Abstract
The contribution of CD8+ T cells to the control of tuberculosis has been studied primarily during acute infection in mouse models. Memory or recall responses in tuberculosis are less well characterized, particularly with respect to the CD8 T-cell subset. In fact, there are published reports that CD8+ T cells do not participate in the memory immune response to Mycobacterium tuberculosis. We examined the CD8+ T-cell memory and local recall response to M. tuberculosis. To establish a memory immunity model, C57BL/6 mice were infected with M. tuberculosis, followed by treatment with anti-mycobacterial drugs and prolonged rest. The lungs of memory immune mice contained CD4+ and CD8+ T cells with the cell surface phenotype characteristic of memory cells (CD69low CD25low CD44high). At 1 week postchallenge with M. tuberculosis via aerosol, ≥30% of both CD4+ and CD8+ T cells in the lungs of immune mice expressed the activation marker CD69 and could be restimulated to produce gamma interferon (IFN-γ). In contrast, <6% of T cells in the lungs of naive challenged mice were CD69+ at 1 week postchallenge, and IFN-γ production was not observed at this time point. CD8+ T cells from the lungs of both naive and memory mice after challenge were cytotoxic toward M. tuberculosis-infected macrophages. Our data indicate that memory and recall immunity to M. tuberculosis is comprised of both CD4+ and CD8+ T lymphocytes and that there is a rapid response of both subsets in the lungs following challenge.
Control of acute tuberculosis in mice involves participation of CD8+ T cells (6, 22, 37), although a recent publication argues for a more important role for this subset in control of persistent infection (38). Recent studies on the development and activation of mycobacterium-specific CD8+ T cells have indicated that substantial numbers of activated CD8+ T lymphocytes with both cytokine-secreting and cytotoxic functions are present in the lungs during the acute phase of infection in mice (13, 19, 33–35). However, whether this acute response culminates in the development of memory CD8+ T-cell populations that can respond robustly to a secondary Mycobacterium tuberculosis challenge is not clear. It is believed that a proper memory response develops after an infection is cleared. CD8+ T cells play a prominent role during memory immune responses in intracellular bacterial infections with Listeria monocytogenes (11) and Chlamydia pneumoniae (31). In the mouse model and in humans, M. tuberculosis can be a persistent or latent infection. To study the memory response in this infection, it is useful to first reduce or eliminate the bacterial burden in the mouse tissues. It has been reported that the treatment of infected mice with antibiotics leads to the development of an immune response capable of reducing the bacterial burden after challenge (4). The major role in combating bacterial challenge during memory immune responses so far has been attributed exclusively to CD4+ T cells (4).
Earlier studies have argued against the participation of memory CD8+ T cells in the recall immune responses in a murine model of tuberculosis (3, 4, 30). However, the development of a memory CD8+ T-cell population during M. tuberculosis infection is supported by the findings that mycobacterium-specific gamma interferon (IFN-γ)-secreting and cytolytic CD8+ T cells can be cultured from the peripheral blood of healthy PPD+ individuals (12, 25, 26, 28). The failure to detect memory M. tuberculosis-specific CD8+ T cells in a number of murine studies appears to be in discordance with the fact that strong CD8+ T-cell mediated immune responses are elicited during the acute stages of the infection (19, 33, 34). Previous studies did not examine lung specific responses or used methods to test T-cell responses that might not provide data about the CD8+ T-cell subset.
The participation of CD8+ T cells in the recall response to M. tuberculosis challenge is important in terms of rational vaccine development and design. Given the recent data on CD8+ T-cell responses during acute infection and newer tools for analyzing T-cell responses in the lungs, we revisited this important question. We hypothesized that acute infection of mice with M. tuberculosis culminates in the development of both CD4+ and CD8+ memory T cells and that both subsets of memory lymphocytes actively participate in the immune response upon secondary infection of immune mice. We present data indicating that memory CD8+ T cells mobilized rapidly to the lungs during secondary M. tuberculosis infection and became activated to an extent similar to that of CD4+ T cells. Our results indicate that significant percentages of both CD4+ and CD8+ T cells producing IFN-γ appear in the lungs of rechallenged mice as early as 1 week postinfection. CD8+ T cells present in the lungs of challenged mice lysed M. tuberculosis-infected macrophages, suggesting that CD8+ T cells contribute to memory immunity by a combination of cytokine production and cytotoxicity.
MATERIALS AND METHODS
Mice.
Eight- to ten-week-old female C57BL/6 mice (Charles River Laboratories) were used and maintained in specific-pathogen-free Biosafety Level 3 facilities. All experimental and animal handling procedures were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh School of Medicine.
Bacteria and infections.
M. tuberculosis (Erdman strain; Trudeau Institute, Saranac Lake, N.Y.) was passed through mice, grown in culture once, and frozen in aliquots. Before infection, an aliquot was thawed, diluted in phosphate-buffered saline (PBS) containing 0.05% Tween 80, and sonicated for 10 s in a cup horn sonicator. Mice were infected intravenously (i.v.) via tail vein with 2 × 105 live bacilli in 100 μl or by aerosol with approximately 100 live bacilli as determined by viable counts on 7H10 agar plates (Difco Laboratories, Detroit, Mich.). For aerosol infections, an Intox Products (Albuquerque, N.M.) nose-only exposure system was used as previously described (33); 107 CFU/ml were placed in the nebulizer and mice were exposed for 20 min, followed by 5 min of air only. This results in the deposition of 30 to 100 CFU/lung. For all studies, three to four mice per time point were used, and each experiment was performed at least twice.
Antibiotic treatment.
The antibiotics were administered in the drinking water as a solution of 0.1 g of isoniazid (Sigma, St. Louis, Mo.) and 15 g of pyrazinamide (Acros Organics) per liter. The antibiotic-containing water was changed twice weekly for the duration of 2 months.
CFU determination.
Organs were homogenized in PBS containing 0.05% Tween 80, and dilutions were plated on 7H10 agar (Difco). Plates were incubated at 37°C in 5% CO2, and colonies were enumerated after 18 to 21 days.
Culture and infection of DC and macrophages.
Dendritic cells (DCs) and macrophages were grown from murine bone marrow precursors and cultured for 5 days using methods previously described (34). For macrophage infection, adherent cells were washed twice with ice-cold PBS (Life Technologies, Grand Island, N.Y.), and media were added containing Dulbecco modified Eagle medium (DMEM), 10% certified fetal bovine serum (FBS), 1 mM sodium pyruvate, and 2 mM l-glutamine (Life Technologies). For DC infection, nonadherent cells were harvested, adjusted to 0.5 × 106 cells/ml in DC media containing recombinant murine granulocyte-macrophage colony-stimulating factor and dispersed into 25-cm2 culture flasks (Costar, Cambridge, Mass.) for infection. For infection of antigen-presenting cells, frozen aliquots were used to start cultures at a concentration of 2.5 × 106/ml in liquid medium (7H9 Middlebrook; Difco, Detroit, Mich.); bacteria were grown in 5% CO2 at 37°C. Four to six-day-old cultures were used to infect cells. Bacteria were washed, resuspended in DMEM medium (Life Technologies), and sonicated for 15 s prior to infection of cell cultures. Cells were infected at a multiplicity of infection (MOI) of 3 to 5; extracellular bacteria were separated from cells by low-speed centrifugation (DCs) or by washing adherent cells twice with PBS (macrophages). After 18 to 36 h of infection, cells were incubated for 10 min on ice and then harvested by forceful pipetting. The percentage of infection was estimated in each experiment by staining aliquots of cells by the Kinyoun method for acid-fast bacteria (Difco). Routinely, 40 to 55% of DCs and 60 to 85% of macrophages were infected.
FACS analysis of cell surface markers.
Lung cells were obtained from mice infected for various periods of time by crushing the organs in cell strainers (Becton Dickinson Labware, Lincoln Park, N.J.) to obtain single cell suspensions. Red blood cells were lysed with NH4Cl-Tris solution, and cells were washed twice. Cells were stained for cell surface markers using antibodies against CD8 (CyChrome Ab clone 53-6.7), CD4 (CyChrome Ab, clone H129.19), CD44 (fluorescein isothiocyanate [FITC] Ab, clone IM7), CD25 (phycoerythrin [PE] Ab, clone PC61), and CD69 (FITC Ab, clone H1.2F3) in PBS containing 20% mouse serum, 0.1% bovine serum albumin, and 0.1% sodium azide for 30 min at 4°C. All antibodies were used at 0.2 μg/106 cells and were obtained from PharMingen (San Diego). Cells were fixed with 4% paraformaldehyde for 4 to 15 h and analyzed by fluorescence-activated cell sorter (FACS) analysis using CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). Cells were gated on the lymphocyte population by size and forward and side scatter.
Intracellular staining.
Single cell suspensions of lungs at various times postinfection were prepared as described above. Staining for intracellular cytokines was performed as described previously (34). Briefly, cells were either stimulated with anti-CD3 (clone 145-2C11, 0.1 μg/ml) and anti-CD28 (clone 37.51, 1 μg/ml) antibodies (PharMingen) or left unstimulated for 5 to 6 h in the presence of 3 μM monensin (Sigma Chemicals, St. Louis, Mo.). At the end of stimulation period, cells were stained for CD4 and CD8, fixed, permeabilized, and stained for intracellular IFN-γ (PE Ab, clone XMG1.2; PharMingen).
Culture of lung cells for CTL assays.
Lung cells from infected mice were obtained as described above and plated in 96-well U-bottom plates (Corning Incorporated, Corning, N.Y.) in DMEM supplemented with 10% certified FBS, 1 mM sodium pyruvate, 2 mM l-glutamine, 25 mM HEPES (Life Technologies), 50 μM 2-mercaptoethanol (Sigma), 30 μg of gentamicin (GIBCO-BRL, Gaithersburg, Md.) per ml, 15 to 20 U of murine recombinant interleukin-2 (rIL-2; Boehringer-Mannheim, Indianapolis, Ind.) per ml, and 1 mM aminoguanidine (Sigma) at 2 × 105 cells/well. DCs infected for 18 to 24 h as described above were added to the cell cultures at 6.5 × 103 to 7 × 103 viable cells/well. After 2 to 3 days of culture, 100 μl of medium was removed from each well and replaced with fresh medium containing IL-2. Cells were cultured for additional 3 to 4 days prior to cytoxic T lymphocyte (CTL) assays. The resultant cultures were typically 65 to 85% CD8+.
Cytotoxicity assays.
Lymphocytes harvested from 5 to 7-day stimulation cultures were tested in a 4-h 51Cr release assay as described previously (33). To prepare targets, macrophages uninfected or infected for 36 to 42 h were harvested as described above and labeled with 100 μl of Na51CrO4 (Amersham) in Teflon jars (Savillex, Minnetonka, Minn.) for 1 h at 37°C. Cells were washed three times with DMEM, added to wells of 96-well U-bottom plates (Corning) at 4 × 103 cells/well, and allowed to adhere for 20 min prior to the addition of T cells. Cultured cells were added at various effector/target ratios in a total volume of 0.1 ml in DMEM supplemented with 10% certified FBS, 1 mM sodium pyruvate, 2 mM l-glutamine, 25 mM HEPES, and 50 μM 2-mercaptoethanol, and the assay was carried out for 4 h. After 4 h, 85 μl of supernatant was removed from each well without disturbing the cells and counted in a gamma counter. Spontaneous release was determined by culturing the target cells in medium alone, and the total release was determined by adding 0.1% Triton-X to target cells. The percent specific lysis was calculated by the formula: 100 × (experimental counts per minute [cpm] − spontaneous cpm/total cpm − spontaneous cpm).
Statistics.
The unpaired t test was used to compare naive and memory mice at each time point. For comparison of bacterial numbers in the lungs (see Fig. 2), log transformation of the CFU numbers was performed prior to analysis. Statistical analysis was performed using the Prism program (GraphPad Software, San Diego, Calif.). P values are shown on each figure, comparing data from naive and memory mice at each time point.
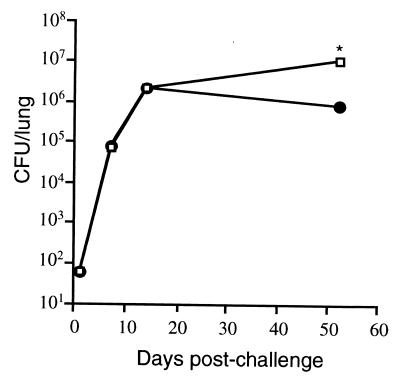
FIG. 2.
Course of M. tuberculosis aerosol challenge in the lungs of memory and naive mice. Memory immune (●) and naive (□) mice were infected with ∼60 M. tuberculosis bacilli via aerosol. The numbers of viable bacilli were determined by plating serial dilutions of lung homogenates onto 7H10 plates and counting the colonies after 3 weeks at 37°C. Each time point represents four mice, and the experiment was repeated once. The standard error bars are too small to see on this graph. ∗, P ≤ 0.001, comparing naive and memory mice at each time point.
RESULTS
Establishment of memory immunity in M. tuberculosis-infected mice.
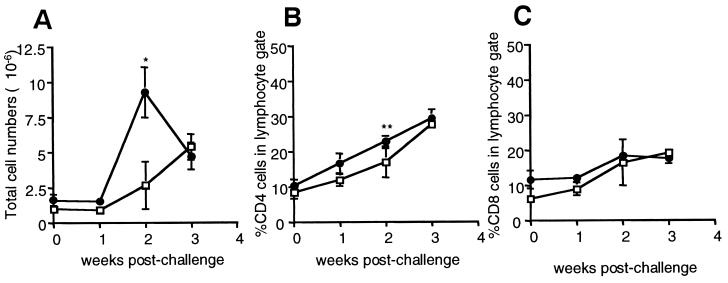
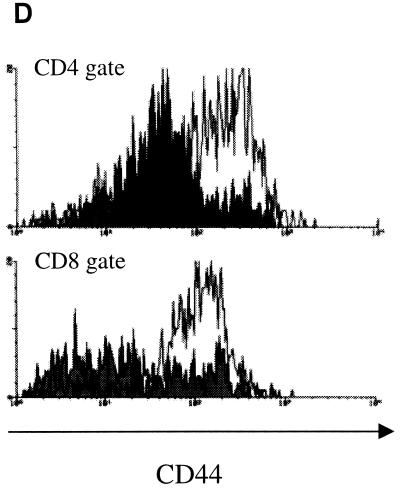
A memory model was established by infecting C57BL/6 mice i.v. with approximately 2 × 105 M. tuberculosis bacilli for 1 month, followed by the administration of the antibiotics isoniazid and pyrazinamide for 2 months. In accordance with previous data (3, 4, 30, 32), this regimen of antimycobacterial drugs led to the reduction of bacterial numbers in the organs of mice to undetectable levels (<102 CFU as assessed by plating whole lung suspensions [data not shown]). Mice then were rested for 4 to 6 months. The apparent elimination of bacteria and the long duration of the rest period ensured that a stable pool of memory lymphocytes was established. At the end of the rest period, the lungs of memory immune mice closely resembled the lungs of naive age-matched mice with low numbers of cells overall and relatively few CD4+ and CD8+ T cells (Fig. 1). Naive lymphocytes are small, recirculating lymphocytes, which do not express detectable levels of activation markers such as CD69 and CD25 and which express low levels of the effector-memory marker CD44. In contrast, memory lymphocytes generally express high levels of CD44 while lacking activation markers CD69 and CD25 (8, 9, 18). The lungs of previously infected and drug-treated mice contained CD4+ and CD8+ T cells with the phenotype of memory lymphocytes (CD69low CD25low CD44high). In contrast, lungs of naive mice contained T cells of the naive CD69low CD25low CD44low phenotype (Fig. 1D; Fig. 3B, day 0; data not shown). Although we cannot exclude the possibility that the memory T cells are present in the blood circulating through the lungs and possibly contaminating our T-cell preparation, our data nonetheless demonstrate that memory lymphocytes were present either in the lung tissue or in the circulation surveying the lung.
FIG. 1.
Characterization of lung cell composition in naive and memory immune mice following M. tuberculosis aerosol challenge. C57BL/6 mice were infected i.v. with 2 × 105 viable M. tuberculosis bacilli (strain Erdman) for 1 month, treated with isoniazid and pyrazinamide for 2 months, and rested for 4 months. Memory immune (●) and naive (□) mice were challenged with ∼60 M. tuberculosis bacilli via aerosol and lungs were harvested and disaggregated at 0, 1, 2, and 3 weeks postinfection. (A) The numbers of viable cells in lungs following challenge were counted by trypan blue exclusion. Each time point represents four to eight mice. ∗, P = 0.008, comparing naive and memory immune mice at each time point. (B and C) Cells harvested from lungs were stained for CD4 (B) and CD8 (C). Cells were gated on lymphocyte population by size and analyzed by flow cytometry. Error bars represent standard error. ∗∗, P = 0.048. (D). Lung cells were harvested from memory immune (white histogram) and age-matched naive (black histogram) mice prior to aerosol infection; stained for CD4 (top panel), CD8 (bottom panel), and CD44; fixed in paraformaldehyde; and analyzed by two-color flow cytometry. Shown are the results from a representative mouse. The experiment was repeated four times.
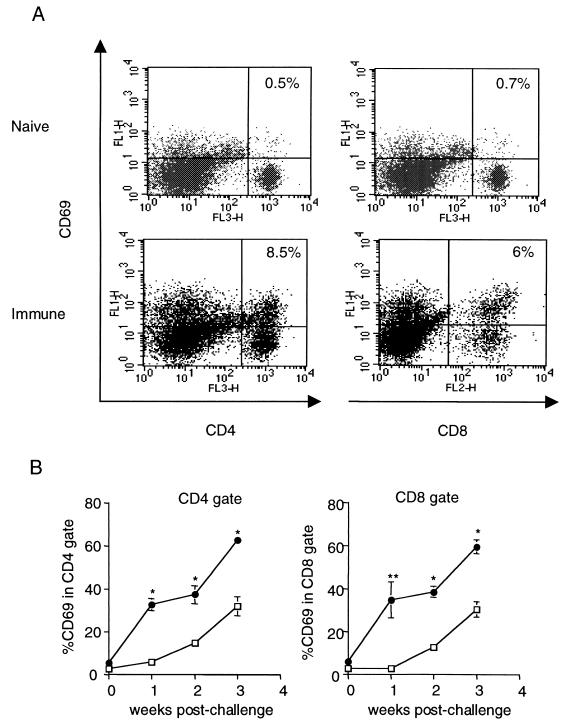
FIG. 3.
Changes in activation profiles of CD4+ and CD8+ T cells in the lungs following M. tuberculosis challenge. Memory immune and naive mice were infected via aerosol, and lung cells were harvested at 0, 1, 2, and 3 weeks postinfection, stained for CD4, CD8, and CD69, gated on lymphocyte population by size, and analyzed by flow cytometry. (A) Expression of CD69, CD4, and CD8 in the lungs of memory and immune mice 1 week postchallenge. Shown is a representative experiment. (B) Cells were harvested from lungs of challenged memory (●) and naive (□) mice at day 0 and at weeks 1, 2, and 3 postchallenge, stained for CD4, CD8, and CD69, and gated on lymphocytes. Cells were further gated on CD4 and CD8, and expression of CD69 in the gates was analyzed. Each time point in panel B represents three to six mice. Error bars represent standard error. ∗, P ≤ 0.0001; ∗∗, P ≤ 0.01, comparing naive and memory mice at each time.
Activation of CD4 and CD8 T cells following M. tuberculosis challenge.
We compared the kinetics of emergence and activation of CD8+ T cells in the lungs of memory immune and naive mice following challenge with M. tuberculosis. At the end of the rest period, memory immune and naive mice were challenged with ∼60 viable M. tuberculosis bacilli via aerosol and lungs were harvested 1, 2, and 3 weeks postchallenge. Previously, memory immune mice were shown to quickly control bacterial numbers in the spleen and lung following a secondary infection delivered i.v. (4), although not when the challenge was delivered via aerosol (16). In our experiments, the bacterial loads in the lungs of immune or naive mice remained comparable for the first 2 weeks following aerosol challenge (Fig. 2). At 2 weeks postchallenge, the lungs of memory immune mice contained ∼106 bacteria, and no further increase in the CFU counts were observed at 7 weeks postchallenge. In contrast, the bacterial loads in the lungs of acutely infected mice continued to increase past 2 weeks postinfection and were ∼10-fold higher than in the lungs of immune mice at 7 weeks postinfection (P < 0.001).
No increase in the total numbers of cells in the lungs was observed in either naive or drug-treated mice during the first week of aerosol challenge (Fig. 1). By 2 weeks postchallenge, the number of cells in the lungs of both groups of mice began to increase, but the lungs of memory immune mice had threefold more cells compared to the lungs of nonimmune mice. The percentages of CD4+ and CD8+ T cells in the lungs of both groups of mice increased by up to 3 weeks postchallenge (Fig. 1). These results indicate that both CD4+ and CD8+ T cells participate in the recall response to M. tuberculosis infection.
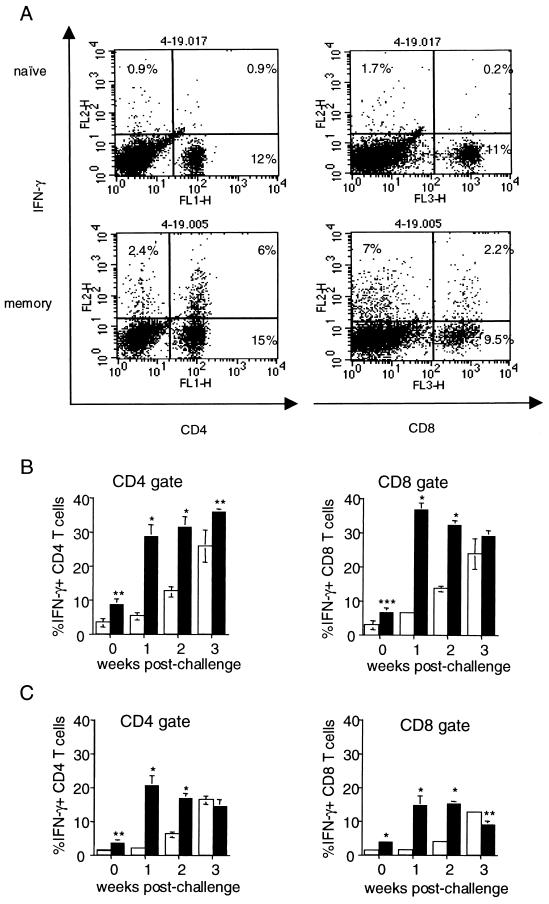
We examined the activation status of T cells in the lungs of infected mice by assessing the expression of the activation marker CD69 on the surface of T cells. CD69 is upregulated in response to engagement of the T-cell receptor by antigen-presenting cells. We found that 32.6% ± 2.8% of CD4+ T cells and 34.6% ± 8.5% of CD8+ T cells were also CD69+ and therefore presumably active in the immune response in the lungs by the first week postchallenge. These numbers continued to increase throughout the duration of the experiment (Fig. 3B). In contrast, lymphocytes harvested from the lungs of infected naive mice did not appear activated until week 2 postinfection; the percentages of CD69-expressing CD4+ and CD8+ T cells in the infected naive lungs at this time point were ∼3-fold less than in the challenged memory lungs (Fig. 3A and B). Since there were more cells in the lungs of immune mice at this time point (Fig. 1), this difference translates into substantially different overall numbers of activated lymphocytes in the lungs of naive and immune mice. The percentages of CD69-expressing T lymphocytes in the lungs of immune mice were higher than the percentages of activated T cells in the naive infected lungs at all time points examined (Fig. 3A and B).
Emergence of cytokine-producing CD8 T cells during primary and memory immune responses.
Protective immune responses during murine and human tuberculosis depend in part on the ability of T cells to produce cytokines such as IFN-γ (15, 20, 23, 29). We previously reported that lungs of naive mice infected i.v. contained mycobacterium-specific CD4+ and CD8+ T cells capable of rapid secretion of this cytokine upon TCR triggering (34). To examine the emergence of cytokine-producing T-cell populations during primary and secondary immune responses to an aerosol challenge, lung cells from naive and immune mice were harvested at various times postchallenge and stimulated briefly (5 h) with anti-CD3 and anti-CD28 antibodies in the presence of monensin, followed by intracellular cytokine staining. This provides an estimate of the number of T cells in the lungs capable of producing IFN-γ in the lungs if properly stimulated (34). Under these conditions, cells from immune or naive mouse lungs did not produce detectable IFN-γ prior to challenge (Fig. 4B, day 0). Following aerosol M. tuberculosis challenge, the cytokine producing CD4+ and CD8+ T-cell populations in the lungs of naive mice developed with a delay compared to that previously reported for i.v.-infected mice (34), and significant percentages of IFN-γ-producing CD4+ and CD8+ T cells did not appear until 3 weeks postinfection (Fig. 4A and B). In marked contrast, CD4+ and CD8+ T cells capable of secreting IFN-γ were present in the lungs of immune mice by as early as 1 week postchallenge; 30 to 35% of both CD4+ and CD8+ T cells in the lungs of challenged immune mice produced IFN-γ in response to TCR stimulation at this and subsequent time points (Fig. 4A and B).
FIG. 4.
Intracellular IFN-γ staining of lung cells from M. tuberculosis-challenged mice. Lung cells from memory and naive mice were harvested at 0, 1, 2, and 3 weeks postchallenge and stimulated with anti-CD3 and anti-CD28 antibodies or left unstimulated in the presence of monensin for 5 to 6 h. Cells were stained for CD4 and CD8, fixed in paraformaldehyde, permeabilized, and stained for intracellular IFN-γ. Cells were gated on lymphocytes by size and analyzed by three-color flow cytometry. (A) Production of IFN-γ by CD4+ and CD8+ T cells harvested from lungs at 1 week postchallenge after anti-CD3 and anti-CD28 antibody stimulation. A representative mouse is shown. Lung cells from M. tuberculosis-challenged immune (filled bars) and naive (open bars) mice were stained for CD4, CD8, and IFN-γ and gated on CD4 or CD8; expression of IFN-γ with (B) or without (C) anti-CD3/anti-CD28 antibody stimulation within each gate was analyzed. Each time point represents four to eight mice. ∗, P ≤ 0.0001; ∗∗, P ≤ 0.01; ∗∗∗, P = 0.02, comparing naive and memory mice at each time point.
In vivo cytokine production by memory CD8+ T cells following challenge.
To assess actual cytokine production by T cells in vivo, we cultured lung cells for 4 to 5 h in the presence of monensin without stimulation and assessed unstimulated ex vivo IFN-γ production by intracellular staining, as previously described (34). Under these conditions, the only IFN-γ-positive cells detected would be cells that were actively secreting cytokine in vivo immediately prior to harvest. Prior to challenge, T cells from the lungs of uninfected mice and immune mice did not produce IFN-γ with or without stimulation (Fig. 4B and C, day 0). Both CD4+ and CD8+ T cells from the lungs of immune mice secreted IFN-γ ex vivo without restimulation as early as 1 week postchallenge: 20.2% ± 2.4% of CD4+ and 14.7% ± 2.7% of CD8+ T cells were IFN-γ positive (Fig. 4C). In contrast, no significant unstimulated cytokine secretion by CD4+ and CD8+ T cells from the lungs of naive infected mice was observed for the first 2 weeks postinfection (Fig. 4C). By the third week postinfection, however, the percentages of cytokine-producing T cells in the lungs of challenged memory immune and naive mice were comparable (Fig. 4C). These results indicate that there is a rapid and robust recall response in the lungs of previously infected mice upon challenge with M. tuberculosis, and this memory response involves both CD4+ and CD8+ T-cell subsets. The data above suggest that similar percentages of memory CD4+ and CD8+ T cells are primed to produce IFN-γ and that each subset is actively secreting this cytokine at the site of infection early in the secondary response.
Cytotoxic function of memory CD8+ T cells.
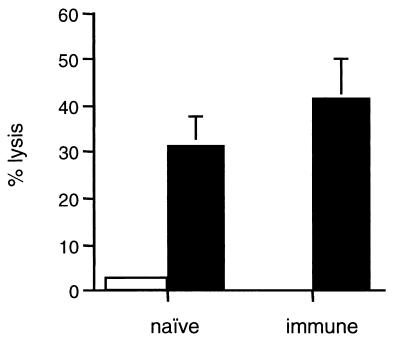
Previously, we demonstrated that lungs of acutely infected mice contain CD8+ CTLs that can recognize and lyse M. tuberculosis-infected macrophages (33). To examine whether the secondary response to M. tuberculosis challenge includes mycobacterium-specific CTLs capable of lysing infected macrophages, the cells harvested from lungs of naive or immune mice at 3 weeks postchallenge were cultured with M. tuberculosis-infected DCs in the presence of IL-2 for 5 days. At the end of the culture period, resultant T-cell cultures were tested for cytotoxicity against M. tuberculosis-infected macrophages (Fig. 5). Although several antigens have been shown to be recognized by mycobacterium-specific CD8+ T cells (14, 28, 35, 39), the antigenic repertoire recognized by CD8+ T cells in tuberculosis remains largely uncharacterized. Therefore, we have used infected macrophages as targets for specific CD8+ CTLs to ensure that complete spectrum of antigens is presented to T cells during the assay. The CD8 T cells cultured from the lungs of both naive and immune challenged mice at this time point (where cell numbers were similar) lysed 30 to 40% of the target cells, with very low lysis (<5%) of uninfected macrophages. These results indicate that mycobacterium-specific CD8+ CTLs are present in the lungs of naive or immune challenged mice by 3 weeks postinfection. The relatively small number of cells in the lungs of the mice at earlier time points, particularly in the infected naive mice, were used for cytokine analyses, which precluded CTL studies. Thus, it remains to be established that memory CTLs are present in the lungs of immune mice earlier postchallenge than in the naive mice. In addition, the contribution of CD8 T cells to protection induced by the memory response remains to be determined.
FIG. 5.
Cytotoxic activity of lung CD8+ T-cell cultures. Lungs from memory immune or naive mice were harvested 3 weeks after challenge and stimulated with M. tuberculosis-infected MHC class II−/− DC for 5 days and used in a 4-h 51Cr release cytotoxicity assay. Targets: uninfected macrophages (open bars) and macrophages infected for 36 h with M. tuberculosis (MOI 4) (filled bars). Shown are the results of a representative experiment at 30:1 effector/target ratio. The experiment was repeated in four different mice of each group at this time point. Error bars represent the standard error.
DISCUSSION
Despite accumulating evidence indicating a role for CD8+ T cells in the acute immune response to M. tuberculosis, the participation of this cell subset in the memory immune response remains controversial. Recently, we have demonstrated that the lungs of acutely infected mice contain CD8+ T cells that secrete IFN-γ, express perforin in vivo, and are cytotoxic for M. tuberculosis-infected macrophages (33, 34). In this study, we provide evidence that M. tuberculosis infection leads to the development of memory CD8+ and CD4+ T-cell populations capable of rapid activation and effector function in the lungs upon challenge of mice. Our data indicate that CD8+ T-cell-mediated responses during secondary infection were significantly heightened compared to primary responses in the lungs. The numbers of CD8+ T cells increased dramatically in the lungs shortly after challenge, and many of these CD8+ T cells were producing IFN-γ.
Memory immunity can be established by subjecting M. tuberculosis-infected mice to a protracted course of antimycobacterial drug therapy. Upon secondary challenge, recall immune responses rapidly develop and result in enhanced control of the infection. There have been a limited number of studies that examined memory responses in M. tuberculosis-infected mice. The early studies (3, 30) used i.v. challenge with high doses of M. tuberculosis, and immune responses were assessed in the spleen and liver. During the first 7 days after challenge, the bacterial growth in these organs of memory immune mice occurred at a slower rate than that observed in the organs of naive mice (3, 30). However, the natural route of infection with M. tuberculosis is via the respiratory tract, and the memory response in the lungs is likely to be most important in controlling infection. A more recent study compared the control of i.v. and aerosol challenges in memory immune mice and found that bacterial numbers in the lungs of mice challenged by aerosol were not controlled until after 2 weeks postinfection (16). We also observed that during the first 2 weeks post-aerosol challenge, the bacterial numbers increased in the lungs of both rechallenged and acutely infected mice at the same rate. However, after the second week postchallenge, memory immune mice established control over infection, while the bacterial numbers in the lungs of acutely infected mice continued to increase. By 7 weeks postchallenge, the bacterial numbers in the lungs of immune mice were 10-fold lower than in the challenged naive mice. The inability of the memory response in the lungs to control the initial replication of a secondary infection remains a challenge for vaccine design, particularly if the goal is to protect humans from airborne infection with M. tuberculosis.
Early reports on the memory response in the spleen following M. tuberculosis infection indicated that CD4 T cells were the primary cell type responsible for control of infection, and an increase in CD8 T cells after challenge was transient (3). These studies concluded that CD8 T cells were not likely to play a significant role in the memory immune response to M. tuberculosis. However, the subsets of cells in the lungs responding to a secondary aerosol challenge were not studied. A recent report suggested that T cells from the spleen and the thoracic lymph nodes recognized different antigens following i.v. or aerosol challenge (16). However, in these studies, antigens were provided for in vitro stimulation in a manner expected to favor CD4 T-cell responses. Responses in the lungs may be very different than in the spleen or liver.
A number of cell surface markers have been widely used to define memory and naive phenotypes of T cells in humans and mice (reviewed in reference 18). Generally, both memory and naive T cells are associated with low levels of expression of activation markers such as CD69 and the IL-2 receptor (CD25) (18). In mice, the level of CD45RB expression is sometimes used to distinguish naive and memory lymphocytes. However, this phenotype is not reliable and can change with activation or cytokine treatment (10). The expression of CD62L, an adhesion molecule, is another marker used for identifying naive T cells, and the loss of CD62L expression correlates with T-cell priming. However, CD62L expression is sometimes regained on memory cells (18). The naive T-cell phenotype is also associated with low expression of CD44, a molecule that mediates binding to hyaluronan and promotes T-cell migration to sites of inflammation. CD44 expression increases upon primary T-cell stimulation and remains high on activated and memory cells for prolonged periods of time (8, 9). We typically observe that once T cells upregulate CD44 expression during acute infection, it remains stable for up to 8 months (unpublished observations), making CD44 an attractive marker for differentiating memory and activated cells from their naive counterparts. Defining memory T lymphocytes as CD69low CD25low CD44high, we detected small numbers of CD4+ and CD8+ T lymphocytes with these characteristics in the lungs and/or circulating blood within the lungs of previously infected, drug-treated mice prior to challenge.
CD69 expression is rapidly upregulated upon triggering of T-cell receptor reaching a peak at 24 to 48 h (5, 7, 27) and thus can be used as an indicator of T cells interacting with antigen-presenting cells. Upon challenge of memory immune mice with M. tuberculosis via aerosol, activation of both CD4+ and CD8+ T cells was observed as early as 1 week postinfection, and the expression of the activation marker CD69 was detected in approximately 30% of both T-cell subsets; this level was maintained for at least 3 weeks. In marked contrast, activated T cells were undetectable in the naive infected mice at this time point. Accelerated responses of activated CD4+ and CD8+ T cells in the lungs of the memory mice following M. tuberculosis challenge could be attributed to the increased frequencies of memory antigen-specific T cells and/or the ability of these cells to respond to lower antigenic doses or inflammatory stimuli compared to T cells undergoing primary activation (1). Importantly, our results demonstrated that activation of memory CD8+ T cells was evident in the lungs of memory immune mice shortly after challenge; the extent of CD8+ T-cell engagement in the memory immune responses was similar to that of CD4+ T cells. Surprisingly, despite the rapid activation of T cells, the cell numbers were similar between naive and memory mice in the first week postchallenge. Previously, a sharp increase in splenic CD4+ and CD8+ T-cell numbers was reported to occur 5 days after the i.v. challenge of immune mice (3). The delayed recruitment of cells to the lungs in our experiments may be related to the low dose of bacterial inoculum delivered. Under these conditions, inflammatory responses sufficient to induce cell migration into the infected lungs might not be established until later in infection.
Previously, cytokine production in the spleens of i.v.-challenged mice has been attributed exclusively to CD4+ T cells (3). In those experiments, splenocytes were stimulated with mycobacterial short-term culture filtrate proteins and the ability of anti-CD4 or anti-CD8 antibodies to block IFN-γ production was assessed. Spleen and thoracic lymph node T cells from memory immune mice were also stimulated with protein or peptide following aerosol challenge and shown to produce IFN-γ (16). However, this choice of stimulation might not be optimal for induction of CD8+ T-cell responses, since CD8+ T cells do not generally respond well to soluble protein antigens. Our results demonstrated that a significant percentage of these cells produced IFN-γ after stimulation with anti-CD3 and anti-CD28 antibodies. Since T-cell stimulations were carried out for only brief periods of time (<5 h), only primed and activated cells could respond to this treatment, as we described previously (34). We observed that 30 to 35% of both CD4+ and CD8+ T cells were capable of secreting this cytokine upon stimulation as early as 1 week postchallenge, while significant cytokine production by T cells from acutely infected mice was not detected until week 3 postinfection. Our previous studies documented that a large percentage of these IFN-γ-producing CD4 and CD8 T cells were mycobacterium specific (34). Therefore, the antimycobacterial memory response was comprised of both cytokine-producing CD4+ and CD8+ T cells, which were readily available once the inflammatory process was initiated. Moreover, memory CD8+ T cells appeared to be actively secreting IFN-γ in the infected lungs, as judged by production of this cytokine ex vivo without restimulation, and the percentage of cytokine-secreting CD8+ and CD4+ T cells was similar. In murine tuberculosis, IFN-γ production is an essential component of the protective immune response; this cytokine activates macrophages to kill intracellular M. tuberculosis (2). The early presence of CD8+ T cells in the lungs of challenged mice secreting IFN-γ suggests that these cells actively participate in the memory response by activating macrophages.
Apart from the ability to make cytokines, CD8+ T cells may also function as CTLs; the development of CD8+ CTLs during acute murine tuberculosis has been documented (17, 24, 33, 35, 36, 39). We demonstrated that, 3 weeks postchallenge, the lungs of memory mice contained CD8+ CTLs capable of recognizing and lysing M. tuberculosis-infected macrophages, indicating that CD8+ T cells may contribute to protective memory immune responses in a dual fashion. Overall, the early emergence in the lungs of memory mice of activated effector CD4+ and CD8+ T-cell populations correlated with the ability of these mice to establish control of bacterial growth after the second week postchallenge.
Our results argue that an initial M. tuberculosis infection primes both a CD4+ and a CD8+ memory T-cell population. Clearly, this is true in humans, where both CD4 and CD8 T cells from the peripheral blood of previously infected individuals recognize mycobacterial antigens (reviewed in reference 21). However, it is more difficult to study the localized responses in the lungs of humans following reinfection with M. tuberculosis, so the populations which mobilize to this site are unknown. In this study we demonstrated that not only CD4+ but also CD8+ T cells are rapidly activated and deployed to the lung upon challenge with M. tuberculosis. These CD8+ T cells secrete IFN-γ and, at least at later time points, can recognize and lyse infected macrophages. Our results support the belief that the design and development of vaccines against tuberculosis should take into account all facets of the immune response, including CD8+ T cells. However, our data confirm that this rapid and initial response in the lungs is not capable of preventing infection or controlling replication for at least 2 weeks postchallenge. Clearly, additional research into lung-specific immunity is necessary to design effective vaccines against tuberculosis.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant AI37859 (J.L.F.) and T32-CA82084 (N.V.S.).
We are grateful to members of the Flynn lab for helpful discussion and to Susan McCarthy and Charles Scanga for careful reading of the manuscript.
REFERENCES
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Almeida G B, Chitale C, Boutsikakis I, Geng J-Y, Doo H, He S, Ho J L. Induction of in vitro human macrophage anti-Mycobacterium tuberculosis activity: requirement for interferon-γ and primed lymphocytes. J Immunol. 1998;160:4490–4499. [PubMed] [Google Scholar]
- 3.Andersen P, Andersen A B, Sorensen A L, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- 4.Andersen P, Heron I. Specificity of a protective memory immune response against Mycobacterium tuberculosis Infect. Immun. 1993;61:844–851. doi: 10.1128/iai.61.3.844-851.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arva E, Andersson B. Kinetics of cytokine release and expression of lymphocyte cell-surface activation markers after in vitro stimulation of human peripheral blood mononuclear cells with Streptococcus pneumoniae. Scand J Immunol. 1999;49:237–243. doi: 10.1046/j.1365-3083.1999.00470.x. [DOI] [PubMed] [Google Scholar]
- 6.Behar S M, Dascher C C, Grusby M J, Wang C R, Brenner M B. Susceptibility of mice deficient in CD1d or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999;189:1973–1980. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biselli R, Matricardi P M, D'Amelio R, Fattorossi A. Multiparametric flow cytometric analysis of the kinetics of surface molecule expression after polyclonal activation of human peripheral blood T lymphocytes. Scand J Immunol. 1992;35:439–447. doi: 10.1111/j.1365-3083.1992.tb02879.x. [DOI] [PubMed] [Google Scholar]
- 8.Budd R C, Cerottini J-C, Horvath C, Bron C, Pedrazzini T, Howe R C, MacDonald R H. Distinction of virgin and memory T lymphocytes. J Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 9.Budd R C, Cerottini J-C, MacDonald R H. Phenotypic identification of memory cytolytic lymphocytes in a subset of Lyt-2+ cells. J Immunol. 1987;138:1009–1013. [PubMed] [Google Scholar]
- 10.Bunce C, Bell E. CD45RC isophorms define two types of CD4 memory T cells, one of which depends on persisting antigen. J Exp Med. 1997;185:767–776. doi: 10.1084/jem.185.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busch D H, Pilip I, Pamer E G. Evolution of a complex T cell receptor repertoire during primary and recall bacterial infections. J Exp Med. 1998;188:61–70. doi: 10.1084/jem.188.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canaday D H, Ziebold C, Noss E H, Chervenak K A, Harding C V, Boom W H. Activation of human CD8+ αβ TCR+ cells by Mycobacterium tuberculosis via an alternate class I MHC antigen processing pathway. J Immunol. 1999;162:372–379. [PubMed] [Google Scholar]
- 13.Caruso A M, Serbina N, Klein E, Triebold K, Bloom B R, Flynn J L. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-γ, yet succumb to tuberculosis. J Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 14.Cho S, Mehra V, Thoma-Uszynski S, Stenger S, Serbina N, Mazzaccaro R, Flynn J L, Barnes P F, Southwood S, Celis E, Bloom B R, Modlin R L, Sette A. Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc Natl Acad Sci USA. 2000;97:12210–12215. doi: 10.1073/pnas.210391497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper A M, Dalton D K, Stewart T A, Griffen J P, Russell D G, Orme I M. Disseminated tuberculosis in IFN-γ gene-disrupted mice. J Exp Med. 1993;178:2243–2248. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper A M, Callahan J E, Keen M, Belisle J T, Orme I M. Expression of memory immunity in the lung following re-exposure to Mycobacterium tuberculosis. Tuberc Lung Dis. 1997;78:67–73. doi: 10.1016/s0962-8479(97)90017-4. [DOI] [PubMed] [Google Scholar]
- 17.De Libero G, Flesch I, Kaufmann S H E. Mycobacteria-reactive Lyt-2+ T cell lines. Eur J Immunol. 1988;18:59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- 18.Dutton R W, Bradley L M, Swain S L. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 19.Feng C, G, Bean A G D, Hooi H, Briscoe H, Britton W J. Increase in gamma interferon-secreting CD8+, as well as CD4+ T cells in lungs following aerosol infection with Mycobacterium tuberculosis. Infect Immun. 1999;67:3242–3247. doi: 10.1128/iai.67.7.3242-3247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flynn J L, Ernst J D. Immune responses in tuberculosis. Curr Opin Immunol. 2000;12:432–436. doi: 10.1016/s0952-7915(00)00116-3. [DOI] [PubMed] [Google Scholar]
- 22.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile J-F, Newport M, Levin M, Blanche S, Seboun E, Fischer A, Casanova J-L. Interferon-γ receptor deficiency in an infant with fatal Bacille Calmette-Guerin infection. New Engl J Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann S H, Vath U, Thole J E, Van Embden J D, Emmrich F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol. 1987;17:351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- 25.Lalvani A, Brookes R, Wilkinson R, Malin A, Pathan A, Andersen P, Dockrell H, Pasvol G, Hill A. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:270–275. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewinsohn D, Alderson M, Briden A, Riddell S, Reed S, Grabstein K. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen presenting cells. J Exp Med. 1998;187:1633–1640. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mardiney M R, Brown M R, Fleisher T A. Measurement of T-cell CD69 expression: a rapid and effectively means to assess mitogen- or antigen-induced proliferative capacity in normals. Cytometry. 1996;26:305–310. doi: 10.1002/(SICI)1097-0320(19961215)26:4<305::AID-CYTO11>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 28.Mohagheghpour N, Gammon D, Kawamura L M, van Vollenhoven A, Benike C J, Engleman E G. CTL response to Mycobacterium tuberculosis: identification of an immunogenic epitope in the 19-kDa lipoprotein. J Immunol. 1998;161:2400. [PubMed] [Google Scholar]
- 29.Newport M J, Huxley C, Huston S, Hawrylowicz C, Oostra B, Williamson R, Levin M. A mutation in the interferon-γ-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 30.Orme I. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988;140:3589–3593. [PubMed] [Google Scholar]
- 31.Pentilla J M, Antilla M, Varkila K, Puolakkainen M, Sarvas M, Makela P H, Rautonen N. Depletion of CD8+ T cells abolishes memory in acquired immunity against Chlamydia pneumoniae in BALB/c mice. Immunology. 1999;97:490–496. doi: 10.1046/j.1365-2567.1999.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scanga C A, Mohan V P, Joseph H, Yu K, Chan J, Flynn J. Reactivation of latent tuberculosis: variations on the Cornell murine model. Infect Immun. 1999;67:4531–4538. doi: 10.1128/iai.67.9.4531-4538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serbina N V, Liu C-C, Scanga C A, Flynn J L. CD8+ cytotoxic T lymphocytes from lungs of M. tuberculosis infected mice express perforin in vivo and lyse infected macrophages. J Immunol. 2000;165:353–363. doi: 10.4049/jimmunol.165.1.353. [DOI] [PubMed] [Google Scholar]
- 34.Serbina N V, Flynn J L. Early emergence of CD8+ T cells primed for production of type 1 cytokines in the lungs of Mycobacterium tuberculosis-infected mice. Infect Immun. 1999;67:3980–3988. doi: 10.1128/iai.67.8.3980-3988.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva C L, Lowrie D B. Identification and characterization of murine cytotoxic T cells that kill Mycobacterium tuberculosis. Infect Immun. 2000;68:3269–3274. doi: 10.1128/iai.68.6.3269-3274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva C L, Silva M F, Pietro R, Lowrie D B. Protection against tuberculosis by passive transfer with T-cell clones recognizing mycobacterial heat shock protein 65. Immunology. 1994;83:341–346. [PMC free article] [PubMed] [Google Scholar]
- 37.Sousa A O, Mazzaccaro R J, Russell R G, Lee F K, Turner O C, Hong S, Van Kaer L, Bloom B R. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc Natl Acad Sci USA. 1999;97:4204–4208. doi: 10.1073/pnas.97.8.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Pinxteren L A H, Cassidy J P, Smedegaard B H C, Agger E M, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur J Immunol. 2000;30:3689–3698. doi: 10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Zhu X, Stauss H J, Ivanyi J, Vordermeier H M. Specificity of CD8+ T cells from subunit vaccinated or infected H-2b mice recognizing the 38-kDa antigen of Mycobacterium tuberculosis. Int Immunol. 1997;9:1669. doi: 10.1093/intimm/9.11.1669. [DOI] [PubMed] [Google Scholar]