-based monitoring could be achieved by combining electrochemical impedance spectroscopy measurements with other microfabricated sensors to detect reduction reactions, oxidation reactions, oxygen levels, and nutrient levels within the system. This would provide enhanced warning if any of these levels reach critical thresholds.
7. Path forward
NASA has a long history of utilizing research and ‘lessons learned’ to improve methods, build upon current procedures, and produce models to develop best practices. Rigorous scientific study, coupled with a continual willingness to examine and improve, are critical to answer important questions in biofilm research. Ultimately, this will provide the best chance of success for long-duration space missions when it comes to biofilm mitigation and the use of microbial sources to sustain life.
CRediT authorship contribution statement
Yo-Ann Vélez Justiniano: Writing – original draft, Writing – review & editing, Supervision. Darla M. Goeres: Writing – original draft, Writing – review & editing, Supervision. Elizabeth L. Sandvik: Writing – original draft, Writing – review & editing. Birthe Veno Kjellerup: Writing – original draft, Writing – review & editing. Tatyana A. Sysoeva: Writing – original draft, Writing – review & editing. Jacob S. Harris: Writing – original draft, Writing – review & editing. Stephan Warnat: Writing – original draft, Writing – review & editing, Supervision. Matthew McGlennen: Writing – original draft, Writing – review & editing. Christine M. Foreman: Writing – original draft, Writing – review & editing, Supervision. Jiseon Yang: Writing – original draft, Writing – review & editing, Supervision. Wenyan Li: Writing – original draft, Writing – review & editing. Chelsi D. Cassilly: Writing – original draft, Writing – review & editing. Katelynn Lott: Writing – original draft. Lauren E. HerrNeckar: Writing – original draft.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Darla M. Goeres reports financial support was provided by NASA Marshall Space Flight Center. Elizabeth L. Sandvik reports financial support was provided by NASA Marshall Space Flight Center. Stephan Warnat reports was provided by NASA Marshall Space Flight Center. Christine M. Foreman reports was provided by NASA Marshall Space Flight Center. Matthew McGlennen reports financial support was provided by NASA Marshall Space Flight Center. Stephan Warnat reports equipment, drugs, or supplieswere provided by the National Science Foundation. First author Yo-Ann Vélez Jutiniano is a guest managing editor in the Biofilms in Space Special issue. Co-authors Darla Goeres and Birthe Veno Kjellerup are senior editors in the Biofilm journal.
Acknowledgements
We thank the ECLSS group at NASA George C. Marshall Space Flight Center and all cooperating institutions. This work was also made possible thanks to the projects NASA 80NSSC20M0264 to SW and CMF, and NASA 80NSSC20P0581 to DMG, SW and CMF (Center for Biofilm Engineering, Montana State University). Biosensor work mentioned was performed in part at the Montana Nanotechnology Facility, a member of the National Nanotechnology Coordinated Infrastructure (NNCI), which is supported by the National Science Foundation (Grant# ECCS-2025391).
Data availability
No data was used for the research described in the article.
References
- 1.Bhattacharya S., Chatterjee A., Mehta S., Pierson D., Ott C.M., Oubre C. National Aeronautics and Space Administration; 2016. Risk of adverse health effects due to host-microorganism interactions human health countermeasures (HHC) element.https://humanresearchroadmap.nasa.gov/Evidence/reports/Host_Micro.pdf [Google Scholar]
- 2.Rensselaer Polytechnic Institute Researchers to send bacteria into orbit aboard space shuttle Atlantis. Science daily. 2010. https://www.sciencedaily.com/releases/2010/05/100510114349.htm
- 3.McLean R.J.C., Cassanto J.M., Barnes M.B., Koo J.H. Bacterial biofilm formation under microgravity conditions. FEMS (Fed Eur Microbiol Soc) Microbiol Lett. 2001;195(2):115–119. doi: 10.1111/j.1574-6968.2001.tb10507.x. [DOI] [PubMed] [Google Scholar]
- 4.Aunins T.R., Erickson K.E., Prasad N., Levy S.E., Jones A., Shrestha S., Mastracchio R., Stodieck L., Klaus D., Zea L., Chatterjee A. Spaceflight modifies Escherichia coli gene expression in response to antibiotic exposure and reveals role of oxidative stress response. Front Microbiol. 2018;9 doi: 10.3389/fmicb.2018.00310. https://doi:10.3389/fmicb.2018.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morales Medina W.R., Eramo A., Tu M., Fahrenfeld N.L. Sewer biofilm microbiome and antibiotic resistance genes as function of pipe material, source of microbes, and disinfection: field and laboratory studies. Environ Sci J Integr Environ Res: Water Res Technol. 2020;6(8):2122–2137. doi: 10.1039/d0ew00265h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franks A.E., Malvankar N., Nevin K.P. Bacterial biofilms: the powerhouse of a microbial fuel cell. Biofuels. 2010;1(4):589–604. doi: 10.4155/bfs.10.25. [DOI] [Google Scholar]
- 7.Singh R., Paul D., Jain R.K. Biofilms: implications in bioremediation. Trends Microbiol. 2006;14(9):389–397. doi: 10.1016/j.tim.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Caplice E., Fitzgerald G.F. Food fermentations: role of microorganisms in food production and preservation. Int J Food Microbiol. 1999;50(1):131–149. doi: 10.1016/S0168-1605(99)00082-3. [DOI] [PubMed] [Google Scholar]
- 9.González-Rivas F., Ripolles-Avila C., Fontecha-Umaña F., Ríos-Castillo A.G., Rodríguez- Jerez J.J. Biofilms in the spotlight: detection, quantification, and removal methods. Compr Rev Food Sci Food Saf. 2018;17(5):1261–1276. doi: 10.1111/1541-4337.12378. [DOI] [PubMed] [Google Scholar]
- 10.Veerachamy S., Yarlagadda T., Manivasagam G., Yarlagadda P.K. Bacterial adherence and biofilm formation on medical implants: a review. Proc IME H J Eng Med. 2014;228(10):1083–1099. doi: 10.1177/0954411914556137. [DOI] [PubMed] [Google Scholar]
- 11.Zea L., Nisar Z., Rubin P., Cortesão M., Luo J., McBride S.A., Moeller R., Klaus D., Müller D., Varanasi K.K., Muecklich F., Stodieck L. Design of a spaceflight biofilm experiment. Acta Astronaut. 2018;148:294–300. doi: 10.1016/j.actaastro.2018.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zea L., McLean R.J.C., Rook T.A., Angle G., Carter D.L., Delegard A., Denvir A., Gerlach R., Gorti S., McIlwaine D., Nur M., Peyton B.M., Stewart P.S., Sturman P., Velez Justiniano Y.A. Potential biofilm control strategies for extended spaceflight missions. Biofilm. 2020;2 doi: 10.1016/j.bioflm.2020.100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovett R. Science. American Association for the Advancement of Science (AAAS); 2019. Space station mold survives 200 times the radiation dose that would kill a human. [DOI] [Google Scholar]
- 14.Blue R.S., Bayuse T.M., Daniels V.R., Wotring V.E., Surech R., Mulcahy R.A., Antonsen E.L. Supplying a pharmacy for NASA exploration spaceflight: challenges and current understanding. Npj Microgravity. 2019;5(1) doi: 10.1038/s41526-019-0075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vroom M.M., Rodriguez-Ocasio Y., Lynch J.B., Ruby E.G., Foster J.S. Modeled microgravity alters lipopolysaccharide and outer membrane vesicle production of the beneficial symbiont Vibrio fischeri. Npj Microgravity. 2021;7(1) doi: 10.1038/s41526-021-00138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volger R., Pettersson G.M., Brouns S.J.J., Rothschild L.J., Cowley A., Lehner B.A.E. Mining Moon & Mars with microbes: biological approaches to extract iron from lunar and martian regolith. Planet Space Sci. 2020;184 doi: 10.1016/j.pss.2020.104850. [DOI] [Google Scholar]
- 17.Lehner B.A.E., Schlechten J., Filosa A., Mazzotta D.G., Spina F., Teeny L., Snyder J., Tjon S.Y., Meyer A.S., Brouns S.J.J., Cowley A., Rothschild L.J. End- to-end mission design for microbial ISRU activities as preparation for a Moon village. Acta Astronaut. 2019;162:216–226. doi: 10.1016/j.actaastro.2019.06.001. [DOI] [Google Scholar]
- 18.Anderson M.S., Barta D., Douglas G., Motil B., Massa G., Fritsche R., Quincy C., Romeyn M., Hanford A. Key gaps for enabling plant growth in FutureMissions. AIAA SPACE and Astronautics Forum and Exposition. 2017 doi: 10.2514/6.2017-5142. [DOI] [Google Scholar]
- 19.NASA NASA's lunar exploration program overview. 2020. https://www.nasa.gov/sites/default/files/atoms/files/artemis_plan-20200921.pdf NASA.
- 20.Schubert W., Plett G., Yavrouian A., Barengoltz J. Viability of bacterial spores exposed to hydrazine. Adv Space Res. 2008;42(6):1144–1149. doi: 10.1016/j.asr.2007.07.031. [DOI] [Google Scholar]
- 21.Hill E.C., Hill G.C. Microbial contamination and associated corrosion in fuels, during storage, distribution and use. Adv Mater Res. 2008;38:257–268. doi: 10.4028/www.scientific.net/amr.38.257. [DOI] [Google Scholar]
- 22.McNamara C.J., Perry T.D., Leard R., Bearce K., Dante J., Mitchell R. Corrosion of aluminum alloy 2024 by microorganisms isolated from aircraft fuel tanks. Biofouling. 2005;21(5–6):257–265. doi: 10.1080/08927010500389921. [DOI] [PubMed] [Google Scholar]
- 23.Pruitt J.M., Carter L., Bagdigian R.M., Kayatin M.J. Upgrades to the ISS water recovery system. 2015. https://ttu-ir.tdl.org/bitstream/handle/2346/64405/ICES_2015_submission_133.pdf?sequence=1&isAllowed=y
- 24.Rauch M.E., Graef H.W., Rozenzhak S.M., Jones S.E., Bleckmann C.A., Kruger R.L., Naik R.R., Stone M.O. Characterization of microbial contamination in United States air force aviation fuel tanks. J Ind Microbiol Biotechnol. 2005;33(1):29–36. doi: 10.1007/s10295-005-0023-x. [DOI] [PubMed] [Google Scholar]
- 25.Adam N., Callahan M., Almengor A., Gilbert N., Harris J., Jimenez J., Hanford A., Toon K. Texas Tech University; 2020. Update on feasibility of UV LEDs in a spacecraft wastewater TankApplication.https://ttu-ir.tdl.org/handle/2346/86481 [Google Scholar]
- 26.Buchovec I., Gricajeva A., Kalėdienė L., Vitta P. Antimicrobial photoinactivation approach based on natural agents for control of bacteria biofilms in spacecraft. Int J Mol Sci. 2020;21(18):6932. doi: 10.3390/ijms21186932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velez Justiniano Y.-A., Carter D., Nur M., Angle G. Texas Tech University; 2020. Developing methods for biofilm control in microgravity for a water recovery system.https://ttu-ir.tdl.org/handle/2346/86332 [Google Scholar]
- 28.Avila-Herrera A., Thissen J., Urbaniak C., Be A.N., Smith J.D., Karouia F., Mehta S., Venkateswaran K., Jaing C. Crewmember microbiome may InfluenceMicrobial compositions of ISS habitable surfaces. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0231838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velez Justiniano Y.-A., Carter D., Sandvik E., Stewart P., Goeres D., Sturman P., Li W., Johnson A., Cioanta I. Texas Tech University; 2021. Biofilm management in a microgravity water recovery system.https://ttu-ir.tdl.org/handle/2346/87082 [Google Scholar]
- 30.NASA Technical Standard, NASA-STD-3001 NASA spaceflight human-system standard. Hum Fact Habitat Environ Health. 2022;2 https://standards.nasa.gove/standard/nasa/nasa-std-3001-vol-1 [Google Scholar]
- 31.Pierson D.L., Botkin D.J., Bruce R.J., Castro V.A., Smith M.J., Oubre C.M., Ott C.M. vol. 6. DHI Publishing, LLC; 2012. (Environmental monitoring: a comprehensive handbook). [Google Scholar]
- 32.Castro S.L., Smith D.J., Ott C.M. A researcher's guide to: international space station – microbial research. Rai A., Hosein N., editors. NASA ISS Prog Sci Off. 2014;1:11–12. https://www.nasa.gov/sites/default/files/files/Microbial-Observatory-Mini-Book -04-28-14-508.pdf [Google Scholar]
- 33.Bijlani S., Singh N.K., Eedara V.V.R., Podile A.R., Mason C.E., Wang C.C.C., Venkateswaran K. Methylobacterium ajmalii sp. nov., isolated from the international space station. Front Microbiol. 2021 doi: 10.3389/fmicb.2021.639396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang J.M., Coil D.A., Neches R.Y., Brown W.E., Cavalier D., Severance M., Hampton- Marcell J.T., Gilbert J.A., Eisen J.A. A microbial survey of the international space station (ISS) PeerJ. 2017;5 doi: 10.7717/peerj.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castro V.A., Thrasher A.N., Healy M., Ott C.M., Pierson D.L. Microbial characterization during the early habitation of the international space station. Microb Ecol. 2004;47(2):119–126. doi: 10.1007/s00248-003-1030-y. [DOI] [PubMed] [Google Scholar]
- 36.Perrin E., Bacci G., Garrelly L., Canganella F., Bianconi G., Biowyse Consortium. Fani R., Mengoni A. Furnishing spaceship environment: evaluation of bacterial biofilms on different materials used inside international space station. Res Microbiol. 2018;169(6):289–295. doi: 10.1016/j.resmic.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Rosenzweig J.A., Ahmed S., Eunson J., Chopra A.K. Low-shear force associated with modeled microgravity and spaceflight does not similarly impact the virulence of notable bacterial pathogens. Appl Microbiol Biotechnol. 2014;98(21):8797–8807. doi: 10.1007/s00253-014-6025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haines S.R., Bope A., Horack J.M., Meyer M.E., Dannemiller K.C. Applied microbiology and biotechnology. Springer Science and Business Media LLC; 2019. Quantitative evaluation of bioaerosols in different particle size fractions in dust collected on the International Space Station (ISS) pp. 7767–7782. Vol. 103, Issue 18. [DOI] [PubMed] [Google Scholar]
- 39.Wang M., Duday D., Scolan E., Perbal S., Prato M., Lasseur C., Hołyńska M. Antimicrobial surfaces for applications on confined inhabited space stations. Adv Mater Interfac. 2021 [Google Scholar]
- 40.Checinska Sielaff A., Urbaniak C., Mohan G.B.M., Stepanov V.G., Tran Q., Wood J.M., Minich J., McDonald D., Mayer T., Knight R., Karouia F., Fox G.E., Venkateswaran K. Characterization of the total and viable bacterial and fungal communities associated with the international space station surfaces. Microbiome. 2019;7(50) doi: 10.1186/s40168-019-0666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balcázar J.L., Subirats J., Borrego C.M. The role of biofilms as environmental reservoirs of antibiotic resistance. Front Microbiol. 2015;6 doi: 10.3389/fmicb.2015.01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams D., Dake J., Gentry G. 42nd international conference on environmental systems. 2012. International space station environmental control and life support system status for the prior year: 2010 – 2011. [DOI] [Google Scholar]
- 43.Williams D.E. International space station temperature and humidity control subsystem verification for node 1. NASA Tech Rep Server (NTRS) 2007 https://ntrs.nasa.gov/citations/20070018272 [Google Scholar]
- 44.Pultarova T. How do you clean a space station? Astronaut thomas pesquet shares orbital spring-cleaning tips. Space. 2021. https://www.space.com/space-station-cleaning- tips-astronaut-thomas-pesquet
- 45.Adlhart C., Verran J., Azevedo N.F., Olmez H., Keinänen-Toivola M.M., Gouveia I., Melo L.F., Crijns F. Surface modification for antimicrobial effects in the healthcare setting: a critical overview. J Hosp Infect. 2018;99(3):239–249. doi: 10.1016/j.jhin.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 46.Boeing Testing an antimicrobial coating in space. Boeing. 2021 https://www.boeing.com/confident-travel/stories/testing-an-antimicrobial-coating-in-space.html [Google Scholar]
- 47.ESA . The European Space Agency; 2021. Keep this surface dirty.https://www.esa.int/ESA_Multimedia/Images/2021/01/Keep_this_surface_dirty [Google Scholar]
- 48.Costerton J.W., Cheng K.J., Geesey G.G., Ladd T.I., Nickel J.C., Dasgupta M., Marrie T.J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41(Issue 1):435–464. doi: 10.1146/annurev.mi.41.100187.002251. Annual Reviews. [DOI] [PubMed] [Google Scholar]
- 49.Costerton J.W., Stewart P.S., Greenberg E.P. Science. American Association for the Advancement of Science (AAAS); 1999. Bacterial biofilms: a common cause of persistent infections; pp. 1318–1322. Vol. 284, Issue 5418. [DOI] [PubMed] [Google Scholar]
- 50.Jamal M., Ahmad W., Andleeb S., Jalil F., Imran M., Nawaz M.A., Hussain T., Ali M., Rafiq M., Kamil M.A. Journal of the Chinese medical association. Ovid Technologies (Wolters Kluwer Health); 2018. Bacterial biofilm and associated infections; pp. 7–11. Vol. 81, Issue 1. [DOI] [PubMed] [Google Scholar]
- 51.Das J.R., Bhakoo M., Jones M.V., Gilbert P. Changes in the biocide susceptibility of Staphylococcus epidermidis and Escherichia coli cells associated with rapid attachment to plastic surfaces. J Appl Microbiol. 1998;84(5):852–858. doi: 10.1046/j.1365-2672.1998.00422.x. [DOI] [PubMed] [Google Scholar]
- 52.Burmølle M., Thomsen T.R., Fazli M., Dige I., Christensen L., Homøe P., Tvede M., Nyvad B., Tolker-Nielsen T., Givskov M., Moser C., Kirketerp-Møller K., Johansen H.K., Høiby N., Jensen P.Ø., Sørensen S.J., Bjarnsholt T. FEMS immunology & medical microbiology. Oxford University Press (OUP); 2010. Biofilms in chronic infections – a matter of opportunity – monospecies biofilms in multispecies infections; pp. 324–336. Vol. 59, Issue 3. [DOI] [PubMed] [Google Scholar]
- 53.Bowler P.G., Duerden B.I., Armstrong D.G. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14(2):244–269. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan J., Bassler B.L. Surviving as a community: antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe. 2019;26(1):15–21. doi: 10.1016/j.chom.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bowler P., Murphy C., Wolcott R. Antimicrobial resistance & infection control. Springer Science and Business Media LLC; 2020. Biofilm exacerbates antibiotic resistance: is this a current oversight in antimicrobial stewardship? Vol. 9, Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karygianni L., Ren Z., Koo H., Thurnheer T. Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol. 2020;28(Issue 8):668–681. doi: 10.1016/j.tim.2020.03.016. Elsevier BV. [DOI] [PubMed] [Google Scholar]
- 57.Yin W., Wang T., Liu L., He J. Biofilms: the microbial ‘protective clothing’ in extreme environments. Int J Mol Sci. 2019;20(14):3423. doi: 10.3390/ijms20143423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandvik E.L., Borgogna T.R., Stewart P.S. In: Antibiofilm strategies. Richter K., Kragh K.N., editors. vol. 11. Springer; Cham: 2022. Antimicrobial and innate immune tolerance mechanisms in biofilms; pp. 17–35. (Springer series on biofilms). Ch 3. [DOI] [Google Scholar]
- 59.Stewart P.S., Parker A.E. vol. 63. American Society for Microbiology; 2019. Measuring antimicrobial efficacy against biofilms: a meta-analysis. (Antimicrobial agents and chemotherapy). Issue 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levin-Reisman I., Ronin I., Gefen O., Braniss I., Shoresh N., Balaban N.Q. Science. American Association for the Advancement of Science (AAAS); 2017. Antibiotic tolerance facilitates the evolution of resistance; pp. 826–830. Vol. 355, Issue 6327. [DOI] [PubMed] [Google Scholar]
- 61.Olsen I. Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol Infect Dis: Off Publ Eur Soc Clin Microbiol. 2015;34(5):877–886. doi: 10.1007/s10096-015-2323-z. [DOI] [PubMed] [Google Scholar]
- 62.Gallego-Hernandez A.L., DePas W.H., Park J.H., Teschler J.K., Hartmann R., Jeckel H., Drescher K., Beyhan S., Newman D.K., Yildiz F.H. vol. 117. 2020. Upregulation of virulence genes promotes Vibrio cholerae biofilm hyperinfectivity; pp. 11010–11017. (Proceedings of the national academy of sciences of the United States of America). 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lebeaux D., Ghigo J.-M., Beloin C. Microbiology and molecular biology reviews. American Society for Microbiology; 2014. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics; pp. 510–543. Vol. 78, Issue 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao L., Zhu W., Tong W. Clogging processes caused by biofilm growth and organic particle accumulation in lab-scale vertical flow constructed wetlands. J Environ Sci. 2009;21(6):750–757. doi: 10.1016/s1001-0742(08)62336-0. [DOI] [PubMed] [Google Scholar]
- 65.Peszynska M., Trykozko A., Iltis G., Schlueter S., Wildenschild D. Advances in water resources. Elsevier BV; 2016. Biofilm growth in porous media: experiments, computational modeling at the porescale, and upscaling; pp. 288–301. vol. 95. [DOI] [Google Scholar]
- 66.Ogawa A., Takakura K., Hirai N., Kanematsu H., Kuroda D., Kougo T., Sano K., Terada S. Materials. MDPI AG; 2020. Biofilm Formation plays a crucial rule in the initial step of carbon steel corrosion in air and water environments; p. 923. Vol. 13, Issue 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.NASA Environmental control and life support system (ECLSS) 2017. https://www.nasa.gov/sites/default/files/atoms/files/g-281237_eclss_0.pdf NASA.
- 68.Henson P., Yates S., Dotson B., Bonk T., Finger B., Kelsey L., Junaedi C., Rich-Emar M. An environmental control and life support system (ECLSS) for deep space and commercial habitats. 2021. https://hdl.handle.net/2346/87157
- 69.Yang J., Barrila J., Roland K.L., Ott C.M., Nickerson C.A. Physiological fluid shear alters the virulence potential of invasive multidrug-resistant non-typhoidal Salmonella typhimurium D23580. Npj Microgravity. 2016;2(1) doi: 10.1038/npjmgrav.2016.21. https://doi:10.1038/npjmgrav.2016.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim W., Tengra F.K., Young Z., Shong J., Marchand N., Chan H.K.…Collins C.H. Spaceflight promotes biofilm formation by Pseudomonas aeruginosa. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0062437. https://doi:10.1371/journal.pone.0062437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson J.W., Ott C.M., zu Bentrup K.H., Ramamurthy R., Quick L., Porwollik S., Cheng P., McClelland M., Tsaprailis G., Radabaugh T., Hunt A., Fernandez D., Richter E., Shah M., Kilcoyne M., Joshi L., Nelman-Gonzalez M., Hing S., Parra M., Dumars P. Space flight alters bacterial gene expression and virulence and reveals a role for global regulator hfq. Proc Natl Acad Sci USA. 2007;104(41):16299–16304. doi: 10.1073/pnas.0707155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nickerson C.A., Ott C.M., Wilson J.W., Ramamurthy R., Pierson D.L. Microbial responses to microgravity and other low-shear environments. Microbiol Mol Biol Rev: MMBR (Microbiol Mol Biol Rev) 2004;68(2):345–361. doi: 10.1128/MMBR.68.2.345-361.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crabbé A., Nielsen-Preiss S.M., Woolley C.M., Barrila J., Buchanan K., McCracken J., Inglis D.O., Searles S.C., Nelman-Gonzalez M.A., Ott C.M., Wilson J.W., Pierson D.L., Stefanyshyn-Piper H.M., Hyman L.E., Nickerson C.A. Spaceflight enhances cell aggregation and random budding in Candida albicans. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0080677. https://doi:10.1371/journal.pone.0080677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nielsen S., White K., Preiss K., Peart D., Gianoulias K., Juel R., Sutton J., McKinney J., Bender J., Pinc G., Bergren K., Gans W., Kelley J., McQuaid M. Growth and antifungal resistance of the pathogenic yeast, Candida albicans, in the microgravity environment of the international space station: an aggregate of multiple flight experiences. Life. 2021;11(4) doi: 10.3390/life11040283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nickerson C.A., Ott C.M., Mister S.J., Morrow B.J., Burns-Keliher L., Pierson D.L. Microgravity as a novel environmental signal affecting Salmonella enterica serovar typhimurium virulence. Infect Immun. 2000;68:3147–3152. doi: 10.1128/iai.68.6.3147-3152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chopra V., Fadl A.A., Sha J., Chopra S., Galindo C.L., Chopra A.K. Alterations in the virulence potential of enteric pathogens and bacterial-host cell interactions under simulated microgravity conditions. J Toxicol Environ Health, Part A. 2006;69:1345–1370. doi: 10.1080/15287390500361792. [DOI] [PubMed] [Google Scholar]
- 77.Mora M., Wink L., Kögler I., Mahnert A., Rettberg P., Schwendner P., Demets R., Cockell C., Alekhova T., Klingl A., Krause R., Zolotariof A., Alexandrova A., Moissl-Eichinger C. Space station conditions are selective but do not alter microbial characteristics relevant to human health. Nat Commun. 2019;10(1) doi: 10.1038/s41467-019-11682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matin A.C., Wang J.H., Keyhan M., Singh R., Benoit M., Parra M.P., Padgen M.R., Ricco A.J., Chin M., Friedericks C.R., Chinn T.N., Cohen A., Henschke M.B., Snyder T.V., Lera M.P., Ross S.S., Mayberry C.M., Choi S., Wu D.T., Tan M.X., Boone T.D., Beasley C.C., Piccini M.E., Spremo S.M. Payload hardware and experimental protocol development to enable future testing of the effect of space microgravity on the resistance to gentamicin of uropathogenic Escherichia coli and its σ s -deficient mutant. Life Sci Space Res. 2017;15:1–10. doi: 10.1016/j.lssr.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 79.Lawal A., Jejelowo O.A., Rosenzweig J.A. The effects of low-shear mechanical stress on Yersinia pestis virulence. Astrobiology. 2010;10(9):881–888. doi: 10.1089/ast.2010.0493. [DOI] [PubMed] [Google Scholar]
- 80.Klaus D., Simske S., Todd P., Stodieck L. Investigation of space flight effects on Escherichia coli and a proposed model of underlying physical mechanisms. Microbiology (Reading, England) 1997;143(Pt 2):449–455. doi: 10.1099/00221287-143-2-449. [DOI] [PubMed] [Google Scholar]
- 81.Vukanti R., Model M.A., Leff L.G. Effect of modeled reduced gravity conditions on bacterial morphology and physiology. BMC Microbiol. 2012;12:4. doi: 10.1186/1471-2180-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kacena M.A., Merrell G.A., Manfredi B., Smith E.E., Klaus D.M., Todd P. Bacterial growth in space flight: logistic growth curve parameters for Escherichia coli and Bacillus subtilis. Appl Microbiol Biotechnol. 1999;51(2):229–234. doi: 10.1007/s002530051386. [DOI] [PubMed] [Google Scholar]
- 83.Guzman A. Going with the fluid flow aboard the international space station. NASA. 2022. https://www.nasa.gov/mission_pages/station/research/benefits/going-with-the-fluid-flow-on-iss Retrieved October 20, 2022, from.
- 84.Singh N.K., Bezdan D., Checinska Sielaff A., Wheeler K., Mason C.E., Venkateswaran K. Multi-drug resistant Enterobacter bugandensis species isolated from the international space station and comparative genomic analyses with human pathogenic strains. BMC Microbiol. 2018;18(1):175. doi: 10.1186/s12866-018-1325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crucian B.E., Choukèr A., Simpson R.J., Mehta S., Marshall G., Smith S.M., Zwart S.R., Heer M., Ponomarev S., Whitmire A., Frippiat J.P., Douglas G.L., Lorenzi H., Buchheim J.-I., Makedonas G., Ginsburg G.S., Ott C.M., Pierson D.L., Frieger S.S., Baecker N. Immune system dysregulation during spaceflight: potential countermeasures for deep space exploration missions. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Dyck K., Pinto R.M., Pully D., Van Dijck P. Microbial interkingdom biofilms and the quest for novel therapeutic strategies. Microorganisms. 2021;9(2):412. doi: 10.3390/microorganisms9020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simões L.C., Simões M., Vieira M.J. Biofilm interactions between distinct bacterial genera isolated from drinking water. Appl Environ Microbiol. 2007;73(19):6192–6200. doi: 10.1128/AEM.00837-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Røder H.L., Raghupathi P.K., Herschend J., Brejnrod A., Knøchel S., Sørensen S.J., Burmølle M. Interspecies interactions result in enhanced biofilm formation by co-cultures of bacteria isolated from a food processing environment. Food Microbiol. 2015;51:18–24. doi: 10.1016/j.fm.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 89.Thompson A.F., English E.L., Nock A.M., Willsey G.G., Eckstrom K., Cairns B., Bavelock M., Tighe S.W., Foote A., Shulman H., Pericleous A., Gupta S., Kadouri D.E., Wargo M.J. Characterizing species interactions that contribute to biofilm formation in a multispecies model of a potable water bacterial community. Microbiology (Reading, England) 2020;166(1):34–43. doi: 10.1099/mic.0.000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burmølle M., Ren D., Bjarnsholt T., Sørensen S.J. Interactions in multispecies biofilms: do they actually matter? Trends Microbiol. 2014;22(2):84–91. doi: 10.1016/j.tim.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 91.Madsen J.S., Sørensen S.J., Burmølle M. Bacterial social interactions and the emergence of community-intrinsic properties. Curr Opin Microbiol. 2018;42:104–109. doi: 10.1016/j.mib.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 92.Hansen M.F., Svenningsen S.L., Røder H.L., Middelboe M., Burmølle M. Big impact of the tiny: bacteriophage-bacteria interactions in biofilms. Trends Microbiol. 2019;27(9):739–752. doi: 10.1016/j.tim.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 93.Sadiq F.A., Burmølle M., Heyndrickx M., Flint S., Lu W., Chen W., Zhao J., Zhang H. Community-wide changes reflecting bacterial interspecific interactions in multispecies biofilms. Crit Rev Microbiol. 2021;47(3):338–358. doi: 10.1080/1040841X.2021.1887079. [DOI] [PubMed] [Google Scholar]
- 94.Røder H.L., Olsen N., Whiteley M., Burmølle M. Unravelling interspecies interactions across heterogeneities in complex biofilm communities. Environ Microbiol. 2020;22(1):5–16. doi: 10.1111/1462-2920.14834. [DOI] [PubMed] [Google Scholar]
- 95.Pastar I., Nusbaum A.G., Gil J., Patel S.B., Chen J., Valdes J., Stojadinovic O., Plano L.R., Tomic-Canic M., Davis S.C. Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peters B.M., Jabra-Rizk M.A., Scheper M.A., Leid J.G., Costerton J.W., Shirtliff M.E. Microbial interactions and differential protein expression in Staphylococcus aureus -Candida albicans dual-species biofilms. FEMS Immunol Med Microbiol. 2010;59(3):493–503. doi: 10.1111/j.1574-695X.2010.00710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Darch S.E., Ibberson C.B., Whiteley M. Evolution of bacterial “frenemies”. mBio. 2017;8(3) doi: 10.1128/mBio.00675-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prensky H., Gomez-Simmonds A., Uhlemann A.C., Lopatkin A.J. Conjugation dynamics depend on both the plasmid acquisition cost and the fitness cost. Mol Syst Biol. 2021;17(3) doi: 10.15252/msb.20209913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barlow M. What antimicrobial resistance has taught us about horizontal gene transfer. Methods Mol Biol. 2009;532:397–411. doi: 10.1007/978-1-60327-853-9_23. [DOI] [PubMed] [Google Scholar]
- 100.De Boever P., Mergeay M., Ilyin V., Forget-Hanus D., Van der Auwera G., Mahillon J. Microgravity science and technology. Springer Science and Business Media LLC; 2007. Conjugation-mediated plasmid exchange between bacteria grown under space flight conditions; pp. 138–144. Vol. 19, Issues 5–6. [DOI] [Google Scholar]
- 101.Kiss J.Z., Wolverton C., Wyatt S.E., Hasenstein K.H., van Loon J.J.W.A. Frontiers in plant science. Frontiers Media SA; 2019. Comparison of microgravity analogs to spaceflight in studies of plant growth and development. vol. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zea L., Prasad N., Levy S.E., Stodieck L., Jones A., Shrestha S., Klaus D. A molecular genetic basis explaining altered bacterial behavior in space. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0164359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zea L., Larsen M., Estante F., Qvortrup K., Moeller R., Dias de Oliveira S.…Klaus D. Phenotypic changes exhibited by E. coli cultured in space. Front Microbiol. 2017;8 doi: 10.3389/fmicb.2017.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sheoran V., Sheoran A.S., Poonia P. Minerals engineering. Elsevier BV; 2009. Phytomining: a review; pp. 1007–1019. Vol. 22, Issue 12. [DOI] [Google Scholar]
- 105.Johnson D.B. Biomining—biotechnologies for extracting and recovering metals from ores and waste materials. Curr Opin Biotechnol. 2014;30:24–31. doi: 10.1016/j.copbio.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 106.Sen P. In: NASA johnson space center. Guzman A., editor. 2021. Researchers successfully biomine vanadium aboard the space station.https://www.nasa.gov/mission_pages/station/research/news/researchers-successfully-biomine-vanadium [Google Scholar]
- 107.Santomartino R., Waajen A.C., de Wit W., Nicholson N., Parmitano L., Loudon C.M., Moeller R., Rettberg P., Fuchs F.M., Van Houdt R., Finster K., Coninx I., Krause J., Koehler A., Caplin N., Zuijderduijn L., Zolesi V., Balsamo M., Mariani A., Pellari S.S., Cockell C.S. No effect of microgravity and simulated Mars gravity on final bacterial cell concentrations on the international space station: applications to space bioproduction. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.579156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cockell C.S., Santomartino R., Finster K., Waajen A.C., Nicholson N., Loudon C.-M., Eades L.J., Moeller R., Rettberg P., Fuchs F.M., Van Houdt R., Leys N., Coninx I., Hatton J., Parmitano L., Kraus J., Koehler A., Caplin N., Zuijderduijn L., Mariani Microbially-enhanced vanadium mining and bioremediation under micro- andMars gravity on the international space station. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.641387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fahrion J., Mastroleo F., Dussap C.-G., Leys N. Frontiers in microbiology. Frontiers Media SA; 2021. Use of photobioreactors in regenerative life support systems for human space exploration. vol. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Verseux C., Baqué M., Lehto K., Vera P.J., Rothschild L.J., Billi D. Sustainable life support on Mars - the potential roles of cyanobacteria. Int J Astrobiol. 2016;15(1):65–92. doi: 10.1017/s147355041500021x. [DOI] [Google Scholar]
- 111.Cockell C.S. Geomicrobiology beyond Earth: microbe–mineral interactions in space exploration and settlement. Trends Microbiol. 2010;18(7):308–314. doi: 10.1016/j.tim.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 112.Kalkus T.J., Averesch N.J., Lehner B.A. 69th international astronautical congress. 2018. The power of life: how biology can help address the long-term energy demands of space colonization.https://www.researchgate.net/publication/349896246_The_power_of_life_how_biology_can_help_address_the_long-term_energy_demands_of_space_colonization [Google Scholar]
- 113.Llorente B., Williams T.C., Goold H.D. The multiplanetary future of plant synthetic biology. Emerg Appl Synth Biol. 2018;9(7):348. doi: 10.3390/genes9070348. https://soi.org/10.3390/genes9070348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lopez J.V., Peixoto R.S., Rosado A.S. Inevitable future: space colonization beyond Earth with microbes first. FEMS (Fed Eur Microbiol Soc) Microbiol Ecol. 2019;95(10) doi: 10.1093/femsec/fiz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shunk G.K., Gomez X.R., Averesch N.J. The Print Server for Biology; 2020. Exploration: radiotrophic fungi can attenuate ionizing radiation aboard the international space station. bioRxiv. [DOI] [Google Scholar]
- 116.Montague M., McArthurIV G.H., Cockwell C.S., Held J., Marshall W., Sherman L.A., Wang N., Nicholson W.L., Tarjan D.R., Cumbers J. The role of synthetic biology for in-situ resource utilization (ISRU) Int J Astrobiol. 2012;12:1135–1142. doi: 10.1089/ast.2012.0829. [DOI] [PubMed] [Google Scholar]
- 117.Du B., Daniels V.R., Vaksman Z., Boyd J.L., Crady C., Putcha L. Evaluation of physical and chemical changes in pharmaceuticals flown on space missions. AAPS J. 2011;13:299–308. doi: 10.1208/s12248-011-9270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cao J.C., Perez-Pinera P., Lowenhaupt K., Wu M.R., Purcell O., de la Fuente-Nunez C., Lu T.K. Versatile and on-demand biologics Co-production in yeast. Nat Commun. 2018;9(1):77. doi: 10.1038/s41467-017-02587-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Perez-Pinera P., Han N.R., Cleto S., Cao J.C., Purcell O., Shah K.A., Lee K., Ram R., Lu T.K. Synthetic biology and microbioreactor platforms for programmable production of biologics at the point-of-care. Nat Commun. 2016;7(1) doi: 10.1038/ncomms12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pardee K., Slomovic S., Nguyen P.Q., Lee J.W., Donghia N., Burrill D., Ferrante T., McSorley F.R., Furuta Y., Vernet A., Collins J.J. Portable, on-demand biomolecular manufacturing. Science Direct. 2016;167:248–259. doi: 10.1016/j.cell.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 121.Sanders G.B. 48th AIAA aerospace science meeting including the new horizons forum and aerospace exposition. 2010. In-situ resource utilization on mars-update from DRA 5.0 study. [DOI] [Google Scholar]
- 122.Schippers A., Hedrich S., Vasters J., Drobe M., Sand W., Willscher S. Biomining: metal recovery from ores with microorganisms. Adv. Biochem. Eng. Biotechnol. 2014;141:1–47. doi: 10.1007/10_2013_216. [DOI] [PubMed] [Google Scholar]
- 123.Jerez C.A. Biomining of metals: how to access and exploit natural resource sustainably. Microb Biotechnol. 2017;10:1191–1193. doi: 10.1111/1751-7915.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Loudon C.M., Nicholson N., Finster K., Leys N., Byloos B., Houdt R.V., Rettberg P., Moeller R., Fuchs F.M., Demets R., Krause J., Vukich M., Mariani A., Cockell C. BioRock: new experiments and hardware to investigate microbe-mineral interactions in space. Int J Astrobiol. 2018;17:303–313. doi: 10.1017/s1473550417000234. [DOI] [Google Scholar]
- 125.Cockell C.S., Santomartino R., Finster K., Waajen A.C., Eades L.J., Moeller R., Rettberg P., Fuchs F.M., Van Houdt R., Leys N., Coninx I., Hatton J., Parmitano L., Krause J., Koehler A., Caplin N., Zuijderduijn L., Mariani A., Pellari S.S., Carubia F. Space station biomining experiment demonstrates rare Earth element extraction in microgravity and Mars gravity. Nat Commun. 2020;11:5523. doi: 10.1038/s41467-020-19276-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Menezes A.A., Montague M.G., Cumbers J., Hogan J.A., Arkin A.P. Grand challenges in space synthetic biology. J R Soc Interface. 2015;12(113) doi: 10.1098/rsif.2015.0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mora M., Perras A., Alekhova T.A., Wink L., Krause R., Aleksandrova A., Novozhilova T., Moissl-Eichinger C. Resilient microorganisms in dust samples of the international space station—survival of the adaptation specialists. Microbiome. 2016;4(1) doi: 10.1186/s40168-016-0217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Checinska A., Probst A.J., Vaishampayan P., White J.R., Kumar D., Stepanov V.G., Fox G.E., Nilsson H.R., Pierson D.L., Perry J., Venkateswaran K. Microbiomes of the dust particles collected from the international space station and spacecraft assembly facilities. Microbiome. 2015;3(1) doi: 10.1186/s40168-015-0116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Be N.A., Avila-Herrera A., Allen J.E., Singh N., Sielaff A.C., Jaing C., Venekateswaran K. Whole metagenome profiles of particulates collected from the international space station. Microbiome. 2017;5(81) doi: 10.1186/s40168-017-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Averesch N.J.H. Choices of microbial systems for in-situ resource utilization on Mars. Front Astronom Space sci. 2021;8 doi: 10.3389/fspas.2021.700370. [DOI] [Google Scholar]
- 131.Subramanian S., Huiszoon R.C., Chu S., Bentley W.E., Ghodssi R. Microsystems for biofilm characterization and sensing - a review. Biofilm. 2020;2 doi: 10.1016/j.bioflm.2019.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim Y.W., Meyer M.T., Berkovich A., Subramanian S., Iliadis A.A., Bentley W.E., Ghodssi R. Sensors and actuators A: physical. Elsevier BV; 2016. A surface acoustic wave biofilm sensor integrated with a treatment method based on the bioelectric effect; pp. 140–149. vol. 238. [DOI] [Google Scholar]
- 133.Song J., Li Y., Yin F., Zhang Z., Ke D., Wang D., Yuan Q., Zhang X.-E. ACS sensors. American Chemical Society (ACS); 2020. Enhanced electrochemical impedance spectroscopy analysis of microbial biofilms on an electrochemically in situ generated graphene interface; pp. 1795–1803. Vol. 5, Issue 6. [DOI] [PubMed] [Google Scholar]
- 134.Gu J.D., Roman M., Esselman T., Mitchell R. The role of microbial biofilms in deterioration of space station candidate materials. Int Biodeterior Biodegrad. 1998;41(1):25–33. [PubMed] [Google Scholar]
- 135.Tabb D., Carter L. 45th international conference on environmental systems. 2015. Water recovery system design to accommodate dormant periods for manned missions.https://ntrs.nasa.gov/citations/20150019532 [Google Scholar]
- 136.Stenzel C. Deployment of precise and robust sensors on board ISS-for scientific experiments and for operation of the station. Anal Bioanal Chem. 2016;408(24):6517–6536. doi: 10.1007/s00216-016-9789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.