Abstract
Neurodegenerative diseases (NDs) have become a significant threat to an aging human society. Numerous studies have been conducted in the past decades to clarify their pathologic mechanisms and search for reliable biomarkers. Magnetic resonance imaging (MRI) is a powerful tool for investigating structural and functional brain alterations in NDs. With the advantages of being non-invasive and non-radioactive, it has been frequently used in both animal research and large-scale clinical investigations. MRI may serve as a bridge connecting micro- and macro-level analysis and promoting bench-to-bed translational research. Nevertheless, due to the abundance and complexity of MRI techniques, exploiting their potential is not always straightforward. This review aims to briefly introduce research progress in clinical imaging studies and discuss possible strategies for applying MRI in translational ND research.
Keywords: Magnetic resonance imaging, Neurodegenerative disease, Translational research, Alzheimer's disease, Parkinson's disease
Background
The aging of the world population is continuously progressing [1]. Among many diseases of the elderly, neurodegenerative diseases (NDs), represented by Alzheimer's disease (AD) and Parkinson's disease (PD), involve > 50 million older adults [2]. They can cause severe dementia, paralysis, and other consequences, imposing heavy burdens on families and society [3, 4]. At present, the clinical treatment of NDs is still mainly based on symptom management. There is a lack of reliable early diagnostic measures and disease-modifying therapies [5].
The understanding of pathophysiological mechanisms is fundamental to lowering the harmfulness of NDs, and the continuous invention of research tools has brought much progress. Taking AD as an example, since its pathology was first discovered in 1906, AD research has experienced many upsurges such as histopathology, biochemistry, and genetics [6]. With modern neuroscience methods, we have gained a richer and more detailed understanding of AD pathology. The medical imaging revolution that began in the 1990s also brought a new dawn to the research on NDs. Among them, positron emission tomography (PET) and magnetic resonance imaging (MRI) have become the core research methods of many longitudinal multicenter projects due to their non-invasive and multimodal advantages, such as the Alzheimer's Disease Neuroimaging Initiative (http://adni.loni.usc.edu), the Parkinson's Progression Markers Initiative (https://www.ppmi-info.org). Imaging plays a crucial role in revealing the dynamic development of a disease and implicating pathological mechanisms. With progress in various fields, a new question is how to integrate micro-and macro-level research to improve diagnosis and treatment.
Specifically, laboratory studies can reveal structural and functional changes at the level of cells, tissues, and neural circuits, but the translation into clinical applications is often uncertain. On the other hand, medical imaging can reveal the trajectory of macroscopic brain degeneration in aging [7] and NDs [8] that is tightly associated with clinical impairments. Nevertheless, the pathologies behind imaging abnormalities are often complex and need careful explanation. By combining macro- and micro-scale research tools, translation from basic disease mechanism studies to clinical scenarios might be more direct. As nuclear imaging is relatively straightforward and some related reviews have already been published [9], we focus on MRI. For this purpose, we first briefly describe current theories on the pathogenesis of ND and the progress of clinical MRI research, using AD and PD as examples. We have no intention of including all the disease mechanisms and imaging methods but only provide information necessary for related discussions. Further, we will mention several possible strategies and directions for conducting translational research using MRI.
Basic Pathologies and Neural Circuit Changes in NDs
AD pathology is characterized by the presence of amyloid plaques, neuritic plaques, and neurofibrillary tangles. The "amyloid cascade hypothesis" is the mainstream theory [10] regarding the development of pathology in AD; it has been suggested that the increase of amyloid-β (Aβ) is key to triggering tau pathology followed by neuronal death. Under the action of genes, environmental toxins, aging, and other factors, Aβ is gradually deposited in the brain and causes tau protein deposition through mechanisms that are not yet fully understood. Tau protein spreads in the brain through synaptic connections [11, 12], starting from the hippocampus and entorhinal cortex and gradually to the posterior cortex. Cognitive impairment is more strongly associated with tau-related brain damage than the Aβ burden [13]. On the circuitry level, the hippocampal network is most important and has been extensively studied [14, 15]. The default mode network (DMN) [16], which was first discovered in human imaging studies and verified in rodents [17], has been consistently reported to be damaged in AD [18].
Apoptotic cell death of dopamine neurons in the substantia nigra (SN) induced by Lewy body deposition is the core pathological mechanism of PD. According to the traditional Braak pathological staging [19], Lewy body pathology starts from the olfactory bulb, medulla oblongata, and pons. It gradually spreads to the midbrain and limbic system, and eventually to the cortical gray matter. While abundant evidence suggests that the pathological α-synuclein aggregates originates in the peripheral nervous system and spreads retrogradely into the brain, a brain-first pattern is also possible [20]. When the loss of SN dopamine neurons reaches as high as 30% or more, it leads to an imbalance of nigrostriatal circuit function, breaking the balance between direct/indirect motor pathways in the basal ganglia–thalamocortical circuit [21], leading to movement disorders. Dysfunction of the cerebellar circuit is associated with the tremor [22] and freezing of gait [23] in PD.
Cerebrovascular degeneration [24], neuroinflammation [25], and glymphatic dysfunction [26, 27] may contribute to the development of NDs, before or after disease onset. During aging, intracranial blood vessels often develop sclerosis, collagen deposition, stenosis, and other pathologies, which lead to the gradual decline of cerebral blood flow (CBF) [28]. On the other hand, the blood-brain barrier (BBB) becomes weak and its permeability gradually increases, causing leakage of blood cells and harmful substances into the brain parenchyma [29]. Homeostatic imbalance and the invasion of exogenous substances may cause immune responses. Misfolded and aggregated proteins in various NDs can also trigger neuroinflammation, characterized by a reactive morphology of both astrocytes and microglial cells [25]. Chronic neuroinflammation can lead to various forms of brain damage (such as demyelination and axon damage) and accelerates neurodegeneration [30]. Research on the glymphatic system [31] has received extensive interest in recent years. Glymphatic dysfunction not only reduces the rate of brain metabolic waste removal and accelerates the accumulation of pathological proteins but also worsens inflammatory responses and further accelerates ND progression [26, 31].
Pathologies often do not exist in isolation [32]. AD is frequently accompanied by vascular pathologies [33]. A neuropathological study [34] showed that 79.9% of patients diagnosed with AD have cerebrovascular pathologies. In patients with dominantly-inherited AD, the burden of white matter hyperintensity (WMH), an imaging sign considered to be associated with small vessel degeneration, increases 6 years before the expected symptom onset [35]. In patients with PD, one-third of those with cognitive impairment and one-half of those with dementia have elevated AD biomarkers in the cerebrospinal fluid (CSF) [36]. The presence of tau can be as high as 100% in LRRK2 PD [37]. Therefore, increasing studies have begun to make simultaneous measurements on common ND pathologies [38], such as Aβ, tau, TDP-43, α-synuclein, and vascular damage. In this way, we can investigate the interaction of different neuropathologies to understand their contribution to disease progression [39].
Brain MRI Methods
As summarized in Fig. 1, many imaging methods have been used for investigating brain degeneration and aiding the diagnosis of ND. Generally, MRI markers are not disease-specific, but their spatial patterns are closely associated with core pathologies. For example, AD-related brain atrophy is prominent in the hippocampus and medial temporal lobe due to early tau pathology in these regions, while PD patients only demonstrate significant brain atrophy in late stages of the disease. In AD, brain iron deposition is associated with amyloid plaques that extensively involve cortical areas. On the other hand, iron deposition in PD is due to dopamine neuronal death and is most marked in the SN. Choosing methods based on the understanding of disease pathologies can maximize the efficiency of experimental designs.
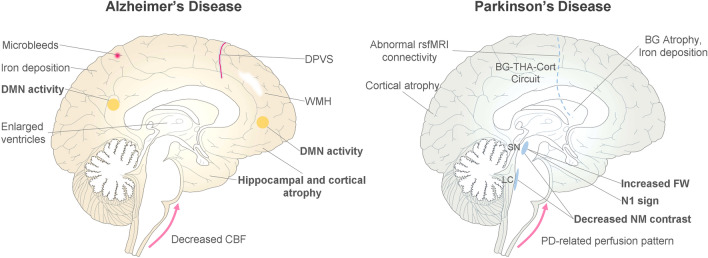
Fig. 1.
Common imaging abnormalities on MR images of AD and PD patients. In AD, brain damage is consistently found in the hippocampus and DMN, with frequent vascular degeneration. In PD patients, imaging abnormalities are more related to the dysfunction of the substantia nigra and BG-THA-Cort circuit. Markers in bold have been extensively tested and have successfully demonstrated clinical value. dPVS: dilated perivascular space; WMH, white matter hyperintensity; DMN, default mode network; CBF, cerebral blood flow; BG, basal ganglia; THA, thalamus; Cort, cortex; N1, nigrosome-1; FW, free water; NM, neuromelanin.
Structural Imaging
Structural MRI has long been used for assessing brain atrophy in NDs. For example, atrophy in the midbrain and pons can differentiate PD from progressive supranuclear palsy [40]. Hippocampal atrophy is the core brain imaging marker of AD. However, it is not a marker with good specificity because many other diseases, such as vascular degeneration, can also lead to hippocampal atrophy [41]. If atrophy in the whole brain is simultaneously considered, the diagnostic accuracy can be much higher. SPARE-AD, a brain atrophy score derived from whole-brain analysis using automated segmentation methods, separates AD from controls with high accuracy [42]. Deep-learning-based methods are excellent for recognizing complex image patterns and may outperform traditional methods [41]. The increase in field strength and imaging resolution has allowed more precise quantification of structural abnormalities. On 7T MRI, the analysis of hippocampal atrophy can be pushed to the subregional level [43], allowing the detection of more subtle and early changes (a background hypothesis is that certain hippocampal subregions are more vulnerable to AD pathology and the hippocampus does not degenerate at a uniform rate).
Different weighting methods can enhance the visualization of various brain structures and lesions. For general purposes such as atrophy assessment, segmentation, and registration, T1-weighted imaging with gray\white matter contrast enhancement is recommended. T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences can demonstrate vascular-related changes, such as WMH and dilation of perivascular spaces (PVSs) commonly seen in older adults. Susceptibility weighted imaging (SWI) can display venopathy [44] and microbleeds, and quantify iron deposition. In AD, iron loading is a prominent feature of activated microglia [45], although how macrophages accumulate, store, and utilize intracellular iron is still not completely understood. In PD, the loss of nigrosome-1 can be visualized on SWI. Appearing as swallowtails or loops, they are valuable markers for PD diagnosis [46, 47].
As brain damage progresses very slowly in NDs, it can take an exceptionally long time for macroscopic changes to occur, and imaging at finer resolution is desired. Restrained by physical rules, the current limit of in vivo human brain imaging is only at the sub-millimeter level. To further understand microscopic changes at the tissue level, the apparent properties of brain tissues within each voxel can be used to infer its composition, which is the basic idea of "Microstructural Imaging" [48]. The typical approach assumes the properties (the most common is water molecule diffusion) of several types of cells and components, sets a mathematical model, and collects data to solve it. Then, disease-related changes at microscopic levels can be inferred. Usually, these models need to be first evaluated on animal brains or ex vivo brains to confirm that the derived parameters well reflect the tissue properties. Despite the fact that that model fitting is never perfect, some parameters have demonstrated very robust associations with clinical manifestations and can be used as valuable biomarkers for ND studies, such as diffusion tensor imaging (DTI) [49], neurite orientation dispersion density imaging [50]), and free water imaging [51].
Functional Imaging
Intuitively, neuronal functions might have already altered before the death of neurons. Therefore, functional imaging is believed to be more sensitive to early brain changes than structural imaging. Blood-oxygen-level dependent (BOLD) imaging is the most widely used functional imaging method. Traditional task-related functional magnetic resonance imaging (fMRI) has good specificity. It can reveal changes in brain activation patterns when patients engage in different tasks, providing information for understanding the degeneration and remodeling of the neural circuits underlying clinical impairments. For example, episodic memory tasks have been used in early AD patients to assess their hippocampal functions [52]. Sensorimotor [53] and executive tasks [54] have been extensively used in PD to demonstrate changes related to dopamine deficiency. Interestingly, functional imaging can be combined with electromyography to reveal brain oscillations associated with resting tremors [55].
Because task-related fMRI requires a high degree of patient cooperation, it is usually difficult to carry out in severe disease conditions and routine clinical practice. The resting-state fMRI does not require patients to perform specific tasks; it detects brain abnormality by measuring fluctuations in the local BOLD signal [56] or associations between different brain regions [57]. The premise that resting-state fMRI can detect disease-related abnormalities is that resting-state activity is associated with the inherent "trait" of disease damage. Alteration of resting-state brain activity in the DMN has been consistently reported in AD patients as well as persons with mild cognitive impairment (MCI) carrying AD pathology [58, 59]. Although the phenomenon is reliable, clinical diagnosis based on resting-state fMRI is difficult due to individual differences in brain recruitment and changes in psychophysical conditions. Since the completion of brain function usually requires the collaborative work of multiple regions, the efficiency of large-scale brain networks is crucial. Functional network analysis based on graph theory is suitable for analyzing complex brain networks by revealing higher-order network properties [60, 61].
Due to the rapidly-changing human brain activity and the inherently low signal-to-noise ratio (SNR) of the BOLD signal, fMRI is often criticized for its low reproducibility. BOLD imaging on 7T MR scanners can achieve a higher SNR and sub-millimeter resolution with customized coils. In recent years, various new noise-reduction methods and multiple comparison correction methods have improved the reliability of fMRI research. In ND research, there is another crucial interference factor in fMRI—neurovascular coupling. Lower blood perfusion and hemodynamic alterations may also change global or regional BOLD signals and mix with signals elicited by neural activity. How to distinguish neuronal from vascular-related alterations has been discussed in some recent reviews and is worthy of further research [62]. Brain atrophy, prominent in the elderly, can exaggerate signal overlap in adjacent cortical gyri. Imaging at higher resolution and the application of surface-based processing methods may alleviate this problem [59].
Vascular and Perfusion Imaging
Aging-related changes in cardiac output, vessel wall pulsatility, and parenchymal resistance can lead to chronic hypoperfusion and accelerate neurodegeneration [63]. General vascular morphometry and plaques can be well displayed with time-of-flight angiography and black-blood vessel wall images. Although narrowing and occlusion of large vessels are not major pathologies of NDs, they can lead to vascular dementia or vascular parkinsonism and need to be considered in clinical practice. Small vessel disease (SVD) is more common in the elderly and dramatically increases with age. Because arterioles and capillaries are too small to be seen on MR images, some indirect imaging signs have been widely used to reflect SVD severity [64], including WMH of presumed vascular origin, lacunae, microbleeds, recent small subcortical infarct, dilated perivascular space (PVS), and superficial siderosis. WMH and cortical microbleeds are quite common in AD patients [65, 66] due to the deposition of Aβ in vessel walls.
The measurement of BBB integrity mainly includes methods based on contrast agents [67] or water diffusion [68]. They assume different water compartments (vascular and brain tissue) in the brain and calculate the exchange rate across the barriers. Increased BBB leakage at the hippocampus has been consistently found in AD [69] and vascular cognitive impairment [70] using dynamic contrast-enhanced (DCE) imaging methods. Because BBB damage in aging and NDs is only mild-to-moderate, it is vital to select the appropriate scanning parameters and mathematical models for accurate measurement [71]. Due to its invasiveness and concerns of Gadolinium deposition in the brain, DCE-MRI is rarely used in large-scale studies. New BBB imaging methods [68, 72] that use labelled water as the tracking agent may be more applicable in community and preclinical cohorts. Nevertheless, BBB alterations measured by water exchange are different from traditional methods. Because water molecules are small, the loss of tight junctions is not necessary for increased permeability. Assessing BBB permeability to substances with different molecular sizes may better reveal disease pathologies [73].
The arterial spin-labelling method can measure blood perfusion in different brain regions without injecting a contrast agent and thus has been widely used in clinical investigations [74, 75]. Notably, aging-related alterations in the vessel wall and brain stiffness can lead to slow blood flow, which may potentially bias blood perfusion measurement. By setting different post-labelling delays and fitting a mathematical model, the arterial arrival time can be calculated, reflecting hemodynamic alterations [76]. Interestingly, hemodynamic changes also influence CSF hydrokinetics associated with brain waste clearance (see below) and further neurodegeneration. Previous studies have shown that AD patients may have significantly reduced CBF in the hippocampus and precuneus [77], while PD patients have increased blood flow in the basal ganglia and decreased blood flow in some cortical regions [78].
Glymphatic Imaging
Although animal studies have consistently found an association between glymphatic dysfunction and ND, validation in humans is rare. Glymphatic clearance can be directly assessed by intrathecal or intravenous injection of Gadolinium contrast agent [79]. After the injection, image acquisition is repeated several times at delays of hours or seconds depending on the purpose. Signal enhancement at different brain sites and cervical lymph nodes are then recorded to reflect the arrival of the contrast agent and the rate of glymphatic clearance [80]. This method is easy to use in animals [81] or human subjects [80], making it a useful tool for translational research. A recent study investigated glymphatic dysfunction in 375 PD patients using this method and found reduced flow through the meningeal lymphatic vessels along the superior sagittal sinus and sigmoid sinus [31]. The foundation of glymphatic clearance is CSF\interstitial fluid motion and exchange, to which MRI is sensitive [82]. Phase‑contrast MRI can detect bulk flow at the midbrain aqueduct and foramen magnum [83], where fluid motion is relatively rapid. Diffusion imaging can reflect slow water molecule motion in the perivascular space [84] and brain parenchymal [85]. Under disease conditions, pathological proteins and cell debris may deposit in PVS and cause compensatory PVS dilation. The dilated PVS can be seen on T2-weighted images and has been widely used as a marker of glymphatic dysfunction. High-resolution imaging and artificial intelligence (AI)-based segmentation methods can better quantify dilated PVS volume and reveal its association with clinical factors [86]. Many new glymphatic imaging techniques are still being developed and have been introduced in some recent reviews [87, 88].
Other Imaging Methods
MRI can also detect brain alterations at the molecular level. Based on the principle that different molecules have different resonant frequencies, MR spectroscopy (MRS) can detect various products of brain metabolism, such as lactate, N-acetylaspartate, glutamine/glutamate. With new imaging sequences, whole-brain MRS could be applied in clinical imaging research, demonstrating distinct spatial patterns of brain metabolites in NDs [89, 90]. Chemical exchange saturation transfer (CEST) imaging can enable the indirect detection of metabolites with exchangeable protons located on proteins. A few attempts have been made to detect proteins associated with different pathological processes in NDs. For example, a significantly lower amide-weighted signal (3.5 ppm) is associated with Aβ deposition in AD mice [91], and lower CEST signals at 1 and 2 ppm are associated with markers of neuroinflammation [92]. Furthermore, ultra-small superparamagnetic iron oxide nanoparticles can be designed to detect specific substances, such as Aβ1–42 peptides in animal models [93], but translation into human subjects may be a long road.
Multimodal, Longitudinal Imaging
As discussed above, the development of human NDs is usually driven by various pathologies with complex interactions. In this regard, multimodal imaging and longitudinal observations are necessary [94]. By analyzing brain alterations from multiple aspects, a whole picture can be put together for inferring underlying pathologies and making clinical decisions. For example, by using quantitative susceptibility imaging to investigate SN degeneration and resting-state fMRI to investigate brain network changes, a link between the primary pathology and downstream mechanisms can be built up [95]. Aging-related WMH lesions, fiber tract damage, and cortical activity can be simultaneously displayed, allowing the understanding of coherent brain structural-functional impairment [96] (Fig. 2). By combining PET with structural MRI, information about pathological molecules and macrostructural changes can be incorporated to understand how AD pathology causes brain atrophy, microbleeds, and clinical impairments [97, 98]. Based on longitudinal data, causality between different pathologies and brain damage can be inferred. However, in clinical research, patients usually cannot hold their heads still during a long scan time. The scan parameters must be optimized to best match the study purpose and patient compliance.
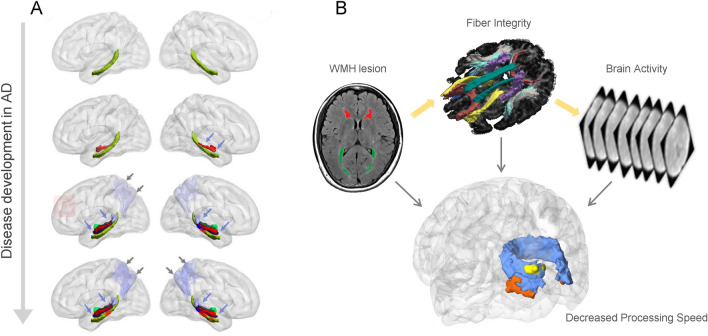
Fig. 2.
Using multimodal imaging to study disease evolution and structural-functional coherent changes. A The severity of AD increases from top to bottom, stratified by AD biomarkers. The green structure represents the cingulum-angular bundle (CAB) tracts. The red segments of the CAB indicate a significant group difference from normal controls. The colorful structures above the green structure represent the hippocampal (HP) subfields. The lavender area in the posterior region represents the precuneus volume. In summary, the degeneration of HP subfields, CAB, and precuneus are aggravated with higher disease stages [94]. B WMH can lead to a slower processing speed in the elderly. By combining information from different modalities, a coherent damaging pattern is seen in the occipital lobe, supporting the WMH–Tract–Function–Behavior link [96].
MRI in Translational Research
Benefiting from its in vivo feature, MRI can be a powerful tool in translational research. With proper validation and interpretation, imaging features can bridge the gap between micro-and macro-level research, or between animal research and clinical application (Fig. 3). On 3T clinical scanners, 0.5–1 mm imaging resolution can be achieved for structural imaging and 2–4 mm for functional imaging. Most MRI methods can be easily implemented in clinical settings, but some are difficult. The success rate of task-fMRI may be low in patients, and molecular imaging methods need extensive preclinical tests before human imaging. Here, we discuss several translational imaging research paradigms for NDs (Fig. 4).
Fig. 3.
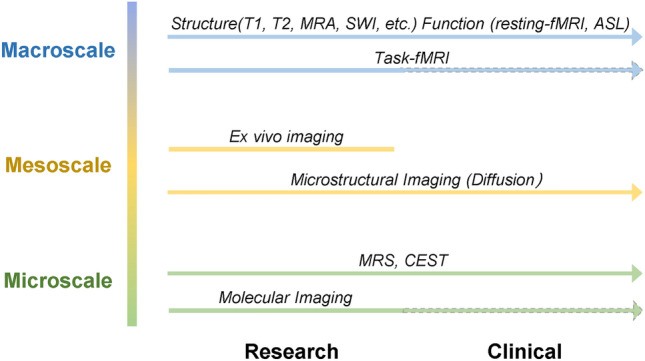
The information that MR imaging techniques provide on different scales and their potential for clinical applications. Task-fMRI may be difficult to implement on patients due to impaired motor or cognitive functions. Molecular imaging may have safety issues and needs extensive pre-clinical studies. MRA, MR angiography; SWI, susceptibility-weighted imaging; ASL, arterial spin labelling; MRS, MR spectroscopy; CEST, chemical exchange saturation transfer.
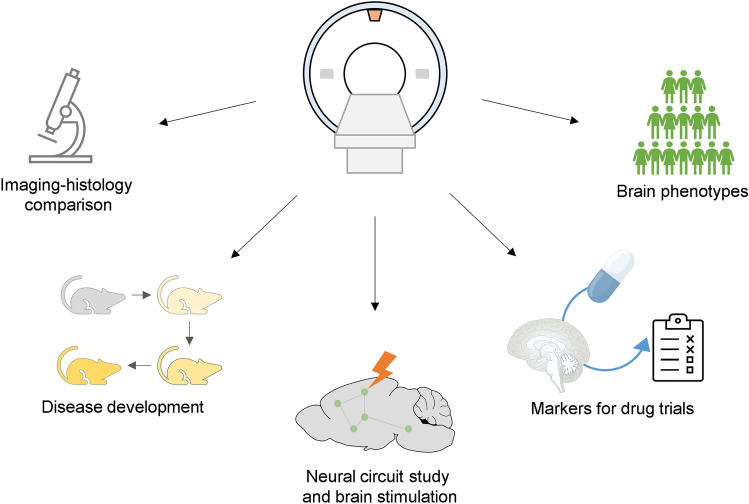
Fig. 4.
Possible applications of MRI in translational ND research.
Imaging-Histology Comparison
While structural imaging is very robust for measuring volumetric and morphometric changes, the restriction of imaging resolution limits the detection of small lesions. For example, microbleeds detected by MRI are ~ten times larger at post-mortem examination [99]. When one microinfarct is seen on MRI, the actual number of such lesions can be substantially higher [100]. Using ultra-high field MRI and optimized scanning parameters could improve detection efficiency. The number of microbleeds [101], microinfarcts [102], dilated PVS [103], and other structures are significantly higher on 7T images than at 3T. Imaging-histology comparison can reveal quantitative differences that can be used when inferring the severity of underlying pathologies in clinical practice. The interpretation of signal changes, common in clinical investigations, may be complex depending on different clinical scenarios. WMH can be induced by increased water content, demyelination, inflammation, or protein deposition [104]. Imaging-histology comparisons in different diseases may show distinct driving factors. By comparing MRI with histological images, the effect of fiber tracts and iron deposition on MR susceptibility can be quantified to guide clinical applications [105].
In some imaging methods that need extensive mathematical modelling (such as diffusion imaging and BBB imaging), the derived imaging parameters are more difficult to interpret. Although DTI has been successfully applied in demonstrating fiber tract changes in various brain diseases, the association between diffusion parameters and specific disease pathologies (such as demyelination and axon damage) is not specific [106]. Furthermore, problems such as fiber crossing [107] and water contamination [108] can lead to substantiate biases. In novel methods aimed at solving these problems, the complexity of the diffusion models increases, and thus simulation and validation of these advanced models are vital. Imaging-histology comparison can provide effective validation [109].
Although the correspondence between MR imaging features and true pathologies is not always simple and straightforward, clarifying their associations can be of tremendous help for understanding disease mechanisms and improving clinical practice. Due to the complexity of diseases, many unique imaging abnormalities are consistently discovered without knowing the specific pathologies [110, 111]. For example, inferior frontal sulcal hyperintensity observed on FLAIR images is supposed to be associated with glymphatic dysfunction [110], because the inferior frontal sulci are just superior to the cribriform plate where some CSF drains to the nasal lymphatics, but the actual pathology remains unknown. Furthermore, lesion patterns and spatial features can provide key information for inferring pathology [112]. The topological connection between microbleeds and venules observed on 7T suggest a contribution of disrupted BBB in venules to microbleeds [112]. The fact that incident lacunae are preferentially localized to the edge of WMH indicates hypoperfusion around WMH lesions [113].
Dynamic Imaging in Animal Models
Through dynamic imaging, association and causalities among different disease pathologies and macroscopic brain changes can be better demonstrated. For example, the association between β-amyloid deposition and decreased CBF can be shown by longitudinal PET\MR imaging in amyloid precursor protein transgenic mouse models [114]. This is of value for revealing early brain degeneration, which is difficult to understand in patients due to late diagnosis. Studies using MRI to visualize the pathophysiology in animal models of amyloidosis have been well summarized in a recent review [115]. In vivo imaging is vital for studying certain pathophysiological processes that are best studied in living animals, such as brain activation or glymphatic function. Because waste clearance is a dynamic process and depends on fluid flow and exchange driven by physiological processes such as respiration and cardiac pulsatility, it needs in vivo observations. Even the structure of the PVS (the major route for waste clearance) may shrink and cannot be accurately measured in post-mortem studies due to fixation methods [116]. By injecting a contrast agent into the ventricles and carrying out continuous MRI scans, researchers can analyze the enhancement in various brain regions at different time points, so the path and speed of waste clearance can be inferred [80]. Using this method, researchers have demonstrated the influences of hypertension [117] and diabetes [81] on glymphatic clearance, and the association between waste clearance and AD pathology [118]. Importantly, this method can also be implemented in human subjects. After intrathecal or intravenous injection of contrast agent, the effects of aging [79], sleep deprivation [119] and NDs [31] on waste clearance have been investigated with great interest.
Neural Circuit Study and Brain Stimulation
MRI is useful for studying neural circuitry. Large fiber bundles can be tracked and visualized with good reliability, especially using advanced models [120]. Thin fiber tracts connecting smaller brain structures can also be analyzed with higher imaging resolution and probabilistic tractography methods [121]. Coordination among different cortical regions and subcortical nuclei can be investigated using functional connectivity analysis based on fMRI. Although not as precise as electrophysiological recordings, since these methods can be used in both animals and humans, they can facilitate comparison and translation across species [122]. With an ultra-high magnetic field and fast imaging acquisition methods, the temporal resolution can be pushed down to 100 ms [123] with full brain coverage, and the spatial resolution can achieve a sub-millimeter level [124]. Unfortunately, these limits cannot be reached at the same time. Choices for spatial and temporal resolution must be made to best match research goals.
These advantages can aid translational research on developing new strategies for deep brain stimulation (DBS). As a useful treatment method for late-stage PD, the optimization of DBS targets and stimulating frequencies have first been performed on rodents [125] and large animals [126]. The modulating effect is well demonstrated by using fMRI to monitor the brain activation elicited. With this knowledge, structural and functional imaging are performed before DBS surgery to predict the treatment effect [127, 128] and guide electrode placement [129]. In recent years, non-invasive brain stimulation has shown good efficacy in treating NDs. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex can improve cognition both in MCI and AD [130]. Stimulation of the motor cortex can improve motor functions in PD. Circuit mapping using structural and functional MR can provide a precise target for rTMS and enhance its therapeutic efficacy [131, 132]. New brain stimulation techniques such as transcranial focused ultrasound can target much deeper brain regions [133], and the activation-stimulation research paradigms have tremendous potential for ND treatment.
MRI in Drug Trials
In drug development, medical imaging can be helpful during participant selection and the evaluation of effectiveness. Due to the long disease course and irreversible neuronal damage, clinical trials on NDs usually need to be performed in early-stage patients to best demonstrate their protective effect. Confirming disease risks by evaluating amyloid deposition and neurodegeneration during patient enrollment can reduce the required sample sizes of AD clinical trials by 45% to 60% [134]. At the same time, the effect of drug treatment and neurodegeneration during a short research period might be too mild to be demonstrated by clinical assessments, and confounded by subjective evaluation biases and the patients' psychophysical conditions. Therefore, many clinical trials have adopted objective imaging parameters as secondary outcomes [135]. In studies on small vessel degeneration, WMH and diffusion imaging parameters [135] have already become essential assessment tools in clinical trials [136, 137]. By applying these markers, the sample size required for a 3-year clinical trial on SVD was dramatically reduced [138] compared to measuring cognitive functions (124 versus 6135 subjects). In PD, SN free water measurements have achieved an effect size similar to nuclear imaging methods in reflecting longitudinal SN degeneration [139], and has been used to assess whether specific drugs have a protective effect [140]. Using neuroimaging markers, the cost of large-scale clinical trials may be significantly reduced. MRI can help monitor and control adverse effects in clinical trials. Amyloid-related imaging abnormalities (ARIA), including ARIA-E (with effusion or edema) and ARIA-H (hemosiderin deposits), have been found in patients taking drugs aimed at removing amyloid plaques from the brain [141]. Evaluating the severity of SVD at baseline could also help to control ARIA.
Brain Phenotype Studies
Brain degeneration and NDs are associated with countless demographic, genetic, environmental, and lifestyle factors that are impossible to simulate in experimental conditions. Large-scale population studies using the non-invasive, non-radiative MRI methods provide opportunities for revealing weak associations and promoting the discovery of new theories. For example, the UK Biobank project plans to collect multimodal brain images, as well as phenotypic and genetic information of 100,000 people. This provides a foundation for understanding how some basic features of the human brain are affected by various risk factors. Genetic-Brain imaging association studies in over 8000 subjects revealed 21 genes [142] important for iron metabolism, axon growth, and brain plasticity. A study focused on aging found heterogeneous brain degeneration patterns showing distinct functional and structural brain changes, which were associated with different genetics, lifestyle, cognition, physical measures, and diseases [143]. Similarly, the association between brain degeneration and genetic variations has been explored in specific disease cohorts [144, 145]. Further research into the genetic and biochemical mechanisms behind these superficial associations may provide new knowledge regarding brain degeneration and disease treatment. In another way, the effect of modulating the related pathways can be easily assessed on animals as they are originally derived from imaging studies.
Investigating brain phenotypes based on large imaging datasets is vital for understanding disease heterogeneity [146, 147] and choosing therapeutic strategies [148]. For example, PD patients may exhibit tremor-dominant or rigidity-dominant motor symptoms, with or without cognitive impairment. Such heterogeneity cannot be fully explained by overall pathology burdens but is ascribed to individual variations in brain organization, regional vulnerability to disease pathology, and diversity of pathology. Brain imaging is powerful for revealing different phenotypes of brain degeneration. Based on brain atrophy patterns, two PD biotypes have been discovered that have distinct clinical symptoms and disease progression rates [149]. Regional radiomics similarity networks derived from structural images have been used to screen MCI subjects with a higher probability of dementia progression [150]. Furthermore, the structural connections of white matter reflect differences in individual brain organization or functional network properties [151, 152]. Compared to subtyping based on clinical features, imaging phenotypes are more objective and stable, but they still need extensive external validation [153]. It is better to consider both disease-specific and subtype-specific features to avoid non-related trivial clustering results when dealing with heterogeneity. For example, a depression-related or cognition-related brain pattern may not be specific to PD patients and could not add additional clinical value.
Future Directions
MRI techniques are still undergoing rapid development. 7T MR scanners with clinical modes have been installed in many universities and hospitals, and several 11.7T scanners are being tested at some research centers. Recently, a 5T imaging system has been announced, which could be more versatile in clinical imaging research. In high-resolution imaging, the impact of head movement is more serious, and the use of prospective head movement correction methods can help achieve more accurate in vivo structural imaging [154]. With increased spatial and temporal resolution, MRI can be used to visualize smaller brain structures, helping detect more covert disease-related brain changes and connect with fundamental research at the tissue and circuit levels. Due to limited accessibility, clinical research using ultra-high-field MR scanners has been mainly performed in small samples. Large cohort studies and clinical applications would be much easier with more facilities installed at clinical sites.
AI may play a crucial role in this new era. With the help of deep-learning-based reconstruction algorithms, the scanning time of multimodal imaging can be significantly reduced [155]. Imaging at higher spatial and temporal resolution, the amount of information to be processed has also increased exponentially. AI-based detection and segmentation algorithms can be very good at identifying brain lesions and providing quantitative measures [156–158], and they also have great potential in diagnosis and prognosis. However, due to limited imaging modalities, most previous studies were focused on using T1 structural images to build classification models. Future studies should consider incorporating other imaging features, such as brain susceptibility in PD and SVD features in AD. Notably, with more complex models, cross-validation in large and multicenter datasets is necessary to validate their generalizability [153]. Data harmonization based on specifically-designed phantoms or post-processing methods is expected to remove inter-vendor variability and improve data consistency [159].
Due to the difficulty in early diagnosis, disease evolution and brain degeneration in the early stages of NDs remain largely unknown. Constructing prodromal cohorts at high risk for specific NDs and performing longitudinal imaging observations are necessary for revealing the trajectories of brain degeneration and discovering diagnostic markers [160]. Multi-scale translational research on animal models could help clarify the association between macro- and micro-level changes and describe the causal interactions among ND pathologies, brain phenotypes, and functional impairments in different stages of disease. With new imaging techniques and clinical imaging discoveries continuously validated, MRI will be more powerful for translational ND research in the future.
Acknowledgements
This review was supported by the National Natural Science Foundation of China (81971577 and 81771820), the Natural Science Foundation of Zhejiang Province (LSZ19H180001), and the 13th Five-year Plan for National Key Research and Development Program of China (2016YFC1306600).
Contributor Information
Peiyu Huang, Email: huangpy@zju.edu.cn.
Minming Zhang, Email: zhangminming@zju.edu.cn.
References
- 1.Population Division, United Nations. World population prospects 2019. UN, 2019.
- 2.Collaborators G2N Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia JP, Wei CB, Chen SQ, Li FY, Tang Y, Qin W, et al. The cost of Alzheimer's disease in China and re-estimation of costs worldwide. Alzheimers Dement. 2018;14:483–491. doi: 10.1016/j.jalz.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Yang WY, Hamilton JL, Kopil C, Beck JC, Tanner CM, Albin RL, et al. Current and projected future economic burden of Parkinson's disease in the US. NPJ Parkinsons Dis. 2020;6:15. doi: 10.1038/s41531-020-0117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang AE. Clinical trials of disease-modifying therapies for neurodegenerative diseases: The challenges and the future. Nat Med. 2010;16:1223–1226. doi: 10.1038/nm.2220. [DOI] [PubMed] [Google Scholar]
- 6.Hardy J. A hundred years of Alzheimer's disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Vinke EJ, de Groot M, Venkatraghavan V, Klein S, Niessen WJ, Ikram MA, et al. Trajectories of imaging markers in brain aging: The Rotterdam Study. Neurobiol Aging. 2018;71:32–40. doi: 10.1016/j.neurobiolaging.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Hansson O. Biomarkers for neurodegenerative diseases. Nat Med. 2021;27:954–963. doi: 10.1038/s41591-021-01382-x. [DOI] [PubMed] [Google Scholar]
- 9.Drzezga A, Barthel H, Minoshima S, Sabri O. Potential clinical applications of PET/MR imaging in neurodegenerative diseases. J Nucl Med. 2014;55:47S–55S. doi: 10.2967/jnumed.113.129254. [DOI] [PubMed] [Google Scholar]
- 10.Korczyn AD. The amyloid cascade hypothesis. Alzheimer's Dement. 2008;4:176–178. doi: 10.1016/j.jalz.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. 2016;19:1085–1092. doi: 10.1038/nn.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs HIL, Hedden T, Schultz AP, Sepulcre J, Perea RD, Amariglio RE, et al. Structural tract alterations predict downstream tau accumulation in amyloid-positive older individuals. Nat Neurosci. 2018;21:424–431. doi: 10.1038/s41593-018-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pontecorvo MJ, Devous MD, Sr, Navitsky M, Lu M, Salloway S, Schaerf FW, et al. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain. 2017;140:748–763. doi: 10.1093/brain/aww334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dautricourt S, de Flores R, Landeau B, Poisnel G, Vanhoutte M, Delcroix N, et al. Longitudinal changes in hippocampal network connectivity in Alzheimer's disease. Ann Neurol. 2021;90:391–406. doi: 10.1002/ana.26168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zott B, Busche MA, Sperling RA, Konnerth A. What happens with the circuit in Alzheimer's disease in mice and humans? Annu Rev Neurosci. 2018;41:277–297. doi: 10.1146/annurev-neuro-080317-061725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raichle ME. The brain's default mode network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 17.Lu HB, Zou QH, Gu H, Raichle ME, Stein EA, Yang YH. Rat brains also have a default mode network. PNAS. 2012;109:3979–3984. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherr M, Utz L, Tahmasian M, Pasquini L, Grothe MJ, Rauschecker JP, et al. Effective connectivity in the default mode network is distinctively disrupted in Alzheimer's disease-A simultaneous resting-state FDG-PET/fMRI study. Hum Brain Mapp. 2021;42:4134–4143. doi: 10.1002/hbm.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkes CH, del Tredici K, Braak H. A timeline for Parkinson's disease. Park Relat Disord. 2010;16:79–84. doi: 10.1016/j.parkreldis.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Borghammer P, van den Berge N. Brain-first versus gut-first Parkinson's disease: A hypothesis. J Parkinsons Dis. 2019;9:S281–S295. doi: 10.3233/JPD-191721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGregor MM, Nelson AB. Circuit mechanisms of Parkinson's disease. Neuron. 2019;101:1042–1056. doi: 10.1016/j.neuron.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Helmich RC, van den Berg KRE, Panyakaew P, Cho HJ, Osterholt T, McGurrin P, et al. Cerebello-cortical control of tremor rhythm and amplitude in Parkinson's disease. Mov Disord. 2021;36:1727–1729. doi: 10.1002/mds.28603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fasano A, Laganiere SE, Lam S, Fox MD. Lesions causing freezing of gait localize to a cerebellar functional network. Ann Neurol. 2017;81:129–141. doi: 10.1002/ana.24845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, et al. Cerebrovascular atherosclerosis correlates with alzheimer pathology in neurodegenerative dementias. Brain. 2012;135:3749–3756. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 26.Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370:50–56. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou WY, Pu TL, Feng WX, Lu M, Zheng Y, Du RH, et al. Blocking meningeal lymphatic drainage aggravates Parkinson's disease-like pathology in mice overexpressing mutated α-synuclein. Transl Neurodegener. 2019;8:7. doi: 10.1186/s40035-019-0147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoquart-Elsankari S, Balédent O, Gondry-Jouet C, Makki M, Godefroy O, Meyer ME. Aging effects on cerebral blood and cerebrospinal fluid flows. J Cereb Blood Flow Metab. 2007;27:1563–1572. doi: 10.1038/sj.jcbfm.9600462. [DOI] [PubMed] [Google Scholar]
- 29.Banks WA, Reed MJ, Logsdon AF, Rhea EM, Erickson MA. Healthy aging and the blood-brain barrier. Nat Aging. 2021;1:243–254. doi: 10.1038/s43587-021-00043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leng FD, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat Rev Neurol. 2021;17:157–172. doi: 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- 31.Ding XB, Wang XX, Xia DH, Liu H, Tian HY, Fu Y, et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson's disease. Nat Med. 2021;27:411–418. doi: 10.1038/s41591-020-01198-1. [DOI] [PubMed] [Google Scholar]
- 32.Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141:2181–2193. doi: 10.1093/brain/awy146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hachinski V, Einhäupl K, Ganten D, Alladi S, Brayne C, Stephan BCM, et al. Preventing dementia by preventing stroke: The Berlin Manifesto. Alzheimers Dement. 2019;15:961–984. doi: 10.1016/j.jalz.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain. 2013;136:2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S, Viqar F, Zimmerman ME, Narkhede A, Tosto G, Benzinger TLS, et al. White matter hyperintensities are a core feature of Alzheimer's disease: Evidence from the dominantly inherited Alzheimer network. Ann Neurol. 2016;79:929–939. doi: 10.1002/ana.24647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montine TJ, Shi M, Quinn JF, Peskind ER, Craft S, Ginghina C, et al. CSF Aβ42and tau in Parkinson's disease with cognitive impairment. Mov Disord. 2010;25:2682–2685. doi: 10.1002/mds.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henderson MX, Sengupta M, Trojanowski JQ, Lee VMY. Alzheimer's disease tau is a prominent pathology in LRRK2 Parkinson's disease. Acta Neuropathol Commun. 2019;7:183. doi: 10.1186/s40478-019-0836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oveisgharan S, Yu L, Poole VN, Evia AM, Barnes LL, Schneider JA, et al. Association of white matter hyperintensities with pathology and progression of Parkinsonism in aging. JAMA Neurol. 2021;78:1494–1502. doi: 10.1001/jamaneurol.2021.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bassil F, Brown HJ, Pattabhiraman S, Iwasyk JE, Maghames CM, Meymand ES, et al. Amyloid-beta (aβ) plaques promote seeding and spreading of alpha-synuclein and tau in a mouse model of lewy body disorders with aβ pathology. Neuron. 2020;105:260–275.e6. doi: 10.1016/j.neuron.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massey LA, Jäger HR, Paviour DC, O'Sullivan SS, Ling H, Williams DR, et al. The midbrain to Pons ratio: A simple and specific MRI sign of progressive supranuclear palsy. Neurology. 2013;80:1856–1861. doi: 10.1212/WNL.0b013e318292a2d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng XY, Provenzano FA, Small SA, Initiative ADN. A deep learning MRI approach outperforms other biomarkers of prodromal Alzheimer's disease. Alzheimers Res Ther. 2022;14:45. doi: 10.1186/s13195-022-00985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davatzikos C, Xu F, An Y, Fan Y, Resnick SM. Longitudinal progression of Alzheimer's-like patterns of atrophy in normal older adults: The SPARE-AD index. Brain. 2009;132:2026–2035. doi: 10.1093/brain/awp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanken AE, Hurtz S, Zarow C, Biado K, Honarpisheh H, Somme J, et al. Associations between hippocampal morphometry and neuropathologic markers of Alzheimer's disease using 7 T MRI. Neuroimage Clin. 2017;15:56–61. doi: 10.1016/j.nicl.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang RT, Huang PY, Jiaerken Y, Wang SY, Hong H, Luo X, et al. Venous disruption affects white matter integrity through increased interstitial fluid in cerebral small vessel disease. J Cereb Blood Flow Metab. 2021;41:157–165. doi: 10.1177/0271678X20904840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kenkhuis B, Somarakis A, de Haan L, Dzyubachyk O, Ijsselsteijn ME, de Miranda NFCC, et al. Iron loading is a prominent feature of activated microglia in Alzheimer's disease patients. Acta Neuropathol Commun. 2021;9:27. doi: 10.1186/s40478-021-01126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng ZH, He NY, Huang P, Li Y, Tang RB, Sethi SK, et al. Imaging the Nigrosome 1 in the substantia nigra using susceptibility weighted imaging and quantitative susceptibility mapping: An application to Parkinson's disease. Neuroimage Clin. 2020;25:102103. doi: 10.1016/j.nicl.2019.102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasuhn J, Neumann A, Strautz R, Dreischmeier S, Lemmer F, Hanssen H, et al. Clinical MR imaging in Parkinson's disease: How useful is the swallow tail sign? Brain Behav. 2021;11:e02202. doi: 10.1002/brb3.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones DK, Alexander DC, Bowtell R, Cercignani M, Dell'Acqua F, McHugh DJ, et al. Microstructural imaging of the human brain with a 'super-scanner': 10 key advantages of ultra-strong gradients for diffusion MRI. NeuroImage. 2018;182:8–38. doi: 10.1016/j.neuroimage.2018.05.047. [DOI] [PubMed] [Google Scholar]
- 49.Teipel SJ, Wegrzyn M, Meindl T, Frisoni G, Bokde ALW, Fellgiebel A, et al. Anatomical MRI and DTI in the diagnosis of Alzheimer's disease: A European multicenter study. J Alzheimers Dis. 2012;31:S33–S47. doi: 10.3233/JAD-2012-112118. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 2012;61:1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- 51.Hoy AR, Koay CG, Kecskemeti SR, Alexander AL. Optimization of a free water elimination two-compartment model for diffusion tensor imaging. NeuroImage. 2014;103:323–333. doi: 10.1016/j.neuroimage.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clément F, Belleville S. Test-retest reliability of fMRI verbal episodic memory paradigms in healthy older adults and in persons with mild cognitive impairment. Hum Brain Mapp. 2009;30:4033–4047. doi: 10.1002/hbm.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wurster CD, Graf H, Ackermann H, Groth K, Kassubek J, Riecker A. Neural correlates of rate-dependent finger-tapping in Parkinson's disease. Brain Struct Funct. 2015;220:1637–1648. doi: 10.1007/s00429-014-0749-1. [DOI] [PubMed] [Google Scholar]
- 54.Cameron IGM, Pari G, Alahyane N, Brien DC, Coe BC, Stroman PW, et al. Impaired executive function signals in motor brain regions in Parkinson's disease. NeuroImage. 2012;60:1156–1170. doi: 10.1016/j.neuroimage.2012.01.057. [DOI] [PubMed] [Google Scholar]
- 55.Dirkx MF, den Ouden H, Aarts E, Timmer M, Bloem BR, Toni I, et al. The cerebral network of Parkinson's tremor: An effective connectivity fMRI study. J Neurosci. 2016;36:5362–5372. doi: 10.1523/JNEUROSCI.3634-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang H, Long XY, Yang YH, Yan H, Zhu CZ, Zhou XP, et al. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. NeuroImage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 57.Smitha KA, Akhil Raja K, Arun KM, Rajesh PG, Thomas B, Kapilamoorthy TR, et al. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. Neuroradiol J. 2017;30:305–317. doi: 10.1177/1971400917697342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mevel K, Chételat G, Eustache F, Desgranges B. The default mode network in healthy aging and Alzheimer's disease. Int J Alzheimer's Dis. 2011;2011:1–9. doi: 10.4061/2011/535816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu ZL, Zeng QZ, Kong LH, Luo X, Li KC, Xu XP, et al. Altered spontaneous brain activity in subjects with different cognitive states of biologically defined Alzheimer's disease: A surface-based functional brain imaging study. Front Aging Neurosci. 2021;13:683783. doi: 10.3389/fnagi.2021.683783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brier MR, Thomas JB, Fagan AM, Hassenstab J, Holtzman DM, Benzinger TL, et al. Functional connectivity and graph theory in preclinical Alzheimer's disease. Neurobiol Aging. 2014;35:757–768. doi: 10.1016/j.neurobiolaging.2013.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 62.Tsvetanov KA, Henson RNA, Rowe JB. Separating vascular and neuronal effects of age on fMRI BOLD signals. Philos Trans R Soc Lond B Biol Sci. 2021;376:20190631. doi: 10.1098/rstb.2019.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, et al. Vascular dysfunction-The disregarded partner of Alzheimer's disease. Alzheimers Dement. 2019;15:158–167. doi: 10.1016/j.jalz.2018.07.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kester MI, Goos JDC, Teunissen CE, Benedictus MR, Bouwman FH, Wattjes MP, et al. Associations between cerebral small-vessel disease and Alzheimer disease pathology as measured by cerebrospinal fluid biomarkers. JAMA Neurol. 2014;71:855–862. doi: 10.1001/jamaneurol.2014.754. [DOI] [PubMed] [Google Scholar]
- 66.Provenzano FA, Muraskin J, Tosto G, Narkhede A, Wasserman BT, Griffith EY, et al. White matter hyperintensities and cerebral amyloidosis: Necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol. 2013;70:455–461. doi: 10.1001/jamaneurol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang CE, Wong SM, van de Haar HJ, Staals J, Jansen JFA, Jeukens CRLPN, et al. Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology. 2017;88:426–432. doi: 10.1212/WNL.0000000000003556. [DOI] [PubMed] [Google Scholar]
- 68.Wang JJ, Fernández-Seara MA, Wang SM, St Lawrence KS. When perfusion meets diffusion: in vivo measurement of water permeability in human brain. J Cereb Blood Flow Metab. 2007;27:839–849. doi: 10.1038/sj.jcbfm.9600398. [DOI] [PubMed] [Google Scholar]
- 69.van de Haar HJ, Burgmans S, Jansen JFA, van Osch MJP, van Buchem MA, Muller M, et al. Blood-brain barrier leakage in patients with early alzheimer disease. Radiology. 2016;281:527–535. doi: 10.1148/radiol.2016152244. [DOI] [PubMed] [Google Scholar]
- 70.Wardlaw JM, Makin SJ, Valdés Hernández MC, Armitage PA, Heye AK, Chappell FM, et al. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: Evidence from a cohort study. Alzheimer's Dement. 2017;13:634–643. doi: 10.1016/j.jalz.2016.09.006. [DOI] [Google Scholar]
- 71.Heye AK, Thrippleton MJ, Armitage PA, Valdés Hernández M, Makin SD, Glatz A, et al. Tracer kinetic modelling for DCE-MRI quantification of subtle blood-brain barrier permeability. NeuroImage. 2016;125:446–455. doi: 10.1016/j.neuroimage.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin ZX, Li Y, Su P, Mao D, Wei ZL, Pillai JJ, et al. Non-contrast MR imaging of blood-brain barrier permeability to water. Magn Reson Med. 2018;80:1507–1520. doi: 10.1002/mrm.27141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin ZX, Sur S, Liu PY, Li Y, Jiang DR, Hou XR, et al. Blood-brain barrier breakdown in relationship to alzheimer and vascular disease. Ann Neurol. 2021;90:227–238. doi: 10.1002/ana.26134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Z, Das SR, Xie SX, Arnold SE, Detre JA, Wolk DA, et al. Arterial spin labeled MRI in prodromal Alzheimer's disease: A multi-site study. Neuroimage Clin. 2013;2:630–636. doi: 10.1016/j.nicl.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rane S, Koh N, Oakley J, Caso C, Zabetian CP, Cholerton B, et al. Arterial spin labeling detects perfusion patterns related to motor symptoms in Parkinson's disease. Parkinsonism Relat Disord. 2020;76:21–28. doi: 10.1016/j.parkreldis.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al-Bachari S, Parkes LM, Vidyasagar R, Hanby MF, Tharaken V, Leroi I, et al. Arterial spin labelling reveals prolonged arterial arrival time in idiopathic Parkinson's disease. Neuroimage Clin. 2014;6:1–8. doi: 10.1016/j.nicl.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Camargo A, Wang Z, Initiative ADN. Longitudinal cerebral blood flow changes in normal aging and the Alzheimer's disease continuum identified by arterial spin labeling MRI. J Alzheimers Dis. 2021;81:1727–1735. doi: 10.3233/JAD-210116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma YL, Huang CR, Dyke JP, Pan H, Alsop D, Feigin A, et al. Parkinson's disease spatial covariance pattern: Noninvasive quantification with perfusion MRI. J Cereb Blood Flow Metab. 2010;30:505–509. doi: 10.1038/jcbfm.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou Y, Cai JS, Zhang WH, Gong XX, Yan SQ, Zhang KM, et al. Impairment of the glymphatic pathway and putative meningeal lymphatic vessels in the aging human. Ann Neurol. 2020;87:357–369. doi: 10.1002/ana.25670. [DOI] [PubMed] [Google Scholar]
- 80.Ringstad G, Eide PK. Cerebrospinal fluid tracer efflux to parasagittal Dura in humans. Nat Commun. 2020;11:354. doi: 10.1038/s41467-019-14195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang Q, Zhang L, Ding GL, Davoodi-Bojd E, Li QJ, Li L, et al. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. 2017;37:1326–1337. doi: 10.1177/0271678X16654702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eide PK, Valnes LM, Lindstrøm EK, Mardal KA, Ringstad G. Direction and magnitude of cerebrospinal fluid flow vary substantially across central nervous system diseases. Fluids Barriers CNS. 2021;18:16. doi: 10.1186/s12987-021-00251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamada S, Ishikawa M, Ito H, Yamamoto K, Yamaguchi M, Oshima M, et al. Cerebrospinal fluid dynamics in idiopathic normal pressure hydrocephalus on four-dimensional flow imaging. Eur Radiol. 2020;30:4454–4465. doi: 10.1007/s00330-020-06825-6. [DOI] [PubMed] [Google Scholar]
- 84.Harrison IF, Siow B, Akilo AB, Evans PG, Ismail O, Ohene Y, et al. Non-invasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with diffusion tensor MRI. eLife. 2018;7:e34028. doi: 10.7554/eLife.34028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang WH, Zhou Y, Wang JN, Gong XX, Chen ZC, Zhang XT, et al. Glymphatic clearance function in patients with cerebral small vessel disease. NeuroImage. 2021;238:118257. doi: 10.1016/j.neuroimage.2021.118257. [DOI] [PubMed] [Google Scholar]
- 86.Wang SY, Huang PY, Zhang RT, Hong H, Jiaerken Y, Lian CF, et al. Quantity and morphology of perivascular spaces: Associations with vascular risk factors and cerebral small vessel disease. J Magn Reson Imag. 2021;54:1326–1336. doi: 10.1002/jmri.27702. [DOI] [PubMed] [Google Scholar]
- 87.Klostranec JM, Vucevic D, Bhatia KD, Kortman HGJ, Krings T, Murphy KP, et al. Current concepts in intracranial interstitial fluid transport and the glymphatic system: Part II-imaging techniques and clinical applications. Radiology. 2021;301:516–532. doi: 10.1148/radiol.2021204088. [DOI] [PubMed] [Google Scholar]
- 88.Klostranec JM, Vucevic D, Bhatia KD, Kortman HGJ, Krings T, Murphy KP, et al. Current concepts in intracranial interstitial fluid transport and the glymphatic system: Part I-anatomy and physiology. Radiology. 2021;301:502–514. doi: 10.1148/radiol.2021202043. [DOI] [PubMed] [Google Scholar]
- 89.Su L, Blamire AM, Watson R, He J, Hayes L, O'Brien JT. Whole-brain patterns of (1)H-magnetic resonance spectroscopy imaging in Alzheimer's disease and dementia with Lewy bodies. Transl Psychiatry. 2016;6:e877. doi: 10.1038/tp.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mazuel L, Chassain C, Jean B, Pereira B, Cladière A, Speziale C, et al. Proton MR spectroscopy for diagnosis and evaluation of treatment efficacy in parkinson disease. Radiology. 2016;278:505–513. doi: 10.1148/radiol.2015142764. [DOI] [PubMed] [Google Scholar]
- 91.Huang JP, Lai JHC, Tse KH, Cheng GWY, Liu Y, Chen ZL, et al. Deep neural network based CEST and AREX processing: Application in imaging a model of Alzheimer's disease at 3 T. Magn Reson Med. 2022;87:1529–1545. doi: 10.1002/mrm.29044. [DOI] [PubMed] [Google Scholar]
- 92.Liu T, Chen YR, Thomas AM, Song XL. CEST MRI with distribution-based analysis for assessment of early stage disease activity in a mouse model of multiple sclerosis: An initial study. NMR Biomed. 2019;32:e4139. doi: 10.1002/nbm.4139. [DOI] [PubMed] [Google Scholar]
- 93.Yang J, Wadghiri YZ, Hoang DM, Tsui W, Sun YJ, Chung E, et al. Detection of amyloid plaques targeted by USPIO-Aβ1-42 in Alzheimer's disease transgenic mice using magnetic resonance microimaging. NeuroImage. 2011;55:1600–1609. doi: 10.1016/j.neuroimage.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li KC, Wang SY, Luo X, Zeng QZ, Jiaerken Y, Xu XP, et al. Progressive memory circuit impairments along with Alzheimer's disease neuropathology spread: Evidence from in vivo neuroimaging. Cereb Cortex. 2020;30:5863–5873. doi: 10.1093/cercor/bhaa162. [DOI] [PubMed] [Google Scholar]
- 95.Guan XJ, Zhang YY, Wei HJ, Guo T, Zeng QL, Zhou C, et al. Iron-related nigral degeneration influences functional topology mediated by striatal dysfunction in Parkinson's disease. Neurobiol Aging. 2019;75:83–97. doi: 10.1016/j.neurobiolaging.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang SY, Jiaerken Y, Yu XF, Shen ZJ, Luo X, Hong H, et al. Understanding the association between psychomotor processing speed and white matter hyperintensity: A comprehensive multi-modality MR imaging study. Hum Brain Mapp. 2020;41:605–616. doi: 10.1002/hbm.24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen YF, Wang JK, Cui CL, Su YS, Jing DL, Wu LY, et al. Evaluating the association between brain atrophy, hypometabolism, and cognitive decline in Alzheimer's disease: A PET/MRI study. Aging. 2021;13:7228–7246. doi: 10.18632/aging.202580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ossenkoppele R, Lyoo CH, Sudre CH, van Westen D, Cho H, Ryu YH, et al. Distinct tau PET patterns in atrophy-defined subtypes of Alzheimer's disease. Alzheimers Dement. 2020;16:335–344. doi: 10.1016/j.jalz.2019.08.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haller S, Scheffler M, Salomir R, Herrmann FR, Gold G, Montandon ML, et al. MRI detection of cerebral microbleeds: Size matters. Neuroradiology. 2019;61:1209–1213. doi: 10.1007/s00234-019-02267-0. [DOI] [PubMed] [Google Scholar]
- 100.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: The invisible lesions. Lancet Neurol. 2012;11:272–282. doi: 10.1016/S1474-4422(11)70307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hütter BO, Altmeppen J, Kraff O, Maderwald S, Theysohn JM, Ringelstein A, et al. Higher sensitivity for traumatic cerebral microbleeds at 7 T ultra-high field MRI: Is it clinically significant for the acute state of the patients and later quality of life? Ther Adv Neurol Disord. 2020;13:1756286420911295. doi: 10.1177/1756286420911295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Veluw SJ, Zwanenburg JJM, Engelen-Lee J, Spliet WGM, Hendrikse J, Luijten PR, et al. In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J Cereb Blood Flow Metab. 2013;33:322–329. doi: 10.1038/jcbfm.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barisano G, Law M, Custer RM, Toga AW, Sepehrband F. Perivascular space imaging at ultrahigh field MR imaging. Magn Reson Imag Clin N Am. 2021;29:67–75. doi: 10.1016/j.mric.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wardlaw JM, Valdés Hernández MC, Muñoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc. 2015;4:e001140. doi: 10.1161/JAHA.114.001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hametner S, Endmayr V, Deistung A, Palmrich P, Prihoda M, Haimburger E, et al. The influence of brain iron and myelin on magnetic susceptibility and effective transverse relaxation—A biochemical and histological validation study. Neuroimage. 2018;179:117–133. doi: 10.1016/j.neuroimage.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 106.Lin M, He HJ, Schifitto G, Zhong JH. Simulation of changes in diffusion related to different pathologies at cellular level after traumatic brain injury. Magn Reson Med. 2016;76:290–300. doi: 10.1002/mrm.25816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raffelt DA, Tournier JD, Smith RE, Vaughan DN, Jackson G, Ridgway GR, et al. Investigating white matter fibre density and morphology using fixel-based analysis. NeuroImage. 2017;144:58–73. doi: 10.1016/j.neuroimage.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Planetta PJ, Ofori E, Pasternak O, Burciu RG, Shukla P, DeSimone JC, et al. Free-water imaging in Parkinson's disease and atypical Parkinsonism. Brain. 2016;139:495–508. doi: 10.1093/brain/awv361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou ZH, Tong QQ, Zhang L, Ding QP, Lu H, Jonkman LE, et al. Evaluation of the diffusion MRI white matter tract integrity model using myelin histology and Monte-Carlo simulations. Neuroimage. 2020;223:117313. doi: 10.1016/j.neuroimage.2020.117313. [DOI] [PubMed] [Google Scholar]
- 110.Zhang JF, Lim HF, Chappell FM, Clancy U, Wiseman S, Valdés-Hernández MC, et al. Relationship between inferior frontal sulcal hyperintensities on brain MRI, ageing and cerebral small vessel disease. Neurobiol Aging. 2021;106:130–138. doi: 10.1016/j.neurobiolaging.2021.06.013. [DOI] [PubMed] [Google Scholar]
- 111.Huang PY, Zhang RT, Jiaerken Y, Wang SY, Yu WK, Hong H, et al. Deep white matter hyperintensity is associated with the dilation of perivascular space. J Cereb Blood Flow Metab. 2021;41:2370–2380. doi: 10.1177/0271678X211002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rotta J, Perosa V, Yakupov R, Kuijf HJ, Schreiber F, Dobisch L, et al. Detection of cerebral microbleeds with venous connection at 7-tesla MRI. Neurology. 2021;96:e2048–e2057. doi: 10.1212/WNL.0000000000011790. [DOI] [PubMed] [Google Scholar]
- 113.Duering M, Csanadi E, Gesierich B, Jouvent E, Hervé D, Seiler S, et al. Incident lacunes preferentially localize to the edge of white matter hyperintensities: Insights into the pathophysiology of cerebral small vessel disease. Brain. 2013;136:2717–2726. doi: 10.1093/brain/awt184. [DOI] [PubMed] [Google Scholar]
- 114.Maier FC, Wehrl HF, Schmid AM, Mannheim JG, Wiehr S, Lerdkrai C, et al. Longitudinal PET-MRI reveals β-amyloid deposition and rCBF dynamics and connects vascular amyloidosis to quantitative loss of perfusion. Nat Med. 2014;20:1485–1492. doi: 10.1038/nm.3734. [DOI] [PubMed] [Google Scholar]
- 115.Ni RQ. Magnetic resonance imaging in animal models of Alzheimer's disease amyloidosis. Int J Mol Sci. 2021;22:12768. doi: 10.3390/ijms222312768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mestre H, Mori Y, Nedergaard M. The brain's glymphatic system: Current controversies. Trends Neurosci. 2020;43:458–466. doi: 10.1016/j.tins.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mortensen KN, Sanggaard S, Mestre H, Lee H, Kostrikov S, Xavier ALR, et al. Impaired glymphatic transport in spontaneously hypertensive rats. J Neurosci. 2019;39:6365–6377. doi: 10.1523/JNEUROSCI.1974-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harrison IF, Ismail O, Machhada A, Colgan N, Ohene Y, Nahavandi P, et al. Impaired glymphatic function and clearance of tau in an Alzheimer's disease model. Brain. 2020;143:2576–2593. doi: 10.1093/brain/awaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eide PK, Vinje V, Pripp AH, Mardal KA, Ringstad G. Sleep deprivation impairs molecular clearance from the human brain. Brain. 2021;144:863–874. doi: 10.1093/brain/awaa443. [DOI] [PubMed] [Google Scholar]
- 120.Lo CY, Wang PN, Chou KH, Wang JH, He Y, Lin CP. Diffusion tensor tractography reveals abnormal topological organization in structural cortical networks in Alzheimer's disease. J Neurosci. 2010;30:16876–16885. doi: 10.1523/JNEUROSCI.4136-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Langley J, Hussain S, Huddleston DE, Bennett IJ, Hu XP. Impact of locus coeruleus and its projections on memory and aging. Brain Connect. 2022;12:223–233. doi: 10.1089/brain.2020.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grandjean J, Derungs R, Kulic L, Welt T, Henkelman M, Nitsch RM, et al. Complex interplay between brain function and structure during cerebral amyloidosis in APP transgenic mouse strains revealed by multi-parametric MRI comparison. Neuroimage. 2016;134:1–11. doi: 10.1016/j.neuroimage.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 123.Hennig J, Kiviniemi V, Riemenschneider B, Barghoorn A, Akin B, Wang F, et al. 15 years MR-encephalography. Magn Reson Mater Phys Biol Med. 2021;34:85–108. doi: 10.1007/s10334-020-00891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang XT, Zhang Y, Roe AW. Ultra-high-field MRI studies of brain structure and function in humans and nonhuman Primates: A collaborative approach to precision medicine. Curr Opin Biomed Eng. 2021;20:100320. doi: 10.1016/j.cobme.2021.100320. [DOI] [Google Scholar]
- 125.Lai HY, Younce JR, Albaugh DL, Kao YCJ, Shih YYI. Functional MRI reveals frequency-dependent responses during deep brain stimulation at the subthalamic nucleus or internal globus pallidus. Neuroimage. 2014;84:11–18. doi: 10.1016/j.neuroimage.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 126.Min HK, Hwang SC, Marsh MP, Kim I, Knight E, Striemer B, et al. Deep brain stimulation induces BOLD activation in motor and non-motor networks: An fMRI comparison study of STN and EN/GPi DBS in large animals. Neuroimage. 2012;63:1408–1420. doi: 10.1016/j.neuroimage.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boutet A, Madhavan R, Elias GJB, Joel SE, Gramer R, Ranjan M, et al. Predicting optimal deep brain stimulation parameters for Parkinson's disease using functional MRI and machine learning. Nat Commun. 2021;12:3043. doi: 10.1038/s41467-021-23311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mosley PE, Paliwal S, Robinson K, Coyne T, Silburn P, Tittgemeyer M, et al. The structural connectivity of subthalamic deep brain stimulation correlates with impulsivity in Parkinson's disease. Brain. 2020;143:2235–2254. doi: 10.1093/brain/awaa148. [DOI] [PubMed] [Google Scholar]
- 129.Xiao YM, Lau JC, Hemachandra D, Gilmore G, Khan AR, Peters TM. Image guidance in deep brain stimulation surgery to treat Parkinson's disease: A comprehensive review. IEEE Trans Biomed Eng. 2021;68:1024–1033. doi: 10.1109/TBME.2020.3006765. [DOI] [PubMed] [Google Scholar]
- 130.Chou YH, Ton That V, Sundman M. A systematic review and meta-analysis of rTMS effects on cognitive enhancement in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2020;86:1–10. doi: 10.1016/j.neurobiolaging.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Heath A, Taylor JL, McNerney MW. rTMS for the treatment of Alzheimer's disease: Where should we be stimulating? Expert Rev Neurother. 2018;18:903–905. doi: 10.1080/14737175.2018.1538792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J, Deng XP, Wu YY, Li XL, Feng ZJ, Wang HX, et al. High-frequency rTMS of the motor cortex modulates cerebellar and widespread activity as revealed by SVM. Front Neurosci. 2020;14:186. doi: 10.3389/fnins.2020.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Folloni D, Verhagen L, Mars RB, Fouragnan E, Constans C, Aubry JF, et al. Manipulation of subcortical and deep cortical activity in the primate brain using transcranial focused ultrasound stimulation. Neuron. 2019;101:1109–1116.e5. doi: 10.1016/j.neuron.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wolz R, Schwarz AJ, Gray KR, Yu P, Hill DLG, Initiative ADN. Enrichment of clinical trials in MCI due to AD using markers of amyloid and neurodegeneration. Neurology. 2016;87:1235–1241. doi: 10.1212/WNL.0000000000003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cash DM, Rohrer JD, Ryan NS, Ourselin S, Fox NC. Imaging endpoints for clinical trials in Alzheimer's disease. Alzheimers Res Ther. 2014;6:87. doi: 10.1186/s13195-014-0087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Smith EE, Markus HS. New treatment approaches to modify the course of cerebral small vessel diseases. Stroke. 2020;51:38–46. doi: 10.1161/STROKEAHA.119.024150. [DOI] [PubMed] [Google Scholar]
- 137.Lam BYK, Leung KT, Yiu B, Zhao L, Biesbroek JM, Au L, et al. Peak width of skeletonized mean diffusivity and its association with age-related cognitive alterations and vascular risk factors. Alzheimers Dement (Amst) 2019;11:721–729. doi: 10.1016/j.dadm.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Benjamin P, Zeestraten E, Lambert C, Ster IC, Williams OA, Lawrence AJ, et al. Progression of MRI markers in cerebral small vessel disease: Sample size considerations for clinical trials. J Cereb Blood Flow Metab. 2016;36:228–240. doi: 10.1038/jcbfm.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Burciu RG, Ofori E, Archer DB, Wu SS, Pasternak O, McFarland NR, et al. Progression marker of Parkinson's disease: A 4-year multi-site imaging study. Brain. 2017;140:2183–2192. doi: 10.1093/brain/awx146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arpin DJ, Mitchell T, Archer DB, Burciu RG, Chu WT, Gao HZ, et al. Diffusion magnetic resonance imaging detects progression in Parkinson's disease: A placebo-controlled trial of rasagiline. Mov Disord. 2022;37:325–333. doi: 10.1002/mds.28838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ketter N, Brashear HR, Bogert J, Di JN, Miaux Y, Gass A, et al. Central review of amyloid-related imaging abnormalities in two phase III clinical trials of bapineuzumab in mild-to-moderate Alzheimer's disease patients. J Alzheimers Dis. 2017;57:557–573. doi: 10.3233/JAD-160216. [DOI] [PubMed] [Google Scholar]
- 142.Elliott LT, Sharp K, Alfaro-Almagro F, Shi SN, Miller KL, Douaud G, et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature. 2018;562:210–216. doi: 10.1038/s41586-018-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Smith SM, Elliott LT, Alfaro-Almagro F, McCarthy P, Nichols TE, Douaud G, et al. Brain aging comprises many modes of structural and functional change with distinct genetic and biophysical associations. eLife. 2020;9:e52677. doi: 10.7554/eLife.52677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shen L, Kim S, Risacher SL, Nho K, Swaminathan S, West JD, et al. Whole genome association study of brain-wide imaging phenotypes for identifying quantitative trait loci in MCI and AD: A study of the ADNI cohort. Neuroimage. 2010;53:1051–1063. doi: 10.1016/j.neuroimage.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saykin AJ, Shen L, Yao XH, Kim S, Nho K, Risacher SL, et al. Genetic studies of quantitative MCI and AD phenotypes in ADNI: Progress, opportunities, and plans. Alzheimers Dement. 2015;11:792–814. doi: 10.1016/j.jalz.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Berg D, Borghammer P, Fereshtehnejad SM, Heinzel S, Horsager J, Schaeffer E, et al. Prodromal Parkinson disease subtypes - key to understanding heterogeneity. Nat Rev Neurol. 2021;17:349–361. doi: 10.1038/s41582-021-00486-9. [DOI] [PubMed] [Google Scholar]
- 147.Badhwar A, McFall GP, Sapkota S, Black SE, Chertkow H, Duchesne S, et al. A multiomics approach to heterogeneity in Alzheimer's disease: Focused review and roadmap. Brain. 2020;143:1315–1331. doi: 10.1093/brain/awz384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marras C, Chaudhuri KR, Titova N, Mestre TA. Therapy of Parkinson's disease subtypes. Neurotherapeutics. 2020;17:1366–1377. doi: 10.1007/s13311-020-00894-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang LB, Cheng W, Rolls ET, Dai FL, Gong WK, Du JN, et al. Association of specific biotypes in patients with Parkinson disease and disease progression. Neurology. 2020;95:e1445–e1460. doi: 10.1212/WNL.0000000000010498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhao K, Zheng Q, Dyrba M, Rittman T, Li A, Che TT, et al. Regional radiomics similarity networks reveal distinct subtypes and abnormality patterns in mild cognitive impairment. Adv Sci (Weinh) 2022;9:e2104538. doi: 10.1002/advs.202104538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guo T, Guan XJ, Zhou C, Gao T, Wu JJ, Song Z, et al. Clinically relevant connectivity features define three subtypes of Parkinson's disease patients. Hum Brain Mapp. 2020;41:4077–4092. doi: 10.1002/hbm.25110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu HT, Zhou C, Bai XQ, Liu XC, Chen JW, Wen JQ, et al. Identifying a whole-brain connectome-based model in drug-naïve Parkinson's disease for predicting motor impairment. Hum Brain Mapp. 2022;43:1984–1996. doi: 10.1002/hbm.25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jin D, Zhou B, Han Y, Ren JJ, Han T, Liu B, et al. Generalizable, reproducible, and neuroscientifically interpretable imaging biomarkers for Alzheimer's disease. Adv Sci (Weinh) 2020;7:2000675. doi: 10.1002/advs.202000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Federau C, Gallichan D. Motion-correction enabled ultra-high resolution in-vivo 7T-MRI of the brain. PLoS ONE. 2016;11:e0154974. doi: 10.1371/journal.pone.0154974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hyun CM, Kim HP, Lee SM, Lee S, Seo JK. Deep learning for undersampled MRI reconstruction. Phys Med Biol. 2018;63:135007. doi: 10.1088/1361-6560/aac71a. [DOI] [PubMed] [Google Scholar]
- 156.Liu SF, Utriainen D, Chai C, Chen YS, Wang L, Sethi SK, et al. Cerebral microbleed detection using Susceptibility Weighted Imaging and deep learning. Neuroimage. 2019;198:271–282. doi: 10.1016/j.neuroimage.2019.05.046. [DOI] [PubMed] [Google Scholar]
- 157.Liu MH, Li F, Yan H, Wang KD, Ma YX, Initiative ADN, et al. A multi-model deep convolutional neural network for automatic hippocampus segmentation and classification in Alzheimer's disease. Neuroimage. 2020;208:116459. doi: 10.1016/j.neuroimage.2019.116459. [DOI] [PubMed] [Google Scholar]
- 158.Yu BL, Li L, Guan XJ, Xu XJ, Liu XL, Yang Q, et al. HybraPD atlas: Towards precise subcortical nuclei segmentation using multimodality medical images in patients with Parkinson disease. Hum Brain Mapp. 2021;42:4399–4421. doi: 10.1002/hbm.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tong QQ, Gong T, He HJ, Wang Z, Yu WW, Zhang JJ, et al. A deep learning-based method for improving reliability of multicenter diffusion kurtosis imaging with varied acquisition protocols. Magn Reson Imaging. 2020;73:31–44. doi: 10.1016/j.mri.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 160.Heinzel S, Berg D, Gasser T, Chen HL, Yao C, Postuma RB, et al. Update of the MDS research criteria for prodromal Parkinson's disease. Mov Disord. 2019;34:1464–1470. doi: 10.1002/mds.27802. [DOI] [PubMed] [Google Scholar]