Abstract
TCRαβ+ CD8α+CD8β− intestinal intraepithelial lymphocytes (CD8αα IEL) are gut T cells that maintain barrier surface homeostasis. Most CD8αα IEL are derived from thymic precursors (IELp) through a mechanism referred to as clonal diversion. In this model, self-reactive thymocytes undergo deletion in the presence of CD28 co-stimulation, but in its absence undergo diversion to the IEL fate. While previous reports showed that IELp were largely β2m dependent, the antigen presenting cells (APC) that drive the development of these cells are poorly defined. We found that both CD80 and CD86 restrain IELp development, and conventional dendritic cells play a prominent role. We sought to define a CD80/86 negative, MHCI positive APC that supports the development to the IEL lineage. Chimera studies showed that MHCI needs to be expressed on hematopoietic APC for selection. As, thymic hematopoietic APC are heterogeneous in their expression of MHCI and co-stimulatory molecules, we identified four thymic APC types that were CD80/86neg/low and MHCI+. However, selective depletion of β2m in individual APC suggested functional redundancy. Thus, while hematopoietic APC play a critical role in clonal diversion, no single APC subset is specialized to promote the CD8αα IEL fate.
Keywords: MHC class I, APC, IEL, thymus
Introduction
Intraepithelial lymphocytes (IEL) are resident T cells located in the gut epithelium, where they protect against harmful pathogens, while also playing a regulatory role in preserving barrier homeostasis [1, 2]. IEL are a heterogeneous population, broadly divided into conventional and unconventional subsets. Conventional IEL, otherwise known as induced IEL, are TCRαβ+CD4+ and TCRαβ+CD8αβ+ cells generated from naïve T cells that have recognized foreign antigens in the periphery. Unconventional IEL, also known as natural IELs, include TCRγδ+ T cells, and TCRαβ+ CD4−CD8α+CD8β− (CD8αα) T cells. Natural IEL derive from thymic precursors, but their developmental pathway(s) remain incompletely defined. In particular, we lack an understanding of the thymic antigen presenting cells that instruct their differentiation and trafficking.
CD8αα IEL largely differentiate from thymic precursors that have received strong stimulation by self-antigens but escaped deletion, in a process termed agonist selection [2–4]. Thymic precursors to CD8αα+ IEL (IELp) are found in the CD4−CD8− double-negative (DN) population of the thymus and are TCRαβ+CD5+H-2Kb+CD122+ [5–9]. This precursor population is itself heterogeneous, with two major subsets that differ in both cell surface markers and T cell receptor (TCR) specificities [8, 9]. The proportionally larger IELp population – Type A IELp – are a nascent population induced by strong TCR signaling, express PD-1, and readily emigrate from the thymus. The smaller population – Type B IELp – contribute little to the emigrating IELp pool in adult mice, and are PD-1−NK1.1+ [9]. Nonetheless, both IELp are capable of giving rise to CD8αα+ IEL in the gut, and recent evidence suggests Type B IELp make a larger contribution to the gut early in life, and become outcompeted by Type A IELp in adulthood [9–11]. Most IELp are restricted to classical and non-classical MHC class I (MHCI) molecules [6, 7, 9]. Strong TCR engagement of MHCI together with CD28 co-stimulation results in death of thymic precursors (negative selection), and co-stimulation-deficient mice (either Cd28−/− or Cd80−/−Cd86−/−) had higher numbers of thymic IELp and intestinal CD8αα+ IEL [9, 12, 13]. Thus, avoidance of clonal deletion, called ‘clonal diversion’, is thought to be a major driver of IELp development. However, little is known about the thymic antigen presenting cells (APC) that lead to this diversion [9, 14]. Given the above, we predict that such APC would express MHC class I and low or no CD80/86.
While medullary thymic epithelial cells (mTEC) and thymic dendritic cells (DC) are known to drive clonal deletion in the thymus, they are not the only APC that mediate selection processes [15]. Here, we sought to determine the role of APC that support positive selection of IELp. Using bone marrow chimeras, we show that IELp require MHC class I on hematopoietic APC (hAPC). However, our data with selective depletion suggest that no single hAPC subset is responsible for IELp selection on its own. Moreover, CD80 co-stimulation alone can limit the Type A IELp fate, even without CD86. Collectively, our findings show that hAPC play a specialized role in clonal diversion, but specific thymic hAPC subsets may play functionally redundant roles.
Results
Hematopoietic APC are required for IELp selection in the thymus
Previous reports indicate that both CD8αα IEL in the gut and their thymic precursors of IEL are β2m-dependent, with 10 fold fewer signaled PD-1+ DN thymocytes in β2m deficient models[6, 7, 9]. However, there are many different thymic APC with β2m dependent MHC molecules expressed on the cell surface, including both hAPC (such as dendritic cells, B cells, macrophages, and eosinophils) and stromal APC (thymic epithelial cells) that can determine the fate of thymocyte selection[15–18]. Therefore, we first sought to differentiate between the roles of hematopoietic and stromal APC for IELp development using bone marrow chimeras. WT or β2m-deficient marrow was used to reconstitute lethally irradiated β2m-deficient or WT recipients, respectively. Using congenic markers to differentiate host- and donor-derived IELp (Fig. 1A; typical IELp gating without congenic markers shown in Supp. Fig. 1), we found that a significantly lower proportion of signaled DN thymocytes developed into mature (CD122+) IELp in mice with β2m-deficient marrow (Fig. 1B). This IELp reduction in these mice where β2m was deficient in hematopoietic cells was reflected in the reduction of both the PD-1+ Type A and NK1.1+ Type B IELp numbers (Fig. 1C).
Figure 1. Positive selection of IELp depends on MHC class I expressed on hematopoietic APC.
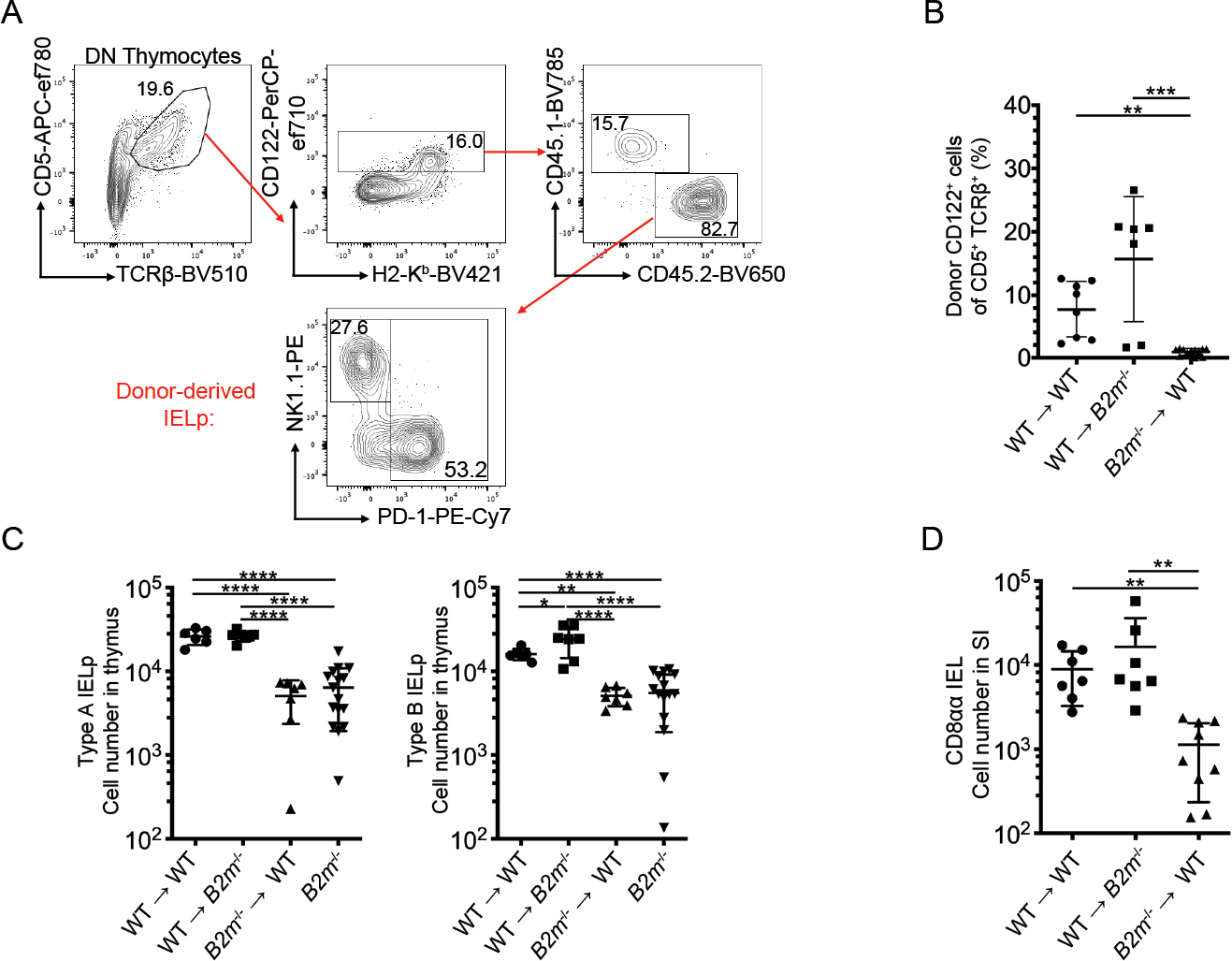
(A) Representative flow cytometry plots of DN thymocytes in bone marrow chimeras (WT → WT shown). Full gating for IELp shown in Supp. Fig. 1. Mature IELp were defined by CD122 expression alone due to the lack of H2-Kb expression in mice with β2m deficient bone marrow. Donor and host cells were differentiated using congenic markers, CD45.1 and CD45.2. Numbers adjacent to the outlined areas indicate the percentage of cells in each. (B) Quantified percentage of donor-derived IELp after WT or B2m−/− bone marrow transfer into lethally irradiated recipients indicated. (C) Absolute number of PD-1+ Type A IELp (left) and NK1.1+ Type B IELp (right) after WT or B2m−/− bone marrow transfer into lethally irradiated recipients indicated. (D) Absolute number of small intestine CD8αα IEL in the indicated bone marrow chimeras. Each symbol in (B-D) represents an individual mouse [n=8 for WT → WT in (B-C), n=7 in (D); n=7 for WT→B2m−/− in (B-D); n=7 for B2m−/− →WT in (B-C), n=9 in (D); n=16 for B2m−/− in (C)]. Data are pooled from at least three independent experiments. Error bars show mean ± SD. *p≤0.05, **p≤0.01, ***p≤0.001, ****p ≤ 0.0001, ANOVA with multiple comparisons.
Our finding of significantly fewer IELp with β2m-deficient hAPC was recapitulated by a reduction in CD8αα+ IEL in the small intestine of these mice (Fig. 1D). Taken together, our data suggest that MHC class I expression is required on hAPC to positively select developing Type A and Type B IELp, and these precursors may rely on a single or select hAPC for this activity.
CD28 co-stimulation drives cells away from the Type A IELp fate
Most T cell precursors that interact strongly via their TCR with self-peptide:MHC undergo apoptosis. However, some cells (including IELp) can escape deletion, and these high-affinity TCR interactions lead to other cell fates in a process termed “agonist selection”[14, 19]. These agonist-selected populations include T regulatory (Treg) cells, which require CD28 co-stimulation by CD80 and/or CD86 for their development and function[20, 21]. In contrast, IELp are agonist-selected cells that are positively selected in the absence of CD28 co-stimulation[9, 13]. For the process of clonal deletion, the ligands for CD28 –CD80 (B7–1) and CD86 (B7–2) – are not equivalently expressed, and several APC throughout the thymus express CD80 alone [22]. Given these patterns, we evaluated the role of costimulatory molecules in IELp development in CD86 knockout (KO) and CD80/CD86 double KO mice. Consistent with previous reports using CD28 deficient mice, the number of Type A IELp increased in the CD80/86 double KO mice [9, 13] (Fig. 2A). Further, the double KO rescued IELp and CD8αα IEL numbers more effectively than CD86 deficiency alone, suggesting both CD80 and CD86 restrain the Type A IELp fate in the thymus(Fig. 2A, 2B).
Figure 2. Co-stimulation by CD80 and CD86 restrains the Type A IELp fate.
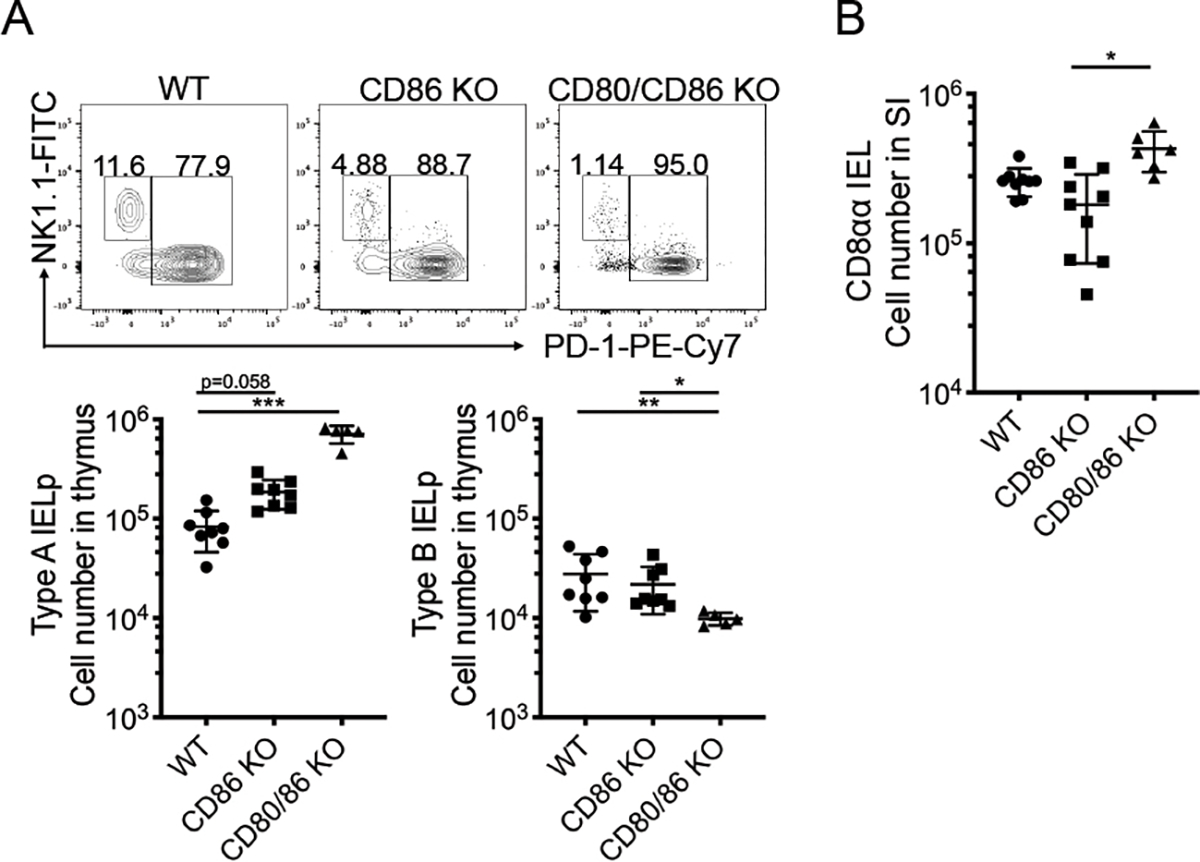
(A) Representative flow cytometry data of expression of NK1.1 (Type B) and PD-1 (Type A) on CD122+ H2-Kb+ mature thymic IELp in WT, CD86 KO, and CD80/86 double KO mice. Numbers adjacent to the outlined areas indicate the percentage of cells in each. On bottom, absolute cell number of Type A IELp (left) and Type B IELp (right) in WT, CD86 KO, and CD80/86 double KO mice. (B) Absolute number of small intestine CD8αα IEL in WT, CD86 KO, and CD80/CD86 KO mice. Each symbol in (A and B) represents an individual mouse [n=8 for WT and CD86 KO in (A), n=9 for WT and CD86 KO in (B); n=5 for CD80/86 KO in (A), n=6 for CD80/86 KO in (B)]. Data are pooled from 4 independent experiments. Error bars show mean ± SD. *p≤0.05, **p≤0.01, ***p≤0.001, ANOVA with multiple comparisons.
In agreement with our previous study indicating that Type B IELp are not rescued in CD28 deficient mice, numbers of thymic Type B IELp were not increased in CD80/86 double KO mice compared to WT and, in fact, trended in the opposite direction[9] (Fig. 2A). Thus, Type B IELp do not appear to be clones diverted from a co-stimulation dependent cell death process, like Type A IELp are.
Several subsets of hematopoietic APC express low levels of CD80
Thymocyte selection is orchestrated by both hematopoietic and stromal derived APC, but these APC subsets express varying levels of co-stimulation, with CD80 being more broadly expressed than CD86 overall[15, 22]. In light of our observation that hAPC were required for IELp selection and that CD80 co-stimulation restrained the Type A IELp fate, we hypothesized that the APC that selects Type A IELp is a hematopoietic cell that expresses MHCI but does not express CD80 or CD86. While the majority of Type A IELp reside in the thymic cortex, some can be found in the medulla [9]. Therefore, we did a comprehensive examination of the various APC found in the thymus. We included not only the well-defined dendritic cell subsets – plasmacytoid DC (pDC) and the two conventional DC (cDC) populations defined by XCR1 (cDC1) or SIRPα (cDC2) – but also B cells and various myeloid cells, as they have all been implicated in thymocyte selection or activation[15, 18, 23–25]. Thymic myeloid populations have been described separately[18, 26–28], so our APC gating strategy was designed to be comprehensive, and included several lineage markers such Siglec F (eosinophils) and Ly6G (neutrophils). As we found thymic F4/80+ cells are CD64+ (data not shown), CD64 was used to define thymic monocytes and macrophages after excluding eosinophils, which are F4/80+ as well (gating for thymic APC shown in Supp. Fig. 2). These myeloid populations, such as thymic eosinophils, are not trivial in the landscape of thymic APC, and are numerically some of the most abundant(Fig. 3A).
Figure 3. Thymic APC have heterogeneous levels of MHC class I and CD80.
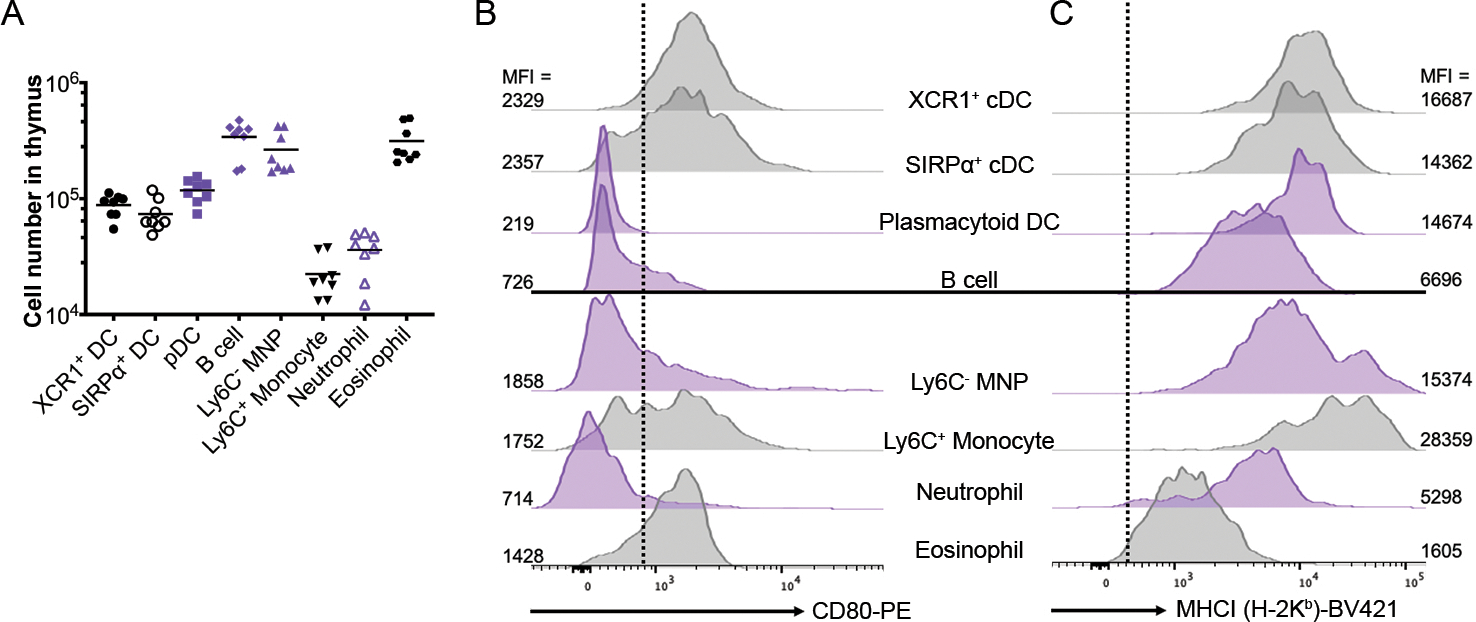
(A) Absolute cell number of thymic APC. Each symbol represents an individual mouse (n=8 for each APC population); horizontal lines indicate the mean. Data are pooled from 3 independent experiments. Representative expression of (B) CD80 and (C) H-2Kb, a classical MHCI molecule on the indicated thymic APC populations. Mean fluorescence intensity (MFI) is listed for each histogram in (B) and (C). Note that all CD86 expressing APC in the thymus also express CD80 ([22] and data not shown), thus CD80 negative APC also lack CD86. Graphs in purple indicate APC populations that are MHCI+ and CD80− (pDC) or CD80low (B cell, Ly6C− MNP, Ly6C+ MNP). (B-C) Representative data showing 2 WT mice from 2 experiments. All data was measured by flow cytometry.
Four populations became appealing candidates for the APC that select Type A IELp: pDC, B cells, Ly6C− mononuclear phagocytes (abbreviated Ly6C− MNP and include thymic macrophages), and neutrophils. These four APC had low levels of CD80 and CD86 expression while expressing moderate to high levels of MHCI (Fig. 3B, 3C). Of these populations, Ly6C− MNP were of particular interest, as they were one of the most abundant APC found in the thymus (Fig. 3A), are of hematopoietic origin, and are localized both within the medulla and the cortex[27, 28]. Conversely, the conventional DC populations expressed high levels of CD80 and MHCI, suggesting that they may oppose self-reactive thymocytes becoming Type A IELp through deletion (Fig. 3B, 3C). Thus, to distinguish the role of the various APC subsets in Type A IELp selection, we employed different Cre-recombinase transgenic strains bred to β2mfl/fl mice to specifically target MHCI deficiency to specific thymic APC (Table I).
Table I:
Conditional deletion strategy1
| Zbtb46 Cre | Itgax Cre | Lyz2 Cre | Cd79a Cre | |
|---|---|---|---|---|
|
| ||||
| XCR1+ DC | + | + | − | − |
| SIRPα+ DC | + | + | − | − |
| pDC | − | + | − | − |
| B cell | − | − | − | + |
| Ly6C− MNP | − | + | + | − |
| Ly6C+ Mono | − | − | + | − |
| Neutrophil | − | + | − | |
| Eosinophil | − | # | − | − |
Where “−“ denotes no effect, “+” denotes significant effect, and “#” denotes mild effect in thymus
Conventional DCs drive cells away from the Type A IELp fate
To determine the contribution of cDC to the Type A IELp fate, we crossed β2mfl/fl mice to zDC (Zbtb46) Cre to create mice that specifically targeted MHCI on SIRPα+ and XCR1+ DC (Supp. Fig. 3A). In these zDC Cre-β2mfl/fl mice, we observed a higher proportion and number of Type A IELp (Figure 4), indicating that normally, thymocyte interactions with cDC skew cells away from the Type A IELp fate. These data fall readily in line with previous work showing that thymic conventional DC contribute to negative selection of self-reactive thymocytes[15, 23, 29] and have high levels of co-stimulation.
Figure 4. Conditional deletion of β2m in conventional DC drive more cells toward the Type A IELp fate.
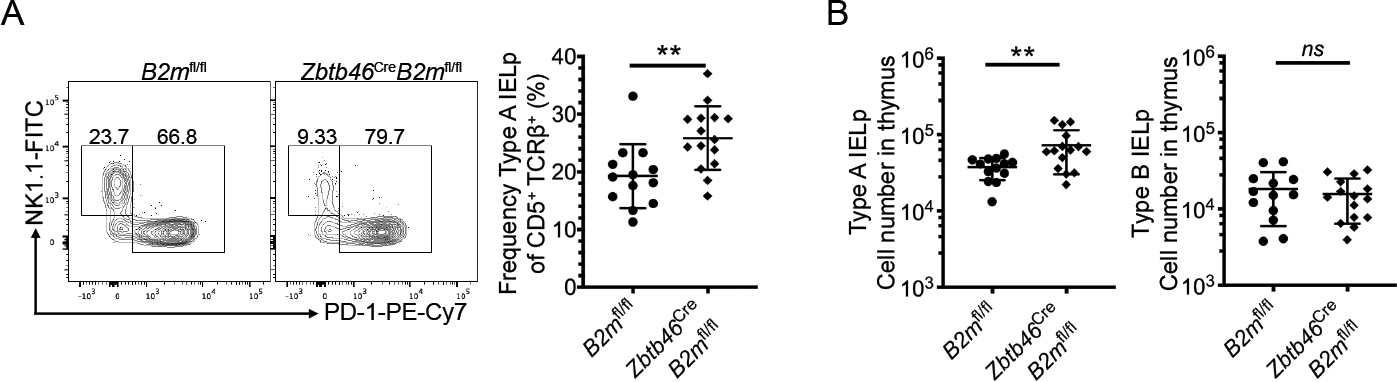
(A) Expression of NK1.1 (Type B IELp) and PD-1 (Type A IELp) on CD122+ H2-Kb+ mature thymic IELp in Zbtb46Cre (zDCCre)-B2mfl/fl and littermate control mice (left, representative data are shown). Numbers adjacent to the outlined areas indicate the percentage of cells in each. Quantified percentage of PD-1+ Type A IELp in zDCCre-B2mfl/fl and B2mfl/fl mice among signaled CD5+ TCRβ+ DN thymocytes (right). (B) Absolute numbers of PD-1+ Type A IELp and NK1.1+ Type B IELp in zDCCre-B2mfl/fl and B2mfl/fl mice. Each symbol in (A & B) represents an individual mouse (n=13 for B2mfl/fl and n=15 for Zbtb46Cre-B2mfl/fl). Data are pooled from 7 independent experiments. Error bars show mean ± SD. **p≤0.01, unpaired two-tailed t test. All data was measured by flow cytometry.
Hematopoietic APC have overlapping roles in selecting Type A IELp
Although CD11c is a well-known marker for dendritic cells, CD11c (Itgax) Cre targets not only cDC, but also pDC and monocytes/macrophages[30]. Using CD11c Cre-β2mfl/fl mice confirmed that this targeting held true for thymic APC, with lack of MHCI on cDC, pDC, and MNP; and a partial reduction on thymic eosinophils and neutrophils (Supp. Fig. 3B). Intriguingly, while cDC in the thymi of CD11c Cre-β2mfl/fl mice had robust MHCI loss, we did not see a replication of increased numbers of Type A IELp like in zDC Cre-β2mfl/fl thymi (Fig. 5A). Therefore, we reasoned that at least one of the other APC affected in the CD11c Cre-β2mfl/fl thymi might support Type A IELp selection. The absence of MHCI on this population could offset the increase in IELp numbers caused by fewer interactions with clonally deleting cDC, making interpretation of this result difficult.
Figure 5. No single APC subset is dedicated to Type A IELp positive selection.
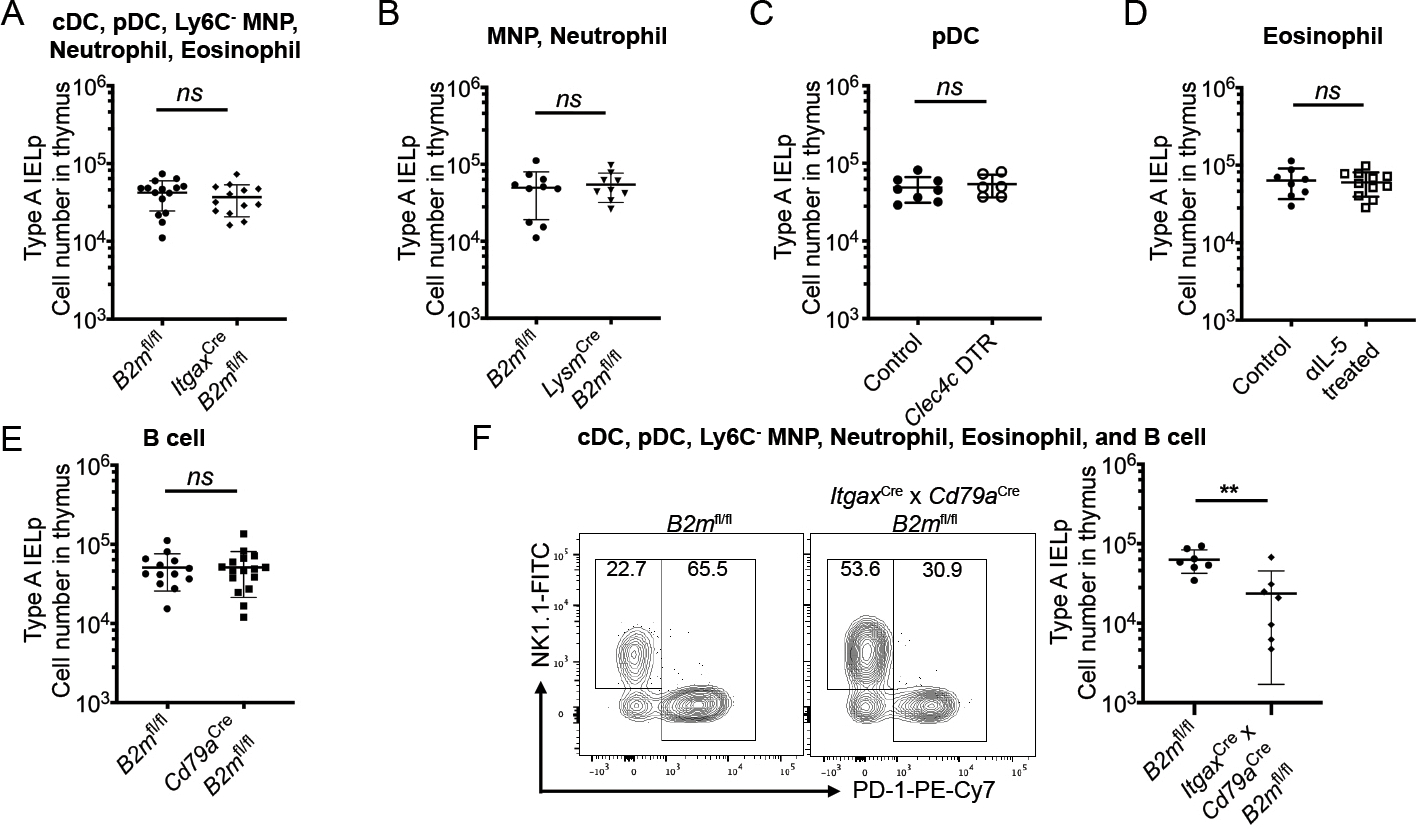
(A) Absolute number of Type A IELp in ItgaxCre (CD11cCre)-B2mfl/fl and littermate controls. Data are pooled from 4 independent experiments (n=15 for B2mfl/fl; n=13 for ItgaxCre-B2mfl/fl. (B) Absolute number of Type A IELp in LysmCre (LysMCre)-B2mfl/fl mice and littermate controls. Data are pooled from 3 independent experiments (n=10 for B2mfl/fl; n=9 for LysmCre-B2mfl/fl). (C) Absolute number of Type A IELp in Clec4c (BDCA2)-DTR+/− mice and littermate controls after a 9d course of DT treatment. Data are pooled from 4 independent experiments (n=8 for control; n=6 for Clec4c DTR). (D) Absolute number of Type A IELp in WT mice treated with αIL-5 (clone TRFK5) or an IgG1 isotype control for 7d. Data are pooled from 3 independent experiments (n=8 for control; n=11 for αIL-5 treated). (E) Absolute number of Type A IELp in Cd79aCre (Mb1Cre)-B2mfl/fl and littermate controls. Data are pooled from 5 independent experiments (n=13 for B2mfl/fl; n=16 for Cd79aCre-B2mfl/fl). (F) Representative data of expression of NK1.1 (Type B) and PD-1 (Type A) on CD122+ H2-Kb+ mature thymic IELp (left). Absolute number of Type A IELp ItgaxCrexCd79aCre-B2mfl/fl and littermate controls (right). Data are pooled from 3 independent experiments (n=7 for B2mfl/fl; n=7 for ItgaxCrexCd79aCre-B2mfl/fl). For (A – F), the graph titles indicate the thymic APC populations affected in the experimental mice (Cre+, BDCA2 DTR+, αIL-5 treated). Each symbol represents an individual mouse. Error bars show mean ± SD. **p≤0.01, ns = not significant, unpaired two-tailed t test.
Thus, to distinguish the role of the myeloid APC subsets, we generated LysM (Lyz2) Cre-β2mfl/fl mice. In these mice, MHCI is targeted on thymic neutrophils, Ly6C+ inflammatory monocytes, and Ly6C− MNP, but not cDC (Supp. Fig. 3B). Both thymic neutrophils and Ly6C− MNP expressed low levels of CD80 on their cell surface, making them attractive candidates for the APC dedicated to Type A IELp selection (Fig. 3B). Nonetheless, the number of Type A IELp was not affected in LysM Cre-β2mfl/fl mice (Fig. 5B). As CD11c Cre perturbed MHCI expression on thymic pDC and eosinophils as well, we depleted these APC subsets specifically. To assess the role of pDC on selection, pDC were ablated in BDCA2 (Clec4c) DTR mice with diphtheria toxin (DT) administration every other day. After 9 days of DT treatment, there was no effect on Type A IELp numbers compared to BDCA2-DTR−/− littermate controls (Fig. 5C). Eosinophils were depleted using an αIL-5 antibody (clone TRFK5). After a 7-day course of αIL-5 treatment, there was no effect on Type A IELp numbers compared to controls (Fig. 5D). Likewise, loss of MHCI on B cells in Mb1 (Cd79a) Cre-β2mfl/fl thymi (Supp. Fig. 3B), did not lead to Type A IELp reduction (Fig. 5E).
Finally, we crossed the CD11c Cre-β2mfl/fl mice to Mb1 Cre to generate CD11c x Mb1 Cre-β2mfl/fl mice. These mice have MHCI depletion on a majority of hAPC. Thymi from these mice were not able to support normal levels of Type A IELp selection, as indicated by reduced numbers of Type A IELp compared to littermate controls (Fig. 5F). Unlike Type A IELp selection, Type B IELp were not affected with the loss of MHCI on any APC subset or subset specific depletion (Supp. Fig. 4). As single Cre models did not affect Type A IELp selection, while the dual Cre model (CD11c Cre x Mb1 Cre-β2mfl/fl) did, our data suggests functional redundancy amongst CD80/86 low APC for Type A IELp selection.
Discussion
Type A IELp, the thymic precursors of CD8αα IEL in the gut, receive strong stimulation by self-antigens, yet escape clonal deletion[2–4, 9]. In this study, we investigated the thymic antigen presenting cells that orchestrate the decision of clonal diversion into IELp instead of deletion. Using β2m deficient bone marrow chimeras, we showed that MHCI expression on hAPC rather than stromal APC was crucial for selection of IELp.
The current and previous studies have shown that CD28-mediated co-stimulation, along with TCR stimulation, is important in determining thymocyte fate into the deletion pathway[9, 12, 13]. CD28 on developing thymocytes can interact with the ligands CD80 (B7–1) and CD86 (B7–2), but these two molecules are distinct – sharing only approximately 25% sequence identity, and are differentially expressed on various APC[22, 31]. We therefore hypothesized that the two B7 molecules serve non-redundant roles in selection. This was supported by our data in CD80/CD86 double knockout mice compared to the CD86 knockout mice. Out data support previous findings that CD80 serves a non-redundant role in mediating clonal deletion in the thymic cortex – the region in which most Type A IELp reside[9, 22]. These distinct roles may be due to differences in which APC express CD80 or CD86. Interestingly, hAPC in the thymus do not generally express CD86 alone, but rather express either CD80 alone or both CD80 and CD86. Alternatively, these distinct roles may be due to differences in the interactions between these ligands and their mutual receptor, as CD86 is predicted to bind more strongly to CD28[32]. Without the CD80/CD86 ligand interactions, CD28 accumulates only half as well at the immunological synapse and only needs CD86 expression for the CD28 accumulation, not CD80[33].
Given that our data showed that hAPC are required for Type A IELp selection and that CD28 co-stimulation restrains this fate, we examined the role of thymic APC that expressed MHCI with little/no CD80, or high levels of CD80. Similar to previous reports, we saw that thymic conventional DC expressed high levels of co-stimulatory molecules, which contributes to their role in deletional tolerance[15, 34]. When we selectively depleted MHCI on cDC, so that class I restricted, self-reactive developing thymocytes could no longer interact with these ‘deleting’ APC, we indeed found that more cells were diverted toward the Type A IELp fate. On the other hand, thymic pDC, Ly6C− MNP, B cells, and neutrophils expressed high levels of MHCI but minimal levels of CD80, suggesting a role in IELp positive selection. However, when we selectively depleted MHCI on each population individually, we did not see a decrease in Type A IELp selection as expected if that population was essential. Instead, we only found a decrease in Type A IELp when MHCI was depleted on the majority of hAPC, suggesting functional redundancy amongst CD80/86 neg/low APC in contributing to the diversion fate.
Like thymic hAPC, DP thymocytes do not express CD80 or CD86, and it could be reasoned that they could potentially select IELp as well. Indeed, iNKT cells, another relatively self-reactive population, are selected on cortical DP thymocytes expressing lipid antigens on CD1d [35]. However, DP thymocytes express very low levels of classical MHCI on their cell surface, making them unlikely partners for the strong TCR signaling seen in Type A IELp. Further, our results showed a decrease in Type A IELp selection when classical MHCI was depleted on APC alone, suggesting that DP thymocytes are not sufficient for their selection. However, DP thymocytes, which represent the largest population of cells in the thymus, have not been formally excluded from contributing to IELp selection, either directly or indirectly. While CD1d itself is β2m dependent and was also likely depleted along with classical MHCI in our cell-specific deletion of β2m, Type A IELp are not dependent on CD1d for selection[9, 36]. Intriguingly, loss of β2m on all hematopoietic cells led to fewer Type B IELp, but MHCI depletion on hematopoietic APC did not yield the same result. This leaves open the possibility that Type B IELp are positively selected by a thymocyte population, like DP thymocytes.
In conclusion, we showed that hAPC play an essential role in clonal diversion into the IELp fate, although it appears there is no single APC that is specialized for ‘diverting’ thymocytes to Type A IELp. In addition, the loss of co-stimulatory molecules on all APC exaggerates Type A IELp selection, while the presence of cDC with high levels of co-stimulatory molecules restrains it.
Materials and methods
Mice
C57BL/6NCrl (B6) and B6.SJL-PtprcaPepcb/BoyCrl (B6.SJL) mice were purchased from the National Cancer Institute. B6.129P2-B2mtm1Unc/DcrJ (B2m−/−), B6.129S4-Cd80tm1Shr Cd86tm2Shr/J (CD80/CD86 KO), B6.Cg-Zbtb46tm3.1(cre)Mnz/J (Zbtb46Cre), B6.Cg-Tg(Itgax-cre)1–1Reiz/J (ItgaxCre), B6.C(Cg)-Cd79atm1(cre)Reth/EhobJ (Cd79aCre), and C57BL/6-Tg(CLEC4C-HBEGF)956Cln/J (Clec4c DTR) mice were obtained from Jackson Laboratories. Cd80floxBACTg/ B6.129S4-Cd80tm1Shr Cd86tm2Shr/J, referred to as CD86 KO mice in this study, were kindly provided by R. J. Hodes (National Institutes of Health) and were described previously[37]. B6.B2mflox/flox (B2mfl/fl) mice were kindly provided by C. N. Morrell (University of Rochester School of Medicine) and were described previously[38]. B6.129P2-Lyz2tm1(cre)Ifo/J (Lyz2Cre) mice were kindly provided by M. Jenkins (University of Minnesota). Except the bone marrow chimeras, mice were used between 6 to 10 weeks of age, and were age-matched with controls in each experiment. Animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
Bone marrow chimeras
For bone marrow chimeras, recipients were depleted of NK cells with PK136, then lethally irradiated and reconstituted with donor bone marrow depleted of T cells. Chimeras were provided water supplemented with neomycin and polymyxin B for 2 weeks. Chimeras involving B2m−/− recipients and bone marrow were analyzed at a minimum of 8 weeks after reconstitution.
Lymphocyte Isolation
Single cell suspensions of thymocytes were isolated by mashing and filtering thymi through 70μM cell strainers (Falcon), or digestion with collagenase D (Roche, 1mg/mL) at 37°C for 30 minutes before mashing and filtering thymi through 70μM cell strainers. Small intestine IEL were isolated as previously described[9] and filtered through 70μM cell strainers. Lymphocytes were enriched by centrifugation over a 80%:40% Percoll gradient.
Flow cytometry
Flow cytometry and analysis adhered to the general guidelines as previously described[39]. Single cell suspensions were incubated with Fc block (Tonbo) for 15 minutes at 4°C before staining with surface antibodies and viability dye for 30 minutes at 37°C or 1 hour at room temperature. Antibodies from BioLegend were: CD4 (RM4–5), CD8b (YTS156.7.7), CD25 (PC61), TCRγδ (GL3), NK1.1 (PK136), CD45.2 (104), B220 (RA3–6B2), CD11c (N418), XCR1 (ZET), SIRPα (P84), CD90.2 (30-H12), CD19 (6D5), Ly6G (1A8), CD64 (X54–5/7.1), CX3CR1 (SA011F11), and CD80 (16–10A1). Antibodies from BD Biosciences were: CD8α (53–6.7), TCRβ (H57–597), H2-Kb (AF6–88.5), Siglec F (E50–2440), and CD86 (GL1). Antibodies from eBioscience were: CD5 (53–7.3), PD-1 (J43), CD122 (TM-b1), F4/80 (BM8), MHC Class II (I-A/I-E, clone M5/114.15.2), and Ly6C (HK1.4). CD11b (M1/70) was purchased from Tonbo. Biotinylated CD1d–PBS57 monomers were obtained from the US National Institutes of Health tetramer core and were incubated with APC streptavidin to tetramerize. Samples were acquired on a BD Fortessa or BD LSRII, and data were analyzed with FlowJo 10.
APC subset depletion treatments
For pDC depletion, Clec4c DTR and littermate controls were injected intraperitoneally (i.p.) with diphtheria toxin (DT; Sigma-Aldrich) in 100uL PBS every other day for 9 days (500ng DT for first injection, 100ng of DT for subsequent injections). For eosinophil depletion, B6 mice were treated i.p. every other day for 7 days with 25μg αIL-5 (TRFK5) or IgG1 isotype control (HRPN) in 100uL PBS. Tissues for treated mice were harvested the day after the final injections.
Statistical Analysis
Data were analyzed using GraphPad Prism software version 8.0. For comparison of two data sets, two-tailed, unpaired t tests were performed. For comparisons of three or more data sets, 1-way ANOVA with multiple comparisons was used for data analysis and calculation of p values. P values ≤ 0.05 were considered significant. Numbers of experimental replicates and additional details are explained in each figure legend.
Supplementary Material
Acknowledgements
Support for this work came from R37AI39560 (KAH) and T32 GM113846 (STL).
Footnotes
Conflict of interests
The authors declare no commercial or financial conflicts of interest.
Data availability statement
Data available on request from the authors.
References:
- 1.Sheridan BS and Lefrancois L, Intraepithelial lymphocytes: to serve and protect. Curr Gastroenterol Rep 2010. 12: 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheroutre H, Lambolez F and Mucida D, The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol 2011. 11: 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leishman AJ, Gapin L, Capone M, Palmer E, MacDonald HR, Kronenberg M and Cheroutre H, Precursors of Functional MHC Class I- or Class II- Restricted CD8αα+ T cells are Positively Selected in the Thymus by Agonist Self-Peptides. Immunity 2002. 16: 355–364. [DOI] [PubMed] [Google Scholar]
- 4.McDonald BD, Jabri B and Bendelac A, Diverse developmental pathways of intestinal intraepithelial lymphocytes. Nat Rev Immunol 2018. 18: 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gangadharan D, Lambolez F, Attinger A, Wang-Zhu Y, Sullivan BA and Cheroutre H, Identification of pre- and postselection TCRalphabeta+ intraepithelial lymphocyte precursors in the thymus. Immunity 2006. 25: 631–641. [DOI] [PubMed] [Google Scholar]
- 6.Mayans S, Stepniak D, Palida SF, Larange A, Dreux J, Arlian BM, Shinnakasu R, Kronenberg M, Cheroutre H and Lambolez F, alphabetaT cell receptors expressed by CD4(−)CD8alphabeta(−) intraepithelial T cells drive their fate into a unique lineage with unusual MHC reactivities. Immunity 2014. 41: 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald BD, Bunker JJ, Ishizuka IE, Jabri B and Bendelac A, Elevated T cell receptor signaling identifies a thymic precursor to the TCRalphabeta(+)CD4(−)CD8beta(−) intraepithelial lymphocyte lineage. Immunity 2014. 41: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golec DP, Hoeppli RE, Henao Caviedes LM, McCann J, Levings MK and Baldwin TA, Thymic progenitors of TCRalphabeta(+) CD8alphaalpha intestinal intraepithelial lymphocytes require RasGRP1 for development. J Exp Med 2017. 214: 2421–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruscher R, Kummer RL, Lee YJ, Jameson SC and Hogquist KA, CD8alphaalpha intraepithelial lymphocytes arise from two main thymic precursors. Nat Immunol 2017. 18: 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klose CSN, Hummel JF, Faller L, d’Hargues Y, Ebert K and Tanriver Y, A committed postselection precursor to natural TCRalphabeta(+) intraepithelial lymphocytes. Mucosal Immunol 2018. 11: 333–344. [DOI] [PubMed] [Google Scholar]
- 11.Ruscher R, Lee ST, Salgado-Barrero O, Breed ER, Isakson SH and Hogquist KA, Intestinal CD8alphaalpha IEL derived from two distinct thymic precursors have staggered ontogeny. J Exp Med 2020. 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Punt JA, Havran W, Abe R, Sarin A and Singer A, T Cell receptor (TCR)-induced death of immature CD4+CD8+ thymocytes by two distinct mechanisms differing in their requirement for CD28 costimulation: Implications for negative selection in the thymus. J Exp Med 1997. 186: 1911–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pobezinsky LA, Angelov GS, Tai X, Jeurling S, Van Laethem F, Feigenbaum L, Park JH and Singer A, Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat Immunol 2012. 13: 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stritesky GL and Hogquist KA, Death diverted, but to what? Nat Immunol 2012. 13: 528–530. [DOI] [PubMed] [Google Scholar]
- 15.Breed ER, Lee ST and Hogquist KA, Directing T cell fate: How thymic antigen presenting cells coordinate thymocyte selection. Semin Cell Dev Biol 2018. 84: 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soga H, Nakamura M, Yagi H, Kayaba S, Ishii T, Gotoh T and Itoh T, Heterogeneity of mouse thymic macrophages: I. immunohistochemical analysis Arch Histol Cytol 1997. 60: 53–63. [DOI] [PubMed] [Google Scholar]
- 17.Delaney JR, Sykulev Y, Eisen HN and Tonegawa S, Differences in the level of expression of class I major histocompatibility complex proteins on thymic epithelial and dendritic cells influence the decision of immature thymocytes between positive and negative selection. Proc Natl Acad Sci U S A 1998. 95: 5235–5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Throsby M, Herbelin A, Pleau JM and Dardenne M, CD11c+ eosinophils in the murine thymus: developmental regulation and recruitment upon MHC class I-restricted thymocyte deletion. J Immunol 2000. 165: 1965–1975. [DOI] [PubMed] [Google Scholar]
- 19.Stritesky GL, Jameson SC and Hogquist KA, Selection of self-reactive T cells in the thymus. Annu Rev Immunol 2012. 30: 95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lio CW, Dodson LF, Deppong CM, Hsieh CS and Green JM, CD28 facilitates the generation of Foxp3(−) cytokine responsive regulatory T cell precursors. J Immunol 2010. 184: 6007–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vang KB, Yang J, Pagan AJ, Li LX, Wang J, Green JM, Beg AA and Farrar MA, Cutting edge: CD28 and c-Rel-dependent pathways initiate regulatory T cell development. J Immunol 2010. 184: 4074–4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breed ER, Watanabe M and Hogquist KA, Measuring Thymic Clonal Deletion at the Population Level. J Immunol 2019. 202: 3226–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH and von Andrian UH, Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol 2006. 7: 1092–1100. [DOI] [PubMed] [Google Scholar]
- 24.Yamano T, Nedjic J, Hinterberger M, Steinert M, Koser S, Pinto S, Gerdes N, Lutgens E, Ishimaru N, Busslinger M, Brors B, Kyewski B and Klein L, Thymic B cells are licensed to present self antigens for central T cell tolerance induction. Immunity 2015. 42: 1048–1061. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Breed ER, Lee YJ, Qian LJ, Jameson SC and Hogquist KA, Myeloid cells activate iNKT cells to produce IL-4 in the thymic medulla. Proc Natl Acad Sci U S A 2019. 116: 22262–22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HJ, Alonzo ES, Dorothee G, Pollard JW and Sant’Angelo DB, Selective depletion of eosinophils or neutrophils in mice impacts the efficiency of apoptotic cell clearance in the thymus. PLoS One 2010. 5: e11439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soga H, Nakamura M, Yagi H, Kayaba S, Ishii T, Gotoh T and Itoh T, Heterogeneity of mouse thymic macrophages: I. immunohistochemical analysis. Arch Histol Cytol 1997. 60: 53–63. [DOI] [PubMed] [Google Scholar]
- 28.Tacke R, Hilgendorf I, Garner H, Waterborg C, Park K, Nowyhed H, Hanna RN, Wu R, Swirski FK, Geissmann F and Hedrick CC, The transcription factor NR4A1 is essential for the development of a novel macrophage subset in the thymus. Sci Rep 2015. 5: 10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry JS, Lio CW, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM and Hsieh CS, Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity 2014. 41: 414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abram CL, Roberge GL, Hu Y and Lowell CA, Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J Immunol Methods 2014. 408: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freeman GJ, Borriello F, Hodes RJ, Reiser H, Gribben JG, Ng JW, Kim J, Goldberg JM, Hathcock K, Laszlo G, A. LL, Wang S, Gray GS, Nadler LM and Sharpe AH, Murine B7–2, an alternative CTLA4 counter-receptor that costimulates T cell proliferation and interleukin 2 production. J Exp Med 1993. 178: 2185–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins M, Ling V and Carreno BM, The B7 family of immune-regulatory ligands. Genome Biol 2005. 6: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pentcheva-Hoang T, Egen JG, Wojnoonski K and Allison JP, B7–1 and B7–2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity 2004. 21: 401–413. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Park J, Foss D and Goldschneider I, Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med 2009. 206: 607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H and Hogquist KA, How Lipid-Specific T Cells Become Effectors: The Differentiation of iNKT Subsets. Front Immunol 2018. 9: 1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Dong M and Wang XG, The implication and significance of Beta 2 microglobulin: A conservative multifunctional regulator. Chin Med J (Engl) 2016. 129: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe M, Fujihara C, Radtke AJ, Chiang YJ, Bhatia S, Germain RN and Hodes RJ, Co-stimulatory function in primary germinal center responses: CD40 and B7 are required on distinct antigen-presenting cells. J Exp Med 2017. 214: 2795–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hilt ZT, Pariser DN, Ture SK, Mohan A, Quijada P, Asante AA, Cameron SJ, Sterling JA, Merkel AR, Johanson AL, Jenkins JL, Small EM, McGrath KE, Palis J, Elliott MR and Morrell CN, Platelet-derived beta2M regulates monocyte inflammatory responses. JCI Insight 2019. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cossarizza A, Chang HD, Radbruch A, Acs A, Adam D, Adam-Klages S, Agace WW, Aghaeepour N, Akdis M, Allez M, Almeida LN, Alvisi G, Anderson G, Andra I, Annunziato F, Anselmo A, Bacher P, Baldari CT, Bari S, Barnaba V, Barros-Martins J, Battistini L, Bauer W, Baumgart S, Baumgarth N, Baumjohann D, Baying B, Bebawy M, Becher B, Beisker W, Benes V, Beyaert R, Blanco A, Boardman DA, Bogdan C, Borger JG, Borsellino G, Boulais PE, Bradford JA, Brenner D, Brinkman RR, Brooks AES, Busch DH, Buscher M, Bushnell TP, Calzetti F, Cameron G, Cammarata I, Cao X, Cardell SL, Casola S, Cassatella MA, Cavani A, Celada A, Chatenoud L, Chattopadhyay PK, Chow S, Christakou E, Cicin-Sain L, Clerici M, Colombo FS, Cook L, Cooke A, Cooper AM, Corbett AJ, Cosma A, Cosmi L, Coulie PG, Cumano A, Cvetkovic L, Dang VD, Dang-Heine C, Davey MS, Davies D, De Biasi S, Del Zotto G, Dela Cruz GV, Delacher M, Della Bella S, Dellabona P, Deniz G, Dessing M, Di Santo JP, Diefenbach A, Dieli F, Dolf A, Dorner T, Dress RJ, Dudziak D, Dustin M, Dutertre CA, Ebner F, Eckle SBG, Edinger M, Eede P, Ehrhardt GRA, Eich M, Engel P, Engelhardt B, Erdei A, Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur J Immunol 2019. 49: 1457–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request from the authors.


