Abstract
While the effects of physical risk factors on MSD development have been a primary focus of musculoskeletal research, psychological stressors and certain personal characteristics (e.g., aging, sex, and obesity) are also associated with increased MSD risk. The psychological and personal characteristics listed above share a common characteristic: all are associated with disruption of the body’s neuroendocrine and immune responses resulting in an impaired healing process. An impaired healing response may result in reduced fatigue life of musculoskeletal tissues due to a diminished ability to keep pace with accumulating damage (ordinarily reparable), and an increased vulnerability of damaged tissue to further trauma owing to the prolonged healing process. Research in engineered self-healing materials suggests that decreased healing kinetics in the presence of mechanical loading can substantially reduce the fatigue life of materials. A model of factors influencing damage accrual and healing is presented.
I. Introduction
Musculoskeletal disorders (MSDs) are comprised of a variety of inflammatory and degenerative conditions in musculoskeletal tissues, which may involve muscles, tendons, ligaments, and peripheral nerves. They are prevalent in society and result in substantial direct and indirect costs for both individuals and industry (National Research Council – Institute of Medicine, 2001; Punnett, et al., 2005; Deeney & O'Sullivan, 2009; Global Burden of Disease Study 2013 Collaborators, 2015; Global Buren of Disease, 2018; Bevan, 2015; Hoy, et al., 2015; Huisstede, Bierma-Zeinstra, Koes, & Verhaar, 2006 Bureau of Labor Statistics, 2019). The association of physical work risk factors and MSDs has been well-studied (e.g., NIOSH, 1997; National Research Council – Institute of Medicine, 2001), and include factors such as high force demands, repetitive work, adoption of non-neutral postures, and/or exposure to vibration (Bongers, De Winter, Kompier, & Hildebrandt, 1993; Bongers, Kremer, & ter Laak, 2002; Deeney & O'Sullivan, 2009; Hauke, Flintrop, Brun, & Rugulies, 2011). Certain personal characteristics are consistently associated with the development of MSDs, including age, sex, and obesity (National Research Council – Institute of Medicine, 2001). MSDs are also associated with psychosocial stress at work, such as high psychological job demands and low job control (Deeney & O'Sullivan, 2009; Davis & Heaney, 2000). Yet, specific mechanisms associated with the increased risk of MSD development from psychological (or psychosocial) stressors and personal characteristics are less well understood than their physical counterparts (Deeney & O'Sullivan, 2009).
It is apparent that musculoskeletal tissues subjected to repeated stress experience tissue damage as the result of a fatigue failure process (e.g., Andarawis-Puri & Flatow, 2011; Barbe, et al., 2013; Brinckmann, et al. 1988; Carter & Hayes, 1976; Cyron & Hutton, 1978; Fung D., et al., 2010; Gallagher & Heberger, 2013; Gallagher, et al., 2007; Schechtman & Bader, 1997; Shepherd & Screen, 2013; Sun, et al., 2010; Weightman, 1976). Fatigue failure is the process by which materials incur cumulative damage development when exposed to repeated stress (Stephens, Fatemi, Stephens, & Fuchs, 2001). Fatigue failure methods have long been employed to evaluate the fatigue life of engineering materials, such as metals, plastics, or composite materials. However, musculoskeletal tissues are also materials, and (like other materials) would also be expected to incur fatigue damage when subjected to repeated stress. Without exception, musculoskeletal tissues tested in vitro or ex vivo display a characteristic fatigue failure response when exposed to repeated stress (e.g., Brinckmann, 1988; Schechtman & Bader, 1997; Thornton & Bailey, 2013; Gallagher, et al., 2007; Carter & Hayes, 1976; Carter, Caler, Spengler, & Frankel, 1981; Weightman, 1976; Shepherd J.H., 2012; Shepherd & Screen, 2013). Results of in vivo animal studies examining the effects of repetitive loading on musculoskeletal tissues also report damage accumulation characteristic of a fatigue failure failure process (e.g., Barbe, et al., 2013; Andarawis-Puri & Flatow, 2011; Fung D., et al., 2010; Fung D., et al., 2009; Sun, et al., 2010). A systematic review of MSD epidemiology studies allowing assessment of a force-repetition interaction found a consistent interaction pattern predicted by fatigue failure theory (Gallagher & Heberger, 2013). Additionally, three recently developed fatigue failure-based risk assessment tools have demonstrated dose-response relationships between fatigue failure damage estimates and multiple low back, upper extremity and shoulder outcomes (Gallagher, Sesek, Schall Jr, & Huangfu, 2017; Gallagher, Schall Jr, Sesek, & Huangfu, 2018; Bani Hani, et al., 2020). Thus, several lines of evidence support the notion that a fatigue failure process is etiologically significant in the development of MSDs.
However, fatigue failure in musculoskeletal tissues differs from the fatigue failure process of inert materials (metals, plastics, etc.) due to the presence of a healing process that can help repair damage incurred. The healing process referred to here is defined as the classic model of wound healing is divided into three sequential, yet overlapping, phases that occur in parallel with hemostasis: (1) inflammatory, (2) proliferative, and (3) remodeling. This healing capacity is clearly extremely important to musculoskeletal health. We will examine the impacts of both damage and healing below.
II. The importance of healing on musculoskeletal tissue fatigue life
The fatigue life of a material (often designated by Nf) is defined as “the number of cycles of stress or strain of a specified character that a given specimen sustains before failure of a specified nature occurs” (Stephens, Fatemi, Stephens, & Fuchs, 2001). Failure could be defined as the initiation of damage, damage reaching a specified size, or complete material failure, for example. In non-biological materials, fatigue life is dependent on two primary factors: the strength of the material and the load stress characteristics. However, biological tissues have an additional factor that likely to influence fatigue life: the ability to repair tissues damaged due to exposure to repeated stress. Clearly, this repair capacity would be expected to extend the fatigue life of the tissue (compared to the absence of such capacity).
A common finding from in vitro or ex vivo material testing of musculoskeletal tissues is that the fatigue life of tissues in these conditions appear to be much lower than that necessary to maintain the health of the material throughout one’s lifetime. For example, in vitro tests on the human extensor digitorum longus indicated that at a stress level of 20 MPa (20% of ultimate tensile strength), the fatigue life of these tendons in vitro was about 300,000 cycles. This is the equivalent of approximately four months of normal walking activity (Schechtman & Bader, 1997). Similar findings have been shown with other musculoskskeletal materials (e.g., Thornton & Bailey, 2013; Shepherd & Screen, 2013; Carter & Hayes, 1976; Brinckmann, Biggemann, & Hilweg, 1988; Gallagher, et al., 2007). Clearly, there must be a reason for the considerable difference between the fatigue life obtained in vitro compared to that observed in vivo. There would appear to be four possible options for this: 1) loads on the tissues are much lower than we believe to be the case, 2) tissue strength in vivo is much greater than that in vitro, 3) the body’s healing process substantially increases fatigue life, or 4) some combination of these factors. There is not much in the way of evidence that the first two options are the case. However, biological tissues have additional factors that are likely to influence fatigue life. One additional factor is the ability to repair tissues damaged due to exposure to repeated stress. Clearly, this repair capacity would be expected to extend the fatigue life of the tissue.
But what happens if the fatigue-life extending healing process becomes disrupted? For example, suppose that there are factors present that slow down the healing process or that impact a portion of the process in a way that reduces the effectiveness of the healing mechanism. There are several reasons to believe that an impaired healing process may have a deleterious effect on the fatigue life of a self-healing tissue. The healing process of soft tissues generally involves the cleaning out of debris from the injured area through phagocytosis and will often be associated with the development of a notch or a groove in the tissue which is gradually filled in from the edges of the wound to the center during the healing process (Gonzalez, Costa, Andrade, & Medrado, 2016). It should be noted that the notched or indented shape of the debrided wound is a shape known to result in a stress concentration and would be expected to be an area vulnerable to additional damage if exposed to sufficient repeated stress. Any process that delays the kinetics of the healing process would extend the period of increased vulnerability for the damaged tissue. This increased vulnerability would be expected to reduce the fatigue life of the healing tissue.
Thus, it would appear fatigue life of a tissue can be influenced by cumulative damage development due to both the traditional fatigue failure process (i.e., increased damage kinetics), but may also be influenced by the kinetics of the repair process. Things that would enhance fatigue life would include avoiding stressful, repetitive loading and/or a more rapid healing process. Factors that would reduce tissue fatigue life would include increased damage kinetics (higher stress and increased repetition) and/or decreased healing kinetics (e.g., an impaired healing response). The fact that these two processes can each influence the fatigue life of musculoskeletal materials suggests that both should be taken into consideration when evaluating musculoskeletal injury risk due to fatigue damage.
The fact that the tissue remodeling and repair processes are constantly at work in the body suggests a potential reason why fatigue life may be extended to such a remarkable degree in musculoskeletal tissues. Through this constant process it would be expected that relatively small areas of damage could be repaired relatively quickly. Repair early in the fatigue failure process can be very effective at reducing the chances of significant damage accumulation (Stephens, Fatemi, Stephens, & Fuchs, 2001; Jones, Rule, Moore, Sottos, & White, 2007; Maiti, Shankar, Geubelle, & Kieffer, 2006). Thus, the turnover of old collagen with new and repair of early microfailures would be expected to lead to significant life extension of these materials. However, the greater the rate of cumulative damage, the more difficult it would be for the healing process to maintain pace with damage development.
Unfortunately, we know little regarding the relationship of these competing processes. However, it would seem that maintenance of musculoskeletal health must involves a dynamic balance between the amount of damage accrual due to fatigue loading versus the amount of healing that can be achieved over a given timeframe (Nash, 1966). Disruption of this balance by either process (or both) would be expected to increase MSD risk. Excessive fatigue loading would increase the rate of damage development and may exceed the normal rate of damage repair (Gallagher & Schall, 2017). On the other side of the ledger, factors that negatively impact repair kinetics would also enhance damage development and reduce fatigue life (Godbout & Glaser, 2006; Gouin & Kiecolt-Glaser, 2011; Guo & DiPietro, 2010), such as from factors related to psychological stress, aging and obesity, as discussed below. An impaired healing capacity could mean, for example, that damage previously repairable by a normal (unimpaired) healing process might instead accumulate (Gallagher & Schall, 2017). An impaired healing response may also extend the period during which a healing tissue, weakened by damage and experiencing a stress concentration in the injured area, would remain vulnerable to the development of additional damage.
III. Psychological (Psychosocial) stress and MSDs
Commonly cited MSD risk factors associated with psychosocial stress include high psychological job demands, low job control, monotonous work and low social support for the worker in the workplace (Deeney & O'Sullivan, 2009; Davis & Heaney, 2000). Job demands include work that is performed under time pressure, work pressure, and/or with low workload variability (NIOSH, 1997). High psychological job demands, or emotionally demanding work have been associated with increased risk of upper extremity MSD complaints in several studies (Smith, Mihashi, Adachi, Koga, & IshitakeT., 2006; Van Den Heuvel, Van Der Beek, Blatter, Hoogendoorn, & Bongers, 2005; Bernard, Sauter, Fine, M., & Hales, 1994; Nicolakakis, Stock, Abrahamowicz, Kline, & Messing, 2017). Low worker job control has also been associated with increased MSD symptoms in the upper extremities (Bernard, Sauter, Fine, M., & Hales, 1994; Hales, et al., 1994; Lagerström, Wenemark, M., & Hjelm, 1995). Jobs that are associated with tedium and little variety are considered monotonous work. Research has demonstrated a relationship between monotonous work and MSDs, including of the neck and shoulder (Harkness, Macfarlane, Nahit, Silman, & Mcbeth, 2003; Ryan & Bampton, 1988; Johansson, et al., 1993). Finally, social support at work is generally defined as how an individual draws support from interpersonal interactions. Examples of low social support at work includes low recognition at work, a lack of promotion prospects, poor support from co-workers and supervisors, hostility at work, and harassment. Low social support has been associated with neck and shoulder MSDs (Aasa, Barnekow-Bergkvist, K.A., & Brulin, 2005) and back pain (Skov, Borg, & Orhede, 1996 ; Nicolakakis, Stock, Abrahamowicz, Kline, & Messing, 2017).
Several theories have been put forth to explain the links between psychosocial factors and MSDs. These include the Biopsychosocial model (Engel, 1977), Hyperventilation theory (Schleifer, Ley, & Spalding, 2002), the Migraine theory (Knardahl, 2002 ), the Muscle Spindle theory (Johansson & Sojka, 1991), the Cinderella hypothesis (Hagg, 1991), and the Nitric Oxide/Oxygen Ratio hypothesis (Eriksen, 2004 ). Most of these theories concentrate on effects such as increased muscle tension and pain (Johansson & Sojka, 1991), decreased blood flow, factors inhibiting the repair of muscle tissue, and prolonged activation of low-threshold motor units (Hauke et al., 2011). While most of these explanations primarily focus on possible psychosocial effects on muscle physiology (Deeney & O'Sullivan, 2009; Hauke, Flintrop, Brun, & Rugulies, 2011), psychosocial factors have also been associated with a number of MSDs involving tendon damage, damage to other musculoskeletal tissues, and/or peripheral nerves (e.g., lateral/medial epicondylitis, low back pain, and carpal tunnel syndrome) (Bugajska, et al., 2013; Thiese, et al., 2020).
Another theory that has been increasingly used to understand how psychological stressors lead to pathophysiological responses in workers is Allostatic Load (i.e., the cost of maintaining Allostasis), proposed by McEwen in 1998 (McEwan, 1998). Allostasis (literally “maintaining stability, or homeostasis, through change”) refers the process of adaptation of an organism to acute stress across all biological systems, as a means to restore homeostasis after a challenge (McEwen, 2000). Biological systems promote and coordinate adaptation using systemic mediators (cortisol, sympathetic and parasympathetic mediators, pro- and anti-inflammatory cytokines, metabolic mediators, and hormones), via a non-linear network in which each mediator regulates other mediators, often in a reciprocal fashion, with the brain typically coordinating these efforts (Karatsoreos & McEwen, 2011; Sterling, 2012). While adaptive acutely, chronic overactivity of a system, such as, cardiovascular, metabolic, immune, hypothalamus-pituitary-adrenal (HPA) axis, sympathetic-adrenal-medullary (SAM) system, and cognitive centers of the brain, in response to chronic or severe stressors (McEwen, 1998; Karatsoreos & McEwen, 2011) can induce a domino effect on the interconnected systems, leading one or more to overcompensate or become dysregulated, and can lead to the eventual disruption of a system, leaving the organism open to stress-related diseases (McEwen & Gianaros, 2011; Juster, McEwen, & SJ., 2010). For example, pro-inflammatory cytokines released from injured cells or macrophages can enter the blood stream and become systemic. This can stimulate production of corticosteroids by the brain that then, in turn, reduce inflammatory cytokine production, as seen in Figure 1. Sympathetic and parasympathetic nervous systems (fight or flight systems) exert differential effects on pro-inflammatory cytokines, with the former stimulating production and the latter inhibiting them. When these responses are unbalanced, appropriate inflammatory responses may be inhibited, or vice versa. Allostatic Load is the accumulated burden (“wear and tear”) on the brain and other systems from trying to re-establish allostasis after exposure to repeated or chronic stressors (McEwen, 1998; McEwen, 2000), while Allostatic Overload occurs when the demands of the stressor exceed the body’s ability to repeatedly adapt, leading to disordered and diseased endpoints (Juster, McEwen, & SJ., 2010). In the current context, an allostatic overload due to psychological stress may result in a diminished healing capacity, which when paired with the physical process of tissue damage (fatigue failure) may result in increased MSD risk. The following sections will examine the effects of psychological stress and certain personal characteristics on healing kinetics and how these factors might influence the development of MSDs.
Figure 1.
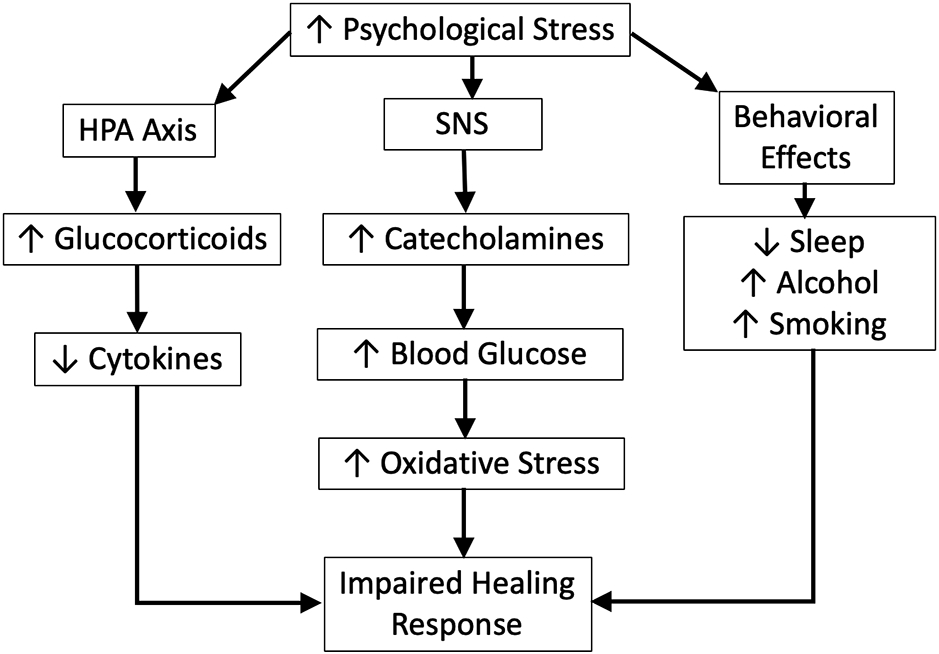
Mechanisms associated with the impaired healing response resulting from psychological stress.
IV. Psychological (Psychosocial) stress and healing
The damage and healing model provided in Figure 1 suggests possible mechanisms by which psychosocial stress may directly impact the development of MSDs through changes in healing responses. Specifically, psychological stress is known to negatively impact the healing of tissues through well-established mechanisms (Chrousos & Gold, 1992; Guo & DiPietro, 2010). This mechanism involves the secretion of various glucocorticoids and catecholamines (e.g., norepinephrine and epinephrine) that inhibit the healing response, as well as reducing sleep time and quality (also known to negatively impact healing), not to mention the development of certain unhealthy behaviors that can lead to additional mechanisms that reduce the effectiveness of healing (such as smoking and alcohol use) (Guo & DiPietro, 2010).
Psychological stress has been shown in many studies to have a significant impact in terms of inhibiting the healing response of tissues (Godbout & Glaser, 2006; Chrousos & Gold, 1992). This has been demonstrated in both animal and human studies. For example, one study found that students facing examination stress during the academic year demonstrated a 40% increase in the time it took to heal a 3.5 mm biopsy punch wound on the hard palate compared to an identical wound placed on the contralateral side during a period of vacation (Marucha, Kiecolt-Glaser, & Favagehi, 1998). All 11 subjects demonstrated a slowed healing response under stress and averaged a 3-day increase in the time to heal. In other research, caregivers operating in stressful situations have demonstrated a similar response (Kiecolt-Glaser, Marucha, Malarkey, Mercado, & Glaser, 1995). Compared with controls, stressed caregivers experienced wound healing averaging 9 days longer (48 versus 39 days). Similarly, individuals living in hostile marital relationships showed a 60% decrease in the rate of wound healing, which was associated with a decrease in IL-1beta, IL-6 and TNF-alpha levels at the wound site (Kiecolt-Glaser, et al., 2005).
A systematic review and meta-analysis examined psychological stress and wound healing in humans (Walburn, Vedhara, Hankins, Rixon, & Weinman, 2009). Of 22 studies accepted for inclusion in the review, 17 found that psychological stress was associated with impaired wound healing or dysregulation of biomarkers associated with wound healing across a variety of experimentally induced wounds and different conceptualizations of psychological stressors. Results of the meta-analysis (involving 12 studies) demonstrated a pooled effect size of r = −0.42 (95% CI = −0.51, −0.32), indicating that greater levels of psychological stress are associated with impaired wound healing.
The delay in healing resulting from stress is partly due to the HPA axis secretion of glucocorticoid hormones. A significant impact of GC compounds is the decreased proliferation and differentiation of immune cells, along with a decrease in gene transcription, and reduce cell adhesion activity vital in the immune healing response (Sternberg, 2006). One potent glucocorticoid (cortisol) acts as a strong anti-inflammatory agent which counters the immune responses involved in the initial phase of healing (Guo & DiPietro, 2010). In this manner, psychological stress can impair the initiation of a normal healing response, resulting in a significant delay in the healing process.
Many of the studies on impaired healing due to psychological stress have dealt with healing of skin or mucosal biopsies. However, it is well-documented that glucocorticoids (such as those secreted as a result of psychological stress) decrease proinflammatory cytokine response in tendons and impair local collagen synthesis (Gouin & Kiecolt-Glaser, 2011). This can lead to tendon atrophy, reduction of tensile strength and decreased load to failure (Balasubramaniam & Prathap, 1972; Dean, et al., 2014; Kapetanos, 1982). A systematic review of the effects of local glucocorticoid administration as a treatment for tendinopathy showed mostly detrimental effects (Dean, et al., 2014). Local glucocorticoid administration reduced collagen synthesis in tendon, and was associated with collagen disorganization and necrosis, all of which adversely affected mechanical properties (including yield stress, yield load and stiffness) (Dean, et al., 2014). This in turn may lead to the development of increased damage accumulation with loading. It may be noted that some research has shown a beneficial healing effect of systemically delivered steroids; however, it appears that this result is dependent on the timing of the administration (it is most effective when administered during the early inflammatory stage) (Blomgran, Hammerman, & Aspenberg, 2017). Unfortunately, the cortisol response to psychosocial stress is not likely to be so carefully timed.
In addition to the effects of stress on glucocorticoids (inhibition of healing processes), psychological stress is also known to adversely impact various behavioral mechanisms that can impair healing, including disturbed sleep, alcohol use, and smoking. Sleep is considered important in the healing process of tissues due in part to the bolus of growth factors secreted during deep sleep (Veldhuis & Iranmanesh, 1996). Disturbed sleep has been shown to negatively impact skin wound healing (Altemus, Rao, Dhabar, & al., 2001; Guo & DiPietro, 2010) as well as muscle repair (Schwarz, Graham, Li, Locke, & Peever, 2013). This may be because disturbed sleep results in elevated serum and tissue levels of glucocorticoids (Guo & DiPietro, 2010). In addition, psychological stress is associated with unhealthy habits, such as increased alcohol use, cigarette smoking, and use of other drugs that can affect healing (Guo & DiPietro, 2010). Heavy alcohol use is associated with decreased cell migration and collagen deposition at the wound site, leading to an impaired healing response (Benveniste & Thut, 1981), similar to smoking (Silverstein, 1992).
Finally, increased catecholamine production, another by-product of psychological stress, is also thought to play a role in inhibiting the rate of wound healing. Studies in mice exposed to various psychological stress modalities have shown that treatment with adrenergic receptor antagonists attenuate the stress-induced impairment of wound healing (Gouin & Kiecolt-Glaser, 2011). These results suggest that catecholamines may play a role in slowing the healing response due to stress (Gouin & Kiecolt-Glaser, 2011).
In summary, there is a substantial amount of research suggesting that increased psychological stress disrupts the body’s healing process, and results in an extended healing process. This would be expected to decrease the fatigue life of musculoskeletal tissues injured due to repeated stress. However, there are several personal characteristics that may also lead to an impaired healing response, which will be discussed in the following section.
V. Effects of Personal Characteristics on wound healing
V.1. Age
It is commonly recognized that individuals of increased age have a healing response that takes significantly longer than individuals of a younger age (Guo & DiPietro, 2010). However, while the healing process takes longer, in healthy aged individuals, the quality of healing is not necessarily impaired (Gosain & DiPietro, 2004). The extended wound healing time appears to be due in part to a delayed inflammatory response (Figure 2). For example, some of the altered wound healing activity in peripheral tissues includes changes in the inflammatory phase, such as, reduced vascular permeability, reduced macrophage infiltration and function, delayed T-cell infiltration, increased pro-inflammatory cytokine and chemokine production, and reduced growth factor production (Swift, Burns, Gray, & DiPietro, 2001). The proliferative and remodelling phases are also affected by age-related changes, including slower collagen deposition and decreased remodelling (Figure 2) (Guo & DiPietro, 2010). At the end of the process, decreased wound strength will often result (Gosain & DiPietro, 2004).
Figure 2.
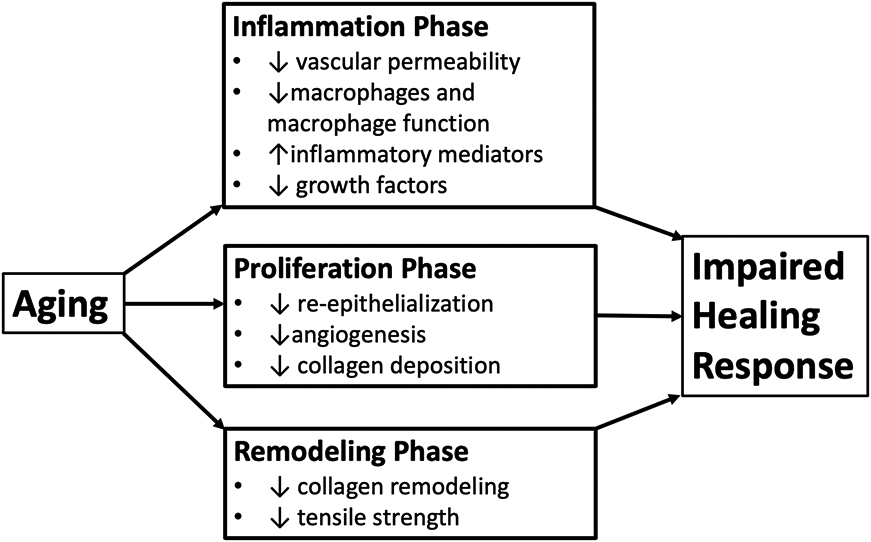
Effect of aging on the healing process (Toy, 2005)
V.2. Sex
The rate of collagen synthesis is a critical factor in the healing of musculoskeletal tissues. Research over the past two decades has demonstrated that a sex difference exists with respect to the rate of collagen synthesis between males and females, with decreased collagen synthesis in the latter (Kjaer, et al., 2009). Females have a lower rate of basal collagen synthesis compared to males, and also demonstrate a decreased collagen synthesis rate compared to males after exposure to exercise (Miller B. , et al., 2006a; Miller B. , et al., 2006b). This decreased collagen synthesis rate has been linked with estrogen levels, as varying levels of estradiol (one of the three estrogen hormones produced by the body) have been associated with a diminished rate of collagen synthesis in females (Hansen, et al., 2008). These findings are supported by in vitro studies in which estradiol receptors have been identified in ligaments (Sciore, Frank, & Hart, 1998), and that estradiol itself can inhibit collagen synthesis in ligaments and tendons (Liu, et al., 1996; Yu, Panossian, Hatch, Liu, & Finerman, 2001). The influence of estradiol levels on collagen synthesis may not be direct, but may be the result of the effect that estradiol has on circulating insulin-like growth factor (IGF-I), a substance directly related with the rate of collagen synthesis (Kjaer, et al., 2009).
V.3. Obesity
Health issues associated with obesity are numerous, and include increased risk of type II diabetes, heart disease, high blood pressure, stroke, sleep apnea, and respiratory problems (Guo & DiPietro, 2010). In addition to these health problems, obese individuals demonstrate an impaired wound healing capability, along with an increased number of complications during the wound healing process (Guo & DiPietro, 2010). Many of these problems stem from a decrease in blood perfusion and ischemia present in adipose tissue. The same tension that causes a wound to experience dehiscence (bursting open) may also contribute to reduced perfusion at the wound site, leading to decreased delivery of oxygen and reducing availability of biochemical compounds necessary to heal the wound (Anaya & Dellinger, 2006; Wilson & Clark, 2004).
Type II Diabetes is strongly associated with obesity and can negatively impact healing kinetics to a great degree (Brem & Tomic-Canic, 2007; Lin, et al., 2017). Hypoxia is often observed in diabetic wounds and can prolong the injury healing time of wounds by increasing the levels of oxygen radicals in diabetics (Guo & DiPietro, 2010). A decrease in the amount of angiogenesis is also commonly observed in diabetics (Brem & Tomic-Canic, 2007). The neuropathy attendant to diabetes probably also increases wound healing times (Guo & DiPietro, 2010). Tendinopathy, tendon ruptures, and impaired tendon healing are all more prevalent among those with diabetes (Maffulli, Longo, Maffulli, Khanna, & Denaro, 2011). High blood glucose levels appear to inhibit proliferation of tendon derived stem cells and increase the rate of cell apoptosis (Lin, et al., 2017). Thus, healing of tendons appears to be significantly delayed in a hyperglycemic environment (Lin, et al., 2017). Figure 3 shows some of the factors that may lead to impaired wound healing in obesity (Guo & DiPietro, 2010).
Figure 3.
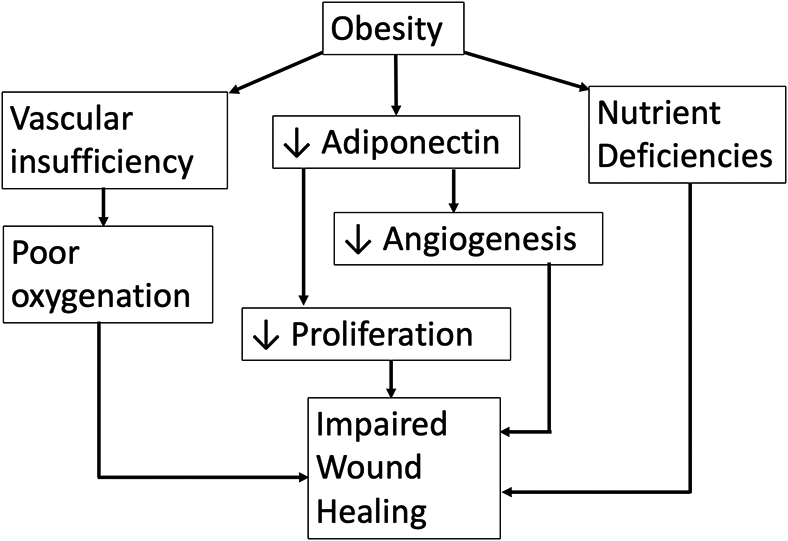
Pathways to impaired wound healing due to obesity.
VI. The impact of healing rate on tissue fatigue life
The evidence presented above suggests that there are several mechanisms that may lead to an impaired healing response when exposed to psychological stress, aging, sex, and/or obesity. Certainly, combinations of these factors may have additive or multiplicative effects in this regard. The question that arises is, “What happens when a biological tissue (exposed to repeated loading and damage accrual) experiences a decrease in healing kinetics?” Unfortunately, the relationship of damage accumulation to healing is exceedingly difficult to ascertain in vivo. In a search of the literature, we found no research that assessed concurrent fatigue damage development and healing rates in musculoskeletal tissues.
However, there is some data that may be relevant to this question from the field of engineered self-healing materials. These are materials that have been inspired by (and designed to mimic) the healing process of biological materials and provide a method of repairing damage accumulated in the material due to fatigue failure. Such studies can provide some insight (clearly with caveats) into the benefits of having a self-healing process on material fatigue life, as well as how the degree of benefit can vary as the result of the rate at which healing occurs.
A fascinating study by Jones and colleagues (2007) provides data on the extension of fatigue life provided by three different rates of healing in a self-healing viscoelastic polymer material. When the material experienced damage (crack formation), microcapsules containing dicyclopentadiene (DCPD) would rupture and fill the crack. This compound was then acted upon by one of three catalyst treatments (each catalyst having different healing rates). The slowest healing treatment used the catalyst as received, the moderate healing condition used the catalyst but in recrystalized form, and the fastest healing process use the catalyst in a freeze-dried state. All specimens were loaded identically using a Kmax of 0676 MPa/m2, R=0.1 and a loading frequency of 5 Hz.
Results of this study demonstrated that the presence of a self-healing process significantly increased fatigue life of the material compared to the control condition (which had no healing capability), even when healing kinetics were relatively slow. Compared with the control condition, the condition with slow healing kinetics exhibited a fatigue life extension of approximately 17 times that of the control condition (1.5 million versus 86,000 cycles). The life extension multipliers of the moderate and fast healing kinetics conditions were 25 (2.2 million cycles) and 32 (2.8 million cycles) times the fatigue life of the control condition, respectively. These data suggest that the ability of a material to self-heal can confer impressive benefits in terms extending a material’s fatigue life.
However, these data also demonstrate that the degree to which material fatigue life is extended is highly dependent on the rate of healing. In this example, the slowest healing process exhibited a fatigue life extension that was only 54% that of the fast-healing condition. Moderate healing kinetics demonstrated about 80% of the fatigue life extension compared to the fast-healing condition (Jones, Rule, Moore, Sottos, & White, 2007). Thus, a dose-response relationship was observed between the rate of healing and the degree to which fatigue life was extended in this self-healing material.
VI.a. Implications
The fatigue life of musculoskeletal tissues may be primarily a function of three parameters: the strength of the material, the load to which it is exposed, and the rate at which damage can be healed. Our “Impaired Healing” hypothesis deals with factors that negatively impact the healing process (psychological stress, aging, sex, and obesity), while the fatigue failure process explains the develop of cumulative loading due to repeated stress. It is the combination of these two processes that will ultimately control the fatigue life of musculoskeletal tissues, according to our model. Changes in any of the parameters above may have a significant impact on musculoskeletal health.
In prior models of the effects of psychosocial stress, emphasis has generally been placed on the loading (damage development) side of the equation, but there are certainly indications that psychological stress may play an influential role in healing kinetics, and this may be a significant reason for the relationship observed between psychological stress and increased MSD prevalence and incidence. In fact, our sense from the literature is that the impact psychological stress has on healing might have a greater impact on the MSD development than the increases in loading that may accrue from such stress. However, it may well be that psychological stress has an influence on both damage accrual and impaired healing.
As discussed earlier, however, it is not just psychological stress that can negatively affect the healing process. Personal characteristics such as age, sex, and obesity are known to influence the healing process. Figure 4 provides an overall model of this relationship, incorporating both cumulative damage development (left side) and factors impairing the healing process (right side). In this model, we will talk in terms of the kinetics of damage development and healing. If the damage kinetics are lower than the healing kinetics, this would be expected to lead to a restorative repair of the musculoskeletal tissue. However, if damage kinetics exceed the healing kinetics, damage will accumulate in the tissue and this accumulation will continue as long as repeated stress is experienced. Damage kinetics are primarily the result of repeated stress (cyclic and creep loading) experienced by the tissues and the resultant process of fatigue failure. The higher the rate of cumulative damage, the greater the risk that damage may exceed the healing capacity. Healing kinetics can be positively influenced by factors such as good quality sleep and exercise (not shown in the model). However, the focus of this article is that psychological stress, aging, obesity and certain disease states (such as diabetes) can negatively impact healing rates. Obviously, lower rates of fatigue damage and a higher rates of healing kinetics are the most desirable condition and would lead to restorative repair of the damage incurred. However, all too often, the damage kinetics exceed the healing capacity, potentially leading to accumulating damage in the tissues and development of MSDs. When examining the model in Figure 4, it should be kept in mind that pathways to impaired healing due to psychological stress, age, and obesity have been provided in Figures 1-3, respectively.
Figure 4.
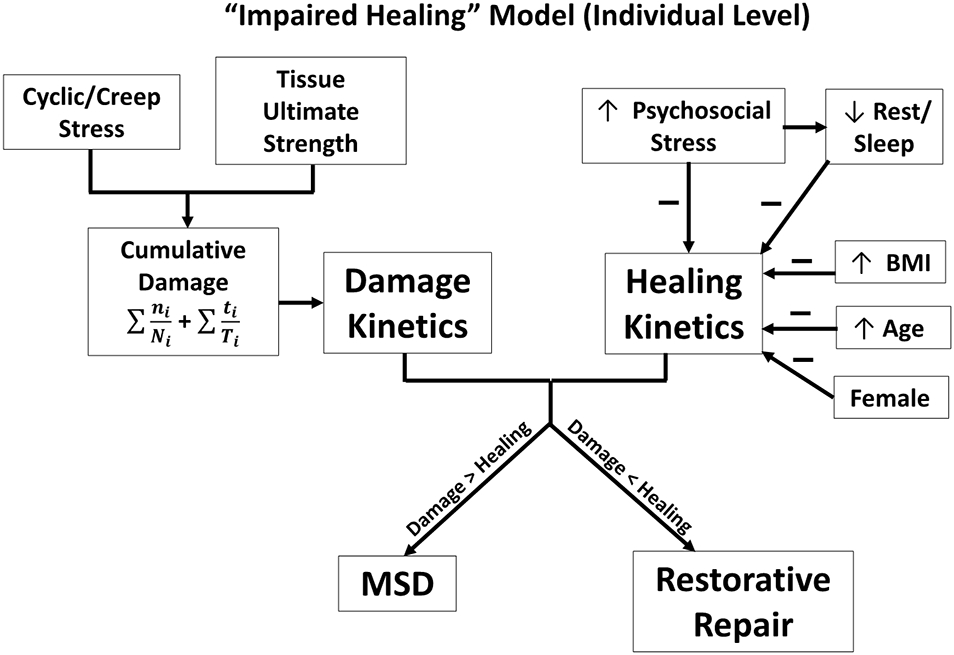
An individual-level model of the relationship of damage versus healing kinetics and selected MSD risk factors.
Because the processes of damage development and healing are generally hidden from view (much likely occurs at the microscopic level), the damage/repair relationship in biological tissues is not easily measured, and much remains to be understood. Many important questions need to be answered. For example, a common finding when performing fatigue failure tests of musculoskeletal tissues in vitro is that the number of cycles to failure (even at lower levels of stress) is much smaller than the materials are known to last during a lifetime (Schechtman & Bader, 1997; Brinckmann, Biggemann, & Hilweg, 1988; Thornton & Bailey, 2013). Certainly, the presence of a healing process would be expected to confer some benefit in this regard, but how much? Another question is: to what degree is rest helpful to the healing process?
VIII. Discussion
Our Impaired Healing Hypothesis suggests that a significant risk for MSDs may be due to an impaired healing response resulting from psychosocial stress, age, sex, and/or BMI has some attendant limitations which should be noted. For example, it is very difficult to measure healing rates of musculoskeletal tissues in the living state. It is much easier to observe and measure the healing rate of skin or mucosal wounds, and this is undoubtedly the reason why most the research on the effects of psychosocial stress on wound healing has focused on these tissues (Guo & DiPietro, 2010). However, as noted earlier, the administration of glucocorticoids on tendon has been found decrease proinflammatory cytokine response at the wound site and inhibit collagen synthesis, both of which would be expected to extend healing time in tendons. The skin wound limitation also applies to research on impaired healing in obese individuals. However, research on impaired healing in females and with age have been demonstrated on musculoskeletal tissues and injuries.
In addition, while engineered self-healing materials were inspired by the biological self-healing process, there are certainly differences in the healing processes between the biological healing process versus the engineered variety. Notably, the engineered healing procedure is more rapid than the biological one. However, the general trends observed regarding the benefits of a healing process (versus none) in extending fatigue life and with different healing rates (faster healing kinetics leading to increased fatigue life under equivalent loading) would seem in line with what would be anticipated in biological tissues. That is, one would expect that having a material that self-repairs would lead to increased fatigue life. Further, the faster the healing kinetics, the greater would be the anticipated benefit to material fatigue life.
One of the key points of this article is that the rate of damage accumulation and the rate of healing are both important determinants of MSD risk. In the ergonomics literature, much greater emphasis has been put on the former compared to the latter. However, when we look at factors apart from those on the physical loading side (i.e., repeated stress), we find that those most commonly associated with MSDs (psychological factors, older age, female sex, and obesity) are all factors that exhibit an impaired rate of healing. Fatigue failure techniques can help us quantify the loading associated with application of repeated stress; however, the healing side of the equation may be equally as important, but more poorly understood, due in large part to the difficulty of quantifying the healing process. However, a clearer understanding of the interplay between the rate of damage accumulation and the rate of healing would appear to be one of the most important research goals in the field of musculoskeletal disorders. Without a clearer picture of the interplay of these two factors, our understanding of the development of MSDs will remain unacceptably deficient.
IX. Conclusion
The fatigue life of musculoskeletal tissues is a likely a function of tissue strength, the stress magnitude and repetition of the loading experienced, and the ability of the healing process to renew or restore damaged tissue. Much research has focused on the physical aspects of this equation; however, the remodeling/healing processes are also an important aspect of musculoskeletal health and deserve attention. Several well-established MSD risk factors such as psychological stress, increasing age, being female, and obesity are known to negatively impact the healing process, which would be expected to reduce fatigue life and increase MSD risk. High levels of fatigue loading in conjunction with factors that impair the healing response would be anticipated to substantially elevate MSD risk. The fatigue failure model is well-positioned to incorporate healing due to its focus on cumulative damage development, as healing would simply account for a decrease (or impaired healing an increase) in cumulative damage development. Unfortunately, our current understanding of the interaction between damage accumulation and healing in musculoskeletal tissues is deficient and would greatly benefit from additional research.
References
- Aasa U, Barnekow-Bergkvist M, K.A. A, & Brulin C (2005). Relationships between work-related factors and disorders in the neck-shoulder and low-back region among female and male ambulance personnel. Journal of Occupational Health, 47, 481–489. [DOI] [PubMed] [Google Scholar]
- Aissa B, Therriault D, Haddad E, & Jamroz W (2012). Self-Healing Materials Systems: Overview of Major Approaches and Recent Developed Technologies. Advances in Materials Science and Engineering, 17 pages. [Google Scholar]
- Altemus M, Rao B, Dhabar F, & al., e. (2001). Stress-induced changes in skin barrier function in healthy women. J Invest Dermatol, 117, 309–217. [DOI] [PubMed] [Google Scholar]
- Anaya D, & Dellinger E (2006). The obese surgical patient: a susceptible host for infection. Surg Infect (Larchmt), 7, 473–480. [DOI] [PubMed] [Google Scholar]
- Andarawis-Puri N, & Flatow E (2011). Tendon fatigue in response to mechanical loading. Journal of Musculoskeletal and Neuronal Interactions, 11, 106–114. [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam P, & Prathap K (1972). The effect of injection of hydrocortisone into rabbit calcaneal tendons. J Bone Joint Surg Br., 54, 729–734. [PubMed] [Google Scholar]
- Bani Hani D, Huangfu R, Sesek R, Schall M Jr., Davis G, & Gallagher S (2020). Development and Validation of a Cumulative Exposure Shoulder Risk Assessment Tool Based on Fatigue Failure Theory. Ergonomics, DOI: 10.1080/00140139. [DOI] [PubMed] [Google Scholar]
- Barbe M, Gallagher S, Massicotte V, Tytell M, Popoff S, & Barr-Gillespie A (2013). The interaction of force and repetition on musculoskeletal and neural tissue responses and sensorimotor behavior in a rat model of work-related musculoskeletal disorders. BMC Musculoskskeletal Disorders, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe M, Hilliard B, Amin M, Harris M, Hobson L, Cruz G, & Popoff S (2020). Blocking CTGF/CCN2 reduces established skeletal muscle fibrosis in a rat model of overuse injury. FASEB J., 34, 6554–6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D Jr., Wyatt H, Thompson H, Peters J, & Hill J (2010). Pedometer-Measured Physical Activity and Health Behaviors in U.S. Adults. Medicine & Science in Sports & Exercise, 42 , 1819–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste K, & Thut P (1981). The effect of chronic alcoholism on wound healing. Proc Exp Biol Med, 166, 568–575. [DOI] [PubMed] [Google Scholar]
- Bernard B, Sauter S, Fine L, M. P, & Hales T (1994). Job task and psychosocial risk factors for work-related muscu- loskeletal disorders among newspaper employees. Scandinavian Journal of Work, Environment and Health, 20, 417–426. [DOI] [PubMed] [Google Scholar]
- Bevan S (2015). Economic impact of musculoskeletal disorders (MSDs) on work in Europe. Best Practice & Research Clinical Rheumatology, 29, 356–373. [DOI] [PubMed] [Google Scholar]
- Blomgran P, Hammerman M, & Aspenberg P (2017). Systemic corticosteroids improve tendon healing when given after the early inflammatory phase. Sci Rep, 7, 12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongers P, De Winter C, Kompier M, & Hildebrandt V (1993). Psychosocial factor at work and musculoskeletal disease. Scandinavian Journal of Work, Environment and Health, 19, 297–312. [DOI] [PubMed] [Google Scholar]
- Bongers P, Kremer A, & ter Laak J (2002). Are psychosocial factors, risk factors for symptoms and signs of the shoulder, elbow, or hand/wrist? A review of the epidemiological literature. American Journal of Industrial Medicine, 41, 315–342. [DOI] [PubMed] [Google Scholar]
- Brem H, & Tomic-Canic M (2007). Cellular and molecular basis of wound healing in diabetes. J Clin Invest, 117, 1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinckmann P, Biggemann M, & Hilweg D (1988). Fatigue Fracture of Human Lumbar Vertebrae. Clinical Biomechanics, 3 (Suppl. 1):, S1–S23. [DOI] [PubMed] [Google Scholar]
- Bugajska J, Zołnierczyk-Zreda D, Jędryka-Góral A, Gasik R, Hildt-Ciupińska K, Malińska M, & Bedyńska S (2013). Psychological factors at work and musculoskeletal disorders: a one year prospective study. Rheumatology international, 33, 2975–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdorf A, Van Riel M, & Brand T (1997). Physical load as a risk factor for musculoskeletal complaints among tank terminal workers. American Industrial Hygiene Association Journal, 58, 489–497. [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics. (2019, June 28). TABLE R19. Number of nonfatal occupational injuries and illnesses involving days away from work by part of body and selected natures of injury or illness, private industry, 2018.". Retrieved from https://www.bls.gov/iif/oshwc/osh/case/cd_r19_2018.htm
- Carter D, & Hayes W (1976). Fatigue life of compact bone--I. Effects of stress amplitude, temperature and density. Journal of Biomechanics, 9, 27–34. [DOI] [PubMed] [Google Scholar]
- Carter D, Caler W, Spengler D, & Frankel V (1981). Fatigue behavior of adult cortical bone: the influence of mean strain and strain range. Acta Orthopaedica Scandinavica, 52, 481–490. [DOI] [PubMed] [Google Scholar]
- Chrousos G, & Gold P (1992). The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Journal of the American Medical Association, 267, 1244–1252. [PubMed] [Google Scholar]
- Cleak M, & Eston R (1992). Muscle soreness, swelling, stiffness and strength loss after intense eccentric exercise. British Journal of Sports Medicine, 26, 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I (2006). The Evolution of Wound Healing. In Veeves A, Giurini J, & Logerfo F, The Diabetic Foot (pp. 51–57). Totowa, NJ: Humana Press. [Google Scholar]
- Cyron B, & Hutton W (1978). The fatigue strength of the lumbar neural arch in spondylolysis. Journal of Bone and Joint Surgery, 60B, 234–238. [DOI] [PubMed] [Google Scholar]
- Davis K, & Heaney C (2000). The relationship between psychosocial work characteristics and low back pain: underlying methodological issues. Clinical Biomechanics, 15, 389–406. [DOI] [PubMed] [Google Scholar]
- Dean B, Lostis E, Oakley T, Rombach I, Morrey M, & Carr A (2014). The risks and benefits of glucocorticoid treatment for tendinopathy: a systematic review of the effects of local glucocorticoid on tendon. Semin Arthritis Rheum., 43, 570–576. [DOI] [PubMed] [Google Scholar]
- Deeney C, & O'Sullivan L (2009). Work related psychosocial risks and musculoskeletal disorders: potential risk factors, causation and evaluation methods. Work, 34, 239–248. [DOI] [PubMed] [Google Scholar]
- Engel G (1977). The need for a new medical model: a challenge for biomedicine. Science, 196 , 129–136. [DOI] [PubMed] [Google Scholar]
- Engstrom T, Johansson J, Jonsson D, & Medbo L (1995). Empirical evaluation of the reformed assembly work at the Volvo Uddevalla plant: Psychosocial effects and performance. International Journal of Industrial Ergonomics, 16, 293–308. [Google Scholar]
- Eriksen W (2004. ). Linking work factors to neck myalgia: the nitric oxide/oxygen ratio hypothesis . Medical Hypotheses, 62, 721–726. [DOI] [PubMed] [Google Scholar]
- Forsman M, Kadefors R, Zhang Q, Birch L, & Palmerud G (1999). Motor-unit recruitment in the trapezius muscle during arm movements and in VDU precision work. International Journal of Industrial Ergonomics, 24, 619–630. [Google Scholar]
- Fung D, Wang V, Andarawis-Puri N, Basta-Pljakic J, Li Y, Laudier D, … Flatow E (2010). Early response to tendon fatigue damage accumulation in a novel in vivo model. Journal of Biomechanics, 43, 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung D, Wang W, Laudier D, Shine J, Basta-Pljakic J, Jepsen KS, & Flatow E (2009). Subrupture tendon fatigue damage. Journal of Orthopaedic Research, 27, 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S, & Heberger J (2013). Examining the interaction of force and repetition on musculoskeletal disorder risk: a systematic literature review. Human Factors, 14, 108–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S, & Schall JM (2017). Musculoskeletal disorders as a fatigue failure process: Evidence, implications and research needs. Ergonomics, 60, 255–269. [DOI] [PubMed] [Google Scholar]
- Gallagher S, Marras W, Litsky A, Burr D, Landoll J, & Matkovic V (2007). A Comparison of Fatigue Failure Responses of Old versus Middle-Aged Lumbar Motion Segments in Simulated Flexed Lifting. Spine, 32, 1832–1839. [DOI] [PubMed] [Google Scholar]
- Gallagher S, Schall M Jr, Sesek R, & Huangfu R (2018). An upper extremity risk assessment tool based on material fatigue failure theory: the Distal Upper Extremity Tool (DUET). Human factors, 60, 1146–1162. [DOI] [PubMed] [Google Scholar]
- Gallagher S, Sesek R, Schall M Jr, & Huangfu R (2017). Development and validation of an easy-to-use risk assessment tool for cumulative low back loading: The Lifting Fatigue Failure Tool (LiFFT). Applied ergonomics, 63, 142–150. [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet, 392, 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Study 2013 Collaborators . (2015). Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet, 386, 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout J, & Glaser R (2006). Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol , 1, 421–427. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Costa T, Andrade Z, & Medrado A (2016). Wound healing - A literature review. . An Bras Dermatol., 91, 614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosain A, & DiPietro L (2004). Aging and wound healing. World J Surg, 28, 321–326. [DOI] [PubMed] [Google Scholar]
- Gouin J-P, & Kiecolt-Glaser J (2011). The impact of psychological stress on wound healing. Immunol Allergy Clin N Am, 31, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, & DiPietro L (2010). Factors affecting wound healing. Journal of Dental Research, 89, 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg G (1991). Static work loads and occupational myalgia – a new explanation model. Electromyographical Kinesiology, 141–144. [Google Scholar]
- Hales T, Sauter S, Peterson M, Fine L, Putz- Anderson V, Schleifer L, … Bernard B (1994). Musculoskeletal disorders among visual display terminal users in a telecommunications company. Ergonomics, 37, 1603–1621. [DOI] [PubMed] [Google Scholar]
- Hansen M, Miller B, Holm LD, Petersen S, Skovgaard D, Frystyk J, … Langberg H (2008). Effect of administration of oral contraceptives in vivo on collagen synthesis in tendon and muscle connective tissue in young women. J Appl Physiol, 586, 3005–3016. [DOI] [PubMed] [Google Scholar]
- Harkness E, Macfarlane G, Nahit E, Silman A, & Mcbeth J (2003). Mechanical and psychosocial factors predict new onset shoulder pain: a prospective cohort study of newly em- ployed workers, Journal of Occupational and Environmental Medicine. Journal of Occupational and Environmental Medicine, 60, 1603–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Adamson C, Eisen E, Kapellusch J, Garg A, Hegmann K, Thiese M, … Silverstein B (2015). Biomechanical risk factors for carpal tunnel syndrome: a pooled study of 2474 workers. Occupation. Occupational and Environmental Medicine, 72, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauke A, Flintrop J, Brun E, & Rugulies R (2011). The impact of work-related psychosocial stressors on the onset of musculoskeletal disorders in specific body regions: a review and meta-analysis of 54 longitudinal studies. Work & Stress, 25, 243–256. [Google Scholar]
- Hoy D, Smith E, Cross M, Sanchez-Riera L, Blyth F, Buchbinder R, … March L (2015). Reflecting on the global burden of musculoskeletal conditions: lessons learnt from the global burden of disease 2010. Ann Rheum Dis, 74, 4–7. [DOI] [PubMed] [Google Scholar]
- Huber G, Nagel K, Skrzypiec D, Klein A, Püschel K, & Morlock M (2016). A description of spinal fatigue strength. Journal of Biomechanics, 49, 875–880. [DOI] [PubMed] [Google Scholar]
- Huisstede B, Bierma-Zeinstra S, Koes B, & Verhaar J (2006). ) Incidence and prevalence of upper-extremity musculoskeletal disorders. A systematic appraisal of the literature. BMC Musculoskeletal Disorders, 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H, & Sojka P (1991). Pathophysiological mechanisms involved in genesis and spread of muscular tension in occupational muscle pain and in chronic musculoskeletal pain syndromes: a hypothesis . Medical Hypotheses, 35 , 196–203. [DOI] [PubMed] [Google Scholar]
- Johansson J, Kadefors R, Rubenowitz S, Klingenstierna U, Lindstrom I, Engstrom T, & Johansson M (1993). Musculoskeletal symptoms, ergonomic aspects and psychosocial factors in two different truck assembly concepts. International Journal of Industrial Ergonomics, 12, 35–48. [Google Scholar]
- Jones A, Rule J, Moore J, Sottos N, & White S (2007). Life extension of self-healing polymers with rapidly growing fatigue cracks. Journal of the Royal Society, Interface, 4 , 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster R, McEwen B, & SJ. L (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev., 35, 2–16. [DOI] [PubMed] [Google Scholar]
- Kapetanos G (1982). The effect of the local corticosteroids on the healing and biomechanical proper- ties of the partially injured tendon. Clin Orthop Relat Res, 170–179. [PubMed] [Google Scholar]
- Karatsoreos I, & McEwen B (2011). Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci., 15, 576–584. [DOI] [PubMed] [Google Scholar]
- Ker R (2002). The implications of the adaptable fatigure quality of tendons for their construction, repair, and function. Comparative Biochemistry and Physiology (part A), 133, 987–1000. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J, Loving T, Stowell J, Malarkey W, Lemeshow S, Dickinson S, & Glaser R (2005). Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry, 62, 1377–1384. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J, Marucha P, Malarkey W, Mercado A, & Glaser R (1995). Slowing of wound healing by psychological stress. Lancet, 346, 1194–1196. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Langberg K, Heinemeier M, Bayer M, Hansen M, … Magnusson S (2009). From mechanical loading to collagen synthesis, strucural changes and function in human tendon. Scandinavian Journal of Medicine and Science in Sport, 19, 500–510. [DOI] [PubMed] [Google Scholar]
- Knardahl S (2002). Psychophysiological mechanisms of pain in computer work: the blood vessel-nociceptor interaction hypothesis . Work and Stress, 16, 179–189. [Google Scholar]
- Lagerström M, Wenemark M, M. H, & Hjelm E (1995). Occupational and individual factors related to musculoskeletal symptoms in five body regions among Swedish nursing per- sonnel, International Archives of Occupational and Environmental Health. International Archives of Occupational and Environmental Health, 68, 27–35. [DOI] [PubMed] [Google Scholar]
- Leask A (2020). Slow train coming: an anti-CCN2 strategy reverses a model of chronic overuse muscle fibrosis. J Cell Commun Signal, 14, 349–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Li Y, Rui Y, Dai G, Shi L, Xu H, … Teng G (2017). The effects of high glucose on tendon-derived stem cells: implications of the pathogenesis of diabetic tendon disorders. Oncotarget, 8, 17518–17528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, al Shaikh R, Panossian V, Yang R, Nelson S, Soleiman N, … Lane J (1996). Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. J Orthop Res, 14, 526–533. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Longo U, Maffulli G, Khanna A, & Denaro V (2011). Achilles tendon ruptures in diabetic patients. Arch Orthop Trauma Surg., 131, 33–38. [DOI] [PubMed] [Google Scholar]
- Maiti S, Shankar C, Geubelle P, & Kieffer J (2006). Continuum and Molecular-Level Modeling of Fatigue Crack Retardation in Self-Healing Polymers . ASME. J. Eng. Mater. Technol , 128, 595–602. [Google Scholar]
- Marucha P, Kiecolt-Glaser J, & Favagehi M (1998). Mucosal Wound Healing Is Impaired by Examination Stress . Psychosomatic Medicine, 60, 362–365. [DOI] [PubMed] [Google Scholar]
- McEwan B (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Acad Sci, 840, 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen B (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci, 840, 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen B (2000). Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology, 22, 108–124. [DOI] [PubMed] [Google Scholar]
- McEwen B, & Gianaros P (2011). Stress- and allostasis-induced brain plasticity. Annu Rev Med. , 62, 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Hansen M, Olesen J, Flyvbjerg A, Schwarz P, Babraj J, … Kjær M (2006a). No effect of menstrual cycle on myofibrillar andconnective tissue synthesis incontracting skeletal muscle. Am J Physiol , 290, E163–E168. [DOI] [PubMed] [Google Scholar]
- Miller B, Hansen M, Olesen J, Schwarz P, Babraj J, Smith K, … Kjær M (2006b). Tendon collagensynthesis at rest and after exercise in women. J Appl Physiol, 102, 542–547. [DOI] [PubMed] [Google Scholar]
- Miner MA (1945). Cumulative Damage In Fatigue. Journal of Applied Mechanics, 16, A159–A164. [Google Scholar]
- Nash JC (1966). Fatigue of Self-Healing Structure: A Generalized Theory of Fatigue Failure. New York: American Society of Mechanical Engineers. [Google Scholar]
- National Research Council – Institute of Medicine. (2001). Musculoskeletal disorders and the workplace: low back and upper extremities. Washington, DC: National Academy Press. [PubMed] [Google Scholar]
- Nicolakakis N, Stock S, Abrahamowicz M, Kline R, & Messing K (2017). Relations between work and upper extremity musculoskeletal problems (UEMSP) and the moderating role of psychosocial work factors on the relation between computer work and UEMSP. Int Arch Occup Environ Health, 90, 751–764. [DOI] [PubMed] [Google Scholar]
- NIOSH. (1997). Musculoskeletal Disorders and Workplace Factors. Cincinnati, OH: National Institute for Occupational Safety and Health. [Google Scholar]
- Palmgren A (1924). Die Lebebsdaue rvon Kugellagern. Z. Ver. Dt. Ing, 68, 339–341. [Google Scholar]
- Paris P, Gomez M, & Anderson W (1961). A rational analytic theory of fatigue. Trends in Engineering, 13, 9. [Google Scholar]
- Punnett L, Prüss-Ütün A, Nelson D, Fingerhut M, Leigh J, Tak S, & Phillips S (2005). Estimating the global burden of low back pain attributable to combined occupational exposures. American Journal of Industrial Medicine, 48, 459–469. [DOI] [PubMed] [Google Scholar]
- Ryan G, & Bampton M (1988). Comparison of data process operators with and without upper limb symptoms. Community Health Studies, 12, 63–68. [DOI] [PubMed] [Google Scholar]
- Sandrey M (2003). Acute and chronic tendon injuries: factors affecting the healing response and treatment. Journal of sport rehabilitation, 12, 70–91. [Google Scholar]
- Schechtman H, & Bader D (1997). In vitro fatigue of human tendons. Journal of Biomechanics, 829–835. [DOI] [PubMed] [Google Scholar]
- Schleifer L, Ley R, & Spalding T (2002). A hyperventilation theory of job stress and musculoskeletal disorders. American Journal of Industrial Medicine, 41, 420–432. [DOI] [PubMed] [Google Scholar]
- Schwarz P, Graham W, Li F, Locke M, & Peever J (2013). Sleep deprivation impairs functional muscle recovery following injury. Sleep Medicine, 14 , e262. [Google Scholar]
- Sciore P, Frank C, & Hart D (1998). Identification of sex hormone receptors in human and rabbit ligaments of the knee by reverse transcription-polymerase chain reaction: evidence that receptors are present in tissue from both male and female subjects . J Orthop Res, 16, 604–610. [DOI] [PubMed] [Google Scholar]
- Shepherd JH, L. K. (2012). The Fatigue Behaviour of Functionally Distinct Bovine Tendons. . BSMB Special Meeting – Tendinopathy – from basic science to treatment. Norwich, UK. [Google Scholar]
- Shepherd J, & Screen H (2013). Fatigue loading of tendon. International Journal of Experimental Pathology, 94, 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein P (1992). Smoking and wound healing. Am J Med, 93, 22S–24S. [DOI] [PubMed] [Google Scholar]
- Skov T, Borg V, & Orhede E (1996. ). Psychosocial and physical risk factors for musculoskeletal disorders of the neck, shoulders, and lower back in salespeople. Occupational and Environmental Medicine, 53, 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Mihashi M, Adachi Y, Koga H, & Ishitake T (2006). A detailed analysis of musculoskeletal disorder risk factors among Japanese nurses,. Journal of Safety Research, 37, 195–200. [DOI] [PubMed] [Google Scholar]
- Snedeker J, & Foolen J (2017). Tendon injury and repair - A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomaterialia, 63, 18–36. [DOI] [PubMed] [Google Scholar]
- Stauber W (2004). Factors involved in strain-induced injury in skeletal muscles and outcomes of prolonged exposures. J Electromyogr Kinesiol., 14, 61–70. [DOI] [PubMed] [Google Scholar]
- Stephens R, Fatemi A, Stephens R, & Fuchs H (2001). Metal Fatigue in Engineering (2nd Edition ed.). New York: John Wiley & Sons. [Google Scholar]
- Sterling P (2012). Allostasis: a model of predictive regulation. Physiology & behavior, 106, 5–15. [DOI] [PubMed] [Google Scholar]
- Sternberg E (2006). Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol, 6, 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockstill J, Harn S, D. S, & Hruska R (1993). Prevalence of upper extremity neuropathy in a clinical dentist population. Journal of the American Dental Association, 124, 67–72. [DOI] [PubMed] [Google Scholar]
- Sun H, Andarawis-Puri N, Li Y, Fung D, Lee J, Wang V, … Flatow EL (2010). Cycle-dependent matric remodeling gene expression response in fatigue-loaded rat patellar tendons. Journal of Orthopaedic Research, 28, 1380–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift M, Burns A, Gray K, & DiPietro L (2001). Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol, 117, 1027–1035. [DOI] [PubMed] [Google Scholar]
- Thiese M, Lu M, Merryweather A, Tang R, Ferguson S, Malloy E, & … Kapellusch J (2020). Psychosocial Factors and Low Back Pain Outcomes in a Pooled Analysis of Low Back Pain Studies. Journal of Occupational and Environmental Medicine, 62, 810–815. [DOI] [PubMed] [Google Scholar]
- Thornton G, & Bailey S (2013). Healing ligaments have shorter lifetime and greater strain rate during fatigue than creep at functional stresses. Journal of Biomechanical Engineering, 135, 1713–1721. [DOI] [PubMed] [Google Scholar]
- Toy L (2005). How much do we understand about the effects of ageing on healing? Journal Of Wound Care, 14, 472. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel S, Van Der Beek A, Blatter B, Hoogendoorn W, & Bongers P (2005). Psychosocial work characteristics in relation to neck and upper limb symptoms. Pain, 114, 47–53. [DOI] [PubMed] [Google Scholar]
- Veldhuis J, & Iranmanesh A (1996). Physiological regulation of the human growth hormone (GH)-insulin-like growth factor type I (IGF-I) axis: predominant impact of age, obesity, gonadal function, and sleep. Sleep, 19(suppl_10) , S221–S224. [DOI] [PubMed] [Google Scholar]
- Walburn J, Vedhara K, Hankins M, Rixon L, & Weinman J (2009). Psychological stress and wound healing in humans: a systematic review and meta-analysis. Journal of psychosomatic research, 67, 253–271. [DOI] [PubMed] [Google Scholar]
- Weightman B (1976). Tensile fatigue of human articular cartilage. Journal of Biomechanics, 9, 193–200. [DOI] [PubMed] [Google Scholar]
- Wikipedia. (2020, August 28). Self-Healing Materials. Retrieved from https://en.wikipedia.org/wiki/Self-healing_material
- Wilson J, & Clark J (2004). Obesity: impediment to postsurgical wound healing. Adv Skin Wound Care, 17, 426–435. [DOI] [PubMed] [Google Scholar]
- Wool R (2008). Self-healing material: a review. Soft Matter, 4, 400–418. [DOI] [PubMed] [Google Scholar]
- Yu W, Panossian V, Hatch J, Liu S, & Finerman G (2001). Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin Orthop, 21, 268–281. [DOI] [PubMed] [Google Scholar]


