Abstract
Background: Fibrotic interstitial lung diseases (F-ILDs) have a high symptom burden with progressive dyspnea as a primary feature. Breathlessness is underrecognized and undertreated primarily due to lack of consensus on how to best measure and manage it. Several nonpharmacologic and pharmacologic strategies are published in the literature, however there is a paucity of real-world data describing their systematic implementation. Objectives: We describe the types of breathlessness interventions and timing of implementation in our multidisciplinary collaborative care (MDC) ILD clinic and the impact of our approach on dyspnea trajectory and acute care use in ILD. Methods: A retrospective, observational study of deceased ILD patients seen in our clinic (2012-2018) was conducted. Patients were grouped by baseline medical research council (MRC) grade and dyspnea interventions from clinic enrolment until death were examined. Healthcare usage in the last 6 months of life was collected through Alberta’s administrative database. Results: Eighty-one deceased ILD patients were identified. Self management advice was provided to 100% of patients. Pulmonary rehabilitation (PR) and home care (HC) referrals were made in 40% and 57% of patients, respectively. Eighty percent were treated with oxygen and 53% with opioids during the study. MDC-initiated referral to PR and HC, oxygen and opioid prescriptions were provided a median of 13, 9, 11, and 4 months prior to death, respectively. Stepwise implementation of interventions was observed more commonly in MRC 1-2 and concurrent implementation in MRC 4-5. Conclusions: Our clinic’s approach allows early and systematic dyspnea management.
Keywords: interstitial lung disease, breathlessness, dyspnea, non-pharmacologic, oxygen, opioid, self-management
Introduction
Dyspnea is a disabling and distressing symptom, prevalent in fibrosing interstitial lung diseases (F-ILD).1 Idiopathic pulmonary fibrosis (IPF), the most common and well studied F-ILD with a life expectancy of only 3 years,2 has a dyspnea prevalence of 90% at diagnosis.3 Despite the high dyspnea burden in F-ILD and its’ impact on both health-related quality of life (HRQOL) and mortality,4-7 healthcare professionals often do not adequately assess or treat it in practice.8,9 Many reasons have been proposed for this care gap.
Firstly, dyspnea is a subjective symptom where experience is shaped by a variety of factors; including physiological, psychological and environmental influences.10,11 Degree of lung function impairment does not consistently predict level of breathlessness and cannot be used as a surrogate for direct dyspnea measures.12,13 Experts have therefore recommended that patients are routinely asked about dyspnea and should rate its severity. Knowledge of dyspnea severity may help guide management.14 Unfortunately, this is uncommon in practice.15
The American Thoracic Society (ATS) statements and guidelines2,16,17 emphasize the urgent need to assess and manage dyspnea in respiratory disease, including IPF. Although they recommend the treatment of associated psychosocial factors, pulmonary rehabilitation, anxiolytics and opioids, they do not provide a treatment algorithm.2,16,17
Recognition that dyspnea is a complex symptom that is undertreated and that a single intervention is unlikely to alleviate the multiple domains of breathlessness18 has led to research efforts focused on the development of breathlessness services. These are multidisciplinary models for managing breathlessness with proven efficacy and cost effectiveness in both malignant and non-malignant disease.19-22 However, such services are not widely available. Physician perspectives, inadequate education and lack of services are all important additional barriers to effective dyspnea management.23 As a result, outside of clinical trials, real-world experience with dyspnea management is limited in the literature.
In November 2012, we implemented a multidisciplinary collaborative care (MDC) ILD clinic model to address these gaps in dyspnea care. We used a pilot tool to assess dyspnea severity and help guide management. We have previously shown that our approach to dyspnea management facilitates patient self-management and mastery, allowing reduction in acute care utilization and healthcare costs in IPF.24-26 In this study, we examine the types of breathlessness interventions and their timing and order of implementation in F-ILD patients managed within this real-world clinic model. We also describe the impact on dyspnea trajectory and acute care use in F-ILD. We hypothesize that our multidisciplinary approach including dyspnea severity assessment will lead to systematic use of non-pharmacologic and pharmacologic dyspnea therapies in F-ILD.
Methods
Study Design
This is a longitudinal, retrospective chart review of deceased ILD patients from our MDC ILD clinic. This is a descriptive study. Our study received ethics approval from the University of Alberta (PRO00059361 and PRO00100883).
Setting
ILD patients received care in the MDC ILD clinic through the Kaye Edmonton Clinic, Alberta Health Services, Edmonton, Alberta, Canada between November 2012, and December 2018. This is a multidisciplinary, specialized clinic that delivers ILD care with a focus on early dyspnea management.
In our MDC clinic model,27 dyspnea assessment and management are undertaken with the support of allied health and home care services. This is initiated by clinicians without formal palliative care training. As a part of patient clinical assessment, an eleven-point numerical rating scale (NRS) was used to measure dyspnea intensity with rest and selected everyday activities (NRS pilot dyspnea tool). All reversible causes of dyspnea were medically optimized. Non-pharmacological and pharmacological breathlessness interventions were subsequently provided as determined by treating physicians based on NRS component scores, desaturations, functional assessment, and clinical judgement at each visit. Our multidisciplinary team composition and roles as well as approach to opioid titrations are described elsewhere.24,28,29 Home care services were provided by Alberta Health Services staff in the community.
Study Population
All deceased ILD patients with at least 1 documented NRS dyspnea score seen in our MDC clinic (November 2012 - December 2018) were eligible for inclusion. F-ILD was defined as a multidisciplinary diagnosis of chronic ILD of any etiology with features of diffuse fibrosing lung disease >10% extent on high-resolution computed tomography (HRCT) chest. IPF diagnosis was made in accordance with the ATS 2011 guidelines.
Data Collection
All data was extracted from patient electronic medical records (EMR). Baseline data collected from the first MDC clinic visit included demographics, comorbidities that could cause dyspnea, smoking status, Medical Research Council (MRC) grade, NRS dyspnea scores, pulmonary function test (PFT) and 6-minute walk test (6MWT). Gender-age-physiology (GAP) stage at referral was calculated. GAP is an index and staging system that uses clinical variables to predict mortality risk in ILD.30
Longitudinal data extracted by clinic visit included: NRS dyspnea scores, MRC grade and applied interventions. Extracted interventions included symptom self-management education (including pacing and energy conservation strategies, breathing techniques, oxygen, and nasal care), pulmonary rehabilitation (PR) referral and completion, home care (HC) referrals, oxygen initiation and titration, and opioid prescription. Self-management education referred to here was provided within clinical encounters by nurses, allied health professionals and physicians. Interventions that occurred between visits were linked to the closest visit.
Date of death was collected from patient EMRs. Patients’ healthcare usage in the last 6 months of life was collected through Alberta’s administrative database which includes Inpatient Discharge Abstract Database (DAD), National Ambulatory Care Reporting System (NACRS) and Practitioner Claims.
Data Analysis
We present summary statistics in the format of mean (standard deviation (SD)) or median (interquartile range (IQR)). Mann-Kendall test was used to test the dyspnea trend overtime. Sunburst diagrams were used to capture the sequence of interventions patients received and the distribution in this population. All analyses were conducted using SAS 9.4 [Cary, NC, USA] and Excel.
Results
A total of 497 ILD patients were seen in MDC ILD clinic between November 2012 and December 2018 and 109 of them died during that timeframe. Eighty-one of the deceased MDC ILD clinic patients had at least 1 NRS score documented and were identified for analysis (80/81 had F-ILD). Median age at first visit was 70 years old (65-76); 62% were male; 53 (65%) had IPF. Ninety-one percent of patients had an MRC ≥3 at baseline. Baseline patient characteristics are reviewed in Table 1.
Table 1.
Patient Characteristics.
| Variable | ||
|---|---|---|
| Patients, n | 81 | |
| Age at first MDC Clinic visit, median (IQR) | 70 (65-76) | |
| Male, n (%) | 50 (61.7) | |
| Final diagnoses, n (%) | IPF | 53 (65.4) |
| CTD-ILD | 10 (12.3) | |
| NSIP | 6 (7.4) | |
| Other | 12 (14.8) | |
| Comorbidities at referral, n (%) | COPD | 11 (13.6) |
| Cardiac disease | 31 (38.3) | |
| Pulmonary hypertension | 19 (23.5) | |
| OSA | 10 (12.3) | |
| Depression | 12 (14.8) | |
| Anxiety | 4 (4.9) | |
| Smoking status at referral, n (%) | Ever smoker | 63 (77.8) |
| Current smoker | 3 (3.7) | |
| GAP stage at baseline, n (%) | I | 16 (19.8) |
| II | 25 (30.9) | |
| III | 8 (9.9) | |
| Unknown | 32 (39.5) | |
| FVC (%predicted) at baseline, median (IQR)a | 60.7 (51.9-75.3) | |
| DLCO (%predicted) at baseline, median (IQR)a | 40.2 (33.2-51.9) | |
| 6MWD in meters at baseline, median (IQR)a | 267.8 (197-393.6) | |
| 6MWT resting SpO2 at baseline, median (IQR)a | 94 (91-96) | |
| 6MWT nadir SpO2 at baseline, median (IQR)a | 83 (80-86) | |
| MRC at baseline, median (IQR) | 4 (3-4) | |
| NRS at baseline, median (IQR)b | Rest | 1 (0-3) |
| Eating | 2 (1-4) | |
| Talking | 3 (1-5) | |
| Dressing | 4 (2-6) | |
| Bathing | 5 (3-7) | |
| Bowel movement | 3 (1-5) | |
| Exercise | 6 (4-8) | |
| Stairs | 7 (5-8) |
Abbreviations: COPD, chronic obstructive pulmonary disease; CTD-ILD, connective tissue disease related ILD; DLCO, diffusing capacity of the lungs for carbon monoxide; FVC, forced vital capacity; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; 6MWD, 6-minute walk distance; 6MWT, 6-minute walk test; MDC, multidisciplinary collaborative care; MRC, medical research council; NRS, numerical rating scale; NSIP, nonspecific interstitial pneumonia; OSA, obstructive sleep apnea; SD, standard deviation; SpO2, peripheral capillary oxygen saturation.
aBaseline FVC, DLCO, 6MWD, 6MWT Resting and Nadir SpO2 were defined as results from the time of or within 3 months of first MDC visit. Not all patients had test results within this period. 66 patients had FVC results, 49 patients had DLCO results, 48 had 6MWD and nadir SpO2 results, 47 had resting SpO2 results.
bBaseline NRS is defined as the first available NRS score.
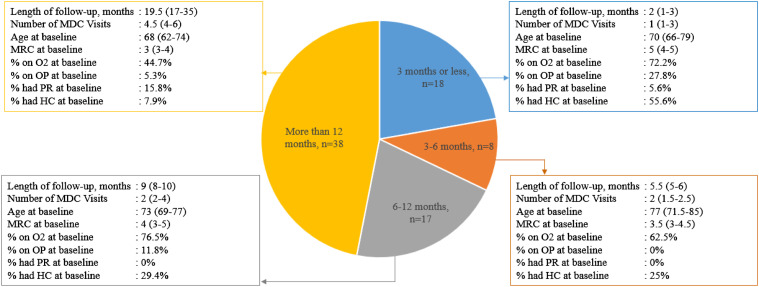
The median number of visits per patient was 3. Forty-seven percent of patients were followed in MDC clinic for more than 12 months and 22% were followed for <3 months. Median length of follow-up was 10 months (5-19). [Figure 1.]
Figure 1.
Patient characteristics grouped by length of follow-up in MDC clinic. All values are presented as median (interquartile range), unless otherwise indicated. Baseline is the first MDC clinic visit. Abbreviations: HC, home care; MDC, multidisciplinary collaborative care; MRC, medical research council, O2, oxygen; OP, opioid; PR, pulmonary rehabilitation.
Summary of Interventions
Non-pharmacologic management
Self-management advice was provided to 100% of patients in MDC clinic. HC referrals were made in 46/81 (57%) and 20/81 (25%) of patients were already enrolled at referral. PR referrals were made in 32/81 (40%) patients and 7/81 (9%) patients had either already been referred or completed PR within a year prior to MDC. Patients with MRC 5 baseline dyspnea were referred to PR least frequently (2/18; 11%).
Oxygen
Sixty-five (80%) patients were treated with home oxygen during the study period. Forty-eight (59%) patients were already using home oxygen at their first MDC clinic visit. Sixteen (20%) patients were never initiated on oxygen. Those who did not receive any oxygen interventions in MDC clinic had been either lost to follow-up, died prior to requiring or qualifying for oxygen, or they declined oxygen.
Seventeen patients had oxygen initiated during their time in MDC clinic. Five patients were started on oxygen during their first MDC clinic visit and of those, 4/5 had exertional desaturations <88% on 6MWT and 1 desaturated to 80% while walking across the room. Oxygen was initiated a median of 11 months (5-18) prior to death. Oxygen was frequently titrated with 54/81 (67%) of patients having had their oxygen titrated at least once. Median number of oxygen titrations per patient was 2 (1-3). (Table 2).
Table 2.
Dyspnea Interventions Grouped by Baseline MRC.
| MRC 1 (n = 4) | MRC 2 (n = 3) | MRC 3 (n = 24) | MRC 4 (n = 32) | MRC 5 (n = 18) | Overall | ||
|---|---|---|---|---|---|---|---|
| Pulmonary rehabilitation | |||||||
| Initiated prior to MDC | Total patients, n (%) | 0 (0) | 0 (0) | 3 (12.5) | 4 (12.5) | 0 (0) | 7 (8.6) |
| Referred prior to MDC, n | 0 | 0 | 1 | 1 | 0 | 2 | |
| Completed ≤1 year prior to MDC, n | 0 | 0 | 2 | 3 | 0 | 5 | |
| Referred during MDC | Total Patients, n | 2 | 3 | 13 | 12 | 2 | 32 |
| Visit # of referral, median (IQR) | 3.5 (1-6) | 1 (1-1) | 1 (1-3) | 1 (1-1) | 1 (1-1) | 1 (1-1) | |
| Months prior to death, median (IQR) | 11 (5-17) | 53 (17-67) | 14 (9-19) | 11 (7-16.5) | 8.5 (7-10) | 13 (8-19) | |
| Home care | |||||||
| Enrolled prior to MDC | Total patients, n (%) | 0 (0) | 0 (0) | 2 (8.3) | 8 (25.0) | 10 (55.6) | 20 (24.7) |
| Referred during MDC | Total Patients, n | 2 | 3 | 17 | 18 | 6 | 46 |
| Visit # of referral, median (IQR) | 6 (5-7) | 5 (3-11) | 2 (1-3) | 1 (1-2) | 1 (1-1) | 1 (1-3) | |
| Months prior to death, median (IQR) | 1.5 (1-2) | 7 (1-22) | 10 (8-17) | 10.5 (7-17) | 6 (2-8) | 9 (4-16) | |
| Oxygen | |||||||
| Initiated prior to MDC | Total patients, n (%) | 0 (0) | 0 (0) | 13 (54.2) | 19 (59.4) | 16 (88.9) | 48 (59.3) |
| Initiated during MDC | Total Patients, n | 3 | 2 | 5 | 6 | 1 | 17 |
| Visit # of initiation, median (IQR) | 4 (2-7) | 3 (2-4) | 3 (2-6) | 1 (1-2) | 2 (2-2) | 2 (1-4) | |
| Months prior to death, median (IQR) | 7 (2-23) | 18.5 (11-26) | 12 (6-23) | 10.5 (3-14) | 5 (5-5) | 11 (5-18) | |
| Patients on O2 at baseline with O2 titration during MDC | Total Patients, n | 0 | 0 | 13 | 18 | 12 | 43 |
| Visit # of titration, median (IQR) | — | — | 1 (1-1) | 1 (1-1) | 1 (1-1) | 1 (1-1) | |
| Months prior to death, median (IQR) | — | — | 15 (8-19) | 12 (6-22) | 3 (2-6.5) | 8 (3-18) | |
| Titration during MDC | Total Patients, n | 3 | 1 | 16 | 22 | 12 | 54 |
| Titrations per patient, median (IQR) | 1 (1-1) | 3 (3-3) | 3 (1-4) | 2 (1-3) | 2 (1-2) | 2(1-3) | |
| Any O2 intervention during MDC | Total patients, n (%) | 3 | 2 | 18 | 24 | 13 | 60 (74.1) |
| Interventions per patient, median (IQR) | 2 (2-2) | 2.5 (1-4) | 2.5 (1-4) | 2 (1.5-3) | 2 (1-2) | 2 (1-3) | |
| Opioid | |||||||
| Initiated prior to MDC | Total patients, n (%) | 0 (0) | 0 (0) | 1 (4.2) | 2 (6.3) | 6 (33.3) | 9 (11.1) |
| Initiated during MDC | Total patients, n (%) | 2 (50.0) | 1 (33.3) | 9 (37.5) | 14 (43.8) | 8 (44.4) | 34 (41.9) |
| Visit # of initiation, median (IQR) | 6.5 (5-8) | 10 (10-10) | 3 (2-5) | 2 (1-3) | 1 (1-1.5) | 2 (1-4) | |
| Months prior to death, median (IQR) | 1 (1-1) | 1(1-1) | 5 (4-10) | 3.5 (1-11) | 4 (2-7) | 4 (1-8) | |
| Patients on OP at baseline with dose adjustment during MDC | Total Patients, n | 0 | 0 | 1 | 1 | 6 | 8 |
| Visit # of dose adjustment, median (IQR) | — | — | 6 | 1 | 1 (1-1) | 1 (1-1) | |
| Months prior to death, median (IQR) | — | — | 2 | 10 | 2.5 (1-3) | 2.5 (1.5-6.5) | |
| Dose adjustment during MDC | Total Patients, n | 0 | 1 | 5 | 9 | 12 | 27 |
| Dose adjustments per patient, median (IQR) | — | 1 (1-1) | 1 (1-2) | 1 (1-2) | 1 (1-2) | 1 (1-2) | |
| Any OP intervention during MDC | Total Patients, n | 2 | 1 | 10 | 15 | 14 | 42 |
| Interventions per patient, median (IQR) | 1(1-1) | 1(1-1) | 1.5 (1-2) | 1 (1-3) | 2 (1-3) | 1.5 (1-2) | |
Abbreviations: IQR, interquartile range; MDC, multidisciplinary collaborative care; MRC, medical research council; O2, oxygen; OP, opioid; #, number.
Opioids
Nine (11%) patients were already using opioids at their first MDC clinic visit. Opioids were initiated in 34/81 (42%) of patients during their enrolment in the MDC clinic with a median initiation time of 4 months (1-8) prior to death. Opioid dose was titrated in 27 patients (33%). (Table 2).
Those not prescribed opioids through the MDC clinic had been referred for lung transplant, had an unanticipated death from an unrelated etiology, death from ILD resulting in hospitalization, hospice placement or palliative home care death or were lost to follow-up.
Order of Interventions
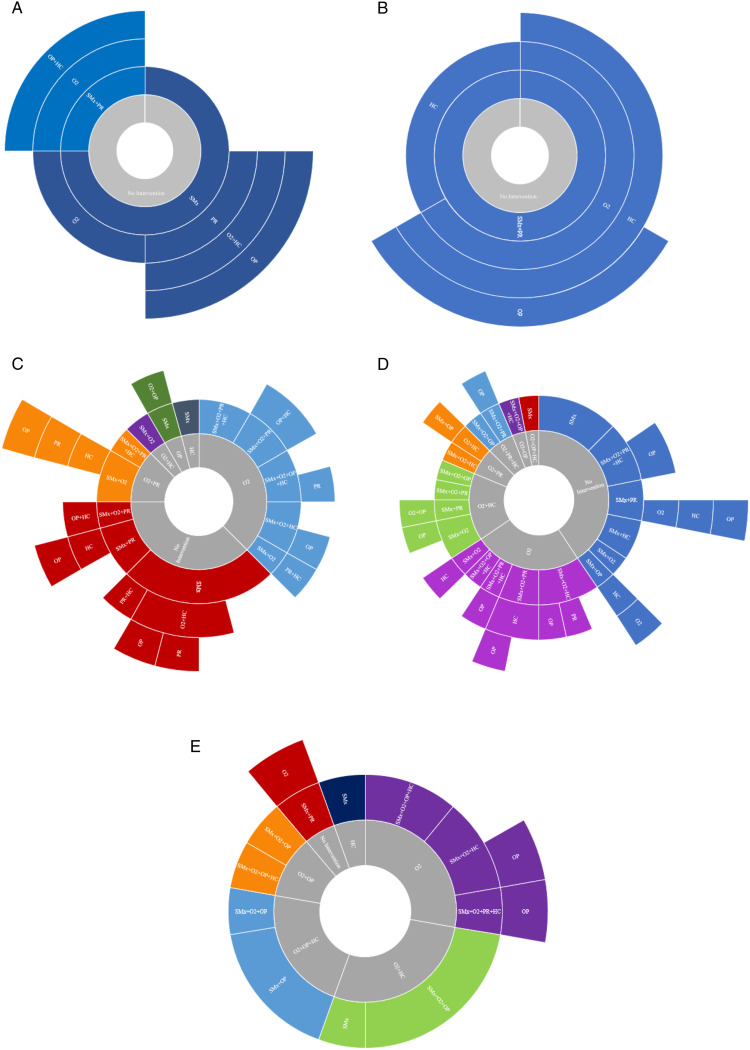
Sunburst diagrams were used to depict the order of dyspnea interventions over time grouped by baseline MRC grade. [Figure 2.]
Figure 2.
(A-E). Pictorial representation of the order of interventions applied in MDC clinic over time grouped by MRC (1-5) at baseline visit. (A): Baseline MRC 1; (B): Baseline MRC 2; (C): Baseline MRC 3; (D): Baseline MRC 4; (E) Baseline MRC 5. The inner grey ring represents baseline treatments from the time of referral. Each successive ring represents consecutive levels/order of therapies initiated in MDC clinic overtime. Only the first time a therapy was applied or adjusted in clinic is included to show the sequential order of types of dyspnea interventions. Rings are subdivided by therapies applied at each level. The size of each subdivision within a ring represents the relative proportion of patients that received particular intervention(s). Abbreviations: HC, home care; MDC, multidisciplinary collaborative care; MRC, medical research council; O2, oxygen; OP, opioid; PR, pulmonary rehabilitation; SMx, symptom self management education.
MRC Grade 1-2 (n = 7): All patients received non-pharmacological interventions (such as symptom management and PR) as their first intervention. None of these patients were on oxygen at baseline; it was not an initial intervention but was eventually initiated in 57% (4) patients, after optimization of non-pharmacological interventions. Opioids were used in 43% (3) and when utilized, were the final new intervention (ie, after all other interventions had been initiated).
MRC Grade 3 (n = 24): As initial intervention(s), all patients received self management education, 58% (14) had oxygen initiated or titrated and 8% (2) were prescribed opioids. Opioids were prescribed/adjusted in 42% (10) and were a final new intervention in 9.
MRC Grade 4 (n = 32): As initial intervention(s), 97% (31) of patients received self management education, 66% (21) had oxygen initiated or titrated and 16% (5) had opioids prescribed. At baseline, 2/32 (6%) were on opioids. Opioids were subsequently prescribed/adjusted in 14/32 (44%) cases; a total of 15/32 (47%) of patients received opioids. It was a final new intervention in 14/32 (44%).
MRC Grade 5 (n = 18): As initial intervention(s) 100% of patients received self management education, 67% (12) had oxygen initiated or titrated and 67% (12) had opioids prescribed or titrated. At baseline 6/18 (33%) were on opioids. Opioids were prescribed in 14/18 (77%) cases and were a final new intervention in all cases it was prescribed.
Dyspnea Trajectory Over Time
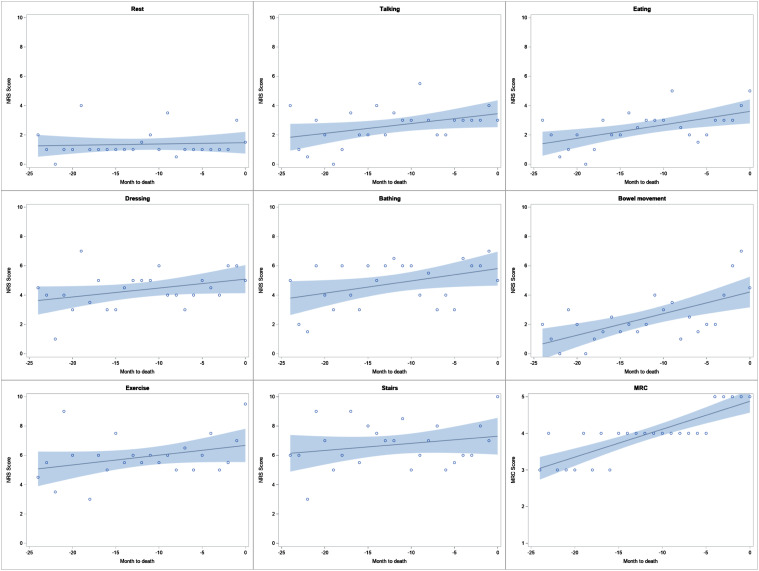
A median of 2 (1-4) NRS dyspnea scores were collected per patient. There was an increasing trend in median NRS scores closer to death with all measured activities, although the change was minimal. [Figure 3.]. This increasing trend was only significant with eating (P = .006) and bowel movements (P = .002) from Mann-Kendall test (Table 3). When assessing each patient individually, the trend was variable with approximately half showing increasing and half showing decreasing trajectory overtime, depending on the activity, and almost all were not significant (Supplemental Table S1).
Figure 3.
Trajectory of median NRS scores with various activities and median MRC scores in the months prior to death. Regression line with 95% confidence limit band. Abbreviations: MRC, medical research council; NRS, numerical rating scale.
Table 3.
Mann-Kendall (Tau) Trend Test Result on the Trajectory of Median NRS Scores With Various Activities and MRC Scores in the Months Prior to Death in Figure 3.
| MRC | Rest | Talking | Eating | BM | Bathing | Dressing | Exercise | Stairs | |
|---|---|---|---|---|---|---|---|---|---|
| Tau | .72 | .10 | .27 | .41 | .46 | .27 | .27 | .24 | .117 |
| P-value | <.0001 | .51 | .07 | .006 | .002 | .07 | .08 | .12 | .43 |
Abbreviations: BM, bowel movement; MRC, medical research council; NRS, numerical rating scale.
Acute Care Utilization and Location of Death
Fifty-eight (72%) patients were hospitalized at least once during the last 6 months of life. Forty-two (52%) patients died in hospital. See Table 4 for details.
Table 4.
Healthcare Usage in the Last 6 Months of Life by Length of Follow up in MDC Clinic.
| ≤3 months (n = 18) | 3-6 months (n = 8) | 6-12 months (n = 17) | >12 months (n = 38) | Total | |
|---|---|---|---|---|---|
| Hospitalizations | |||||
| % patients with hospitalization (n) | 72.2% (13) | 75% (6) | 82.3% (14) | 65.8% (25) | 71.6% (58) |
| % patients die in hospital (n) | 44.4% (8) | 37.5% (3) | 64.7% (11) | 52.6% (20) | 51.9% (42) |
| Number of hospitalizations/pt, median (IQR) | 2 (1-2) | 1 (1-1) | 2 (1-3) | 2 (1-3) | 2 (1-3) |
| Total LOS/pt (days), median (IQR) | 23 (13-35) | 13.5 (4-43) | 16.5 (7-36) | 19 (9-28) | 19 (8-35) |
| Total acute LOS/pt (days), median (IQR) | 23 (13-35) | 13.5 (4-43) | 14 (7-24) | 18 (9-25) | 17.5 (8-34) |
| Total RIW/pt, median (IQR) | 5.9 (1.4-9.2) | 2.95 (.8-5.5) | 2.9 (1.1-4.7) | 3.4 (1.8-4.7) | 3.5 (1.3-6.2) |
| Emergency department visits | |||||
| % patients with ED visits (n) | 72.2% (13) | 50% (4) | 70.6% (12) | 73.7% (28) | 70.4% (57) |
| Number of ED visits/pt, median (IQR) | 3 (2-4) | 1.5 (1-2) | 3 (1-5) | 2.5 (2-4) | 2 (2-4) |
| Total stay in minutes/pt, median (IQR) | 1150 (608-4338) | 945.5 (257.5-1451.5) | 2596 (1669-3847) | 1880 (959-3675) | 1749 (716-3626) |
| Total RIW/pt, median (IQR) | .25 (.15-.28) | .16 (.1-.19) | .30 (.11-.44) | .24 (.18-.38) | .25 (.15-.37) |
| Primary physician visits | |||||
| % patients with GP visit (n) | 94.4% (17) | 100% (8) | 70.6% (12) | 86.8% (33) | 86.4% (70) |
| Number of GP visits/pt, median (IQR) | 5 (2-12) | 4 (1.5-9.5) | 4 (3-9.5) | 6 (3-12) | 5 (3-11) |
Abbreviations: ED, emergency department; GP, general practitioner; IQR, interquartile range; LOS, length of stay; pt, patient; MDC, multidisciplinary collaborative care; RIW, Resource Intensity Weighta,b.
aCanadian Institute for Health Information. Resource Indicators: DAD Resource Intensity Weights And Expected Length Of Stay | CIHI. Canadian Institute for Health Information. https://www.cihi.ca/en/resource-indicators-dad-resource-intensity-weights-and-expected-length-of-stay. Accessed April 3, 2022.
bCanadian Institute for Health Information. CMG+ CIHI. https://www.cihi.ca/en/cmg. Published 2019. Accessed April 3, 2022.
Discussion
We show that dyspnea assessment and a multidisciplinary treatment approach can facilitate iterative breathlessness management in a real-world ILD clinic. To our knowledge, this is the first detailed description of the timeline and order of implementation of breathlessness interventions in F-ILD in a real-world setting. We show high rates of early implementation of non-pharmacologic therapies, oxygen, and opioids compared to historical cohorts. Therapies were applied either stepwise or concurrently to manage dyspnea, which differed within and between baseline MRC grades. When breathlessness management is informed by dyspnea severity ratings with rest and everyday activities, treatment heterogeneity is observable within and across MRC grades. Our results suggest that this approach attenuated worsening in dyspnea severity and may have reduced hospitalizations at end-of-life.
In a US hospitalist survey, 95% of respondents said dyspnea severity assessment impacts their decision to intensify treatments, but in reality <3% reported using a numerical scale to measure severity.15 All of our patients had at least 1 documented NRS dyspnea score.
At baseline 37% of our patients had not been prescribed any dyspnea interventions by referring providers (100% of MRC 1-2, 39% of MRC 3-4 and 6% of MRC 5 patients). When prescribed, the most common baseline treatments from referring providers were oxygen (59%), opioids (11%) and PR (9%). This suggests that dyspnea management remains suboptimal.
Our clinic’s approach, resulted in patients with baseline MRC grades 1-2 receiving a gradual, stepwise implementation of interventions; with non-pharmacologic interventions being introduced upfront and other interventions following overtime. Comparatively, patients with baseline MRC 3-5 grade dyspnea often had multiple interventions implemented concurrently. Notably, the number and types of interventions applied concurrently differed between patients within the same baseline MRC grade, which may reflect the complex and individual nature of the dyspnea experience and exertional hypoxemia.
Non-pharmacologic strategies, the foundation of breathlessness management,31 are increasingly highlighted in the ILD literature.32,33 A qualitative study of 13 IPF patients described a patient-reported benefit from symptom self-management education, with 80% reporting self-efficacy in symptom management.26 Nonetheless, they are rarely prescribed in F-ILD in practice.34 In contrast, 100% of patients in our cohort received self-management education from clinicians that included pacing, energy conservation, breathing techniques, oxygen strategies and nasal care.26
Oxygen is another important strategy for exertional dyspnea and hypoxemia that can occur frequently and early in course of ILD.2 Recent studies suggest that ambulatory oxygen may improve dyspnea, walk distance and HRQOL in the short-term for patients with F-ILD.35-38 In the absence of evidence informed guidelines on oxygen use for exertional hypoxemia in ILD, most experts agree that exertional desaturations <88% with symptoms should be sufficient criteria to start ambulatory oxygen,2 but varying oxygen funding criteria pose barriers to access. In an Australian ILD registry, 50% patients had baseline isolated exertional hypoxemia but only 29% were prescribed oxygen.38 In our study, 59% (48) of patients were on oxygen at baseline, with an additional 6% (5) started on oxygen at first visit. Of those on oxygen at baseline, 88% had it titrated at first visit.
There is no consensus on how to titrate oxygen and monitor ongoing therapy.39 IPF patients and their caregivers have indicated the need for guidance with oxygen use.40 Exertional hypoxemia also has prognostic significance further supporting the importance of improving oxygen management.41 In a large US survey, only 29% of patients reported adjusting flow rates based on oximeter readings.42 In our cohort, 83% (54/65) of patients prescribed oxygen received titrations to maintain nadir exertional SpO2 >88% when possible. The median number of oxygen titrations per patient was 2 (1-3). We hypothesize that frequent titrations and oxygen self management education helped improve breathlessness management in our cohort.
Opioids are recommended in the ATS IPF guidelines for dyspnea management.2 In a study of palliative care needs in progressive fibrotic interstitial lung disease by Bajwah et al, 49% (22/45) of patients received opioids.34 In a recent Swedish population-based study of ILD patients 43% of patients received opioids, but only 6.4% of prescriptions were for breathlessness43 suggesting that opioids are under prescribed in this population. In our cohort, 53% of patients received opioids in MDC clinic and it was an initial intervention in 8% (2/24) MRC 3 and 34% (17/50) MRC 4-5 patients.
Although there is a lack of real-world data in ILD regarding timing of specific breathlessness interventions, there is agreement that dyspnea management is inadequate and that palliative care interventions should be implemented early.9,32 MDC-initiated referral to PR and HC, oxygen and opioid prescriptions were recorded a median of 13, 9, 11 and 4 months prior to death respectively. This contrasts with prior studies where non-pharmacologic management was rarely provided, if at all34; oxygen was initiated 4 months prior to death44 and opioids were rarely prescribed for breathlessness even in the last 3 months of life.43 In a study by Rajala et al,45 71% of IPF patients were prescribed opioids only during the last week of life. This suggests that knowledge of dyspnea severity, coupled with a structured management algorithm, can lead to early and improved dyspnea treatment.
The trajectory of median NRS dyspnea scores in our cohort was noted to increase closer to death with all measured activities, although the rate of increase was minimal and mostly non-significant. In contrast, Rajala et al46 showed worsening dyspnea over 2 years prior to death, with a significantly rapid rise at end-of-life in IPF. Our approach may have helped slow down the worsening of dyspnea.
We believe the variation in individual dyspnea trends (ie, increasing and decreasing dyspnea scores) likely reflects both the inherent diversity in dyspnea between individual patients as well as the differences in time between visits for each patient (Supplemental Table S1). Some patients' follow ups, based on clinical need, occurred soon after intervention and thus reflected the response to the interventions with greater fidelity compared to other patients with a longer follow up interval. Disease and dyspnea progression during this period may have influenced the impact of intervention(s). This is a real-world study, unlike a clinical trial where an intervention and its follow up are tightly linked.
Dyspnea is a frequent driver of hospitalizations in F-ILD. Compared with historical cohorts, fewer of our patients were hospitalized towards end of life, however the limitation of our study is the lack of a control group. Fifty-two percent of our patients died in hospital, which is significantly less than the previously described 76-80% hospital deaths reported in UK and Finnish cohorts.34,45 In our cohort, 72% of patients were hospitalized for a median of 19 days (mean 29 days) during the last 6 months of their life compared to 93% for a mean of 30 days in the Finnish study.45 We suggest that our strategies helped decrease breathlessness severity that drives hospitalizations.47 This is in keeping with a 2018 review from our clinic that showed implementation of symptom-based therapy (including dyspnea) reduced acute care utilization and hospital deaths.24
We propose that a dyspnea management algorithm that includes self-management education for all patients; PR wherever possible; HC as appropriate; oxygen to target nadir exertional oxygen saturations ≥88% and opioids when other therapies are optimized, personalized by the NRS pilot dyspnea scale may improve care.
There were several limitations to our study. It is subject to all the limitations and biases associated with any retrospective study. Firstly, our cohort had a small sample size and more advanced F-ILD than most registry studies with more patients in GAP II-III stage and baseline MRC grade 4. The frequency of visits was determined by clinical needs and patient wishes, which may have affected the timing and sequence of dyspnea interventions. Although every patient completed at least 1 NRS dyspnea score, not all patients had an NRS documented at every visit. Additionally, as patients approached end-of-life with worsening dyspnea, many stopped performing high intensity activities, such as climbing stairs which reduced NRS data points. Provincial qualification requirements lead to limitations in oxygen access in some cases. As we only collected intervention data from MDC clinic visits, there may have been interventions applied outside clinic that were not captured in our study.
Conclusion
Dyspnea severity assessment and a multidisciplinary clinical approach can guide the algorithmic implementation of dyspnea interventions. Such an approach can result in earlier use of non-pharmacologic and pharmacologic therapies at high rates. Heterogeneity in the stepwise or concurrent implementation of dyspnea interventions within and across MRC grades may reflect the varying severity of dyspnea experienced within each MRC grade and the importance of measuring it. An approach that includes dyspnea assessment and systematic management may also help to reduce hospitalizations and hospital deaths in F-ILD.
Supplemental Material
Supplemental Material for A Retrospective, Descriptive Study of Dyspnea Management in a Multidisciplinary Interstitial Lung Disease Clinic by Laura van den Bosch, Ting Wang, Jeffrey A. Bakal, Janice Richman-Eisenstat, and Meena Kalluri in American Journal of Hospice and Palliative Medicine®
Acknowledgments
Study data were collected and managed using REDCap* electronic data capture tools hosted and supported by the Women and Children’s Health Research Institute at the University of Alberta. *Paul A. Harris, Robert Taylor, Robert Thielke, Jonathon Payne, Nathaniel Gonzalez, Jose G. Conde, Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009 Apr;42(2):377-81.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Laura van den Bosch https://orcid.org/0000-0002-9113-8309
Meena Kalluri https://orcid.org/0000-0003-4645-6292
References
- 1.Johannson KA, Kolb M, Fell CD, et al. Evaluation of patients with fibrotic interstitial lung disease: a Canadian thoracic society position statement. Can J Respir Crit Care Sleep Med. 2017;1:133-141. doi: 10.1080/24745332.2017.1359056. [DOI] [Google Scholar]
- 2.Raghu G, Collard HR, Egan JJ, et al. An Official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157(1):199-203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 4.Nishiyama O, Taniguchi H, Kondoh Y, et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis. What is the main contributing factor? Respir Med. 2005;99(4):408-414. doi: 10.1016/j.rmed.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Martinez TY, Pereira CAC, dos Santos ML, Ciconelli RM, Guimarães SM, Martinez JAB. Evaluation of the short-form 36-item questionnaire to measure health- related quality of life in patients with idiopathic pulmonary fibrosis. Chest. 2000;117(6):1627-1632. doi: 10.1378/chest.117.6.1627. [DOI] [PubMed] [Google Scholar]
- 6.Pesola GR, Ahsan H. Dyspnea as an independent predictor of mortality. Clin Respir J. 2016;10(2):142-152. doi: 10.1111/crj.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishiyama O, Taniguchi H, Kondoh Y, et al. A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur Respir J. 2010;36(5):1067-1072. doi: 10.1183/09031936.00152609. [DOI] [PubMed] [Google Scholar]
- 8.Bajwah S, Higginson IJ, Ross JR, et al. The palliative care needs for fibrotic interstitial lung disease: a qualitative study of patients, informal caregivers and health professionals. Palliat Med. 2013;27(9):869-876. doi: 10.1177/0269216313497226. [DOI] [PubMed] [Google Scholar]
- 9.Mularski RA, Reinke LF, Carrieri-Kohlman V, et al. An official American thoracic society workshop report: assessment and palliative management of dyspnea crisis. Ann Am Thorac Soc. 2013;10(5):S98-S106. doi: 10.1513/AnnalsATS.201306-169ST. [DOI] [PubMed] [Google Scholar]
- 10.In- B . Dyspnea mechanisms, assessment, and management: a consensus statement. Am J Respir Crit Care Med. 1999;159:321-340. [DOI] [PubMed] [Google Scholar]
- 11.Hayen A, Herigstad M, Pattinson KTS. Understanding dyspnea as a complex individual experience. Maturitas. 2013;76(1):45-50. doi: 10.1016/j.maturitas.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Agusti A, Calverley PMA, Calverley PM, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11(1):122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gronseth R, Vollmer WM, Hardie JA, et al. Predictors of dyspnoea prevalence: Results from the BOLD study. Eur Respir J. 2014;43(6):1610-1620. doi: 10.1183/09031936.00036813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahler DA, Selecky PA, Harrod CG, et al. American college of chest physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010;137(3):674-691. doi: 10.1378/CHEST.09-1543. [DOI] [PubMed] [Google Scholar]
- 15.Stefan MS, Au DH, Mularski RA, et al. Hospitalist attitudes toward the assessment and management of dyspnea in patients with acute cardiopulmonary diseases. J Hosp Med. 2015;10(11):724-730. doi: 10.1002/jhm.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanken PN, Terry PB, DeLisser HM, et al. An official American thoracic society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177(8):912-927. doi: 10.1164/rccm.200605-587ST. [DOI] [PubMed] [Google Scholar]
- 17.Parshall MB, Schwartzstein RM, Adams L, et al. An official American thoracic society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higginson IJ. Refractory breathlessness: oxygen or room air? Lancet. 2010;376(9743):746-748. doi: 10.1016/S0140-6736(10)61346-3. [DOI] [PubMed] [Google Scholar]
- 19.Farquhar MC, Prevost AT, McCrone P, et al. The clinical and cost effectiveness of a Breathlessness Intervention Service for patients with advanced non-malignant disease and their informal carers: mixed findings of a mixed method randomised controlled trial. Trials. 2016;17(1):185-216. doi: 10.1186/S13063-016-1304-6/TABLES/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farquhar MC, Prevost AT, McCrone P, et al. Is a specialist breathlessness service more effective and cost-effective for patients with advanced cancer and their carers than standard care? Findings of a mixed-method randomised controlled trial. BMC Med. 2014;12(1):194-213. doi: 10.1186/S12916-014-0194-2/TABLES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higginson IJ, Bausewein C, Reilly CC, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med. 2014;2(12):979-987. doi: 10.1016/S2213-2600(14)70226-7. [DOI] [PubMed] [Google Scholar]
- 22.Bausewein C, Schunk M, Schumacher P, Dittmer J, Bolzani A, Booth S. Breathlessness services as a new model of support for patients with respiratory disease. Chron Respir Dis. 2018;15(1):48-59. doi: 10.1177/1479972317721557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocker G, Young J, Donahue M, Farquhar M, Simpson C. Perspectives of patients, family caregivers and physicians about the use of opioids for refractory dyspnea in advanced chronic obstructive pulmonary disease. CMAJ. 2012;184:E497-E504. doi: 10.1503/cmaj.111758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalluri M, Claveria F, Ainsley E, Haggag M, Armijo-Olivo S, Richman-Eisenstat J. Beyond idiopathic pulmonary fibrosis diagnosis: multidisciplinary care with an early integrated palliative approach is associated with a decrease in acute care utilization and hospital deaths. J Pain Symptom Manage. 2018;55:420-426. doi: 10.1016/j.jpainsymman.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Kalluri M, Lu-Song J, Younus S, et al. Health care costs at the end of life for patients with idiopathic pulmonary fibrosis. evaluation of a pilot multidisciplinary collaborative interstitial lung disease clinic. Ann Am Thorac Soc. 2020;17(6):706-713. doi: 10.1513/AnnalsATS.201909-707OC. [DOI] [PubMed] [Google Scholar]
- 26.Kalluri M, Younus S, Archibald N, Richman-Eisenstat J, Pooler C. Action plans in idiopathic pulmonary fibrosis: a qualitative study “I do what i can do. BMJ Support Palliat Care. 2021:1-8. doi: 10.1136/bmjspcare-2020-002831. [DOI] [PubMed] [Google Scholar]
- Kalluri M. Specialty palliative care program ILD. Respir Med 2021;303-331. doi: 10.1007/978-3-030-81788-6_16. [DOI] [Google Scholar]
- 28.Kalluri M, Richman-Eisenstat J. Breathing is not an option; dyspnea is. J Palliat Care. 2014;30(3):188-191. doi: 10.1177/082585971403000309. [DOI] [PubMed] [Google Scholar]
- 29.Kalluri M, Richman-Eisenstat J. Early and integrated palliative care to achieve a home death in idiopathic pulmonary fibrosis. J Pain Symptom Manage. 2017;53(6):1111-1115. doi: 10.1016/J.JPAINSYMMAN.2016.12.344. [DOI] [PubMed] [Google Scholar]
- 30.Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684-695. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 31.Spathis A, Booth S, Moffat C, et al. The breathing, thinking, functioning clinical model: a proposal to facilitate evidence-based breathlessness management in chronic respiratory disease. NPJ Prim Care Respir Med. 2017;27(1):1-6. doi: 10.1038/s41533-017-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreuter M, Bendstrup E, Russell A-M, et al. Palliative care in interstitial lung disease: living well. Lancet Respir Med. 2017;5(12):968-980. doi: 10.1016/S2213-2600(17)30383-1. [DOI] [PubMed] [Google Scholar]
- 33.Prihartadi AS, Licastro IG, Pearson M, Johnson MJ, Luckett T, Swan F. Non-medical devices for chronic breathlessness: use, barriers and facilitators for patients, carers and clinicians - a scoping review. BMJ Support Palliat Care. 2021:bmjspcare-2021-002962. doi: 10.1136/BMJSPCARE-2021-002962 [DOI] [PubMed] [Google Scholar]
- 34.Bajwah S, Higginson IJ, Ross JR, et al. Specialist palliative care is more than drugs: a retrospective study of ILD patients. Lung. 2012;190(2):215-220. doi: 10.1007/s00408-011-9355-7. [DOI] [PubMed] [Google Scholar]
- 35.Visca D, Montgomery A, De Lauretis A, et al. Ambulatory oxygen in interstitial lung disease. Eur Respir J. 2011;38:987-990. doi: 10.1183/09031936.00190710. [DOI] [PubMed] [Google Scholar]
- 36.Dowman LM, McDonald CF, Bozinovski S, et al. Greater endurance capacity and improved dyspnoea with acute oxygen supplementation in idiopathic pulmonary fibrosis patients without resting hypoxaemia. Respirology. 2017;22:957-964. doi: 10.1111/resp.13002. [DOI] [PubMed] [Google Scholar]
- 37.Visca D, Mori L, Tsipouri V, et al. Effect of ambulatory oxygen on quality of life for patients with fibrotic lung disease (AmbOx): a prospective, open-label, mixed-method, crossover randomised controlled trial. Lancet Respir Med. 2018;6:759-770. doi: 10.1016/S2213-2600(18)30289-3. [DOI] [PubMed] [Google Scholar]
- 38.Khor YH, Smith DJF, Johannson KA, Renzoni E. Oxygen for interstitial lung diseases. Curr Opin Pulm Med. 2020;26(5):464-469. doi: 10.1097/MCP.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 39.Lim RK, Humphreys C, Morisset J, Holland AE, Johannson KA. Oxygen in patients with fibrotic interstitial lung disease: an international Delphi survey. Eur Respir J. 2019;54(2):1900421. doi: 10.1183/13993003.00421-2019. [DOI] [PubMed] [Google Scholar]
- 40.Sampson C, Gill BH, Harrison NK, Nelson A, Byrne A. The care needs of patients with idiopathic pulmonary fibrosis and their carers (CaNoPy): results of a qualitative study. BMC Pulm Med. 2015;15(1):1-7. doi: 10.1186/S12890-015-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khor YH, Gutman L, Abu Hussein N, et al. Incidence and prognostic significance of hypoxemia in fibrotic interstitial lung disease: an international cohort study. Chest. 2021:994-1005. doi: 10.1016/J.CHEST.2021.04.037. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs SS, Lederer DJ, Garvey CM, et al. Optimizing home oxygen therapy an official american thoracic society workshop report. Ann Am Thorac Soc. 2018;15(12):1369-1381. doi: 10.1513/AnnalsATS.201809-627WS. [DOI] [PubMed] [Google Scholar]
- 43.Genberg J, Davies JM, Ahmadi Z, et al. Indications and patterns of use of benzodiazepines and opioids in severe interstitial lung disease: a population-based longitudinal study. ERJ Open Res. 2021;7(1):00716-02020. doi: 10.1183/23120541.00716-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olson AL, Graney B, Baird S, et al. Tracking dyspnea up to supplemental oxygen prescription among patients with pulmonary fibrosis. BMC Pulm Med. 2017;17(1):1-7. doi: 10.1186/S12890-017-0497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajala K, Lehto JT, Saarinen M, Sutinen E, Saarto T, Myllärniemi M. End-of-life care of patients with idiopathic pulmonary fibrosis. BMC Palliat Care. 2016;15(1):1-6. doi: 10.1186/s12904-016-0158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajala K, Lehto JT, Sutinen E, Kautiainen H, Myllärniemi M, Saarto T. Marked deterioration in the quality of life of patients with idiopathic pulmonary fibrosis during the last two years of life. BMC Pulm Med. 2018;18(1):172. doi: 10.1186/S12890-018-0738-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens JP, Sheridan AR, Bernstein HB, et al. A multidimensional profile of dyspnea in hospitalized patients. Chest. 2019;156(3):507-517. doi: 10.1016/J.CHEST.2019.04.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for A Retrospective, Descriptive Study of Dyspnea Management in a Multidisciplinary Interstitial Lung Disease Clinic by Laura van den Bosch, Ting Wang, Jeffrey A. Bakal, Janice Richman-Eisenstat, and Meena Kalluri in American Journal of Hospice and Palliative Medicine®