Abstract
Diabetes mellitus (DM) is associated with musculoskeletal complications—including tendon dysfunction and injury. Patients with DM show altered foot and ankle mechanics that have been attributed to tendon dysfunction as well as impaired recovery post-tendon injury. Despite the problem of DM-related tendon complications, treatment guidelines specific to this population of individuals are lacking. DM impairs tendon structure, function, and healing capacity in tendons throughout the body, but the Achilles tendon is of particular concern and most studied in the diabetic foot. At macroscopic levels, asymptomatic, diabetic Achilles tendons may show morphological abnormalities such as thickening, collagen disorganization, and/or calcific changes at the tendon enthesis. At smaller length scales, DM affects collagen sliding and discrete plasticity due to glycation of collagen. However, how these alterations translate to mechanical deficits observed at larger length scales is an area of continued investigation. In addition to dysfunction of the extracellular matrix, tendon cells such as tenocytes and tendon stem/progenitor cells show significant abnormalities in proliferation, apoptosis, and remodeling capacity in the presence of hyperglycemia and advanced glycation end-products, thus contributing to the disruption of tendon homeostasis and healing. Improving our understanding of the effects of DM on tendons—from molecular pathways to patients—will progress toward targeted therapies in this group at high risk of foot and ankle morbidity.
Keywords: Achilles, tendinopathy, tendinosis, diabetes mellitus
Introduction
Diabetes mellitus (DM) is a leading cause of disability and mortality, affecting 422 million adults worldwide. 1 DM is currently the most expensive chronic condition in the US, with a total economic cost of $327 billion in 2017. 2 As of 2020, more than 34 million people in the US of all ages have diabetes. 3 DM is a result of chronic endocrine dysfunction and hyperglycemia and is associated with impairment of the vascular, immune, neurological, and musculoskeletal systems.4,5 There is a clear link between metabolic dysfunction found in DM and musculoskeletal pathologies. For example, DM accelerates the progression and severity of osteoarthritis and increases the risk of bone fracture.6,7 DM leads to peripheral neuropathy and also directly affects the muscles, resulting in muscle atrophy and muscle cell death. 8
In addition to its effect on other musculoskeletal tissues, DM also impairs tendon homeostasis and repair following injury. The effect of DM on tendons is evidenced by an increased incidence of tendon disorders and a higher prevalence of tendinopathy in patients with DM compared to their age-and sex-matched peers without DM.9,10 The etiology of Achilles tendon dysfunction includes a spectrum of pathologic changes associated with an overuse injury, training errors, inflammatory disorders, and intrinsic disease or degeneration. Tendon disorders in DM are characterized by impaired tendon structure, function, and healing capacity that lead to tendon-limited joint range of motion and three times higher risk of tendon injury relative to individuals without DM.11-13 Specific to the diabetic foot, dysfunction of the Achilles tendon is thought to be one of the factors initiating the cascade to lower limb loss,14-18 making the Achilles tendon a target for interventions aimed at resolving foot ulceration.19,20
The purpose of this review is to provide an overview of the clinical presentation of individuals with diabetes-related tendon dysfunction, particularly relating to the diabetic foot, as well as outline mechanisms contributing to diabetes-related impairments in tendon homeostasis and healing.
Clinical Manifestations of Diabetes on Tendon Homeostasis and Healing in the Diabetic Foot
Tendons are spring-like connective tissues that store and return energy into the movement system while transferring muscle forces to the bone to generate movement. Given this role, tendons are a critical component in limb and joint motion, and tendon dysfunction is associated with impaired mobility and a reduced quality of life. Tendon disorders can also be painful, further impacting a person’s ability to be active and complete daily living, occupational, and recreational activities. For individuals with diabetes, an inability to move efficiently is particularly concerning as regular physical activity is a mainstay of glycemic control and diabetes management. 21
Tendon appearance on clinical diagnostic imaging
Diabetes is associated with an increased frequency of Achilles tendon abnormalities on diagnostic imaging in asymptomatic individuals, including tendon thickening, collagen disorganization, calcific changes at the tendon enthesis, and alterations in mechanical behavior.22-25 Sonographic abnormalities have been reported in asymptomatic patients, with defects focused on the enthesis observed in 25.7% of patients with diabetes compared to 11.7% of patients without diabetes. 26
In addition to focal tendon defects, some studies using ultrasound and magnetic resonance imaging (MRI) have reported thickened Achilles tendons in individuals with diabetes compared to the tendons of individuals without diabetes, though other studies have not detected differences in tendon size.16,22,27-30 In some instances, Achilles thickening has been presumed to result in higher tendon stiffness and may contribute to limitations in ankle dorsiflexion.19,20 However, studies of tendon morphological changes have not included an assessment of tendon material properties, which complicates this interpretation of the clinical implications of tendon thickening. For example, tendon thickening is also characteristic of tendon degenerative changes (or tendinosis),31-34 which is generally associated with reductions in tissue stiffness in non-diabetic tendinopathy.31,35
Similar to the tendon degenerative changes observed with Achilles tendinosis, diabetic tendinopathy is also characterized by disorganization of the collagen. Collagen organization of the Achilles tendon assessed via ultrasound imaging—including quantitative techniques like ultrasound tissue characterization—has been shown to be more disorganized in the tendon tissue of patients with diabetes.28,36 An ex vivo study using quantitative polarized light imaging during tensile testing on tendons from patients with diabetes found strong relationships between collagen disorganization and reduced tendon linear modulus. 37 Taken together, it may be that collagen disorganization at larger length scales of tendon architecture (ie, fascicle level) contributes to reductions in the linear modulus 38 and elasticity on ultrasound shear wave elastography 24 assessed ex vivo and in vivo, respectively, in the Achilles tendons of individuals with diabetes.
Patient presentation and treatment considerations
Approximately 80% of individuals attending outpatient physical therapy have diabetes, prediabetes, or diabetes risk factors, 39 indicating the significance of diabetes on the development of musculoskeletal disorders. The clinical presentation and treatment of tendon dysfunction associated with diabetes can broadly be divided into two categories: (1) tendon injury (ie, tendinopathy, tendon rupture) with impaired healing and (2) chronically altered tendon homeostasis.
Diabetes is an important consideration in the care of individuals with tendon injuries. Individuals with diabetes are at an increased risk of developing tendinopathy (eg, tendon pain), and pain reduction during tendon rehabilitation is blunted in individuals with metabolic syndrome. 40 In addition to the direct effects of diabetes on tendon homeostasis that may contribute to injury, diabetes is also associated with peripheral neuropathy and obesity, which can further alter loads on the tendon, contributing to tendon dysfunction and impaired response to treatment.4,40-43
In the context of tendon rupture, individuals with diabetes are often excluded from clinical trials, likely because diabetes could impact wound and tendon healing in unknown ways. Still, individuals with diabetes are known to be at increased risk of requiring hospitalization after Achilles tendon rupture, 13 which may indicate higher rates of rupture and/or complications (such as wound infection) when compared to individuals without diabetes. In addition, animal models of Achilles tendon rupture have suggested impaired healing post-rupture, evidenced by larger reductions in tendon stiffness due to reduced collagen expression in diabetic compared with non-diabetic rats. 44 In humans, diabetes is associated with higher self-reported symptoms and functional limitations during recovery from Achilles tendon rupture compared to individuals without diabetes. 45
In the absence of pain or acute tendon injury, individuals with diabetes may experience downstream effects of impaired tendon homeostasis, potentially leading to ulceration and amputation. Specifically, thickening and increased tendon stiffness of the Achilles tendon restrict ankle dorsiflexion range of motion.14-16,19,20,46,47 During tasks like walking, restricted ankle dorsiflexion alters foot mechanics and changes plantar pressure on the foot.15,17-19 Giacommozzi and colleagues have reported a combination of plantar fascia thickness, Achilles tendon thickness, and great toe extension to account for 70.1% of the total variance in vertical force through the metatarsals during walking gait. Increased plantar pressures contribute to ulceration, including non-healing plantar wounds requiring surgical intervention.
Achilles tendon lengthening procedures have been used as a way to intervene, improving ankle range of motion, reducing plantar flexor power and plantar pressures, and allowing plantar wounds to heal.19,20,48,49 For example, Maluf and colleagues reported a transient 27% decrease in peak forefoot pressure after Achilles tendon lengthening (assessed at 3 weeks post-wound closure). However, peak forefoot pressure returned by 8 months post-wound closure. 19 While participants did demonstrate an average increase of 8 degrees of dorsiflexion range of motion at the 3 week time point, it was a reduction in plantar flexor power rather than dorsiflexion range of motion that was significantly correlated to forefoot pressures. 19 It is important to note that there are a host of other factors that contribute to this cascade (eg, circulation changes, 50 loss of foot structural integrity,29,51 chronic low-grade inflammation) 52 beyond a purely mechanical framework.
Mechanisms Underlying Tendon Dysfunction in Individuals With Diabetes
Effect of glycation on collagen and tendon mechanical behavior
Increased protein glycation and the formation of advanced glycation end-products (AGEs) in the collagenous tissues of patients with diabetes is thought to be one of the main drivers of tissue dysfunction.53-56 AGEs are a heterogeneous group of compounds formed by a non-enzymatic reaction between reducing sugars and free amino groups of proteins and lipids, called the Maillard reaction.57-60 In a collagen-rich extracellular matrix, AGEs can form crosslinks between collagen fibrils, 37 which can then alter biomechanical properties,38,61 thermal stability, 62 enzymatic degradation, and collagen packing.55,63 AGE-crosslinks can persist for the full lifetime of the protein to which they are bound, which presents a substantial concern in tendon tissue due to the relatively slow rate of collagen turnover. 64
Tendons are organized in a hierarchical fashion: collagen molecules are combined in parallel to form fibrils, which are packed together to form fibers, which then form fascicles and are combined to form the whole tendon. 65 An important attribute of tendons is the ability to decrease strain experienced by each tendon substructure compared to the next largest structure along the length scale, known as strain attenuation. Strain attenuation enables tendons to reduce the accumulation of microdamage and increase the maximum strain that can be supported before failure.66,67 Due to decreased collagen sliding, glycated tendons tend to exhibit less strain attenuation, increasing strain on individual fibers and fibrils and lowering the maximum strain on whole tendons, potentially leading to increased microdamage at these smaller length scales during normal tendon loading.37,68
At smaller length scales, AGEs such as collagenous form non-enzymatic crosslinks within and between collagen molecules, adding excessive stability and preventing the motion between collagen fibrils. 69 Veres et al revealed diabetes-related impairments in discrete plasticity at the nanoscale level. Discrete plasticity is a phenomenon by which tendons prevent overload-induced rupture of collagen via a characteristic nanoscale damage motif observed as kinking along the length of collagen fibrils, produced by rupture and repeated sub-rupture of tendons (Figure 1).70,71 Repeated damage at the nanoscale level provides cues for cellular repair and remodeling. 72 In vitro, ribose-induced AGE formation significantly inhibited the formation of discrete plasticity damage, revealing another mechanism that might be involved in the disease progression of diabetic tendinopathy. 73 At the fiber level, altered collagen alignment due to decreased D-period length was observed with increased accumulation of AGEs, which impacted tensile behavior by reducing collagen fibril viscoelasticity and altering fibril failure mode. 74
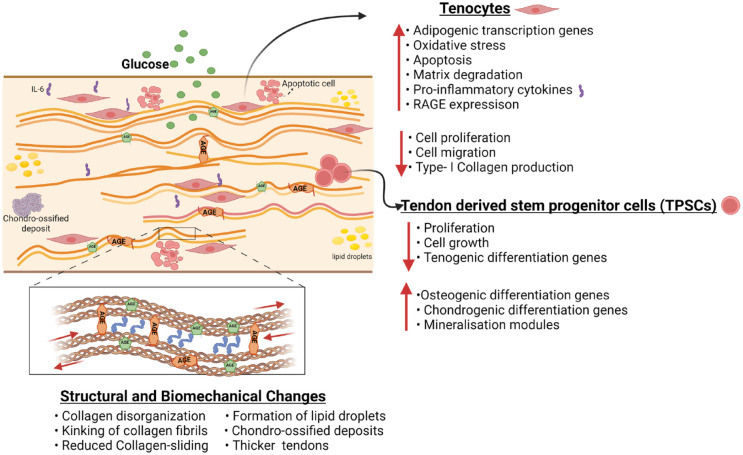
Figure 1.
Proposed structural and functional changes in tendon in diabetes. Diabetes has been suggested to result in a host of tendon changes—including alterations in tenocyte and tendon derived stem progenitor cells (TSPCs) along with structural and biochemical changes. This figure outlines diabetes-related alterations in tendon tissue that have been identified in the literature, though, additional studies confirming these findings and investigating interactions between cellular and mechanical changes are warranted.
Several studies have observed altered biomechanical properties in tendons in animal models as well as in individuals with DM, although reported results have been inconsistent. For example, contradictory results have been reported for tendon stiffness in diabetic conditions. On one hand, loss of collagen type I will reduce tendon stiffness,24,38,75 while on the other hand, accumulation of AGEs leads to dysfunctional matrix stiffening.23,22,76,77 A study by Li et al 69 found that stiffness changes at the tissue level were not due to increased stiffness at the fibril level but rather due to changes in larger-scale hierarchical structures. Thus, the observed difference in stiffness suggests that the relationship between AGE content and tendon tensile mechanics may be masked by multifactorial collagen disorganization at larger length scales. 37 A detailed report on changes in biomechanical properties in diabetic tendinopathy is reviewed elsewhere. 78
Overall, the mechanisms by which collagen glycation affects tendon mechanics have been elucidated at smaller length scales by ex vivo methods. However, standardization of currently available methods to assess tendon in vivo—including non-invasive approaches like ultrasound—and further development of in vivo testing methods is necessary to detect and confirm the effect of AGEs changes at larger length scales. In addition to the pathophysiological reasons underpinning contradictory findings related to the effect of diabetes on tendon mechanical behavior, methodological factors may also be contributing to the lack of consistency, including differences in the method of mechanical testing, type/region of tendon tested, and the selection of a diabetes model for evaluation.
Effects of hyperglycemia and AGEs on tendon cell behavior
The effect of DM on the functional and mechanical behavior of tendons is also reflected in alterations to the cellular environment. The most common cells in tendons are tenocytes and tendon stem/progenitor cells (TSPCs), which play a vital role in tendon homeostasis, maintenance, remodeling, and repair. 79 A hyperglycemic environment and diabetic conditions can negatively alter tendon cells and subsequently cause structural and functional changes in the tendons of patients with diabetes that accelerate the progression of tendinopathy.
Tenocytes are primary tendon cellular components that remodel the extracellular matrix (ECM) and maintain tissue function by generating collagen, proteins and proteoglycans that remodel and repair the ECM.80,81 Several in vitro studies have found that tenocytes cultured in high glucose environments exhibit decreased cell proliferation 82 and cell migration, 83 yet increased apoptosis.84,85 Hyperglycemic conditions have been shown to induce the formation of AGEs, which is not only limited to crosslinking of collagen in the ECM but extends to altered cellular function via the signaling role of AGEs.86,87 Specifically, AGEs are associated with several signaling cascades within many cell types by virtue of their interaction with the receptor for AGE (RAGE).88-90 Activation of AGE-RAGE can induce apoptosis, 91 regulate the expression of pro-inflammatory markers,89,92-99 and lead to degradation of the ECM 100 (Figure 1). Patel et al investigated the effect of AGEs in the presence and absence of high glucose on the mitochondrial function of tenocytes. This study reported that high glucose did impact some mitochondrial functions; however, AGEs were primarily responsible for decreasing ATP production, electron transport efficiency, and proliferation as well as altering the regulation of gene expression associated with ECM remodeling, energy metabolism, and apoptosis. 101 These results suggest that AGEs might affect different functions within the tissue compared to hyperglycemia. However, more mechanistic studies are required to reveal the different pathways by which AGEs affect downstream signaling.
Diabetes-related impairment of tenocyte signaling has been suggested to affect nearly all components of the ECM, though there is limited literature in this area to date, particularly for non-collagenous components. Tendon ECM is primarily composed of type I collagen, though other ECM components (eg, elastin, proteoglycans) may play an important role in tendon function. 102 Rat tenocytes cultured in high glucose show decreased expression of type I collagen expression and increased expression of type III collagen. 82 Burner et al 103 found that high glucose treatment of tenocytes leads to reduced mRNA expression of proteoglycan protein backbones and affects the expression of several proteoglycans, including biglycan, versican, fibromodulin, decorin, and lumican. Ueda et al 82 showed that high glucose (12 mmol/L) stimulated production of various ECM enzymes such as NADPH oxidase (NOX-1, NOX-4), matrix metalloproteinase (MMP-1, MMP-2), tissue inhibitors of MMPs (TIMP-1, TIMP-2), as well as increased interleukin-6 (1L-6) production and ROS accumulation, suggesting an overall increase in oxidative stress and matrix degradation. Mokuhara et al 104 found dehydroepiandrosterone (DHEA), an adrenal steroid, to have a protective effect against high glucose-induced overexpression of catabolic enzymes such as NOX1, NOX-4, Il-6, MMP1-2, and TIMP1-2, Col III in tenocytes in both in vitro tenocytes and in vivo Achilles tendon models, suggesting the effectiveness of DHEA as an antioxidant and anti-inflammatory steroid. Overall, these studies demonstrate that high glucose concentrations and AGEs can profoundly affect matrix organization and turnover. Often overlooked, the exact levels of glucose that can reach the tendon also remain unclear. 105 Thus, further in vivo studies are required to deduce the appropriate glucose concentration in the tendon environment in cases of hyperglycemia of different severity. In addition, fewer studies have explored the effects of insulin treatment and antiglycation compounds in reversing these effects.
Hyperglycemia also reduces the suppression of tendon-related genes in tenocytes and increases the expression of adipogenic transcription factors such as PPARγ and C/EBPs. 83 The adipogenic transdifferentiation of tenocytes under high glucose conditions might promote the formation of lipid deposits in the tissue and further impair the functional and biomechanical properties of the tendons of patients with diabetes. A more detailed account of the effect of hyperglycemia and AGEs on tenocytes is provided in the review. 78
In addition to tenocytes, a small niche population of tendon stem progenitor cells (TSPCs) has been identified in many species.79,106 TSPCs possess stem cell characteristics and play a significant role in tendon repair and regeneration.107,108 Diabetes-related TSPC alterations are related to enhanced transdifferentiation or lower repair and renewal capacity. Diabetic TSPCs show reduced expression of CD44, 109 a glycoprotein involved in cell growth, survival, differentiation, and motility processes, compared to healthy TSPCs. Lower expression of CD44 might result in decreased self-regeneration and self-repair abilities in diabetic tendinopathy.110,111 TSPCs from diabetic rat patellar tendons show decreased proliferation when cultured in a high glucose environment compared to TSPCs from healthy controls. 112 However, in vitro treatment of TSPCs with insulin leads to significantly increased proliferation. 113 In vitro hyperglycemic treatment also increased apoptosis of TSPC, 114 which might correspond to the exhaustion of TSPCs during in vivo progression of diabetic tendinopathy.
Alterations in TSPC gene expression may also contribute to abnormal cell differentiation toward a chondrocyte-like cellular phenotype and higher rates of cellular abnormalities in diabetic compared with non-diabetic conditions. TSPCs in the presence of diabetes, show increased expression of osteochondrogenic markers such as bone morphogenetic protein-1 (BMP-1), osteopontin (OPN), osteocalcin (OCN), Col II, Sox-9, and alkaline phosphatase (ALP), and reduced expression of tendon markers such as Col I and TNMD. 115 In addition, in vitro insulin treatment of TSPCs increased the formation of mineralization modules and increased the expression of osteogenic genes such as Runx2, ALP, and osteonectin (OSN).112,116 Thus, TSPCs derived from diabetic rat tendons demonstrate enhanced osteogenic and chondrogenic differentiation and suppressed tenogenic differentiation, which are likely to affect the tendon repair and facilitate the progression of diabetic tendinopathy. An in vitro study by Xu et al, reported that increased AGEs reduced cell viability, increased apoptosis and autophagy, induced senescence, and enhanced ossification in mouse Achilles TSPCs (Figure 1). The same study also revealed that pioglitazone, a PPARγ agonist, ameliorated the dysfunction in TSPCs caused by AGEs. 117
Further studies should focus on the underlying molecular pathways involved in the erroneous differentiation of these cells. An understanding of the mechanistic effects of AGEs versus hyperglycemia will enable the development of targeted therapies. In addition, the role of inflammation and metabolism in processes of tendon homeostasis, tendinopathy, and tendon healing needs to be investigated for therapeutic purposes.
Current Challenges and Future Opportunities
In summary, diabetes is associated with substantial tendon-related complications—from the molecular to the patient level. From a clinical standpoint, there is a paucity of information to guide clinical decision-making for patients with diabetic tendinopathy. Moving forward, studies specifically designed to investigate the tendon-care needs of individuals with diabetes would help inform the diagnosis and management of diabetic tendinopathy along with improve our understanding of how tendon health factors into the foot and ankle management of this high-risk group.
In vitro studies have shown that hyperglycemia and AGEs can affect tendon mechanics and cell behavior, but the degree to which these specific alterations may contribute to diabetic tendinopathy and impaired healing in vivo is still unknown. While collagen glycation plays a significant role in affecting tendon mechanics and cellular functions, identifying which AGEs could be used as biomarkers could prove useful in early diagnosis of diabetic tendinopathy.
While animal models of diabetes are similar to human cases, a lack of early-stage clinical samples makes it challenging to recapitulate tendon dysfunction in patients with diabetes. Moreover, changes in tendon associated with different models are not well-characterized to attribute to model-specific changes that are not consistent with other models. Characterizing the human condition and improving our understanding of the strengths and limitations of animal models of diabetic tendinopathy is required for better translation of the results to develop effective therapeutic targets.
Acknowledgments
Figure was created with Biorender.com.
Footnotes
Abbreviations: AGEs, advanced glycation end-products; ATP, adenosine triphosphate; Col I, Collagen type I; Col III, collagen type III; DHEA, dehydroepiandrosterone; DM, diabetes; ECM, extracellular matrix; IL, interleukin; MMP, matrix metalloproteinase; MRI, magnetic resonance imaging; mRNA, messenger ribonucleic acid; NADPH, nicotinamide adenine dinucleotide phosphate; NOX, NADPH oxidase; RAGE, receptor for advanced glycation end-products; ROS, reactive oxygen species; TIMP, metallopeptidases; TSPCs, tendon stem progenitor cells; PPARγ, peroxisome proliferator-activated receptor gamma; C/EBPs, CCAAT-enhancer-binding proteins; CD44, cluster of differentiation antigen-44; BMP, bone morphogenetic protein; OPN, osteopontin; OCN, osteocalcin; ALP, alkaline phosphatase; TNMD, tenomodulin; Runx, Runt-related transcription factor; OSN, osteonectin.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Rachana Vaidya  https://orcid.org/0000-0002-6120-5056
https://orcid.org/0000-0002-6120-5056
Jennifer A. Zellers  https://orcid.org/0000-0002-0601-1355
https://orcid.org/0000-0002-0601-1355
References
- 1. IDF Congress 2019: shaping the future of diabetes. Diabetes Res Clin Pract. 2019;158:107954. [DOI] [PubMed] [Google Scholar]
- 2. Lin J, Thompson TJ, Cheng YJ, et al. Projection of the future diabetes burden in the United States through 2060. Popul Health Metr. 2018;16(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 4. Ranger TA, Wong AMY, Cook JL, Gaida JE. Is there an association between tendinopathy and diabetes mellitus? A systematic review with meta-analysis. Br J Sports Med. 2016;50(16):982-989. [DOI] [PubMed] [Google Scholar]
- 5. Sapra A, Bhandari P. Diabetes mellitus. In: StatPearls. Treasure Island, FL: StatPearls; 2022. https://www.ncbi.nlm.nih.gov/books/NBK551501/ [Google Scholar]
- 6. Tchetina EV, Markova GA, Sharapova EP. Insulin resistance in osteoarthritis: similar mechanisms to type 2 diabetes mellitus. J Nutr Metab. 2020;2020:4143802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H, Ba Y, Xing Q, Du JL. Diabetes mellitus and the risk of fractures at specific sites: a meta-analysis. BMJ Open. 2019;9(1):e024067. doi: 10.1136/bmjopen-2018-024067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bohnert KL, Hastings MK, Sinacore DR, et al. Defining the cellular basis for poor muscle performance in diabetic peripheral neuropathy. Foot Ankle Orthop. 2019;4(4):2473011419S00112. doi: 10.1177/2473011419S00112. [DOI] [Google Scholar]
- 9. Cannata F, Vadalà G, Ambrosio L, et al. The impact of type 2 diabetes on the development of tendinopathy. Diabetes Metab Res Rev. 2021;37(6):e3417. doi: 10.1002/dmrr.3417. [DOI] [PubMed] [Google Scholar]
- 10. Dimitri-Pinheiro S, Pimenta M, Cardoso-Marinho B, Torrão H, Soares R, Karantanas A. Diabetes: a silent player in musculoskeletal interventional radiology response. Porto Biomed J. 2021;6(1):e112. doi: 10.1097/j.pbj.0000000000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nichols AEC, Oh I, Loiselle AE. Effects of type II diabetes mellitus on tendon homeostasis and healing. J Orthop Res. 2020;38(1):13-22. doi: 10.1002/jor.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dean BJF, Dakin SG, Millar NL, Carr AJ. Review: emerging concepts in the pathogenesis of tendinopathy. Surgeon. 2017;15(6):349-354. doi: 10.1016/j.surge.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zakaria M, Davis W, Davis T. Incidence and predictors of hospitalization for tendon rupture in type 2 diabetes: the Fremantle diabetes study. Diabetic Med. 2014;31(4):425-430. doi: 10.1111/dme.12344. [DOI] [PubMed] [Google Scholar]
- 14. Cronin NJ, Peltonen J, Ishikawa M, et al. Achilles tendon length changes during walking in long-term diabetes patients. Clin Biomech (Bristol, Avon). 2010;25(5):476-482. doi: 10.1016/j.clinbiomech.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 15. D’Ambrogi E, Giacomozzi C, Macellari V, Uccioli L. Abnormal foot function in diabetic patients: the altered onset of Windlass mechanism. Diabet Med. 2005;22(12):1713-1719. doi: 10.1111/j.1464-5491.2005.01699.x. [DOI] [PubMed] [Google Scholar]
- 16. Giacomozzi C, D’Ambrogi E, Uccioli L, Macellari V. Does the thickening of Achilles tendon and plantar fascia contribute to the alteration of diabetic foot loading? Clin Biomech (Bristol, Avon). 2005;20(5):532-539. doi: 10.1016/j.clinbiomech.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 17. Lott DJ, Zou D, Mueller MJ. Pressure gradient and subsurface shear stress on the neuropathic forefoot. Clin Biomech (Bristol, Avon). 2008;23(3):342-348. doi: 10.1016/j.clinbiomech.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zou D, Mueller MJ, Lott DJ. Effect of peak pressure and pressure gradient on subsurface shear stresses in the neuropathic foot. J Biomech. 2007;40(4):883-890. doi: 10.1016/j.jbiomech.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 19. Maluf K, Mueller M, Strube M, Engsberg J, Johnson J. Tendon Achilles lengthening for the treatment of neuropathic ulcers causes a temporary reduction in forefoot pressure associated with changes in plantar flexor power rather than ankle motion during gait. J Biomech. 2004;37(6):897-906. doi: 10.1016/j.jbiomech.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 20. Mueller MJ, Sinacore DR, Hastings MK, Lott DJ, Strube MJ, Johnson JE. Impact of Achilles tendon lengthening on functional limitations and perceived disability in people with a neuropathic plantar ulcer. Diabetes Care. 2004;27(7):1559-1564. doi: 10.2337/diacare.27.7.1559. [DOI] [PubMed] [Google Scholar]
- 21. Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16(suppl 1):3-63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 22. Abate M, Schiavone C, Di Carlo L, Salini V. Achilles tendon and plantar fascia in recently diagnosed type II diabetes: role of body mass index. Clin Rheumatol. 2012;31(7):1109-1113. doi: 10.1007/s10067-012-1955-y. [DOI] [PubMed] [Google Scholar]
- 23. Couppe C, Svensson RB, Kongsgaard M, et al. Human Achilles tendon glycation and function in diabetes. J Appl Physiol. 2016;120(2):130-137. doi: 10.1152/japplphysiol.00547.2015. [DOI] [PubMed] [Google Scholar]
- 24. Evranos B, Idilman I, Ipek A, Polat SB, Cakir B, Ersoy R. Real-time sonoelastography and ultrasound evaluation of the Achilles tendon in patients with diabetes with or without foot ulcers: a cross sectional study. J Diabetes Complications. 2015;29(8):1124-1129. doi: 10.1016/j.jdiacomp.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 25. Ursini F, Arturi F, D’Angelo S, et al. High prevalence of Achilles tendon enthesopathic changes in patients with type 2 diabetes without peripheral neuropathy. J Am Podiatr Med Assoc. 2017;107(2):99-105. doi: 10.7547/16-059. [DOI] [PubMed] [Google Scholar]
- 26. Abate M, Salini V, Antinolfi P, Schiavone C. Ultrasound morphology of the Achilles in asymptomatic patients with and without diabetes. Foot Ankle Int. 2014;35(1):44-49. doi: 10.1177/1071100713510496. [DOI] [PubMed] [Google Scholar]
- 27. Akturk M, Ozdemir A, Maral I, Yetkin I, Arslan M. Evaluation of Achilles tendon thickening in type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2007;115(2):92-96. doi: 10.1055/s-2007-955097. [DOI] [PubMed] [Google Scholar]
- 28. Batista F, Nery C, Pinzur M, et al. Achilles tendinopathy in diabetes mellitus. Foot Ankle Int. 2008;29(5):498-501. doi: 10.3113/fai-2008-0498. [DOI] [PubMed] [Google Scholar]
- 29. Hastings MK, Mueller MJ, Woodburn J, et al. Acquired midfoot deformity and function in individuals with diabetes and peripheral neuropathy. Clin Biomech (Bristol, Avon). 2016;32:261-267. doi: 10.1016/j.clinbiomech.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Papanas N, Courcoutsakis N, Papatheodorou K, Daskalogiannakis G, Maltezos E, Prassopoulos P. Achilles tendon volume in type 2 diabetic patients with or without peripheral neuropathy: MRI study. Exp Clin Endocrinol Diabetes. 2009;117(10):645-648. doi: 10.1055/s-0029-1224121. [DOI] [PubMed] [Google Scholar]
- 31. Corrigan P, Cortes DH, Pontiggia L, Silbernagel KG. The degree of tendinosis is related to symptom severity and physical activity levels in patients with midportion Achilles tendinopathy. Int J Sports Phys Ther. 2018;13(2):196-207. [PMC free article] [PubMed] [Google Scholar]
- 32. Gibbon WW, Cooper JR, Radcliffe GS. Distribution of sonographically detected tendon abnormalities in patients with a clinical diagnosis of chronic Achilles tendinosis. J Clin Ultrasound. 2000;28(2):61-66. doi: 10.1002/(sici)1097-0096(200002)28:2<61::aid-jcu1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 33. Ohberg L, Lorentzon R, Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: normalised tendon structure and decreased thickness at follow up. Br J Sports Med. 2004;38(1):8-11. doi: 10.1136/bjsm.2001.000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zellers JA, Bley BC, Pohlig RT, Alghamdi NH, Silbernagel KG. Frequency of pathology on diagnostic ultrasound and relationship to patient demographics in individuals with insertional Achilles tendinopathy. Int J Sports Phys Ther. 2019;14(5):761-769. [PMC free article] [PubMed] [Google Scholar]
- 35. Arya S, Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol. 2010;108(3):670-675. doi: 10.1152/japplphysiol.00259.2009. [DOI] [PubMed] [Google Scholar]
- 36. de Jonge S, Rozenberg R, Vieyra B, et al. Achilles tendons in people with type 2 diabetes show mildly compromised structure: an ultrasound tissue characterisation study. Br J Sports Med. 2015;49(15):995-999. doi: 10.1136/bjsports-2014-093696. [DOI] [PubMed] [Google Scholar]
- 37. Zellers JA, Eekhoff JD, Walk RE, Hastings MK, Tang SY, Lake SP. Human Achilles tendon mechanical behavior is more strongly related to collagen disorganization than advanced glycation end-products content. Sci Rep. 2021;11(1):24147. doi: 10.1038/s41598-021-03574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guney A, Vatansever F, Karaman I, Kafadar I, Oner M, Turk C. Biomechanical properties of Achilles tendon in diabetic vs. non-diabetic patients. Exp Clin Endocrinol Diabetes. 2015;123(7):428-432. doi: 10.1055/s-0035-1549889. [DOI] [PubMed] [Google Scholar]
- 39. Kirkness CS, Marcus RL, Lastayo PC, Asche CV, Fritz JM. Diabetes and associated risk factors in patients referred for physical therapy in a national primary care electronic medical record database. Phys Ther. 2008;88(11):1408-1416. doi: 10.2522/ptj.20080129. [DOI] [PubMed] [Google Scholar]
- 40. Park YH, Kim W, Kim JY, Choi GW, Kim HJ. Clinical impact of metabolic syndrome on eccentric exercises for chronic insertional Achilles tendinopathy [published online ahead of print November 2021]. J Foot Ankle Surg. doi: 10.1053/j.jfas.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 41. Gaida JE, Ashe MC, Bass SL, Cook JL. Is adiposity an under-recognized risk factor for tendinopathy? A systematic review. Arthritis Care Res. 2009;61(6):840-849. doi: 10.1002/art.24518. [DOI] [PubMed] [Google Scholar]
- 42. Franceschi F, Papalia R, Paciotti M, et al. Obesity as a risk factor for tendinopathy: a systematic review. Int J Endocrinol. 2014;2014:670262. doi: 10.1155/2014/670262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Macchi M, Spezia M, Elli S, Schiaffini G, Chisari E. Obesity increases the risk of tendinopathy, tendon tear and rupture, and postoperative complications: a systematic review of clinical studies. Clin Orthop Relat Res. 2020;478(8):1839-1847. doi: 10.1097/corr.0000000000001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ahmed AS, Schizas N, Li J, et al. Type 2 diabetes impairs tendon repair after injury in a rat model. J Appl Physiol. 2012;113(11):1784-1791. doi: 10.1152/japplphysiol.00767.2012. [DOI] [PubMed] [Google Scholar]
- 45. Cramer A, Jacobsen NC, Hansen MS, Sandholdt H, Hölmich P, Barfod KW. Diabetes and treatment with orally administrated corticosteroids negatively affect treatment outcome at follow-up after acute Achilles tendon rupture. Knee Surg Sports Traumatol Arthrosc. 2021;29(5):1584-1592. doi: 10.1007/s00167-020-06371-0. [DOI] [PubMed] [Google Scholar]
- 46. Costa ML, Logan K, Heylings D, Donell ST, Tucker K. The effect of Achilles tendon lengthening on ankle dorsiflexion: a cadaver study. Foot Ankle Int. 2006;27(6):414-417. doi: 10.1177/107110070602700605. [DOI] [PubMed] [Google Scholar]
- 47. Van Bael K, Van der Tempel G, Claus I, et al. Gastrocnemius fascia release under local anaesthesia as a treatment for neuropathic foot ulcers in diabetic patients: a short series. Acta Chir Belg. 2016;116(6):367-371. doi: 10.1080/00015458.2016.1192378. [DOI] [PubMed] [Google Scholar]
- 48. Colen LB, Kim CJ, Grant WP, Yeh J-T, Hind B. Achilles tendon lengthening: friend or foe in the diabetic foot? Plast Reconstr Surg. 2013;131(1):37e-43e. doi: 10.1097/PRS.0b013e3182729e0b. [DOI] [PubMed] [Google Scholar]
- 49. Phillips S, Shah A, Staggers JR, et al. Anatomic evaluation of percutaneous Achilles tendon lengthening. Foot Ankle Int. 2018;39(4):500-505. doi: 10.1177/1071100717745559. [DOI] [PubMed] [Google Scholar]
- 50. Zellers JA, Edalati M, Eekhoff J, Lake SP, Zheng J, Hastings MK. Relationship between ex vivo tissue diffusion tensor indexes and material properties in posterior tibialis tendon. Foot Ankle Orthop. 2019;4(4):2473011419S00455. doi: 10.1177/2473011419S00455. [DOI] [Google Scholar]
- 51. Cheuy VA, Hastings MK, Commean PK, Mueller MJ. Muscle and joint factors associated with forefoot deformity in the diabetic neuropathic foot. Foot Ankle Int. 2016;37(5):514-521. doi: 10.1177/1071100715621544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dakin SG, Newton J, Martinez FO, et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br J Sports Med. 2018;52(6):359-367. doi: 10.1136/bjsports-2017-098161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vlassara H, Palace M. Diabetes and advanced glycation endproducts. J Int Med. 2002;251(2):87-101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 54. Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014;14(1):453. doi: 10.1007/s11892-013-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ahmed N. Advanced glycation endproducts—role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67(1):3-21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 56. Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129-146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 57. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813-820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 58. Goh S-Y, Cooper ME. The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metabo. 2008;93(4):1143-1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 59. Kalousova M, Skrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res. 2002;51(6):597-604. [PubMed] [Google Scholar]
- 60. Poulsen MW, Hedegaard RV, Andersen JM, et al. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol. 2013;60:10-37. doi: 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 61. Karim L, Vashishth D. Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS ONE. 2012;7(4):e35047. doi: 10.1371/journal.pone.0035047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Andreassen TT, Seyer-Hansen K, Bailey AJ. Thermal stability, mechanical properties and reducible cross-links of rat tail tendon in experimental diabetes. Biochim Biophys Acta. 1981;677(2):313-317. doi: 10.1016/0304-4165(81)90101-x. [DOI] [PubMed] [Google Scholar]
- 63. Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998;106(1):1-56. 10.1016/S0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 64. Heinemeier KM, Schjerling P, Heinemeier J, Magnusson SP, Kjaer M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb (14)C. FASEB J. 2013;27(5):2074-2079. doi: 10.1096/fj.12-225599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10(6):312-320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- 66. Fang F, Lake SP. Multiscale strain analysis of tendon subjected to shear and compression demonstrates strain attenuation, fiber sliding, and reorganization. J Orthop Res. 2015;33(11):1704-1712. doi: 10.1002/jor.22955. [DOI] [PubMed] [Google Scholar]
- 67. Konow N, Azizi E, Roberts TJ. Muscle power attenuation by tendon during energy dissipation. Proc Biol Sci. 2012;279(1731):1108-1113. doi: 10.1098/rspb.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Eekhoff JD, Fang F, Lake SP. Multiscale mechanical effects of native collagen cross-linking in tendon. Connect Tissue Res. 2018;59(5):410-422. doi: 10.1080/03008207.2018.1449837. [DOI] [PubMed] [Google Scholar]
- 69. Li Y, Fessel G, Georgiadis M, Snedeker JG. Advanced glycation end-products diminish tendon collagen fiber sliding. Matrix Biol. 2013;32(3-4):169-177. doi: 10.1016/j.matbio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 70. Veres SP, Harrison JM, Lee JM. Repeated subrupture overload causes progression of nanoscaled discrete plasticity damage in tendon collagen fibrils. J Orthop Res. 2013;31(5):731-737. doi: 10.1002/jor.22292. [DOI] [PubMed] [Google Scholar]
- 71. Veres SP, Harrison JM, Lee JM. Mechanically overloading collagen fibrils uncoils collagen molecules, placing them in a stable, denatured state. Matrix Biol. 2014;33:54-59. doi: 10.1016/j.matbio.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 72. Veres SP, Brennan-Pierce EP, Lee JM. Macrophage-like U937 cells recognize collagen fibrils with strain-induced discrete plasticity damage. J Biomed Mater Res A. 2015;103(1):397-408. doi: 10.1002/jbm.a.35156. [DOI] [PubMed] [Google Scholar]
- 73. Lee JM, Veres SP. Advanced glycation end-product cross-linking inhibits biomechanical plasticity and characteristic failure morphology of native tendon. J Appl Physiol. 2019;126(4):832-841. doi: 10.1152/japplphysiol.00430.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gautieri A, Passini FS, Silván U, et al. Advanced glycation end-products: mechanics of aged collagen from molecule to tissue. Matrix Biol. 2017;59:95-108. doi: 10.1016/j.matbio.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 75. Connizzo BK, Bhatt PR, Liechty KW, Soslowsky LJ. Diabetes alters mechanical properties and collagen fiber re-alignment in multiple mouse tendons. Ann Biomed Eng. 2014;42(9):1880-1888. doi: 10.1007/s10439-014-1031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Studentsova V, Mora KM, Glasner MF, Buckley MR, Loiselle AE. Obesity/type II diabetes promotes function-limiting changes in murine tendons that are not reversed by restoring normal metabolic function. Sci Rep. 2018;8(1):9218. doi: 10.1038/s41598-018-27634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Petrovic M, Deschamps K, Verschueren SM, et al. Altered leverage around the ankle in people with diabetes: a natural strategy to modify the muscular contribution during walking? Gait Posture. 2017;57:85-90. doi: 10.1016/j.gaitpost.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 78. Lu P-P, Chen M-H, Dai G-C, Li Y-J, Shi L, Rui Y-F. Understanding cellular and molecular mechanisms of pathogenesis of diabetic tendinopathy. World J Stem Cells. 2020;12(11):1255-1275. doi: 10.4252/wjsc.v12.i11.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219-1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 80. Maffulli N, Ewen SW, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic Achilles tendons produce greater quantities of type III collagen than tenocytes from normal Achilles tendons. An in Vitro Model of Human Tendon Healing. Am J Sports Med. 2000;28(4):499-505. doi: 10.1177/03635465000280040901. [DOI] [PubMed] [Google Scholar]
- 81. Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. J Anat. 2008;212(3):211-228. doi: 10.1111/j.1469-7580.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ueda Y, Inui A, Mifune Y, et al. The effects of high glucose condition on rat tenocytes in vitro and rat Achilles tendon in vivo. Bone Joint Res. 2018;7(5):362-372. doi: 10.1302/2046-3758.75.Bjr-2017-0126.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wu Y-F, Huang Y-T, Wang H-K, Yao C-CJ, Sun J-S, Chao Y-H. Hyperglycemia augments the adipogenic transdifferentiation potential of tenocytes and is alleviated by cyclic mechanical stretch. Int J Mol Sci. 2018;19(1):90. doi: 10.3390/ijms19010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wu YF, Wang HK, Chang HW, Sun J, Sun JS, Chao YH. High glucose alters tendon homeostasis through downregulation of the AMPK/Egr1 pathway. Sci Rep. 2017;7:44199. doi: 10.1038/srep44199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Poulsen R, Knowles H, Carr A, Hulley P. Cell differentiation versus cell death: extracellular glucose is a key determinant of cell fate following oxidative stress exposure. Cell Death & Disease. 2014;5(2):e1074. doi: 10.1038/cddis.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Burr SD, Stewart JA., Jr. Extracellular matrix components isolated from diabetic mice alter cardiac fibroblast function through the AGE/RAGE signaling cascade. Life Sci. 2020;250:117569. doi: 10.1016/j.lfs.2020.117569. [DOI] [PubMed] [Google Scholar]
- 87. Vaidya R, Church A, Karim L. Effect of type 2 diabetes on bone cell behavior. In: Gefen A, ed. The Science, Etiology and Mechanobiology of Diabetes and its Complications. Cambridge, MA: Academic Press; 2021:313-326. [Google Scholar]
- 88. Basta G, Lazzerini G, Massaro M, et al. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105(7):816-822. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- 89. Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411-429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pietkiewicz J, Seweryn E, Bartyś A, Gamian A. [Receptors for advanced glycation end products and their physiological and clinical significance]. Postepy Hig Med Dosw. 2008;62:511-523. [PubMed] [Google Scholar]
- 91. Kasper M, Funk RH. Age-related changes in cells and tissues due to advanced glycation end products (AGEs). Arch Gerontol Geriatr. 2001;32(3):233-243. doi: 10.1016/s0167-4943(01)00103-0. [DOI] [PubMed] [Google Scholar]
- 92. Lui PPY, Yung PSH. Inflammatory mechanisms linking obesity and tendinopathy. J Orthop Translat. 2021;31:80-90. doi: 10.1016/j.jot.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Arnalich F, Hernanz A, López-Maderuelo D, et al. Enhanced acute-phase response and oxidative stress in older adults with type II diabetes. Horm Metab Res. 2000;32(10):407-412. doi: 10.1055/s-2007-978662. [DOI] [PubMed] [Google Scholar]
- 94. Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63(4):582-592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 95. Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067-2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 96. Kwan C-K, Fu S-C, Yung PS-H. A high glucose level stimulate inflammation and weaken pro—resolving response in tendon cells—a possible factor contributing to tendinopathy in diabetic patients. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2020;19:1-6. doi: 10.1016/j.asmart.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327-334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 98. Ramya R, Coral K, Bharathidevi SR. RAGE silencing deters CML-AGE induced inflammation and TLR4 expression in endothelial cells. Exp Eye Res. 2021;206:108519. doi: 10.1016/j.exer.2021.108519. [DOI] [PubMed] [Google Scholar]
- 99. Spindler MP, Ho AM, Tridgell D, et al. Acute hyperglycemia impairs IL-6 expression in humans. Immun Inflamm Dis. 2016;4(1):91-97. doi: 10.1002/iid3.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Panwar P, Butler GS, Jamroz A, Azizi P, Overall CM, Brömme D. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2018;65:30-44. doi: 10.1016/j.matbio.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 101. Patel SH, Yue F, Saw SK, et al. Advanced glycation end-products suppress mitochondrial function and proliferative capacity of Achilles tendon-derived fibroblasts. Sci Rep. 2019;9(1):12614. doi: 10.1038/s41598-019-49062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Siadat SM, Zamboulis DE, Thorpe CT, Ruberti JW, Connizzo BK. Tendon extracellular matrix assembly, maintenance and dysregulation throughout life. Adv Exp Med Biol. 2021;1348:45-103. doi: 10.1007/978-3-030-80614-9_3. [DOI] [PubMed] [Google Scholar]
- 103. Burner T, Gohr C, Mitton-Fitzgerald E, Rosenthal AK. Hyperglycemia reduces proteoglycan levels in tendons. Connect Tissue Res. 2012;53(6):535-541. doi: 10.3109/03008207.2012.710670. [DOI] [PubMed] [Google Scholar]
- 104. Mukohara S, Mifune Y, Inui A, et al. In vitro and in vivo tenocyte-protective effectiveness of dehydroepiandrosterone against high glucose-induced oxidative stress. BMC Musculoskelet Disord. 2021;22(1):519. doi: 10.1186/s12891-021-04398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Izumi S, Otsuru S, Adachi N, Akabudike N, Enomoto-Iwamoto M. Control of glucose metabolism is important in tenogenic differentiation of progenitors derived from human injured tendons. PLoS ONE. 2019;14(3):e0213912. doi: 10.1371/journal.pone.0213912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shi L, Lu P-P, Dai G-C, Li Y-J, Rui Y-F. Advanced glycation end productions and tendon stem/progenitor cells in pathogenesis of diabetic tendinopathy. World J Stem Cells. 2021;13(9):1338-1348. doi: 10.4252/wjsc.v13.i9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhang X, Lin Y-C, Rui Y-F, et al. Therapeutic roles of tendon stem/progenitor cells in tendinopathy. Stem Cells Int. 2016;2016:4076578. doi: 10.1155/2016/4076578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ni M, Lui PPY, Rui YF, et al. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J Orthop Res. 2012;30(4):613-619. doi: 10.1002/jor.21559. [DOI] [PubMed] [Google Scholar]
- 109. Ansorge HL, Beredjiklian PK, Soslowsky LJ. CD44 deficiency improves healing tendon mechanics and increases matrix and cytokine expression in a mouse patellar tendon injury model. J Orthop Res. 2009;27(10):1386-1391. doi: 10.1002/jor.20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wu P-T, Su W-R, Li C-L, et al. Inhibition of CD44 induces apoptosis, inflammation, and matrix metalloproteinase expression in tendinopathy. J Biol Chem. 2019;294(52):20177-20184. doi: 10.1074/jbc.RA119.009675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhou Z, Akinbiyi T, Xu L, et al. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell. 2010;9(5):911-915. doi: 10.1111/j.1474-9726.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Patel SH, Sabbaghi A, Carroll CC. Streptozotocin-induced diabetes alters transcription of multiple genes necessary for extracellular matrix remodeling in rat patellar tendon. Connect Tissue Res. 2018;59(5):447-457. doi: 10.1080/03008207.2018.1470168. [DOI] [PubMed] [Google Scholar]
- 113. Durgam SS, Altmann NN, Coughlin HE, Rollins A, Hostnik LD. Insulin enhances the in vitro osteogenic capacity of flexor tendon-derived progenitor cells. Stem Cells Int. 2019;2019:1602751. doi: 10.1155/2019/1602751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lin Y-C, Li Y-J, Rui Y-F, et al. The effects of high glucose on tendon-derived stem cells: implications of the pathogenesis of diabetic tendon disorders. Oncotarget. 2017;8(11):17518. doi: 10.18632/oncotarget.15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kim D-H, Noh S-U, Chae S-W, Kim S-J, Lee Y-T. Altered differentiation of tendon-derived stem cells in diabetic conditions mediated by macrophage migration inhibitory factor. Int J Mol Sci. 2021;22(16):8983. doi: 10.3390/ijms22168983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Via AG, McCarthy MB, Francke M, Oliva F, Mazzocca AD, Maffulli N. Hyperglycemia induces osteogenic differentiation of bone marrow derived stem cells: an in vitro study. Muscles Ligaments Tendons J. 2018;8(1):1-7. doi: 10.5935/abc.20170176 [DOI] [Google Scholar]
- 117. Xu L, Xu K, Wu Z, et al. Pioglitazone attenuates advanced glycation end products-induced apoptosis and calcification by modulating autophagy in tendon-derived stem cells. J Cell Mol Med. 2020;24(3):2240-2251. doi: 10.1111/jcmm.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]