Abstract
Colorectal cancer (CRC) is the second most lethal cancer worldwide and the prognosis of metastatic CRC (mCRC) remains poor. Recent advancements in translational research have led to the identification of several new therapeutic targets and improved the treatment outcome of patients with tumours harbouring BRAF V600E mutation, (HER2) ErBB2 alterations, NTRK gene fusions and KRAS(G12C) mutation. Improved understanding towards the mechanism of resistance to targeted therapy such as anti-epidermal growth factor receptor antibodies and the evolving role of therapeutic monitoring with circulating tumour DNA (ctDNA) has enabled the longitudinal tracking of clonal evolution during treatment and the individualization of subsequent treatments. To broaden the community-based implementation of precision oncology in directing targeted therapies for patients with gastrointestinal cancers including mCRC, the feasibility of ‘Master Protocols’ that utilizes ctDNA-based genotyping platforms is currently being evaluated. Such protocols encompass both observational and interventional clinical trials of novel targeted therapies conducted within a large clinical trial network. In this review, we will discuss the latest developments in targeted therapies, and therapeutic strategies for overcoming acquired drug resistance in patients with mCRC.
Keywords: BRAF inhibitor, colorectal cancer, EGFR inhibitor, personalized medicine, precision oncology, targeted therapy
Introduction
Colorectal cancer (CRC) is the third most prevalent (10%) and second most lethal (9.4%) cancer worldwide.1 Although only 21% have distant metastasis on initial presentation,2 20–35% of resected stage II–III patients experience recurrence within 5 years, with most of them being distant recurrence.3 Over the last two decades, different treatment modalities for metastatic CRC (mCRC) have emerged. The combination of fluoropyrimidines with oxaliplatin or irinotecan has increased median overall survival (OS) to nearly 20 months.4,5 The addition of anti-vascular endothelial growth factor (VEGF) in unselected patients and anti-epidermal growth factor receptor (EGFR) in KRAS (Kirsten rat sarcoma virus)-wild type (wt) patients further prolonged OS.6,7 The integration of multi-modal therapy including surgical resection, radiofrequency ablation, targeted radiotherapy and notably, neoadjuvant chemotherapy in liver metastasis has made oligometastatic disease a potentially curable condition in carefully selected patients. With all the aforementioned advances, survival for mCRC has improved over the past decade from a 5-year survival of 10.3% in 1996–2003 to 14.3% in 2010–2016.8
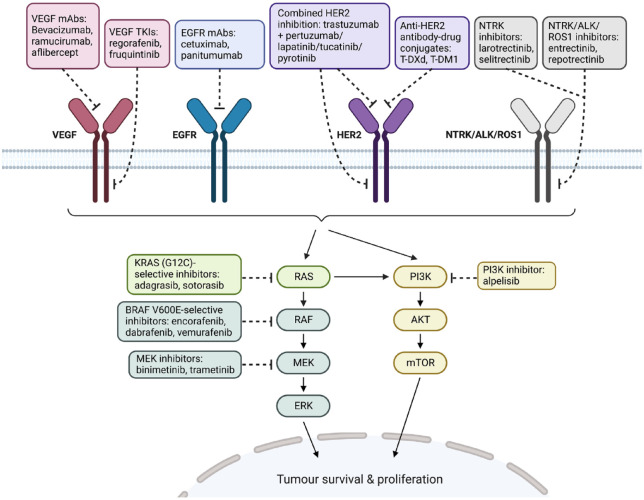
The uniqueness of CRC is characterized by its inter-metastatic and intra-tumour heterogeneity, which contributes to the complexity of the disease.9 The comprehensive genome-wide profiling of CRC by The Cancer Genome Atlas has allowed a more thorough understanding of the disease.10 Several novel actionable targets including BRAF (serine/threonine-protein kinase B-Raf) V600E mutation, ErBB2 (V-erb-b2 erythroblastic leukaemia viral oncogene homolog 2) alterations, KRAS(G12C) mutation and gene fusions such as NTRK (neurotrophic tyrosine receptor kinase), ALK (anaplastic lymphoma kinase) and ROS-1 (c-ros oncogene 1) have been identified over the past decade (Figure 1). The identification of tumour-sidedness as a predictive biomarker of response to EGFR antibodies and the use of circulating tumour DNA (ctDNA) in guiding the re-challenge of EGFR antibodies have significantly influenced the contemporary management of mCRC. In this review, we will discuss the recent advancements in targeted therapies and their impact on the clinical outcome of patients with mCRC.
Figure 1.
Selected summary of actionable targets and their targeted therapies with demonstrable clinical efficacy in mCRC.
Source: Adapted from ‘HER2 Signalling Pathway’, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates.
ALK, anaplastic lymphoma kinase; BRAF, serine/threonine-protein kinase B-Raf; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; HER2, human epidermal growth factor receptor 2; KRAS, Kirsten rat sarcoma virus; mAbs, monoclonal antibodies; mCRC, metastatic colorectal cancer; MEK, mitogen-activated protein kinase kinase; mTOR, mammalian target of rapamycin; NTRK, neurotrophic tyrosine receptor kinase; PI3K, phosphoinositide 3-kinase; ROS1, c-ros oncogene 1; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan or DS-8201; TKIs, tyrosine kinase inhibitors; VEGF, vascular endothelial growth factor.
EGFR inhibition
The role of the EGFR receptor tyrosine kinase (RTK) in CRC carcinogenesis has been recognized for more than 30 years – by downstream signalling through mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)/AKT and JAK (Janus kinase)/STAT (activator of transcription) pathways, EGFR promotes cancer cell survival and proliferation and was recognized as an actionable target in mCRC.11 The EGFR monoclonal antibodies cetuximab and panitumumab have been shown to be efficacious across different lines of therapy for RAS-wt mCRC.12,13
In first-line treatment for patients with RAS-wt mCRC, the addition of cetuximab or panitumumab improved progression-free survival (PFS) [hazard ratio (HR) = 0.65, p < 0.00001], OS (HR = 0.83, p = 0.07) and objective response rates (ORR) (relative risk = 1.55, p = 0.0009) compared with chemotherapy alone.14 To cite an example, the addition of panitumumab to FOLFOX4 increased median OS from 19.7 to 23.9 months (p = 0.072).7 EGFR antibodies have been combined with doublet or triplet regimens containing 5-fluorouracil (5-FU), leucovorin, oxaliplatin and/or irinotecan with manageable safety profiles and efficacy.15–17 However, oral capecitabine is not a recommended chemo-backbone for EGFR antibodies due to overlapping toxicity and reduced survival benefit.18 The TRIPLETE phase III study randomized patients to panitumumab in combination with either the modified (m)FOLFOXIRI or mFOLFOX regimen. The result has been recently reported, showing that four-drug regimen did not improve PFS (12.7 versus 12.3 months, p = 0.277) nor ORR (73% versus 76%, p = 0.526) over the three-drug regimen, with notably higher rates of grade 3–4 adverse events (AEs) (69% versus 57%).19
Maintenance therapy with EGFR antibodies has been shown to improve PFS in some studies20,21; however, it remains unclear whether they should be used alone or with fluoropyrimidines.22,23 While fluoropyrimidine-based therapy has been used as maintenance for mCRC, the recent PANAMA trial has established a modest PFS improvement with the addition of panitumumab to maintenance fluoropyrimidine over fluoropyrimidine alone (8.8 versus 5.7 months, p = 0.014).24
Downstream activation of the MAPK pathway, including alterations in EGFR, KRAS, NRAS (neuroblastoma RAS viral oncogene homolog) and BRAF, is the main cause of both innate and acquired resistance to EGFR antibodies in mCRC.25,26 KRAS, NRAS and BRAF V600E mutations were found in 35.9%, 4.1% and 6.8% of mCRC patients, respectively,27 and studies have shown that mutations in KRAS exon 2–4, NRAS exon 2–4 and BRAF V600E confer resistance to EGFR antibodies.12,28 Current guidelines therefore support extended RAS and BRAF mutation testing upon the diagnosis of mCRC.29 In addition, genetic alterations, including HER2 amplification, PIK3CA mutation and PTEN (phosphatase and tensin homolog) inactivation, have all been linked to EGFR antibody resistance in clinical studies.30–35
EGFR antibody re-challenge
Re-challenge therapy is defined as retreatment following a treatment-free interval of the particular drug in question, where the tumour displayed initial response/disease control and then developed resistance during treatment.36 The strategy was first reported by Santini et al., where 39 patients were re-challenged with irinotecan-based cetuximab therapy after initial response/disease stabilization and then clinical resistance to the same therapy.37 Subsequently, several phase II trials were conducted and demonstrated that re-challenging patients with cetuximab or panitumumab have modest benefits in later lines of therapy (Table 1).38–43 However, the factors that may predict benefit from EGFR antibody re-challenge are needed as the ORR ranged from 2.9% to 21% in these trials38–41 and there are other competing options in later lines such as regorafenib.44 The CRICKET study investigated the use of ctDNA in detecting the presence RAS and BRAF mutation in patients prior to re-treatment with EGFR antibodies, and found that 12 out of the 25 evaluable patients had detectable RAS mutations in ctDNA. Patients without detectable RAS mutation in ctDNA had significantly longer PFS compared with those with detectable RAS mutations following EGFR antibody re-challenge (4.0 versus 1.9 months, p = 0.03).38 This finding supports the hypothesis that RAS-wt cancer clones that are sensitive to EGFR antibodies may be restored during a period of treatment break.
Table 1.
Selected past and ongoing prospective clinical trials on anti-EGFR re-challenge in metastatic CRC.
| Trial | Phase | Sample size | Treatment arm(s) | Previous treatments | ORR (%) | mPFS (months) | mOS (months) | Remarks |
|---|---|---|---|---|---|---|---|---|
| CRICKET38 | II | 28 | Cetuximab + irinotecan | 1L cetuximab + irinotecan-based chemotherapy; 2L bevacizumab + oxaliplatin-based chemotherapy | 21 | 3.4 | 9.8 | ctDNA RAS-wt patients had prolonged mPFS (4.0 versus 1.9 months) |
| E-RECHALLENGE39 | II | 33 | Cetuximab + irinotecan | Fluoropyrimidine, oxaliplatin, irinotecan, cetuximab and bevacizumab | 15.6 | 2.9 | 8.6 | ctDNA RAS-wt patients had prolonged mPFS (7.0 versus 2.9 months) |
| JACCRO CC-0840 | II | 34 | Cetuximab + irinotecan | 1L cetuximab + irinotecan/oxaliplatin-based chemotherapy; 2L oxaliplatin/irinotecan-based chemotherapy | 2.9 | 2.4 | 8.2 | Patients with anti-EGFR-free interval >372 days had prolonged mPFS (4.6 versus 2.1 months) and mOS (14.1 versus 6.3 months) |
| JACCRO CC-0941 | II | 25 | Panitumumab + irinotecan | 1L panitumumab + FOLFOX/FOLFIRI; 2L bevacizumab/panitumumab + chemotherapy | 8.3 | 3.1 | 8.9 | Patients with anti-EGFR-free interval >372 days had prolonged mPFS (4.42 versus 2.51 months) and mOS (15.84 versus 7.33 months) |
| CHRONOS42 | II | 27 | Panitumumab | Previous anti-EGFR in 1L (63%), 2L (15%) or >2L (22%) | 30 | 3.7 | – | Only patients with ctDNA RAS/BRAF/EGFR-wt were enrolled |
| CAVE43 | II | 77 | Cetuximab + avelumab | 1L anti-EGFR + chemotherapy, at least one >1L therapy | 7.8 | 3.6 | 11.6 | ctDNA RAS/BRAF-wt patients had prolonged mPFS (4.1 versus 3.0 months) and mOS (17.3 versus 10.4 months); 4% were MSI-high tumours |
| REMARRY & PURSUIT45 (UMIN000036424) (jRCTs031190096) | II | 50 | Panitumumab + irinotecan | Fluoropyrimidine, oxaliplatin, irinotecan and anti-EGFR | 14 | 3.6 | – | ctDNA RAS-wt patients had better ORR (16% versus 0%); patients with anti-EGFR-free interval >365 days had better ORR (44.4% versus 7.3%) |
| FIRE-4 (NCT02934529) | III | 550 | Cetuximab + irinotecan-based chemotherapy versus regorafenib | 1L cetuximab + FOLFIRI; 2L bevacizumab + FOLFOX | – | – | – | – |
| CAPRI II GOIM (NCT05312398) | II | 200 | Cetuximab + irinotecan versus TAS-102 or regorafenib | 1L cetuximab + FOLFIRI; 2L cetuximab + FOLFOX versus bevacizumab + FOLFOX | – | – | – | Patients will be selected for suitable second- and third-line therapies according to ctDNA RAS-BRAF status |
| PULSE (NCT03992456) | II | 120 | Panitumumab versus TAS-102 or regorafenib | – | – | – | – | ctDNA analysis included |
| PARERE (NCT04787341) | II | 214 | Panitumumab followed by regorafenib versus regorafenib followed by panitumumab | 1L anti-EGFR-based therapy; previous 5-FU, oxaliplatin, irinotecan and anti-angiogenics | – | – | – | Only ctDNA RAS/BRAF-wt patients will be enrolled |
| VELO (EudraCT: 2018-001600-12) | II | 112 | Panitumumab + TAS-102 versus TAS-102 alone | – | – | – | – | – |
| NCT03524820 | II | 60 | Cetuximab ± chemotherapy | 1L anti-EGFR + chemotherapy; other 2L treatments | – | – | – | – |
ctDNA, circulating tumour DNA; EGFR, epidermal growth factor receptor; mOS, median overall survival; mPFS, median progression-free survival; MSI, microsatellite instability; ORR, objective response rate; wt, wild type; 1L, first-line therapy; 2L, second-line therapy; 5-FL, 5-fluorouracil.
The JACCRO CC-08 study showed that the treatment-free intervals between EGFR antibody treatment may affect subsequent response to EGFR antibody re-challenge. Patients with EGFR antibody-free intervals of more than 1 year had prolonged median PFS (4.6 versus 2.1 months) and OS (14.1 versus 6.3 months) compared with their counterparts.40 However, some studies have yielded conflicting results.39,42 In the recently reported CHRONOS study, 36 (69%) out of 52 patients were found to be RAS/BRAF-wt in ctDNA analyses. An ORR of 30% was achieved among the 27 evaluable patients – which is relatively high compared with historic rates of up to 20% as discussed previously. Interestingly, after disease progression with anti-EGFR re-challenge, de novo MET amplification was detected on ctDNA in three patients, suggesting MET amplification being a potential therapeutic target for patients progressing on anti-EGFR therapy. The authors have also noted that the presence of resistance-conferring mutations and objective responses were independent of the EGFR antibody-free interval, suggesting that ctDNA maybe more effective in selecting patients for re-challenge therapy.42
Several ongoing phase II/III trials will confirm the benefit of re-challenge therapy in RAS-wt ctDNA-selected patients while looking further into other molecular predictors (Table 1) (UMIN000036424, jRCTs031190096, NCT02934529). In the preliminary reports of the REMARRY and PURSUIT trials (UMIN000036424, jRCTs031190096), although EGFR antibody re-challenge did not meet the pre-specified primary endpoint of ORR (14%), the results confirmed the utility of ctDNA and EGFR antibody-free interval in selecting patients for re-challenge therapy. Five out of 31 patients (16%) with ctDNA RAS-wt at progression during prior EGFR antibody therapy responded to re-challenge, while none of the seven patients who were ctDNA RAS-mt responded (p = 0.25). Furthermore, patients with an EGFR antibody-free interval of over 1 year had a significantly higher ORR (44.4% versus 7.3%, p = 0.0037).45 The ongoing FIRE-4 phase III randomized controlled trial (RCT) will compare the efficacy of re-challenge cetuximab-chemotherapy treatment with regorafenib in the third-line setting, following disease progression after first-line cetuximab plus FOLFIRI and second-line bevacizumab plus FOLFOX (NCT02934529).
VEGF inhibition
The anti-VEGF antibody bevacizumab was one of the first biologics alongside with cetuximab approved for the indication of treating patients with mCRC, regardless of the RAS mutational status.46,47,48 Several meta-analyses have demonstrated that bevacizumab could be combined safely with doublet chemotherapy comprising of 5-FU, capecitabine, oxaliplatin and/or irinotecan with survival gains as first-line treatment.49–51 In a pooled meta-analysis of 1697 patients from five RCTs which investigated the optimal chemotherapy regimen to be combined with bevacizumab, the triplet FOLFOXIRI backbone was found to have longer PFS (HR = 0.74, p < 0.001) and OS (HR = 0.81, p < 0.001) compared with FOLFIRI when combined with bevacizumab, at the expense of higher rates of toxicity such as neutropenia, febrile neutropenia, mucositis and diarrhoea.51 First-line FOLFOXIRI with bevacizumab is now one of the standard therapeutic options in medically fit patients with mCRC.29 The role of bevacizumab in maintenance therapy remains unclear. Although significant PFS benefits were observed in bevacizumab plus fluoropyrimidine in some studies,52,53 an individual-patient data meta-analysis showed that the PFS difference between the bevacizumab monotherapy and observation group was limited to less than 1 month (9.6 versus 8.9 months, p < 0.0001).54 Since fluoropyrimidine–bevacizumab and fluoropyrimidine alone are regarded as some of the popularly used maintenance approaches following initial chemotherapy, the ongoing BEVAMAINT study will formally compare the two strategies (NCT04188145).
In second-line therapy, VEGF inhibitors including the anti-VEGFR2 antibody ramucirumab and the recombinant fusion protein aflibercept combined with FOLFIRI demonstrated survival benefits.55,56 Bevacizumab is the only agent to date which have been shown prospectively to be safe and effective when combined with oral capecitabine-based regimen.57–59 In the AXEPT non-inferiority trial, 650 Asian patients were randomized to mXELIRI (or FOLFIRI) with or without bevacizumab. The median OS of the mXELIRI group was non-inferior to the FOLFIRI group (16.8 versus 15.4 months), and the mXELIRI group had an encouraging safety profile with numerically lower incidences of grade 3–4 AEs compared with the latter (54% versus 72%).60 The oral tyrosine kinase inhibitor (TKI) fruquintinib selectively binds to VEGFR1–3 and the recent FRESCO-2 international RCT has demonstrated fruquintinib as a viable option for refractory mCRC. Compared with placebo, fruquintinib improved OS (7.4 months versus 4.8 months, p < 0.001) and PFS (3.7 months versus 1.8 months, p < 0.001) with tolerable safety profile (grade ⩾3 AE 62.7% versus 50.4%).61
Patient selection for targeted therapy in the first-line treatment of microsatellite stable metastatic CRC: new studies
Primary tumour sidedness
A meta-analysis of the FIRE-3, CALGB/SWOG 80405 and PEAK trials compared the efficacy of first-line chemotherapy with EGFR antibodies or with bevacizumab, after stratifying patients into having left-sided or right-sided RAS-wt primary tumours. The use of EGFR antibodies was more favourable in patients with left-sided primaries in terms of OS and ORR after exclusion of BRAF-mutant patients, whereas bevacizumab demonstrated better PFS in right-sided tumours.62 In another meta-analysis of the CRYSTAL and PRIME trials, only patients with left-sided tumours significantly benefited from first-line usage of chemotherapy and EGFR antibodies compared with chemotherapy alone.62 The DEEPER study showed that the depth of response to cetuximab when combined with mFOLFOXIRI was significantly superior than when combined with bevacizumab in patients with left-sided primary tumours.63 Recently, the PARADIGM trial provided the first ever prospective evidence of panitumumab in providing greater OS benefit and higher objective response over bevacizumab when combined with mFOLFOX6 in both the intention-to-treat cohort (all RAS-wt tumours, n = 802) and in patients with left-sided primary tumours (n = 604). The median OS was 37.9 months versus 34.3 months (HR = 0.82, 95% CI: 0.68–0.99, p = 0.031), and the ORR was 80.2% versus 68.6% favouring the panitumumab plus mFOLFOX6 arm.64 Anti-EGFR blockade combined with doublet or triplet chemotherapy is therefore considered as the standard of care for patients with left-sided, RAS-WT mCRC. However, the optimal treatment for right-sided tumours is yet to be defined, and the prognosis of this population remains poor. As studies showed that the benefit from bevacizumab therapy is independent of primary tumour sidedness,65,66 bevacizumab plus chemotherapy is a reasonable treatment option for right-sided RAS-wt tumours.
Optimal treatment sequence
Preclinical studies have suggested that VEGF expression increases upon onset of anti-EGFR resistance,67 whereas exposure to bevacizumab induces elevated VEGF-A levels which activates STAT-3 and VEGFR2 signalling, leading to resistance to cetuximab.68 A number of retrospective studies reported consistent findings, as first-line chemotherapy with EGFR antibody followed by second-line chemotherapy with anti-VEGF achieved more favourable survival benefits than the reverse treatment sequence.69–71 The STRATEGIC-1 phase III trial is a RCT which investigated the impact of drug sequencing on the primary endpoint of disease control in treatment-naïve patients with mCRC. This study randomized 263 patients with RAS/BRAF-wt mCRC, to FOLFIRI-cetuximab followed by mFOLFOX6-bevacizumab (Arm A), or first-line oxaliplatin-based regimen in a ‘stop-and-go’ manner with bevacizumab, followed by second-line FOLFIRI-bevacizumab and third-line anti-EGFR with or without irinotecan (Arm B). Although the study did not meet its primary endpoint, the findings were consistent with the FIRE-3 and PARADIGM study since the use of EGFR antibody-based chemotherapy in the first line (arm A) resulted in higher tumour responses and a trend for better OS (37.8 versus 34.4 months, p = 0.121).72 The ongoing phase III CR-SEQUENCE study will evaluate the optimal treatment sequence with panitumumab and bevacizumab combined with chemotherapy in RAS-wt left-sided tumours (NCT03635021).
Uncommon molecular groups in metastatic CRC
BRAF V600E mutation
BRAF mutations are found in 7.1–12.5% of mCRCs27,73–75 and the majority are V600E hotspot mutations.73 The mutation is thought to play a fundamental role in the serrated pathway of CRC pathogenesis – it is closely linked to epigenetic hypermethylation changes of promotor CpG islands associated with silencing of tumour suppressors. CpG island methylation, along with BRAF downstream MAPK pathway signalling which exert synergistic effects in BRAF-mt tumours.76 BRAF mutations are mutually exclusive from RAS mutations,77 and are more commonly found in microsatellite instability (MSI)-high tumours.74 BRAF-mt mCRCs are also associated with poorer survival outcomes and several unfavourable characteristics, such as proximal tumour location, higher T staging, poor differentiation and higher rates of peritoneal and distant lymph node metastases.46,74,78 Compared with BRAF V600E mutations, non-V600E mutations tend to confer better prognosis with distinct clinicopathological characteristics.79
Conventional first-line therapy for BRAF mutants
Since BRAF activates the MAPK pathway downstream of EGFR, RAS-wt/BRAF-mt tumours are less responsive to EGFR inhibitors.28 The FIRE-4.5 (AIO KRK-0116) study is a randomized phase II study of BRAF-mt/RAS-wt patients who had treatment-naïve mCRC, in which 108 patients were randomized to FOLFOXIRI with cetuximab or with bevacizumab. The bevacizumab arm was associated with better ORR (66.7% versus 52.0%) and median PFS (8.3 versus 5.9 months) compared with the cetuximab group.80 Bevacizumab plus triplet or doublet chemotherapy should therefore be considered the preferred option for the first-line treatment of BRAF-mt mCRC before the era of BRAF inhibition.29,48
However, it is debatable whether bevacizumab plus triplet FOLFOXIRI chemotherapy brings more benefit than doublet FOLFOX/FOLFIRI chemotherapy in BRAF-mt patients. The TRIBE study contributed the first piece of evidence to the controversy, as a small subgroup analysis with 28 BRAF-mt patients of the phase III TRIBE study showed a trend of longer OS in bevacizumab plus FOLFOXIRI versus FOLFIRI.46 The analysis was however underpowered, and the results were later challenged by an individual patient data meta-analysis from five studies, which showed no difference in survival of bevacizumab with triplets versus doublets in BRAF mutants (HR = 1.11). In addition, FOLFOXIRI plus bevacizumab was associated with significantly higher rates of grade 3–4 gastrointestinal and haematological AEs; thus, the authors concluded that FOLFOX, instead of FOLFOXIRI, should be regarded as the standard-of-care chemotherapy regimen to combine with bevacizumab in BRAF-mt mCRC patients.51 The recent result from the CAIRO5 study adds to the controversy by taking into account of tumour-sidedness. In this study, 294 patients with BRAF V600E-mt and/or right-sided primary tumours were randomized to receive either first-line FOLFOX or FOLFIRI plus bevacizumab (arm A) or FOLFOXIRI plus bevacizumab (arm B). Patients in arm B had significantly longer PFS (9.0 versus 10.6 months, p = 0.02) and ORR (32.0% versus 52.1%, p < 0.001) at the expense of more frequent grade ⩾3 toxicities (58.5% versus 75.0%, p = 0.003).81 FOLFOXIRI-bevacizumab may therefore be considered in right-sided BRAF-mt patients if intensified chemotherapy could be tolerated.
Targeting the MAPK pathway
Unlike melanoma, BRAF inhibitor has a modest single agent response rate of 5% in patients BRAF-mt mCRC.82 This may be in part due to MAPK activation as a potential resistance mechanism against BRAF inhibition, thus prompting the use of multi-drug combinations targeting BRAF, EGFR and the MAPK pathway.83 The role of dual BRAF and EGFR inhibition in BRAF-mt mCRC patients was first demonstrated in a pilot trial by Yaeger et al.,84 where two out of 15 patients had partial responses to vemurafenib and panitumumab. Several studies further assessed the efficacy and safety profile of different combinations of BRAF, EGFR and MEK inhibitors (Table 2).85–90 The phase III BEACON trial was the first to demonstrate the superiority of encorafenib-based, chemotherapy-free regimens over the standard EGFR-antibody plus chemotherapy in patients with previously treated BRAF-mt mCRC. Both the doublet (encorafenib plus cetuximab) and triplet (encorafenib, cetuximab plus binimetinib) were superior to the control arm (cetuximab plus irinotecan-based chemotherapy) in terms of median OS (9.3 months versus 5.9 months) and ORR (26.8% for triplet, 19.5% for doublet and 1.8% for control arm). Although the triplet group had higher rates of grade ⩾3 AEs (65.8%) than the doublet group (57.4%), both arms showed similar toxicity profiles compared with the control group (64.2%).85 Furthermore, a recent study reported that patients with tumours of the Consensus Molecular Subtype 4 (CMS4) and BRAF mutant 1 (BM1) having higher response rates with the triplet than the doublet therapy.91 Therefore, while it is generally agreed that the doublet (encorafenib plus cetuximab) is currently preferred over the triplet regimen given the similar impact on OS, certain high-risk subgroups may potentially be more suitable for triplet therapy.
Table 2.
Selected past and ongoing prospective clinical trials on targeted therapy and immunotherapy in BRAF V600E metastatic CRC.
| Trial | Phase | Sample size | Treatment arm(s) | Line of treatment (and MSI status) | ORR (%) | mPFS (months) | mOS (months) |
|---|---|---|---|---|---|---|---|
| Combined BRAF, EGFR ± MEK inhibition | |||||||
| Yaeger et al.84 | I | 15 | Vemurafenib + panitumumab | ⩾2L | 13 | 3.2 | 7.6 |
| Corcoran et al.89 | I | 20 | Dabrafenib + panitumumab | ⩾1L | 10 | 3.5 | 13.2 |
| 91 | Dabrafenib + panitumumab + trametinib | ⩾1L | 21 | 4.2 | 9.1 | ||
| BEACON CRC85 | III | 220 | Encorafenib + cetuximab (versus cetuximab + irinotecan-based chemotherapy) | ⩾2L | 19.5 (versus 1.8) | 4.3 (versus 1.5) | 9.3 (versus 5.9) |
| 224 | Encorafenib + cetuximab + binimetinib (versus cetuximab + irinotecan-based chemotherapy) | ⩾2L | 26.8 (versus 1.8) | 4.5 (versus 1.5) | 9.3 (versus 5.9) | ||
| ANCHOR CRC87 (NCT03693170) | II | 92 | Encorafenib + cetuximab + binimetinib | 1L | 47.8 | 5.8 | 17.2 |
| BREAKWATER (NCT04607421) | III | 765 (in 3 arms) | Encorafenib + cetuximab versus encorafenib + cetuximab + FOLFOX (versus bevacizumab + FOLFOX/FOLFIRI/XELOX) | 1L | – | – | – |
| Immunotherapy plus BRAF doublet inhibition | |||||||
| Corcoran et al.92 | II | 20 | Dabrafenib + trametinib + spartalizumab | Unspecified (MSI-unselected) | 35 | – | – |
| Morris et al.93 | II | 23 | Encorafenib + cetuximab + nivolumab | ⩾2L (MSS patients) | 48 | 7.4 | 15.1 |
| SWOG 2107 (NCT05308446) | II | 84 (in 2 arms) | Encorafenib + cetuximab + nivolumab (versus encorafenib + cetuximab) | ⩾2L (MSS patients) | – | – | – |
| SEAMARK (NCT05217446) | II | 104 (in 3 arms) | Encorafenib + cetuximab + pembrolizumab (versus pembrolizumab alone) | 1L (MSI-high patients) | – | – | – |
| Combined BRAF, EGFR and PI3K inhibition | |||||||
| Tabernero et al.94 | II | 52 | Encorafenib + cetuximab + alpelisib (versus encorafenib + cetuximab) | ⩾2L | 27 (versus 22) | 5.4 (versus 4.2) | 15.2 (versus not reached) |
| BRAF and EGFR inhibition plus irinotecan | |||||||
| SWOG S140690 | II | 50 | Vemurafenib + cetuximab + irinotecan versus cetuximab + irinotecan | 2L/3L | 17 (versus 4) | 4.2 (versus 2.0) | 9.6 (versus 5.9) |
BRAF, serine/threonine-protein kinase B-Raf; CRC, colorectal cancer; EGFR, epidermal growth factor receptor; MEK, mitogen-activated protein kinase kinase; mOS, median overall survival; mPFS, median progression-free survival; MSI, microsatellite instability; MSS, microsatellite stability; ORR, objective response rate; PI3K, phosphoinositide 3-kinase.
The phase III BREAKWATER (NCT04607421) and phase II single-arm ANCHOR CRC87 (NCT03693170) studies are two notable clinical trials which evaluate BRAF-EGFR and/or MEK inhibitors in the first-line treatment of BRAF-mt mCRC. In the ANCHOR CRC study, an ORR of 47.8% was observed in the 92 evaluable patients who received encorafenib–binimetinib–cetuximab, with a median OS of 17.2 months a grade ⩾3 AEs rate of 69.5%.87 Apart from encorafenib-based therapies, regimens including other BRAF inhibitors such as dabrafenib–panitumumab–trametinib, dabrafenib–panitumumab and vemurafenib–cetuximab–irinotecan have also demonstrated increased survival and tumour response rates in clinical studies, at the expense of higher toxicities.89,90 Encorafenib-based regimens have been included in the latest NCCN guideline (June 2022), but not the other regimens at this juncture.29
Other strategies for BRAF-mutant mCRC
Recently, the addition of programmed death receptor-1 (PD-1) inhibitor to BRAF-inhibitors has been investigated in several trials, as preclinical studies found that the combination demonstrates down-regulation of mismatch repair (MMR) and up-regulation of error-prone polymerases, which induces DNA damage and hypermutability, and ultimately triggers a proficient MMR-to-dMMR phenotypic switch.95 A single-arm phase II study demonstrated an ORR of 35% with combined dabrafenib, trametinib and the PD-1 antibody spartalizumab in 20 patients with BRAF V600E-mt microsatellite status-unselected patients.92 In another phase I/II trial, 23 treatment refractory microsatellite stable (MSS) BRAF-mt patients were treated with encorafenib, cetuximab and nivolumab and 48% attained radiographic response.88 Based on these results, the phase II SWOG 2107 multi-centre RCT has is evaluating encorafenib plus cetuximab with or without nivolumab in chemo-refractory BRAF-mt/MSS patients (NCT05308446), while the phase II SEAMARK study will evaluate encorafenib plus cetuximab with or without pembrolizumab in treatment-naïve BRAF-mt/MSI-H patients (NCT05217446).
As PI3K/AKT activation has also been identified as a mechanism of acquired resistance to BRAF inhibitors in preclinical studies,96 a phase II trial compared the triplet encorafenib–cetuximab–alpelisib with the doublet (encorafenib–cetuximab) in patients with chemotherapy-refractory BRAF-mt mCRC. The addition of PI3K inhibitor alpelisib resulted in a non-significant trend of higher median PFS from 4.2 to 5.4 months (p = 0.064) and confirmatory studies are needed.94
HER2 alterations
HER2 or ErBB2 amplification was one of the earliest-identified therapeutic markers of solid tumours. It is found in around 1.1–5.8% of patients with mCRC especially in KRAS/BRAF-wt patients.97–102 ErBB2 amplification leads to HER2 RTK overexpression, it is then activated by dimerization with another receptor of the ErbB family, resulting in transphosphorylation of tyrosine residues within the cytoplasmic domain, thus leading to downstream signal transduction along the MAPK, PI3K/AKT and JAK/STAT pathway.103 In mCRC, HER2 overexpression is associated with left-sided primary, more frequent lung metastases and higher number of metastases.30,97,98
Combined HER2 inhibition
Unlike HER2-positive breast cancer, trastuzumab alone is ineffective in HER2-altered mCRC. The HERACLES study is the first to evaluate dual anti-HER2 strategies in HER-2 altered mCRC using trastuzumab and the TKI lapatinib.104 Among the 32 evaluable HER2-positive mCRC patients heavily pre-treated with chemotherapy and EGFR inhibitors, the respective median PFS and OS were 4.7 and 10 months, while the ORR was 28%, which is seemingly better than the reported activity of standard third-line therapies such as regorafenib or TAS-102.105 Central nervous system (CNS) recurrence occurred in 19% of the patients, compared with historic rates of 1–4% in mCRC patients. The higher incidence of CNS metastasis maybe due to the propensity of ErBB2 amplification driving CNS spread, or the limited intracranial activity of anti-HER2 therapies.105
Other combinations of anti-HER2 therapies have also been investigated in recent years (Table 3). As preclinical evidence demonstrated greater specificity of tucatinib to HER2 compared with lapatinib,106 the efficacy of tucatinib–trastuzumab in pre-treated patients has been investigated in the phase II MOUNTAINEER study, which reported an ORR of 38.1% and disease control rate (DCR) of 71.4% at a median follow-up of 20.7 months. Median PFS and OS were 8.2 and 24.1 months, respectively, among the 86 patients. Diarrhoea was the most common treatment-related AE (TRAE) of the combination, yet grade 3 diarrhoea were noted in only 3% of the patients.107
Table 3.
Selected past and ongoing prospective clinical trials on targeted therapy in HER2-positive metastatic CRC.
| Trial | Phase | Sample size | Treatment arm(s) | Line of treatment | ORR (%) | mPFS (months) | mOS (months) |
|---|---|---|---|---|---|---|---|
| Combined HER2 inhibition | |||||||
| HERACLES108 | II | 32 | Trastuzumab + lapatinib | ⩾2L | 28 | 4.7 | 10.0 |
| MOUNTAINEER107 | II | 86 | Trastuzumab + tucatinib | ⩾2L | 38.1 | 8.2 | 24.1 |
| HER2-FUSCC-G109 | II | 11 | Trastuzumab + pyrotinib | ⩾2L | 45.5 | 7.8 | 15.0 |
| Yuan et al.110 | II | 11 | Trastuzumab + pyrotinib | ⩾1L | 27 | – | – |
| MyPathway111 | II | 84 | Pertuzumab + trastuzumab | ⩾2L | 26.2 | – | – |
| TRIUMPH112 | II | 27 | Pertuzumab + trastuzumab | ⩾2L | 30 | 4.0 | 10.1 |
| TAPUR113 | II | 28 | Pertuzumab + trastuzumab | ⩾2L | 14 | 4.0 | – |
| NSABP FC-11114 | II | 21 | Neratinib + cetuximab | ⩾2L (prior anti-HER2 not allowed) | 33 | – | – |
| S1613 (NCT03365882) | II | 240 (in 2 arms) | Pertuzumab + trastuzumab versus cetuximab + irinotecan | ⩾2L | – | – | – |
| Anti-HER2 antibody–drug conjugates | |||||||
| HERACLES-B115 | II | 31 | Pertuzumab + T-DM1 | ⩾2L | 9.7 | 4.1 | |
| DESTINY-CRC01116 | II | 53 | T-DXd | ⩾3L | 45.3 | 6.9 | 15.5 |
| DESTINY-CRC02 (NCT04744831) | II | 122 | T-DXd | ⩾2L (prior anti-HER2 allowed) | – | – | – |
CRC, colorectal cancer; HER2, human epidermal growth factor receptor 2; mOS, median overall survival; mPFS, median progression-free survival; ORR, objective response rate; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan or DS-8201; 2L, second-line therapy; 3L, third-line therapy.
Preliminary reports from two phase II studies on pyrotinib–trastuzumab for HER2-positive mCRC have been published recently.109,110 Pyrotinib is a novel irreversible TKI targeting HER2 and EGFR and was shown to be more efficacious than lapatinib when combined with capecitabine in metastatic breast cancer.117 Among the 11 mCRC patients in the HER2-FUSCC-G study, 45.5% responded to pyrotinib–trastuzumab, and ORR was even higher in RAS-wt patients (55.6%). An acceptable grade 3-4 TRAE rate of 36.4% was reported.109
The pertuzumab–trastuzumab combination along with chemotherapy is currently considered a first-line option in patients with HER2-positive metastatic breast cancer.118 While pertuzumab itself inhibits dimerization between HER2 and HER3, it has a synergistic effect when combined with trastuzumab.119 The ongoing MyPathway study evaluated this combination in 84 HER2-amplified mCRC patients and reported an ORR of 26.2%. Notably, only one of 16 (6.3%) KRAS-mt patients responded to the combination, suggesting limited activity of pertuzumab–trastuzumab in KRAS-mt tumours.111 At a median follow-up of 7.3 months, estimated median PFS and OS were 2.9 and 11.5 months, respectively. Grade 3–4 TRAEs were reported in 37% of the patients.120 The TRIUMPH and TAPUR phase II studies which evaluated the efficacy of pertuzumab plus trastuzumab in HER2-amplified/RAS-wt mCRC, also reported similar findings to that of the MyPathway study.112,113
Anti-HER2 antibody–drug conjugates
Based on the activity of the antibody–drug conjugate trastuzumab–emtansine (T-DM1) in combination with pertuzumab in metastatic breast cancer, the HERACLES-B trial evaluated this three-drug regimen in 31 patients with chemo-refractory HER2-positive mCRC. The trial however did not reach its primary endpoint of ORR (9.7%). It should be noted that since cytotoxic emtansine was included in the regimen, a lower dose of T-DM1 was delivered. The trial reported a median PFS comparable to other anti-HER2 combination therapies (4.1 months) and only 6.5% of patients experienced grade 3 TRAEs.115
The DESTINY-CRC01 trial evaluates the efficacy of trastuzumab–deruxtecan (DS-8201/T-DXd) in 86 mCRC patients who had received two or more lines of therapy, among which 53 were HER2-positive, which was defined as immunohistochemistry (IHC) 3+, or 2+ and in situ hybridization (ISH)-positive. More than 30% of the cohort had prior anti-HER2 therapy. The HER2-positive cohort showed an impressive ORR of 45.3%, and median PFS and OS were 6.9 and 15.5 months, respectively, at a median follow-up of 62.4 weeks; ⩾Grade 3 TRAEs were however considerably high (65.1%), with the most common ones being haematological and gastrointestinal AEs. Notably, eight patients (9.3%) had interstitial lung disease related to T-DXd, and three of them resulted in treatment-related deaths.116 Therefore, the phase II DESTINY-CRC02 trial was recently initiated to assess the efficacy of trastuzumab–deruxtecan at two different doses in HER2-overexpressing advanced CRC121 (NCT04744831).
Predicting anti-HER2 response and resistance
Several trials have identified the magnitude of ErBB2 amplification or HER2 overexpression as a predictive biomarker of anti-HER2 therapy.108,116,120 The HERACLES trial tested gene copy number by quantitative real-time PCR and identified that patients with tissue copy number (tCN) ⩾9.45 had prolonged PFS.108 The MyPathway trial measured HER2 tCN by next-generation sequencing and found that more patients with higher copy number responded to pertuzumab–traztuzumab.120 Subgroup analysis in the DESTINY-CRC01 trial found that patients with IHC 3+ achieved the highest ORR of 57.5%, compared with only 7.7% of patients with IHC2+ and ISH positive.116
Molecular analyses of ctDNA samples from the HERACLES trial revealed that the MAPK pathway was involved in resistance to anti-HER2 therapies, as alterations in RAS/RAF were detected in 86% of treatment-refractory patients but only in 14% of responders. KRAS, BRAF, HER2, EGFR, PIK3CA and PTEN alterations have also been detected at disease progression, which suggest both MAPK and PI3K/AKT pathways playing a role in acquired resistance against anti-HER2 therapy.122 Similar findings were also documented in the MyPathway trial, as lower ORRs were seen in KRAS-mt (8% versus 40%) and PIK3CA-mt (13% versus 43%) patients compared with their counterparts who are wild-typed.120
Owing to the relatively small subset of HER2-positive mCRC, clinical research on subsequent treatment strategies following acquired anti-HER2 resistance is limited. However, T-DXd may be potential strategy to overcome acquired resistance, as responses are seen in patients who had prior anti-HER2 therapy.116 The main past and ongoing studies on HER2 inhibition are summarized in Table 3.
NTRK and other actionable gene fusions
Although kinase gene fusions represent only about 0.9% of CRCs, they are considered an actionable group of therapeutic targets. The most frequently detected fusions were NTRK fusions, while other fusions including BRAF, RET, FGFR (fibroblast growth factor receptor), ROS1 and ALK. Higher incidences of gene fusions were found in MSI-high and RAS/BRAF-wt tumours,123 and the clinical characteristics of patients harbouring these gene fusions are reminiscent of those in BRAF mutations, including older age, right-sided primary, higher rates of lymphatic spread and lower rates of liver metastases.124,125 As current evidence suggests that gene fusions may be both a negative prognostic factor that predict resistance to EGFR inhibitor, novel strategies targeting gene fusions is an attractive field of research to improve the clinical outcomes for this subset of patients.34,35,124,125
First-generation NTRK inhibitors
Owing to the scarcity of gene fusions, most trials targeting gene fusions are designed as tumour-agnostic basket trials. Three phase I studies (LOXO-TRK-14001, SCOUT and NAVIGATE) evaluated the efficacy of the NTRK inhibitor larotrectinib in NTRK-positive metastatic non-CNS primary solid tumours and were included in a pooled analysis. Among the eight patients with colon cancer (out of 153 patients with solid tumours), ORR was 50% and median duration of response was 3.7 months. In a safety population of 260 patients treated regardless of kinase fusion status, 13.5% of them developed grade 3–4 TRAEs.126 In another integrated analysis of three phase I/II studies STARTRK-1, ALKA-372-001 and STARTRK-2 which evaluated the efficacy of the NTRK/ALK/ROS-1 inhibitor entrectinib, among the four patients (out of 54 patients with solid tumours) with CRC, 1 (25%) achieved objective response. In the overall safety-evaluable population comprising of 355 patients, entrectinib toxicity was generally tolerable as only 4% of patients discontinued treatment due to TRAEs.127 With their established efficacy and safety profile, the US Food and Drug Administration (FDA) has granted approval to larotrectinib and entrectinib for all solid tumours that harbour NTRK gene fusions, and current guidelines also support larotrectinib and entrectinib as subsequent treatment options for NTRK-positive mCRC.29
Overcoming resistance with second-generation NTRK inhibitors
Two different mechanisms of resistance to NTRK inhibitors, namely ‘on-target’ and ‘off-target’ resistance, have been described. The on-target resistance is the most common mechanism of resistance to the first-generation NTRK inhibitors larotrectinib and entrectinib, which are caused by mutations that decrease binding affinity of NTRK inhibitors to the kinase domain. Notably, the mutations contributing to on-target resistance to NTRK inhibitors are paralogous to mutations found in lung cancer patients resistant to ALK and ROS1 inhibitors.128,129 To address on-target resistance to NTRK inhibitors, the second-generation NTRK inhibitors selitrectinib (LOXO-195) and repotrectinib (TPX-0005) were designed and are currently being evaluated in phase I/II studies. A phase I study for selitrectinib was initiated following a case report of confirmed PR in an advanced NTRK-positive CRC patient who developed acquired G595R resistance to previous larotrectinib.130 A tumour agnostic cohort of 31 NTRK-positive patients who failed prior NTRK inhibition was recruited. Among 20 patients with TRK kinase mutations identified, 9 (45%) achieved complete response (CR)/ partial response (PR), and 6 (30%) had stable disease (SD).131 The NTRK/ALK/ROS1 inhibitor repotrectinib is currently being evaluated in the phase II tumour-agnostic TRIDENT-1 study. To date, among six NTRK-positive patients pretreated with NTRK inhibitors, three (50%) of them achieved objective response.132,133
On the other hand, off-target resistance consists of downstream activation of MAPK signalling including BRAF V600E and KRAS mutations or alterations in other RTKs such as MET (mesenchymal–epithelial transition) amplification.128,134 These genetic alterations are mostly identified in patients with gastrointestinal cancers and may account for the poorer response to larotrectinib and entrectinib in CRC compared with other cancers.126,127 Combinations of different TKIs were therefore postulated as a method to overcome off-target resistance.
KRAS(G12C)-selective inhibitors
Treatment for KRAS-mt mCRC is considered challenging as they are refractory to anti-EGFR therapy and no targeted therapy selective against KRAS mutations has been approved for mCRC. Numerous efforts to target KRAS have failed due to its high affinity with guanosine-5′-triphosphate and the absence of suitable binding pockets for drugs.135 However, the KRAS(G12C) mutant has been recently found to harbour a cysteine residue suitable for binding of covalent inhibitors, which led to the development of KRAS(G12C)-selective inhibitors.136 Following the promising results from the phase II portion of the CodeBreaK100 trial, the KRAS(G12C) inhibitor sotorasib (AMG510) was first approved by the FDA for KRAS(G12C)-mutated non-small-cell lung cancer (NSCLC).137 Since KRAS(G12C) mutation is only present in around 3% of all mCRCs,138 studies for KRAS(G12C) inhibition in mCRC are currently limited.
Several KRAS(G12C) inhibitors have been evaluated in phase I/II trials. Recently, a pre-specified analysis of the phase II portion of the CodeBreak100 study was released. Among 62 KRAS(G12C)-mt patients who received sotorasib, 9.7% and 72.6% achieved PR and SD, respectively. Median PFS and OS were 4.0 and 10.6 months, respectively, and grade ⩾3 TRAEs were seen in only 10% of the patients. The responses are relatively modest compared with patients with NSCLC where an ORR of 37.1%, median PFS of 6.8 months and OS 12.5 months are noted. This discrepancy maybe due to139 activation of other RTKs including EGFR which may bypass KRAS(G12C) blockade.140 Given the successful use of dual inhibition of BRAF and EGFR in BRAF-mt mCRC,85 concomitant KRAS(G12C) and EGFR blockade has been suggested to overcome resistance to KRAS(G12C) inhibition in CRC.139 The KRYSTAL-1 multicohort phase I/II study tested this postulation by evaluating the efficacy of adagrasib (MRTX849) alone or in combination with cetuximab in KRAS(G12C) mutant mCRC. In all, 44 and 32 patients were recruited to adagrasib monotherapy group and adagrasib plus cetuximab group, respectively. Both groups exhibited tolerable toxicity profiles. In adagrasib monotherapy group, the ORR and DCR were 19% and 86%, respectively, while the median PFS was 5.6 months. In the cetuximab–adagrasib group, a higher ORR of 46% and 100% DCR were observed, and median PFS was 6.9 months.141 As adagrasib plus cetuximab demonstrated promising clinical activity, the combination is currently being evaluated in the phase III KRYSTAL-10 trial in the second-line setting (NCT04793958). Despite these promising results, acquired resistance to adagrasib may be associated with the presence KRAS alterations, mutations along the MAPK and PI3K pathways and gene fusions involving ALK, RET, BRAF, RAF1 and FGFR3.142 The diversity of mechanisms provides a strong rationale to support the use of KRAS(G12C) inhibitors with other targeted therapies. Of note, the phosphatase Src homology region 2-containing protein tyrosine phosphatase 2 (SHP2) has been identified to be a common node that mediates signalling from multiple RTKs to RAS. Co-inhibition of KRAS(G12C) and SHP2 is able to suppress RAS signalling and induce tumour response in vitro and in vivo.143 The combination is further investigated in phase I/II trials NCT04185883 (CodeBreaK101), NCT04330664 (KRYSTAL-2) and NCT04699188 (KontRASt-01). Other combinations involving inhibition of EGFR, PD-1 and MEK are also explored in trials NCT04793958 (KRYSTAL-10), NCT03785249 (KRYSTAL-1), NCT04449874 and NCT04185883 (Code BreaK101).
Conclusion and future directions
The treatment paradigm of mCRC has been rapidly evolving over the past few years. Historically, targeted therapies in mCRC are more active when combined with chemotherapy backbones, but recent studies have shown that chemotherapy-free regimens can improve survival in certain molecular subgroups such as patient with BRAF-mt and MSI-H mCRC. RAS-mt MSS tumours represent a significant proportion of mCRC, yet most of them could not benefit from targeted therapy (with the possible exception of KRAS(G12D) mutant mCRC) and immunotherapy. Transforming immune-cold to immune hot tumours has therefore come under the spotlight recently, and VEGF inhibitors and/or chemotherapy combined with immune-checkpoint inhibitors maybe an effective method of re-invigorating the immune system. These hypotheses are currently being tested in several phase I-II studies.144–148
The advent of ctDNA analysis may further improve the precision of individualizing targeted therapies for patients with mCRC. Current evidence supports the use of ctDNA in tracking clonal resistance against EGFR antibody therapies and therefore should be used to identify patients who are suitable for EGFR antibody re-challenge therapy – as supported by recently reported European Society of Medical Oncology guideline.149 The use of ctDNA in tracking clonal resistance against other targeted therapies against BRAF mutation and NTRK fusions needs to be further evaluated, while the role of ctDNA in predicting early response to targeted therapies remains unclear. In early stage CRC, ctDNA has been shown to be a reliable marker in detecting minimal residual disease after surgical resection and identifying patients who may benefit more from adjuvant chemotherapy.150,151 Furthermore, ctDNA may be very useful in selecting patients for clinical trials given its convenience compared with tissue genotyping, and has been shown to significantly increase trial enrolment rates without compromising treatment efficacy in the SCRUM-Japan GOZILA studies.152
The traditional phase I to phase II-III model of clinical trial designs remains to be one of the causes hindering the development of targeted therapy in mCRC. The novel ‘Master Protocol’ clinical trial design may overcome such limitations, such as umbrella trials that evaluate multiple treatments in one disease. The COLOMATE study is an ongoing, seamless adaptive protocol that primarily uses ctDNA (Guardant 360) to screen patients with secondary resistance to targeted therapies, where patients will be enrolled into three separate clinical trials depending on their ctDNA genotype: Patients with ctDNA showing HER-2 alteration will be enrolled into a study involving tucatinib, trastuzumab and TAS-102; patients with BRAF V600E mutation in ctDNA are re-challenged with encorafenib, cetuximab and binimetinib; patients without ctDNA RAS mutations are re-challenged with panitumumab153 (PULSE study; NCT03992456).
As the number of new targeted therapies are being discovered and evaluated in patients with mCRC, the priorities of research in the next decade should include (1) the development of novel strategies to overcome secondary drug resistance; (2) to refine the detection limit, accuracy and optimal timing of ctDNA in monitoring emerging drug resistance during treatment and in directing subsequent therapies; (3) to evaluate targeted therapies in other clinical settings such as in locally advanced rectal cancer and in the postoperative adjuvant treatment following resection of oligometastases in mCRC; (4) to optimize the safe and effective combination of targeted therapy with immunotherapy and other treatment modalities such as radiotherapy and (5) to evaluate relevance of the CMS subtyping of mCRC in determining response to certain targeted therapies.
Acknowledgments
We would like to express our sincere thanks to Ms. Alice Kong for clerical assistance.
Footnotes
ORCID iDs: Ambrose H. N. Wong  https://orcid.org/0000-0002-8039-2643
https://orcid.org/0000-0002-8039-2643
Brigette Ma  https://orcid.org/0000-0003-4802-1102
https://orcid.org/0000-0003-4802-1102
Contributor Information
Ambrose H. N. Wong, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
Brigette Ma, State Key Laboratory of Translational Oncology, Sir YK Pao Centre for Cancer, Department of Clinical Oncology, Hong Kong Cancer Institute, Hong Kong SAR, China.
Rashid N. Lui, Department of Clinical Oncology, and Division of Gastroenterology and Hepatology, Department of Medicine and Therapeutics, Institute of Digestive Disease, The Chinese University of Hong Kong, 9/F, Prince of Wales Hospital, Shatin, New Territories, Hong Kong SAR, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Ambrose HN Wong: Conceptualization; Writing – original draft; Writing – review & editing.
Brigette Ma: Conceptualization; Supervision; Writing – original draft; Writing – review & editing.
Rashid N Lui: Conceptualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This review is supported in part by the Kingboard Precision Oncology Program and the Charlie Lee Precision Immuno-oncology program, The Chinese University of Hong Kong, Hong Kong SAR.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020; 70: 145–164. [DOI] [PubMed] [Google Scholar]
- 3. Bockelman C, Engelmann BE, Kaprio T, et al. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol 2015; 54: 5–16. [DOI] [PubMed] [Google Scholar]
- 4. Cassidy J, Tabernero J, Twelves C, et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol 2004; 22: 2084–2091. [DOI] [PubMed] [Google Scholar]
- 5. Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004; 22: 23–30. [DOI] [PubMed] [Google Scholar]
- 6. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–2342. [DOI] [PubMed] [Google Scholar]
- 7. Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010; 28: 4697–4705. [DOI] [PubMed] [Google Scholar]
- 8. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2017. Bethesda, MD: National Cancer Institute, https://seer.cancer.gov/csr/1975_2017/ (2020, accessed 9 December 2022). [Google Scholar]
- 9. Molinari C, Marisi G, Passardi A, et al. Heterogeneity in colorectal cancer: a challenge for personalized medicine? Int J Mol Sci 2018; 19: 3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salomon DS, Brandt R, Ciardiello F, et al. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 1995; 19: 183–232. [DOI] [PubMed] [Google Scholar]
- 12. Sorich MJ, Wiese MD, Rowland A, et al. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol 2015; 26: 13–21. [DOI] [PubMed] [Google Scholar]
- 13. Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol 2014; 15: 569–579. [DOI] [PubMed] [Google Scholar]
- 14. Pietrantonio F, Cremolini C, Petrelli F, et al. First-line anti-EGFR monoclonal antibodies in panRAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2015; 96: 156–166. [DOI] [PubMed] [Google Scholar]
- 15. Kawai S, Takeshima N, Hayasaka Y, et al. Comparison of irinotecan and oxaliplatin as the first-line therapies for metastatic colorectal cancer: a meta-analysis. BMC Cancer 2021; 21: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cremolini C, Antoniotti C, Lonardi S, et al. Activity and safety of cetuximab plus modified FOLFOXIRI followed by maintenance with cetuximab or bevacizumab for RAS and BRAF wild-type metastatic colorectal cancer: a randomized phase 2 clinical trial. JAMA Oncol 2018; 4: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Modest DP, Martens UM, Riera-Knorrenschild J, et al. FOLFOXIRI plus panitumumab as first-line treatment of RAS wild-type metastatic colorectal cancer: the randomized, open-label, phase II VOLFI study (AIO KRK0109). J Clin Oncol 2019; 37: 3401–3411. [DOI] [PubMed] [Google Scholar]
- 18. Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011; 377: 2103–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rossini D, Antoniotti C, Lonardi S, et al. Upfront modified fluorouracil, leucovorin, oxaliplatin, and irinotecan plus panitumumab versus fluorouracil, leucovorin, and oxaliplatin plus panitumumab for patients with RAS/BRAF wild-type metastatic colorectal cancer: the phase III TRIPLETE study by GONO. J Clin Oncol 2022; 40: 2878–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boige V, Francois E, Ben Abdelghani M, et al. Maintenance treatment with cetuximab versus observation in RAS wild-type metastatic colorectal cancer: results of the randomized phase II PRODIGE 28-time UNICANCER study. J Clin Oncol 2021; 39: 15. [Google Scholar]
- 21. Lopes da Silva L, Nazareth Aguiar P, D’Alpino R, et al. Maintenance therapy following an anti-EGFR-based induction regimen in metastatic colorectal cancer (mCRC): a network meta-analysis of clinical trials. J Clin Oncol 2022; 40: 128. [Google Scholar]
- 22. Aranda E, Garcia-Alfonso P, Benavides M, et al. First-line mFOLFOX plus cetuximab followed by mFOLFOX plus cetuximab or single-agent cetuximab as maintenance therapy in patients with metastatic colorectal cancer: phase II randomised MACRO2 TTD study. Eur J Cancer 2018; 101: 263–272. [DOI] [PubMed] [Google Scholar]
- 23. Pietrantonio F, Morano F, Corallo S, et al. Maintenance therapy with panitumumab alone vs panitumumab plus fluorouracil-leucovorin in patients with RAS wild-type metastatic colorectal cancer: a phase 2 randomized clinical trial. JAMA Oncol 2019; 5: 1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Modest DP, Karthaus M, Fruehauf S, et al. Panitumumab plus fluorouracil and folinic acid versus fluorouracil and folinic acid alone as maintenance therapy in RAS wild-type metastatic colorectal cancer: the randomized PANAMA trial (AIO KRK 0212). J Clin Oncol 2022; 40: 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Di Nicolantonio F, Vitiello PP, Marsoni S, et al. Precision oncology in metastatic colorectal cancer - from biology to medicine. Nat Rev Clin Oncol 2021; 18: 506–525. [DOI] [PubMed] [Google Scholar]
- 26. Parseghian CM, Napolitano S, Loree JM, et al. Mechanisms of innate and acquired resistance to anti-EGFR therapy: a review of current knowledge with a focus on rechallenge therapies. Clin Cancer Res 2019; 25: 6899–6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levin-Sparenberg E, Bylsma LC, Lowe K, et al. A systematic literature review and meta-analysis describing the prevalence of KRAS, NRAS, and BRAF gene mutations in metastatic colorectal cancer. Gastroenterology Res 2020; 13: 184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer 2015; 51: 587–594. [DOI] [PubMed] [Google Scholar]
- 29. National Comprehensive Cancer Network. Colon cancer (version 2.2022), https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (2022, accessed 9 December 2022).
- 30. Sartore-Bianchi A, Amatu A, Porcu L, et al. HER2 positivity predicts unresponsiveness to EGFR-targeted treatment in metastatic colorectal cancer. Oncologist 2019; 24: 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raghav K, Loree JM, Morris JS, et al. Validation of HER2 amplification as a predictive biomarker for anti-epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. JCO Precis Oncol 2019; 3: 1–13. [DOI] [PubMed] [Google Scholar]
- 32. Martin V, Landi L, Molinari F, et al. HER2 gene copy number status may influence clinical efficacy to anti-EGFR monoclonal antibodies in metastatic colorectal cancer patients. Br J Cancer 2013; 108: 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Therkildsen C, Bergmann TK, Henrichsen-Schnack T, et al. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: a systematic review and meta-analysis. Acta Oncol 2014; 53: 852–864. [DOI] [PubMed] [Google Scholar]
- 34. Cremolini C, Morano F, Moretto R, et al. Negative hyper-selection of metastatic colorectal cancer patients for anti-EGFR monoclonal antibodies: the PRESSING case-control study. Ann Oncol 2017; 28: 3009–3014. [DOI] [PubMed] [Google Scholar]
- 35. Morano F, Corallo S, Lonardi S, et al. Negative hyperselection of patients with RAS and BRAF wild-type metastatic colorectal cancer who received panitumumab-based maintenance therapy. J Clin Oncol 2019; 37: 3099–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mauri G, Pizzutilo EG, Amatu A, et al. Retreatment with anti-EGFR monoclonal antibodies in metastatic colorectal cancer: systematic review of different strategies. Cancer Treat Rev 2019; 73: 41–53. [DOI] [PubMed] [Google Scholar]
- 37. Santini D, Vincenzi B, Addeo R, et al. Cetuximab rechallenge in metastatic colorectal cancer patients: how to come away from acquired resistance? Ann Oncol 2012; 23: 2313–2318. [DOI] [PubMed] [Google Scholar]
- 38. Cremolini C, Rossini D, Dell’Aquila E, et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol 2019; 5: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nakamura M. Phase II study of cetuximab rechallenge in patients with RAS wild-type metastatic colorectal cancer: E-rechallenge trial. Ann Oncol 2019; 30: vi116. [Google Scholar]
- 40. Masuishi T, Tsuji A, Kotaka M, et al. Phase 2 study of irinotecan plus cetuximab rechallenge as third-line treatment in KRAS wild-type metastatic colorectal cancer: JACCRO CC-08. Br J Cancer 2020; 123: 1490–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsuji A, Nakamura M, Watanabe T, et al. Phase II study of third-line panitumumab rechallenge in patients with metastatic wild-type KRAS colorectal cancer who obtained clinical benefit from first-line panitumumab-based chemotherapy: JACCRO CC-09. Target Oncol 2021; 16: 753–760. [DOI] [PubMed] [Google Scholar]
- 42. Sartore-Bianchi A, Pietrantonio F, Lonardi S, et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial. Nat Med 2022; 28: 1612–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martinelli E, Martini G, Famiglietti V, et al. Cetuximab rechallenge plus avelumab in pretreated patients with RAS wild-type metastatic colorectal cancer: the phase 2 single-arm clinical CAVE trial. JAMA Oncol 2021; 7: 1529–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013; 381: 303–312. [DOI] [PubMed] [Google Scholar]
- 45. Kagawa Y, Kotani D, Bando H, et al. Plasma RAS dynamics and anti-EGFR rechallenge efficacy in patients with RAS/BRAF wild-type metastatic colorectal cancer: REMARRY and PURSUIT trials. J Clin Oncol 2022; 40: 3518. [Google Scholar]
- 46. Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 2015; 16: 1306–1315. [DOI] [PubMed] [Google Scholar]
- 47. Passardi A, Nanni O, Tassinari D, et al. Effectiveness of bevacizumab added to standard chemotherapy in metastatic colorectal cancer: final results for first-line treatment from the ITACa randomized clinical trial. Ann Oncol 2015; 26: 1201–1207. [DOI] [PubMed] [Google Scholar]
- 48. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27: 1386–1422. [DOI] [PubMed] [Google Scholar]
- 49. Baraniskin A, Buchberger B, Pox C, et al. Efficacy of bevacizumab in first-line treatment of metastatic colorectal cancer: a systematic review and meta-analysis. Eur J Cancer 2019; 106: 37–44. [DOI] [PubMed] [Google Scholar]
- 50. Botrel TEA, Clark LGO, Paladini L, et al. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: a systematic review and meta-analysis. BMC Cancer 2016; 16: 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cremolini C, Antoniotti C, Stein A, et al. Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol 2020; 38: 3314–3324. [DOI] [PubMed] [Google Scholar]
- 52. Hegewisch-Becker S, Graeven U, Lerchenmuller CA, et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol 2015; 16: 1355–1369. [DOI] [PubMed] [Google Scholar]
- 53. Simkens LH, Van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet 2015; 385: 1843–1852. [DOI] [PubMed] [Google Scholar]
- 54. Salvatore L, Bria E, Sperduti I, et al. Bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: a meta-analysis of individual patients’ data from 3 phase III studies. Cancer Treat Rev 2021; 97: 102202. [DOI] [PubMed] [Google Scholar]
- 55. Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol 2015; 16: 499–508. [DOI] [PubMed] [Google Scholar]
- 56. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012; 30: 3499–3506. [DOI] [PubMed] [Google Scholar]
- 57. Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013; 14: 29–37. [DOI] [PubMed] [Google Scholar]
- 58. Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007; 25: 1539–1544. [DOI] [PubMed] [Google Scholar]
- 59. Masi G, Salvatore L, Boni L, et al. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Ann Oncol 2015; 26: 724–730. [DOI] [PubMed] [Google Scholar]
- 60. Xu R-H, Muro K, Morita S, et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): a multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol 2018; 19: 660–671. [DOI] [PubMed] [Google Scholar]
- 61. Dasari NA, Lonardi S, Garcia-Carbonero R, et al. LBA25 FRESCO-2: a global phase III multiregional clinical trial (MRCT) evaluating the efficacy and safety of fruquintinib in patients with refractory metastatic colorectal cancer. Ann Oncol 2022; 33: S1391–S1392. [Google Scholar]
- 62. Holch JW, Ricard I, Stintzing S, et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer 2017; 70: 87–98. [DOI] [PubMed] [Google Scholar]
- 63. Satake H, Tsuji A, Tanaka C, et al. Tumor response of FOLFOXIRI plus cetuximab versus bevacizumab in RAS wild-type metastatic colorectal cancer: the subgroup-analysis of DEEPER trial (JACCRO CC-13). J Clin Oncol 2022; 40: 109.34929133 [Google Scholar]
- 64. Yoshino T, Watanabe J, Shitara K, et al. Panitumumab (PAN) plus mFOLFOX6 versus bevacizumab (BEV) plus mFOLFOX6 as first-line treatment in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC): results from the phase 3 PARADIGM trial. J Clin Oncol 2022; 40: LBA1. [Google Scholar]
- 65. Tapia Rico G, Price T, Tebbutt N, et al. Right or left primary site of colorectal cancer: outcomes from the molecular analysis of the AGITG MAX trial. Clin Colorectal Cancer 2019; 18: 141–148. [DOI] [PubMed] [Google Scholar]
- 66. Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst 2015; 107: dju427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chebib R, Verlingue L, Cozic N, et al. Angiogenesis inhibition in the second-line treatment of metastatic colorectal cancer: a systematic review and pooled analysis. Semin Oncol 2017; 44: 114–128. [DOI] [PubMed] [Google Scholar]
- 68. Derangere V, Fumet JD, Boidot R, et al. Does bevacizumab impact anti-EGFR therapy efficacy in metastatic colorectal cancer? Oncotarget 2016; 7: 9309–9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Modest DP, Stintzing S, Von Weikersthal LF, et al. Impact of subsequent therapies on outcome of the FIRE-3/AIO KRK0306 trial: first-line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild-type tumors in metastatic colorectal cancer. J Clin Oncol 2015; 33: 3718–3726. [DOI] [PubMed] [Google Scholar]
- 70. Peeters M, Forget F, Karthaus M, et al. Exploratory pooled analysis evaluating the effect of sequence of biological therapies on overall survival in patients with RAS wild-type metastatic colorectal carcinoma. ESMO Open 2018; 3: e000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu CC, Hsu CW, Hsieh MC, et al. Optimal sequence and second-line systemic treatment of patients with RAS wild-type metastatic colorectal cancer: a meta-analysis. J Clin Med 2021; 10: 5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chibaudel B, Dourthe L-M, Andre T, et al. STRATEGIC-1: multi-line therapy trial in unresectable wild-type KRAS/NRAS/BRAF metastatic colorectal cancer—A GERCOR-PRODIGE randomized open-label phase III study. J Clin Oncol 2022; 40: 3504. [Google Scholar]
- 73. Innocenti F, Ou FS, Qu X, et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J Clin Oncol 2019; 37: 1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011; 117: 4623–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chu JE, Johnson B, Kugathasan L, et al. Population-based screening for BRAF (V600E) in metastatic colorectal cancer reveals increased prevalence and poor prognosis. Clin Cancer Res 2020; 26: 4599–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010; 138: 2088–2100. [DOI] [PubMed] [Google Scholar]
- 77. De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 2010; 11: 753–762. [DOI] [PubMed] [Google Scholar]
- 78. Clancy C, Burke JP, Kalady MF, et al. BRAF mutation is associated with distinct clinicopathological characteristics in colorectal cancer: a systematic review and meta-analysis. Colorectal Dis 2013; 15: e711–e718. [DOI] [PubMed] [Google Scholar]
- 79. Jones JC, Renfro LA, Al-Shamsi HO, et al. (Non-V600) BRAF mutations define a clinically distinct molecular subtype of metastatic colorectal cancer. J Clin Oncol 2017; 35: 2624–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stintzing S, Heinrich K, Tougeron D, et al. Randomized study to investigate FOLFOXIRI plus either bevacizumab or cetuximab as first-line treatment of BRAF V600E-mutant mCRC: the phase-II FIRE-4.5 study (AIO KRK-0116). J Clin Oncol 2021; 39: 3502. [Google Scholar]
- 81. Punt CJA, Bond MJG, Bolhuis K, et al. FOLFOXIRI + bevacizumab versus FOLFOX/FOLFIRI + bevacizumab in patients with initially unresectable colorectal liver metastases (CRLM) and right-sided and/or RAS/BRAFV600E-mutated primary tumor: phase III CAIRO5 study of the Dutch Colorectal Cancer Group. J Clin Oncol 2022; 40: LBA3506. [Google Scholar]
- 82. Kopetz S, Desai J, Chan E, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol 2015; 33: 4032–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ahronian LG, Sennott EM, Van Allen EM, et al. Clinical acquired resistance to RAF inhibitor combinations in BRAF-mutant colorectal cancer through MAPK pathway alterations. Cancer Discov 2015; 5: 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yaeger R, Cercek A, O’Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res 2015; 21: 1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tabernero J, Grothey A, Van Cutsem E, et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol 2021; 39: 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kopetz S, Grothey A, Yaeger R, et al. BREAKWATER: randomized phase 3 study of encorafenib (enco) + cetuximab (cet) ± chemotherapy for first-line treatment (tx) of BRAF V600E-mutant (BRAFV600) metastatic colorectal cancer (mCRC). J Clin Oncol 2022; 40: TPS211. [Google Scholar]
- 87. Van Cutsem E, Taieb J, Yaeger R, et al. O-10 ANCHOR CRC: results from a single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E–mutant metastatic colorectal cancer. Ann Oncol 2021; 32: S222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Morris VK, Parseghian CM, Escano M, et al. Phase I/II trial of encorafenib, cetuximab, and nivolumab in patients with microsatellite stable, BRAFV600E metastatic colorectal cancer. J Clin Oncol 2022; 40: 12.34752147 [Google Scholar]
- 89. Corcoran RB, Andre T, Atreya CE, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAF(V600E)-mutant colorectal cancer. Cancer Discov 2018; 8: 428–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kopetz S, Guthrie KA, Morris VK, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J Clin Oncol 2021; 39: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kopetz S, Murphy DA, Pu J, et al. Molecular correlates of clinical benefit in previously treated patients (pts) with BRAF V600E-mutant metastatic colorectal cancer (mCRC) from the BEACON study. J Clin Oncol 2021; 39: 3513. [Google Scholar]
- 92. Corcoran R, Giannakis M, Allen J, et al. SO-26 clinical efficacy of combined BRAF, MEK, and PD-1 inhibition in BRAFV600E colorectal cancer patients. Ann Oncol 2020; 31: S226–S227. [Google Scholar]
- 93. Morris VK, Parseghian CM, Escano M, et al. Phase I/II trial of encorafenib, cetuximab, and nivolumab in patients with microsatellite stable (MSS), BRAFV600E metastatic colorectal cancer. J Clin Oncol 2022; 40: 3598. [Google Scholar]
- 94. Tabernero J, Geel RV, Guren TK, et al. Phase 2 results: encorafenib (ENCO) and cetuximab (CETUX) with or without alpelisib (ALP) in patients with advanced BRAF-mutant colorectal cancer (BRAFm CRC). J Clin Oncol 2016; 34: 3544.27573652 [Google Scholar]
- 95. Russo M, Crisafulli G, Sogari A, et al. Adaptive mutability of colorectal cancers in response to targeted therapies. Science 2019; 366: 1473–1480. [DOI] [PubMed] [Google Scholar]
- 96. Mao M, Tian F, Mariadason JM, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res 2013; 19: 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Seo AN, Kwak Y, Kim DW, et al. HER2 status in colorectal cancer: its clinical significance and the relationship between HER2 gene amplification and expression. PLoS One 2014; 9: e98528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nam SK, Yun S, Koh J, et al. BRAF, PIK3CA, and HER2 oncogenic alterations according to KRAS mutation status in advanced colorectal cancers with distant metastasis. PLoS One 2016; 11: e0151865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Richman SD, Southward K, Chambers P, et al. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol 2016; 238: 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Raghav KPS, Loree JM, Morris J, et al. Detection and description of ERBB2 amplification using circulating cell free tumor DNA (ctDNA) genomic analysis in metastatic colorectal cancer (mCRC). J Clin Oncol 2018; 36: 661. [Google Scholar]
- 101. Ingold Heppner B, Behrens HM, Balschun K, et al. HER2/neu testing in primary colorectal carcinoma. Br J Cancer 2014; 111: 1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ross JS, Fakih M, Ali SM, et al. Targeting HER2 in colorectal cancer: the landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer 2018; 124: 1358–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Guarini C, Grassi T, Pezzicoli G, et al. Beyond RAS and BRAF: HER2, a new actionable oncotarget in advanced colorectal cancer. Int J Mol Sci 2021; 22: 6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Leto SM, Sassi F, Catalano I, et al. Sustained inhibition of HER3 and EGFR is necessary to induce regression of HER2-amplified gastrointestinal carcinomas. Clin Cancer Res 2015; 21: 5519–5531. [DOI] [PubMed] [Google Scholar]
- 105. Tosi F, Sartore-Bianchi A, Lonardi S, et al. Long-term clinical outcome of trastuzumab and lapatinib for HER2-positive metastatic colorectal cancer. Clin Colorectal Cancer 2020; 19: 256–262. [DOI] [PubMed] [Google Scholar]
- 106. Kulukian A, Lee P, Taylor J, et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol Cancer Ther 2020; 19: 976–987. [DOI] [PubMed] [Google Scholar]
- 107. Strickler J, Cercek A, Siena S, et al. LBA-2 Primary analysis of MOUNTAINEER: a phase 2 study of tucatinib and trastuzumab for HER2-positive mCRC. Ann Oncol 2022; 33: S375–S376. [Google Scholar]
- 108. Sartore-Bianchi A, Trusolino L, Martino C, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol 2016; 17: 738–746. [DOI] [PubMed] [Google Scholar]
- 109. Li W, Chang J, Xu M, et al. Anti-HER2 therapy with pyrotinib and trastuzumab in refractory HER2-positive metastatic colorectal cancer: a preliminary report from HER2-FUSCC-G study. J Clin Oncol 2022; 40: 97. [DOI] [PubMed] [Google Scholar]
- 110. Yuan Y, Fu X, Ying J, et al. Dual-targeted therapy with pyrotinib and trastuzumab for HER2-positive advanced colorectal cancer: preliminary results from a multicenter phase 2 trial. J Clin Oncol 2021; 39: e15554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Meric-Bernstam F, Hainsworth J, Bose R, et al. MyPathway HER2 basket study: pertuzumab (P) + trastuzumab (H) treatment of a large, tissue-agnostic cohort of patients with HER2-positive advanced solid tumors. J Clin Oncol 2021; 39: 3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nakamura Y, Okamoto W, Kato T, et al. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: a phase 2 trial. Nat Med 2021; 27: 1899–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gupta R, Garrett-Mayer E, Halabi S, et al. Pertuzumab plus trastuzumab (P+T) in patients (Pts) with colorectal cancer (CRC) with ERBB2 amplification or overexpression: results from the TAPUR Study. J Clin Oncol 2020; 38: 132. [Google Scholar]
- 114. Jacobs SA, George TJ, Kolevska T, et al. NSABP FC-11: a phase II study of neratinib (N) plus trastuzumab (T) or N plus cetuximab (C) in patients (pts) with “quadruple wild-type” metastatic colorectal cancer (mCRC) based on HER2 status. J Clin Oncol 2022; 40: 3564.35679525 [Google Scholar]
- 115. Sartore-Bianchi A, Lonardi S, Martino C, et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phase II HERACLES-B trial. ESMO Open 2020; 5: e000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yoshino T, Di Bartolomeo M, Raghav KPS, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing metastatic colorectal cancer (mCRC): final results from a phase 2, multicenter, open-label study (DESTINY-CRC01). J Clin Oncol 2022; 40: 119. [Google Scholar]
- 117. Xu B, Yan M, Ma F, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 2021; 22: 351–360. [DOI] [PubMed] [Google Scholar]
- 118. National Comprehensive Cancer Network. Breast cancer (version 4.2022), https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (2022, accessed 9 December 2022).
- 119. Richard S, Selle F, Lotz JP, et al. Pertuzumab and trastuzumab: the rationale way to synergy. An Acad Bras Cienc 2016; 88: 565–577. [DOI] [PubMed] [Google Scholar]
- 120. Meric-Bernstam F, Hurwitz H, Raghav KPS, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 2019; 20: 518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Raghav KPS, Yoshino T, Guimbaud R, et al. Trastuzumab deruxtecan in patients with HER2-overexpressing locally advanced, unresectable, or metastatic colorectal cancer (mCRC): a randomized, multicenter, phase 2 study (DESTINY-CRC02). J Clin Oncol 2021; 39: TPS3620. [Google Scholar]
- 122. Siravegna G, Lazzari L, Crisafulli G, et al. Radiologic and genomic evolution of individual metastases during HER2 blockade in colorectal cancer. Cancer Cell 2018; 34: 148–162. [DOI] [PubMed] [Google Scholar]
- 123. Cocco E, Benhamida J, Middha S, et al. Colorectal carcinomas containing hypermethylated MLH1 promoter and wild-type BRAF/KRAS are enriched for targetable kinase fusions. Cancer Res 2019; 79: 1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Pietrantonio F, Di Nicolantonio F, Schrock AB, et al. ALK, ROS1, and NTRK rearrangements in metastatic colorectal cancer. J Natl Cancer Inst 2017; 109: djx089. [DOI] [PubMed] [Google Scholar]
- 125. Pietrantonio F, Di Nicolantonio F, Schrock AB, et al. RET fusions in a small subset of advanced colorectal cancers at risk of being neglected. Ann Oncol 2018; 29: 1394–1401. [DOI] [PubMed] [Google Scholar]
- 126. Hong DS, DuBois SG, Kummar S, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol 2020; 21: 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020; 21: 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann Oncol 2019; 30: viii23–viii30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 2018; 15: 731–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Drilon A, Nagasubramanian R, Blake JF, et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov 2017; 7: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hyman D, Kummar S, Farago A, et al. Abstract CT127: phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi) Cancer Res 2019; 79: CT127. [Google Scholar]
- 132. Drilon A, Ou SI, Cho BC, et al. Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent- front mutations. Cancer Discov 2018; 8: 1227–1236. [DOI] [PubMed] [Google Scholar]
- 133. Cho BC, Doebele RC, Lin J, et al. MA11.07 phase 1/2 TRIDENT-1 study of repotrectinib in patients with ROS1+ or NTRK+ advanced solid tumors. J Thorac Oncol 2021; 16: S174–S175. [Google Scholar]
- 134. Cocco E, Schram AM, Kulick A, et al. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat Med 2019; 25: 1422–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. McCormick F. KRAS as a therapeutic target. Clin Cancer Res 2015; 21: 1797–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Janes MR, Zhang J, Li LS, et al. Targeting KRAS mutant cancers with a covalent G12C-specific inhibitor. Cell 2018; 172: 578–589. [DOI] [PubMed] [Google Scholar]
- 137. Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med 2021; 384: 2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. AACR Project GENIE Consortium. AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov 2017; 7: 818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Fakih MG, Kopetz S, Kuboki Y, et al. Sotorasib for previously treated colorectal cancers with KRAS(G12C) mutation (CodeBreaK100): a prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol 2022; 23: 115–124. [DOI] [PubMed] [Google Scholar]
- 140. Amodio V, Yaeger R, Arcella P, et al. EGFR blockade reverts resistance to KRAS(G12C) inhibition in colorectal cancer. Cancer Discov 2020; 10: 1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Klempner SJ, Weiss J, Pelster M, et al. LBA24 KRYSTAL-1: updated efficacy and safety of adagrasib (MRTX849) with or without cetuximab in patients with advanced colorectal cancer (CRC) harboring a KRASG12C mutation. Ann Oncol 2022; 33: S1391. [Google Scholar]
- 142. Awad MM, Liu S, Rybkin II, et al. Acquired resistance to KRAS(G12C) inhibition in cancer. N Engl J Med 2021; 384: 2382–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ryan MB, Fece de la Cruz F, Phat S, et al. Vertical pathway inhibition overcomes adaptive feedback resistance to KRAS(G12C) inhibition. Clin Cancer Res 2020; 26: 1633–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Cremolini C, Rossini D, Antoniotti C, et al. LBA20 FOLFOXIRI plus bevacizumab (bev) plus atezolizumab (atezo) versus FOLFOXIRI plus bev as first-line treatment of unresectable metastatic colorectal cancer (mCRC) patients: results of the phase II randomized AtezoTRIBE study by GONO. Ann Oncol 2021; 32: S1294–S1295. [Google Scholar]
- 145. Lenz H-J, Parikh AR, Spigel DR, et al. Nivolumab (NIVO) + 5-fluorouracil/leucovorin/oxaliplatin (mFOLFOX6)/bevacizumab (BEV) versus mFOLFOX6/BEV for first-line (1L) treatment of metastatic colorectal cancer (mCRC): phase 2 results from CheckMate 9X8. J Clin Oncol 2022; 40: 8–8.34694897 [Google Scholar]
- 146. Damato A, Bergamo F, Antonuzzo L, et al. Phase II study of nivolumab in combination with FOLFOXIRI/bevacizumab as first-line treatment in patients with advanced colorectal cancer RAS/BRAF mutated (mut): NIVACOR trial (GOIRC-03-2018). J Clin Oncol 2022; 40: 3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kim RD, Tehfe M, Kavan P, et al. Pembrolizumab (pembro) plus mFOLFOX7 or FOLFIRI for metastatic colorectal cancer (CRC) in KEYNOTE-651: long-term follow-up of cohorts B and D. J Clin Oncol 2022; 40: 3521. [Google Scholar]
- 148. Fang X, Zhong C, Zhu N, et al. A phase 2 trial of sintilimab (IBI 308) in combination with CAPEOX and bevacizumab (BBCAPX) as first-line treatment in patients with RAS-mutant, microsatellite stable, unresectable metastatic colorectal cancer. J Clin Oncol 2022; 40: 3563. [Google Scholar]
- 149. Pascual J, Attard G, Bidard FC, et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO Precision Medicine Working Group. Ann Oncol 2022; 33: 750–768. [DOI] [PubMed] [Google Scholar]
- 150. Dasari A, Morris VK, Allegra CJ, et al. ctDNA applications and integration in colorectal cancer: an NCI Colon and Rectal–Anal Task Forces whitepaper. Nat Rev Clin Oncol 2020; 17: 757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Tie J, Cohen JD, Lahouel K, et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med 2022; 386: 2261–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Nakamura Y, Taniguchi H, Ikeda M, et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med 2020; 26: 1859–1864. [DOI] [PubMed] [Google Scholar]
- 153. Yoshino T. COLOMATE challenge to overcome resistance in metastatic colorectal cancer. Oncology (Williston Park) 2021; 35: 656. [DOI] [PubMed] [Google Scholar]