Abstract
The contribution of glutamyl transpeptidase (GGT) (γ-glutamyltransferase [EC 2. 3. 2. 2]) to Helicobacter pylori virulence was investigated in piglets and mice using GGT-deficient isogenic strains. All animals became colonized. However, the bacterial load was significantly lower for mutant bacteria than for parent strains. These results suggest that GGT activity provides an advantage to H. pylori in colonization.
Helicobacter pylori is a gram-negative spiral bacterium that causes gastritis and ulcers and is associated with gastric cancer (14, 24, 28, 31). The mechanisms by which H. pylori colonizes and persists within the gastric mucosa are poorly understood. Elucidating the mechanisms involved in both survival and virulence of pathogenic bacteria is often facilitated through the use of animal models. Until recently, most H. pylori animal models were cost and space prohibitive for many researchers. These models included nonhuman primates (4, 5, 8, 13, 17), gnotobiotic piglets (11, 12, 21), and the domestic cat (16). Of these, the gnotobiotic piglet has been one of the most widely used and trusted models for H. pylori infection. Recently, the study of H. pylori immunity and pathogenesis has been greatly facilitated by the development of several murine models of H. pylori infection (19, 22, 23, 32).
The expansion of the number of animal models should facilitate the characterization of putative virulence and colonization factors. One such potential virulence factor is the glutamyl transpeptidase (GGT) (γ-glutamyltransferase [EC 2. 3. 2. 2]) enzyme. In mammalian tissues, GGT activity has been well studied and includes such functions as transpeptidation reactions and glutathione synthesis (35). However, GGT expression and activity in bacteria has been poorly characterized. Recently, the ggt gene for H. pylori was identified and sequenced by Chevalier et al. (7), making it one of only several bacterial species in which the ggt gene has been characterized (18, 34, 38). Chevalier et al. reported that deletion of GGT had no deleterious effect on the ability of H. pylori to grow in culture, but GGT is essential for H. pylori infection of the mouse (7). The present study was performed to extend the findings of Chevalier et al. by employing isogenic, GGT-deficient H. pylori mutants in the gnotobiotic-piglet model. However, we now report, using two distinct animal models, that although GGT activity may provide some advantage to H. pylori in colonization of the gastric mucosa, it is not essential for initial colonization or maintenance of chronic infection.
The ggt::aph insertional mutation was constructed utilizing the sequence of the ggt gene (open reading frame 1118) from the database of The Institute for Genomic Research. The 5′ end of the gene was amplified with primer GTHPF4 (CATCGTCTCTTGTAATGAG) and primer GTHPR5 (CGACGAGATCTCGCTGCCGAAGCGATGCG). The 3′ end of the gene was amplified with primer GTHPF5 (CGACGAGATCTCTCCCGAACTTGGCGGCG) and primer GTHPR4 (GCATCATGTAAGTTATAAGCG). Each fragment was cloned into pCRscript plasmids (Stratagene, La Jolla, Calif.). These fragments were constructed to contain a BglII site at the 3′ end of the 5′ fragment and the 5′ end of the 3′ fragment to allow a 1-kb insertion of the kanamycin resistance gene aph from Tn903 (29) (PCR amplified with the addition of flanking BamHI and BglII sites), resulting in a 100-bp deletion and the loss of all enzyme activity. Confirmation of the mutant construction was performed by both GGT assay and PCR amplification utilizing primer GTHPF6 (GTGAAATCTTGGGGCTGAAACCGC) and primer GTHPR7 (CAAGGGCAAGTCGCTGAGC), which flanked the insertion. Transformation of H. pylori strains (the piglet-adapted strain KE26695 [12] and the mouse-adapted strain HpM5 developed by one of the authors [T.G.B.]) was performed by modification of the method described by Wang et al. (37). The DNA harboring the disrupted ggt gene was combined with H. pylori and spotted onto a brucella agar plate overnight. The cells were then resuspended in 100 μl of phosphate-buffered saline and plated on new plates containing 20 μg of kanamycin/ml. Kanamycin-resistant colonies were then assayed by PCR for the presence of the disrupted ggt gene and for lack of GGT activity. For development of a ureB-deficient strain, a plasmid containing a ureB::aph knockout, pEJ22, was obtained from the laboratory of Andrew Wright at Tufts University.
The assay for GGT activity was adapted from the method of Meister et al. (26). Briefly, reaction buffer consisting of 20 mM Gly-Gly, 300 μM l-γ-glutamyl-ρ-nitroanilide, and 60 mM Tris (pH 8.0) was prepared. One-tenth the volume of H. pylori cells at A600 of 1.0 was added for a final A600 of 0.1. Reactions proceeded at 37°C for 60 min, and the release of ρ-nitroanilide was monitored by the A405. Additionally, a fluorimetric assay protocol was adapted from the method of Forman et al. (15). Briefly, in each well of a 96-well plate, 70 μl of 70 μM γ-Glu-7-amino-4-methyl coumarin (AMC) was combined with 30 μl of H. pylori cells at an A600 of 0.05 for a final γ-Glu-AMC concentration of 50 μM and a final A600 for H. pylori of 0.015. The chemicals employed in these assays were purchased from Sigma (St. Louis, Mo.).
Caesarian-derived gnotobiotic piglets from date-mated specific-pathogen-free sows (21) were inoculated with 109 CFU of H. pylori in 2.0 ml of brucella broth at an age of 3 days and terminated either 6 or 20 days postinfection. The stomachs were opened, and a small strip from the lesser curvature, incorporating portions of the cardia, fundus, antrum, and pyloris, was removed for histopathological evaluation. The remaining stomachs were used for bacterial reisolation, in which the mucosa was removed from the muscularis, weighed, and homogenized in brucella broth (10% [wt/vol]). Quantitative 10-fold bacterial titrations were performed in triplicate (two replicates per dilution) onto Skirrow's agar plates. Gross lesions were recorded, and samples of formalin-fixed gastric cardia, fundus, antrum, and pyloris were prepared for histopathological evaluation. For histopathology, 6-μm-thick section replicates, stained with hematoxylin and eosin and the Warthin-Starry stain for demonstration of organisms, were examined by light microscopy. Each section was assigned a qualitative score of 0 to 4, as described previously (20).
Six- to 8-week-old C57BL/6 mice (Harlan Sprague Dawley, Inc., Indianapolis, Ind.), housed in microisolator cages, were inoculated by gastric intubation with 750 μl of culture grown to an OD450 of 0.1 on two consecutive days and terminated either 28 or 49 days postinfection. Two biopsy specimens (2 by 2 mm) were surgically removed from the gastric antrum of each mouse, homogenized in 200 μl of brucella broth, and cultured on Skirrow's agar plates under microaerobic conditions at 37°C for 96 h. Additionally, for histological evaluation, a narrow strip of tissue was surgically removed from the greater curvature of the stomach from the duodenum to the gastric cardia of each necropsied mouse. The tissues were fixed in 10% buffered formalin and processed for histological examination at University Hospitals in Cleveland, Ohio. For all experiments, differences in bacterial load between control and experimental groups were evaluated by analysis of variance. The differences were considered statistically significant if the P values were less than 0.05.
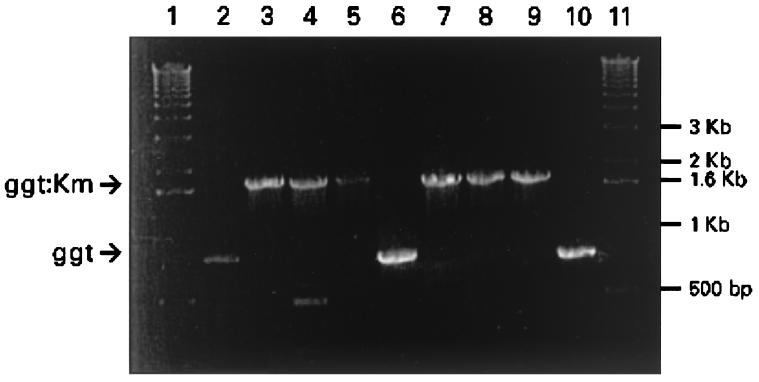
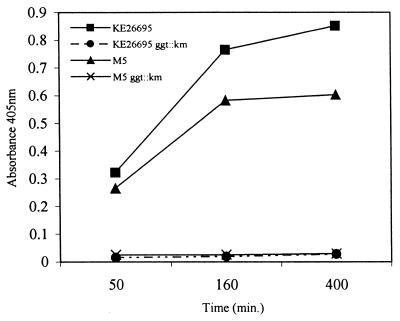
Cloning of the ggt gene into Escherichia coli resulted in a high level of GGT enzyme compared to that of E. coli without the plasmid (data not shown). Although E. coli normally carries the ggt gene, activity is not detectable. A kanamycin-resistant insertional ggt mutant of H. pylori was constructed as described above and analyzed by PCR. As shown in Fig. 1, lane 6, amplification of the ggt gene from strain KE26695 yielded the predicted fragment size of 750 bp. Amplification of the ggt::aph mutation, however, yielded a 1.7-kb fragment (Fig. 1, lane 7) corresponding to the size predicted for the ggt construct containing the kanamycin resistance cassette. H. pylori transformants created with plasmid containing the ggt::aph mutation were compared to parent strains to evaluate the levels of GGT activity. No GGT activity was detected in H. pylori strains harboring the ggt insertional mutation by either our colorimetric assay (Fig. 2) or the more sensitive fluorimetric assay (data not shown). The isogenic H. pylori control strains possessed the predicted wild-type levels of activity. The lack of GGT activity had no effect on the in vitro growth kinetics of the transformants compared to the parent strain. These data, combined with our PCR analysis, indicate successful deletion of GGT activity in our animal-adapted H. pylori strains.
FIG. 1.
PCR amplification of the ggt gene from H. pylori strains. Lanes 1 and 11, 1-kb ladder markers; lane 2, HpM5; lane 3, M5 ggt::aph; lane 4, M5 ggt::aph from mouse 31; lane 5, M5 ggt::aph from mouse 44; lane 6, KE26695; lane 7, KE26695 ggt::aph; lane 8, KE26695 ggt::aph from pig 312; lane 9, KE26695 ggt::aph from pig 313; lane 10, KE26695 from pig 316. The wild-type ggt 800-bp band and the ggt::aph 1.7-kb band are indicated by arrows.
FIG. 2.
GGT enzyme assay. The GGT activity was measured as the A405 of ρ-nitroanilide released by KE26695 and M5 and by ggt mutant derivatives.
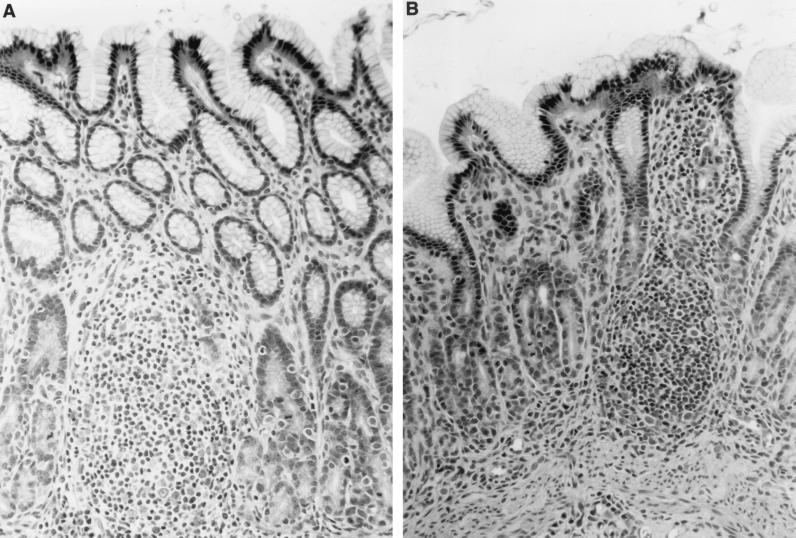
Separately housed gnotobiotic piglets were orally inoculated with either KE26695 or KE26695 ggt::aph as described above. The piglets were terminated at either 6 or 20 days postinoculation, and the gastric tissue was analyzed for bacterial load and pathology as described above. Piglets inoculated with parental KE26695 yielded 9.9 × 106 to 18.6 × 106 CFU of bacteria per gram (mean, 14.25 × 106 CFU/g) (Table 1) at 6 days postinoculation, comparable to levels reported previously (20). The recovered organisms were confirmed to be H. pylori by colony morphology and biochemical assays for urease and catalase and were shown to express the parental-type GGT by PCR and enzyme activity (see below). In contrast, piglets inoculated with KE26695 ggt::aph had significantly reduced numbers of bacteria, with greater than 2-log unit reduction in colonization 6 days after inoculation (<0.085 × 106 CFU/g) (Table 1). By 20 days after infection, the colonization levels in the KE26695 ggt::aph-inoculated piglets (1.64 × 106 CFU/g) approached those in the control groups from both 6 and 20 days after infection. The recovered organisms were confirmed to be H. pylori as described above and were shown to retain the ggt mutation, as detailed below. Histopathological evaluation revealed antral-dominant lymphoplasmacytic inflammation and lymphoid follicle formation typical of H. pylori gastritis (Fig. 3) in this species (20). The lesions, ranging from 0 to 2 (on a scale of 0 to 4) did not differ appreciably between the parental-strain-infected piglets and the ggt mutant-infected piglets.
TABLE 1.
Recovery of H. pylori parental strain KE26695 and the isogenic mutant KE26695 ggt::aph from gastric mucosae of gnotobiotic piglets
| Time and strain | Piglet no. | No. of bacteria (106 CFU/g)
|
% Parental recovery | |
|---|---|---|---|---|
| H. pylori | Group mean | |||
| 6 days postinoculation | ||||
| KE26695 | 1 | 18.6 | 14.25 | 100 |
| 2 | 9.93 | |||
| KE26695 ggt::aph | 3 | <0.001 | <0.085 | 0.05 |
| 4 | 0.17 | |||
| 20 days postinoculation | ||||
| KE26695 | 5 | 5.64 | 100 | |
| KE26695 ggt::aph | 6 | 3.24 | 1.64 | 29.6 |
| 7 | 0.5 | |||
FIG. 3.
Photomicrograph of gastric mucosa and lamina propria from a gnotobiotic piglet infected with KE26695 ggt::aph 20 days previously. (A) Developing lymphoid follicle. There are also lymphocytic infiltrates within the lamina propria (B). The tissues were stained with hematoxylin and eosin. Magnification, ×270.
A mouse model of H. pylori infection was also employed to determine if there was a significant difference in colonization requirements between animal models. Mouse-adapted HpM5 efficiently and reproducibly infects mice. This strain was chosen due to the difficulty in transforming other mouse-adapted strains, such as the Sidney strain (22). Kanamycin-resitant transformants were screened by PCR to verify the presence of the ggt insertional mutation (Fig. 1, lanes 2 and 3). As described above for KE26695, no GGT activity was observed in the HpM5 ggt mutant strain by either of our assays (Fig. 2). Mice were inoculated with the H. pylori strains as described above and terminated on days 28 and 49 postinoculation. These time points were chosen to increase the likelihood of recovering organisms from the gastric mucosa. All mice inoculated with mouse-adapted HpM5 were culture positive at both time points (Table 2). H. pylori was also recovered from 100% of the mice inoculated with HpM5 carrying the ggt knockout mutation at both 28 and 49 days postinoculation. However, while the bacterial counts per biopsy specimen increased over time for mice inoculated with either HpM5 or HpM5 ggt::aph, the number of bacteria in mice inoculated with the ggt knockout was significantly lower than in mice inoculated with wild-type HpM5. This observation was consistent among time points, although the disparity decreased (28 days, P = 0.0002; 49 days, P = 0.024). No bacteria were recovered from mice inoculated with M5 lacking the urease B subunit at either time point, verifying urease as a virulence factor for H. pylori. Two H. pylori isolates from the stomachs of piglets and two from mice infected with isogenic strains were tested both for the presence of the ggt mutation and for GGT activity. Both the genotype (Fig. 1) and phenotype (data not shown) of the mutants remained as expected during animal passage.
TABLE 2.
Recovery of H. pylori parental strain HpM5 and the isogenic mutant HpM5 ggt::aph from gastric mucosae of C57BL/6 mice
| Strain |
H. pylori organismsa
|
|||
|---|---|---|---|---|
| No. 28 days postinoculationb | % Parental recovery | No. 49 days postinoculationc | % Parental recovery | |
| HpM5 | 879 (224) | 100 | 3,811 (1,278) | 100 |
| HpM5 ggt::aph | 37 (13) | 8.4 | 1,191 (409) | 31.2 |
| HpM5 ureB::aph | 0 (0) | 0 | 0 (0) | 0 |
Data are presented as mean CFU per biopsy and standard error.
Eight mice per group.
Seven mice per group.
Our results indicate that the presence of GGT confers an advantage upon H. pylori in colonizing the gastric mucosa. Early identification and clinical classification of H. pylori showed GGT being expressed at unusually high levels (25). The presence of unusually high levels of this enzyme in H. pylori led Chevalier et al. to further examine the role of GGT in H. pylori viability and survival in the host (7). Our results are consistent with those of Chevalier et al. in showing that GGT is not essential for H. pylori viability, as both isogenic ggt-deficient H. pylori strains grew normally in vitro despite the demonstrated lack of GGT activity. However, our results differ from those in the previous report in that, although GGT activity did provide an advantage for increasing the bacterial load, GGT-deficient H. pylori strains successfully infected both gnotobiotic piglets and mice.
Animal models have been used to test the contributions of several H. pylori virulence factors in colonization and pathogenicity. Urease-deficient H. pylori generated via chemical mutagenesis fails to colonize gnotobiotic piglets (9), and a ureA mutant of Helicobacter mustelae was unable to colonize the ferret stomach (3). Similar studies using isogenic mouse-adapted H. pylori strains confirmed this observation in mice (33, 36). Motility has also been shown to be essential based on studies demonstrating that strains with limited motility had poor colonization rates in the piglet model (12). A study performed using recombinant flagellin-negative H. mustelae confirmed the importance of motility in establishing chronic infection (2). The role of vacuolating toxin (VacA) in promoting more severe symptoms (reviewed in reference 30) was tested by using toxigenic and nontoxigenic isogenic H. pylori mutants in the piglet model, which showed that the presence of VacA does not enhance H. pylori-associated gastritis (10). Another protein believed to correlate with increased H. pylori pathogenicity in humans is the 120-kDa cytotoxin-associated protein (CagA), which is also a marker for a pathogenicity island, a 40-kb segment of DNA possessing up to 26 genes which is present in a large percentage of H. pylori strains (1, 6). Since strains lacking the cag pathogenicity island are isolated from a substantial percentage of H. pylori-infected individuals, this segment of DNA does not possess genes essential for colonization.
The present study used two distinct animal models of H. pylori infection to assess the importance of GGT activity in H. pylori virulence. Our results with gnotobiotic piglets indicated that after a 6-day infection with ggt-deficient H. pylori, colonization, although very much reduced in comparison to infection with the isogenic wild-type KE26695, was accomplished in 100% of our animals. Similar results were obtained for the ggt mutant after a 20-day infection, although the numbers were not as low as those observed at 6 days of infection. We extended our study by repeating it using a ggt-deficient mouse-adapted strain of H. pylori. The results complemented the piglet model. H. pylori could be isolated from 100% of the mice 28 days postinoculation with the HpM5 ggt::aph strain although the bacterial load was significantly lower than that from mice infected with the parent strain. When the mice were examined at 49 days, the ggt mutant was recovered at higher numbers than at 28 days but still significantly reduced in comparison to infection with HpM5.
The complementary results obtained in these two animal model systems are reassuring, since there is no ideal model for studying H. pylori infection other than humans. However, these results are in conflict with those observed by Chevalier et al. (7) in a murine model of H. pylori infection in which ggt-negative H. pylori was unable to infect the gastric mucosa. Although methods for bacterial culture and quantification from gastric biopsies were similar, several differences in experimental design may explain the difference in our results. First, Chevalier et al. used H. pylori SS1, a mouse-adapted strain recently used for vaccine models (22). We employed one of our own mouse-adapted isolates, HpM5, which is readily transformable and consistently colonizes C57BL/6 mice. Both H. pylori SS1 (22) and HpM5 (data not shown) are cagA+ and vacA+ by PCR analysis. Since deletion of GGT activity in our strain resulted in decreased bacterial loads, it is possible that in other strains of H. pylori such a deletion could completely ablate the ability to infect the host.
Second, Chevalier et al. inoculated outbred Swiss mice whereas we utilized inbred C57BL/6 mice. Although studies comparing mouse strain susceptibilities have focused primarily on disease (22, 27, 32), we have noticed differences among mouse strains with regard to the ease of infection with H. pylori when testing our other mouse-adapted strains. When seven different strains of mice were inoculated simultaneously with H. pylori, we were unable to subsequently culture organisms from gastric biopsy specimens from several mouse strains (unpublished observations). It is possible that Swiss mice are less amenable to hearty infection than C57BL/6 mice and that the loss of GGT activity that results in reduced bacterial loads in C57BL/6 mice may result in either a failure to colonize Swiss mice or colonization below the level of detection. These experiments underscore the concept that there is a considerable amount of variation among mouse-adapted H. pylori strains and H. pylori animal models in general.
Evidence obtained regarding the importance of specific virulence factors may be strengthened through the use of multiple models. The consistency in results that we observed in mice and piglets demonstrates that GGT activity provides at least some advantage to H. pylori, as ggt mutants colonized at levels that were significantly lower than those of wild-type isogenic strains of H. pylori, particularly at early time points. However, while GGT activity does provide some advantage, it is not essential for the establishment of chronic infection of the gastric mucosa.
Acknowledgments
This research was partially supported by grant DK-46461 from the National Institutes of Health.
We thank Michael Nguyen and Larry Dick for assistance with the GGT assays and Rachel Cahill for critical review of the manuscript.
REFERENCES
- 1.Akopyants N, Clifton S W, Kersulyte D, Crabtree J E, Youree B E, Reece C A, Bukanov N O, Drazek E S, Roe B A, Berg D E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 2.Andrutis K A, Fox J G, Schauer D B, Marini R P, Li X, Yan L, Josenhans C, Suerbaum S. Infection of the ferret stomach by isogenic flagellar mutant strains of Helicobacter mustelae. Infect Immun. 1997;65:1962–1966. doi: 10.1128/iai.65.5.1962-1966.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrutis K A, Fox J G, Schauer D B, Marini R P, Murphy J C, Yan L, Solnick J V. Inability of an isogenic urease-negative mutant strain of Helicobacter mustelae to colonize the ferret stomach. Infect Immun. 1995;63:3722–3725. doi: 10.1128/iai.63.9.3722-3725.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baskerville A, Newell D G. Naturally occurring chronic gastritis and C. pylori infection in the Rhesus monkey: a potential model for gastritis in man. Gut. 1988;29:465–472. doi: 10.1136/gut.29.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bronsdon M A, Schoenknecht F D. Campylobacter pylori isolated from the stomach of the monkey Macaca nemestrina. J Clin Microbiol. 1988;26:1725–1728. doi: 10.1128/jcm.26.9.1725-1728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chevalier C, Thiberge J-M, Ferrero R, Labigne A. Essential role of Helicobacter pylori γ-glutamyltranspeptidase for the colonization of the gastric mucosa of mice. Mol Microbiol. 1999;31:1359–1372. doi: 10.1046/j.1365-2958.1999.01271.x. [DOI] [PubMed] [Google Scholar]
- 8.Dubois A, Fiala N, Heman-Ackah L M, Drazek E S, Tarnawski A, Fishbein W N, Perez-Perez G I, Blazer M J. Natural gastric infection with Helicobacter pylori in monkeys: model for spiral bacteria infection in humans. Gastroenterology. 1994;106:1405–1417. doi: 10.1016/0016-5085(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 9.Eaton K A, Brooks C L, Morgan D R, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets Infect. Immun. 1991;59:2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton K A, Cover T L, Tummuru M K, Blaser M J, Krakowka S. Role of vacuolating cytotoxin in gastritis due to Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1997;65:3462–3464. doi: 10.1128/iai.65.8.3462-3464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton K A, Morgan D R, Krakowka S. Persistence of Helicobacter pylori in conventionalized piglets. J Infect Dis. 1990;161:1299–1301. doi: 10.1093/infdis/161.6.1299. [DOI] [PubMed] [Google Scholar]
- 12.Eaton K A, Morgan D R, Krakowka S. Campylobacter pylori virulence factors in gnotobiotic piglets. Infect Immun. 1989;57:1119–1125. doi: 10.1128/iai.57.4.1119-1125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Euler A R, Zurenko G E, Moe J B, Ulrich R G, Yagi Y. Evaluation of two monkey species (Macaca mulatta and Macaca fascicularis) as possible models for human Helicobacter pylori disease. J Clin Microbiol. 1990;28:2285–2290. doi: 10.1128/jcm.28.10.2285-2290.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eurogast Study Group. An international association between Helicobacter pylori infection and gastric cancer. Lancet. 1993;341:1359–1362. [PubMed] [Google Scholar]
- 15.Forman H J, Ming Shi M, Iwamoto T, Liu R-M, Robinson T W. Measurement of γ-glutamyl transpeptidase and γ-glutamylcysteine synthetase activities in cells. Methods Enzymol. 1995;252:66–71. doi: 10.1016/0076-6879(95)52009-0. [DOI] [PubMed] [Google Scholar]
- 16.Fox J G, Batchelder M, Marini R, Yan L, Handt L, Li X, Shames B, Hayward A, Campbell J, Murphy J C. Helicobacter pylori-induced gastritis in the domestic cat. Infect Immun. 1995;63:2674–2681. doi: 10.1128/iai.63.7.2674-2681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazell S L, Eichberg J W, Lee D R, Alpert L, Evans D G, Evans D J, Jr, Graham D Y. Selection of the chimpanzee over the baboon as a model for Helicobacter pylori infection. Gastroenterology. 1992;103:848–854. doi: 10.1016/0016-5085(92)90016-r. [DOI] [PubMed] [Google Scholar]
- 18.Ishiye M, Yamashita M, Niwa M. Molecular cloning of the gamma-glutamyltranspeptidase gene from a Pseudomonas strain. Biotechnol Prog. 1993;9:323–331. doi: 10.1021/bp00021a012. [DOI] [PubMed] [Google Scholar]
- 19.Karita M, Li Q, Cantero D, Okita K. Establishment of a small animal model for human Helicobacter pylori infection using germ-free mouse. Am J Gastroenterol. 1994;89:208–213. [PubMed] [Google Scholar]
- 20.Krakowka S, Eaton K A. Helicobacter pylori infection in gnotobiotic piglets. A model of human gastric bacterial disease. In: Schook T A, editor. Advances in swine in biomedical research. New York, N.Y: Plenum Press; 1996. pp. 779–810. [Google Scholar]
- 21.Krakowka S, Morgan D M, Kraft W G, Leunk R D. Establishment of gastric Campylobacter pylori infection in the neonatal gnotobiotic piglet. Infect Immun. 1987;55:2789–2796. doi: 10.1128/iai.55.11.2789-2796.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee A, O'Rourke J, De Ungria M C, Robertson B, Daskalopoulos G, Dixon M F. A standardised mouse model of Helicobacter pylori infection. Introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 23.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 24.Marshall B J, Warren J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 25.Megraud F, Bonnet F, Garnier M, Lamouliatte H. Characterization of “Campylobacter pyloridis” by culture, enzymatic profile, and protein content. Infect Immun. 1985;22:1007–1010. doi: 10.1128/jcm.22.6.1007-1010.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meister A, Tate S S, Griffith O W. γ-Glutamyl transpeptidase. Methods Enzymol. 1981;77:237–253. doi: 10.1016/s0076-6879(81)77032-0. [DOI] [PubMed] [Google Scholar]
- 27.Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect Immun. 1996;64:238–245. doi: 10.1128/iai.64.1.238-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 29.Oka A, Sugisaki H, Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981;147:217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- 30.Papini E, Satin B, de Bernard M, Molinari M, Arico B, Galli C, Telford J R, Rappuoli R, Montecucco C. Action site and cellular effects of cytotoxin VacA produced by Helicobacter pylori. Folia Microbiol. 1998;43:279–284. doi: 10.1007/BF02818613. [DOI] [PubMed] [Google Scholar]
- 31.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobcter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 32.Sakagami T, Dixon M, O'Rourke J, Howlett R, Alderuccio F, Vella J, Shimoyama T, Lee A. Atrophic gastric changes in both H. felis and H. pylori infected mice are host dependent and separate from antral gastritis. Gut. 1996;39:639–648. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skouloubris S, Thiberge J-M, Labigne A, De Reuse H. The Helicobacter pylori Urel protein is not involved in urease activity but is essential for bacterial survival in vivo. Infect Immun. 1998;66:4517–4521. doi: 10.1128/iai.66.9.4517-4521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki H, Kumagai H, Echigo T, Tochikura T. DNA sequence of the Escherichia coli K-12 gamma-glutamyltranspeptidase gene, ggt. Bacteriology. 1989;171:5169–5172. doi: 10.1128/jb.171.9.5169-5172.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tate S S, Meister A. Gamma-glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem. 1981;39:357–368. doi: 10.1007/BF00232585. [DOI] [PubMed] [Google Scholar]
- 36.Tsuda M, Karita M, Morshed M G, Okita K, Nakazawa T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect Immun. 1994;62:3586–3589. doi: 10.1128/iai.62.8.3586-3589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Roos K P, Taylor D I. Transformation of Helicobacter pylori by chromosomal metronidazole resistance and by a plasmid with a selectable chloramphenicol resistance marker. J Gen Microbiol. 1993;139:2485–2493. doi: 10.1099/00221287-139-10-2485. [DOI] [PubMed] [Google Scholar]
- 38.Xu K, Strauch M A. Identification, sequence, and expression of the gene encoding gamma-glutamyltranspeptidase in Bacillus subtilis. J Bacteriol. 1996;178:4319–4322. doi: 10.1128/jb.178.14.4319-4322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]