Abstract
Hydrochorea and Balizia were established to accommodate four and three species, respectively, that were previously included in different ingoid genera, based primarily on differences in fruit morphology. Both genera have Amazonia as their centre of diversity, extending to Central America and the Brazilian Atlantic Rainforest. Previous phylogenetic evidence showed Balizia to be paraphyletic with respect to Hydrochorea, and species of Hydrochorea and Balizia were placed in a large unresolved polytomy with species of Jupunba. Here we present a new phylogenomic analysis based on 560 exons, from which 686 orthologous alignments were derived for gene tree inference. This analysis confirms a paraphyletic Balizia in relation to Hydrochorea, together with two African species formerly placed in Albizia nested within the clade. Jupunbamacradenia was resolved as sister to the clade combining those taxa. However, quartet support is low for several of the branches at the base of the clade combining the genera Jupunba, Balizia and Hydrochorea, suggesting that rapid initial divergence in this group led to extensive incomplete lineage sorting and consequently poor phylogenetic resolution. Because of these phylogenomic complexities, we decided to use morphology as the main guide to consider Hydrochorea as a distinct genus from Jupunba, and Balizia as a new synonym for Hydrochorea. The taxonomic treatment includes the study of collections from various herbaria and fieldwork expeditions. We present a re-circumscribed Hydrochorea accommodating a total of 10 species, including six new combinations, five new synonyms, one new taxonomic status, two corrections of nomenclature category for lectotypes, and a second step lectotype and three new lectotypes. A new species from the Brazilian Amazon is described and illustrated. An identification key for all species of Hydrochorea is presented, together with comments and illustrations.
Keywords: Albizia , Balizia , Cathormion , Fabaceae, nomenclature, taxonomy
Introduction
Rupert C. Barneby and James W. Grimes established a new generic system for most of the ingoid mimosoids of the Americas in a landmark monographic series (Barneby and Grimes 1996, 1997; Barneby 1998). In it they created seven new genera, including Hydrochorea Barneby & J.W. Grimes and Balizia Barneby & J.W. Grimes, which were established to accommodate four and three species, respectively, that were previously included (as many ingoid species have been) in several different genera such as Albizia Durazz., Arthrosamanea Britton & Rose, Cathormion Hassk. and Pithecellobium Mart., among others (Lewis and Rico Arce 2005; Brown 2008). While Barneby and Grimes (1996: p. 35) stated that Hydrochorea and Balizia “have arisen from common ancestry”, these were nonetheless treated as separate genera based on differences in fruit morphology that were ascribed to adaptation to different habitats and seed dispersal strategies: Hydrochorea was defined based on lomentiform fruits adapted to water-borne dispersal in seasonally inundated habitats, while Balizia was described as a genus of “terra firme” forest (even though at least two of its three species were mentioned to also often occur on riverbanks), and recognized mainly based on having indehiscent or follicular fruits, with a septate endocarp but not lomentiform. Barneby and Grimes (1996) recognized Balizia, Hydrochorea, and Abarema Pittier s.l. as closely related genera, distinguished by fruit morphology (Iganci and Morim 2009, 2012). However, Abarema was shown to be polyphyletic (Iganci et al. 2016) and the type species of the genus, Abaremacochliacarpos (Gomes) Barneby & J.W. Grimes is placed in the Inga clade, together with the recently described A.diamantina E. Guerra, M.P. Morim & Iganci (Guerra et al. 2016, 2019). The other species of Abarema s.l. were segregated in the two genera Jupunba Britton & Rose and Punjuba Britton & Rose (Soares et al. 2021). These findings question all the former classifications of those taxa, which were mostly based on fruit morphology, and call for further studies aiming to better understand fruit and seed morphology in the context of the evolution of dispersal strategies and ecological adaptations.
Recent phylogenetic evidence (Iganci et al. 2016; Koenen et al. 2020b; Soares et al. 2021; Ringelberg et al. 2022; and a new analysis presented here) has shown Balizia to be paraphyletic with respect to Hydrochorea. Furthermore, two African species that were formerly placed in several genera including Albizia Durazz. and Cathormion Hassk. were also shown to be most closely related to Hydrochorea (Koenen et al. 2020b), and their general morphological features and ecology are virtually indistinguishable from Neotropical species of Hydrochorea.
Besides the advances in phylogenetic and phylogenomic methods, recent fieldwork collecting programmes have greatly contributed to herbarium collections of Amazonian taxa (Milliken et al. 2010; Cardoso et al. 2017; Ulloa et al. 2017; BFG 2021), and furthermore, the Reflora Program has led to the online availability of nearly all Brazilian plant collections (Pearce et al. 2020; BFG 2021), providing excellent opportunities for synoptic taxonomic revisions.
Here we present a taxonomic update including a new generic circumscription of Hydrochorea based on phylogenomic and morphological evidence, along with a nomenclatural review presented as a synopsis of the genus, which includes new combinations, new synonyms, and the description of a newly discovered species from the Upper Rio Negro. We include an identification key, illustrations, and distribution maps for the 10 species now accommodated in Hydrochorea.
Materials and methods
Taxonomy
Standard herbarium taxonomy practices were used for analysis of all species studied in the present work. The collections (including digital images) of the following herbaria were analysed: A, BM, BR, CTBS, E, F, G, GH, HUEFS, IAN, INPA, K, MG, MO, NY, P, PEL, R, RB, SP, US and Z (Thiers 2022). Fieldwork was carried out especially in the Upper Rio Negro region of Amazonian Brazil, where we collected four species of Balizia and Hydrochorea, including a species new to science. All the new collections were incorporated into the RB herbarium, in the Rio de Janeiro Botanical Garden, and duplicates were sent to partner institutions. Fresh leaf samples were stored in silica gel for total DNA extraction. We also visited the collections in NY to study the specimens that Barneby and Grimes (1996) worked with for their taxonomic account. Combined with studying the large number of new collections that have been made in the past 25 years since publication of the Barneby and Grimes (1996) taxonomic account, we were able to review their taxonomic decisions based on the relatively limited herbarium material available to them at the time.
Online databases were used to view digital images of specimens including types, especially the Reflora Virtual Herbarium (REFLORA -Herbário Virtual 2022), National Institute of Science and Technology (INCT-Splink 2022), and JSTOR (2022). Geographic distributions of each species were inferred based on specimen labels and literature (Barneby and Grimes 1996; Soares 2015). The morphological characters were described following Beentje (2016) and Iganci and Morim (2009, 2012), for habit, leaves, inflorescences, flowers, pods, and seeds. Original descriptions of all taxa were analysed, and nomenclature was revised according to the International Code of Nomenclature of algae, fungi and plants (the Shenzen code; Turland et al. 2018). Except for the new species described here, and the two African species now placed in Hydrochorea, all other species have been described earlier in detail by specialists (Barneby and Grimes 1996). Thus, for those species, here we only present new combinations, taxonomic notes and a reference to the literature where the complete description is available. Synonyms are accepted following Barneby and Grimes (1996) and are only listed here when either lectotypification or nomenclatural correction is needed.
Exon selection, matrix assembly and phylogenomic analysis
To better evaluate the evidence for monophyly of the studied genera, or lack thereof, we have performed new analyses based on a selection of exons with flanking non-coding regions derived from the sequencing data of Koenen et al. (2020b) and Ringelberg et al. (2022), including network analyses and quantification of supporting bipartitions across gene trees for alternative topologies. The sequencing methods are described in those publications and here we only briefly describe our methods when they differ from Koenen et al. (2020b) and Ringelberg et al. (2022). In the original Mimobaits probe design (Koenen et al. 2020b), exons were predicted, and flanking untranslated regions (UTRs) were also (partially) included. From this reference exon set, we selected all of those that are longer than 500bp, which for initial or terminal exons includes the UTR. Read data of Koenen et al. (2020b) and Ringelberg et al. (2022) for the accessions of the Jupunba clade plus six outgroup accessions were mapped against these exons and non-matching reads discarded. Read quality filtering and de novo assembly methods followed Koenen et al. (2020b), and after clustering the assembled contigs to the reference sequences of the exons, initial alignments and gene trees were inferred using MAFFT (Katoh et al. 2005) and RAxML (Stamatakis 2014), respectively. Then, mono- and paraphyletic groups per species were collapsed to select a single allele in case multiple alleles were reconstructed (Yang and Smith 2014), followed by cutting long internal branches to splice potential paralogs into separate alignments using the cut_long_branches.py script of Yang and Smith (2014). The resulting clusters were then realigned with MAFFT and used to infer multilabeled gene trees using RAxML. Finally, gene trees with a single tip per species were extracted from these gene trees using the maximum inclusion (MI) method of Yang and Smith (2014) and used in species tree analysis in ASTRAL-III (Zhang et al. 2018) with default settings and nodes with less than 10% bootstrap support collapsed as suggested by the authors of the software. A filtered supernetwork was constructed in SplitsTree4 (Huson 1998) from the same set of gene trees but with nodes with less than 50% bootstrap support collapsed and with the mintrees parameter set to 100 (25% of the total number of gene trees). Quantification of supporting bipartitions across gene trees for alternative topologies followed the same methodology as Koenen et al. (2020a).
Results
Our herbarium taxonomic work has resulted in the synopsis presented below. This includes a total of six new combinations, including a new status for a species that had been treated at varietal rank by Barneby and Grimes (1996), as well as the description of a new species. Balizia and its sections are placed in the synonymy of Hydrochorea and two new heterotypic synonyms, one at species and one at varietal rank, are proposed, as well as two lectotype corrections. A second step lectotype and three new lectotypes are designated.
Phylogenomic analysis
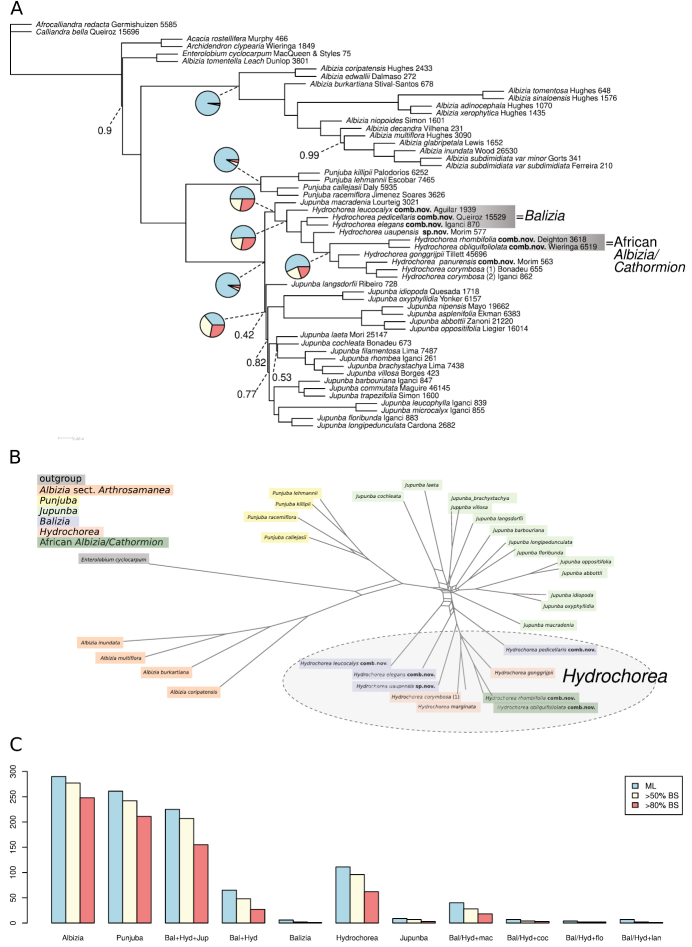
A total of 560 exons were selected for gene tree inference, from which 398 MI gene trees were extracted after clustering and filtering (Suppl. materials 1, 2). The species tree based on these gene trees (Fig. 1A, Suppl. material 3) does not provide qualitatively different results from those of Ringelberg et al. (2022), showing a paraphyletic Balizia relative to Hydrochorea together with the two African Cathormion/Albizia species. All currently recognised species of Balizia and Hydrochorea, including the type species of both genera, were sampled, with the exception of H.marginata, although this species was included in the studies of Iganci et al. (2016) and Soares et al. (2021). Also, Jupunbamacradenia (Pittier) M.V.B. Soares, M.P. Morim & Iganci was found as sister of the clade combining these genera. However, quartet support is low for several of the branches at the base of the clade combining the genera Jupunba, Balizia and Hydrochorea, suggesting that rapid initial divergence in this group led to extensive incomplete lineage sorting and consequently poor phylogenetic resolution. This is further reinforced by the filtered supernetwork (Fig. 1B), which also clearly shows the paraphyly of Balizia, and shows that Hydrochorea and Balizia together form a group that is separate from Jupunba, but with a complex network structure indicative of incomplete lineage sorting. When the monophyly of these genera and their sister-group relationships are evaluated based on the number of compatible bipartitions across gene trees, it is clear that Albiziasect.Arthrosamanea (Britton & Rose) Barneby & J.W. Grimes, Punjuba and Hydrochorea (including the two African Cathormion/Albizia species) are each monophyletic, while Balizia and Jupunba are only supported to be monophyletic by a small number of gene trees (Fig. 1C). Support for the sister-group relationship of Jupunbamacradenia with Balizia + Hydrochorea is supported by c. 10% of the gene trees. This is higher than what is found for some other species of Jupunba that are sister to Balizia + Hydrochorea in some gene trees, but nonetheless the separation between these genera is not very clear likely due to significant incomplete lineage sorting.
Figure 1.
Phylogenomics of the Jupunba clade A ASTRAL-III species tree based on 398 gene trees, posterior probability values are shown only for those nodes for which support is lower than 1.0, and pie charts on several crucial nodes indicate alternative quartet support B filtered super-network of the same gene tree set with the genus Hydrochorea as circumscribed in this study indicated by a grey ellipse C bar graphs indicating numbers of compatible bipartitions across the same gene tree set in the maximum-likelihood estimate (ML) and when only taking into account bipartitions that receive at least 50 or 80% bootstrap support. The abbreviations that are used are Bal = Balizia, Hyd = Hydrochorea, and Jup = Jupunba. Note that the taxonomy of Albiziasect.Arthrosamanea is updated in this volume by Aviles Peraza et al. (2022), where new binomials in the genus Pseudalbizzia are presented for the majority of the species of this section.
Discussion
In this study, we have made an in-depth investigation of the generic delimitation issues surrounding the genera Balizia, Cathormion and Hydrochorea, to reconcile morphological characters of the group with phylogenetic relationships and to propose a revised classification. While the uncovered phylogenomic complexity adds further difficulty to the goal of achieving a stable classification for these taxa, we conclude that the taxa with either indehiscent, follicular or lomentiform fruits, that are septate between the seeds at least in the endocarp, are preferably all classified within a recircumscribed Hydrochorea, separate from the genus Jupunba which is characterized by dehiscent fruits that are never septate between the seeds. Extensive incomplete lineage sorting surrounding the early evolution of these genera means that they are phylogenomically not well separated (Fig. 1), but in order to ensure both diagnosability and stability of names, we believe keeping these as separate genera is justified (i.e., transferring some species to Hydrochorea to account for the non-monophyly of Balizia is preferable to moving all taxa to a morphologically heterogeneous Jupunba).
One of the most interesting aspects of Hydrochorea is the evolution of its fruit morphology and dehiscence in adaptation to water-borne seed dispersal, which presumably led to its distribution in riparian, swamp and periodically inundated forests on both sides of the Atlantic, as trans-oceanic dispersal is presumed to be relatively likely in hydrochorous plants. Much attention was traditionally given to pod morphology in mimosoids, in attempts to classify the ingoid genera, as one of the most easily observable characters to visually distinguish the taxa (Barneby and Grimes 1996; Lewis and Rico-Arce 2005). Lomentiform pods are typical of most species of Hydrochorea, but are also found in Albizia s.s. (Albiziadolichadena I.C. Nielsen, Albiziamoniliformis (DC.) F. Muell., Albiziarosulata (Kosterm.) I.C. Nielsen and Albiziaumbellata (Vahl) E.J.M. Koenen) and Albiziasect.Arthrosamanea (Barneby and Grimes 1996; Aviles Peraza et al. 2022). The craspedia, as found in Mimosa L., Adenopodia C. Presl and Entada Adans., are somewhat similar, but in those genera there is a replum (a persistent framework formed by the upper and lower suture of the craspedium) that stays attached to the infructescence after the 1-seeded articles have been shed. The fruit of Cathormionaltissimum (Hook. f.) Hutch. & Dandy (which is transferred to a new genus by Koenen 2022) is also similar, but the fruit of that species differs in containing aerenchymous tissue on the seminiferous nuclei to promote floating. These various lomentiform ingoid fruits also differ in whether they break up while still attached to the tree as is the case in most species of Hydrochorea and some species of Albiziasect.Arthrosamanea, or whether the fruit falls from the tree entire and only tardily breaks up into 1-multiple seeded articles afterwards, as appears to be the case in most of the other genera. Phylogenetic evidence clearly indicates that these fruits have all evolved independently from one another (Ringelberg et al. 2022), presumably in response to adaptation to riparian and periodically inundated habitats. The homoplasious nature of these similar fruits has led to many species having been moved around between different genera, based on their fruit morphology (Nielsen 1981). We note that the legume fruit appears to be amenable to the evolution of lomentiform fragmentation, given the similar craspedia that were independently derived at least twice and, moreover, lomentiform fruits are also present in several lineages of subfamily Papilionoideae.
Recent advances in molecular systematics of ingoid legumes also demonstrated the pod morphology to be less informative than previously thought (Souza et al. 2013; Iganci et al. 2016; Koenen et al. 2020b; Soares et al. 2021; Souza et al. 2022). Barneby and Grimes (1996) stated that Balizia and Hydrochorea closely resemble Albizia but are distinguished by indeterminate inflorescence-axes and vegetative branches arising from sylleptic and proleptic buds, pinnate leaflet venation, and truncate ovaries. The authors also highlighted the strong similarities in flower morphology shared between Abarema s.l., Balizia and Hydrochorea.
Iganci et al. (2016) found the species of Hydrochorea and Balizia in a large unresolved polytomy together with Jupunba species. The Bayesian results of Soares et al. (2021) are in line with the generic delimitation proposed here, with 1.0 posterior probability supporting Jupunba, Hydrochorea and Balizia as monophyletic considering matK sequences only, and a monophyletic Jupunba and paraphyletic Hydrochorea in relation to Balizia when considering ETS sequences only. However, there was no bootstrap support for a monophyletic Jupunba and neither for the clade uniting Hydrochorea and Balizia. The results of Ringelberg et al. (2022) show that J.macradenia is more closely related to Hydrochorea and Balizia than to other species of Jupunba, in contrast to the phylogenetic position of the same accession in Soares et al. (2021), but in accordance with the phylogenomic results presented here. Notably, Soares et al. (2021) included three accessions of J.macradenia, which were firmly nested in a monophyletic Jupunba, suggesting that further phylogenomic analyses with more accessions included will need to be carried out to further test the monophyly of Jupunba.
Phylogenetic evidence (Iganci et al. 2016; Koenen et al. 2020b; Soares et al. 2021; Ringelberg et al. 2022; and a new analysis presented here) that shows Balizia to be paraphyletic with respect to Hydrochorea, as well as the field discovery of a new species that is morphologically intermediate between the two genera, with crypto-lomentiform pods that resemble more the follicle of Baliziapedicellaris (DC.) Barneby & J.W. Grimes than the lomentiform pods of Hydrochorea, but a species of seasonally inundated forest with hydrochorous seed dispersal, prompted us to decide that the two genera are best combined. Furthermore, two African species that were formerly placed in several genera, including Albizia and Cathormion, were shown to be closely related to Hydrochorea based on phylogenomic evidence (Koenen et al. 2020b). These species are therefore best accommodated within Hydrochorea, as their general morphological features and ecology are virtually indistinguishable from Neotropical species of Hydrochorea.
Koenen et al. (2020b) and Ringelberg et al. (2022) also show that Albiziasect.Arthrosamanea is placed in the Jupunba clade, being more closely related to Jupunba, Punjuba, Balizia and Hydrochorea than to Albizia s.s. These results are reinforced by Aviles Peraza et al. (2022) who proposed nomenclatural updates to solve this situation based on more extensive sampling of Albiziasect.Arthrosamanea. In our study (Fig. 1), we have still referred to these species as Albiziasect.Arthrosamanea but we refer the reader to Aviles Peraza et al. (2022) for presentation of a new taxonomy of the group.
Based on parsimony analysis of morphological characters only, Barneby and Grimes (1996) recognized pollen polyads comprising 16 grains as a synapomorphy for the Abarema alliance, while other alliances that they recognized (e.g., the Samanea, Chloroleucon and Inga alliances) presented a polymorphic polyad number. Indeed, Guinet and Grimes (1997) studied all Neotropical species of Hydrochorea and Balizia for their polyads and we observed that the nested African species Cathormionobliquifoliolatum (De Wild.) G.C.C. Gilbert & Boutique (Deighton 3618, K) and Cathormionrhombifolium (Benth.) Keay (Germain 87, K) also have 16-celled polyads. Polyad morphology was also highlighted as diagnostic for the recognition of Afrocalliandra E.R. Souza & L.P. Queiroz, a genus segregated from Calliandra Benth. (Souza et al. 2013), and more attention should be given to this character in future studies. Furthermore, the taxa in the Jupunba clade share the simultaneous presence of vegetative and reproductive branches (sylleptic), a character considered by Grimes (1999) as uncommon amongst the ingoid legumes. Thus, possessing polyads with 16 grains and sylleptic branches could circumscribe either the clade comprised of Hydrochorea, Jupunba and Punjuba, or a more conservative Jupunba s.l. Forthcoming studies, including more samples of unstable taxa in current molecular analyses, new field collections and advances in phylogenomic analysis, will hopefully resolve this question.
We did not include Hydrochoreaacreana (J.F. Macbr.) Barneby & J.W. Grimes in our synopsis and the name is here considered as incertae sedis. Pods from this species were not known to Barneby and Grimes (1996), and herbarium specimens are difficult to identify when flowers and especially fruits are unavailable. The type specimen (Krukoff 5631, NY334624) includes flowers arranged in a large terminal panicle composed of umbelliform pseudoracemes of capitula, differing from the axillary to terminal inflorescences in Hydrochorea and Jupunba that are not paniculate, and always have sylleptic branches present. Citing fruiting Central American collections, Hydrochoreaacreana was combined into Abarema s.l. by Rico-Arce (1999) as Abaremaacreana (J.F. Macbr.) L. Rico. However, the Central American collections cited by Rico-Arce (1999) were identified by Barneby and Grimes as either Abaremamacradenia (Pittier) Barneby & J.W. Grimes or Abaremaadenophora (Ducke) Barneby & J.W. Grimes, with which we agree. Interestingly, a specimen from Acre which was collected in 1995 (Oliveira 691; NY00662831) and was presumably not seen by Barneby and Grimes (1996), but was identified as A.acreana by L. Rico-Arce, does include unripe pods and this material is likely conspecific with the type material of H.acreana. However, as discussed before, fruit morphology often has been shown to be rather misleading in mimosoid taxonomy due to homoplasy, and we point out the differences in inflorescence structure from Jupunba as discussed above. Soares et al. (2021) resolved H.acreana as sister to Albiziasubdimidiata (Splitg.) Barneby & J.W. Grimes, and not closely related to Hydrochorea nor Jupunba, but these analyses were based on ETS sequences only and morphologically the material does not bear much resemblance to species of Albiziasect.Arthrosamanea, in which Albiziasubdimidiata is placed. Given the taxon’s unusual combination of morphological characters, it may well represent an isolated lineage that merits recognition as a distinct genus; this decision is pending further study.
Taxonomic treatment
Hydrochorea
Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74(1): 23. 1996.
EBB162E5-9FC3-52D9-A745-335989AF21FE
Figure 2.
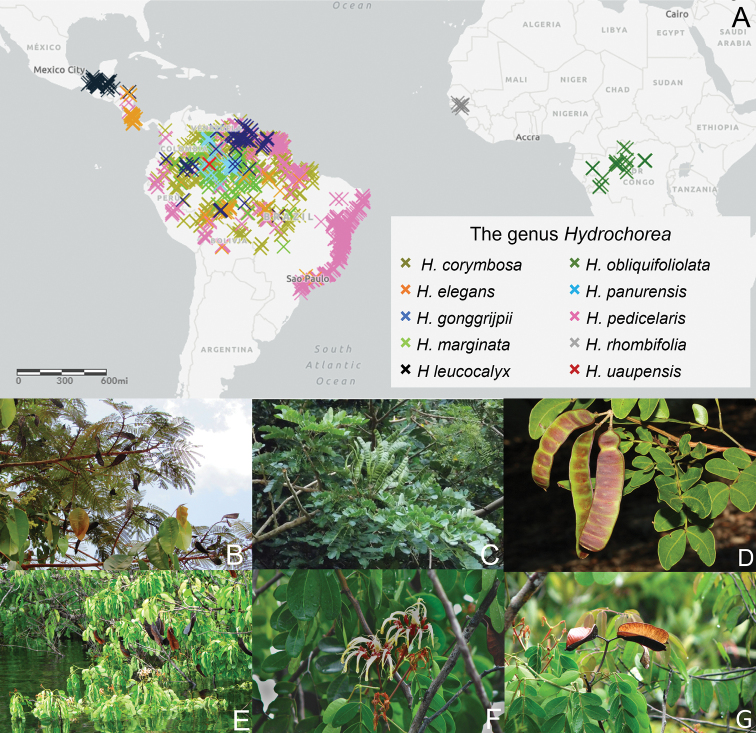
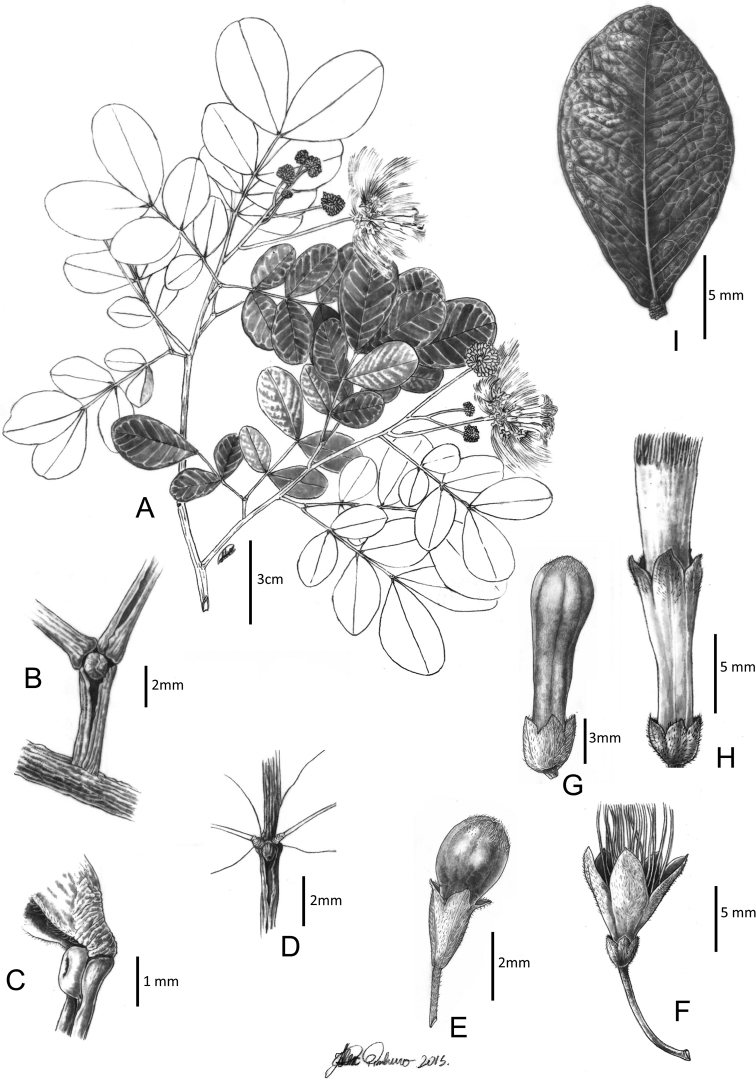
The genus Hydrochorea Barneby & J.W. Grimes A The amphi-atlantic geographic distribution of HydrochoreaBHydrochoreapedicellaris (DC.) M.V.B. Soares, Iganci & M.P. Morim foliage and fruits CHydrochoreacorymbosa (Rich.) Barneby & J.W. Grimes foliage and fruits DHydrochoreapanurensis (Spruce ex Benth.) M.V.B. Soares, M.P. Morim & Iganci foliage and fruits EHydrochoreauaupensis M.P. Morim, Iganci & E.J.M. Koenen in habitat, with foliage and fruits F Flowers of H.uaupensis after rain G mature fruits of H.uaupensis. B, C from M.V.B Soares D from D. Cardoso E–G from J.R.V. Iganci.
Figure 3.
The genus Hydrochorea Barneby & J.W. Grimes (continued). Species from the Americas A flowering branch of Hydrochoreacorymbosa (Rich.) Barneby & J.W. Grimes B close-up of inflorescence of H.corymbosaC discolorous leaves of H.corymbosaD close-up of inflorescence of Hydrochoreapanurensis (Spruce ex Benth.) M.V.B. Soares, M.P. Morim & Iganci E unripe lomentiform pod of H.panurensisF close-up of inflorescence of Hydrochoreapedicellaris (DC.) M.V.B. Soares, Iganci & M.P. Morim, with a few peripheral flowers removed to expose sessile terminal flowers G unripe pods of H.pedicellarisH dehisced follicular pods of H.pedicellaris showing papery septate endocarp I detail of primary rachis of H.pedicellaris showing interpinnal extra-floral nectaries J inflorescence of Hydrochoreauaupensis M.P. Morim, Iganci & E.J.M. Koenen showing large sessile central flower and pedicellate peripheral flowers K unripe crypto-lomentiform pod and seed enveloped by septate endocarp of H.uaupensis; African species L inflorescence of Hydrochoreaobliquifoliolata (De Wild.) E.J.M. Koenen M pinnae of Hydrochorearhombifolia (Benth.) E.J.M. Koenen showing rhombic leaflets. A–E, J, K Erik Koenen F-I Colin Hughes L Jan Wieringa M William Hawthorne. Vouchers A–CJ.R.V. Iganci 862D, EM.P. Morim 563F–IL.P. Queiroz 15529J, KM.P. Morim 577LJ.J. Wieringa 6519M unvouchered.
Balizia Barneby & J.W. Grimes, syn. nov., Mem. New York Bot. Gard. 34(1). 23. 1996. Type: Baliziapedicellaris (DC.) Barneby & J.W. Grimes.
Balizia sect. Leucosamanea Barneby & J.W. Grimes, syn. nov., Mem. New York Bot. Gard. 34(1). 36. 1996. Type: Balizialeucocalyx (Britton & Rose) Barneby & J.W. Grimes.
Balizia Barneby & J.W. Grimes sect. Balizia syn. nov., Mem. New York Bot. Gard. 34 (1). 37. 1996. Type: Baliziapedicellaris (DC.) Barneby & J.W. Grimes.
Type.
Hydrochoreacorymbosa (Rich.) Barneby & J.W. Grimes.
Description.
Shrubs and trees, unarmed; branches grey to brown pilosulous to glabrescent, cylindrical; stipules persistent or caducous. Leaves bipinnate, with 1–15 pairs of pinnae; petiole canaliculate or cylindrical, grey to brown pilosulous or glabrous; nectaries sessile to stipitate, orbicular, patelliform, or cupuliform, the first either near mid-petiole or between the first pinnae pair, and often along the leaf rachis, between the leaflet pairs; leaflets 2–33 pairs per pinna, petiolate to subsessile, rhombic-ovate, rhombic-lanceolate, rhombic-oblong, rhombic-obovate, ovate, elliptic, oblong, lanceolate or oblanceolate, grey to brown pilosulous, ciliate or glabrous, concolorous or more often discolorous, venation pinnate. Inflorescence consisting of umbelliform capitula or corymbiform racemes, arising singly or fasciculate from the axils of coeval or hysteranthous leaves, bracts generally caducous; bracteoles persistent or caducous. Flowers heteromorphic, pedicellate in peripheral flowers, mostly pentamerous, and sessile in the larger terminal flower, 5–8-merous; calyx green, gamosepalous, campanulate, or tubular, pubescent, ciliate or glabrous; corolla pinkish to reddish, yellowish or whitish, gamopetalous, infundibuliform, campanulate, or tubular, glabrous, puberulent, ciliate or pilose at the apex; androecium with (10–)12–60(–75) stamens; filaments white, greenish or roseate, fused into a tube, included in peripheral flowers or exserted beyond the corolla in the terminal flower; stemonozone present, anthers dorsifixed; ovary superior, sessile, truncate at the apex, usually pubescent or sometimes glabrous. Fruits sessile or shortly stipitate, straight or slightly recurved, either lomentiform, the seeds released in one-seeded articles, or woody and indehiscent, the exocarp with transverse fibres and the endocarp hard and septate, or follicular, with similar exocarp but the septate endocarp papyraceous and shed along with the seeds, or crypto-lomentiform with follicular dehiscence, the exocarp smooth and the endocarp remaining attached to the seeds forming 1-seeded articles. Seeds with a hard testa, with pleurogram complete or narrowly U-shaped.
Distribution and habitat.
North America (Mexico), Central America (Belize, Costa Rica, Guatemala, Honduras and Nicaragua), South America (Brazil, Bolivia, Colombia, Ecuador, French Guiana, Guyana, Peru, Suriname and Venezuela) and Africa (Congo Basin and West Africa) (Fig. 2A). Hydrochorea species occur in riparian habitats, inundated and non-inundated wet tropical forests of the Orinoco and Amazon basins, pre-Andean Amazonia along the Nor-Yungas and Pando in Bolivia, Vaupés in Colombia and Huánuco in Peru, Central Brazilian Savanna, the Atlantic Rainforest of Brazil and extending to northern South America in Venezuela and the Guianas and the Gulf-Caribbean lowlands until Mexico, and one species in coastal tidal swamp forests in Upper Guinea (West Africa) and one species in riparian and seasonally inundated forests in the Congo Basin.
Note.
Since the names Hydrochorea and Balizia were published in the same publication (Barneby and Grimes 1996), neither has priority, although Hydrochorea was treated as genus 1 and Balizia as genus 2, Hydrochorea thus appearing first in the publication. The name Hydrochorea is here chosen to represent the recircumscribed genus, especially since the name is appropriate for most of its species, and most Balizia species are also thought to frequently use water-borne seed dispersal, and all but one species (B.elegans) are reported to often occur along river-banks. The name Balizia, being an anagram of Albizia, is less appropriate given that several of its species have previously been placed in Albizia and therefore the name may suggest close kinship, while actually being most closely related to the genus Jupunba.
Identification key to the species of Hydrochorea
| 1a | Species from Congo Basin and West Africa | 2 |
| 2a | Adaxial leaflet surface shiny, abaxial leaflet surface glabrous, apart from the ciliate midrib or with few scattered short hairs especially on and near the midrib; calyx and corolla green to greenish white, corolla lobes glabrous or with a few short white hairs around the apex, Congo Basin (Democratic Republic of Congo, Central African Republic and Gabon) | 6. H.obliquifoliolata |
| 2b | Adaxial leaflet surface dull, abaxial leaflet surface pilose with varying density of hairs (rarely nearly glabrous); calyx and corolla white, upper half of corolla lobes rusty pilose to villous, West Africa (Senegal, Guinea-Bissau, Guinea, and Sierra Leone) | 9. H.rhombifolia |
| 1b | Species from North, Central and South America | 3 |
| 3a | Pinnae 1–jugate on every leaf (seldom 2-jugate and then the true petiole very short) | 4 |
| 4a | Calyx covering the corolla in bud; flowers glabrous, terminal flower with tubular calyx | 7. H.panurensis |
| 4b | Calyx not covering the corolla in bud; flowers puberulous, terminal flower with campanulate calyx | 5. H.marginata |
| 3b | Pinnae 2– or more jugate (seldom 1–jugate on some leaves of the same individual) | 5 |
| 5a | Leaflets up to 10 pairs per pinna | 6 |
| 6a | Pinnae 1–2 jugate, leaflets ovate to rhombic-ovate, corolla of peripheral flowers up to 1.5 mm long; follicular crypto-lomentiform fruit | 10. H.uaupensis |
| 6b | Pinnae (2–)3–6-jugate; leaflets rhombic-oblong, rhombic-ovate or rhombic-lanceolate; corolla of peripheral flowers with more 1.5 mm long; fruit indehiscent or lomentiform | 7 |
| 7a | Leaflets rhombic-oblong; corolla of peripheral flowers more than 7 mm long | 4. H.leucocalyx |
| 7b | Leaflets rhombic-ovate to rhombic-lanceolate; corolla of peripheral flowers up to 6 mm long | 1. H.corymbosa |
| 5b | Leaflets in more than 10 pairs per pinna | 8 |
| 8a | Corolla of peripheral flowers 8–10 mm long, fruit indehiscent, not lomentiform | 2. H.elegans |
| 8b | Corolla of peripheral flowers up to 7.5 mm long, fruit lomentiform or follicular | 9 |
| 9a | Pinnae 3–5-jugate; fruit lomentiform | 3. H.gonggrijpii |
| 9b | Pinnae 6–17-jugate; fruit follicular, with septate endocarp and transverse fibers in the exocarp | 8. H.pedicellaris |
1. Hydrochorea corymbosa
(Rich.) Barneby & J.W. Grimes, New York Bot. Gard. 74(1): 27. 1996.
DAD0E817-D749-5B44-A35A-CA0660B3173F
Figure 4.
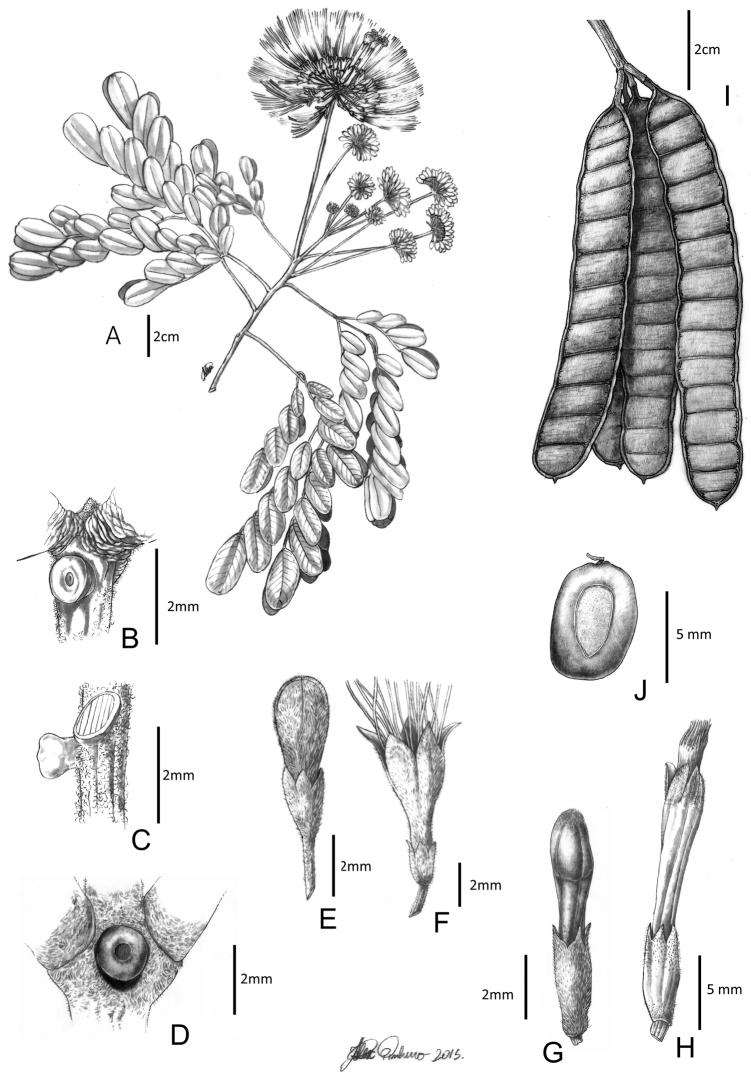
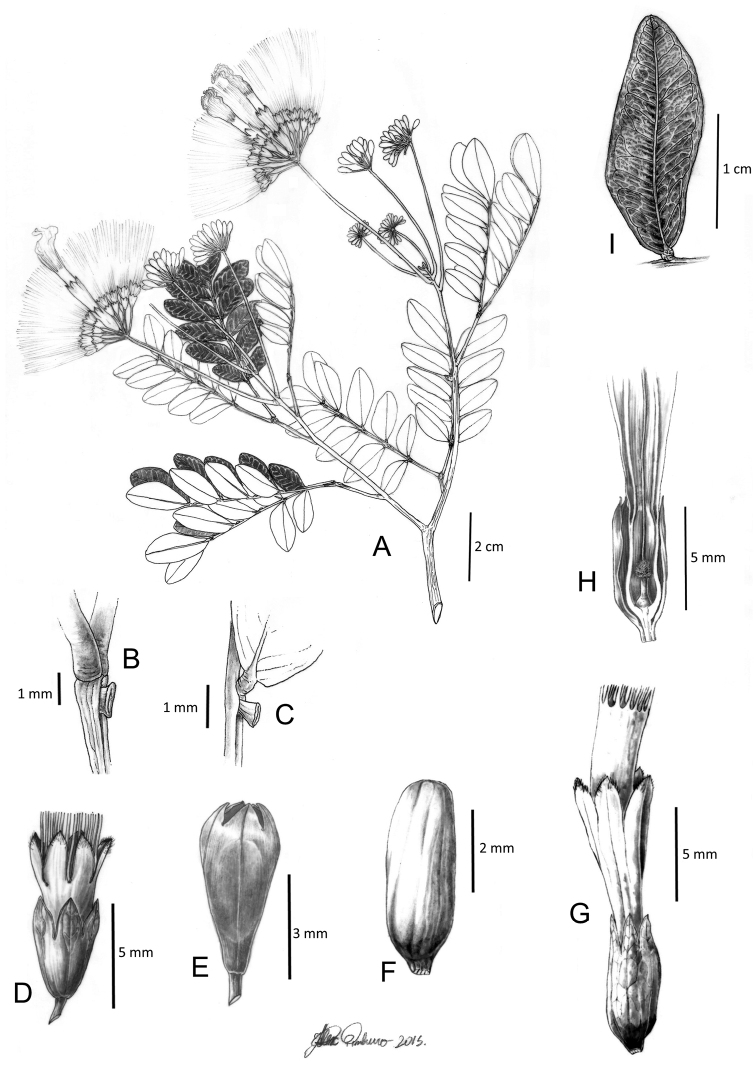
Hydrochoreacorymbosa (Rich.) Barneby & J.W. Grimes A branch with inflorescences B–D extra-floral nectaries E peripheral flower bud F peripheral flower G terminal flower bud H terminal flower I fruit J seed. A, E–H from M.V.B. Soares 75B–D from M.V.B. Soares 180I–J from M.V.B. Soares 174. Illustration by Alex Pinheiro.
Pithecellobium subcorymbosum Hoehne [as Pithecolobium], Comiss. Linhas Telegr. Estratég., Mato Grosso-Amazonas, Bot. 8: 18, Ic. 133. 1919. Type: Brazil, Mato Grosso, São Luiz de Cáceres, nas margens do rio Paraguai, perto da Campina, Hoehne 4582 (lectotype, designated here from amongst the syntypes: R! [R000003169]; isolectotype: SP).
Basionym.
Mimosacorymbosa Rich., Actes Soc. d’Hist. Nat. Paris 1: 113. 1792.
Type material.
French Guiana, frequens in sylvis ripariis fluvii Kourou, Louis Claude Richard s.n. (lectotype, designated by Barneby and Grimes 1996, p. 27, as holotype, here corrected: P [P02142909] digital image!).
Distribution and habitat.
Bolivia, Brazil, Colombia, Ecuador, French Guiana, Guyana, Paraguay, Peru, Venezuela. Hydrochoreacorymbosa occurs in periodically or permanently inundated riparian forest, gallery forest, and open vegetation, up to 480 m elevation (Barneby and Grimes 1996).
Notes.
Hydrochoreacorymbosa is morphologically similar to H.gonggrijpii by its leaves with (2–)3–6 pairs of pinnae (3–5 pairs of pinnae in H.gonggrijpii), but differs by presenting (4–)5–11(–14) leaflet pairs per pinnae (vs. (12–)14–35 in H.gonggrijpii). Hydrochoreacorymbosa has a wide distribution in the Brazilian Amazon, and displays wide morphological plasticity. Barneby and Grimes (1996) recognised the specimen Louis Claude Richard s.n. (P02142909) as holotype, although the species protologue did not present a type specimen. The specimen does represent original material that the author associated with the taxon, being a specimen collected by the author and annotated as Mimosacorymbosa. Thus, Louis Claude Richard s.n. (P02142909) is here corrected to lectotype (Art. 9.3, 9.4, 9.8 and 9.10; Turland et al. 2018).
Selected specimens examined.
Brazil, Amazonas: São Gabriel da Cachoeira, entre Assunção do Içana e Camarão, mato de Igapó, margem do rio, 10 July 2012, J.R.V. Iganci 862 (RB). Bolivia, Pando: Federico Roman, bordo del Río Abuna, 18 November 2006, S. Altamirano & H. Ramos 4293 (K). Colombia, Vaupés: Mitú and Vicinity, lower rio Kubiyú, 26 September 1976, Zarucchi 2147 (INPA). Ecuador, Francisco de Orellana: Estación Científica Yasuní, Río Tiputini, este de la Carretera Repsol-YPF, km 7 desvío hacia el pozo Tivacuno, Laguna Herradura, 20 April 1999, G. Villa 177 (K). Guyana: Potaro-Siparuni, riparian zone lower Kuribrong, April 2010, Zartman et al. 8002 (INPA). Peru, Loreto: Jenaro Herrena, Cano Supay, flooded forest along cano, 23 May 2002, T.D. Pennington et al. 17430 (K). Venezuela, Amazonas: Departamento Rio Negro, middle part of the Río Baria, 21 July 1984, G. Davidse 27570 (K).
2. Hydrochorea elegans
(Ducke) M.V.B. Soares, Iganci & M.P. Morim comb. nov.
C88905FF-CD0D-5E1C-8F7A-C41EA9C07A4E
urn:lsid:ipni.org:names:77303827-1
Balizia elegans (Ducke) Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74(1): 40 1996. Albiziaelegans (Ducke) L. Rico, Novon, 9(4): 556. 1999.
Albizia duckeana L. Rico, syn. nov., Kew Bull. 55(2): 404. 2000. Type: based on Pithecellobiumelegans Ducke.
Basionym.
Pithecellobiumelegans Ducke [as Pithecolobium], Arch. Jard. Bot. Rio de Janeiro 3: 64. 1922.
Type material.
Brazil, in silvis non inundatis, prope Alcobaca (Tocantins), A. Ducke 16271 (lectotype, designated by Barneby and Grimes 1996, p. 40: MG [MG00016271], digital image!; isolectotypes: G [G00359898] digital image!, MG, P [P03093819] digital image!, R [R000002384] digital image!, RB [RB10177]!, US [US1040853] digital image!, US [US00000336] digital image!, US [US00610722] digital image!).
Distribution and habitat.
Bolivia, Brazil, Costa Rica, Ecuador, Honduras, Nicaragua, Peru. Hydrochoreaelegans occurs in primary rain forest, up to 350 m elevation (Barneby and Grimes 1996).
Notes.
Hydrochoreaelegans has a morphological affinity with H.pedicellaris, as already pointed out by Ducke (1922) and by Barneby and Grimes (1996). However, the corolla of peripheral flowers is larger (8–10 mm long) in H.elegans than in H.pedicellaris (up to 7.5 mm long). Ducke (1922) and Barneby and Grimes (1996) also commented on the similarity between the fruit of both species, but the fruits of H.elegans are indehiscent (vs. follicular dehiscence in H.pedicellaris). Hydrochoreaelegans has a disjunct distribution between hylaean Brazil and Costa Rica and Nicaragua.
Selected specimens examined.
Brazil, Rondônia: Porto Velho, área do Reservatório da Usina Hidrelétrica de Samuel, 15 June 1986, C.A.C. Ferreira 7458 (K). Costa Rica, Limón: Talamanca, Fila Carbon, Finca de Pedro Bolivar, 25 May 1999, O. Valerde 1175 (K).
3. Hydrochorea gonggrijpii
(Kleinhoonte) Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74 (1): 25. 1996.
24CE013B-BE4C-5C34-9CDD-75A09762713F
Figure 5.
Hydrochoreagonggrijpii (Kleinhoonte) Barneby & J.W. Grimes A branch with inflorescences B, C extra-floral nectaries D peripheral flower bud E peripheral flower F terminal flower bud G terminal flower H leaflet. A–H from Fróes 28045. Illustration by Alex Pinheiro.
Basionym.
Pithecellobium [as Pithecolobium] gonggrijpii Kleinhoonte Recueil Trav. Bot. Néerl. 22: 414. 1926.
Type material.
Suriname, im Reservat der Zanderij I, die nummerierten Baume n. 102 (Herb. [Acad.Rhenotraiect.J n. 1529, im Dez. 1915, und n. 4350bl. im Juli 1919) und n. 141 (Herb. n. 4357, bl. Im Juli 1919.)” 141, 10/VII/1919”, Forest Bureau 4357 (lectotype, designated here from amongst the syntypes: IAN [IAN49436]!; isolectotypes: A [A00064017] digital image!, BR [BR0000005170067] digital image!, K [K000527996]!, K [K000527995]!, MO [MO954361] digital image!, NY [NY00334660] digital image!, NY [NY00334661] digital image!, NY [NY00334662] digital image!, P [P01818508] digital image!, U [U U0003385] digital image!, U [U0003384] digital image!, US [US00629380] digital image!).
Distribution and habitat.
Brazil, Colombia, Guyana, French Guiana, Suriname, Venezuela. Hydrochoreagonggrijpii occurs along riverbanks, gallery forest margins, and low-lying swamp forests, at 40–1400 m elevation (Barneby and Grimes 1996).
Notes.
In the nomenclatural treatment of H.gonggrijpiiBarneby and Grimes (1996: p. 25) maintained the specimens “Surinam: im Reservat der Zanderij I, die nummerierten Baume n. 102 (Herb. [Acad. Rhenotraiect. J n. 1529, im Dec. 1915, und n. 4350 bl. im Juli 1919) und n. 141 (Herb. n. 4357, bl. im Juli 1919.)” as syntypes. In the present work, the specimen Forest Bureau 4357 (IAN49436) is designated as lectotype (Art. 9.3, Turland et al. 2018).
Selected specimens examined.
Brazil, Amazonas: Presidente Figueiredo, Cachoeira do boto, 21 September 2007, Carvalho-Sobrinho et al 1632 (RB). Colombia, Vaupés: Mitú and vicinity, lower Río Kubiyú, along river, 26 September 1976, J.L. Zarucchi s.n. (K). Suriname. Plantas de Tafelberg (Table Mountain), 10 August 1944, Maguire 24273 (RB). Venezuela, Bolivar: Distrito Piar, gallery forest bordering savana, vicinity of Guadequen (Buadequen), Río Acanán (affluent of Río Carrao), Cerros Los Hermanos, 20 May 1986, Lat 5°26'N, Long 62°17'W, alt 470 meters, J.A. Steyermark et al. 131865 (NY).
4. Hydrochorea leucocalyx
(Britton & Rose) Iganci, M.V.B. Soares & M.P. Morim comb. nov.
77D73681-6358-51F0-A90A-DEB01B38B288
urn:lsid:ipni.org:names:77303828-1
Balizia leucocalyx (Britton & Rose) Barneby & J.W. Grimes, in Mem. New York Bot. Gard. 74(1): 85. 1996.
Basionym.
Samanealeucocalyx Britton & Rose, N. Amer. Fl. 23: 34. 1928.
Type material.
Mexico. Tabasco, El Limon, J. N. Rovirosa 976 (lectotype, designated by Barneby and Grimes 1996, p. 36, as holotype, here corrected: US [US13198371] digital image!, clastotypus (fragm. + photo): NY [NY00003824] digital image!).
Distribution and habitat.
Belize, Guatemala, Honduras, Mexico. Hydrochorealeucocalyx occurs in wet tropical forests, often along riverbanks, seldom in anthropogenic pastures, up to 400 m elevation (Barneby and Grimes 1996).
Notes.
Amongst the species of Hydrochorea, H.leucocalyx is one of the few that does not occur in Amazonia. It has affinities with the new species described in this treatment (see H.uaupensis) and is mainly distinguished by the lomentiform indehiscent fruit (vs. the cryptoloment in H.uaupensis). Barneby and Grimes (1996) recognised the specimen J. N. Rovirosa 976 as holotype, but in the species protologue (Britton and Rose 1928), the authors did not indicate the herbarium where the type specimen was deposited. Thus, following Art. 9.10 of the International Code of Botanical Nomenclature (Turland et al. 2018), the specimen J. N. Rovirosa 976 (US13198371) is here corrected to lectotype.
Selected specimens examined.
Honduras: 7 September 1932, W.S. Schipp 1024 (K). Mexico, Chiapas: km 12 carretera Pénjamo-Chancalá, 8 June 1960, J.P. Chavelas et al. s.n. (K).
5. Hydrochorea marginata
(Spruce ex Benth.) Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74(1): 29. 1996.
8172C4D2-1911-5DD1-9299-9AD42912A6C2
Figure 6.
Hydrochoreamarginata (Spruce ex Benth.) Barneby & J.W. Grimes A branch with inflorescences B–D extra-floral nectaries E peripheral flower bud F peripheral flower G terminal flower bud H terminal flower I leaflet. A–D, F, H, I from A. Carlos et al. 066E, G from C. Ferreira et al. 7260. Illustration by Alex Pinheiro.
Basionym.
Pithecellobium [as Pithecolobium] marginatum Spruce ex Benth., Trans. Linn. Soc. London 30: 586. 1875.
Type material.
Brazil, Barra, by a stream [Prov. Rio Negro], Spruce 1658 (lectotype, designated by Barneby and Grimes 1996, p. 31: K [K000528011]!; isolectotypes: E [E00313848] digital image!, F [V0058733F] digital image!], G [G275450] digital image!, P [P03094432] digital image!, P [P03094430] digital image!).
Distribution and habitat.
Brazil and Venezuela. Hydrochoreamarginata occurs in Amazonia, in flooded areas and along riverbanks and lake shores.
Notes.
Barneby and Grimes (1996) considered H.marginata to comprise three varieties, H.marginatavar.panurensis (Benth.) Barneby & J.W. Grimes, H.marginatavar.scheryi Barneby & J.W. Grimes, and Hydrochoreamarginatavar.marginata. Hydrochoreamarginatavar.panurensis is recognized at the species level in this treatment, and H.marginatavar.scheryi is placed as a synonym of H.panurensis because we identified no morphological diagnostic characters that support them as independent taxa. Both these taxonomic decisions are discussed under H.panurenis.
Selected specimens examined.
Brazil: Amazonas, Rio Negro between Moreira and Rio Arirahá, 13 October 1971, G.T. Prance 15206 (NY).
6. Hydrochorea obliquifoliolata
(De Wild.) E.J.M. Koenen comb.nov.
B04306BD-20E4-5F5B-8322-447CFD1C201F
urn:lsid:ipni.org:names:77303829-1
Pithecellobium obliquifoliolatum (De Wild.) J. Léonard, in Compt. Rend. Sem. Agric. Yangambi Comm. No. 67, 868 (1947).
Pithecellobium obliquifoliolatum (De Wild.) Aubrév., Fl. Forest. Soudano-Guin. 290 (1950), in obs., Aubrev. in Not. Syst., ed. Humbert, xiv. 57 (1950) nom. illeg.;
Arthrosamanea obliquifoliolata (De Wild.) G.C.C. Gilbert & Boutique, Fl. Congo Belge & Ruanda-Urundi iii. 194 (1952).
Cathormion obliquifoliolatum (De Wild.) G.C.C. Gilbert & Boutique, Bull. Soc. Roy. Bot. Belgique 90: 309 (1958).
Basionym.
Albiziaobliquifoliolata De Wild., Bull. Jard. Bot. État Bruxelles 7: 253 (1920).
Type material.
Democratic Republic of the Congo, Congo Belge, Eala, Laurent 1823 (lectotype, designated here: BR [BR0000008916334]!; isolectotype: BR [BR0000008916662]!).
Description.
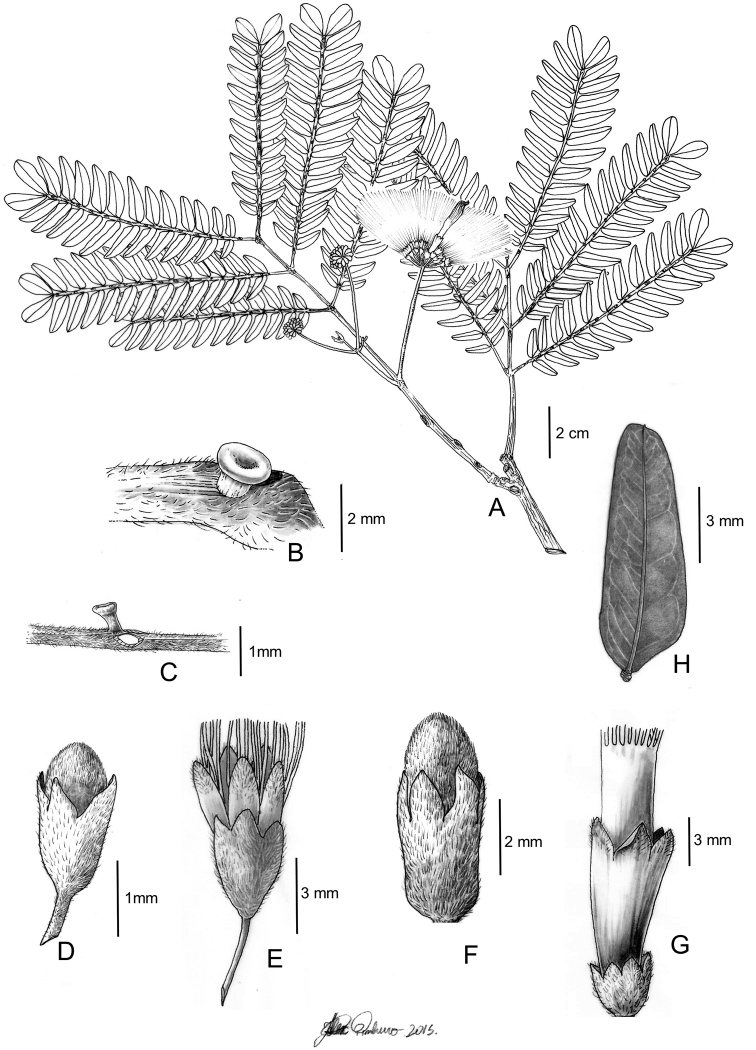
Trees up to 30 m in height and up to 1m DBH, the bark with both small scattered and long transverse linear lenticels, the indumentum consisting of a dense rusty to golden-brown pubescence covering the young twigs, petiole and primary rachis, with more sparse pubescence on peduncles and pinna-rachises except for dense rows of hairs at the margins of the otherwise glabrous canaliculate adaxial side of the pinna rachises, often also the canaliculate primary rachis of the leaf sparsely pubescent to glabrous adaxially. Stipules linear deltoid to falcate, 2–3 mm long, adaxially glabrous except at apex, densely pubescent, caducous. Leaves with (1–)2(–3) pairs of pinnae, petiole pulvinate and slightly flattened at base, (1.5–)2–3.5(–4.5) cm long, with a sessile concave circular to triangular nectary at the apex, c. 0.8–1.5 mm in diameter, rachis usually canaliculate adaxially, (0–)1.5–3(–6) cm long, if the leaf 3-jugate then usually with an inter-pinnal nectary, similar to the petiolar one, in between the middle pair of pinnae, apical nectary usually lacking, pinnae distinctly pulvinate, and usually with minute paraphyllidia at the apex of the pulvini, pinna-rachises canaliculate adaxially, the groove glabrous, c. (3–)4–11(–15) cm long, with short stipitate circular to elliptical cupular or trumpet-shaped nectaries c. (0.2–)0.5–1 mm in diameter. Leaflets in (3–)5–7(–8) pairs per pinna, subsessile on a c. 0.5 mm long pulvinule, widely spaced so that the margins do not overlap, bicolorous, dark green and shiny above, pale dull green beneath, rhomboid and often distinctly curved towards pinna apices, base asymmetrically obtuse or slightly oblique and the apex rounded or shallowly emarginate, sometimes mucronate, (1.4–)2.2–3.5(–4.7) × (0.6–)1.1–1.8(–2.2) cm, except the apical pair that is asymmetrically elliptic with oblique base and emarginate apex, (1.8–)2.7–4.8(–5.5) × (1.0–)1.5–2.5 cm, venation pinnate with (6–)12–16(–18) secondary veins brochidodromous, prominent on both surfaces or prominulous to slightly sunken on upper surface, and reticulate tertiary venation, often prominent on upper surface, obscure beneath, margins and midrib ciliate on both surfaces, lamina glabrous but for a few short scattered appressed hairs. Inflorescences (10–)15–20 flowered umbelliform capitula, on long slender peduncles arising 1–2 from axillary buds of coeval or caducous leaves, held above the foliage, the axillary meristems usually not continuous beyond the peduncles and aborted prior to fruit set, dimorphic with a single enlarged terminal flower and often one dispositioned peripheral flower c. 0.5 cm below the others, on peduncles 4–8(–12) cm long. Bracts linear to spatulate, sometimes bilobed at apex, c. 2–3.5 × 0.5 mm, pubescent with longer hairs at apex. Peripheral flowers on pedicels 2–6 mm long, calyx pentamerous, green to greenish white, c. 3–4 mm long, the deltoid lobes c. 0.5 × 0.5 mm, glabrous, corolla pentamerous, green to greenish white, c. 5–6 mm long, the lobes c. 2–3 × 2 mm, glabrous or with short white hairs around the apices of the lobes, androecium consisting of c. 20–30 stamens, c. 2.1–2.5 cm long, the filaments white to pale green at apex, fused into a tube c. 3 mm long, with dorsifixed pale yellowish green anthers, pollen in 16-celled plano-compressed disc-shaped polyads, pistil c. 2.5–2.8 cm long, ovary c. 3 mm long, pubescent in upper half, the pale green to white style emerging from it at an angle of c. 45°, with a green funnel-shaped stigma, extending beyond the stamens. Terminal flower sessile to subsessile, similar to peripheral flowers but broadly campanulate and larger, calyx c. 3.5–5 mm and corolla c. 7.5–9 mm long, the filaments thicker and staminal tube c. 8–10 mm long, exserted well beyond the corolla tube. Pods falcate and weakly articulated, base often tapering into a c. 5 mm long stipe, (3–)6–12 seeded with a thin papery fruit wall and slightly thickened rim, dark brown to black outside when ripe, light brown inside, (3.7–)5.5–9.6 × 1.2–1.4 cm, breaking up into 1-seeded articles 0.4–0.7(–1.0) cm long, the basal and apical articles up to 1 cm long, seed c. 6.5 × 4.5 × 0.5 mm, the testa hard, light brown with a darker brown closed elliptic pleurogram, c. 4 × 2 mm.
Distribution and habitat.
Gabon, Central African Republic, Congo-Brazzaville, Democratic Republic of the Congo. Hydrochoreaobliquifoliolata occurs in the Congo Basin, and is a species of swamp forests, seasonally inundated forests and riverbanks.
Notes.
The similarities to Cathormionrhombifolium, the other African species that is here transferred to Hydrochorea, are discussed below.
Selected specimens examined.
Gabon: Ogooué-Lolo, road Okondja to Bambidie and Lastoursville, 21 km SW of Okondja, 7 February 2008, J.J. Wieringa 6519 (BR, K). Democratic Republic of Congo: Yafunda, rive guache, près d’Isangi, 8 September 1938, J. Louis 11175 (BR). Boendu, August 1938, Du Bois 904 (BR), G. Couteaux 55 (BR). Bolomba, 7 November 1957, C. Évrard 2746 (BR). Bongoy, 4 January 1958, C. Évrard 3191 (BR). Botsima, route station-village, 28 January 1991, J.B.M.M. Dhetchuvi 321 (BR). Yangambi, île Tutuku en face du plateau de l’Isalowe, 3 January 1940, R.G.A. Germain 87 (BR). Bokondji, 28 September 1959, De Wanckel 162 (BR).
7. Hydrochorea panurensis
(Spruce ex Benth.) M.V.B. Soares, M.P. Morim & Iganci comb. nov.
10C8808B-B354-5266-BEA8-4C4BECB054E5
urn:lsid:ipni.org:names:77303830-1
Figure 7.
Hydrochoreapanurensis (Spruce ex Benth.) M.V.B. Soares, M.P. Morim & Iganci A branch with inflorescences B, C extra-floral nectaries D peripheral flower E peripheral flower bud F terminal flower bud G terminal flower H terminal flower longitudinal section I leaflet. A–I from Wurdack & Adderley 43618. Illustration by Alex Pinheiro.
Hydrochorea marginata var. panurensis (Benth.) Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74: 32. 1996. Type: based on Pithecellobiumpanurense Spruce ex Benth., syn. nov.
Hydrochorea marginata var. scheryi Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74: 32. 1996. Type: Venezuela, at Sanariapo, Territorio Federal Amazonas, Llewellyn Williams 15953 (lectotype first step, designated by Barneby and Grimes 1996, p. 32, as holotype, here corrected: F; lectotype second step, here designated: F [V0058706F], digital image!; isotypes: F [V0058707F] digital image!), G [G00365761] digital image!, GH [GH00060404] digital image! K [K0005279] digital image!, MO [MO954357] digital image!, MO [MO954358] digital image!), NY [NY00334620] digital image! US [US00000401] digital image!, US [US00385625] digital image!, VEN [VEN2237] digital image!, syn. nov.
Basionym.
Pithecellobium [as Pithecolobium] panurense Spruce ex Benth., Trans. Linn. Soc. London 30: 586. 1875.
Type material.
Brazil, in silvis ‘Gapó’ ad flumen Uaupés prope Panuré, prov. do Alto Amazonas, Spruce 2425 (lectotype first step, designated by Barneby and Grimes 1996, p. 32, as holotype, here corrected: K; lectotype second step, here designated: K [K000528008]!; isolectotypes: E [E00313845] digital image!, F, [V0058739F] digital image!, G [G00365356] digital image!, G [G00365687] digital image!, GH [GH00063965] digital image!, K [K000528007]!, NY [NY334690], P [P03094382] digital image!, P [P03094383] digital image!, RB [RB00708599]!).
Distribution and habitat.
Brazil, Venezuela. Hydrochoreapanurensis occurs in seasonally flooded Amazonian sites along rocky stream banks and ecotone with gallery forests.
Notes.
Ducke (1949) considered Pithecellobiumpanurense as a form of P.marginatum, considering the vegetative characters to be more important taxonomically than the differences between the flowers of those taxa. Barneby and Grimes (1996) moved P.panurense to Hydrochorea as H.marginatavar.panurensis. However, Hydrochoreapanurense has diagnostic floral characters which distinguish it from H.marginata. The floral buds of H.panurense have the corolla covered by the calyx; the flowers are glabrous and the terminal flower has a tubular calyx (vs. calyx not covering the corolla in the floral buds, pubescent flowers, and calyx of the terminal flower campanulate in H.marginata). Barneby and Grimes (1996) also described H.marginatavar.scheryi, distinguishing it from H.marginatavar.panurensis only by the pedicel of the peripheral flowers, this 4–5 mm long. (vs. 6.5–13 mm long in H.marginatavar.panurensis). Barneby and Grimes (1996) emphasized, however, that this is the only character to distinguish between the two taxa. In the present study we consider H.marginatavar.scheryi to be a synonym of H.panurensis.
A second step lectotypification is designated here for both H.panurensis and H.marginatavar.scheryi, since the material that Barneby and Grimes (1996) recognised as holotypes consists of two sheets in both cases and they did not select lectotypes from amongst these sheets (Art. 9.10,9.17; Turland et al. 2018).
Selected specimens examined.
Brazil, Amazonas: Barcelos, Serra do Araçá, Rio Araçá à 13 h de Barcelos, 28 July 1985, Silva 389 (INPA); São Gabriel da Cachoeira. Margem do Rio Içana em direção a comunidade Camarão, 0°48'35.8"N, 67°32'10"W, 19 July 2012, Morim, M.P., Iganci, J.R.V, Bonadeu F & Koenen, E. 563 (RB, Z). Venezuela, Amazonas: Rio Casiquiare, 11 November 1959, Wurdack & Adderley 43407 (IAN).
8. Hydrochorea pedicellaris
(DC.) M.V.B. Soares, Iganci & M.P. Morim comb. nov.
71652CE9-BE1B-57EE-B55B-E1AE26D7B1B1
urn:lsid:ipni.org:names:77303831-1
Balizia pedicellaris (DC.) Barneby & J.W. Grimes, in Mem. New York Bot. Gard. 74(1): 85. 1996.
Albizia pedicellaris (DC.) L. Rico, in Novon 9(4): 555. 1999.
Basionym.
Ingapedicellaris DC., Prodr. 2: 441. 1825.
Type material.
French Guiana, “...in Cayenna” (lectotype, designated by Barneby and Grimes 1996, p. 36, as holotype, here corrected: G-DC = F Neg. 6972, digital image!).
Distribution and habitat.
Bolivia, Brazil, Colombia, Ecuador, French Guiana, Guyana, Peru, Suriname, Venezuela. Hydrochoreapedicellaris occurs in non-inundated primary rainforest in Amazonia, in the lowlands of the Atlantic Rainforest, and in gallery forests, up to 200 m elevation, and occasionally at 700–800 m elevation in Bolivia, Ecuador and eastern Brazil (Barneby and Grimes 1996).
Notes.
Hydrochoreapedicellaris is the only species of the genus that occurs in a range of environments including areas of the Brazilian Atlantic Forest. It has an affinity with H.elegans (see comment under that species), but when it is in fruit it is easily recognized by its follicular dehiscence, and an exocarp with deep, transversal fissures. Barneby and Grimes (1996) recognised a specimen in the G-DC herbarium as holotype, but in the species protologue (De Candolle 1825), the author did not indicate a type specimen. Thus, the specimen at G-DC should be considered a lectotype, here corrected (Art. 9.10; Turland et al. 2018).
Selected specimens examined.
Bolivia, La Paz: Province of Larecaja, Tuiri, 12 September 1989, B. Krukoff 10886 (K). Brazil, Amazonas: São Gabriel da Cachoeira, Rio Içana, na comunidade Camarão, terra firme, 0°37'23"N, 67°26'57"W, 20 July 2012, Iganci, J.R.V, Morim, M.P., Bonadeu F., Koenen, E. 870 (RB); Espírito Santo: Linhares Fragmento em frente a casa do Reis, Sítio Santo Domingo, Restinga arbórea de cordões arenosos, 19°21'6"S, 39°43'31"W, 13 March 2007, R.D. Ribeiro et al. 812 (RB). Guyana: Territorio Federal Delta Amacuro, 29 May 1964, L.M. Berti 225 (K). Peru: Palcazú, Pasco Oxapampa, localidad Mayro, 20 May 2010, R. Vásquez et al. 36546 (K). Suriname: Zenderij, November 1944, M. Koeleroe 237 (RB). Venezuela: Altiplanicie de Nuria, 15 July 1960, J.A. Steyermark 86335 (K).
9. Hydrochorea rhombifolia
(Benth.) E.J.M. Koenen comb. nov.
492FC025-6480-5E32-AF87-6D60DD90AD63
urn:lsid:ipni.org:names:77303832-1
Feuilleea rhombifolia (Benth.) Kuntze, Revis. Gen. Pl. 1: 189 (1891).
Cathormion rhombifolium (Benth.) Keay, Kew Bull. 8(4): 489 (1953).
Basionym.
Albiziarhombifolia Benth., London J. Bot. 3: 87 (1844).
Type material.
Guinée, Conakry, Heudelot 735 (lectotype designated here from amongst the syntypes: K [K000043955]!; isolectotypes: K [K000043954]!, K [K000043949]!, P [P00418271] digital image!, P [P00418272] digital image!, P [P00418270] digital image!).
Description.
Trees or shrubs up to 12 m tall, the young stems, all leaf-axes and peduncles puberulent-tomentulose with rusty brown hairs. Stipules deltoid, c. 1 mm long, puberulent-tomentulose, caducous. Leaves with 2–3 pairs of pinnae, petiole pulvinate, ventrally flattened above pulvinule and with central groove in upper half, 2–3.5(–8.5) cm long, rachis ventrally grooved, 1.5–4(–12.5) cm long, pinna rachises pulvinate, ventrally grooved, (3.2–)4–6(–12) cm long. Nectaries present at the petiole apex just below the first pair of pinnae as well as just below each further pair of pinnae, sessile or shortly stipitate on stipe to 0.5 mm, cupular or sometimes concave, circular and 0.8–2.2 mm in diameter, and between the upper 2–3 pairs of leaflets, trumpet-shaped and then on a short stipe 0.5 mm or cupular and (sub)sessile, the lower ones circular and the upper ones elliptical, 0.8–1.5 × 0.8–1.1 mm. Minute paraphyllidia sometimes present at the apex of the pinna-pulvinus. Leaflets in 4–6 pairs per pinna, closely spaced, bicoloured leaflets often with partly overlapping margins, bright green above and pale green beneath, dull on both surfaces, rhomboid with a pulvinate sessile oblique base and rounded to slightly emarginate apex, increasing in size towards pinna apex, (1.1–)1.7–3.5(–5.1) × (0.5–)1.2–1.8(–2.3) cm, except for the apical pair which has a less oblique to nearly acute base, (2.1–)2.5–4.5(–5.7) × (1.1–)1.5–2.5(–3.2) cm; venation pinnate with 8–12(–18) secondary veins brochidodromous, tertiary venation reticulate, prominulous on both surfaces, midribs ciliate on both sides, the lower leaflet surface pilose with a variable density of brownish to white hairs, rarely almost glabrous, sometimes villose particularly near the midrib giving a rusty orange-brown appearance. Inflorescences umbelliform capitula, axillary to co-eval leaves on peduncles (4.5–)5–9.5 cm long, dimorphic with 6–16 peripheral flowers and 1–2 terminal flower(s) with elongated exserted staminal tubes. Bracts spatulate, c. 1.8 mm long, puberulent with minute rusty hairs, caducous. Peripheral flowers on pedicels of 1–4 mm, calyx pentamerous, white, 3–3.5 mm long, fused, the deltoid lobes 1–1.3 mm long, glabrous or with few minute hairs, corolla pentamerous, white, 6–8 mm long, fused in the lower half, glabrous, pilose to villose in the upper half, androecium 1.6–2.3 cm long, consisting of 20–28 stamens with white filaments fused at the base into a short tube of c. 2 mm, anthers dorsifixed, pollen in 16-celled plano-compressed disc-shaped polyads, gynoecium with a c. 2 mm long ovary, pubescent on the upper half, the 1.6–2.5 cm long white style emerging from it at an angle of c. 45°, with a funnel-shaped stigma, extending beyond the stamens. Terminal flower(s) similar but larger and more robust in appearance, calyx c. 4.5 mm long with c. 1.5 mm long lobes, corolla c. 9 mm long, androecium with 30–36 stamens that are thicker and fused into a tube 7–10 mm long, exserted well beyond the corolla tube, and with a sunken nectariferous disk below the base of the ovary, gynoecium otherwise similar to that of the peripheral flowers. Pods straight to falcate, 6–12-seeded with a thin papery fruit wall and thickened rim, dark brown outside when ripe, whitish grey inside, (4.5–)7–12.5 × 1.4–1.9 cm, breaking up into 1-seeded articles 0.6–1.1 cm long, seed c. 7 × 4.5 × 2 mm, the testa hard, light brown with a wide lighter brown closed pleurogram.
Distribution and habitat.
Known from the tidal riverine systems near the coast from Senegal to Sierra Leone. Hydrochorearhombifolia occurs often abundantly, in permanent or tidal swamp forest, including on the edge of mangrove swamps, and in gallery forests.
Notes.
Bentham (1844) described Albiziarhombifolia, before designation of holotypes was required by the International Code of Botanical Nomenclature. Keay (1953) made the new combination Cathormionrhombifolium and cited the holotype as being at Kew. However, there are three specimens of Heudelot 735 at K, the type that was cited by Bentham, leaving it ambiguous as to which one of these represents the holotype. Therefore, the specimen from Herbarium Benthamianum (the oldest deposited specimen dating to 1854) is here designated as a lectotype: it has leaves and flowers, and is more richly annotated than the other two specimens.
Hydrochoreaobliquifoliolata and H.rhombifolia are morphologically very similar and have sometimes been confused in herbaria, despite their clearly different geographical distributions. The species are readily separated by the darker appearance of the leaflets of H.obliquifoliolata, which have a distinct shine on the upper surface and the lower surface usually (sub-)glabrous (vs. a usually rusty pilose lower leaflet surface in H.rhombifolia). The leaflets of H.rhombifolia are also more closely spaced than those of H.obliquifoliolata, the latter not having overlapping margins. Furthermore, the flower colour of the two species is clearly different (as per the key), a characteristic which remains apparent when comparing dried flowering specimens in the herbarium, and the corolla lobes of H.obliquifoliolata are glabrous or with a few short apical hairs (vs. pilose to villous on the upper half in H.rhombifolia).
Selected specimens examined.
Sierra Leone: Mange, 7 February 1939, F.C. Deighton 3618 (K), Rokupr, 25 May 1953, F.C. Deighton 5925 (K), Kasanko (Mafore), 3 December 1950, T.S. Jones 52 (K), near Tassin and Kukum, 17 January 1892, G.F. Scott Elliot 4418 (K); Guinée-Bissau: Gabu, Ponte do rio Colufe, 10 June 1949, Espirito Santo 2500 (K).
10. Hydrochorea uaupensis
M.P. Morim, Iganci & E.J.M. Koenen sp. nov.
3BE35DAE-0FD1-5E19-B087-AEDA50DF6775
urn:lsid:ipni.org:names:77303833-1
Figure 8.
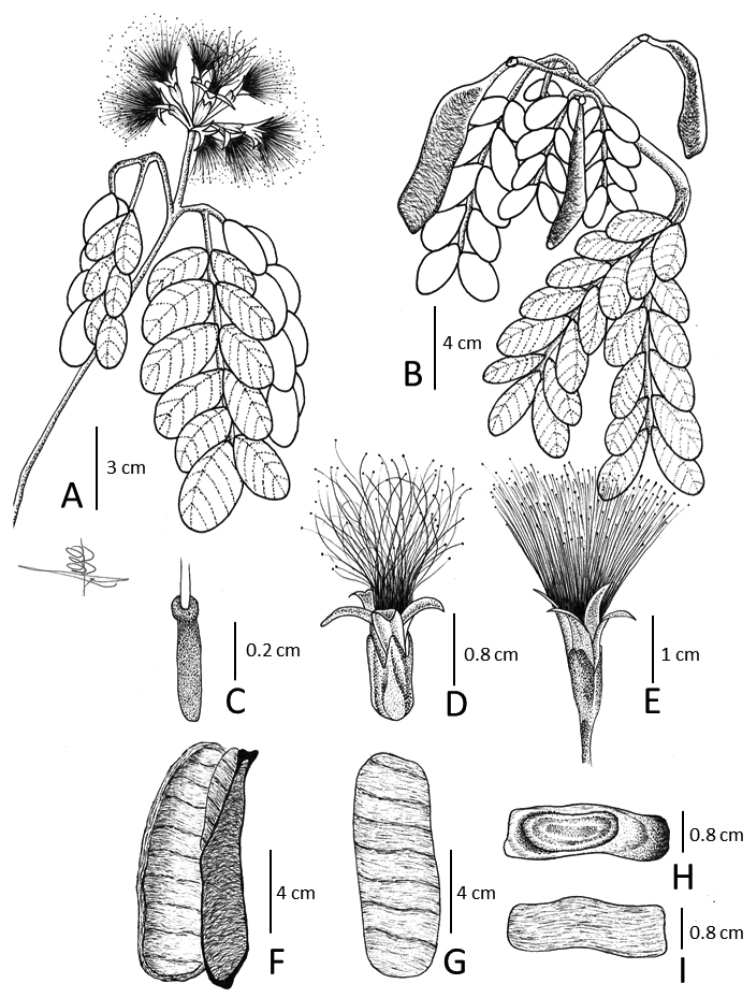
Hydrochoreauaupensis M.P. Morim, Iganci & E.J.M. Koenen A branch with inflorescences B branch with fruits C ovary D terminal flower E peripheral flower F dehisced fruit G detail of fruit endocarp forming 1-seeded articles H, I monospermous articles. A–I from M.P. Morim et al. 577. Illustration by João Augusto Castor Silva.
Diagnosis.
Hydrochoreauaupensis is morphologically similar in appearance to H.leucocalyx (Britton & Rose) Iganci, M.V.B. Soares & M.P. Morim by its leaflets and inflorescence, however it differs by having a red or green calyx, pink corolla, 1–2 pairs of pinnae, and crypto-lomentiform fruits (vs. white calyx and corolla, 3–5(–6) pairs of pinnae and indehiscent fruits in H.leucocalyx).
Type material.
Brazil, Amazonas, São Gabriel da Cachoeira. Igarapé Tibuari, afluente do Vaupés 0°05'5"N, 67°23’16"W, 23 July 2012, fl. and fr., M.P. Morim, J.R.V. Iganci, F. Bonadeu, E. Koenen 577 (holotype: RB [RB00728413]!; isotypes: HUEFS!, INPA!, K!, MBM!, MG!, MO!, NY!, PEL!, S!, US!, Z!).
Description.
Trees 2–6 m tall, trunk not observed, partially underwater during seasonal inundation. Branches, leaf axes and peduncles sparsely pubescent to glabrescent. Stipules linear, to 1.2 cm long, densely pubescent on outer surface. Leaves with 1–2 pairs of pinnae; petiole including pulvinus 1.5–4.5 cm, cylindrical; rachis 0 or 3–4(–9) cm, glabrous, canaliculate; extrafloral nectaries borne between first or both pairs of pinnae, sessile, patelliform and smaller nectaries usually present between the leaflet pairs; pinnae 3–5 jugate; leaflets subsessile on pulvinules, chartaceous, c. 2–4(–6) × 1.5–2(–3) cm, rhomboid or ovate, apex emarginate or obtuse, sometimes with a minute mucro, base asymmetrically oblique to acute; adaxial and abaxial surfaces glabrous, discolorous, adaxial surface sometimes lustrous; venation pinnate with c. 11–17 secondary veins, tertiary venation reticulate and prominent on both surfaces when leaflets dry. Inflorescences dimorphic, umbelliform with 7–10 peripheral flowers and an enlarged sessile terminal flower, peduncle 4–6(–8) cm. Bracts and bracteoles not seen. Flowers with a reddish or green calyx, pink corolla and white filaments, the flower buds oblong, ca. 8 mm long, with the corolla concealed by the calyx prior to anthesis, peripheral flowers on pedicels 0.7–1.5 cm, calyx campanulate, c. 9 mm long, 5-angulate due to prominently raised veins, sparsely puberulent or ciliate at the apex of the lobes, corolla tubular, with a prominulous midvein on the lobes, c. 1.5 cm long, sparsely puberulent on the upper half of the lobes, stamens c. 50–60, the filaments white, c. 3 cm long, exserted from the corolla ca. 2 cm; ovary glabrous, 3–4 mm, sub-truncate to truncate at the apex, style 3.5–4 cm, stigma funnel-shaped; terminal flower similar to peripheral flowers but more robust and c. 5 mm wide at base, calyx c. 1.2 cm long, corolla 1.6 cm long, stamens ca. 75, ca. 3.5 cm long. Pods typically 1–3 per infructescence, crypto-lomentiform, up to 15-seeded, oblong, slightly curved, lignescent, c. 9.5 × 2.5 cm excluding a ca. 5 mm long mucro, dehiscence follicular, the smooth exocarp and transversely fibrous mesocarp continuous, the endocarp septate, enveloping the seeds which are released in monospermous articles. Seeds not seen in mature state, oblong, c. 1.6 × 0.4–0.7 cm, pleurogram extending from apex to base, c. 1.3 × 0.3–0.4 cm, closed.
Distribution and habitat.
Brazil. Known only from the Upper Rio Negro region in the Brazilian Amazon (Amazonas state), in seasonally inundated “campinarana” vegetation.
Phenology.
Flowering and fruiting in July.
Etymology.
The specific epithet refers to the type locality, near the River Uaupés, in the state of Amazonas, Brazil. The indigenous people living in this area (e.g., the Tucanos) were known as Uaupés, and later the river took the same name.
Notes.
Hydrochoreauaupensis is only known from Amazonas state, Brazil, where it was collected at “Igarapé Tibuari”, in the municipality of São Gabriel da Cachoeira, during fieldwork in July 2012. The species grows in open vegetation on white sand, known in Brazil as campinarana in the Amazon Domain. During times of flood, only the treetops are exposed above the water line. A second herbarium collection from close to the type locality (Rio Tourí, afl. do Rio Negro, igapó; R.L. Fróes 28691, IAN [IAN78279]), of which we have only seen an image, is here tentatively included under H.uaupensis because the fruit and leaves match the type material and the flowers are described as pink on the specimen label. Since these two occurrence records are close to the borders with Colombia and Venezuela, the species is to be expected in those two countries.
The phylogenetic position of H.uaupensis, as the sister lineage of the clade composed of Hydrochorea sensu Barneby and Grimes (1996) and the African Hydrochorea spp., provides ample support for this as a distinct taxon and a species new to science, as it does not form a sister pair with any other known species. Furthermore, this phylogenetic position is in line with the fruit morphology of the species being intermediate between Balizia and Hydrochorea, adding further support, along with the paraphyly of Balizia, for not maintaining these as distinct genera.
Conservation status.
Data deficient. The species is known only from two adjacent localities in the Upper Rio Negro region of Amazonas state, Brazil. More collections are needed to assess the species’ conservation status.
Conclusion
Our results provide significant advances in the generic delimitation of Hydrochorea and related taxa, as well as broadening our understanding of ongoing diversification in these taxa. The uncertain phylogenomic position of Jupunbamacradenia, and other species of Jupunba, sharing a relatively large number of incompletely sorted genes with Hydrochorea, leads to further difficulties in our ability to delimit genera in a group where classification has been notoriously unstable. Nevertheless, given the complex evolutionary patterns across the genome presented by the Jupunba clade taxa, we decided to use morphology as our main guide for taxonomic decisions, re-circumscribing Hydrochorea to include ten species to account for the paraphyly of Balizia, while incomplete lineage sorting surrounding the divergence between Hydrochorea and Jupunba does not falsify these two genera as natural groups. Furthermore, not transferring all these species to Jupunba, although a cautious decision, avoids the publication of more new names while safeguarding morphological diagnosability. The species treated here as Hydrochorea form a morphologically homogeneous group in terms of vegetative and floral characters, although the fruits are variable as observed in other mimosoid genera (e.g., Aviles Peraza et al. 2022). Nevertheless, fruit type is useful for the identification of some species of Hydrochorea. Quantitative characters, such as the number of pinnae per leaf and number of leaflets per pinna, also can be useful for identification of some species, but we commonly observed overlap in these characters between species and even variation on the same individual.
Supplementary Material
Acknowledgements
The authors are grateful to the curators of all herbaria consulted, with special thanks to RB, NY and K. We thank Francismeire Bonadeu MSc., for her contributions during fieldwork and for preparing herbarium labels; the National Indian Foundation (FUNAI) for providing permits for research inside indigenous areas in Amazonia; the indigenous community Baniwa, for receiving us during fieldwork along the Içana River, in the Brazilian Amazon; Eimear NicLughada, for sending high resolution specimen images from K; Alex Pinheiro and João Augusto Castor Silva for the illustrations. JRVI thanks the Brazilian National Council for Scientific and Technological Development (CNPq-310075/2020-3; 311847/2021-8) for the research grants received. We thank Domingos Cardoso and Marcelo Simon for sharing fieldwork photographs and herbarium specimen images. MVBS thanks the Federal University of Amazonas (UFRA), the Museu Paraense Emílio Goeldi (MPEG), and the Federal University of Rio Grande do Sul (UFRGS), for infrastructure support of this work; the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES - Office for the Advancement of Higher Education; Grant no. PNADB 922/2010) for a grant supporting an MSc. scholarship and travel to consult herbaria; the Brazilian National Council for Scientific and Technological Development (CNPq-141414/2016-2) for a PhD. scholarship. EJMK wishes to thank Gwilym Lewis for acting as SYNTHESYS+ host at K. EJMK was supported by the Swiss National Science Foundation (Early.Postdoc.Mobility fellowship P2ZHP3_199693) and received further support from the SYNTHESYS+ project http://www.synthesys.info/ which is financed by European Community Research Infrastructure Action under the H2020 Integrating Activities Programme, Project number 823827. We would like to thank Gwilym Lewis and Marcelo Simon for their reviews that provided very useful comments that have improved the manuscript.
Appendix 1
Vouchers and European Nucleotide Archive (ENA, https://www.ebi.ac.uk/ena/) accession numbers for molecular phylogenetic data. Note that the taxonomy of Albiziasect.Arthrosamanea is updated in this volume by Aviles Peraza et al. (2022), where new binomials in the genus Pseudalbizzia Britton & Rose are presented for the majority of the species of this section.
Acaciarostellifera Benth., Murphy 466 (MEL): ERS11697109; Afrocalliandraredacta (J.H. Ross) E.R. Souza & L.P. Queiroz, Germishuizen 5585 (PRE): ERS11697117; Albiziaadinocephala Britton & Rose, Hughes 1070 (FHO): ERS11697120; Albiziaburkartiana Barneby & J.W. Grimes, Stival-Santos 678 (RB): ERS4812854 ; Albiziacoripatensis (Rusby) Schery, Hughes 2433 (FHO): ERS11697123; Albiziadecandra (Ducke) Barneby & J.W. Grimes, Vilhena 231 (NY): ERS11697124; Albiziaedwallii (Hoehne) Barneby & J.W. Grimes, Dalmaso 272 (RB): ERS4812856 ; Albiziaglabripetala (H.S. Irwin) G.P. Lewis & P.E. Owen, Lewis 1652 (K): ERS11697125; Albiziainundata (Mart.) Barneby & J.W. Grimes, Wood 26530 (K): ERS4812859; Albiziamultiflora (Kunth) Barneby & J.W. Grimes, Hughes 3090 (FHO): ERS11697127; Albizianiopoides (Spruce ex Benth.) Burkart, Simon 1601 (CEN): ERS11697128; Albiziasinaloensis Britton & Rose, Hughes 1576 (K): ERS11697130; Albiziasubdimidiatavar.minor Barneby & J.W. Grimes, Gorts 341 (K): ERS11697131; Albiziasubdimidiata(Splitg.)Barneby & J.W. Grimesvar.subdimidiata, Ferreira 210 (K): ERS11697129; Albiziatomentella Miq., Leach & Dunlop 3801 (L): ERR9867596; Albiziatomentosa Standl., Hughes 648 (K): ERS11697132; Albiziaxerophytica J.Linares, Hughes 1435 (K): ERS11697133; Archidendronclypearia (Jack) I.C. Nielsen, Wieringa 1849 (WAG): ERS11697136; Calliandrabella Benth., Queiroz 15696 (HUEFS): ERS11697156; Enterolobiumcyclocarpum (Jacq.) Griseb., Macqueen & Styles 75 (K): ERS11697204; Hydrochoreacorymbosa (Rich.) Barneby & J.W. Grimes (1), Bonadeu 655 (RB): ERS4812902 ; Hydrochoreacorymbosa (Rich.) Barneby & J.W. Grimes (2), Iganci 862 (RB): ERS4812903 ; Hydrochoreaelegans (Ducke) Iganci, M.V.B. Soares & M.P. Morim, Iganci 870 (RB): ERS11697146; Hydrochoreagonggrijpii (Kleinh.) Barneby & J.W. Grimes, Tillett 45696 (K): ERS11697223; Hydrochorealeucocalyx (Britton & Rose) Iganci, M.V.B. Soares & M.P. Morim, Aguilar 1939 (NY): ERS11697147; Hydrochoreapanurensis (Spruce ex Benth.) M.V.B. Soares, M.P. Morim & Iganci, Morim 563 (NY): ERS11697224; Hydrochoreaobliquifoliolata (De Wild.) E.J.M. Koenen, Wieringa 6519 (WAG): ERS4812863 ; Hydrochoreapedicellaris (DC.) Iganci, M.V.B. Soares & M.P. Morim, Queiroz 15529 (HUEFS): ERS4812877 ; Hydrochorearhombifolia (Benth.) E.J.M. Koenen, Deighton 3618 (K): ERS11697168; Hydrochoreauaupensis M.P. Morim, Iganci & E.J.M. Koenen, Morim 577 (RB): ERS4812878 ; Jupunbaabbottii Britton & Rose, Zanoni 21220 (NY): ERS11697071; Jupunbaasplenifolia Britton & Rose, Ekman 6383 (NY): ERS11697072; Jupunbabarbouriana (Standl.) M.V.B. Soares, M.P. Morim & Iganci, Iganci 847 (RB): ERS11697073; Jupunbabrachystachya (DC.) M.V.B. Soares, M.P. Morim & Iganci, Lima 7438 (RB): ERS11697074; Jupunbacochleata (Willd.) M.V.B. Soares, M.P. Morim & Iganci, Bonadeu 673 (RB): ERS11697076; Jupunbacommutata (Barneby & J.W. Grimes) M.V.B. Soares, M.P. Morim & Iganci, Maguire 46145 (NY): ERS11697077; Jupunbafilamentosa, (Benth.) M.V.B. Soares, M.P. Morim & Iganci Lima 7487 (RB): ERS11697079; Jupunbafloribunda (Spruce ex Benth.) M.V.B. Soares, M.P. Morim & Iganci, Iganci 883 (RB): ERS11697080; Jupunbaidiopoda (S.F. Blake) M.V.B. Soares, M.P. Morim & Iganci, Quesada 1718 (NY): ERS11697081; Jupunbalaeta (Benth.) M.V.B. Soares, M.P. Morim & Iganci, Mori 25147 (NY): ERS11697083; Jupunbalangsdorfii (Benth.) M.V.B. Soares, M.P. Morim & Iganci, Ribeiro 728 (RB): ERS11697084; Jupunbaleucophylla (Spruce ex Benth.) M.V.B. Soares, M.P. Morim & Iganci, Iganci 839 (RB): ERS11697086; Jupunbalongipedunculata (H.S. Irwin) M.V.B. Soares, M.P. Morim & Iganci, Cardona 2682 (NY): ERS11697088; Jupunbamacradenia (Pittier) M.V.B. Soares, M.P. Morim & Iganci, Lourteig 3021 (NY): ERS11697089; Jupunbamicrocalyx (Spruce ex Benth.) M.V.B. Soares, M.P. Morim & Iganci, Iganci 855 (RB): ERS11697090; Jupunbanipensis Britton & Rose, Mayo 19662 (NY): ERS11697091; Jupunbaoppositifolia Britton & Rose, Liegier 16014 (NY): ERS11697092; Jupunbaoxyphyllidia (Barneby & J.W. Grimes) M.V.B. Soares, M.P. Morim & Iganci, Yonker 6157 (NY): ERS11697093; Jupunbarhombea (Benth.) M.V.B. Soares, M.P. Morim & Iganci, Iganci 261 (RB): ERS11697087; Jupunbatrapezifolia Moldenke, Simon 1600 (CEN): ERS4812839 ; Jupunbavillosa (Iganci & M.P. Lima) M.V.B. Soares, M.P. Morim & Iganci, Borges 423 (RB): ERS11697095; Punjubacallejasii (Barneby & J.W. Grimes) M.V.B. Soares, M.P. Morim & Iganci, Daly 5935 (NY): ERS11697075; Punjubakillipii Britton & Rose, Palodorios 6252 (NY): ERS11697082; Punjubalehmannii Britton & Rose ex Britton & Killip, Escobar 7465 (NY): ERS11697085; Punjubaracemiflora Britton & Rose, Jimenez & Soares 3626 (USJ): ERS11697094.
Citation
Soares MVB, Koenen EJM, Iganci JRV, Morim MP (2022) A new generic circumscription of Hydrochorea (Leguminosae, Caesalpinioideae, mimosoid clade) with an amphi-Atlantic distribution. In: Hughes CE, de Queiroz LP, Lewis GP (Eds) Advances in Legume Systematics 14. Classification of Caesalpinioideae Part 1: New generic delimitations. PhytoKeys 205: 401–437. https://doi.org/10.3897/phytokeys.205.82775
Funding Statement
Brazilian National Council for Scientific and Technological Development (CNPq-310075/2020-3) Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES - Office for the Advancement of Higher Education; Grant no. PNADB 922/2010) Brazilian National Council for Scientific and Technological Development (CNPq-141414/2016-2) Swiss National Science Foundation (Early.Postdoc.Mobility fellowship P2ZHP3_199693 to EJMK) SYNTHESYS+ project http://www.synthesys.info/ which is financed by European Community Research Infrastructure Action under the H2020 Integrating Activities Programme, Project number 823827
Footnotes
These authors contributed equally to this work.
Supplementary materials
Supplementary data file S1
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Marcos Vinicius Batista Soares, Erik Jozef Mathieu Koenen3, João Ricardo Vieira Iganci, Marli Pires Morim
Data type
zip. archiv.
Explanation note
Alignments of 560 nuclear loci in FASTA format (zipped).
Supplementary data file S2
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Marcos Vinicius Batista Soares, Erik Jozef Mathieu Koenen3, João Ricardo Vieira Iganci, Marli Pires Morim
Data type
zip. archiv.
Explanation note
Gene trees of 560 nuclear loci in Newick format (zipped).
Supplementary data file S3
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Marcos Vinicius Batista Soares, Erik Jozef Mathieu Koenen3, João Ricardo Vieira Iganci, Marli Pires Morim
Data type
zip. archiv.
Explanation note
MI gene trees (one tip per accession) of 398 nuclear loci with nodes with < 10% bootstrap support collapsed, in Newick format.
References
- Aviles Peraza G, Koenen EJM, Riina R, Hughes CE, Ringelberg JJ, Carnevali Fernández-Concha G, Ramírez Morillo IM, Can Itza LL, Tamayo-Cen I, Ramírez Prado JH, Cornejo X, Mattapha S, Duno de Stefano R. (2022) Re-establishment of the genus Pseudalbizzia (Leguminosae, Caesalpinioideae: mimosoid clade): the New World species formerly placed in Albizia. In: Hughes CE, de Queiroz LP, Lewis GP. (Eds) Advances in Legume Systematics 14. Classification of Caesalpinioideae Part 1: New generic delimitations.PhytoKeys 205: 371–400. 10.3897/phytokeys.205.76821 [DOI] [PMC free article] [PubMed]
- Barneby RC. (1998) Silk tree, guanacaste, monkey’s earring: A generic system of the synandrous Mimosaceae of the Americas. Part II. Calliandra. Memoirs of the New York Botanical Garden 74(3).
- Barneby RC, Grimes J. (1996) Silk tree, guanacaste, monkey’s earring: A generic system of the synandrous Mimosaceae of the Americas. Part I. Abarema, Albizia, and allies. Memoirs of the New York Botanical Garden 74(1).
- Barneby RC, Grimes JW. (1997) Silk tree, guanacaste, monkey’s earring: A generic system of the synandrous Mimosaceae of the Americas. Part II. Pithecellobium, Cojoba, and Zygia. Memoirs of the New York Botanical Garden 74(2).
- Beentje H. (2016) The Kew plant glossary: an Illustrated dictionary of plant terms, 2nd edn. Royal Botanic Gardens, Kew.
- Bentham G. (1844) Notes on Mimoseae, with a short synopsis of species. London. Le Journal de Botanique 3: 195–226. [Google Scholar]
- BFG [The Brazil Flora Group] (2021) Brazilian Flora 2020: Leveraging the power of collaborative scientific network, Taxon online first. 10.1002/tax.12640 [DOI]
- Britton NL, Rose JN. (1928) North American Flora, Volume. 23. The Bronx: New York Botanical Garden, 34.
- Brown GK. (2008) Systematics of the tribe Ingeae (Leguminosae-Mimosoideae) over the past 25 years. Muelleria 26: 27–42. [Google Scholar]
- Cardoso D, Särkinen T, Alexander S, Amorim AM, Bittrich V, Celis M, Daly DC, Fiaschi P, Funk VA, Giacomin LL, Goldenberg R, Heiden G, Iganci J, Kelloff CL, Knapp S, Lima HC, Machado AFP, Santos RM, Mello-Silva R, Michelangeli FA, Mitchell J, Moonlight P, Moraes PRL, Mori SA, Nunes TS, Pennington TD, Pirani JR, Prance GT, Queiroz LP, Rapini A, Riina R, Rincon CAV, Roque N, Shimizu G, Sobral M, Stehmann JR, Stevens WD, Taylor CM, Trovó M, van den Berg C, van der Werff H, Viana PL, Zartman CE, Forzza RC. (2017) Amazon plant diversity revealed by a taxonomically verified species list. Proceedings of the National Academy of Sciences of the United States of America 114(40): 10695–10700. 10.1073/pnas.1706756114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Candolle AP. (1825) Leguminosae. Prodromus Systematis Naturalis Regni Vegetabilis (DC.) 2: 441.
- Ducke A. (1922) Plantes nouvelles ou peu connus de la région Amazonienne. Archivos do Jardim Botânico do Rio de Janeiro 3: 64.
- Ducke A. (1949) Leguminosas da Amazônia Brasileira. Boletim do Instituto Agronômico do Norte 37.
- Grimes (1999) Inflorescence Morphology, Heterochrony, and Phylogeny in the Mimosoid Tribes Ingeae and Acacieae (Leguminosae: Mimosoideae). Botanical Review 65: 317–347. 10.1007/BF02857753 [DOI] [Google Scholar]
- Guerra E, Morim MP, Iganci JRV. (2016) A new species of Abarema (Fabaceae) from Brazil. Phytotaxa 289(1): 77–82. 10.11646/phytotaxa.289.1.6 [DOI] [Google Scholar]
- Guerra E, Andrade BO, Morim MP, Iganci JRV. (2019) Taxonomic Delimitation of Species Complexes: A Challenge for Conservation; First Steps with the Abaremacochliacarpos Complex. Systematic Botany 44(4): 818–825. 10.1600/036364419X15710776741422 [DOI] [Google Scholar]
- Guinet P, Grimes JW. (1997) A summary of pollen characteristics of some new world members of the Pithecellobium-complex. Memoirs of the New York Botanical Garden 74(2): 151–161. [Google Scholar]
- Huson DH. (1998) SplitsTree: Analyzing and visualizing evolutionary data. Bioinformatics (Oxford, England) 14(1): 68–73. 10.1093/bioinformatics/14.1.68 [DOI] [PubMed] [Google Scholar]
- Iganci JRV, Morim MP. (2009) Three new species of Abarema (Leguminosae, Mimosoideae) from south-eastern Brazil. Kew Bulletin 64(2): 271–277. 10.1007/s12225-009-9110-x [DOI] [Google Scholar]
- Iganci JRV, Morim MP. (2012) Abarema (Fabaceae, Mimosoideae) in the Atlantic Domain, Brazil. Botanical Journal of the Linnean Society 168(4): 473–486. 10.1111/j.1095-8339.2012.01222.x [DOI] [Google Scholar]
- Iganci JR, Soares MVB, Guerra E, Morim MP. (2016) A preliminary molecular phylogeny of the Abarema alliance (Leguminosae) and implications for taxonomic rearrangement. International Journal of Plant Sciences 177(1): 34–43. 10.1086/684078 [DOI] [Google Scholar]
- INCT-Splink (2022) Erythrinafalcata. http://inct.splink.org.br
- JSTOR (2022) JSTOR. https://plants.jstor.org
- Katoh K, Kuma KI, Toh H, Miyata T. (2005) MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Research 33(2): 511–518. 10.1093/nar/gki198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay RWJ. (1953) Revision of the “Flora of West Tropical Africa.”: V. Kew Bulletin 8(4): 487–492. 10.2307/4117351 [DOI] [Google Scholar]
- Koenen EJM. (2022) Osodendron gen. nov. (Leguminosae, Caesalpinioideae), a new genus of mimosoid legumes of tropical Africa. In: Hughes CE, de Queiroz LP, Lewis GP. (Eds) Advances in Legume Systematics 14. Classification of Caesalpinioideae Part 1: New generic delimitations.PhytoKeys 205: 453–470. 10.3897/phytokeys.205.82821 [DOI] [PMC free article] [PubMed]
- Koenen EJM, Ojeda DI, Steeves R, Migliore J, Bakker FT, Wieringa JJ, Kidner C, Hardy OJ, Pennington RT, Bruneau A, Hughes CE. (2020a) Large‐scale genomic sequence data resolve the deepest divergences in the legume phylogeny and support a near‐simultaneous evolutionary origin of all six subfamilies. The New Phytologist 225(3): 1355–1369. 10.1111/nph.16290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen EJM, Kidner C, de Souza ÉR, Simon MF, Iganci JR, Nicholls JA, Brown GK, de Queiroz LP, Luckow M, Lewis GP, Pennington RT, Hughes CE. (2020b) Hybrid capture of 964 nuclear genes resolves evolutionary relationships in the mimosoid legumes and reveals the polytomous origins of a large pantropical radiation. American Journal of Botany 107(12): 1710–1735. 10.1002/ajb2.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milliken W, Lewis GP, Rico Arce ML. (2005) Tribe Ingeae. In: Lewis G, Schrire B, Mackinder B, Lock M (Eds) Legumes of the world. Royal Botanic Gardens, Kew, 193–213.
- Milliken W, Zappi D, Sasaki D, Hopkins M, Pennington RT. (2010) Amazon vegetation: How much don’t we know and how much does it matter? Kew Bulletin 65(4): 691–709. 10.1007/s12225-010-9236-x [DOI]
- Nielsen I. (1981) Ingeae. In: Polhill RM, Raven PH. (Eds) Advances in Legume Systematics pt.2. Royal Botanic Gardens, Kew, London, 173–190.
- Pearce TR, Antonelli A, Brearley FQ, Couch C, Forzza RC, Gonçalves SC, Magassouba S, Morim MP, Mueller GM, Nic Lughadha E, Obreza M, Sharrock S, Simmonds MSJ, Tambam BB, Utteridge TMA, Breman E. (2020) International collaboration between collections-based institutes for halting biodiversity loss and unlocking the useful properties of plants and fungi. Plants, People, Planet 2(5): 515–534. 10.1002/ppp3.10149 [DOI] [Google Scholar]
- REFLORA - Herbário Virtual (2022) Herbário Virtual. http://reflora.jbrj.gov.br/reflora/herbarioVirtual/
- Rico Arce ML. (1999) New combinations in Mimosaceae. Novon 9(4): 554–556. 10.2307/3392164 [DOI] [Google Scholar]
- Ringelberg JJ, Koenen EJM, Iganci JR, de Queiroz LP, Murphy DJ, Gaudeul M, Bruneau A, Luckow M, Lewis GP, Hughes CE. (2022) Phylogenomic analysis of 997 nuclear genes reveals the need for extensive generic re-delimitation in Caesalpinioideae (Leguminosae). In: Hughes CE, de Queiroz LP, Lewis GP. (Eds) Advances in Legume Systematics 14. Classification of Caesalpinioideae Part 1: New generic delimitations.PhytoKeys 205: 3–58. 10.3897/phytokeys.205.85866 [DOI] [PMC free article] [PubMed]
- Soares MVB. (2015) Sistemática do Gênero Hydrochorea Barneby & J.W. Grimes (Leguminosae, Mimosoideae). Dissertation. Universidade Federal Rural da Amazônia e Museu Paraense Emílio Goeldi, Brazil.
- Soares MVB, Guerra E, Morim MP, Iganci JRV. (2021) Reinstatement and recircumscription of Jupunba and Punjuba (Fabaceae) based on phylogenetic evidence. Botanical Journal of the Linnean Society 196(4): 456–479. 10.1093/botlinnean/boab007 [DOI] [Google Scholar]
- Souza ER, Lewis GP, Forest F, Schnadelbach AS, Van Der Berg C, Queiroz LP. (2013) Phylogeny of Calliandra (Leguminosae-Mimosoideae) based on nuclear and plastid molecular markers. Taxon 62(6): 1201–1220. 10.12705/626.2 [DOI] [Google Scholar]
- Souza ER, Luz ARM, Rocha L, Lewis GP. (2022) Molecular and Morphological Analysis Supports the Separation of Robrichia as a Genus Distinct from Enterolobium (Leguminosae: Caesalpinioideae: Mimosoid Clade). Systematic Botany 47(1): 268–277. 10.1600/036364422X16442669847067 [DOI] [Google Scholar]
- Stamatakis A. (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiers B. (2022) [continuously updated]. Index herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/science/ih [accessed 20.01.2022]
- Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber WH, Li D-Z, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF [Eds] (2018) International Code of Nomenclature for algae, fungi, and plants (ShenzhenCode) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Koeltz Botanical Books, Glasshütten. 10.12705/Code.2018 [DOI]
- Ulloa CU, Acevedo-Rodríguez P, Beck S, Belgrano MJ, Bernal R, Berry PE, Brako L, Celis M, Davidse G, Forzza RC, Robbert Gradstein S, Hokche O, León B, León-Yánez S, Magill RE, Neill DA, Nee M, Raven PH, Stimmel H, Strong MT, Villaseñor JL, Zarucchi JL, Zuloaga FO. (1614–1617) Jørgensen (2017) An integrated assessment of the vascular plant species of the Americas. Science 358(6370): 1614–1617. 10.1126/science.aao0398 [DOI] [PubMed] [Google Scholar]
- Yang Y, Smith SA. (2014) Orthology inference in non-model organisms using transcriptomes and low-coverage genomes: Improving accuracy and matrix occupancy for phylogenomics. Molecular Biology and Evolution 31(11): 3081–3092. 10.1093/molbev/msu245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Rabiee M, Sayyari E, Mirarab S. (2018) ASTRAL-III: Polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics 19(S6): 153. 10.1186/s12859-018-2129-y [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data file S1
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Marcos Vinicius Batista Soares, Erik Jozef Mathieu Koenen3, João Ricardo Vieira Iganci, Marli Pires Morim
Data type
zip. archiv.
Explanation note
Alignments of 560 nuclear loci in FASTA format (zipped).
Supplementary data file S2
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Marcos Vinicius Batista Soares, Erik Jozef Mathieu Koenen3, João Ricardo Vieira Iganci, Marli Pires Morim
Data type
zip. archiv.
Explanation note
Gene trees of 560 nuclear loci in Newick format (zipped).
Supplementary data file S3
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Marcos Vinicius Batista Soares, Erik Jozef Mathieu Koenen3, João Ricardo Vieira Iganci, Marli Pires Morim
Data type
zip. archiv.
Explanation note
MI gene trees (one tip per accession) of 398 nuclear loci with nodes with < 10% bootstrap support collapsed, in Newick format.