Abstract
The association between irritable bowel syndrome (IBS) and psychiatric and mood disorders may be more fundamental than was previously believed. Prenatal, perinatal, postnatal, and early-age conditions can have a key role in the development of IBS. Subthreshold mental disorders (SMDs) could also be a significant source of countless diverse diseases and may be a cause of IBS development. We hypothesize that stress-induced implicit memories may persist throughout life by epigenetic processes in the enteric nervous system (ENS). These stress-induced implicit memories may play an essential role in the emergence and maintenance of IBS. In recent decades, numerous studies have proven that hypnosis can improve the primary symptoms of IBS and also reduce noncolonic symptoms such as anxiety and depression and improve quality of life and cognitive function. These significant beneficial effects of hypnosis on IBS may be because hypnosis allows access to unconscious brain processes.
Keywords: IBS, Subthreshold mental disorders, Gut-brain axis, ENS with Implicit epigenetic long-term memory, Hypnotherapy
1. Introduction
There is growing evidence that psychiatric and mood disorders play a key role in the development of irritable bowel syndrome (IBS) [1–4]. IBS, a condition affecting the colon, is almost mystical because no anatomical cause can be detected on laboratory tests, X-rays, or biopsies. Although IBS is not life-threatening, it has a significant economic impact as well as a huge impact on quality of life and mental health [5,6].
We hypothesize that stress-induced implicit memories in the enteric nervous system (ENS) may persist throughout life by epigenetic mechanisms [7]; Furness, 2000). These stress-induced memories may play a key role in the emergence and maintenance of IBS. Namely, it is possible that IBS starts in the gut, and the gut probably drives psychological alterations in a main group of cases with functional gastrointestinal disorders (FGIDs) [8].
We also point out that the significant beneficial effects of hypnosis on IBS may be because hypnosis allows access to unconscious brain processes that modulate stress-associated unconscious (implicit) information via the gut-brain axis (GBA) [9–11]. As a result, subjective pain perception (visceral hypersensitivity, somatization) decreases in the CNS as well as the stress-related inflammatory response in the gut.
2. The enteric nervous system and the gut-brain axis
The enteric nervous system (ENS) is the nervous system of the gastrointestinal (GI) tract that has evolved over hundreds of millions of years. Recently, Spencer et al. [12] showed how the ENS produces propulsion along the gut that behaves similarly to other neural networks in the brain and spinal cord. Spencer et al. [12] revealed how all different neurochemical classes of myenteric neurons are temporally and spatially activated along the colon and generate propulsive contractions. This study confirmed again that the ENS is the ‘first brain’ in the evolutionary process.
The ENS is a very complex, autonomous nervous system that develops before and independently of the central nervous system (CNS) [13]. Although the ENS can function independently, the ENS and CNS communicate bidirectionally [14]. The ENS is the largest part of the peripheral nervous system that regulates and coordinates all bowel functions, and contains approximately 200–600 million neurons in humans [15,16]. The diversity of neuronal and glial subtypes and neurotransmitters in the ENS is as rich as that in the CNS [17]. The ENS is a network of neurons and glial cells located within the gut wall that is essential for control of gastrointestinal function. The ENS communicates with other cell types, including intestinal epithelial, endocrine, and immune cells, which influence numerous physiological responses at the level of the gut [14]. During embryonic development, neurons and glial cells of the ENS are mainly derived from pluripotent stem cells of the vagal neural crest that invade, proliferate, and migrate through the intestinal wall until the entire bowel is colonized [18,19]. The intestinal microbiome interacts with the ENS and affects intestinal physiology [20].
The gut-brain axis (GBA) is a bidirectional communication system between the gastrointestinal tract and brain. The GBA (broadly defined) includes the CNS; neuroendocrine system; neuroimmune systems; the hypothalamic pituitary adrenal axis (HPA); sympathetic and parasympathetic arms of the autonomic nervous system (ANS); ENS; vagus nerve; and gut microbiota and its metabolites, such as essential vitamins, secondary bile acids, amino acids, short-chain fatty acids (SCFAs), neurotransmitters, and hormones [21,22]. Kano et al. [23] found that IBS involves corticotropin-releasing hormone (CRH)-dependent dysregulation of the GBA. This supports the conceptualization of IBS as a disorder of brain-gut interactions with stress response systems, such as the ANS and HPA axis, serving as an important interface.
Through the GBA, these pathways and metabolites can influence behaviour, memory, learning, locomotion, stem cell proliferation and differentiation, neurotransmission pathways, activity-dependent synaptic plasticity, brain development, mental and neurodegenerative disorders, hormonal and immune system pathways, etc. [[24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]]. Microbe-derived metabolites can also modulate host metabolism via epigenetic regulators [36,37]. In addition, Kaelberer et al. [38] demonstrated the existence of a direct link between enteroendocrine (EC) cells and the brain. Namely, EC cells synapse with vagal neurons to transduce millisecond-long intestinal luminal signals by glutamate as a neurotransmitter. Furthermore, intestinal microbiota changes brain mechanisms that aid in threat processing [39].
Exposure to various factors, such as diet, toxins, drugs, antibiotics, pathogens, and psychological stress, can perturb the normal microbiota that causes dysbiosis, impairing communication via the GBA [[40], [41], [42]]. Impaired communication between the gut and brain axis can produce numerous intestinal and extraintestinal disorders (obesity, type 2 diabetes, glucose intolerance, insulin resistance, acne, atopic dermatitis, psoriasis, colorectal cancer, nonalcoholic fatty liver disease, IBS, Crohn's disease, ulcerative colitis, fibromyalgia, chronic pain, stroke, lung disease, celiac disease, and metabolic syndrome) as well as disorders of the nervous system [[43], [44], [45], [46], [47], [48]]. There is increasing evidence that the GBA axis plays a key role in the maintenance of brain homeostasis as well as in the development of major neurological and psychiatric disorders, such as Parkinson's disease (PD), Alzheimer's disease (AD), multiple sclerosis (MS), autism spectrum disorder (ASD), and major depressive disorder (MDD) [46,49,50]. It seems that the gut microbiota can affect almost all aspects of human development and health [51].
3. The enteric nervous system may have long-term memory
Studies have suggested that the ENS may be able of learning and memorizing [7,52,53]. Recently, Schemann et al. [7] proposed that the ENS may perform memorization and implicit learning; thus, it may work similarly to a “little brain” in the gut. One of the main comments by Gershon [54] on Schemann's statement [7] was that enteric neurons cannot survive for a lifetime, since 88% of ENS may be lost every two weeks [55]. Thus, the rapid exchange of enteric neurons is difficult to reconcile with the notion that the ENS has circuits that are able to cope with complex processes such as learning and memory [54].
However, there are studies that do not suggest such a high degree of neurogenesis [20,56] in the ENS, as reported by Kulkarni et al. [55]; and we should consider that the origin of differentiating cells is currently under debate. In addition, the latest studies question the 70-year-old paradigm, the Hebbian hypothesis of neuroscience plasticity, according to which the brain learns by modifying the strength of synapses [57,58]. Hodassman et al. [57] proposed that the neuron is not a binary unit that can fire or not and that a single neuron can realize learning algorithms that previously required an artificially complex network of thousands of interconnected neurons and synapses.
Furthermore, several studies have suggested that long-term memory (LTM) may exist by epigenetic mechanisms at the cellular level [[59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69]These findings support the notion that simple stress-induced implicit LTM may also persist throughout life by epigenetic mechanisms in the ENS.
4. Irritable bowel syndrome
Irritable bowel syndrome (IBS) is the most common functional gastrointestinal disorder (FGID) [70] that has no organic cause and cannot be detected by routine laboratory tests. The aetiology and pathogenesis of IBS are multifactorial. Hence, the underlying pathogenesis of IBS is complex, and the molecular pathophysiology is far from understood. According to Van Oudenhove et al. [71,72]: “It is generally accepted that functional gastrointestinal disorders (FGIDs) result from complex and reciprocal interactions between biological, psychological, and social factors, rather than from linear monocausal etiopathogenetic processes.”
According to the ROME III criteria in 2006, patients with IBS belong to one of the three predominant subtypes: IBS with constipation (IBS–C); IBS with diarrhoea (IBS-D); mixed IBS with alternating diarrhoea and constipation (IBS-M); and IBS-U is an unsubtyped IBS for people who do not fit into the IBS-D, IBS-C, and IBS-M types [73]. In 2016, the Rome IV criteria defined IBS as an FGID in which recurrent abdominal pain is associated with defecation or a change in bowel habits. This produced greater heterogeneity within diagnostic categories [74]. Namely, there was a 3-fold increase in the rate of a functional dyspepsia FD/IBS overlap diagnosis. However, the diagnosis of IBS is still controversial and is partly made on the basis of the exclusion of other diseases.
IBS substantially impairs quality of life, and the overall health-care costs are high. The prevalence of IBS ranges from 5% to 20%, depending on the country and criteria used to diagnose IBS [75]. If the incidence of IBS remains unchanged, projections of global population growth alone indicate that many more people will suffer from IBS worldwide in the future [76]. IBS is more frequent in women than men [77]. Various predisposing factors have been suggested for IBS, such as sex, age, psychological stress factors, early life events, gastrointestinal infections, altered gut microbiota, asthma and atopic disorders, diet, socioeconomic, family, and environmental factors, among others [[78], [79], [80]].
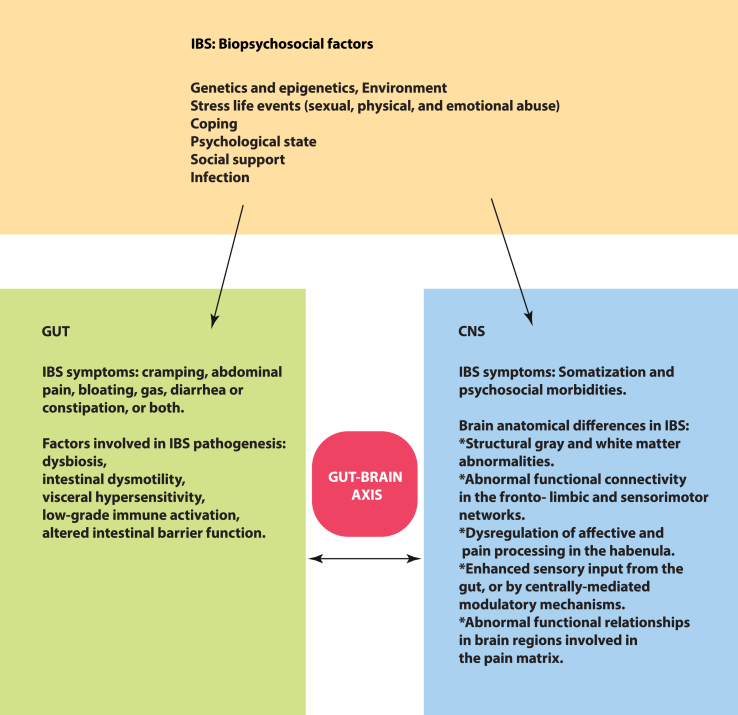
IBS is now considered a disorder of altered brain-gut interactions, where a biopsychosocial model helps in understanding the symptoms (see Fig. 1) [81]. The biopsychosocial model of IBS is useful in understanding FGIDs as complex conditions that involve complex interplay between biological, psychological, and (psycho)social factors. A 2019 meta-analysis [3] found that PTSD is significantly associated with an increased likelihood of IBS. This supports a biopsychosocial understanding and model for IBS. In addition, there is growing evidence that dysregulation in immune function is associated with IBS and could contribute to either aetiology or symptoms. Mast cells are immune cells in the gastrointestinal tract that are regarded as key components in inflammatory reactions and IBS pathophysiology [82,83].
Fig. 1.
Simple illustration about the biopsychosocial model of IBS with IBS symptoms and factors involved in IBS pathogenesis in the gut and CNS.
Current approaches in IBS treatment include dietary, probiotic, pharmacological (depending on the subtype, including antidepressants, antibiotics, peripherally restricted opioids, cannabis), psychotherapeutic (cognitive behavioral therapy and gut-directed hypnotherapy), and microbiota transplantation approaches, among others [[84], [85], [86], [87], [88], [89]]. Unfortunately, the present diagnostic criteria for FGIDs, as well as disease severity, frequency, duration, and treatment efficacy, are based solely on subjective patient reports without objective biomarkers [90,91].
5. IBS: comorbidity with mental diseases and subthreshold mental disorders
Although the role and importance of psychological factors in IBS are debated, a growing number of studies suggest that psychiatric and mood disorders may play a key role in the development and maintenance of IBS. Thirty to forty percent of patients with IBS have comorbid depression or anxiety disorder [92,93]. Approximately 60% of those seeking medical treatment for FGIDs suffer from a psychiatric illness [94]. Suicidal ideation occurs in 15–38% of patients with IBS [95]. A systematic review and meta-analysis found that the prevalence of IBS varies from 1% to more than 45%, according to the geographic location of the population under study [96]. Kabra and Nadkarni [97] found that the prevalence of depression and anxiety in IBS patients was 37.1 and 31.4%, respectively, in an Indian population. Newer meta-analysis studies show that depression and anxiety are significantly higher in IBS patients than in healthy controls [98,99]. A multivariate analysis of 769 IBS patients by Midenfjord et al. [2] revealed that the prevalence of depression and anxiety was 25.7% and 44.9%, respectively, in IBS patients. Hu et al. [100] conducted a network meta-analysis of 18 studies with 7095 participants. The authors found that IBS-C had the highest incidence of depression (38%) and anxiety (40%), followed by IBS-D, IBS-M, and IBS-U. In addition, IBS-M was associated with higher levels of depression and anxiety, and IBS-C had the highest prevalence of depression and anxiety. According to a systematic review and meta-analysis by Zamani et al. [101]; IBS patients presented threefold increased odds of either anxiety or depression compared to healthy controls. A 2019 meta-analysis by Ng et al. [3] contained a total of 648,375 subjects, specifically examining the association between PTSD and IBS, and found that PTSD was significantly associated with an increased likelihood of IBS. Interestingly, a large Korean study by Lee et al. [102] reported that the incidence rate of IBS was higher in patients with mild depression than in those with severe depression. Referring to the biopsychosocial model, Person and Keefer [103] wrote: “In this model, gastrointestinal disease can be seen as more than just an isolated pathophysiological process, but in the context of psychosocial factors that may be a key to the genesis and maintenance of the illness."
Subthreshold mental disorders (SMDs) (that do not meet full diagnostic criteria for mental disorders) are attracting increased attention in scientific research because SMDs can be an important source of countless other diseases and poor quality of life and thus may also be a cause of IBS development [[104], [105], [106], [107], [108], [109], [110], [111], [112], [113]]. SMDs are often undiagnosed, although the prevalence of SMDs is higher than that of diagnosed mental disorders [104]. Furthermore, SMDs include the same symptoms as diagnosed mental disorders, except that they differ in the number, duration, severity, and frequency of symptoms and exclusion criteria [107]. It is very probable that many people may have SMDs for a shorter or longer period of time or throughout their lives without diagnosis [112,114]. Therefore, SMDs could be a significant source of countless diverse diseases and may also be a cause of IBS development.
6. IBS: structural and functional brain changes
Although various studies have investigated structural and functional brain changes associated with IBS, the IBS-associated exact neural substrate is still unclear. Seminowicz et al. [115] found that IBS is associated with decreased grey matter density (GMD), including the medial prefrontal and ventrolateral prefrontal cortex, the posterior parietal cortex, the ventral striatum, and the thalamus. They proposed that morphometric alterations take place mainly in brain networks concerned with attention and emotion modulation, as well as in cortio-limbic pontine pain modulatory systems, and to a lesser degree, in networks processing interoceptive information.
The study by Zhao et al. [116] found that elderly IBS patients presented reduced fractional anisotropy (FA) in the callosum, upper corona, fornix, internal capsule, and caudex cerebri, indicating that white matter abnormalities occurred in IBS patients with grey matter abnormalities. Weng et al. [117]; by resting-state functional magnetic resonance imaging (rsfMRI), observed that IBS patients presented widely perturbed functional connectivity density (FCD). Furthermore, some areas with altered FCD had abnormal functional relationships in brain regions involved in the pain matrix of IBS patients.
Labus et al. [118] found increases and decreases in GMV and alterations in regional network properties in predominantly premenopausal female IBS patients. These changes could reflect different pathophysiological components of the disease processes underlying IBS symptoms. Namely, increased sensitivity to somatic and visceral stimuli (higher GMV in S1) was associated with an increase in emotional arousal (lower GMV in the hippocampus in IBS) and other chronic pain-related mechanisms (lower GMV in insula and cingulate cortices, differences in insula, cingulate, thalamus and brain stem network properties).
IBS is strongly associated with a high level of somatization and psychosocial morbidities [71,72,119]. In magnetic resonance imaging (MRI) experiments, Grinsvall et al. [120] found that somatization level was associated with differences in local grey matter covariance, mainly in regions of the prefrontal cortex, insula, and cerebellum in IBS. The authors proposed that prefrontal mechanisms may be more important than insular mechanisms in the neurobiological sensitization process associated with IBS high somatization.
Icenhour et al. [121]; by rsfMRI, studied resting-state functional connectivity (rsFC) within the default mode network (DMN), salience and sensorimotor networks in IBS patients with and without visceral hypersensitivity compared to healthy controls. Namely, the authors investigated group differences in FC in insular and cingulate subregions, thalamus, and amygdala, since it was demonstrated that these brain regions are activated by visceral stimulation [122]. Icenhour et al. [121] proposed that group differences related to visceral sensitivity under resting conditions could be caused by enhanced sensory input from the gut or by centrally mediated modulatory mechanisms.
The habenula is a small epithalamic complex nucleus that connects the limbic forebrain and midbrain. The habenula is involved in nociception, sleep-wake cycles, reproductive behaviour, and mood and has been implicated in the pathogenesis of various psychiatric disorders and pain [123,124]. Mao et al. [125] investigated habenular function in IBS patients by rsfMRI. They showed that IBS patients had altered rsfMRI and effective connectivity of the habenula associated with pain. These results may indicate the dysregulation of affective and pain processing in these disease conditions.
MRI investigations of patients with IBS with depressive symptoms (DEP-IBS) by Li et al. [32,33] suggest that depressive symptoms can act as mediators between gastrointestinal symptoms and GMV in the left insula, right medial prefrontal cortex, and right middle frontal gyrus, whereas gastrointestinal symptoms can act as mediators between depression and GMV in these areas. This suggests that convergent syndromic atrophy develops in the pain and emotional systems of patients with DEP-IBS. Li et al. [126,127], using rsfMRI, found that patients with DEP-IBS present abnormal functional connectivity (FC) in brain areas associated with the fronto-limbic and sensorimotor networks, especially the insula and supplementary motor area (SMA), which can elucidate the vicious circle between negative emotions and gastrointestinal symptoms in IBS.
7. IBS: prenatal, perinatal, postnatal, and early age stress, familial clustering and transfer across generations
According to foetal programming theory, exposure to prenatal suboptimal intrauterine conditions in later adulthood can predispose a person to developing chronic diseases [128]. However, there is growing evidence that prenatal, perinatal, postnatal, and early age conditions could predispose to developing chronic diseases in later adulthood [[129], [130], [131], [132]]. Maternal prenatal and postnatal anxiety and depression, neonatal maternal separation, and early adverse life events (EALs), such as sexual and physical abuse or emotional trauma, are significant vulnerability factors that predispose individuals to gastrointestinal diseases and development of adult IBS [133,134]. Maternal depression and anxiety are most common during pregnancy and the postpartum period [135]. Maternal prenatal stress influences the neurodevelopment and birth outcomes of offspring [136].
We identified neonatal maternal separation (MS) as an essential risk factor for neurodevelopmental disorders [137]. There is also growing evidence that perinatal, postnatal, and early age conditions can have an essential role in the development of IBS. Various studies have pointed out that MS, as an early adverse life event, can be particularly dangerous to newborns and induce visceral hypersensitivity [[138], [139], [140], [141], [142]].
Various studies have pointed out that IBS can accumulate in families and be inherited across generations [[143], [144], [145]]. Aguas et al. [146] found that the incidence of IBS was higher in first-degree blood relatives than in spouses of patients. The authors also suggested that genetic and psychological factors are more important than environmental factors. van den Wijngaard et al. [147] showed that MS-produced visceral hypersensitivity could be transferred across generations. In addition, this transfer depended on maternal care, which suggests that the MS model can be used to determine early life adverse triggers and mechanisms relevant to IBS. Twin studies also emphasize that genetic and psychological processes may explain familial clustering of IBS and that both heredity and social learning contribute to its aetiology [148–150]. A recent population-based twin cohort study [148] demonstrated that familial as well as intrauterine factors have significant roles in the cooccurrence of IBS and symptoms of anxiety and depression. The authors also revealed that both genetic factors and intrauterine factors can influence the associations between IBS, anxiety, and depression. In addition, IBS presents a higher concordance rate among monozygotic compared to dizygotic twins [151,152].
In their large cohort study, Waehrens et al. [153] revealed that many perinatal and familial factors could be independently associated with an increased risk of IBS. This suggests that perinatal and familial factors may have an important long-term role in the development of IBS. The increased risk of IBS among first-degree, second-degree, and third-degree relatives suggests an important genetic component of the family grouping of IBS [153]. Nevertheless, nongenetic factors also contribute to the increased risk among spouses, and the genetic component plays an essential role in the familial clustering of IBS [154]. According to Gazouli et al. [155]; environmental factors such as childhood trauma, physical and psychological stress, pathogens, and perturbed gut microbiota have key roles in the development of IBS.
8. Hypnosis
Hypnosis can be considered an altered state of consciousness (trancelike state) that allows the individual to recall memories or instruct the individual to change a particular behaviour. Using electroencephalography (EEG) and imaging methods, hypnosis can be well distinguished from other states of consciousness, such as normal wakefulness, deep relaxation, sleep, or meditation [156].
Numerous studies have revealed that hypnosis is a nonpharmacological and cost-effective method that can be used as an effective and safe method for the management of diverse conditions, such as pain, anxiety, mood disorders, sleep problems, depression and anxiety, wound healing, haemorrhage, stress and pain associated with medical and surgical procedures, and gastrointestinal disorders [9,[157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174]].
At present, the hypnosis-induced neurophysiological mechanisms are not yet well understood. Although various models have been suggested to explain these effects, the biopsychosocial model of hypnosis (biological, psychological, and social factors and their interactions) may serve as a better integration model [175].
According to neuroimaging studies, hypnosis suggestions create an altered functional association. These subjective changes are associated with corresponding changes in specific psychological functions within brain regions. Although there are methodological and subjective differences, numerous experiments suggest that a hypnotic state includes the insular cortex, anterior cingulate cortex (ACC), thalamus, increased pituitary-mesencephalic brainstem, increased activation of the occipital and dorsolateral prefrontal cortex (DLPFC), amygdaloid memory system, and decreased activation of the precuneus [9,[176], [177], [178]]. In addition, recently, we [9] and others [179] pointed out that hypnosis may produce changes in neuroplasticity and that epigenetic mechanisms may underlie these changes in synaptic plasticity.
Since hypnosis allows access to the unconscious brain and could modulate unconscious (implicit) information, it could be a significant adjuvant technique that is not used in everyday practice and is basically just being researched [10,180,181].
9. Hypotherapy and IBS
Since 1984, when Whorwell et al. published their outcomes of a small clinical trial in the Lancet that showed that hypnotherapy could relieve several of the symptoms of IBS, there has been an increased interest in researching the effectiveness of psychological treatment for IBS. Numerous experiments and studies have demonstrated the significant efficacy of hypnosis in the treatment of IBS [85,[182], [183], [184], [185], [186], [187], [188], [189], [190], [191], [192], [193]].
Gut-focused hypnotherapy (GFH) can not only improve the primary symptoms of IBS but also reduce noncolon symptoms, anxiety, and depression and improve quality of life and cognitive function [194,195]. Hypnotherapy is particularly effective in children with IBS [189,196,197]. Hasan et al. [198] demonstrated that Skype hypnotherapy, regarding IBS, produced significant improvements and was almost as effective as face-to-face treatment (outcomes of Skype hypnotherapy were 65% and face-to-face treatment was 76%). Rutten et al. [199] performed a randomized controlled trial to compare the cost-effectiveness of individual hypnotherapy performed by a qualified therapist with gut-directed hypnotherapy (GHT) by means of CD-recorded self-exercises at home in children with IBS or functional abdominal pain (FAP). They found that hypnotherapy with CD was comparable to (or slightly lower) hypnotherapy with a therapist. Peter et al. [200] studied the microbial composition of patients with IBS and psychological distress before and after GHT. The authors found that GHT reduced IBS symptoms and psychological burden. Nevertheless, only small changes were found in intestinal microbiota composition. The authors proposed that GHT may act by means of central nervous impact and further factors that are basically independent of microbiota composition, modulating the brain-gut axis, perhaps alterations in vagus nerve functioning and microbiota metabolism. Shahbazi et al. [201] compared the efficacy of standard medical treatment alone and hypnotherapy plus standard medical treatment on quality of life in IBS patients. The authors found that psychological intervention, especially hypnotherapy, in addition to standard medical therapy, can contribute to improving quality of life, pain and fatigue, and psychiatric disorders in treatment-resistant patients with IBS. In addition, therapeutic costs, hospital stays, and days off work can be reduced, and patient efficiency increased. Shahbazi et al. [201] suggested that gastroenterologists, psychologists, nurses, and psychiatrists cooperate in treating IBS patients. Hasan et al. [183] performed the largest randomized study of gut-focused hypnotherapy (GFH) to date. The authors found that six sessions of hypnotherapy produced similar levels of improvement in IBS symptoms compared with 12 sessions. However, the main limitation of this randomized study was the lack of long-term follow-up data to determine the persistence of the effects of abbreviated hypnotherapy, which should be examined in the future. The relevant studies supporting the utility of hypnotherapy in IBS treatment are shown in Table 1 [85,[182], [183], [184], [185], [186], [187], [188], [189],191,[193], [194], [195], [196], [197],199,202].
Table 1.
Relevant studies supporting the utility of hypnotherapy in IBS treatment.
| STUDY | OBJECTIVES OF THE STUDY | RESULTS |
|---|---|---|
| Whorwell et al. [202] | 30 patients with severe refractory IBS were randomly allocated to treatment with either hypnotherapy or psychotherapy and placebo. | The psychotherapy produced a small improvement in abdominal pain, abdominal distension, and general well-being but not in bowel habit. The hypnotherapy presented a dramatic improvement in all features and no relapses were recorded during the 3-month follow-up period. |
| Donnet et al. [194] | Recorded 150 IBS patients' perceptions and expectations of hypnotherapy as well as their symptom response. | Hypnotherapy improved symptoms and resulted in a wide range of additional benefits. Expectation did not necessarily influence the outcome. Recording IBS symptoms alone does not fully capture the patient's experience of treatment. |
| Sasegbon et al. [182] | Forty-four Asian patients with refractory IBS received 12-sessions of gut-directed hypnotherapy (GDH) using the Manchester protocol. | GDH was highly effective with similar response rates to outcomes in other IBS populations regardless of the ethnicity of the therapist. |
| Hasan et al. [183] | Randomized study of gut-focused hypnotherapy (GFH). 450 subjects with IBS were allocated to either 6 or 12 weekly sessions. | Compared with 12 sessions, 6 sessions of GFH produced similar levels of improvement in IBS symptoms, noncolonic symptoms, anxiety, depression, and quality of life. |
| Hoekman et al. [85] | Randomized, controlled, open-label trial to compare the effectiveness of hypnotherapy vs standard medical treatment (SMT) for IBS- type symptoms in 80 IBD patients. | The effectiveness of SMT and hypnotherapy for IBS-type symptoms was comparable in IBD patients. |
| Black et al. [184] | Searching the medical literature through January 2020 for randomized controlled trials assessing the efficacy of psychological therapies for adults with IBS, compared with each other or a control intervention. | Several psychological therapies are efficacious for IBS, although none were superior to another. CBT (cognitive behavioral therapy)- based interventions and gut-directed hypnotherapy had the largest evidence base and were the most efficacious long term. |
| Vasant et al. [185], | 32 young patients with IBS received 12 sessions of gut-focused hypnotherapy (GFH). | GFH was very effective in children and adolescents with IBS. Early intervention with GFH in childhood IBS may reduce the subsequent burden of this problem in adults. |
| Rutten et al. [199] | A systematic review to compare the effectiveness of gut-directed hypnotherapy (GDH) by means of home-based self-exercises using an audio CD with that of individual hypnotherapy (iHT) performed by qualified therapists. 132 children were assigned to the CD group and 128 to the iHT group. | The long-term effectiveness of home-based hypnotherapy with an audio CD is noninferior to iHT performed by therapists in pediatric IBS or functional abdominal pain (FAP). Treatment with hypnosis using an audio CD provides an attractive treatment option for these children. |
| Peters et al. [186] | A review of published literature and a systematic review of clinical trials about gut- directed hypnotherapy. | Gut-directed hypnotherapy has durable efficacy in patients with IBS and possibly ulcerative colitis. |
| Miller et al. [195] | 1000 consecutive patients with IBS received 12 sessions of hypnotherapy over 3 months. | The results of this audit confirmed that 3 months of hypnotherapy produced significant improvement in symptoms in this large group of refractory IBS patients, who were continuing to experience troubling symptoms despite multiple conventional interventions. |
| Gerson et al. [187] | Testing whether group gut-focused hypnotherapy would improve IBS in 75 patients. | The group hypnotherapy was effective in patients with IBS. |
| Rutten et al. [196] | A systematic review to assess the efficacy of gut-directed hypnotherapy in pediatric functional abdominal pain (FAP) and IBS patients. | The therapeutic effects of HT seem superior to standard medical care in children with FAP or IBS. It remains difficult to quantify the exact benefits. The need for more high-quality research is evident. |
| Lindfors et al. [188] | 208 patients received gut-directed hypnotherapy that was retrospectively evaluated. | This long-term follow-up study indicated that gut-directed hypnotherapy in refractory IBS is an effective treatment option with long- lasting effects, also when given outside highly specialized hypnotherapy centers. |
| Vlieger et al. [197] | This follow-up study investigated the long- term effects of gut-directed hypnotherapy vs. standard medical treatment plus standard supportive therapy (SMT) in 52 participants. | This follow-up study clearly demonstrated that the beneficial effects of gut-directed hypnotherapy are sustained over a period of 5 years in children with functional abdominal pain (FAP) or IBS. |
| Vlieger et al. [189] | 53 pediatric patients, aged 8–18 years, with functional abdominal pain (FAP) or IBS, were randomized to either hypnotherapy or standard supportive therapy (SMT). | Hypnotherapy was more effective, with a significantly greater reduction in pain scores compared with SMT. |
| Gonsalkorale et al. [191] | 204 patients prospectively completed questionnaires scoring symptoms, quality of life, anxiety, and depression before, immediately after, and up to six years following hypnotherapy. | This study demonstrated that the beneficial effects of hypnotherapy for IBS last at least five years. |
| Palsson et al. [193] | Patients with severe IBS received seven biweekly hypnosis sessions and used hypnosis audiotapes at home. | Rectal pain thresholds, rectal smooth muscle tone, and autonomic functioning were unaffected by hypnosis. Somatization and psychological distress showed large decreases. Hypnosis improved IBS symptoms through reductions in psychological distress and somatization. |
10. Summary with hypothesis
The ENS is the “first brain’ in the evolutionary process that is specifically a complex, autonomous nervous system that develops before and independently of the CNS [12,13]. The diversity of neuronal and glial subtypes and neurotransmitters in the ENS is similarly rich as in the CNS [17]. It was proposed that the ENS may perform implicit learning and memorization, and therefore, it may work similarly to a “little brain” in the gut [7,52,53].
Numerous studies have suggested that psychiatric and mood disorders may play a key role in the development and maintenance of IBS [2,[92], [93], [94],97].
SMDs could also be a considerable source of IBS development [104–113].
IBS develops mainly during the prenatal, perinatal, postnatal, and early adolescent stages, when the developing ENS is still very vulnerable to stress factors, which produces dysfunctions of the ENS and contributes to the development of chronic diseases in later adulthood [[129], [130], [131], [132],203,204]. Maternal prenatal and postnatal anxiety and depression, neonatal maternal separation (MS), and early adverse life events such as sexual and physical abuse or emotional trauma are substantial vulnerability factors that predispose to gastrointestinal diseases and development of adult IBS [133–142].
IBS can accumulate in families and be inherited across generations [143–145]. MS-induced visceral hypersensitivity can be transferred across generations [147]. The incidence of IBS is higher in first-degree blood relatives than in spouses of patients, and genetic and psychological factors can be more important than environmental factors [146]. Twin studies emphasize that genetic and psychological processes may explain familial clustering of IBS and that both heredity and social learning contribute to its aetiology [[148], [149], [150],205,206].
Quoted from Hurwitz [207]; “Somatization is the psychological mechanism whereby psychological distress is expressed in the form of physical symptoms”. IBS is strongly associated with somatization and psychosocial morbidities [71,72,119]. It is not surprising that somatization is often associated with psychological distress [208,209,210].
Various studies have demonstrated the significant efficacy of hypnosis in the treatment of IBS [85,[183], [184], [185], [186], [187], [188], [189], [190], [191], [192], [193]]. Gut-directed hypnotherapy can not only improve the primary symptoms of IBS but also reduce noncolon symptoms, anxiety, and depression and improve quality of life and cognitive function [194,195]. Hypnotherapy is particularly effective in children with IBS [189,196,197]. The favourable effects of hypnosis on IBS may be because hypnosis allows access to unconscious brain processes that could modulate stress-associated unconscious (implicit) information originating from the ENS [9,10,180,181] and the mind-brain axis in patients with IBS [11].
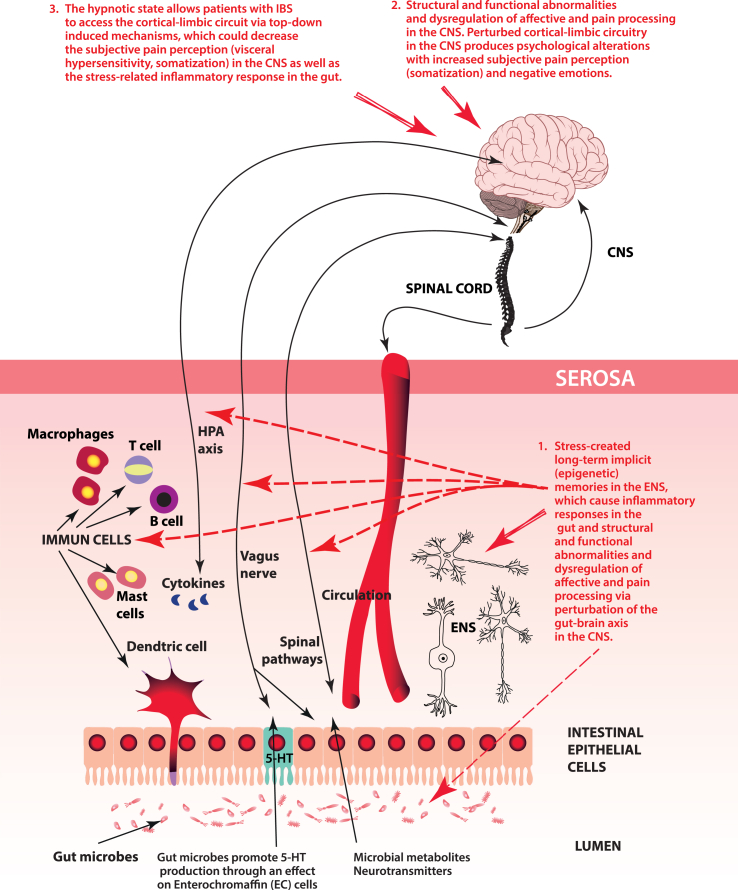
HYPOTHESIS (see Fig. 2): In most cases, mainly during prenatal, perinatal, postnatal, and early age states, (when the developing ENS and CNS are still very vulnerable) epigenetically inherited or/and psychologically induced stress (maternal separation, depression, anxiety, etc.) produce stress-induced long-term implicit memories in the ENS. These stress-induced memories may persist throughout life by epigenetic processes in the ENS. The ENS and CNS normally communicate bidirectionally via the gut-brain axis; however, the ENS can also function independently.
Fig. 2.
Visualization of the key aspects of our hypothesis.
Stress-induced long-term implicit ENS information can be transmitted to the CNS via the gut-brain axis, causing structural and functional abnormalities and dysregulation of affective and pain processing in the CNS. As a result, perturbed cortical-limbic circuits in the CNS produce psychological alterations with increased subjective pain perception (somatization) and negative emotions. The CNS tries to decrease the effect of the stress-induced signal from the ENS, although the ENS continuously sends stress-induced long-term (implicit) epigenetic information to the CNS via the gut-brain axis.
Under the hypnotic state, patients with IBS can access their emotional level, i.e., the cortical-limbic circuit, via top-down induced processes, which could decrease subjective pain perception (visceral hypersensitivity, somatization) in the CNS as well as the stress-related inflammatory response in the gut. This hypothesis may partially explain why there is no real effective treatment for IBS (i.e., it due to the stress-related long-term implicit information from ENS) and why hypnosis may be one of the most effective methods for alleviating the symptoms of IBS.
Funding
This work was not supported by any funding.
Author contributors
I.B. developed the concept. N.Cs-N. and I.B. contributed to the writing of the manuscript.
Declaration of competing interest
The authors report no financial and non-financial conflicts of interests. The authors alone are responsible for all the content.
Acknowledgments
The authors are grateful for the critical comments by Prof. Michael Schemann.
Contributor Information
N. Császár-Nagy, Email: noemi.csaszar@pszichoszamoca.hu.
I. Bókkon, Email: bokkoni@yahoo.com.
References
- 1.Chen X.F., Guo Y., Lu X.Q., Qi L., Xu K.H., Chen Y., Li G.X., Ding J.P., Li J. Aberrant intraregional brain activity and functional connectivity in patients with diarrhea-predominant irritable bowel syndrome. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.721822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Midenfjord I., Polster A., Sjövall H., Törnblom H., Simrén M. Anxiety and depression in irritable bowel syndrome: exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neuro Gastroenterol. Motil. 2019;31(8) doi: 10.1111/nmo.13619. [DOI] [PubMed] [Google Scholar]
- 3.Ng Q.X., Soh A.Y.S., Loke W., Venkatanarayanan N., Lim D.Y., Yeo W.S. Systematic review with meta-analysis: the association between post-traumatic stress disorder and irritable bowel syndrome. J. Gastroenterol. Hepatol. 2019;34(1):68–73. doi: 10.1111/jgh.14446. [DOI] [PubMed] [Google Scholar]
- 4.Lydiard R.B. Irritable bowel syndrome, anxiety, and depression: what are the links? J. Clin. Psychiatr. 2001;62(Suppl 8):38–45. ; discussion 46-47. [PubMed] [Google Scholar]
- 5.Canavan C., West J., Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2014;40:1023–1034. doi: 10.1111/apt.12938. [DOI] [PubMed] [Google Scholar]
- 6.Kopczyńska M., Mokros Ł., Pietras T., Małecka-Panas E. Quality of life and depression in patients with irritable bowel syndrome. Przeglad Gastroenterol. 2018;13(2):102–108. doi: 10.5114/pg.2018.75819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schemann M., Frieling T., Enck P. To learn, to remember, to forget-how smart is the gut? Acta Physiol. 2020;228(1) doi: 10.1111/apha.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koloski N.A., Jones M., Talley N.J. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment. Pharmacol. Ther. 2016;44(6):592–600. doi: 10.1111/apt.13738. [DOI] [PubMed] [Google Scholar]
- 9.Császár N., Scholkmann F., Bókkon I. Implications on hypnotherapy: neuroplasticity, epigenetics, and pain. Neurosci. Biobehav. Rev. 2021;131:755–764. doi: 10.1016/j.neubiorev.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Császár N., Salari V., Scholkmann F., Kapócs G., Bókkon I. The “hidden observer” as the cognitive unconscious during hypnosis. Act. Nerv. Super. (2007) 2016;58:51–61. [Google Scholar]
- 11.De Benedittis G. Hypnobiome: a new, potential frontier of hypnotherapy in the treatment of irritable bowel syndrome-A narrative review of the literature. Int. J. Clin. Exp. Hypn. Jul. 2022;6:1–14. doi: 10.1080/00207144.2022.2094269. [DOI] [PubMed] [Google Scholar]
- 12.Spencer N.J., Travis L., Wiklendt L., Costa M., Hibberd T.J., Brookes S.J., Dinning P., Hu H., Wattchow D.A., Sorensen J. Long range synchronization within the enteric nervous system underlies propulsion along the large intestine in mice. Commun. Biol. 2021;4(1):955. doi: 10.1038/s42003-021-02485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furness J.B., Stebbing M.J. The first brain: species comparisons and evolutionary implications for the enteric and central nervous systems. Neuro Gastroenterol. Motil. 2018;30(2) doi: 10.1111/nmo.13234. [DOI] [PubMed] [Google Scholar]
- 14.Rao M., Gershon M.D. Enteric nervous system development: what could possibly go wrong? Nat. Rev. Neurosci. 2018;19(9):552–565. doi: 10.1038/s41583-018-0041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider S., Wright C.M., Heuckeroth R.O. Unexpected roles for the second brain: enteric nervous system as master regulator of bowel function. Annu. Rev. Physiol. 2019;81:235–259. doi: 10.1146/annurev-physiol-021317-121515. [DOI] [PubMed] [Google Scholar]
- 16.Furness J.B. Enteric nervous system. Scholarpedia. 2007;2:4064. [Google Scholar]
- 17.Furness J.B. The Enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 18.Gogolou A., Frith T.J.R., Tsakiridis A. Generating enteric nervous system progenitors from human pluripotent stem cells. Curr. Protoc. 2021;1(6):e137. doi: 10.1002/cpz1.137. [DOI] [PubMed] [Google Scholar]
- 19.Lake J.I., Heuckeroth R.O. Enteric nervous system development: migration, differentiation, and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305(1):G1–G24. doi: 10.1152/ajpgi.00452.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawolski V., Schmidt M.H.H. Neuron-glia interaction in the developing and adult enteric nervous system. Cells. 2020;10(1):47. doi: 10.3390/cells10010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stopińska K., Radziwoń-Zaleska M., Domitrz I. The microbiota-gut-brain axis as a key to neuropsychiatric disorders: a mini review. J. Clin. Med. 2021;10(20):4640. doi: 10.3390/jcm10204640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 23.Kano M., Muratsubaki T., Van Oudenhove L., Morishita J., Yoshizawa M., Kohno K., Yagihashi M., Tanaka Y., Mugikura S., Dupont P., Ly H.G., Takase K., Kanazawa M., Fukudo S. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-09635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarubbo F., Cavallucci V., Pani G. The influence of gut microbiota on neurogenesis: evidence and hopes. Cells. 2022;11(3):382. doi: 10.3390/cells11030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuervo-Zanatta D., Garcia-Mena J., Perez-Cruz C. Gut microbiota alterations and cognitive impairment are sexually dissociated in a transgenic mice model of Alzheimer's disease. J. Alzheimers Dis. 2021;82(s1):S195–S214. doi: 10.3233/JAD-201367. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H., Chen Y., Wang Z., Xie G., Liu M., Yuan B., Chai H., Wang W., Cheng P. Implications of gut microbiota in neurodegenerative diseases. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.785644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murciano-Brea J., Garcia-Montes M., Geuna S., Herrera-Rincon C. Gut microbiota and neuroplasticity. Cells. 2021;10(8):2084. doi: 10.3390/cells10082084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutsch A., Kantsjö J.B., Ronchi F. The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saji N. Association between the gut microbiome and cognitive function. Brain Nerve. 2020;72(3):241–250. doi: 10.11477/mf.1416201513. [DOI] [PubMed] [Google Scholar]
- 30.Luca M., Chattipakorn S.C., Sriwichaiin S., Luca A. Cognitive-behavioural correlates of dysbiosis: a review. Int. J. Mol. Sci. 2020;21(14):4834. doi: 10.3390/ijms21144834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tooley K.L. Effects of the human gut microbiota on cognitive performance, brain structure and function: a narrative review. Nutrients. 2020;12(10):3009. doi: 10.3390/nu12103009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J., Yuan B., Li G., Lu X., Guo Y., Yang Y., Liang M., Ding J., Zhou Q. Convergent syndromic atrophy of pain and emotional systems in patients with irritable bowel syndrome and depressive symptoms. Neurosci. Lett. 2020;723 doi: 10.1016/j.neulet.2020.134865. [DOI] [PubMed] [Google Scholar]
- 33.Li Y., Ning L., Yin Y., Wang R., Zhang Z., Hao L., Wang B., Zhao X., Yang X., Yin L., Wu S., Guo D., Zhang C. Age-related shifts in gut microbiota contribute to cognitive decline in aged rats. Aging (Albany NY) 2020;12(9):7801–7817. doi: 10.18632/aging.103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu J., Lu L., Yu Y., Cluette-Brown J., Martin C.R., Claud E.C. Effects of intestinal microbiota on brain development in humanized gnotobiotic mice. Sci. Rep. 2018;8(1):5443. doi: 10.1038/s41598-018-23692-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16(6):341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagu P., Parashar A., Behl T., Mehta V. Gut microbiota composition and epigenetic molecular changes connected to the pathogenesis of Alzheimer's disease. J. Mol. Neurosci. 2021;71(7):1436–1455. doi: 10.1007/s12031-021-01829-3. [DOI] [PubMed] [Google Scholar]
- 37.Kaur H., Singh Y., Singh S., Singh R.B. Gut microbiome-mediated epigenetic regulation of brain disorder and application of machine learning for multi-omics data analysis. Genome. 2021;64(4):355–371. doi: 10.1139/gen-2020-0136. [DOI] [PubMed] [Google Scholar]
- 38.Kaelberer M.M., Buchanan K.L., Klein M.E., Barth B.B., Montoya M.M., Shen X., Bohórquez D.V. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361:6408. doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall C.V., Harrison B.J., Iyer K.K., Savage H.S., Zakrzewski M., Simms L.A., Radford-Smith G., Moran R.J., Cocchi L. Microbiota links to neural dynamics supporting threat processing. Hum. Brain Mapp. 2022;43(2):733–749. doi: 10.1002/hbm.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasan N., Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019 doi: 10.7717/peerj.7502. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karl J.P., Hatch A.M., Arcidiacono S.M., Pearce S.C., Pantoja-Feliciano I.G., Doherty L.A., Soares J.W. Effects of psychological, environmental and physical stressors on the gut microbiota. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02013. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon M.Y., Yoon S.S. Disruption of the gut ecosystem by antibiotics. Yonsei Med. J. 2018;59:4–12. doi: 10.3349/ymj.2018.59.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gebrayel P., Nicco C., Al Khodor S., Bilinski J., Caselli E., Comelli E.M., Egert M., Giaroni C., Karpinski T.M., Loniewski I., Mulak A., Reygner J., Samczuk P., Serino M., Sikora M., Terranegra A., Ufnal M., Villeger R., Pichon C., Konturek P., Edeas M. Microbiota medicine: towards clinical revolution. J. Transl. Med. 2022;20(1):111. doi: 10.1186/s12967-022-03296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawkins K.G., Casolaro C., Brown J.A., Edwards D.A., Wikswo J.P. The microbiome and the gut-liver-brain axis for central nervous system clinical pharmacology: challenges in specifying and integrating in vitro and in silico models. Clin. Pharmacol. Ther. 2020;108(5):929–948. doi: 10.1002/cpt.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scriven M., Dinan T.G., Cryan J.F., Wall M. Neuropsychiatric disorders: influence of gut microbe to brain signalling. Diseases. 2018;6(3):78. doi: 10.3390/diseases6030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ojeda J., Ávila A., Vidal P.M. Gut microbiota interaction with the central nervous system throughout life. J. Clin. Med. 2021;10(6):1299. doi: 10.3390/jcm10061299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yarandi S.S., Peterson D.A., Treisman G.J., Moran T.H., Pasricha P.J. Modulatory effects of gut microbiota on the central nervous system: how gut could play a role in neuropsychiatric health and diseases. J. Neurogastroenterol. Motil. 2016;22:201–212. doi: 10.5056/jnm15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Palma G., Collins S.M., Bercik P., Verdu E.F. The microbiota-gut-brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? J. Physiol. 2014;592(14):2989–2997. doi: 10.1113/jphysiol.2014.273995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pellegrini C., Antonioli L., Calderone V., Colucci R., Fornai M., Blandizzi C. Microbiota-gut-brain axis in health and disease: is NLRP3 inflammasome at the crossroads of microbiota-gut-brain communications? Prog. Neurobiol. 2020;191 doi: 10.1016/j.pneurobio.2020.101806. [DOI] [PubMed] [Google Scholar]
- 50.Suganya K., Koo B.S. Gut-brain axis: role of gut microbiota on neurological disorders and how probiotics/prebiotics beneficially modulate microbial and immune pathways to improve brain functions. Int. J. Mol. Sci. 2020;21(20):7551. doi: 10.3390/ijms21207551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohajeri M.H., Brummer R.J.M., Rastall R.A., Weersma R.K., Harmsen H.J.M., Faas M., Eggersdorfer M. The role of the microbiome for human health: from basic science to clinical applications. Eur. J. Nutr. 2018;57(Suppl 1):1–14. doi: 10.1007/s00394-018-1703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Annahazi A., Schemann M. The enteric nervous system: “A little brain in the gut”. Neuroform. 2020;26(1):31–42. [Google Scholar]
- 53.Furness J.B., Clerc N., Kunze W.A. Memory in the enteric nervous system. Gut. 2000 Dec;47(Suppl 4):iv60–i62. doi: 10.1136/gut.47.suppl_4.iv60. Suppl 4. discussion iv76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gershon M.D. The thoughtful bowel. Acta Physiol. 2020;228(1) doi: 10.1111/apha.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kulkarni S., Micci M.A., Leser J., Shin C., Tang S.C., Fu Y.Y., Liu L., Li Q., Saha M., Li C., Enikolopov G., Becker L., Rakhilin N., Anderson M., Shen X., Dong X., Butte M.J., Song H., Southard-Smith E.M., Kapur R.P., Bogunovic M., Pasricha P.J. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E3709–E3718. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joseph N.M., He S., Quintana E., Kim Y.G., Núñez G., Morrison S.J. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J. Clin. Invest. 2011;121(9):3398–3411. doi: 10.1172/JCI58186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hodassman S., Vardi R., Tugendhaft Y., Goldental A., Kanter I. Efficient dendritic learning as an alternative to synaptic plasticity hypothesis. Sci. Rep. 2022;12(1):6571. doi: 10.1038/s41598-022-10466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mikulasch F.A., Rudelt L., Priesemann V. Local dendritic balance enables learning of efficient representations in networks of spiking neurons. Proc. Natl. Acad. Sci. U.S.A. 2021;118(50) doi: 10.1073/pnas.2021925118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abraham W.C., Jones O.D., Glanzman D.L. Is plasticity of synapses the mechanism of long-term memory storage? NPJ Sci. Learn. 2019;4:9. doi: 10.1038/s41539-019-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trettenbrein P.C. The demise of the synapse as the locus of memory: a looming paradigm shift? Front. Syst. Neurosci. 2016;10:88. doi: 10.3389/fnsys.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zovkic I.B., Guzman-Karlsson M.C., Sweatt J.D. Epigenetic regulation of memory formation and maintenance. Learn. Mem. 2013;20(2):61–74. doi: 10.1101/lm.026575.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borodinova A.A., Balaban P.M. Epigenetic regulation as a basis for long-term changes in the nervous system: in search of specificity mechanisms. Biochemistry (Mosc.) 2020;85(9):994. doi: 10.1134/S0006297920090023. 966. [DOI] [PubMed] [Google Scholar]
- 63.Levenson J.M., Sweatt J.D. Epigenetic mechanisms in memory formation. Nat. Rev. Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- 64.Arshavsky Y.I. Neurons versus networks: the interplay between individual neurons and neural networks in cognitive functions. Neuroscientist. 2017;23(4):341–355. doi: 10.1177/1073858416670124. [DOI] [PubMed] [Google Scholar]
- 65.Arshavsky Y.I. The seven sins" of the Hebbian synapse: can the hypothesis of synaptic plasticity explain long-term memory consolidation? Prog. Neurobiol. 2006;80(3):99–113. doi: 10.1016/j.pneurobio.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Arshavsky Y.I. Cellular and network properties in the functioning of the nervous system: from central pattern generators to cognition. Brain Res. Brain Res. Rev. 2003;41(2–3):229–267. doi: 10.1016/s0165-0173(02)00249-7. [DOI] [PubMed] [Google Scholar]
- 67.Bédécarrats A., Chen S., Pearce K., Cai D., Glanzman D.L. RNA from trained Aplysia can induce an epigenetic engram for long-term sensitization in untrained Aplysia. eNeuro. 2018;5(3):38. doi: 10.1523/ENEURO.0038-18.2018. ENEURO 18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryan T.J., Roy D.S., Pignatelli M., Arons A., Tonegawa S. Memory. Engram cells retain memory under retrograde amnesia. Science. 2015;348(6238):1007–1013. doi: 10.1126/science.aaa5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dias B.G., Ressler K.J. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 2014;17(1):89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Creed F., Ratcliffe J., Fernandez L., Tomenson B., Palmer S., Rigby C., Guthrie E., Read N., Thompson D. Health-related quality of life and health care costs in severe, refractory irritable bowel syndrome. Ann. Intern. Med. 2001;134(9 Pt 2):860–868. doi: 10.7326/0003-4819-134-9_part_2-200105011-00010. [DOI] [PubMed] [Google Scholar]
- 71.Van Oudenhove L., Crowell M.D., Drossman D.A., Halpert A.D., Keefer L., Lackner J.M., Murphy T.B., Naliboff B.D., Levy R.L. Biopsychosocial aspects of functional gastrointestinal disorders. Gastroenterology. 2016;S0016–5085(16):218. doi: 10.1053/j.gastro.2016.02.027. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Oudenhove L., Törnblom H., Störsrud S., Tack J., Simrén M. Depression and somatization are associated with increased postprandial symptoms in patients with irritable bowel syndrome. Gastroenterology. 2016;150(4):866–874. doi: 10.1053/j.gastro.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 73.Lacy B.E., Patel N.K. Rome criteria and a diagnostic approach to irritable bowel syndrome. J. Clin. Med. 2017;6(11):99. doi: 10.3390/jcm6110099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edwards T., Friesen C., Schurman J.V. Classification of pediatric functional gastrointestinal disorders related to abdominal pain using Rome III vs. Rome IV criterions. BMC Gastroenterol. 2018;18(1):41. doi: 10.1186/s12876-018-0769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sperber A.D., Dumitrascu D., Fukudo S., Gerson C., Ghoshal U.C., Gwee K.A., Hungin A.P.S., Kang J.Y., Minhu C., Schmulson M., Bolotin A., Friger M., Freud T., Whitehead W. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 2017;66(6):1075–1082. doi: 10.1136/gutjnl-2015-311240. [DOI] [PubMed] [Google Scholar]
- 76.Black C.J., Ford A.C. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020;17(8):473–486. doi: 10.1038/s41575-020-0286-8. [DOI] [PubMed] [Google Scholar]
- 77.Oka P., Parr H., Barberio B., Black C.J., Savarino E.V., Ford A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(10):908–917. doi: 10.1016/S2468-1253(20)30217-X. [DOI] [PubMed] [Google Scholar]
- 78.Aguilera-Lizarraga J., Florens M.V., Viola M.F., Jain P., Decraecke L., Appeltans I., Cuende-Estevez M., Fabre N., Van Beek K., Perna E., Balemans D., Stakenborg N., Theofanous S., Bosmans G., Mondelaers S.U., Matteoli G., Ibiza Martínez S., Lopez-Lopez C., Jaramillo-Polanco J., Talavera K., Alpizar Y.A., Feyerabend T.B., Rodewald H.R., Farre R., Redegeld F.A., Si J., Raes J., Breynaert C., Schrijvers R., Bosteels C., Lambrecht B.N., Boyd S.D., Hoh R.A., Cabooter D., Nelis M., Augustijns P., Hendrix S., Strid J., Bisschops R., Reed D.E., Vanner S.J., Denadai-Souza A., Wouters M.M., Boeckxstaens G.E. Local immune response to food antigens drives meal-induced abdominal pain. Nature. 2021;590(7844):151–156. doi: 10.1038/s41586-020-03118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Creed F. Review article: the incidence and risk factors for irritable bowel syndrome in population-based studies. Aliment. Pharmacol. Ther. 2019;50(5):507–516. doi: 10.1111/apt.15396. [DOI] [PubMed] [Google Scholar]
- 80.Devanarayana N.M., Rajindrajith S. Irritable bowel syndrome in children: current knowledge, challenges and opportunities. World J. Gastroenterol. 2018;24(21):2211–2235. doi: 10.3748/wjg.v24.i21.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chong P.P., Chin V.K., Looi C.Y., Wong W.F., Madhavan P., Yong V.C. The microbiome and irritable bowel syndrome - a review on the pathophysiology, current research and future therapy. Front. Microbiol. 2019;10:1136. doi: 10.3389/fmicb.2019.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ng Q.X., Soh A.Y.S., Loke W., Lim D.Y., Yeo W.S. The role of inflammation in irritable bowel syndrome (IBS) J. Inflamm. Res. 2018;11:345–349. doi: 10.2147/JIR.S174982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Uranga J.A., Martínez V., Abalo R. Mast cell regulation and irritable bowel syndrome: effects of food components with potential nutraceutical use. Molecules. 2020;25(18):4314. doi: 10.3390/molecules25184314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sebastián Domingo J.J. Irritable bowel syndrome. Med. Clin. 2022;158(2):76–81. doi: 10.1016/j.medcli.2021.04.029. [DOI] [PubMed] [Google Scholar]
- 85.Hoekman D.R., Vlieger A.M., Stokkers P.C., Mahhmod N., Rietdijk S., de Boer N.K., de Meij T.G., Frankenhuis C., D'Haens G.R., Benninga M.A. Hypnotherapy for irritable bowel syndrome-type symptoms in patients with quiescent inflammatory bowel disease: a randomized, controlled trial. J. Crohns Colitis. 2021;15(7):1106–1113. doi: 10.1093/ecco-jcc/jjaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Włodarczyk J., Szałwińska P., Waśniewska A., Fichna J. Experimental therapies in irritable bowel syndrome. Folia Med. Cracov. 2021;61(1):5–17. [PubMed] [Google Scholar]
- 87.Arokiadoss A., Weber H.C. Targeted pharmacotherapy of irritable bowel syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2021;28(2):214–221. doi: 10.1097/MED.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 88.Holvoet T., Joossens M., Vázquez-Castellanos J.F., Christiaens E., Heyerick L., Boelens J., Verhasselt B., van Vlierberghe H., De Vos M., Raes J., De Looze D. Fecal microbiota transplantation reduces symptoms in some patients with irritable bowel syndrome with predominant abdominal bloating: short- and long-term results from a placebo-controlled randomized trial. Gastroenterology. 2021;160(1):145–157. doi: 10.1053/j.gastro.2020.07.013. e8. [DOI] [PubMed] [Google Scholar]
- 89.Radziwon C.D., Lackner J.M. Cognitive behavioral therapy for IBS: how useful, how often, and how does it work? Curr. Gastroenterol. Rep. 2017;19(10):49. doi: 10.1007/s11894-017-0590-9. [DOI] [PubMed] [Google Scholar]
- 90.Drossman D.A. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;S0016–5085(16):223–227. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 91.Drossman D.A., Hasler W.L. Rome IV-Functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150(6):1257–1261. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 92.Tollefson G.D., Tollefson S.L., Pederson M., Luxenberg M., Dunsmore G. Comorbid irritable bowel syndrome in patients with generalized anxiety and major depression. Ann. Clin. Psychiatr. 1991;3:215–220. [PubMed] [Google Scholar]
- 93.Lydiard R.B., Falsetti S.A. Experience with anxiety and depression treatment studies: implications for designing irritable bowel syndrome clinical trials. Am. J. Med. 1999;107(5A):65S–73S. doi: 10.1016/s0002-9343(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 94.Levy R.L., Olden K.W., Naliboff B.D., Bradley L.A., Francisconi C., Drossman D.A., Creed F. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology. 2006;130(5):1447–1458. doi: 10.1053/j.gastro.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 95.Miller V., Hopkins L., Whorwell P.J. Suicidal ideation in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2004;2(12):1064–1068. doi: 10.1016/s1542-3565(04)00545-2. [DOI] [PubMed] [Google Scholar]
- 96.Lovell R.M., Ford A.C. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. 2012;10:712–721. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 97.Kabra N., Nadkarni A. Prevalence of depression and anxiety in irritable bowel syndrome: a clinic based study from India. Indian J. Psychiatr. 2013;55:77–80. doi: 10.4103/0019-5545.105520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Q.E., Wang F., Qin G., Zheng W., Ng C.H., Ungvari G.S., Yuan Z., Mei S., Wang G., Xiang Y.T. Depressive symptoms in patients with irritable bowel syndrome: a meta-analysis of comparative studies. Int. J. Biol. Sci. 2018;14(11):1504–1512. doi: 10.7150/ijbs.25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee S.P., Sung I.K., Kim J.H., Lee S.Y., Park H.S., Shim C.S. The effect of emotional stress and depression on the prevalence of digestive diseases. J. Neurogastroentero. Motil. 2015;21(2):273–282. doi: 10.5056/jnm14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu Z., Li M., Yao L., Wang Y., Wang E., Yuan J., Wang F., Yang K., Bian Z., Zhong L.L.D. The level and prevalence of depression and anxiety among patients with different subtypes of irritable bowel syndrome: a network meta-analysis. BMC Gastroenterol. 2021;21(1):23. doi: 10.1186/s12876-020-01593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zamani M., Alizadeh-Tabari S., Zamani V. Systematic review with meta-analysis: the prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019;50(2):132–143. doi: 10.1111/apt.15325. [DOI] [PubMed] [Google Scholar]
- 102.Lee C., Doo E., Choi J.M., Jang S.H., Ryu H.S., Lee J.Y., Oh J.H., Park J.H., Kim Y.S. Brain-Gut Axis Research Group of Korean Society of Neurogastroenterology and Motility. The increased level of depression and anxiety in irritable bowel syndrome patients compared with healthy controls: systematic review and meta-analysis. J. Neurogastroenterol. Motil. 2017;23(3):349–362. doi: 10.5056/jnm16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Person H., Keefer L. Psychological comorbidity in gastrointestinal diseases: update on the brain-gut-microbiome axis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;107 doi: 10.1016/j.pnpbp.2020.110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mokhtar N.M., Bahrudin M.F., Abd Ghani N., Abdul Rani R., Raja Ali R.A. Prevalence of subthreshold depression among constipation-predominant irritable bowel syndrome patients. Front. Psychol. 2020;11:1936. doi: 10.3389/fpsyg.2020.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stasi C., Nisita C., Cortopassi S., Corretti G., Gambaccini D., De Bortoli N., Fani B., Simonetti N., Ricchiuti A., Dell'Osso L., Marchi S., Bellini M. Subthreshold psychiatric psychopathology in functional gastrointestinal disorders: can it be the bridge between gastroenterology and psychiatry? Gastroenterol. Res. Pract. 2017 doi: 10.1155/2017/1953435. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Staudacher H.M., Mikocka-Walus A., Ford A.C. Common mental disorders in irritable bowel syndrome: pathophysiology, management, and considerations for future randomised controlled trials. Lancet Gastroenterol. Hepatol. 2021;6(5):401–410. doi: 10.1016/S2468-1253(20)30363-0. [DOI] [PubMed] [Google Scholar]
- 107.Biella M.M., Borges M.K., Strauss J., Mauer S., Martinelli J.E., Aprahamian I. Subthreshold depression needs a prime time in old age psychiatry? A narrative review of current evidence. Neuropsychiatric Dis. Treat. 2019;15:2763–2772. doi: 10.2147/NDT.S223640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Williams S.Z., Chung G.S., Muennig P.A. Undiagnosed depression: a community diagnosis. SSM Popul Health. 2017;3:633–638. doi: 10.1016/j.ssmph.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vaccaro R., Borrelli P., Abbondanza S., Davin A., Polito L., Colombo M., Francesca Vitali S., Villani S., Guaita A. Subthreshold depression and clinically significant depression in an Italian population of 70-74-Year-Olds: prevalence and Association with Perceptions of Self. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/3592359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haller H., Cramer H., Lauche R., Gass F., Dobos G.J. The prevalence and burden of subthreshold generalized anxiety disorder: a systematic review. BMC Psychiatr. 2014;14:128. doi: 10.1186/1471-244X-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bertha E.A., Balázs J. Subthreshold depression in adolescence: a systematic review. Eur. Child Adolesc. Psychiatr. 2013;22(10):589–603. doi: 10.1007/s00787-013-0411-0. [DOI] [PubMed] [Google Scholar]
- 112.Meeks T.W., Vahia I.V., Lavretsky H., Kulkarni G., Jeste D.V. A tune in "a minor" can "b major": a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J. Affect. Disord. 2011;129(1–3):126–142. doi: 10.1016/j.jad.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Geiselmann B., Linden M., Helmchen H. Psychiatrists' diagnoses of subthreshold depression in old age: frequency and correlates. Psychol. Med. 2001;31(1):51–63. doi: 10.1017/s0033291799002883. [DOI] [PubMed] [Google Scholar]
- 114.Adams K.B., Moon H. Subthreshold depression: characteristics and risk factors among vulnerable elders. Aging Ment. Health. 2009;13:682–692. doi: 10.1080/13607860902774501. [DOI] [PubMed] [Google Scholar]
- 115.Seminowicz D.A., Labus J.S., Bueller J.A., Tillisch K., Naliboff B.D., Bushnell M.C., Mayer E.A. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139(1):48–57. doi: 10.1053/j.gastro.2010.03.049. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao L., Wang Y., Zhang Y. Microstructural changes in the brain in elderly patients with irritable bowel syndrome. Aging Med. (Milton) 2018;1(2):141–148. doi: 10.1002/agm2.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weng Y., Qi R., Liu C., Ke J., Xu Q., Wang F., Zhang L.J., Lu G.M. Disrupted functional connectivity density in irritable bowel syndrome patients. Brain Imaging Behav. 2017;11(6):1812–1822. doi: 10.1007/s11682-016-9653-z. [DOI] [PubMed] [Google Scholar]
- 118.Labus J.S., Dinov I.D., Jiang Z., Ashe-McNalley C., Zamanyan A., Shi Y., Hong J.Y., Gupta A., Tillisch K., Ebrat B., Hobel S., Gutman B.A., Joshi S., Thompson P.M., Toga A.W., Mayer E.A. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain. 2014;155(1):137–149. doi: 10.1016/j.pain.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Patel P., Bercik P., Morgan D.G., Bolino C., Pintos-Sanchez M.I., Moayyedi P., Ford A.C. Irritable bowel syndrome is significantly associated with somatisation in 840 patients, which may drive bloating. Aliment. Pharmacol. Ther. 2015;41(5):449–458. doi: 10.1111/apt.13074. [DOI] [PubMed] [Google Scholar]
- 120.Grinsvall C., Van Oudenhove L., Dupont P., Ryu H.J., Ljungberg M., Labus J.S., Törnblom H., Mayer E.A., Simrén M. Altered structural covariance of insula, cerebellum and prefrontal cortex is associated with somatic symptom levels in irritable bowel syndrome (IBS) Brain Sci. 2021;11(12):1580. doi: 10.3390/brainsci11121580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Icenhour A., Witt S.T., Elsenbruch S., Lowén M., Engström M., Tillisch K., Mayer E.A., Walter S. Brain functional connectivity is associated with visceral sensitivity in women with irritable bowel syndrome. Neuroimage Clin. 2017;15:449–457. doi: 10.1016/j.nicl.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tillisch K., Mayer E.A., Labus J.S. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fakhoury M. The habenula in psychiatric disorders: more than three decades of translational investigation. Neurosci. Biobehav. Rev. 2017;83:721–735. doi: 10.1016/j.neubiorev.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 124.Namboodiri V.M., Rodriguez-Romaguera J., Stuber G.D. The habenula. Curr. Biol. 2016;26(19):R873–R877. doi: 10.1016/j.cub.2016.08.051. [DOI] [PubMed] [Google Scholar]
- 125.Mao C.P., Chen F.R., Huo J.H., Zhang L., Zhang G.R., Zhang B., Zhou X.Q. Altered resting-state functional connectivity and effective connectivity of the habenula in irritable bowel syndrome: a cross-sectional and machine learning study. Hum. Brain Mapp. 2020;41(13):3655–3666. doi: 10.1002/hbm.25038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li J., He P., Lu X., Guo Y., Liu M., Li G., Ding J. A resting-state functional magnetic resonance imaging study of whole-brain functional connectivity of voxel levels in patients with irritable bowel syndrome with depressive symptoms. J. Neurogastroenterol. Motil. 2021;27(2):248–256. doi: 10.5056/jnm20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li L., Solvi C., Zhang F., Qi Z., Chittka L., Zhao W. Gut microbiome drives individual memory variation in bumblebees. Nat. Commun. 2021;12(1):6588. doi: 10.1038/s41467-021-26833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gluckma P.D., Hanson M.A. The Fetal Matrix. Evolution, Development and Disease. Cambridge Universiy Press; Cambridge, UK: 2005. Predictive adaptive responses and human disease; pp. 78–102. [Google Scholar]
- 129.Aktar E., Qu J., Lawrence P.J., Tollenaar M.S., Elzinga B.M., Bögels S.M. Fetal and infant outcomes in the offspring of parents with perinatal mental disorders: earliest influences. Front. Psychiatr. 2019;10:391. doi: 10.3389/fpsyt.2019.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang H.J., Xu X., Xie R.H., Rui Y.Y., Zhang P.A., Zhu X.J., Xu G.Y. Prenatal maternal stress induces visceral hypersensitivity of adult rat offspring through activation of cystathionine-β-synthase signaling in primary sensory neurons. Mol. Pain. 2018;14 doi: 10.1177/1744806918777406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu R.T. Childhood adversities and depression in adulthood: current findings and future directions. Clin. Psychol. 2017;24:140–153. doi: 10.1111/cpsp.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hodgson D.M., Nakamura T., Walker A.K. Prophylactic role for complementary and alternative medicine in perinatal programming of adult health. Complement. Med. Res. 2007;14:92–101. doi: 10.1159/000100958. [DOI] [PubMed] [Google Scholar]
- 133.Bradford K., Shih W., Videlock E.J., Presson A.P., Naliboff B.D., Mayer E.A., Chang L. Association between early adverse life events and irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2012;10(4):385–390. doi: 10.1016/j.cgh.2011.12.018. e1-e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chitkara D.K., van Tilburg M.A., Blois-Martin N., Whitehead W.E. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am. J. Gastroenterol. 2008;103(3):765–774. doi: 10.1111/j.1572-0241.2007.01722.x. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rees S., Channon S., Waters C.S. The impact of maternal prenatal and postnatal anxiety on children's emotional problems: a systematic review. Eur. Child Adolesc. Psychiatr. 2019;28(2):257–280. doi: 10.1007/s00787-018-1173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Walsh K., McCormack C.A., Webster R., Pinto A., Lee S., Feng T., Krakovsky H.S., O'Grady S.M., Tycko B., Champagne F.A., Werner E.A., Liu G., Monk C. Maternal prenatal stress phenotypes associate with fetal neurodevelopment and birth outcomes. Proc. Natl. Acad. Sci. U.S.A. 2019;116(48):23996–24005. doi: 10.1073/pnas.1905890116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Császár N., Bókkon I. Mother-newborn separation at birth in hospitals: a possible risk for neurodevelopmental disorders? Neurosci. Biobehav. Rev. 2017;84:337–351. doi: 10.1016/j.neubiorev.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 138.Tao E., Long G., Yang T., Chen B., Guo R., Ye D., Fang M., Jiang M. Maternal separation induced visceral hypersensitivity evaluated via novel and small size distention balloon in post-weaning mice. Front. Neurosci. 2022;15 doi: 10.3389/fnins.2021.803957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yi L., Zhang H., Sun H., Zhou L., Chen Y., Xuan L., Jiang Y., Xu S. Maternal separation induced visceral hypersensitivity from childhood to adulthood. J. Neurogastroenterol. Motil. 2017;23(2):306–315. doi: 10.5056/jnm16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moloney R.D., O'Leary O.F., Felice D., Bettler B., Dinan T.G., Cryan J.F. Early-life stress induces visceral hypersensitivity in mice. Neurosci. Lett. 2012;512:99–102. doi: 10.1016/j.neulet.2012.01.066. [DOI] [PubMed] [Google Scholar]
- 141.Tjong Y.W. Neonatal maternal separation elevates thalamic corticotrophin releasing factor type 1 receptor expression response to colonic distension in rat. Neuroendocrinol. Lett. 2010;31:215–220. [PubMed] [Google Scholar]
- 142.Coutinho S.V., Plotsky P.M., Sablad M., Miller J.C., Zhou H., Bayati A.I., McRoberts J.A., Mayer E.A. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282(2):G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 143.Saito Y.A. The role of genetics in IBS. Gastroenterol. Clin. N. Am. 2011;40(1):45–67. doi: 10.1016/j.gtc.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saito Y.A., Zimmerman J.M., Harmsen W.S., De Andrade M., Locke G.R., 3rd, Petersen G.M., Talley N.J. Irritable bowel syndrome aggregates strongly in families: a family-based case-control study. Neuro Gastroenterol. Motil. 2008;20(7):790–797. doi: 10.1111/j.1365-2982.2007.1077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kalantar J.S., Locke G.R., 3rd, Zinsmeister A.R., Beighley C.M., Talley N.J. Familial aggregation of irritable bowel syndrome: a prospective study. Gut. 2003;52(12):1703–1707. doi: 10.1136/gut.52.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aguas M., Garrigues V., Bastida G., Nos P., Ortiz V., Fernandez A., Ponce J. Prevalence of irritable bowel syndrome (IBS) in first-degree relatives of patients with inflammatory bowel disease (IBD) J. Crohns Colitis. 2011;5(3):227–233. doi: 10.1016/j.crohns.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 147.van den Wijngaard R.M., Stanisor O.I., van Diest S.A., Welting O., Wouters M.M., Cailotto C., de Jonge W.J., Boeckxstaens G.E. Susceptibility to stress induced visceral hypersensitivity in maternally separated rats is transferred across generations. Neuro Gastroenterol. Motil. 2013;25(12):e780–e790. doi: 10.1111/nmo.12202. [DOI] [PubMed] [Google Scholar]
- 148.Bengtson M.B., Aamodt G., Vatn M.H., Harris J.R. Co-occurrence of IBS and symptoms of anxiety or depression, among Norwegian twins, is influenced by both heredity and intrauterine growth. BMC Gastroenterol. 2015;15:9. doi: 10.1186/s12876-015-0237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Levy R.L., Jones K.R., Whitehead W.E., Feld S.I., Talley N.J., Corey L.A. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology. 2001;121(4):799–804. doi: 10.1053/gast.2001.27995. [DOI] [PubMed] [Google Scholar]
- 150.Morris-Yates A., Talley N.J., Boyce P.M., Nandurkar S., Andrews G. Evidence of a genetic contribution to functional bowel disorder. Am. J. Gastroenterol. 1998;93(8):1311–1317. doi: 10.1111/j.1572-0241.1998.440_j.x. [DOI] [PubMed] [Google Scholar]
- 151.Lembo A., Zaman M., Jones M., Talley N.J. Influence of genetics on irritable bowel syndrome, gastro-oesophageal reflux and dyspepsia: a twin study. Aliment. Pharmacol. Ther. 2007;25:1343–1350. doi: 10.1111/j.1365-2036.2007.03326.x. [DOI] [PubMed] [Google Scholar]
- 152.Bengtson M.B., Rønning T., Vatn M.H., Harris J.R. Irritable bowel syndrome in twins: genes and environment. Gut. 2006;55(12):1754–1759. doi: 10.1136/gut.2006.097287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Waehrens R., Li X., Sundquist J., Sundquist K., Zöller B. Perinatal and familial risk factors for irritable bowel syndrome in a Swedish national cohort. Scand. J. Gastroenterol. 2018;53(5):559–566. doi: 10.1080/00365521.2017.1398345. [DOI] [PubMed] [Google Scholar]
- 154.Waehrens R., Zöller B., Sundquist J., Sundquist K., Pirouzifard M. A Swedish national adoption study of risk of irritable bowel syndrome (IBS) BMJ Open Gastroenterol. 2017;4(1) doi: 10.1136/bmjgast-2017-000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gazouli M., Wouters M.M., Kapur-Pojskić L., Bengtson M.B., Friedman E., Nikčević G., Demetriou C.A., Mulak A., Santos J., Niesler B. Lessons learned--resolving the enigma of genetic factors in IBS. Nat. Rev. Gastroenterol. Hepatol. 2016;13(2):77–87. doi: 10.1038/nrgastro.2015.206. [DOI] [PubMed] [Google Scholar]
- 156.Peter B. In: International Encyclopedia of the Social and Behavioral Sciences. second ed. Volume 11. Wright D.E., editor. Elsevier; Oxford: 2015. Hypnosis; pp. 458–464. [Google Scholar]
- 157.Sine H., Achbani A., Filali K. The effect of hypnosis on the intensity of pain and anxiety in cancer patients: a systematic review of controlled experimental trials. Cancer Invest. 2022;40(3):235–253. doi: 10.1080/07357907.2021.1998520. [DOI] [PubMed] [Google Scholar]
- 158.Bicego A., Rousseaux F., Faymonville M.E., Nyssen A.S., Vanhaudenhuyse A. Neurophysiology of hypnosis in chronic pain: a review of recent literature. Am. J. Clin. Hypn. 2022;64(1):62–80. doi: 10.1080/00029157.2020.1869517. [DOI] [PubMed] [Google Scholar]
- 159.Roberts R.L., Rhodes J.R., Elkins G.R. Effect of hypnosis on anxiety: results from a randomized controlled trial with women in postmenopause. J. Clin. Psychol. Med. Settings. 2021;28(4):868–881. doi: 10.1007/s10880-021-09810-3. [DOI] [PubMed] [Google Scholar]
- 160.Elkins G., Otte J., Carpenter J.S., Roberts L., Jackson L.S., Kekecs Z., Patterson V., Keith T.Z. Hypnosis intervention for sleep disturbance: determination of optimal dose and method of delivery for postmenopausal women. Int. J. Clin. Exp. Hypn. 2021;69(3):323–345. doi: 10.1080/00207144.2021.1919520. [DOI] [PubMed] [Google Scholar]
- 161.Cozzolino M., Celia G., Rossi K.L., Rossi E.L. Hypnosis as sole anesthesia for dental removal in a patient with multiple chemical sensitivity. Int. J. Clin. Exp. Hypn. 2020;68(3):371–383. doi: 10.1080/00207144.2020.1762494. [DOI] [PubMed] [Google Scholar]
- 162.Beevi Z., Low W.Y., Hassan J. The Effectiveness of hypnosis intervention in alleviating postpartum psychological symptoms. Am. J. Clin. Hypn. 2019;61(4):409–425. doi: 10.1080/00029157.2018.1538870. [DOI] [PubMed] [Google Scholar]
- 163.Chiu L., Lee H.W., Lam W.K. The effectiveness of hypnotherapy in the treatment of Chinese psychiatric patients. Int. J. Clin. Exp. Hypn. 2018;66(3):315–330. doi: 10.1080/00207144.2018.1461472. [DOI] [PubMed] [Google Scholar]
- 164.Chamine I., Atchley R., Oken B.S. Hypnosis intervention effects on sleep outcomes: a systematic review. J. Clin. Sleep Med. 2018;14(2):271–283. doi: 10.5664/jcsm.6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Johnson A.J., Marcus J., Hickman K., Barton D., Elkins G. Anxiety reduction among breast-cancer survivors receiving hypnotic relaxation therapy for hot flashes. Int. J. Clin. Exp. Hypn. 2016;64(4):377–390. doi: 10.1080/00207144.2016.1209042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chester S.J., Stockton K., De Youngm A., Kipping B., Tyack Z., Griffin B., Chester R.L., Kimble R.M. Effectiveness of medical hypnosis for pain reduction and faster wound healing in pediatric acute burn injury: study protocol for a randomized controlled trial. Trials. 2016;17(1):223. doi: 10.1186/s13063-016-1346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Becker P.M. Hypnosis in the management of sleep disorders. Sleep Med. Clin. 2015;10(1):85–92. doi: 10.1016/j.jsmc.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 168.Peretz B., Bercovich R., Blumer S. Using elements of hypnosis prior to or during pediatric dental treatment. Pediatr. Dent. 2013;35(1):33–36. [PubMed] [Google Scholar]
- 169.Abdeshahi S.K., Hashemipour M.A., Mesgarzadeh V., Shahidi Payam A., Halaj Monfared A. Effect of hypnosis on induction of local anaesthesia, pain perception, control of haemorrhage and anxiety during extraction of third molars: a case-control study. J. Cranio-Maxillo-Fac. Surg. 2013;41:310–315. doi: 10.1016/j.jcms.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 170.Xu Y., Cardeña E. Hypnosis as an adjunct therapy in the management of diabetes. Int. J. Clin. Exp. Hypn. 2008;56(1):63–72. doi: 10.1080/00207140701673050. [DOI] [PubMed] [Google Scholar]
- 171.Hammond D.C. Review of the efficacy of clinical hypnosis with headaches and migraines. Int. J. Clin. Exp. Hypn. 2007;55(2):207–219. doi: 10.1080/00207140601177921. [DOI] [PubMed] [Google Scholar]
- 172.Michalak E.E., Lam R.W. Breaking the myths: new treatment approaches for chronic depression. Can. J. Psychiatr. 2002;47(7):635–643. doi: 10.1177/070674370204700705. [DOI] [PubMed] [Google Scholar]
- 173.Yapko M. Hypnosis in treating symptoms and risk factors of major depression. Am. J. Clin. Hypn. 2001;44(2):97–108. doi: 10.1080/00029157.2001.10403465. [DOI] [PubMed] [Google Scholar]
- 174.Spiegel D., Cardena E. New uses of hypnosis in the treatment of posttraumatic stress disorder. J. Clin. Psychiatr. 1990;51(Suppl):39–43. discussion 44-46. [PubMed] [Google Scholar]
- 175.Jensen M.P., Adachi T., Tomé-Pires C., Lee J., Osman Z.J., Miró J. Mechanisms of hypnosis: toward the development of a biopsychosocial model. Int. J. Clin. Exp. Hypn. 2015;63:34–37. doi: 10.1080/00207144.2014.961875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Clark J. Neuroscience Implications of using hypnoanalysis: two case studies. Act. Nerv. Super. 57. 2015:49–58. [Google Scholar]
- 177.Del Casale A., Ferracuti S., Rapinesi C., Serata D., Sani G., Savoja V., Kotzalidis G.D., Tatarelli R., Girardi P. Neurocognition under hypnosis: findings from recent functional neuroimaging studies. Int. J. Clin. Exp. Hypn. 2012;60(3):286–317. doi: 10.1080/00207144.2012.675295. [DOI] [PubMed] [Google Scholar]
- 178.Rainville P., Hofbauer R.K., Bushnell M.C., Duncan G.H., Price D.D. Hypnosis modulates activity in brain structures involved in the regulation of consciousness. J. Cognit. Neurosci. 2002;14(6):887–901. doi: 10.1162/089892902760191117. [DOI] [PubMed] [Google Scholar]
- 179.Cozzolino M., Cocco S., Piezzo M., Celia G., Costantini S., Abate V., Capone F., Barberio D., Girelli L., Cavicchiolo E., Ascierto P.A., Madonna G., Budillon A., De Laurentiis M. A psychosocial genomics pilot study in oncology for verifying clinical, inflammatory and psychological effects of mind-body transformations-therapy (MBT-T) in breast cancer patients: preliminary results. J. Clin. Med. 2021;10:136. doi: 10.3390/jcm10010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Landry M., Appourchaux K., Raz A. Elucidating unconscious processing with instrumental hypnosis. Front. Psychol. 2014;5:785. doi: 10.3389/fpsyg.2014.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kihlstrom J.F. The cognitive unconscious. Science. 1987;237:1445–1452. doi: 10.1126/science.3629249. [DOI] [PubMed] [Google Scholar]
- 182.Sasegbon A., Hasan S.S., Whorwell P.J., Vasant D.H. Experience and clinical efficacy of gut-directed hypnotherapy in an Asian population with refractory irritable bowel syndrome. JGH Open. 2022;6(7):447–453. doi: 10.1002/jgh3.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hasan S.S., Whorwell P.J., Miller V., Morris J., Vasant D.H. Six vs 12 Sessions of gut-focused hypnotherapy for irritable bowel syndrome: a randomized trial. Gastroenterology. 2021;160(7):2605–2607. doi: 10.1053/j.gastro.2021.02.058. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Black C.J., Thakur E.R., Houghton L.A., Quigley E.M.M., Moayyedi P., Ford A.C. Efficacy of psychological therapies for irritable bowel syndrome: systematic review and network meta-analysis. Gut. 2020;69(8):1441–1451. doi: 10.1136/gutjnl-2020-321191. [DOI] [PubMed] [Google Scholar]
- 185.Vasant D.H., Hasan S.S., Cruickshanks P., Whorwell P.J. Gut-focused hypnotherapy for children and adolescents with irritable bowel syndrome. Frontline Gastroenterol. 2020;12(7):570–577. doi: 10.1136/flgastro-2020-101679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Peters S.L., Muir J.G., Gibson P.R. Review article: gut-directed hypnotherapy in the management of irritable bowel syndrome and inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015;41(11):1104–1115. doi: 10.1111/apt.13202. [DOI] [PubMed] [Google Scholar]
- 187.Gerson C.D., Gerson J., Gerson M.J. Group hypnotherapy for irritable bowel syndrome with long-term follow-up. Int. J. Clin. Exp. Hypn. 2013;61(1):38–54. doi: 10.1080/00207144.2012.700620. [DOI] [PubMed] [Google Scholar]
- 188.Lindfors P., Unge P., Nyhlin H., Ljótsson B., Björnsson E.S., Abrahamsson H., Simrén M. Long-term effects of hypnotherapy in patients with refractory irritable bowel syndrome. Scand. J. Gastroenterol. 2012;47(4):414. doi: 10.3109/00365521.2012.658858. 220. [DOI] [PubMed] [Google Scholar]
- 189.Vlieger A.M., Menko-Frankenhuis C., Wolfkamp S.C., Tromp E., Benninga M.A. Hypnotherapy for children with functional abdominal pain or irritable bowel syndrome: a randomized controlled trial. Gastroenterology. 2007;133(5):1430–1436. doi: 10.1053/j.gastro.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 190.Wilson S., Maddison T., Roberts L., Greenfield S., Singh S., Birmingham IBS Research Group Systematic review: the effectiveness of hypnotherapy in the management of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2006;24(5):769–780. doi: 10.1111/j.1365-2036.2006.03028.x. [DOI] [PubMed] [Google Scholar]
- 191.Gonsalkorale W.M., Miller V., Afzal A., Whorwell P.J. Long term benefits of hypnotherapy for irritable bowel syndrome. Gut. 2003;52(11):1623–1629. doi: 10.1136/gut.52.11.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Guthrie E., Whorwell P.J. In: Irritable Bowel Syndrome: Diagnosis and Treatment. Camilleri M., Spiller R., editors. WB Saunders; London: 2002. Psychotherapy and hypnotherapy in IBS; pp. 151–159. (Chapter 16) [Google Scholar]
- 193.Palsson O.S., Turner M.J., Johnson D.A., Burnett C.K., Whitehead W.E. Hypnosis treatment for severe irritable bowel syndrome: investigation of mechanism and effects on symptoms. Dig. Dis. Sci. 2002;47(11):2605–2614. doi: 10.1023/a:1020545017390. [DOI] [PubMed] [Google Scholar]
- 194.Donnet A.S., Hasan S.S., Whorwell P.J. Hypnotherapy for irritable bowel syndrome: patient expectations and perceptions. Therap. Adv. Gastroenterol. 2022;15 doi: 10.1177/17562848221074208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Miller V., Carruthers H.R., Morris J., Hasan S.S., Archbold S., Whorwell P.J. Hypnotherapy for irritable bowel syndrome: an audit of one thousand adult patients. Aliment. Pharmacol. Ther. 2015;41(9):844–855. doi: 10.1111/apt.13145. [DOI] [PubMed] [Google Scholar]
- 196.Rutten J.M., Reitsma J.B., Vlieger A.M., Benninga M.A. Gut-directed hypnotherapy for functional abdominal pain or irritable bowel syndrome in children: a systematic review. Arch. Dis. Child. 2013;98:252–257. doi: 10.1136/archdischild-2012-302906. [DOI] [PubMed] [Google Scholar]
- 197.Vlieger A.M., Rutten J.M., Govers A.M., Frankenhuis C., Benninga M.A. Long-term follow-up of gut-directed hypnotherapy vs. standard care in children with functional abdominal pain or irritable bowel syndrome. Am. J. Gastroenterol. 2012;107:627–631. doi: 10.1038/ajg.2011.487. [DOI] [PubMed] [Google Scholar]
- 198.Hasan S.S., Pearson J.S., Morris J., Whorwell P.J. Skype hypnotherapy for irritable bowel syndrome: effectiveness and comparison with face-to-face treatment. Int. J. Clin. Exp. Hypn. 2019;67(1):69–80. doi: 10.1080/00207144.2019.1553766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rutten J.M.T.M., Vlieger A.M., Frankenhuis C., George E.K., Groeneweg M., Norbruis O.F., Tjon A.Ten W., van Wering H.M., Dijkgraaf M.G.W., Merkus M.P., Benninga M.A. Home-based hypnotherapy self-exercises vs individual hypnotherapy with a therapist for treatment of pediatric irritable bowel syndrome, functional abdominal pain, or functional abdominal pain syndrome: a randomized clinical trial. JAMA Pediatr. 2017;171(5):470–477. doi: 10.1001/jamapediatrics.2017.0091. [DOI] [PubMed] [Google Scholar]
- 200.Peter J., Fournier C., Keip B., Rittershaus N., Stephanou-Rieser N., Durdevic M., Dejaco C., Michalski M., Moser G. Intestinal microbiome in irritable bowel syndrome before and after gut-directed hypnotherapy. Int. J. Mol. Sci. 2018;19(11):3619. doi: 10.3390/ijms19113619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shahbazi K., Solati K., Hasanpour-Dehkordi A. Comparison of hypnotherapy and standard medical treatment alone on quality of life in patients with irritable bowel syndrome: a randomized control trial. J. Clin. Diagn. Res. 2016;10(5):OC01–4. doi: 10.7860/JCDR/2016/17631.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Whorwell P.J., Prior A., Faragher E.B. Controlled trial of hypnotherapy in the treatment of severe refractory irritable-bowel syndrome. Lancet. 1984;324(8414):1232–1234. doi: 10.1016/s0140-6736(84)92793-4. [DOI] [PubMed] [Google Scholar]
- 203.Chanpong A., Borrelli O., Thapar N. Recent advances in understanding the roles of the enteric nervous system. Fac. Rev. 2022;11:7. doi: 10.12703/r/11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Low E.X.S., Mandhari M.N.K.A., Herndon C.C., Loo E.X.L., Tham E.H., Siah K.T.H. Parental, perinatal, and childhood risk factors for development of irritable bowel syndrome: a systematic review. J. Neurogastroenterol. Motil. 2020;26(4):437–446. doi: 10.5056/jnm20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lundgaard Donovan L., Henningsen K., Flou Kristensen A., Wiborg O., Nieland J.D. Lichota, J. Maternal separation followed by chronic mild stress in adulthood is associated with concerted epigenetic regulation of ap-1 complex genes. J. Pers. 2021;11(3):209. doi: 10.3390/jpm11030209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Guan L., Shi X., Tang Y., Yan Y., Chen L., Chen Y., Gao G., Lin C., Chen A. Contribution of amygdala histone acetylation in early life stress-induced visceral hypersensitivity and emotional comorbidity. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.843396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hurwitz T.A. Somatization and conversion disorder. Can. J. Psychiatr. 2004;49(3):172–178. doi: 10.1177/070674370404900304. [DOI] [PubMed] [Google Scholar]
- 208.De Gucht V., Maes S. Explaining medically unexplained symptoms: toward a multidimensional, theory-based approach to somatization. J. Psychosom. Res. 2006;60:349–352. doi: 10.1016/j.jpsychores.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 209.De Gucht V., Fischler B. Somatization: a critical review of conceptual and methodological issues. Psychosomatics. 2002;43(1):1–9. doi: 10.1176/appi.psy.43.1.1. [DOI] [PubMed] [Google Scholar]
- 210.Dinan T.G., Stilling R.M., Stanton C., Cryan J.F. Collective unconscious: how gut microbes shape human behavior. J. Psychiatr. Res. 2015;63:1–9. doi: 10.1016/j.jpsychires.2015.02.021. [DOI] [PubMed] [Google Scholar]