Abstract
Gut microbiota regulates neurotransmission, neurogenesis, neuroinflammation, and neuroendocrine signaling. The aim of the present review is to analyze the literature concerning gut microbiota dysregulation and mood symptoms, with the specific hypothesis that such alterations play a role in the onset of mood disorders. Here, in fact, we review recent research focusing on how gut microbiota dysregulation influences the onset of mood disorders and on possible pathophysiological mechanisms involved in this interaction. We pay specific attention to the relationship between gut microbiota dysregulation and inflammatory state, Th17 differentiation, neuroactive factors, and TRP metabolism. The association between gut microbiota dysregulation and mood disorders is critically analyzed under a clinical point of view, also focusing on the emergence of mood symptoms in the context of medical conditions.
These latter correlations may enable an interdisciplinary perspective in the clinical approach to such symptoms, as well as new treatment strategies, such as nutritional interventions, psychobiotics, antibiotics, as well as fecal microbiota transplantation.
Keywords: Gut microbiota dysregulation, Brain, Inflammation, Mood disorders, Affective symptoms
Graphical abstract
Highlights
-
•
Gut microbiota mediates communication between gut and brain.
-
•
Neuroinflammation contributes to the development of mood disorders.
-
•
Gut microbiota dysregulation influences the onset of mood symptoms through chronic inflammatory processes.
1. Introduction
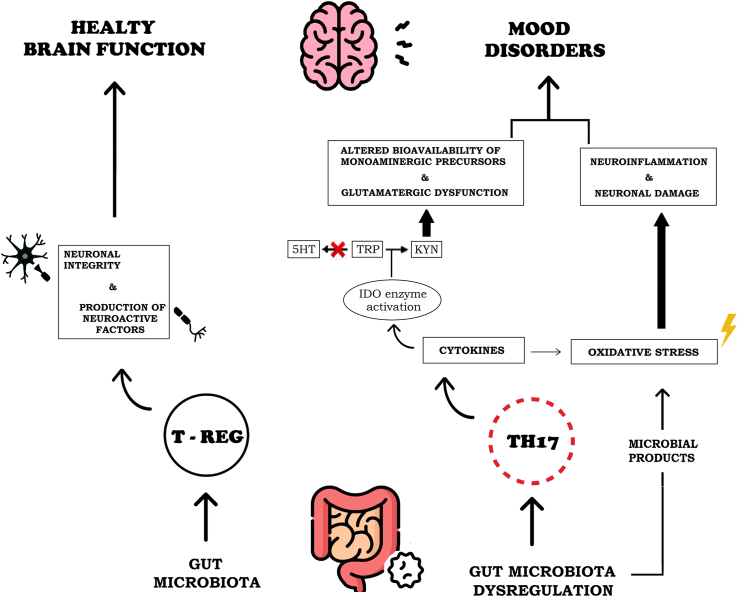
The human lower gastrointestinal tract hosts a high density and diversity of commensal microorganisms. In particular, there are trillions of bacteria that compose the so-called gut microbiota (Strandwitz, 2018), which influences human homeostasis through both the metabolization of nutrients and the production of metabolites, also ensuring the gastrointestinal barrier integrity (Wang and Wang, 2016; Cocchi and Gabrielli, 2019). In line with the literature, we assume that there is a bidirectional communication between the gut and the brain, which is mediated by gut microbiota. This interaction is also referred to as microbiota-gut-brain-axis (Margolis et al., 2021). Particularly, the gut microbiota influences the central nervous system (CNS) by activating the vagus nerve, by producing microbial antigens that recruit inflammatory cells, and by enteroendocrine signaling from epithelial intestinal cells (Wang and Wang, 2016; Cocchi and Gabrielli, 2019). Through these pathways, the gut microbiota regulates neurotransmission, neurogenesis, neuroinflammation, and neuroendocrine signaling, thus playing a role in the modulation of several neuropsychiatric disorders (Cocchi and Gabrielli, 2019). Some experimental studies, in fact, showed that potential alterations in the gut bacterial ecosystem, known as dysbiosis, and the subsequent chronic inflammatory processes, can influence the onset of psychiatric disease such as mood disorders (Konjevod et al., 2021). We define dysbiosis as an interruption of the physiological balance of the intestinal microbiota, both in composition and function (Petra et al., 2015; Levy et al., 2017). This condition may affect gut permeability and the development of low-grade chronic inflammation (Telle-Hansen et al., 2018). The latter mechanisms it can be considered a risk factor for the occurrence of mood disorders, particularly major depressive disorder (MDD) and bipolar disorder (BD) (Zalar et al., 2018; Cocchi and Gabrielli, 2019; Averina et al., 2020; Rutsch et al., 2020). MDD and BD are two impactful psychiatric diseases with an high prevalence worldwide and whose pathophysiology has not been fully understood yet (Malhi and Mann, 2018; Jucevičiute et al., 2019). In particular, BD affects about 1% of the world's population, but the prevalence rises to 2–4% when considering the whole BD spectrum (Grande et al., 2016). According to the World Health Organization, MDD is a significant cause of disability, and more than 300 million people suffer from this disorder worldwide (De Aguiar Neto and Rosa, 2019). Although various treatments are available, approximately one-third of subjects suffering from MDD do not respond to conventional therapies (Miller and Raison, 2016). Moreover, current treatments for subjects with BD are often unsatisfactory for maintaining symptoms control (Benedetti et al., 2020). Several studies have shown that neuroinflammation contributes to the development of MDD e BD (Colpo et al., 2018) (for outline of the possible relationship between mood disorders and gut microbiota dysregulation see Fig. 1).
Fig. 1.
Outline of the possible relationship between mood disorders and gut microbiota dysregulation.
An increase of inflammatory mediators was demonstrated in subjects affected by these conditions (Felger, 2017). Similarly, depressive-like behaviors were induced by administering cytokines to laboratory animals and humans (Raison et al., 2006; Haroon et al., 2012). Moreover, typical symptoms of mood disorders may appear in different medical conditions, especially during inflammatory diseases (Hashmi et al., 2013). Treatment of mood disorders is hampered by the difficulty to identify pathophysiological mechanisms that respond to distinct treatments: inflammatory depression, for example, may present specific clinical correlates and biological underpinnings (Suneson et al., 2021). The relationship between gut microbiota and inflammation, as well as their possible link with the development of neuropsychiatric disorders, has interested a lot of research in recent years (Foster et al., 2021; Simpson et al., 2021; Tremblay et al., 2021). The majority of research focusing on psychiatric conditions investigated the role of inflammation in MDD, paying less attention to other diseases classified within the mood spectrum (Angst and Cassano, 2005). Furthermore, mood symptoms may be present in a number of psychiatric conditions that do not necessarily comply with Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) criteria for mood disorders, as well as in a number of medical diseases, such as neurodegenerative and inflammatory diseases (Menculini et al., 2021). A better understanding of the molecular pathways involved in the onset of mood symptoms and mood disorders may allow to detect more homogeneous subpopulations of subjects suffering from these conditions. This would help to identify new therapeutic targets in the context of precision medicine, thus improving the overall clinical management of such conditions. On the basis of these premises, this review explores the possible role of gut microbiota dysregulation in the pathophysiology of mood disorders, evaluated as a spectrum, and mood symptoms. Particularly, dysbiosis-related neuroinflammatory mechanisms will be considered. We hypothesize that gut microbiota dysregulation can play a role in the onset of several neuropsychiatric disorders, especially mood disorders.
2. Materials and methods
We conducted a literature search of the electronic database MEDLINE/PubMed/Index Medicus, variously combining the following terms: Gut Microbiota; Dysbiosis; Gut Microbiota Dysregulation; Brain; Bowel Disease; Inflammation; Mood Disorders; Affective Symptoms. Two independent investigators (FB e AM) performed the literature search, title/abstract screening and full text screening. The reference list of selected articles underwent further screening in order to search for additional literature. We included in our review original studies reporting data about the possible relationship between gut microbiota dysregulation and inflammatory state, Th17 differentiation, neuroactive factors, and TRP metabolism. Furthermore, we included literature exploring the relationship between gut microbiota dysregulation and mood disorders or mood symptoms, with specific interest in the prevalence and possible clinical impact of this association. Research conducted from any time to September 30, 2021 were considered for inclusion. Articles not presenting original data, letters to the editor, commentaries, case reports were excluded. No language restriction was applied.
3. Results
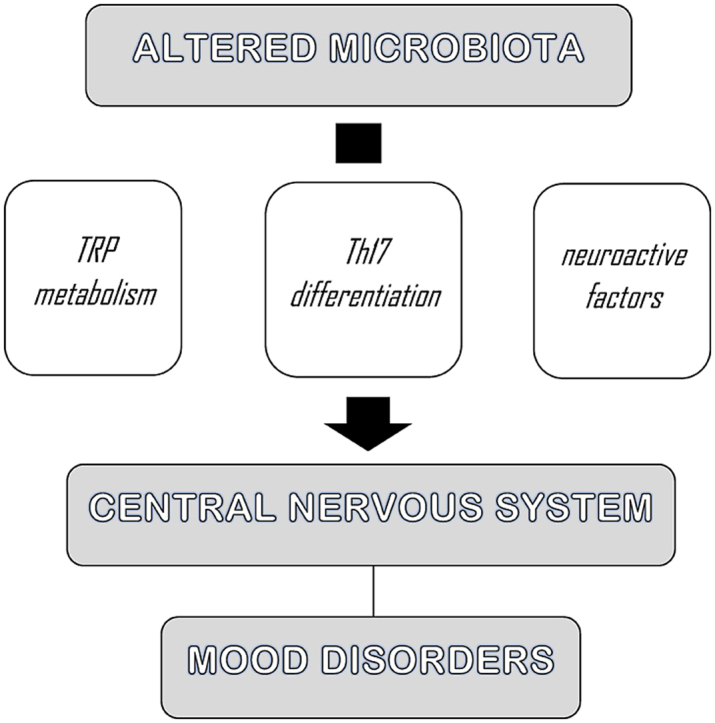
We present data from the literature following a narrative approach. Results will be divided into chapters of interest: Gut microbiota dysregulation and inflammatory state, Gut microbiota dysregulation and Th17 differentiation, Gut microbiota dysregulation and neuroactive factors, Gut microbiota dysregulation and TRP metabolism, Gut microbiota dysregulation and mood disorders, Mood symptoms in medical conditions related to gut microbiota dysregulation.
3.1. Gut microbiota dysregulation and inflammatory state
Mood disorders, as elucidated in several studies, can develop after the chronic exposure to high levels of pro-inflammatory cytokines (Iwata et al., 2013). Cytokines are soluble inflammatory mediators that play an important role in brain development and can promote healthy brain function by supporting neuronal integrity, neurogenesis and synaptic remodeling (Felger and Lotrich, 2013). Moreover, the dysruption of neurotransmitter systems that may be caused by cytokines plays a relevant role in the onset of mood disorders (Felger, 2017). To note, cytokines influence brain pathways by: (a) altering the metabolism of neurotransmitters such as serotonin (5HT), dopamine (DA), and glutamate; (b) impacting on glial cells, especially by damaging oligodendrocytes; (c) impacting on neuroendocrine function, particularly inducing glucocorticoid receptor resistance and subsequent overproduction of pro-inflammatory cytokines and (d) impacting on synaptic plasticity through alterations of growth factors like Brain-Derived Neurotrophic Factor (BDNF) (Raison et al., 2006; Haroon et al., 2012; Hashmi et al., 2013). The correlation between mood disorders and inflammation was based on the following data emerging from the literature: (1) “organic” inflammatory diseases are associated with higher rates of MDD or BD (Hashmi et al., 2013; Jucevičiute et al., 2019); (2) subjects treated with cytokines display a higher risk of developing depressive symptoms (Capuron et al., 2002; Haroon et al., 2012); (3) mood disorders are associated with increased inflammatory markers and (4) anti-inflammatory drugs showed an antidepressant effect in subjects suffering from mood disorders (Colpo et al., 2018; Ghasemi, 2019). In fact, previous literature demonstrated an increase of cytokines in the peripheral blood and in the cerebrospinal fluid of depressed subjects when compared to controls (Miller and Raison, 2016). Similarly, a correlation between the level of cytokines in the cerebrospinal fluid and severity of depression was demonstrated (Haroon et al., 2012). In addition, past research underlined that subjects suffering from BD exhibit increased levels of pro-inflammatory cytokines and decreased anti-inflammatory T Regulatory (T Reg) lymphocytes during manic phases when compared not only to healthy controls, but also to euthymic BD subjects in (Brambilla et al., 2014). These results indicate that the pro-inflammatory state in BD may exacerbate during manic episodes and the severity of mood episodes could be linked to the levels of pro-inflammatory markers (Barbosa et al., 2014). Therefore, all the biological factors that could trigger and perpetuate inflammation might be implicated in the development of the above mentioned psychiatric disorders (Felger and Lotrich, 2013).
Gut microbiota dysregulation deserves particular attention, since the increase in pro-inflammatory bacteria and a reduction in immunomodulatory ones (Lee, 2018) causes a state of general low-grade inflammation (Galland, 2014; Bander et al., 2020). In physiological conditions, commensal bacteria play an important role in modulating the immune response since they influence pathways driven by Nod Like Receptor family pyrin domain containing 3 (NLRP3) and by inteleukin-1 beta (IL-1β) (Inserra et al., 2018). Moreover, they regulate the development of T lymphocytes, promoting their differentiation into anti-inflammatory T Reg lymphocytes instead of pro-inflammatory T Helper 17 (Th17) lymphocytes (Haroon et al., 2012).
Activation of dendritic cells and other immune cell types in the context of gut microbiota dysregulation leads to the production of pro-inflammatory cytokines such as IL-1β and interleukin-6 (IL-6), which can cause the orphan receptor transcription factor's (ROR) expression and also stimulate differentiation of the naive T cell into the highly pro-inflammatory Th17 cell subset (Capuron and Miller, 2011). Additionally, numerous stimuli in the context of gut microbiota dysregulation, e.g., pro-inflammatory cytokines, microbial products, or oxidative stress, can activate the nuclear factor k chain transcription in B cells (NF-kB) pathway (Serra et al., 2019). This cellular signaling pathway is involved in low-grade intestinal inflammation, because activated NF-kB regulates the expression of genes encoding pro-inflammatory cytokines or enzymes, such as cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) (Surh et al., 2001). Overstimulation of iNOS leads to excessive nitric oxide (NO) production that is involved in neurotoxicity and attenuation of monoamines biosynthesis. It has been hypothesized that dysregulation of the NO signaling pathway may contribute to the onset of mood disorders (Ghasemi, 2019). In particular, levels of nitrate and nitrite were demonstrated to increase in subjects with MDD, and inhibitors of NO signaling exert antidepressant-like effects in various animal models (Ghasemi, 2019). Moreover, some studies indicated that higher plasma levels of NO in MDD subjects were normalized after treatment with some antidepressant drugs (Herken et al., 2007).
3.2. Gut microbiota dysregulation and Th17 differentiation
When alterations in the gut microbiota is occur, a chronic inflammatory status is maintained by the preferential differentiation of T cells into a Th17 phenotype (Capuron and Miller, 2011). Additionally, Th17 lymphocytes are strong regulators of brain function, since they impact on multiple cells (including neurons, astrocytes, and microglia) that contribute to neuroinflammation (Beurel and Lowell, 2018). Th17 cells promote demyelinating pathology in the CNS through the induction of oligodendrocyte apoptosis caused by the production of interleukin 17 (IL-17) (Beurel and Lowell, 2018); they are also able to cause neuronal death through intracellular increase of Ca2+ levels (Siffrin et al., 2010). In addition, Th17 cells attract neutrophils and other immune cells to the brain, leading to further recruitment of these same Th17 cells and consequent neuronal damage (Beurel and Lowell, 2018). Th17 cells are also primarily present in the gut but they are not detectable in the blood, so a possible source of peripheral Th17 cells could be the lamina propria of the small intestine (Beurel and Lowell, 2018).
The role of Th17 cells in depression has been recently studied, due to the growing evidence that inflammation may promote depressive symptoms. Indeed, many pro-inflammatory cytokines, including IL-6, tumor necrosis factor alpha (TNFα) and IL-1β that were demonstrated to be altered in the blood of depressed subjects are known to promote Th17 cell differentiation (Beurel and Lowell, 2018).
In addition, antidepressants drugs are able to normalize serum levels of inflammatory cytokines by maintaining the balance between T Reg cells and Th17 cells, confirming that imbalance in T cell differentiation (and the resulting inflammatory state) is one of the determinants of the physiopathology underlying mood disorders (Beurel and Lowell, 2018).
Further studies highlighted that the levels of Th17 cells presents a 3-fold increase in the brains of mice who exhibit depressive behavior (J. Kim et al., 2021), while the blockade of the transcription factor ROR is sufficient to make mice resistant to the induction of depression (Beurel and Lowell, 2018). In addition, the administration of interleukin-17A is able to promote depressive behavior in mice and to increase inflammatory mediators in brain areas associated with depression (e.g. hippocampus and prefrontal cortex), while administration of anti-interleukin-17A antibody leads to a reduction in depression-like symptoms (Nadeem et al., 2017).
3.3. Gut microbiota dysregulation and neuroactive factors
The composition of gut bacterial ecosystem may influence the onset of psychiatric disorders by alterating the bioavailability of monoaminergic precursors and neuroactive factors (Painold et al., 2019). Indeed, the association between neurotransmitters and gut microbiota has been previously studied and it was demonstrated that most of them can be produced by commensal gut bacteria, which are considered for this reason psychobiotics (Strandwitz, 2018).
We define psychobiotics as a group of probiotics that affect CNS functions and behaviors mediated by the gut-brain axis via immune, humoral, neural, and metabolic pathways. Not only may psychobiotics improve the gastrointestinal function, but they also demonstrated antidepressant and anxiolytic properties (Cheng et al., 2019).
Probiotics are living microorganisms that can confer health benefits to the host: they exert their therapeutic effect by reconstituting the gastrointestinal barrier and they are also involved in the modulation of the immune and inflammatory response by promoting the production of regulatory T cells, by inhibiting the production of pro-inflammatory cytokines, and by increasing the expression of anti-inflammatory mediators (Mangiola et al., 2016).
Particularly, Lactobacillus produces acetylcholine and gamma-aminobutyric acid (GABA), Bifidobacterium synthesizes GABA, Escherichia produces norepinephrine (NA), 5-HT, and DA, Streptococcus and Enterococcus synthesize 5-HT, and Bacillus produces DA and NA (Painold et al., 2019). The role of gut bacteria on tryptophan (TRP) metabolism, whose bioavailability is impaired both in subjects with gut microbiota dysregulation and in those with mood disorders, was also emphasized (Jenkins et al., 2016).
3.4. Gut microbiota dysregulation and TRP metabolism
TRP is a precursor of serotonin and other neuroactive metabolites, so its deficiency necessarily influences brain functions and contributes to the onset of behavioral modifications (Lindseth et al., 2015).
The gut microbiota influences three main pathways of TRP metabolism, since it can be converted into 5HT, kynurenine (KYN), or indole (Q. Wang et al., 2015). In the context of dysbiosis, chronic cytokine production causes activation of indoleamine 2,3 dioxygenase (IDO) enzyme which metabolizes TRP into KYN, a substance strongly implicated in inflammatory mechanisms (Grifka-Walk et al., 2021). It can be further metabolized to generate neuroactive compounds such as 3-hydroxykinenine (3-HK), quinolytic acid (QA) or chinurenic acid (Kna). These compounds can also generate free radicals responsible for oxidative stress, while QA is an agonist of the glutamate receptor N-Methyl-D-aspartate (NMDA) and it has been evidenced that the increased activity of this receptor causes excitotoxicity (Vaslin et al., 2007). In contrast, Kna was hypothesized to play a neuroprotective role, as an NMDA receptor antagonist (Agus et al., 2018). Therefore, TRP degradation along the KYN pathway generates compounds that act as NMDA receptor agonists or antagonists, and this may contribute to the alteration of glutamatergic neurotransmission (Dantzer et al., 2008). Moreover, in recent years several studies have shown that the dysregulation of glutamatergic system contributes to the pathophysiology of mood disorders (Henter et al., 2021).
3.5. Gut microbiota dysregulation and mood disorders
As already elucidated, changes in the gut microbiota composition can be associated with alterations in CNS functions (Forsythe et al., 2010). Some studies showed that gut bacterial ecosystem of subjects suffering from psychiatric disorders is different from those of healthy controls (Evans et al., 2017; Bear et al., 2020). Evans et al. (2017), compared the gut microbiota of subjects diagnosed with BD to those of healthy controls, observing a depletion of Faecalibacterium in BD subjects; in particular, this bacterial genus is a recognized suppressor of the inflammatory response and lower levels of this microorganism were also observed in depressed subjects (Inserra et al., 2018). Human studies demonstrated the lower abundance of anti-inflammatory bacteria, such as Lactobacillus and Bifidobacterium, in subjects with MDD (Amirkhanzadeh Barandouzi et al., 2020). Moreover, Bifidobacterium, a species that is associated with suppression of inflammatory pathways through inhibition of NF-Kb, was also found to be reduced in stressed mice (Inserra et al., 2018). In animal models, Lactobacillus and Bifidobacterium can decrease the severity of depressive symptoms, also exerting a positive impact on memory, learning and cognition (Serra et al., 2019). On the other side, one study showed that Lactobacillus was seen increased in stressed mice and BD subjects with high IL-6 levels, but findings should be critically revised after considering the study limitations (Painold et al., 2019). Inserra et al. (2018) have shown that proteobacteria are increased in subjects suffering from MDD. Particularly, this has been demonstrated for Proteus mirabilis, which triggers NLRP3 activation and IL-1β production has seen to be increased in MDD subjects and for proteobacteria components alone (such as lipopolysaccharide of Pseudomonas that can trigger depressive symptoms through inflammasome activation) (In et al., 2018). In addition, Bacteroidetes are prevalent in subjects suffering from depression, particularly the genera Parabacteroides and Alistipes that metabolize TRP and that alter its bioavailability with consequences on serotonergic balances (Inserra et al., 2018). It has been shown that the gut microbiota influences immune function and that rates of MDD and BD could be limited through correction of immunological imbalances (Subramaniapillai et al., 2017). Therefore, the regulation of dysbiosis and exploitation of the gut microbiota's ability to induce tolerance through T Reg cells could prevent and treat mood disorders (Capuron and Miller, 2011).
3.6. Mood symptoms in medical conditions related to gut microbiota dysregulation
The diseases that underpin inflammatory processes as putative pathophysiological mechanisms are characterized by an increased risk of developing a comorbid mood disorder (Ghasemi, 2019). Indeed, subjects with autoimmune and inflammatory diseases may present different psychopathological manifestations (such as mood symptoms). At the same time, subjects suffering from MDD or BD have a higher incidence of these medical conditions, thus suggesting common pathophysiology (Iwata et al., 2013; Barbosa et al., 2014), which is potentially related to alterations in the gut microbiota. There is a strong relationship between alterations in the gut microbiota composition and the onset of inflammatory diseases (Lee, 2018). In subjects suffering from rheumatoid arthritis (RA), psoriasis, multiple sclerosis (MS), and inflammatory bowel disease (IBD) the gut microbiota composition differs from healthy controls, and evidence suggests that the lack of balance between Th17 and Treg in favor of the first could be the etiopathogenetic mechanism that drives the development of these autoimmune diseases (Horta-Baas et al., 2017; Lee, 2018). In addition, the gut microbiota influences brain function through the gut-brain axis: it regulates the development and function of microglia and astrocytes, which mediate neurophysiological processes including neural development, neurotransmission, neuroinflammation, and blood-brain barrier integrity, also contributing to the pathogenesis and resolution of CNS lesions (Fung et al., 2017). In most of the major neurodegenerative diseases, such as Alzheimer's disease (AD), Parkinson's disease (PD) and Huntington's disease (HD) an alteration in the composition of the gut microbiota has been demonstrated (Wasser et al., 2020). For example, Keshavarzian et al. (2015) showed that proinflammatory Proteobacteria were significantly more abundant in mucosa of PD subjects than controls. On the other side, anti-inflammatory bacteria from the genus Faecalibacterium were significantly more abundant in the mucosa of controls than PD subjects (Keshavarzian et al., 2015). Moreover, differences in bacterial abundance including increased Bacteroidetes, and decreased Bifidobacterium were showed in the microbiome of AD subjects (Vogt et al., 2017). In these neurological conditions, psychiatric, and particularly mood symptoms, occur with a high prevalence (Galts et al., 2019; Menculini et al., 2021). Thus, the occurrence of gut microbiota alterations both in inflammatory and neurodegenerative diseases, as well as in subjects with mood symptoms, could explain the high comorbidity between these medical conditions and psychiatric disorders. However, it is still difficult to identify the molecular mechanisms underlying the onset of mood symptoms in the context of these medical illness. Further research on medical conditions in which mood symptoms occur may add knowledge about the pathophysiology of mood disorders and help tailoring appropriate treatment strategies for these conditions.
4. Discussion
In this narrative review, we first examined relationships between gut microbiota dysregulation and inflammation, also analyzing the effects of these disruption on Th17 regulation. We then took into account the link between gut microbiota dysregulation and neuroactive factors, among which the most important seem to be those with the metabolism of tryptophan. Moreover, we reported evidence on the relationship between gut microbiota dysregulation and mood disorders, as well as with mood symptoms occurring in the context of medical diseases. In all the considered aspects, the link between inflammation (expressed in the form of dysbiosis, prevalence of T lymphocytes, and metabolism of neurotransmitters) and mood disorders appears to be narrow.
On these bases, therapeutic options should be oriented towards the underlying biological mechanisms, in order to increase precise and evidence-based treatments (Oakley-Browne, 2001). Multiple treatments targeting the gut microbiota are under investigation. Indeed, if the bacterial species involved in gut microbiota dysregulation underlying the emergence of psychiatric symptoms were adequately identified, their overabundance could be therapeutically modulated through nutritional interventions, use of complete psychobiotics with all their metabolites (probiotics and prebiotics), pharmacological inhibition of specific inflammatory mediators, use of antibiotics, and fecal microbiota transplantation (Inserra et al., 2018). The main purpose of these therapeutic strategies is to regulate the silent inflammatory process that originates in the gut and to restore immunomodulating immune defenses (Cocchi and Gabrielli, 2019).
However, it should be emphasized that inflammation contributes to the pathophysiology of some, but not all cases of mood disorders and that some studies have failed to find an association between these conditions and inflammatory processes or have found opposite correlations for different pro-inflammatory mediators (Raison et al., 2006). Moreover, it is not possible to identify the link between specific behavioral symptoms and the over-production of specific cytokines yet, as well as the action of inflammatory mediators in a well-defined area of the CNS. In addition, some studies have shown that CNS activity under stressful conditions can affect the composition of the gut microbiota by altering the growth of bacterial species and the production of microbial compounds, so gut microbiota dysregulation could be a consequence and not necessarily a cause of mood disorders (Galland, 2014). Therefore, we need to take in consideration the limitations of our review, since findings from the included studies could be due to incidental and indirect effects and not to a causality link. In example, in animal models (Beurel et al., 2013) mood symptoms could depend also on other factors, such as the overall physical condition linked to the presence of a digestive syndrome, or stress-induced behaviors like learned helplessness (Ménard et al., 2016). Although there are many mice models of depression, the periods of time or stressors used for creating such models were limited, so for example in some cases (Kim et al., 2021) a new model for mouse depression was created to improve assessment of rodent depression and make it more representative of human behaviors. Moreover, most animal models of depression were developed using male rodents and were later applied to their female counterparts. Consequently, some models could be less appropriate to study human female depression (Ménard et al., 2016). In addition, limitations of this review also include the small sample size of considered studies and the heterogeneity of the types of articles included (e.g., clinical studies of varying size, case reports, clinical evidence). Despite neurobiological mechanisms linking pro-inflammatory cytokines and mood disorders have been not fully characterized, some findings based on animal models are consistent with human studies, which demonstrate the link between depression and NFκB signaling (Miklowitz and Chung, 2016; Caviedes et al., 2017). Subsequently, the role of dysbiosis-related inflammation in the etiopathogenesis of mood disorders still remains a subject of study (Dantzer et al., 2008; Bauer and Teixeira, 2019). Moreover, the heterogeneity of mood disorders may partially be ascribed to the symptom-based diagnostic approach operated by the DSM-5. This categorical approach puts different heterogeneous populations into the same category and ignores the different pathophysiological pathways underlying psychiatric symptoms (Sakamoto et al., 2021). To overcome these limitations, a dimensional approach that considers an objective biological measure using body fluids biomarkers, genetic aspects, or multimodal imaging may be useful to detect a more homogeneous subpopulation of subjects suffering from mood disorders or mood symptoms. This approach may allow us to provide individualized treatment in the perspective of precision medicine. Over the last few decades, the development of precision medicine in the field of psychiatry helped focusing on tailored treatments that fit the individual, physiological, and genetic profile of subjects (König et al., 2017; Perna et al., 2020). Compared to other medical disciplines, several goals should still be reached in precision psychiatry. There are various possible causes for this evidence including inaccurate diagnostic criteria, incomplete understanding of the involved molecular pathology, absence of available clinical tools and, finally, individual characteristics (Perna et al., 2020). There are several reasons to consider the role of gut microbiota dysregulation in the development of mood disorders under the perspective of precision psychiatry: (1) to identify biomarkers related to biological differences that allow us to categorize clinical populations and improve the ability to provide individuals the most appropriate treatment, (2) to identify at-risk individuals in order to develop early intervention strategies, (3) to provide novel targets for drug development, and (4) to facilitate the expansion and the new development of microbiome-targeted therapies including, but not limited to, diet, prebiotics, and probiotics (Foster, 2020). Further research is needed to elucidate the role of gut bacterial composition in the onset of mood disorders, as well as to identify predictors of treatment response and to understand how therapeutic strategies targeting the gut microbiota may impact on the gut-brain axis in subjects suffering from BD and MDD (Inserra et al., 2018; Painold et al., 2019). Therefore, it is still necessary to promote further studies in order to identify the molecular mechanisms at the origin of the link between inflammation and mood disorders, to study the neurobiological mechanisms underlying the behavioral effects induced by cytokines, and to identify personal microbiome's profile and understand the role of basic nutritional and inflammatory status in the onset of mood disorders (Maes et al., 2009). This could help discover new biomarkers of inflammatory and neurodegenerative processes, in order to identify at-risk subjects and to predict response to treatment (Felger and Lotrich, 2013; Mangiola et al., 2016; Inserra et al., 2018).
5. Conclusion
Our hypothesis that gut microbiota dysregulation can play a role in the onset of several neuropsychiatric disorders, especially mood disorders, is supported by recent research in this field. The gut microbiota and its alterations would seem to play a relevant role in the development of psychiatric disorders, especially mood disorders. Therefore, our hypothesis could be a guide for further experimental studies. Animal models do not perfectly reproduce natural progression of human disorders and therefore it is crucial to increase the number of studies based on humans. Current literature on the subject is still emerging, focusing on both molecular etiopathogenesis and its clinical correlates. These growing evidences could increase knowledge on neurobiology, but could also be relevant in a precision psychiatry perspective to improve intervention strategies.
CRediT authorship contribution statement
Agnese Minuti: Writing – review & editing, Writing – original draft, Investigation, Data curation. Francesca Brufani: Writing – review & editing, Writing – original draft, Investigation, Data curation. Giulia Menculini: Methodology, Validation, Formal analysis. Patrizia Moretti: Supervision, Conceptualization. Alfonso Tortorella: Project administration, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Hips which may be considered as potential competing interests.
Acknowledgements
None.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crneur.2022.100044.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Agus A., Planchais J., Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018 doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Amirkhanzadeh Barandouzi Z., Starkweather A.R., Henderson W.A., Gyamfi A., Cong X.S. Altered composition of gut microbiota in depression: a systematic review. Front. Psychiatr. 2020;11 doi: 10.3389/fpsyt.2020.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst J., Cassano G. The mood spectrum: improving the diagnosis of bipolar disorder. Bipolar Disord. 2005;7(4) doi: 10.1111/j.1399-5618.2005.00210.x. Supplement. [DOI] [PubMed] [Google Scholar]
- Averina O.V., Zorkina Y.A., Yunes R.A., Kovtun A.S., Ushakova V.M., Morozova A.Y., Kostyuk G.P., Danilenko V.N., Chekhonin V.P. Bacterial metabolites of human gut microbiota correlating with depression. Int. J. Mol. Sci. 2020 doi: 10.3390/ijms21239234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bander Z. Al, Nitert M.D., Mousa A., Naderpoor N. International Journal of Environmental Research and Public Health. 2020. The gut microbiota and inflammation: an overview. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa I.G., Machado-Vieira R., Soares J.C., Teixeira A.L. 2014. The Immunology of Bipolar Disorder. NeuroImmunoModulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M.E., Teixeira A.L. Inflammation in psychiatric disorders: what comes first? Ann. N. Y. Acad. Sci. 2019;1437(Issue 1) doi: 10.1111/nyas.13712. [DOI] [PubMed] [Google Scholar]
- Bear T.L.K., Dalziel J.E., Coad J., Roy N.C., Butts C.A., Gopal P.K. Advances in Nutrition. 2020. The role of the gut microbiota in dietary interventions for depression and anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Aggio V., Pratesi M.L., Greco G., Furlan R. Frontiers in Psychiatry. 2020. Neuroinflammation in bipolar depression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E., Harrington L.E., Jope R.S. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol. Psychiatr. 2013;73(7) doi: 10.1016/j.biopsych.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E., Lowell J.A. Brain, Behavior, and Immunity. 2018. Th17 cells in depression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P., Bellani M., Isola M., Bergami A., Marinelli V., Dusi N., Rambaldelli G., Tansella M., Maria Finardi A., Martino G., Perlini C., Furlan R. Increased M1/decreased M2 signature and signs of Th1/Th2 shift in chronic patients with bipolar disorder, but not in those with schizophrenia. Transl. Psychiatry. 2014;4 doi: 10.1038/tp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L., Hauser P., Hinze-Selch D., Miller A.H., Neveu P.J. Treatment of cytokine-induced depression. Brain Behav. Immun. 2002;16(5) doi: 10.1016/S0889-1591(02)00007-7. [DOI] [PubMed] [Google Scholar]
- Capuron L., Miller A.H. Pharmacology and Therapeutics. 2011. Immune system to brain signaling: neuropsychopharmacological implications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviedes A., Lafourcade C., Soto C., Wyneken U. BDNF/NF-κB signaling in the neurobiology of depression. Curr. Pharmaceut. Des. 2017;23(21) doi: 10.2174/1381612823666170111141915. [DOI] [PubMed] [Google Scholar]
- Cheng L.H., Liu Y.W., Wu C.C., Wang S., Tsai Y.C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019;27(Issue 3) doi: 10.1016/j.jfda.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi M., Gabrielli F. Italian Society of Experimental Biology; 2019. Brain & Anxiety, Microbiota e Bacterial metabolites. [Google Scholar]
- Colpo G.D., Leboyer M., Dantzer R., Trivedi M.H., Teixeira A.L. Immune-based strategies for mood disorders: facts and challenges. Expert Rev. Neurother. 2018;18(Issue 2) doi: 10.1080/14737175.2018.1407242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. Nature Reviews Neuroscience. 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Aguiar Neto F.S., Rosa J.L.G. Depression biomarkers using non-invasive EEG: a review. Neurosci. Biobehav. Rev. 2019;105 doi: 10.1016/j.neubiorev.2019.07.021. [DOI] [PubMed] [Google Scholar]
- Evans S.J., Bassis C.M., Hein R., Assari S., Flowers S.A., Kelly M.B., Young V.B., Ellingrod V.E., McInnis M.G. The gut microbiome composition associates with bipolar disorder and illness severity. J. Psychiatr. Res. 2017 doi: 10.1016/j.jpsychires.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C. Imaging the role of inflammation in mood and anxiety-related disorders. Curr. Neuropharmacol. 2017;15 doi: 10.2174/1570159x15666171123201142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Lotrich F.E. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. 2013. Neuroscience. [DOI] [PMC free article] [PubMed]
- Forsythe P., Sudo N., Dinan T., Taylor V.H., Bienenstock J. Brain, Behavior, and Immunity. 2010. Mood and gut feelings. [DOI] [PubMed] [Google Scholar]
- Foster J.A. Decoding microbiome research for clinical psychiatry. Can. J. Psychiatr. 2020;65(Issue 1) doi: 10.1177/0706743719890725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J.A., Baker G.B., Dursun S.M. The relationship between the gut microbiome-immune system-brain Axis and major depressive disorder. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.721126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.C., Olson C.A., Hsiao E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017 doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland L. The gut microbiome and the brain. J. Med. Food. 2014;17(12) doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galts C.P.C., Bettio L.E.B., Jewett D.C., Yang C.C., Brocardo P.S., Rodrigues A.L.S., Thacker J.S., Gil-Mohapel J. Depression in neurodegenerative diseases: common mechanisms and current treatment options. Neurosci. Biobehav. Rev. 2019;102 doi: 10.1016/j.neubiorev.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Ghasemi M. Nitric oxide: antidepressant mechanisms and inflammation. Adv. Pharmacol. 2019;86 doi: 10.1016/bs.apha.2019.04.004. [DOI] [PubMed] [Google Scholar]
- Grande I., Berk M., Birmaher B., Vieta E. Bipolar disorder. Lancet. 2016;387(10027) doi: 10.1016/S0140-6736(15)00241-X. [DOI] [PubMed] [Google Scholar]
- Grifka-Walk H.M., Jenkins B.R., Kominsky D.J. Amino acid Trp: the far out impacts of host and commensal tryptophan metabolism. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.653208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E., Raison C.L., Miller A.H. Neuropsychopharmacology. 2012. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi A.M., Butt Z., Umair M. Journal of the Pakistan Medical Association. 2013. Is depression an inflammatory condition? A review of available evidence. [PubMed] [Google Scholar]
- Henter I.D., Park L.T., Zarate C.A. Novel glutamatergic modulators for the treatment of mood disorders: current status. CNS Drugs. 2021;35(Issue 5) doi: 10.1007/s40263-021-00816-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herken H., Gurel A., Selek S., Armutcu F., Ozen M.E., Bulut M., Kap O., Yumru M., Savas H.A., Akyol O. Adenosine deaminase, nitric oxide, superoxide dismutase, and Xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch. Med. Res. 2007;38(2) doi: 10.1016/j.arcmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Horta-Baas G., Romero-Figueroa M.D.S., Montiel-Jarquín A.J., Pizano-Zárate M.L., García-Mena J., Ramírez-Durán N. Journal of Immunology Research. 2017. Intestinal dysbiosis and rheumatoid arthritis: a link between gut microbiota and the pathogenesis of rheumatoid arthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inserra A., Rogers G.B., Licinio J., Wong M.L. BioEssays. 2018. The microbiota-inflammasome hypothesis of major depression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M., Ota K.T., Duman R.S. 2013. The Inflammasome: Pathways Linking Psychological Stress, Depression, and Systemic Illnesses. Brain, Behavior, and Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins T.A., Nguyen J.C.D., Polglaze K.E., Bertrand P.P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016 doi: 10.3390/nu8010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucevičiute N., Žilaitiene B., Aniuliene R., Vanagiene V. The link between thyroid autoimmunity, depression and bipolar disorder. Open Med. 2019;14(Issue 1):52–58. doi: 10.1515/med-2019-0008. De Gruyter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A., Green S.J., Engen P.A., Voigt R.M., Naqib A., Forsyth C.B., Mutlu E., Shannon K.M. Colonic bacterial composition in Parkinson's disease. Mov. Disord. 2015;30(10) doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- Kim J., Suh Y.H., Chang K.A. Interleukin-17 induced by cumulative mild stress promoted depression-like behaviors in young adult mice. Mol. Brain. 2021;14(1) doi: 10.1186/s13041-020-00726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König I.R., Fuchs O., Hansen G., von Mutius E., Kopp M.V. What is precision medicine? Eur. Respir. J. 2017;50(Issue 4) doi: 10.1183/13993003.00391-2017. [DOI] [PubMed] [Google Scholar]
- Konjevod M., Nikolac Perkovic M., Sáiz J., Svob Strac D., Barbas C., Rojo D. Metabolomics analysis of microbiota-gut-brain axis in neurodegenerative and psychiatric diseases. J. Pharmaceut. Biomed. Anal. 2021;194 doi: 10.1016/j.jpba.2020.113681. [DOI] [PubMed] [Google Scholar]
- Lee G.R. International Journal of Molecular Sciences. 2018. The balance of th17 versus treg cells in autoimmunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M., Kolodziejczyk A.A., Thaiss C.A., Elinav E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017;17(Issue 4) doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- Lindseth G., Helland B., Caspers J. Archives of Psychiatric Nursing; 2015. The Effects of Dietary Tryptophan on Affective Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Yirmyia R., Noraberg J., Brene S., Hibbeln J., Perini G., Kubera M., Bob P., Lerer B., Maj M. Metabolic Brain Disease. 2009. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. [DOI] [PubMed] [Google Scholar]
- Malhi G.S., Mann J.J. Depression. Lancet. 2018;392(10161):2299–2312. doi: 10.1016/S0140-6736(18)31948-2. Lancet Publishing Group. [DOI] [PubMed] [Google Scholar]
- Mangiola F., Ianiro G., Franceschi F., Fagiuoli S., Gasbarrini G., Gasbarrini A. World Journal of Gastroenterology. 2016. Gut microbiota in autism and mood disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis K.G., Cryan J.F., Mayer E.A. The microbiota-gut-brain Axis: from motility to mood. Gastroenterology. 2021;160(Issue 5) doi: 10.1053/j.gastro.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menculini G., Chipi E., Paoletti F.P., Gaetani L., Nigro P., Simoni S., Mancini A., Tambasco N., di Filippo M., Tortorella A., Parnetti L. Insights into the pathophysiology of psychiatric symptoms in central nervous system disorders: implications for early and differential diagnosis. Int. J. Mol. Sci. 2021;22(Issue 9) doi: 10.3390/ijms22094440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz D.J., Chung B. Family-focused Therapy for bipolar disorder: reflections on 30 Years of research. Fam. Process. 2016;55(3) doi: 10.1111/famp.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. Nature Reviews Immunology. 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard C., Hodes G.E., Russo S.J. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321 doi: 10.1016/j.neuroscience.2015.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem A., Ahmad S.F., Al-Harbi N.O., Fardan A.S., El-Sherbeeny A.M., Ibrahim K.E., Attia S.M. 2017. IL-17A Causes Depression-like Symptoms via NFκB and p38MAPK Signaling Pathways in Mice: Implications for Psoriasis Associated Depression. Cytokine. [DOI] [PubMed] [Google Scholar]
- Oakley-Browne M.A. EBM in practice: Psychiatry. Med. J. Aust. 2001;174(Issue 8) doi: 10.5694/j.1326-5377.2001.tb143344.x. [DOI] [PubMed] [Google Scholar]
- Painold A., Mörkl S., Kashofer K., Halwachs B., Dalkner N., Bengesser S., Birner A., Fellendorf F., Platzer M., Queissner R., Schütze G., Schwarz M.J., Moll N., Holzer P., Holl A.K., Kapfhammer H.P., Gorkiewicz G., Reininghaus E.Z. A step ahead: exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019 doi: 10.1111/bdi.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna G., Alciati A., Daccò S., Grassi M., Caldirola D. Personalized psychiatry and depression: the role of sociodemographic and clinical variables. Psychiatry Investigation. 2020;17(Issue 3) doi: 10.30773/pi.2019.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petra A.I., Panagiotidou S., Hatziagelaki E., Stewart J.M., Conti P., Theoharides T.C. Gut-microbiota-brain Axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin. Therapeut. 2015;37(Issue 5) doi: 10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Capuron L., Miller A.H. Trends in Immunology. 2006. Cytokines sing the blues: inflammation and the pathogenesis of depression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch A., Kantsjö J.B., Ronchi F. The gut-brain Axis: how microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto S., Zhu X., Hasegawa Y., Karma S., Obayashi M., Alway E., Kamiya A. Inflamed brain: targeting immune changes and inflammation for treatment of depression. Psychiatr. Clin. Neurosci. 2021;75(10) doi: 10.1111/pcn.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra D., Almeida L.M., Dinis T.C.P. The impact of chronic intestinal inflammation on brain disorders: the microbiota-gut-brain Axis. Mol. Neurobiol. 2019;56(Issue 10) doi: 10.1007/s12035-019-1572-8. [DOI] [PubMed] [Google Scholar]
- Siffrin V., Radbruch H., Glumm R., Niesner R., Paterka M., Herz J., Leuenberger T., Lehmann S.M., Luenstedt S., Rinnenthal J.L., Laube G., Luche H., Lehnardt S., Fehling H.J., Griesbeck O., Zipp F. Vivo Imaging of Partially Reversible Th17 Cell-Induced Neuronal Dysfunction in the Course of Encephalomyelitis. Immunity. 2010 doi: 10.1016/j.immuni.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Simpson C.A., Diaz-Arteche C., Eliby D., Schwartz O.S., Simmons J.G., Cowan C.S.M. The gut microbiota in anxiety and depression – a systematic review. Clin. Psychol. Rev. 2021;83 doi: 10.1016/j.cpr.2020.101943. [DOI] [PubMed] [Google Scholar]
- Strandwitz P. Brain Research. 2018. Neurotransmitter modulation by the gut microbiota. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniapillai M., Carmona N.E., Rong C., McIntyre R.S. 2017. Inflammation: Opportunities for Treatment Stratification Among Individuals Diagnosed with Mood Disorders. Dialogues in Clinical Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suneson K., Lindahl J., Hårsmar S.C., Söderberg G., Lindqvist D. Inflammatory depression—mechanisms and non-pharmacological interventions. Int. J. Mol. Sci. 2021;22(Issue 4) doi: 10.3390/ijms22041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh Y.J., Chun K.S., Cha H.H., Han S.S., Keum Y.S., Park K.K., Lee S.S. 2001. Molecular Mechanisms Underlying Chemopreventive Activities of Anti-inflammatory Phytochemicals: Down-Regulation of COX-2 and iNOS through Suppression of NF-Κb Activation. Mutation Research - Fundamental and Molecular Mechanisms of Mutagenesis; pp. 480–481. [DOI] [PubMed] [Google Scholar]
- Telle-Hansen V.H., Holven K.B., Ulven S.M. Impact of a healthy dietary pattern on gut microbiota and systemic inflammation in humans. Nutrients. 2018;10(11) doi: 10.3390/nu10111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay A., Lingrand L., Maillard M., Feuz B., Tompkins T.A. The effects of psychobiotics on the microbiota-gut-brain axis in early-life stress and neuropsychiatric disorders. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2021;105 doi: 10.1016/j.pnpbp.2020.110142. [DOI] [PubMed] [Google Scholar]
- Vaslin A., Puyal J., Borsello T., Clarke P.G.H. Excitotoxicity-related endocytosis in cortical neurons. J. Neurochem. 2007;102(3) doi: 10.1111/j.1471-4159.2007.04564.x. [DOI] [PubMed] [Google Scholar]
- Vogt N.M., Kerby R.L., Dill-McFarland K.A., Harding S.J., Merluzzi A.P., Johnson S.C., Carlsson C.M., Asthana S., Zetterberg H., Blennow K., Bendlin B.B., Rey F.E. Gut microbiome alterations in Alzheimer's disease. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.X., Wang Y.P. Gut microbiota-brain axis. Chinese Med J. 2016;129(19):2373–2380. doi: 10.4103/0366-6999.190667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Liu D., Song P., Zou M.H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Frontiers Bioscience - Landmark. 2015;20(7) doi: 10.2741/4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser C.I., Mercieca E.-C., Kong G., Hannan A.J., McKeown S.J., Glikmann-Johnston Y., Stout J.C. Gut dysbiosis in Huntington's disease: associations among gut microbiota, cognitive performance and clinical outcomes. Brain Communications. 2020;2(2) doi: 10.1093/braincomms/fcaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalar B., Haslberger A., Peterlin B. The role of microbiota in depression - a Brief review. Psychiatr. Danub. 2018 doi: 10.24869/spsih.2018.136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.