Abstract
Background:
We studied a smart insulin pen cap that can be plugged to several brand of insulin pens, to track insulin administration via smart-phone Bluetooth technology, with alarm/reminder system aiming.
Methods:
This pilot randomized, cross-over design study assessed the use of a smart insulin pen cap in improving adherence, glycemic control and patient satisfaction in insulin-treated patients with poorly controlled type 2 diabetes. Eighty patients on basal insulin ± oral agents with hemoglobin A1C (HbA1c) between 7.0% and 12.0% were randomized to a 12-week active phase receiving alarms/reminders and a 12-week control/masked phase without feedback. We assessed differences between groups on treatment adherence, insulin omission, and mistiming of insulin injections, HbA1c, treatment satisfaction (using Diabetes Treatment Satisfaction Questionnaire Status).
Results:
Compared to the control/masked phase, the active phase resulted in lower mean daily blood glucose (147.0 ± 34 vs 157.6 ± 42 mg/dL, P < .01); and greater reduction in HbA1c from baseline (−0.98% vs −0.72%, P = .006); however, no significant differences in treatment adherence, insulin omission or insulin mistiming were observed. High patient satisfaction scores were reported in both active and control phases, with DTSQc of 15.5 ± 3.7 and 14.9 ± 3.6, respectively. Statistical models showed no residual effect after cross-over between active and control phases.
Conclusions:
The results of this pilot study indicates that this smart insulin pen cap was effective in improving glycemic control with overall good satisfaction in insulin treated patients with type 2 diabetes. Future studies are needed to confirm its potential for improving care in insulin treated patients with diabetes.
Keywords: Smart Insulin Pen Cap, insulin administration, type 2 diabetes, glycemic control, treatment satisfaction
Introduction
Based on recent reports from the Center for Disease Control and Prevention, over 31 million U.S. adults had diabetes in 2018, representing 13% of the U.S. adult population. 1 Most patients with type 2 diabetes are initially managed with oral antidiabetic agents, but as β-cell function declines and the disease progresses, insulin therapy is frequently needed to achieve and maintain glycemic control. More than 7 million Americans with diabetes use one or more insulin formulation. 2 Current clinical guidelines recommend adding basal insulin when other oral or injectable antidiabetic agents fail to achieve recommended glycemic targets. 3
The results of randomized controlled trials and meta-analyses have reported that less than half of individuals treated with basal insulin alone or in combination with oral antidiabetic agents achieved an HbA1C target of <7.0%.4-6 Among factors that could explain the low rate of optimal response to insulin therapy, poor adherence to insulin administration has been reported in up to half of patients with diabetes,7-9 with one-third of patients reporting an average of 3 episodes of insulin omission within the previous month.8,10 Barriers to patients’ poor adherence with insulin therapy include socio-economic factors, difficulty remembering to take medications, medication regimen complexity, multiple daily dosing of medications, weight gain, cost, and fear of side effects, 11 as well as injections being time consuming, and forgetfulness. 12 Thus, there is a need for novel strategies and tools to increase adherence to prescribed insulin regimens.
Recent diabetes technology tools have shown promise in improving glycemic control and adherence to insulin administration, including the use of electronic reminders, mobile communication technology such as phone short message services, 13 smartphones and wrist-worn smartwatch, 14 and a variety of insulin pens with memory functions and electronic display.15-17 Smart insulin pens may provide an additional resource for patients and health care providers to overcome problems such as poor insulin adherence, incorrect insulin initiation and titration, and medication errors. 18 However, these devices are not interchangeable among different insulin pen devices, and do not have alarm systems to alert patients about insulin delays and missing doses.
Insulclock®19 is a smart insulin pen cap plugged to several brands of insulin pen devices to help track date, time and dosage of the last injection, type of insulin used, and temperature range. The smart insulin pen cap has an alarm system with visual and sound alerts designed to assist preventing insulin omissions and mistiming. A recent pilot study using this device reported improvement in glycemic control, reduction in glycemic variability, and improved adherence and satisfaction in patients with type 1 diabetes. 20 We conducted a pilot randomized cross-over controlled trial to assess the use of the smart insulin pen cap on treatment adherence, glycemic control, and patient’s satisfaction among insulin treated patients with poorly controlled type 2 diabetes.
Methods
Study Design and Participants
We conducted a pilot, prospective, randomized (1:1), 26-weeks, cross-over clinical trial in insulin-treated patients with inadequate glycemic control. We included adult patients with type 2 diabetes, between 18 and 80 years of age, with HbA1c between 7.0% and ≤12%, while receiving treatment with basal insulin, including NPH, glargine, or detemir, at a total daily dose of ≤0.5 U/Kg/day, as monotherapy or in combination with oral antidiabetic agents. All patients in the study were self-injecting insulin for more than 3 months prior to participation. We excluded patients treated with glargine U300 insulin, degludec, or treated with basal-bolus (basal + prandial) insulin regimen during the previous 3 months, as well as patients with a history of diabetic ketoacidosis during the previous 6 months, or history of hypoglycemia unawareness. We also excluded patients with estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2, liver disease (defined as alanine aminotransferase levels >3 times above upper limit of normal), pregnancy, dementia, and patients receiving corticosteroid therapy.
Randomization and Procedures
All participants were screened from the Grady Memorial Hospital Diabetes Center and Emory University Midtown endocrinology service, Atlanta, Georgia. The Emory Institutional Review Board approved the study. This trial was registered with ClinicalTrials.gov, NCT 03224234. After explaining the study’s procedures in detail by study personnel, patients provided written informed consent before starting the clinical trial.
Prior to randomization, for patients without smartphones (n: 10), the study provided a new unlocked smartphone (Kiocera DuraForce) with WIFI capability. After providing informed consent, the smart insulin pen cap app (see Supplemental Material and https://insulclock.com/professional/) was installed by research personnel on each participant’s smartphone, and both smart insulin pen cap and cellphones were paired. Participants were instructed on how to use the cell phones and smart insulin pen cap. Insulin pens (Lantus®) were provided at no cost to all participants during the study period, and were titrated per protocol. In addition, participants were trained on the use of glucose meter, capillary glucose testing, and keeping a glucose diary. Participants were instructed on the use of insulin pens [glargine U100 insulin pens (Lantus SoloStar®)] and data collection diaries. Diabetes education, insulin and smart insulin pen cap were provided to participants at no charge.
During a 2-week run-in period and before randomization, participants were switched to glargine insulin and were asked to test capillary blood glucose before meals and bedtime. Those who tested glucose more than 3 times daily and were able to charge the smart insulin pen cap every 1-2 days during the run-in period, continued in the study, and were trained on the use and maintenance of the device. Subjects were also trained on how to use the smart insulin pen cap application, and device connectivity. Those subjects failing to use the device properly during the run-in period were not included in the clinical trial.
After the run-in period, participants were randomly assigned in a 1:1 ratio to the active intervention with feedback and alarm notifications or to a control group using a masked device without alarm notifications. We conducted a block randomization stratified by HbA1c ≤ 8.0% or >8.0%, with each phase lasting 12 weeks. Participants in the active/intervention phase received daily information on their smartphones on insulin administration (time and dosing), as well as reminders in the event of missing doses. After completing the first phase, patients crossed over to the second phase (Figure 1). Participants and investigators were not masked to treatment allocation. Basal insulin was titrated to a target fasting and pre-meal glucose of 70-130 mg/dL.
Figure 1.
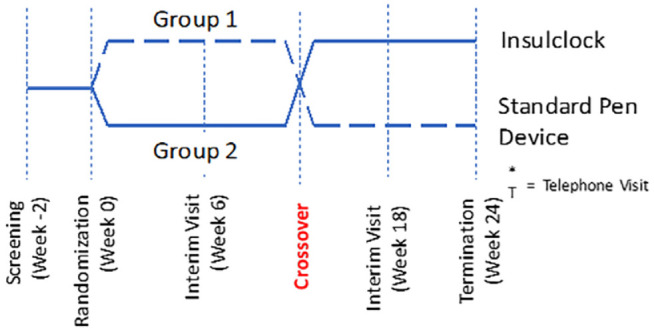
Study design (cross-over, randomized, controlled trial).
Study Outcomes
We assessed differences between groups in treatment adherence including omission and mistiming of insulin injections, glycemic control, as measured by fasting glucose, treatment satisfaction by Diabetes Treatment Satisfaction Questionnaire (DTSQs C) survey. For insulin injections recorded as given/administered, we defined mistiming if not given within 2 hours on the expected daily time of administration. Similarly, dose omissions were defined as doses not documented in the diary or not recorded by the device. Cumulative treatment adherence was defined as proportion of expected injections completed, as captured by the number of weekly basal insulin doses administered, and classified as highly adherent (>85% of completed doses, equivalent to missing 1 dose per week), moderately adherent (>60-85% of completed doses, equivalent to missing 1-3 doses per week, poorly adherent (15-60% of completed doses, missing 4-5 doses per week, not adherent (<15% of completed doses, missing 6 doses per week). For assessment of treatment satisfaction, we used the Diabetes Treatment Satisfaction Questionnaire Status (DTSQc)21,22 administered at baseline (week 0) and at weeks 12-14 and 26 to assess relative changes in the DTSQ between interventions. Results are expressed on a scale of ranging from +3, +2, +1. 0, −1, −2, −3 according to the degree of satisfaction by treatment group (see Supplemental materials).
Statistical Analysis
This study followed the standard 2 by 2 cross-over study design. We summarized continuous outcomes by mean and standard deviation and categorical outcomes by count and percentage. For continuous outcomes, we assessed crossover residual effect and the effect of intervention based on the Grizzle model. 23 For binary outcomes, we assessed the effect of intervention based on Prescott’s test. 24 To evaluate the change of continuous outcomes from baseline, we used paired t-tests. The level of statistical significance was set at P < .05. All statistical analyses were performed using the SAS software for Windows, version 9.2 (SAS Institute, Cary, SC, USA).
Results
Between September 2017 and March 2019, a total of 88 participants (mean age 55.7 ± 11 years, mean body mass index (BMI) 32.4 ± 7.6 kg/m2, and mean HbA1c 9.2% ± 1.5 consented to participate in the study. After the run-in period and excluding screen failures (n 8), 40 participants were randomized to the active phase with real-time feedback and 40 patients to the control group without feedback. After the initial 12-week active phase on the intervention, patients were crossed over and followed for an additional 12 weeks. Patients had similar clinical characteristics at baseline, without significant differences in age, ethnicity, BMI, duration of diabetes, annual income, mean baseline glucose, and HbA1c concentration (Table 1).
Table 1.
Baseline Characteristics of Participants (by Study Phases).
| Active followed by control masked | Control masked followed by active | P Value | |
|---|---|---|---|
| Age, years | 54.5 ± 9.7 | 57 ± 12.2 | .32 |
| Sex | .025 | ||
| Female, n (%) | 17 (43) | 27 (68) | |
| Male, n (%) | 23 (58) | 13 (33) | |
| Body weight, kg | 99.4 ± 25.4 | 87.7 ± 23.8 | .06 |
| Body mass index, kg/m2 | 33.3 ± 7.04 | 31.5 ± 8.09 | .16 |
| Race, Blacks, n (%) | 37 (93%) | 36 (90%) | .24 |
| Duration of diabetes, median, years (Q1, Q3) | 10 (4.5, 18.5) | 10 (5-15) | .67 |
| HbA1c, % | 9.12 ± 1.71 | 9.34 ± 1.35 | .35 |
| Annual income under $20,000/year, n (%) | 26 (65) | 27 (68) | .81 |
| Anti-diabetic medications, n (%) | |||
| Metformin | 28 (70) | 30 (75) | .81 |
| Secretagogues | 4 (10) | 7 (18) | .52 |
| Dipeptidyl-peptidase IV inhibitors (DPPIVi) | 10 (25) | 6 (15) | .40 |
| Sodium-glucose loop transporters 2 inhibitor (SGLT2i) | 6 (15) | 5 (13) | 1.0 |
| Thiazolidiones (TZD) | (2.5) | 1 (2.5) | 1.0 |
| Basal insulin (Glargine, Detemir, NPH) | 40 (100) | 40 (100) | 1.0 |
All data are presented in mean ± SD, unless otherwise stated.
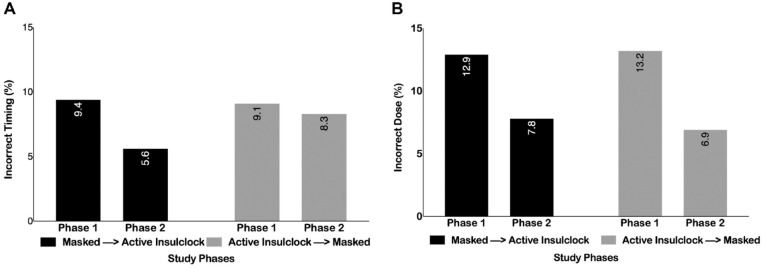
Overall, 53% of patients were highly adherent reporting >85% of completed doses, 19% were moderately adherent (>60-85% of completed doses), and 24% were poorly adherent (15-60% of completed doses), and 3.8% were not adherent (<15% of completed doses). Participants on average missed 23.6% of insulin doses, administered an average of 10.6% of incorrect doses, and an average of 7.9% of doses at the incorrect time. There was a reduction in the proportion of patients with missed dosing (22% vs 24%, P = .04), for patients starting the active phase and crossing to the control phase. Similarly, there were no differences in the proportion of patients with mistiming of insulin injections between groups (9.36% vs 9.10%, P = .70 and 5.6% vs 8.3%, P = .57, respectively) (Figure 2A and B).
Figure 2.
(A) Proportion of patients with incorrect time of insulin dose in each group and by study phases. (B) Proportion of patients with incorrect doses in each group and by study phases.
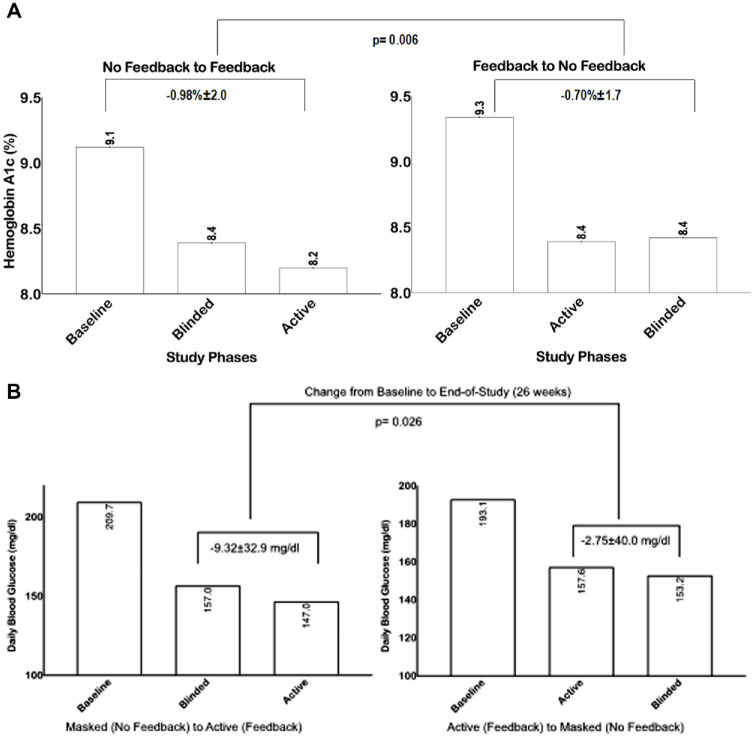
Overall, both groups had significant improvement on HbA1c during the study period (Figure 3A). Those starting with the active phase had a mean HbA1c improvement from 9.3% ± 1.3% to 8.4% ± 1.9% (P: .003) after 12 weeks, and no further improvement after switching to the masked phase (for 12 weeks), from 8.4% ± 1.9% to 8.4% ± 1.6% (P = .9). Participants starting with the masked phase had a mean HbA1c improvement from 9.1% ± 1.7% to 8.4% ± 2.0% after 12 weeks (P: .0035), and further improvement after switching to the active phase (for 12 weeks), from 8.4% ± 2.0% to 8.2% ± 1.5% (P = .9) (end-of-study, week 26). Overall, there was a small but significant difference in HbA1c reduction from baseline in the active vs. control phase (−0.98% ± 2.0% vs −0.70% ± 1.7%, P = .006).
Figure 3.
(A) Changes in Hemoglobin A1c from baseline to end-of-study in each group and by study phases. (B) Changes in daily blood glucose from baseline to end-of-study in each group and by study phases.
Changes in mean daily blood glucose concentration during the study period is shown in Figure 3B. Those starting with active phase had an improvement on mean daily glucose, from 193.13 ± 82 mg/dl to 157.56 ± 42 mg/dl after 12 weeks (P: .59), without significant deterioration after switching to the masked phase (for 12 weeks), from 157.56 ± 42 mg/dl to 153.16 ± 37 mg/dl after 12 weeks (P: .4). Patients starting with the masked phase had an improvement on mean daily glucose, from 209.70 ± 76 mg/dl to 156.95 ± 48 mg/dl after 12 weeks (P: .59), and further improvement after switching to the active phase (for 12 weeks), from 156.95 ± 47 mg/dl to 147.03 ± 35 mg/dl (P: .5 (end-of-study, week 26). Overall, there was a significant difference in mean daily blood glucose reduction from baseline in the active vs. control phase (−9.32 ± 32 mg/dl vs −2.75 mg/dl, P = .008). Similar findings were observed on mean fasting blood glucose with a significant difference in blood glucose reduction from baseline in the active vs. control phase (P = .02). There were no differences on the rates of severe hyperglycemia >300 mg/dl or hypoglycemia <70 mg/dl between groups.
Overall, patients were equally satisfied with the device during active and control phase, with a total DTSQc score of 15.5 ± 3.7 and 14.9 ± 3.6/18 points, respectively. These results indicate that most patients were satisfied with the intervention. During follow-up, no patients were dissatisfied with the device.
Discussion
The results of this pilot randomized controlled cross-over trial of insulin-treated patients with uncontrolled type 2 diabetes indicate that the smart insulin pen cap resulted in small but significant improvement in glycemic control with overall good patient satisfaction. Our results are similar to a recent pilot study using this smart insulin pen cap in patients with type 1 diabetes. 20 These reports builds on the information available on the efficacy and safety of electronic reminders and technology devices aiming to improve and facilitate care in insulin treated patients with diabetes.
Despite significant expansion on our insulin portfolio during the past decade, a large proportion of patients with type 2 diabetes receiving basal insulin do not achieve target HbA1c <7.0%.4,6,25 In a contemporary, study of electronic medical records of over 1 million patients with type 2 diabetes in the US, 72% failed to achieve HbA1c targets of <7% at 6 months. 5 Similarly, in a review of 11 randomized clinical trials using glargine or human basal insulin (NPH), 49% failed to achieve a target HbA1c <7% at 6 months. 5 In agreement with these studies, we observed a tendency toward HbA1c reduction from baseline during the study period. The modest difference between the active and control phases in the present study may be the result of short duration on intervention, as well as the closed follow-up and free insulin and supplies provided to both groups.
Prior studies have reported low adherence to insulin regimens due to complexity of treatment, limited access and costs.7-10 Use of mobile health technology is an emergent area, with promising results to improve treatment adherence, satisfaction and glycemic control in patients with diabetes, including: use of electronic reminders, mobile short message services, 13 smartphones and wrist-worn smartwatch with alarms and reminders, 14 and the development of smart insulin pens devices with memory functions and message display.15-17 Our study indicates that incorporating a tracking device on insulin administration, using smartphone technology, was well received by participants, with a tendency towards improved glycemic control measures.
In a recent survey, the most common factor to reduce insulin discontinuation was increased staff support and feedback from healthcare team. 26 In our study, we found that facilitating insulin administration using mobile health technology, glycemic control was improved in patients with poorly controlled type 2 diabetes. Our results are in concordance with recent reports of a smart connected insulin pen, NovoPen 6®, showing improvement of additional glycemic control markers such as time-in-target range (TIR) among patients with type 1 diabetes. 27 While these results are a step forward, most smart insulin pens are not interchangeable among brands, compared to this smart insulin pen cap that can be added-on to most commercially available insulins in Europe and the United States. In fact, a recent study demonstrated that this smart insulin pen cap used on Humalog KwikPen among patients with type 1 diabetes in Spain resulted in improved mean glucose, time in range and time-above range, along with improvements on treatment satisfaction. 20
This pilot study using this smart insulin pen represents a potential new resource to improve glycemic control in patients with uncontrolled diabetes, but several limitations are noted. We included patients with type 2 diabetes with long-term insulin use prior to participation, which could explain the lack of difference in adherence with insulin regimen. The study team also provided smartphones, insulin and supplies to all patients at no cost, which may have resulted in higher rates of treatment adherence and satisfaction in both groups. Similarly, the frequent telephones and frequent in-person visits allowed close supervision, may have influenced glycemic control and overall high treatment satisfaction in both groups. The difference in HbA1c was statistically significant, but of small clinical relevance. Future real-world studies using the device in larger number of patients with type 1 and type 2 diabetes are needed to confirm our preliminary results.
In conclusion, this pilot cross-over randomized controlled trial using a mobile health technology showed that this smart insulin pen cap was safe, with a tendency towards improvement in glycemic control parameters, and with overall high satisfaction rates. Future larger studies are needed to confirm these findings in a diverse population of patients with diabetes.
Supplemental Material
Supplemental material, sj-pdf-1-dst-10.1177_19322968211033837 for Efficacy of a Smart Insulin Pen Cap for the Management of Patients with Uncontrolled Type 2 Diabetes: A Randomized Cross-Over Trial by Rodolfo J. Galindo, Clementina Ramos, Saumeth Cardona, Priyathama Vellanki, Georgia M. Davis, Omolade Oladejo, Bonnie Albury, Neil Dhruv, Limin Peng and Guillermo E. Umpierrez in Journal of Diabetes Science and Technology
Acknowledgments
Part of this study was previously presented at the 80th Scientific Sessions of the American Diabetes Association, Virtual program, 2020.
Footnotes
Abbreviations: BMI, body mass index; DTSQc, diabetes treatment satisfaction questionnaire status; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; NPH, neutral protamine hagedorn insulin.
Author Contribution: RJG conducted the study, analyzed the data and results, and drafted the initial manuscript. CR, SC, OO, BA, BD conducted the study. PV, FJP, GMD conducted the study, reviewed the data and analysis, critically reviewed and contributed to the manuscript. LP performed the statistical analysis. GEU wrote the initial research protocol and designed the study, conducted the study, analyzed the data, reviewed and drafted the manuscript. GEU is the guarantor of this work and, as such, had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RJG is supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institute of Health under Award Numbers P30DK111024 and 1K23DK123384-01. RJG received unrestricted research support (to Emory University) for investigator-initiated studies from Novo Nordisk and Dexcom, and consulting fees from Abbott Diabetes Care, Sanofi, Novo Nordisk, Eli Lilly and Valeritas. GEU is partly supported by research grants from the NIH/NATS UL1 TR002378 from the Clinical and Translational Science Award program, and 1P30DK111024-01 from NIH and National Center for Research Resources. GEU has received unrestricted research support for research studies (to Emory University) from Merck, Novo Nordisk, and Dexcom Inc. FJP, GMD, and PV are supported by NIH grants: 1K23GM128221-01A1, 1K23DK122199 - 01A1, and 3K12HD085850-03S1. FJP received consulting fees from Merck, Boehringer Ingelheim, Lilly, and AstraZeneca, and Sanofi, and research support from Merck and Dexcom Inc. PV has received consulting fees from Merck, and Boehringer Ingelheim. No other potential conflict of interest relevant to this article was reported.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was an investigator-initiated study funded by Insulcloud, Inc. The terms of this agreement were reviewed and approved by Emory University in accordance with its conflict-of-interest policies. The funding source was not involved in the study design, data interpretation, statistical analysis, manuscript preparation, or the decision to submit the manuscript for publication.
ORCID iDs: Rodolfo J. Galindo  https://orcid.org/0000-0002-9295-3225
https://orcid.org/0000-0002-9295-3225
Saumeth Cardona  https://orcid.org/0000-0003-1295-1754
https://orcid.org/0000-0003-1295-1754
Omolade Oladejo  https://orcid.org/0000-0002-6150-6705
https://orcid.org/0000-0002-6150-6705
Guillermo E. Umpierrez  https://orcid.org/0000-0002-3252-5026
https://orcid.org/0000-0002-3252-5026
Supplemental Material: Supplemental material for this article is available online.
References
- 1. CDC. National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. [Google Scholar]
- 2. Cefalu WT, Dawes DE, Gavlak G, et al. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care. 2018;41(6):1299-1311. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S98-S110. [DOI] [PubMed] [Google Scholar]
- 4. Esposito K, Chiodini P, Bellastella G, Maiorino MI, Giugliano D. Proportion of patients at HbA1c target <7% with eight classes of antidiabetic drugs in type 2 diabetes: systematic review of 218 randomized controlled trials with 78 945 patients. Diabetes Obes Metab. 2012;14(3):228-233. [DOI] [PubMed] [Google Scholar]
- 5. Blonde L, Brunton SA, Chava P, et al. Achievement of target A1C <7.0% (<53 mmol/mol) by U.S. type 2 diabetes patients treated with basal insulin in both randomized controlled trials and clinical practice. Diabetes Spectr. 2019;32(2):93-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dalal MR, Grabner M, Bonine N, Stephenson JJ, DiGenio A, Bieszk N. Are patients on basal insulin attaining glycemic targets? Characteristics and goal achievement of patients with type 2 diabetes mellitus treated with basal insulin and physician-perceived barriers to achieving glycemic targets. Diabetes Res Clin Pract. 2016;121:17-26. [DOI] [PubMed] [Google Scholar]
- 7. Stolpe S, Kroes MA, Webb N, Wisniewski T. A systematic review of insulin adherence measures in patients with diabetes. J Manag Care Spec Pharm. 2016;22(11):1224-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brod M, Rana A, Barnett AH. Adherence patterns in patients with type 2 diabetes on basal insulin analogues: missed, mistimed and reduced doses. Curr Med Res Opin. 2012;28(12):1933-1946. [DOI] [PubMed] [Google Scholar]
- 9. Tiv M, Viel JF, Mauny F, et al. Medication adherence in type 2 diabetes: the ENTRED study 2007, a French population-based study. PLoS One. 2012;7(3):e32412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peyrot M, Rubin RR, Polonsky WH, Best JH. Patient reported outcomes in adults with type 2 diabetes on basal insulin randomized to addition of mealtime pramlintide or rapid-acting insulin analogs. Curr Med Res Opin. 2010;26(5):1047-1054. [DOI] [PubMed] [Google Scholar]
- 11. Sarbacker GB, Urteaga EM. Adherence to insulin therapy. Diabetes Spectr. 2016;29(3):166-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farsaei S, Radfar M, Heydari Z, Abbasi F, Qorbani M. Insulin adherence in patients with diabetes: risk factors for injection omission. Prim Care Diabetes. 2014;8(4):338-345. [DOI] [PubMed] [Google Scholar]
- 13. Celik S, Cosansu G, Erdogan S, et al. Using mobile phone text messages to improve insulin injection technique and glycaemic control in patients with diabetes mellitus: a multi-centre study in Turkey. J Clin Nurs. 2015;24(11-12):1525-1533. [DOI] [PubMed] [Google Scholar]
- 14. Arsand E, Muzny M, Bradway M, Muzik J, Hartvigsen G. Performance of the first combined smartwatch and smartphone diabetes diary application study. J Diabetes Sci Technol. 2015;9(3):556-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klausmann G, Hramiak I, Qvist M, Mikkelsen KH, Guo X. Evaluation of preference for a novel durable insulin pen with memory function among patients with diabetes and health care professionals. Patient Prefer Adherence. 2013;7:285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo X, Sommavilla B, Vanterpool G, Qvist M, Bethien M, Lilleore SK. Evaluation of a new durable insulin pen with memory function among people with diabetes and healthcare professionals. Expert Opin Drug Deliv. 2012;9(4):355-356. [DOI] [PubMed] [Google Scholar]
- 17. Danne T, Forst T, Deinhard J, Rose L, Moennig E, Haupt A. No effect of insulin pen with memory function on glycemic control in a patient cohort with poorly controlled type 1 diabetes: a randomized open-label study. J Diabetes Sci Technol. 2012;6(6):1392-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Munshi MN, Slyne C, Greenberg JM, et al. Nonadherence to insulin therapy detected by bluetooth-enabled pen cap is associated with poor glycemic control. Diabetes Care. 2019;42(6):1129-1131. [DOI] [PubMed] [Google Scholar]
- 19. Gomez-Peralta F, Abreu C, Gomez-Rodriguez S, Ruiz L. Insulclock: a novel insulin delivery optimization and tracking system. Diabetes Technol Ther. 2019;21(4):209-214. [DOI] [PubMed] [Google Scholar]
- 20. Gomez-Peralta F, Abreu C, Gomez-Rodriguez S, et al. Efficacy of insulclock in patients with poorly controlled type 1 diabetes mellitus: a pilot, randomized clinical trial. Diabetes Technol Ther. 2020;22(9):686-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bradley C. Diabetes treatment satisfaction questionnaire: change version for use alongside status version provides appropriate solution where ceiling effects occur. Diabetes Care. 1999;22(3):530-532. [DOI] [PubMed] [Google Scholar]
- 22. Bradley C, Lewis KS. Measures of psychological well-being and treatment satisfaction developed from the responses of people with tablet-treated diabetes. Diabet Med. 1990;7(5):445-451. [DOI] [PubMed] [Google Scholar]
- 23. Grizzle JE. The two-period change-over design an its use in clinical trials. Biometrics. 1965;21:467-480. [PubMed] [Google Scholar]
- 24. Senn SS. Cross-Over Trials in Clinical Research. 2nd ed. Wiley; 2020. [Google Scholar]
- 25. Schmittdiel JA, Uratsu CS, Karter AJ, et al. Why don’t diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med. 2008;23(5):588-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roborel de Climens A, Pain E, Boss A, Shaunik A. Understanding reasons for treatment discontinuation, attitudes and education needs among people who discontinue type 2 diabetes treatment: results from an online patient survey in the USA and UK. Diabetes Ther. 2020;11(8):1873-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adolfsson P, Hartvig NV, Kaas A, Moller JB, Hellman J. Increased time in range and fewer missed bolus injections after introduction of a smart connected insulin pen. Diabetes Technol Ther. 2020;22(10):709-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-dst-10.1177_19322968211033837 for Efficacy of a Smart Insulin Pen Cap for the Management of Patients with Uncontrolled Type 2 Diabetes: A Randomized Cross-Over Trial by Rodolfo J. Galindo, Clementina Ramos, Saumeth Cardona, Priyathama Vellanki, Georgia M. Davis, Omolade Oladejo, Bonnie Albury, Neil Dhruv, Limin Peng and Guillermo E. Umpierrez in Journal of Diabetes Science and Technology