Abstract
Collections of pathogenic fungi found on spiders from Thailand were selected for a detailed taxonomic study. Morphological comparison and phylogenetic analyses of the combined ITS, LSU, tef1, rpb1 and rpb2 sequence data indicated that these specimens formed new independent lineages within the Cordycipitaceae, containing two new genera occurring on spiders, i.e. Jenniferiagen. nov. and Polystromomycesgen. nov. Two new species in Jenniferia, J.griseocinereasp. nov. and J.thomisidarumsp. nov., are described. Two strains, NHJ 03510 and BCC 2191, initially named as Akanthomycescinereus (Hevansiacinerea), were shown to be part of Jenniferia. By including sequences of putative Hevansia species from GenBank, we also revealed Parahevansia as a new genus with the ex-type strain NHJ 666.01 of Pa.koratensis, accommodating specimens previously named as Akanthomyceskoratensis (Hevansiakoratensis). One species of Polystromomyces, Po.araneaesp. nov., is described. We established an asexual-sexual morph connection for Hevansianovoguineensis (Cordycipitaceae) with ex-type CBS 610.80 and proposed a new species, H.minutasp. nov. Based on characteristics of the sexual morph, Hevansia and Polystromomyces share phenotypic traits by producing stipitate ascoma with fertile terminal heads; however, they differ in the shape and colour of the stipes. Meanwhile, Jenniferia produces non-stipitate ascoma with aggregated superficial perithecia forming a cushion. A new morphology of ascospores in Jenniferia is described, illustrated and compared with other species in Cordycipitaceae.
Keywords: Cordycipitaceae, Hevansia , Jenniferia , Parahevansia , Polystromomyces , spider pathogenic fungi
Introduction
Members of Cordycipitaceae (Hypocreales, Ascomycota) are parasitic on spiders (Araneae) and several orders of insects from larva to adult states (Sung et al. 2007; Shrestha et al. 2016). Several species of this family are recognised for their economic importance, such as Cordycepsmilitaris (L.) Fr., a famous traditional Chinese medicine, edible mushroom and source of bioactive compounds (Wu et al. 2021) and others that are being used or developed as biopesticides against different insect pests (Wang et al. 2019; Sun et al. 2020). Seventeen genera are established in this family from combined molecular phylogenetic and morphological evidence (Zare and Gams 2016; Kepler et al. 2017; Mongkolsamrit et al. 2018, 2020b; Thanakitpipattana et al. 2020; Wang et al. 2020; Zhang et al. 2020). Recently, the genera Pseudogibellula Samson & H.C. Evans and Pleurodesmospora Samson, W. Gams & H.C. Evans were clarified, based on molecular phylogenetic analyses and confirmed to be members of Cordycipitaceae (Chen et al. 2021b; Mongkolsamrit et al. 2021), suggesting that the taxonomic diversity of this family is still under-explored.
Arthropod pathogenic fungi in Cordycipitaceae have a distinctive fleshy texture and pallid (white to yellow) to brightly coloured stipitate stromata with loosely embedded or superficial perithecia. Species with these features include Cordycepsmilitaris (L.) Fr., Blackwellomycespseudomilitaris (Hywel-Jones & Sivichai) Spatafora & Luangsa-ard, Flavocilliumbifurcatum H. Yu, Y.B. Wang, Y. Wang, Q. Fan & Zhu L. Yang and Samsoniellainthanonensis Mongkols., Noisrip., Thanakitp., Spatafora & Luangsa-ard (Sung et al. 2007; Kepler et al. 2017; Mongkolsamrit et al. 2018; Wang et al. 2020). Nonetheless, some Cordycipitaceae species are characterised by possessing non-stipitate ascomata, such as Akanthomycesthailandicus Mongkols., Spatafora & Luangsa-ard and Gibellulacebrennini Tasan., Kuephadungphan & Luangsa-ard (Mongkolsamrit et al. 2018; Kuephadungphan et al. 2020), which are parasitic on the spiders, Hyperdermiumpulvinatum J.F. White, R.F. Sullivan, Bills & Hywel-Jones and H.caulium (Berk. & M.A. Curtis) P. Chaverri & K.T. Hodge occurring on scale insects (Sullivan et al. 2000; Chaverri et al. 2008).
Hevansia and Gibellula were separated from other genera, based on monophyletic clades in the Cordycipitaceae (Kepler et al. 2017). Hevansia was erected with H.novoguineensis (synonym: Akanthomycesnovoguineensis Samson & B.L. Brady) as the type species infecting spiders collected from Papua New Guinea (Samson and Brady 1982). Hevansia and Gibellula species are specialised parasites on spiders that inhabit the undersides of leaves. However, the asexual morph of Hevansia differs from Gibellula in the production of phialides in monolayer with mono- or polyphialidic conidiogenous cells, whereas species in Gibellula produce the primary synnemata bearing predominantly aspergillus-like conidiophores or occasionally growing penicillate or granulomanus-like conidiophores (Samson and Brady 1982; Samson and Evans 1992; Kuephadungphan et al. 2020, 2022).
At present, most of the species in Hevansia have been described, based on asexual morphs that were reported from China, Papua New Guinea, Sri Lanka, Taiwan and Thailand (Samson and Evans 1974; Samson and Brady 1982; Hywel-Jones 1996; Hsieh et al. 1997; Huang et al. 2000). Hevansianelumboides, the only species from Japan, has been accepted and described, based on sexual characters producing short stipes with fertile terminal heads, immersed perithecia and ascospores disarticulating into part-spores (Kobayasi and Shimizu 1977; Kepler et al. 2017). The sexual morph of Gibellula is well-known for forming a torrubiella-like state and ascospores that disarticulate into part-spores. Species in Gibellula have been reported from several countries including China, Ecuador, Ghana, Taiwan and Thailand (Samson and Evans 1992; Hsieh et al. 1997; Kuephadungphan et al. 2020; Chen et al. 2021a).
From surveys of arthropod pathogenic fungi in Thailand’s national parks, collections of pathogens on spiders were found on the underside of leaves from forest plants. Based on the macroscopic features of the sexual morph, some specimens possess non-stipitate ascomata with aggregated superficial perithecia forming a cushion. In contrast, some specimens have stipes with fertile heads at the terminal part arising from the spiders’ abdominal region which closely match with H.nelumboides. Asexually reproductive species that produce several synnemata on spiders were also included in this study. The goal of these investigations is to elucidate the phylogenetic and taxonomic placement of these collections of parasitic fungi on spiders through multi-locus molecular phylogenetic analyses and the observation of diagnostic macro- and micro-morphological characteristics. Additionally, this work has allowed us to refine the diagnostic characters of the species classification of Hevansia.
Materials and methods
Specimen collection and isolation
The fungal specimens were collected in forests during the rainy season from 2009 to 2020. The specimens of fungi occurring on spiders found on the underside of living leaves of forest plants were collected carefully to preserve host and fungal structures, then were put in plastic boxes and carried to the laboratory for isolation. The materials were examined under a stereomicroscope (Olympus SZ61). The protocol for isolation from sexual and asexual morphs followed previous studies (Luangsa-ard et al. 2018; Mongkolsamrit et al. 2018). The cultures were grown on potato dextrose agar (PDA; freshly diced potatoes 200 g, dextrose 20 g, agar 15 g, in 1 litre distilled water) and deposited at the BIOTEC Culture Collection (BCC), Thailand. The specimens were dried in an electric food dryer (50–55 °C) overnight and stored in plastic boxes before storage at the BIOTEC Bangkok Herbarium (BBH), National Biobank of Thailand. The identification of the spider hosts was conducted after cultures of fungal pathogens were acquired. The spider hosts were identified, based on morphological characteristics, such as eyes, cephalic regions and legs (Deeleman-Reinhold 2001).
Morphological observation
Important macroscopic and microscopic features of the fungal specimens were observed using a stereomicroscope (Olympus CX31) and a compound microscope (Olympus SZ61). The fungal materials, including perithecia, asci, ascospores, phialides and conidia, were mounted on microscope slides and stained in lactophenol cotton blue solution for observation. The characteristics of these materials (shape and size) were determined and measured according to Mongkolsamrit et al. (2018, 2020b). Cultures were grown on oatmeal agar (OA, Difco, oatmeal 60 g, agar 12.5 g, in 1 litre distilled water) and PDA agar plates at 25 °C under light/dark condition (L:D = 14:10) for 21 days, depending on the sporulation in culture. The colours of the specimens and colonies grown on OA and PDA were described and codified following the Royal Horticultural Society (RHS 2015).
DNA extraction, amplification and sequencing
Genomic DNA was extracted from the mycelia of 10–14 days old cultures on PDA using a modified cetyltrimethyl ammonium bromide (CTAB) method as previously described in Mongkolsamrit et al. (2009). Nuclear loci were sequenced, including the nuc rDNA region encompassing the internal transcribed spacers (ITS), ITS1 and ITS2, the partial gene regions of the nuc 28S rDNA (Large Subunit Ribosomal DNA: LSU), the translation elongation factor-1α gene (tef1), the largest (rpb1) and second largest (rpb2) subunits of RNA polymerase II. Polymerase chain reaction (PCR) primers used to amplify these markers were ITS5 and ITS4 for ITS (White et al. 1990), LROR and LR5 for LSU (Vilgalys and Hester 1990; Rehner and Samuels 1994), 983F and 2218R for tef1 (Rehner and Buckley 2005), CRPB1 and RPB1Cr for rpb1 (Castlebury et al. 2004), RPB2-5F2 and RPB2-7Cr for rpb2 (Liu et al. 1999; O’Donnell et al. 2007). The thermocycler conditions for PCR amplifications used in this study followed the method described in Sung et al. (2007). The purified PCR products were sequenced with PCR amplification primers for Sanger dideoxy sequencing. The sequences obtained in this study were deposited in GenBank (Table 1).
Table 1.
List of taxa included in the phylogenetic analyses and their GenBank accession numbers.
| Species | Code | Host/ Substratum | GenBank accession numbers | References | ||||
|---|---|---|---|---|---|---|---|---|
| ITS | LSU | tef1 | rpb1 | rpb2 | ||||
| Akanthomycesaculeatus | HUA 772 | Lepidoptera; Sphingidae | — | — | Sanjuan et al. (2014) | |||
| A.aculeatus | HUA 186145T | — | — | — | — | Kepler et al. (2017) | ||
| A.kanyawimiae | TBRC 7244T | Araneae; spider | — | — | Mongkolsamrit et al. (2018) | |||
| A.lecanii | CBS 101247 | Homoptera | — | — | Sung et al. (2001); Spatafora et al. (2007) | |||
| A.sulphureus | TBRC 7248T | Araneae; spider | Mongkolsamrit et al. (2018) | |||||
| A.thailandicus | TBRC 7245T | Araneae; spider | — | — | Mongkolsamrit et al. (2018) | |||
| A.waltergamsii | TBRC 7252T | Araneae; spider | Mongkolsamrit et al. (2018) | |||||
| Ascopolyporuspolychrous | P.C. 546 | Plant | — | — | Chaverri et al. (2005) | |||
| A.villosus | ARSEF 6355 | Plant | — | — | Bischoff et al. (2005); Chaverri et al. (2005) | |||
| Beauveriabassiana | ARSEF 1564T | Lepidoptera | — | Rehner et al. (2011) | ||||
| B.bassiana | ARSEF 7518 | Hymenoptera | — | Rehner et al. (2011) | ||||
| Blackwellomycesaurantiacus | BCC 85060T | Lepidoptera | Mongkolsamrit et al. (2020b) | |||||
| B.aurantiacus | BCC 85061 | Lepidoptera | Mongkolsamrit et al. (2020b) | |||||
| B.pseudomilitaris | BCC 1919T | Lepidoptera | — | — | Kepler et al. (2017) | |||
| B.pseudomilitaris | BCC 2091 | Lepidoptera | — | — | Kepler et al. (2017) | |||
| Cordycepsaraneae | BCC 85066T | Arachnid; Araneae | Mongkolsamrit et al. (2020b) | |||||
| C.inthanonensis | BCC 55812T | Lepidoptera | — | Mongkolsamrit et al. (2020b) | ||||
| C.inthanonensis | BCC 56302 | Lepidoptera | Mongkolsamrit et al. (2020b) | |||||
| C.kuiburiensis | BCC 90322T | Araneidae | — | Crous et al. (2019) | ||||
| C.militaris | OSC 93623 | Lepidoptera | — | Sung and Spatafora (2004); Spatafora et al. (2007); Kepler et al. (2012) | ||||
| C.militaris | YFCC 6587 | Lepidoptera | — | Wang et al. (2020) | ||||
| C.nidus | HUA 186125T | Araneidae | — | — | Chirivı´ et al. (2017) | |||
| C.piperis | CBS 116719 | Hemiptera | — | Chaverri et al. (2005); Bischoff et al. (2004); Johnson et al. (2009); Kepler et al. (2017) | ||||
| Engyodontiumaranearum | CBS 309.85 | Arachnida | — | Sung et al. (2001); Kepler et al. (2017) | ||||
| Flavocilliumbifurcatum | YFCC 6101T | Lepidoptera; Noctuidae | — | Wang et al. (2020) | ||||
| Gamszareahumicola | CGMCC3 19303T | Soil | — | Zhang et al. (2020) | ||||
| G.humicola | LC 12462 | Soil | — | Zhang et al. (2020) | ||||
| Gibellulacebrennini | BCC 39705 | Arachnida; Cebrenninuscf.magnus | Kuephadungphan et al. (2020) | |||||
| G.cebrennini | BCC 53605T | Arachnida; Cebrenninuscf.magnus | Kuephadungphan et al. (2020) | |||||
| G.clavuliferavar.alba | ARSEF 1915 | Arachnida | — | Chaverri et al. (2005); Spatafora et al. (2007); Crous et al. (2019) | ||||
| G.gamsii | BCC 25798 | Arachnida; Araneida | Kuephadungphan et al. (2019) | |||||
| G.gamsii | BCC 27968T | Arachnida; Araneida | — | Kuephadungphan et al. (2019) | ||||
| G.scorpioides | BCC 43298 | Arachnida, Portia sp. | Kuephadungphan et al. (2020) | |||||
| G.scorpioides | BCC 47976 T | Arachnida, Portia sp. | Kuephadungphan et al. (2020) | |||||
| Hevansiaarachnophila | NHJ 2633 | Arachnida | Ridkaew et al. Unpublished data (2009); Kuephadungphan Unpublished data (2018) | |||||
| H.arachnophila | NHJ 2465 | Arachnida | — | Kuephadungphan Unpublished data (2018); this study | ||||
| H.minuta | BCC 47519T | Araneae, Meotipa sp. | This study | |||||
| H.minuta | BCC 47520 | Araneae, Meotipa sp. | This study | |||||
| H.nelumboides | TNS 16306 | Araneidae | — | — | — | Kepler et al. (2017) | ||
| H.novoguineensis | BCC 2190 | Arachnida | — | — | — | Kepler et al. (2017) | ||
| H.novoguineensis | BCC 42675 | Araneae | — | This study | ||||
| H.novoguineensis | BCC 49323 | Araneae | — | This study | ||||
| H.novoguineensis | CBS 610.80T | Arachnida | — | Mongkolsamrit et al. (2020b) | ||||
| H.cf.novoguineensis | BCC 2093 | Arachnida | — | — | Kepler et al. (2017) | |||
| H.cf.novoguineensis | NHJ 4314 | Arachnida | — | — | Johnson et al. (2009) | |||
| H.cf.websteri | BCC 23860 | Arachnida | — | — | Kuephadungphan et al. (2019) | |||
| H.cf.websteri | BCC 36541 | Arachnida | Kuephadungphan Unpublished data (2018) | |||||
| Hyperdermiumpulvinatum | P.C. 602 | Hemiptera | — | — | Chaverri et al. (2005) | |||
| Jenniferiacinerea | BCC 2191 | Arachnida, Amyciaea sp. | — | — | Kuephadungphan et al. (2019) | |||
| J.cinerea | NHJ 03510T | Araneae, Amyciaea sp. | Johnson et al. (2009); Ridkaew et al. Unpublished data (2009) | |||||
| J.griseocinerea | BCC 42062T | Araneae, Diaea sp. | This study | |||||
| J.griseocinerea | BCC 42063 | Araneae, Diaea sp. | This study | |||||
| J.griseocinerea | BCC 54893 | Araneae, Diaeacf.dorsata | — | This study | ||||
| J.griseocinerea | BCC 57821 | Araneae, Diaeacf.dorsata | — | This study | ||||
| J.thomisidarum | BCC 48932 | Araneae, Diaeacf.dorsata | — | This study | ||||
| J.thomisidarum | BCC 49257 | Araneae, Diaeacf.dorsata | — | — | This study | |||
| J.thomisidarum | BCC 54482 | Araneae, Diaeacf.dorsata | — | — | This study | |||
| J.thomisidarum | BCC 66224 | Araneae, Diaeacf.dorsata | — | This study | ||||
| J.thomisidarum | BCC 37881T | Araneae, Diaeacf.dorsata | This study | |||||
| J.thomisidarum | BCC 37882 | Araneae, Diaeacf.dorsata | This study | |||||
| Lecanicilliumantillanum | CBS 350.85T | Agaric | — | Sung et al. (2001); Chaverri et al. (2005); Spatafora et al. (2007) | ||||
| L.aranearum | CBS 726.73a | Arachnid, Araneae | — | Sung et al. (2001); Sung et al. (2007) | ||||
| Liangiasinensis | YFCC 3103T | Beauveriayunnanensis | — | Wang et al. (2020) | ||||
| L.sinensis | YFCC 3104 | Beauveriayunnanensis | — | Wang et al. (2020) | ||||
| Neotorrubiellachinghridicola | BCC 39684 | Orthopterida | — | Thanakitpipattana et al. (2020) | ||||
| N.chinghridicola | BCC 80733T | Orthopterida | — | — | Thanakitpipattana et al. (2020) | |||
| Parahevansiakoratensis | NHJ 666.01 | Arachnida | — | — | Ridkaew et al. Unpublished data (2009) | |||
| Pa.koratensis | NHJ 2662 | Lepidoptera | Ridkaew et al. Unpublished data (2009); this study | |||||
| Pleurodesmosporalepidopterorum | DY 10501T | Lepidoptera | — | Chen et al. (2021b) | ||||
| P.lepidopterorum | DY 10502 | Lepidoptera | — | — | Chen et al. (2021b) | |||
| Polystromomycesaraneae | BCC 93301T | Arachnida | This study | |||||
| Pseudogibellulaformicarum | BCC 84257 | Ophiocordycepsflavida | — | Mongkolsamrit et al. (2021) | ||||
| P.formicarum | CBS 433.73 | Pahothyreustarsatus | — | Vu et al. (2019); Mongkolsamrit et al. (2021); | ||||
| Samsoniellaaurantia | TBRC 7271T | Lepidoptera | Mongkolsamrit et al. (2018) | |||||
| S.aurantia | TBRC 7272 | Lepidoptera | — | Mongkolsamrit et al. (2018) | ||||
| Simplicilliumlanosoniveum | CBS 704.86 | Hemileiavastatrix | — | Sung et al. (2001); Spatafora et al. (2007) | ||||
| S.lanosoniveum | CBS 101267 | Hemileiavastatrix | — | Sung et al. (2001); Spatafora et al. (2007) | ||||
The accession numbers marked in bold font refer to sequences new in this study or have been generated by our group in Thailand. Tex-type species.
Sequence alignment and phylogenetic analyses
The DNA sequences generated in this study were examined for ambiguous bases and corrected using BioEdit v. 7.2.5 (Hall 1999), then submitted to GenBank. Sequences of ITS, LSU, tef1, rpb1 and rpb2, of closely-related taxa for the analyses were taken from previous studies as shown in Table 1. The phylogenetic analyses for combined and single-locus alignments were performed using RAxML-HPC2 on XSEDE v. 8.2.12 (Stamatakis 2014) in CIPRES Science Gateway portal, with GTRGAMMA+I model and 1000 bootstrap iterations. Bayesian Inference (BI) of the phylogenetic relationship was performed in MrBayes v. 3.2.7a (Ronquist et al. 2012), with best-fit models selected using MrModeltest v. 2.2 (Nylander 2004). The best model was GTR + G + I. Markov Chain Monte Carlo (MCMC) simulations were run for 2,000,000 generations, sampling every 1000 and discarding the first 10% as burn-in. The remaining 20,001 trees were used to calculate the posterior probability values. RAxML and BI output were imported into TreeView v. 1.6.6 to visualise the phylogenetic tree (Page 1996).
Results
Molecular phylogeny
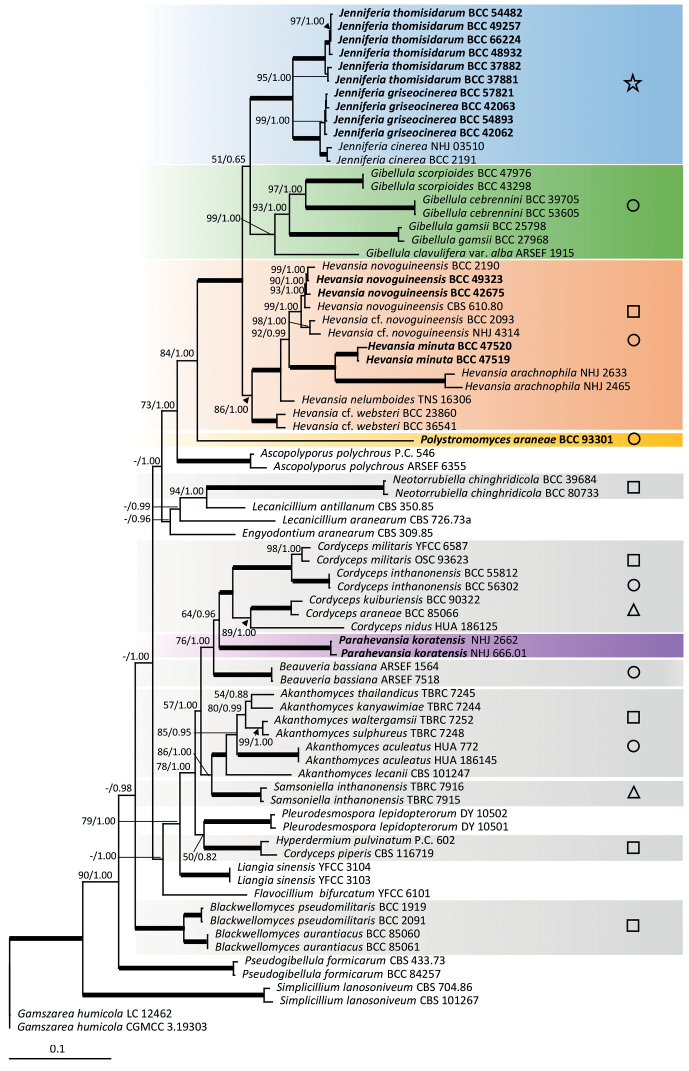
We generated 65 new sequences (15 ITS, 15 LSU, 15 tef1, 7 rpb1 and 13 rpb2) from living cultures (Table 1). Gamszareahumicola Z.F. Zhang & L. Cai (3.19303 and LC 12462) was used as an outgroup. The combined dataset from 77 specimens, with multi-locus sequences totalling an alignment length of 4231 characters with gaps (ITS 656, LSU 841, tef1 921, rpb1 764 and rpb2 1049) was analysed. The maximum-likelihood phylogenetic analyses resulted in a multi-locus tree with maximum likelihood bootstrap values (MLB) shown in Fig. 1 and in single-locus trees (Suppl. material 1: Figs S1–S5). The nodes were also evaluated with Bayesian posterior probabilities (BPP). Bold lines in the tree represent 100% of MLB and 1.00 of BPP.
Figure 1.
RAxML tree of Hevansia, Jenniferia, Parahevasia, Polystromomyces and related genera in the Cordycipitaceae from a combined ITS, LSU, tef1, rpb1 and rpb2 dataset. Numbers at the major nodes represent Maximum Likelihood Bootstrap (MLB) and Bayesian Posterior Probabilities (BPP). Bold lines in the tree represent 100% of MLB and 1.00 of BPP. Symbols on the right-hand side correspond to the types of ascospore morphologies found in each genus that are observed in natural specimens of Cordycipitaceae described in Fig. 2.
The phylogenetic analyses supported Hevansia as a monophyletic clade with maximum support (MLB = 86 / BPP = 1.00), including ex-type H.novoguineensis (CBS 610.80) from Papua New Guinea as the type species. The strain BCC 42675, isolated from a sexual morph from Thailand, clustered with H.novoguineensis (CBS 610.80) with high support (MLB = 93 / BPP = 1.00), revealing a sexual morph connection to this species. Two strains from Thailand (BCC 2093, NHJ 4314) formed a sister clade to the clade containing the ex-type strain of H.novoguineensis with maximum support for the separating node (MLB = 98 / BPP = 1.00). This separation was observed with the phylogenetic signal from only LSU, tef1, while the other markers either did not have sufficient sample coverage for comparison (ITS, rpb1: Suppl. material 1: Figs S1 and S4) or did not recover this separation (rpb2: Suppl. material 1: Fig. S5). These two specimens were thus named as H.cf.novoguineensis herein. Two unknown Hevansia strains from both an asexual state (BCC 47520) and a sexual state (BCC 47519) were found as a well-supported clade (MLB = 100 / BPP = 1.00) within Hevansia, but separated from H.novoguineensis, which was also recovered by all single-locus phylogenies. These two Hevansia strains were thus proposed as a new species, Hevansiaminuta. Furthermore, two strains of H.arachnophila (NHJ 2465, NHJ 2633) and two strains of H.cf.websteri (BCC 23860, BCC 36541) were included in our phylogenetic analyses and shown to belong to Hevansia. Additionally, a strain formerly named as Hevansiakoratensis (NHJ 666.01 (BCC 1485)) and a strain previously recognised as H.websteri (NHJ 2662 (BCC 2113)) formed together an independent clade with strong support (MLB = 100/ BPP = 1.00), out of the Hevansia clade and in the proximity of Cordyceps species. Hence, this clade does not belong to Hevansia and is proposed as a new genus named Parahevansia (Fig. 1).
The combined-genes phylogenetic tree revealed one important terminal monophyletic clade close to Gibellula with total support (MLB = 100 / BPP = 1.00), Fig. 1. This clade is proposed as a new genus named Jenniferia. The genus Jenniferia formed a monophyletic clade separated from Hevansia and Gibellula for all the markers used in this study (Suppl. material 1: Figs S1–S5). Jenniferia contains two novel species, Jenniferiagriseocinerea and J.thomisidarum and includes J.cinerea, which is proposed as a new combination of H.cinerea to this genus. Jenniferiagriseocinerea is distinguished from J.cinerea, based on the separated monophyletic clades in the multi-locus phylogeny (Fig. 1). The separation between the two species was recovered in most of the single-locus phylogenies (tef1, rpb1 and rpb2, but not ITS nor LSU: Suppl. material 1: Figs S1–S5).
The combined-genes analysis also revealed a deep taxon from a unique specimen (BCC 93301), branched as sister to the three genera occurring on spider egg sac (Gibellula, Hevansia and Jenniferia), which was thus proposed as a new genus Polystromomyces. The branching of this specimen had high support (MLB = 84 / BPP = 1.00) and was found consistently amongst different markers (Suppl. material 1: Figs S1–S5). This taxon was never within the three main genera occurring on spiders (Gibellula, Hevansia and Jenniferia), supporting the status of a different genus. Polystromomyces contains a new species, Po.araneae.
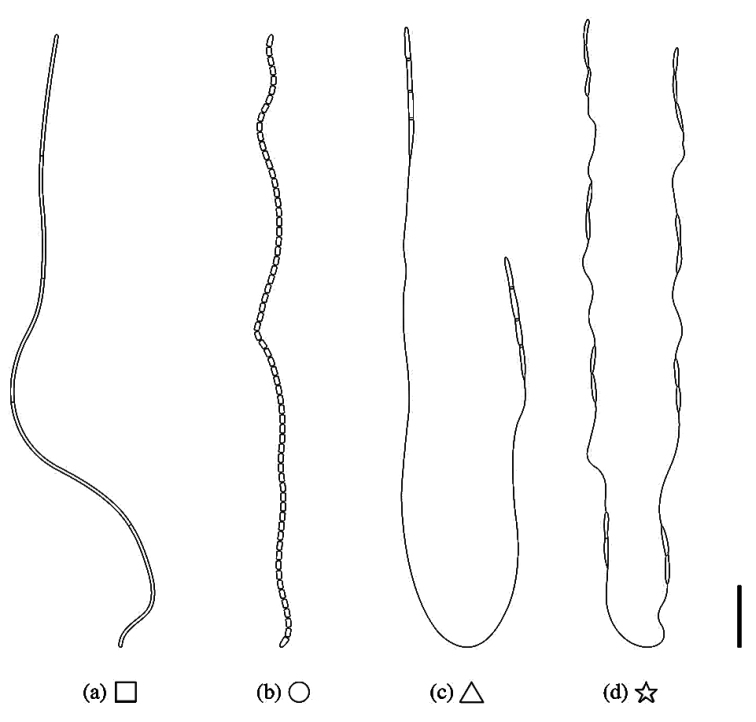
Overview of types of ascospores in Cordycipitaceae
Different types of ascospore morphologies were observed in natural specimens of Cordycipitaceae as shown in Fig. 2. Three types observed previously include: (a) filiform, multiseptate, whole ascospores, (b) filamentous, multiseptate ascospores disarticulating into part-spores and (c) bola-shaped, whole ascospores, non-disarticulating, characterised by a thread-like structure connected to fusiform, terminal, multi-septate parts at both ends, resembling a skipping rope. We observed a new type of ascospore morphology in Jenniferia as shown in Fig. 2(d), in which septate part-spores are alternately connected with thread-like structures along the whole ascospore. The ascospore morphologies shown in Fig. 2a, b and d were observed on spider-pathogenic fungi in this study.
Figure 2.
The types of ascospores morphologies observed in natural specimens of Cordycipitaceae: a filiform, multiseptate, whole ascospores (square) b filamentous, multiseptate ascospores disarticulating into part-spores (circle) c bola-shaped, whole ascospores (triangle) and d whole ascospores with septate part-spores alternately connected with thread-like structures (star). Scale bars: 10 µm (a, b); 20 µm (c, d).
Taxonomy
. Hevansia
Luangsa-ard, Hywel-Jones & Spatafora, in Kepler, Luangsa-ard, Hywel-Jones, Quandt, Sung, Rehner, Aime, Henkel, Sanjuan, Zare, Chen, Li, Rossman, Spatafora, Shrestha, IMA Fungus 8: 348 (2017). Emend. S. Mongkolsamrit, W. Noisripoom & K. Tasanathai
225C50F2-9050-5AEE-87B9-9F07392DE3AC
≡ Akanthomycesnovoguineensis Samson & B.L. Brady, Trans. Br. mycol. Soc. 79: 571 (1982).
Type species.
Hevansianovoguineensis (Samson & B.L. Brady) Luangsa-ard, Hywel-Jones & Spatafora, IMA Fungus 8: 349 (2017).
Emended generic description
(modified from Kepler et al. 2017).Circumscription: The sexual morph characteristics in genus are emended, based on three species H.minuta, H.nelumboides and H.novoguineensis producing sexual morph as members of Hevansia lineage in Fig. 1. Sexual morph: Stromata arising from dorsal abdomen, stipe 1–10 mm, fertile part at the terminal of stipe, ca. 1–3 × 1–2 mm, white to cream. Perithecia immersed, narrowly ovoid. Asci cylindrical with thickened caps, 8-spored, ascospores hyaline, filiform, whole or disarticulating into part-spores. Asexual morph: Synnemata erect, simple or branched, solitary to numerous, cylindrical to clavate, mycelium covering host, white, cream to ash-grey or brownish-white. Phialides in a monolayer, sparsely scattered or crowded, on mycelium or on a basal cell, smooth-walled, cylindrical, globose, obovoid, with distinct necks. Conidia one-celled, smooth-walled, hyaline, occasionally in a short chain, clavate, cylindrical, fusiform to narrowly obclavate. Colony on PDA white, reverse cream, orange to pale red. Some species produce pale red pigment diffusing in the medium.
Notes.
Two specimens of H.arachnophila (NHJ 2465, NHJ 2633) were described by Hywel-Jones (1996). While the type strain of H.websteri (NHJ 2661) and living cultures are unavailable, available sequences of H.arachnophila and two strains of H.websteri (BCC 23860, BCC 36541) were retrieved from the GenBank nucleotide database and used in this study. The phylogenetic tree revealed that H.arachnophila and H.websteri (BCC 23860, BCC 36541) belong to the genus Hevansia (Fig. 1). The two strains of H.websteri (BCC 23860, BCC 36541) were not designated as type, nor as neotype. These strains (BCC 23860, BCC 36541) were thus named as Hevansiacf.websteri. Hevansialongispora and H.ovalongata were not included in the phylogenetic study because multi-locus sequence data are unavailable. To better resolve the genus Hevansia, H.longispora, H.ovalongata and H.websteri should be recollected from the locality and designated as neotypes and studied for their phylogenetic affinity to other Hevansia species in the future. However, H.longispora, H.ovalongata and H.websteri were accepted in Hevansia following complete and well-illustrated descriptions by Hywel-Jones (1996), Hsieh et al. (1997) and Huang et al. (2000).
. Hevansia novoguineensis
(Samson & B.L. Brady) Luangsa-ard, Hywel-Jones & Spatafora
A576212B-CED9-5739-8231-AFC78C231622
Figure 3.
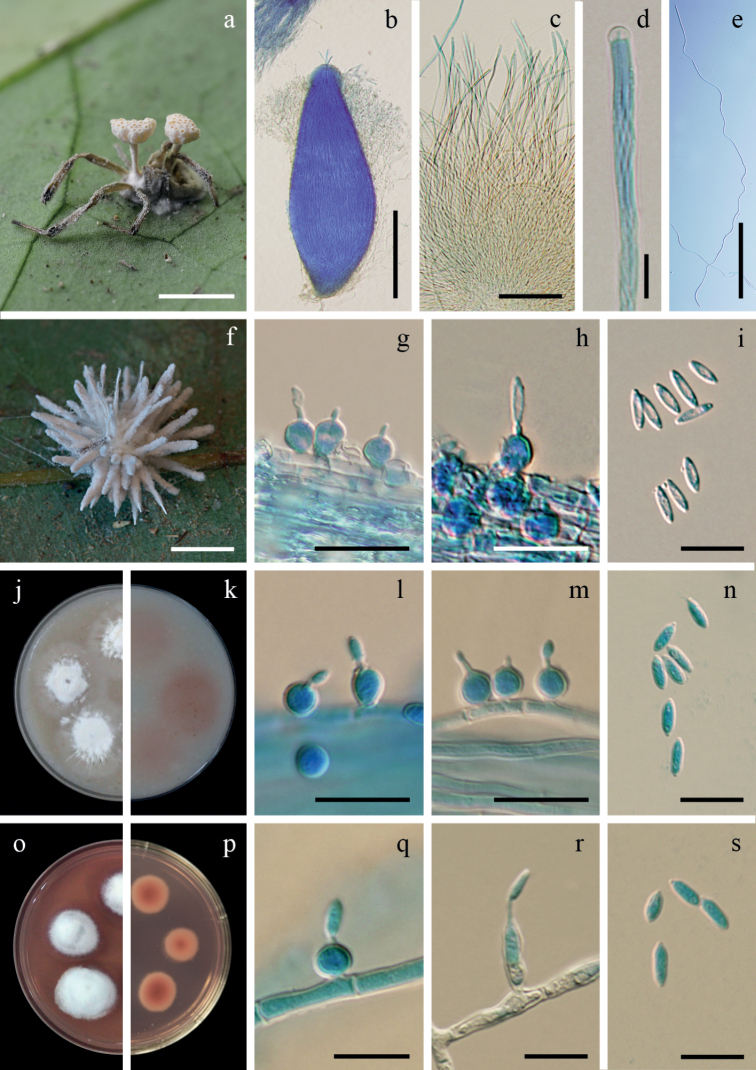
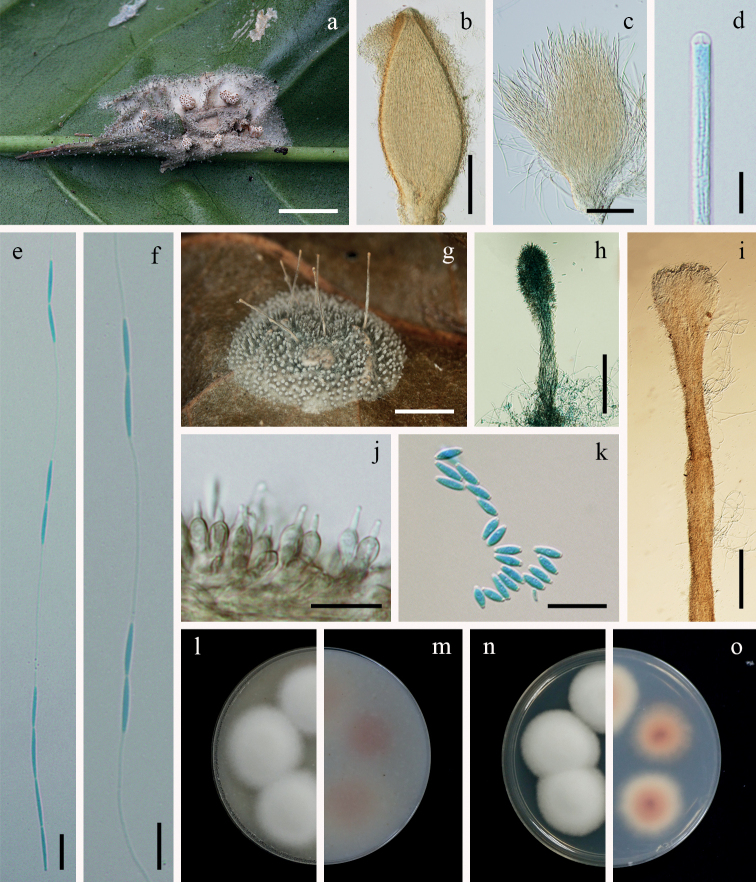
Hevansianovoguineensisa fungus on a spider (BBH 32171) b perithecium c asci d ascus tip e filiform, whole ascospore f fungus on a spider (BBH 31299) g–i phialides with conidia on synnema j, k colonies on OA at 21 days (j obverse, k reverse) l–n phialides with conidia on OAo, p colonies on PDA at 21 days with purplish-red pigment diffusing in agar medium (o obverse, p reverse) q–s phialides with conidia on PDA. Scale bars: 5 mm (a, f); 200 µm (b); 100 µm (c, e); 10 µm (d, g–i, l–n, q, r, s).
Remark.
The description below is based on natural specimens collected in Thailand.
Description.
Spider hosts covered by light yellow to pale yellow (158A–B) mycelium. Sexual morph: Stromata stipitate, solitary or multiple. Stipes cylindrical, arising from the dorsal region of the host, white to pale yellow, 3–5 mm long, 0.5–1 mm broad. Fertile heads produce at the terminal of stipes, disc-shaped, upper surface slightly convex, 1–3 × 1–2 mm. Perithecia completely immersed, narrowly ovoid, 500–750 × 200–300 µm, ostioles strong orange yellow (163B). Asci cylindrical, 8-spored, 350–450 µm long, 5–7 µm broad, with cap 3–5 µm thick. Ascospores hyaline, filiform, whole ascospores, 400–460 × 1–1.5 µm. Asexual morph: Synnemata multiple, cylindrical, occasionally acuminate apex, white, up to 8 mm long, 50–200 µm broad. Conidiogenous cells phialidic, scattered along with the synnemata. Phialides solitary, globose to subglobose, arising from the mycelium, (4)5–5.5(6) × (4)5–5.5(6) µm, with distinct necks, 0.5–1.5 × 0.5–1 µm. Conidia hyaline, fusoid or fusiform-elliptical, (2)6–8(10) × 1–2(2.5) µm.
Culture characteristics.
Colonies on OA attaining a diam. of 18–20 mm in 21 days, cottony with high mycelium density in the middle of colonies, mycelium with low density around the margin of colonies, flattened, white, reverse deep pink (180D). Sparse synnemata with conidiogenous cells producing conidia observed after 30 days, white, on the edge of a colonies. Phialides solitary, globose to subglobose, (4)5.5–6.5(7) × 3.5–5(5.5) µm, distinct necks, 1–3 × 0.5–1 µm. Conidia hyaline, fusoid, fusiform-elliptical, (2)6–10(13) × 1–3 µm.
Colonies on PDA attaining a diam. of 7–9(10) mm in 21 days, cottony with high mycelium density, white, moderate purplish-red to dark purplish-pink (186B–C) pigment diffusing in the medium, reverse moderate red (180 A–B). Sporulation observed after 30 days with absence of synnemata. Phialides arising from aerial hyphae, solitary, mostly globose to subglobose, occasionally cylindrical, (4)5.5–11.5(15) × 2–3.5(5) µm, distinct necks, 0.5–2 × 0.5–1 µm. Conidia hyaline, fusoid, fusiform-elliptical, cylindrical, (2)6–9.5(11) × 1–3 µm.
Host.
Spiders (Araneae, Theridiidae).
Habitat.
Specimens were found on the underside of dicot leaves of forest plants.
Materials examined.
Thailand, Nakhon Ratchasima Province, Khao Yai National Park, 14°26'20.72"N, 101°22'20.02"E, on spider (Web builder, Araneae) attached to the underside of a dicot leaf of forest plants, 10 June 2010, K. Tasanathai, P. Srikitikulchai, S. Mongkolsamrit, R. Ridkaew, MY6026.01 (BBH 32171, BCC 42675) isolated from ascospores; idem, 6 April 2010, K. Tasanathai, S. Mongkolsamrit, T. Chohmee, A. Khonsanit, R. Ridkaew, MY6988.01 (BBH 31299, BCC 49323) isolated from conidia; Kamphaeng Phet, Khlong Lan National Park, 16°7'46.84"N, 99°16'53.11"E, on spider (Web builder, Araneae, Theridiidae) attached to the underside of a dicot leaf of forest plants, 6 November 2007, K. Tasanathai, S. Mongkolsamrit, P. Srikitikulchai, B. Thongnuch, R. Ridkaew, A. Khonsanit, W. Chaygate, MY2770 (BBH 22744, BCC 28581), MY2771 (BBH 22745, BCC 28582), MY2775 (BBH 22747, BBC 28585).
Notes.
Hevansianovoguineensis is morphologically similar to H.nelumboides, both species producing fertile heads at the terminal end of stipes. The perithecia are completely immersed. However, H.novoguineensis differs from H.nelumboides in producing whole ascospores. Hevansianelumboides produces multiseptated ascospores disarticulating into part-spores (Kobayasi and Shimizu 1977; Shimizu 1994). Based on natural specimens, the conidia from Thai specimens are shorter than those reported for specimens from Papua New Guinea (2–10 × 1–2.5 µm vs. 10.5–17.5 × 1.5–3 µm) (Samson and Brady 1982). In addition, there are other species producing the fertile heads at the terminal end of stipes infecting ants (Hymenoptera), for example, Ophiocordycepsbinata (H.C. Evans & Samson) J.P.M. Araújo, H.C. Evans & D.P. Hughes, O.pseudolloydii (H.C. Evans & Samson) G.H. Sung, J.M. Sung, Hywel-Jones & Spatafora and O.lloydii (H.S. Fawc.) G.H. Sung, J.M. Sung, Hywel-Jones & Spatafora (Araújo et al. 2020). Ophiocordycepsbinata is most similar to H.novoguineensis by producing disc-shaped fertile heads, while fertile heads in O.pseudolloydii and O.lloydii are subglobose.
Currently accepted species of Hevansia
. Hevansia arachnophila
(Petch) Luangsa-ard, Hywel-Jones & Spatafora, IMA Fungus 8: 348 (2017).
EF3845AA-278A-5760-A8DF-E72428F2B4B4
≡ Trichosterigmaarachnophilum Petch [as ‘arachnophila’], Trans. Br. mycol. Soc. 8: 215 (1923).
≡ Hirsutellaarachnophila (Petch) Petch, Trans. Br. mycol. Soc. 9: 93 (1923).
≡ Akanthomycesarachnophilus (Petch) Samson & H.C. Evans, Acta bot. neerl. 23: 33 (1974).
. Hevansia longispora
(B. Huang, S.B. Wang, M.Z. Fan & Z.Z. Li) Luangsa-ard, Hywel-Jones & Spatafora, IMA Fungus 8: 349 (2017).
97F91D8A-BB49-5780-830B-C98A6F04CB77
≡ Akanthomyceslongisporus B. Huang, S.B. Wang, M.Z. Fan & Z.Z. Li, Mycosystema 19: 172 (2000).
. Hevansia nelumboides
(Kobayasi & Shimizu) Luangsa-ard, Hywel-Jones & Spatafora, IMA Fungus 8: 349 (2017).
AD4FB252-0B1D-57F4-A55B-2262A48798D2
≡ Cordycepsnelumboides Kobayasi & Shimizu, Kew Bull. 31: 557 (1977).
. Hevansia ovalongata
(L.S. Hsieh, Tzean & W.J. Wu) Luangsa-ard, Hywel-Jones & Spatafora, IMA Fungus 8: 349 (2017).
C42167C3-2222-5179-B209-B09C3EF0000B
≡ Akanthomycesovalongatus L.S. Hsieh, Tzean & W.J. Wu, Mycologia 89: 321 (1997).
. Hevansia websteri
(Hywel-Jones) Luangsa-ard, Hywel-Jones & Spatafora, IMA Fungus 8: 349 (2017).
6CBB45B2-7A32-583D-AF41-C8EB28BAC6EA
≡ Akanthomyceswebsteri Hywel-Jones, Mycol. Res. 100: 1068 (1996).
. Hevansia minuta
Tasanathai, Noisripoom & Mongkolsamrit sp. nov.
ECA6F486-376A-5132-898F-534D03CD1CFE
843088
Figure 4.
Hevansiaminutaa fungus on a spider (BBH 30490) b perithecia c ascus d ascus tip e filiform, whole ascospore f, g phialides with conidia h, i colonies on OA at 21 days (h obverse, i reverse) j, k colonies on PDA at 21 days (j obverse, k reverse). Scale bars: 5 mm (a), 100 µm (b), 50 µm (c), 10 µm (d, f, g), 20 µm (e).
Typification.
Thailand, Chumphon Province, Heo Lom Waterfall, 9°43'45.04"N, 98°40'52.71"E, on spider (Web builder, Araneae, Theridiidae, Meotipa sp.) attached to the underside of a dicot leaf of forest plants, 30 May 2011, K. Tasanathai, P. Srikitikulchai, A. Khonsanit, K. Sansatchanon, D. Thanakitpipattana, MY6537.01 (BBH 30490, holotype), ex-type culture BCC 47519 isolated from ascospores.
Etymology.
Refers to the small stroma of this species.
Description.
Spider host covered by white mycelium. Sexual morph: Stromata stipitate, arising from the dorsal region of the host, solitary, cylindrical to enlarging apically, white to cream, 10 mm long, 1 mm broad. Fertile head oval, ca. 2–2.5 mm long, ca. 1.5 mm broad. Perithecia completely immersed, narrowly ovoid, 400–500 × 100–170 µm. Asci cylindrical, 8-spored, 325–450 × 3–5 µm, with cap 2–5 µm thick. Ascospores hyaline, filiform, whole ascospores, 320–450 × 0.5–1.5 µm. Asexual morph: Conidiogenous cells phialidic scattered along with the stipe. Phialides solitary, globose to ovoid, arising from the mycelium, 5–7 × 5–6 µm, distinct necks, 1–2 × 0.5–1 µm. Conidia hyaline, fusiform, 2–7 × 2–3 µm.
Culture characteristics.
Colonies on OA attaining a diam. of 15–18(20) mm in 21 days, cottony with high mycelium density, white. Conidia and reproductive structures not observed.
Colonies on PDA attaining a diam. of 8–9(10) mm in 21 days, cottony with high mycelium density, white, reverse pale yellow (161C–D). Conidia and reproductive structures not observed.
Host.
Spiders (Araneae, Theridiidae, Meotipa sp.).
Habitat.
Specimens were found on the underside of dicot leaves of forest plants.
Additional materials examined.
Thailand, Chumphon Province, Heo Lom Waterfall, 9°43'45.04"N, 98°40'52.71"E, on spider (Web builder, Araneae, Theridiidae, Meotipa sp.) attached to the underside of a dicot leaf of forest plants, 30 May 2011, K. Tasanathai, P. Srikitikulchai, A. Khonsanit, K. Sansatchanon, D. Thanakitpipattana, MY06537.02 (BBH 30490, paratype), ex-paratype culture BCC 47520 isolated from conidia.
Notes.
Hevansiaminuta differs significantly from H.novoguineensis and H.nelumboides in the shape of the fertile heads, which is oval in H.minuta and disc-shaped, slightly convex on the upper surface in H.novoguineensis and H.nelumboides. Additionally, H.minuta differs from H.novoguineensis in the size of the perithecia. In H.minuta, perithecia are smaller than those reported for H.novoguineensis (400–500 × 100–170 µm vs. 500–750 × 200–300 µm) (Table 2). Synnema in H.minuta was not observed in the natural specimen, while the other species in Hevansia produce synnemata (Table 3). Hevansiaminuta does not produce pigment in culture. Meanwhile, H.novoguineensis produces a purplish-red pigment diffusing in PDA plates.
Table 2.
Morphological comparisons of sexual morphs in Hevansia, Jenniferia and Polystromomyces.
| Species | Host | Stromata | Fertile part | Perithecia | Asci | Ascospores | References |
|---|---|---|---|---|---|---|---|
| Hevansiaminuta | Spider (Theridiidae, Meotipa sp.) | Stipitate, solitary, white to cream, 10 mm long, 1 mm broad | Oval, ca. 2–2.5 mm long, ca. 1.5 mm broad | Immersed, narrowly ovoid, 400–500 × 100–170 µm | Cylindrical, 325–450 × 3–5 µm | Filiform, whole ascospores, 320–450 × 0.5–1.5 µm | This study |
| H.nelumboides | Spider | Stipitate, white, 4 mm long, 0.4 mm broad | Disc-shaped, 2 × 0.8 mm | Immersed, fusoid-ellipsoidal, 535–545 × 180–190 µm | 400–450 × 5–6 µm | Part-spores, ca. 5 × 1 µm | Kobayasi and Shimizu (1977) |
| H.novoguineensis | Spider (Theridiidae) | Stipitate, solitary, or multiple, cylindrical, white to pale yellow, 3–5 mm long, 0.5–1 mm broad | Disc-shaped, upper surface slightly convex, 1–3 × 1–2 mm | Immersed, narrowly ovoid, 500–750 × 200–300 µm | Cylindrical, 350–450 × 5–7 µm | Filiform, whole ascospores, 400–460 × 1–1.5 µm | This study |
| Jenniferiagriseocinerea | Spider (Thomisidae, Diaeacf.dorsata, Diaea sp.) | Non-stipitate | Perithecia aggregated in clusters forming a cushion | Superficial, ovoid, 650–850 × 250–320 µm | Cylindrical, 375–460 × 5–6 µm | Whole ascospores with septate part-spores alternately connected with thread-like structures, up to 400 µm long, each cell narrowly fusiform, 10–15 × 1–2 µm, filiform regions, 35–45 × 0.2–0.8 µm | This study |
| J.thomisidarum | Spider (Thomisidae, Diaeacf.dorsata) | Non-stipitate | Perithecia aggregated in clusters forming a cushion | Superficial, obpyriform, 850–1100 × 300–400 µm | Cylindrical, 520–700 × 4–6 µm | Whole ascospores with septate part-spores alternately connected with thread-like structures, up to 680 µm long, each cell narrowly fusiform, 10–20 × 1–2 µm, filiform regions, 30–50 × 0.2–0.8 µm | This study |
| Polystromomycesaraneae | Spider egg sac | Stipitate, multiple, moderate yellow, 8–12 mm long, 1–3 mm broad | Disc-shaped, upper surface slightly convex, 3–4 × 2–3.5 mm | Immersed, narrowly ovoid, 1000–1400 × 200–350 µm | Cylindrical, 400–1000 µm long, 3.5–6 µm | Part-spores, cylindrical, 2–6 × 1–3 µm | This study |
Table 3.
Morphological comparisons of asexual morphs in Hevansia, Jenniferia and Parahevansia.
| Species | Host | Synnemata | Phialides | Conidia | References |
|---|---|---|---|---|---|
| Hevansiaarachnophila | Spider | Simple, solitary (rarely two or three together), cylindrical, cream, up to 6 mm long, 45–100 µm broad | Globose, 3–4.5 µm broad, with distinct necks, 1–2 × 0.5 µm | Cymbiform, 3.5–6 × 1–1.5 µm | Hywel-Jones (1996) |
| H.longispora | Spider | Multiple, clavate, brown, 250–700 µm long | Ellipsoid to cylindrical, 7–15 × 2–4 µm | Cylindrical to fusiform, 8.8–14.8 × 2–3 µm | Huang et al. (2000) |
| H.minuta | Spider (Theridiidae, Meotipa sp.) | Non-synnemata | Globose to ovoid, 5–7 × 5–6 µm with distinct necks, 1–2 × 0.5 µm | Fusiform, 2–7 × 2–3 µm | This study |
| H.nelumboides | Spider | NA | Elongate | Ovoid, 5 × 3 µm | Kobayasi and Shimizu (1977) |
| H.novoguineensis | Spider (Theridiidae) | Multiple, cylindrical, occasionally acuminate apex, white, up to 8 mm long, 50–200 µm broad | Globose to subglobose, 4–6 × 4–6 µm, with distinct necks, 0.5–1.5 × 0.5–1 µm | Fusoid or fusiform-elliptical, 2–10 × 1–2.5 µm | This study |
| H.novoguineensis | Spider | Multiple, slender, acuminate apex, white to pale yellow, 3.5 mm long, 50–150 µm broad | Globose to ovoid, 5–6.5 × 4–6 µm broad, with distinct necks, 2–3 × 0.8–1.5 µm | Cylindrical, curved or slightly fusiform, 10.5–17.5 × 1.5–3 µm | Samson and Brady (1982) |
| H.ovalongata | Spider | Multiple, simple, or branch, white to greyish-orange, 2.2–9 mm long, 112–520 µm broad | Globose to subglobose, cylindrical, or ellipsoid, 6–8.7 × 4–6.4 µm, with distinct necks, 1.4–3.2 × 0.8–1.8 µm | Ellipsoid, obovate to oblong, 6–10.3 × 2.4–4.4 µm | Hsieh et al. (1997) |
| H.websteri | Spider | Simple, cylindrical, cream-white, up to 12 mm long, 50–70 µm broad | Ellipsoid, 4.5–8.5 × 2–3.5 µm, with distinct necks, 1.5–3 × 0.5 µm | Cylindrical, 4–7 × 1–1.5 µm | Hywel-Jones (1996) |
| Jenniferiacinerea | Spider (Thomisidae, Amyciaea sp.) | Multiple, clavate, grey, up to 3 mm long, 60–70 µm broad | Cylindrical, 3.5–6.5 × 1.5–2 µm, with distinct necks, 2–2.5 × 0.5 µm | Clavate, 3.5–5.5 × 1–1.5 µm | Hywel-Jones (1996) |
| J.griseocinerea | Spider (Thomisidae, Diaeacf.dorsata, Diaea sp.) | Two types of synnemata, long synnemata, cylindrical with blunt end, grey to pale brown, 2.5–5 mm long, 100–150 µm broad, middle of long synnemata, 50–80 μm broad; short synnemata, cylindrical, pale grey to dark grey, up to 450 µm long, 20–50 µm broad | Flask-shaped, 5–10 × 3–5 µm, with distinct necks, 2–3.5 × 0.5–1 µm | Fusiform, 3–6 × 1–2 µm | This study |
| J.thomisidarum | Spider (Thomisidae, Diaeacf.dorsata) | Multiple, cylindrical to clavate, greyish-brown, up to 800 µm long, 30–100 µm broad | Cylindrical, 7–16 × 2–5 µm, with distinct necks, 1–5 × 1–1.5 µm | Fusiform, cylindrical, 3–12 × 1–3 µm | This study |
| Parahevansiakoratensis | Spider (Salticidae) | Multiple, simple, brown at the sterile base, becoming grey white, up to 6 mm long, 50 µm broad | Obovoid to ellipsoid, 4–5.5 × 3–3.5 µm, with distinct necks, 2.5–3 × 0.5–1 µm | Clavate, 4.5–5.5 × 1–1.5 µm | Hywel-Jones (1996) |
NA, information not provided in the original description.
Key to the species of Hevansia
Based on sexual state characters
| 1 | Ascospores filamentous, disarticulating into part-spores, immersed perithecia, solitary or multiple stipes | H.nelumboides |
| – | Ascospores filiform, whole ascospores, immersed perithecia, solitary or multiple stipes | 2 |
| 2 | Ascospores 320–450 × 0.5–1.5 µm, solitary stipe | H.minuta |
| – | Ascospores 400–460 × 1–1.5 µm, solitary or multiple stipes | H.novoguineensis |
Based on asexual state characters
| 1 | Phialides mostly arising from the mycelium, globose to subglobose | 2 |
| – | Phialide arising on basal cells, obovoid, ellipsoid, cylindrical | 3 |
| 2 | Conidia cymbiform, 3.5–6 × 1–1.5 µm | H.arachnophila |
| – | Conidia fusiform, 2–7 × 2–3 µm | H.minuta |
| – | Conidia cylindrical, fusoid, fusiform-elliptical, (from Thailand, 2–10 × 1–2.5 µm); occasionally curved, (Papua New Guinea, 10.5–17.5 × 1.5–3 µm | H.novoguineensis |
| – | Conidia oblong, obovate or broadly ellipsoidal 6–10.3 × 2.4–4.4 µm | H.ovalongata |
| 3 | Conidia cylindrical to fusiform 8.8–14.8 × 2–3 µm | H.longispora |
| – | Conidia cylindrical, 4–7 × 1–1.5 µm | H.websteri |
. Jenniferia
Mongkolsamrit, Noisripoom & Tasanathai gen. nov.
2227B7ED-AEED-5B7E-9122-8FD0A7A83CC7
843089
Type species.
Jenniferiathomisidarum Mongkolsamrit, Noisripoom & Tasanathai.
Etymology.
In honour of Dr. Jennifer Luangsa-ard, for her support and guidance in arthropod pathogenic fungi research.
Description.
Spider hosts covered with pale yellow to dark greyish-yellow mycelium. Sexual morph: Stromata non-stipitate. Perithecia growing in subiculum, superficial, aggregated in clusters forming a cushion. Asci cylindrical with thickened caps. Ascospores hyaline, septate part-spores alternately connected with thread-like structures along the whole ascospore (Fig. 2d). Asexual morph: Synnemata arising from all parts of host, numerous, cylindrical to clavate. Conidiogenous cells phialidic, producing along the synnemata or upper part of the synnemata. Phialides flask-shaped with distinct necks. Conidia hyaline, fusiform or cylindrical.
Notes.
Jenniferia is strongly supported as a monophyletic clade by having unique morphological characteristics of perithecia and ascospores. In sexual morph specimens, this genus produces aggregated superficial perithecia forming a cushion with septate part-spores alternately connected with thread-like structures along the whole ascospore (Fig. 2d), which are not seen in any allied genera of the family.
. Jenniferia cinerea
(Hywel-Jones) Mongkolsamrit & Noisripoom comb. nov.
8CFCE167-3F92-5435-A4AF-DA39A3B346E5
843090
Figure 5.
Jenniferiacinereaa, b fungus on a spider (BBH 2649, NHJ 03510, BCC 6839) c, d fungus on a spider (BBH 4896, NHJ 05984, BCC 2191). Scale bars: 5 mm (b, d).
≡ Akanthomycescinereus Hywel-Jones, Mycol. Res. 100: 1068 (1996).
≡ Hevansiacinerea (Hywel-Jones) Luangsa-ard, Hywel-Jones & Spatafora, IMA Fungus 8: 349 (2017).
Description and illustration.
See Hywel-Jones (1996).
Host.
Spiders (Araneae, Thomisidae, Amyciaea sp.).
Habitat.
Specimens were found on the underside of dicot leaves and bamboo leaves of forest plants.
Material examined.
Thailand, Ranong Province, Khlong Nakha Wildlife Sanctuary, 9°27'34.52"N, 98°30'16.15"E, on spider (Araneae), 21 April 1994, Hywel-Jones NL, Nasit R, Plomhan R, Sivichai S, Thienhirun S, NHJ 3531 holotype, holotype damaged and no culture living, Neotype designated here: THAILAND, Ranong Province, Khlong Nakha Wildlife Sanctuary, 9°27'34.52"N, 98°30'16.15"E, on spider (Non-web builder, Araneae, Thomisidae, Amyciaea sp.), 21 April 1994, Hywel-Jones NL, Nasit R, Plomhan R, Sivichai S, Thienhirun S, NHJ 03510 (BBH 2649, holotype), ex-type culture BCC 6839.
Notes.
Based on the asexual morph of species in Jenniferia, they share similar characteristics in producing grey mycelium covering the spider host and multiple cylindrical synnemata from all parts of the host. The phylogenetic analysis supported J.cinerea as a sibling species to J.griseocinerea, but they have differences in producing synnemata. Jenniferiacinerea produces long synnemata, while J.griseocinerea produces short and long synnemata (Fig. 6). Jenniferiacinerea was not found as a sexual morph, whereas both J.griseocinerea and J.thomisidarum were found with sexual and asexual morphs (Tables 2 and 3). The shape of conidia in J.cinerea is clavate, but conidia in J.griseocinerea are fusiform and in J.thomisidarum are fusiform to cylindrical (Table 3). The spider hosts of J.cinerea from both specimens presented herein are identified as Amyciaea sp. belonging to the family Thomisidae.
Figure 6.
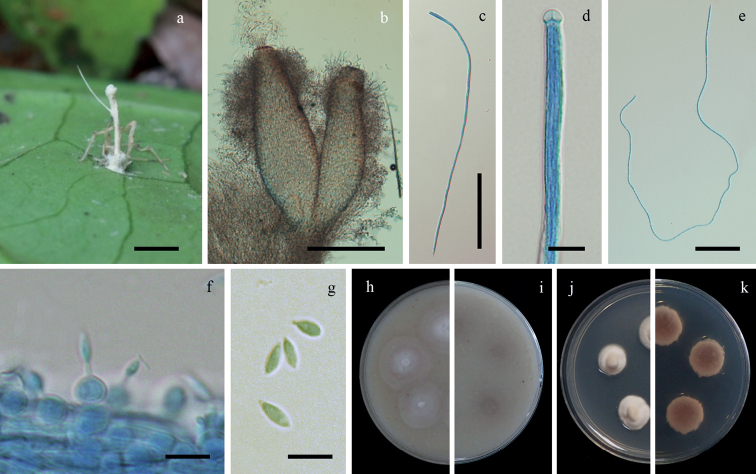
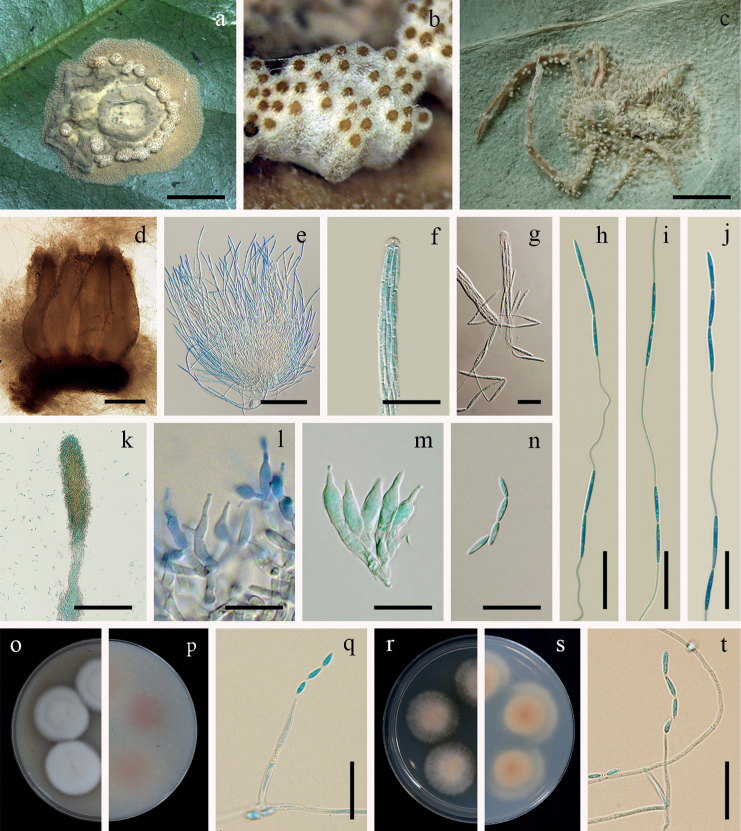
Jenniferiagriseocinereaa fungus on a spider (BBH 29656) b perithecium c asci d ascus tip e, f whole ascospores with septate part-spores alternately connected with thread-like structures g fungus on a spider (BBH 33219) h short synnema i long synnema j phialides k conidia l, m colonies on OA at 21 days (l obverse, m reverse) n, o colonies on PDA at 21 days (n obverse, o reverse). Scale bars: 2 mm (a, g); 200 µm (b); 100 µm (c, h, i); 10 µm (d, e, f, j, k).
. Jenniferia griseocinerea
Tasanathai, Noisripoom & Mongkolsamrit sp. nov.
972B0606-DB8B-5815-92DA-78F28FC60841
843091
Typification.
Thailand, Nakhon Ratchasima Province, Khao Yai National Park, 14°26'20.72"N, 101°22'20.02"E, on spider (Non-web builder, Araneae, Thomisidae, Diaea sp.) attached to the underside of a dicot leaf of forest plants, 31 May 2010, K. Tasanathai, P. Srikitikulchai, S. Mongkolsamrit, T. Chohmee, A. Khonsanit, R. Somnuk, K. Sansatchanon, MY6006.01 (BBH 29656, holotype), ex-type culture BCC 42062 isolated from ascospores.
Etymology.
Named after the colour of the fresh specimens, from the Latin ‘griseo’, referring to dark grey and ‘cinerea’ meaning ash grey.
Description.
Spider hosts covered by yellowish-grey mycelium (156C). Sexual morph: Stromata non-stipitate. Perithecia growing in subiculum, aggregated in clusters, superficial, ovoid, 650–850 × 250–320 µm, ostiole pale brown. Asci cylindrical, 8-spored, 375–460 µm long, 5–6 µm broad, with cap 2–6 µm thick. Ascospores hyaline, whole ascospores with septate part-spores alternately connected with thread-like structures, four-terminal cells on each end with six alternating pairs of cells and filaments, sixteen cells per ascospore, up to 400 µm long, each cell narrowly fusiform, 10–15 × 1–2 µm, filiform regions, 35–45 × 0.2–0.8 µm. Asexual morph: Two types of synnemata were produced from all parts of the hosts. Several long synnemata, grey becoming pale brown at terminal ends, cylindrical with blunt end, 2.5–5 mm long, 100–150 μm broad, middle of long synnemata, 50–80 μm broad. Conidiogenous cells producing along long synnemata. Short synnemata, pale grey to dark grey, cylindrical, up to 450 µm long, 20–50 µm broad. Conidiogenous cells producing at the upper part of synnemata. Phialides flask-shaped at the base, 5–10 × 3–5 µm, tapering into distinct necks, 2–3.5 × 0.5–1 µm. Conidia hyaline, fusiform, 3–6 × 1–2 µm.
Culture characteristics.
Colonies on OA attaining a diam. of 18–20 mm in 21 days, cottony with high mycelium density, white, reverse pale yellow (165D). Conidia and reproductive structures not observed.
Colonies on PDA attaining a diam. of (16)17–20 mm in 21 days, cottony with high mycelium density, white, reverse pale yellow (165D). Conidia and reproductive structures not observed.
Host.
Spiders (Araneae, Thomisidae, Diaeacf.dorsata, Diaea sp.).
Habitat.
Specimens were found on the underside of dicot leaves of forest plants.
Additional materials examined.
Thailand, Nakhon Ratchasima Province, Khao Yai National Park, 14°26'20.72"N, 101°22'20.02"E, on spider (Non-web builder, Araneae, Thomisidae, Diaea sp.) attached to the underside of a dicot leaf of forest plants, 31 May 2010, K. Tasanathai, P. Srikitikulchai, S. Mongkolsamrit, T. Chohmee, A. Khonsanit, R. Somnuk, K. Sansatchanon, MY6006.02 (BBH 29656, paratype) ex-paratype culture BCC 42063 isolated from conidia; idem, on spider (Non-web builder, Araneae, Thomisidae, Diaeacf.dorsata) attached to the underside of a dicot leaf of forest plants, 8 November 2012, S. Mongkolsamrit, A. Khonsanit, W. Noisripoom, P. Srikitikulchai, R. Somnuk, MY8241 (BBH 33219) culture BCC 57821 isolated from conidia; idem, 9 August 2012, K. Tasanathai, S. Mongkolsamrit, A. Khonsanit, W. Noisripoom, K. Sansatchanon, MY7627 (BBH 36128) culture BCC 54893 isolated from conidia.
Notes.
Based on the multi-gene phylogenetic analyses presented in Fig. 1, Jenniferiagriseocinerea is closely related to J.cinerea. It shares similarity with J.cinerea in the production of several cylindrical synnemata arising from all parts of the spider host. However, J.griseocinerea differs from J.cinerea in producing long and short synnemata, while J.cinerea produces only long synnemata. The shape of phialides in J.griseocinerea from the specimens differs from J.cinerea and J.thomisidarum. Phialides in J.griseocinerea are flask-shaped, while phialides in J.cinerea and J.thomisidarum are cylindrical. Conidia in J.griseocinerea and J.thomisidarum are fusiform, occasionally cylindrical in J.thomisidarum. The conidia in J.griseocinerea are shorter than those reported for J.thomisidarum (3–6 × 1–2 µm vs. 3–12 × 1–3 µm) (Table 3).
. Jenniferia thomisidarum
Mongkolsamrit, Noisripoom & Tasanathai sp. nov.
070A1D30-91F8-599B-AC8C-CF07E933BA18
843092
Figure 7.
Jenniferiathomisidaruma fungus on a spider (BBH 29502) b perithecia c fungus on a spider (BBH 30660) d perithecia e asci f, g ascus tip h–j whole ascospores with septate part-spores alternately connected with thread-like structures k synnema with conidiogenous cells l, m phialides n conidia o, p colonies on OA at 21 days (o obverse, p reverse) q phialide with conidia on OAr, s colonies on PDA at 21 days (r obverse, s reverse) t phialide with conidia on PDA. Scale bars: 2 mm (a, c); 300 µm (d); 200 µm (e); 100 µm (k); 10 µm (f, g, l, m, n); 20 µm (h, i, j, q, t).
Typification.
Thailand, Nakhon Ratchasima Province, Khao Yai National Park, 14°26'20.72"N, 101°22'20.02"E, on spider (Non-web builder, Araneae, Thomisidae, Diaeacf.dorsata) attached to the underside of a dicot leaf of forest plants, 23 July 2009, K. Tasanathai, P. Srikitikulchai, S. Mongkolsamrit, R. Ridkaew, MY5032.01 (BBH 29502, holotype), ex-type culture BCC 37881 isolated from ascospores.
Etymology.
Named after the host belonging to the family Thomisidae (Araneae).
Description.
Spider hosts covered by dense greyish-brown mycelium (199C–D). Sexual morph: Stromata non-stipitate. Perithecia growing in subiculum, aggregated in clusters, superficial, obpyriform, 850–1100 × 300–400 µm, ostiole pale brown. Asci cylindrical, 8-spored, 520–700 µm long, 4–6 µm broad, with cap 2–6 µm thick. Ascospores hyaline, whole ascospores with septate part-spores alternately connected with thread-like structures, three-terminal cells on each end with six alternating pairs of cells and filament, eighteen cells per ascospore, up to 680 µm long, each cell narrowly fusiform, 10–20 × 1–2 µm, filiform regions, 30–50 × 0.2–0.8 µm. Asexual morph: Synnemata arising from the mycelial mat, numerous, greyish-brown, cylindrical to clavate, erect up to 800 µm long, 30–100 µm broad. Conidiogenous cells producing at the upper part of synnemata, mostly monophialidic or some polyphialidic. Phialides cylindrical, (7)10–15(16) × 2–4(5) µm, tapering into a distinct neck, (1)1.5–3.5(5) × 1–1.5 µm. Conidia hyaline, fusiform, cylindrical, (3)8.5–10.5(12) × 1–3 µm.
Culture characteristics.
Colonies on OA attaining a diam. of (12)14–15 mm in 21 days, cottony with high mycelium density, white, reverse pale orange (165D), poor sporulation. Phialides arising from aerial hyphae, solitary, awl-shaped, lecanicillium-like, 20–40 × 1–2 µm. Conidia in chains, hyaline, fusiform, cylindrical, smooth, (3)7.5–10.5(12) × (1.5)2–2.5(3) µm.
Colonies on PDA attaining a diam. of 8–10 mm in 21 days, cottony with high mycelium density in the middle of colonies, mycelium with low density around the margin of colonies, pale orange, reverse moderate orange (167D), poor sporulation. Phialides arising from aerial hyphae, solitary, awl-shaped, lecanicillium-like, 10–35 × 1–2 µm. Conidia in the chains, hyaline, fusiform, cylindrical, smooth, (3)6.5–9.5(10) × (1.5)2–2.5(3) µm.
Host.
Spiders (Araneae, Thomisidae, Diaeacf.dorsata) .
Habitat.
Specimens were found on the underside of dicot leaves of forest plants.
Additional materials examined.
Thailand, Nakhon Ratchasima Province, Khao Yai National Park, 14°26'20.72"N, 101°22'20.02"E, on spider (Non-web builder, Araneae, Thomisidae, Diaeacf.dorsata) attached to the underside of a dicot leaf of forest plants, 23 July 2009, K. Tasanathai, P. Srikitikulchai, S. Mongkolsamrit, R. Ridkaew, MY5032.02 (BBH 29502, paratype), ex-paratype culture BCC 37882 isolated from conidia; idem, 7 August 2011, K. Tasanathai, P. Srikitikulchai, S. Mongkolsamrit, A. Khonsanit, W. Noisripoom, K. Sansatchanon, MY6813 (BBH 30660, culture BCC 48932); idem, 3 August 2011, K. Tasanathai, P. Srikitikulchai, S. Mongkolsamrit, A. Khonsanit, W. Noisripoom, K. Sansatchanon, MY6866 (BBH 30690), culture BCC 49257; idem, 9 August 2012, K. Tasanathai, S. Mongkolsamrit, A. Khonsanit, W. Noisripoom, MY7598 (BBH 32822), culture BCC 54482; MY7599 (BBH 32823), culture BCC 54483; MY7600 (BBH 32824), culture BCC 32824; idem, 26 June 2012, K. Tasanathai, P. Srikitikulchai, S. Mongkolsamrit, A. Khonsanit, W. Noisripoom, K. Sansatchanon, R. Somnuk, MY8636 (BBH 35789), culture BCC 64182; idem, 7 August 2013, P. Srikitikulchai, S. Mongkolsamrit, A. Khonsanit, W. Noisripoom, MY8878 (BBH 336396), culture BCC 66224.
Notes. In sexual morph specimens found in nature, Jenniferiathomisidarum resembles J.griseocinerea by the formation of non-stipitate ascomata. The perithecia of both species are superficial and aggregate in clusters, challenging the identification of the species rank. The ascospores are of the same type by septate part-spores alternately connected with thread-like structures along the whole ascospore (Fig. 2d). Ascospores in J.thomisidarum are longer than those reported for J.griseocinerea (Table 2). Jenniferiathomisidarum differs from J.griseocinerea in the size and shape of the perithecia and asci. In J.thomisidarum, perithecia and asci are larger and longer than those reported for J.griseocinerea (850–1100 × 300–400 µm vs. 650–850 × 250–320 µm; 520–700 × 4–6 µm vs. 375–460 × 5–6 µm). The perithecia in J.thomisidarum are obpyriform, while perithecia in J.griseocinerea are ovoid.
Key to the species of Jenniferia
Based on sexual state characters
| 1 | Ascospores septate part-spores alternately connected with thread-like structures along the whole ascospore, non-stipitate ascomata, superficial perithecia up to 400 µm long | J.griseocinerea |
| – | Ascospores septate part-spores alternately connected with thread-like structures along the whole ascospore, non-stipitate ascomata, superficial perithecia to 680 µm long | J.thomisidarum |
Based on asexual state characters
| 1 | Synnemata, multiple, two types of synnemata, long synnemata cylindrical with a blunt end, short synnemata | J.griseocinerea |
| – | Synnemata, multiple, one type of synnemata | 2 |
| 2 | Conidia 3.5–5.5 × 1–1.5 µm, clavate | J.cinerea |
| – | Conidia, 3–12 × 1–3 µm, fusiform, cylindrical | J.thomisidarum |
. Parahevansia
Mongkolsamrit & Noisripoom gen. nov.
F9747AA2-1142-5E66-9992-A11B85ED9B2F
844040
Type species.
Parahevansiakoratensis (Hywel-Jones) Mongkolsamrit & Noisripoom, comb. nov., Mycol. Res. 100: 1067 (1996).
Etymology.
Morphologically resembling the genus Hevansia, but being phylogenetically distinct.
Description.
Asexual morph: Synnemata arising from all parts of host, numerous, simple, brown at the sterile base becoming grey white with fertile part. Conidiogenous cells phialidic producing upper part of the synnemata. Phialides in a monolayer, single on basal lateral cells of synnemata, crowded, obovoid to ellipsoid with distinct necks. Conidia in chain, hyaline, smooth-walled, clavate.
Notes.
Parahevansiakoratensis, the type species of this genus, was originally described as species of Akanthomyces (Hywel-Jones, 1996) and later transferred to Hevansia (Kepler et al. 2017). Our multi-gene phylogenetic analyses supported Parahevansia as a monophyletic clade with strong support (MLB = 100 / BPP = 1.00, Fig. 1). Therefore, we introduced Parahevansia as a new genus that accommodates a single species, Pa.koratensis.
. Parahevansia koratensis
(Hywel-Jones) Mongkolsamrit & Noisripoom, comb. nov.,
FCB48883-0BD3-50D6-BDAA-22316B1C4B9B
844041
≡ Akanthomyceskoratensis Hywel-Jones, Mycol. Res. 100: 1068 (1996).
≡ Hevansiakoratensis (Hywel-Jones) Luangsa-ard, Hywel-Jones & Spatafora, IMA Fungus 8: 349 (2017).
Typification.
Thailand, Nakhon Ratchasima Province, Khao Yai National Park, 14°26'20.72"N, 101°22'20.02"E, on spider (Araneae, Salticidae), 12 December 1991, N.L. Hywel-Jones, NHJ 666.01 holotype.
Description and illustration.
See Hywel-Jones (1996).
Host.
Spider (Araneae, Salticidae).
Habitat.
Specimens were found on the underside of dicot leaves of forest plants.
Notes.
Both Parahevansiakoratensis and H.novoguineensis occur on spiders and both produce white mycelium with reddish pigment diffusing in agar media (Hywel-Jones 1996). However, the sporulation of H.novoguineensis is produced on media, while no sporulation on media in Pa.koratensis was observed. Based on the phylogenetic tree (Fig. 1), NHJ 2662 clustered with the ex-type strain NHJ 666.01 of Pa.koratensis. The insect host of the strain NHJ 2662 was recorded as a Lepidoptera larva. This result shows that Pa.koratensis is parasitic on spiders and Lepidoptera larva.
. Polystromomyces
Mongkolsamrit, Noisripoom, Sakolrak & Himaman gen. nov.
9A09E9CA-2810-5CD3-BC01-3DD610D02B1B
843093
Type species.
Polystromomycesaraneae Mongkolsamrit, Noisripoom, Sakolrak & Himaman.
Etymology.
From Latin “poly” (many), referring to many stromata of the fungus on the host.
Description.
Sexual morph: Stromata stipitate, multiple, pale yellow mycelium covering the host. Stipes arising from spider egg sac, cylindrical at the base, slightly enlarged midway to the terminal end of the stipe below the fertile head. Fertile heads produce at the terminal stipes, disc-shaped, upper surface slightly convex. Perithecia completely immersed, ovoid. Asci cylindrical. Ascospores hyaline, filiform, disarticulating into part-spores. Colony on PDA and OA, white, producing microcycle conidiation.
Notes.
Polystromomyces contains a new species, Po.araneae. It shares similarity with species in Hevansia in producing multiple stipes with fertile heads at the apex. This specimen is found on a spider egg sac (Araneae) attached to the underside of a dicot leaf. There is no record of the asexual morph on the specimen.
. Polystromomyces araneae
Mongkolsamrit, Noisripoom, Sakolrak & Himaman sp. nov.
664FD2BB-9690-5008-B84D-3AC9DC881EE1
843094
Figure 8.
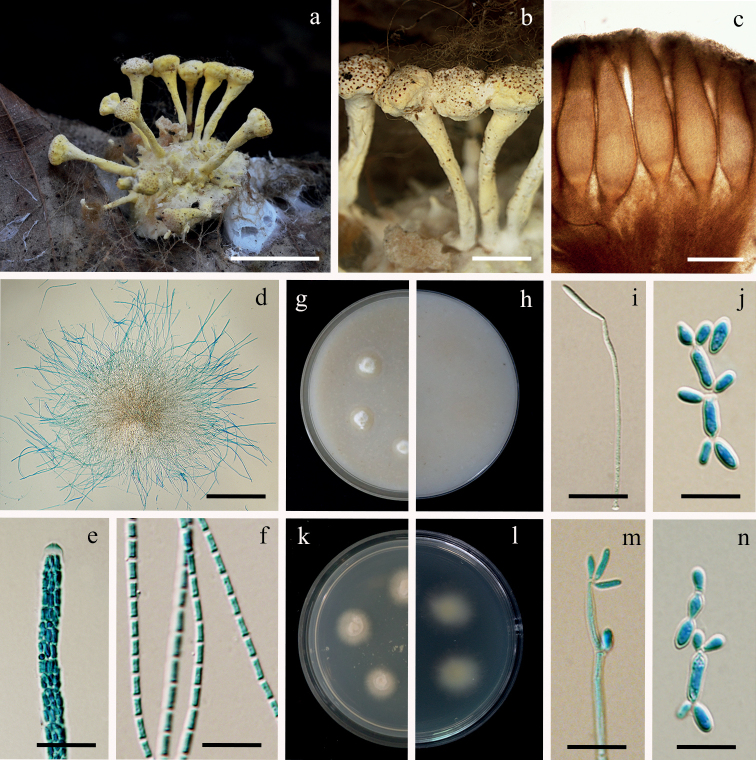
Polystromomycesaraneaea fungus on a spider egg sac (BBH 49054) b fertile heads c perithecia d asci e ascus tip f part-spores g, h colonies on OA at 21 days (g obverse, h reverse) i conidium formation from a hypha on OAj microcycle conidiation on OAk, l colonies on PDA at 21 days (k obverse, l reverse) m conidia formation from a hypha on PDA n microcycle conidiation on PDA. Scale bars: 10 mm (a); 3 mm (b); 200 µm (c, d); 10 µm (e, f, i, j, m, n).
Typification.
Thailand, Tak Province, Umphang Wildlife Sanctuary, 15°55'36.33"N, 98°45'12.15"E, on spider egg sac (Araneidaesensu lato) attached to the underside of a dicot leaf, 6 December 2020, B. Sakolrak, MY12684 (BBH 49054, holotype), ex-type culture BCC 93301 isolated from ascospores.
Etymology.
From Latin, “aranea” refers to a spider host.
Description.
Hosts covered by dense pale yellow mycelium (162D). Sexual morph: Stromata stipitate, arising from the host, multiple, cylindrical at the base, slightly enlarged midway to the terminal stipe below the fertile head, moderate yellow (162A–B), 8–12 mm long, 1–3 mm broad. Fertile head disc-shaped, upper surface slightly convex, 3–4 × 2–3.5 mm. Perithecia completely immersed, narrowly ovoid, 1000–1400 × 200–350 µm, ostiole pale brownish-orange (165B). Asci cylindrical, 8-spored, 400–1000 µm long, 3.5–6 µm broad, with cap 2–5 µm thick. Ascospores hyaline, dissociating into 128 part-spores, cylindrical, 2–6 × 1–3 µm.
Culture characteristics.
Colonies on OA attaining a diam. of 8–10 mm in 21 d, mycelium sparse, white, reverse pale yellow (161C). Conidia forming on vegetative hyphae or by microcyclic conidiation, hyaline, clavate to cylindrical, 2–10 × 1–5 µm.
Colonies on PDA attaining a diam. of 8–10 mm in 20 d, mycelium sparse, white, reverse pale yellow (161C). Conidia forming on vegetative hyphae or by microcyclic conidiation, hyaline, clavate to cylindrical, 2–12 × 1–5 µm.
Host.
Spider egg sac.
Habitat.
Specimen was found on the underside of a dicot leaf of a forest plant.
Notes.
Based on natural specimens, Po.araneae closely resembles H.nelumboides and H.novoguineensis by producing fertile heads at the end of the stipes. The perithecia of these species are completely immersed. The ascospores of Po.araneae and H.nelumboides are filamentous, multiseptate ascospores disarticulating into part-spores, whereas H.novoguineensis produces filiform, whole ascospores. However, Po.araneae differs from H.nelumboides in the size of the perithecia. In Po.araneae, perithecia are larger than those reported for H.nelumboides (1000–1400 × 200–350 µm vs. 535–545 × 180–190 µm) (Table 2). Polystromomycesaraneae produces microcycle conidiation from conidia on culture, while the microcyclic sporulation is often seen in discharged ascospores in Metarhiziumphuwiangense Luangsa-ard, Mongkols., Himaman, Thanakitp. & Samson and Purpureomyceskhaoyaiensis (Hywel-Jones) Luangsa-ard, Samson & Thanakitp (Mongkolsamrit et al. 2020a).
Discussion
In this study, we conducted comparative morphological studies and phylogenetic analyses of spider parasitic fungi belonging to Hevansia, Jenniferia, Parahevansia and Polystromomyces. Kepler et al. (2017) established Hevansia with two species, i.e. H.nelumboides and H.novoguineensis, based on a split inferred from molecular data. Our molecular analyses revealed the sexual-asexual link between the Thai material (BCC 42675) and the ex-type culture of H.novoguineensis (CBS 610.80) and a novel species, H.minuta (Fig. 1). The sexual morph morphological characters in Hevansia (observed in H.novoguineensis, H.minuta and H.nelumboides) include stipes with terminal fertile heads arising from the dorsal region of their spider hosts (Figs 3a and 4a, this study; Fig. 3J in Kepler et al. 2017). Hevansiacf.novoguineensis (BCC 2093 and NHJ 4314) formed a subclade genetically close to H.novoguineensis, but the herbarium materials of these strains were not available for comparison. Considering that H.cf.novoguineensis formed a sister clade to H.novoguineensis (Fig. 1), but this relation was not consistently found between the markers, we propose that H.novoguineensis is a species complex and that H.cf.novoguineensis could potentially be considered as a different species if more molecular markers could unambiguously demonstrate its separation from the clade containing the ex-type strain.
In this study, the genus Polystromomyces is established with a single species (Po.araneae); it formed the basal lineage to Hevansia, Jenniferia and Gibellula and shared the same ecological habitat (on the underside of dicot leaves of forest plants). Polystromomycesaraneae shares morphological similarity to Hevansia by producing multiple stromata with fertile heads at the terminal part of stipes. Notably, Po.araneae can be distinguished from Hevansia by the shape of stipes. The stipes in Polystromomyces are cylindrical at the base and slightly enlarged midway to the terminal below the disc-shaped fertile heads. In contrast, the stipes of Hevansia are connected in a cylindrical arrangement with the fertile heads, resembling lotus seed pods on stems.
The novel genus Jenniferia was proposed to accommodate Jenniferiacinerea, J.griseocinerea and J.thomisidarum. Based on the natural specimens, the sexual morph of species within Jenniferia produce non-stipitate ascomata. The lack of stipe is a shared trait amongst pathogenic fungi species on spiders in Cordycipitaceae, such as Gibellula spp., Akanthomycesthailandicus and A.sulphureus, forming a torrubiella-like sexual morph (Mongkolsamrit et al. 2018; Kuephadungphan et al. 2020). However, species in Jenniferia described here can be easily distinguished from species in Gibellula spp., A.thailandicus and A.sulphureus by the superficial and aggregated perithecia in clusters forming a cushion (a distinctive character of Jenniferia), causing species in this genus to be easily recognisable in the field.
We reviewed valid species according to a current classification through molecular data combined with the observation of ascospore micro-morphology. Many studies revealed that cordycipitaceous fungi produced three types of ascospore morphology shown through the illustration and description in Figs 1 and 2(a–c). The filiform whole ascospores type (Fig. 2a) with the shape of thread is observed in Akanthomycessulphureus, Blackwellomyces spp., Cordycepskuiburiensis, Hyperdermium (e.g. H.bertonii, H.pulvinatum) and Neotorrubiellachinghridicola (Mongkolsamrit et al. 2018, 2020b; Crous et al. 2019; Sullivan et al. 2000; Thanakitpipattana et al. 2020). The presence of multiseptate ascospores disarticulating into part-spores (Fig. 2b) can be seen in several genera, such as Akanthomyces (e.g. A.thailandicus, A.pyralidarum and A.noctuidarum), Beauveria (e.g. B.asiatica, B.gryllotalpidicola), Cordyceps (e.g. C.militaris, C.inthanonensis and C.nidus) and also includes species in Gibellula (Mains 1958; Chiriví et al. 2017; Mongkolsamrit et al. 2018, 2020b; Aini et al. 2020; Kuephadungphan et al. 2020). The bola-ascospores morphology was noted in the description of Cordycepsbifusispora O.E. Erikss. and Cordycepsninchukispora (C.H. Su & H.H. Wang) G.H. Sung, J.M. Sung, Hywel-Jones & Spatafora (Fig. 2c) by Eriksson (1982) and Su and Wang (1986), respectively. Many Cordyceps species producing bola-ascospores were reported from Thailand and China (Tasanathai et al. 2016; Mongkolsamrit et al. 2018, 2020b; Wang et al. 2020). Samsoniella, a recent established genus also produces bola-ascospores (Mongkolsamrit et al. 2018; Wang et al. 2020). Examination of our specimens of Jenniferia revealed that its ascospores possess a unique shape not seen before in Cordycipitaceae. In this study, we are introducing another ascospore morphology (Fig. 2d), which is an autapomorphic character within Jenniferia that can be used to identify at the genus level.
There are two types of phialides in species of Hevansia. Some species produce globose to subglobose phialides with a distinct neck along the synnemata (e. g. H.arachnophila, H.minuta, H.novoguineensis and H.ovalongata), whereas other species produce phialides on the basal cells along the synnemata (e.g. H.longispora and H.websteri). These characters can be informative for recognising species of Hevansia. All species in Jenniferia produce the asexual morph and only two species are occasionally found producing sexual and asexual morphs on the same specimens, i.e. J.griseocinerea and J.thomisidarum. The Jenniferia asexual morph in nature differs from species in Hevansia in possessing pale grey to ash grey synnemata scattered over the body and legs of its host. Notably, J.griseocinerea significantly differs by producing two types of synnemata (Fig. 6g–i). In contrast, the anamorph of Hevansia (e.g. H.novoguineensis) produces white synnemata arising from the host (Fig. 3f).
Spider hosts associated with the Jenniferia species were identified as Diaeacf.dorsata for all specimens of J.griseocinerea and J.thomisidarum, except one specimen of J.griseocinerea that was identified as Diaea sp. Meanwhile, Amyciaea sp. is found as the host of J.cinerea. Jenniferia is, thus, up to now exclusively associated with the spider genera Diaea and Amyciaea in the family Thomisidae. A review by Shrestha et al. (2019) reported pathogenic fungi on spiders found in Thomisidae and includes Gibellula spp. on Tmarus spp. (Costa 2014), Torrubiellaalbolanata on a thomisid spider (Petch 1944) and T.neofusiformis on a thomisid spider (Kobayasi and Shimizu 1982). Recently, an additional species occurring on Thomisidae was found, including Gibellulacebrennini associated with Cebrenninuscf.magnus (Kuephadungphan et al. 2020).
Hevansia species are specialised parasites on spiders. Parahevansia, proposed as a new genus that accommodates Pa.koratensis (≡ Akanthomyceskoratensis), is parasitic on a salticid spider (Salticidae) and Lepidoptera larva (Hywel-Jones 1996; Shrestha et al 2019, in this study). Polystromomycesaraneae occurs on the spider egg sac (Araneidaesensu lato) attached to the underside of a dicot leaf. Cordycepsaraneae Mongkols., Tasan., Noisrip., Himaman & Luangsa-ard has also been reported on spider egg sac inhabiting the leaf litter (Mongkolsamrit et al. 2020b). Although Po.araneae is most similar to H.nelumboides and H.novoguineensis by producing stipes with fertile heads at the terminal, the two latter species are found on adult spiders.
Supplementary Material
Acknowledgements
This research was supported by the Platform Technology Management Section, National Center for Genetic Engineering and Biotechnology (BIOTEC), Grant No. P19-50231. We thank Nalun Chaichanyut for photos of the specimens. We are indebted to the Department of National Parks, Wildlife and Plant Conservation for their cooperation and support of our research project. We would like to thank Dr. Philip James Shaw for the thoughtful editing of the manuscript and the three anonymous reviewers and editors whose suggestions and comments helped improve the manuscript.
Citation
Mongkolsamrit S, Noisripoom W, Tasanathai K, Kobmoo N, Thanakitpipattana D, Khonsanit A, Petcharad B, Sakolrak B, Himaman W (2022) Comprehensive treatise of Hevansia and three new genera Jenniferia, Parahevansia and Polystromomyces on spiders in Cordycipitaceae from Thailand. MycoKeys 91: 113–149. https://doi.org/10.3897/mycokeys.91.83091
Supplementary materials
Figures S1–S5
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Suchada Mongkolsamrit, Wasana Noisripoom, Kanoksri Tasanathai, Noppol Kobmoo, Donnaya Thanakitpipattana, Artit Khonsanit, Booppa Petcharad, Baramee Sakolrak, Winanda Himaman
Data type
Pdf file
Explanation note
RAxML trees of Hevansia, Jenniferia, Parahevansia, Polystromomyces and related genera in the Cordycipitaceae from different molecular markers.
References
- Aini AN, Mongkolsamrit S, Wijanarka W, Thanakitpipattana D, Luangsa-ard JJ, Budiharjo A. (2020) Diversity of Akanthomyces on moths (Lepidoptera) in Thailand. MycoKeys 71: 1–22. 10.3897/mycokeys.71.55126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo JPM, Evans HC, Fernandes IO, Ishler MJ, Hughes DP. (2020) Zombie-ant fungi cross continents: II. Myrmecophilous hymenostilboid species and a novel zombie lineage. Mycologia 112(6): 1138–1170. 10.1080/00275514.2020.1822093 [DOI] [PubMed] [Google Scholar]
- Bischoff JF, White Jr JF. (2004) Torrubiellapiperis sp. nov. (Clavicipitaceae, Hypocreales), a new teleomorph of the Lecanicillium complex. Studies in Mycology 50: 89–94. [Google Scholar]
- Bischoff JF, Chaverri P, White Jr JF. (2005) Clarification of the host substrate of Ascopolyporus and description of Ascopolyporusphilodendrus sp. nov. Mycologia 97(3): 710–717. 10.1080/15572536.2006.11832800 [DOI] [PubMed] [Google Scholar]
- Castlebury LA, Rossman AY, Sung GH, Hyten AS, Spatafora JW. (2004) Multigene phylogeny reveals new lineage for Stachybotryschartarum, the indoor air fungus. Mycological Research 108(8): 864–872. 10.1017/S0953756204000607 [DOI] [PubMed] [Google Scholar]
- Chaverri P, Bischoff J, Evans H, Hodge K. (2005) Regiocrella, a new entomopathogenic genus with a pycnidial anamorph and its phylogenetic placement in the Clavicipitaceae. Mycologia 97(6): 1225–1237. 10.1080/15572536.2006.11832732 [DOI] [PubMed] [Google Scholar]
- Chaverri P, Liu M, Hodge KT. (2008) A monograph of the entomopathogenic genera Hypocrella, Moelleriella, and Samuelsia gen. nov. (Ascomycota, Hypocreales, Clavicipitaceae), and their aschersonia-like anamorphs in the Neotropics. Studies in Mycology 60: 1–66. 10.3114/sim.2008.60.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Wang T, Lin Y, Huang B. (2021a) Gibellulaflava sp. nov. (Cordycipitaceae, Hypocreales), a new pathogen of spider from China. Phytotaxa 527(2): 125–133. 10.11646/phytotaxa.527.2.5 [DOI] [Google Scholar]
- Chen WH, Han YF, Liang JD, Tian WY, Liang ZQ. (2021b) Multi-gene phylogenetic evidence indicates that Pleurodesmospora belongs in Cordycipitaceae (Hypocreales, Hypocreomycetidae) and Pleurodesmosporalepidopterorum sp. nov. on pupa from China. MycoKeys 80: 45–55. 10.3897/mycokeys.80.66794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiriví J, Danies G, Sierra R, Schauer N, Trenkamp S, Restrepo S, Sanjuan T. (2017) Metabolomic profile and nucleoside composition of Cordycepsnidus sp. nov. (Cordycipitaceae): A new source of active compounds. PLoS ONE 12(6): e0179428. 10.1371/journal.pone.0179428 [DOI] [PMC free article] [PubMed]
- Costa PP. (2014) Gibellula spp. associadas a aranhas da Mata do Paraíso, Viçosa-MG (M.Sc.), Minas Gerais, Brazil, Universidade Federal de Viçosaans.
- Crous PW, Wingfield MJ, Lombard L, Roets F, Swart WJ, Alvarado P, Carnegie AJ, Moreno G, Luangsa-ard J, Thangavel R, Alexandrova AV, Baseia IG, Bellanger JM, Bessette AE, Bessette AR, Delapeña-Lastra S, García D, Gené J, Pham THG, Heykoop M, Malysheva E, Malysheva V, Martín MP, Morozova OV, Noisripoom W, Overton BE, Rea AE, Sewall BJ, Smith ME, Smyth CW, Tasanathai K, Visagie CM, Adamík S, Alves A, Andrade JP, Aninat MJ, Araújo RVB, Bordallo JJ, Boufleur T, Baroncelli R, Barreto RW, Bolin J, Cabero J, Cabo M, Cafà G, Caffot MLH, Cai L, Carlavilla JR, Chávez R, Decastro RRL, Delgat L, Deschuyteneer D, Dios MM, Domínguez LS, Evans HC, Eyssartier G, Ferreira BW, Figueiredo CN, Liu F, Fournier J, Galli-Terasawa LV, Gil-Durán C, Glienke C, Gonçalves MFM, Gryta H, Guarro J, Himaman W, Hywel-Jones N, Iturrieta-González I, Ivanushkina NE, Jargeat P, Khalid AN, Khan J, Kiran M, Kiss L, Kochkina GA, Kolaík M, Kubátová A, Lodge DJ, Loizides M, Luque D, Manjón JL, Marbach PAS, Massolajr NS, Mata M, Miller AN, Mongkolsamrit S, Moreau P-A, Morte A, Mujic A, Navarro-Ródenas A, Németh MZ, Nóbrega TF, Nováková A, Olariaga I, Ozerskaya SM, Palma MA, Petters-Vandresen DAL, Piontelli E, Popov ES, Rodríguez A, Requejo Ó, Rodrigues ACM, Rong IH, Roux J, Seifert KA, Silva BDB, Sklená F, Smith JA, Sousa JO, Souza HG, Desouza JT, Vec K, Tanchaud P, Tanney JB, Terasawa F, Thanakitpipattana D, Torres-Garcia D, Vaca I, Vaghefi N, Vaniperen AL, Vasilenko OV, Verbeken A, Yilmaz N, Zamora JC, Zapata M, Jurjević Ž, Groenewald JZ. (2019) Fungal Planet description sheets: 951–1041. Persoonia 43(1): 223–425. 10.3767/persoonia.2019.43.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeleman-Reinhold CL. (2001) Forest spiders of South East Asia: with a revision of the sac and ground spiders (Araneae: Clubionidae, Corinnidae, Liocranidae, Gnaphosidae, Prodidomidae and Trochanterriidae. Brill, Leiden, 591 pp. 10.1163/9789004475588 [DOI] [Google Scholar]
- Eriksson O. (1982) Cordycepsbifusispora spec. nov. Mycotaxon 15: 185–188. [Google Scholar]
- Hall T. (1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hsieh L, Tzean S, Wu WJ. (1997) The Genus Akanthomyces on Spiders from Taiwan. Mycologia 89(2): 319–324. 10.1080/00275514.1997.12026788 [DOI] [Google Scholar]
- Huang B, Wang S, Fan M, Li Z. (2000) Two species of Akanthomyces from Dabieshan mountains. Junwu Xuebao 19: 172–174. [Google Scholar]
- Hywel-Jones NL. (1996) Akanthomyces on spiders in Thailand. Mycological Research 100(9): 1065–1070. 10.1016/S0953-7562(96)80214-0 [DOI] [Google Scholar]
- Johnson D, Sung GH, Hywel-Jones NL, Luangsa-Ard JJ, Bischoff JF, Kepler RM, Spatafora JW. (2009) Systematics and evolution of the genus Torrubiella (Hypocreales, Ascomycota). Mycological Research 113(3): 279–289. 10.1016/j.mycres.2008.09.008 [DOI] [PubMed] [Google Scholar]
- Kepler RM, Sung GH, Ban S, Nakagiri A, Chen MJ, Huang B, Li Z, Spatafora JW. (2012) New teleomorph combinations in the entomopathogenic genus Metacordyceps. Mycologia 104(1): 182–197. 10.3852/11-070 [DOI] [PubMed] [Google Scholar]
- Kepler RM, Luangsa-ard JJ, Hywel-Jones NL, Quandt CA, Sung GH, Rehner SA, Aime MC, Henkel TW, Sanjuan T, Zare R, Chen M, Li Z, Rossman AY, Spatafora JW, Shrestha B. (2017) A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 8(2): 335–353. 10.5598/imafungus.2017.08.02.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayasi Y, Shimizu D. (1977) Some species of Cordyceps and its allies on spiders. Kew Bulletin 31(3): 557–566. 10.2307/4119402 [DOI] [Google Scholar]
- Kobayasi Y, Shimizu D. (1982) Monograph of the genus Torrubiella. Bulletin of the National Science Museum, Tokyo 8: 43–78. [Google Scholar]
- Kuephadungphan W, Macabeo APG, Luangsa-ard JJ, Tasanathai K, Thanakitpipattana D, Phongpaichit S, Yuyama K, Stadler M. (2019) Studies on the biologically active secondary metabolites of the new spider parasitic fungus Gibellulagamsii. Mycological Progress 18(1–2): 135–146. 10.1007/s11557-018-1431-4 [DOI] [Google Scholar]
- Kuephadungphan W, Tasanathai K, Petcharad B, Khonsanit A, Stadler M, Luangsa-ard JJ. (2020) Phylogeny- and morphology-based recognition of new species in the spider-parasitic genus Gibellula (Hypocreales, Cordycipitaceae) from Thailand. MycoKeys 72: 17–42. 10.3897/mycokeys.72.55088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuephadungphan W, Petcharad B, Tasanathai K, Thanakitpipattana D, Kobmoo N, Khonsanit A, Samson RA, Luangsaard JJ. (2022) Multi-locus phylogeny unmasks hidden species within the specialised spider parasitic fungus, Gibellula (Hypocreales, Cordycipitaceae). Studies in Mycology 101(1): 245–286. 10.3114/sim.2022.101.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Whelen S, Hall BD. (1999) Phylogenetic relationships among Ascomycetes: Evidence from an RNA polymerse II subunit. Molecular Biology and Evolution 16(12): 1799–1808. 10.1093/oxfordjournals.molbev.a026092 [DOI] [PubMed] [Google Scholar]
- Luangsa-ard J, Tasanathai K, Thanakitpipattana D, Khonsanit A, Stadler M. (2018) Novel and interesting Ophiocordyceps spp. (Ophiocordycipitaceae, Hypocreales) with superficial perithecia from Thailand. Studies in Mycology 89(1): 125–142. 10.1016/j.simyco.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains EB. (1958) North American Entomogenous Species of Cordyceps. Mycologia 50(2): 169–222. 10.1080/00275514.1958.12024722 [DOI] [Google Scholar]
- Mongkolsamrit S, Luangsa-ard JJ, Spatafora JW, Sung GH, Hywel-Jones NL. (2009) A combined ITS rDNA and beta-tubulin phylogeny of Thai species of Hypocrella with nonfragmenting ascospores. Mycological Research 113(6–7): 684–699. 10.1016/j.mycres.2009.02.004 [DOI] [PubMed] [Google Scholar]
- Mongkolsamrit S, Noisripoom W, Thanakitpipattana D, Wutikhun T, Spatafora JW, Luangsa-ard J. (2018) Disentangling cryptic species with isaria-like morphs in Cordycipitaceae. Mycologia 110(1): 230–257. 10.1080/00275514.2018.1446651 [DOI] [PubMed] [Google Scholar]
- Mongkolsamrit S, Khonsanit A, Thanakitpipattana D, Tasanathai K, Noisripoom W, Lamlertthon S, Himaman W, Houbraken J, Samson RA, Luangsa-ard J. (2020a) Revisiting Metarhizium and the description of new species from Thailand. Studies in Mycology 95: 171–251. 10.1016/j.simyco.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongkolsamrit S, Noisripoom W, Tasanathai K, Khonsanit A, Thanakitpipattana D, Himaman W, Kobmoo N, Luangsa-ard JJ. (2020b) Molecular phylogeny and morphology reveal cryptic species in Blackwellomyces and Cordyceps (Cordycipitaceae) from Thailand. Mycological Progress 19(9): 957–983. 10.1007/s11557-020-01615-2 [DOI] [Google Scholar]
- Mongkolsamrit S, Noisripoom W, Pumiputikul S, Boonlarppradab C, Samson RA, Stadler M, Becker K, Luangsa-ard JJ. (2021) Ophiocordycepsflavida sp. nov. (Ophiocordycipitaceae), a new species from Thailand associated with Pseudogibellulaformicarum (Cordycipitaceae), and their bioactive secondary metabolites. Mycological Progress 20(4): 477–492. 10.1007/s11557-021-01683-y [DOI] [Google Scholar]
- Nylander JAA. (2004) MrModeltest 2.0. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala.
- O’Donnell K, Sarver BA, Brandt M, Chang DC, Noble-Wang J, Park BJ, Sutton DA, Benjamin L, Lindsley M, Padhye A, Geiser DM, Ward TJ. (2007) Phylogenetic diversity and microsphere array-based genotyping of human pathogenic Fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. Journal of Clinical Microbiology 45(7): 2235–2248. 10.1128/JCM.00533-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RD. (1996) TreeView: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357–358. 10.1093/bioinformatics/12.4.357 [DOI] [PubMed] [Google Scholar]
- Petch T. (1944) Notes on entomogenous fungi. Transactions of the British Mycological Society 27(1–2): 81–93. 10.1016/S0007-1536(44)80014-6 [DOI] [Google Scholar]
- Rehner SA, Buckley E. (2005) A beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97(1): 84–98. 10.3852/mycologia.97.1.84 [DOI] [PubMed] [Google Scholar]
- Rehner SA, Samuels GJ. (1994) Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98(6): 625–634. 10.1016/S0953-7562(09)80409-7 [DOI] [Google Scholar]
- Rehner SA, Minnis AM, Sung GH, Luangsa-ard JJ, Devotto L, Humber RA. (2011) Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 103(5): 1055–1073. 10.3852/10-302 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Mark P, Ayres D, Darling A, Höhna S, Larget B, Liu L, Suchard M, Huelsenbeck J. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3): 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal Horticultural Society (RHS) (2015) Colour Chart 6th edn. Royal Horticultural Society: London, UK.
- Samson RA, Brady BL. (1982) Akanthomycesnovoguineensis sp. nov. Transactions of the British Mycological Society 79(3): 571–572. 10.1016/S0007-1536(82)80065-X [DOI] [Google Scholar]
- Samson RA, Evans HC. (1974) Note on entomopathogenous fungi from Ghana. II. The genus Akanthomyces. Acta Botanica Neerlandica 23(1): 28–35. 10.1111/j.1438-8677.1974.tb00913.x [DOI] [Google Scholar]
- Samson RA, Evans HC. (1992) New species of Gibellula on spiders (Araneida) from South America. Mycologia 84(3): 300–314. 10.1080/00275514.1992.12026143 [DOI] [Google Scholar]
- Sanjuan T, Tabima J, Restrepo S, Læssøe T, Spatafora J, Molano A. (2014) Entomopathogens of Amazonian stick insects and locusts are members of the Beauveria species complex (Cordycepssensu stricto). Mycologia 106(2): 260–275. 10.3852/13-020 [DOI] [PubMed] [Google Scholar]
- Shimizu D. (1994) Color iconography of vegetable wasps and plant worms (in Japanese). Seibundo Shinkosha, Tokyo.
- Shrestha B, Tanaka E, Hyun MW, Han JG, Kim CS, Jo JW, Han SK, Oh J, Sung GH. (2016) Coleopteran and Lepidopteran Hosts of the Entomopathogenic Genus Cordycepssensu lato. Journal of Mycology 7648219: 1–14. 10.1155/2016/7648219 [DOI] [Google Scholar]
- Shrestha B, Kubátová A, Tanaka E, Oh J, Yoon DH, Sung JM, Sung GH. (2019) Spider-pathogenic fungi within Hypocreales (Ascomycota): Their current nomenclature, diversity, and distribution. Mycological Progress 18(8): 983–1003. 10.1007/s11557-019-01512-3 [DOI] [Google Scholar]
- Spatafora JW, Sung GH, Sung JM, Hywel-Jones NL, White Jr JF. (2007) Phylogenetic evidence for an animal pathogen origin of ergot and the grass endophytes. Molecular Ecology 16(8): 1701–1711. 10.1111/j.1365-294X.2007.03225.x [DOI] [PubMed] [Google Scholar]
- Stamatakis A. (2014) RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics (Oxford, England) 30(9): 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CH, Wang HH. (1986) Phytocordyceps, a new genus of the Clavicipitaceae. Mycotaxon 26: 337–344. [Google Scholar]
- Sullivan RF, Bills GF, Hywel-Jones NL, White Jr JF. (2000) Hyperdermium: A new clavicipitalean genus for some tropical epibionts of dicotyledonous plants. Mycologia 92(5): 908–918. 10.1080/00275514.2000.12061236 [DOI] [Google Scholar]
- Sun T, Shen Z, Shaukat M, Du C, Ali S. (2020) Endophytic isolates of Cordycepsfumosorosea to enhance the growth of Solanummelongena and reduce the survival of Whitefly (Bemisiatabaci). Insects 11(2): 78. 10.3390/insects11020078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung GH, Spatafora JW. (2004) Cordycepscardinalis sp. nov., a new species of Cordyceps with an east Asian-eastern North American distribution. Mycologia 96(3): 658–666. 10.1080/15572536.2005.11832962 [DOI] [PubMed] [Google Scholar]
- Sung GH, Spatafora JW, Zare R, Hodge KT, Gams W. (2001) A revision of Verticilliumsect.Prostrata. II. Phylogenetic analyses of SSU and LSU nuclear rDNA sequences from anamorphs and teleomorphs of the Clavicipitaceae. Nova Hedwigia 72(3–4): 311–328. 10.1127/nova.hedwigia/72/2001/311 [DOI] [Google Scholar]
- Sung GH, Hywel-Jones NL, Sung JM, Luangsa-ard JJ, Shrestha B, Spatafora JW. (2007) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Studies in Mycology 57: 5–59. 10.3114/sim.2007.57.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasanathai K, Thanakitpipattana D, Noisripoom W, Khonsanit A, Kumsao J, Luangsa-ard JJ. (2016) Two new Cordyceps species from a community forest in Thailand. Mycological Progress 15(3): e28. 10.1007/s11557-016-1170-3 [DOI]
- Thanakitpipattana D, Tasanathai K, Mongkolsamrit S, Khonsanit A, Lamlertthon S, Luangsa-ard J. (2020) Fungal pathogens occurring on Orthopterida in Thailand. Persoonia 44(1): 140–160. 10.3767/persoonia.2020.44.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172(8): 4238–4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu D, Groenewald M, de Vries M, Gehrmann T, Stielow B, Eberhardt U, Al-Hatmi A, Groenewald JZ, Cardinali G, Houbraken J, Boekhout T, Crous PW, Robert V, Verkley GJM. (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92(1): 135–154. 10.1016/j.simyco.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xu J, Wang X, Qiu B, Cuthbertson AGS, Du C, Wu J, Ali S. (2019) Isariafumosorosea-based zero-valent iron nanoparticles affect the growth and survival of sweet potato whitefly, Bemisiatabaci (Gennadius). Pest Management Science 75(8): 2174–2181. 10.1002/ps.5340 [DOI] [PubMed] [Google Scholar]
- Wang YB, Wang Y, Fan Q, Duan DE, Zhang GD, Dai RQ, Dai YD, Zeng WB, Chen ZH, Li DD, Tang DX, Xu ZH, Sun T, Nguyen TT, Tran NL, Dao VM, Zhang CM, Huang LD, Liu YJ, Zhang XM, Yang DR, Sanjuan T, Liu XZ, Yang ZL, Yu H. (2020) Multigene phylogeny of the family Cordycipitaceae (Hypocreales): New taxa and the new systematic position of the Chinese cordycipitoid fungus Paecilomyceshepiali. Fungal Diversity 103(1): 1–46. 10.1007/s13225-020-00457-3 [DOI] [Google Scholar]
- White TJ, Bruns T, Lee S. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ. (Eds) PCR protocols: a guide to methods and applications.Academic Press Inc, New York, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Wu X, Wu T, Huang A, Shen Y, Zhang X, Song W, Wang S, Ruan H. (2021) New insights into the biosynthesis of typical bioactive components in the traditional Chinese medicinal fungus Cordycepsmilitaris. Frontiers in Bioengineering and Biotechnology 9: e801721. 10.3389/fbioe.2021.801721 [DOI] [PMC free article] [PubMed]
- Zare R, Gams W. (2016) More white verticillium-like anamorphs with erect conidiophores. Mycological Progress 15(10–11): 993–1030. 10.1007/s11557-016-1214-8 [DOI] [Google Scholar]
- Zhang ZF, Zhou SY, Eurwilaichitr L, Ingsriswang S, Raza M, Chen Q, Zhao P, Liu F, Cai L. (2020) Culturable mycobiota from Karst caves in China II, with descriptions of 33 new species. Fungal Diversity 106(1): 29–136. 10.1007/s13225-020-00453-7 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S5
Copyright notice: This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Suchada Mongkolsamrit, Wasana Noisripoom, Kanoksri Tasanathai, Noppol Kobmoo, Donnaya Thanakitpipattana, Artit Khonsanit, Booppa Petcharad, Baramee Sakolrak, Winanda Himaman
Data type
Pdf file
Explanation note
RAxML trees of Hevansia, Jenniferia, Parahevansia, Polystromomyces and related genera in the Cordycipitaceae from different molecular markers.