Abstract
BACKGROUND
Information on liver involvement in patients with coronavirus disease 2019 is currently fragmented.
AIM
To highlight the pathological changes found during the autopsy of severe acute respiratory syndrome coronavirus 2 positive patients.
METHODS
A systematic literature search on PubMed was carried out until June 21, 2022.
RESULTS
A literature review reveals that pre-existing liver disease and elevation of liver enzyme in these patients are not common; liver enzyme elevations tend to be seen in those in critical conditions. Despite the poor expression of viral receptors in the liver, it seems that the virus is able to infect this organ and therefore cause liver damage. Unfortunately, to date, the search for the virus inside the liver is not frequent (16% of the cases) and only a small number show the presence of the virus. In most of the autopsy cases, macroscopic assessment is lacking, while microscopic evaluation of livers has revealed the frequent presence of congestion (42.7%) and steatosis (41.6%). Less frequent is the finding of hepatic inflammation or necrosis (19%) and portal inflammation (18%). The presence of microthrombi, frequently found in the lungs, is infrequent in the liver, with only 12% of cases presenting thrombotic formations within the vascular tree.
CONCLUSION
To date, the greatest problem in interpreting these modifications remains the association of the damage with the direct action of the virus, rather than with the inflammation or alterations induced by hypoxia and hypovolemia in patients undergoing oxygen therapy and decompensated patients.
Keywords: Liver, COVID-19, Autopsy, Immunohistochemistry, In situ hybridization, Immunofluorescence
Core Tip: A literature review, about liver pathology in coronavirus disease 2019 (COVID-19) patients, demonstrates the presence of liver damage, which is represented mainly by congestion, steatosis, hepatic inflammation and necrosis, and portal inflammation. The problem to date is whether the damage is COVID-19 related (meaning from direct virus damage/inflammatory related/systemic pathology related) or drug induced. However, this demonstration involves the need to be careful during drug treatment in patients with altered liver enzyme values to prevent further clinical worsening.
INTRODUCTION
The new coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been well studied in relation to pulmonary and cardiac histologic manifestations, but little is yet known regarding hepatic manifestations. COVID-19 has, in fact, to be considered a systemic infectious and inflammatory disease with histological changes also in other organs apart from its main target represented by the lungs. Liver involvement to date is recognized and defined as any liver damage occurring during the course of the disease or its treatment[1], meaning that liver damage can be caused by direct cytotoxicity or inflammatory response and hypoxic/cardiovascular changes, or it may be drug-induced[2-4]. SARS-CoV-2 liver tropism is also well studied, with many authors demonstrating the presence of angiotensin converting enzyme 2 (ACE2) receptor and transmembrane serine protease 2 in the liver, mainly expressed on cholangiocytes, where the levels of expression are similar to those on alveolar cells, though they are only minimally expressed on hepatocytes. No ACE2 expression was demonstrated on sinusoidal endothelial cells or Kupffer cells, apart from Wanner et al[5] who demonstrated minimal expression of ACE2 on Kupffer cells through immunofluorescence and Pirisi et al[6] who demonstrated the presence of virus-like particles in endothelial cells of hepatic sinusoids. Curiously, in patients with liver fibrosis/cirrhosis and in cases of hypoxia, the expression of ACE2 is increased, therefore pre-existing liver injury or hypoxic conditions, common in patients with COVID-19, could favor SARS-CoV-2 liver tropism[4,7-9]. Liver infection could also be explained by its immunological role and the proximity to the digestive organs, which exhibit a strong SARS-CoV-2-tropism, that could favor the entry of the virus through the portal system. Hepatic macrophages (mainly Kupffer cells) and sinusoidal endothelial cells have a key role in the activation of the immune response through pathogen recognition receptors, thus favoring virus entry[10].
The incidence of liver injury in COVID-19 patients is seen in 14%-53% cases[9,11,12] mainly demonstrated through abnormal liver function enzymes. In the literature, only a small number of studies focus on liver damage and even fewer on histological changes in patients who died with or from COVID-19. The purpose of this review is to summarize the results of studies in the literature and evaluate the biochemical and histological changes in the liver, demonstrating that the execution of autopsies is not obsolete, but represents a fundamental tool to create a bridge between clinical manifestations and cytological damage.
MATERIALS AND METHODS
A systematic literature search on PubMed was carried out until June 21, 2022. No time restrictions were applied. The review was conducted using MeSH terms, Boolean operators, and free-text terms to broaden the research. Studies focusing on autopsies of COVID-19 deaths and in particular on liver pathology were initially searched using the terms “((COVID-19) AND (autopsy) AND ((death) OR (liver))” in title, abstract, and keywords. Study design included case reports, case series, and retrospective and prospective studies. Reviews were excluded in order not to create duplication of data, but were analyzed to search for any studies not resulting from the search in the database. No unpublished or gray literature was searched. A total of 526 articles were found in the database. The evaluation of references during full text screening allowed the inclusion of further seven studies. After evaluation of abstracts and full text, 46 articles were included because of their compliance with the inclusion criteria. We also conducted a relevant search using Reference Citation Analysis (https://www.referencecitationanalysis.com/) database to supplement and improve the highlights of the latest cutting-edge research results. Data from each included study were extracted using Microsoft Excel spreadsheets, including information on authors, publishing year, nation, sample size, gender, age, type of autopsy, laboratory results, pre-existing liver disease, macroscopic and microscopic results, additional staining, cause of death, medications, and search of the virus in the liver (Table 1).
Table 1.
Literature review results
|
Ref.
|
Country
|
Cases (n)
|
Age (mean, range)
|
Sex
|
Type of autopsy
|
Pre-existing liver disease or other diseases
|
Laboratory findings
|
Macroscopic results
|
Microscopic results
|
Additional stainings
|
Cause of death
|
Medications
|
Hospitalization
|
Virus identification in liver
|
| Aguiar et al[13], 2020 | Switzerland | 1 | 31 | F | Complete | Obesity | NR | Nutmeg appearance | Microabscesses | None | Respiratory failure in COVID-19 | None | Home death | No search |
| Arslan et al[14], 2021 | Turkey | 7 | 56, 43-68 | M 6, F 1 | Partial | 3 obesity, 2 hypertension, 1 in hemodialysis | NR | NR | 4 mild steatosis, 1 biliary microhamartoma | None | Respiratory failure in COVID-19 | NR | 5 hospitalized, 2 NR | No search |
| Barton et al[15], 2020 | United States | 2 | 59, 42-77 | M 2 | Complete | 2 obesity, 1 hypertension and 1 myotonic muscular dystrophy | NR | Case 1: weight: 2232 g, steatosis. Case 2: weight: 1683 g, cirrhosis | Nr | None | 1 respiratory failure in COVID-19, 1 complications of hepatic cirrhosis | NR | 1 hospitalized and 1 home death | No search |
| Beigmohammadi et al[16], 2021 | Iran | 7 | 68, 46-84 | M 5, F 2 | Core-biopsy | 4 hypertension, 1 immunocompromised and 1 valvular hearth disease | NR | NR | 7 congestion, 7 steatosis, 7 portal inflammation, 7 hepatitis, 4 ballooning degeneration of hepatocytes, 2 bile plugs, 7 focal confluent necrosis, 4 focal hepatocyte drop out | Masson’s trichrome: 1 case of mild fibrosis | NR | 7 were treated with hydroxycholorquine and 6 with antivirals | All hospitalized | No search |
| Bradley et al[17], 2020 | United States | 14 | 74, 42-84 | M 6, F 8 | 7 partial and 7 complete | 5 obesity, 8 hypertension, 4 heart failure, 8 CKD | NR | Congestion | 10 congestion, 9 steatosis, 1 toxic or metabolic disease, 4 centrilobular necrosis, 3 periportal inflammation | None | 12 respiratory failure in COVID-19, 2 cardiovascular failure | NR | All hospitalized | 2 positive and 1 negative PCR-test, 11 not tested. 14 negative IHC and TEM |
| Bryce et al[18], 2020 | United States | 92 | NR | NR | Complete | 28 fatty liver disease | NR | NR | 8 cirrhosis, 57 early organizing thrombi in portal venules and terminal hepatic venules, 41 congestion with some cases showing hemophagocytosis | None | NR | NR | NR | No search |
| Bösmüller et al[19], 2020 | Germany | 4 | 72, 59-79 | M 3, F 1 | Complete-no brain | 2 HIV, 2 hypertension | 3 NR 1 with normal values | 1 hepatosplenomegaly and 1 yellowish surface | Case 1: congestion and activation of Kupffer cells. Case 2: macrophages activation with signs hemophagocytosis | None | 1 respiratory failure in COVID-19, 3 MOF | 1 had a MOF and was intubated, 1 was treated with meropenem, 1 was treated with ECMO | 3 hospitalized and 1 home death | Negative PCR-test |
| Bugra et al[20], 2021 | Turkey | 100 | 55, 7-98 | M 80, F 20 | Partial | NR | NR | NR | 84 inflammation, 54 steatosis, 19 glycogenisation, 9 centilobulary necrosis, 18 autolysis, 45 congestion, 7 endotheliitis, 1 cirrhosis, 2 fibrin thrombosis, 2 bridging necrosis, 1 granulomatous inflammation, 23 cholestasis | CD3+ in portal space | 74 respiratory failure in COVID-19 and 26 NR | NR | 25 hospitalized, 55 home dead, 6 falling from height, 5 car accidents and 9 NR | No search |
| Chornenkyy et al[21], 2021 | United States | 8 | 58, 18-81 | M 3, F 5 | Complete | 2 chronic liver disease (1 HCV and 1 autoimmune hepatitis), 6 obesity, 4 hypertension | Peak AST: 146 (20-1470) and ALT: 214 (10-9961) | Yellowish surface and congestion | 3 periportal fibrosis, 2 necrosis, 5 inflammation, 7 portal inflammation, 6 congestion, 4 steatosis, 6 acute hepatitis | NR | NR | NR | All hospitalized | 4 positive and 4 negative PCR-test |
| Danics et al[22], 2021 | Hungary | 100 | 75, 40-102 | M 50, F 50 | Complete | 36 obesity, 6 liver diseases, 85 hypertension | 41 elevated AST values and 27 elevated ALT values | Average weight 1544 g (range 520-3046 g) | 63 steatosis, 43 portal fibrosis, 4 cirrhosis, 11 centrolobular necrosis, 87 congestion, 52 hepatocellular cholestasis | None | NR | NR | All hospitalized | No search |
| Del Nonno et al[23], 2021 | Italy | 3 | 69, 63-76 | M 2, F 1 | Complete | NR | Admission AST: 63 (31-128) and ALT: 41 (19-84) | NR | All cases showed steatosis, portal inflammation, portal fibrosis, focal lobular inflammation, zonal necrosis and congestion | IHC: CD8+ in portal inflammation, CD34 positive staining in the portal tract vasculature and sinusoids, Perl's staining for iron demonstrated iron deposits into hepatocytes | All respiratory failure in COVID-19 | 1 NR, one with immunosuppressor (tocilizumab) and one with antibiotics + morphine. All had O2 therapy (1 CPAP and 2 venturi mask) | All hospitalized | Negative PCR-test and IHC detection (nucleocapsid and nucleoprotein) |
| Edler et al[24], 2020 | Germany | 80 | 79, 52-96 | M 46, F 34 | Complete | 6 obesity, 4 cirrhosis | NR | NR | Congestion | None | 76 respiratory failure in COVID-19, 1 pericardial tamponade, 1 sepsis and 2 cardiovascular failure | 17 with NIV | 51 hospitalized, 13 in nursing care homes, 12 home deaths, 1 in a hotel and 3 NR | No search |
| Elsoukkary et al[25], 2020 | United States | 32 | 68, 30-100 | M 22, F 10 | Partial | 17 hypertension, 12 obesity | AST: 567 (18-6000) and ALT: 387 (12-4885) | NR | 9 steatosis, 6 portal inflammation, 3 bridging fibrosis and/or cirrhosis | None | NR | 19 hydroxychloroquine and antibiotics, 9 only antibiotics | All hospitalized | No search |
| Evert et al[26], 2021 | Germany | 8 | 62, 44-73 | M 4, F 4 | Complete | 7 obesity, 1 liver cirrhosis, 5 hypertension | NR | NR | 7 cholestasis, 7 single-cell necrosis, 5 fatty degeneration with 2 showing marked steatosis, 2 mild fibrosis, 1 cirrhosis | None | 8 MOF | All did NIV, dialysis and antibiotics. 5 had ECMO | All hospitalized | 3 positive PCR-test |
| Falasca et al[27], 2020 | Italy | 22 | 68, 27-92 | M 15, F 7 | Complete | 1 obesity | NR | Congestion | 11 inflammation, 10 congestion, 12 steatosis | None | All respiratory failure in COVID-19 | NR | All hospitalized | No search |
| Fassan et al[28], 2020 | Italy | 26 | 82, 61-97 | M 14, F 11 | Complete | 5 obesity, 1 HCV-related cirrhosis | NR | NR | 1 cirrhosis, 22 congestion, 5 centrilobular parenchymal atrophy, 2 fibrosis, 5 sinusoidal diffuse microthrombi, 3 portal vein thrombosis, 2 centroacinar necrosis, 26 activation of Kupffer cells, 1 portal inflammation, 9 steatosis | None | NR | NR | NR | Negative ISH |
| Greuel et al[29], 2021 | Germany | 6 | 35, 26-46 | M 3 F 3 | Complete | 1 obesity, 2 right cardiac insufficiency, 1 Ewing sarcoma | 4 elevated AST and ALT values | NR | 1 severe cholestasis, 1 focal ischemic damage 2 steatosis | None | 3 MOF, 1 acute mesenteric ischemia, 1 cardiovascular failure, 1 hemorrhagic shock | 5 had ECMO and NIV | All hospitalized | Negative PCR-test |
| Grosse et al[30], 2020 | Austria | 14 | 82, 55-94 | M 9, F 5 | Complete | 1 liver cirrhosis, 8 hypertension | Admission AST: 49 (12-98) and ALT: 25 (7-87) | NR | 13 steatosis, 14 congestion, 12 portal lymphoid infiltration, 4 portal fibrosis | None | 2 bronchopneumonia, 12 NR | 12 had antibiotics | All hospitalized | No search |
| Hanley et al[31], 2020 | United Kingdom | 10 | 73, 52-79 | M 7, F 3 | Complete | 5 obesity, 4 hypertension | NR | Average weight 1432 g (range 1012-2466) and 3 hepatomegaly | 7 steatosis, 3 cirrhosis or bridging fibrosis | None | NR | 4 NIV | All hospitalized | 3 positive PCR-test (e gene) |
| Hirayama et al[32], 2021 | United Kingdom | 19 | 71, 42-94 | M 11, F 8 | Complete | 5 obesity, 8 hypertension | NR | NR | 12 steatosis, 5 congestion, 4 cirrhosis, 3 portal inflammation | None | NR | NR | All hospitalized | No search |
| Hooper et al[33], 2021 | United States-Brazil | 135 | 61 | M 80, F 55 | 36 core-biopsy and 99 partial | 34 obesity, 5 liver disease, 86 hypertension | NR | NR | 41 necrosis, 37 steatosis, 19 inflammation, 7 fibrosis, 6 congestion, 5 cirrhosis, 3 cholestasis | None | 101 respiratory failure in COVID-19, 6 cardiovascular failure, 28 NR | NR | All hospitalized | No search |
| Ihlow et al[34], 2021 | Germany | 1 | 88 | F | Complete | None | Peak AST: 1690 and ALT: 1632 | Subtotal liver dystrophy | Necrosis, cirrhosis, portal inflammation | IHC for ACE2, TMPESS2 and cathepsin L: strong membranous signals in intrahepatic bile duct epithelium | Acute liver failure | Antibiotics | Hospitalized | ISH positive in the bile duct epithelium and positive PCR-test |
| Lacy et al[35], 2020 | United States | 1 | 58 | F | Complete | Obesity | NR | Weight 1990 g | Steatosis and congestion | None | Respiratory failure in COVID-19 | NR | Home death | No search |
| Lagana et al[36], 2020 | United States | 40 | 70, 66-80 | M 28, F 12 | NR | 2 chronic liver disease, 1 alcohol-related cirrhosis, 1 liver transplant with acute rejection and 1 with anti-HBV core antibody positivity | n = 33 Admission AST: 63 (43-92) and ALT: 32 (19 - 55). Peak AST: 102 (54-294) and ALT: 68 (32-258) | 2 fibrosis and 1 had abscesses, 37 with steatosis and congestion | 20 lobular necroinflammation, 20 portal inflammation, 10 lobular apoptosis, 30 steatosis, 32 congestion, 16 centrilobular necrosis, 15 cholestasis | None | NR | 22 steroids, 19 hydroxychloroquine, and 6 received tocilizumab | All hospitalized | 11 positive and 9 negative PCR-test |
| Lax et al[37], 2020 | Austria | 11 | 82, 75-91 | M 8, F 3 | Partial | 2 obesity, 9 hypertension, 1 Hodgkin lymphoma and 1 bladder carcinoma | AST: 66 (17-189) and ALT: 41 (19-98) | NR | 11 steatosis, 8 congestion, 7 necrosis, 10 Kupffer cell proliferation, 6 portal fibrosis, 8 inflammation, 8 ductular proliferation | None | Pulmonary arterial thrombosis | 2 NIV, 9 AIRVO and 9 had antibiotics | All hospitalized | No search |
| Malik et al[38], 2021 | India | 1 | 31 | F | Complete | None | NR | Congestion | Congestion, mild chronic inflammatory infiltrate in some portal tract, and occasional lymphocytic aggregate adjacent to central vein | None | Respiratory failure in COVID-19 | None | Hospitalized | Positive PCR-test |
| Menter et al[39], 2020 | Switzerland | 21 | 76, 53-96 | M 17, F 4 | 17 complete and 4 partial | 2 chronic liver disease, 21 hypertension, 6 obesity | n = 10 AST: 67.2 (22-214) | NR | 7 steatosis, 5 necrosis, 3 ASH/NASH | None | Respiratory failure in COVID-19 | NR | All hospitalized | No search |
| Nunes et al[40], 2021 | South Africa | 75 | 60, 49-68 | M 29, F 46 | Core- biopsy | 41 hypertension, 20 HIV | NR | NR | 33 portal inflammation, 24 steatosis, 40 sinusoidal inflammation, 10 lobular hepatitis, 9 Kupffer cell activation, 11 spotty necrosis, 4 confluent necrosis, 6 fibrosis, 26 congestion, 7 fibrin-platelet thrombi | None | NR | NR | All hospitalized | No search |
| Oprinca[41], 2020 | Romania | 3 | 59, 27-79 | M 3 | 1 complete and 2 partial | 1 choledochal preampular intraluminal obstruction | NR | Case 1: choledochal preampullary intraluminal obstruction, case 2: normal, case 3: hepatomegaly and cirrhosis | Case 1: congestion, steatosis, periportal fibrosis and portal inflammation, case 2: nothing, case 3: bridging fibrosis and portal inflammation | None | 2 respiratory failure in COVID-19, 1 shock hemorrhagic | Case 1: antibiotics, corticosteroids and assisted oxygenation. Case 2: none (home death). Case 3: none | 2 hospitalized, 1 NR | No search |
| Rapkiewicz et al[42], 2020 | United States | 7 | NR, 44-65 | M 3, F 4 | Complete | 5 obesity and 7 hypertension | NR | NR | 6 steatosis, 1 cirrhosis, 6 platelet-fibrin microthrombi in sinusoids, 2 necrosis | None | Cardiovascular failure | 5 azithromycina and hydroxychloroquine and O2 NIV | 5 hospitalized, 2 home deaths | No search |
| Remmelink et al[43], 2020 | Belgium | 17 | 72, 62-77 | M 12, F 5 | Complete | 2 cirrhosis, 1 liver transplant, 10 hypertension | NR | 5 hepatomegaly | 7 congestion, 1 steato-necrosis, 10 steatosis, 1 cholestasis, 3 chronic hepatitis, 2 cirrhosis, 1 centro-obular necrosis | None | 9 respiratory failure in COVID-19, 7 MOF and 1 NR | 11 had mechanical ventilation | All hospitalized | 14 positive and 3 negative PCR-test |
| Ren et al[44], 2021 | China | 1 | 53 | F | Complete | None | Admission AST: 27 and ALT: 24. Peak AST: 83 and ALT: 93 | Normal | Nothing remarkable | None | Respiratory failure with bacterial infection | She treated herself at home with Chinese herb medicine. In hospital intensive oxygen and supportive measurements, extensive antibiotics and antiviral | Hospitalized | Positive PCR-test |
| Schmit et al[45], 2020 | Belgium | 14 | 63, 50-83 | M 10, F 4 | Complete | 1 HIV, 1 non-alcoholic steatohepatitis, 1 HCV-hepatitis, 6 obesity | Admission AST: 54 (15-188) and ALT: 30 (7-62). Peak AST: 2610 (15-24176) and ALT: 854 (10-7245) | Average weight 1988 g (range 1280-3220 g). 8 cases yellowish appearance 6 nutmeg appearance, 2 indurated consistency, 1 hepatocellular carcinoma, 1 normal | 11 centrilobular necrosis, 9 steatosis, 8 lobular inflammation, 12 portal inflammation, 4 fibrosis, 5 bile duct proliferation, 5 cholestasis, 5 iron overload | None | 13 NR and 1 acute mesenteric ischemia | 8 hydroxychloroquine and antibiotics, 4 with antibiotics, 2 with hydroxychloroquine | All hospitalized | No search |
| Schweitzer et al[46], 2020 | Switzerland | 1 | 50 | M | Complete | HIV | NR | Reduced consistency | Steatosis and liver dystrophy | None | Respiratory failure in COVID-19 | None | Home death | No search |
| Shishido-Hara et al[47], 2021 | Japan | 1 | 75 | M | Complete | None | NR | Normal | Portal inflammation | None | Severe hemorrhage | Anti-viral therapy, antibiotics, O2 therapy | Hospitalized | No search |
| Sonzogni et al[48], 2020 | Italy | 48 | 71, 32-86 | M 22, F 8 | 30 partial and 18 complete - no brain | 7 obesity | 47 elevated values | NR | 24 lobular inflammation, 32 portal inflammation, 18 confluent necrosis 18, 26 steatosis, 48 vascular thrombosis (35 portal, 13 sinusoidal), 37 fibrosis | None | NR | NR | All hospitalized | No search |
| Suess et al[49], 2020 | Switzerland | 1 | 59 | M | Complete | None | NR | NR | Steatosis and some single necrotic hepatocytes | None | Respiratory failure in COVID-19 | NR | Home death | No search |
| Tehrani et al[50], 2022 | Iran | 5 | 71, 55-85 | M 3, F 2 | Partial | None | AST: 275 (106-528) and ALT: 392 (168-978) | NR | Congestion, hepatocytes mildly expanding and bile plugs | None | 4 respiratory failure in COVID-19 and 1 cardiovascular failure | 1 hydroxychloroquine and antibiotics, 2 with hydroxychloroquine and anti-viral therapy, 1 only anti-viral therapy, 1 anti-viral therapy + antibiotics | All hospitalized | No search |
| Tian et al[51], 2020 | China | 4 | 73, 59-81 | M 3, F 1 | Core-biopsy | 1 cirrhosis and 1 hypertension | AST: 36,4 (30-48.8) and ALT: 16 (11-25.5) | NR | Case 1: congestion, glycogen accumulation and focal steatosis, case 2: regenerative nodules and fibrous bands, lobular inflammation and Kupffer cell activation, cases 3: Kupffer cell activation, case 4: periportal and centrilobular necrosis | None | Respiratory failure in COVID-19 | Antibiotics, antiviral therapy assisted oxygenation | All hospitalized | 1 positive and 2 negative PCR-test, 1 was not tested |
| Varga et al[52], 2020 | Switzerland | 1 | 58 | F | NR | Obesity and hypertension | NR | NR | Endotheliitis and necrosis | None | MOF | Dialysis | Hospitalized | No search |
| Wang et al[53], 2020 | China | 2 | 50 and 79 | M 1, F 1 | Core-biopsy | NR | Case 1 peak ALT and AST of 70 U/L and 111 U/L, respectively. Case 2 peak ALT and AST of 76 and 236 U/L | NR | Case 1: apoptotic hepatocytes, steatosis, lobular inflammation, portal inflammation, case 2: apoptotic bodies, steatosis, portal inflammation | IHC: case 1 increased CD68 + cells in hepatic sinusoids and infrequent CD4+. Case 2: many CD68+ cells in sinusoids | 1 respiratory failure in COVID-19 and 1 septic shock | Both had antiviral therapy and antibiotics | All hospitalized | 2 positive TEM (viral particles exist without membrane-bound vesicles) |
| Wang et al[54], 2020 | China | 1 | 75 | F | Core-biopsy | Chronic cardiac insufficiency, hypertension | Elevated AST and ALT values | NR | Necrosis, activated histiocytes, occasional apoptotic hepatocytes, steatosis and cholestasis | None | MOF | NR | Hospitalized | Negative ISH |
| Xu et al[55], 2020 | China | 1 | 50 | M | Core-biopsy | NR | NR | NR | Steatosis | None | Respiratory failure in COVID-19 | Antibiotics, antiviral therapy and oxygenation | Hospitalized | No search |
| Yadav et al[56], 2022 | India | 21 | 61, 25-84 | M 15, F 6 | Complete | 6 obesity, 1 hepatitis B, 1 multiple myeloma | Admission AST: 95.4 (18.9-760.4) and ALT: 52,1 (13,2-229,2). Peak AST: 162,6 (19,8-760,4) and ALT: 75 (21.8-229.2) | NR | 20 portal inflammation, 17 steatosis, 9 lobular inflammation, 1 fibrosis, 1 vascular thrombosis, 1 necrosis | None | 10 MOF, 1 multiple injuries, 6 septic shock, 3 cardiovascular failure, 1 respiratory failure in COVID-19 | 11 treated with antibiotics, 7 antibiotics and antiviral therapy | All hospitalized | 11 positive, 9 negative PCR-test, 1 not tested |
| Youd et al[57], 2020 | United Kingdom | 9 | 72, 33-88 | M 4, F 5 | Complete | 3 obesity | NR | 4 congestion, 1 steatosis and 4 normal | NR | None | Respiratory failure in COVID-19 | NR | 9 deaths in community settings | No search |
| Zhao et al[58], 2020 | United States | 17 | 65, 44-85 | M 10, F 7 | Complete | 5 hyperlipidemia, 1 cirrhosis | 12 elevated AST and ALT values. Peak AST: 1903 (24-13592) and ALT 1059 (13-6136) | Weight 17694 g (1000-2600 g) | 12 platelet-fibrin microthrombi, 5 histiocyte activation, 12 steatosis, 5 lobular inflammation, 8 portal inflammation, 10 necrosis | CD68 stain confirmed histiocytic hyperplasia | NR | NR | All hospitalized | 5 positive IHC (spike protein) in the histiocytes in the portal tracts. Negative IHC in endothelial cells and hepatocytes |
F: Female; Male: M; HCV: Hepatitis C virus; NIV: Non-invasive ventilation; ECMO: Extracorporeal membrane oxygenation; COVID-19: Coronavirus disease 2019; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ISH: In situ hybridization; MOF: Multi-organ failure; PCR: Polymerase chain reaction; TEM: Transmission electron microscopy; NR: Not reported; IHC: Immunohistochemistry.
RESULTS
Demographics
A total of 11 case reports and 35 case series were analyzed, with a total of 994 autopsy cases of COVID-19 patients. Studies were from all over the world: One from Hungary, Romania, Japan, South Africa, and United States in association with Brazil each, two from Austria, Belgium, India, Iran, and Turkey each, three from the United Kingdom, four from Italy, five from Germany, Switzerland, and China each, and nine from the United States. Gender was specified in 882 cases, of whom 54% (540) were male and 35% (342) were female. Age ranged from 18 to 102 years with a mean age of 53 years. Age distribution is summarized in Figure 1.
Figure 1.
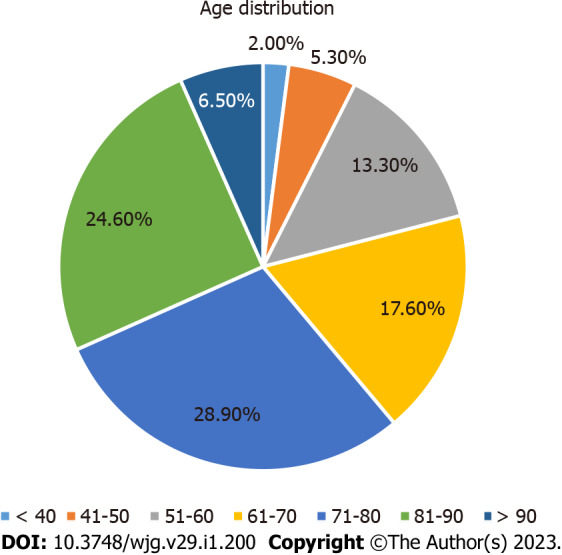
Age distribution.
Liver disease
Pre-existing liver diseases were described in 61 (6%) cases, comprising 28 cases of fatty liver disease, 19 cases of chronic liver disease, 11 cases of cirrhosis, and 1 case each of hepatitis B and C. In 161 cases, body mass index (BMI) was over 30 kg/m2.
Laboratory findings
Laboratory values of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were described in 350 cases, with only 1 case described with values within the ranges of normality. The description of the laboratory values differed somewhat between the various studies, with only 5 studies (55 cases) reporting AST and ALT values at admission and 8 (64 cases) reporting the maximum values during hospitalization. Additional 4 reports for AST (51 cases) and 5 papers for ALT (61 cases) described the laboratory values without specifying the timing of the sampling. Data is summarized in Table 2. Abnormal AST and ALT values were described in 105 and 91 additional cases, respectively.
Table 2.
Laboratory findings
|
Laboratory findings
|
Mean (UI/L)
|
Range (UI/L)
|
| Admission values (n = 53) | ||
| AST | 58 | 12-760 |
| ALT | 34 | 7-229 |
| Peak values (n = 64) | ||
| AST | 868 | 15-24176 |
| ALT | 509 | 10-9961 |
| Non specified | ||
| AST (n = 61) | 202 | 17-6000 |
| ALT (n = 51) | 209 | 11-4885 |
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase.
Hospitalization and medications
For the subsequent analysis of the macroscopic and microscopic findings, it was decided to evaluate whether the patients were hospitalized and whether drug therapies capable of causing liver alterations, such as antibiotics, antivirals, and quinine, were administered. In 861 cases the place where the death took place was described. In 752 cases the patient was hospitalized and died in hospital, 76 cases died at home, 22 cases died in community settings, and 11 cases were not hospitalized and died in other circumstances such as car accidents and falls from a height. In 133 cases a hospital stay or the place of death was not described. Medication administration was described in 201 cases, of which 22 were administered with hydroxychloroquine only. In 41 cases quinine was administered together with an antibiotic or antiviral, in 17 cases antibiotics and antivirals were given, and in 56 only an antibiotic was administered. In 766 cases the administration of hepatotoxic drugs was not reported.
Type of autopsy
Autopsies were performed in all 994 cases; in 508 (51%) cases autopsies were complete, of which 2% (22) had a complete autopsy without the evaluation of the brain to avoid the risk of COVID-19 infection, in 38% (372) of the cases a core biopsy was performed, in 51 (5%) cases a partial autopsy was carried out, and in 41 cases information about the type of autopsy performed was not reported.
Macroscopic results
Macroscopic results were described in only 265 (27%) cases. The most frequent finding, in 79 cases, was the presence of congestion, followed by steatosis in 39 cases. A nutmeg or yellow aspect of the liver surface was seen in 16 cases, a fibrosis-indurated consistency in 6 cases, and only 1 case showed the macroscopic presence of cancer. Lastly, 11 livers were described of increased size (hepatomegaly) and 10 livers as normal. For 144 patients weight was reported; mean weight was 1805 g with a range from 520 to 3220 g.
Microscopic results
Microscopic results were described in 983 (99%) cases. The two most frequent findings were congestion, in 420 cases, and steatosis, in 409 cases. Four cases were described as normal. All findings are described in Table 3.
Table 3.
Microscopic findings
|
Microscopic findings
|
n
(%)
|
| Hepatic necrosis | 190 (19) |
| Hepatic inflammation | 190 (19) |
| Portal inflammation | 178 (18) |
| Fibrosis | 149 (15) |
| Microthrombi | 121 (12) |
| Cholestasis | 114 (11) |
| Hemophagocytosis | 51 (5) |
| Bile plugs | 2 (0.2) |
| Endotheliitis | 7 (0.7) |
| Autolysis | 20 (2) |
| Iron overload | 5 (0.5) |
| Other (abscess, ductal proliferation and granulomatosis) | 15 (1.5) |
Cause of death
Cause of death was reported for 440 (44%) cases. The most frequent cause of death was respiratory failure in COVID-19, seen in 355 (81%) cases, followed by multi-organ failure in 33 cases, cardiovascular failure in 22, pulmonary thrombosis in 11, and sepsis in 8. The remaining 11 cases died respectively of hemorrhagic shock (3 cases), acute liver failure (2 cases), acute mesenteric ischemia (2 cases), bronchopneumonia (2 cases), and one case each of cardiac tamponade and multiple injuries.
Virus search
The search for the presence of SARS-CoV-2 was performed in only 162 (16%) cases. Of these 105 were tested by real-time reverse-transcription polymerase chain reaction (RT-PCR) and found positive in 53 cases, 34 cases were tested by immunohistochemistry (IHC) and all found negative, 28 were tested by in situ hybridization (ISH) and found negative in all cases, and lastly, 16 were tested by transmission electron microscopy and found positive in 2 cases.
DISCUSSION
A total of 994 autopsy cases of COVID-19 patients with liver assessment were found in the literature. As expected, more than half of the deceased were males and age distribution was highly variable, with a predominance of subjects in the age group 60-90 (71.1%).
Pre-existing liver disease was rare (6%-literature data shows a frequency of 2%-11%), with only 16.2% of the cases presenting obesity (BMI > 30 kg/m2)[7]. Obesity, in association with diabetes and hypertension, is a prominent risk factor for severe disease and could predispose to nonalcoholic fatty liver disease (NAFLD), a metabolic syndrome which is known to suppress the pro-inflammatory M1 macrophages favoring the progression of virus infection[2,8,11]. NAFLD seems to be identified with a higher prevalence in patients with severe COVID-19 and predisposes to higher liver enzymes at admission and at discharge[59]. To date the fact that pre-existing liver disease is an independent risk factor for poor outcome is still debated; for some authors patients with liver diseases are not over-represented in hospital casuistry[4,60-62], while for others the presence of a pre-exiting illness is index of a greater probability of a bad outcome[7,63-65]. This does not count in the case of cirrhosis, seen in only 1% in this review, which is known to be an important predictor of mortality, with a mortality rate of 31%[2,61]. It appears that in the case of cirrhosis those who survive the first insult have a re-admission rate in hospital similar to those with cirrhosis, but without COVID-19, indicating that beyond the acute phase SARS-CoV-2 does not change the natural history of the disease[4]. There are currently few data regarding the mortality rate associated with alcohol liver disease as an independent risk factor, mainly related to the difficulties of correlating liver damage or elevation of liver enzymes to alcohol consumption. To date, it seems that alcohol liver disease increases the mortality risk by 1.8 fold[61].
Laboratory findings have not been collected in a homogeneous way, with 27 papers not reporting any data, 5 reporting AST and ALT values at admission, and 8 reporting the maximum values during hospitalization, and 4 reports for AST and 5 papers for ALT described the laboratory values without specifying the timing of the sampling. Abnormal values, without specifying the laboratory values, were described in 5 articles. From literature data it appears that liver enzyme abnormalities have a wide range, occurring in 14%-76% of the cases[4,5,7,11,66]. This great range, as Marjot et al[4] pointed out, could be attributed to different limits of the definition of normal values. It is still debated whether elevated liver enzymes are associated with a greater risk of mortality, because patients with worst outcomes tend to be monitored in intensive care units, while those with mild symptoms are not strictly monitored. Thus, the use of abnormal laboratory findings at admission as a predictor of poor outcome is still not sure. Liver enzyme elevation mainly affects AST and ALT, indicating hepatocellular damage rather than cholestatic, despite a greater expression of ACE2 receptor in cholangiocytes[3]. As the study of Wong et al[67] pointed out, the odd ratio of elevated AST and ALT levels in COVID-19 patients is 3.4 and 2.5, respectively.
Due to the presence of such fragmented laboratory data, it is difficult to draw conclusions about the trend of laboratory values during hospitalization, although some authors have found a tendency of increased values during hospitalization, in particular in those in critical conditions[9,11,12,68,69]. Whether enzyme elevation is induced directly by the virus or because of the inflammation, congestion, or medications is still not clear. Certainly, many of the drugs used in COVID-19 positive patients turn out to be hepatotoxic such as hydroxychloroquine and antivirals such as ritonavir, lopinavir, and remdesivir[8,66]. The meta-analysis by Wong et al[67] and Cai et al[66] suggests that liver injury is higher in studies with high usage of lopinavir/ritonavir, despite that their hepatotoxic role is still to be described in patients without pre-existing liver disease, while there was no evidence of a higher risk of liver injury for those treated with antibiotics, nonsteroidal anti-inflammatory drugs, ribavirin, herbal medications, and interferon.
The literature review highlighted the presence of a great discrepancy in the autopsy protocols, with only half of the autopsies performed as complete (full autopsies), while the other half as partial. Macroscopic evaluation of the liver was not frequent, while microscopic assessment was present in almost every case (99%). As expected, congestion and steatosis were the most frequent findings. The congestion can be traced back to the presence in these patients of cardiovascular dysfunction due to the massive inflammation and cytokine storm linked to the infection. The presence of steatosis needs a more complex analysis; lipid accumulation due to SARS-CoV-2 has to be differentiated from pre-existing modifications, typical of patient with metabolic syndrome. COVID-19 lipid accumulation can be explained because of the cytopathic effect of the coronavirus, which induces endoplasmatic stress and lipogenesis[2]. Transcriptomic profiling of COVID-19 patients by Wanner et al[5] demonstrated an upregulation of cellular processes involved in lipid/cholesterol synthesis. Furthermore, corticosteroid therapy, widely used in the treatment of COVID-19, is known to be associated with steatosis or glycogenosis[2].
Hepatic necrosis and inflammation can be multifactorial; they can be induced by a cytopathic direct effect of the virus, because of inflammatory storm or hypoxic hepatitis, or may be drug induced. These hepatic changes are the third most frequent finding in liver autopsies of COVID-19 patients[70]. Differentiating the different causes from a pathological point of view is impossible, also in consideration of the fact that they can overlap one another. In addition, patients with pre-existing liver diseases, such as chronic liver disease, have an increased risk of drug-induced hepatic damage, therefore in those patients the use of hepatotoxic treatments should be weighted. Liver damage in critically ill patients is known and is linked to the so-called hypoxic hepatitis, which is caused by underlying cardiac dysfunction and respiratory failure that decrease the blood flow and oxygenation inducing cellular stress. Moreover, damage could even be mediated by reperfusion, which promotes the production of reactive oxygen species, leading to damage. This process can be highlighted in some cases as a picture of endotheliitis[2,3,11]. Massive inflammation is common in COVID-19 patients and macrophage activation is evidenced by the presence of hemophagocytosis in liver tissue.
Unlike what is reported by Marjot et al[4], the frequency of thrombotic phenomena of the hepatic vascular tree is lower, with 12% of cases instead of 29%. As Kleiner[70] noted, death could occur long after the acute phase of liver damage, so the histological changes do not always represent a reliable image of what happened in acute damage, but are the result of damage and reparative modifications. Therefore, to better understand the acute damage, it could be of help to perform a liver biopsy in patients with liver damage. Obviously, it is understood that the execution of such an invasive examination is not a priority in the treatment of these patients, but it could be performed in those cases where the hepatic injury dominates the clinical picture.
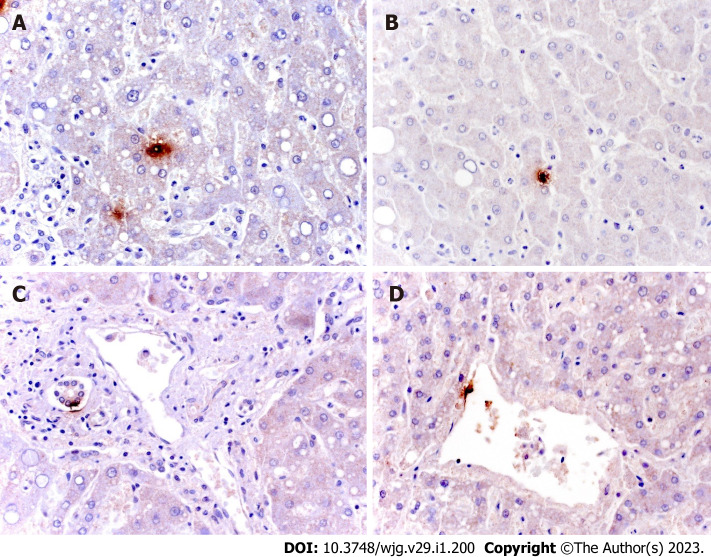
Despite the presence of hepatic injury, the presence of SARS-CoV-2 in the liver has been sought infrequently (16% of the cases). Most studies have exploited the RT-PCR to search for the viral genome, but only a few have applied other techniques (IHC, ISH, and transmission electron microscopy) to identify the cells in which the viral proteins were expressed (Figure 2A and B). It is not surprising that by using RT-PCR a greater number of cases resulted positive, because this type of analysis uses a homogenized tissue, which also contains vessels and immunity cells. However, the few available data allow us to confirm the fact that the virus can be found mainly in Kupffer cells, endothelial cells of centrolobulare veins, and cholangiocytes (Figure 2C and D). Note that Wanner et al[5] demonstrated that, when comparing the levels of SARS-CoV-2 RNA copies per cell between airway samples and autopsy livers biopsies, the levels of RNA show similar ranges, but with lower median RNA in liver specimens.
Figure 2.
Immunohistochemical staining for severe acute respiratory syndrome coronavirus 2 in liver tissue. A and B: Spot localization of virus in samples of initial hepatic necrosis and in Kupffer cells; C and D: Spot localization of isolated ductular and endothelial cells (mouse, GeneTex GTX632604, 1A9 clone, 1:100).
CONCLUSION
Postmortem investigations remain the gold standard to investigate the effects of SARS-CoV-2 in different organs and apparatuses. It is well known that the absence of postmortem investigations in the first wave of the pandemic has failed to provide a valuable contribution to the correct management and treatment of patients. On the other hand, the execution of clinical and forensic autopsies has disclosed several important aspects of the disease, clarifying morphological and virologic features and promoting unexplored therapeutic approaches and new frontiers of research[71-74]. Despite the limited number of performed autopsies worldwide, to date there is no doubt that the liver is a target for the virus, despite minimal viral receptor expression. However, liver damage is not always directly linked to the action of the virus, but can be secondary to inflammation or even simply caused by the therapy administered during hospitalization. Therefore, it is important to monitor patients who use hepatotoxic drugs, to avoid worsening of the liver functions, which can affect the patient’s outcome.
ARTICLE HIGHLIGHTS
Research background
Hepatic histologic manifestations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are far to be completely investigated. Many authors demonstrated the presence of angiotensin converting enzyme 2 receptor in the liver as well as transmembrane serine protease 2.
Research motivation
Liver injury was demonstrated in 14%-53% of cases of patients with SARS-CoV-2 infection. In the first wave of the pandemic few autopsies were performed and only few authors can provide a wide casistic. Authors started to study the histologic manifestations of coronavirus disease 2019 (COVID-19) in the lungs, heart, and liver, too.
Research objectives
The objectives of the study were to summarize the biochemical and histological changes in the liver and to promote the leading role of autopsy in the pandemic.
Research methods
Authors provide a systematic review focusing on autopsy studies of COVID-19 deaths and in particular on liver pathology.
Research results
Forty-six articles corresponding to the inclusion criteria were included, with only 994 autopsy cases of COVID-19 patients. Congestion and steatosis were the main histopathological findings, followed by hepatic necrosis, hepatic and portal inflammation, and fibrosis. The most frequent cause of death was respiratory failure, pulmonary thrombosis, and sepsis. Acute liver failure was indicated as the cause of death in two cases.
Research conclusions
The review of the literature highlighted the presence of a great discrepancy in the autopsy protocols, with only half of the autopsies performed as complete (full autopsies), while the other half as partial. Macroscopic and microscopic evaluation of the liver was not always performed or described. Despite the presence of hepatic injury, the presence of SARS-CoV-2 in the liver has been sought infrequently (16% of the cases).
Research perspectives
Much more effort needs to be addressed to completely investigate liver toxicity from COVID-19. Autopsies had a leading role during the pandemic and were important to understand the physiopathology of SARS-CoV-2 infection and should be always considered to improve scientific research.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest for this article.
PRISMA 2009 Checklist statement: The review followed the PRISMA 2009 checklist statement.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 13, 2022
First decision: October 30, 2022
Article in press: December 21, 2022
Specialty type: Pathology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bataga SM, Romania; He F, China S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Zhang H
Contributor Information
Martina Zanon, Department of Medical Surgical and Health Sciences, University of Trieste, Trieste 34149, Italy.
Margherita Neri, Department of Medical Sciences, University of Ferrara, Ferrara 44121, Italy.
Stefano Pizzolitto, Department of Pathology, Santa Maria della Misericordia University Hospital, Udine 33100, Italy.
Davide Radaelli, Department of Medical Surgical and Health Sciences, University of Trieste, Trieste 34149, Italy.
Monica Concato, Department of Medical Surgical and Health Sciences, University of Trieste, Trieste 34149, Italy.
Michela Peruch, Department of Medical Surgical and Health Sciences, University of Trieste, Trieste 34149, Italy.
Stefano D'Errico, Department of Medical Surgical and Health Sciences, University of Trieste, Trieste 34149, Italy. sderrico@units.it.
References
- 1.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278–1281. doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 2.Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20–32. doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang RX, Zheng RD, Fan JG. Etiology and management of liver injury in patients with COVID-19. World J Gastroenterol. 2020;26:4753–4762. doi: 10.3748/wjg.v26.i32.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marjot T, Webb GJ, Barritt AS 4th, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348–364. doi: 10.1038/s41575-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wanner N, Andrieux G, Badia-I-Mompel P, Edler C, Pfefferle S, Lindenmeyer MT, Schmidt-Lauber C, Czogalla J, Wong MN, Okabayashi Y, Braun F, Lütgehetmann M, Meister E, Lu S, Noriega MLM, Günther T, Grundhoff A, Fischer N, Bräuninger H, Lindner D, Westermann D, Haas F, Roedl K, Kluge S, Addo MM, Huber S, Lohse AW, Reiser J, Ondruschka B, Sperhake JP, Saez-Rodriguez J, Boerries M, Hayek SS, Aepfelbacher M, Scaturro P, Puelles VG, Huber TB. Molecular consequences of SARS-CoV-2 liver tropism. Nat Metab. 2022;4:310–319. doi: 10.1038/s42255-022-00552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pirisi M, Rigamonti C, D'Alfonso S, Nebuloni M, Fanni D, Gerosa C, Orrù G, Venanzi Rullo E, Pavone P, Faa G, Saba L, Boldorini R. Liver infection and COVID-19: the electron microscopy proof and revision of the literature. Eur Rev Med Pharmacol Sci. 2021;25:2146–2151. doi: 10.26355/eurrev_202102_25120. [DOI] [PubMed] [Google Scholar]
- 7.Warner FJ, Rajapaksha H, Shackel N, Herath CB. ACE2: from protection of liver disease to propagation of COVID-19. Clin Sci (Lond) 2020;134:3137–3158. doi: 10.1042/CS20201268. [DOI] [PubMed] [Google Scholar]
- 8.Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231–1240. doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammed A, Paranji N, Chen PH, Niu B. COVID-19 in Chronic Liver Disease and Liver Transplantation: A Clinical Review. J Clin Gastroenterol. 2021;55:187–194. doi: 10.1097/MCG.0000000000001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lizardo-Thiebaud MJ, Cervantes-Alvarez E, Limon-de la Rosa N, Tejeda-Dominguez F, Palacios-Jimenez M, Méndez-Guerrero O, Delaye-Martinez M, Rodriguez-Alvarez F, Romero-Morales B, Liu WH, Huang CA, Kershenobich D, Navarro-Alvarez N. Direct or Collateral Liver Damage in SARS-CoV-2-Infected Patients. Semin Liver Dis. 2020;40:321–330. doi: 10.1055/s-0040-1715108. [DOI] [PubMed] [Google Scholar]
- 11.Parker GA, Picut CA. Liver immunobiology. Toxicol Pathol. 2005;33:52–62. doi: 10.1080/01926230590522365. [DOI] [PubMed] [Google Scholar]
- 12.Gracia-Ramos AE, Jaquez-Quintana JO, Contreras-Omaña R, Auron M. Liver dysfunction and SARS-CoV-2 infection. World J Gastroenterol. 2021;27:3951–3970. doi: 10.3748/wjg.v27.i26.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiel MI, El Jamal SM, Paniz-Mondolfi A, Gordon RE, Reidy J, Bandovic J, Advani R, Kilaru S, Pourmand K, Ward S, Thung SN, Schiano T. Findings of Hepatic Severe Acute Respiratory Syndrome Coronavirus-2 Infection. Cell Mol Gastroenterol Hepatol. 2021;11:763–770. doi: 10.1016/j.jcmgh.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguiar D, Lobrinus JA, Schibler M, Fracasso T, Lardi C. Inside the lungs of COVID-19 disease. Int J Legal Med. 2020;134:1271–1274. doi: 10.1007/s00414-020-02318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arslan MN, Büyük Y, Ziyade N, Elgörmüş N, Şirin G, Çoban İ, Gökşen ME, Daş T, Akçay A. COVID-19 autopsies of Istanbul. Ir J Med Sci. 2022;191:529–541. doi: 10.1007/s11845-021-02602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 Autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beigmohammadi MT, Jahanbin B, Safaei M, Amoozadeh L, Khoshavi M, Mehrtash V, Jafarzadeh B, Abdollahi A. Pathological Findings of Postmortem Biopsies From Lung, Heart, and Liver of 7 Deceased COVID-19 Patients. Int J Surg Pathol. 2021;29:135–145. doi: 10.1177/1066896920935195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB, Albrecht R, Hernandez T, Stock A, Zhao Z, AlRasheed MR, Chen J, Li L, Wang D, Corben A, Haines GK 3rd, Westra WH, Umphlett M, Gordon RE, Reidy J, Petersen B, Salem F, Fiel MI, El Jamal SM, Tsankova NM, Houldsworth J, Mussa Z, Veremis B, Sordillo E, Gitman MR, Nowak M, Brody R, Harpaz N, Merad M, Gnjatic S, Liu WC, Schotsaert M, Miorin L, Aydillo Gomez TA, Ramos-Lopez I, Garcia-Sastre A, Donnelly R, Seigler P, Keys C, Cameron J, Moultrie I, Washington KL, Treatman J, Sebra R, Jhang J, Firpo A, Lednicky J, Paniz-Mondolfi A, Cordon-Cardo C, Fowkes ME. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;34:1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bösmüller H, Traxler S, Bitzer M, Häberle H, Raiser W, Nann D, Frauenfeld L, Vogelsberg A, Klingel K, Fend F. The evolution of pulmonary pathology in fatal COVID-19 disease: an autopsy study with clinical correlation. Virchows Arch. 2020;477:349–357. doi: 10.1007/s00428-020-02881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bugra A, Das T, Arslan MN, Ziyade N, Buyuk Y. Postmortem pathological changes in extrapulmonary organs in SARS-CoV-2 rt-PCR-positive cases: a single-center experience. Ir J Med Sci. 2022;191:81–91. doi: 10.1007/s11845-021-02638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chornenkyy Y, Mejia-Bautista M, Brucal M, Blanke T, Dittmann D, Yeldandi A, Boike JR, Lomasney JW, Nayar R, Jennings LJ, Pezhouh MK. Liver Pathology and SARS-CoV-2 Detection in Formalin-Fixed Tissue of Patients With COVID-19. Am J Clin Pathol. 2021;155:802–814. doi: 10.1093/ajcp/aqab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danics K, Pesti A, Törő K, Kiss-Dala N, Szlávik J, Lakatos B, Radnai A, Balázs T, Bacskai M, Dobi D, Várkonyi T, Glasz T, Lotz G, Kiss A, Schaff Z, Vályi-Nagy I. A COVID-19-association-dependent categorization of death causes in 100 autopsy cases. Geroscience. 2021;43:2265–2287. doi: 10.1007/s11357-021-00451-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Nonno F, Nardacci R, Colombo D, Visco-Comandini U, Cicalini S, Antinori A, Marchioni L, D'Offizi G, Piacentini M, Falasca L. Hepatic Failure in COVID-19: Is Iron Overload the Dangerous Trigger? Cells. 2021;10 doi: 10.3390/cells10051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edler C, Schröder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, Klein A, Langenwalder F, Lütgehetmann M, Meißner K, Püschel K, Schädler J, Steurer S, Mushumba H, Sperhake JP. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsoukkary SS, Mostyka M, Dillard A, Berman DR, Ma LX, Chadburn A, Yantiss RK, Jessurun J, Seshan SV, Borczuk AC, Salvatore SP. Autopsy Findings in 32 Patients with COVID-19: A Single-Institution Experience. Pathobiology. 2021;88:56–68. doi: 10.1159/000511325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evert K, Dienemann T, Brochhausen C, Lunz D, Lubnow M, Ritzka M, Keil F, Trummer M, Scheiter A, Salzberger B, Reischl U, Boor P, Gessner A, Jantsch J, Calvisi DF, Evert M, Schmidt B, Simon M. Autopsy findings after long-term treatment of COVID-19 patients with microbiological correlation. Virchows Arch. 2021;479:97–108. doi: 10.1007/s00428-020-03014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falasca L, Nardacci R, Colombo D, Lalle E, Di Caro A, Nicastri E, Antinori A, Petrosillo N, Marchioni L, Biava G, D'Offizi G, Palmieri F, Goletti D, Zumla A, Ippolito G, Piacentini M, Del Nonno F. Postmortem Findings in Italian Patients With COVID-19: A Descriptive Full Autopsy Study of Cases With and Without Comorbidities. J Infect Dis. 2020;222:1807–1815. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fassan M, Mescoli C, Sbaraglia M, Guzzardo V, Russo FP, Fabris R, Trevenzoli M, Pelizzaro F, Cattelan AM, Basso C, Navalesi P, Farinati F, Vettor R, Dei Tos AP. Liver histopathology in COVID-19 patients: A mono-Institutional series of liver biopsies and autopsy specimens. Pathol Res Pract. 2021;221:153451. doi: 10.1016/j.prp.2021.153451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greuel S, Ihlow J, Dragomir MP, Streit S, Corman VM, Haberbosch L, Winkler D, Meinhardt J, Aschman T, Schneider J, Trotsyuk I, Kunze CA, Maurer L, Radbruch H, Heppner FL, Horst D, Elezkurtaj S. COVID-19: Autopsy findings in six patients between 26 and 46 years of age. Int J Infect Dis. 2021;108:274–281. doi: 10.1016/j.ijid.2021.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grosse C, Grosse A, Salzer HJF, Dünser MW, Motz R, Langer R. Analysis of cardiopulmonary findings in COVID-19 fatalities: High incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc Pathol. 2020;49:107263. doi: 10.1016/j.carpath.2020.107263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R, Al-Sarraj S, Abdolrasouli A, Swann OC, Baillon L, Penn R, Barclay WS, Viola P, Osborn M. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirayama Y, Daniels NF, Evans S, Clarke D, Purvis S, Oliver C, Woodmansey S, Staniforth J, Soilleux EJ. High Prevalence of Pre-Existing Liver Abnormalities Identified Via Autopsies in COVID-19: Identification of a New Silent Risk Factor? Diagnostics (Basel) 2021;11 doi: 10.3390/diagnostics11091703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hooper JE, Padera RF, Dolhnikoff M, da Silva LFF, Duarte-Neto AN, Kapp ME, Lacy JM, Mauad T, Saldiva PHN, Rapkiewicz AV, Wolf DA, Felix JC, Benson P, Shanes E, Gawelek KL, Marshall DA, McDonald MM, Muller W, Priemer DS, Solomon IH, Zak T, Bhattacharjee MB, Fu L, Gilbert AR, Harper HL, Litovsky S, Lomasney J, Mount SL, Reilly S, Sekulic M, Steffensen TS, Threlkeld KJ, Zhao B, Williamson AK. A Postmortem Portrait of the Coronavirus Disease 2019 (COVID-19) Pandemic: A Large Multi-institutional Autopsy Survey Study. Arch Pathol Lab Med. 2021;145:529–535. doi: 10.5858/arpa.2020-0786-SA. [DOI] [PubMed] [Google Scholar]
- 35.Ihlow J, Seelhoff A, Corman VM, Gruber AD, Dökel S, Meinhardt J, Radbruch H, Späth-Schwalbe E, Elezkurtaj S, Horst D, Herbst H. COVID-19: a fatal case of acute liver failure associated with SARS-CoV-2 infection in pre-existing liver cirrhosis. BMC Infect Dis. 2021;21:901. doi: 10.1186/s12879-021-06605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lacy JM, Brooks EG, Akers J, Armstrong D, Decker L, Gonzalez A, Humphrey W, Mayer R, Miller M, Perez C, Arango JAR, Sathyavagiswaran L, Stroh W, Utley S. COVID-19: Postmortem Diagnostic and Biosafety Considerations. Am J Forensic Med Pathol. 2020;41:143–151. doi: 10.1097/PAF.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, Del Portillo A, De Michele S, de Gonzalez AK, Saqi A, Khairallah P, Chong AM, Park H, Uhlemann AC, Lefkowitch JH, Verna EC. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147–2155. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome : Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malik Y, Singh K, Yadav S, Vashist YK, Garg A, Kumar S, Sharma G. COVID-19: Asymptomatic Carrier: An Autopsy Case Report. Int J Appl Basic Med Res. 2021;11:120–124. doi: 10.4103/ijabmr.IJABMR_579_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nunes MC, Hale MJ, Mahtab S, Mabena FC, Dludlu N, Baillie VL, Thwala BN, Els T, du Plessis J, Laubscher M, Mckenzie S, Mtshali S, Menezes C, Serafin N, van Blydenstein S, Tsitsi M, Dulisse B, Madhi SA. Clinical characteristics and histopathology of COVID-19 related deaths in South African adults. PLoS One. 2022;17:e0262179. doi: 10.1371/journal.pone.0262179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oprinca GC, Muja LA. Postmortem examination of three SARS-CoV-2-positive autopsies including histopathologic and immunohistochemical analysis. Int J Legal Med. 2021;135:329–339. doi: 10.1007/s00414-020-02406-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, Hochman JS, Reynolds HR. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Remmelink M, De Mendonça R, D'Haene N, De Clercq S, Verocq C, Lebrun L, Lavis P, Racu ML, Trépant AL, Maris C, Rorive S, Goffard JC, De Witte O, Peluso L, Vincent JL, Decaestecker C, Taccone FS, Salmon I. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24:495. doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren L, Liu Q, Wang R, Chen R, Ao Q, Wang X, Zhang J, Deng F, Feng Y, Wang G, Zhou Y, Li L, Liu L. Clinicopathologic Features of COVID-19: A Case Report and Value of Forensic Autopsy in Studying SARS-CoV-2 Infection. Am J Forensic Med Pathol. 2021;42:164–169. doi: 10.1097/PAF.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmit G, Lelotte J, Vanhaebost J, Horsmans Y, Van Bockstal M, Baldin P. The Liver in COVID-19-Related Death: Protagonist or Innocent Bystander? Pathobiology. 2021;88:88–94. doi: 10.1159/000512008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schweitzer W, Ruder T, Baumeister R, Bolliger S, Thali M, Meixner E, Ampanozi G. Implications for forensic death investigations from first Swiss post-mortem CT in a case of non-hospital treatment with COVID-19. Forensic Imaging. 2020;21:200378. [Google Scholar]
- 48.Shishido-Hara Y, Furukawa K, Nishio M, Honda K, Tando S, Yaoi T, Kawamoto M, Maehara Y, Nakaya T, Itoh K. An autopsy case of COVID-19 with a sudden death: Clinico-pathological comparison. Clin Case Rep. 2022;10:e5961. doi: 10.1002/ccr3.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suess C, Hausmann R. Gross and histopathological pulmonary findings in a COVID-19 associated death during self-isolation. Int J Legal Med. 2020;134:1285–1290. doi: 10.1007/s00414-020-02319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shirazi Tehrani A, Tabatabaei Mirakabad FS, Abdollahifar MA, Mollazadehghomi S, Darabi S, Forozesh M, Rezaei-Tavirani M, Mahmoudiasl GR, Ahrabi B, Azimzadeh Z, Allah Abbaszadeh H. Severe Acute Respiratory Syndrome Coronavirus 2 Induces Hepatocyte Cell Death, Active Autophagosome Formation and Caspase 3 Up-Regulation in Postmortem Cases: Stereological and Molecular Study. Tohoku J Exp Med. 2022;256:309–319. doi: 10.1620/tjem.2022.J007. [DOI] [PubMed] [Google Scholar]
- 52.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang XX, Shao C, Huang XJ, Sun L, Meng LJ, Liu H, Zhang SJ, Li HJ, Lv FD. Histopathological features of multiorgan percutaneous tissue core biopsy in patients with COVID-19. J Clin Pathol. 2021;74:522–527. doi: 10.1136/jclinpath-2020-206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yadav J, Goel G, Purwar S, Saigal S, Tandon A, Joshi A, Patel B, Js S, S M, Singh J, Shankar P, Arora A, Singh S. Clinical, Virological, and Pathological Profile of Patients Who Died of COVID-19: An Autopsy-Based Study From India. Cureus. 2022;14:e23538. doi: 10.7759/cureus.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Youd E, Moore L. COVID-19 autopsy in people who died in community settings: the first series. J Clin Pathol. 2020;73:840–844. doi: 10.1136/jclinpath-2020-206710. [DOI] [PubMed] [Google Scholar]
- 59.Zhao CL, Rapkiewicz A, Maghsoodi-Deerwester M, Gupta M, Cao W, Palaia T, Zhou J, Ram B, Vo D, Rafiee B, Hossein-Zadeh Z, Dabiri B, Hanna I. Pathological findings in the postmortem liver of patients with coronavirus disease 2019 (COVID-19) Hum Pathol. 2021;109:59–68. doi: 10.1016/j.humpath.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Fang X, Cai Z, Wu X, Gao X, Min J, Wang F. Comorbid Chronic Diseases and Acute Organ Injuries Are Strongly Correlated with Disease Severity and Mortality among COVID-19 Patients: A Systemic Review and Meta-Analysis. Research (Wash D C) 2020;2020:2402961. doi: 10.34133/2020/2402961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vila-Corcoles A, Satue-Gracia E, Vila-Rovira A, de Diego-Cabanes C, Forcadell-Peris MJ, Hospital-Guardiola I, Ochoa-Gondar O, Basora-Gallisa J. COVID19-related and all-cause mortality risk among middle-aged and older adults across the first epidemic wave of SARS-COV-2 infection: a population-based cohort stuJune 2020.dy in Southern Catalonia, Spain, March- BMC Public Health. 2021;21:1795. doi: 10.1186/s12889-021-11879-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One. 2020;15:e0243191. doi: 10.1371/journal.pone.0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong YJ, Tan M, Zheng Q, Li JW, Kumar R, Fock KM, Teo EK, Ang TL. A systematic review and meta-analysis of the COVID-19 associated liver injury. Ann Hepatol. 2020;19:627–634. doi: 10.1016/j.aohep.2020.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Y, Li H, Guo X, Yoshida EM, Mendez-Sanchez N, Levi Sandri GB, Teschke R, Romeiro FG, Shukla A, Qi X. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatol Int. 2020;14:621–637. doi: 10.1007/s12072-020-10074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barnes E. Infection of liver hepatocytes with SARS-CoV-2. Nat Metab. 2022;4:301–302. doi: 10.1038/s42255-022-00554-4. [DOI] [PubMed] [Google Scholar]
- 70.Kleiner DE. Liver Biopsy Shines a Light on COVID-19-Related Liver Injury. Cell Mol Gastroenterol Hepatol. 2021;11:881–882. doi: 10.1016/j.jcmgh.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D'Errico S, Zanon M, Montanaro M, Radaelli D, Sessa F, Di Mizio G, Montana A, Corrao S, Salerno M, Pomara C. More than Pneumonia: Distinctive Features of SARS-Cov-2 Infection. From Autopsy Findings to Clinical Implications: A Systematic Review. Microorganisms. 2020;8 doi: 10.3390/microorganisms8111642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Macor P, Durigutto P, Mangogna A, Bussani R, D'Errico S, Zanon M, Pozzi N, Meroni P, Tedesco F. Multi-organ complement deposition in COVID-19 patients. medRxiv. 2021 doi: 10.3390/biomedicines9081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cipolloni L, Sessa F, Bertozzi G, Baldari B, Cantatore S, Testi R, D'Errico S, Di Mizio G, Asmundo A, Castorina S, Salerno M, Pomara C. Preliminary Post-Mortem COVID-19 Evidence of Endothelial Injury and Factor VIII Hyperexpression. Diagnostics (Basel) 2020;10 doi: 10.3390/diagnostics10080575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frisoni P, Neri M, D'Errico S, Alfieri L, Bonuccelli D, Cingolani M, Di Paolo M, Gaudio RM, Lestani M, Marti M, Martelloni M, Moreschi C, Santurro A, Scopetti M, Turriziani O, Zanon M, Scendoni R, Frati P, Fineschi V. Cytokine storm and histopathological findings in 60 cases of COVID-19-related death: from viral load research to immunohistochemical quantification of major players IL-1β, IL-6, IL-15 and TNF-α. Forensic Sci Med Pathol. 2022;18:4–19. doi: 10.1007/s12024-021-00414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]