Abstract
Context:
The incidental detection of localized renal masses has been rising steadily, but a significant proportion of these tumors are benign or indolent and, in most cases, do not require treatment. At the present time, a majority of patients with an incidentally detected renal tumor undergo treatment for the presumption of cancer, leading to a significant number of unnecessary surgical interventions that can result in complications including loss of renal function. Thus, there exists a clinical need for improved tools to aid in the pretreatment characterization of renal tumors to inform patient management.
Objective:
To systematically review the evidence on noninvasive, imaging-based tools for solid renal mass characterization.
Evidence acquisition:
The MEDLINE database was systematically searched for relevant studies on novel imaging techniques and interpretative tools for the characterization of solid renal masses, published in the past 10 yr.
Evidence synthesis:
Over the past decade, several novel imaging tools have offered promise for the improved characterization of indeterminate renal masses. Technologies of particular note include multiparametric magnetic resonance imaging of the kidney, molecular imaging with targeted radiopharmaceutical agents, and use of radiomics as well as artificial intelligence to enhance the interpretation of imaging studies. Among these, 99mTc-sestamibi single photon emission computed tomography/computed tomography (CT) for the identification of benign renal oncocytomas and hybrid oncocytic chromophobe tumors, and positron emission tomography/CT imaging with radiolabeled girentuximab for the identification of clear cell renal cell carcinoma, are likely to be closest to implementation in clinical practice.
Conclusions:
A number of novel imaging tools stand poised to aid in the noninvasive characterization of indeterminate renal masses. In the future, these tools may aid in patient management by providing a comprehensive virtual biopsy, complete with information on tumor histology, underlying molecular abnormalities, and ultimately disease prognosis.
Patient summary:
Not all renal tumors require treatment, as a significant proportion are either benign or have limited metastatic potential. Several innovative imaging tools have shown promise for their ability to improve the characterization of renal tumors and provide guidance in terms of patient management.
Keywords: Renal cell carcinoma, Kidney cancer, Multiparametric magnetic, resonance imaging, Girentuximab, Radiomics, Virtual biopsy, 99mTc-sestamibi, PET, SPECT, Machine learning, Artificial intelligence
1. Introduction
In recent decades, there has been a steady increase in the incidental detection of small renal masses (SRMs) [1]. With up to 70% of SRMs ultimately being diagnosed as renal cell carcinoma (RCC), this trend has led to an increase in the overall prevalence of this malignancy. Furthermore, because the majority of incidental cases of RCC are localized to the kidney, there has been an overall downward stage migration associated with this disease [2]. Unfortunately, this has not translated to improved rates of cancer-specific survival, suggesting that many newly diagnosed cases of SRMs are benign or of low malignant potential and likely do not require treatment [3].
The increasing detection of SRMs has also led to a rise in unnecessary treatment of benign tumors of the kidney, such as oncocytomas and angiomyolipomas (AMLs). This is because these tumors can be difficult to distinguish from renal cancers on the basis of conventional imaging modalities such as ultrasound, computed tomography (CT), and magnetic imaging resonance (MRI) [4,5]. It is estimated that ~25% of renal masses of <4 cm are preoperatively misclassified as malignant and are needlessly surgically excised [6,7]. A considerable number of patients could therefore be spared from unnecessary interventions and the associated risk of complications or loss of renal function.
Currently, renal mass biopsy (RMB) is considered the gold standard for determining the presurgical histology of an SRM. Adoption of this diagnostic procedure, however, has been hampered by concerns over a high nondiagnostic rate of 10–15% and 10% erroneous diagnoses due to tumor heterogeneity [8–11]. Additionally, RMB is not always feasible due to the anatomic tumor location, and it remains an intrinsically invasive procedure despite a relatively low complication rate [8,9]. Thus, the vast majority of SRMs are removed surgically without prior knowledge of the tumor’s underlying histology.
Considering the issues outlined above, there remains a significant need for improvements in the pretreatment characterization of SRMs. Several novel imaging techniques as well as methods of image processing offer promise to noninvasively determine the histology of SRMs—a so-called “virtual biopsy”. Technologies of particular note include multiparametric MRI (mpMRI) of the kidney, molecular imaging with radiopharmaceutical agents, and radiomics as well as artificial intelligence (AI) to enhance image interpretation. In this collaborative review, we provide a summary of these novel and emerging imaging tools for the noninvasive characterization of solid renal masses with an emphasis on topics that are most likely to gain traction in clinical practice.
2. Evidence acquisition
With the consensus of the authors, three main areas of research focusing on improved characterization of solid renal masses were deemed applicable and subsequently explored further: mpMRI of the kidney, molecular imaging with radiopharmaceutical agents, and advanced image processing and interpretation methods (ie, radiomics and AI). A search of the MEDLINE database was conducted to identify original studies related to these three topics. Keywords included “kidney neoplasm”, “renal tumor”, “renal mass”, “magnetic resonance imaging”, “molecular imaging”, “positron emission tomography”, “single photon emission tomography”, “radiomics”, “machine learning”, and “artificial intelligence” along with derivative terms (Supplementary material). Articles were included up to July 1, 2021, and the initial search was limited to studies and investigations published in the past 10 yrs. Articles on the characterization of metastatic disease or response to therapy were excluded as they fall outside of the scope of the current review. The retrieved articles were screened by title and abstract using Rayyan (Rayyan Systems Inc., Cambridge, MA, USA). References cited in the retrieved articles were subsequently searched to identify additional manuscripts of interest that were not included in the initial search to ensure completeness. Figure 1 shows the flowchart of study selection according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses criteria. A total of 149 articles underwent rigorous full-text review by the first author. Articles with the highest level of evidence were evaluated and selected with the consensus of the other authors.
Fig. 1 –

PRISMA flowchart showing study selection. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses.
3. Evidence synthesis
3.1. Multiparametric MRI of the kidney
Currently, contrast-enhanced CT (CECT) is the most commonly used imaging modality for assessing renal lesions. Enhancement on CT is indicative of a solid renal mass; however, little else about the lesion can reliably be determined on this basis. Although there are some features on CECT that can be suggestive of a tumor’s histology, these findings typically lack the specificity necessary to make informed clinical decisions. For example, a renal tumor with a central stellate region of relatively low level of enhancement is suggestive of an oncocytoma, but this can easily be confused with central necrosis of an RCC. Similarly, the timing or pattern of contrast enhancement on multiphase CECT has been explored as a means of distinguishing between RCC subtypes, but due the considerable overlap of these features between the various renal tumor histologies this has seen little in the way of clinical adoption [4,5,12–16].
MRI offers several advantages over CT for the characterization of renal masses. First, MRI offers improved contrast resolution and eliminates concerns regarding ionizing radiation. More importantly, however, various MRI sequences can be combined to form a multiparametric study that can more reliably probe the underlying histology of a renal mass [4]. mpMRI combines anatomic information from the T1- and T2-weighted imaging sequences with other MRI sequences, such as chemical-shift imaging to display micro- and macroscopic fat, diffusion-weighted imaging (DWI) to assess tumor cell density, and dynamic contrast enhancement to display tumor vascularity [17]. Limitations of mpMRI include its relatively higher costs than CT and significantly longer examination times [18].
Contemporary mpMRI protocols can offer anatomic insight and provide qualitative, semiquantitative, and fully quantitative imaging biomarkers that correlate with histological subtype, tumor grade, and clinical behavior. In one study, Sasiwimonphan et al [19] reported a sensitivity of 73%, a specificity of 99%, and an accuracy of 96% for the differentiation of fat-poor AMLs (fpAMLs) from RCC using a T2 tumor to renal cortex signal intensity ratio of <0.9 combined with either an arterial-to-delayed enhancement ratio of >1.5 or a signal intensity index of >20% along with a T1 signal intensity ratio of >1.5. Similar observations have been made using mpMRI to diagnose fpAMLs, and a recent systematic review reported a sensitivity of 83% and a specificity of 93% for this diagnosis [20].
Kay et al [21] developed and implemented an mpMRI protocol with a predefined diagnostic algorithm in which several imaging sequences were consecutively analyzed to predict RCC histology among SRMs (Fig. 2). In this study, seven reviewers independently reviewed mpMRI images of 109 renal masses using this algorithm as a guideline for the most likely histopathological diagnosis, but were allowed to over-rule the algorithm ad libitum. Using this qualitative diagnostic framework, the authors observed a sensitivity of 85% and a specificity of 76% for the prediction of clear cell RCC (ccRCC). Similarly, the authors reported a sensitivity of 80% and a specificity of 94% for the prediction of papillary RCC (pRCC). Performance of the reviewers was poorest with regard to the diagnosis of benign renal masses and chromophobe RCC (chrRCC). Overall, the average accuracy of the reviewers was 65% when using the algorithm but only 43% when the algorithm was not followed.
Fig. 2 –
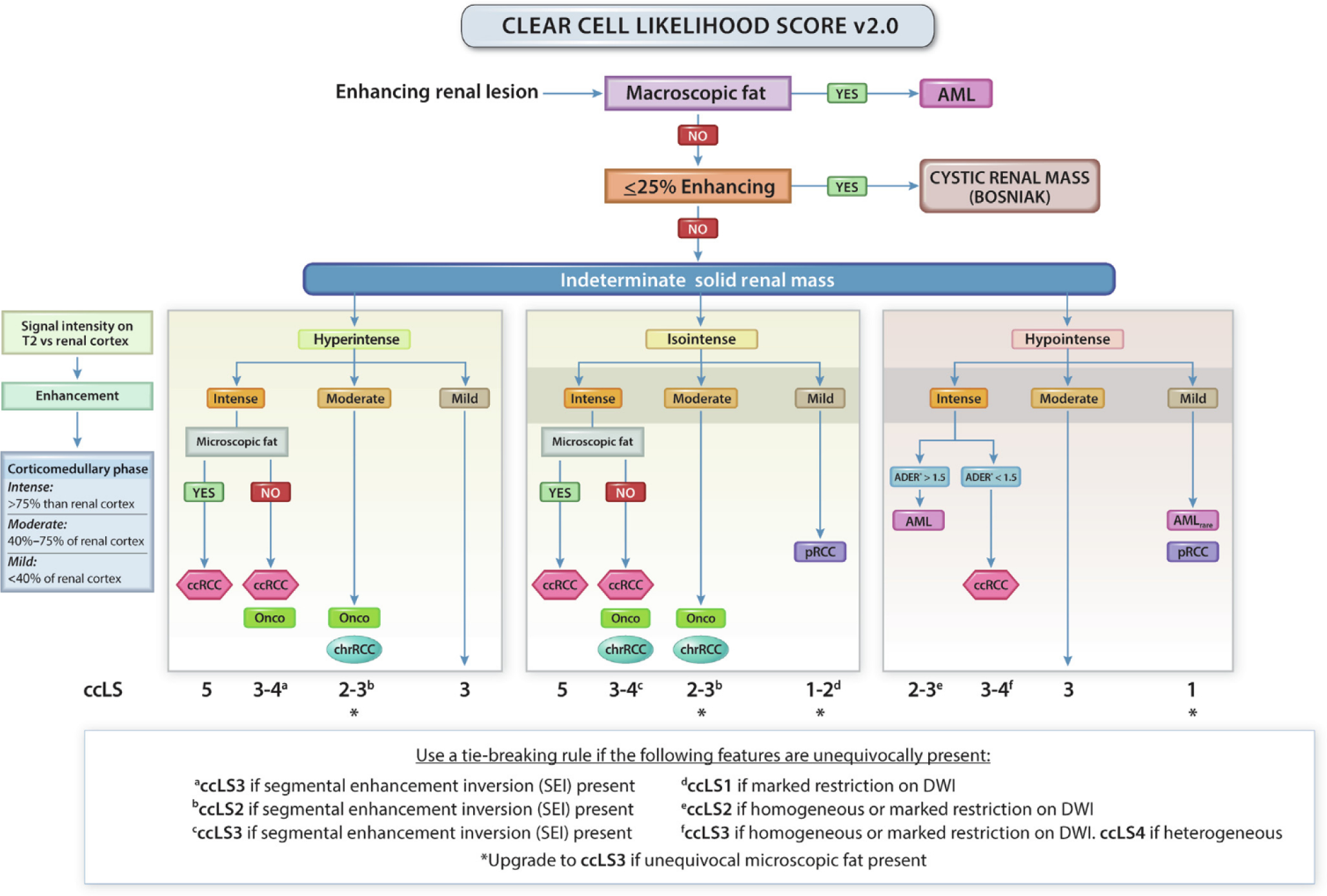
Clear cell likelihood score (ccLS) algorithm version 2.0. Presence of enhancement in ≥25% of the mass volume and absence of macroscopic fat represent eligibility criteria for the use of the ccLS algorithm. Signal intensity on T2-weighted single-shot fast/turbo spin echo imaging (T2) relative to renal cortex, enhancement during the corticomedullary phase relative to renal cortex, and presence of microscopic fat are the major criteria evaluated in every mass. Restriction in DWI, segmental enhancement inversion, and arterial-to-delayed enhancement ratio (ADER) are ancillary findings, which are assessed when indicated in the algorithm. AML = angiomyolipoma; ccRCC = clear cell renal cell carcinoma; chrRCC = chromophobe renal cell carcinoma; DWI = diffusion weighted imaging; Onco = oncocytoma; pRCC = papillary renal cell carcinoma. (Figure was reproduced with permission from Pedrosa and Cadeddu [120].)
Using this same algorithm, Canvasser et al [22] developed a subjective scale, known as the the clear cell likelihood score (ccLS), for the prediction of ccRCC histology. This scoring system assigns a given SRM a score of 1 to 5 (very unlikely to very likley) to indicate the reader’s level of certainty that the tumor represents a ccRCC. Using this system, the authors found that scores ≥4 had a sensitivity of 78% and a specificity of 80%, while scores ≥3 had values of 95% and 58%, respectively. Steinberg et al [23] reported similar results after prospectively assigning ccLSs to 454 renal masses. In this study, the authors found a positive predictive value of 91% for ccLS ≥4 and a negative predictive value of 94% for ccLS ≤2. Although these results do not provide information on malignant versus nonmalignant tumor histology or tumor aggressiveness, they could inform subsequent SRM management. For example, Canvasser and coworkers [22] found that an approach where ccLS 1–2 SRMs would undergo active surveillance, equivocal ccLS 3 lesions would be biopsied, and ccLS 4–5 tumors would be treated surgically, would decrease the rate of RMB to 20% while limiting the rate of unnecessary treatment of oncocytomas and AMLs to 4.5% and 1.7% of the cohort, respectivly.
Quantitative analysis of MRI acquisitions have also shown promise in renal mass characterization. In a recent systematic review, the use of apparent diffusion coefficient values derived from DWI sequences was found to have moderate accuracy for the prediction of malignant versus benign histology with pooled values of sensitivity and specificity of 86% and 78%, respectively [24]. Moreover, DWI can aid in the distinction of high-grade from low-grade ccRCC with moderate test accuracy, but is not able to accurately differentiate between RCC subtypes [24,25]. Similarly, preliminary data in 34 patients using arterial spin labeled MRI, a technique to quantitatively assess tissue perfusion without the use of an exogenous contrast agent, revealed higher tumor perfusion in oncocytomas than in RCC, and lower perfusion in pRCC than in non-pRCC, which could contribute to the characterization of indeterminate renal masses [26].
3.2. Molecular imaging
Molecular imaging is a branch of radiology that provides for the in vivo visualization and quantification of various normal and diseased processes within the body at the cellular and molecular levels. Most commonly, this involves the use of a radiopharmaceutical agent that binds to or is taken up by a target tissue of interest. This differs from conventional imaging modalities such as CT or ultrasound, which more simply allow for the visualization of physical structures within the body based on size and density. A molecular imaging test designed to target a cellular process specific to a renal tumor subtype (eg, an overexpressed protein or upregulated metabolic pathway) could not only allow for the detection of sites of disease, but, in the case of SRM imaging, possibly also provide critical information on a given tumor’s underlying histology. To date, a handful of radiolabeled molecular probes have been studied with this application in mind. Two radiotracers in particular–99mTc-sesmtamibi and radiolabeled girentuximab–stand poised to be incorporated into routine clinical practice for this purpose in the near future.
3.2.1. Single photon emission computed tomography/CT with 99mTc-sestamibi
A promising approach for the characterization of indeterminate renal masses involves the use of 99mTc-sestamibi, which is a lipophilic cationic mitochondrial imaging agent that is currently widely used for myocardial and parathyroid imaging [27,28]; 99mTc-sestamibi accumulates in cells with high mitochondrial content and low multidrug resistance pump (MDR) expression [27]. A significantly increased mitochondrial mass and decreased MDR expression are characteristic of renal oncocytomas. The high mitochondrial content found within oncocytomas is the result of pathogenic mutations leading to the accumulation of respiration defective mitochondria [29]. Conversely, ccRCCs are relatively devoid of mitochondria and have high MDR expression [30]. Other RCC subtypes tend to have varying amounts of mitochondria, but MDR activity outweighs their presence [27]. On this basis, it was first hypothesized that single photon emission computed tomography (SPECT) imaging with 99mTc-sestamibi can aid in the discernment of benign versus malignant renal tumors (Fig. 3).
Fig. 3 –

Differentiation of renal tumors on the basis of 99mTc-sestamibi uptake. (A) Axial contrast-enhanced CT, (B) axial 99mTc-sestamibi SPECT, and (C) axial 99mTc-sestamibi SPECT/CT images of a patient with a right renal mass (red arrow heads). Uptake in the mass is heterogeneous but overall similar to kidney background. There is relative photopenia in the less enhancing central portion of the tumor. The patient underwent percutaneous biopsy that demonstrated an oncocytic neoplasm and was then enrolled in active surveillance. (D) Axial contrast-enhanced CT, (E) axial 99mTc-sestamibi SPECT, and (F) axial 99mTc-sestamibi SPECT/CT images of a patient with a right renal mass (red arrow heads). There is no appreciable radiotracer uptake in the tumor. The patient underwent a partial nephrectomy with surgical pathology revealing a clear cell renal cell carcinoma. CT = computed tomography; SPECT = single photon emission computed tomography.
In a first report on this concept, Gormley et al [28] showed that a single large renal oncocytoma displayed uptake of the 99mTc-sestamibi radiotracer, whereas five other renal tumors were devoid of radiotracer signal. Next, Rowe et al [30] showed that oncocytomas exhibited 99mTc-sestamibi uptake on SPECT/CT at or near the renal background level, whereas RCCs appeared as photopenic defects. In a prospective study of 99mTc-sestamibi SPECT/CT that included 50 patients with a solitary renal mass imaged prior to surgery, Gorin et al [31] reported sensitivity of 87.5% and specificity of 95.2% for the diagnosis of renal oncocytomas and hybrid oncocytic/chromophobe tumors (HOCTs). Two false-positive tumors were identified as eosinophilic variants of chrRCC, which are typically indolent [32]. These findings were subsequently externally validated by Tzortzakakis et al [33] who studied 27 patients harboring 31 tumors, and found that 11 of 12 (91.6%) oncocytomas and three of three (100%) HOCTs were correctly identified with 99mTc-sestamibi SPECT/CT. They noted two false positives, one pRCC with slight uptake, and one AML. Additional validation of this imaging modality has recently been performed by Sistani et al [34] and Asi et al [35].
The implementation of 99mTc-sestamibi SPECT/CT in the diagnostic workup of indeterminate renal tumors could potentially spare a substantial number of patients from unnecessary interventions. A recent decision analysis that simulated lifetime health utilities and direct medical costs from a US health payer perspective suggested that the use of 99mTc-sestamibi SPECT/CT as the initial diagnostic test for newly diagnosed SRMs followed by RMB to confirm benign or indolent tumors would be a cost-effective approach for the management of these tumors compared with RMB alone or upfront surgery [36]. Moreover, in this modeling study, the combination of 99mTc-sestamibi SPECT/CT followed by RMB led to the fewest treated benign/indolent tumors with an acceptably low number of untreated malignant SRMs.
The findings presented above, combined with the fact that 99mTc-sestamibi is already approved worldwide for use in myocardial and parathyroid imaging, make this imaging test poised for widespread clinical adoption in the near term. Moreover, SPECT/CT is a relatively inexpensive imaging modality and access to these scanners is relatively commonplace, making the adoption of this imaging technique quite feasible.
3.2.2. Positron emission tomography/CT with radiolabeled girentuximab
A second molecular imaging tool of particular promise for the characterization of solid renal masses utilizes one of several radiolabeled forms of the humanized monoclonal antibody girentuximab. Girentuximab, formerly known as the G250 antibody, selectively binds with high affinity to the cell surface zinc metalloenzyme carbonic anhydrase IX (CAIX). Under normal physiological conditions, CAIX is upregulated in response to hypoxic conditions and serves to catalyze the reversible hydration of carbon dioxide. CAIX is overexpressed in approximately 95% of ccRCCs, following the loss or inactivation of the Von Hippel-Lindau (VHL) tumor suppressor gene, which is a hallmark event in the pathogenesis of this tumor [37,38]. Of note, CAIX is also overexpressed in the recently described clear cell papillary RCC, which represents 2–4% of renal cortical tumors and follows a more indolent disease course [39].
Girentuximab can be labeled with a number of different radionuclides and has been studied as both a diagnostic and a therapeutic agent for ccRCC [40]. In 2007, Divgi et al [37] performed a prospective study of 124I-girentuximab positron emission tomography (PET)/CT, in which 26 patients with an indeterminate renal mass were imaged prior to surgery. Remarkably, this imaging test correctly identified 15 of 16 (93.8%) ccRCCs without any false-positive results. Following this successful pilot study, the same group conducted the prospective, multicenter, REDECT trial, comparing the accuracy of 124I-girentuximab PET/CT versus conventional CECT for the diagnosis of ccRCC among 195 patients with an indeterminate renal mass scheduled for surgery [41]. In this positive trial, 124I-girentuximab PET/CT correctly identified cases of ccRCCs with reported sensitivity and specificity values of 86.2% and 85.9%, as compared to 75.5% and 46.8% for conventional CECT.
Following the results of the REDECT trial, a confirmatory study of PET imaging with girentuximab was requested by the US Food and Drug Administration prior to the regulatory approval of this novel imaging agent [42]. This second multicenter prospective study, known as the ZIRCON trial, is currently underway at 30 sites worldwide (ClinicalTrials.gov identifier NCT03849118). It is worth noting that in this trial, an 89Zr-labeled form of girentuximab is being studied in place of the 124I-labeled antibody (Fig. 4). This is because studies have suggested that labeling girentuximab with 89Zr leads to higher tumor uptake and retention, and perhaps more sensitive detection of ccRCC than the 124I-labeled radiotracer [43,44]. This observed difference stems from the fact that 89Zr becomes “charge trapped” in the cytoplasm of tumor cells after internalization and breakdown of the antibody. In contrast, free 124I is released into circulation. As a result, imaging with 89Zr-girentuximab PET/CT results in a superior signal-to-noise ratio than the 124I-labeled version of the antibody. SPECT/CT in combination with girentuximab has also been investigated. Muselaers et al [45] found a positive predictive value of 94% for the detection of ccRCC with 111In-girentuximab SPECT/CT in a study that included 22 patients. However, PET offers distinct advantages over SPECT in terms of superior image resolution and shorter image acquisition time [46].
Fig. 4 –

Differentiation of a renal tumor on the basis of 89Zr-girentuximab uptake. (A) Axial contrast-enhanced CT, (B) axial 89Zr-girentuximab PET, and (C) axial fused 89Zr-girentuximab PET/CT images in a patient with a left renal mass (red arrow heads). There is clear uptake in the mass, with very little background uptake. The patient underwent a partial nephrectomy with surgical pathology revealing a clear cell renal cell carcinoma. CT = computed tomography; PET = positron emission tomography.
Despite these encouraging data, it is worth noting that there are several factors that may present a barrier to the widespread clinical adoption of intact monoclonal antibodies as molecular imaging probes. For example, the long residence of antibodies in the bloodstream is associated with relatively high background levels and requires a several-day interval from the time of injection to image acquisition [47]. In the case of girentuximab, this interval is 5–7 d, which introduces logistical hurdles to test administration. In contrast, 99mTc-sestamibi SPECT/CT imaging is performed only ~75 min after the administration of this small molecule radiotracer [30]. The use of low-molecular-weight molecular imaging agents targeting CAIX could overcome this limitation and are currently being investigated [48]. Future endeavors may also focus on the development of dual radiotracers employed in a single imaging acquisition. For instance, a dual-radiotracer SPECT imaging test with 99mTc-sestamibi and 111In-girentuximab could potentially classify an indeterminate renal mass as oncocytoma/HOCT, ccRCC, or other tumor histology with a single imaging study.
3.2.3. Other radiotracers
3.2.3.1. PET/CT with 18F-FDG.
The most widely used PET radiotracer in the field of oncology is 2-deoxy-2-[18F] fluoro-D-glucose (18F-FDG), which targets malignant cells exhibiting cytosolic aerobic glycolysis. However, this radiotracer has only a limited role in the evaluation of primary renal masses due to its physiological renal excretion and high background activity in the kidney. For example, a meta-analysis by Wang et al [49] reported pooled sensitivity of only 62% for 18F-FDG PET for renal lesion detection. Furthermore, this sensitivity does not seem to improve significantly with the use of modern PET/CT [49–51]. In addition, 18F-FDG is largely unable to aid in distinguishing between the various renal tumor histologies due to the fact that this radiotracer is nonspecifically taken up by any tumor displaying an increase in aerobic glycolysis. However, some studies have shown that semiquantitative and quantitative analyses of 18F-FDG PET uptake are associated with the presence of higher nuclear grade or sarcomatoid features, and thus prognosis [51–57].
3.2.3.2. Prostate-specific membrane antigen–targeted PET/CT.
Despite the specificity implied by its name, the cell surface protein prostate-specific membrane antigen (PSMA) is expressed in the neovasculature of a number of solid tumors, including RCC. Although a majority of studies evaluating PSMA-targeted imaging of RCC have focused on patients with metastatic disease [58–62], some have investigated the clinical utility of this class of imaging agents for the noninvasive characterization of solid renal masses [63,64]. For example, using quantitative 68Ga-PSMA-11 PET/CT kinetics, Golan et al [63] demonstrated increased radiotracer uptake and slower washout in malignant versus benign renal tumors. Moreover, Gao et al [64] found that increased 68Ga-PSMA-11 uptake was associated with the presence of higher nuclear grade and adverse pathological features, including tumor necrosis and sarcomatoid or rhabdoid features. However, the diagnostic performance of this imaging test for the evaluation of primary renal masses remains limited due to poor tumor-to-background ratios and is likely more applicable for imaging of metastatic lesions or evaluation of response to systemic therapy.
3.2.3.3. PET/CT with 11C-acetate.
PET/CT with 11-labeled acetate targets rapidly dividing cancer cells due to its incorporation into cell membrane precursors [65]. It has been found to be potentially useful for the differentiation of AMLs from RCC when 11-acetate PET/CT is combined with a separately performed 18F-FDG PET/CT study, with sensitivity of 94% and specificity of 98% [66]. Markedly increased 11C-acetate uptake was observed in the AMLs, whereas all were 18F-FDG negative. In the same study, the authors found that all chrRCC showed 11-acetate uptake but no 18F-FDG uptake, whereas the inverse pattern was observed in pRCC [66]. However, the need for two separate PET studies and the short half-life of 11C limit the clinical applicability of this imaging test.
A summary of novel imaging tests for renal mass characterization is provided in Table 1. Multiple other radiotracers and ligands are under preclinical investigation, but have been studied only in a few patients and are far from translation into clinical practice.
Table 1 –
Summary of novel imaging tests for renal mass characterization.
| Imaging method | Potential use | Key features | Limitations |
|---|---|---|---|
|
| |||
| Multiparametric MRI | Differentiation of tumor histology | Improved contrast resolution compared with contrast-enhanced CT | Longer examination times compared with contrast-enhanced CT |
| No concern for ionizing radiation | High cost | ||
| Use with the clear cell likelihood score (ccLS) predicts the likelihood of clear cell RCC histology | |||
| 99mTc-sestamibi SPECT/CT | Diagnosis of oncocytomas and hybrid oncocytic/chromophobe tumors | Accumulates in cells with high mitochondrial content and low multidrug resistance pump expression | Low spatial resolution makes it difficult to image small and entirely intrarenal tumors |
| Readily available and inexpensive | |||
| 124I or 89Zr girentuximab PET/CT | Diagnosis of clear cell RCC | Targets carbonic anhydrase IX, which is a sensitive and specific marker for clear cell RCC | Long interval between radiotracer administration and PET/CT scan (3–7 d) |
| Better contrast and spatial resolution than SPECT/CT | Lacks regulatory approval | ||
| High cost | |||
| 18F-FDG PET/CT | Nuclear grade prediction | Targets malignant cells exhibiting aerobic glycolysis | High background activity in the kidney limits the potential for primary renal mass characterization |
| Readily available | |||
| PSMA-targeted 68Ga-PSMA-11 and 18F-DCFPyL PET/CT | Differentiation of benign from malignant histology | PSMA is expressed in the neovasculature of most solid malignant tumors | High background activity in the kidney limits the potential for primary renal mass characterization |
| Prediction of adverse pathological features | PSMA-targeted PET is readily available in most countries | ||
| 11C-acetate PET/CT | Differentiation of AML vs RCC | Targets rapidly dividing cancer cells due to its incorporation into cell membrane precursors | Need for use in combination with a separately performed 18F-FDG PET limits clinical implementation |
AML = angiomyolipoma; CT = computed tomography; FDG = 2-deoxy-2-fluoro-D-glucose; MRI = magnetic resonance imaging; PET = positron emission tomography; PSMA = prostate-specific membrane antigen; RCC = renal cell carcinoma; SPECT = single photon emission computed tomography.
Imaging methods are listed in the order of mention in the article’s main text.
3.3. Radiomics and AI
Analytic methods for more in-depth analysis of medical images have been a topic of increasing interest. As a broad concept, radiomics involves high-throughput extraction of quantitative information from medical images. Briefly, after imaging processing and standardization, a region or volume of interest is identified and subsequently segmented either manually or semiautomatically [67]. Radiomic features are then generated from the pixel/voxel data within these segmented volumes. While many radiomic features have been described, the Image Biomarker Standardization Initiative recently standardized a set of 169 radiomic features, including morphological characteristics, features related to intensity, and texture features derived from gray-level and neighborhood gray tone/level matrixes [67]. Although these radiomic features were not specifically developed for renal tumors, standardization of radiomic features might enable verification of radiomic software and improve the reproducibility of these studies.
Predefined quantitative radiomic features can be used to predict histological tumor characteristics. Alternatively, these can be fed into machine learning algorithms, a type of AI that can parse data, learn from data, and make decisions based on what has been learned. Machine learning models can combine radiomic features with demographic, histological, genomic, or transcriptomic features to predict patient outcomes. In turn, deep learning can be regarded as a subcategory of machine learning, in which a layered structure of algorithms, a so-called artificial neural network, is used. Deep learning algorithms extract information directly from raw images without the need of segmentation and generate features adaptive to a certain problem to capture the full range of information provided by the images [68,69]. These analytic methods can be used for renal mass subtyping, nuclear grade prediction, and prediction of molecular features. Moreover, these data can be combined in integrative multi-omics biomarker models to further increase their predictive accuracy. Figure 5 shows a schematic representation of a typical radiomic workflow.
Fig. 5 –

Schematic representation of a radiomic workflow.
3.3.1. Renal mass sub typing
Radiomic models based on CT texture analysis, as well as AI-derived deep learning classifiers based on routine CT or MRI images, have shown a fair degree of diagnostic accuracy for the differentiation of benign versus malignant renal masses [70–76]. In one example, Xi et al [76] developed a residual convolutional neural network (ResNet)-based deep learning model for this clinical application that incorporated data from clinical variables and conventional MRI sequences (T2 weighted and T1 postcontrast). Their model was developed using data from 1162 renal masses (655 malignant and 507 benign) from a multicenter cohort divided into training, validation, and test sets. An analysis of clinical data alone by the deep learning model resulted in an area under the curve (AUC) of 0.54 for discerning benign from malignant renal tumors. The individual analyses of T1-postcontrast and T2-weighted sequences yielded AUCs of 0.59 and 0.70, respectively. Incorporation of all three data inputs resulted in superior performance by the deep learning model as compared to a radiomics-based classifier (AUC of 0.76 vs 0.54). Additionally, the model outperformed expert radiologists in terms of sensitivity (92% vs 80%), specificity (41% vs 35%), and overall accuracy (70% vs 60%).
Several radiomic models based on quantitative CT texture features have been found to be helpful in the differentiation of fpAML from RCC, which currently represents a common imaging dilemma [77–83]. In a study of 41 fpAMLs and 130 RCCs, a CT texture analysis machine learning classifier outperformed expert radiologists in the differentiation of fpAML from RCC, ccRCC, and non-ccRCC, with AUCs of 0.96, 0.97, and 0.89 versus 0.67, 0.68, and 0.64, respectively [81]. Similarly, MRI texture features may be useful for the differentiation of fpAML from ccRCC [84]. Interestingly, Lee et al [85] showed that a deep learning approach outperformed conventional machine learning in the differentiation of fpAML from ccRCC, improving the sensitivity from 69% to 77% and specificity from 71% to 76%. Deep learning models were also able to distinguish oncocytomas from chrRCC in a small study on 20 renal masses, with reported sensitivity of 100% and specificity of 89% [86].
Typically, ccRCC has a more heterogeneous radiological appearance than other renal tumors [87,88]. Therefore, radiomic models based on CT and MRI texture analysis may be able to distinguish ccRCC from non-ccRCC subtypes [87–89]. Moreover, these texture features could also be useful in the differentiation of type I from the more aggressive type II pRCC [90,91]. Duan et al [90] studied 62 pRCCs, and reported sensitivity and specificity of 89% and 80%, respectively, for the differentiation of type I from type II pRCC using a CT texture analysis model. Vendrami et al [91] found that type I and type II pRCCs had significantly different MRI texture features, but this did not translate into an improved discriminative ability when added to a model using only qualitative MRI features. Interestingly, Schieda et al [92] also found that increased heterogeneity on CT texture analysis correlated with the presence of sarcomatoid features in ccRCC, which, in turn, correlates with worse prognosis.
3.3.2. Nuclear grade prediction
Multiple radiomic studies have focused on the evaluation of tumor aggressiveness through the prediction of nuclear grade. Both CT- and MRI-based radiomic approaches have been shown to accurately predict the presence of high-versus low-grade ccRCC, often through the use of texture analysis [93–100]. Cui et al [97] studied 460 patients with pathologically proven ccRCC, and developed and externally validated mpMRI- and multiphase CT-based machine learning models that could reliably distinguish high- from low-grade ccRCC, resulting in test accuracies of 73% and 79%, respectively. A deep learning approach based on conventional MRI was able to accurately distinguish high- from low-grade RCC in a multi-institutional cohort of 376 patients harboring 430 RCCs, with a test accuracy of 88% [101]. Notably, these advanced radiomic pipelines were developed based on resected nephrectomy specimens, which may introduce selection bias thereby limiting applicability in the setting of indeterminate renal masses.
3.3.3. Radiogenomics
The search for prognostic and predictive biomarkers to individualize and guide treatment approaches has been an area of great research interest in recent years [102–104]. The emerging field of radiogenomics investigates the association of quantitative and radiomic features with gene mutations and gene expression–based molecular signatures. These surrogate imaging biomarkers could tackle the issues of tumor heterogeneity and sampling bias that are associated with tissue-based biomarkers [11].
An example of the application of radiogenomics for renal mass characterization can be found in a recent report by Udayakumar and coworkers [105]. In this study, the authors investigated the correlation between contrast enhancement pattern on MRI with gene expression profiles in cases of localized ccRCC. Interestingly, they found that areas of high enhancement correlated with higher angiogenic gene expression in corresponding tissue samples acquired by multiregion tumor sampling. Conversely, areas of low enhancement seemed to be enriched in immune-related gene expression signatures. In a separate cohort of patients with metastatic ccRCC, higher enhancement scores, reflecting increased angiogenesis, were associated with better outcomes with antiangiogenic therapy.
In another study, Jamshidi et al [106] showed the feasibility of developing a noninvasive image-based surrogate of a prognostic molecular assay for renal tumors. More specifically, the authors developed a radiomic tool based on single CT images of patients with ccRCC that achieved 70% agreement with the results of a previously validated prognostic gene expression assay. The surrogate radiomic tool was able to predict disease-specific survival in this cohort of patients allowing for it to serve in place of the invasive tissue-based assay. Similarly, Yin et al [107] studied whether 18F-FDG PET/MRI could serve as a surrogate biomarker for ccRCC molecular tumor subtype, as defined by the 34-gene ClearCode34 classifier. The authors’ radiomic model achieved a correct classification rate of 87% for distinguishing between the two prognostically distinct molecular subtypes of ccRCC (ie, ccA vs ccB).
In addition to correlation with gene expression signatures, radiogenomics can be used to predict the presence of somatic gene mutation status. In one example, The Cancer Genome Atlas—Renal Cell Carcinoma Imaging Research Group conducted a multi-institutional, multireader, study that found a significant association between imaging features on CT and/or MRI with BAP1 and MUC4 mutational status in cases of ccRCC [108]. In their study, ill-defined tumor margins and presence of calcifications were associated with BAP1 mutations, which are strongly correlated with unfavorable outcomes [102,108,109]. Additionally, exophytic growth pattern was associated with MUC4 mutations, which is potentially correlated with improved survival [108,109]. Radiogenomics models that implement machine learning have also been shown to predict VHL, BAP1, and PBRM1 mutational status in several studies using publicly available datasets [110–113]. VHL mutation and inactivation, which are associated with higher angiogenic gene expression, is characteristic of ccRCC, and the loss of PBRM1 is associated with improved survival [102,109]. Using quantitative CT features in a radiogenomics model, Chen et al [113] reported an AUC of >0.85 for the prediction of all three gene mutations. In turn, Marigliano et al [114] found a significant association between one CT texture analysis feature and the tumoral expression of miRNA 21–5p, which is involved in RCC tumorigenesis and is associated with poor prognosis [109,115,116].
Despite the encouraging data presented above, it is worth noting that the low prevalence of many of these molecular abnormalities limits the utility of radiogenomics in clinical practice at the present time. Moreover, gene-based changes in several radiomic features might not be consistent across imaging phases, and the clinical relevance of the radiogenomics models in these studies is rarely tested directly. Regardless, with the vast amount of genomic and transcriptomic data rapidly becoming available, this field is likely to evolve in the near future.
3.3.4. Current limitations and future directions in radiomics
Although the field of radiomics is currently moving at an unprecedented pace, and the potential of radiomic and AI applications seems limitless, there are several key challenges to overcome prior to the more widespread study and clinical adoption of radiomic and AI-based tools for renal mass characterization. For example, development of radiomic applications is inherently dependent on the provided datasets, which often suffer from a significant selection bias since these do not include all histological subtypes. Moreover, imaging data are often collected from surgically treated tumors in a retrospective fashion, which might lead to an over-representation of more aggressive tumors selected for surgical treatment. Pooling of multiple large datasets might overcome some of these limitations and allow for an adequate representation of rarer histological subtypes.
Indeed, generalizability and reproducibility remain a key issue among radiomic studies and need to be addressed prior to possible widespread clinical implementation [117]. Kocak et al [118] assessed 30 studies involving AI in renal mass characterization and found that only 10% of studies performed a generalizability assessment using independent or external validation. The same was concluded by Ursprung et al [119], who assessed the methodological quality of 57 radiomic studies. Of these, only three used external validation sets and one used a publicly available validation dataset. None of the studies shared code, regions of interest, or images. Moreover, scanner models as well as acquisition and reconstruction protocols are highly variable between and within institutions and impede generalizability. Manual segmentation of a region of interest is labor intensive, and successful automated segmentation methods, preferably three dimensional, are currently lacking. Highly reproducible segmentation techniques would be needed for widespread adoption of radiomic applications. While deep learning models do not require this guided segmentation of a region or volume of interest, these unsupervised approaches sacrifice critical clinical knowledge, which might lead to overfitting of these predictive models and indicates the need for structured quality assessment. Overall, this means that while the feasibility of radiomic and Al applications seems apparent, the reproducibility is seriously lacking and may halt the integration of radiomics into routine clinical practice. Al methods may be less dependent on variations among scanners and protocols, although such algorithms can function as “black boxes” in which the output is not explainable in human terms, which may also slow adoption.
4. Conclusions
A number of novel imaging tools are currently under investigation to improve the noninvasive characterization of solid renal masses. Several of these tools are poised for widespread clinical adoption and will likely serve to augment or possibly replace RMB in the workup of solid renal masses, thus providing a comprehensive virtual biopsy. Among these tools, 99mTc-sestamibi SPECT/CT for the identification of renal oncocytomas/HOCTs and PET/CT with radiolabeled girentuximab for the identification of ccRCC are, by far, closest to implementation in routine clinical practice. Ultimately, it is likely that regardless of the imaging techniques used, radiomics and AI-powered interpretative tools will be required to maximize the diagnostic accuracy and utility of these tests. Furthermore, these advanced interpretative tools stand to possibly allow for more in-depth characterization of solid renal masses in terms of tumor grade, underlying genomic abnormalities, and clinical behavior.
Supplementary Material
Footnotes
Financial disclosures: Eduard Roussel certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Eduard Roussel has received an unconditional research grant from Ipsen and Pfizer. Ivan Pedrosa served as a scientific advisor for both Bayer Healthcare and Merck, for which he received honoraria; served as a scientific advisor for Health Tech International, for which he received stock options; and is a coinventor of patents with Philips Healthcare, for which he receives no royalties. Steven Rowe receives salary support and serves as a consultant to Lantheus Medical Imaging, Inc.; owns stock in, has licensed patents to, and serves as a consultant to Precision Molecular, Inc.; and owns stock in and has licensed patents to Plenary AI, Inc. Michael Gorin serves as a paid consultant to Ambu A/S; Blue Earth Diagnostics, Inc; Corbin Clinical Resources, Inc. (Perineologic); Galvanize Therapeutics; KOELIS, Inc.; Lanthius Medical Imaging, Inc; and Simulated Inanimate Models, LLC. Dr. Gorin also has licensed a patent to Precision Molecular, Inc. for which he receives royalties and owns stock in Simulated Inanimate Models, Inc. All other authors have nothing to disclose.
Appendix A: Peer Review Summary
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eururo.2022.01.040.
References
- [1].Capitanio U, Bensalah K, Bex A, et al. Epidemiology of renal cell carcinoma. Eur Urol 2019;75:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67:519–30. [DOI] [PubMed] [Google Scholar]
- [3].Smaldone MC, Egleston B, Hollingsworth JM, et al. Understanding treatment disconnect and mortality trends in renal cell carcinoma using tumor registry data. Med Care 2017;55:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kang SK, Huang WC, Pandharipande PV, Chandarana H. Solid renal masses: what the numbers tell us. Am J Roentgenol 2014;202:1196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sevcenco S, Heinz-Peer G, Ponhold L, et al. Utility and limitations of 3-Tesla diffusion-weighted magnetic resonance imaging for differentiation of renal tumors. Eur J Radiol 2014;83:909–13. [DOI] [PubMed] [Google Scholar]
- [6].Johnson DC, Vukina J, Smith AB, et al. Preoperatively misclassified, surgically removed benign renal masses: a systematic review of surgical series and United States population level burden estimate. J Urol 2015;193:30–5. [DOI] [PubMed] [Google Scholar]
- [7].Kim JH, Li S, Khandwala Y, Chung KJ, Park HK, Chung BI. Association of prevalence of benign pathologic findings after partial nephrectomy with preoperative imaging patterns in the United States from 2007 to 2014. JAMA Surg 2019;154:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Marconi L, Dabestani S, Lam TB, et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol 2016;69:660–73. [DOI] [PubMed] [Google Scholar]
- [9].Patel HD, Johnson MH, Pierorazio PM, et al. Diagnostic accuracy and risks of biopsy in the diagnosis of a renal mass suspicious for localized renal cell carcinoma: systematic review of the literature. J Urol 2016;195:1340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ball MW, Bezerra SM, Gorin MA, et al. Grade heterogeneity in small renal masses: potential implications for renal mass biopsy. J Urol 2015;193:36–40. [DOI] [PubMed] [Google Scholar]
- [11].Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bertolotto M, Cicero C, Perrone R, Degrassi F, Cacciato F, Cova MA. Renal masses with equivocal enhancement at CT: characterization with contrast-enhanced ultrasound. AJR Am J Roentgenol 2015;204:W557–65. [DOI] [PubMed] [Google Scholar]
- [13].Kim JK, Kim TK, Ahn HJ, Kim CS, Kim K-R, Cho K-S. Differentiation of subtypes of renal cell carcinoma on helical CT scans. Am J Roentgenol 2002;178:1499–506. [DOI] [PubMed] [Google Scholar]
- [14].Rosenkrantz AB, Hindman N, Fitzgerald EF, Niver BE, Melamed J, Babb JS. MRI features of renal oncocytoma and chromophobe renal cell carcinoma. AmJ Roentgenol 2010;195:W421–7. [DOI] [PubMed] [Google Scholar]
- [15].Egbert ND, Caoili EM, Cohan RH, et al. Differentiation of papillary renal cell carcinoma subtypes on CT and MRI. Am J Roentgenol 2013;201:347–55. [DOI] [PubMed] [Google Scholar]
- [16].Yang C-W, Shen S-H, Chang Y-H, et al. Are there useful CT features to differentiate renal cell carcinoma from lipid-poor renal angiomyolipoma? AmJ Roentgenol 2013;201:1017–28. [DOI] [PubMed] [Google Scholar]
- [17].Shinagare AB, Davenport MS, Park H, et al. Lexicon for renal mass terms at CT and MRI: a consensus of the Society of Abdominal Radiology Disease-focused Panel on Renal Cell Carcinoma. Abdom Radiol 2021;46:703–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Siegel C Re: Characterizing solid renal neoplasms with MRI in adults. J Urol 2015;193:1152. [DOI] [PubMed] [Google Scholar]
- [19].Sasiwimonphan K, Takahashi N, Leibovich BC, Carter RE, Atwell TD, Kawashima A. Small (<4 cm) renal mass: differentiation of angiomyolipoma without visible fat from renal cell carcinoma utilizing MR imaging. Radiology 2012;263:160–8. [DOI] [PubMed] [Google Scholar]
- [20].Wilson MP, Patel D, Murad MH, Mclnnes MDF, Katlariwala P, Low G. Diagnostic performance of MRI in the detection of renal lipid-poor angiomyolipomas: a systematic review and meta-analysis. Radiology 2020;296:511–20. [DOI] [PubMed] [Google Scholar]
- [21].Kay FU, Canvasser NE, Xi Y, et al. Diagnostic performance and interreader agreement of a standardized MR imaging approach in the prediction of small renal mass histology. Radiology 2018;287:543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Canvasser NE, Kay FU, Xi Y, et al. Diagnostic accuracy of multiparametric magnetic resonance imaging to identify clear cell renal cell carcinoma in cT1a renal masses. J Urol 2017;198:780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Steinberg RL, Rasmussen RG, Johnson BA, et al. Prospective performance of clear cell likelihood scores (ccLS) in renal masses evaluated with multiparametric magnetic resonance imaging. Eur Radiol 2021;31:314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kang SK, Zhang A, Pandharipande PV, Chandarana H, Braithwaite RS, Littenberg B. DWI for renal mass characterization: systematic review and meta-analysis of diagnostic test performance. Am J Roentgenol 2015;205:317–24. [DOI] [PubMed] [Google Scholar]
- [25].Cornelis F, Tricaud E, Lasserre AS, et al. Multiparametric magnetic resonance imaging for the differentiation of low and high grade clear cell renal carcinoma. Eur Radiol 2015;25:24–31. [DOI] [PubMed] [Google Scholar]
- [26].Lanzman RS, Robson PM, Sun MR, et al. Arterial spin-labeling MR imaging of renal masses: correlation with histopathologic findings. Radiology 2012;265:799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rowe SP, Gorin MA, Solnes LB, et al. Correlation of 99mTc-sestamibi uptake in renal masses with mitochondrial content and multi-drug resistance pump expression. EJNMMI Res 2017;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gormley TS, Van Every MJ, Moreno AJ. Renal oncocytoma: preoperative diagnosis using technetium 99m sestamibi imaging. Urology 1996;48:33–9. [DOI] [PubMed] [Google Scholar]
- [29].Joshi S, Tolkunov D, Aviv H, et al. The genomic landscape of renal oncocytoma identifies a metabolic barrier to tumorigenesis. Cell Rep 2015;13:1895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rowe SP, Gorin MA, Gordetsky J, et al. Initial experience using 99mTc-MIBI SPECT/CT for the differentiation of oncocytoma from renal cell carcinoma. Clin Nucl Med 2015;40:309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gorin MA, Rowe SP, Baras AS, et al. Prospective evaluation of 99mTc-sestamibi SPECT/CT for the diagnosis of renal oncocytomas and hybrid oncocytic/chromophobe tumors. Eur Urol 2016;69:413–6. [DOI] [PubMed] [Google Scholar]
- [32].Abu-Ghanem Y, Powles T, Capitanio U, et al. The impact of histological subtype on the incidence, timing, and patterns of recurrence in patients with renal cell carcinoma after surgery–results from RECUR consortium. Eur Urol Oncol 2021;4:473–82. [DOI] [PubMed] [Google Scholar]
- [33].Tzortzakakis A, Gustafsson O, Karlsson M, Ekström-Ehn L, Ghaffarpour R, Axelsson R. Visual evaluation and differentiation of renal oncocytomas from renal cell carcinomas by means of 99mTc-sestamibi SPECT/CT. EJNMMI Res 2017;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sistani G, Bjazevic J, Kassam Z, Romsa J, Pautier S. Evaluation and risk stratification of renal masses with 99mTc-sestamibi SPECT/CT. J Urol 2020;203:e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Asi T, Tuncali MÇ, Tuncel M, et al. The role of Tc-99m MIBI scintigraphy in clinical T1 renal mass assessment: does it have a real benefit? Urol Oncol Semin Orig Investig 2020;38, 937.e11–7. [DOI] [PubMed] [Google Scholar]
- [36].Su ZT, Patel HD, Huang MM, et al. Cost-effectiveness analysis of 99mTc-sestamibi SPECT/CT to guide management of small renal masses. Eur Urol Focus 2020;203:e1224–5. [DOI] [PubMed] [Google Scholar]
- [37].Divgi CR, Pandit-Taskar N, Jungbluth AA, et al. Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-CG250) and PET in patients with renal masses: a phase I trial. Lancet Oncol 2007;8:304–10. [DOI] [PubMed] [Google Scholar]
- [38].Stillebroer AB, Mulders PFA, Boerman OC, Oyen WJG, Oosterwijk E. Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Eur Urol 2010;58:75–83. [DOI] [PubMed] [Google Scholar]
- [39].Weng S, DiNatale RG, Silagy A, et al. The clinicopathologic and molecular landscape of clear cell papillary renal cell carcinoma: implications in diagnosis and management. Eur Urol 2021;79:468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lau J, Lin K-S, Bénard F. Past, present, and future: development of theranostic agents targeting carbonic anhydrase IX. Theranostics 2017;7:4322–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Divgi CR, Uzzo RG, Gatsonis C, et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: results from the REDECT trial. J Clin Oncol 2013;31:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wilex AG. Wilex AG and the FDA agree on the further development of REDECTANE(R) 2012. https://www.dgap.de/dgap/News/corporate/wilex-and-the-fda-agree-the-further-development-redectaner/?newsID=734856.
- [43].Cheal SM, Punzalan B, Doran MG, et al. Pairwise comparison of 89Zr- and 124I-labeled cG250 based on positron emission tomography imaging and nonlinear immunokinetic modeling: in vivo carbonic anhydrase IX receptor binding and internalization in mouse xenografts of clear-cell renal cell carcinoma. Eur J Nucl Med Mol Imaging 2014;41:985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Merkx RIJ, Lobeek D, Konijnenberg M, et al. Phase I study to assess safety, biodistribution and radiation dosimetry for 89Zr-girentuximab in patients with renal cell carcinoma. Eur J Nucl Med Mol Imaging 2021;48:3277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Muselaers CHJ, Boerman OC, Oosterwijk E, Langenhuijsen JF, Oyen WJG, Mulders PFA. Indium-111-labeled Girentuximab ImmunoSPECT as a diagnostic tool in clear cell renal cell carcinoma. Eur Urol 2013;63:1101–6. [DOI] [PubMed] [Google Scholar]
- [46].Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun 2008;29:193–207. [DOI] [PubMed] [Google Scholar]
- [47].Garousi J, Huizing FJ, Vorobyeva A, et al. Comparative evaluation of affibody- and antibody fragments-based CAIX imaging probes in mice bearing renal cell carcinoma xenografts. Sci Rep 2019;9:14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Minn I, Koo SM, Lee HS, et al. [64Cu]XYIMSR-06: a dual-motif CAIX ligand for PET imaging of clear cell renal cell carcinoma. Oncotarget 2016;7:56471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang HY, Ding HJ, Chen JH, et al. Meta-analysis of the diagnostic performance of [18F]FDG-PET and PET/CT in renal cell carcinoma. Cancer Imaging 2012;12:464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Özülker T, Özülker F, Özbek E, Özpaçaci T. A prospective diagnostic accuracy study of F-18 fluorodeoxyglucose-positron emission tomography/computed tomography in the evaluation of indeterminate renal masses. Nucl Med Commun 2011;32:265–72. [DOI] [PubMed] [Google Scholar]
- [51].Gündoğan C, Çermik TF, Erkan E, et al. Role of contrast-enhanced 18F-FDG PET/CT imaging in the diagnosis and staging of renal tumors. Nucl Med Commun 2018;39:1174–82. [DOI] [PubMed] [Google Scholar]
- [52].Singh H, Arora G, Nayak B, et al. Semi-quantitative F-18-FDG PET/computed tomography parameters for prediction of grade in patients with renal cell carcinoma and the incremental value of diuretics. Nucl Med Commun 2020;41:485–93. [DOI] [PubMed] [Google Scholar]
- [53].Takahashi M, Kume H, Koyama K, et al. Preoperative evaluation of renal cell carcinoma by using 18F-FDG PET/CT. Clin Nucl Med 2015;40:936–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhao Y, Wu C, Li W, et al. 2-[18F]FDG PET/CT parameters associated with WHO/ISUP grade in clear cell renal cell carcinoma. Eur J Nucl Med Mol Imaging 2021;48:570–9. [DOI] [PubMed] [Google Scholar]
- [55].Nakajima R, Abe K, Kondo T, Tanabe K, Sakai S. Clinical role of early dynamic FDG-PET/CT for the evaluation of renal cell carcinoma. Eur Radiol 2016;26:1852–62. [DOI] [PubMed] [Google Scholar]
- [56].Noda Y, Kanematsu M, Goshima S, et al. 18-F fluorodeoxyglucose uptake in positron emission tomography as a pathological grade predictor for renal clear cell carcinomas. Eur Radiol 2015;25:3009–16. [DOI] [PubMed] [Google Scholar]
- [57].Zhu H, Zhao S, Zuo C, Ren F. FDG PET/CT and CT findings of renal cell carcinoma with sarcomatoid differentiation. Am J Roentgenol 2020;215:645–51. [DOI] [PubMed] [Google Scholar]
- [58].Gorin MA, Rowe SP, Hooper JE, et al. PSMA-targeted 18F-DCFPyL PET/CT imaging of clear cell renal cell carcinoma: results from a rapid autopsy. Eur Urol 2017;71:145–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rowe SP, Gorin MA, Hammers HJ, et al. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann Nucl Med 2015;29:877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Meyer AR, Carducci MA, Denmeade SR, et al. Improved identification of patients with oligometastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann Nucl Med 2019;33:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Mittlmeier LM, Unterrainer M, Rodler S, et al. (18)F-PSMA-1007 PET/CT for response assessment in patients with metastatic renal cell carcinoma undergoing tyrosine kinase or checkpoint inhibitor therapy: preliminary results. Eur J Nucl Med Mol Imaging 2021;48:2031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gühne F, Seifert P, Theis B, Steinert M, Freesmeyer M, Drescher R. PSMA-PET/CT in patients with recurrent clear cell renal cell carcinoma: histopathological correlations of imaging findings. Diagnostics (Basel, Switzerland) 2021:11:1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Golan S, Aviv T, Groshar D, et al. Dynamic 68 Ga-PSMA-11 PET/CT for the primary evaluation of localized renal mass: a prospective study. J Nucl Med 2021;62:773–8. [DOI] [PubMed] [Google Scholar]
- [64].Gao J, Xu Q, Fu Y, et al. Comprehensive evaluation of 68Ga-PSMA-11 PET/CT parameters for discriminating pathological characteristics in primary clear-cell renal cell carcinoma. Eur J Nucl Med Mol Imaging 2021;48:561–9. [DOI] [PubMed] [Google Scholar]
- [65].Grassi I, Nanni C, Allegri V, et al. The clinical use of PET with (11)C-acetate. Am J Nucl Med Mol Imaging 2012;2:33–47. [PMC free article] [PubMed] [Google Scholar]
- [66].Ho C, Chen S, Ho KMT, et al. Dual-tracer PET/CT in renal angiomyolipoma and subtypes of renal cell carcinoma. Clin Nucl Med 2012;37:1075–82. [DOI] [PubMed] [Google Scholar]
- [67].Zwanenburg A, Vallières M, Abdalah MA, et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 2020;295:328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Goodfellow I, Bengio Y, Courville A. Deep learning. The MIT Press; 2016. [Google Scholar]
- [69].Lee M, Wei S, Anaokar J, Uzzo R, Kutikov A. Kidney cancer management 3.0: can artificial intelligence make us better? Curr Opin Urol 2021;31:409–15. [DOI] [PubMed] [Google Scholar]
- [70].Deng Y, Soule E, Cui E, et al. Usefulness of CT texture analysis in differentiating benign and malignant renal tumours. Clin Radiol 2020;75:108–15. [DOI] [PubMed] [Google Scholar]
- [71].Varghese BA, Chen F, Hwang DH, et al. Differentiation of predominantly solid enhancing lipid-poor renal cell masses by use of contrast-enhanced CT: evaluating the role of texture in tumor subtyping. Am J Roentgenol 2018;211:W288–96. [DOI] [PubMed] [Google Scholar]
- [72].Uhlig J, Biggemann L, Nietert MM, et al. Discriminating malignant and benign clinical T1 renal masses on computed tomography. Medicine (Baltimore) 2020;99:e19725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Oberai A, Varghese B, Cen S, et al. Deep learning based classification of solid lipid-poor contrast enhancing renal masses using contrast enhanced CT. Br J Radiol 2020;93:20200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zabihollahy F, Schieda N, Krishna S, Ukwatta E. Automated classification of solid renal masses on contrast-enhanced computed tomography images using convolutional neural network with decision fusion. Eur Radiol 2020;30:5183–90. [DOI] [PubMed] [Google Scholar]
- [75].Tanaka T, Huang Y, Marukawa Y, et al. Differentiation of small (≤4 cm) renal masses on multiphase contrast-enhanced CT by deep learning. Am J Roentgenol 2020;214:605–12. [DOI] [PubMed] [Google Scholar]
- [76].Xi IL, Zhao Y, Wang R, et al. Deep learning to distinguish benign from malignant renal lesions based on routine MR imaging. Clin Cancer Res 2020;26:1944–52. [DOI] [PubMed] [Google Scholar]
- [77].Ma Y, Cao F, Xu X, Ma W. Can whole-tumor radiomics-based CT analysis better differentiate fat-poor angiomyolipoma from clear cell renal cell carcinoma: compared with conventional CT analysis? Abdom Radiol 2020;45:2500–7. [DOI] [PubMed] [Google Scholar]
- [78].Nie P, Yang G, Wang Z, et al. A CT-based radiomics nomogram for differentiation of renal angiomyolipoma without visible fat from homogeneous clear cell renal cell carcinoma. Eur Radiol 2020;30:1274–84. [DOI] [PubMed] [Google Scholar]
- [79].Yang R, Wu J, Sun L, et al. Radiomics of small renal masses on multiphasic CT: accuracy of machine learning–based classification models for the differentiation of renal cell carcinoma and angiomyolipoma without visible fat. Eur Radiol 2020;30:1254–63. [DOI] [PubMed] [Google Scholar]
- [80].You MW, Kim N, Choi HJ. The value of quantitative CT texture analysis in differentiation of angiomyolipoma without visible fat from clear cell renal cell carcinoma on four-phase contrast-enhanced CT images. Clin Radiol 2019;74:547–54. [DOI] [PubMed] [Google Scholar]
- [81].Cui E-M, Lin F, Li Q, et al. Differentiation of renal angiomyolipoma without visible fat from renal cell carcinoma by machine learning based on whole-tumor computed tomography texture features. Acta Radiol 2019;60:1543–52. [DOI] [PubMed] [Google Scholar]
- [82].Lee HS, Hong H, Jung DC, Park S, Kim J. Differentiation of fat-poor angiomyolipoma from clear cell renal cell carcinoma in contrast-enhanced MDCT images using quantitative feature classification. Med Phys 2017;44:3604–14. [DOI] [PubMed] [Google Scholar]
- [83].Yan L, Liu Z, Wang G, et al. Angiomyolipoma with minimal fat. Acad Radiol 2015;22:1115–21. [DOI] [PubMed] [Google Scholar]
- [84].Li H, Li A, Zhu H, et al. Whole-tumor quantitative apparent diffusion coefficient histogram and texture analysis to differentiation of minimal fat angiomyolipoma from clear cell renal cell carcinoma. Acad Radiol 2019;26:632–9. [DOI] [PubMed] [Google Scholar]
- [85].Lee H, Hong H, Kim J, Jung DC. Deep feature classification of angiomyolipoma without visible fat and renal cell carcinoma in abdominal contrast-enhanced CT images with texture image patches and hand-crafted feature concatenation. Med Phys 2018;45:1550–61. [DOI] [PubMed] [Google Scholar]
- [86].Baghdadi A, Aldhaam NA, Elsayed AS, et al. Automated differentiation of benign renal oncocytoma and chromophobe renal cell carcinoma on computed tomography using deep learning. BJU Int 2020;125:553–60. [DOI] [PubMed] [Google Scholar]
- [87].Deng Y, Soule E, Samuel A, et al. CT texture analysis in the differentiation of major renal cell carcinoma subtypes and correlation with Fuhrman grade. Eur Radiol 2019;29:6922–9. [DOI] [PubMed] [Google Scholar]
- [88].Leng S, Takahashi N, Gomez Cardona D, et al. Subjective and objective heterogeneity scores for differentiating small renal masses using contrast-enhanced CT. Abdom Radiol 2017;42:1485–92. [DOI] [PubMed] [Google Scholar]
- [89].Zhang G-M-Y, Shi B, Xue H-D, Ganeshan B, Sun H, Jin Z-Y. Can quantitative CT texture analysis be used to differentiate subtypes of renal cell carcinoma? Clin Radiol 2019;74:287–94. [DOI] [PubMed] [Google Scholar]
- [90].Duan C, Li N, Niu L, et al. CT texture analysis for the differentiation of papillary renal cell carcinoma subtypes. Abdom Radiol 2020;45:3860–8. [DOI] [PubMed] [Google Scholar]
- [91].Vendrami CL, Velichko YS, Miller FH, et al. Differentiation of papillary renal cell carcinoma subtypes on MR1: qualitative and texture analysis. Am J Roentgenol 2018;211:1234–45. [DOI] [PubMed] [Google Scholar]
- [92].Schieda N, Thornhill RE, Al-Subhi M, et al. Diagnosis of sarcomatoid renal cell carcinoma with CT: evaluation by qualitative imaging features and texture analysis. Am J Roentgenol 2015;204:1013–23. [DOI] [PubMed] [Google Scholar]
- [93].Han D, Yu Y, Yu N, et al. Prediction models for clear cell renal cell carcinoma ISUP/WHO grade: comparison between CT radiomics and conventional contrast-enhanced CT. Br J Radiol 2020;93:20200131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Nazari M, Shiri I, Hajianfar G, et al. Noninvasive Fuhrman grading of clear cell renal cell carcinoma using computed tomography radiomic features and machine learning. Radiol Med 2020;125:754–62. [DOI] [PubMed] [Google Scholar]
- [95].Sun X, Liu L, Xu K, et al. Prediction of ISUP grading of clear cell renal cell carcinoma using support vector machine model based on CT images. Medicine (Baltimore) 2019;98:e15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Shu J, Tang Y, Cui J, et al. Clear cell renal cell carcinoma: CT-based radiomics features for the prediction of Fuhrman grade. Eur J Radiol 2018;109:8–12. [DOI] [PubMed] [Google Scholar]
- [97].Cui E, Li Z, Ma C, et al. Predicting the ISUP grade of clear cell renal cell carcinoma with multiparametric MR and multiphase CT radiomics. Eur Radiol 2020;30:2912–21. [DOI] [PubMed] [Google Scholar]
- [98].Sun J, Pan L, Zha T, Xing W, Chen J, Duan S. The role of MRI texture analysis based on susceptibility-weighted imaging in predicting Fuhrman grade of clear cell renal cell carcinoma. Acta Radiol 2021;62:1104–11. [DOI] [PubMed] [Google Scholar]
- [99].Stanzione A, Ricciardi C, Cuocolo R, et al. MRI radiomics for the prediction of Fuhrman grade in clear cell renal cell carcinoma: a machine learning exploratory study. J Digit Imaging 2020;33:879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yaşar S, Voyvoda N, Voyvoda B, Özer T. Using texture analysis as a predictive factor of subtype, grade and stage of renal cell carcinoma. Abdom Radiol 2020;45:3821–30. [DOI] [PubMed] [Google Scholar]
- [101].Zhao Y, Chang M, Wang R, et al. Deep learning based on MRI for differentiation of low- and high-grade in low-stage renal cell carcinoma. J Magn Reson Imaging 2020;52:1542–9. [DOI] [PubMed] [Google Scholar]
- [102].Kotecha RR, Motzer RJ, Voss MH. Towards individualized therapy for metastatic renal cell carcinoma. Nat Rev Clin Oncol 2019;16:621–33. [DOI] [PubMed] [Google Scholar]
- [103].Roussel E, Verbiest A, Kinget L, et al. Molecular subtypes and gene expression signatures as prognostic features in fully resected clear cell renal cell carcinoma: a tailored approach to adjuvant trials. Clin Genitourin Cancer 2021;19:e382–94. [DOI] [PubMed] [Google Scholar]
- [104].Roussel E, Kinget L, Verbiest A, et al. Molecular underpinnings of glandular tropism in metastatic clear cell renal cell carcinoma: therapeutic implications. Acta Oncol 2021;60:1499–506. [DOI] [PubMed] [Google Scholar]
- [105].Udayakumar D, Zhang Z, Xi Y, et al. Deciphering intratumoral molecular heterogeneity in clear cell renal cell carcinoma with a radiogenomics platform. Clin Cancer Res 2021;27:4794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Jamshidi N, Jonasch E, Zapala M, et al. The radiogenomic risk score: construction of a prognostic quantitative, noninvasive image-based molecular assay for renal cell carcinoma. Radiology 2015;277:114–23. [DOI] [PubMed] [Google Scholar]
- [107].Yin Q, Hung S-C, Rathmell WK, et al. Integrative radiomics expression predicts molecular subtypes of primary clear cell renal cell carcinoma. Clin Radiol 2018;73:782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Shinagare AB, Vikram R, Jaffe C, et al. Radiogenomics of clear cell renal cell carcinoma: preliminary findings of The Cancer Genome Atlas–Renal Cell Carcinoma (TCGA–RCC) Imaging Research Group. Abdom Imaging 2015;40:1684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Creighton CJ, Morgan M, Gunaratne PH, et al. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kocak B, Durmaz ES, Ates E, Ulusan MB. Radiogenomics in clear cell renal cell carcinoma: machine learning–based high-dimensional quantitative CT texture analysis in predicting PBRM1 mutation status. Am J Roentgenol 2019;212:W55–63. [DOI] [PubMed] [Google Scholar]
- [111].Ghosh P, Tamboli P, Vikram R, Rao A. Imaging-genomic pipeline for identifying gene mutations using three-dimensional intra-tumor heterogeneity features. J Med Imaging (Bellingham, Wash) 2015;2:41009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Li ZC, Zhai G, Zhang J, et al. Differentiation of clear cell and non-clear cell renal cell carcinomas by all-relevant radiomics features from multiphase CT: a VHL mutation perspective. Eur Radiol 2019;29:3996–4007. [DOI] [PubMed] [Google Scholar]
- [113].Chen X, Zhou Z, Hannan R, et al. Reliable gene mutation prediction in clear cell renal cell carcinoma through multi-classifier multi-objective radiogenomics model. Phys Med Biol 2018;63:215008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Marigliano C, Badia S, Bellini D, et al. Radiogenomics in clear cell renal cell carcinoma: correlations between advanced CT imaging (texture analysis) and MicroRNAs expression. Technol Cancer Res Treat 2019;18:153303381987845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Verbiest A, van Hoef V, Rodriguez-Antona C, et al. MicroRNA expression profiles in molecular subtypes of clear-cell renal cell carcinoma are associated with clinical outcome and repression of specific mRNA targets. PLoS One 2020;15:e0238809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kinget L, Roussel E, Lambrechts D, et al. MicroRNAs possibly involved in the development of bone metastasis in clear-cell renal cell carcinoma. Cancers 2021;13:1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kocak B, Durmaz ES, Erdim C, Ates E, Kaya OK, Kilickesmez O. Radiomics of renal masses: systematic review of reproducibility and validation strategies. Am J Roentgenol 2020;214:129–36. [DOI] [PubMed] [Google Scholar]
- [118].Kocak B, Kaya OK, Erdim C, Kus EA, Kilickesmez O. Artificial intelligence in renal mass characterization: a systematic review of methodologic items related to modeling, performance evaluation, clinical utility, and transparency. Am J Roentgenol 2020;215:1113–22. [DOI] [PubMed] [Google Scholar]
- [119].Ursprung S, Beer L, Bruining A, et al. Radiomics of computed tomography and magnetic resonance imaging in renal cell carcinoma–a systematic review and meta-analysis. Eur Radiol 2020;30:3558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Pedrosa I, Cadeddu JA. How we do it: managing the indeterminate renal mass with the MRI clear cell likelihood score. Radiology. In press, 10.1148/radiol.210034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


