Abstract
Rationale
Risk of asthma hospitalization and its disparities associated with air pollutant exposures are less clear within socioeconomically disadvantaged populations, particularly at low degrees of exposure.
Objectives
To assess effects of short-term exposures to fine particulate matter (particulate matter with an aerodynamic diameter of ⩽2.5 μm [PM2.5]), warm-season ozone (O3), and nitrogen dioxide (NO2) on risk of asthma hospitalization among national Medicaid beneficiaries, the most disadvantaged population in the United States, and to test whether any subpopulations were at higher risk.
Methods
We constructed a time-stratified case-crossover dataset among 1,627,002 hospitalizations during 2000–2012 and estimated risk of asthma hospitalization associated with short-term PM2.5, O3, and NO2 exposures. We then restricted the analysis to hospitalizations with degrees of exposure below increasingly stringent thresholds. Furthermore, we tested effect modifications by individual- and community-level characteristics.
Measurements and Main Results
Each 1-μg/m3 increase in PM2.5, 1-ppb increase in O3, and 1-ppb increase in NO2 was associated with 0.31% (95% confidence interval [CI], 0.24–0.37%), 0.10% (95% CI, 0.05 − 0.15%), and 0.28% (95% CI, 0.24 − 0.32%) increase in risk of asthma hospitalization, respectively. Low-level PM2.5 and NO2 exposures were associated with higher risk. Furthermore, beneficiaries with only one asthma hospitalization during the study period or in communities with lower population density, higher average body mass index, longer distance to the nearest hospital, or greater neighborhood deprivation experienced higher risk.
Conclusions
Short-term air pollutant exposures increased risk of asthma hospitalization among Medicaid beneficiaries, even at concentrations well below national standards. The subgroup differences suggested individual and contextual factors contributed to asthma disparities under effects of air pollutant exposures.
Keywords: asthma, air pollutants, disadvantaged population, disparities
At a Glance Commentary
Scientific Knowledge on the Subject
Evidence on the effects of short-term exposures to air pollutants and asthma is lacking among the disadvantaged population.
What This Study Adds to the Field
We assessed the effects of short-term exposures to fine particulate matter, warm-season ozone, and nitrogen dioxide on the risk of asthma hospitalization among national Medicaid beneficiaries, the most disadvantaged population in the United States, and identified individual and contextual factors that contributed to asthma disparities under the effects of air pollutant exposures.
Asthma is a prevalent, noncommunicable respiratory disease characterized by airway obstruction and lung function decrement (1). In the United States, there are approximately 25 million patients diagnosed with asthma, imposing a substantial burden of healthcare utilization (2). Starting in the 1980s, the prevalence and incidence of asthma have increased in almost all age, sex, racial/ethnic, and socioeconomic groups (3). The prominence of environmental exposures among asthma risk factors has long suggested important roles for ambient air pollutants, particularly fine particulate matter (particulate matter with an aerodynamic diameter of ⩽2.5 μm [PM2.5]), ozone (O3), and nitrogen dioxide (NO2) (4). Evidence suggests that short-term exposures to such pollutants can trigger asthma attacks and worsen the symptoms, leading to rescue medication use, emergency department visit, hospitalization, or even death (5–11).
Under the Clean Air Act, the U.S. Environmental Protection Agency (EPA) is required to update the National Ambient Air Quality Standards (NAAQS) every 5 years for the protection of public health, particularly for vulnerable populations and communities (12). However, few studies have investigated the role of air pollutant exposures on asthma among populations in low socioeconomic positions, who have poor access and quality of healthcare and usually face greater health consequences (13). It is also unclear whether within the disadvantaged population certain individual and community characteristics may increase the susceptibility of asthma attack to the effect of air pollutants (14, 15). Furthermore, evidence for the effect of air pollutants on asthma at degrees of exposure below the NAAQS is lacking.
We analyzed 1,627,002 inpatient claims with asthma among Medicaid fee-for-service beneficiaries <65 years of age during the years 2000–2012 to assess the risk of asthma hospitalization and its disparities associated with short-term exposures to PM2.5, O3, and NO2, three major air pollutants regulated by the EPA. Medicaid is the single largest federal–state jointly funded insurance program that provided health coverage to an annual average of 47 million Americans during the study period, including low-income adults and children and individuals with disabilities (16); the characteristics and size of this cohort allow for investigating the susceptibility in the impact of air pollution within a socioeconomically disadvantaged population.
Methods
This study was approved by the institutional review board at Harvard T. H. Chan School of Public Health.
Inpatient Data
From the Center for Medicare and Medicaid Services, we obtained Medicaid fee-for-service inpatient claims among all the beneficiaries residing in the contiguous United States during 2000–2012. For each claim, we extracted 1) a unique identification code for every beneficiary; 2) inpatient admission date; 3) International Classification of Diseases, Ninth Revision (ICD-9) principal diagnosis code at discharge (17); 4) patient demographic characteristics including sex, race/ethnicity, and age; and 5) ZIP Code of residence. The ZIP Code of residence was used to spatially link each claim with exposures and covariates. We restricted the analysis to urgent and emergent hospital admissions for asthma, defined as having a principal diagnosis of International Classification of Diseases, Ninth Revision, code 493, and excluded scheduled admissions. To avoid potential selection bias, we also excluded admissions for patients aged ⩾65 years who enrolled in both Medicare and Medicaid because the Medicare was always the primary payer for those admissions, and therefore the Medicaid file may not contain complete records for all admissions for patients aged ⩾65 years (18). The Medicaid inpatient claims were not available for Maine during 2005–2010 nor for Kansas in 2010. In total, we analyzed 1,627,002 asthma hospitalizations.
Exposure Assessment
We implemented ensemble predictions of three machine learning models (random forest, gradient boosting, and neural network) to estimate the daily 24-hour average PM2.5, 8-hour maximum O3, and 1-hour maximum NO2 (in accordance with averaging times in NAAQS [19]) at the centroids of 1-km2 grid cells across the contiguous United States. As predictors we considered air monitoring data, satellite aerosol optical depth, meteorological conditions, chemical transport model simulations, and land-use variables. The ensemble models were calibrated using monitoring data, with 10-fold cross-validated r2 on held out monitors of 0.86 for PM2.5, 0.86 for O3, and 0.79 for NO2. More details were published elsewhere (20–22).
With these high-resolution predictions at 1-km2 grid cells, we estimated degrees of air pollution in each ZIP Code by averaging the predictions at grid cells whose centroids were inside the polygonal area for general ZIP Codes, or assigning the prediction at the nearest grid cell for other ZIP Codes that do not have polygon representations, for example, an apartment building, a military base, or a post office. More details are provided in Section 1 in the online supplement. The ZIP Code–level air pollution estimations were then linked to each hospitalization according to the ZIP Code of residence and admission date and were considered as proxy measurements of pollutant exposures.
For each pollutant, we examined the 7-day moving average exposure more than a week before each hospitalization (lag 0–6 d) and exposures at single lag days (from lag 0 to 6 d). For O3, following the previous literature (23, 24), we restricted the analysis to hospitalizations occurred in warmer months between April and September.
Meteorological Variables
Daily surface air temperature and specific humidity data were obtained from National Aeronautics and Space Administration’s Phase 2 of the North American Land Data Assimilation System with a 12-km2 spatial resolution (25), which were linked to each hospitalization according to the ZIP Code of residence and admission date.
Community-Level Variables
Annual averaged population density at ZIP Code Tabulation Areas (ZCTA) was linearly interpolated and extrapolated by year using U.S. Census 2000 and 2010 Summary Files (26–28). Annual averaged body mass index (BMI) and percentage of ever-smokers at counties were obtained from the Behavioral Risk Factor Surveillance System (29). The distance from the centroid of each ZCTA to the nearest hospital for each year was calculated using the Dartmouth Atlas of Health Care data (30), which were considered as a proxy for the average distance to the nearest hospital. These variables were linked to each hospitalization according to the ZIP Code of residence and the year of admission. To evaluate whether beneficiaries living in socioeconomically disadvantaged communities experienced disproportionately higher risk associated with air pollution exposures, we obtained the national area deprivation index (ADI) from the Neighborhood Atlas website, which was considered as a composite metric of neighborhood disadvantage level. The ADI incorporated ZCTA-level education, employment, housing quality, and poverty originally drawn from the U.S. Census and American Community Survey (31). Because only 2015 and 2019 ADI were available, the 2015 ADI was linked to each hospitalization according to the ZIP Code of residence (32).
Statistical Analysis
We used a time-stratified case-crossover design to estimate the percent change in the risk of asthma hospitalization associated with each 1-μg/m3 increase of PM2.5, 1-ppb increase of O3, and 1-ppb increase of NO2. The case-crossover design has been widely used in environmental epidemiology for studying health outcomes with abrupt onset, such as asthma and myocardial infarction (5, 8, 9, 23, 33).
We constructed a time-stratified case-crossover dataset as follows. A case day was defined as the date of admission. For each case day, we identified control days as days with the same day of the week (before and after the case day), month, and year as the case day. We matched each patient’s degree of exposure on the case day with that patient’s degrees of exposure on control days. This self-matching eliminated any potential confounding by individual variables that were unlikely to vary within a month, such as individual-level sex, race/ethnicity, age, socioeconomic status, smoking status, lipid concentrations, diet, education, and BMI, as well as community-level factors, such as population density, greenness, access to pharmacy and grocery store, proximity to highways, and so on; matching on day of the week eliminated potential confounding that varied within a week, such as weekday/weekend differences in amounts of air pollution and admission rate, with bidirectional selection for controls before and after the case to eliminate long-term time trends (34); and matching on month and year eliminated potential confounding by seasonal variation and long-term time trend, respectively (35). The resulting case-to-control ratio for the current study was 1.0:3.4.
For each pollutant, we used conditional logistic regressions to estimate associations between short-term exposures at the moving average of lag 0–6 days or at single lag days and the risk of hospitalization for asthma, adjusting for potential confounding of air temperature and specific humidity during lag 0–6 days (23, 24), as well as the exposure the day after admission (lead 1 d). The lead 1 exposure served as a negative exposure control, i.e., a proxy for potential time-varying confounders, such as other air pollutants, meteorological patterns, physical activity, etc., which may be correlated with the admission and the exposures before admission and, thus, confound the associations, but also likely to be correlated with exposure the day after the admission (lead 1 exposure) as well (36). Bonferroni correction was used to adjust for multiple comparisons for the three concurrent exposures. To capture the potential nonlinearity of the confounding effects, covariates were modeled using penalized cubic splines each with up to nine degrees of freedom (37). Computational details are provided in Section 2 in the online supplement.
To assess the risk of asthma hospitalization associated with lag 0–6 exposure at low degrees of exposure, for each pollutant, we restricted the analysis to days (both cases and controls) within the case-crossover dataset in which the lag 0–6 exposures were below increasingly stringent thresholds, including those well below the NAAQS (35 μg/m3 for PM2.5, 70 ppb for O3, and 100 ppb for NO2) (19).
To assess whether certain subpopulations among Medicaid beneficiaries faced higher risk, for each pollutant, we fitted separate regressions for each subgroup divided by age group (0–4, 5–12, 13–18, 19–34, or 35–64 yr), sex (female or male), race/ethnicity (White, Black, Hispanic/Latino, or other), or the total number of asthma hospitalizations of a patient during the study period (single or multiple). In addition, to assess differential effects of air pollutants between communities, for each pollutant, we fitted separate regressions for each subgroup divided by the upper or lower quartile of community-level population density, average BMI, percentage of ever-smokers, distance to the nearest hospital, or degree of neighborhood disadvantage (ADI). We used independent sample t tests to compare subgroup differences. Moreover, because major mechanisms of asthma progression and severity vary considerably across the life course (1), for each pollutant, we performed further separate analysis by age group for each subgroup of sex, race/ethnicity, the total number of asthma hospitalizations during the study period, and community-level characteristics.
Sensitivity Analyses
We assessed the robustness of the main results by fitting a three-pollutant model with three negative exposure controls and conducting sensitivity analyses with respect to the exposure time window for air temperature and specific humidity (at lag 0–1 or lag 0–4). We also conducted analysis for full-year O3 exposure at lag 0–6 days without restricting to warmer months. For the subgroup who had only one asthma hospitalization during the study period, we refitted the model after excluding beneficiaries with the hospitalization occurred in the first 3 years of the study period (i.e., washout period), who may have unstable asthma and hence have had hospitalizations before entering the study.
Results
We analyzed 1,627,002 asthma hospitalizations of 852,395 Medicaid fee-for-service beneficiaries <65 years of age residing in the contiguous United States during 2000–2012. The population was composed of more children aged ⩽18 years (62%), more females (53%), and mostly White (35%) and Black (35%) individuals, and 34% of the population had at least two asthma hospitalizations during the study period (Table 1). Section 3 of the online supplement shows the admission count for each state and the cumulative number of admissions in each year. Table 2 summarized descriptive statistics for PM2.5, O3, and NO2 concentrations and community-level characteristics. The average daily concentrations of PM2.5, warm-season O3, and NO2 were 10.4 μg/m3, 45.9 ppb, and 17.1 ppb, respectively. The daily concentrations were mostly below the NAAQS. Section 4 in the online supplement shows spatial and temporal patterns of the pollutant concentrations during the study period.
Table 1.
Demographic Characteristics of Medicaid Beneficiaries Admitted to Hospital for Asthma during 2000–2012
| Characteristics | n | % |
|---|---|---|
| Population | 852,395 | 100 |
| Admissions | 1,627,002 | |
| Age at first admission, yr | ||
| 0–4 | 314,042 | 37 |
| 5–12 | 173,786 | 20 |
| 13–18 | 42,673 | 5 |
| 19–34 | 75,117 | 9 |
| 35–64 | 246,777 | 29 |
| Sex | ||
| Female | 449,043 | 53 |
| Male | 403,148 | 47 |
| Unknown | 204 | 0 |
| Race/Ethnicity | ||
| White | 298,453 | 35 |
| Black | 298,886 | 35 |
| Hispanic/Latino | 160,891 | 19 |
| Other | 94,165 | 11 |
| Individuals with ⩾2 admissions | 293,884 | 34 |
Table 2.
Summary Statistics for Daily Concentrations of Fine Particulate Matter, Warm-Season Ozone, and Nitrogen Dioxide and Annual Community Characteristics across All ZIP Codes in the Contiguous United States, 2000–2012
| Mean ± SD | 5th Percentile | 25th Percentile | Median | 75th Percentile | 95th Percentile | |
|---|---|---|---|---|---|---|
| PM2.5, μg/m3 | 10.4 ± 6.7 | 2.8 | 5.8 | 9.0 | 13.4 | 22.8 |
| O3, ppb | 45.9 ± 12.2 | 26.1 | 37.6 | 45.8 | 53.8 | 66.3 |
| NO2, ppb | 17.1 ± 12.0 | 3.9 | 8.3 | 13.9 | 22.8 | 40.8 |
| Population density, persons/mile2 | 96.7 ± 456.5 | 0.1 | 1.1 | 4.7 | 50.2 | 319.2 |
| Average BMI, kg/m2 | 28.5 ± 3.0 | 26.0 | 27.0 | 27.8 | 28.9 | 33.6 |
| Percent of ever-smokers, % | 46.2 ± 7.2 | 36.5 | 41.7 | 45.0 | 50.4 | 58.8 |
| Distance to the nearest hospital, km | 13.9 ± 12.2 | 1.0 | 4.1 | 11.9 | 20.0 | 35.2 |
| Neighborhood disadvantage level, percentile rank* | 58.5 ± 25.1 | 11.0 | 39.0 | 61.1 | 78.0 | 92.8 |
Definition of abbreviations: BMI = body mass index; NO2 = nitrogen dioxide; O3 = ozone; PM2.5 = particulate matter with an aerodynamic diameter of ⩽2.5 μm.
The degree of neighborhood disadvantage was measured as national percentile rankings at ZIP code level from 1 to 100. A ranking of 100 indicated the highest degree of disadvantage.
We found statistically significantly positive associations between exposures to PM2.5, warm-season O3, and NO2 at lag 0–6 days and risk of asthma hospitalization: for each 1-μg/m3 increase in PM2.5, 1-ppb increase in warm-season O3, and 1-ppb increase in NO2, the percentage increase in risk of asthma admission was 0.31% (95% confidence interval [CI], 0.24–0.37%), 0.10% (95% CI, 0.05–0.15%), and 0.28% (95% CI, 0.24–0.32%), respectively. The single-lagged association remained positive over lag 0–6 days for PM2.5 and NO2 and was significantly positive during lag 1–3 days for warm-season O3 (Figure 1). At concentrations below the NAAQS, we found higher risk of admission associated with lag 0–6 exposures to PM2.5 and NO2. The association was mixed at low concentrations for warm-season O3 (Figure 2).
Figure 1.
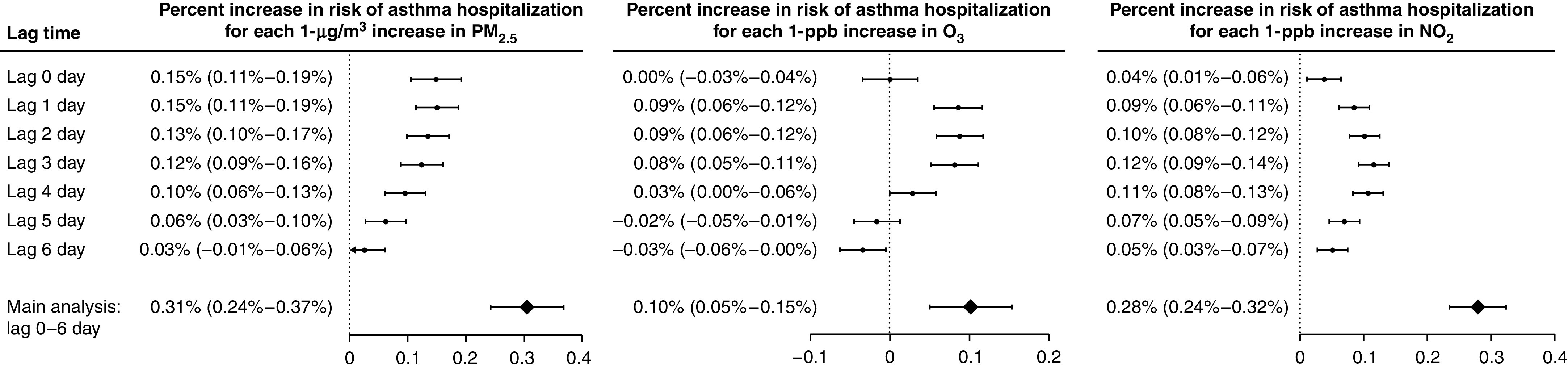
Percent increases (and Bonferroni-corrected 95% confidence intervals) in risk of asthma hospitalization associated with 1-μg/m3 increase in PM2.5, 1-ppb increase in warm-season ozone (O3), and 1-ppb increase in nitrogen dioxide (NO2) at single lag days and at the moving average of lag 0–6 days. PM2.5 = particulate matter with an aerodynamic diameter of ⩽2.5 μm.
Figure 2.
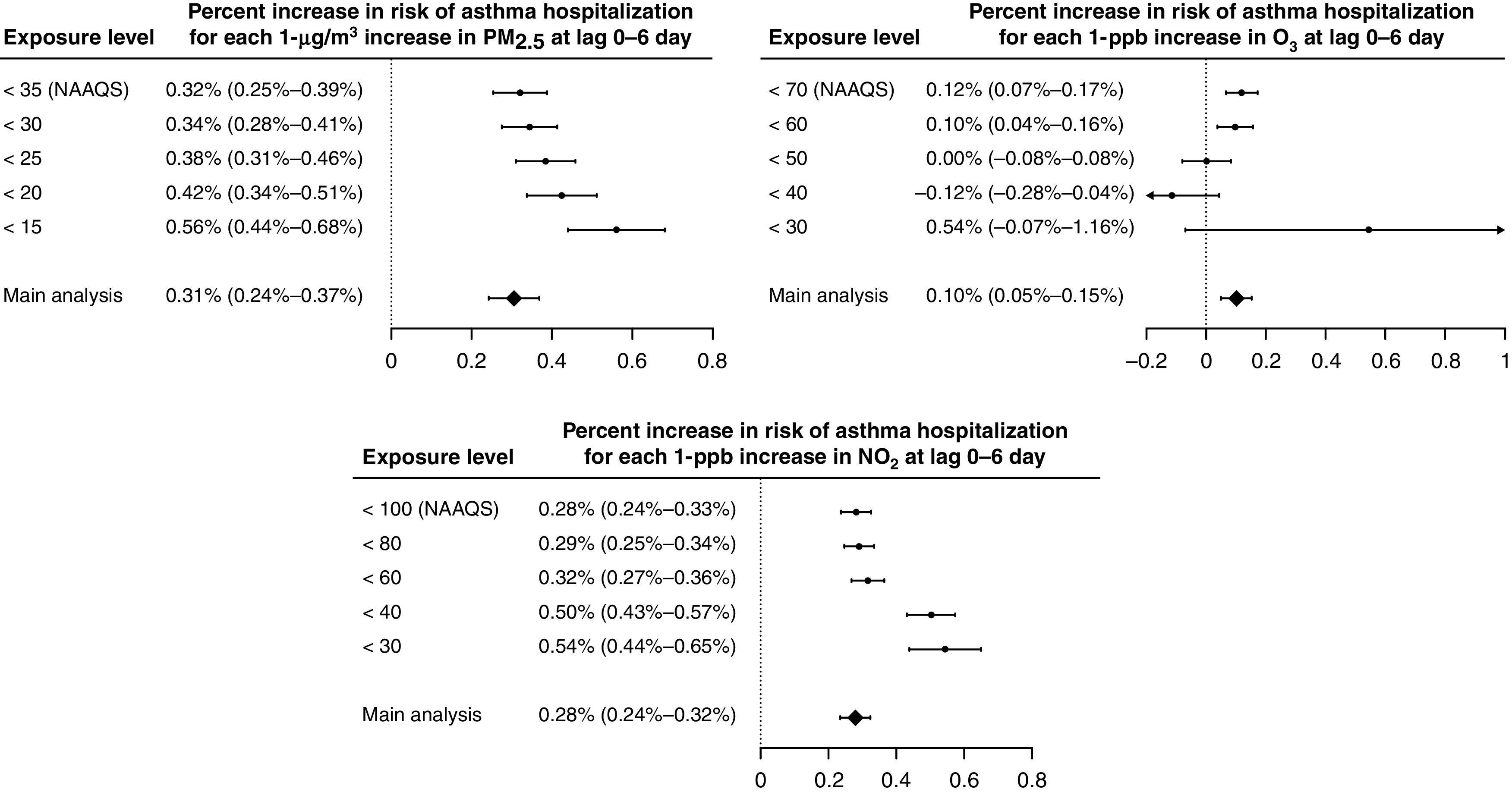
Percent increases (and Bonferroni-corrected 95% confidence intervals) in risk of asthma hospitalization associated with 1-μg/m3 increase in PM2.5, 1-ppb increase in warm-season ozone (O3), and 1-ppb increase in nitrogen dioxide (NO2) at the moving average of lag 0–6 days, when restricting the analysis to hospitalizations with lag 0–6 exposures below increasingly stringent thresholds, including those well below the National Ambient Air Quality Standards (NAAQS). PM2.5 = particulate matter with an aerodynamic diameter of ⩽2.5 μm.
Among subgroups of individual-level characteristics, we found consistently and significantly higher risk of asthma hospitalization for beneficiaries who had only one asthma admission during the study period for the three exposures at lag 0–6 days (Figure 3). For warm-season O3, the risks for the 0 − 4-year and 5 − 12-year age groups were not statistically significant. For NO2, the 0 − 4-year and 5 − 12-year age groups were at higher risk than the overall population. No consistent differences were found between subgroups of sex or race/ethnicity.
Figure 3.
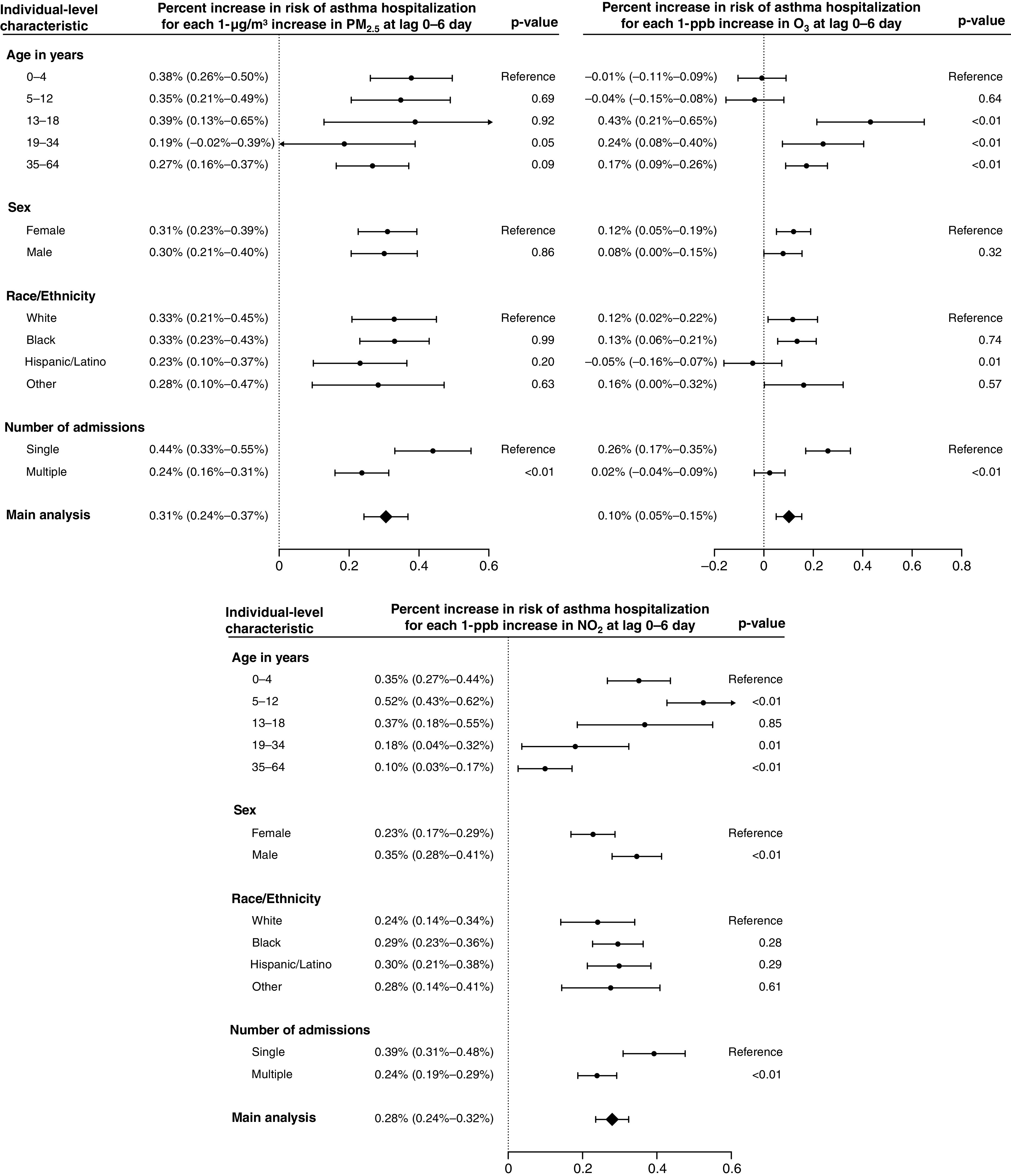
Percent increases (and Bonferroni-corrected 95% confidence intervals) in risk of asthma hospitalization associated with 1-μg/m3 increase in PM2.5, 1-ppb increase in warm-season ozone (O3), and 1-ppb increase in nitrogen dioxide (NO2) at the moving average of lag 0–6 days for each subgroup of individual-level characteristics. P values for the independent sample t tests were used to compare subgroup differences. Further separate analysis by age group was performed with results provided in Figure E5 in the online supplement. PM2.5 = particulate matter with an aerodynamic diameter of ⩽2.5 μm.
Among subgroups of community-level characteristics, we found consistently and significantly higher risk of asthma hospitalization for beneficiaries living in ZIP Codes with lower population density (⩽25th percentile), higher average BMI (⩾75th percentile), or longer distance to the nearest hospital (⩾75th percentile) for the three exposures at lag 0–6 days (Figure 4). We also found consistently higher risk for beneficiaries in more disadvantaged communities with higher ADI (⩾75th percentile), although the subgroup difference was statistically significant for PM2.5 and O3 and was marginally significant for NO2 (P = 0.06). Further separate analysis by age showed that within those communities that experienced higher risk from the exposures, the associations were consistently higher for all age groups than the overall population (Figure E6).
Figure 4.
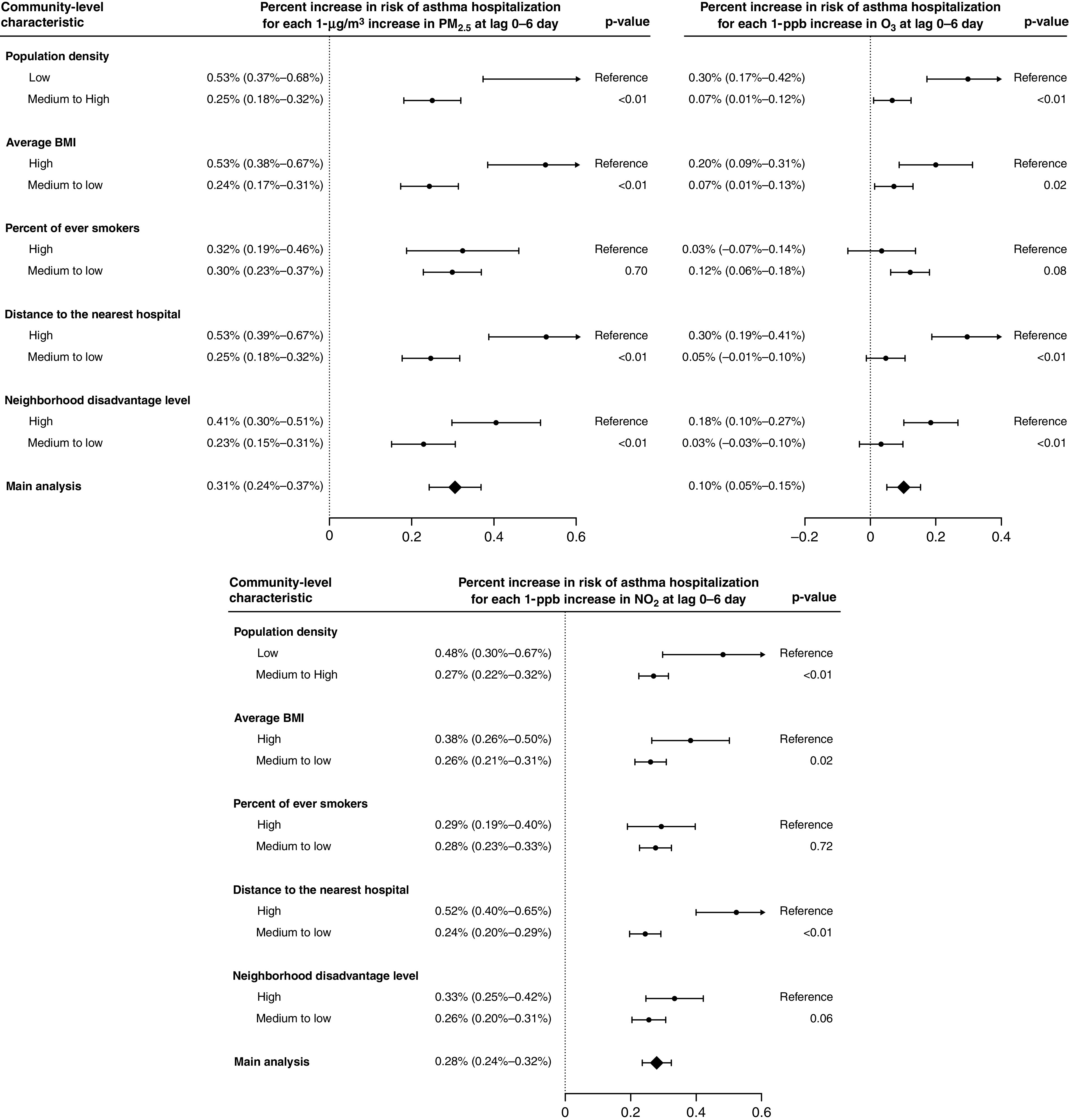
Percent increases (and Bonferroni-corrected 95% confidence intervals) in risk of asthma hospitalization associated with 1-μg/m3 increase in PM2.5, 1-ppb increase in warm-season ozone (O3), and 1-ppb increase in nitrogen dioxide (NO2) at the moving average of lag 0–6 days for each subgroup of community-level characteristics. “Low” represents subgroups within the bottom 25% of the characteristics, and “High” represents subgroups within the top 25% of the characteristics. P values for the independent sample t tests were used to compare subgroup differences. Further separate analysis by age group was performed with results provided in Figure E6. BMI = body mass index; PM2.5 = particulate matter with an aerodynamic diameter of ⩽2.5 μm.
In the three-pollutant model, the effect estimates for PM2.5 and NO2 attenuated but remained significant; for O3, the point estimate went beyond the null, and the direction of point estimate was reversed. The results remained robust after adjustment for lag 0–1 or lag 0–4 of air temperature and specific humidity (Figure E7). The effect estimate for full-year O3 attenuated to the null but remained statistically significant (0.07%; 95% CI, 0.03–0.11%). After excluding beneficiaries with asthma admissions in the first 3 years of the study period, the effect estimates for the three exposures remained consistently higher for those with only one admission during the study period than those with multiple admissions (Figure E8).
Discussion
In December 2020, the EPA decided to retain the current NAAQS for PM2.5 and O3 without revision (38, 39). In September 2021, conversely, the WHO sharply tightened its global air quality guidelines to concentrations that are well below the NAAQS. In accordance with the Clean Air Act, the EPA is responsible for improving the nation’s air quality and setting standards that provide public health protection for all, including at-risk groups (12). Our study linked PM2.5, warm-season O3, and NO2 with 1.6 million asthma hospitalizations of Medicaid fee-for-service beneficiaries. Using a time-stratified case-crossover design, we found that short-term exposures to PM2.5, warm-season O3, and NO2 were all associated with increased risk of asthma hospitalization, even at degrees of exposure well below current NAAQS. In particular, the effect size estimates were larger when PM2.5 was <25 μg/m3 and when NO2 was below 40 ppb than above the current NAAQS. The single-lagged associations suggest that the adverse effects remain a week after exposures, consistent with results from Rosenquist and colleagues (5) and O’Connor and colleagues (6). Overall, by focusing on one of the most socioeconomically disadvantaged populations in the United States, our findings indicate that improving air quality will not only better protect the most vulnerable population but also decrease healthcare use by preventing hospitalizations.
Our findings suggest that asthma susceptibility to the pollutants differed by severity. The consistently higher risk of asthma hospitalization associated with three exposures for beneficiaries with only one asthma admission during the study period than those with multiple admissions suggests that for people with severe asthma with frequent hospitalizations, outdoor air pollution played a less important role than other factors such as aeroallergens, environmental tobacco smoke, or nonadherence to controller medications, etc. (17). The findings of no consistent differences between subgroups of sex or race/ethnicity suggest that the burdens of air pollution were equally distributed across these subgroups within the socioeconomically disadvantaged population, consistent with findings by Liu and colleagues (8), Garcia and colleagues (10), and Nardone and colleagues (14).
At the community level, we found consistently higher risk of asthma hospitalization associated with the three exposures for ZIP Codes with lower population density, higher average BMI, longer distance to the nearest hospital, or greater neighborhood deprivation. These identified differences in susceptibility were consistent with results of Guarnieri and colleagues (4), Schikowski and colleagues (7), and Delfino and colleagues (11), suggesting certain contextual factors contributed to asthma disparities in the impact of air pollution. First, lower population density typically characterizes rural areas where air pollution sources (e.g., agriculture, industry, and natural processes), building characteristics, and activity patterns are different, resulting in inequitable burden from air pollutant exposures on acute exacerbation of asthma (40). Second, the higher risk for communities with higher BMI indicates that unhealthy diets and physical inactivity may increase the susceptibility to adverse effects of air pollution (4). As shown in literature (41), diets high in antioxidants such as fruits and vegetables are likely to prevent oxidative stress in pathways through which particulate matters and gases affect the severity of asthma. Third, longer distance to the nearest hospital indicates less access to healthcare services, which increases the risk of hospitalization. Indeed, timely and effective outpatient care of asthma is the key of preventing adverse outcomes and reducing the risk of hospitalization (1). Finally, greater neighborhood deprivation combined broad factors that may make the residents more susceptible to asthma attacks, such as the lack of pharmacy access, poor job opportunities, increased occupational hazards, poor housing quality, unhealthy lifestyle, etc. (42), the overall effects of which exacerbated inequality and vulnerability within the Medicaid. All these community-level factors are relatively independent and were weakly correlated with the exposures (see Section 5 in the online supplement), suggesting that they play different roles differentiating the susceptibility to air pollutants. The consistently higher risk for all age groups within each at-risk community suggest that these contextual factors modify the susceptibility throughout the life course.
In the three-pollutant model, including the three pollutant exposures and their negative exposure controls likely introduced overcontrol bias, which pulled the effect estimates toward the null and even reversed the direction of the O3 estimate (43). In a single-pollutant model, because the negative exposure control served as a proxy for all residual time-varying confounders, the two other pollutants had been indirectly adjusted for. Therefore, in the main analysis, we fitted single-pollutant models and selected the negative exposure control as appropriate variable for which to control to provide more reliable estimates (44). However, the PM2.5 and NO2 effects remained in the three-pollutant model with three negative controls.
Our study has several strengths. First, the analysis of more than 1.6 million asthma hospitalizations among the national Medicaid population allowed for an unprecedented degree of generalizability of the effects of major air pollutants within a vulnerable population. Second, subgroup analyses by individual- and community-level characteristics captured both individual and contextual factors that contributed to asthma disparities within this population in the impact of air pollutant exposures, providing better mechanistic understanding and evidence base for strategies targeting the specific subpopulations and communities. Third, the adjustment for negative exposure control reduced the bias owing to unmeasured confounding.
Our study showed that the effect of warm-season ozone on children aged 0–12 years was not statistically significant. The age-dependent associations of ozone exposure and asthma hospitalization have been observed in other studies, in which short-term ozone exposure has less significant or even protective effects in young children (45). One explanation is that in addition to introducing a proinflammatory response, ozone has antiviral effects, reducing or controlling respiratory viral infection, a major cause of asthma exacerbation in young children (46). Age could also influence the inhalation intake of air pollution and its effect on the respiratory tract (47), which was further complicated by the different physicochemical properties of the three pollutants (12). However, limited by available data sources and current understanding of ozone-induced respiratory pathology in childhood, this result should be interpreted cautiously. Clearly, further investigations into the potential underlying mechanisms are warranted.
This study also has limitations. First, we could not fully capture all disadvantaged Americans because Medicaid did not cover low-income single individuals without children and its eligibility varies by state. Second, although the analysis for the total population had high enough statistical power, some of the subgroup analysis may be underpowered, which may be the reason why we did not find an effect or a difference between subgroups. Third, the use of community-based measurements to gauge the characteristics was subject to measurement error. Further validity assessments by comparing with other surveys or using self-reported data would be valuable.
In sum, we found increased risk of asthma hospitalization associated with short-term exposures to PM2.5, warm-season O3, and NO2 among national Medicaid beneficiaries <65 years of age, even at amounts well below the national standards. In subgroup analyses, we found that asthma susceptibility to the effects of air pollutant exposures differed by severity and certain community-level characteristics, suggesting the importance of addressing both individual and contextual influences in protecting disadvantaged populations.
Footnotes
Supported by NIH grant R01 ES032418, the United States Environmental Protection Agency (EPA) grant RD-8358720, and NIH National Institute of Environmental Health Sciences grant ES-000002. This manuscript's contents are solely the responsibility of the grantee and do not necessarily represent the official views of the EPA. Furthermore, the EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Author Contributions: Y.W. contributed to study design, data preparation, data analysis, data interpretation, and drafting of the manuscript. X.Q. contributed to data preparation, data analysis, and drafting of the manuscript. M.B.S., M.D.Y., and K.Y. contributed to data preparation and data interpretation. L.L., A.A.P., C.W., P.K., A.Z., and F.D. contributed to formulation of the idea and review of the manuscript. J.D.S. contributed to formulation of the idea, study design, and review of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202107-1596OC on January 24, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet . 2018;391:783–800. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- 2.Most recent national asthma data. Atlanta, GA: Centers for Disease Control and Prevention; 2019. [updated 2021 Mar 31; accessed 2022 Mar 4]. Available from: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm [Google Scholar]
- 3.Asthma surveillance data Atlanta, GA: Centers for Disease Control and Prevention; 2019. [updated 2021 Sep 16; accessed 2022 Mar 4]. Available from: https://www.cdc.gov/asthma/default.htm. [Google Scholar]
- 4. Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet . 2014;383:1581–1592. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosenquist NA, Metcalf WJ, Ryu SY, Rutledge A, Coppes MJ, Grzymski JJ, et al. Acute associations between PM2.5 and ozone concentrations and asthma exacerbations among patients with and without allergic comorbidities. J Expo Sci Environ Epidemiol . 2020;30:795–804. doi: 10.1038/s41370-020-0213-7. [DOI] [PubMed] [Google Scholar]
- 6. O’Connor GT, Neas L, Vaughn B, Kattan M, Mitchell H, Crain EF, et al. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J Allergy Clin Immunol . 2008;121:1133–1139.e1. doi: 10.1016/j.jaci.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 7. Schikowski T, Schaffner E, Meier F, Phuleria HC, Vierkötter A, Schindler C, et al. Improved air quality and attenuated lung function decline: modification by obesity in the SAPALDIA cohort. Environ Health Perspect . 2013;121:1034–1039. doi: 10.1289/ehp.1206145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Y, Pan J, Zhang H, Shi C, Li G, Peng Z, et al. Short-term exposure to ambient air pollution and asthma mortality. Am J Respir Crit Care Med . 2019;200:24–32. doi: 10.1164/rccm.201810-1823OC. [DOI] [PubMed] [Google Scholar]
- 9. Orellano P, Quaranta N, Reynoso J, Balbi B, Vasquez J. Effect of outdoor air pollution on asthma exacerbations in children and adults: systematic review and multilevel meta-analysis. PloS One . 2017;12:e0174050. doi: 10.1371/journal.pone.0174050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia E, Berhane KT, Islam T, McConnell R, Urman R, Chen Z, et al. Association of changes in air quality with incident asthma in children in California, 1993-2014. JAMA . 2019;321:1906–1915. doi: 10.1001/jama.2019.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delfino RJ, Chang J, Wu J, Ren C, Tjoa T, Nickerson B, et al. Repeated hospital encounters for asthma in children and exposure to traffic-related air pollution near the home. Ann Allergy Asthma Immunol . 2009;102:138–144. doi: 10.1016/S1081-1206(10)60244-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.1990 Clean Air Act amendment summary: Title I. Provisions for attainment and maintenance of national ambient air quality standards Washington, D.C: United States Environmental Protection Agency; 1990. [updated 2021 Dec 8; accessed 2022 Mar 4]. Available from: https://www.epa.gov/clean-air-act-overview/1990-clean-air-act-amendment-summary-title-i. [Google Scholar]
- 13. McMaughan DJ, Oloruntoba O, Smith ML. Socioeconomic status and access to healthcare: interrelated drivers for healthy aging. Front Public Health . 2020;8:231. doi: 10.3389/fpubh.2020.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nardone A, Neophytou AM, Balmes J, Thakur N. Ambient air pollution and asthma-related outcomes in children of color of the USA: a scoping review of literature published between 2013 and 2017. Curr Allergy Asthma Rep . 2018;18:29. doi: 10.1007/s11882-018-0782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sullivan K, Thakur N. Structural and social determinants of health in asthma in developed economies: a scoping review of literature published between 2014 and 2019. Curr Allergy Asthma Rep . 2020;20:5. doi: 10.1007/s11882-020-0899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medicaid & CHIP enrollment data. Baltimore, MD: Centers for Medicare & Medicaid Services; [accessed 2022 Mar 4]. Available from: https://www.medicaid.gov/medicaid/national-medicaid-chip-program-information/medicaid-chip-enrollment-data/index.html. [Google Scholar]
- 17. Cloutier MM, Baptist AP, Blake KV, Brooks EG, Bryant-Stephens T, DiMango E, et al. Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC) 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol . 2020;146:1217–1270. doi: 10.1016/j.jaci.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gluckman TJ, Spinelli KJ, Wang M, Yazdani A, Grunkemeier G, Bradley SM, et al. Trends in diagnosis related groups for inpatient admissions and associated changes in payment from 2012 to 2016. JAMA Netw Open . 2020;3:e2028470. doi: 10.1001/jamanetworkopen.2020.28470. [DOI] [PubMed] [Google Scholar]
- 19.National ambient air quality standards table. Washington, D.C: United States Environmental Protection Agency; 2016. [updated 2021 Feb 10; accessed 2022 Mar 4]. Available from: https://www.epa.gov/criteria-air-pollutants/naaqs-table. [Google Scholar]
- 20. Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, et al. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int . 2019;130:104909. doi: 10.1016/j.envint.2019.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Requia WJ, Di Q, Silvern R, Kelly JT, Koutrakis P, Mickley LJ, et al. An ensemble learning approach for estimating high spatiotemporal resolution of ground-level ozone in the contiguous United States. Environ Sci Technol . 2020;54:11037–11047. doi: 10.1021/acs.est.0c01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, et al. Assessing NO2 concentration and model uncertainty with high spatiotemporal resolution across the contiguous United States using ensemble model averaging. Environ Sci Technol . 2020;54:1372–1384. doi: 10.1021/acs.est.9b03358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Q, Dai L, Wang Y, Zanobetti A, Choirat C, Schwartz JD, et al. Association of short-term exposure to air pollution with mortality in older adults. JAMA . 2017;318:2446–2456. doi: 10.1001/jama.2017.17923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lam HC, Li AM, Chan EY, Goggins WB., III The short-term association between asthma hospitalisations, ambient temperature, other meteorological factors and air pollutants in Hong Kong: a time-series study. Thorax . 2016;71:1097–1109. doi: 10.1136/thoraxjnl-2015-208054. [DOI] [PubMed] [Google Scholar]
- 25.NLDAS-2 model data description/information. Washington, D.C: National Aeronautics and Space Administration; [updated 2021 Nov 18; accessed 2022 Mar 4]. Available from: https://ldas.gsfc.nasa.gov/index.php/nldas/v2/models [Google Scholar]
- 26.U.S. Census Bureau 2000. Summary file 3 [accessed 2022 Mar 4]. Available from: https://www.census.gov/data/datasets/2000/dec/summary-file-3.html.
- 27.U.S. Census Bureau. 2010. Census demographic profile summary file [accessed 2022 Mar 4]. Available from: https://www.census.gov/data/datasets/2010/dec/demographic-profile-with-geos.html.
- 28.National Research Council Using the American Community Survey: benefits and challenges. Washington, D.C: The National Academies Press; 2007. [Google Scholar]
- 29.Behavioral Risk Factor Surveillance System: BRFSS 2013 Survey Data and Documentation. Atlanta, GA: Centers for Disease Control and Prevention; 2013. [updated 2013 Jul 23; accessed 2022 Mar 4]. Available from: https://www.cdc.gov/brfss/annual_data/annual_2013.html [Google Scholar]
- 30. Dartmouth Atlas of Health Care: study of nation's health system uncovers large disparities. Ga Hosp Today . 1996;40:1–4. [PubMed] [Google Scholar]
- 31. Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible: the Neighborhood Atlas. N Engl J Med . 2018;378:2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neighborhood Atlas. Madison, WI: University of Wisconsin School of Medicine and Public Health; 2015. [accessed 2022 Mar 4]. Available from: https://www.neighborhoodatlas.medicine.wisc.edu/. [Google Scholar]
- 33. Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol . 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 34. Navidi W. Bidirectional case-crossover designs for exposures with time trends. Biometrics . 1998;54:596–605. [PubMed] [Google Scholar]
- 35. Levy D, Lumley T, Sheppard L, Kaufman J, Checkoway H. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology . 2001;12:186–192. doi: 10.1097/00001648-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 36. Sanderson E, Macdonald-Wallis C, Davey Smith G. Negative control exposure studies in the presence of measurement error: implications for attempted effect estimate calibration. Int J Epidemiol . 2018;47:587–596. doi: 10.1093/ije/dyx213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood SN. Generalized additive models: an introduction with R. Boca Raton, FL: CRC Press; [Google Scholar]
- 38.EPA. 40 CFR Part 50, review of the national ambient air quality standards for particulate matter, final decision. 2020. [accessed 2022 Mar 4]. Available from: https://www.epa.gov/sites/default/files/2020-12/documents/frl-10018-11_12_4_2020_admin.pdf.
- 39.EPA. Ozone national ambient air quality standards, final decision. 2020. [accessed 2022 Mar 4]. Available from: https://www.epa.gov/ground-level-ozone-pollution/final-decision-retain-ozone-national-ambient-air-quality-standards.
- 40.Cooper N, Green D, Knibbs LD.Inequalities in exposure to the air pollutants PM 2.5 and NO 2 in Australia Environ Res Lett 201914115005. [Google Scholar]
- 41. Guilleminault L, Williams EJ, Scott HA, Berthon BS, Jensen M, Wood LG. Diet and asthma: is it time to adapt our message? Nutrients . 2017;9:1227. doi: 10.3390/nu9111227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goode JV, Owen J, Page A, Gatewood S. Community-based pharmacy practice innovation and the role of the community-based pharmacist practitioner in the United States. Pharmacy (Basel) . 2019;7:106. doi: 10.3390/pharmacy7030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grätz M. When less conditioning provides better estimates: overcontrol and collider bias in research on intergenerational mobility. Presented at the Spring Meeting of the Research Committee 28 of the International Sociological Association. March 21–23, 2019, Frankfurt, Germany. [Google Scholar]
- 44. VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol . 2019;34:211–219. doi: 10.1007/s10654-019-00494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dai Y, Qiu H, Sun S, Yang Y, Lin H, Tian L. Age-dependent effect of ambient ozone on emergency asthma hospitalizations in Hong Kong. J Allergy Clin Immunol . 2018;141:1532–1534.e5. doi: 10.1016/j.jaci.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 46. Wolcott JA, Zee YC, Osebold JW. Exposure to ozone reduces influenza disease severity and alters distribution of influenza viral antigens in murine lungs. Appl Environ Microbiol . 1982;44:723–731. doi: 10.1128/aem.44.3.723-731.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Strosnider HM, Chang HH, Darrow LA, Liu Y, Vaidyanathan A, Strickland MJ. Age-specific associations of ozone and fine particulate matter with respiratory emergency department visits in the United States. Am J Respir Crit Care Med . 2019;199:882–890. doi: 10.1164/rccm.201806-1147OC. [DOI] [PubMed] [Google Scholar]


