Abstract
To initiate invasion of the mosquito midgut, Plasmodium ookinetes secrete chitinolytic activity to penetrate the peritrophic matrix surrounding the blood meal. While ookinetes of the avian malaria parasite Plasmodium gallinaceum appear to secrete products of two chitinase genes, to date only one chitinase gene, PfCHT1, has been identified in the nearly completed Plasmodium falciparum strain 3D7 genome database. To test the hypothesis that the single identified chitinase of P. falciparum is necessary for ookinete invasion, the PfCHT1 gene was disrupted 39 bp upstream of the stop codon. PfCHT1-disrupted parasites had normal gametocytogenesis, exflagellation, and ookinete formation but were markedly impaired in their ability to form oocysts in Anopheles freeborni midguts. Confocal microscopy demonstrated that the truncated PfCHT1 protein was present in mutant ookinetes but that the concentration of mutant PfCHT1 within the apical end of the ookinetes was substantially reduced. These data suggest that full-length PfCHT1 is essential for intracellular trafficking and secretion and that the PfCHT1 gene product is necessary for ookinetes to invade the mosquito midgut.
The Plasmodium ookinete is the developmental stage of the malaria parasite that invades the mosquito midgut. After gametocytes are taken up by a mosquito during ingestion of a blood meal, male and female gametes fuse to form the fertilized zygote. Over the subsequent 15 to 24 h, the round zygote elongates to form the invasive ookinete. The ookinete penetrates the acellular, chitin-containing peritrophic matrix (PM) surrounding the blood meal (18) and then invades the midgut epithelium (10, 25). The ookinete secretes chitinolytic activity that it uses to penetrate the PM (11, 22). The Streptomyces-produced chitinase inhibitor allosamidin prevents Plasmodium ookinetes from traversing the PM, thereby preventing invasion of the mosquito midgut (17). The latter observation suggests that Plasmodium chitinases may be targets for blocking malaria transmission in human populations.
Two Plasmodium chitinase genes have been identified and molecularly cloned: PgCHT1 of the avian malaria parasite Plasmodium gallinaceum (22) and PfCHT1 of the lethal human malaria parasite Plasmodium falciparum (21). Biochemical and antibody data suggest that P. gallinaceum ookinetes secrete the products of at least two chitinase genes (22). Anion-exchange chromatography of P. gallinaceum ookinete extracts has demonstrated at least two distinct chitinase activities with different pH optima, sensitivity to allosamidin, and apparent molecular masses, as detected with antibodies that recognize the conserved active site of chitinases. The Km values of PfCHT1 and PgCHT2 are similar to each other and distinct from that of PgCHT1 (21). A monoclonal antibody developed against recombinant P. falciparum chitinase recognizes the second P. gallinaceum chitinase (provisionally termed PgCHT2 [22]) but not native or recombinant PgCHT1 (R. C. Langer and J. M. Vinetz, unpublished observations). In contrast, only one P. falciparum chitinase gene can be identified in the nearly completed P. falciparum genome database, and low-stringency Southern blot analysis is consistent with a single-copy gene (21). Further, PgCHT1 and PfCHT1 have substantially different primary sequences and domain structures (21). PgCHT1 has a proenzyme domain which can be proteolytically removed by ookinete-produced proteases under in vitro conditions in axenic medium (22) and a carboxy-terminal cysteine-rich putative chitin-binding domain. In contrast, PfCHT1 lacks proenzyme and putative chitin-binding domains and is likely secreted as an already active enzyme (21). The pH optimum, allosamidin sensitivity, and molecular mass of PfCHT1 are similar to those of PgCHT2 (22). Therefore, available evidence indicates a disparate number of chitinase genes between P. falciparum and P. gallinaceum and suggests that PfCHT1 and PgCHT1 are paralogs, not orthologs.
To test whether the single identified P. falciparum chitinase gene, PfCHT1, is necessary for P. falciparum ookinetes to invade the mosquito midgut, this gene was targeted for disruption. The ability of the resulting mutant parasites to invade the mosquito midgut was analyzed. The results presented here are consistent with the presence in P. falciparum of a single chitinase gene whose role is critical for human malaria transmission.
MATERIALS AND METHODS
Construction of a PfCHT1 gene disruption plasmid.
Nucleotides 153 to 1095 of the coding sequence of PfCHT1 were PCR amplified using primers that added NotI and PstI restriction sites to the 5′ and 3′ ends of the PCR product (GCGGCGGCCGCAAAGGAATTATTCAAGGTTATTATC [NotI restriction site is underlined]; GCGCTGCAGCATTATGTGCAGCATTATCAGAAGATAAAGAC [PstI restriction site is underlined]). The partial PfCHT1 sequence was ligated into plasmid pHDWT (6) (Fig. 1A), which contains the human dihydrofolate reductase gene as a selectable marker to generate construct pPfCHT1KO1.
FIG. 1.
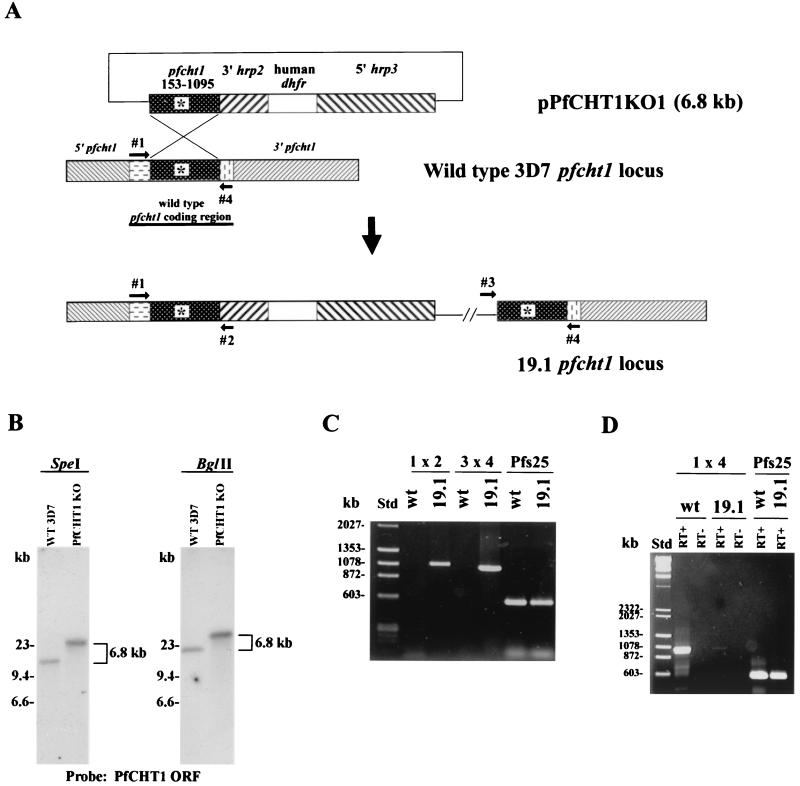
Design and experimental verification of PfCHT1 gene disruption in P. falciparum strain 3D7. (A) A PCR-amplified partial coding sequence corresponding to nucleotides 153 to 1095 of PfCHT1 (stippled box labeled pfcht1) was inserted into plasmid pHDWT, which contains the human dihydrofolate reductase gene as a selectable marker under the control of the 5′ untranslated sequence of Pfhrp3 and the 3′ untranslated sequence of Pfhrp2. The 6.8-kb disruption plasmid is indicated as pPfCHT1KO1. Primers 1 and 4 are from the 5′ and 3′ ends, respectively, of the PfCHT1 coding region not included in the disruption construct. Primer 2 is from the 3′ Pfhrp2 untranslated region, and primer 3 is from the pBluescript plasmid backbone. The wild-type 3D7 PfCHT1 locus on chromosome 12 is diagrammed before and after (labeled as 19.1) the predicted integration event. The asterisk indicates the chitinase enzymatic active site. (B) Southern blot analysis of the PfCHT1 locus in wild-type (WT) 3D7 and mutant 19.1. Genomic DNA was digested with either SpeI or BglII and probed with the digoxigenin-labeled PfCHT1 coding sequence; chemiluminescence was used for development of the blot. ORF, open reading frame. (C) PCR analysis of wild-type 3D7 (wt) and mutant 19.1 with pairs of oligonucleotide primers schematically depicted in panel A. PCR of the Pfs25 gene encoding the 25-kDa P. falciparum zygote-ookinete surface protein was performed as a positive control to demonstrate the presence of amplifiable DNA. Std, molecular size standards. (D) Reverse transcriptase PCR was performed using RNA extracted from wild-type 3D7 or 19.1 gametocytes. RT+ and RT− indicate the presence and absence of reverse transcriptase in the reaction mixture, respectively. Primers to amplify Pfs25 were used as a positive control to demonstrate equivalent amounts of Pfs25 RNA in the wild-type 3D7 and 19.1 RNA samples.
Transfection.
Asexual blood-stage parasites and transfectants of the P. falciparum gametocyte-producing strain 3D7 were cultivated in leukocyte-free human red blood cells in complete medium (RPMI 1640 with l-glutamine [Life Technologies, Gaithersburg, Md.], 50 mg of hypoxanthine/liter, 10 mg of gentamicin/liter, 25 mM HEPES, 0.225% NaHCO3, and 10% heat-inactivated human serum). Transfectants were produced by electroporation of parasites with 100 μg of pHDWT-PfCHT1153-1095 plasmid DNA that had been purified with Maxi-Prep columns (Qiagen, Chatsworth, Calif.) as described previously (6). Briefly, 109 red cells with synchronized ring-stage parasitemia at 5% were transfected in incomplete Cytomix (5) using electroporator settings of 0.31 kV and 960 μF (Gene Pulser II; Bio-Rad, Hercules, Calif.). Drug selection was initiated 48 h after electroporation with 5 nM WR99210 (5) and maintained at that concentration thereafter. The medium was changed daily for the first 8 days after electroporation to removed lysed cells and debris and was then changed every other day until ring-stage parasites were microscopically detected (on day 23). One transformant parasite line, 19.1, was chosen for further study, as it retained the ability to form gametocytes.
Molecular analysis of transformants.
Southern blotting, PCR, and reverse transcriptase PCR were performed according to standard procedures. DNA was extracted from asexual blood-stage parasites of either wild-type 3D7 or the mutant 19.1 (24). RNA was extracted from stage V gametocyte-containing cultures using Trizol (Life Technologies) and was twice treated with RNase-free DNase I (Roche Molecular Biochemicals, Indianapolis, Ind.). Diagnostic PCR primers were as follows: primer 1, GAATCAAGAAAAAACCCGAGAG; primer 2, CCTAATCATGTAAATCTTAAATTTTTC; primer 3, AATTAACCCTCACTAAAGGGAAC; and primer 4, GTAAAGATTCTACGAAATATTCAATTGC.
Membrane feeding assay.
P. falciparum parasites were switched to RPMI medium containing 10% human AB serum in place of 0.5% Albumax (Life Technologies) prior to initiation of sexual stage studies. Gametocytes were fed to Anopheles freeborni mosquitoes in Parafilm-covered membrane feeders, and oocyst counts were determined according to standard methods (14).
Confocal immunofluorescence microscopy of P. falciparum ookinetes.
In vitro-cultivated P. falciparum gametocytes were fed to A. freeborni mosquitoes through a membrane feeder. After 30 h, individual midguts were dissected, placed in 5 μl of phosphate-buffered saline (PBS) on a glass slide, macerated, and smeared; the suspension was allowed to air dry. After fixation in 100% methanol at −20°C for 1 h, slides were equilibrated in PBS and blocked in 10% bovine serum albumin in PBS for 10 min at room temperature. Two primary antibodies were added to the slides for 30 min in a humidified chamber at 37°C (1/10 dilution of a supernatant of hybridoma 1C3, a mouse monoclonal antibody against PfCHT1, and a 1/2,000 dilution of a polyclonal rabbit antibody raised against a Pfs25-Pfs28 fusion protein [9]). After three PBS washes, a 1/200 dilution of secondary antibodies (Alexa red-labeled anti-rabbit immunoglobulin G and Alexa green-labeled anti-mouse immunoglobulin G [Molecular Probes, Eugene, Oreg.]) was added for an additional 30 min. After washing with PBS, 15 μl of Vectashield antifading reagent (Molecular Probes) was added, and the slides were mounted with coverslips and sealed. Images were collected with a Zeiss Axiophot 2 immunofluorescence microscope or a Leica TCS-NT/SP confocal microscope. For confocal microscopy, z stacks of images were collected in 0.203-μm increments. Images were processed using Leica TCS-NT/SP software (version 1.6.551) and Imaris 3.0.2 software (Bitplane AG, Basel, Switzerland).
RESULTS
Disruption of the carboxy terminus of the P. falciparum ookinete-secreted chitinase, PfCHT1.
Plasmid pPfCHT1KO1 was constructed to disrupt the P. falciparum chitinase gene PfCHT1 just after the catalytic domain (Fig. 1A). This construct was based on the predictions that (i) a putative chitin-binding domain would be identified downstream of the catalytic active site and (ii) the primary structures of PfCHT1 and PgCHT1 would be similar. Subsequently, the complete PfCHT1 gene was shown to lack a carboxy-terminal putative chitin-binding domain (21). Therefore, pPfCHT1KO1 would be predicted to disrupt PfCHT1 39 bp upstream of the stop codon, producing a gene product truncated by 13 amino acids from the carboxy terminus.
pPfCHT1KO1 was electroporated into P. falciparum (strain 3D7). Episomally transformed parasites were selected by inclusion in the culture medium of 5 nM WR99210 (which selects for the human dihydrofolate reductase marker present in pPfCHT1KO1) (5). One transformed line, 19.1, was obtained that produced gametocytes in vitro. Subsequent propagation of this line led to the outgrowth of rapidly growing parasites in which the episomal plasmid had been integrated into the P. falciparum nuclear genome.
Southern analysis demonstrated a single-copy insertion of the 6.8-kb mutant construct into the 19.1 mutant line (Fig. 1B). Using a partial PfCHT1 coding sequence to probe SpeI and BglII digests of wild-type 3D7 genomic DNA, we observed single bands of ∼16 and ∼19 kb, respectively. These bands were increased by ∼6.8 kb in the 19.1 mutant line, indicating the integration of a single copy of the pPfCHT1KO1 plasmid. The mutant plasmid pPfCHT1KO1 does not contain SpeI and BglII restriction sites. No band corresponding to an episomal form of the plasmid could be detected by Southern blotting even after overexposure (data not shown).
PCR analysis confirmed plasmid integration by homologous recombination into the PfCHT1 gene locus (Fig. 1C). PCR amplification (see Materials and Methods) with primers 1 (from the 5′ end of the chromosomal copy of the PfCHT1 coding region not present in the disruption construct) and 2 (from the 3′ flanking region of hrp2) generated a 1,085-bp product specific for the recombinant PfCHT1 locus. Similarly, primers 3 (from the pBluescript plasmid backbone sequence) and 4 (from the 3′ end of the chromosomal copy of the PfCHT1 coding region not present in the disruption construct) generated the expected 1,034-bp product from 19.1 but not from wild-type 3D7 genomic DNA (Fig. 1C). Because leaky mRNA expression of zygote- and ookinete-specific genes is known to occur in gametocytes (for example, the gene encoding the surface molecule Pfs25 [1]), we used RNA extracted from stage V gametocyte-containing cultures to compare PfCHT1 expression in wild-type and 19.1 parasites. Reverse transcriptase PCR using primers 1 and 4 amplified the expected 1,030-bp product from wild-type 3D7 gametocytes; a faint band representing wild-type PfCHT1 cDNA was seen in 19.1 gametocytes (Fig. 1D). Approximately equal quantities of template were present, as indicated by the band produced in the Pfs25 control (Fig. 1D). Taken together, these data demonstrate that the PfCHT1 gene in 19.1 was disrupted at the 3′ end and that transcription of the full-length native PfCHT1 gene was essentially eliminated in the 19.1 parasite line. Examination of the recombinant locus indicated that 19.1 expresses a mutant form of PfCHT1 truncated by 13 amino acids but with the addition of 3 amino acids encoded by the plasmid before the presence of a stop codon encoded by the plasmid.
Disruption of the PfCHT1 gene markedly impairs parasite invasion of the mosquito midgut.
To assess the effect of the PfCHT1 gene disruption on the ability of 19.1 to form oocysts in the mosquito midgut, wild-type 3D7 and 19.1 gametocytes were fed on four separate occasions to A. freeborni using a membrane feeding assay (14). Gametocytogenesis of wild-type 3D7 was indistinguishable from that of 19.1, and the numbers of exflagellation centers (approximately one or two every ×40 field) observed in vitro were similar in all experiments. At 24 h after feeding, similar numbers (geometric means of 2.9 to 2.4 ookinetes per midgut in 3D7 versus 2.2 to 1.8 in 19.1) of morphologically normal (Fig. 2), Pfs25- or Pfs28-expressing ookinetes were observed in midguts of wild-type and 19.1 parasites, indicating no difference between the strains in their ability to develop normally to the ookinete stage. However, in all four experiments, the ability of 19.1 to form oocysts in the mosquito midgut was markedly reduced compared to that of its wild-type parent, 3D7 (Table 1). Compared with wild-type 3D7, which had a high rate of mosquito infectivity (Table 1), 19.1 was completely unable to form oocysts in experiments 1 to 3. In experiment 4, 19.1 produced a small number of breakthrough oocysts, yet still its infectivity was markedly reduced compared to that of wild-type 3D7.
FIG. 2.
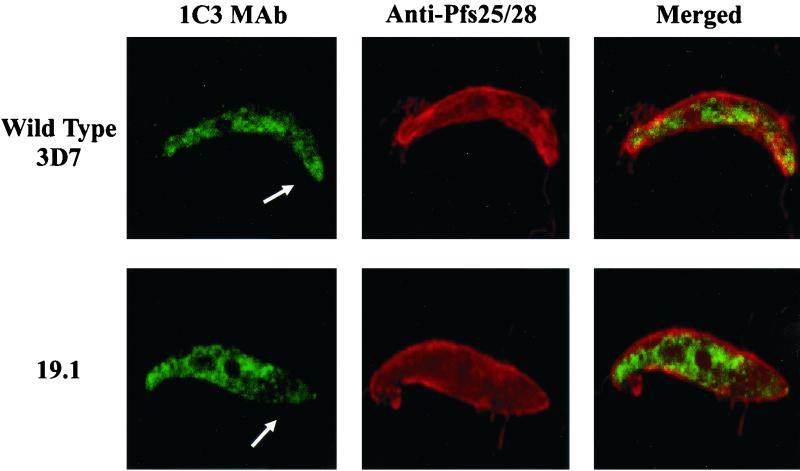
Confocal microscopy of wild-type 3D7 and 19.1 ookinetes. The presence of the P. falciparum zygote-ookinete surface proteins and PfCHT1 was simultaneously assessed in ookinetes found in A. freeborni midguts 30 h after ingestion of a blood meal. MAb, monoclonal antibody. The arrow indicates the apical end of an ookinete.
TABLE 1.
Oocyst counts from membrane feeds of P. falciparum wild-type 3D7 and PfCHT1 mutant 19.1 parasite lines given to A. freeborni mosquitoesa
| Expt | Parasite | Geometric mean no. of oocysts (range) | No. infected/no. dissected |
|---|---|---|---|
| 1 | 3D7 | 1.05 (0–7) | 16/22 |
| 19.1 | 0 | 0/22 | |
| 2 | 3D7 | 14.6 (1–36) | 25/25 |
| 19.1 | 0 | 0/25 | |
| 3 | 3D7 | 0.77 (0–4) | 16/28 |
| 19.1 | 0 | 0/17 | |
| 4 | 3D7 | 3.5 (1–13) | 20/20 |
| 19.1 | 0.37 (0–3)b | 8/20 |
Membrane feeds were set up with 0.5% stage V gametocytes. Equivalent numbers of exflagellation centers (approximately one per one or two ×40 fields) were present in both 3D7 parasites and 19.1 parasites. P values for all comparisons, as determined by the Mann-Whitney test, were <0.01.
Seven midguts contained one oocyst; one midgut contained three oocysts.
Plasmid rescue and PCR were performed to address the possibility that 19.1 oocyst formation in experiment 4 was a result of low-level outgrowth of revertant parasites that had reconstituted the wild-type PfCHT1 gene. Even though overexposure of the Southern blot (Fig. 1B) failed to demonstrate a band corresponding to an episomally replicating plasmid, approximately 40 to 60 colonies of mutant plasmid pfCHT1KO1 were isolated per microgram of total DNA extracted from parasites after experiment 4 (compared to 104 CFU per μg of genomic DNA reported for parasites episomally transformed with the human dihydrofolate resistance plasmid pHD22Y) (5). PCR analysis also confirmed the presence of wild-type 3D7 genomic DNA at the PfCHT1 locus in this preparation of 19.1 DNA (made at the time of the fourth membrane feeding experiment). Spontaneous excision of a disruption plasmid from the site of chromosomal integration has been previously observed (19, 20) and appears to be the mechanism by which a small population of wild-type parasites was maintained in the 19.1 mutant parasite line.
To assess whether the addition of exogenous chitinase to an infectious blood meal fed to mosquitoes would reverse the PfCHT1 mutant phenotype (phenotypic complementation), both Streptomyces griseus chitinase and recombinant, enzymatically active PfCHT1 were added to wild-type 3D7 and 19.1 membrane feeds. In three separate experiments, no oocysts appeared in the 19.1 feeds, and the number of oocysts produced by wild-type 3D7 parasites either was markedly reduced or was zero (data not shown). Dissections of mosquito midguts confirmed the absence of a PM. These experiments suggested that these preparations of exogenous chitinase independently reduced parasite infectivity for mosquitoes, but the ability of exogenous chitinase to reverse the PfCHT1 mutant phenotype could not be assessed.
Carboxy-terminal disruption of PfCHT1 reduces the concentration of chitinase in the apical end of the ookinete.
Confocal microscopy using monoclonal antibody 1C3 raised against recombinant PfCHT1 (12) demonstrated the presence of a PfCHT1 epitope in both wild-type 3D7 and 19.1 parasites (Fig. 2). In more than 15 wild-type 3D7 ookinetes observed (we examined thousands of oil immersion fields of midguts taken from five mosquitoes in membrane feeding assay 3 [Table 1], where oocysts were enumerated), PfCHT1 was consistently seen throughout the cytoplasm, was concentrated in the apical end, and was readily seen extracellularly in the midgut milieu, consistent with a previous report (12). In contrast, in more than 15 ookinetes observed from mosquito infections with 19.1 parasites, there was a consistent absence of PfCHT1 concentrated both in the apical end of the parasite and extracellularly (Fig. 2).
DISCUSSION
Our data demonstrate that a carboxy-terminal truncation of the chitinase PfCHT1 markedly impaired the ability of P. falciparum to invade the A. freeborni mosquito midgut. In view of earlier data suggesting that Plasmodium ookinetes secrete chitinase to penetrate the chitin-containing PM (11, 17, 21, 22), it is likely that the PfCHT1 truncation prevented the malaria parasite from penetrating the PM.
The results of this study directly support the hypothesis that P. falciparum ookinetes require the single identified chitinase gene, PfCHT1, to form oocysts within the mosquito midgut. While these findings are consistent with our finding of only a single chitinase gene in the P. falciparum strain 3D7 genome database (90 to 95% raw data complete as of November 2000), these results do not by themselves rule out the possibility that there is another P. falciparum chitinase gene. It appears that the reference strain of P. falciparum, 3D7, is dependent upon its single chitinase gene for invasion of the mosquito midgut. It is conceivable, however, that 3D7 could have lost genomic DNA containing an additional chitinase gene. Such genomic loss in 3D7 has been observed for the asexual stage-specific genes orfP and orgGap, which were present in all tested field isolates but absent in 3D7 (15).
The precise mechanism by which the PfCHT1 truncation prevented oocyst formation is not clear but appears to be related to the inability of the ookinete to concentrate truncated PfCHT1 in the apical complex and secrete the protein extracellularly. An apparently quantitative difference in the apical localization of PfCHT1 in 3D7 versus 19.1 ookinetes (Fig. 2) supports this conclusion. While monoclonal antibody 1C3 detected PfCHT1 in both wild-type 3D7 and 19.1 mutant parasites, within 19.1 ookinetes the 1C3 epitope was found throughout the cytoplasm but little was observed concentrated within the apical end of the ookinetes. Several considerations support the specificity of the PfCHT1 immunostaining in these experiments. First, a monoclonal antibody, 1C3, was used for staining. Second, we have obtained recent data localizing the 1C3 epitope to a minimal 20-amino-acid linear region within the central portion of PfCHT1 that is predicted to be present in the expressed truncated chitinase gene product in the 19.1 mutant line (Langer and Vinetz, unpublished observations). Third, computational analysis by the SMART algorithm (16) indicates that the 1C3 epitope is not within a low-complexity amino acid sequence, and BLAST analysis has failed to identify any homologies in the P. falciparum genome database (data not shown).
Our results do not distinguish whether the truncation of the carboxy-terminal 13 amino acids of PfCHT1 leads to an improperly folded protein or to the loss of a specific secretion targeting signal. We have attempted to express the 13-amino-acid truncated version of PfCHT1 under conditions identical to those that successfully yielded enzymatically active, full-length PfCHT1, but the mutant protein was not expressed in appreciable quantities (Y.-L. Tsai and J. M. Vinetz, unpublished observations). In 19.1 parasites, PfCHT1 was not concentrated in the apical end of the ookinete; in contrast, in wild-type 3D7 parasites, PfCHT1 is clearly concentrated in the apical end prior to secretion. Since chitinase secretion occurs through the apical end of the ookinete (12) and is mediated by a micronemal pathway, our data suggest that 19.1 ookinetes were unable to form oocysts in the mosquito midgut because of the inability to transport or secrete enzymatically active PfCHT1.
In the first three membrane feeding experiments, 19.1 was unable to form oocysts. These experiments with 19.1 were run in parallel with experiments with its wild-type parent, 3D7, and were controlled for numbers of gametocytes and exflagellation centers. Equivalent numbers of morphologically mature ookinetes expressing the proper surface antigens were found in feeds of both 3D7 and 19.1 parasites. The numbers of oocysts found in wild-type-fed mosquito midguts in these experiments were similar to those found in previous experiments using P. falciparum in membrane feeding assays (9). In experiment 4, 19.1 produced a small number of oocysts in some of the mosquitoes, although in markedly lower numbers than 3D7. One potential explanation for this finding is that a wild-type parasite could have been produced in the 19.1 parasite line by a self-excision reversion event where the mutant plasmid was excised from the chromosomal insertion site as a recombination event. Another explanation is that PfCHT1 might not be absolutely necessary for oocyst formation in A. freeborni mosquitoes, although in light of previous evidence that allosamidin prevents P. falciparum ookinete invasion of the A. freeborni midgut (17), this possibility seems unlikely. A third explanation is that some minor proportion of functional, truncated PfCHT1 might be secreted to account for the breakthrough oocysts. The data presented here are most consistent with the hypothesis that the truncation of 13 amino acids from the carboxy terminus of PfCHT1 led to the inability of 19.1 to invade the mosquito midgut.
We found that the addition of exogenous chitinases—either S. griseus chitinase or enzymatically active, Escherichia coli-produced, recombinant PfCHT1—did not reverse the inability of PfCHT1 mutant parasites to form oocysts. This observation stands in contrast to previous findings where the addition of S. griseus chitinase to an infectious blood meal reversed the effect of allosamidin in preventing ookinete invasion (17). In fact, the addition of either chitinase to infectious blood meals of wild-type 3D7 or mutant 19.1 reduced infectivity. Previous observations have noted the increased sensitivity of early sexual stage P. gallinaceum parasites to proteases in the mosquito midgut (8). The absence of the PM could lead to an abnormally early exposure of sexual stage parasites within the blood meal to mosquito midgut proteases, which would result in lower oocyst counts. In addition, others have observed that in the absence of the PM, a decrease in the number of oocysts can be observed, at least for P. gallinaceum (17). An alternative possibility is that the truncated PfCHT1 acts as a dominant-negative mutation and interrupts another important biological function in ookinetes, such as protein secretion. The addition of an exogenous chitinase would not reverse such a dominant-negative mutation. However, the reduction in oocyst numbers in wild-type parasites because of the absence of the PM would not by explained by this mechanism. Genetic complementation experiments to reconstitute the disrupted PfCHT1 locus or to episomally complement the disrupted PfCHT1 gene are under way to address the possibility that the truncated PfCHT1 acts as a dominant-negative mutant.
Two Plasmodium ookinete-secreted chitinase genes have been identified to date, those for PfCHT1 and a chitinase of P. gallinaceum, PgCHT1. Substantial biochemical and immunological data strongly suggest that P. gallinaceum ookinetes secrete products of at least two different chitinase genes (22). The PfCHT1 gene is found on chromosome 12, whose sequence is complete. If a second chitinase gene were to be found in the P. falciparum genome, it would likely be present in a syntenic relationship, since it would be assumed that the two genes arose by gene duplication. This feature is true of at least two sexual stage families, Pfs25 and Pfs28, whose genes are located on chromosome 10 (with a similar arrangement of the two genes found in P. gallinaceum [3]); Pfs230 and Pfs230 II, whose genes are located on chromosome 2 (7); and several other Plasmodium gene families as well (for example, the genes for plasmepsins PfPM1 and PfPMII on chromosome 14 [2]). Therefore, our current phylogenetic comparison of P. gallinaceum and P. falciparum chitinases shows a discrepancy in gene number and the ortholog-paralog relationship. This finding is even more curious given that three independent molecular analyses indicated that P. falciparum is most closely related to avian malaria parasites (4, 13, 23). It is tempting to speculate that as P. falciparum changed hosts from bird to primate in evolutionary history and perhaps even underwent a change in arthropod vector from culicine to anopheline mosquito, a genetic loss occurred such that one chitinase gene was lost from the P. falciparum genome. Alternatively, it is possible that the P. falciparum reference strain 3D7, with which these PfCHT1 gene disruption studies were performed (and for which the genome database is available), has lost DNA, including a potential second chitinase gene, during in vitro cultivation. Nonetheless, the results presented here suggest that transmission-blocking strategies (both immunological and pharmacological) aimed at the single identified P. falciparum chitinase gene may be appropriate.
ACKNOWLEDGMENTS
Y.-L.T. and R.E.H. contributed equally to this work.
We thank David Keister and Olga Muratova for help with membrane feeding, Owen Schwartz for confocal microscopy, and David Kaslow and Louis Miller for advice and support in initiating this project.
R.C.L. was supported by U.S. Public Health Service grant T32-AI07536 from the National Institute of Allergy and Infectious Diseases. J.M.V. is a Culpeper Medical Sciences Scholar supported by the Rockefeller Brothers Fund. This work was also supported by U.S. Public Health Service grant RO1-AI 45999 from the National Institute of Allergy and Infectious Diseases (to J.M.V.).
REFERENCES
- 1.Babiker H A, Abdel-Wahab A, Ahmed S, Suleiman S, Ranford-Cartwright L, Carter R, Walliker D. Detection of low level Plasmodium falciparum gametocytes using reverse transcriptase polymerase chain reaction. Mol Biochem Parasitol. 1999;99:143–148. doi: 10.1016/s0166-6851(98)00175-3. [DOI] [PubMed] [Google Scholar]
- 2.Carlton J M, Galinski M R, Barnwell J W, Dame J B. Karyotype and synteny among the chromosomes of all four species of human malaria parasite. Mol Biochem Parasitol. 1999;101:23–32. doi: 10.1016/s0166-6851(99)00045-6. [DOI] [PubMed] [Google Scholar]
- 3.Duffy P, Kaslow D. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect Immun. 1997;65:1109–1113. doi: 10.1128/iai.65.3.1109-1113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escalante A A, Freeland D E, Collins W E, Lal A A. The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochondrial genome. Proc Natl Acad Sci USA. 1998;95:8124–8129. doi: 10.1073/pnas.95.14.8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fidock D A, Wellems T E. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc Natl Acad Sci USA. 1997;94:10931–10936. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fidock D A, Nomura T, Wellems T. Cycloguanil and its parent compound proguanil demonstrate distinct activities against Plasmodium falciparum malaria parasites transformed with human dihydrofolate reductase. Mol Pharmacol. 1998;54:1140–1147. doi: 10.1124/mol.54.6.1140. [DOI] [PubMed] [Google Scholar]
- 7.Gardner J M, Tettelin H, Carucci D J, Cummings L M, Aravind L, Koonin E V, Shallom S, Mason T, Yu K, Fujii C, Pederson J, Shen K, Jing J, Aston C, Lai Z, Schwartz D C, Pertea M, Salzberg S, Zhou L, Sutton G G, Clayton R, White O, Smith H O, Fraser C M, Hoffman S L. Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum. Science. 1998;282:1126–1132. doi: 10.1126/science.282.5391.1126. [DOI] [PubMed] [Google Scholar]
- 8.Gass R F, Yeates R A. In vitro damage of cultured ookinetes of Plasmodium gallinaceum by digestive proteinases from susceptible Aedes aegypti. Acta Trop. 1979;36:243–252. [PubMed] [Google Scholar]
- 9.Gozar M M, Price V L, Kaslow D C. Saccharomyces cerevisiae-secreted fusion proteins Pfs25 and Pfs28 elicit potent Plasmodium falciparum transmission-blocking antibodies in mice. Infect Immun. 1998;66:59–64. doi: 10.1128/iai.66.1.59-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han Y S, Thompson J, Kafatos F C, Barillas-Mury C. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. EMBO J. 2000;19:6030–6040. doi: 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber M, Cabib E, Miller L H. Malaria parasite chitinase and penetration of the mosquito peritrophic membrane. Proc Natl Acad Sci USA. 1991;88:2807–2810. doi: 10.1073/pnas.88.7.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langer R C, Hayward R, Tsuboi T, Tachibana M, Torii M, Vinetz J M. Micronemal transport of Plasmodium ookinete chitinases to the electron-dense area of the apical complex for extracellular secretion. Infect Immun. 2000;68:6461–6465. doi: 10.1128/iai.68.11.6461-6465.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCutchan T F, Kissinger J C, Touray M G, Rogers M J, Li J, Sullivan M, Braga E M, Krettli A U, Miller L H. Comparison of circumsporozoite proteins from avian and mammalian malarias: biological and phylogenetic implications. Proc Natl Acad Sci USA. 1996;93:11889–11894. doi: 10.1073/pnas.93.21.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quakyi I A, Carter R, Rener J, Kumar N, Good M F, Miller L H. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987;139:4213–4217. [PubMed] [Google Scholar]
- 15.Sallicandro P, Paglia M G, Hashim S O, Silvestrini F, Picci L, Gentile M, Mulaa F, Alano P. Repetitive sequences upstream of the pfg27/25 gene determine polymorphism in laboratory and natural lines of Plasmodium falciparum. Mol Biochem Parasitol. 2000;110:247–257. doi: 10.1016/s0166-6851(00)00274-7. [DOI] [PubMed] [Google Scholar]
- 16.Schultz J, Copley R R, Doerks T, Ponting C P, Bork P. SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 2000;28:231–234. doi: 10.1093/nar/28.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahabuddin M, Toyoshima T, Aikawa M, Kaslow D C. Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc Natl Acad Sci USA. 1993;90:4266–4270. doi: 10.1073/pnas.90.9.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sieber K P, Huber M, Kaslow D, Banks S M, Torii M, Aikawa M, Miller L H. The peritrophic membrane as a barrier: its penetration by Plasmodium gallinaceum and the effect of a monoclonal antibody to ookinetes. Exp Parasitol. 1991;72:145–156. doi: 10.1016/0014-4894(91)90132-g. [DOI] [PubMed] [Google Scholar]
- 19.Sultan A, Thathy V, Frevert U, Robson K, Crisanti A, Nussenzweig V, Nussenzweig R, Menard R. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 20.Templeton T J, Kaslow D C, Fidock D A. Developmental arrest of the human malaria parasite Plasmodium falciparum within the mosquito midgut via CTRP gene disruption. Mol Microbiol. 2000;36:1–9. doi: 10.1046/j.1365-2958.2000.01821.x. [DOI] [PubMed] [Google Scholar]
- 21.Vinetz J M, Dave S K, Specht C, Brameld K, Hayward R E, Fidock D A. The chitinase PfCHT1 from the human malaria parasite Plasmodium falciparum lacks proenzyme and chitin-binding domains and displays unique substrate preferences. Proc Natl Acad Sci USA. 1999;96:14061–14066. doi: 10.1073/pnas.96.24.14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinetz J M, Valenzuela J G, Specht C A, Aravind L, Langer R C, Ribeiro J M, Kaslow D C. Chitinases of the avian malaria parasite Plasmodium gallinaceum, a class of enzymes necessary for parasite invasion of the mosquito midgut. J Biol Chem. 2000;275:10331–10341. doi: 10.1074/jbc.275.14.10331. [DOI] [PubMed] [Google Scholar]
- 23.Waters A P, Higgins D G, McCutchan T F. Plasmodium falciparum appears to have arisen as a result of lateral transfer between avian and human hosts. Proc Natl Acad Sci USA. 1991;88:3140–3144. doi: 10.1073/pnas.88.8.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wellems T E, Walliker D, Smith C L, do Rosario V E, Maloy W L, Howard R J, Carter R, McCutchan T F. A histidine-rich protein gene marks a linkage group favored strongly in a genetic cross of Plasmodium falciparum. Cell. 1987;49:633–642. doi: 10.1016/0092-8674(87)90539-3. [DOI] [PubMed] [Google Scholar]
- 25.Zieler H, Dvorak J A. Invasion in vitro of mosquito midgut cells by the malaria parasite proceeds by a conserved mechanism and results in death of the invaded midgut cells. Proc Natl Acad Sci USA. 2000;97:11516–11521. doi: 10.1073/pnas.97.21.11516. [DOI] [PMC free article] [PubMed] [Google Scholar]