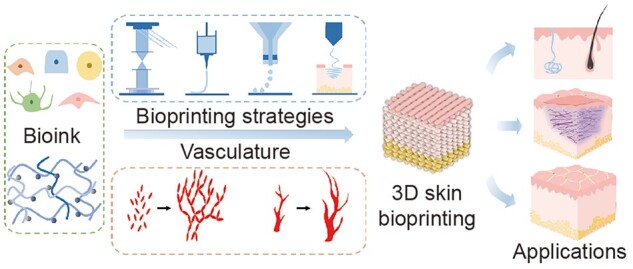
Abstract
Even with many advances in design strategies over the past three decades, an enormous gap remains between existing tissue engineering skin and natural skin. Currently available in vitro skin models still cannot replicate the three-dimensionality and heterogeneity of the dermal microenvironment sufficiently to recapitulate many of the known characteristics of skin disorder or disease in vivo. Three-dimensional (3D) bioprinting enables precise control over multiple compositions, spatial distributions and architectural complexity, therefore offering hope for filling the gap of structure and function between natural and artificial skin. Our understanding of wound healing process and skin disease would thus be boosted by the development of in vitro models that could more completely capture the heterogeneous features of skin biology. Here, we provide an overview of recent advances in 3D skin bioprinting, as well as design concepts of cells and bioinks suitable for the bioprinting process. We focus on the applications of this technology for engineering physiological or pathological skin model, focusing more specifically on the function of skin appendages and vasculature. We conclude with current challenges and the technical perspective for further development of 3D skin bioprinting.
Keywords: 3D bioprinting, skin, stem cells, regeneration
Graphical Abstract
Introduction
Human skin is a complex three-layered structure formed by the combined, functional organization of multiple cell types. The cells in these layers are highly specialized and gathered to perform distinctive functions [1]. Various skin-related injuries are drastically increasing due to traumatic damage and disease [2]. Currently, the clinical treatments for the repair or replacement of missing or malfunctioning human skin are limited by the availability of healthy donor tissue and immune rejection of donated tissue [3, 4]. In the search for alternatives to conventional treatment strategies for skin replacement and repair, tissue engineering approaches are being explored as a promising solution.
In the past three decades, skin tissue engineering has shown great promise in the application of wound healing strategies and tissue regeneration. In some practice, such as deep burns and wounds, artificial skin substitutes are upcoming alternatives to traditional treatment. Recent advancements in stem cell biology and various innovative biomaterials have further provided a tremendous springboard for researchers in developing and manipulating tissue-engineered skin for improved skin regeneration and wound healing. However, low adherence to the wound bed, the inability to reproduce skin appendages and inadequate vascularization limits their utilization for restoration of normal skin anatomy on a routine basis [5, 6].
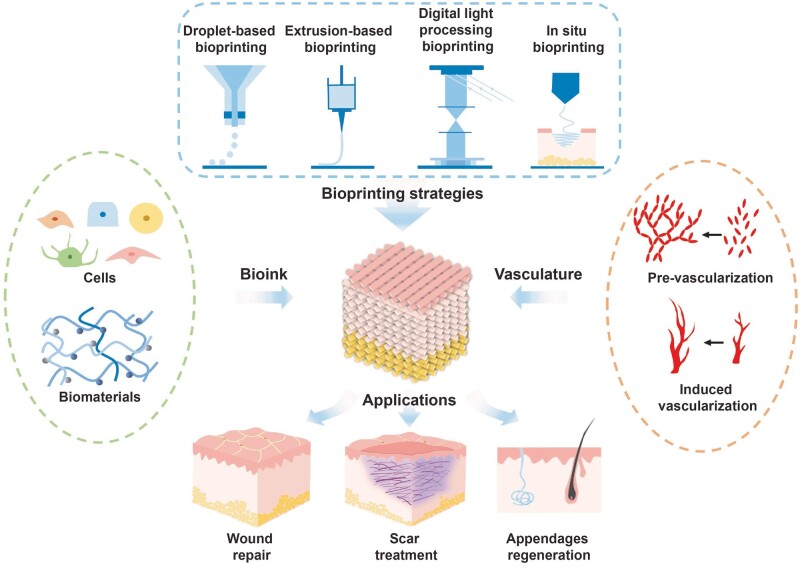
Three-dimensional (3D) bioprinting is a cutting-edge technology to fabricate precisely controlled architecture with highly reproducibility and repeatability through a layer-by-layer building process [7, 8]. Therefore, in the skin tissue engineering field, it can provide an excellent alternative for biomimetic scaffold fabrication by accurately positioning multiple cell types as well as biochemical and biophysical cues simultaneously into complex multi-layer architectures that better represent the structural and functional complexity of skin.
Although still in its infancy considering the complexity and functionality, 3D bioprinting approaches have been widely studied for skin tissue engineering and regeneration, both in research and clinical applications [9–11]. When engineering a 3D skin models in vitro, the requirements of cells, bioink and bioprinting process must be considered in a biomimetic and spatiotemporal manner. In this manner, 3D structural characteristics and physical properties can substantially enhance the biological performance and function through appropriate cell–cell and cell–extracellular matrix (ECM) communication. Therefore, in this review, we focus on general principles of cell and bioink choice or prepare techniques, and other essential elements pertaining to the application of 3D bioprinting technologies for generating physiological or pathological skin model. We propose a strategy of bioprinting the representative microenvironmental characteristics of native skin and further regenerating skin with appendages. Furthermore, we present recent advances in 3D skin with vascular network integration and discuss current challenges and exciting opportunities of 3D skin bioprinting toward recreating the complex, heterogeneous architecture of functional skin that further fundamental research and translational medicine (Fig. 1).
Figure 1.
Schematic diagram of essential elements pertaining to the application of skin bioprinting.
Bioinks for skin bioprinting
Bioinks play a critical role in 3D bioprinting to recapitulate the physiological and pathological environment. They are designed elaborately with a hydrogel form of biomaterials loaded with specific cell types [12]. Yet, creating an equivalent that faithfully mimics the intricate composition, structure and function of skin is still a challenge [13]. This puts forward high demands on the selection of both biomaterials and cells for bioinks. In this section, suitable biomaterials and desired properties are briefly summarized at the beginning. Then different cells used for skin bioprinting are reviewed.
Biomaterials
Category
Natural polymers and synthetic polymers are the common biomaterials used for 3D bioprinting [14]. Many natural polymers existed in natural ECM or extracted from marine organisms and natural substances have been widely used in skin bioprinting such as gelatin, collagen, alginate, chitosan and fibrin [15]. They mostly have a high water content, which is similar to the native ECM. Excellent biocompatibility, degradability and low immunogenicity make them preferable in the applications of skin bioprinting. However, the poor mechanical properties and potential immunogenic reactions restrict their applications [11]. Synthetic polymers can be generally classified into nonbiodegradable and biodegradable polymers [16]. Nonbiodegradable synthetic polymers, among which polyethylene glycol (PEG) is the most widely exploited, are more frequently used for engineering bone and cartilage instead of engineering skin [16]. Biodegradable synthetic polymers, such as poly(lactic acid) and poly(ε-caprolactone) (PCL), can degrade naturally at a certain rate [17]. Controllable mechanical properties and structure stability are the outstanding advantages of synthetic polymers [18]. In practical applications, synthetic materials are often modified and blended with natural materials to enhance their properties. Table 1 provides a brief summary of the characteristics, disadvantages and applications of several common natural polymers.
Table 1.
Characteristics and applications of common polymers in skin bioprinting
| Category | Polymers | Gelation mechanism | Biodegradability | Cell binding domains | Disadvantages | Applications |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Common usage | Target structure | Cells | Bioprinting strategy | Ref. | ||||||
| Natural polymers | Gelatin |
|
Yes | Yes |
|
|
|
|
|
|
| Collagen | Thermal | Yes | Yes |
|
|
|
|
|
||
| Alginate | Ionic | No | No |
|
|
|
|
|
||
| Chitosan | pH | Yes | Yes |
|
|
|
|
|
||
| Fibrin | Thrombin | Yes | Yes |
|
Fibrin | Epidermis/dermis | KCs/Fbs | Extrusion | [29] | |
| Synthetic polymers | PEG | Thermoplastic | NO | NO |
|
|
|
|
|
|
| PLA | Thermoplastic | Yes | No |
|
PLA/chitosan/HA | Dermis | Fbs | Extrusion | [32] | |
| PCL | Thermoplastic | Yes | No |
|
PCL/collagen | Epidermis/dermis | KCs/Fbs | Inkjet | [33] | |
AECs, amniotic epithelial cells; CMC, carboxymethyl cellulose; Eps, epidermal progenitors; Fbs, fibroblasts; GelMA, gelatin methacryloyl; HA, hyaluronic acid; KCs, keratinocytes; MCs, melanocytes; MSCs, mesenchymal stem cells; NFC, nano-fibrillated cellulose; PCL, poly(ε-caprolactone); PEG, polyethylene glycol; PLA, poly(lactic acid); PLGA, poly(lactic-co-glycolic acid).
In addition, the highly dynamic wound healing process and the complex microenvironment of skin diseases make higher requirements on biomaterials [34, 35]. Bioinks based on decellularized extracellular matrix (dECM), which can retain part of the structural and functional properties of natural ECM, have been considered a promising choice in recent years [36]. Since dECM is obtained from heterogeneous natural tissues, its greatest advantage distinguished from other biomaterials is that it can provide a greater diversity of structural, chemical and biological cues [37, 38]. It also shows good biocompatibility and printability [39]. Several bioinks based on dECM perform well in the construction of skin substitutes [10, 40–42]. However, removal of histocompatibility complexes from native tissues by physical, chemical or biological means is a necessary step to avoid immune and rejection reactions after transplantation, which inevitably leads to the destruction of the microstructure and bioactive substances in ECM [43]. Optimization of the decellularization process is still a challenge to be overcome in the future.
Physical and chemical properties
Biomaterials not only act as temporary ECM scaffolds, affecting the biological behavior of cells, but also determine whether printing can be carried out successfully [44]. Ideality, during the printing process, bioinks should have strong plasticity and ensure cell viability. Upon completion of printing, it is important to maintain structural stability for a certain period of time and regulate the behavior of cells of the printed construction. When the printed tissue is transplanted on wound or defect of organism, it is necessary to ensure the interaction and integration between them [45]. In view of the above, biomaterials for skin bioprinting should be evaluated according to the following properties: (i) biocompatibility and degradability; (ii) printability; and (iii) biomechanical and biochemical properties [46].
First and foremost, the biomaterials used for bioink should be hypotoxic to encapsulated cells and implanted host as well as their degradation products [44]. In addition to direct toxic damage, negative immune responses should also be avoided. Immune rejection is a great challenge for the in vivo application of skin substitutes [44]. As for the skin wound treatment, biomaterials with excellent biocompatibility could avoid excessive inflammatory reactions and infections. In this term, using biomaterials with bio-inertia or adding bioactive substances to bioinks may be effective [47]. Immunomodulatory biomaterials, which can induce positive immune responses to accelerate wound healing on their own, have also gained attention in recent years. Griffin et al. described a scaffold containing microporous annealed particle for wound treatment. It showed the adaptive immune response from this scaffold induced cutaneous regenerative healing [48]. Meanwhile, biomaterials should have adhesive sites with cells for their adhesion, proliferation and differentiation. Alginate is a commonly used natural polymer due to its good biocompatibility and printability [49]. It can be easily cross-linked into hydrogels by divalent cations, but it needs to be used with other biomaterials since the lack of adhesive sites with cells. De Santis et al. described a tissue-specific hybrid bioink composed of alginate and dECM. The presence of dECM provided adhesive sites for cells and significantly increased the viability and proliferation of epithelial and endothelial cells (ECs). In vivo, 3D-bioprinted constructs promoted angiogenesis and minimized the foreign body response [50]. On the other hand, degradability is the key to accelerate wound repair after implantation by cell delivery and intercellular communication [44]. From this aspect, biomaterials with tunable degradation rates similar to natural ECM are preferred. Alginate/gelatin bioink, performing excellent printability and structural fidelity, is widely used in skin bioprinting [51]. However, the absence of enzymes in mammals that can break down alginate scaffold causes alginate residues in the body, limiting the biological behavior of cells [52]. Huang et al. developed a degradable alginate/gelatin bioink by adding alginate lyase and coordinated the degradation rate and skin wound healing. Through this approach, 3D-bioprinted constructs demonstrated better degradability both in vitro and in vivo. Cellular behaviors, such as cell adhesion, extension and proliferation were also promoted [52].
Printability ensures that the bioink forms a stable model consistent with the predetermined structure without damaging the cells [53]. Although the density and size of cells in bioinks might affect the printability [13], the biomaterials primarily determine the printability. Printability of bioink is defined by the rheological properties of its main biomaterials in hydrogel phase [53]. Bioinks need to be injected smoothly under certain pressure in the printing process and maintain stable structure after printing [54]. Viscosity is one of the basic parameters to evaluate the rheological properties of biomaterials, it refers to the resistance of a fluid to flow upon application of stress [55, 56]. While higher viscosity means stiffer bioink, it may cause damage to cells. Lower viscosity is more friendly to cells, but makes it difficult to form stable structures [13]. Balancing a proper viscosity is an important aspect for the preparation of bioinks. Another important property of biomaterials is shear thinning. Under the high shear rate inside nozzle during printing, shear thinning causes a decrease in viscosity to avoid clogging, as well as avoid damaging cells by high shear stress [57]. The reduction of shear rate after printing in turn increases the viscosity of bioinks, thus ensuring the fidelity of the printed structure. Many approaches have been used to adjust rheological properties and enhance the printability of bioinks, among which changing the concentration or ratio of different biomaterials is more popular [58]. Huang et al. found that the printability of bioink can also been influenced by the solvent through tuning the ionic strength. By adjusting formulations to customize bioink with different solvent ionic strength, the printing performance of gelatin–alginate bioink and the behavior of the embedded stem cells was optimized [59].
The biomaterials used in bioinks are not simply provide scaffolds, they also deliver biomechanical and biological signals for cell survival, which are essential for the function of skin [9]. The distribution and density of ECM proteins in the skin varies across regions, stages of wound healing and pathological states [60], which leads to differences in the biomechanical properties of the skin. Recent studies have declared that mechanosensitive proteins inside cells can sense biomechanical changes of the environment, which influence long-term cellular processes such as differentiation and fibrosis through continuous sensing and cellular memory [61]. Meanwhile, the behavior of cells also generates mechanical forces and deformations in biological materials, which in turn influence the surrounding cells and microenvironment. Therefore, the category of biomaterials should be appropriately selected and mixed according to the required biomechanical properties when preparing different skin substitutes. There are many approaches to optimize the biomechanical properties of bioink, such as utilizing functionalized polymers, supramolecular hydrogels and surface modifications [58]. However, these methods are more complex and costly. Huang et al. explored an easy-to-use method of mechanical enhancement by adding bioactive glass nanoparticles to gelatin–alginate bioink. The mechanical strength, printability and cellular behavior were improved in the bioprinted constructs [62, 63]. Although many physical cues, such as geometry, porosity and topology [64], have been shown to affect various aspects of cell behavior, viscoelasticity is an important factor of mechanical properties for biomaterials to be considered in recent researches. Viscoelasticity is a near-universal feature of living tissues and ECMs [61]. The viscoelastic natural skin exhibits both elastic solid properties that restore the original structure and viscous liquid characteristics that dissipate energy during deformation, which is more critical for applications in vivo rather than in vitro studies [65]. Moreover, viscoelasticity also influences the smoothness of the printing process and the stability of the printed structure [13]. Therefore, viscoelasticity is an important factor to screen suitable biomaterials for skin substitutes bioprinting.
In addition to complex biomechanical signals, cells are also regulated by biochemical signals from neighboring cells or the surrounding microenvironment [65]. The addition of chemokines and cytokines is an effective means to improve the biochemical properties of bioink. However, the abundance of bioactives in natural skin ECM cannot be mimicked by the addition of one or several factors [43], which leads to more attention is focused on the natural cocktails of microenvironmental factors, such as dECM, exosome and platelet-rich plasma (PRP). Zhao et al. constructed a multi-layered skin structure by PRP-containing bioink used for wound treatment. Compared with unmodified bioink, the PRP-containing bioink facilitated vital physiological processes including ECM synthesis, macrophage polarization and angiogenesis [66]. Wang et al. reported that a methylcellulose–chitosan hydrogel loaded with placental mesenchymal stem cell (MSC)-derived exosomes can promote skin wound healing in diabetic mice by enhancing vascularization and reducing apoptosis. In addition to neovascularization, hair follicles and glands were observed in the healed region [67].
Seed cells
As mentioned above, seed cell is important component of bioinks. Skin cells are positioned at high degrees of heterogeneity in a sophisticated microenvironment [68]. To emulate native human skin, the suitable cell type should be determined based on the fundamental structure of skin.
As the largest organ of the human body, skin has developed a three-layered construction [69]. The epidermis can readily regenerate after minor insults, in which keratinocyte is the main cell type [65]. The dermis, mainly comprised of fibroblasts, provides structural support to the epidermis and embedded appendages [69]. The hypodermis, which is mainly consisted of lipocytes, can buffer impact force, store energy and secrete bioactive substances [70]. The presence of appendages (hair follicles, sweat glands, sebaceous glands and nerves, for instance) further increases the structure complexity of skin. Besides keratinocytes and fibroblasts, there are also melanocytes, vascular ECs and immune cells in the skin [69]. Multiple cell types interact with each other and secrete ECM proteins to maintain normal tissue homeostasis and coordinate wound repair [71]. Cellular diversity and spatial structural heterogeneity combine to create the prominent functions of skin such as barrier, thermoregulation and sensation, metabolism, as well as immunity [72].
The basic strategy of skin tissue engineering is to recapitulate epidermis and dermis, which makes fibroblasts and keratinocytes, alone or together, the primary choice [9]. Keratinocytes live across the epidermal region with varying degrees of differentiation [73]. Keratins, a differentiation protein expressed by keratinocytes, help to provide structural supports and to regulate the cell growth and differentiation [74]. Fibroblasts are responsible for the production of ECM, providing skin with sufficient strength and good ductility [75]. Keratinocytes and fibroblasts play an important role in wound healing and pathological development of skin diseases [76]. Though the combination of epidermal keratinocyte and dermal fibroblast is better than being individually used [11], skin equivalents containing only two types of cells simply restore the structure of skin. Great efforts have been made to explore better printing strategies for biomimetic skin models since Lee et al. first deposited keratinocytes and fibroblasts in a stratified arrangement using 3D bioprinting in 2009 [77]. Nowadays, more types of functional cells are applied in the research of wound healing, pigmentation, vascularization, appendage regeneration and even the construction of pathological microenvironment in 3D-bioprinted skin.
Melanocytes are given great hope to construct 3D-printed skin with pigmentation. They reside in the stratum basal layer and produce pigment, which helps to protect the skin from ultraviolet rays [78]. Melanocytes are also part of the skin immunological response and display extensive interactions with other immune cells [79]. The use of melanocytes has long been explored in the construction of skin substitutes, but uneven pigmentation is still a great challenge. Recently, Wei et al. fabricated a uniformly pigmented human skin model using melanocytes, keratinocytes and fibroblasts. The 3D-bioprinted skin showed resembling morphology and similar pigmentation as the natural skin [21]. It might help to address the psychological and physical problems caused by abnormal pigment after skin substitutes transplantation on patients [78].
Under natural conditions, functional cells in the skin obtain nutrients and oxygen through the adjacent vascular system to maintain physiological functions [46]. When skin is injured, blood vessels transport immune cells and bioactive substances to the wound to regulate the regional microenvironment [69]. Furthermore, the reconstruction of blood vessels using vascular ECs within a skin equivalent can improve the longevity of the model. In practice, by combining ECs with other skin cells, researchers have created perfusable vascularized human skin equivalents [80], vascularized pathological models such as atopic dermatitis model [81] and diabetic skin model [82], and even vascularized skin models that support regeneration of the appendages [83]. It is no exaggeration that the use of ECs to construct vascularized skin models is a great breakthrough in 3D bioprinting technology.
The skin equivalents constructed from the above cell types have shown good results in in vitro experiments, but for in vivo application, the host immune response must be taken into account [55]. It is difficult to obtain cells from the damaged host autologously, and the excessive cell demand and long culture time limit the clinical application. Therefore, an alternative cell with low immunogenicity and good proliferation or differentiation capacity is needed. Stem cells are the most promising cells to solve these problems [84]. Many types of stem cells have been applied into skin tissue engineering, such as MSCs, adipose‐derived stem cells (ADSCs) and induced pluripotent stem cells (iPSCs). They can regulate the microenvironment and promote wound repairing, vascularization and appendage regeneration through their strong differentiation and secretion abilities [84]. However, it necessary to fully understand the differentiation process when undifferentiated stem cells are used as a source of differentiated cell types. Multiple factors may lead to deviations in therapeutic outcomes, even tumorigenesis [85]. In addition, ethical issues are obstacles that must be faced for stem cell applications [86].
In general, research on different cells for the construction of 3D-bioprinted skin has been successful preliminary, but there is still a lot of work to fully restore the dynamically varying microenvironment. It is a great challenge to precisely regulate the biological behavior of cells so that they can play their proper role without side effects. Furthermore, it is necessary to choose the appropriate cell types flexibly according to the heterogeneity of tissues, the complexity of microenvironment and the differences of individuals.
Strategies for skin bioprinting
The need for a satisfactory, permanent physiologic replacement of skin as well as a credible and effective model in vitro for scientific researches have long been recognized. Initially, skin substitutes mainly consisted of a single type of cell and/or biomaterials with simple structure. Further development mainly focuses on optimizing the process of the skin bioengineering and shortening the lengthy preparation time [9, 87]. Although giant progress has been gained, limitations such as tedious construction steps, lengthy culture time, high cost, low cell viability and vulnerable to infection constrain further scientific studying and clinical application of bioengineered skin [9, 10]. As an advanced, versatile and innovative biofabricating technology, 3D bioprinting provides a promising platform to create highly sophisticated and multicomponent structures to promote skin tissue engineering for wound healing and disease modeling.
Bioprinting strategies for skin constructs mainly include droplet-based bioprinting, extrusion-based bioprinting, digital light processing bioprinting, in situ bioprinting, cell spheroid-laden bioprinting, etc. The advantages and disadvantages of these bioprinting strategies are summarized in Table 2.
Table 2.
Comparison of various strategies for skin bioprinting
| Bioprinting strategy | Advantages | Disadvantages |
|---|---|---|
| Inkjet bioprinting | Widely used | Thermal and mechanical stress to cells |
| High printing speed | Limited printable materials | |
| High resolution | Low cell concentration | |
| High cell viability | ||
| Low cost | ||
| Laser-assisted bioprinting | Non-contacting process | Limited printable materials |
| Nozzle free | High cost | |
| High precision | Time-consuming | |
| High concentration and high viability of cells | ||
| Electrohydrodynamic jet printing | High precision | High cost |
| High structural integrity | Limited printable materials | |
| Extrusion-based bioprinting | Widely used | Limited printing accuracy |
| Good compatibility with materials | The need for gelation and shear thinning properties of materials | |
| Digital light processing bioprinting | High printing speed and consistency | The need for photocuring properties of materials |
| High structural integrity and mechanical property | ||
| High precision | ||
| In situ bioprinting | ||
| Automated | Automated fabrication process | Complicated scanning modality |
| In situ cross-linking | Sufficient room for operation | |
| Minimal invasion | Low degree of freedom | |
| Handheld | Low cost | Experience-dependence |
| Portability | Low resolution | |
| Convenient for sterilization | Non-uniform deposition | |
| Cell spheroid-laden bioprinting | Pre-aggregation of cells | Limited size of spheroids |
| Precise positioning and arrangement of cells | Complex steps for construction | |
| 4D bioprinting | Recreation of spatiotemporal factors | Limited printable materials |
| Bioprinted skin-on-a-chip | Presence of biochemical and biomechanical cues | Complex process for construction |
| Presence of multi-cell/multi-organ interactions | ||
| Microfluidics-assisted extrusion bioprinting | Precise deposition of hydrogels | Complex process for construction |
| Good repeatability | ||
Droplet-based bioprinting
Droplet-based bioprinting originates from inkjet printing technology, which could be traced back in the 1950s when the first practical inkjet device was invented [88]. According to the principle of droplet formation, droplet-based bioprinting can be mainly classified into three major categories, namely inkjet bioprinting, laser-assisted bioprinting and electrohydrodynamic jet bioprinting [89, 90].
Inkjet bioprinting can be divided into two categories: continuous-inkjet bioprinting and drop-on-demand inkjet bioprinting. In brief, continuous-inkjet bioprinting relies on the inherent tendency of bioink solution to flow through a nozzle under pressure and subsequently shatter into continuous droplets owing to Rayleigh–Plateau instability. Electrically conductive drops of bioink can be easily steered to their specific locations under the influence of electric or magnetic force. For drop-on-demand inkjet bioprinting, pressure pulses are introduced through a thermal or a piezoelectric or an electrostatic actuator. Continuous-inkjet bioprinters generate droplet at a relatively faster rate, while drop-on-demand inkjet bioprinters generate droplets when required [7, 88]. Inkjet bioprinting has been frequently used in skin engineering and regenerative medicine [9, 88]. Albanna et al. creatively combined inkjet bioprinting with wound scanning imaging technology to ensure the accurate delivery of appropriate materials and cells to specific areas of the wound. Results showed that in situ inkjet bioprinting of autologous skin cells accelerated wound healing of extensive full-thickness wound [91]. Kim et al. modified a 3D bioprinting system by combining inkjet and extrusion printing modules to construct a vascularized skin model where the epidermal compartment was fabricated via inkjet bioprinting. This bioprinted skin model with a functional transwell system revealed favorable biological characteristics including a stabilized fibroblast-stretched dermis and stratified epidermis layers after 14 days, providing a cost-efficient and attractive research platform [33].
Laser-assisted bioprinting relies on laser-induced driving force to propel the cell-laden bioink onto a collector substrate, including laser-guided direct writing and laser-induced forward transfer (LIFT) as well as emerging technologies derived mainly from LIFT (absorbing film-assisted LIFT, biological laser processing, etc.) [89, 92]. This technology is versatile to fabricate tissue constructs with high resolution, high cell density and viability in a nozzle-free and non-contact way. Limitations include high demands on physical properties of bioink, high cost and time-consuming [7, 9]. Koch et al. successfully created a simple skin substitute with fibroblasts and keratinocytes embedded in collagen hydrogel via laser-assisted bioprinting. The bioprinted skin substitute precisely mimicked natural skin cell localization and gap junction formation [93].
Electrohydrodynamic jet printing employs an electric field to pull the bioink through the orifice obviating the need for a substantially high pressure. The electric field leads to the accumulation of mobile ions in the bioinks as well as the deformation of suspended bioink meniscus at the orifice. Bioink droplets are ejected when the electrostatic stresses overcome the surface tension at the orifice [88, 90, 94]. Recent studies of electrohydrodynamic printing in the field of skin engineering and regenerative medicine mainly focus on the fabrication of skin scaffolds and novel percutaneous therapy devices such as microneedles and thermotherapy patches [95–98].
Extrusion-based bioprinting
Extrusion-based bioprinting is the most popular and widely used bioprinting technology to create 3D cell-laden constructs for research and clinical practice [7, 9]. Commercial extrusion-based bioprinter usually includes a bioink dispersing system with one or more printheads, a temperature control module and a program control platform. Extrusion-based bioprinting employs pneumatic pressure or mechanical (usually piston or screw) force to disperse a premixed cell-laden bioink from the nozzle to a collector plate. The printhead together with printing cartridge provides the movement in x-, y- and z-axis. Filaments are generated according to pre-designed 3D CAD models layer-by-layer [9, 89]. Extrusion-based bioprinting demands viscous bioinks so as to maintain the shape of the construct before chemical, thermal or photo-cross-linked [99, 100].
Extrusion-based bioprinting has many advantages such as high cell viability, high compatibility, great flexibility and versatility. Notably, other bioengineering technologies, e.g. coaxial bioprinting, multi-material bioprinting and microfabrication technologies, can be combined with extrusion-based bioprinting for skin bioengineering [33, 82, 101, 102]. Micro-extrusion bioprinting has boosted models with great potential to emulate the microstructure of natural skin [103]. The pioneering work of Huang et al. demonstrated successful regeneration of sweat glands in the extrusion-bioprinted niche [24, 104]. Other applications of extrusion-based bioprinting, including cutaneous wound healing, vascularized substitutes formation, skin appendages regeneration and skin disease modeling were elaborated by recent fantastic reviews [58, 105, 106].
Digital light processing bioprinting
Digital light processing bioprinting, as a kind of photocuring-based bioprinting, utilizes photosensitive polymers and precisely controlled lighting to obtain constructs with high resolution and stable structure [89]. Recent researches on photoactivated materials and bioprinting strategies have been reviewed by Lee et al. In brief, exciting progress has been made in the field of photocuring materials, especially in the construction of cell-laden scaffolds [107]. Digital light processing bioprinting has a considerable advantage in printing speed and consistency, and improves structural integrity and mechanical property [9]. Zhou et al. employed digital light processing bioprinting to fabricate a functional skin substitute with integrated vasculature. The well-formed interconnected microchannels facilitated gas/nutrient exchange which supported better cell behaviors in vitro, and the deposition of well-organized collagen fibers as well as the regeneration of skin appendages indicated the faster and better healing process in vivo [83].
In situ bioprinting
Significant progress has been made in developing 3D bioprinting technologies and versatile bioinks to facilitate complex tissue regeneration. However, most of the traditional bioprinting strategies form bioprinted constructs with specific shapes on flat surfaces, resulting in the unmatched bioprinted constructs to the defect site. Besides, bioprinted constructs in petri dish do not adhere properly to the host tissue [108, 109]. In situ bioprinting, also known as in vivo bioprinting or intraoperative bioprinting, literally directs depositing bioinks inside the defect in vivo and has been introduced as an alternative strategy for translation of bioprinting from bench to bedside [110, 111]. Samandari et al. systemically reviewed the emerging in situ bioprinting strategies of various kinds and specific requirements of bioinks for in situ bioprinting [108]. With an in situ bioprinter, the operator can immediately apply the treatment by manufacturing customized constructs which accurately match the defect geometry and in situ cross-linked to enhance the mechanical strength. Skin was identified as an optimal target organ to perform in situ bioprinting without open surgery [111]. In situ bioprinting can be classified into two major categories, namely automated in situ bioprinting and handheld in situ bioprinting.
Automated in situ bioprinting is similar to most conventional bioprinting strategies in terms of the automated mode. Once the computer-aided design is loaded into the software, the bioprinter automatically fabricates a 3D scaffold. The automated system ensures high printing accuracy, rapid fabrication rate, minimal invasion and human errors [108, 110, 112, 113]. The drawbacks are the complicated scanning modality, more room for operation and low degree of freedom. Modulations such as adaptive bioprinting and error compensation before bioprinting have been adopted to solve these problems. Various bioprinting strategies, such as extrusion-based bioprinting, inkjet bioprinting and stereolithography bioprinting, have been adapted for automated in situ printing for the treatment of skin wounds (Table 3).
Table 3.
Application of in situ bioprinting in skin regeneration and wound repair
| Printing strategy | Scanning approach | Bioink | Laden cells | Models | Ref. |
|---|---|---|---|---|---|
| Automatic | |||||
| Extrusion-based bioprinting | NA | Fibrinogen + collagen | AFSCs, BMSCs | Murine full-thickness skin wound model | [118] |
| NA | Thiolated HA + thiolated gelatin + PEGDA | AFSCs | Murine full-thickness skin wound model | [119] | |
| (For soft tissue repair) droplet-based bioprinting | 3D laser scanner | (For soft tissue repair) fibrinogen + collagen + KGF | (For soft tissue repair) dermal fibroblasts | Murine composite skin and calvarial bone defect | [114] |
| Inkjet-based bioprinting | 3D laser scanner | Fibrinogen + collagen | Dermal fibroblasts, epidermal keratinocytes | Murine and porcine full-thickness skin wounds | [91] |
| Two-photon bioprinting | NA | HCC-PEG | Muscular fibroblasts | Printing inside murine normal dermis | [111] |
| Handheld | |||||
| Extrusion-based bioprinting | Not required | GelMA + VEGF | NA | Porcine full-thickness skin wound model | [109] |
| Fibrinogen + HA | UMSCs | Porcine full-thickness skin burn injury model | [116] | ||
| Alginate + collagen; fibrinogen + HA + collagen | (For in vitro experiments) dermal fibroblasts, epidermal keratinocytes |
Murine and porcine full-thickness skin wounds | [117] | ||
| Alginate + gelatin + PRP | Dermal fibroblasts, epidermal stem cells | Murine full-thickness skin wound model | [66] | ||
AFSCs, amniotic fluid-derived stem cells; BMSCs, bone marrow-derived mesenchymal stem cells; GelMA, gelatin methacryloyl; HA, hyaluronic acid; HCC, 7-hydroxycoumarin-3-carboxylate; KGF, keratinocyte growth factor; NA, not applicable; PEG, polyethylene glycol; PRP, platelet-rich plasma; UMSCs, umbilical cord-derived mesenchymal stromal cells; VEGF, vascular endothelial growth factor.
Handheld in situ bioprinting is recognized as a simplified and promising bioprinting strategy, which is more economical, portable, minimal-invasive and easy to be sterilized [108]. Several handheld in situ printers have been developed to treat skin wounds, most of which rely on extrusion-based bioprinting (Table 3). As a manually operated and experience-dependent technology, handheld in situ printing has its inherent weaknesses such as lower resolution, non-uniform deposition and lack of precision in reconstruction of anatomically correct shapes [108, 114]. Alternative strategies, e.g. employing a human-controlled robotic-assisted bioprinting system, have been developed as remedies for handheld in situ bioprinting [115–117].
Cell spheroid-laden bioprinting
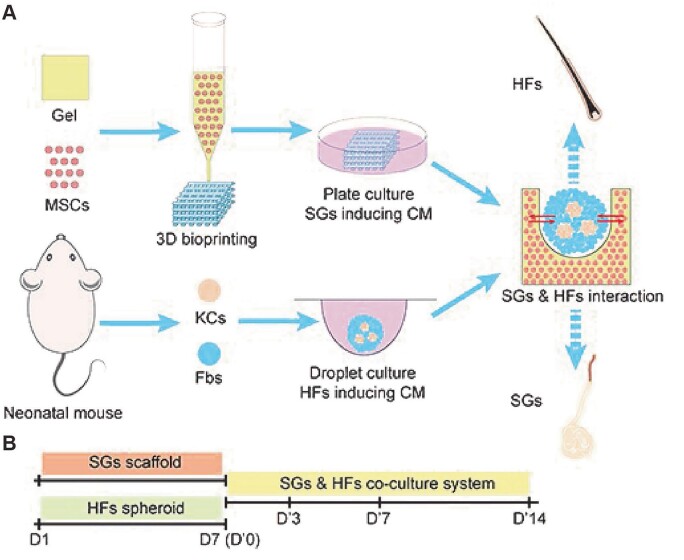
Owing to the distinct characteristics including self-renewal ability and high proliferative potential for different lineage differentiation, a variety of stem cells have been applied to bioprint 3D skin substitutes [103]. The precise positioning and arrangement of stem cells are essential for the reconstruction of complex tissue architecture [120, 121]. Cell spheroids and aggregations have advantages to mimic the native structure and microenvironment [120, 122, 123]. Combining cell spheroids with 3D bioprinting technologies, cell spheroid-laden bioprinting has increasingly been employed to answer a wide variety of clinical and biomedical inquiries ranging from pathophysiological and pharmacology research to tissue engineering and regeneration [102, 122–124]. Recent progress of cell spheroid-laden bioprinting in skin engineering mainly focuses on appendages regeneration. For example, a novel sweat gland scaffold was fabricated via extrusion-based bioprinting with hair follicle spheroids seeded on top, realizing the symbiosis of sweat glands and hair follicles in vitro (Fig. 2) [125].
Figure 2.
Establishment of 3D skin constructs with multiple appendages. (A) Schematic diagram showing the procedure to establish 3D skin constructs in vitro. (B) Time points used in inducing SGs and HFs separately and SG–HF co-culture. Adapted with permission from Zhang et al. [125].
Other strategies
In addition to the strategies reviewed above, other emerging technologies, for instance, four-dimensional (4D) bioprinting [126–128], bioprinted skin-on-a-chip [129–131], and microfluidics-assisted extrusion bioprinting [9, 132–134], are being or to be applied for skin tissue engineering and wound healing researches as well. Derived from 3D bioprinting, 4D bioprinting may have the potential to recreate the spatiotemporal changes in tissue geometry and transformations in the spatial distribution of cells and ECM [128]. Recently, Douillet et al. developed a versatile in vitro tool by means of patterning fibroblasts on collagen gels via laser-based 4D bioprinting to investigate different aspects of matrix remodeling. This model contributed to a better understanding of morphogenetic mechanisms, dermal organizations and remodeling process in healing [126]. In terms of wound healing, pH and temperature-responsive bioprinted multifunctional dressings were fabricated to provide signals of wound inflammation as well as release the drugs as needed [135, 136]. Skin-on-a-chip technology combines advancements in tissue engineering and microfluidics to reproduce critical functions of natural skin. The key features of the skin-on-a-chip include the presence of biochemical and biomechanical cues and the integration of multiple cell types to model the intricate interactions. Mori et al. fabricated ECs-coated vascular channels within a cultured skin equivalent to form a skin-on-a-chip. The skin-on-a-chip, which contained vascular channels with good delivery and absorption functions, proved to be a promising platform for drug development, cosmetics testing and skin biology research [130]. Microfluidics-assisted extrusion bioprinting has been explored for the precise fabrication of skin constructs in recent decades. Leng et al. developed a 3D bioprinting system based on a microfluidics approach to print human fibroblasts-laden alginate hydrogel into a sheet-like format to fabricate skin tissue-like structures. Further results indicated a faster healing and keratinization in a murine wound model [137]. Cheng et al. developed a handheld bioprinter with a microfluidic printhead for uniform distribution of MSC-containing fibrin gel directly to the wound, which promoted re-epithelialization, dermal cell proliferation and neovascularization in a porcine full-thickness burn model [116].
Applications of 3D skin bioprinting
3D-bioprinted skin constructs for wound healing
Wound healing process is a coordinated cascade of interacted events to restore the skin integrity, involving an integrated action of multiple cells and bioactive molecules [9, 11, 138]. Typically, wound healing process can be sectioned into four overlapping and consistent phases, namely hemostasis, inflammation, proliferation and remodeling. After injury, platelets and the clotting cascade are quickly activated, releasing cytokines, inflammatory and growth factors. Fibrin along with platelets, red blood cells and inflammatory cells forms a temporary protective layer at the wound site [87]. Neutrophils and monocyte–macrophages as well as various growth factors and cytokines take part in the removal of debris and foreign matter [139]. The tissue repair process starts with the formation of granulation tissue and is intensified by the migration and proliferation of fibroblasts, keratinocytes and ECs, namely collagen deposition, epithelialization and angiogenesis. At the final stage of wound healing, collagen is rearranged to form regularly oriented collagen bundles under the effect of mechanical force and other factors. In the meanwhile, the fibroblast differentiates into the myofibroblast phenotype, which is considered a characteristic feature of scarring [140, 141].
Skin wound is common in daily life. However, injuries to large surface areas, such as massive burns and extensive trauma, as well as refractory wounds with underlying diseases complicate the healing process. 3D-bioprinted skin constructs are considered to be ideal models for the research of skin cell behavior and wound repair process under different conditions. Douillet et al. fabricated an in vitro model that could replicate fibroblast–myofibroblast dynamic remodeling. Fibroblasts were patterned on collagen gels with laser-assisted bioprinting and the results showed that the maturation of anisotropic fibroblast patterns, instead of myofibroblasts, resulted in collagen anisotropic reorganization [126]. Sarmin et al. employed dECM-enhanced multicellular (immortalized keratinocytes, dermal fibroblasts and THP-1 monocytes) fibrin bioink to develop a flexible and tunable model for wound healing. The bioink was deposited on a sectionalized silicone frame and Pluronic F127 was employed as a sacrificial wound template to generate wounds of varying sizes and shapes [142]. Kim et al. fabricated a diabetic skin model with structural similarities and diabetic properties by combining inkjet, extrusion and coaxial bioprinting with a PCL transwell system. Human epidermal keratinocytes, diabetic dermal fibroblasts, diabetic preadipocytes and human umbilical vein endothelial cells (HUVECs) were encapsulated in different bioinks respectively. To create a wound, a printing needle was located at the center of the model. Typical hallmarks in diabetes, such as insulin resistance, adipocyte hypertrophy, inflammatory reactions, vascular dysfunction and slow re-epithelization after wound, were successfully observed in the diabetic skin model [82].
The restoration of skin structure and function remains an intractable problem to date. 3D bioprinting offers an attractive and competitive solution to construct patient-specific skin grafts with multi-layered biomimetic structures [143]. Jin et al. utilized acellular dermal matrix-enhanced gelatin methacryloyl (GelMA) bioink to develop a novel 3D full-thickness skin construct where human epidermal (HaCaT) cells, fibroblasts and HUVECs were, respectively, encapsulated into different concentrations of GelMA to simulate the stratified structure of the natural skin. The bioprinted functional skin accelerated wound healing and re-epithelization, and stimulated dermal ECM secretion and angiogenesis [144]. Zhao et al. employed PRP-integrated alginate–gelatin bioink to fabricate 3D skin constructs embedded with dermal fibroblasts and epidermal stem cells via in situ extrusion bioprinting. The skin constructs accelerated wound closure, modulated the inflammation process and initiated angiogenesis at the wound site [145]. Compared to conventional skin tissue engineering approaches, 3D bioprinting offers many advantages in complex structure construction, spatial integration and reproducibility. In addition, bioprinted skin constructs may help to improve regeneration and decrease intervention, thus conducive to obtain better clinical outcomes [9, 87].
3D skin bioprinting for appendages regeneration
Skin appendages, including sweat glands, hair follicles and sebaceous glands, are responsible for maintaining skin barrier function, regulating body temperature and aesthetic function [146]. Abundant researches revealed the origination and regulatory mechanism of skin appendages with their microenvironment [147–149]. In vitro-induced differentiation of skin appendages and symbiotic culture of them have been achieved in labs, and multifarious bioengineered skin equivalents seeded with induced stem cells or spheroids have been constructed, which promoted the functional regeneration of skin tissues at the wound site [24, 125, 146, 150]. Notably, recent studies have reported remarkable results of the reconstruction of skin with functional appendages via 3D bioprinting [24, 151, 152].
Sweat gland
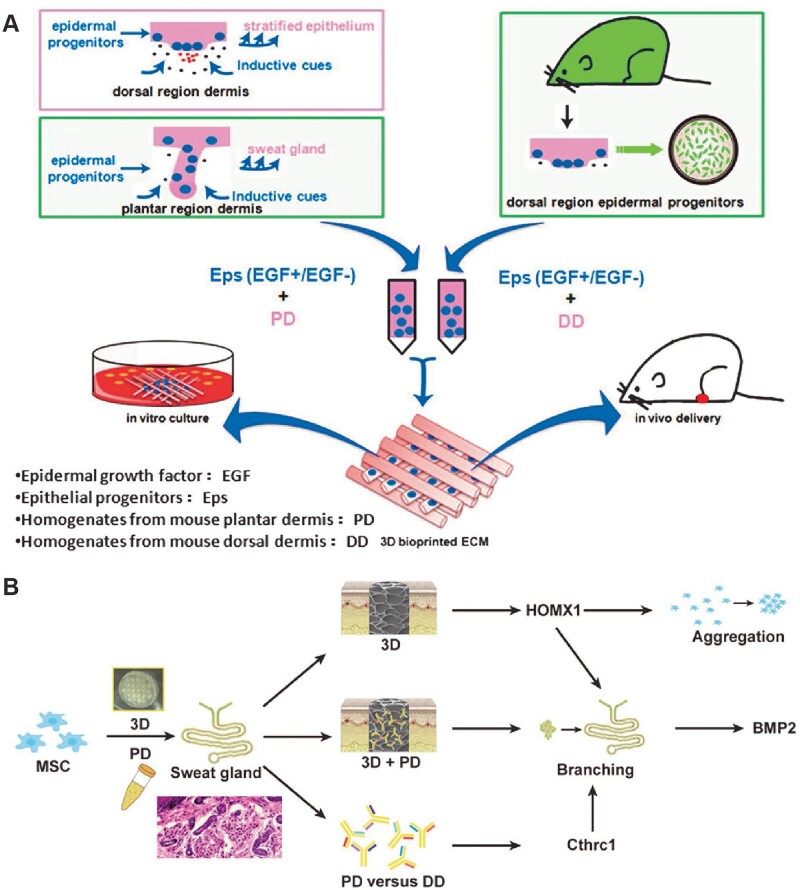
Sweat glands can be divided into eccrine sweat glands and apocrine sweat glands, of which the former is mainly responsible for thermoregulation and sweating. Patients with extensive trauma and deep burn would suffer from sweat gland regeneration disorder and endure the pain of being unable to sweat and dissipate heat in hot environment. In terms of in vitro reconstruction of the sweat glands, Huang et al. initiatively fabricated 3D-bioprinted ECM containing mouse PD homogenate to create an inductive niche for sweat gland differentiation of mouse epidermal progenitor cells. Meanwhile, in vivo transplantation of the bioprinted constructs resulted in functional restoration of sweat glands at the burned paws of mice (Fig. 3A) [24, 104]. Yao et al. employed extrusion-based bioprinting to construct a sweat gland microenvironment in vitro to drive MSCs differentiation into functional sweat gland. Biochemical and structural cues were identified as two critical impacts of 3D-printed matrix on MSCs fate decision into the glandular lineage. In addition, the results revealed that Collagen Triple Helix Repeat Containing 1 (CTHRC1), a critical biochemical regulator for sweat gland specification, and heme oxygenase 1 (Hmox1), an important gene involved in MSCs differentiation and biomechanical activation, were two essential factors to boost sweat gland gene expression profile (Fig. 3B) [153]. Song et al. comprehensively reviewed recent progress in bioprinting-assisted sweat gland reconstruction and innovatively outlined different aspects of the biomimetic microenvironment indispensable for sweat gland regeneration [106].
Figure 3.
In vitro bioprinted microenvironment for sweat gland regeneration. (A) Schematic of the printing process with epidermal progenitors and ECM incorporated in composite hydrogels to mimic the microenvironment for sweat gland regeneration. (B) The graphic illustration of 3D-bioprinted matrix directed MSC differentiation. CTHRC1 is the main biochemical cues during SG development, and structural cues up-regulated the expression of Hmox1 synergistically initiated branching morphogenesis of SG. Adapted with permission from Huang et al. and Yao et al. [24, 153].
Hair follicle
Hair follicles are composed of hair papillae, hair matrix, root sheath and hair bulges. In vitro reconstruction and in vivo regeneration of hair follicles have always been a hot issue. Recent studies revealed a road map for the development of hair follicles, providing guidance for the construction of hair follicles. In terms of bioprinting for hair follicles, Zhang et al. reported the symbiosis of sweat glands and hair follicles in vitro: 3D-bioprinted porous construct containing induced sweat gland cells was seeded with hanging drop cultured hair follicle spheroids to permit interdependent regeneration of sweat glands and hair follicles. The mutual inhibitory effect was revealed in this 3D-bioprinted skin constructs, indicating the sequential genesis of multiple appendages in skin regeneration (Fig. 2) [125]. Kang et al. fabricated a multi-layered scaffold for dermal papilla cells to regenerate entire hair follicles in vivo via 3D bioprinting. Fibroblasts, HUVECs, dermal papilla cells and epidermal cells were encapsulated in gelatin–alginate hydrogel, respectively, to bioprint each layer of the scaffold to simulate the microenvironment required for hair follicle reconstruction [152]. Nanmo et al. proposed an efficient approach for the scalable and automated preparation of highly hair-inductive grafts by multiple nozzle bioprinting [151]. Lian et al. employed coaxial vertical bioprinting to reconstruct human hair follicles in a multi-segment filament manner. Human dermal papilla cell-laden GelMA and human keratinocyte-laden gelatin were simultaneously but independently embedded in human dermal fibroblast-laden GelMA, thus providing complex microenvironments for the regeneration of hair follicles [101].
Sebaceous gland
Sebaceous glands are composed of numerous acini branching off a central duct [154]. The regeneration of sebaceous glands has also attracted attention in recent years. In terms of in vitro reconstruction of the sebaceous glands, Chen et al. employed poly-caprolactone, which was coated with decellularized matrix of ADSCs, to fabricate tarsal plate scaffolds via 3D printing technology. The biocompatible scaffolds were seeded with immortalized human SZ95 sebocytes. In vivo experiments revealed excellent sebocytes proliferation and lipogenesis on the scaffolds, which made it a feasible strategy for tarsal plate regeneration [155].
3D skin-bioprinted models for in vitro medical searches
Animal models and two-dimensional (2D) cultured cells are not always effective at discovering human pathophysiological and toxicological responses. Numerous instances of significant differences between the disease phenotypes observed in animal models and humans have been reported [76, 156, 157]. Cells in 2D petri dish could not exactly mimic native cell behavior and the complexity interactions with the microenvironment in vivo [158]. In comparison, in vitro physiological and pathological 3D skin models can overcome these limitations and replicate in vivo biomechanical and biochemical cues, providing an efficacious and promising platform for mechanism/therapeutic research and pharmaceutical/cosmetic screening.
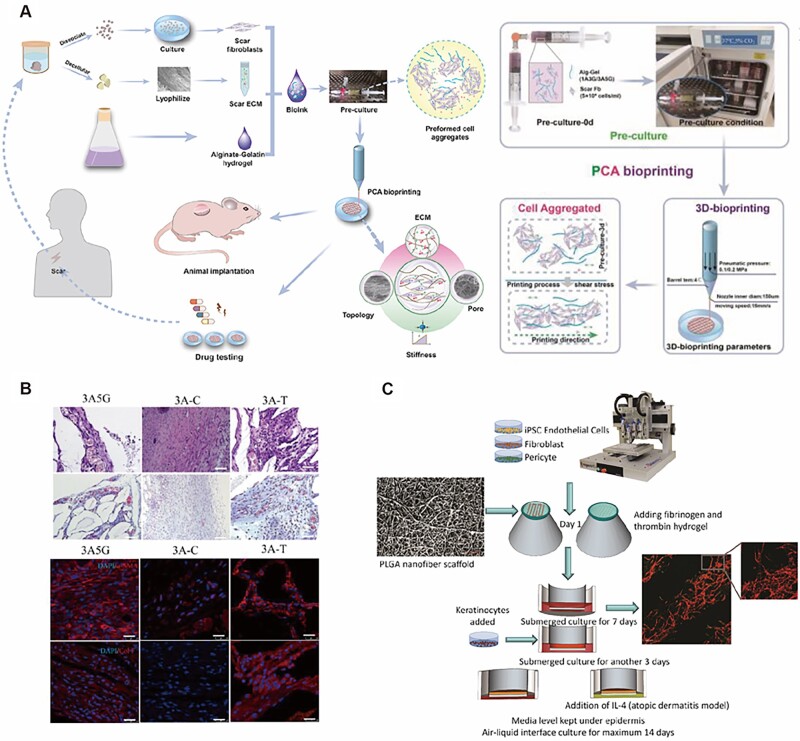
As an emerging fabrication method allowing automated, standardized, massive, and faster production, 3D bioprinting is employed to create multifarious skin disease models, such as hypertrophic scar models, atopic dermatitis models and melanoma models. Yao et al. fabricated scar dECM-enhanced alginate–gelatin hydrogel to construct a 3D functional human hypertrophic scar model by preformed cellular aggregates bioprinting (Fig. 4A). Gene and protein expression showed that this model exhibited characteristics of early-stage hypertrophic scar. Responsiveness to clinical anti-scarring drugs demonstrated its potential for drug testing (Fig. 4B) [159]. Liu et al. employed human keratinocytes, fibroblasts, pericytes and iPSC-derived ECs to fabricate a vascularized full-thickness skin equivalent via 3D bioprinting, in which interleukin-4 was added to generate the atopic dermatitis model. Specific clinical hallmarks present in the models, such as spongiosis and hyperplasia, differential expression of differentiation proteins and increased levels of pro-inflammatory cytokines, demonstrated its successful construction. Anti-inflammatory corticosteroid and Janus Kinase inhibitors for atopic dermatitis resulted in morphology and functional restoration in this bioprinted models (Fig. 4C) [81]. Duan et al. used 3D bioprinting to construct GelMA-polyethylene (glycol) diacrylate composite scaffolds for human malignant melanoma cell growth. The results showed a high aggregation, expansion and differentiation of melanoma cells on the bioprinted scaffolds [160]. Similarly, Jeong et al. constructed a bioprinted collagen scaffolds which could improve the maintenance and survival rate of cryopreserved patients-derived melanoma explants, providing an alternative way to in vitro construct melanoma models [161].
Figure 4.
Modeling human hypertrophic scars and atopic dermatitis with 3Dbioprinting. (A) Schematic illustration of the whole process of fabrication of scar model and the details of PCA bioprinting. (B) HE (scale bar = 200 μm), Masson images (scale bar = 200 μm) and the expression of myofibroblasts markers of scar tissue in different groups (α-SMA, col I: red, DAPI: blue, scale bar = 100 μm). 3A5G: 3A5G+SFb+scar ECM, 3A–C: 3ASS+ cobimetinib, 3A–T: 3ASS+ triamcinolone acetonide. (C) Bioprinting steps of vascularized full-thickness skin equivalent and diseased condition. Poly(lactic-co-glycolic acid) nanofibers scaffold was taken using Keyence 3D laser scanning confocal microscope (scale bar: 20 μm). Vascularization in the dermis equivalent after 1 week of culture (CD31, red, laminin, green, scale bar: 200 μm). Adapted with permission from Liu et al. [81] and Bin et al. [159].
3D-bioprinted skin models and skin-on-a-chip models enable the pharmaceutical or cosmetic screening in a high-throughput, high-fidelity, cost-efficient and time-saving manner. Both pharmaceuticals and cosmetics must be assessed for potential toxic and allergic effects prior to the further testing such as pharmacokinetic and pharmacodynamic studies [162–164]. Especially, with the total ban of animal trials for cosmetic purposes in growing numbers of countries and regions, there is a strong demand for skin substitutes that could serve as an alternative to animal experiments [164]. Various engineered skin models have been developed to meet the urgent need [132, 163]. Some skin-on-a-chip models combine the basic components with perfusion pumps or even with other organ-on-a-chip models or organoids to examine multi-cell and multi-organ interactions especially in more complicated cases [129, 134]. Although most of them remain to be further validated in terms of both the fabrication process and the efficacy in toxicity and allergenicity testing, they offer the possibility of measuring the impact of subjects on skin main compartments with high throughput and efficiency.
Vascularization of 3D-bioprinted skin substitutes
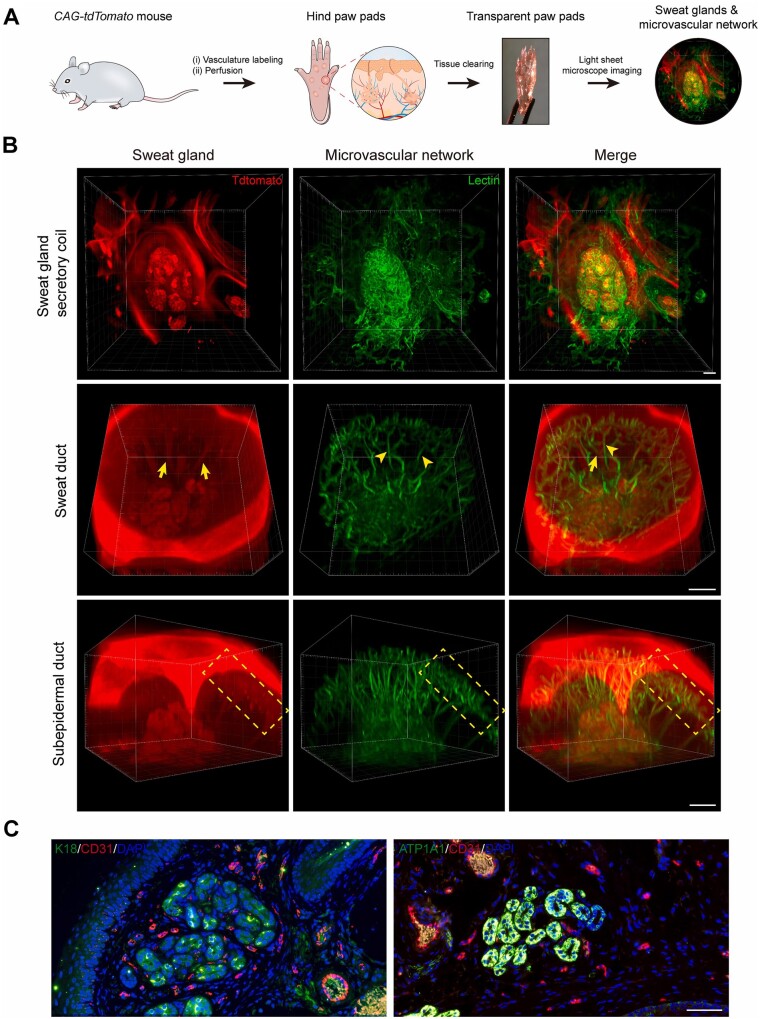
The complex and mature vasculature of the skin structure creates a dynamic microenvironment, which is critical to the function of skin tissue in both physiological and pathological conditions [165]. The role of blood vessels in the skin is not limited to the transport of nutrients; in the case of hair follicles, the interactions with blood vessels regulate the activation of hair follicle stem cells to maintain homeostasis, which may have positive implications for hair follicle regeneration [166]. Recently, Huang et al. further found that blood vessels are inextricably associated with the morphogenesis and regeneration of sweat glands via tissue clearing technique (Fig. 5) [167], which suggested that vascularization may be an indispensable step in the construction of skin substitutes containing functional appendages. Moreover, dynamic development of the vascular system is present at every stage of the wound healing process, and abnormalities at any stage can lead to abnormal healing [69, 168]. Changes of the vascular system also contribute to the development of many skin diseases, especially autoimmune diseases and skin tumors [169, 170]. Therefore, researches in skin bioprinting have increasingly focused on the realization of vascularization to realistically reproduce the skin microenvironment in recent years.
Figure 5.
Determine the anatomy of SGs and their surrounding microvasculature using tissue clearing technique and histological staining. (A) Schematic showing 3D imaging of the SG-vasculature anatomical structure by using the tissue-clearing technique. (B) Light-sheet microscopy images showing the close proximity of SGs and their vascular beds after optical clearing. Arrows and arrowheads indicate SGs and microvessels, respectively. Dashed boxes highlight the subepidermal ducts and nearby vascular arcades. (C) Conventional histological sections confirming the capillaries (CD31) closely surround the SGs (K18 and ATP1A1). CD31, red; K18, ATP1A1, green; DAPI, blue. Scale bars: (B) 100 μm; (C) 50 μm. Adapted with permission from Yuan et al. [167].
Many methods have been explored to construct vascularized 3D printed skin, which can be roughly divided into pre-vascularization in vitro and induced vascularization in vivo [171]. The most common method for pre-vascularization in vitro is performed by using sacrificial materials. In other words, sacrificial biomaterials are first embedded in the printed structure, followed by dissolving the sacrificial biomaterials to form hollow channels, and finally perfusing vascular ECs, which can adhere to the channel surface and form vascular-like structures [172]. More recently, coaxial bioprinting, one technique of extrusion-based bioprinting for stereoscopic concentric structure, makes large-scale fabrication of vascularized tissue a reality without complex bioink removal procedures [173, 174]. Hong et al. described a vascularized construct by using gelatin–PEG–tyramine prepolymer containing fibroblasts as a shell layer encircling gelatin-based bioink containing ECs via coaxial bioprinting. After in vitro incubation, the construct formed an ECs-lined hollow structure and maintained for up to 8 days in vitro, which provided new ideas for bioprinting of perfusable multicellular blood vessel in skin substitutes [175].
However, blood vessels formed directly by bioprinting in vitro relatively deviate from the complex network of microvessels in the skin in terms of diameter and morphology [165, 176]. Inducing formation of blood vessels in vivo by adding growth factors or modulating mechanical and topological cues of the printed tissue after transplanted is expected to overcome this challenge. Vascular endothelial growth factor [177], basic fibroblast growth factor [178], platelet-derived growth factor [179] and stem cell-derived exosomes [180] have been proved to promote neovascularization effectively. Meanwhile, ECs can sense the mechanical signals and microtopography of the 3D environment and change their behavior [181]. Stiffer matrix inhibits migration of ECs and microvascular assembly [182, 183]. Another research showed that uniaxially aligned scaffolds can improve pre-vascularization using porous freeze-dried collagen scaffolds compared with randomly oriented pores [184].
The self-assembly ability of ECs also deserves to be taken into account. Baltazar et al. described an implantable multi-layered vascularized bioengineered skin graft using 3D bioprinting. The 3D printed construct is composed of human keratinocytes, fibroblasts, ECs and placental pericytes. In vitro, the placental pericytes and ECs self-assemble into interconnected microvascular networks. After implanted, the networks inosculated with mouse microvessels within the wound bed and become perfused in 4 weeks [185]. What cannot be ignored is that inducing neovascularization in vivo is less controllable than constructing vascular channels in vitro directly before transplanted, potentially leading to excessive vascularization and causing side effects.
All of the above developments have contributed to the advancement of vascularized printed skin, but there are still many issues that need to be addressed. The vascular system within the skin is composed of a network that span multiple orders of magnitude in diameter [186]. It contains arterial, venous and microcirculatory systems with different structure, but most of the current methods for pre-vascularization in vitro only construct a tubular single-cell layer [187]. Recapitulating the complex multiscale vascular architecture is still a great challenge. In addition to being a transport channel, blood vessels should also act as a tight barrier, which is necessary to be evaluated after constructed [181]. The issue of the source of ECs deserves equal attention because of the limited amount of autologous supply and the low efficiency of induction using stem cells. Finally, the construction of skin disease models containing specific vascular alterations remains a major obstacle. There is still a long way for the exploration of vascularized skin models.
Limitations
Numerous 3D skin bioprinting strategies have been reported in the recent decades, which could be regarded as a giant leap for skin tissue engineering. However, few of them have been applied for clinical translation. It is still a long way to finally achieve the functional regeneration of skin by 3D bioprinting. There are several limitations of current 3D skin bioprinting research and development. First, the biocompatibility and mechanical strength of bioink is compromised by the pursuit of perfect printability. For example, collagen owns perfect skin compatibility but poor extrusion property and mechanical strength, which should be modified by mixing printable biomaterials with less compatibility, like gelatin and sodium alginate [188]. Therefore, it is still a challenge to discover perfect biomaterial or blend biomaterials with both promising printability and compatibility for 3D skin bioprinting. Second, there are no efficient vascularization strategies for bioprinted skin substitutes. Vascularization is the first to determine the survival of bioprinted skin substitutes on recipient wound bed. To date, existing vascularization strategies for 3D skin bioprinting, however, could not achieve vascular anastomosis between skin substitute and recipient wound bed within 72 h, indicates lower survival rate of bioprinted skin than skin grafts [189]. Finally, 3D-bioprinted full-functional skin substitute with nerve, vessel and lymph integration is still a theoretical model in lab. The full-function relies on the coordination of bioprinted skin appendages with recipient nerve, vessel and lymph [190]. For example, bioprinted sweat glands embedded in skin substitutes could be functionalized by recipient neural control and sufficient blood supply for exocrine sweat [191]. It is the main difficulty to figure out the interaction between skin appendages and nerve/vessel in future research.
Additionally, the problems in clinical translation of 3D skin bioprinting, such as ethical issues of cell therapy, large-scale production, preservation and transportation, should be taken into consideration during the development of 3D-bioprinted skin substitutes. The solutions to above problems will contribute to the marketing of 3D skin bioprinting in the future decades.
Future perspectives
The need for a satisfactory, permanent physiologic replacement of skin as well as a credible and effective model in vitro for scientific researches have long been recognized. Significant advances in wound repair have been made since 1980s with various skin substitutes being developed for extensive burn injury [7, 9, 192, 193]. Although giant progress has been gained, limitations constrain further clinical translation of bioprinted skin, such as compromised biocompatibility, low mechanical strength, insufficient vascularization and dysfunction.
As the development of material science and advanced manufacturing industry, further 3D skin bioprinting research will mainly focus on optimizing function and developing standard system. Biomimetic bioinks would be discovered to mimic structural and functional heterogeneity of native skin [99, 100]. Efforts such as combining with growth factors delivery and stem cell therapy will achieve better functional outcomes [194–197]. Integration of functional modules to 3D-bioprinted skin should be feasible and customized. Quality standard system of bioprinted skin substitutes and clinical pathway of would treatment with 3D skin bioprinting should be established and completed. Finally, 3D skin bioprinting will be the pioneer to bridges the gap between 3D bioprinting and clinical translation.
Contributor Information
Mengde Zhang, Research Center for Tissue Repair and Regeneration affiliated to the Medical Innovation Research Department, PLA General Hospital and PLA Medical College, 28 Fu Xing Road, Beijing 100853, China.
Chao Zhang, Research Center for Tissue Repair and Regeneration affiliated to the Medical Innovation Research Department, PLA General Hospital and PLA Medical College, 28 Fu Xing Road, Beijing 100853, China; School of Medicine, Nankai University, 94 Wei Jing Road, Tianjin 300071, China.
Zhao Li, Research Center for Tissue Repair and Regeneration affiliated to the Medical Innovation Research Department, PLA General Hospital and PLA Medical College, 28 Fu Xing Road, Beijing 100853, China.
Xiaobing Fu, Research Center for Tissue Repair and Regeneration affiliated to the Medical Innovation Research Department, PLA General Hospital and PLA Medical College, 28 Fu Xing Road, Beijing 100853, China; School of Medicine, Nankai University, 94 Wei Jing Road, Tianjin 300071, China.
Sha Huang, Research Center for Tissue Repair and Regeneration affiliated to the Medical Innovation Research Department, PLA General Hospital and PLA Medical College, 28 Fu Xing Road, Beijing 100853, China.
Funding
This study was supported by the Science Fund for National Defense Distinguished Young Scholars (2022-JCJQ-ZQ-016), Key Basic Research Projects of the Foundation Strengthening Plan (2022-JCJQ-ZD-096-00), National Key Research and Development Program of China (2022YFA1104604), National Natural Science Foundation of China (32000969) and Key Support Program for Growth Factor Research (SZYZ-TR-03).
Conflicts of interest statement. The authors declare that they have no conflict of interest.
References
- 1. Brohem CA, Cardeal LB, Tiago M, Soengas MS, Barros SB, Maria-Engler SS.. Artificial skin in perspective: concepts and applications. Pigment Cell Melanoma Res 2011;24:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun BK, Siprashvili Z, Khavari PA.. Advances in skin grafting and treatment of cutaneous wounds. Science 2014;346:941–5. [DOI] [PubMed] [Google Scholar]
- 3. Groeber F, Holeiter M, Hampel M, Hinderer S, Schenke-Layland K.. Skin tissue engineering—in vivo and in vitro applications. Adv Drug Deliv Rev 2011;63:352–66. [DOI] [PubMed] [Google Scholar]
- 4. Pereira RF, Bártolo PJ.. Traditional therapies for skin wound healing. Adv Wound Care (New Rochelle) 2016;5:208–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U.. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res 2017;58:81–94. [DOI] [PubMed] [Google Scholar]
- 6. Bhardwaj N, Chouhan D, Mandal BB.. Tissue engineered skin and wound healing: current strategies and future directions. Curr Pharm Des 2017;23:3455–82. [DOI] [PubMed] [Google Scholar]
- 7. Vijayavenkataraman S, Yan WC, Lu WF, Wang CH, Fuh JYH.. 3D bioprinting of tissues and organs for regenerative medicine. Adv Drug Deliv Rev 2018;132:296–332. [DOI] [PubMed] [Google Scholar]
- 8. Li J, Chen M, Fan X, Zhou H.. Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med 2016;14:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Daikuara LY, Chen X, Yue Z, Skropeta D, Wood FM, Fear MW, Wallace GG.. 3D bioprinting constructs to facilitate skin regeneration. Adv Funct Mater 2022;32:2105080. [Google Scholar]
- 10. Kim BS, Kwon YW, Kong JS, Park GT, Gao G, Han W, Kim MB, Lee H, Kim JH, Cho DW.. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials 2018;168:38–53. [DOI] [PubMed] [Google Scholar]
- 11. Vijayavenkataraman S, Lu WF, Fuh JY.. 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes. Biofabrication 2016;8:032001. [DOI] [PubMed] [Google Scholar]
- 12. Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT.. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020;226:119536. [DOI] [PubMed] [Google Scholar]
- 13. Ning L, Gil CJ, Hwang B, Theus AS, Perez L, Tomov ML, Bauser-Heaton H, Serpooshan V.. Biomechanical factors in three-dimensional tissue bioprinting. Appl Phys Rev 2020;7:041319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zorlutuna P, Vrana NE, Khademhosseini A.. The expanding world of tissue engineering: the building blocks and new applications of tissue engineered constructs. IEEE Rev Biomed Eng 2013;6:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scognamiglio C, Soloperto A, Ruocco G, Cidonio G.. Bioprinting stem cells: building physiological tissues one cell at a time. Am J Physiol Cell Physiol 2020;319:C465–80. [DOI] [PubMed] [Google Scholar]
- 16. Zhu J, Marchant RE.. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices 2011;8:607–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Kankala RK, Ou C, Chen A, Yang Z.. Advances in hydrogel-based vascularized tissues for tissue repair and drug screening. Bioact Mater 2022;9:198–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bedell M, Navara A, Du Y, Zhang S, Mikos A.. Polymeric systems for bioprinting. Chem Rev 2020;120:10744–92. [DOI] [PubMed] [Google Scholar]
- 19. Chen X, Yue Z, Winberg PC, Lou YR, Beirne S, Wallace GG.. 3D bioprinting dermal-like structures using species-specific ulvan. Biomater Sci 2021;9:2424–38. [DOI] [PubMed] [Google Scholar]
- 20. Liu P, Shen H, Zhi Y, Si J, Shi J, Guo L, Shen SG.. 3D bioprinting and in vitro study of bilayered membranous construct with human cells-laden alginate/gelatin composite hydrogels. Colloids Surf B Biointerfaces 2019;181:1026–34. [DOI] [PubMed] [Google Scholar]
- 21. Ng WL, Qi JTZ, Yeong WY, Naing MW.. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 2018;10:025005. [DOI] [PubMed] [Google Scholar]
- 22. Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, Yoo SS, Dai G, Karande P.. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods 2014;20:473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi Y, Xing TL, Zhang HB, Yin RX, Yang SM, Wei J, Zhang WJ.. Tyrosinase-doped bioink for 3D bioprinting of living skin constructs. Biomed Mater 2018;13:035008. [DOI] [PubMed] [Google Scholar]
- 24. Huang S, Yao B, Xie J, Fu X.. 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater 2016;32:170–7. [DOI] [PubMed] [Google Scholar]
- 25. Wang S, Xiong Y, Chen J, Ghanem A, Wang Y, Yang J, Sun B.. Three dimensional printing bilayer membrane scaffold promotes wound healing. Front Bioeng Biotechnol 2019;7:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zidaric T, Milojevic M, Gradisnik L, Stana Kleinschek K, Maver U, Maver T.. Polysaccharide-based bioink formulation for 3D bioprinting of an in vitro model of the human dermis. Nanomaterials (Basel) 2020;10:733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Intini C, Elviri L, Cabral J, Mros S, Bergonzi C, Bianchera A, Flammini L, Govoni P, Barocelli E, Bettini R, McConnell M.. 3D-printed chitosan-based scaffolds: an in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohydr Polym 2018;199:593–602. [DOI] [PubMed] [Google Scholar]
- 28. Ng WL, Yeong WY, Naing MW.. Development of polyelectrolyte chitosan-gelatin hydrogels for skin bioprinting. Procedia CIRP 2016;49:105–12. [Google Scholar]
- 29. Cubo N, Garcia M, Del Canizo JF, Velasco D, Jorcano JL.. 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication 2016;9:015006. [DOI] [PubMed] [Google Scholar]
- 30. Hafezi F, Shorter S, Tabriz AG, Hurt A, Elmes V, Boateng J, Douroumis D.. Bioprinting and preliminary testing of highly reproducible novel bioink for potential skin regeneration. Pharmaceutics 2020;12:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kwak H, Shin S, Lee H, Hyun J.. Formation of a keratin layer with silk fibroin-polyethylene glycol composite hydrogel fabricated by digital light processing 3D printing. J Ind Eng Chem 2019;72:232–40. [Google Scholar]
- 32. Azadmanesh F, Pourmadadi M, Zavar Reza J, Yazdian F, Omidi M, Haghirosadat BF.. Synthesis of a novel nanocomposite containing chitosan as a three-dimensional printed wound dressing technique: emphasis on gene expression. Biotechnol Prog 2021;37:e3132. [DOI] [PubMed] [Google Scholar]
- 33. Kim BS, Lee JS, Gao G, Cho DW.. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 2017;9:025034. [DOI] [PubMed] [Google Scholar]
- 34. Nystrom A, Bruckner-Tuderman L.. Matrix molecules and skin biology. Semin Cell Dev Biol 2019;89:136–46. [DOI] [PubMed] [Google Scholar]
- 35. Wells A, Nuschke A, Yates CC.. Skin tissue repair: matrix microenvironmental influences. Matrix Biol 2016;49:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dzobo K, Motaung K, Adesida A.. Recent trends in decellularized extracellular matrix bioinks for 3D printing: an updated review. Int J Mol Sci 2019;20:4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim B, Das S, Jang J, Cho D.. Decellularized extracellular matrix-based bioinks for engineering tissue- and organ-specific microenvironments. Chem Rev 2020;120:10608–61. [DOI] [PubMed] [Google Scholar]
- 38. Choudhury D, Tun HW, Wang T, Naing MW.. Organ-derived decellularized extracellular matrix: a game changer for bioink manufacturing? Trends Biotechnol 2018;36:787–805. [DOI] [PubMed] [Google Scholar]
- 39. Kim BS, Kim H, Gao G, Jang J, Cho DW.. Decellularized extracellular matrix: a step towards the next generation source for bioink manufacturing. Biofabrication 2017;9:034104. [DOI] [PubMed] [Google Scholar]
- 40. Jorgensen AM, Chou Z, Gillispie G, Lee SJ, Yoo JJ, Soker S, Atala A.. Decellularized skin extracellular matrix (dsECM) improves the physical and biological properties of fibrinogen hydrogel for skin bioprinting applications. Nanomaterials (Basel) 2020;10:1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Q, Johnson JA, Dunne LW, Chen Y, Iyyanki T, Wu Y, Chang EI, Branch-Brooks CD, Robb GL, Butler CE.. Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps. Acta Biomater 2016;35:166–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parmaksiz M, Elcin AE, Elcin YM.. Decellularized bSIS-ECM as a regenerative biomaterial for skin wound repair. Methods Mol Biol 2019;1879:175–85. [DOI] [PubMed] [Google Scholar]
- 43. Abaci A, Guvendiren M.. Designing decellularized extracellular matrix-based bioinks for 3D bioprinting. Adv Healthc Mater 2020;9:e2000734. [DOI] [PubMed] [Google Scholar]
- 44. Mandrycky C, Wang Z, Kim K, Kim DH.. 3D bioprinting for engineering complex tissues. Biotechnol Adv 2016;34:422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heid S, Boccaccini AR.. Advancing bioinks for 3D bioprinting using reactive fillers: a review. Acta Biomater 2020;113:1–22. [DOI] [PubMed] [Google Scholar]
- 46. Ng WL, Wang S, Yeong WY, Naing MW.. Skin bioprinting: impending reality or fantasy? Trends Biotechnol 2016;34:689–99. [DOI] [PubMed] [Google Scholar]
- 47. Chen FM, Liu X.. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci 2016;53:86–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Griffin DR, Archang MM, Kuan CH, Weaver WM, Weinstein JS, Feng AC, Ruccia A, Sideris E, Ragkousis V, Koh J, Plikus MV, Di Carlo D, Segura T, Scumpia PO.. Activating an adaptive immune response from a hydrogel scaffold imparts regenerative wound healing. Nat Mater 2021;20:560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pahlevanzadeh F, Mokhtari H, Bakhsheshi-Rad HR, Emadi R, Kharaziha M, Valiani A, Poursamar SA, Ismail AF, RamaKrishna S, Berto F.. Recent trends in three-dimensional bioinks based on alginate for biomedical applications. Materials (Basel) 2020;13:3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. De Santis MM, Alsafadi HN, Tas S, Bolukbas DA, Prithiviraj S, Da Silva IAN, Mittendorfer M, Ota C, Stegmayr J, Daoud F, Konigshoff M, Sward K, Wood JA, Tassieri M, Bourgine PE, Lindstedt S, Mohlin S, Wagner DE.. Extracellular-matrix-reinforced bioinks for 3D bioprinting human tissue. Adv Mater 2021;33:e2005476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Somasekharan LT, Raju R, Kumar S, Geevarghese R, Nair RP, Kasoju N, Bhatt A.. Biofabrication of skin tissue constructs using alginate, gelatin and diethylaminoethyl cellulose bioink. Int J Biol Macromol 2021;189:398–409. [DOI] [PubMed] [Google Scholar]
- 52. Yao B, Hu T, Cui X, Song W, Fu X, Huang S.. Enzymatically degradable alginate/gelatin bioink promotes cellular behavior and degradation in vitro and in vivo. Biofabrication 2019;11:045020. [DOI] [PubMed] [Google Scholar]
- 53. Gao T, Gillispie GJ, Copus JS, Pr AK, Seol YJ, Atala A, Yoo JJ, Lee SJ.. Optimization of gelatin-alginate composite bioink printability using rheological parameters: a systematic approach. Biofabrication 2018;10:034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim HN, Kang DH, Kim MS, Jiao A, Kim DH, Suh KY.. Patterning methods for polymers in cell and tissue engineering. Ann Biomed Eng 2012;40:1339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jain P, Kathuria H, Dubey N.. Advances in 3D bioprinting of tissues/organs for regenerative medicine and in-vitro models. Biomaterials 2022;287:121639. [DOI] [PubMed] [Google Scholar]
- 56. Malda J, Visser J, Melchels FP, Jungst T, Hennink WE, Dhert WJ, Groll J, Hutmacher DW.. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater 2013;25:5011–28. [DOI] [PubMed] [Google Scholar]
- 57. Freeman S, Calabro S, Williams R, Jin S, Ye K.. Bioink formulation and machine learning-empowered bioprinting optimization. Front Bioeng Biotechnol 2022;10:913579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang Y, Yuan X, Yao B, Zhu S, Zhu P, Huang S.. Tailoring bioinks of extrusion-based bioprinting for cutaneous wound healing. Bioact Mater 2022;17:178–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li Z, Huang S, Liu Y, Yao B, Hu T, Shi H, Xie J, Fu X.. Tuning alginate-gelatin bioink properties by varying solvent and their impact on stem cell behavior. Sci Rep 2018;8:8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Watt FM, Fujiwara H.. Cell–extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol 2011;3:a005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, Shenoy VB.. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020;584:535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wei L, Li Z, Li J, Zhang Y, Yao B, Liu Y, Song W, Fu X, Wu X, Huang S.. An approach for mechanical property optimization of cell-laden alginate–gelatin composite bioink with bioactive glass nanoparticles. J Mater Sci Mater Med 2020;31:103. [DOI] [PubMed] [Google Scholar]
- 63. Li J, Zhang Y, Enhe J, Yao B, Wang Y, Zhu D, Li Z, Song W, Duan X, Yuan X, Fu X, Huang S.. Bioactive nanoparticle reinforced alginate/gelatin bioink for the maintenance of stem cell stemness. Mater Sci Eng C Mater Biol Appl 2021;126:112193. [DOI] [PubMed] [Google Scholar]
- 64. Li J, Liu Y, Zhang Y, Yao B, Enhejirigala, Li Z, Song W, Wang Y, Duan X, Yuan X, Fu X, Huang S.. Biophysical and biochemical cues of biomaterials guide mesenchymal stem cell behaviors. Front Cell Dev Biol 2021;9:640388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yan WC, Davoodi P, Vijayavenkataraman S, Tian Y, Ng WC, Fuh JYH, Robinson KS, Wang CH.. 3D bioprinting of skin tissue: from pre-processing to final product evaluation. Adv Drug Deliv Rev 2018;132:270–95. [DOI] [PubMed] [Google Scholar]
- 66. Zhao M, Wang J, Zhang J, Huang J, Luo L, Yang Y, Shen K, Jiao T, Jia Y, Lian W, Li J, Wang Y, Lian Q, Hu D.. Functionalizing multi-component bioink with platelet-rich plasma for customized in-situ bilayer bioprinting for wound healing. Mater Today Bio 2022;16:100334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang C, Liang C, Wang R, Yao X, Guo P, Yuan W, Liu Y, Song Y, Li Z, Xie X.. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater Sci 2019;8:313–24. [DOI] [PubMed] [Google Scholar]
- 68. Rognoni E, Watt FM.. Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol 2018;28:709–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC.. Wound healing: a cellular perspective. Physiol Rev 2019;99:665–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bruggen MC, Stingl G.. Subcutaneous white adipose tissue: the deepest layer of the cutaneous immune barrier. J Dtsch Dermatol Ges 2020;18:1225–7. [DOI] [PubMed] [Google Scholar]
- 71. Wang Z, Qi F, Luo H, Xu G, Wang D.. Inflammatory microenvironment of skin wounds. Front Immunol 2022;13:789274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rippa AL, Kalabusheva EP, Vorotelyak EA.. Regeneration of dermis: scarring and cells involved. Cells 2019;8:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Roig-Rosello E, Rousselle P.. The human epidermal basement membrane: a shaped and cell instructive platform that aging slowly alters. Biomolecules 2020;10:1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Werner S, Keller L, Pantel K.. Epithelial keratins: biology and implications as diagnostic markers for liquid biopsies. Mol Aspects Med 2020;72:100817. [DOI] [PubMed] [Google Scholar]
- 75. Plikus MV, Wang X, Sinha S, Forte E, Thompson SM, Herzog EL, Driskell RR, Rosenthal N, Biernaskie J, Horsley V.. Fibroblasts: origins, definitions, and functions in health and disease. Cell 2021;184:3852–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stanton DN, Ganguli-Indra G, Indra AK, Karande P.. Bioengineered efficacy models of skin disease: advances in the last 10 years. Pharmaceutics 2022;14:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lee W, Debasitis JC, Lee VK, Lee JH, Fischer K, Edminster K, Park JK, Yoo SS.. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 2009;30:1587–95. [DOI] [PubMed] [Google Scholar]
- 78. Pontiggia L, Van Hengel IA, Klar A, Rutsche D, Nanni M, Scheidegger A, Figi S, Reichmann E, Moehrlen U, Biedermann T.. Bioprinting and plastic compression of large pigmented and vascularized human dermo-epidermal skin substitutes by means of a new robotic platform. J Tissue Eng 2022;13:20417314221088513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Speeckaert R, Belpaire A, Speeckaert M, van Geel N.. The delicate relation between melanocytes and skin immunity: a game of hide and seek. Pigment Cell Melanoma Res 2022;35:392–407. [DOI] [PubMed] [Google Scholar]
- 80. Kim BS, Gao G, Kim JY, Cho DW.. 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin. Adv Healthc Mater 2019;8:e1801019. [DOI] [PubMed] [Google Scholar]
- 81. Liu X, Michael S, Bharti K, Ferrer M, Song MJ.. A biofabricated vascularized skin model of atopic dermatitis for preclinical studies. Biofabrication 2020;12:035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kim BS, Ahn M, Cho WW, Gao G, Jang J, Cho DW.. Engineering of diseased human skin equivalent using 3D cell printing for representing pathophysiological hallmarks of type 2 diabetes in vitro. Biomaterials 2021;272:120776. [DOI] [PubMed] [Google Scholar]
- 83. Zhou F, Hong Y, Liang R, Zhang X, Liao Y, Jiang D, Zhang J, Sheng Z, Xie C, Peng Z, Zhuang X, Bunpetch V, Zou Y, Huang W, Zhang Q, Alakpa EV, Zhang S, Ouyang H.. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials 2020;258:120287. [DOI] [PubMed] [Google Scholar]
- 84. Mazini L, Rochette L, Admou B, Amal S, Malka G.. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci 2020;21:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Agudo J. Immune privilege of skin stem cells: what do we know and what can we learn? Exp Dermatol 2021;30:522–8. [DOI] [PubMed] [Google Scholar]
- 86. Peng YJ, Huang X, Zhou Q.. Ethical and policy considerations for human embryo and stem cell research in China. Cell Stem Cell 2020;27:511–4. [DOI] [PubMed] [Google Scholar]
- 87. Chouhan D, Dey N, Bhardwaj N, Mandal BB.. Emerging and innovative approaches for wound healing and skin regeneration: current status and advances. Biomaterials 2019;216:119267. [DOI] [PubMed] [Google Scholar]
- 88. Gudapati H, Dey M, Ozbolat I.. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials 2016;102:20–42. [DOI] [PubMed] [Google Scholar]
- 89. Gu Z, Fu J, Lin H, He Y.. Development of 3D bioprinting: from printing methods to biomedical applications. Asian J Pharm Sci 2020;15:529–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kim M, Kim G.. 3D multi-layered fibrous cellulose structure using an electrohydrodynamic process for tissue engineering. J Colloid Interface Sci 2015;457:180–7. [DOI] [PubMed] [Google Scholar]
- 91. Albanna M, Binder KW, Murphy SV, Kim J, Qasem SA, Zhao W, Tan J, El-Amin IB, Dice DD, Marco J, Green J, Xu T, Skardal A, Holmes JH, Jackson JD, Atala A, Yoo JJ.. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci Rep 2019;9:1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Guillemot F, Souquet A, Catros S, Guillotin B, Lopez J, Faucon M, Pippenger B, Bareille R, Remy M, Bellance S, Chabassier P, Fricain JC, Amedee J.. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater 2010;6:2494–500. [DOI] [PubMed] [Google Scholar]
- 93. Koch L, Deiwick A, Schlie S, Michael S, Gruene M, Coger V, Zychlinski D, Schambach A, Reimers K, Vogt PM, Chichkov B.. Skin tissue generation by laser cell printing. Biotechnol Bioeng 2012;109:1855–63. [DOI] [PubMed] [Google Scholar]
- 94. He J, Zhang B, Li Z, Mao M, Li J, Han K, Li D.. High-resolution electrohydrodynamic bioprinting: a new biofabrication strategy for biomimetic micro/nanoscale architectures and living tissue constructs. Biofabrication 2020;12:042002. [DOI] [PubMed] [Google Scholar]
- 95. Angkawinitwong U, Courtenay AJ, Rodgers AM, Larrañeta E, McCarthy HO, Brocchini S, Donnelly RF, Williams GR.. A novel transdermal protein delivery strategy via electrohydrodynamic coating of PLGA microparticles onto microneedles. ACS Appl Mater Interfaces 2020;12:12478–88. [DOI] [PubMed] [Google Scholar]
- 96. Haj-Ahmad R, Khan H, Arshad MS, Rasekh M, Hussain A, Walsh S, Li X, Chang MW, Ahmad Z.. Microneedle coating techniques for transdermal drug delivery. Pharmaceutics 2015;7:486–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sangnim T, Limmatvapirat S, Nunthanid J, Sriamornsak P, Sittikijyothin W, Wannachaiyasit S, Huanbutta K.. Design and characterization of clindamycin-loaded nanofiber patches composed of polyvinyl alcohol and tamarind seed gum and fabricated by electrohydrodynamic atomization. Asian J Pharm Sci 2018;13:450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lee S, Kim SW, Ghidelli M, An HS, Jang J, Bassi AL, Lee SY, Park JU.. Integration of transparent supercapacitors and electrodes using nanostructured metallic glass films for wirelessly rechargeable, skin heat patches. Nano Lett 2020;20:4872–81. [DOI] [PubMed] [Google Scholar]
- 99. Decante G, Costa JB, Silva-Correia J, Collins MN, Reis RL, Oliveira JM.. Engineering bioinks for 3D bioprinting. Biofabrication 2021;13:032001. [DOI] [PubMed] [Google Scholar]
- 100. Hull SM, Brunel LG, Heilshorn SC.. 3D bioprinting of cell-laden hydrogels for improved biological functionality. Adv Mater 2022;34:e2103691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lian L, Zhou C, Tang G, Xie M, Wang Z, Luo Z, Japo J, Wang D, Zhou J, Wang M, Li W, Maharjan S, Ruelas M, Guo J, Wu X, Zhang YS.. Uniaxial and coaxial vertical embedded extrusion bioprinting. Adv Healthc Mater 2022;11:e2102411. [DOI] [PubMed] [Google Scholar]
- 102. Murphy SV, Atala A.. 3D bioprinting of tissues and organs. Nat Biotechnol 2014;32:773–85. [DOI] [PubMed] [Google Scholar]
- 103. Ong CS, Yesantharao P, Huang CY, Mattson G, Boktor J, Fukunishi T, Zhang H, Hibino N.. 3D bioprinting using stem cells. Pediatr Res 2018;83:223–31. [DOI] [PubMed] [Google Scholar]
- 104. Liu N, Huang S, Yao B, Xie J, Wu X, Fu X.. 3D bioprinting matrices with controlled pore structure and release function guide in vitro self-organization of sweat gland. Sci Rep 2016;6:34410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Askari M, Afzali Naniz M, Kouhi M, Saberi A, Zolfagharian A, Bodaghi M.. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: a comprehensive review with focus on advanced fabrication techniques. Biomater Sci 2021;9:535–73. [DOI] [PubMed] [Google Scholar]
- 106. Song W, Yao B, Zhu D, Zhang Y, Li Z, Huang S, Fu X.. 3D-bioprinted microenvironments for sweat gland regeneration. Burns Trauma 2022;10:tkab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lee M, Rizzo R, Surman F, Zenobi-Wong M.. Guiding lights: tissue bioprinting using photoactivated materials. Chem Rev 2020;120:10950–1027. [DOI] [PubMed] [Google Scholar]
- 108. Samandari M, Mostafavi A, Quint J, Memic A, Tamayol A.. In situ bioprinting: intraoperative implementation of regenerative medicine. Trends Biotechnol 2022;40:1229–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nuutila K, Samandari M, Endo Y, Zhang Y, Quint J, Schmidt TA, Tamayol A, Sinha I.. In vivo printing of growth factor-eluting adhesive scaffolds improves wound healing. Bioact Mater 2022;8:296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Singh S, Choudhury D, Yu F, Mironov V, Naing MW.. In situ bioprinting – bioprinting from benchside to bedside? Acta Biomater 2020;101:14–25. [DOI] [PubMed] [Google Scholar]
- 111. Urciuolo A, Poli I, Brandolino L, Raffa P, Scattolini V, Laterza C, Giobbe GG, Zambaiti E, Selmin G, Magnussen M, Brigo L, De Coppi P, Salmaso S, Giomo M, Elvassore N.. Intravital three-dimensional bioprinting. Nat Biomed Eng 2020;4:901–15. [DOI] [PubMed] [Google Scholar]
- 112. Zhu Z, Guo SZ, Hirdler T, Eide C, Fan X, Tolar J, McAlpine MC.. 3D printed functional and biological materials on moving freeform surfaces. Adv Mater 2018;30:e1707495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chen Y, Zhang J, Liu X, Wang S, Tao J, Huang Y, Wu W, Li Y, Zhou K, Wei X, Chen S, Li X, Xu X, Cardon L, Qian Z, Gou M.. Noninvasive in vivo 3D bioprinting. Sci Adv 2020;6:eaba7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Moncal KK, Gudapati H, Godzik KP, Heo DN, Kang Y, Rizk E, Ravnic DJ, Wee H, Pepley DF, Ozbolat V, Lewis GS, Moore JZ, Driskell RR, Samson TD, Ozbolat IT.. Intra-operative bioprinting of hard, soft, and hard/soft composite tissues for craniomaxillofacial reconstruction. Adv Funct Mater 2021;31:2010858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Samandari M, Alipanah F, Majidzadeh AK, Alvarez MM, Trujillo-de Santiago G, Tamayol A.. Controlling cellular organization in bioprinting through designed 3D microcompartmentalization. Appl Phys Rev 2021;8:021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cheng RY, Eylert G, Gariepy JM, He S, Ahmad H, Gao Y, Priore S, Hakimi N, Jeschke MG, Gunther A.. Handheld instrument for wound-conformal delivery of skin precursor sheets improves healing in full-thickness burns. Biofabrication 2020;12:025002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hakimi N, Cheng R, Leng L, Sotoudehfar M, Ba PQ, Bakhtyar N, Amini-Nik S, Jeschke MG, Günther A.. Handheld skin printer: in situ formation of planar biomaterials and tissues. Lab Chip 2018;18:1440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, Soker S.. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med 2012;1:792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Skardal A, Murphy SV, Crowell K, Mack D, Atala A, Soker S.. A tunable hydrogel system for long-term release of cell-secreted cytokines and bioprinted in situ wound cell delivery. J Biomed Mater Res B Appl Biomater 2017;105:1986–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sun W, Zhang J, Qin Y, Tang H, Chen Y, Lin W, She Y, Zhang K, Yin J, Chen C.. A simple and efficient strategy for preparing a cell-spheroid-based bioink. Adv Healthc Mater 2022;11:e2200648. [DOI] [PubMed] [Google Scholar]
- 121. Zhuang P, Sun AX, An J, Chua CK, Chew SY.. 3D neural tissue models: from spheroids to bioprinting. Biomaterials 2018;154:113–33. [DOI] [PubMed] [Google Scholar]
- 122. Decarli MC, Amaral R, Santos DPD, Tofani LB, Katayama E, Rezende RA, Silva J, Swiech K, Suazo CAT, Mota C, Moroni L, Moraes AM.. Cell spheroids as a versatile research platform: formation mechanisms, high throughput production, characterization and applications. Biofabrication 2021;13:032002. [DOI] [PubMed] [Google Scholar]
- 123. Kinney MA, Hookway TA, Wang Y, McDevitt TC.. Engineering three-dimensional stem cell morphogenesis for the development of tissue models and scalable regenerative therapeutics. Ann Biomed Eng 2014;42:352–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tasoglu S, Demirci U.. Bioprinting for stem cell research. Trends Biotechnol 2013;31:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhang Y, Enhejirigala, Yao B, Li Z, Song W, Li J, Zhu D, Wang Y, Duan X, Yuan X, Huang S, Fu X.. Using bioprinting and spheroid culture to create a skin model with sweat glands and hair follicles. Burns Trauma 2021;9:tkab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Douillet C, Nicodeme M, Hermant L, Bergeron V, Guillemot F, Fricain JC, Oliveira H, Garcia M.. From local to global matrix organization by fibroblasts: a 4D laser-assisted bioprinting approach. Biofabrication 2022;14:025006. [DOI] [PubMed] [Google Scholar]
- 127. Lee YB, Jeon O, Lee SJ, Ding A, Wells D, Alsberg E.. Induction of 4D spatiotemporal geometric transformations in high cell density tissues via shape changing hydrogels. Adv Funct Mater 2021;31:2010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zhou W, Qiao Z, Nazarzadeh Zare E, Huang J, Zheng X, Sun X, Shao M, Wang H, Wang X, Chen D, Zheng J, Fang S, Li YM, Zhang X, Yang L, Makvandi P, Wu A.. 4D-printed dynamic materials in biomedical applications: chemistry, challenges, and their future perspectives in the clinical sector. J Med Chem 2020;63:8003–24. [DOI] [PubMed] [Google Scholar]
- 129. Zoio P, Oliva A.. Skin-on-a-chip technology: microengineering physiologically relevant in vitro skin models. Pharmaceutics 2022;14:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mori N, Morimoto Y, Takeuchi S.. Skin integrated with perfusable vascular channels on a chip. Biomaterials 2017;116:48–56. [DOI] [PubMed] [Google Scholar]
- 131. Nahak BK, Mishra A, Preetam S, Tiwari A.. Advances in organ-on-a-chip materials and devices. ACS Appl Bio Mater 2022;5:3576–607. [DOI] [PubMed] [Google Scholar]
- 132. Ng WL, Yeong WY.. The future of skin toxicology testing – three-dimensional bioprinting meets microfluidics. Int J Bioprint 2019;5:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Idaszek J, Costantini M, Karlsen TA, Jaroszewicz J, Colosi C, Testa S, Fornetti E, Bernardini S, Seta M, Kasarełło K, Wrzesień R, Cannata S, Barbetta A, Gargioli C, Brinchman JE, Święszkowski W.. 3D bioprinting of hydrogel constructs with cell and material gradients for the regeneration of full-thickness chondral defect using a microfluidic printing head. Biofabrication 2019;11:044101. [DOI] [PubMed] [Google Scholar]
- 134. Sutterby E, Thurgood P, Baratchi S, Khoshmanesh K, Pirogova E.. Microfluidic skin-on-a-chip models: toward biomimetic artificial skin. Small 2020;16:e2002515. [DOI] [PubMed] [Google Scholar]
- 135. Mostafalu P, Tamayol A, Rahimi R, Ochoa M, Khalilpour A, Kiaee G, Yazdi IK, Bagherifard S, Dokmeci MR, Ziaie B, Sonkusale SR, Khademhosseini A.. Smart bandage for monitoring and treatment of chronic wounds. Small 2018;14:e1703509. [DOI] [PubMed] [Google Scholar]
- 136. Mirani B, Pagan E, Currie B, Siddiqui MA, Hosseinzadeh R, Mostafalu P, Zhang YS, Ghahary A, Akbari M.. An advanced multifunctional Hydrogel-Based dressing for wound monitoring and drug delivery. Adv Healthc Mater 2017;6:10.1002/adhm.201700718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Leng L, Amini-Nik S, Ba Q, Jeschke M. Skin printer: microfluidic approach for skin regeneration and wound dressings. In 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences, 27-31 October 2013, Freiburg, Germany.
- 138. Singer AJ, Clark RAF.. Mechanisms of disease – cutaneous wound healing. N Engl J Med 1999;341:738–46. [DOI] [PubMed] [Google Scholar]
- 139. Kratofil RM, Shim HB, Shim R, Lee WY, Labit E, Sinha S, Keenan CM, Surewaard BGJ, Noh JY, Sun Y, Sharkey KA, Mack M, Biernaskie J, Deniset JF, Kubes P.. A monocyte–leptin–angiogenesis pathway critical for repair post-infection. Nature 2022;609:166–73. [DOI] [PubMed] [Google Scholar]
- 140. Darby IA, Zakuan N, Billet F, Desmoulière A.. The myofibroblast, a key cell in normal and pathological tissue repair. Cell Mol Life Sci 2016;73:1145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN.. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet 2016;388:1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sarmin A, El Moussaid N, Suntornnond R, Tyler E, Kim Y-H, Di Cio S, Megone W, Pearce O, Gautrot J, Dawson J, Connelly J.. Multi-scale analysis of the composition, structure, and function of decellularized extracellular matrix for human skin and wound healing models. Biomolecules 2022;12:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ali Zahid A, Chakraborty A, Shamiya Y, Ravi SP, Paul A.. Leveraging the advancements in functional biomaterials and scaffold fabrication technologies for chronic wound healing applications. Mater Horiz 2022;9:1850–65. [DOI] [PubMed] [Google Scholar]
- 144. Jin R, Cui Y, Chen H, Zhang Z, Weng T, Xia S, Yu M, Zhang W, Shao J, Yang M, Han C, Wang X.. Three-dimensional bioprinting of a full-thickness functional skin model using acellular dermal matrix and gelatin methacrylamide bioink. Acta Biomater 2021;131:248–61. [DOI] [PubMed] [Google Scholar]
- 145. Zhao H, Xu J, Yuan H, Zhang E, Dai N, Gao Z, Huang Y, Lv F, Liu L, Gu Q, Wang S.. 3D printing of artificial skin patches with bioactive and optically active polymer materials for anti-infection and augmenting wound repair. Mater Horiz 2022;9:342–9. [DOI] [PubMed] [Google Scholar]
- 146. Weng T, Wu P, Zhang W, Zheng Y, Li Q, Jin R, Chen H, You C, Guo S, Han C, Wang X.. Regeneration of skin appendages and nerves: current status and further challenges. J Transl Med 2020;18:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chu SY, Chou CH, Huang HD, Yen MH, Hong HC, Chao PH, Wang YH, Chen PY, Nian SX, Chen YR, Liou LY, Liu YC, Chen HM, Lin FM, Chang YT, Chen CC, Lee OK.. Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nat Commun 2019;10:1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Morita R, Sanzen N, Sasaki H, Hayashi T, Umeda M, Yoshimura M, Yamamoto T, Shibata T, Abe T, Kiyonari H, Furuta Y, Nikaido I, Fujiwara H.. Tracing the origin of hair follicle stem cells. Nature 2021;594:547–52. [DOI] [PubMed] [Google Scholar]
- 149. Lu CP, Polak L, Keyes BE, Fuchs E.. Spatiotemporal antagonism in mesenchymal-epithelial signaling in sweat versus hair fate decision. Science 2016;354:aah6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Abaci HE, Coffman A, Doucet Y, Chen J, Jackow J, Wang E, Guo Z, Shin JU, Jahoda CA, Christiano AM.. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat Commun 2018;9:5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Nanmo A, Yan L, Asaba T, Wan L, Kageyama T, Fukuda J. (2022) Bioprinting of hair follicle germs for hair regenerative medicine. Acta Biomater. https://doi.org/10.1016/j.actbio.2022.06.021 [DOI] [PubMed] [Google Scholar]
- 152. Kang D, Liu Z, Qian C, Huang J, Zhou Y, Mao X, Qu Q, Liu B, Wang J, Hu Z, Miao Y. (2022) 3D bioprinting of a gelatin-alginate hydrogel for tissue-engineered hair follicle regeneration. Acta Biomater. https://doi.org/10.1016/j.actbio.2022.03.011 [DOI] [PubMed] [Google Scholar]
- 153. Yao B, Wang R, Wang Y, Zhang Y, Hu T, Song W, Li Z, Huang S, Fu X.. Biochemical and structural cues of 3D-printed matrix synergistically direct MSC differentiation for functional sweat gland regeneration. Sci Adv 2020;6:eaaz1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zouboulis CC, Yoshida GJ, Wu Y, Xia L, Schneider MR.. Sebaceous gland: milestones of 30-year modelling research dedicated to the “brain of the skin”. Exp Dermatol 2020;29:1069–79. [DOI] [PubMed] [Google Scholar]
- 155. Chen L, Yan D, Wu N, Zhang W, Yan C, Yao Q, Zouboulis CC, Sun H, Fu Y.. 3D-printed poly-caprolactone scaffolds modified with biomimetic extracellular matrices for tarsal plate tissue engineering. Front Bioeng Biotechnol 2020;8:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Shanks N, Greek R, Greek J.. Are animal models predictive for humans? Philos Ethics Humanit Med 2009;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kim J, Koo BK, Knoblich JA.. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol 2020;21:571–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Fitzgerald KA, Malhotra M, Curtin CM, O’Brien FJ, O’Driscoll CM.. Life in 3D is never flat: 3D models to optimise drug delivery. J Control Release 2015;215:39–54. [DOI] [PubMed] [Google Scholar]
- 159. Bin Y, Dongzhen Z, Xiaoli C, Jirigala E, Wei S, Zhao L, Tian H, Ping Z, Jianjun L, Yuzhen W, Yijie Z, Xiaobing F, Sha H.. Modeling human hypertrophic scars with 3D preformed cellular aggregates bioprinting. Bioact Mater 2022;10:247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Duan J, Cao Y, Shen Z, Cheng Y, Ma Z, Wang L, Zhang Y, An Y, Sang S.. 3D bioprinted GelMA/PEGDA hybrid scaffold for establishing an in vitro model of melanoma. J Microbiol Biotechnol 2022;32:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Jeong YM, Bang C, Park M, Shin S, Yun S, Kim CM, Jeong G, Chung YJ, Yun WS, Lee JH, Jin S.. 3D-printed collagen scaffolds promote maintenance of cryopreserved patients-derived melanoma explants. Cells 2021;10:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Mathes SH, Ruffner H, Graf-Hausner U.. The use of skin models in drug development. Adv Drug Deliv Rev 2014;69–70:81–102. [DOI] [PubMed] [Google Scholar]
- 163. Madiedo-Podvrsan S, Belaidi JP, Desbouis S, Simonetti L, Ben-Khalifa Y, Collin-Djangone C, Soeur J, Rielland M.. Utilization of patterned bioprinting for heterogeneous and physiologically representative reconstructed epidermal skin models. Sci Rep 2021;11:6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Chen L, Li N, Liu Y, Faquet B, Alepee N, Ding C, Eilstein J, Zhong L, Peng Z, Ma J, Cai Z, Ouedraogo G.. A new 3D model for genotoxicity assessment: EpiSkinTM micronucleus assay. Mutagenesis 2021;36:51–61. [DOI] [PubMed] [Google Scholar]
- 165. Randall MJ, Jungel A, Rimann M, Wuertz-Kozak K.. Advances in the biofabrication of 3D skin in vitro: healthy and pathological models. Front Bioeng Biotechnol 2018;6:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Li KN, Jain P, He CH, Eun FC, Kang S, Tumbar T.. Skin vasculature and hair follicle cross-talking associated with stem cell activation and tissue homeostasis. Elife 2019;8:e45977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Yuan X, Duan X, Enhejirigala, Li Z, Yao B, Song W, Wang Y, Kong Y, Zhu S, Zhang F, Liang L, Zhang M, Zhang C, Kong D, Zhu M, Huang S, Fu X.. Reciprocal interaction between vascular niche and sweat gland promotes sweat gland regeneration. Bioact Mater 2023;21:340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Moreira HR, Marques AP.. Vascularization in skin wound healing: where do we stand and where do we go? Curr Opin Biotechnol 2022;73:253–62. [DOI] [PubMed] [Google Scholar]
- 169. Lee HJ, Hong YJ, Kim M.. Angiogenesis in chronic inflammatory skin disorders. Int J Mol Sci 2021;22:12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Mabeta P. Paradigms of vascularization in melanoma: clinical significance and potential for therapeutic targeting. Biomed Pharmacother 2020;127:110135. [DOI] [PubMed] [Google Scholar]
- 171. Phua QH, Han HA, Soh BS.. Translational stem cell therapy: vascularized skin grafts in skin repair and regeneration. J Transl Med 2021;19:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Shao L, Gao Q, Xie C, Fu J, Xiang M, He Y.. Directly coaxial 3D bioprinting of large-scale vascularized tissue constructs. Biofabrication 2020;12:035014. [DOI] [PubMed] [Google Scholar]
- 173. Mohan TS, Datta P, Nesaei S, Ozbolat V, Ozbolat IT.. 3D coaxial bioprinting: process mechanisms, bioinks and applications. Prog Biomed Eng (Bristol) 2022;4:022003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Liang Q, Gao F, Zeng Z, Yang J, Wu M, Gao C, Cheng D, Pan H, Liu W, Ruan C.. Coaxial scale‐up printing of diameter‐tunable biohybrid hydrogel microtubes with high strength, perfusability, and endothelialization. Adv Funct Mater 2020;30:2001485. [Google Scholar]
- 175. Hong S, Kim JS, Jung B, Won C, Hwang C.. Coaxial bioprinting of cell-laden vascular constructs using a gelatin-tyramine bioink. Biomater Sci 2019;7:4578–87. [DOI] [PubMed] [Google Scholar]
- 176. Song D, Xu Y, Liu S, Wen L, Wang X.. Progress of 3D bioprinting in organ manufacturing. Polymers (Basel) 2021;13:3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Kérourédan O, Bourget J-M, Rémy M, Crauste-Manciet S, Kalisky J, Catros S, Thébaud NB, Devillard R.. Micropatterning of endothelial cells to create a capillary-like network with defined architecture by laser-assisted bioprinting. J Mater Sci Mater Med 2019;30:28. [DOI] [PubMed] [Google Scholar]
- 178. He S, Xia T, Wang H, Wei L, Luo X, Li X.. Multiple release of polyplexes of plasmids VEGF and bFGF from electrospun fibrous scaffolds towards regeneration of mature blood vessels. Acta Biomater 2012;8:2659–69. [DOI] [PubMed] [Google Scholar]
- 179. Damanik FFR, Rothuizen CT, Lalai R, Khoenkhoen S, van Blitterswijk C, Rotmans JI, Moroni L.. Long-term controlled growth factor release using layer-by-layer assembly for the development of in vivo tissue-engineered blood vessels. ACS Appl Mater Interfaces 2022;14:28591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. An Y, Lin S, Tan X, Zhu S, Nie F, Zhen Y, Gu L, Zhang C, Wang B, Wei W, Li D, Wu J.. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif 2021;54:e12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Fleischer S, Tavakol DN, Vunjak-Novakovic G.. From arteries to capillaries: approaches to engineering human vasculature. Adv Funct Mater 2020;30:1910811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Saunders RL, Hammer DA.. Assembly of human umbilical vein endothelial cells on compliant hydrogels. Cell Mol Bioeng 2010;3:60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wu Y, Al-Ameen MA, Ghosh G.. Integrated effects of matrix mechanics and vascular endothelial growth factor (VEGF) on capillary sprouting. Ann Biomed Eng 2014;42:1024–36. [DOI] [PubMed] [Google Scholar]
- 184. Berdichevski A, Birch MA, Markaki AE.. Collagen scaffolds with tailored pore geometry for building three-dimensional vascular networks. Mater Lett 2019;248:93–6. [Google Scholar]
- 185. Baltazar T, Merola J, Catarino C, Xie CB, Kirkiles-Smith NC, Lee V, Hotta S, Dai G, Xu X, Ferreira FC, Saltzman WM, Pober JS, Karande P.. Three dimensional bioprinting of a vascularized and perfusable skin graft using human keratinocytes, fibroblasts, pericytes, and endothelial cells. Tissue Eng Part A 2020;26:227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Weng T, Zhang W, Xia Y, Wu P, Yang M, Jin R, Xia S, Wang J, You C, Han C, Wang X.. 3D bioprinting for skin tissue engineering: current status and perspectives. J Tissue Eng 2021;12:20417314211028574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Abaci HE, Guo Z, Coffman A, Gillette B, Lee WH, Sia SK, Christiano AM.. Human skin constructs with spatially controlled vasculature using primary and iPSC-derived endothelial cells. Adv Healthc Mater 2016;5:1800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Marques CF, Diogo GS, Pina S, Oliveira JM, Silva TH, Reis RL.. Collagen-based bioinks for hard tissue engineering applications: a comprehensive review. J Mater Sci Mater Med 2019;30:32. [DOI] [PubMed] [Google Scholar]
- 189. Oualla-Bachiri W, Fernandez-Gonzalez A, Quinones-Vico MI, Arias-Santiago S.. From grafts to human bioengineered vascularized skin substitutes. Int J Mol Sci 2020;21:8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Olejnik A, Semba JA, Kulpa A, Dańczak-Pazdrowska A, Rybka JD, Gornowicz-Porowska J.. 3D bioprinting in skin related research: recent achievements and application perspectives. ACS Synth Biol 2022;11:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Zhang M, Li H, Chen L, Fang S, Xie S, Lin C.. Three-dimensional reconstructed eccrine sweat glands with vascularization and cholinergic and adrenergic innervation. J Mol Histol 2018;49:339–45. [DOI] [PubMed] [Google Scholar]
- 192. O’Connor N, Mulliken J, Banks-Schlegel S, Kehinde O, Green H.. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet 1981;317:75–8. [PubMed] [Google Scholar]
- 193. Burke JF, Yannas IV, Quinby WC, Bondoc CC, Jung WK.. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg 1981;194:413–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Tan SH, Ngo ZH, Sci DB, Leavesley D, Liang K.. Recent advances in the design of three-dimensional and bioprinted scaffolds for full-thickness wound healing. Tissue Eng Part B Rev 2022;28:160–81. [DOI] [PubMed] [Google Scholar]
- 195. Anitua E, Nurden P, Prado R, Nurden AT, Padilla S.. Autologous fibrin scaffolds: when platelet- and plasma-derived biomolecules meet fibrin. Biomaterials 2019;192:440–60. [DOI] [PubMed] [Google Scholar]
- 196. Bian D, Wu Y, Song G, Azizi R, Zamani A.. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther 2022;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Las Heras K, Igartua M, Santos-Vizcaino E, Hernandez RM.. Chronic wounds: current status, available strategies and emerging therapeutic solutions. J Control Release 2020;328:532–50. [DOI] [PubMed] [Google Scholar]