Abstract
Recent experimental evidence on patients with disorders of consciousness revealed that observing brain-heart interactions helps to detect residual consciousness, even in patients with absence of behavioral signs of consciousness. Those findings support hypotheses suggesting that visceral activity is involved in the neurobiology of consciousness, and sum to the existing evidence in healthy participants in which the neural responses to heartbeats reveal perceptual and self-consciousness. More evidence obtained through mathematical modeling of physiological dynamics revealed that emotion processing is prompted by an initial modulation from ascending vagal inputs to the brain, followed by sustained bidirectional brain-heart interactions. Those findings support long-lasting hypotheses on the causal role of bodily activity in emotions, feelings, and potentially consciousness. In this paper, the theoretical landscape on the potential role of heartbeats in cognition and consciousness is reviewed, as well as the experimental evidence supporting these hypotheses. I advocate for methodological developments on the estimation of brain-heart interactions to uncover the role of cardiac inputs in the origin, levels, and contents of consciousness. The ongoing evidence depicts interactions further than the cortical responses evoked by each heartbeat, suggesting the potential presence of non-linear, complex, and bidirectional communication between brain and heartbeat dynamics. Further developments on methodologies to analyze brain-heart interactions may contribute to a better understanding of the physiological dynamics involved in homeostatic-allostatic control, cognitive functions, and consciousness.
Keywords: Physiological modeling, Brain-heart interplay, Heartbeat-evoked responses, Heart rate variability, Interoception, Consciousness
Graphical abstract
Highlights
-
•
Neural responses to heartbeats reveal residual consciousness after brain damage.
-
•
Brain-heart interactions (BHI) may be involved in the neurobiology of consciousness.
-
•
Emotions and feelings imply bidirectional BHI and are prompted by ascending vagal inputs.
-
•
Developments on BHI measurement may help to uncover further links with consciousness.
-
•
Hypotheses on the role of BHI in consciousness should consider complex interactions.
Abbreviations
- Heartbeat-evoked responses
HERs
- Heartbeat-evoked potentials
HEPs
- Heart-rate variability
HRV
- Minimally conscious state
MCS
- Unresponsive wakefulness syndrome
UWS
1. Theoretical background on the role of the heart in cognition
The physiological substrates of emotions and consciousness remain under debate. Emotions are seen as physiological phenomena promoting certain behaviors based on instincts (Adolphs et al., 2019) and previous experiences (Barrett, 2017). While emotions involve the physiological changes, feelings correspond to the subjective meta-representation of these perceived physiological changes (Damasio, 1999). Consciousness, from a very minimalistic point of view, refers to the awareness of the self and the external world (Chalmers, 1995). Long lasting theories of human emotions support that feelings involve the brain monitoring and representation of bodily activity, i.e., the brain reads the ongoing signaling from visceral inputs (for a review, see Pace-Schott et al., 2019). In the same direction, recent theoretical developments on consciousness have rooted the neurobiology of conscious experiences to the monitoring of bodily activity as well (Azzalini et al., 2019; Park and Blanke, 2019a; Park and Tallon-Baudry, 2014). However, in consciousness research the questions outnumber the answers, and a consensus on whether visceral activity is required for consciousness is not in place yet, together with the debate on what consciousness exactly is (Del Pin et al., 2020), whether consciousness is required for emotions (Engelen and Mennella, 2020; LeDoux and Brown, 2017), and what are the functions of consciousness (Cleeremans and Tallon-Baudry, 2022). In this paper, the theory and the experimental evidence on the role of the heart in cognitive functioning are discussed, together with the links to different theories on the neurobiology of consciousness.
The monitoring of visceral activity is part of the processes that contribute to adaptation to changes in the environment (Öhman and Wiens, 2003). For instance, this monitoring can stimulate specific behaviors that would allow to find shelter or food in extreme conditions. The processes involved in the physiological adjustments to maintain optimal functioning are referred as homeostasis, whereas allostasis involves the processes in which the systems anticipate future needs (Smith et al., 2017). Therefore, allostasis requires cognitive functions, such as subjective perception, understanding, learning and memorizing (Smith et al., 2017). Hence, homeostatic-allostatic regulations require of the different processes involved in the sensing of the state of the body. The correlations in cognitive functioning and changes in autonomic tone have been considered in neurovisceral integration models (Thayer et al., 2009; Thayer and Lane, 2009), in which autonomic nervous system markers reflect the self-regulation and adaptability to changes in the environment (Thayer and Lane, 2000). In this direction, the polyvagal theory states that the substrates of adaptative behavior are grounded in the autonomous nervous system, where vagal activity is involved in higher-order responses associated to emotion regulation or social engagement, whereas sympathetic activity regulates stressful “flight or fight” responses (Porges, 2007).
According to Damasio's somatic marker hypothesis, the meta-representation of bodily states in the brain may constitute feelings, which may influence our decision making, and contribute to form the self (Damasio, 1999). Research on interoception aims to describe the mechanisms involved in the sensing of inner bodily signals and their influence on brain dynamics (Azzalini et al., 2019; Khalsa et al., 2018). Interoception includes the sensing of cardiac, respiratory, and gut signaling (Cameron, 2001). In a broader definition, interoception considers the physiological condition of the entire body (Craig, 2002), which may comprise as well thermosensation, pain, and affective touch. The functions associated with interoceptive processes include homeostatic-allostatic regulations, but also low and high order cognitive functions (Tsakiris and Critchley, 2016). In this direction, theoretical neuroscience proposals state that the brain makes predictions based on the integration of exteroceptive and interoceptive information (Seth, 2013), i.e. from inner and outer body, and these interoceptive processes may shape exteroceptive and metacognitive awareness, and vice versa (Nikolova et al., 2021). From this embodied view of cognition, the ascending inputs are required for allostatic regulations, as stated in theories of predictive coding (Allen and Friston, 2018; Barrett and Simmons, 2015; Seth, 2013; Seth and Tsakiris, 2018). In this framework, the brain is constantly adjusting a prediction model to minimize unexpected inputs (Friston, 2010).
Interoceptive mechanisms and pathways have been studied, with some theoretical disagreements. Some are associating bodily states as a contribution to cognitive states, but not necessarily having a central role in consciousness, and these mechanisms would converge in the insula (Craig, 2002, 2009). However, experimental evidence suggests that the insula is not necessary for cardiac interoception and it may be mediated by somatosensory pathways (Khalsa et al., 2009). Therefore, interoception would be part of a multi-pathway and multi-sensory integration (Seth and Tsakiris, 2018), in which sensory, proprioceptive, vestibular, and visceral signals are used by the brain for self-awareness. Further hypotheses have been raised, in which bodily states may be considered as central factors to constitute the self, in which the neural monitoring of ascending visceral inputs would be inherent for subjectivity (Azzalini et al., 2019; Park and Tallon-Baudry, 2014; Tallon-Baudry et al., 2018).
These assumptions would mean that bodily regulation is not separated from cognition, and certainly, that the mind is not separate from the body. The following sections present an overview on the experimental evidence supporting that visceral inputs, in particular heartbeats, have an active participation in cognition and consciousness. In addition, recommendations are provided on how to measure, from a methodological point of view, the brain-heart interactions that may shed light on the role of visceral activity.
2. Behavioral correlates with cardiac phase and interoceptive abilities
The theoretical developments involving cardiac activity in cognition are supported by the increasing evidence on the role of the heart in cognitive functioning. The cardiac cycle consists of two phases; the diastole, in which the heart muscle relaxes and refills with blood; and the systole, the period of muscle contraction and blood pumping. Existing evidence on the behavioral correlates with the phase of the cardiac cycle suggest that the heart timings may serve as inputs in the brain for optimization processes. For instance, some studies have shown group-wise reduced perception/detection when stimuli are presented at systole, as compared to diastole. For instance, in visual (Salomon et al., 2016), auditory (Schulz et al., 2020), and somatosensory detection tasks (Edwards et al., 2009; Motyka et al., 2019). This evidence supports that the brain enhances sensory processing at diastole, suggesting that afferent signals from the baroreceptors occurring at systole attenuate cortical excitability (for a review, see Skora et al., 2022). Nevertheless, there is evidence in the other direction. For instance, saccades during visual search (Galvez-Pol et al., 2020; Ohl et al., 2016), visual attention (Pramme et al., 2014, 2016), active information sampling (Kunzendorf et al., 2019), and reaction/motor excitability (Al et al., 2021b; Larra et al., 2020; Palser et al., 2021; Rae et al., 2018; Ren et al., 2022) are enhanced when stimuli are presented at systole. This evidence depicts that the brain switches to optimize different cognitive processes, depending on cardiac phase. In this direction, passive perception may be enhanced at diastole, and active processes such as attention, active sampling, and action, may be enhanced at systole. Therefore, interoceptive signals might contribute to optimal plasticity. Still, whether the timing of perception or action relates to the cardiac cycle remains speculative.
Interoceptive awareness refers to the capability to access, recognize, and respond to visceral signals, and interoceptive ability refers to the performance of a subject when endeavoring interoceptive awareness. The evidence linking interoceptive abilities, or damaged interoceptive brain networks, and their impact in behavior support the relevance of visceral activity in cognition (Craig, 2003). However, part of this literature has been challenged because of the biased measurements of interoceptive abilities. The level of interoceptive awareness has been mostly quantified through different tasks of heartbeats’ sensation (Fittipaldi et al., 2020; Legrand et al., 2022; Schandry, 1981; Suksasilp and Garfinkel, 2022). The validity of some of those tasks has been questioned because of different biases related to the specificity of the measurements (Brener and Ring, 2016; Zamariola et al., 2018), and because of confounding factors (Buot et al., 2021; Candia-Rivera et al., 2022c). The reported links between atypical or disrupted interoceptive ability include different pathological conditions (Barrett and Simmons, 2015; Khalsa et al., 2018), but also in abnormal emotion regulation (Füstös et al., 2013), emotion sensitivity/recognition (Terasawa et al., 2014), emotion categorization (Murphy et al., 2018), and overall emotional processing (Adolfi et al., 2017). The insula has been widely associated to interoceptive awareness networks (Craig, 2009). The involvement of the insula in perceptual awareness is supported by the evidence from neurodegeneration and its consequences, such as disrupted affective touch (Kirsch et al., 2020), visual awareness (Salomon et al., 2018) and emotional processing (Adolfi et al., 2017). Furthermore, relationships have been reported between the insula with decision making performance (Werner et al., 2013) and interoceptive abilities and decision making performance (Herman and Tsakiris, 2021). It has been hypothesized as well that there is a close interaction between interoception and our interactions with the external world, for instance, in perspective-taking and impulsive behavior (Baiano et al., 2021a, 2021b).
Whether the ability to accurately sense visceral activity can be used as a marker of health remains to be confirmed (Desmedt et al., 2022). Before this can be done, an important challenge in current interoception research is to design methods to enable more comprehensive assessments of interoception based on behavior and self-reported measures, which may in turn lead to uncover their relationships with physiological processes (Crucianelli et al., 2022; Suksasilp and Garfinkel, 2022).
3. Heart-shaped brain dynamics: from interoception to conscious processing
Given the acknowledged limitations of behavioral and self-reported measures to quantify interoceptive abilities, the search of objective interoceptive markers through neural correlates has been extensively performed (Coll et al., 2021). The neural responses to heartbeats, namely heartbeat-evoked responses (HERs for generic use, or HEPs for heartbeat-evoked potentials as measured from EEG), have been proposed as measures of interoception, and repeatedly associated to interoceptive abilities since their proposal as a methodology (Pollatos and Schandry, 2004; Schandry et al., 1986). HERs are associated as well to the focus of attention, either exteroceptive or interoceptive (Petzschner et al., 2019). Recent evidence on heart-transplanted patients depicts that HERs correlate with the ability of heartbeats’ sensation, which is initially reduced after surgery, and recovered after one year (Salamone et al., 2020). Hence, that functional relationship supports that HERs can be used as markers of brain-heart interactions because of their relationship with the recovery of the brain-heart communication.
Beyond interoceptive abilities, HERs have been associated to perceptual awareness. HERs predict perception in a visual detection task (Park et al., 2014), indicating that neural responses to heartbeats shape visual conscious experience, and provide a differential activation in cortical areas. Similarly, HERs correlate with somatosensory perception in a tactile detection task (Al et al., 2020, 2021a), confirming that HERs reflect the integration of internal signals during conscious perception. HERs also relate to the conscious detection of auditory irregularities (Banellis and Cruse, 2020; Candia-Rivera et al., 2021d), showing that cortical potentials locked to heartbeats reflect human expectations of the external world. HERs covary with different aspects of self-related cognition, including self-relatedness of spontaneous thoughts (Babo-Rebelo et al., 2016a, 2016b), self vs other distinction in imagination (Babo-Rebelo et al., 2019), bodily-self-identification of the full body (Park et al., 2016), and face (Sel et al., 2017). Finally, HERs contribute to consistency in choices in a preference-based task (Azzalini et al., 2021), suggesting that HERs reflect different dimensions of subjectivity.
Emotions and feelings have been historically thought to involve the processing of bodily activity (Pace-Schott et al., 2019), and more recently, to depend on the brain's ability to infer the ongoing changes in the environment using inner and outer-body information, namely active inference (Seth, 2013). Various studies have tried to explain different aspects of emotions through HERs, with most of them pointing to a possible relationship with arousal, but without a clear convergence (Coll et al., 2021; Park and Blanke, 2019b). However, evidence from a large cohort showed that HERs modulations in healthy controls, as compared to a diversity of neurodegenerative conditions, correlate with the enhanced emotion recognition performed after a heartbeats sensation task (Salamone et al., 2021). Those results may indicate that HERs and interoception have a functional contribution in the priming of emotions processing.
The existing experimental evidence on HERs is found at a high diversity of latencies with respect to the cardiac cycle, as well as from different brain or scalp locations (Coll et al., 2021). These differences may be the result of the multiple mechanisms for the transduction of cardiac information to the brain. These mechanisms can occur at different phases of the cardiac cycle, but also through different anatomical pathways (Azzalini et al., 2019). The ascending communication from the viscera comprises two core pathways: One is the parasympathetic or vagal afferent through the jugular ganglia that projects to the nucleus tractus solitarii in the brainstem (Craig, 2002), which mostly carries information from mechanic and chemical signaling (Saper, 2002). The second is the sympathetic afferent through dorsal root ganglia that projects to the brain through the spinal cord (Craig, 2002), usually associated to thermal and pain signaling (Barone et al., 1995; Saper, 2002). At the brain level, the information can be transduced directly to the cortex (Park and Blanke, 2019b), or processed in subcortical structures such as the medial nucleus tractus solitarii, the parabrachial nucleus, and the ventromedial nucleus of the thalamus, to later project to higher brain regions, such as the hypothalamus, insula, anterior cingulate cortex, and somatosensory cortex (Critchley and Harrison, 2013; Jänig, 1996; Saper, 2002; Shinder and Newlands, 2014). The descending communication from the central nervous system has the function of control and regulation of different visceral processes, often triggered by an initial ascending signaling (Jänig and Häbler, 2000; Taggart et al., 2016). The descending pathways include vagal and spinal efferents, but also non-neural pathways, such as vascular or lymphatic, and these neural and non-neural pathways may interact as well (Chen et al., 2021).
4. The neurobiology of consciousness: biomarkers comprising the heart
Finding valid neural correlates of consciousness is challenging, starting from the identification of the neural process specific to the subjective experience of a particular content (Aru et al., 2012). Hereafter, the validation of biomarkers has several obstacles, especially for clinical use. Optimal biomarker identification may comprise the use of pattern-recognition for the brain signatures of interest; an assessment of the biomarker's prediction ability; and a long exploration for replicability and generalization at independent contexts, with independent samples (Woo et al., 2017).
Many theories have been established to hypothesize the origin of consciousness. The theoretical landscape is broad, with a lack of convergence between theories (Del Pin et al., 2021; Signorelli et al., 2021). Theories of consciousness range from the sole existence of inner mental states, to the access to higher-order cognition (detailed discussions can be found elsewhere, e.g., Block, 2005; Blumenfeld, 2021; Brown et al., 2019; Chalmers, 1995; Dehaene and Naccache, 2001; Lamme and Roelfsema, 2000; Nieder, 2021; Oizumi et al., 2014; Park and Tallon-Baudry, 2014; Thompson and Varela, 2001). From an embodied point of view, Varela's work suggested a radical–embodiment approach to study the neuroscience of consciousness, highlighting that the crucial processes for consciousness come across the brain–body interactions with the environment, rather than being exclusively in the brain (Shear and Varela, 1999; Thompson and Varela, 2001). In this direction, the Neural Subjective Frame (Park and Tallon-Baudry, 2014; Tallon-Baudry et al., 2018) considers that the constant neural update of the inner visceral states constitute a first-person perspective for conscious experiences. Experimental evidence shows that the heart is functionally involved in emotions, perception and self-related cognition: HERs prime emotions processing (Salamone et al., 2021), predict perceptual consciousness (Al et al., 2020; Park et al., 2014), reflect bodily consciousness (Park et al., 2016; Sel et al., 2017) and the self vs. other distinction (Babo-Rebelo et al., 2019), self-relatedness of spontaneous thoughts (Babo-Rebelo et al., 2016a, 2016b), and shape action and decision (Al et al., 2021b; Azzalini et al., 2021). As cardiac signals are intrinsically originated within the body, they would contribute for this first-person perspective. Therefore, cardiac signals would contribute to consciousness.
Whether a biomarker can reflect or relate to the neurobiology of consciousness can be tested experimentally in patients with disorders of consciousness, after severe brain damage (Hermann et al., 2021). In these patients, the gray zone of (un)consciousness is between Unresponsive Wakefulness Syndrome (UWS) and Minimally Conscious State (MCS) diagnosis. While both diagnoses correspond to noncommunicating patients, UWS presents only reflex-like responses to stimuli (Laureys et al., 2010), though MCS presents fluctuating but reproducible signs of non-reflex behavior (Giacino et al., 2002). Therefore, distinguishing MCS from UWS patients is the main challenge because consciousness does not necessarily translate into overt behavior (Hermann et al., 2021; Tsuchiya et al., 2015). Experimental evidence in disorders of consciousness showed that automated classifications based on brain-heart interactions can distinguish between states-of-consciousness after severe brain damage (Raimondo et al., 2017). It was later confirmed that indeed HERs detect residual consciousness in these patients (Candia-Rivera et al., 2021a). Moreover, HERs can discriminate patients with metabolic cerebral activity compatible with consciousness but showing no behavioral signs of consciousness, namely non-behavioral MCS (Candia-Rivera et al., 2021a). Further evidence showed that MCS patients may show a distinctive HER in resting state, which could be visually differentiated to the average of EEG segments non-locked to heartbeats, whereas UWS patients may not show this distinction (Candia-Rivera and Machado, 2021). Comparing HERs in the most extreme cases, such as brain dead or coma vs locked-in patients or healthy participants, has shown characteristic differences suggesting that the presence of consciousness may cause well-defined HERs, as compared to the absolute absence of consciousness (Candia-Rivera and Machado, 2021). MCS patients also show differentiated HERs compared to UWS patients under the processing of auditory irregularities, suggesting that the integration of external and internal information can reflect states-of-consciousness (Candia-Rivera et al., 2021d).
The raising evidence on HERs in disorders of consciousness supports that visceral activity may be involved in the neurobiology of consciousness (Park and Tallon-Baudry, 2014; Tallon-Baudry et al., 2018), but also potentially linking different theories of consciousness (Safron, 2020; Sattin et al., 2020). HERs can distinguish states-of-consciousness in resting state (Candia-Rivera et al., 2021a; Candia-Rivera and Machado, 2021), probing again that consciousness does not translate into overt behavior (Tsuchiya et al., 2015), and that consciousness could be detected with biomarkers reflecting the existence of inner mental states. HERs reflect consciousness after processing auditory irregularities (Candia-Rivera et al., 2021d). This would mean that the heart is involved in the conscious processing of information, in which long-term memory access occurs according to the Global Neuronal Workspace (Dehaene and Naccache, 2001). But also, consciousness would involve information integration from the available interoceptive and exteroceptive inputs for anticipation processes (Nikolova et al., 2021). The theoretical work on consciousness and complexity states that the presence of complex brain activity is specific in space and time (Sarasso et al., 2021); this statement certainly applies as well to the specificity of heartbeats providing a differentiated cortical activation limited to the cardiac cycle.
In addition to the evidence on brain-heart interactions reflecting states-of-consciousness (Candia-Rivera et al., 2021a, 2021d; Candia-Rivera and Machado, 2021; Raimondo et al., 2017; Riganello et al., 2019), abundant experimental evidence shows that the integration of exteroceptive and interoceptive information, beyond the heart, relate to perceptual and self-awareness (Al et al., 2020; Banellis and Cruse, 2020; Boehme et al., 2019; Candia-Rivera et al., 2021d; Ciaunica et al., 2022; Crucianelli et al., 2018; Grund et al., 2021; Marshall et al., 2020, 2022; Park et al., 2014; Pfeiffer and Lucia, 2017; Suzuki et al., 2013). The biomarkers of brain-heart interactions have a great potential to measure and understand the different dimensions leading to a subjective, conscious experience. Future research has to uncover the relationships of brain-heart interactions with other mechanisms of interoception, as well as with other existing markers of consciousness.
5. Consciousness and complexity in the brain-heart communication scheme
On the brain side, brain complexity is rather high in presence of consciousness, as compared to different altered states-of-consciousness (Demertzi et al., 2019; Sarasso et al., 2021). The thalamus has been hypothesized to arbitrate the complex neural interactions that may give rise to cognition and awareness, given the thalamocortical connections that may promote feedforward/feedback processing modes (Shine, 2021), as well as where the dynamic balance between segregation and integration in the brain would be crucial (Shine, 2019). The role of the thalamocortical and corticocortical communications in the loss of consciousness has been shown through deep brain stimulation to the thalamus, which triggers a consciousness recovery in anesthetized non-human primates (Tasserie et al., 2022). Validated experimental evidence has supported the hypothesis that brain complexity-related measures can describe different states-of-consciousness, for instance, the consciousness indices based on the neural responses to transcranial magnetic stimulation (Casali et al., 2013; Casarotto et al., 2016), or information complexity measures (Engemann et al., 2018, Sitt et al., 2014). These evidences suggest that altered consciousness would be translated in changes of the complexity of brain signaling, or in changes in the regions in which these complex patterns are found (Sarasso et al., 2021).
On the heart side, the existence of complex patterns on heart-rate variability (HRV) has been reported at different contexts (Sassi et al., 2015). Experimental evidence has shown HRV complexity as a potential marker of states-of-consciousness (Riganello et al., 2018a, 2018b; Sattin et al., 2021). The brain structures associated with autonomic activity, and part of the central autonomic network, span from high-order regions (e.g., medial prefrontal cortex and insula), the forebrain (e.g., hypothalamus and amygdala), and several nuclei in the medulla (e.g., nucleus of tractus solitarius, nucleus ambiguous, parabrachial Kolliker fuse nucleus) (Beissner et al., 2013; Silvani et al., 2016; Valenza et al., 2019). More recently, additional brain regions have been associated to heartbeat control, as shown in brain imaging correlates with HRV complexity patterns at rest (Riganello et al., 2018b; Valenza et al., 2020). Interestingly, approximate entropy, a measure of HRV complexity, correlates in both temporal gyrus in resting state (Valenza et al., 2020), which right-lateralization has been associated with overt vs covert consciousness in MCS patients (Candia-Rivera et al., 2021a), but also with the regions distinguishing between self vs social touch (Boehme et al., 2019). Yet, whether those correlations have a functional relationship remains to be confirmed.
Beyond the experimental evidence discussed in the previous section, showing the contribution of heartbeats in consciousness, the raising evidence on brain-heart interactions suggest that HRV may play a role in consciousness as well. Autonomic activity can shape and constrain the ongoing brain dynamics supporting changes in conscious awareness (Munn et al., 2021; Wainstein et al., 2021). Importantly, the changes in sympathetic activity precede the brain network organization, suggesting causal interactions (Munn et al., 2021). Sympathovagal modulations towards brain oscillations shape emotional and physical arousal (Candia-Rivera et al., 2022a, 2022b). The locus coeruleus in the brainstem, which regulates arousal and autonomic function, also shapes neuronal excitability across the brain (Oyarzabal et al., 2022; Tumati et al., 2021; Wainstein et al., 2022), suggesting further links between autonomic activity and the orchestrating of neuronal dynamics during conscious processing. The links of high order structures with HRV have been shown in multiple experimental conditions. For instance, the changes in HRV can be encoded in the prefrontal cortex (orbitofrontal cortex and dorsal anterior cingulate cortex), as registered in non-human primates (Fujimoto et al., 2021), but also in humans, as shown in changes of HRV after prefrontal transcranial magnetic stimulation (Iseger et al., 2020). The parallel fluctuations in heart rate and prefrontal activity relate to decision time in a reward-guided task in non-human primates (Fujimoto et al., 2021). Furthermore, the conscious processing of auditory stimuli leads to synchronized HRV patterns across different participants (Madsen and Parra, 2022; Pérez et al., 2021), and a desynchronized HRV may be associated to unconsciousness or reduced attention to stimuli (Pérez et al., 2021). The experimental evidence supports as well the involvement of parallel HRV and brain dynamics to identify disorders in consciousness (Candia-Rivera et al., 2021a, 2021d; Raimondo et al., 2017; Riganello et al., 2019, 2021).
Functional brain-heart interactions are considered to involve complex interactions because both cardiac and brain oscillations are time-variant, frequency-dependent, and follow non-linear fluctuations (Schiecke et al., 2019). Considering neural correlates of complexity and consciousness, a compatible hypothesis would be that there is a complex interaction between brain activity and ascending bodily signals which would causally give origin to conscious experiences. These causal interactions potentially imply that ascending inputs contain coded information, for instance in HRV. In the first-person perspective of consciousness, the heart is rather seen as a pacemaker, in which the heartbeats are the signals sent to the brain indicating that there is a body, but such signals would not carry coded information, meaning that bodily states (e.g., high or low heart rate) would not have a specific contribution in implementing consciousness (Azzalini et al., 2019; Park and Blanke, 2019b). Note that some emotion theories support that the brain reads and decodes the information provided from bodily inputs (Pace-Schott et al., 2019). One could hypothesize that changes in the heart rate are related to a posteriori process, therefore to the content of consciousness, and that conscious processing modulates the changes in HRV. However, consciousness may be necessary to feel emotions (Engelen and Mennella, 2020; LeDoux and Brown, 2017), and emotional processing is initiated by changes in cardiac vagal activity in emotion elicitation, as shown through mathematical modeling (Candia-Rivera et al., 2022b). Upcoming research in the field needs to discern whether brain-heart interactions have a causal role to subjective experiences; or whether brain-heart interactions correspond to a consequence of the brain processing for the contents of consciousness.
6. Uncovering brain-heart interactions through physiological modeling
Experimental evidence has shown that the heart is continuously providing information to the brain, and the importance of analyzing cardiac signals in parallel with brain activity has been acknowledged in recent years (Azzalini et al., 2019; Petzschner et al., 2021; Quigley et al., 2021). The brain structures involved in brain-heart interactions comprise two relevant brain networks: the central autonomic network, including the insula, which is involved in autonomic control (Silvani et al., 2016; Thayer and Lane, 2009; Valenza et al., 2019, 2020), and the default mode network, which participates in the neural monitoring of cardiac activity for self-related cognition, and conscious perception (Babo-Rebelo et al., 2016a; Park et al., 2014). Moreover, both networks present some overlap (Thayer et al., 2012), which would support the idea of bidirectional brain-heart interactions. Indeed, the connections between the ascending and descending pathways are yet unknown, but also the interactions with non-neural pathways (Critchley and Harrison, 2013). The ascending interplay, from body to brain, is associated to the neural monitoring of bodily activity, which may contribute from basic physiological functions to high order cognition (Quigley et al., 2021). The descending interplay, from brain to body, is associated to neural control of bodily activity including voluntary and non-voluntary action. In particular, heartbeats are able to reach extensive brain regions, not only the brainstem nuclei but also cortical and subcortical regions (Park and Blanke, 2019b), at the same time heartbeat control from the central autonomic network includes cortical, forebrain and medullar regions (Beissner et al., 2013; Silvani et al., 2016; Valenza et al., 2019). The pathways for a bidirectional communication are diverse (Critchley and Harrison, 2013), and the main source of communication is through the autonomic nervous system pathways, either sympathetic or parasympathetic branches (Chen et al., 2021).
As discussed in previous sections, machine learning algorithms detect residual consciousness using from HERs (Candia-Rivera et al., 2021a). Nevertheless, in some specific cases HERs do not reflect the state of the brain-heart communication because of the high presence of delta oscillations that may hide the responses to heartbeats when computing HERs (Candia-Rivera and Machado, 2021). Despite the correlation between high delta power and unconsciousness, the absence of consciousness cannot be confirmed based on delta power (Frohlich et al., 2021). This may imply that ancillary analysis on brain-heart interactions has to be performed in cases of high delta power in brain oscillations. The research on the neural circuits involved in brain-heart interactions is limited given the challenge of the methodological strategies (Chen et al., 2021). Some have tried to measure brain-heart interactions through hemodynamics, as mutual information flow between prefrontal cortex and the brain stem suggest bidirectional interactions with the autonomous nervous system (Pfurtscheller et al., 2021), specifically 0.1 Hz hemodynamic oscillations has shown to precede changes in HRV (Pfurtscheller et al., 2017, 2018). For EEG, a plethora of methods has been proposed to study brain-heart interactions in a non-invasive manner: From different strategies to quantify the transient neural responses to heartbeats; brain-heart oscillations synchronization measures; to mathematical modeling inferring possible causality and directionality of the parallel brain-heart oscillations (for a review, see Candia-Rivera et al., 2021c). While the neural responses to heartbeats are assumed to measure of ascending brain-heart interactions, the synchronization measures do not consider possible directionality of brain-heart interactions. Consequently, some methods to infer the directionality of brain-heart interactions have been proposed, such as Granger Causality (Faes et al., 2015; Greco et al., 2019), Transfer Entropy (Catrambone et al., 2021), and Conditional Entropy (Kumar et al., 2020). However, these methodologies rely on the measurements of causal modulations without considering the physiological priors, which could emerge from casual and not causal co-varying of brain-heart oscillations, as occurs in machine learning models trained with a pattern-based logic (Ramezanian-Panahi et al., 2022). A proposed solution is the modeling of bidirectional interactions through a generative approach (Ramezanian-Panahi et al., 2022; Ramstead et al., 2022), considering the ongoing modulations between brain and cardiac oscillations (Candia-Rivera et al., 2021b, 2022a), in which two physiologically-inspired models of synthetic ECG and EEG series are coupled considering their mutual influences in the ongoing oscillations at different latencies, on the basis of a generative signal.
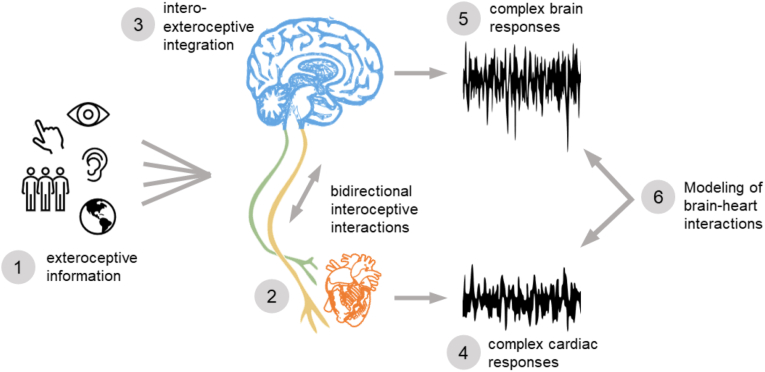
The study of functional bidirectional brain-heart interactions is of interest given the typical assumptions on ascending and descending interplay. For instance, the descending interplay to the heart is usually associated to arousal only (Azzalini et al., 2019). However, the analysis of the bidirectional interactions of the brain with peripheral cardiac activity showed that the reported arousal was correlated with ascending modulations from cardiac vagal activity (Candia-Rivera et al., 2022b). The hypothesis introduced here is that the physiological and cognitive processes involved in interoception, and subjectivity, would involve a dynamic and cyclic information exchange. In Fig. 1 the proposed framework for the analysis of bidirectional brain-heart interactions for the analysis of interoceptive-exteroceptive integration is presented. The brain processes the information coming from exteroceptive inputs: senses, social interactions, and environment. The information processing occurs in parallel with the ongoing bidirectional interoceptive communications between the brain and other organs. The information integration from the available sources, exteroceptive and/or interoceptive inputs, occurs in the brain. The integration results in complex brain responses, but also HRV complexity because of the ongoing bidirectional brain-heart interactions. The presence of complex activity patterns in brain and heart may not be arbitrary but coordinated with possible causal interactions. The proposed alternative approach considers the estimation, through mathematical modeling, of the possible causal interactions between the two complex systems.
Fig. 1.
The interoceptive-exteroceptive integration: information flux in the neural circuits that may reflect conscious experiences. (1) The processing of information coming from exteroceptive inputs occurs in parallel with (2) the bidirectional communication between the brain and interoceptive channels. (3) The brain integrates the information received from the available sources, exteroceptive and/or interoceptive. The result of these integrations are (4) complex autonomic responses and (5) complex brain responses. An alternative approach to study consciousness is to (6) model possible causal interactions between the complex cardiac activity and brain dynamics.
Methodological advancements are needed for understanding the dynamic physiological processes involved in the integration of multisensory information emerging from within and outside the body– which may be necessary to form the sense of self, others, environment, and potentially consciousness. Whether consciousness arises from the integration of information, or whether consciousness facilitates integration remains under debate (Brogaard et al., 2021). Certainly, the integration of interoceptive signals plays a key role in subjective experiences, constituting the self and bodily self-consciousness (Azzalini et al., 2019; Ciaunica and Crucianelli, 2019; Klein et al., 2021; Park and Blanke, 2019a). From a theoretical and philosophical perspective, the predictive processing framework proposes that experiences of embodied selfhood emerge from interoceptive afferents and top-down inferences (Seth and Tsakiris, 2018). Grasping to understand the perceptual and self-awareness processes could contribute to enlighten the nature of minimal forms of being perceptually aware of oneself, inner mental states, and being conscious.
7. Conclusions
The bidirectional brain-heart interactions involve the collection and integration of information, interpretation, and control of bodily activity. Many of these processes are inherent in physiological and cognitive functions, from homeostatic and allostatic control to high order cognition. The theoretical perspectives that consider heartbeat dynamics propose different mechanisms, but whether brain-heart interactions are involved in the neurobiology of consciousness, from its origin to its contents, remains to be broadly acknowledged. The developments in the understanding of the communication pathways and mutual modulation mechanisms between central and visceral systems may unravel some of these matters. As stated by Francisco Varela more than two decades ago: “The processes crucial for consciousness cut across the brain–body–world divisions rather than being located simply in the head. Evaluating this proposal could set the agenda for embodied cognitive science in coming years”. Certainly, the evaluation of consciousness from an embodied cognitive science perspective could set the agenda of the neuroscience of consciousness research.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
I would like to thank Paula C. Salamone, Francesco Massimo, and the two anonym reviewers, for their invaluable feedback to produce this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crneur.2022.100050.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adolfi F., Couto B., Richter F., Decety J., Lopez J., Sigman M., Manes F., Ibáñez A. Convergence of interoception, emotion, and social cognition: a twofold fMRI meta-analysis and lesion approach. Cortex. 2017;88:124–142. doi: 10.1016/j.cortex.2016.12.019. [DOI] [PubMed] [Google Scholar]
- Adolphs R., Mlodinow L., Barrett L.F. What is an emotion? Curr. Biol. 2019;29:R1060–R1064. doi: 10.1016/j.cub.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al E., Iliopoulos F., Forschack N., Nierhaus T., Grund M., Motyka P., Gaebler M., Nikulin V.V., Villringer A. Heart–brain interactions shape somatosensory perception and evoked potentials. Proc. Natl. Acad. Sci. USA. 2020;117:10575–10584. doi: 10.1073/pnas.1915629117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al E., Iliopoulos F., Nikulin V.V., Villringer A. Heartbeat and somatosensory perception. Neuroimage. 2021;238 doi: 10.1016/j.neuroimage.2021.118247. [DOI] [PubMed] [Google Scholar]
- Al E., Stephani T., Engelhardt M., Villringer A., Nikulin V. Cardiac activity impacts cortical motor excitability. 2021. [DOI] [PMC free article] [PubMed]
- Allen M., Friston K.J. From cognitivism to autopoiesis: towards a computational framework for the embodied mind. Synthese. 2018;195:2459–2482. doi: 10.1007/s11229-016-1288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aru J., Bachmann T., Singer W., Melloni L. Distilling the neural correlates of consciousness. Neurosci. Biobehav. Rev. 2012;36:737–746. doi: 10.1016/j.neubiorev.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Azzalini D., Buot A., Palminteri S., Tallon-Baudry C. Responses to heartbeats in ventromedial prefrontal cortex contribute to subjective preference-based decisions. J. Neurosci. 2021;41:5102–5114. doi: 10.1523/JNEUROSCI.1932-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzalini D., Rebollo I., Tallon-Baudry C. Visceral signals shape brain dynamics and cognition. Trends Cognit. Sci. 2019;23:488–509. doi: 10.1016/j.tics.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Babo-Rebelo M., Buot A., Tallon-Baudry C. Neural responses to heartbeats distinguish self from other during imagination. Neuroimage. 2019;191:10–20. doi: 10.1016/j.neuroimage.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babo-Rebelo M., Richter C.G., Tallon-Baudry C. Neural responses to heartbeats in the default network encode the self in spontaneous thoughts. J. Neurosci. 2016;36:7829–7840. doi: 10.1523/JNEUROSCI.0262-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babo-Rebelo M., Wolpert N., Adam C., Hasboun D., Tallon-Baudry C. Is the cardiac monitoring function related to the self in both the default network and right anterior insula? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371 doi: 10.1098/rstb.2016.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiano C., Job X., Santangelo G., Auvray M., Kirsch L.P. Interactions between interoception and perspective-taking: current state of research and future directions. Neurosci. Biobehav. Rev. 2021;130:252–262. doi: 10.1016/j.neubiorev.2021.08.007. [DOI] [PubMed] [Google Scholar]
- Baiano C., Santangelo G., Senese V.P., Di Mauro G., Lauro G., Piacenti M., Conson M. Linking perception of bodily states and cognitive control: the role of interoception in impulsive behaviour. Exp. Brain Res. 2021;239:857–865. doi: 10.1007/s00221-020-06022-3. [DOI] [PubMed] [Google Scholar]
- Banellis L., Cruse D. Skipping a beat: heartbeat-evoked potentials reflect predictions during interoceptive-exteroceptive integration. Cerebral Cortex Commun. 2020;1 doi: 10.1093/texcom/tgaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone F.C., De Coronado I.Z., Wayner M.J. Gastric distension modulates hypothalamic neurons via a sympathetic afferent path through the mesencephalic periaqueductal gray. Brain Res. Bull. 1995;38:239–251. doi: 10.1016/0361-9230(95)00096-W. [DOI] [PubMed] [Google Scholar]
- Barrett L.F. The theory of constructed emotion: an active inference account of interoception and categorization. Soc. Cognit. Affect Neurosci. 2017;12:1–23. doi: 10.1093/scan/nsw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L.F., Simmons W.K. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 2015;16:419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F., Meissner K., Bär K.-J., Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. 2013;33:10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block N. Two neural correlates of consciousness. Trends Cognit. Sci. 2005;9:46–52. doi: 10.1016/j.tics.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. Brain mechanisms of conscious awareness: detect, pulse, switch, and wave. Neuroscientist. 2021 doi: 10.1177/10738584211049378. 10738584211049378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme R., Hauser S., Gerling G.J., Heilig M., Olausson H. Distinction of self-produced touch and social touch at cortical and spinal cord levels. Proc. Natl. Acad. Sci. USA. 2019;116:2290–2299. doi: 10.1073/pnas.1816278116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener J., Ring C. Towards a psychophysics of interoceptive processes: the measurement of heartbeat detection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371 doi: 10.1098/rstb.2016.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogaard B., Chomanski B., Gatzia D.E. Consciousness and information integration. Synthese. 2021;198:763–792. doi: 10.1007/s11229-020-02613-3. [DOI] [Google Scholar]
- Brown R., Lau H., LeDoux J.E. Understanding the higher-order approach to consciousness. Trends Cognit. Sci. 2019;23:754–768. doi: 10.1016/j.tics.2019.06.009. [DOI] [PubMed] [Google Scholar]
- Buot A., Azzalini D., Chaumon M., Tallon-Baudry C. Does stroke volume influence heartbeat evoked responses? Biol. Psychol. 2021;165 doi: 10.1016/j.biopsycho.2021.108165. [DOI] [PubMed] [Google Scholar]
- Cameron O.G. Interoception: the inside story—a model for psychosomatic processes. Psychosom. Med. 2001;63:697–710. doi: 10.1097/00006842-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Candia-Rivera D., Annen J., Gosseries O., Martial C., Thibaut A., Laureys S., Tallon-Baudry C. Neural responses to heartbeats detect residual signs of consciousness during resting state in postcomatose patients. J. Neurosci. 2021;41:5251–5262. doi: 10.1523/JNEUROSCI.1740-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia-Rivera D., Catrambone V., Barbieri R., Valenza G. Functional assessment of bidirectional cortical and peripheral neural control on heartbeat dynamics: a brain-heart study on thermal stress. Neuroimage. 2022;251:119023. doi: 10.1016/j.neuroimage.2022.119023. [DOI] [PubMed] [Google Scholar]
- Candia-Rivera D., Catrambone V., Barbieri R., Valenza G. Integral pulse frequency modulation model driven by sympathovagal dynamics: synthetic vs. real heart rate variability. Biomed. Signal Process Control. 2021;68 doi: 10.1016/j.bspc.2021.102736. [DOI] [Google Scholar]
- Candia-Rivera D., Catrambone V., Thayer J.F., Gentili C., Valenza G. Cardiac sympathetic-vagal activity initiates a functional brain–body response to emotional arousal. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2119599119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia-Rivera D., Catrambone V., Valenza G. The role of electroencephalography electrical reference in the assessment of functional brain–heart interplay: from methodology to user guidelines. J. Neurosci. Methods. 2021;360 doi: 10.1016/j.jneumeth.2021.109269. [DOI] [PubMed] [Google Scholar]
- Candia-Rivera D., Machado C. The differences of heartbeat-evoked responses and EEG non-locked to heartbeats may shed light in identifying disorders of consciousness. 2021. [DOI]
- Candia-Rivera D., Raimondo F., Perez P., Naccache L., Tallon-Baudry C., Sitt J.D. Processing of slow-global auditory regularities causes larger neural responses to heartbeats in patients under minimal consciousness state, compared to unresponsive wakefulness syndrome. 2021. [DOI]
- Candia-Rivera D., Sappia M.S., Horschig J.M., Colier W.N.J.M., Valenza G. Effects of neural monitoring and control of visceral signals on heart rate, respiration rate, and frontal hemodynamics. 2022. [DOI]
- Casali A.G., Gosseries O., Rosanova M., Boly M., Sarasso S., Casali K.R., Casarotto S., Bruno M.-A., Laureys S., Tononi G., Massimini M. A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3006294. 198ra105-198ra105. [DOI] [PubMed] [Google Scholar]
- Casarotto S., Comanducci A., Rosanova M., Sarasso S., Fecchio M., Napolitani M., Pigorini A., G Casali A., Trimarchi P.D., Boly M., Gosseries O., Bodart O., Curto F., Landi C., Mariotti M., Devalle G., Laureys S., Tononi G., Massimini M. Stratification of unresponsive patients by an independently validated index of brain complexity. Ann. Neurol. 2016;80:718–729. doi: 10.1002/ana.24779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catrambone V., Talebi A., Barbieri R., Valenza G. Time-resolved brain-to-heart probabilistic information transfer estimation using inhomogeneous point-process models. IEEE (Inst. Electr. Electron. Eng.) Trans. Biomed. Eng. 2021 doi: 10.1109/TBME.2021.3071348. 1–1. [DOI] [PubMed] [Google Scholar]
- Chalmers D.J. Facing up to the problem of consciousness. J. Conscious. Stud. 1995;2:200–219. [Google Scholar]
- Chen W.G., Schloesser D., Arensdorf A.M., Simmons J.M., Cui C., Valentino R., Gnadt J.W., Nielsen L., Hillaire-Clarke C.St, Spruance V., Horowitz T.S., Vallejo Y.F., Langevin H.M. The emerging science of interoception: sensing, integrating, interpreting, and regulating signals within the self. Trend Neurosci. Special Iss.: Neurosci. Interocep. 2021;44:3–16. doi: 10.1016/j.tins.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaunica A., Crucianelli L. Minimal self-awareness: from within A developmental perspective. J. Conscious. Stud. 2019;26:207–226. [Google Scholar]
- Ciaunica A., Seth A., Limanowski J., Hesp C., Friston K.J. I overthink—therefore I am not: an active inference account of altered sense of self and agency in depersonalisation disorder. Conscious. Cognit. 2022;101 doi: 10.1016/j.concog.2022.103320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeremans A., Tallon-Baudry C. Consciousness matters: phenomenal experience has functional value. Neurosci. Conscious. 2022;2022(1) doi: 10.1093/nc/niac007. 2022, niac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll M.-P., Hobson H., Bird G., Murphy J. Systematic review and meta-analysis of the relationship between the heartbeat-evoked potential and interoception. Neurosci. Biobehav. Rev. 2021;122:190–200. doi: 10.1016/j.neubiorev.2020.12.012. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel — now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig A.D. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13:500–505. doi: 10.1016/S0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Harrison N.A. Visceral influences on brain and behavior. Neuron. 2013;77:624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Crucianelli L., Enmalm A., Ehrsson H.H. Interoception as independent cardiac, thermosensory, nociceptive, and affective touch perceptual submodalities. Biol. Psychol. 2022;172 doi: 10.1016/j.biopsycho.2022.108355. [DOI] [PubMed] [Google Scholar]
- Crucianelli L., Krahé C., Jenkinson P.M., Fotopoulou A.K. Interoceptive ingredients of body ownership: affective touch and cardiac awareness in the rubber hand illusion. Cortex. 2018;104:180–192. doi: 10.1016/j.cortex.2017.04.018. [DOI] [PubMed] [Google Scholar]
- Damasio A. Harcourt College Publishers; Fort Worth, TX, US: 1999. The Feeling of what Happens: Body and Emotion in the Making of Consciousness, the Feeling of what Happens: Body and Emotion in the Making of Consciousness. [Google Scholar]
- Dehaene S., Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition. 2001;79:1–37. doi: 10.1016/s0010-0277(00)00123-2. [DOI] [PubMed] [Google Scholar]
- Del Pin S.H., Skóra Z., Sandberg K., Overgaard M., Wierzchoń M. Comparing theories of consciousness: why it matters and how to do it. Neurosci Conscious. 2021;2021(2) doi: 10.1093/nc/niab019. 2021, niab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pin S.H., Skóra Z., Sandberg K., Overgaard M., Wierzchoń M. Comparing theories of consciousness: object position, not probe modality, reliably influences experience and accuracy in object recognition tasks. Conscious. Cognit. 2020;84 doi: 10.1016/j.concog.2020.102990. [DOI] [PubMed] [Google Scholar]
- Demertzi A, Tagliazucchi E, Dehaene S, Deco G, Barttfeld P, Raimondo F, Martial C, Fernández-Espejo D, Rohaut B, Voss HU, Schiff ND, Owen AM, Laureys S, Naccache L, Sitt JD. Human consciousness is supported by dynamic complex patterns of brain signal coordination. Sci Adv. 2019;5(2):eaat7603. doi: 10.1126/sciadv.aat7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt O., Van Den Houte M., Walentynowicz M., Dekeyser S., Luminet O., Corneille O. How does heartbeat counting task performance relate to theoretically-relevant mental health outcomes? A meta-analysis. Collabra: Psychology. 2022;8 doi: 10.1525/collabra.33271. [DOI] [Google Scholar]
- Edwards L., Ring C., McIntyre D., Winer J.B., Martin U. Sensory detection thresholds are modulated across the cardiac cycle: evidence that cutaneous sensibility is greatest for systolic stimulation. Psychophysiology. 2009;46:252–256. doi: 10.1111/j.1469-8986.2008.00769.x. [DOI] [PubMed] [Google Scholar]
- Engemann Denis A., Federico Raimondo, King Jean-Rémi, Rohaut Benjamin, Gilles Louppe, Faugeras Frédéric, Annen Jitka, et al. Robust EEG-based cross-site and cross-protocol classification of states of consciousness. Brain. 2018;141(11):3179–3192. doi: 10.1093/brain/awy251. [DOI] [PubMed] [Google Scholar]
- Engelen T., Mennella R. What is it like to Be an emotion researcher? 2020. [DOI]
- Faes L., Marinazzo D., Jurysta F., Nollo G. Linear and non-linear brain-heart and brain-brain interactions during sleep. Physiol. Meas. 2015;36:683–698. doi: 10.1088/0967-3334/36/4/683. [DOI] [PubMed] [Google Scholar]
- Fittipaldi S., Abrevaya S., Fuente A. de la, Pascariello G.O., Hesse E., Birba A., Salamone P., Hildebrandt M., Martí S.A., Pautassi R.M., Huepe D., Martorell M.M., Yoris A., Roca M., García A.M., Sedeño L., Ibáñez A. A multidimensional and multi-feature framework for cardiac interoception. Neuroimage. 2020;212 doi: 10.1016/j.neuroimage.2020.116677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- Frohlich J., Toker D., Monti M.M. Consciousness among delta waves: a paradox? Brain. 2021;144:2257–2277. doi: 10.1093/brain/awab095. [DOI] [PubMed] [Google Scholar]
- Fujimoto A., Murray E.A., Rudebeck P.H. Interaction between decision-making and interoceptive representations of bodily arousal in frontal cortex. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2014781118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füstös J., Gramann K., Herbert B.M., Pollatos O. On the embodiment of emotion regulation: interoceptive awareness facilitates reappraisal. Soc. Cognit. Affect Neurosci. 2013;8:911–917. doi: 10.1093/scan/nss089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez-Pol A., McConnell R., Kilner J.M. Active sampling in visual search is coupled to the cardiac cycle. Cognition. 2020;196 doi: 10.1016/j.cognition.2019.104149. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Ashwal S., Childs N., Cranford R., Jennett B., Katz D.I., Kelly J.P., Rosenberg J.H., Whyte J., Zafonte R.D., Zasler N.D. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- Greco A., Faes L., Catrambone V., Barbieri R., Scilingo E.P., Valenza G. Lateralization of directional brain-heart information transfer during visual emotional elicitation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019;317:R25–R38. doi: 10.1152/ajpregu.00151.2018. [DOI] [PubMed] [Google Scholar]
- Grund M., Al E., Pabst M., Dabbagh A., Stephani T., Nierhaus T., Gaebler M., Villringer A. Respiration, heartbeat, and conscious tactile perception. J. Neurosci. 2021;42(4):643–656. doi: 10.1523/JNEUROSCI.0592-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A.M., Tsakiris M. The impact of cardiac afferent signaling and interoceptive abilities on passive information sampling. Int. J. Psychophysiol. 2021;162:104–111. doi: 10.1016/j.ijpsycho.2021.02.010. [DOI] [PubMed] [Google Scholar]
- Hermann B., Sangaré A., Munoz-Musat E., Salah A.B., Perez P., Valente M., Faugeras F., Axelrod V., Demeret S., Marois C., Pyatigorskaya N., Habert M.-O., Kas A., Sitt J.D., Rohaut B., Naccache L. Importance, limits and caveats of the use of “disorders of consciousness” to theorize consciousness. Neurosci. Conscious. 2021;2021(2):niab048. doi: 10.1093/nc/niab048. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseger T.A., Bueren N.E.R., Kenemans J.L., Gevirtz R., Arns M. A frontal-vagal network theory for Major Depressive Disorder: implications for optimizing neuromodulation techniques. Brain Stimul.: Basic Translate. Clinic. Res. Neuromod. 2020;13:1–9. doi: 10.1016/j.brs.2019.10.006. [DOI] [PubMed] [Google Scholar]
- Jänig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol. Psychol. Intercepp. Behave. 1996;42:29–51. doi: 10.1016/0301-0511(95)05145-7. [DOI] [PubMed] [Google Scholar]
- Jänig W., Häbler H.J. Specificity in the organization of the autonomic nervous system: a basis for precise neural regulation of homeostatic and protective body functions. Prog. Brain Res. 2000;122:351–367. doi: 10.1016/s0079-6123(08)62150-0. [DOI] [PubMed] [Google Scholar]
- Khalsa S.S., Adolphs R., Cameron O.G., Critchley H.D., Davenport P.W., Feinstein J.S., Feusner J.D., Garfinkel S.N., Lane R.D., Mehling W.E., Meuret A.E., Nemeroff C.B., Oppenheimer S., Petzschner F.H., Pollatos O., Rhudy J.L., Schramm L.P., Simmons W.K., Stein M.B., Stephan K.E., Van den Bergh O., Van Diest I., von Leupoldt A., Paulus M.P., Ainley V., Al Zoubi O., Aupperle R., Avery J., Baxter L., Benke C., Berner L., Bodurka J., Breese E., Brown T., Burrows K., Cha Y.-H., Clausen A., Cosgrove K., Deville D., Duncan L., Duquette P., Ekhtiari H., Fine T., Ford B., Garcia Cordero I., Gleghorn D., Guereca Y., Harrison N.A., Hassanpour M., Hechler T., Heller A., Hellman N., Herbert B., Jarrahi B., Kerr K., Kirlic N., Klabunde M., Kraynak T., Kriegsman M., Kroll J., Kuplicki R., Lapidus R., Le T., Hagen K.L., Mayeli A., Morris A., Naqvi N., Oldroyd K., Pané-Farré C., Phillips R., Poppa T., Potter W., Puhl M., Safron A., Sala M., Savitz J., Saxon H., Schoenhals W., Stanwell-Smith C., Teed A., Terasawa Y., Thompson K., Toups M., Umeda S., Upshaw V., Victor T., Wierenga C., Wohlrab C., Yeh H., Yoris A., Zeidan F., Zotev V., Zucker N. Interoception and mental health: a roadmap. Biological psychiatry: cognitive neuroscience and neuroimaging. Interocep Mental Health. 2018;3:501–513. doi: 10.1016/j.bpsc.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa S.S., Rudrauf D., Feinstein J.S., Tranel D. The pathways of interoceptive awareness. Nat. Neurosci. 2009;12:1494–1496. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch L.P., Besharati S., Papadaki C., Crucianelli L., Bertagnoli S., Ward N., Moro V., Jenkinson P.M., Fotopoulou A. Damage to the right insula disrupts the perception of affective touch. Elife. 2020;9 doi: 10.7554/eLife.47895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A.S., Dolensek N., Weiand C., Gogolla N. Fear balance is maintained by bodily feedback to the insular cortex in mice. Science. 2021;374:1010–1015. doi: 10.1126/science.abj8817. [DOI] [PubMed] [Google Scholar]
- Kumar M., Singh D., Deepak K.K. Identifying heart-brain interactions during internally and externally operative attention using conditional entropy. Biomed. Signal Process Control. 2020;57 doi: 10.1016/j.bspc.2019.101826. [DOI] [Google Scholar]
- Kunzendorf S., Klotzsche F., Akbal M., Villringer A., Ohl S., Gaebler M. Active information sampling varies across the cardiac cycle. Psychophysiology. 2019;56 doi: 10.1111/psyp.13322. [DOI] [PubMed] [Google Scholar]
- Lamme V.A., Roelfsema P.R. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- Larra M.F., Finke J.B., Wascher E., Schächinger H. Disentangling sensorimotor and cognitive cardioafferent effects: a cardiac-cycle-time study on spatial stimulus-response compatibility. Sci. Rep. 2020;10:4059. doi: 10.1038/s41598-020-61068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S., Celesia G.G., Cohadon F., Lavrijsen J., León-Carrión J., Sannita W.G., Sazbon L., Schmutzhard E., von Wild K.R., Zeman A., Dolce G., the European Task Force on Disorders of Consciousness Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med. 2010;8:68. doi: 10.1186/1741-7015-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E., Brown R. A higher-order theory of emotional consciousness. Proc. Natl. Acad. Sci. USA. 2017;114:E2016–E2025. doi: 10.1073/pnas.1619316114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand N., Nikolova N., Correa C., Brændholt M., Stuckert A., Kildahl N., Vejlø M., Fardo F., Allen M. The heart rate discrimination task: a psychophysical method to estimate the accuracy and precision of interoceptive beliefs. Biol. Psychol. 2022;168 doi: 10.1016/j.biopsycho.2021.108239. [DOI] [PubMed] [Google Scholar]
- Madsen J., Parra L.C. Cognitive processing of a common stimulus synchronizes brains, hearts, and eyes. PNAS Nexus. 2022;1:pgac020. doi: 10.1093/pnasnexus/pgac020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A.C., Gentsch A., Schütz-Bosbach S. Interoceptive cardiac expectations to emotional stimuli predict visual perception. Emotion. 2020;20:1113–1126. doi: 10.1037/emo0000631. [DOI] [PubMed] [Google Scholar]
- Marshall A.C., Gentsch-Ebrahimzadeh A., Schütz-Bosbach S. From the inside out: interoceptive feedback facilitates the integration of visceral signals for efficient sensory processing. Neuroimage. 2022;251 doi: 10.1016/j.neuroimage.2022.119011. [DOI] [PubMed] [Google Scholar]
- Motyka P., Grund M., Forschack N., Al E., Villringer A., Gaebler M. Interactions between cardiac activity and conscious somatosensory perception. Psychophysiology. 2019;56 doi: 10.1111/psyp.13424. [DOI] [PubMed] [Google Scholar]
- Munn B.R., Müller E.J., Wainstein G., Shine J.M. The ascending arousal system shapes neural dynamics to mediate awareness of cognitive states. Nat. Commun. 2021;12:6016. doi: 10.1038/s41467-021-26268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J., Catmur C., Bird G. Alexithymia is associated with a multidomain, multidimensional failure of interoception: evidence from novel tests. J. Exp. Psychol. Gen. 2018;147:398–408. doi: 10.1037/xge0000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieder A. Consciousness without cortex. Current Opinion in Neurobiology, Evolution of Brains and Computation. 2021;71:69–76. doi: 10.1016/j.conb.2021.09.010. [DOI] [PubMed] [Google Scholar]
- Nikolova N., Waade P.T., Friston K.J., Allen M. What might interoceptive inference reveal about consciousness? Rev.Phil.Psych. 2021 doi: 10.1007/s13164-021-00580-3. [DOI] [Google Scholar]
- Ohl S., Wohltat C., Kliegl R., Pollatos O., Engbert R. Microsaccades are coupled to heartbeat. J. Neurosci. 2016;36:1237–1241. doi: 10.1523/JNEUROSCI.2211-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A., Wiens S. Handbook of Affective Sciences, Series in Affective Science. Oxford University Press; New York, NY, US: 2003. On the automaticity of autonomic responses in emotion: an evolutionary perspective; pp. 256–275. [Google Scholar]
- Oizumi M., Albantakis L., Tononi G. From the phenomenology to the mechanisms of consciousness: integrated Information Theory 3.0. PLoS Comput. Biol. 2014;10 doi: 10.1371/journal.pcbi.1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarzabal E.A., Hsu L.-M., Das M., Chao T.-H.H., Zhou J., Song S., Zhang W., Smith K.G., Sciolino N.R., Evsyukova I.Y., Yuan H., Lee S.-H., Cui G., Jensen P., Shih Y.-Y.I. Chemogenetic stimulation of tonic locus coeruleus activity strengthens the default mode network. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abm9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott E.F., Amole M.C., Aue T., Balconi M., Bylsma L.M., Critchley H., Demaree H.A., Friedman B.H., Gooding A.E.K., Gosseries O., Jovanovic T., Kirby L.A.J., Kozlowska K., Laureys S., Lowe L., Magee K., Marin M.-F., Merner A.R., Robinson J.L., Smith R.C., Spangler D.P., Van Overveld M., VanElzakker M.B. Physiological feelings. Neurosci. Biobehav. Rev. 2019;103:267–304. doi: 10.1016/j.neubiorev.2019.05.002. [DOI] [PubMed] [Google Scholar]
- Palser E.R., Glass J., Fotopoulou A., Kilner J.M. Relationship between cardiac cycle and the timing of actions during action execution and observation. Cognition. 2021;217 doi: 10.1016/j.cognition.2021.104907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.-D., Bernasconi F., Bello-Ruiz J., Pfeiffer C., Salomon R., Blanke O. Transient modulations of neural responses to heartbeats covary with bodily self-consciousness. J. Neurosci. 2016;36:8453–8460. doi: 10.1523/JNEUROSCI.0311-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.-D., Blanke O. Coupling inner and outer body for self-consciousness. Trends Cognit. Sci. 2019;23:377–388. doi: 10.1016/j.tics.2019.02.002. [DOI] [PubMed] [Google Scholar]
- Park H.-D., Blanke O. Heartbeat-evoked cortical responses: underlying mechanisms, functional roles, and methodological considerations. Neuroimage. 2019;197:502–511. doi: 10.1016/j.neuroimage.2019.04.081. [DOI] [PubMed] [Google Scholar]
- Park H.-D., Correia S., Ducorps A., Tallon-Baudry C. Spontaneous fluctuations in neural responses to heartbeats predict visual detection. Nat. Neurosci. 2014;17:612–618. doi: 10.1038/nn.3671. [DOI] [PubMed] [Google Scholar]
- Park H.-D., Tallon-Baudry C. The neural subjective frame: from bodily signals to perceptual consciousness. Phil. Trans. R. Soc. B. 2014;369 doi: 10.1098/rstb.2013.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez P., Madsen J., Banellis L., Türker B., Raimondo F., Perlbarg V., Valente M., Niérat M.-C., Puybasset L., Naccache L., Similowski T., Cruse D., Parra L.C., Sitt J.D. Conscious processing of narrative stimuli synchronizes heart rate between individuals. Cell Rep. 2021;36(11):109692. doi: 10.1016/j.celrep.2021.109692. [DOI] [PubMed] [Google Scholar]
- Petzschner F.H., Garfinkel S.N., Paulus M.P., Koch C., Khalsa S.S. Computational models of interoception and body regulation. Trend Neurosci. Special Iss.: Neurosci. Interocep. 2021;44:63–76. doi: 10.1016/j.tins.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzschner F.H., Weber L.A., Wellstein K.V., Paolini G., Do C.T., Stephan K.E. Focus of attention modulates the heartbeat evoked potential. Neuroimage. 2019;186:595–606. doi: 10.1016/j.neuroimage.2018.11.037. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C., Lucia M.D. Cardio-audio synchronization drives neural surprise response. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G., Blinowska K.J., Kaminski M., Schwerdtfeger A.R., Rassler B., Schwarz G., Klimesch W. Processing of fMRI-related anxiety and bi-directional information flow between prefrontal cortex and brain stem. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-01710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G., Schwerdtfeger A., Brunner C., Aigner C., Fink D., Brito J., Carmo M.P., Andrade A. Distinction between neural and vascular BOLD oscillations and intertwined heart rate oscillations at 0.1 Hz in the resting state and during movement. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G., Schwerdtfeger A., Seither-Preisler A., Brunner C., Aigner C.S., Calisto J., Gens J., Andrade A. Synchronization of intrinsic 0.1-Hz blood-oxygen-level-dependent oscillations in amygdala and prefrontal cortex in subjects with increased state anxiety. Eur. J. Neurosci. 2018;47:417–426. doi: 10.1111/ejn.13845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O., Schandry R. Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology. 2004;41:476–482. doi: 10.1111/1469-8986.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- Porges S.W. The polyvagal perspective. Biol. Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramme L., Larra M.F., Schächinger H., Frings C. Cardiac cycle time effects on selection efficiency in vision. Psychophysiology. 2016;53:1702–1711. doi: 10.1111/psyp.12728. [DOI] [PubMed] [Google Scholar]
- Pramme L., Larra M.F., Schächinger H., Frings C. Cardiac cycle time effects on mask inhibition. Biol. Psychol. 2014;100:115–121. doi: 10.1016/j.biopsycho.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Quigley K.S., Kanoski S., Grill W.M., Barrett L.F., Tsakiris M. Functions of interoception: from energy regulation to experience of the self. Trend Neurosci. Special Iss.: Neurosci. Interocep. 2021;44:29–38. doi: 10.1016/j.tins.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C.L., Botan V.E., Gould van Praag C.D., Herman A.M., Nyyssönen J.A.K., Watson D.R., Duka T., Garfinkel S.N., Critchley H.D. Response inhibition on the stop signal task improves during cardiac contraction. Sci. Rep. 2018;8:9136. doi: 10.1038/s41598-018-27513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo F., Rohaut B., Demertzi A., Valente M., Engemann D.A., Salti M., Slezak D.F., Naccache L., Sitt J.D. Brain–heart interactions reveal consciousness in noncommunicating patients. Ann. Neurol. 2017;82:578–591. doi: 10.1002/ana.25045. [DOI] [PubMed] [Google Scholar]
- Ramezanian-Panahi M., Abrevaya G., Gagnon-Audet J.-C., Voleti V., Rish I., Dumas G. Generative models of brain dynamics. Front Artificial Intelligence. 2022;5 doi: 10.3389/frai.2022.807406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramstead M.J.D., Seth A.K., Hesp C., Sandved-Smith L., Mago J., Lifshitz M., Pagnoni G., Smith R., Dumas G., Lutz A., Friston K., Constant A. From generative models to generative passages: a computational approach to (neuro) phenomenology. Rev. Phil. Psych. 2022 doi: 10.1007/s13164-021-00604-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q., Marshall A.C., Kaiser J., Schütz-Bosbach S. Response inhibition is disrupted by interoceptive processing at cardiac systole. Biol. Psychol. 2022;170 doi: 10.1016/j.biopsycho.2022.108323. [DOI] [PubMed] [Google Scholar]
- Riganello F., Chatelle C., Schnakers C., Laureys S. Heart Rate Variability as an indicator of nociceptive pain in disorders of consciousness? J. Pain Symptom Manag. 2018;57:47–56. doi: 10.1016/j.jpainsymman.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Riganello F., Larroque S.K., Bahri M.A., Heine L., Martial C., Carrière M., Charland-Verville V., Aubinet C., Vanhaudenhuyse A., Chatelle C., Laureys S., Di Perri C. A heartbeat away from consciousness: heart rate variability entropy can discriminate disorders of consciousness and is correlated with resting-state fMRI brain connectivity of the central autonomic network. Front. Neurol. 2018:9. doi: 10.3389/fneur.2018.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganello F., Larroque S.K., Di Perri C., Prada V., Sannita W.G., Laureys S. Measures of CNS-autonomic interaction and responsiveness in disorder of consciousness. Front. Neurosci. 2019;13:530. doi: 10.3389/fnins.2019.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganello F., Vatrano M., Carozzo S., Russo M., Lucca L.F., Ursino M., Ruggiero V., Cerasa A., Porcaro C. The timecourse of electrophysiological brain–heart interaction in DoC patients. Brain Sci. 2021;11:750. doi: 10.3390/brainsci11060750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safron A. An integrated world modeling theory (IWMT) of consciousness: combining integrated information and global neuronal workspace theories with the free energy principle and active inference framework; toward solving the hard problem and characterizing agentic causation. Front Artificial Intelligence. 2020;3 doi: 10.3389/frai.2020.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone P.C., Legaz A., Sedeño L., Moguilner S., Fraile-Vazquez M., Campo C.G., Fittipaldi S., Yoris A., Miranda M., Birba A., Galiani A., Abrevaya S., Neely A., Caro M.M., Alifano F., Villagra R., Anunziata F., Oliveira M.O. de, Pautassi R.M., Slachevsky A., Serrano C., García A.M., Ibañez A. Interoception primes emotional processing: multimodal evidence from neurodegeneration. J. Neurosci. 2021;41:4276–4292. doi: 10.1523/JNEUROSCI.2578-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone P.C., Sedeño L., Legaz A., Bekinschtein T., Martorell M., Adolfi F., Fraile-Vazquez M., Rodríguez Arriagada N., Favaloro L., Peradejordi M., Absi D.O., García A.M., Favaloro R., Ibáñez A. Dynamic neurocognitive changes in interoception after heart transplant. Brain Communications. 2020;2 doi: 10.1093/braincomms/fcaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R., Ronchi R., Dönz J., Bello-Ruiz J., Herbelin B., Faivre N., Schaller K., Blanke O. Insula mediates heartbeat related effects on visual consciousness. Cortex. 2018;101:87–95. doi: 10.1016/j.cortex.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Salomon R., Ronchi R., Dönz J., Bello-Ruiz J., Herbelin B., Martet R., Faivre N., Schaller K., Blanke O. The insula mediates access to awareness of visual stimuli presented synchronously to the heartbeat. J. Neurosci. 2016;36:5115–5127. doi: 10.1523/JNEUROSCI.4262-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper C.B. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Sarasso S., Casali A.G., Casarotto S., Rosanova M., Sinigaglia C., Massimini M. Consciousness and complexity: a consilience of evidence. Neurosci. Conscious. 2021:niab023. doi: 10.1093/nc/niab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi R., Cerutti S., Lombardi F., Malik M., Huikuri H.V., Peng C.-K., Schmidt G., Yamamoto Y., Reviewers D., Gorenek B., Lip G.Y.H., Grassi G., Kudaiberdieva G., Fisher J.P., Zabel M., Macfadyen R. Advances in heart rate variability signal analysis: joint position statement by the e-cardiology ESC working group and the European heart rhythm association co-endorsed by the asia pacific heart rhythm society. Europace. 2015;17:1341–1353. doi: 10.1093/europace/euv015. [DOI] [PubMed] [Google Scholar]
- Sattin D., Duran D., Visintini S., Schiaffi E., Panzica F., Carozzi C., Rossi Sebastiano D., Visani E., Tobaldini E., Carandina A., Citterio V., Magnani F.G., Cacciatore M., Orena E., Montano N., Caldiroli D., Franceschetti S., Picozzi M., Matilde L. Analyzing the loss and the recovery of consciousness: functional connectivity patterns and changes in heart rate variability during propofol-induced anesthesia. Front. Syst. Neurosci. 2021;15 doi: 10.3389/fnsys.2021.652080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattin D., Leonardi M., Picozzi M. The autonomic nervous system and the brainstem: a fundamental role or the background actors for consciousness generation? Hypothesis, evidence, and future directions for rehabilitation and theoretical approaches. Brain Behave. 2020;10 doi: 10.1002/brb3.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18:483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Schandry R., Sparrer B., Weitkunat R. From the heart to the brain: a study of heartbeat contingent scalp potentials. Int. J. Neurosci. 1986;30:261–275. doi: 10.3109/00207458608985677. [DOI] [PubMed] [Google Scholar]
- Schiecke K., Schumann A., Benninger F., Feucht M., Baer K.-J., Schlattmann P. Brain-heart interactions considering complex physiological data: processing schemes for time-variant, frequency-dependent, topographical and statistical examination of directed interactions by convergent cross mapping. Physiol. Meas. 2019;40 doi: 10.1088/1361-6579/ab5050. [DOI] [PubMed] [Google Scholar]
- Schulz A., Vögele C., Bertsch K., Bernard S., Münch E.E., Hansen G., Naumann E., Schächinger H. Cardiac cycle phases affect auditory-evoked potentials, startle eye blink and pre-motor reaction times in response to acoustic startle stimuli. Int. J. Psychophysiol. 2020;157:70–81. doi: 10.1016/j.ijpsycho.2020.08.005. [DOI] [PubMed] [Google Scholar]
- Sel A., Azevedo R.T., Tsakiris M. Heartfelt self: cardio-visual integration affects self-face recognition and interoceptive cortical processing. Cerebr. Cortex. 2017;27:5144–5155. doi: 10.1093/cercor/bhw296. [DOI] [PubMed] [Google Scholar]
- Seth A.K. Interoceptive inference, emotion, and the embodied self. Trends Cognit. Sci. 2013;17:565–573. doi: 10.1016/j.tics.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Seth A.K., Tsakiris M. Being a beast machine: the somatic basis of selfhood. Trends Cognit. Sci. 2018;22:969–981. doi: 10.1016/j.tics.2018.08.008. [DOI] [PubMed] [Google Scholar]
- Shear J., Varela F.J. Imprint Academic; 1999. The View from within: First-Person Approaches to the Study of Consciousness. [Google Scholar]
- Shinder M.E., Newlands S.D. Sensory convergence in the parieto-insular vestibular cortex. J. Neurophysiol. 2014;111:2445–2464. doi: 10.1152/jn.00731.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J.M. The thalamus integrates the macrosystems of the brain to facilitate complex, adaptive brain network dynamics. Prog. Neurobiol. 2021;199 doi: 10.1016/j.pneurobio.2020.101951. [DOI] [PubMed] [Google Scholar]
- Shine J.M. Neuromodulatory influences on integration and segregation in the brain. Trends Cognit. Sci. 2019;23:572–583. doi: 10.1016/j.tics.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Signorelli C.M., Szczotka J., Prentner R. Explanatory profiles of models of consciousness - towards a systematic classification. Neurosci. Conscious. 2021 doi: 10.1093/nc/niab021. 2021, niab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvani A., Calandra-Buonaura G., Dampney R.A.L., Cortelli P. Brain-heart interactions: physiology and clinical implications. Philos Trans. A Math Phys. Eng. Sci. 2016;374 doi: 10.1098/rsta.2015.0181. [DOI] [PubMed] [Google Scholar]
- Sitt J.D., King J.-R., El Karoui I., Rohaut B., Faugeras F., Gramfort A., Cohen L., Sigman M., Dehaene S., Naccache L. Large Scale Screening of Neural Signatures of Consciousness in Patients in a Vegetative or Minimally Conscious State. Brain. 2014;137(8):2258–2270. doi: 10.1093/brain/awu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skora L.I., Livermore J.J.A., Roelofs K. The functional role of cardiac activity in perception and action. Neurosci. Biobehav. Rev. 2022;137 doi: 10.1016/j.neubiorev.2022.104655. [DOI] [PubMed] [Google Scholar]
- Smith R., Thayer J.F., Khalsa S.S., Lane R.D. The hierarchical basis of neurovisceral integration. Neurosci. Biobehav. Rev. 2017;75:274–296. doi: 10.1016/j.neubiorev.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Suksasilp C., Garfinkel S.N. Towards a comprehensive assessment of interoception in a multi-dimensional framework. Biol. Psychol. 2022;168 doi: 10.1016/j.biopsycho.2022.108262. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Garfinkel S.N., Critchley H.D., Seth A.K. Multisensory integration across exteroceptive and interoceptive domains modulates self-experience in the rubber-hand illusion. Neuropsychologia. 2013;51:2909–2917. doi: 10.1016/j.neuropsychologia.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Taggart P., Critchley H., van Duijvendoden S., Lambiase P.D. Significance of neuro-cardiac control mechanisms governed by higher regions of the brain. Automatic Neurosci. Central Peripheral Nerve Influence. Cardiac Func. Health Dis. 2016;199:54–65. doi: 10.1016/j.autneu.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C., Campana F., Park H.-D., Babo-Rebelo M. The neural monitoring of visceral inputs, rather than attention, accounts for first-person perspective in conscious vision. Cortex. 2018;102:139–149. doi: 10.1016/j.cortex.2017.05.019. [DOI] [PubMed] [Google Scholar]
- Tasserie J., Uhrig L., Sitt J.D., Manasova D., Dupont M., Dehaene S., Jarraya B. Deep brain stimulation of the thalamus restores signatures of consciousness in a nonhuman primate model. Sci. Adv. 2022;8:eabl5547. doi: 10.1126/sciadv.abl5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa Y., Moriguchi Y., Tochizawa S., Umeda S. Interoceptive sensitivity predicts sensitivity to the emotions of others. Cognit. Emot. 2014;28:1435–1448. doi: 10.1080/02699931.2014.888988. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Ahs F., Fredrikson M., Sollers J.J., Wager T.D. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Hansen A.L., Saus-Rose E., Johnsen B.H. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Lane R.D. Vol. 33. 2009. Claude Bernard and the heart–brain connection: further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews, The Inevitable Link between Heart and Behavior: New Insights from Biomedical Research and Implications for Clinical Practice; pp. 81–88. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Lane R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thompson E., Varela F.J. Radical embodiment: neural dynamics and consciousness. Trends Cognit. Sci. 2001;5:418–425. doi: 10.1016/S1364-6613(00)01750-2. [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Critchley H. Interoception beyond homeostasis: affect, cognition and mental health. Phil. Trans. Biol. Sci. 2016;371 doi: 10.1098/rstb.2016.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya N., Wilke M., Frässle S., Lamme V.A.F. No-report paradigms: extracting the true neural correlates of consciousness. Trends Cognit. Sci. 2015;19:757–770. doi: 10.1016/j.tics.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Tumati S., Paulus M.P., Northoff G. Out-of-step: brain-heart desynchronization in anxiety disorders. Mol. Psychiatr. 2021;26:1726–1737. doi: 10.1038/s41380-021-01029-w. [DOI] [PubMed] [Google Scholar]
- Valenza G., Passamonti L., Duggento A., Toschi N., Barbieri R. Uncovering complex central autonomic networks at rest: a functional magnetic resonance imaging study on complex cardiovascular oscillations. J. R. Soc. Interface. 2020;17 doi: 10.1098/rsif.2019.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza G., Sclocco R., Duggento A., Passamonti L., Napadow V., Barbieri R., Toschi N. The central autonomic network at rest: uncovering functional MRI correlates of time-varying autonomic outflow. Neuroimage. 2019;197:383–390. doi: 10.1016/j.neuroimage.2019.04.075. [DOI] [PubMed] [Google Scholar]
- Wainstein G., Müller E.J., Taylor N., Munn B., Shine J.M. The role of the locus coeruleus in shaping adaptive cortical melodies. Trends Cognit. Sci. 2022;26(6):527–538. doi: 10.1016/j.tics.2022.03.006. [DOI] [PubMed] [Google Scholar]
- Wainstein G., Rojas-Líbano D., Medel V., Alnæs D., Kolskår K.K., Endestad T., Laeng B., Ossandon T., Crossley N., Matar E., Shine J.M. The ascending arousal system promotes optimal performance through mesoscale network integration in a visuospatial attentional task. Network Neurosci. 2021;5:890–910. doi: 10.1162/netn_a_00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner N.S., Schweitzer N., Meindl T., Duschek S., Kambeitz J., Schandry R. Interoceptive awareness moderates neural activity during decision-making. Biol. Psychol. 2013;94:498–506. doi: 10.1016/j.biopsycho.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Woo C.-W., Chang L.J., Lindquist M.A., Wager T.D. Building better biomarkers: brain models in translational neuroimaging. Nat. Neurosci. 2017;20:365–377. doi: 10.1038/nn.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamariola G., Maurage P., Luminet O., Corneille O. Interoceptive accuracy scores from the heartbeat counting task are problematic: evidence from simple bivariate correlations. Biol. Psychol. 2018;137:12–17. doi: 10.1016/j.biopsycho.2018.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.