Abstract
Helicobacter pylori-induced gastritis is an essential precursor lesion for the development of peptic ulcers or gastric adenocarcinoma. We demonstrate that nonresponsiveness to H. pylori SS1 infection is dominantly inherited in mice. F1 hybrid crosses between a nonresponder mouse and three responder strains all possessed the nonresponder phenotype. Secretion of interleukin-10 but not gamma interferon was associated with nonresponsiveness to infection.
Half of the world's population is infected with the stomach-dwelling bacterium Helicobacter pylori (18). Why some individuals develop symptomatic disease, such as peptic ulceration or gastric adenocarcinoma (4, 9), while most H. pylori-infected hosts present with asymptomatic gastritis is not fully understood. The various disease manifestations are clearly multifactorial, with bacterial and environmental factors being important (7, 8, 15), but host genetic factors also exert significant influence (2, 20).
We and others have shown previously that Helicobacter felis infection of inbred mice with different genetic backgrounds induces a dichotomy of inflammatory responses (12, 20, 21, 23). Most mice respond to H. felis infection with corpal gastritis, but some strains such as BALB/c and CBA do not develop inflammation; we term these mice nonresponders. We further dissected the basis of this nonresponsiveness using F1 hybrid mice, crossing three responder strains with nonresponder CBA/Ca mice. Infection of these mice with H. felis demonstrated that the nonresponder phenotype was dominantly inherited, and we hypothesized that “suppressive” mechanisms exist which can inhibit the normal inflammatory response induced by H. felis infection (23).
Here we report that dominant inheritance of nonresponsiveness to H. pylori infection also occurs in mice. Additionally, we examined the cellular response which associates with the nonresponder phenotype, using both H. pylori (the human pathogen) and H. felis, which induces greater inflammation in responder mice.
Mice of the parental strains CBA/Ca, C3H/He, C57BL/6, and SJL and the F1 hybrid strains CBA × C57BL/6, CBA × C3H/He, and SJL × CBA (maternal × paternal) were bred in the School of Microbiology and Immunology Animal Facility, University of New South Wales. Protocols involving animal experimentation were approved by the Animal Care and Ethics Committee at the University of New South Wales. Eight females of each strain were infected with H. pylori SS1 as previously described (17), with four noninfected controls. Six months postinfection, mice were sacrificed and gastritis was assessed histologically on blinded sections stained with hematoxylin and eosin (13). Each stomach was graded for activity (neutrophils) and mononuclear inflammatory cells in the antrum and body as follows: 1, mild multifocal; 2, mild widespread or moderate multifocal; 3, mild widespread and moderate multifocal or severe multifocal; 4, moderate widespread; 5, moderate widespread and severe multifocal; 6, severe widespread. The total number of lymphoid follicles and gland abscesses in each section was counted.
Examination revealed neutrophilic and mononuclear cell infiltration into the gastric tissue of infected C3H/He, C57BL/6, and SJL mice (Table 1). Infected mice of these strains also presented with gland abscesses, and SJL mice developed significant lymphoid aggregates. All these parameters were significantly increased from those of CBA/Ca and all F1 hybrid mice (Kruskal-Wallis; P < 0.05), which had either extremely mild or no gastritis (Table 1). There was no significant difference between CBA/Ca mice and the F1 hybrids. Thus, the inflammatory phenotype of all F1 strains was the same as that of the nonresponder CBA/Ca parent and different from those of their respective responder parents.
TABLE 1.
Histopathological gradings of parental and F1 hybrid mice with or without H. pylori infection.a
| Mouse strain | Infection status | Antrum
|
Body
|
No.
(mean ± SD) of:
|
|||
|---|---|---|---|---|---|---|---|
| Activity | CI | Activity | CI | Lymphoid aggregates | Gland abscesses | ||
| CBA | Control | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 0 |
| Infected | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 0 | |
| CBA × C3H/He | Control | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 0 |
| Infected | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 0 | |
| CBA × C57BL/6 | Control | 0 (0–1) | 0 (0) | 0 (0) | 0 (0) | 0 | 0 |
| Infected | 0 (0) | 0 (0–1) | 0 (0) | 0 (0–1) | 0 | 0 | |
| SJL × CBA | Control | 0 (0) | 0 (0–1) | 0 (0) | 0 (0) | 0 | 0 |
| Infected | 0 (0–1) | 0 (0) | 0 (0–2) | 0 (0–1) | 0.1 ± 0.3 | 0 | |
| C3H/He | Control | 0 (0–1) | 0 (0) | 0 (0) | 0.5 (0–1) | 0.2 ± 0.4 | 0 |
| Infected | 1 (0–3)* | 1 (0–2)* | 1 (0–3)* | 1 (0–2)* | 0.3 ± 0.5 | 0.7 ± 1.0* | |
| C57BL/6 | Control | 0 (0–1) | 0 (0) | 0 (0) | 0 (0) | 0 | 0 |
| Infected | 1 (0–3)* | 0.5 (0–2)* | 1 (0–4)* | 1 (0–3)* | 0 | 0.9 ± 1.4 | |
| SJL | Control | 0 (0) | 0 (0) | 0 (0–1) | 0 (0) | 0 | 0.2 ± 0.4 |
| Infected | 2 (0–3)* | 1 (0–4)* | 2.5 (1–3)* | 2 (1–3)* | 0.6 ± 0.7* | 1.1 ± 1.2* | |
Values for neutrophil infiltration (activity) and mononuclear cell infiltration (CI) are from a 6-point scale: 1, mild multifocal; 2, mild widespread or moderate multifocal; 3, mild widespread and moderate or severe multifocal; 4, moderate widespread; 5, moderate widespread and severe multifocal; 6, severe widespread. Data are nonparametric and are expressed as median values (ranges). *, significantly greater than value for noninfected controls (P < 0.05).
A Th1 immune response is responsible for cell-mediated immunity, is proinflammatory, and is marked by the production of cytokines including gamma interferon (IFN-γ) and interleukin-12 (IL-12). IL-12 is a key cytokine in the induction of a Th1 response leading to the production of IFN-γ. IFN-γ has been shown to be produced in the stomachs of both humans and mice infected with H. pylori (3, 11, 22) and is almost certainly a key factor in driving Helicobacter-induced gastritis. IL-10 can inhibit the production of IL-12 and thus downregulate the proinflammatory Th1-type response (10).
Thus, the IFN-γ and IL-10 cytokine response to Helicobacter infection was assessed in CBA/Ca, C57BL/6, and their (CBA × C57BL/6)F1 hybrid mice (Walter Eliza Hall Institute, Melbourne, Australia). Ten females of each strain were infected with H. felis or H. pylori or left uninfected for 3 months. Histopathological examination, as described above, confirmed that in contrast to the responder C57BL/6 mice, the nonresponder CBA/Ca and (CBA × C57BL/6)F1 hybrid mice developed no gastritis whether infected with H. felis or H. pylori (data not shown).
Spleen cell suspensions were depleted of red cells by hypotonic shock in water, and remaining splenocytes were cultured at 106/ml in complete medium (RPMI 1640 medium [Gibco BRL, Gaithersburg, Md.) with 10% fetal calf serum, 2 mM glutamine, 50 IU of penicillin/ml, 50 μg of streptomycin [Trace Biosciences, Castle Hill, New South Wales, Australia] per ml, and 2.5 μg of amphotericin B [Bristol-Myers Squibb, Princeton, N.J.] per ml) with or without H. pylori or H. felis lysate at 5 μg/ml. After 2 days of incubation at 37°C with 5% CO2, the supernatants were collected for the assessment of cytokines by standard enzyme-linked immunosorbent assay. Maxisorp immunoplates (Nunc, Roskilde, Denmark) were coated with anticytokine antibodies (Pharmingen, San Diego, Calif.) in bicarbonate buffer (pH 9.6). Wells were blocked with 1% (wt/vol) bovine serum albumin in phosphate-buffered saline. Culture supernatants or serial dilutions of recombinant IL-10 (Pharmingen) or IFN-γ (Sigma, St. Louis, Mo.) in complete medium were added to wells in duplicate. Biotinylated anticytokine antibodies (Pharmingen) in phosphate-buffered saline–bovine serum albumin were added, followed by streptavidin-alkaline phosphatase (Zymed, South San Francisco, Calif.). Color was developed by the addition of Sigma 104 phosphatase substrate tablets (Sigma) dissolved in diethanolamine buffer. Absorbance was read at 405 nm. A standard curve was plotted for each recombinant cytokine, from which cytokine levels in supernatants were determined.
Cytokine production was observed only following culture with Helicobacter antigens, and interestingly, the infectious status appeared to be irrelevant. There was virtually no difference in the cytokine patterns whether from uninfected or H. felis- or H. pylori-infected mice. This suggests that the host mounts an inherent response upon the first exposure to Helicobacter antigens, whether in the form of lysate or viable whole bacteria. This initial response apparently dictates the type of long-term immune response that develops.
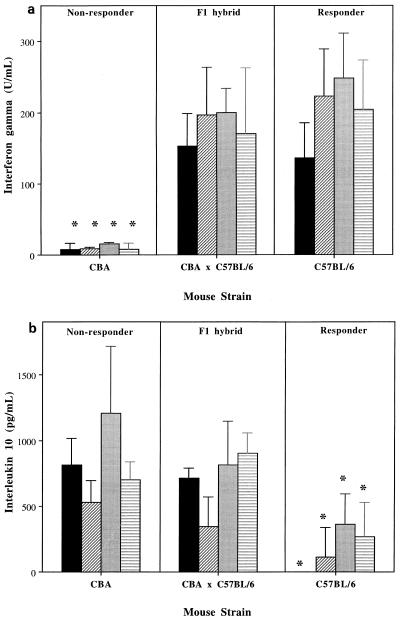
Nonresponder CBA/Ca mice produced very low levels of IFN-γ but large amounts of IL-10 (Fig. 1). Responder C57BL/6 mice produced high levels of IFN-γ but little or no IL-10. Nonresponder F1 hybrid CBA × C57BL/6 mice produced high levels of IFN-γ, significantly more than the CBA/Ca parent (one-way analysis of variance; P < 0.001) but not significantly more or less than the C57BL/6 mice. Inverse to this, F1 mice produced high levels of IL-10 like the CBA/Ca parent but significantly more than the C57BL/6 mice (P < 0.05). Thus, regarding cytokine production, the nonresponder F1 mice were true hybrids, secreting IL-10 like the CBA/Ca nonresponders, but surprisingly also produced IFN-γ levels indistinguishable from those of their responder C57BL/6 parents. IFN-γ would classically be expected to produce inflammation and has been shown to mask the detection of Th2 cytokines in Helicobacter-immunized mice (19). This raises the possibility that IL-10 can be a dominant factor in controlling Helicobacter-induced gastritis. The production of IFN-γ was apparently irrelevant with its proinflammatory effects clearly overridden, possibly by the inhibitory activity of IL-10.
FIG. 1.
Production of IFN-γ and IL-10 following in vitro stimulation of mice with Helicobacter antigens. Data shown represent the mean ± standard deviation for IFN-γ (a) and IL-10 (b) by enzyme-linked immunosorbent assay. All supernatants from cells cultured in medium alone were negative for both cytokines (not shown). Hf, H. felis; Hp, H. pylori. ∗, significantly less cytokine than the equivalent group of the other two strains (P < 0.05). ■, Uninfected and Hf, stimulated; ▨, Uninfected and Hp stimulated; ░⃞, Hf infected and Hf stimulated; ▤, Hp infected and Hp stimulated.
The same situation may exist in humans, with several studies showing the production of both IL-10 and IFN-γ in response to H. pylori (1, 14). Bodger et al. reported higher production of IL-10 in H. pylori-infected individuals than in noninfected persons and those with Helicobacter-negative gastritis. IL-10 was associated with the severity of inflammation; the authors proposed that the increased secretion of this cytokine may be part of the host's attempt to control the inflammation (6).
Evidence from knockout mice deficient in IL-10 also suggests a role for this cytokine in controlling the inflammatory response to infection with Helicobacter hepaticus (16) and H. felis (5). There may be unknown consequences of the complete lack of IL-10 for immune development in these mice; thus, an advantage of the F1 hybrid model is that the mice used are immunocompetent. The demonstration of an association of cytokine profile with inflammatory response supports the knockout mouse data and allows greater confidence in concluding that IL-10 is an important factor controlling Helicobacter-induced gastritis.
In summary, we have confirmed that host genetic mechanisms that influence inflammation in H. felis-infected mice also control responses to the human pathogen H. pylori. Using these models, we have demonstrated that production of IL-10 but not of IFN-γ is associated with the nonresponsiveness of certain strains of mice to Helicobacter infection. Determining the basis of unresponsiveness will allow us to better understand H. pylori pathogenesis and may provide vital information regarding individuals at risk of developing the more severe complications of an H. pylori infection.
Acknowledgments
This work was partly funded by the National Health and Medical Research Council and CSL Ltd., Melbourne, Australia.
We thank Nathan Moss for help with statistical analyses.
REFERENCES
- 1.Aihara M, Imagawa K, Funakoshi Y, Ohmoto Y, Kikuchi M. Effects of rebamipide on production of several cytokines by human peripheral blood mononuclear cells. Dig Dis Sci. 1998;43:S160–S166. [PubMed] [Google Scholar]
- 2.Azuma T, Ito S, Sato F, Yamazaki Y, Miyaji H, Ito Y, Suto H, Kuriyama M, Kato T, Kohli Y. The role of the HLA-DQA1 gene in resistance to atrophic gastritis and gastric adenocarcinoma induced by Helicobacter pyloriinfection. Cancer. 1998;82:1013–1018. doi: 10.1002/(sici)1097-0142(19980315)82:6<1013::aid-cncr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Bamford K B, Fan X J, Crowe S E, Leary J F, Gourley W K, Luthra G K, Brooks E G, Graham D Y, Reyes V E, Ernst P B. Lymphocytes in the human gastric mucosa during Helicobacter pylorihave a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 4.Bell G D, Bate C M, Axon A T R, Tildesley G, Martin J L, Taylor M D, Richardson P D I. Symptomatic and endoscopic duodenal ulcer relapse rates 12 months following Helicobacter pylorieradication treatment with omeprazole and amoxycillin with or without metronidazole. Aliment Pharmacol Ther. 1996;10:637–644. doi: 10.1046/j.1365-2036.1996.36178000.x. [DOI] [PubMed] [Google Scholar]
- 5.Berg D J, Lynch N A, Lynch R G, Lauricella D M. Rapid development of severe hyperplastic gastritis with gastric epithelial dedifferentiation in Helicobacter felis-infected IL-10(−/−) mice. Am J Pathol. 1998;152:1377–1386. [PMC free article] [PubMed] [Google Scholar]
- 6.Bodger K, Wyatt J I, Heatley R V. Gastric mucosal secretion of interleukin-10: relations to histopathology, Helicobacter pyloristatus, and tumour necrosis factor-α secretion. Gut. 1997;40:739–744. doi: 10.1136/gut.40.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Censini S, Lange C, Xiang Z Y, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. Cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen A J, Roe F J C. Evaluation of the aetiological role of dietary salt exposure in gastric and other cancers in humans. Food Chem Toxicol. 1997;35:271–293. doi: 10.1016/s0278-6915(96)00114-7. [DOI] [PubMed] [Google Scholar]
- 9.Correa P, Miller M. Helicobacter pyloriand gastric atrophy—cancer paradoxes. J Natl Cancer Inst. 1995;87:1731–1732. doi: 10.1093/jnci/87.23.1731. [DOI] [PubMed] [Google Scholar]
- 10.D'Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchiere G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delios M M, Manghetti M, Decarli M, Costa F, Baldari C T, Burroni D, Telford J L, Romagnani S, Delprete G. T helper 1 effector cells specific for Helicobacter pyloriin the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 12.Dey A, Yokota K, Kosavashi K, Oguma K, Hirai Y, Akagi T. Antibody and cytokine responses in Helicobacter pylori-infected various mouse strains. Acta Med Okayama. 1998;52:41–48. doi: 10.18926/AMO/31337. [DOI] [PubMed] [Google Scholar]
- 13.Eaton K A, Radin M J, Krakowka S. An animal model of gastric ulcer due to bacterial gastritis in mice. Vet Pathol. 1995;32:489–497. doi: 10.1177/030098589503200506. [DOI] [PubMed] [Google Scholar]
- 14.Karttunen R A, Karttunen T J, Yousfi M M, Elzimaity H, Graham D Y, Elzaatari F. Expression of mRNA for interferon-gamma, interleukin-10, and interleukin-12 (p40) in normal gastric mucosa and in mucosa infected with Helicobacter pylori. Scand J Gastroenterol. 1997;32:22–27. doi: 10.3109/00365529709025058. [DOI] [PubMed] [Google Scholar]
- 15.Kato I, Tominaga S, Matsumoto K. A prospective study of stomach cancer among a rural Japanese population: a 6-year survey. Jpn J Cancer Res. 1992;83:568–575. doi: 10.1111/j.1349-7006.1992.tb00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kullberg M C, Ward J M, Gorelick P L, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticustriggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee A, O'Rourke J, De Ungria M C, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pyloriinfection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell H M. The epidemiology of Helicobacter pylori. Bailliere's Clin Infect Dis. 1997;4:257–281. [Google Scholar]
- 19.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-medicated immune responses display a predominant TH1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 20.Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felisinfection of inbred and congenic mouse strains. Infect Immun. 1996;64:238–245. doi: 10.1128/iai.64.1.238-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakagami T, Dixon M, O'Rourke J, Howlett R, Alderuccio F, Vella J, Shimoyama T, Lee A. Atrophic gastric changes in both Helicobacter felis and Helicobacter pyloriinfected mice are host dependent and separate from antral gastritis. Gut. 1996;39:639–648. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawai N, Kita M, Kodama T, Tanahashi T, Yamaoka Y, Tagawa Y-I, Iwakura Y, Imanishi J. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect Immun. 1999;67:279–285. doi: 10.1128/iai.67.1.279-285.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutton P, Wilson J, Genta R, Torrey D, Savinainen A, Pappo J, Lee A. A genetic basis for atrophy: dominant nonresponsiveness and helicobacter induced gastritis in F-1 hybrid mice. Gut. 1999;45:335–340. doi: 10.1136/gut.45.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]