Abstract
Purpose
The childhood-to-adolescence transition is a notable period of change including pubertal development, neurodevelopment, and psychopathology onset, that occurs in divergent patterns between sexes. This study examined the effects of sex and puberty on cortical thickness (CT) in children and explored whether CT changes over time related to emergence of psychopathology in early adolescence.
Methods
We used longitudinal data (baseline ages 9–10 and Year 2 [Y2] ages 11–12) from the ABCD Study (n = 9985). Linear and penalized function-on-function regressions modeled the impact of puberty, as it interacts with sex, on CT. Focusing on regions that showed sex differences, linear and logistic regressions modeled associations between change in CT and internalizing problems and suicide ideation.
Results
We identified significant sex differences in the inverse relation between puberty and CT in fifteen primarily posterior brain regions. Nonlinear pubertal effects across age were identified in the fusiform, isthmus cingulate, paracentral, and precuneus. All effects were stronger for females relative to males during this developmental window. We did not identify associations between CT change and early adolescent clinical outcomes.
Conclusion
During this age range, puberty is most strongly associated with regional changes in CT in females, which may have implications for the later emergence of psychopathology.
Keywords: Cortical thickness, Neurodevelopment, Puberty, Sex differences, Developmental psychopathology, Suicidal ideation
1. Introduction
Childhood and adolescence are marked by large-scale neurodevelopmental shifts that enable successful functioning in adulthood. The sex-specific hormonal changes which occur in puberty, and their timing, are critical in shaping neurodevelopment (Herting and Sowell, 2017). Nevertheless, the dynamics of how sex, puberty, and age interact to impact neurodevelopment remain poorly understood, likely because disentangling these complexities requires large samples.
1.1. Brain structure in adolescence and across puberty
Puberty is the transition from childhood to adulthood marked by physical changes (i.e., development of secondary sexual characteristics, growth spurts), along with attainment of reproductive capacity and gonadal maturation during gonadarche (Abreu and Kaiser, 2016). Sex differences in pubertal onset and tempo are evident: females generally experience pubertal onset earlier (8–12 years old) and demonstrate linear increases in pubertal development with age, as compared to males whose pubertal onset begins later (9–14 years old) and demonstrates a quadratic-like trajectory (Vijayakumar et al., 2021). These sex-specific patterns suggest that, at a given age, pubertal-specific impacts may unfold differently for male and female youth.
Nonlinear changes in cortical gray matter volume and surface area occur during the adolescent transition. Pre-adolescents show increases in gray matter volume and surface area, followed by decreases in later adolescence, with varied patterns by region (Giedd et al., 1999, Wierenga et al., 2014). Frontal and parietal gray matter peak during early adolescence followed by net decreases with age, while temporal lobe gray matter peaks in late adolescence followed by a slight decline. The occipital lobe shows linear increases in volume over time extending into early adulthood (Ball et al., 2019). Total cortical volume consistently shows decreases over time beginning in adolescence, with a slightly accelerated decrease throughout the adolescent period (Herting and Sowell, 2017, Vijayakumar et al., 2018). Though on average cortical volume, thickness, and surface area all show negative change rates with age, cortical thinning is the dominant contributor to cortical volume reduction during adolescence (Tamnes et al., 2017, Wierenga et al., 2014). Whether pubertal stage and sex differences impact these patterns of neurodevelopment is of great interest.
Sex differences in neurodevelopment are evident between the ages of 4–20-years-old (Giedd et al., 1999, Wierenga et al., 2014). Cortical gray matter volume, specifically in the frontal and parietal lobes, peaks earlier in females (11.0 years and 10.2 years, respectively) compared to males (12.1 years and 11.8 years respectively), although males demonstrate larger total brain volumes and absolute cortical gray matter volumes (Giedd et al., 1999, Koolschijn and Crone, 2013, Lenroot et al., 2007). Cortical thickness (CT) reflects the distance between the surface of white matter and the pial surface. There is evidence of spatial heterogeneity of CT change between sexes; although males demonstrate increased cortex thickness in a number of frontal regions by age 9, by age 22 they demonstrate accelerated cortical thinning compared to females (Raznahan et al., 2010). The same pattern is observed for total brain, cerebral gray, and white matter volumes when brain maturation was examined between the ages of 8–30 (Koolschijn and Crone, 2013). Additionally, females show more rapid cortical thinning compared to males in the inferior parietal, posterior temporal, and bilateral occipital regions (Raznahan et al., 2010). While sex differences in regional CT across age are clear, it is less clear whether puberty is a primary driver of these differences.
Cross-sectional research has consistently evidenced negative associations between global gray matter volume/thickness and pubertal stage, yet inconsistencies emerge when accounting for age (Vijayakumar et al., 2018). A recent longitudinal study suggests that, when factoring in pubertal status in addition to age, linear puberty-related changes are seen across much of the cortex, with cortical regions showing an average 1.29% reduction in thickness per developmental stage (e.g., measured through Tanner staging) (Marshall and Tanner, 1969, Marshall and Tanner, 1970, Vijayakumar et al., 2021). There also appears to be an interaction between pubertal tempo and age in relation to CT, where, between the ages of 8–14 years, males with faster pubertal tempo show more rapid cortical thinning over time (Vijayakumar et al., 2021). It remains unclear how pubertal development may impact CT differentially given chronological age, as opposed to irrespective of age, and whether sex differences influence this association.
1.2. Puberty, brain structure, and clinical outcomes
The adolescent period represents a window of increased risk for internalizing psychopathology and suicidality. Depressive and anxiety disorders are diagnosed in 1–2% of children, as compared to 8–20% of adolescents (Bitsko et al., 2018, Fleming and Offord, 1990, Lewinsohn, 1998). Further, suicide is the second leading cause of death for youth ages 10–19 (Centers for Disease Control and Prevention, National Center for Health Statistics, 2021). Recent large-scale data show that, for children aged 9–10, the lifetime prevalence of suicidal ideation and attempt is about 14% and 1%, respectively (Lawrence et al., 2021).
During the adolescent transition, shifts occur in the patterns of psychopathology among male and female youth. Depression, suicidal ideation, and non-fatal suicide attempts are approximately equally prevalent across male and female children cross-culturally (Rescorla et al., 2007), though recent evidence shows that males may be more likely to demonstrate clinically elevated internalizing and externalizing symptoms at ages 9–10 (Loso et al., 2021). During adolescence, however, female youth become more likely to experience depression (Rutter et al., 2003), non-fatal suicidal thoughts and behaviors (Cha et al., 2018), and internalizing symptoms broadly (Hayward and Sanborn, 2002). Thus, there may be complex, sex-specific pubertal processes that shape neurodevelopment in adolescence and contribute to the emergence of sex-specific patterns of psychopathology.
Pubertal sex hormones, alongside genetic and environmental influences, are thought to modulate trajectories of brain development to produce sex-specific vulnerabilities for psychopathology (López-Ojeda and Hurley, 2021). For example, sex moderates the association between puberty and internalizing symptoms in 9–10-year-old children, predicting greater symptom severity for females as compared to males (McNeilly et al., 2022). Adolescence may be a particularly important time for investigating mechanisms driving sex differences in the development of internalizing symptoms, as youth are undergoing neurodevelopment in key emotional regulation regions (Spear, 2009), including the amygdala, anterior cingulate cortex (ACC), medial prefrontal cortex (PFC), and striatum. Importantly, this developmental process expresses multifinality; while all youth undergo these pubertal changes, few develop psychopathology. As such, Ho and colleagues (2021) theorize that it is not the pubertal processes that produce vulnerability for psychopathology (specifically referring to suicidality), but rather individual differences in neurobiological sensitivity to hormonal changes associated with puberty. In addition, the timing of pubertal processes and their impacts on neurodevelopment are critical to consider, as earlier pubertal onset is associated with heightened risk for psychopathology (Barendse et al., 2021, Ullsperger and Nikolas, 2017), including anxiety, depression, and withdrawn behaviors (McNeilly et al., 2022).
Few studies have examined sex differences in, or the impacts of pubertal stage on, the relation between neurodevelopment and psychopathology. Among children ages 6–10 years, lower than average gray matter volume, particularly in frontal brain regions, was associated with broad indexes of psychopathology (Snyder et al., 2017). Conversely, research from a large sample of 9–10-year-olds found greater global CT among youth with externalizing or internalizing diagnoses compared to healthy counterparts (Yu et al., 2021). Further research on internalizing psychopathology and suicidality implicates neuroanatomical abnormalities in regions associated with emotion regulation and visual processing. Internalizing symptoms have been linked to reduced gray matter volumes in the insula in middle childhood (Snyder et al., 2017) but decreased thinning in the orbital frontal cortex in late childhood (Pfeifer and Allen, 2021, Whittle et al., 2020). In adolescence, internalizing symptoms are associated with greater fusiform gyrus CT for male and female youth and superior frontal gyrus volumes for male, but not female, youth (Smolker et al., 2022). Further, less surface area of frontal, temporal, and parietal regions (Gifuni et al., 2021), as well as smaller volumes in the right superior temporal gyrus (Pan et al., 2015), are cross-sectionally associated with histories of suicidal behavior in adolescence. What remains to be examined is whether change in cortical structure over time is associated with the emergence of clinical problems in adolescence, clarifying the directional nature of these links.
1.3. The current study
To address the gaps in knowledge highlighted above, the present study has two primary aims. Aim 1 seeks to understand the effects of pubertal development on CT as they might differ based on sex assigned at birth and age. To do so, we model a sex by puberty interaction to predict CT accounting for chronological age through two different methods: Aim 1a) including age as a statistical covariate, which means that the interaction effect is modeled as being invariant across age, and Aim 1b) modeling pubertal development and CT both as a function of age, which allows for the interaction effect of sex and pubertal stage to be nonlinear and vary in effect size across age. Aim 2 seeks to characterize the association between cortical development and the emergence of sex-different patterns of clinical problems in early adolescence. To do so, Aim 2 focuses on the regions of interest (ROIs) that demonstrate significant sex by puberty interactions from Aim 1a to model longitudinal change in ROI CT, as it interacts with sex and pubertal change, as a predictor of internalizing problems and suicidal ideation.
2. Method
2.1. Data Source
This study examined data from two visits, Baseline and Year 2 (Y2), from the ongoing Adolescent Brain Cognitive Development (ABCD) Study (data release 4.0, http://dx.doi.org/10.15154/1523041), which samples youth longitudinally. At Baseline (recruited in 2016–2018), 11,878 youth 9–10 years of age were recruited across 22 imaging sites (Garavan et al., 2018).
2.2. Participants
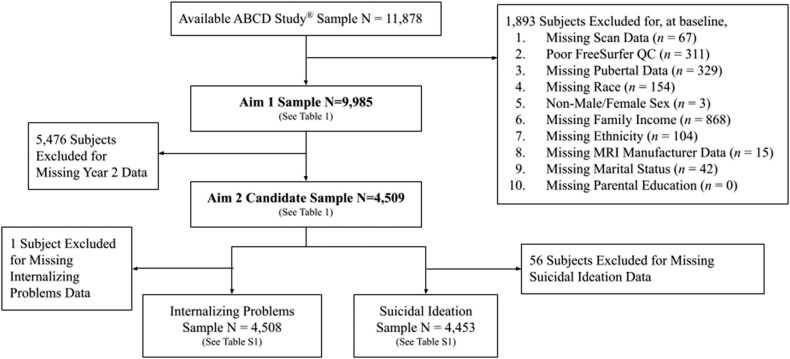
Participants with full covariate data at Baseline, acceptable quality MRI data, and pubertal data for at least one time point (to preserve statistical power) were included for Aim 1 (N = 9985; Table 1). Participants in Aim 2 were further limited to those with full MRI and pubertal data at both timepoints (n = 4509; Table S1). See Fig. 1 for a consort diagram of study sample selection. Missing data descriptives for the Aim 1 and 2 samples are reported in the Supplemental Results.
Table 1.
Aim 1 Sample Demographics and Clinical Characteristics Disaggregated by Sex.
| Female | Male | p-value | |
|---|---|---|---|
| Baseline | (N = 4794) | (N = 5191) | |
| Age in months | |||
| Mean±SD (Min, Max) | 118.818 ± 7.458 (107,132) | 119.217 ± 7.510 (107,132) | 0.06 |
| Pubertal Developmenta | |||
| Mean±SD (Min, Max) | 2.162 ± 0.910 (1,5) | 1.351 ± 0.596 (1,5) | < 0.001 |
| Missing, N(%) | 43 (0.9%) | 53 (1.0%) | |
| Caregiver Marital Status | 1 | ||
| Married, N(%) | 3334 (69.5%) | 3693 (71.1%) | |
| Widowed | 38 (0.8%) | 43 (0.8%) | |
| Divorced | 435 (9.1%) | 476 (9.2%) | |
| Separated | 178 (3.7%) | 173 (3.3%) | |
| Never Married | 559 (11.7%) | 540 (10.4%) | |
| Living with Partner | 250 (5.2%) | 266 (5.1%) | |
| Highest Caregiver Education | |||
| Mean±SD (Min, Max) | 17.812 ± 2.562 (3,24) | 17.832 ± 2.551(3,24) | |
| Race/Ethnicityb | |||
| White, N(%) | 3709 (77.4%) | 4111 (79.2%) | 0.229 |
| Asian | 312 (6.5%) | 318 (6.1%) | 1 |
| Black/African American | 961 (20.0%) | 961 (18.5%) | 0.443 |
| Native American | 167 (3.5%) | 172 (3.3%) | 1 |
| Pacific Islander | 27 (0.6%) | 38 (0.7%) | 1 |
| Other | 298 (6.2%) | 314 (6.0%) | 1 |
| Hispanic/Latinx | 887 (18.5%) | 989 (19.1%) | 1 |
| Internalizing Problems | |||
| Mean±SD (Min, Max) | 5.058 ± 5.508 (0,42) | 5.053 ± 5.508 (0,42) | 1 |
| Suicidal Ideation | |||
| Present, N(%) | 380 (7.9%) | 483 (9.3%) | 0.131 |
| Year 2 | (n = 2210) | (n = 2590) | |
| Age in months | |||
| Mean±SD (Min, Max) | 142.671 ± 7.525 (128,162) | 143.294 ± 7.688 (127,160) | 0.038 |
| Pubertal Developmenta | |||
| Mean±SD (Min, Max) | 3.193 ± 0.813 (1,5) | 1.917 ± 0.866 (1,5) | < 0.001 |
| Caregiver Marital Status | 0.749 | ||
| Married, N(%) | 1542 (69.8%) | 1888 (72.9%) | |
| Widowed | 23 (1.0%) | 22 (0.8%) | |
| Divorced | 211 (9.5%) | 253 (9.8%) | |
| Separated | 85 (3.8%) | 84 (3.2%) | |
| Never Married | 242 (11.0%) | 228 (8.8%) | |
| Living with Partner | 107 (4.8%) | 115 (4.4%) | |
| Highest Caregiver Education | |||
| Mean±SD (Min, Max) | 17.848 ± 2.471 (6,24) | 17.946 ± 2.453 (5,24) | 1 |
| Race/Ethnicityb | |||
| White, N(%) | 1741 (78.8%) | 2135 (82.4%) | 0.125 |
| Asian | 136 (6.2%) | 145 (5.6%) | 1 |
| Black/African American | 409 (18.5%) | 394 (15.2%) | 0.021 |
| Native American | 87 (3.9%) | 82 (3.2%) | 1 |
| Pacific Islander | 18 (0.8%) | 16 (0.6%) | 1 |
| Hispanic/Latinx | 391 (17.7%) | 483 (18.6%) | 1 |
| Internalizing Problems | |||
| Mean±SD (Min, Max) | 5.189 ± 5.813 (0,40) | 4.679 ± 5.353 (0,50) | 0.013 |
| Missing N | 0 (0.0%) | 1 (0.0%) | |
| Suicidal Ideation | |||
| Present, N(%) | 195 (8.8%) | 168 (6.5%) | 0.021 |
| Missing N | 14 (0.01%) | 15 (0.01%) |
Table Caption. Sample descriptors and results from 2-tailed chi-squared and independent samples t-test analyses with Bonferroni correction applied to determine sex differences. * p < 0.05 (difference between males and females). a While some subjects are missing one of Baseline or Y2, none are missing pubertal data at both time points. b For race/ethnicity, the ABCD Study permits subjects to select more than one racial/ethnic affiliation, thus race/ethnicity counts are representative of the total number of subjects that identify with a given race/ethnicity and do not equal total sample size.
Fig. 1.
Consort Diagram of Study Samples by Aim. Figure Caption. Excluded data categories are listed sequentially and represent non-overlapping subjects (e.g., if a participant is missing both Total Family Income and Marital Status data, they are only represented in the Total Family Income category).
2.3. Measures and materials
2.3.1. Demographics
Demographic variables were reported by each parent or guardian (herein referred to as parent) at Baseline and Y2 and included chronological age in months, youth sex assigned at birth, youth race and ethnicity, combined total family income, highest household/parent education, and parent/guardian marital status.
2.3.2. Pubertal development
The Pubertal Developmental Scale (PDS), originally designed as a self-report questionnaire (Petersen et al., 1988), was completed by youth and their (Barch et al., 2018). For our project, we focused on parents’ reports of their youth’s pubertal development at Baseline and Y2. The PDS includes sex-specific questions about height, body hair, skin changes, voice changes (males), breast growth (females), and menarche (females) on a four-point scale ranging from has not started (1) to seems complete (4). Pubertal stage, comparable to Tanner staging (ranging from Tanner I to Tanner V; Emmanuel and Bokor, 2022), was calculated based on parent-reported PDS scores given that parent reports may be more valid at these early ages (Cheng et al., 2021; Herting et al., 2021; Schlossberger et al., 1992) and that there is a large amount of missing data from self-report at Baseline and Y2 (Cheng et al., 2021). This approach is consistent with other studies using ABCD puberty data (e.g., Demidenko et al., 2021; McNeilly et al., 2022; Thijssen et al., 2020; 2022).
2.3.3. Clinical problems
The Child Behavior Checklist (CBCL) was completed by the parent at Baseline and Y2 and indicates specific problems each youth has exhibited in the past six months on a 4-point Likert scale, ranging from not true (0) to very true (3)(Achenbach and Ruffle, 2000; Barch et al., 2018). The current analyses relied on raw scores from the CBCL Internalizing Problems Scale, as is recommended by the instrument authors to preserve the full range of variation (Achenbach & Rescorla, 2001; see also Thurber & Sheehan, 2012).
The computerized Kiddie Schedule for Affective Disorders and Schizophrenia for DSM-5 (KSADS-COMP) was administered to both youth and the parent to assess for clinical symptom profiles (Barch et al., 2018, Kaufman et al., 1997, Kaufman et al., 2021). Suicidal ideation at Baseline and Y2 was coded according to youth endorsement, as youth reports may be more reliable than parent reports and are associated with neural indexes (Wiglesworth et al., 2021), of current or past thoughts in any of the following five domains: passive, active but non-specific, active with a specific method in mind, active with intent, and active with a plan.
2.3.4. Brain imaging
Tabulated structural MRI data provided by the ABCD Study for Baseline and Y2 MRI scans (Casey et al., 2018) was used. We examined CT of 34 ROIs defined by FreeSurfer automatic parcellations (Desikan et al., 2006, Fischl and Dale, 2000). CT values were averaged across hemispheres as we had no a priori hypotheses about lateralization of effects given extant research. Data were included if they met ABCD standards for Freesurfer quality control (Hagler et al., 2019).
2.4. Data analysis
2.4.1. Overview of models and covariates
All analyses controlled for standard covariates recommended by the ABCD Consortium, including Baseline age, sex, race, ethnicity, total family income, parental education, and parent marital status. Linear mixed effect regression (LMER) models used nested random effects to account for the nested structure of participant within family group within ABCD research site. Random effects could not be incorporated into the penalized function-on-function regression (PFFR, see 2.4.2) or logistic regression analyses (see 2.4.3) due to methodological limitations and overfitting of the data, respectively. Instead, MRI manufacturer was included as an additional covariate in PFFR and logistic regression analyses to account for the variance across research sites that is attributable to different scanning platforms (e.g., Siemens, General Electric, Philips). The Bonferroni correction was used to adjust for multiple comparisons across the included ROIs. Additional details specific to each analysis are provided below.
2.4.2. Aim 1
To address Aim 1, which was to model sex differences in the effect of pubertal stage on CT both controlling for and as a function of age, we took a two-pronged approach. First, LMER was used to understand these effects controlling for age (Aim 1a), with the main effects of pubertal stage and sex and their interaction as the primary predictors, and including fixed effects to control for the standard covariates. CT at Baseline and Y2, nested within individuals, was our outcome measure. The LMER, however, assumes that the main effects of PDS and sex do not change based on age. PFFR (Ivanescu et al., 2015) was used to explore whether allowing for non-linear patterns of effects across age illuminated sex differences in different ROIs (Aim 1b). The PFFR modeling framework assumes that pubertal stage and CT are each a sex-specific smooth and potentially nonlinear function of age. PFFR then relates these two functions, thereby allowing us to characterize the association between pubertal stage and CT as a function of age and how this age-varying association differs between sexes. Age-varying coefficient functions relating CT within a region with pubertal stage, sex, and the pubertal stage by sex interaction (to identify sex differences in pubertal effects) were estimated using the pffr() function provided in the R package refund (Goldsmith et al., 2021). Next, Wald-type testing was applied to obtain an F-statistic for the statistical significance of each of these coefficient functions, i.e., if the coefficient function is zero across all ages. Greater values for the F-statistic for the coefficient function for the pubertal stage by sex interaction would indicate greater differences in the effect of puberty on CT for male versus female youth. As the effect may vary across ages, the F-statistic essentially represents the aggregate difference across all ages.
2.4.3. Aim 2
To address Aim 2, which was to model the associations between CT development and the emergence of clinical problems, we conducted a series of LMER and logistic regression analyses. Specifically, we focused on the ROIs identified from Aim 1a that showed significant sex differences in the effects of puberty on CT. Our primary predictor in each model was the change in CT of those ROIs (from Baseline-Y2) as well as the three-way interaction between change in CT, pubertal development (e.g., change from Baseline-Y2), and sex. Each ROI was modeled independently and our dependent variables were Y2 clinical problems, including internalizing problems (LMER) and history of suicidal ideation (logistic regressions). In addition to the previously outlined covariates, we controlled for the respective clinical problem at Baseline, time-since-Baseline, Baseline pubertal stage, and Baseline CT of the ROI and its interaction with sex. We used McFadden’s pseudo R2 (McFadden, 1973) and the conditional R2 (Nakagawa & Schielzeth, 2013) to assess the quality of fit for the logistic regression models and the LMER models, respectively.
3. Results
3.1. Aim 1: effects of puberty on cortical thickness
Descriptives can be found in Table 1 and additional information regarding missing data patterns and subgroup descriptives can be found in the Supplemental Results.
3.1.1. Aim 1a linear mixed effect regression analyses
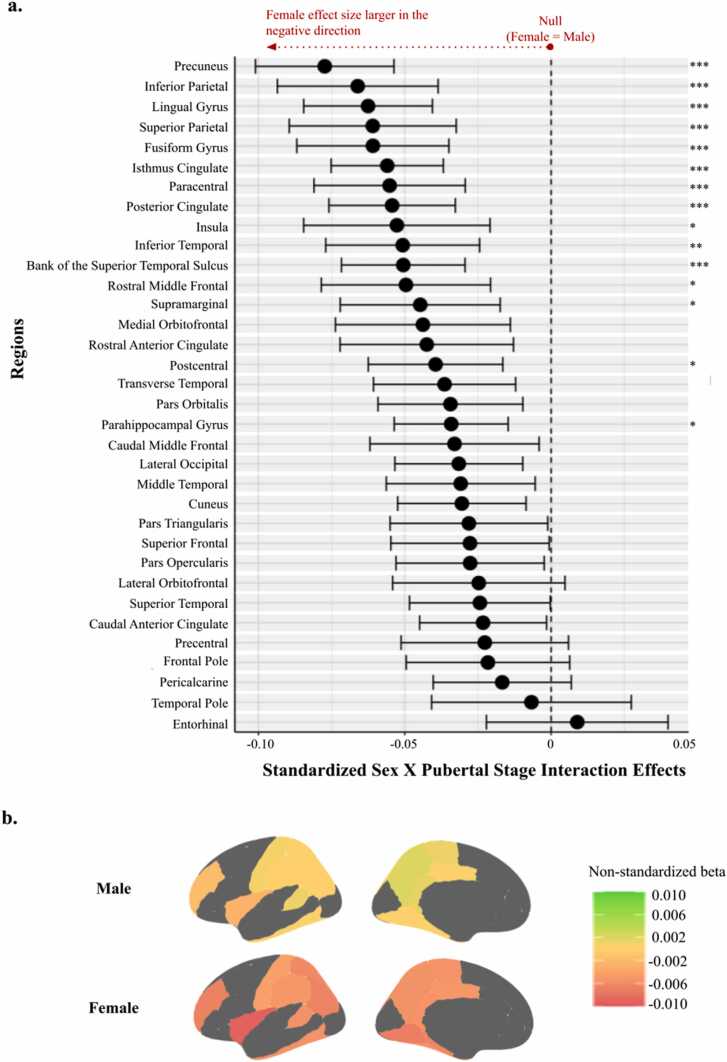
There were no main effects of puberty on CT (Figs. S1, S2). However, pubertal stage interacted with sex to significantly predict CT in 15 ROIs (Fig. 2). Specifically, small negative effects indicating that pubertal stage is inversely associated with CT, which was stronger for female youth than for male youth, were found in the superior temporal sulcus (B=-0.051), fusiform (B=-0.061), inferior parietal (B=-0.066), inferior temporal (B=-0.051), insula (B=-0.053), isthmus cingulate (B=-0.056), lingual (B=-0.063), parahippocampal (B=-0.034), paracentral (B=-0.055), postcentral (B=-0.039), posterior cingulate (B=-0.054), precuneus (B=-0.077), rostral middle frontal (B=-0.050), superior parietal (B=-0.061), and supramarginal (B=-0.045) regions (95% CI and p-values in Table S2).
Fig. 2.
Interaction Effects Between Sex and Pubertal Stage to Predict Cortical Thickness Adjusting for Age. Figure Caption. 2a: Forest plot of the standardized beta coefficients and 95% confidence intervals of the sex by pubertal stage interaction effects, as they are associated with cortical thickness. Dashed vertical line corresponds to the null value (i.e., equal size of effects for male and female youth). Values further to the left of the null indicate where effect sizes for female youth are more negative (i.e., the effect of puberty on cortical thickness– where higher puberty is associated with lower cortical thickness– is stronger) than effect sizes for male youth. *** p < 0.001, ** p < 0.01, * p < 0.05 following Bonferroni correction. 2b: Spatial mappings of the effect of puberty on cortical thickness in regions with significant interaction effects.
3.1.2. Aim 1b penalized function-on-function regression analyses
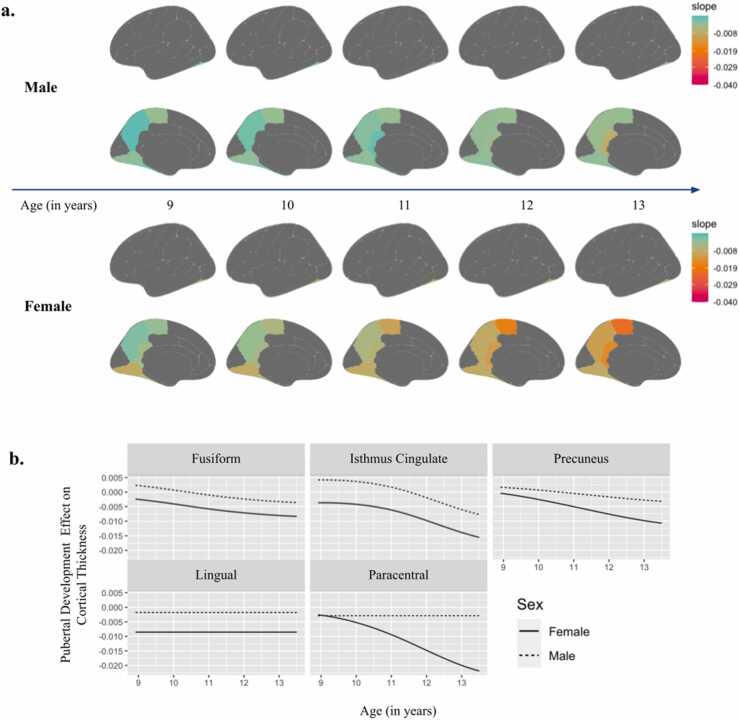
In PFFR analyses, where pubertal stage and CT were modeled as a function of age, the main effect of pubertal stage was significantly associated with CT in seven regions (Fig. S2, S3). Additional significant sex by pubertal stage interactions were identified in five ROIs when modeling pubertal stage and CT as a function of age (Fig. 3; all p’s < 0.05, see Table S3). While the effects of puberty on CT in the lingual gyrus appeared to be flat (age-invariant) for both sexes, the effect was stronger for females as compared to males (F=10.934). The effect of pubertal stage on CT in the fusiform gyrus (F=11.578), isthmus cingulate (F=11.160), and precuneus (F=6.277) was nonlinear across age (e.g., age-variant), but generally demonstrated that more advanced pubertal stage was associated with thinner cortices for both sexes, with the effect being stronger for females than for males. In contrast, patterns diverged in the paracentral gyrus (F=5.196), where for males the effect of pubertal stage was age-invariant (e.g., flat across age), and for females, the negative effect of pubertal stage on CT was nonlinear and strengthened with age. These effects are illustrated in Fig. 3b and summary values of non-standardized effects can be found in Table S4.
Fig. 3.
Interaction Effects Between Sex and Pubertal Stage to Predict Cortical Thickness Modeled as a Function of Age. Figure caption. Results from the penalized function-on-function regression analysis where significant sex-by-puberty interaction effects predicted cortical thickness, modeled as a function of age. Slopes indicate non-standardized beta coefficients. 3a: Spatial mapping of pubertal effects by sex, thresholded to display only significant regions of interest based on interaction effects. 3b: Graphical depiction of interaction effects across age in significant regions of interest.
3.2. Aim 2: cortical thickness change and clinical outcomes
3.2.1. Aim 2 descriptives
Descriptives in the Aim 2 subsample largely mirrored those in the larger sample both at Baseline and Y2 (Table S1). There were no significant differences in internalizing problems or suicide ideation between male and female youth at Baseline. At Y2, female youth had higher internalizing problems (mean=5.170) and were more likely to endorse suicide ideation (8.7%) than male youth (mean=4.668 and 6.5%, respectively).
3.2.2. Aim 2 linear mixed effect and logistic regression analyses
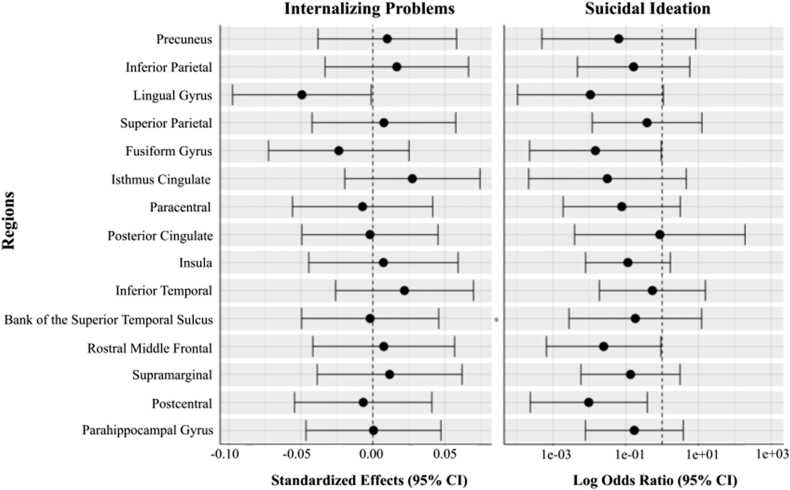
There were no significant three-way interactions to predict Y2 internalizing problems or suicide ideation (Tables S4, S5). To prioritize simplicity and interpretability in the models, we removed the three-way interaction and examined model results while retaining all two-way interactions. We did not identify any significant interaction effects between CT change and sex in predicting internalizing problems or suicide ideation (Fig. 4; Table S7, S23). We also did not identify any significant main effects of CT change to predict either outcome (Fig. S4). Though not of primary interest, more advanced pubertal stage at Baseline significantly predicted higher Y2 internalizing problems, but not suicide ideation, across all models (Tables S8-S38 for full model results).
Fig. 4.
Interaction Effects of Cortical Thickness Change by Sex on Clinical Outcomes. Figure Caption. Forest plots depicting the interaction between biological sex and cortical thickness change to predict internalizing problems and suicidal ideation. Dashed vertical line corresponds to the null value. The horizontal whiskers correspond to the 95% confidence intervals of the standardized beta coefficients (internalizing problems models) and log odds ratios (suicide ideation models).
4. Discussion
Understanding brain changes that children undergo as they traverse the tumultuous years bridging childhood and adolescence is critical, both for advancing foundational knowledge about the human condition, and for understanding the neural underpinnings of differential risk for psychopathology in adolescence. Here we report sex differences in how pubertal development impacts CT across time in a large, longitudinal sample of children entering the pre-adolescent and early adolescent period. Posterior brain regions were the most frequently represented among the fifteen ROIs that evidenced significant sex differences in effects of puberty on CT adjusting for age. Effects for four of these ROIs, all of which were medial posterior cortex regions, showed significant nonlinear effects when modeling puberty and CT as a function of age. Our results demonstrated that in these regions, the inverse association between pubertal development and CT, where advanced pubertal stages bring thinner cortices, was stronger for female youth than male youth of these ages. Among the regions demonstrating sex differences in this age group, there were no significant links between cortical development and early adolescent psychopathology. Indeed, when considering our primary constructs of interest (CT, sex, pubertal development, and age), the only significant relationship was Baseline pubertal stage predicting Y2 internalizing problems. While identified effects are small based on traditional heuristics, significant effects in Aim 1 were predominantly average with respect to effect size benchmarks for the ABCD Study (Owens et al., 2021).
The extant literature highlights the complex, spatially heterogeneous interactions between sex, age, and pubertal timing that relate to CT during late childhood and early adolescent development. The current analyses add nuance to this literature. While past research has identified a linear association between cortical thinning and increased Tanner stage (Vijayakumar et al., 2021), we found no evidence for effects of pubertal stage on CT that were both sex- and age-invariant. Instead, we identified a number of sex differences in these effects and as well as nonlinear effects that varied across age. Our results further underscore the spatially heterogeneous nature of these findings, with sex differences found in pubertal effects on CT in posterior brain regions and sex-adjusted, but age-variant, pubertal effects on CT found almost entirely in frontal brain regions (Fig. S2, S3). Developmental timing may be one factor driving this identified pattern, as posterior brain regions generally mature earlier in the life course (Gogtay et al., 2004, Tamnes et al., 2010) and frontal regions undergo protracted maturation well into early adulthood (Giedd et al., 1999, Tamnes et al., 2010). Further, it remains an important context that female and male youth in our sample are misaligned on pubertal status based on age.
Interestingly, these sex-different regions largely encompass pathways relevant for social perception (e.g., visual regions, social-cognition) and encoding episodic memories. Five of the fifteen sex-different effects involve regions associated with visual processing; the lingual gyrus, implicated in visual recognition and episodic memory consolidation (Kukolja et al., 2016), fusiform gyrus, implicated in object and face perception (Weiner and Zilles, 2016), inferior temporal gyrus, implicated in high level object recognition, salience allocation, and other more advanced aspects of visual perception (Herath et al., 2001), inferior parietal cortex which regulates directed attention to stimuli (Singh-Curry and Husain, 2009) with particular relevance for language and social cognition (Bzdok et al., 2016), and parahippocampal cortex, implicated in visuospatial processing (Aminoff et al., 2013). Further, the middle frontal cortex has been implicated in mediating broader social functioning such as response selection and social monitoring (Rajah et al., 2011). Two of the identified regions, the posterior cingulate cortex (Mar, 2011) and superior temporal sulcus (Beauchamp, 2015), have been implicated in instantiating theory of mind, a set of skills that impact social development and interactions (Hughes and Leekam, 2004). Among the sex-different regions are also those that facilitate self-referential thought, including the posterior cingulate cortex, thought to support internally-directed cognition (Leech and Sharp, 2014) and the precuneus, a node of the default mode network which is thought to instantiate self-referential thoughts (Raichle et al., 2001). Finally, some of the identified regions facilitate the manipulation of working and episodic memories, among them are the parahippocampal gyrus, implicated in episodic memory (Aminoff et al., 2013), superior parietal cortex, shown to be crucial for manipulating information in working memory (Koenigs et al., 2009), isthmus cingulate, the tract that connects the posterior cingulate to the parahippocampal gyrus which has been implicated in episodic memory (Nielsen et al., 2005), and precuneus, which is involved in the recall of episodic memories (Raichle, 2015).
Sex differences showing a stronger relationship between advanced pubertal stage and thinner cortices in female versus male youth may help explain why female youth experience earlier maturation (e.g., synaptic pruning) of these regions than their male peers, given the cumulative effects of pubertal hormones on cortical structures as they develop. This process may be quite consequential for female youth undergoing the transition to adolescence as the functioning and development of these regions are likely critical to effective social perception, communication, and interactions through building appropriate social schemas and self-concept (e.g., Pfeifer and Peake, 2011). These functions are especially crucial for youth as they navigate the complex and ever-changing demands of seeking and obtaining social support from peers, a process that increases in salience in adolescence (Berndt, 1982) and might be related to changes in neurobiology following pubertal onset (Nelson et al., 2005). Greater sensitivity to the impact of pubertal development in these posterior brain regions may in part underlie phenomena in the stress-response and peer relations literatures, such as the “tend-and-befriend” theory which posits that female adolescents and early adults rely more heavily on social connections than their male peers in responding to stressors (Byrd-Craven et al., 2015), as well as findings that female adolescents are more sensitive to social evaluation than males (Rose and Rudolph, 2006). Moreover, gender socialization may be a mechanism bidirectionally associated with brain development through social demands placed on individuals based on their sex (despite gender identity); such demands may be greater for female, as compared to male, youth and increase in intensity in adolescence (Brooks-Gunn and Petersen, 1983, Wichstrøm, 1999), thus continuing to interact with brain development over time.
Given the great distress and dysfunction associated with psychopathology and the safety concerns associated with suicidality specifically, identifying those at most risk for these outcomes is crucial to averting suffering and death. However, we were unable to detect patterns of cortical development in this age group that were significantly linked to clinical problems at ages 11–12 that were strong enough to maintain significance after accounting for multiple comparisons. These null findings may be a function of our sample, who have not yet reached the age for the typical onset of mood disorders and suicidality. Nevertheless, the preliminary signals we were able to identify may be important threads to follow up on in future research in this sample as they progress through adolescence, as many of the ROIs highlighted (e.g., lingual gyrus, isthmus cingulate, precuneus, fusiform gyrus) have been implicated in research on internalizing symptoms, depression, and suicidality in adolescence and adulthood (Cheng et al., 2018, Jung et al., 2014, Ries et al., 2009, Smolker et al., 2022, Westlund Schreiner et al., 2019, Wiglesworth et al., 2021).
4.1. Strengths, limitations, and future directions
A notable strength of the ABCD Study is its status as the largest epidemiologically informed, longitudinal brain development study of this age group to date, providing the opportunity to examine relatively nuanced developmental patterns. Yet, the present study only begins to account for the complexities of puberty. Our assessment of pubertal status relies on parent reports through the PDS. The gold standard to assess pubertal status is Tanner staging through a physical examination by a trained clinician (Dorn and Biro, 2011). Though the PDS has limitations compared to Tanner staging (e.g., captures less information about the early stages of puberty), this form of assessment is valid, non-invasive, and requires fewer resources to conduct (Cheng et al., 2021).
Future research may include other estimates of pubertal development, such as pubertal hormones, and do so beginning at younger ages. These hormones may also act as mechanisms driving associations between puberty and cortical development. For example, increased estradiol in females between ages 10–14 is associated with greater thinning in middle temporal regions (Herting and Sowell, 2017). Complex age by sex interactions are found in the association between testosterone and CT in select regions (e.g., left post cingulate cortex, dorsolateral prefrontal cortex, somatosensory cortex), where there is change in strength or direction across age (sample age range 4–22 years; for the Brain Development Cooperative Group., 2013, Nguyen et al., 2013). Children in our study are at the typical age for entering gonadarche (the second stage of puberty that begins the cascade of physical changes; Dorn et al., 2006; Rosenfield et al., 2009), which offers the potential for capturing the full spectrum of this pubertal process. Processes jump started in adrenarche, which onsets around age 6, may be of interest in mechanistically setting the stage for adolescent neurodevelopment. Indeed, increased production of adrenal hormones during adrenarche is associated with structural brain development in adolescence (Herting and Sowell, 2017, Vijayakumar et al., 2021) as well as psychopathology risk, progression, and treatment (Byrne et al., 2017, Cumberland et al., 2021). In addition, testosterone and estradiol concentration and the distribution of hormone receptors may influence cortical development (e.g., Bramen et al., 2012; Peper et al., 2009), though more work is needed to examine this possibility considering the potential importance of account for age and sex variability in effects.
Another strength of our study includes the use of a flexible statistical tool that accommodates multiple functional predictors as well as both linear and nonlinear effects. In using this PFFR approach, we were able to model the complex associations between a number of relevant variables over time. Contrasting our LMER and PFFR results emphasizes the usefulness of new approaches that allow for modeling age-invariance in potentially disentangling the associations between puberty and cortical maturation. However, this statistical approach has some limitations in that it is unable to incorporate random effects to account for the nested structure of the data. As such, it is possible that the p-values reported by the PFFR are too small, thus increasing the risk for false-positives. Our logistic regression models have similar limitations, as using a random effect to account for site effect in those models led to instabilities in model fit, likely due to the small number of those who expressed suicide ideation. However, in both sets of analyses, we accounted for a portion of the site effect using the MRI platform as a proxy variable and used a Bonferroni correction for our p-values, which is more conservative than other statistical corrections. Variability of the pubertal stage data is another statistical limitation; although there is greater variability in pubertal stage at Baseline than there is in pubertal development from Baseline to Y2, both were critical to consider in our Aim 2 analyses.
The large study sample afforded us the possibility to examine clinical outcomes of interest prior to the typical onset of internalizing psychopathology and suicidal ideation, despite the sample being recruited from the general population rather than being enriched for psychopathology. However, given that participants were not recruited to form a high-risk sample, it is important to acknowledge that there was relatively limited variability of parent reported internalizing problems, and there were a relatively small percentage of youth who endorsed suicidal ideation. Moreover, we only examined unidirectional effects. Research would benefit from exploring the potential for bidirectional effects between brain maturation and psychopathology across development. Early childhood internalizing psychopathology predicts CT of the insula, subgenual cingulate, and inferior parietal cortex (Luking et al., 2021) and early onset of major depressive disorder during childhood is associated with lower brain volume and greater cortical thinning (Luby et al., 2016). There is a particular urgency to continue to work toward early identification of those at risk for suicidality given that adolescents who attempt suicide are most likely to do so within one to two years of the onset of suicidal ideation (Glenn et al., 2017). Given the significant individual difference that exists in pubertal timing, hormone levels, and neurodevelopment, future work might benefit from employing methods that explore the impact differences along these dimensions within individuals (e.g., see Bendezú et al., 2022 for approach).
5. Conclusions
These analyses shed light on the dynamic, age- and sex-varying effects of puberty on brain development in the years bridging late childhood to early adolescence. Sex differences in how pubertal development impacts CT across age were identified in posterior regions of the brain, where the effect of pubertal development on thickness (more advanced pubertal stage being associated with thinner cortex) were stronger for female youth than male youth. The current findings offer new insights that begin to disentangle the complex effects of puberty, sex, and age in brain development during this critical pre-adolescent to adolescent window, and although the results did not relate to psychopathology at this early stage of development, the findings suggest avenues for future research investigating the neurodevelopmental patterns predisposing youth for developing psychopathology later in adolescence.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the children, adolescents, and family members who participated in the study. This project was supported by NIH grant R01MH122473 (Author K.R.C.). Author A.W. is funded by the National Science Foundation Graduate Research Fellowship Program. Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from Data Release Version 4.0. DOIs can be found at http://dx.doi.org/10.15154/1523041.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2022.101195.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
The authors do not have permission to share data but the code used for this study is available upon request from the authors.
References
- Abreu A.P., Kaiser U.B. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016;4(3):254–264. doi: 10.1016/S2213-8587(15)00418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach T.M., Ruffle T.M. The child behavior checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr. Rev. 2000;21(8):265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- Aminoff E.M., Kveraga K., Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 2013;17(8):379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AnonCenters for Disease Control and Prevention, National Center for Health Statistics. (2021). Underlying Cause of Death 1999–2020 on CDC WONDER Online Database.〈http://wonder.cdc.gov/ucd-icd10.html〉.
- Ball G., Beare R., Seal M.L. Charting shared developmental trajectories of cortical thickness and structural connectivity in childhood and adolescence. Hum. Brain Mapp. 2019;40(16):4630–4644. doi: 10.1002/hbm.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M., Albaugh M.D., Avenevoli S., Chang L., Clark D.B., Glantz M.D., Hudziak J.J., Jernigan T.L., Tapert S.F., Yurgelun-Todd D., Alia-Klein N., Potter A.S., Paulus M.P., Prouty D., Zucker R.A., Sher K.J. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev. Cogn. Neurosci. 2018;32:55–66. doi: 10.1016/j.dcn.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendse M.E.A., Byrne M.L., Flournoy J.C., McNeilly E.A., Guazzelli Williamson V., Barrett A.-M.Y., Chavez S.J., Shirtcliff E.A., Allen N.B., Pfeifer J.H. Multimethod assessment of pubertal timing and associations with internalizing psychopathology in early adolescent girls. J. Psychopathol. Clin. Sci. 2021;131(1):14. doi: 10.1037/abn0000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp M.S. The social mysteries of the superior temporal sulcus. Trends Cogn. Sci. 2015;19(9):489–490. doi: 10.1016/j.tics.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezú J.J., Thai M., Wiglesworth A., Cullen K.R., Klimes-Dougan B. Adolescent stress experience–expression–physiology correspondence: links to depression, self-injurious thoughts and behaviors, and frontolimbic neural circuity. J. Affect. Disord. 2022;300:269–279. doi: 10.1016/j.jad.2021.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt T.J. The features and effects of friendship in early adolescence. Child Dev. 1982;53(6):1447–1460. doi: 10.2307/1130071. [DOI] [Google Scholar]
- Bitsko R.H., Holbrook J.R., Ghandour R.M., Blumberg S.J., Visser S.N., Perou R., Walkup J.T. Epidemiology and impact of health care provider–diagnosed anxiety and depression among US children. J. Dev. Behav. Pediatr.: JDBP. 2018;39(5):395–403. doi: 10.1097/DBP.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen J.E., Hranilovich J.A., Dahl R.E., Chen J., Rosso C., Forbes E.E., Dinov I.D., Worthman C.M., Sowell E.R. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J., Petersen A.C., editors. Girls at Puberty: Biological and Psychosocial Perspectives. Springer; US: 1983. [DOI] [Google Scholar]
- Byrd-Craven J., Auer B.J., Kennison S.M. Sex differences in salivary cortisol responses to sex-linked stressors: a test of the tend-and-befriend model. Adapt. Hum. Behav. Physiol. 2015;1(4):408–420. doi: 10.1007/s40750-014-0013-1. [DOI] [Google Scholar]
- Byrne M.L., Whittle S., Vijayakumar N., Dennison M., Simmons J.G., Allen N.B. A systematic review of adrenarche as a sensitive period in neurobiological development and mental health. Dev. Cogn. Neurosci. 2017;25:12–28. doi: 10.1016/j.dcn.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D., Hartwigsen G., Reid A., Laird A.R., Fox P.T., Eickhoff S.B. Left inferior parietal lobe engagement in social cognition and language. Neurosci. Biobehav. Rev. 2016;68:319–334. doi: 10.1016/j.neubiorev.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M., Soules M.E., Teslovich T., Dellarco D.V., Garavan H., Orr C.A., Wager T.D., Banich M.T., Speer N.K., Sutherland M.T., Riedel M.C., Dick A.S., Bjork J.M., Thomas K.M., Dale A.M. The Adolescent Brain Cognitive Development (ABCD) study: imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha C.B., Franz P.J., M. Guzmán E., Glenn C.R., Kleiman E.M., Nock M.K. Annual Research Review: Suicide among youth - epidemiology, (potential) etiology, and treatment. J. Child Psychol. Psychiatry. 2018;59(4):460–482. doi: 10.1111/jcpp.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T.W., Magis-Weinberg L., Guazzelli Williamson V., Ladouceur C.D., Whittle S.L., Herting M.M., Uban K.A., Byrne M.L., Barendse M.E.A., Shirtcliff E.A., Pfeifer J.H. A researcher’s guide to the measurement and modeling of puberty in the ABCD Study® at baseline. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.608575. 〈https://www.frontiersin.org/article/10.3389/fendo.2021.608575〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W., Rolls E.T., Qiu J., Yang D., Ruan H., Wei D., Zhao L., Meng J., Xie P., Feng J. Functional connectivity of the precuneus in unmedicated patients with depression. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging. 2018;3(12):1040–1049. doi: 10.1016/j.bpsc.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Cumberland A.L., Hirst J.J., Badoer E., Wudy S.A., Greaves R.F., Zacharin M., Walker D.W. The enigma of the adrenarche: identifying the early life mechanisms and possible role in postnatal brain development. Int. J. Mol. Sci. 2021 doi: 10.3390/ijms22094296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dorn L.D., Biro F.M. Puberty and its measurement: a decade in review. J. Res. Adolesc. 2011;21(1):180–195. doi: 10.1111/j.1532-7795.2010.00722.x. [DOI] [Google Scholar]
- Dorn L.D., Dahl R.E., Woodward H.R., Biro F. Defining the boundaries of early adolescence: a user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Appl. Dev. Sci. 2006;10(1):30–56. doi: 10.1207/s1532480xads1001_3. [DOI] [Google Scholar]
- Emmanuel, M., & Bokor, B.R. (2022). Tanner Stages. In StatPearls. StatPearls Publishing. 〈http://www.ncbi.nlm.nih.gov/books/NBK470280/〉. [PubMed]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J.E., Offord D.R. Epidemiology of childhood depressive disorders: a critical review. J. Am. Acad. Child Adolesc. Psychiatry. 1990;29(4):571–580. doi: 10.1097/00004583-199007000-00010. [DOI] [PubMed] [Google Scholar]
- Garavan H., Bartsch H., Conway K., Decastro A., Goldstein R.Z., Heeringa S., Jernigan T., Potter A., Thompson W., Zahs D. Recruiting the ABCD sample: design considerations and procedures. Dev. Cogn. Neurosci. 2018;32:16–22. doi: 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10) doi: 10.1038/13158. Article 10. [DOI] [PubMed] [Google Scholar]
- Gifuni A.J., Chakravarty M.M., Lepage M., Ho T.C., Geoffroy `Marie-Claude, Lacourse E., Gotlib I.H., Turecki G., Renaud J., Jollant F. Brain cortical and subcortical morphology in adolescents with depression and a history of suicide attempt. J. Psychiatry Neurosci. 2021;46(3):E347–E357. doi: 10.1503/jpn.200198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn C.R., Lanzillo E.C., Esposito E.C., Santee A.C., Nock M.K., Auerbach R.P. Examining the course of suicidal and nonsuicidal self-injurious thoughts and behaviors in outpatient and inpatient adolescents. J. Abnorm. Child Psychol. 2017;45(5):971–983. doi: 10.1007/s10802-016-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay, N., Giedd, J.N., Lusk, L., Hayashi, K.M., Greenstein, D., Vaituzis, A.C., Nugent, T.F., Herman, D.H., Clasen, L.S., Toga, A.W., Rapoport, J.L., & Thompson, P.M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174–8179. 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed]
- Goldsmith J., Scheipl F., Huang L., Wrobel J., Di C., Gellar J., Harezlak J., McLean M.W., Swihart B., Xiao L., Crainiceanu C., Reiss P.T. Refund: regression with functional data. R. Package Version. 2021;0:1–24. 〈https://CRAN.R-project.org/package=refund〉 [Google Scholar]
- Hagler D.J., Hatton SeanN., Cornejo M.D., Makowski C., Fair D.A., Dick A.S., Sutherland M.T., Casey B.J., Barch D.M., Harms M.P., Watts R., Bjork J.M., Garavan H.P., Hilmer L., Pung C.J., Sicat C.S., Kuperman J., Bartsch H., Xue F., Dale A.M. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage. 2019;202 doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward C., Sanborn K. Puberty and the emergence of gender differences in psychopathology. J. Adolesc. Health. 2002;30(4, Supplement 1):49–58. doi: 10.1016/S1054-139X(02)00336-1. [DOI] [PubMed] [Google Scholar]
- Herath P., Kinomura S., Roland P.E. Visual recognition: evidence for two distinctive mechanisms from a PET study. Hum. Brain Mapp. 2001;12(2):110–119. doi: 10.1002/1097-0193(200102)12:2<110::AID-HBM1008>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., Sowell E.R. Puberty and structural brain development in humans. Front. Neuroendocrinol. 2017;44:122–137. doi: 10.1016/j.yfrne.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C., Leekam S. What are the links between theory of mind and social relations? Review, reflections and new directions for studies of typical and atypical development. Soc. Dev. 2004;13(4):590–619. doi: 10.1111/j.1467-9507.2004.00285.x. [DOI] [Google Scholar]
- Ivanescu A.E., Staicu A.-M., Scheipl F., Greven S. Penalized function-on-function regression. Comput. Stat. 2015;30(2):539–568. doi: 10.1007/s00180-014-0548-4. [DOI] [Google Scholar]
- Jung J., Kang J., Won E., Nam K., Lee M.-S., Tae W.S., Ham B.-J. Impact of lingual gyrus volume on antidepressant response and neurocognitive functions in Major Depressive Disorder: a voxel-based morphometry study. J. Affect. Disord. 2014;169:179–187. doi: 10.1016/j.jad.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Kobak K., Birmaher B., de Lacy N. KSADS-COMP perspectives on child psychiatric diagnostic assessment and treatment planning. J. Am. Acad. Child Adolesc. Psychiatry. 2021;60(5):540–542. doi: 10.1016/j.jaac.2020.08.470. [DOI] [PubMed] [Google Scholar]
- Koenigs M., Barbey A.K., Postle B.R., Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J. Neurosci. 2009;29(47):14980–14986. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn P.C.M.P., Crone E.A. Sex differences and structural brain maturation from childhood to early adulthood. Dev. Cogn. Neurosci. 2013;5:106–118. doi: 10.1016/j.dcn.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukolja J., Göreci D.Y., Onur Ö.A., Riedl V., Fink G.R. Resting-state fMRI evidence for early episodic memory consolidation: Effects of age. Neurobiol. Aging. 2016;45:197–211. doi: 10.1016/j.neurobiolaging.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Lawrence H.R., Burke T.A., Sheehan A.E., Pastro B., Levin R.Y., Walsh R.F.L., Bettis A.H., Liu R.T. Prevalence and correlates of suicidal ideation and suicide attempts in preadolescent children: a US population-based study. Transl. Psychiatry. 2021;11(1):489. doi: 10.1038/s41398-021-01593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Gogtay N., Greenstein D.K., Wells E.M., Wallace G.L., Clasen L.S., Blumenthal J.D., Lerch J., Zijdenbos A.P., Evans A.C., Thompson P.M., Giedd J.N. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn P. Major depressive disorder in older adolescents: prevalence, risk factors, and clinical implications. Clin. Psychol. Rev. 1998;18(7):765–794. doi: 10.1016/S0272-7358(98)00010-5. [DOI] [PubMed] [Google Scholar]
- López-Ojeda W., Hurley R.A. Sexual dimorphism in brain development: influence on affective disorders. J. Neuropsychiatry Clin. Neurosci. 2021 doi: 10.1176/appi.neuropsych.20100269. [DOI] [PubMed] [Google Scholar]
- Loso H.M., Dube S.L., Chaarani B., Garavan H., Albaugh M., Ivanova M., Potter A. Sex differences in psychopathology in a large cohort of nine and ten-year-olds. Psychiatry Res. 2021;302 doi: 10.1016/j.psychres.2021.114026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J.L., Belden A.C., Jackson J.J., Lessov-Schlaggar C.N., Harms M.P., Tillman R., Botteron K., Whalen D., Barch D.M. Early childhood depression and alterations in the trajectory of gray matter maturation in middle childhood and early adolescence. JAMA Psychiatry. 2016;73(1):31. doi: 10.1001/jamapsychiatry.2015.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking K.R., Jirsaraie R.J., Tillman R., Luby J.L., Barch D.M., Sotiras A. Timing and type of early psychopathology symptoms predict longitudinal change in cortical thickness from middle childhood into early adolescence. Biol. Psychiatry.: Cogn. Neurosci. Neuroimaging. 2021 doi: 10.1016/j.bpsc.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar R.A. The neural bases of social cognition and story comprehension. Annu. Rev. Psychol. 2011;62(1):103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly E.A., Saragosa-Harris N., Mills K., Dahl R., Magis-Weinberg L. Reward sensitivity and internalizing symptoms during the transition to puberty: An examination of 9-and 10-year-olds in the ABCD Study. Dev. Cogn. Neurosci. 2022;58:101172. doi: 10.1016/j.dcn.2022.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McCLURE E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35(2):163–174. doi: 10.1017/S0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nguyen T.-V., McCracken J., Ducharme S., Botteron K.N., Mahabir M., Johnson W., Israel M., Evans A.C., Karama S., for the Brain Development Cooperative Group. Testosterone-related cortical maturation across childhood and adolescence. Cereb. Cortex. 2013;23(6):1424–1432. doi: 10.1093/cercor/bhs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.-V., McCracken J.T., Ducharme S., Cropp B.F., Botteron K.N., Evans A.C., Karama S. Interactive effects of dehydroepiandrosterone and testosterone on cortical thickness during early brain development. J. Neurosci. 2013;33(26):10840–10848. doi: 10.1523/JNEUROSCI.5747-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen F.Å., Balslev D., Hansen L.K. Mining the posterior cingulate: segregation between memory and pain components. NeuroImage. 2005;27(3):520–532. doi: 10.1016/j.neuroimage.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Owens M.M., Potter A., Hyatt C.S., Albaugh M., Thompson W.K., Jernigan T., Yuan D., Hahn S., Allgaier N., Garavan H. Recalibrating expectations about effect size: a multi-method survey of effect sizes in the ABCD study. PLoS One. 2021;16(9) doi: 10.1371/journal.pone.0257535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L.A., Ramos L., Segreti A., Brent D.A., Phillips M.L. Right superior temporal gyrus volume in adolescents with a history of suicide attempt. Br. J. Psychiatry. 2015;206(4):339–340. doi: 10.1192/bjp.bp.114.151316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper J.S., Brouwer R.M., Schnack H.G., van Baal G.C., van Leeuwen M., van den Berg S.M., Delemarre-Van de Waal H.A., Boomsma D.I., Kahn R.S., Hulshoff Pol H.E. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34(3):332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Petersen A.C., Crockett L., Richards M., Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Allen N.B. Puberty initiates cascading relationships between neurodevelopmental, social, and internalizing processes across adolescence. Biol. Psychiatry. 2021;89(2):99–108. doi: 10.1016/j.biopsych.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Peake S.J. Self-development: Integrating cognitive, socioemotional, and neuroimaging perspectives. Dev. Cogn. Neurosci. 2011;2(1):55–69. doi: 10.1016/j.dcn.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015;38(1):433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Raichle, M.E., MacLeod, A.M., Snyder, A.Z., Powers, W.J., Gusnard, D.A., & Shulman, G.L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98(2), 676–682. 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed]
- Rajah M.N., Languay R., Grady C.L. Age-related changes in right middle frontal gyrus volume correlate with altered episodic retrieval activity. J. Neurosci. 2011;31(49):17941–17954. doi: 10.1523/JNEUROSCI.1690-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan, A., Lee, Y., Stidd, R., Long, R., Greenstein, D., Clasen, L., Addington, A., Gogtay, N., Rapoport, J.L., & Giedd, J.N. (2010). Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proceedings of the National Academy of Sciences of the United States of America, 107(39), 16988–16993. https://doi.org/10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed]
- Rescorla L., Achenbach T., Ivanova M.Y., Dumenci L., Almqvist F., Bilenberg N., Bird H., Chen W., Dobrean A., Döpfner M., Erol N., Fombonne E., Fonseca A., Frigerio A., Grietens H., Hannesdottir H., Kanbayashi Y., Lambert M., Larsson B., Verhulst F. Behavioral and emotional problems reported by parents of children ages 6 to 16 in 31 societies. J. Emot. Behav. Disord. 2007;15(3):130–142. doi: 10.1177/10634266070150030101. [DOI] [Google Scholar]
- Ries M.L., Wichmann A., Bendlin B.B., Johnson S.C. Posterior cingulate and lateral parietal gray matter volume in older adults with depressive symptoms. Brain Imaging Behav. 2009;3(3):233–239. doi: 10.1007/s11682-009-9065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A.J., Rudolph K.D. A review of sex differences in peer relationship processes: potential trade-offs for the emotional and behavioral development of girls and boys. Psychol. Bull. 2006;132(1):98. doi: 10.1037/0033-2909.132.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfield R.L., Lipton R.B., Drum M.L. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009;123(1):84–88. doi: 10.1542/peds.2008-0146. [DOI] [PubMed] [Google Scholar]
- Rutter M., Caspi A., Moffitt T.E. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J. Child Psychol. Psychiatry. 2003;44(8):1092–1115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- Singh-Curry V., Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47(6):1434–1448. doi: 10.1016/j.neuropsychologia.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolker H.R., Snyder H.R., Hankin B.L., Banich M.T. Gray-matter morphometry of internalizing-symptom dimensions during adolescence. Clin. Psychol. Sci. 2022 doi: 10.1177/21677026211071091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H.R., Hankin B.L., Sandman C.A., Head K., Davis E.P. Distinct patterns of reduced prefrontal and limbic gray matter volume in childhood general and internalizing psychopathology. Clin. Psychol. Sci. 2017;5(6):1001–1013. doi: 10.1177/2167702617714563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Dev. Psychopathol. 2009;21(1):87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Østby Y., Fjell A.M., Westlye L.T., Due-Tønnessen P., Walhovd K.B. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb. Cortex. 2010;20(3):534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Herting M.M., Goddings A.-L., Meuwese R., Blakemore S.-J., Dahl R.E., Güroğlu B., Raznahan A., Sowell E.R., Crone E.A., Mills K.L. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J. Neurosci. 2017;37(12):3402–3412. doi: 10.1523/JNEUROSCI.3302-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger J.M., Nikolas M.A. A meta-analytic review of the association between pubertal timing and psychopathology in adolescence: Are there sex differences in risk. Psychol. Bull. 2017;143(9):903. doi: 10.1037/bul0000106. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Op de Macks Z., Shirtcliff E.A., Pfeifer J.H. Puberty and the human brain: Insights into adolescent development. Neurosci. Biobehav. Rev. 2018;92:417–436. doi: 10.1016/j.neubiorev.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., Youssef G.J., Allen N.B., Anderson V., Efron D., Hazell P., Mundy L., Nicholson J.M., Patton G., Seal M.L., Simmons J.G., Whittle S., Silk T. A longitudinal analysis of puberty‐related cortical development. NeuroImage. 2021;228 doi: 10.1016/j.neuroimage.2020.117684. [DOI] [PubMed] [Google Scholar]
- Weiner K.S., Zilles K. The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia. 2016;83:48–62. doi: 10.1016/j.neuropsychologia.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlund Schreiner M., Klimes-Dougan B., Cullen K.R. Neural correlates of suicidality in adolescents with major depression: resting-state functional connectivity of the precuneus and posterior cingulate cortex. Suicide Life-Threat. Behav. 2019;49(3):899–913. doi: 10.1111/sltb.12471. [DOI] [PubMed] [Google Scholar]
- Whittle S., Vijayakumar N., Simmons J.G., Allen N.B. Internalizing and externalizing symptoms are associated with different trajectories of cortical development during late childhood. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59(1):177–185. doi: 10.1016/j.jaac.2019.04.006. [DOI] [PubMed] [Google Scholar]
- Wichstrøm L. The emergence of gender difference in depressed mood during adolescence: the role of intensified gender socialization. Dev. Psychol. 1999;35(1):232. doi: 10.1037/0012-1649.35.1.232. [DOI] [PubMed] [Google Scholar]
- Wierenga L.M., Langen M., Oranje B., Durston S. Unique developmental trajectories of cortical thickness and surface area. NeuroImage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Wiglesworth A., Falke C.A., Fiecas M., Luciana M., Cullen K.R., Klimes-Dougan B. Brain signatures in children who contemplate suicide: learning from the large-scale ABCD study. Psychol. Med. 2021:1–10. doi: 10.1017/S0033291721004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, G., Wu, X., Liu, Z., Becker, B., Zhang, K., Kuang, N., Kang, J., Dong, G., Zhao, X.-M., Schumann, G., Feng, J., Sahakian, B.J., Robbins, T.W., Palaniyappan, L., & Zhang, J. (2021). Common and Disorders-Specific Cortical Thickness Alterations in Internalizing, Externalizing and Thought Disorders over a 2-year Period in the Preadolescents of the ABCD Study (p. 2021.09.02.21263005). medRxiv. 10.1101/2021.09.02.21263005. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The authors do not have permission to share data but the code used for this study is available upon request from the authors.