Abstract
Background
Very low-calorie ketogenic diet (VLCKD) has shown to significantly reduce body weight and fat mass, as well as inflammation. These effects are supported by nutritional ketosis, which triggers the utilization of the ketone body as an energy source. Medium-chain fatty acids (MCTs) might serve as potential enhancers of ketone bodies production with a greater effect on weight loss. Nevertheless, no clinical studies have evaluated the effect of MCTs supplementation in addition to VLCKD. Therefore, the present study aimed to evaluate whether the supplementation with MCTs can induce a greater weight reduction during the ketogenic phase of VLCKD.
Methods
In this retrospective study, 263 women with overweight/obesity (body mass index, BMI: 35.7 ± 5.3 kg/m2) aged 37.5 ± 14.2 years followed one of these dietary protocols for 45 days: (a) Control group, 83 participants (31.6%) (VLCKD without MCTs), (b) VLCKD + MCTs group, 86 participants (32.7%) (MCTs supplementation − 20 g/day- during VLCKD starting from the first day of the active phase), (c) VLCKD + earlyMCTs, 94 participants (35.7%) (MCTs supplementation − 20 g/day-starting from 5 days before the beginning of the VLCKD active phase. Anthropometric measures, body composition, and c-reactive protein (CRP) concentrations were collected at the beginning and at the end (45 days) of the VLCKD intervention.
Results
MCTs supplementation significantly decreased body weight, BMI, and waist circumference as compared to the control group, with a greater effect in the VLCKD + earlyMCTs group. A two-fold decrease in fat mass and an increase in muscle mass were observed in the VLCKD + earlyMCTs group as compared to the control group. As for inflammation, hs-CRP concentrations (assessed as absolute percent change) were significantly lower in the VLCKD + MCTs group (p = 0.009) and the VLCKD + earlyMCTs group (p = 0.011) than in the control group. A logistic regression model showed that VLCKD + earlyMCTs increase the likelihood of improvement of BMI classes (OR: 1.85, 95% CI 1.02–3.36) also after adjusting for the potential confounding factors.
Conclusion
MCTs supplementation (20 g/day) may be a useful tool to enhance the beneficial effect of VLCKD on the reduction of body weight and fat mass. In particular, MCTs supplementation before the beginning of the VLCKD active phase might facilitate ketosis thus contributing to the effectiveness of the nutritional intervention.
Keywords: Obesity, Nutritional ketosis, VLCKD, Ketogenic diet, Ketone bodies, Medium-chain fatty acids (MCTs), Inflammation, Diet
Introduction
Obesity is recognized as a chronic disease that associates with several comorbidities, such as type 2 diabetes mellitus (T2D), hypertension, dyslipidemia, cardiovascular diseases (mainly coronary heart disease and stroke), sleep disturbance, and some cancers [1–4]. These comorbidities—also known as non-communicable diseases (NCDs)—reduce the quality of life and life span and increase public health costs [3, 5].
Although several strategies have been developed to obtain weight loss, the trend of obesity is dramatically increasing, particularly among young adults and middle-income countries [6, 7].
In addition to lifestyle factors such as physical inactivity, smoking, and alcohol intake, also diet has been established as a highly modifiable risk factor for obesity and NCDs [8].
Among dietary approaches, very low-calorie ketogenic diet (VLCKD) has been appointed as one of the most effective interventions for body weight loss [9, 10]. In addition, it has shown to reduce inflammation and insulin resistance which represent two main triggers for the onset of NCDs [11].
VLCKD consists in a multistep protocol with three main stages: active phase, dietary re-education, and maintenance [12]. The active stage is the most important stage of VLCKD since it allows the achievement of 80% of the target weight loss, with a duration ranging 30–45 days depending on the individual response. Rapid weight loss is obtained through a great energy restriction (600–800 kcal/day) and a sharply sustained nutritional ketosis [12].
Nutritional ketosis occurs when carbohydrate intake is < 50 g/day and, because of carbohydrate restriction, it enhances the oxidation of the fatty acids in the adipose tissue for energy purposes [13, 14]. Indeed, acetyl-CoA is the precursor of ketone bodies (acetoacetate, β-hydroxybutyrate, and acetone) which are used as an alternative fuel in various tissues.
Interestingly, only fatty acids with carbon chain lengths ≤ 8 can cross the inner membrane of the mitochondria independent of carnitine palmitoyl transferase I [15]. In this contest, fatty acids C8 (caprylic acid) might have a stronger ketogenic effect compared to C10 (capric acid) and C12 (lauric acid) [16]. Clinical evidence demonstrated that 20 g of C8 produces a significantly stronger ketogenic response than 10 g of C8 [15]. However, Norgren and colleagues [17] reported that to minimize potential side effects, the dose of C8 should be limited to 15–20 g per intake. Triglycerides containing medium-chain fatty acids (MCTs) consist in fatty acids with a carbon backbone with 6–12 carbon atoms linked to glycerol [18, 19]. After ingestion with the diet, MCTs are digested by intestinal lipases and absorbed in the gut as triglycerides containing long-chain fatty acids (LCTs, > 12 carbon atoms) [19]. Unlike LCTs, the fatty acids contained in MCTs can bind albumin and skip the formation of chylomicrons. Therefore, MCTs skip the hydrolysis by plasma lipoprotein-lipase and the consequent deposition in adipose tissue [20]. Then, MCTs directly reach the liver where they can be metabolized more quickly by mitochondrial β-oxidation [21]. However, unlike LCTs, MCTs do not require carnitine-mediated transport to enter the mitochondria. Moreover, MCTs, especially C8 and C10, can also be oxidized in peroxisomes, thus representing a more available source of energy than LCTs [21]. Several studies showed that MCTs supplementation increases β-hydroxybutyrate concentrations with a dose-dependent relationship [13, 22–24]. Consequently, MCTs might endorse “nutritional ketosis” during ketogenic diets [24].
Over a faster metabolism and less deposition in adipocytes, MCTs can significantly influence energy balance, favouring body weight loss independently of dietary energy intake [25, 26].
The mechanisms behind this effect are not completely understood possibly due to the high heterogeneity of studies available so far. Some studies have shown that MCTs might increase thermogenesis and, consequently, affect energy expenditure. Furthermore, the replacement of LCTs with MCTs was associated with a greater reduction of adipose tissue in animal models as well as in humans [27, 28]. This effect could be mediated by the specific action of MCTs on a G-protein coupled receptor (GPR84) in the adipose tissue [27]. In addition, MCTs might increase satiety feelings thus limiting food intake while favouring body weight control [28–31]. Indeed, hyperketonaemia can enhance the anorexigenic effect at the hypothalamic level [29, 30]. Furthermore, some studies suggested that MCTs can modulate the secretion of some gastrointestinal hormones involved in hunger/satiety feelings (ghrelin and YY peptide, respectively) [29, 31].
To date, it is unclear whether the use of MCTs might increase the acute ketogenic response. Nevertheless, a 30 day clinical trial reported that the consumption of caprylic acid (C8; 6 g twice a day) increased plasma β-hydroxybutyrate concentration from ~ 0.1 mmol/L to ~ 0.2 mmol/L [32].
To the best of our knowledge, no previous studies investigated the potential effects of MCTs supplementation during VLCKD for a greater reduction of body weight and fat mass in individuals with overweight and obesity.
Against this background, the present study aimed to evaluate whether the supplementation with MCTs can induce a greater weight reduction during the ketogenic phase of VLCKD. For this purpose, we retrospectively investigated the effect of MCTs supplementation in addition to diet vs diet alone in a group of individuals with overweight/obesity undergoing a VLCKD. Over the effect on weight loss, we evaluated whether the association between VLCKD and MCTs supplementation may also affect inflammatory status as demonstrated for VLCKD alone.
Methods
Study design and setting
This study was conducted in compliance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement checklist [33]. Data from consecutive participants undergoing a VLCKD protocol for weight loss at Centro Italiano per la cura e il Benessere del paziente con Obesità (C.I.B.O.) of the Federico II University Hospital (Naples, Italy) were retrospectively collected between September 2021 and September 2022. All participants provided written consent after being informed about the study design. The study has been approved by the Local Ethical Committee (n. 50/20). The present analyses included a total of two time points: at baseline and during the VLCKD active stage after 45 days with the collection of anthropometric measures and body composition.
Participants
We included 263 healthy participants as individuals with diseases, such as T2D, might have different metabolic responses [16]. Individuals eligible for this study presented the following features: women aged 18–69 years, body mass index (BMI) 25.0–50.9 kg/m2 at the beginning of the nutritional treatments. We excluded individuals presenting one or more of the following characteristics: (a) new onset physiological or pathological conditions that represent contraindications for VLCKD (i.e., pregnancy/breastfeeding, individuals with diabetes on insulin therapy, liver or kidney insufficiency, etc.) [10], (b) poor adherence to the dietary intervention (negative test for ketonuria or food behaviours not included in the dietary program referred by the participant); (c) individuals needing treatment with anti-obesity drugs or referred to bariatric surgery; (d) use of drugs, supplements or nutraceuticals that affect energy expenditure or weight loss during the intervention.
Nutritional intervention
Participants included in the present study underwent a VLCKD with the use of replacement meals (New Penta, Cuneo, Italy) following a 3-stages protocol (active phase, re-education, and maintenance) [12]. After the nutritional status assessment, the dietary plan was prescribed by the endocrinologist and planned by a skilled nutritionist. The VLCKD provided a total daily energy intake < 800 kcal, with 13% carbohydrates (< 30 g/day); 43% protein (1.2–1.5 g/kg ideal body weight); 44% lipids (mainly from extra-virgin olive oil). According to the international recommendations, participants were prescribed a multi-vitamin and saline supplement (complex B vitamins, vitamins C and E, potassium, sodium, magnesium, calcium, and omega-3 fatty acids; PentaCal, Penta, s.r.l., Cuneo, Italy), as reported in previous studies [34–36]. In all participants, physical activity (at least 30 min/day aerobic exercise) was assessed using a YES/NO response, as reported in previous studies [37–39].
Participants followed one of these dietary protocols: (a) Control group, VLCKD without MCTs; b) VLCKD + MCTs group, MCTs supplementation (20 g/day) during VLCKD starting from the first day of the active phase; c) VLCKD + earlyMCTs, MCTs supplementation (20 g/day) starting from 5 days before the beginning of the VLCKD active phase.
To reduce potential confounding factors related to the oil composition, all participants in the VLCKD + MCTs and VLCKD + earlyMCTs groups used only 100% MCT oil (Kanso MCToil 100%, for composition details, see: https: //www.kanso.com/en/p/oil-mct-100). MCTs supplementation (20 g/day) provided 163.8 kcal and 18.2 g of fat.
To improve compliance with the recommendations for diet and physical activity, participants were contacted by phone calls by a skilled nutritionist each week. Moreover, the participants were advised to measure blood β-hydroxybutyrate by test strips (Optium Xceed Blood Glucose and Ketone Monitoring System; Abbott Laboratories, Chicago, IL, USA) at fasting in the morning and to notify the results to the nutritionist.
Assessment of anthropometric measures and body composition
Anthropometric measures were collected by a single skilled nutritionist at each visit between 8 a.m. and 10 a.m. Weight, height, and waist circumference were detected in participants wearing light clothing and no shoes, after an overnight fast, according to standard procedures [40, 41]. Weight and height were used to calculate BMI (kg/m2) [42]. BMI was classified according to the WHO criteria [43]: normal weight (18.5–24.9 kg/m2); Overweight (25.0–29.9 kg/m2); Obesity class I (30.0–34.9 kg/m2); Obesity class II (35.0–39.9 kg/m2); Obesity class III (≥ 40.0 kg/m2). All measurements were taken while the subject was standing upright with the feet together and the arms hanging closely by the sides, with the subject standing and breathing normally, as previously reported [36–38].
Body composition was evaluated by bioelectrical impedance analysis (BIA). BIA was performed by a phase-sensitive BIA system (an 800 A current with a frequency of 50 kHz BIA 101 RJL, Akern Bioresearch, Florence, Italy) [44, 45] with BIATRODES electrodes (Akern Srl; Florence, Italy), according to the standard procedures of the European Society of Parenteral and Enteral Nutrition (ESPEN) [46]. All measurements were performed under strictly standardized conditions by the same certified skilled nutritionist and with the same device to avoid inter-observer and inter-device variability as reported in previous studies [36, 47, 48]. Briefly, the device was routinely checked with resistors and capacitors of known values. Reliability for intraday and interday measurements by the same observer was < 2% for resistance (R), < 2.5% for reactance (Xc), and < 3.3% for R, < 2.8% for Xc, respectively. The coefficients of variation (CVs) of repeated measurements of R and Xc at 50 kHz were determined in 10 females by the same observer: CVs were 1.4% for R and 1.3% for Xc.
Assessment of C-reactive protein concentrations
In a subgroup of participants (n = 207), information on c-reactive protein (CRP) was retrieved from electronic medical records. During each visit, fasting blood samples were collected in the morning (8.00–10.00 a.m.), and stored at − 80 °C until processing. Serum high-sensitivity (hs) CRP concentrations were analyzed by CardioPhase® (Siemens Healthcare Diagnostics, Marburg, Germany), based on particle-enhanced immunonephelometry. The CV of intra-and interassay was < 7%.
Statistical analyses
The data distribution was evaluated by Kolmogorov-Smirnov test and variables not normally distributed were normalized by logarithmic transformation. Skewed variables (waist circumference, R, and muscle mass) were back transformed for presentation in tables and figures. Continuous variables were expressed as mean ± standard deviation (SD) whereas categorical variables were reported as numbers and percentages (%). The effect of MCTs supplementation was evaluated as absolute changes (45 days minus baseline). Differences between groups were analyzed by analysis of variance (one-way ANOVA) and post hoc analyses for multiple comparisons (Bonferroni). Differences between categorical variables were assessed by χ2 (chi-square) test. A logistic regression model was used to estimation of the likelihood of BMI changes of WHO classes with MCTs supplementation. BMI improvement was investigated as a dichotomous variable (yes/no) and no MCTs supplementation was designated as reference for ease of comparability. Estimates of the logistic regression coefficients were reported as odds ratios (OR). The analysis was conducted in six steps: Model 1 not adjusted; Model 2 adjusted for age; Model 3 adjusted for age and body weight at baseline; Model 4: adjusted for age, body weight at baseline, and percentage of fat mass at baseline. A p value < 0.05 was considered significant. Statistical analysis was performed according to standard methods using the Statistical Package for Social Sciences software 26.0 (SPSS/PC; SPSS, Chicago, IL, USA).
Results
A total of 263 participants were included in the analyses, with 83 participants (31.6%) in the control group (VLCKD alone without any integration with MCTs), while 86 participants (32.7%) in the VLCKD + MCTs group, and 94 participants (35.7%) in the VLCKD + earlyMCTs group. All individuals of the three groups were evaluated at baseline and at the 45th day (the end of the active phase of VLCKD). At baseline, the three groups did not differ for demographic and anthropometric features, as well as for body composition (Table 1). Absolute changes after the intervention were reported in Table 2. MCTs supplementation significantly decreased body weight, BMI, and waist circumference as compared to the control group, with a greater effect in the VLCKD + earlyMCTs group (p < 0.001). MCTs supplementation significantly also affected body composition (Table 2). A two-fold decrease in fat mass and an increase of muscle mass were observed in the VLCKD + earlyMCTs group as compared to the control group (p < 0.001). Fat mass and muscle mass were also different when comparing the two groups with MCTs supplementation. Of interest, from baseline, no participant in the three groups changed their physical activity levels during the 45 days of VLCKD (χ2 = 4.22, p = 0.121). All dietary interventions significantly decreased the prevalence of higher BMI classes (obesity class III and obesity class III) from baseline to 45 days of VLCKD active phase while lower BMI classes increased (obesity class I, overweight, and normal weight) (Table 2). Although no significant difference was observed (p = 0.623), VLCKD + earlyMCTs induced a threefold increase of normal weight participants (n = 6, 6.4%) than the other two dietary interventions (2 participants for both groups) (Table 2).
Table 1.
Demographic and anthropometric characteristics, and body composition in the three groups at baseline
| Parameters | Control group (n = 83, 31.6%) | VLCKD + MCTs (n = 86, 32.7%) | VLCKD + earlyMCTs (n = 94, 35.7%) | p for ANOVA |
|---|---|---|---|---|
| Age (years) | 40.1 ± 15.2 | 36.8 ± 14.1 | 35.9 ± 13.1 | 0.130 |
| Physical activity (yes) | 32 (38.6%) | 21 (22.3%) | 33 (38.4%) | χ2 = 4.22, p = 0.121 |
| Body weight (kg) | 92.5 ± 14.9 | 98.5 ± 16.5 | 95.7 ± 17.5 | 0.059 |
| BMI (kg/m2) | 35.1 ± 5.1 | 35.9 ± 5.2 | 36.0 ± 5.5 | 0.475 |
| 25.0–29.9 kg/m2 | 13 (15.7%) | 11 (12.8%) | 16 (17.0%) | χ2 = 0.64, p = 0.725 |
| 30–34.9 kg/m2 | 31 (37.3%) | 31 (36.0%) | 23 (24.5%) | χ2 = 4.16, p = 0.125 |
| 35–39.9 kg/m2 | 23 (27.7%) | 23 (26.7%) | 37 (39.4%) | χ2 = 4.14, p = 0.126 |
| ≥ 40.0 kg/m2 | 16 (19.3%) | 21 (24.4%) | 18 (19.1%) | χ2 = 0.95, p = 0.622 |
| Waist circumference (cm) | 106.3 ± 13.7 | 105.8 ± 15.6 | 102.2 ± 16.4 | 0.147 |
| R (Ω) | 481.6 ± 68.6 | 467.9 ± 68.1 | 483.8 ± 80.5 | 0.296 |
| Xc (Ω) | 47.6 ± 9.8 | 45.9 ± 8.8 | 45.3 ± 9.4 | 0.245 |
| FM (%) | 41.6 ± 6.4 | 42.4 ± 7.3 | 43.0 ± 7.1 | 0.403 |
| Muscle mass (%) | 27.2 ± 4.3 | 27.8 ± 5.4 | 26.8 ± 4.8 | 0.390 |
Data are expressed as mean ± SD or n (%). One-way ANOVA and post hoc test for multiple comparisons (Bonferroni) and χ2 (chi-square) test
VLCKD Very low-calorie ketogenic diet, MCTs Medium chain fatty acids, BMI body mass index, R resistance, Xc reactance, FM fat mass
Table 2.
Absolute changes in anthropometric characteristics and body composition in the three groups
| Parameters | Control group (n = 83, 31.6%) | VLCKD + MCTs (n = 86, 32.7%) | VLCKD + earlyMCTs (n = 94, 35.7%) | p for ANOVA |
|---|---|---|---|---|
| Body weight (kg) | − 4.8 ± 2.64 | − 7.2 ± 1.9a | − 8.8 ± 2.9a,b | < 0.001 |
| BMI (kg/m2) | − 1.8 ± 0.9 | − 2.6 ± 0.6a | − 3.3 ± 1.1a,b | < 0.001 |
| 18.5–24.9 kg/m2 | 2 (2.4%) | 2 (2.3%) | 6 (6.4%) | χ2 = 2.66, p = 0.263 |
| 25.0–29.9 kg/m2 | 23 (27.7%) | 25 (29.1%) | 24 (25.5%) | χ2 = 0.29, p = 0.865 |
| 30–34.9 kg/m2 | 28 (33.7%) | 26 (30.2%) | 35 (37.2%) | χ2 = 0.98, p = 0.611 |
| 35–39.9 kg/m2 | 24 (28.9%) | 24 (27.9%) | 19 (20.2%) | χ2 = 2.16, p = 0.340 |
| ≥ 40.0 kg/m2 | 6 (7.2%) | 9 (10.5%) | 10 (10.6%) | χ2 = 0.73, p = 0.693 |
| Waist circumference (cm) | − 4.4 ± 5.7 | − 7.3 ± 5.4a | − 8.1 ± 4.9a | < 0.001 |
| R (Ω) | 6.1 ± 36.4 | 8.9 ± 30.7 | − 1.1 ± 49.5 | 0.227 |
| Xc (Ω) | 2.8 ± 6.4 | 3.8 ± 5.4 | 5.0 ± 6.7 | 0.059 |
| FM (%) | − 2.5 ± 2.7 | − 3.7 ± 2.6a | − 5.1 ± 3.8a,b | < 0.001 |
| Muscle mass (%) | 1.1 ± 2.1 | 1.8 ± 1.9 | 2.7 ± 2.6a,b | < 0.001 |
Data are expressed as mean ± SD
A p-value in bold type denotes a significant difference (p < 0.05)
VLCKD Very low-calorie ketogenic diet, MCTs Medium chain fatty acids, BMI body mass index, R resistance, Xc reactance, FM fat mass
ap < 0.05 vs control; one-way ANOVA and post hoc test for multiple comparisons (Bonferroni)
bp < 0.05 vs VLCKD + MCTs; one-way ANOVA and post hoc test for multiple comparisons (Bonferroni).
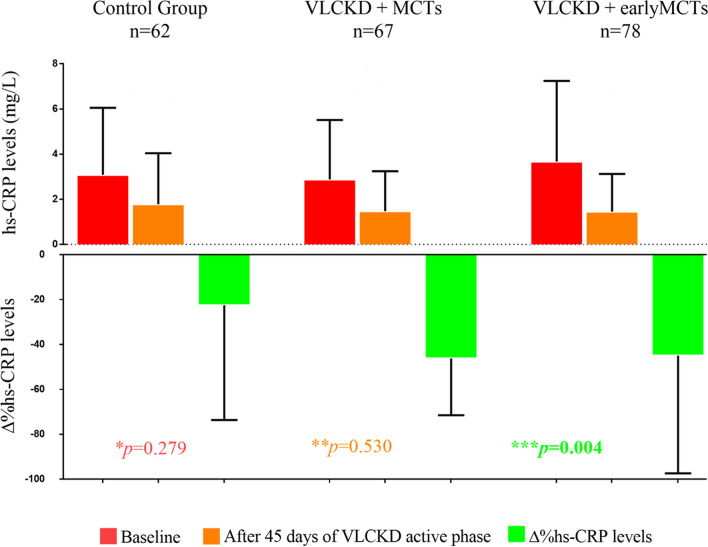
Inflammatory status was assessed in a subgroup of 207 subjects, being 62 participants in the control group, 67 participants in the VLCKD + MCTs group, and 78 participants in the VLCKD + early MCTs group. Hs-CRP concentrations did not differ among the three groups at baseline (control group: 3.1 ± 2.9 mg/L, VLCKD + MCTs: 2.9 ± 2.6 mg/L, VLCKD + earlyMCTs: 3.7 ± 3.5 mg/L; p = 0.279) as well as at 45 days of VLCKD active phase (control group: 1.8 ± 2.3 mg/L, VLCKD + MCTs: 1.5 ± 1.8 mg/L, VLCKD + earlyMCTs: 1.4 ± 1.7 mg/L; p = 0.530) (Fig. 1). However, as compared to the control group, CRP concentrations (evaluated as absolute percent change) were significantly lower in both the VLCKD + MCTs group (∆% = − 22.5 ± 51.2 vs − 46.2 ± 25.3; p = 0.009), and the VLCKD + earlyMCTs group (∆% = − 22.5 ± 51.2 vs − 45.0 ± 52.4; p = 0.011) (Fig. 1).Findings from the logistic regression modeling in the whole population were shown in Table 3.
Fig. 1.
Changes in hs-CRP concentrations in the three study groups. One-way ANOVA and post hoc test for multiple comparisons (Bonferroni). A p-value in bold type denotes a significant difference (p < 0.05). * hs-CRP concentrations in the three groups at baseline. ** hs-CRP concentrations in the three groups after 45 days of VLCKD active phase. *** The absolute percent change of hs-CRP concentrations in the three groups
Table 3.
Logistic regression analyses on the likelihood of BMI improvement * after MCTs supplementation, adjusted for possible confounders
| Intervention | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Control group (no MCTs) | ref | ref | ref | ref |
| VLCKD + MCTs | 1.53 (0.84–2.77) p: 0.131 | 1.59 (0.87–2.91) p: 0.162 | 1.49 (0.83–2.70) p: 0.181 | 1.48 (0.82–2.67) p: 0.190 |
| VLCKD + earlyMCTs | 1.98 (1.08–3.63) p: 0.028 | 1.96 (1.06–3.60) p: 0.031 | 1.90 (1.04–3.48) p: 0.037 | 1.85 (1.02–3.36) p: 0.043 |
*BMI improvement was evaluated as change of BMI classes according to WHO classification after the intervention. Model 1: unadjusted; Model 2: adjusted for age; Model 3: adjusted for age and body weight at baseline; Model 3: adjusted for age, body weight at baseline, and fat mass at baseline
A p-value in bold type denotes a significant difference (p < 0.05)
VLCK Very low-calorie ketogenic diet, MCTs Medium chain fatty acids, BMI body mass index, OR odds ratios, CI confidence interval
MCTs supplementation starting at the beginning of the VLCKD active phase (VLCKD + MCTs) did not influence the likelihood to improve BMI classes. As for VLCKD + earlyMCTs intervention, participants presented a high likelihood of improvement of BMI classes (OR: 1.85, 95% CI 1.02–3.36) also after adjusting for the potential confounding factors (age, body weight at baseline, and fat mass at baseline).
Discussion
The present study showed that individuals undergoing a VLCKD with daily MCTs supplementation (20 g/day) obtained a higher body weight loss than individuals supplied with VLCKD alone. Weight loss translated into a significant reduction in BMI, waist circumference, and fat mass. These effects were greater when MCTs supplementation started 5 days before the beginning of the VLCKD active phase than on the first day of the dietary protocol.
Our results are in line with previous studies focusing on supplementation with MCTs during energy-restricted diets [25, 26]. Indeed, two meta-analyses [25, 26] showed that the isoenergetic substitution of LCTs with MCTs during energy-restricted dietary interventions resulted in a small reduction in body weight (− 0.5 to − 0.7 kg) and waist circumference (− 1.5 to − 1.8 cm) in middle-aged individuals with overweight/obesity. However, when considering studies involving very low-calorie diets (< 800 kcal/day) with MCTs supplementation the mean weight reduction was similar to that observed in our study (on average − 8 kg).
As reported above, MCTs are metabolised differently from LCTs, since they can reach the liver after being absorbed in the intestine and are largely oxidized and not stored [23]. In addition, MCTs have been suggested to increase thermogenesis and reduce fat deposition, thus contributing to weight loss [27, 28]. Indeed, Hill and colleagues [49] demonstrated that MCTs s increased thermogenesis by 50% after a 6 day supplementation. Therefore, this mechanism might explain the greater effect on body weight loss that we observed in the group starting MCTs supplementation prior to the VLCKD.
As for the effect on body composition, MCTs supplementation significantly reduce fat mass whereas muscle mass was increased only in the earlyMCTs group as compared to VLCKD alone.
It is known that during nutritional ketosis, ketone bodies can be used as the main energy source thus limiting protein breakdown for energy purposes [50]. On the other hand, high doses of MCTs have been shown to stimulate lipolysis by increasing lipoprotein lipase activity in animal models [51, 52]. Nevertheless, the mechanisms underlying the effect of MCTs on body composition in humans need further clarification.
In the management of obesity and its metabolic comorbidities, VLCKD has been proposed also to reduce systemic inflammation by virtue of its antioxidant and anti-inflammatory effects [53, 54]. Interestingly, in our study all groups undergoing the VLCKD presented a reduction of CRP concentrations, a well-known marker of inflammation. This result confirms those obtained in previous studies demonstrating the anti-inflammatory effects of the VLCKD in the short [36, 54] and -long-term in individuals with obesity [55]. Indeed, VLCKD has shown to reduce inflammation through several mechanisms, i.e.by inhibiting activation of the nuclear factor kappa-light-chain-enhancer of activated B cells, and the inflammatory nucleotide-binding, leucine-rich-containing family, pyrin domain-containing-3, and inhibiting histone deacetylases [56]. Notably, the earlyMCTs group experienced the greatest reduction as compared to the other groups, likely due to the overflow of ketone bodies [57]. Unfortunately, we did not perform a quantitative measurement of ketone bodies and we were not able to test this hypothesis.
Our study has some strengths and limitations. To the best of our knowledge, this is the first study evaluating the effect of MCTs supplementation in addition to VLCKD in individuals with overweight/obesity. In addition, this study was performed in a large population in a real-life setting.
Weaknesses included the single-centre recruitment with potential selection bias. Nevertheless, to increase the homogeneity of the study population, we included only women to avoid potential gender differences in body composition and CRP concentrations. In addition, we did not evaluate the long-term effect of MCTs supplementation. However, the short study duration increased participants’ compliance to the treatment. Finally, we did not analyse other inflammatory markers, but CRP is a reliable inflammatory biomarker in different clinical settings [58].
Another limitation might be the transferability of these results to other populations. Our study focused on young adult women with overweight/obesity. Previous studies reported that MCTs supplementation did not increase ketone bodies in middle-aged and elderly subjects [59]. Therefore, further studies of the ketogenic effect of MCTs in different populations are warranted.
Conclusion
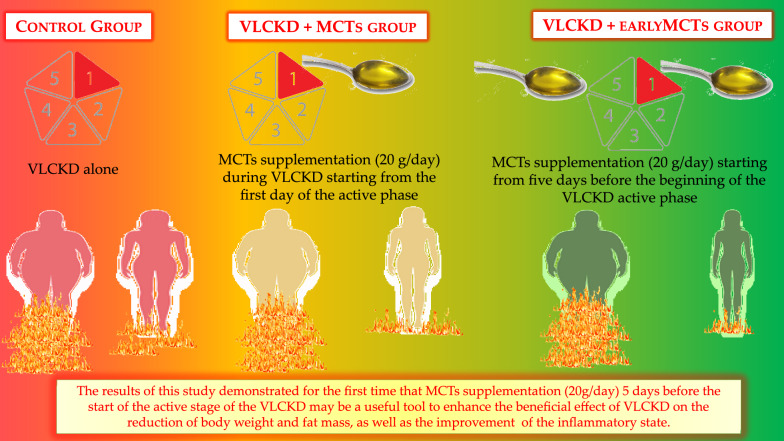
The results of the present study demonstrated for the first time that MCTs supplementation (20 g/day) during the active stage of the VLCKD may be a useful tool to enhance the beneficial effect of VLCKD on the reduction of body weight and fat mass, as well as the improvement of the inflammatory state. In particular, MCTs supplementation 5 days before the beginning of the VLCKD active phase might facilitate the transition into ketosis thus contributing to the effectiveness of the nutritional intervention and enhancing its beneficial effects (Fig. 2). However, further studies extending the observations to subsequent stages of the VLCKD are mandatory. In addition, VLCKD with early MCTs supplementation (5 days before the onset of the active phase) should be compared with other hypocaloric dietary programs to confirm its role in the enhancement of weight loss and reduced inflammation by virtue of the increase of ketosis. Finally, this study underlines the pivotal role of the nutritionist in the management and correct planning of the VLCKD.
Fig. 2.
MCTs supplementation during the active stage of the VLCKD. The MCTs supplementation (20 g/day) 5 days before the beginning of the VLCKD active phase might facilitate the transition into ketosis thus contributing to the effectiveness of the nutritional intervention enhancing its beneficial effects on weight loss, body composition modulation and inflammatory status
Acknowledgements
The authors thank the study participants.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- CRP
C-reactive protein
- LCTs
Long-chain fatty acids
- MCTs
Medium-chain fatty acids
- OR
Odds ratio
- T2D
Type 2 diabetes mellitus
- VLCKD
Very low-calorie ketogenic diet
Author contributions
LB designed the study. CV and LV provided data collection. CV performed the statistical analysis. CV and LV wrote the manuscript. SS, AC, GM, and LB revised the paper. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Kanso Company contributed to the payment of open access publication charges for this manuscript. There are no other conflicts of interest to declare.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
The study has been approved by the Local Ethical Committee (n.50/20) and carried out in accordance with the Declaration of Helsinki for experiments that involved humans. The aim of the study was clearly explained to all the study participants and a written informed consent was obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Claudia Vetrani and Ludovica Verde are equally contributed to this work
Contributor Information
Claudia Vetrani, Email: claudia.vetrani@unipegaso.it.
Giovanna Muscogiuri, Email: giovanna.muscogiuri@unina.it.
Luigi Barrea, Email: luigi.barrea@unipegaso.it.
References
- 1.Jastrebof AM, Kotz CM, Kahan S, Kelly AS, Heymsfeld SB. Obesity as a disease: the obesity society 2018 position statement. Obesity. 2019;27:7–9. doi: 10.1002/oby.22378. [DOI] [PubMed] [Google Scholar]
- 2.De Lorenzo A, Gratteri S, Gualtieri P, Cammarano A, Bertucci P, Di Renzo L. Why primary obesity is a disease? J Transl Med. 2019;17:169. doi: 10.1186/s12967-019-1919-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai H, Alsalhe TA, Chalghaf N, Riccò M, Bragazzi NL, Wu J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the global burden of disease study. PLoS Med. 2020;17(7):e1003198. doi: 10.1371/journal.pmed.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muscogiuri G, Barrea L, Aprano S, Framondi L, Di Matteo R, Laudisio D, Pugliese G, Savastano S, Colao A. On behalf of the opera prevention project sleep quality in obesity: does adherence to the mediterranean diet matter? Nutrients. 2020;12(5):1364. doi: 10.3390/nu12051364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GBD 2019 Risk Factors Collaborators Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GBD 2017 Diet Collaborators Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;393:1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muscogiuri G, El Ghoch M, Colao A, Hassapidou M, Yumuk V, Busetto L. Obesity management task force (OMTF) of the european association for the study of obesity (EASO) european guidelines for obesity management in adults with a very low-calorie ketogenic diet: a systematic review and meta-analysis. Obes Facts. 2021;14(2):222–245. doi: 10.1159/000515381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caprio M, Infante M, Moriconi E, Armani A, Fabbri A, Mantovani G, Mariani S, Lubrano C, Poggiogalle E, Migliaccio S, Donini LM, Basciani S, Cignarelli A, Conte E, Ceccarini G, Bogazzi F, Cimino L, Condorelli RA, La Vignera S, Calogero AE, Gambineri A, Vignozzi L, Prodam F, Aimaretti G, Linsalata G, Buralli S, Monzani F, Aversa A, Vettor R, Santini F, Vitti P, Gnessi L, Pagotto U, Giorgino F, Colao A, Lenzi A. Cardiovascular endocrinology club of the Italian society of endocrinology very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: systematic review and consensus statement from the Italian society of endocrinology (SIE) J Endocrinol Invest. 2019;42(11):1365–1386. doi: 10.1007/s40618-019-01061-2. [DOI] [PubMed] [Google Scholar]
- 11.Mohammadifard N, Haghighatdoost F, Rahimlou M, Rodrigues APS, Gaskarei MK, Okhovat P, de Oliveira C, Silveira EA, Sarrafzadegan N. The effect of ketogenic diet on shared risk factors of cardiovascular disease and cancer. Nutrients. 2022;14(17):3499. doi: 10.3390/nu14173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muscogiuri G, Barrea L, Laudisio D, Pugliese G, Salzano C, Savastano S, Colao A. The management of very low-calorie ketogenic diet in obesity outpatient clinic: a practical guide. J Transl Med. 2019;17(1):356. doi: 10.1186/s12967-019-2104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey CJDC, Schofield GM, Williden M. The use of nutritional supplements to induce ketosis and reduce symptoms associated with keto-induction: a narrative review. PeerJ. 2018;6:e4488. doi: 10.7717/peerj.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sukkar SG, Muscaritoli M. A clinical perspective of low carbohydrate ketogenic diets: a narrative review. Front Nutr. 2021;8:642628. doi: 10.3389/fnut.2021.642628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khabbush A, Orford M, Tsai YC, Rutherford T, O'Donnell M, Eaton S, Heales SJR. Neuronal decanoic acid oxidation is markedly lower than that of octanoic acid: a mechanistic insight into the medium-chain triglyceride ketogenic diet. Epilepsia. 2017;58(8):1423–1429. doi: 10.1111/epi.13833. [DOI] [PubMed] [Google Scholar]
- 16.Lin TY, Liu HW, Hung TM. The ketogenic effect of medium-chain triacylglycerides. Front Nutr. 2021;8:747284. doi: 10.3389/fnut.2021.747284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norgren J, Sindi S, Sandebring-Matton A, Kåreholt I, Daniilidou M, Akenine U, Nordin K, Rosenborg S, Ngandu T, Kivipelto M. Ketosis after intake of coconut oil and caprylic acid-with and without glucose: a cross-over study in healthy older adults. Front Nutr. 2020;15(7):40. doi: 10.3389/fnut.2020.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clegg ME, Golsorkhi M, Henry CJ. Combined medium-chain triglyceride and chilli feeding increases diet-induced thermogenesis in normal-weight humans. Eur J Nutr. 2013;52(6):1579–1585. doi: 10.1007/s00394-012-0463-9. [DOI] [PubMed] [Google Scholar]
- 19.Lee YY, Tang TK, Lai OM. Health benefits, enzymatic production, and application of medium-and long-chain triacylglycerol (MLCT) in food industries: a review. J Food Sci. 2012;77(8):R137–R144. doi: 10.1111/j.1750-3841.2012.02793.x. [DOI] [PubMed] [Google Scholar]
- 20.Rego Costa AC, Rosado EL, Soares-Mota M. Influence of the dietary intake of medium chain triglycerides on body composition, energy expenditure and satiety: a systematic review. Nutr Hosp. 2012;27(1):103–108. doi: 10.1590/S0212-16112012000100011. [DOI] [PubMed] [Google Scholar]
- 21.Wanten GJ, Naber AH. Cellular and physiological effects of medium-chain triglycerides. Mini Rev Med Chem. 2004;4(8):847–857. doi: 10.2174/1389557043403503. [DOI] [PubMed] [Google Scholar]
- 22.St-Onge MP, Ross R, Parsons WD, Jones PJ. Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes Res. 2003;11(3):395–402. doi: 10.1038/oby.2003.53. [DOI] [PubMed] [Google Scholar]
- 23.Krotkiewski M. Value of VLCD supplementation with medium chain triglycerides. Int J Obes Relat Metab Disord. 2001;25(9):1393–1400. doi: 10.1038/sj.ijo.0801682. [DOI] [PubMed] [Google Scholar]
- 24.Harvey CJ, Schofield GM, Williden M, McQuillan JA. The effect of medium chain triglycerides on time to nutritional ketosis and symptoms of keto-induction in healthy adults: a randomised controlled clinical trial. J Nutr Metab. 2018;2018:2630565. doi: 10.1155/2018/2630565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bueno NB, de Melo IV, Florêncio TT, Sawaya AL. Dietary medium-chain triacylglycerols versus long-chain triacylglycerols for body composition in adults: systematic review and meta-analysis of randomized controlled trials. J Am Coll Nutr. 2015;34(2):175–183. doi: 10.1080/07315724.2013.879844. [DOI] [PubMed] [Google Scholar]
- 26.Mumme K, Stonehouse W. Effects of medium-chain triglycerides on weight loss and body composition: a meta-analysis of randomized controlled trials. J Acad Nutr Diet. 2015;115(2):249–263. doi: 10.1016/j.jand.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 27.Hara T, Kashihara D, Ichimura A, Kimura I, Tsujimoto G, Hirasawa A. Role of free fatty acid receptors in the regulation of energy metabolism. Biochim Biophys Acta. 2014;1841(9):1292–1300. doi: 10.1016/j.bbalip.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Swarnamali H, Ranasinghe P, Hills AP, Jayawardena R. Coconut oil consumption and bodyweight reduction: a systematic review and meta-analysis. Minerva Endocrinol. 2021 doi: 10.23736/S2724-6507.21.03654-X. [DOI] [PubMed] [Google Scholar]
- 29.St-Onge MP, Jones PJ. Physiological effects of medium-chain triglycerides: potential agents in the prevention of obesity. J Nutr. 2002;132(3):329–332. doi: 10.1093/jn/132.3.329. [DOI] [PubMed] [Google Scholar]
- 30.Deemer SE, Plaisance EP, Martins C. Impact of ketosis on appetite regulation-a review. Nutr Res. 2020;77:1–11. doi: 10.1016/j.nutres.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Maher T, Clegg ME. Dietary lipids with potential to affect satiety: mechanisms and evidence. Crit Rev Food Sci Nutr. 2019;59(10):1619–1644. doi: 10.1080/10408398.2017.1423277. [DOI] [PubMed] [Google Scholar]
- 32.Courchesne-Loyer A, Fortier M, Tremblay-Mercier J, Chouinard-Watkins R, Roy M, Nugent S, Castellano CA, Cunnane SC. Stimulation of mild, sustained ketonemia by medium-chain triacylglycerols in healthy humans: estimated potential contribution to brain energy metabolism. Nutrition. 2013;29(4):635–640. doi: 10.1016/j.nut.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 33.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE initiative the strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 34.Barrea L, Verde L, Vetrani C, Marino F, Aprano S, Savastano S, Colao A, Muscogiuri G. VLCKD: a real time safety study in obesity. J Transl Med. 2022;20(1):23. doi: 10.1186/s12967-021-03221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrea L, de Alteriis G, Muscogiuri G, Vetrani C, Verde L, Camajani E, Aprano S, Colao A, Savastano S. Impact of a very low-calorie ketogenic diet (VLCKD) on changes in handgrip strength in women with obesity. Nutrients. 2022;14(19):4213. doi: 10.3390/nu14194213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrea L, Muscogiuri G, Aprano S, Vetrani C, de Alteriis G, Varcamonti L, Verde L, Colao A, Savastano S. Phase angle as an easy diagnostic tool for the nutritionist in the evaluation of inflammatory changes during the active stage of a very low-calorie ketogenic diet. Int J Obes. 2022;46(9):1591–1597. doi: 10.1038/s41366-022-01152-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrea L, Tarantino G, Somma CD, Muscogiuri G, Macchia PE, Falco A, Colao A, Savastano S. Adherence to the mediterranean diet and circulating levels of sirtuin 4 in obese patients: a novel association. Oxid Med Cell Longev. 2017;2017:6101254. doi: 10.1155/2017/6101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muscogiuri G, Barrea L, Aprano S, Framondi L, Di Matteo R, Laudisio D, Pugliese G, Savastano S, Colao A. On behalf of the opera prevention project. chronotype and adherence to the mediterranean diet in obesity: results from the opera prevention project. Nutrients. 2020;12(5):1354. doi: 10.3390/nu12051354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vetrani C, Barrea L, Verde L, Docimo A, Aprano S, Savastano S, Colao A, Muscogiuri G. Vitamin D and chronotype: is there any relationship in individuals with obesity? J Endocrinol Invest. 2022 doi: 10.1007/s40618-022-01973-6. [DOI] [PubMed] [Google Scholar]
- 40.Barrea L, Vetrani C, Altieri B, Verde L, Savastano S, Colao A, Muscogiuri G. The importance of being a ‘lark’ in post-menopausal women with obesity: a ploy to prevent type 2 diabetes mellitus? Nutrients. 2021;13(11):3762. doi: 10.3390/nu13113762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrea L, Verde L, Vetrani C, Savastano S, Colao A, Muscogiuri G. Chronotype: a tool to screen eating habits in polycystic ovary syndrome? Nutrients. 2022;14(5):955. doi: 10.3390/nu14050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madden AM, Smith S. Body composition and morphological assessment of nutritional status in adults: a review of anthropometric variables. J Hum Nutr Diet. 2016;29:7–25. doi: 10.1111/jhn.12278. [DOI] [PubMed] [Google Scholar]
- 43.WHO | World Health Organization. Body mass index—BMI n.d. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (Accessed July 15 2022).
- 44.Bioelectrical impedance analysis in body composition measurement National institutes of health technology assessment conference statement. Am J Clin Nutr. 1996;64(3 Suppl):524S–532S. doi: 10.1093/ajcn/64.3.524S. [DOI] [PubMed] [Google Scholar]
- 45.Bera TK. Bioelectrical impedance methods for noninvasive health monitoring: a review. J Med Eng. 2014;2014:381251. doi: 10.1155/2014/381251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis—part II: utilization in clinical practice. Clin Nutr. 2004;23(6):1430–1453. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Barrea L, Muscogiuri G, Laudisio D, Di Somma C, Salzano C, Pugliese G, de Alteriis G, Colao A, Savastano S. Phase angle: a possible biomarker to quantify inflammation in subjects with obesity and 25(OH)D deficiency. Nutrients. 2019;11(8):1747. doi: 10.3390/nu11081747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrea L, Macchia PE, Di Somma C, Napolitano M, Balato A, Falco A, Savanelli MC, Balato N, Colao A, Savastano S. Bioelectrical phase angle and psoriasis: a novel association with psoriasis severity, quality of life and metabolic syndrome. J Transl Med. 2016;14(1):130. doi: 10.1186/s12967-016-0889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill JO, Peters JC, Yang D, Sharp T, Kaler M, Abumrad NN, Greene HL. Thermogenesis in humans during overfeeding with medium-chain triglycerides. Metabolism. 1989;38(7):641–648. doi: 10.1016/0026-0495(89)90101-7. [DOI] [PubMed] [Google Scholar]
- 50.Manninen AH. Very-low-carbohydrate diets and preservation of muscle mass. Nutr Metab. 2006;3:9. doi: 10.1186/1743-7075-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller WC, Bryce GR, Conlee RK. Adaptations to a high-fat diet that increase exercise endurance in male rats. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(1):78–83. doi: 10.1152/jappl.1984.56.1.78. [DOI] [PubMed] [Google Scholar]
- 52.Miyamoto J, Ohue-Kitano R, Mukouyama H, Nishida A, Watanabe K, Igarashi M, Irie J, Tsujimoto G, Satoh-Asahara N, Itoh H, Kimura I. Ketone body receptor GPR43 regulates lipid metabolism under ketogenic conditions. Proc Natl Acad Sci USA. 2019;116(47):23813–23821. doi: 10.1073/pnas.1912573116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forsythe CE, Phinney SD, Fernandez ML, Quann EE, Wood RJ, Bibus DM, Kraemer WJ, Feinman RD, Volek JS. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008;43(1):65–77. doi: 10.1007/s11745-007-3132-7. [DOI] [PubMed] [Google Scholar]
- 54.Monda V, Polito R, Lovino A, Finaldi A, Valenzano A, Nigro E, et al. Short-term physiological effects of a very low-calorie ketogenic diet: effects on adiponectin levels and inflammatory states. Int J Mol Sci. 2020;21(9):3228. doi: 10.3390/ijms21093228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreno B, Bellido D, Sajoux I, Goday A, Saavedra D, Crujeiras AB, Casanueva FF. Comparison of a very low-calorie-ketogenic diet with a standard low-calorie diet in the treatment of obesity. Endocrine. 2014;47(3):793–805. doi: 10.1007/s12020-014-0192-3. [DOI] [PubMed] [Google Scholar]
- 56.Barrea L, Caprio M, Watanabe M, Cammarata G, Feraco A, Muscogiuri G, Verde L, Colao A, Savastano S. Could very low-calorie ketogenic diets turn off low grade inflammation in obesity? emerging evidence. Crit Rev Food Sci Nutr. 2022;4:1–17. doi: 10.1080/10408398.2022.2054935. [DOI] [PubMed] [Google Scholar]
- 57.Qi J, Gan L, Fang J, Zhang J, Yu X, Guo H, Cai D, Cui H, Gou L, Deng J, Wang Z, Zuo Z. Beta-hydroxybutyrate: a dual function molecular and immunological barrier function regulator. Front Immunol. 2022;13:805881. doi: 10.3389/fimmu.2022.805881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nimptsch K, Konigorski S, Pischon T. Diagnosis of obesity and use of obesity biomarkers in science and clinical medicine. Metabolism. 2019;92:61–70. doi: 10.1016/j.metabol.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Freemantle E, Vandal M, Tremblay Mercier J, Plourde M, Poirier J, Cunnane SC. Metabolic response to a ketogenic breakfast in the healthy elderly. J Nutr Health Aging. 2009;13(4):293–298. doi: 10.1007/s12603-009-0026-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.