Abstract
Background:
Prior meta-analyses report a 2–4-fold increased risk of later cardiovascular disease among women with a history of hypertensive disorders of pregnancy (HDP). Given HDP’s vascular underpinnings, it is hypothesized to also be a risk factor for later dementia. We aim to summarize the evidence for the impact of HDP on dementia, and consider unique associations between HDP and dementia subtypes.
Methods:
Observational studies on the relationship between HDP and dementia were identified from online electronic databases, to July 1, 2021 (PROSPERO identifier:CRD42020185630). We included observational studies published in English. Exposure among women was any HDP and HDP subtypes: gestational hypertension, preeclampsia/eclampsia, or other/unspecified HDP. Outcome was any dementia and dementia subtypes: Alzheimer’s disease, vascular dementia, or other/unspecified dementias.
RESULTS:
For our primary analyses, we included 5 cohort studies with a total of 183,874 women with and 2,309,705 women without HDP. Pooled analysis found a 38% higher risk of all-cause dementia among women with, versus without, any type of HDP (aHR, 1.38; 95% CI: 1.18 to 1.61; P<0.01). When examining association by HDP and dementia subtypes, we found that women with, versus without, any type of HDP had over a three-fold higher risk of vascular dementia (aHR: 3.14; 95% CI: 2.32 to 4.24; P <0.01).
CONCLUSIONS:
Our findings indicate that maternal history of HDP is an important risk factor for later development of vascular and all-cause dementia. Further research among more racially/ethnically diverse populations quantifying HDP’s effect on all-cause dementia, and specifically vascular dementia, is warranted.
Keywords: hypertension, pregnancy-induced, pre-eclampsia, eclampsia, dementia, vascular, Alzheimer disease, cognitive dysfunction, chronic disease, epidemiology, pregnancy, mediation analysis
INTRODUCTION
Hypertensive Disorders of Pregnancy (HDP), including preeclampsia, eclampsia, HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome, gestational hypertension, and chronic hypertension represent a spectrum of pregnancy complications that have no known cure other than delivery.1–5 HDP affects up to 8% of all pregnancies,6 15% of parous women,7 and is a leading cause of maternal and perinatal morbidity and mortality.8 An exaggerated inflammatory response leading to endothelial dysfunction is a known pathophysiologic finding in clinically apparent HDP.9 This underlying pathology suggests the possibility that women with HDP may be predisposed to other conditions characterized by chronic inflammation.10–14 Research over the last decade has demonstrated that women who develop HDP are at increased risk for long-term cardiovascular and metabolic disorders. Consequently, the American Heart Association and American College of Obstetricians and Gynecologists now include HDP as a risk factor for future cardiovascular disease.12, 15
Recently, researchers have suggested that women with a history of HDP may also have increased risk for later-life adverse neurologic conditions with a hypertensive vascular etiology similar to cardiovascular disease, such as vascular dementia16; or a shared etiology with Alzheimer’s disease via proteinopathy.17 The relationship between underlying chronic hypertension and future risk of brain injury and dementia has been well established,18, 19 even in younger adults ≤40 years.20 What is not well established is whether a poor underlying vascular risk profile equally increases risk for both HDP and dementia or whether an HDP pregnancy itself produces neurological damage that then predisposes a woman to an augmented dementia risk. The “chicken or the egg” conundrum as reported in relation to cardiovascular disease (does cardiovascular disease risk cause HDP or does HDP harm a previously healthy woman causing later cardiovascular disease, or both)21 is also applicable to investigations assessing HDP and dementia. To date, the “chicken or the egg” conundrum with respect to dementia 22 has not been resolved.
The hypothesized adverse relationship between hypertensive pregnancies and later cognitive functioning has also been demonstrated in human studies assessing memory and speed processing. In a study of over 1200 women who participated in the Family Blood Pressure Project Genetic Epidemiology Network of Arteriopathy study and completed a pregnancy history questionnaire (2000–2004), women with histories of hypertensive pregnancies had worse processing speeds and smaller brain volumes compared to those with normotensive pregnancies.23 In a pilot case-control study, authors found that after accounting for age, parity, and education, women with previous HDP had significantly lower auditory-verbal memory skills compared to those without previous HDP.24 And in a recent prospective cohort study among 596 women participating in the Generation R Study, a history of HDP (driven predominately by gestational hypertension) was associated with poorer working memory and verbal learning 15 years after adjusting for ethnicity, education level, and pre-pregnancy BMI.25
Prior epidemiologic research evaluating risk for dementia among women with, and without, HDP has produced inconsistent findings with some studies showing an inverse 26 or null association 27–29 while others demonstrating a positive association.30–32 Inconsistent findings may be attributed to multiple factors including varying study design, sample size, case definitions for HDP (such as whether HDP includes women with chronic hypertension) and dementia, and whether specific phenotypes were assessed, and confounding factor adjustments.
OBJECTIVE
Our objective was to perform a systematic review and meta-analysis to determine an evidence-based consensus as to whether a history of ≥1 HDP pregnancy increases a woman’s risk for later all-cause dementia, and whether associations between specific HDP subtypes (preeclampsia/eclampsia, gestational hypertension, or other/unspecified HDP) and dementia subtypes (Alzheimer’s disease, vascular dementia, or other/unspecified dementia) may vary.
METHODS
Search strategy
This systematic review was registered and accepted for inclusion in PROSPERO, the International Prospective Register of Systematic Reviews (ID: CRD42020185630). The systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. MEDLINE® and Embase® were searched for studies investigating dementia outcomes of women with and without HDP published between 2000 and 2021. Our study only used existing, de-identified data reported in published manuscripts, and thus exempt from IRB approval.
Study selection
Studies were included if they: 1) were original research with full-length article available; 2) were published in English; 3) provided enough information to calculate risk estimates (odds ratio, risk ratio or hazard ratio); and 4) were cohort (retrospective or prospective) or case-control studies. Studies were excluded if they were conference abstracts, review articles, case series, qualitative studies, or editorials. In studies using overlapping subjects,27,32 we chose the most recent study with the larger sample.32 Secondary screening involved review of full texts to assess for eligibility and potential duplication of patients. For completeness after secondary screening was performed two authors (H.M. and K.S.) independently reviewed and selected the articles in compliance with the inclusion/exclusion criteria in a three-step fashion 1) title and abstract screening; 2) review of full texts of all selected studies, and 3) a manual check of the references of studies included. At each stage, disagreement was resolved by consensus or arbitration.
Exposure and Outcomes
The exposure was HDP and HDP subtypes: gestational hypertension, preeclampsia/eclampsia, or other (i.e., HELLP)/unspecified HDP .2, 3 We included 1) studies that clearly excluded, or took measures to exclude, women with chronic hypertension at baseline;30, 31 2) studies that reported or adjusted for pre-pregnancy hypertension;27, 32 and 3) studies that did not address chronic hypertension at baseline (the two studies that relied on participant recall of hypertensive pregnancies).26, 28 Outcomes were all-cause dementia and dementia subtypes: Alzheimer’s disease, vascular dementia, or other/unspecified dementia (e.g., frontotemporal dementia, Lewy body dementia). We included studies that only assessed all-cause dementia,28, 29 only Alzheimer’s disease,26, 30 or both all-cause dementia and dementia subtypes.27, 31, 32 Studies only assessing cognitive impairment were excluded. Included studies were not explicitly clear how they handled mixed dementia pathology, but it is assumed that both Alzheimer’s disease and vascular dementia could be the sole diagnosis or potentially comorbid with another dementia subtype. Database search terms included a combination of relevant keywords. Using Boolean operators, the search aimed to identify papers that discussed both HDP and dementia. The search terms used in combination were 1) ‘preeclampsia’, ‘pre-eclampsia’, ‘hypertensive disorders of pregnancy’, ‘HELLP’, and ‘gestational hypertension’; and 2) ‘Alzheimers’, ‘dementia’, ‘vascular dementia’.
Data extraction
Data from included studies were extracted into a standardized form detailing the first author, year of publication, country, study period, sample source (population- or hospital-based), study design, exposures and outcomes assessed, sample size of exposed/non-exposed, adjustments or matches made, and reported risk estimate (95% CI). For studies only reporting two-way contingency tables, risk estimates and 95% CI were calculated and converted to HRs.33 For cohort studies, total follow-up time was extracted. Two reviewers (H.M and K.S.) independently extracted and evaluated the data for each included article. Disagreements were resolved through discussion.
Assessment of risk of bias
Two reviewers (H.M. and K.S.) independently assessed study quality using the Newcastle Ottawa Scale (NOS).34 Different assessments were applied to case control versus cohort studies. Cohort studies were evaluated by 1) representativeness of exposed cohort; 2) selection of non-exposed cohort; 3) ascertainment of exposure; 4) demonstration that outcome of interest was not present at start of study; 5) comparability of exposed and unexposed cohorts on the basis of the design or analysis; 6) assessment of outcome; 7) whether follow-up was long enough for outcomes to occur; and 8) adequacy of follow up of cohorts. Case control studies were evaluated by 1) adequacy of case definition; 2) representativeness of the cases; 3) selection of controls (i.e,. community, hospital, no description); 4) definition of controls (i.e. description of history of disease [endpoint]); 5) comparability of cases and controls on the basis of the design or analysis; 6) exposure assessment; 7) ascertainment methodology(ies) for cases and controls; and, 8) non-response rate. As per NOS protocol,34 each item can be awarded a maximum of one star except comparability which was allowed a maximum of two stars (number 5 for both cohort and case control). Total score for each study was obtained by summing up stars for each item with 1–3 stars indicating high risk, 4–6 stars medium risk, and 7–9 stars low risk of bias. Disagreements were resolved through discussion. Primary analyses included studies with low to medium risk of bias. A sensitivity analyses was conducted including all studies, regardless of bias risk score.
Data synthesis and analysis
We used R versions 4.0.1 and Rstudio 1.2.1335 35 to conduct both fixed-effect and random-effects models with the generic inverse-variance weight method to generate pooled adjusted hazard ratios (HR). Random-effects meta-analysis was done to account for significant heterogeneity between studies and avoid a situation in which our pooled estimate is disproportionately influenced by only one study.36 We also conducted a “leave one out” analysis to examine the influence of individual studies on the overall HRs by repeating the meta-analysis after the exclusion of each included study.
Between-study heterogeneity was measured via Higgin’s & Thompson’s I2 and τ2, and assessed by Q test. Restricted maximum-likelihood was used in estimating τ2. The publication bias was evaluated by both funnel plots and Egger test. A 2-tailed was used as the threshold for statistical significance. Given that there were multiple exposures and outcomes in the majority of studies, we implemented separate models to examine the association between HDP and all-cause dementia and dementia subtypes including Alzheimer’s disease, vascular dementia, and other/unspecified dementias. We also evaluated whether all-cause dementia was associated with HDP subtypes including gestational hypertension or preeclampsia/eclampsia. Limited counts preventing us from evaluating all dementia subtypes by HDP subtypes.
For our main analyses, we used effect estimates that considered baseline sociodemographic and reproductive history confounding factors including maternal age, education, marital status, race/ethnicity, and parity. The majority of studies assessed time-fixed confounders (e.g., maternal age at index pregnancy), but one study allowed the HDP exposure to vary over time and treated parity, maternal age, and residence (surrogate of socioeconomic status) as time varying.31 The two studies relying on participant recall of pregnancy complications adjusted for a minimal set of covariates: age and BMI at time of recall interview for one;26 and age, BMI, smoking and education at the time of the recall interview for the other.,28 Via variable adjustment, three studies additionally considered potential mediating factors such as mid-life cardiovascular disease, stroke, chronic kidney disease, diabetes, and hypertension.27, 31, 32 While traditional adjustment for intermediary factors may induce bias,37, 38 to determine the influence of the mediating factor adjustment, we compared pooled hazard ratios for these three studies adjusting for baseline factors versus adjusting for both baseline confounders and mediating factors.
RESULTS
Study selection and characteristics
Out of 51 initial search results, 6 were eligible for inclusion in our quantitative synthesis/meta-analysis (Figure 1).26, 28–32 Of the 6 included studies, 1 was a case-control study and the other 5 were either retrospective or ambispective cohort studies. The included studies were done in the following countries: Denmark, Sweden, the Netherlands, and the United States. Further details of the included studies such as study design, demographics, sample size, and specific exposure and outcome diagnosis are shown in Table 1.
Figure 1:
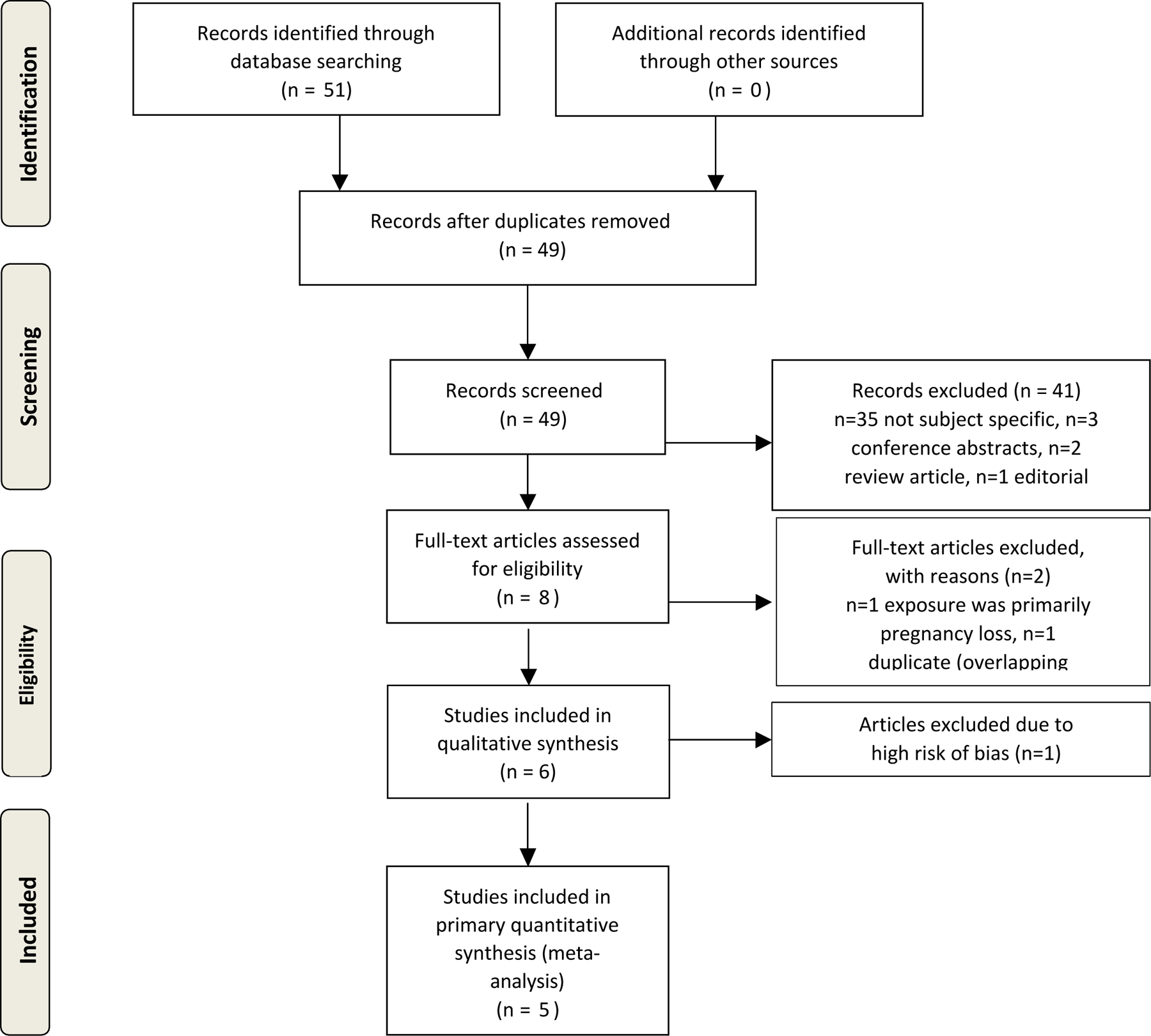
Flow diagram of the study selection process
Table 1:
Characteristics of studies included in the systematic review
| Study | Country | Age at baseline/Follow-up Time1 | Study Period | Sample source | Study design | Type of HDP exposure | Type of Dementia outcome | Sample size of exposed or cases | Sample size of non-exposed or controls | Confounder control |
|---|---|---|---|---|---|---|---|---|---|---|
| Abheiden et al., 2015 | Netherlands | Maternal age, not reported, mean follow up age 64–67 years (± 10 years | Not reported | Hospital-based | Case-control | GH, PE, E (SR) | AD (EA) | 104 (AD) | 129 (no AD) | Age and BMI at time of survey |
| Basit et al., 2018 | Denmark | 95.4% <45 years at start of follow-up/mean follow-up 21.1 years (IQR: 11.3–23.4 years) | Exposure: 1978–2015 Outcome: 1994–2017 | Population-based | Retrospective cohort | Time-varying PE, E, HELLP (MVR) | AD, vascular dementia, and other/unspecified dementia (MVR) | 50,068 HDP | 1,127,937 no HDP | Maternal birth year, and time dependent parity, region of most recent delivery, and mid-life cardiovascular disease, stroke, chronic kidney disease, hypertension, and diabetes |
| Theilen et al., 2016 | United States | Maternal age 26.0 ± 5.9 years/total follow-up of 73 years (mean not reported) | Exposure and Outcome 1939–2012 | Population-based | Retrospective cohort | GH, PE, HELLP, E (MVR) | Death from AD (MVR) | 60,580 HDP | 123,140 no HDP | Neonatal sex, gestational age at delivery, maternal and paternal education, maternal race–ethnicity, and maternal marital status at baseline |
| Nelander et al.,2015 | Sweden | Maternal age not reported, age at end of follow-up was 82 years ± 6 years. | Exposure <1958 Outcome 1998–2010 | Population-based | Ambispective cohort | GH, PE (SR) | All-cause dementia (MVR) | 419 HDP | 2646 no HDP | Maternal age, education, smoking, and BMI at time of interview |
| Fields et al., 2017 | United States | Mean maternal age 24 years (IQR 22, 260/mean follow up age 59 (IQR 56–63 years) | Exposure 1976–1982 Outcome 2016–2017 | Population-based | Retrospective cohort | PE (MVR) | MCI/Dementia (EA) | 40 HDP | 40 no HDP | Maternal age and parity at baseline. |
| Andolf et al., 2020 | Sweden | Not reported | Exposure 1973–1993 Outcome Up to 2013 | Population-based | Retrospective cohort | GH, PE (MVR) | AD, VaD, Other Dementia (MVR) | 20,096 GH 52,671 PE | 1,055,942 no HDP | Maternal education, parity, ethnicity, & neonatal sex (baseline) and cardiovascular disease (follow-up) |
AD: Alzheimers disease; BMI: body mass index; E: Eclampsia HDP: Hypertensive disorder of pregnancy; GH: Gestational hypertension; HELLP: haemolysis, elevated liver enzymes, and low platelets; MVR: medical or vital records; PE: Preeclampsia; SR: self-report; EA: expert assessed; VaD: Vascular Dementia
Note mean/median age at baseline and mean/median age at follow-up or follow-up years is approximate, as most studies reported by HDP status, not overall.
Risk of bias of included studies
As per the Newcastle-Ottawa Quality Assessment Scale, the one case control study had a high risk of bias26 whereas the remaining five cohort studies had a low risk of bias.28–32 (Table 2). The case control study26 had thoroughly vetted cases, via cerebral MRI scans reviewed by experienced an neuroradiologist, but had several weaknesses including poor selection of controls (partners of male Alzheimer’s disease patients who did not receive the same screening as cases) and ascertainment of exposure (relying on recall of HDP exposure among dementia cases and non-dementia controls). The 5 cohort studies ascertained HDP exposure from secure medical records or structured interviews.28–32 Additionally, the 5 cohort studies selected the non-exposed cohort from the same community as the exposed cohort, and adjusted for ≥1 factor to improve comparability of the exposed and non-exposed cohort. The majority of studies used medical or birth registries to identify HDP and/or dementia;26, 28–32 however 2 studies relied on self-report of HDP,26, 28 while another 2 studies relied on expert assessment for dementia assessment.26, 29
Table 2.
Study quality assessment overview (1 case-control study; 5 cohort studies)
| Study Case-control | Is the case definition adequate | Representa-tiveness of the cases | Selection of Controls | Definition of Controls | Comparability | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-Response rate | Total |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Abheiden et al., 2015 | * | - | - | * | - | - | - | * | 3 |
|
| |||||||||
| Study Cohort | Representative-ness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | Total |
|
| |||||||||
| Basit et al., 2018 | * | * | * | * | * | * | - | * | 7 |
| Theilen et al., 2016 | * | * | * | * | ** | - | - | * | 7 |
| Nelander et al.,2015 | - | * | * | * | * | * | * | * | 7 |
| Fields et al., 2017 | * | * | * | * | * | * | * | * | 8 |
| Andolf et al., 2020 | * | * | * | * | * | * | * | * | 8 |
Our primary analysis included data from the five low-bias-risk cohort studies (183,874 women with HDP and 2,309,705 without). We conducted a sensitivity analysis that included data from the five low-bias-risk cohort studies in addition to the one high-bias-risk case control study (183,978 with and 2,493,812 without HDP).
Egger’s regression test of intercept did not indicate the presence of funnel plot asymmetry, suggesting no evidence of substantial publication bias (primary analysis: (intercept, 1.092; 95% CI, −0.57 to −2.75; P=0.29) sensitivity analysis: intercept, −0.063; 95% CI, −2.89 to −2.76; P=0.97) (Figure S1 and S2).
Pooled analyses of HDP and dementia
For our primary analysis of the 5 cohort studies, women with, versus without, HDP had a 38% increased risk of all-cause dementia in the random effects model (adjusted hazard ratio [aHR], 1.38; 95% confidence interval [95% CI]: 1.18 to 1.61) after accounting for socio-demographic and reproductive history confounding factors (Figure 2). There was modest between-study heterogeneity (, p=0.21). In our “leave-one-out” sensitivity analysis, we found that removing the study Andolff 2020 reduced study heterogeneity to 21% and resulted in a near 50% increase of all-cause dementia in women with HDP (aHR:1.49, 95% CI: 1.26 to 1.77) (Figure S3).
Figure 2:
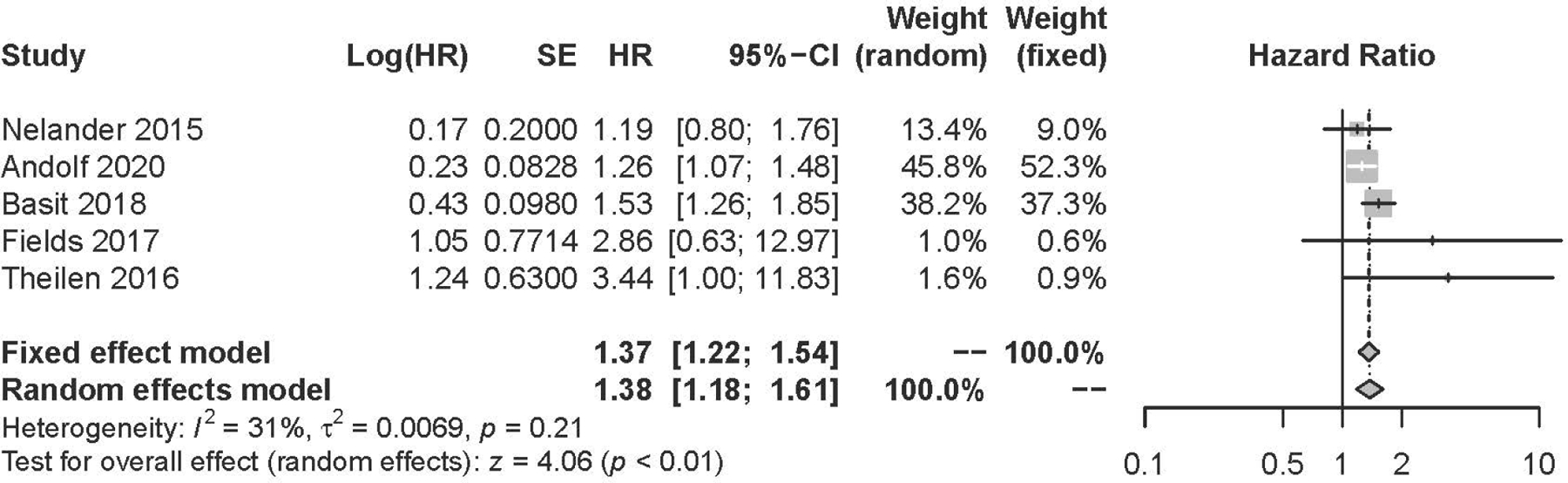
Pooled Hazard Ratio (Forest Plot) for the Risk of All-Cause Dementia with All-Cause Hypertensive Disorders of Pregnancy
Subgroup analysis
Dementia subtype:
For our primary analysis of the 5 cohort studies, women with HDP versus without HDP had a 40% increased risk of Alzheimer’s disease in the random effects model (adjusted hazard ratio [aHR], 1.40; 95% confidence interval [95% CI]: 1.14 to 1.74) (Figure 3). Between-study heterogeneity was low (, p=0.31). Pooled analyses demonstrated a three-fold higher risk of vascular dementia among women with versus without exposure to HDP (aHR: 3.14; 95% CI: 2.32 to 4.24, ) (Figure 3). There was a modest association between HDP and other dementia from the two eligible studies with appropriate other dementia outcomes (aHR, 1.22; 95% CI, 0.98 to 1.53; ) (Figure 3).
Figure 3:
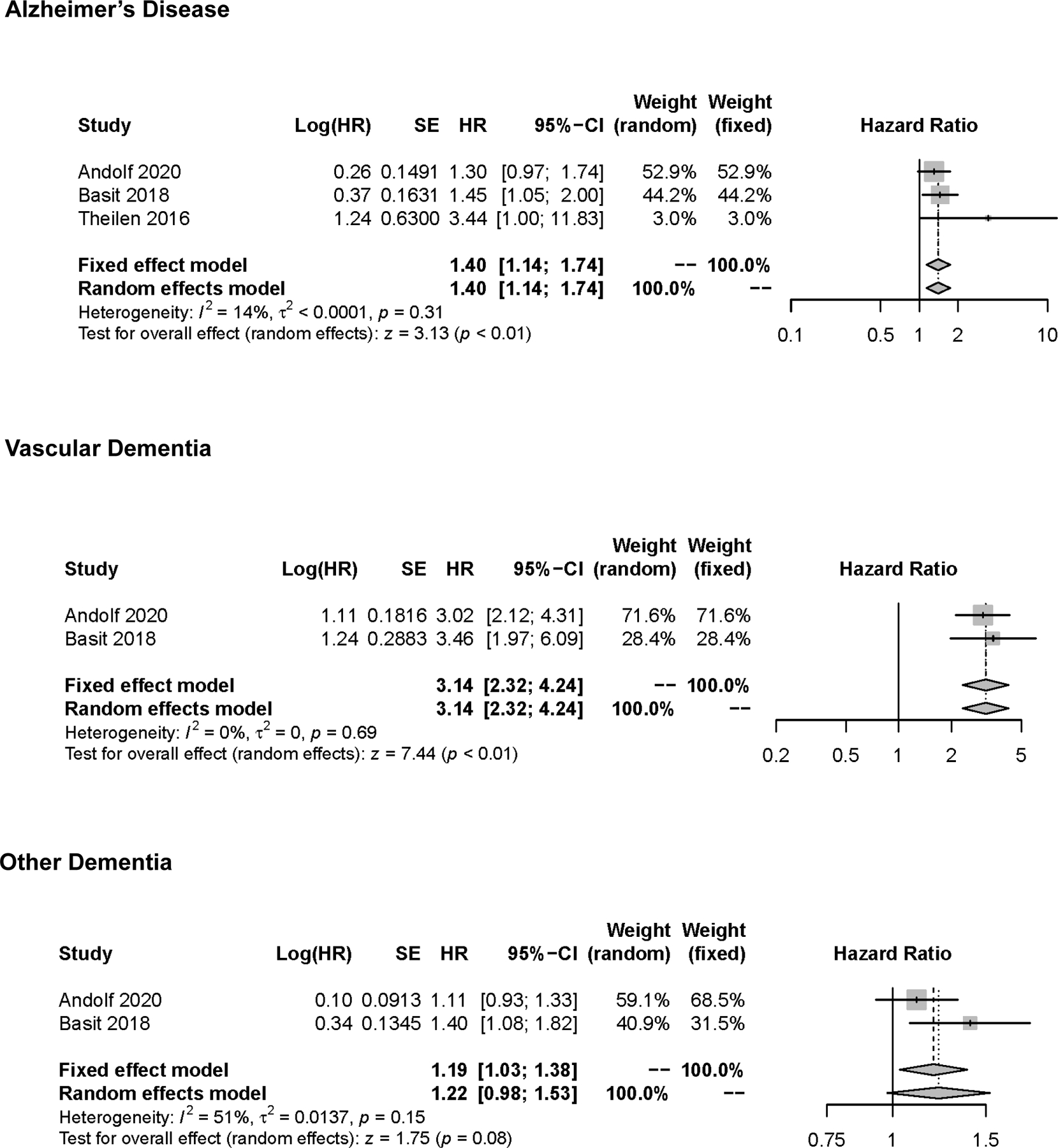
Pooled Hazard Ratio (Forest Plot) for the Risk of Alzheimer’s Disease, Vascular Dementia, and Other/Unspecified Dementia with Hypertensive Disorders of Pregnancy
HDP subtype:
Modeling separately according to different HDP subtypes revealed little difference in estimates between preeclampsia/eclampsia (aHR, 1.47; 95% CI, 0.99 to 2.20; , p<0.01) or gestational hypertension (aHR, 1.25; 95% CI: 1.08 to 1.45; , p=0.79) and all-cause dementia (Figure 4).
Figure 4:
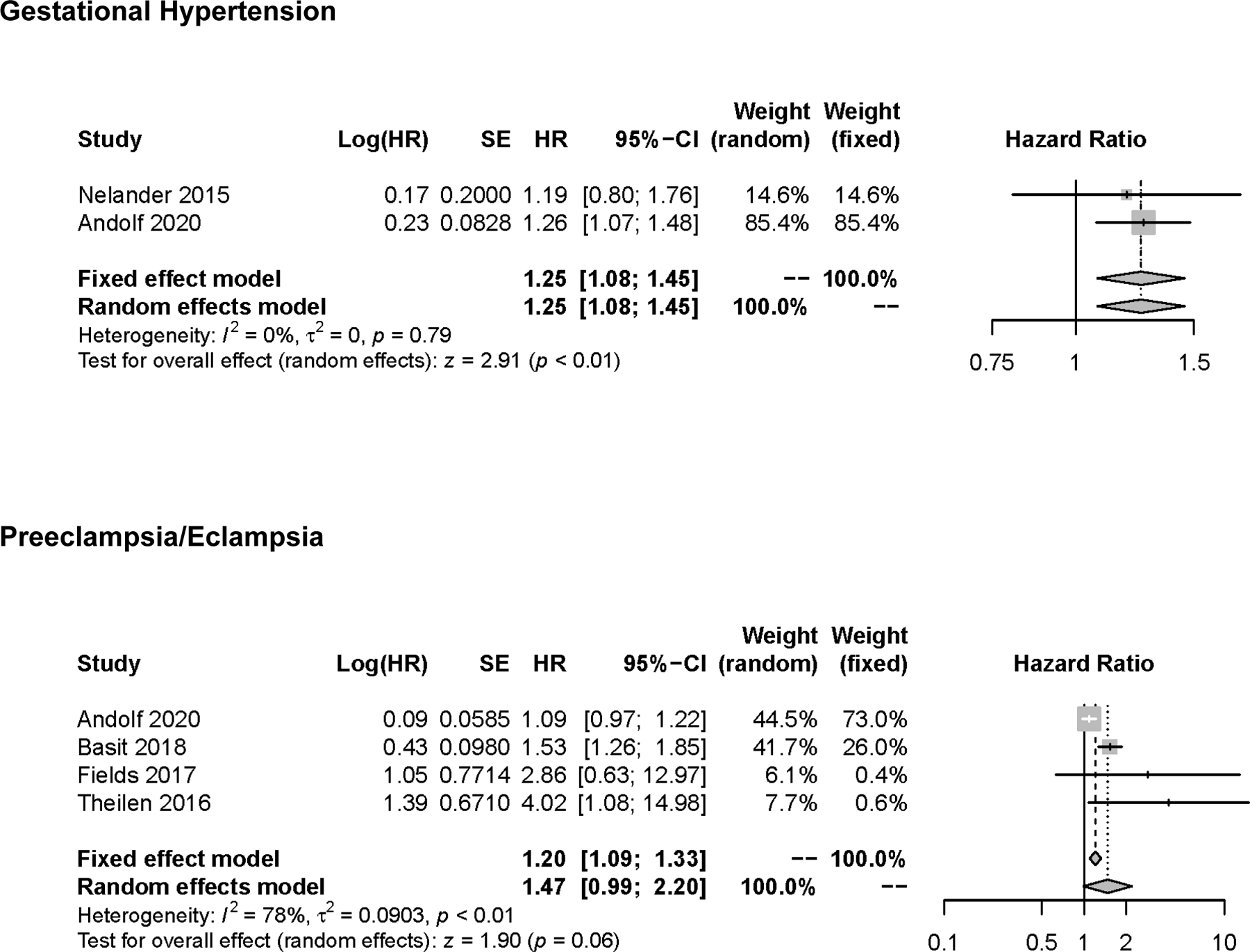
Pooled Hazard Ratio (Forest Plot) for the Risk of All-Cause Dementia with Gestational Hypertension or Preeclampsia/Eclampsia.
Sensitivity analysis including one case control study
Inclusion of the one high-bias-risk case control study that examined HDP and Alzheimer’s disease resulted in an attenuation of the estimate for all-cause dementia (aHR, 1.26; 95% CI: 0.84 to 1.90; , p<0.01) and Alzheimer’s disease subtype (aHR, 1.22; 95% CI: 0.65 to 2.26; , p<0.01)with a notable increase in study heterogeneity (Figures S4–S7).
Baseline confounder and mediator adjusted models
Among the studies that adjusted for both baseline and cardio-metabolic mediating factors,31, 32 the pooled analysis showed a hazard ratio of 1.16 (95% CI: 1.02, 1.32; ) for all-cause dementia, 1.30 (95% CI: 1.05, 1.62; ) for Alzheimer’s disease, and 2.17 (95% CI: 1.60, 2.93; ) for vascular dementia. A null association was found for other dementia (aHR: 1.02, 95% CI: 0.88, 1.18, ).
COMMENT
Principal findings
In this systematic review and meta-analysis of 6 studies of nearly 2.5 million women, we found that while there was a modest 20–40% increased risk of Alzheimer’s disease or other/unspecified dementia among women with a history of HDP, the risk increased to over 200% for vascular dementia. This increased association was found after adjusting for important sociodemographic confounding factors including maternal age at delivery of index pregnancy, parity, education, race/ethnicity, and marital status. Among the two studies 31, 32 that took into account baseline chronic hypertension and further adjusted for intermediary cardio-metabolic disorders, results remained robust for all-cause dementia, Alzheimer’s disease, and vascular dementia. Given the known association between a woman’s history of HDP and cerebral white matter lesions,16 future prospective studies using formal mediation analyses versus simple adjustment for intermediary factors are warranted to help determine direct and indirect effects of HDP on later dementia risk.39
While prior meta-analyses have shown HDP to be associated with adverse cardiovascular events,40, 41 especially within the first ten years after a preeclamptic pregnancy,42 we are unique in this first systematic review and meta-analysis of HDP and dementia to quantify the 3-fold increased risk of vascular dementia 20–40 years or more after the preeclamptic or other HDP pregnancy after accounting for important baseline confounders.
Comparison with Existing Literature and Potential Mechanisms:
Our findings of a 3-fold increased risk of vascular dementia among women with a history of HDP is in line with what prior systematic reviews and meta-analyses have reported for HDP and cardiovascular disease.40–42 Specifically, women with a history of preeclampsia have been found to have a 4-fold increase risk of incident heart failure and a 2-fold increased risk in coronary heart disease, stroke and death after accounting (either through adjustment or exclusion) for sociodemographic factors and baseline cardio-metabolic health including history of diabetes mellitus or gestational diabetes mellitus and smoking. 42 Given the strong evidence for the effect of HDP on cardiovascular disease mortality, selection bias due to differential enrollment22 is a concern for those studies for which baseline enrollment occurred decades after exposure.26, 28 The majority of studies included in this review were retrospective cohort studies29–32 of administrative health databases beginning follow-up at first pregnancy, thereby limiting selection bias due to differential enrollment. Additionally, while selection bias due to differential survival of included women may be problematic for some of the studies that did not address it, competing risk analyses were appropriately conducted 22 for the two largest included studies, further mitigating this potential bias.
The association between HDP and cardiovascular disease is not surprising given shared risk factors including obesity and unfavorable levels of total cholesterol, low-density lipoprotein, and triglycerides.13, 43 Also not surprising is the link between HDP and vascular dementia, given findings from several longitudinal epidemiologic studies showing increased risk of dementia with these same cardiovascular disease risk factors.10, 44–48
Whether these underlying shared risk factors of HDP and cardiovascular disease and vascular dementia largely explain the associations found, or whether HDP is an independent risk factor due to endothelial changes, which directly harms the mother’s circulatory system during pregnancy, making her vulnerable to vascular disease later in life, is unknown. However, it is likely a combination of these two scenarios.21
Strengths and Limitations
The strength of our study is the relatively large sample size with nearly 2.5 million women included, all completed within the past six years. Additionally, given time lag between pregnancies and subsequent dementia, the majority of the studies were relatively well-conducted ambi/retrospective cohort studies,29–32 using data from large population-based medical/vital record registries and accounting for multiple baseline confounding factors.
Nevertheless, our study had its limitations. First, the small number of studies available resulted in modest heterogeneity, potentially due to variation in study period and geography - this may have impacted exposure and outcome assessments. Improvements over the last decade in capturing previously undetected dementia within administrative healthcare records.49 Additionally, Scandinavian compared to US health care systems may better capture EHR dementia diagnoses given universal access to care. Studies may have differed in how HDP exposure was defined, especially given the time range for capture, which dated back to 1939. While the two largest studies to date report efforts to validate their HDP diagnoses with inpatient records30 and/or self-report,31 we still cannot rule out potential misclassification bias in these or the other studies.
Second, several of the studies lacked follow-up of older women, including the largest study among 1.2 million at-risk women for which only 10% of women were over 64 years of age,31 and thus the outcome is biased towards early-onset dementia, which is patho-physiologically distinct from late-onset dementia49
Finally, generalizability of our study is restricted largely to non-Hispanic, white women from Scandinavia or the United States. Of the two US studies,29,30 only one study took into account maternal race/ethnicity (n=21,781 [12%] Hispanic and n=1189 [1%] black women) in their adjusted models.30 Future research among underrepresented minorities, especially among black women, who are disproportionally affected by HDP and also experience higher rates of dementia, 50–52 is critically needed for improved generalizability.
Conclusions and implications
Over 183,000 women with HDP were included in this meta-analysis of 5 cohort studies. We found that HDP was associated with a 1.2 to 1.4-fold increased risk of Alzheimer’s disease and other dementia and a 3-fold increased risk of future vascular dementia. Our findings are in line with current recommendations for patient education and regular monitoring of cardiovascular risk factors in women with a history of HDP. However, limitations of existing studies, including inability to exclude women with chronic hypertension in the majority of the studies, prevent us from knowing whether there is a direct causal pathway between HDP and dementia, notably vascular dementia, or whether an inherent, underlying poor cardiovascular risk profile is the true culprit.
Of the five studies included in our primary meta-analysis model, the majority controlled for key sociodemographic factors such as maternal age, parity, race/ethnicity, education, and marital status, with one additionally accounting for pre-pregnancy BMI and smoking status. Future epidemiologic research should focus on controlling for additional confounders known to impact risk of HDP and dementia including maternal age, subfertility and prior pregnancy complications, BMI, hypertension (both before and after pregnancy), hypercholesterolemia, diabetes mellitus, family health history, lifestyle factors such as smoking, heavy alcohol use, and physical inactivity; as well as psychological factors including depression and anxiety. More studies like Basit et al,31 that treat history of HDP, as well as related confounders, as time dependent variables, such that women can contribute person time in more than one exposure category are needed to better understand the relationship between HDP and future dementia risk. Additionally, while several studies adjusted for mid-life cardiovascular disease and diabetes, a formal mediation analysis39 quantifying the degree to which these mid-life factors, in addition to mid-life depression and anxiety,48 factor into the relationship between HDP and dementia has yet to be done. Until we better understand the causal pathway between underlying risk profiles, pregnancy complications, mid-life health, and subsequent dementia, optimal targeted care for prevention will remain elusive, even if this is of high importance to women worldwide. Finally, given evidence for the association between other related pregnancy complications (including gestational diabetes, preterm birth, and fetal growth restriction) and future cardiovascular disease, further research on how these complications, individually or in combination with HDP impact future dementia risk, is warranted.
Supplementary Material
Funding:
This work was supported in part by the National Institute of Aging (NIA) grant: “Hypertensive Disorders of Pregnancy and Subsequent Risk of Vascular Dementia, Alzheimer’s Disease, or Related Dementia: A Retrospective Cohort Study Taking into Account Mid-Life Mediating Factors” (Project K01AG058781; PI: Karen Schliep).
Footnotes
PROSPERO Registration: ID: CRD42020185630 registered on 05/07/2020.
Conference Presentation: A portion of this paper was presented at 34th Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research (SPER). June 21–22nd, 2021.
Condensation: In this systematic review and meta-analysis, we found hypertensive disorders of pregnancy to be associated with a 3-fold increased hazard of vascular dementia.
Disclosures: None
Data availability:
Data extracted from included studies, data used for all analysis, and analytic code available upon request from Corresponding Author.
REFERENCES
- 1.Buhimschi IA, Nayeri UA, Zhao G, Shook LL, Pensalfini A, Funai EF, Bernstein IM, Glabe CG, Buhimschi CS. Protein misfolding, congophilia, oligomerization, and defective amyloid processing in preeclampsia. Sci Transl Med 2014;6: 245ra92. [DOI] [PubMed]
- 2.Mammaro A, Carrara S, Cavaliere A, Ermito S, Dinatale A, Pappalardo EM, Militello M, Pedata R. Hypertensive disorders of pregnancy. J Prenat Med 2009;3:1–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol 2013;25:124–132. [DOI] [PubMed] [Google Scholar]
- 4.Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol 2020. Jun;135(6):e237–e260. [DOI] [PubMed] [Google Scholar]
- 5.Braunthal S, Brateanu A. Hypertension in pregnancy: Pathophysiology and treatment. SAGE Open Med 2019;7:2050312119843700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duley L The global impact of pre-eclampsia and eclampsia. Semin Perinatol 2009;33:130–137. [DOI] [PubMed] [Google Scholar]
- 7.Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, Lawlor DA. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation 2012;125:1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011;123:2856–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granger JP, Alexander BT, Bennett WA, Khalil RA. Pathophysiology of pregnancy-induced hypertension. Am J Hypertens 2001;14:178S–185S. [DOI] [PubMed] [Google Scholar]
- 10.Beeri MS, Ravona-Springer R, Silverman JM, Haroutunian V. The effects of cardiovascular risk factors on cognitive compromise. Dialogues Clin Neurosci 2009;11:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aukes AM, Wessel I, Dubois AM, Aarnoudse JG, Zeeman GG. Self-reported cognitive functioning in formerly eclamptic women. Am J Obstet Gynecol 2007;197:365 e1–6. [DOI] [PubMed] [Google Scholar]
- 12.ACOG. “American College of Obstetricians and Gynecologists. Hypertension in Pregnancy” (2013).
- 13.Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol 2009;114:961–970. [DOI] [PubMed] [Google Scholar]
- 14.Louis J, Saade G. Pregnancy as a Window to Future Health. Introduction. Semin Perinatol 2015;39:253. [DOI] [PubMed] [Google Scholar]
- 15.Parikh NI, Gonzalez JM, Anderson CAM, Judd SE, Rexrode KM, Hlatky MA, Gunderson EP, Stuart JJ, Vaidya D; American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and the Stroke Council. Adverse Pregnancy Outcomes and Cardiovascular Disease Risk: Unique Opportunities for Cardiovascular Disease Prevention in Women: A Scientific Statement From the American Heart Association. Circulation 2021;143:e902–e916. [DOI] [PubMed] [Google Scholar]
- 16.Wiegman MJ, Zeeman GG, Aukes AM, et al. Regional distribution of cerebral white matter lesions years after preeclampsia and eclampsia. Obstet Gynecol 2014;123:790–5. [DOI] [PubMed] [Google Scholar]
- 17.Cheng S, Banerjee S, Daiello LA, Nakashima A, Jash S, Huang Z, Drake JD, Ernerudh J, Berg G, Padbury J, Saito S, Ott BR, Sharma S. Novel blood test for early biomarkers of preeclampsia and Alzheimer’s disease. Sci Rep 2021;11:15934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouch L, Cestac P, Sallerin B, Piccoli M, Benattar-Zibi L, Bertin P, Berrut G, Corruble E, Derumeaux G, Falissard B, Forette F, Pasquier F, Pinget M, Ourabah R, Danchin N, Hanon O, JS Vidal; S.AGES investigators. Visit-to-Visit Blood Pressure Variability Is Associated With Cognitive Decline and Incident Dementia: The S.AGES Cohort. Hypertension 2020;76:1280–1288. [DOI] [PubMed] [Google Scholar]
- 19.Iadecola C Hypertension and dementia. Hypertension 2014;64:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, Carmichael O, Wolf PA, DeCarli C. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol 2012;11:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith GCS. Long-Term Mortality After Hypertensive Disease of Pregnancy. Obstet Gynecol 2016;128:231–33. [DOI] [PubMed] [Google Scholar]
- 22.Weuve J, Proust-Lima C, Power MC, Gross AL, Hofer SM, Thiébaut R, Chêne G, Glymour MM, Dufouil C; MELODEM Initiative. Guidelines for reporting methodological challenges and evaluating potential bias in dementia research. Alzheimers Dement 2015;11:1098–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mielke MM, Milic NM, Weissgerber TL, White WM, Kantarci K, Mosley TH, Windham BG, Simpson BN, Turner ST, Garovic VD. Impaired Cognition and Brain Atrophy Decades After Hypertensive Pregnancy Disorders. Circ Cardiovasc Qual Outcomes 2016;9:S70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brusse I, Duvekot J, Jongerling J, Steegers E, De Koning I. Impaired maternal cognitive functioning after pregnancies complicated by severe pre-eclampsia: a pilot case-control study. Acta Obstet Gynecol Scand 2008;87:408–412. [DOI] [PubMed] [Google Scholar]
- 25.Adank MC, Hussainali RF, Oosterveer LC, Ikram MA, Steegers EAP, Miller EC, Schalekamp-Timmermans S. Hypertensive Disorders of Pregnancy and Cognitive Impairment: A Prospective Cohort Study. Neurology 2022;96:e709–e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abheiden CN, van Doornik R, Aukes AM, van der Flier WM, Scheltens P, de Groot CJ. Hypertensive Disorders of Pregnancy Appear Not to Be Associated with Alzheimer’s Disease Later in Life. Dement Geriatr Cogn Dis Extra 2015;5:375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andolf EG, Sydsjo GC, Bladh MK, Berg G, Sharma S. Hypertensive disorders in pregnancy and later dementia: a Swedish National Register Study. Acta Obstet Gynecol Scand 2017;96:464–71. [DOI] [PubMed] [Google Scholar]
- 28.Nelander M, Cnattingius S, Akerud H, Wikstrom J, Pedersen NL, Wikstrom AK. Pregnancy hypertensive disease and risk of dementia and cardiovascular disease in women aged 65 years or older: a cohort study. BMJ Open 2016;6:e009880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fields JA, Garovic VD, Mielke MM, Kantarci K, Jayachandran M, White WM, Butts AM, Graff-Radford J, Lahr BD, Bailey KR, Miller VM. Preeclampsia and cognitive impairment later in life. Am J Obstet Gynecol 2017;217:74.e1–74.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theilen LH, Fraser A, Hollingshaus MS, Schliep KC, Varner MW, Smith KR, Esplin MS. All-Cause and Cause-Specific Mortality After Hypertensive Disease of Pregnancy. Obstet Gynecol 2016;128:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basit S, Wohlfahrt J, Boyd HA. Pre-eclampsia and risk of dementia later in life: nationwide cohort study. BMJ 2018;363:k4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andolf E, Bladh M, Moller L, Sydsjo G. Prior placental bed disorders and later dementia: a retrospective Swedish register-based cohort study. BJOG 2020;127:1090–1099. [DOI] [PubMed] [Google Scholar]
- 33.Shor E, Roelfs D, Vang ZM. The “Hispanic mortality paradox” revisited: Meta-analysis and meta-regression of life-course differentials in Latin American and Caribbean immigrants’ mortality. Soc Sci Med 2017;186:20–33. [DOI] [PubMed] [Google Scholar]
- 34.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, & Tugwell P (2000). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses
- 35.Harrer M, Cuijpers P, Furukawa T, Ebert D. Doing meta-analysis in R: a hands-on guide. Chapman and Hall/CRC, 2021.
- 36.Hammer A, Rositch A, Qeadan F, Gravitt PE, Blaakaer J. Age‐specific prevalence of HPV 16/18 genotypes in cervical cancer: a systematic review and meta‐analysis. Int J Cancer 2016;138:2795–2803. [DOI] [PubMed] [Google Scholar]
- 37.Lu H, Cole SR, Platt RW, Schisterman EF. Revisiting Overadjustment Bias. Epidemiology 2021;32:e22–e23. [DOI] [PubMed] [Google Scholar]
- 38.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009;20:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.VanderWeele TJ. Explanation in causal inference : methods for mediation and interaction New York: Oxford University Press. [Google Scholar]
- 40.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J 2008;156:918–930. [DOI] [PubMed] [Google Scholar]
- 41.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol 2013;28:1–19. [DOI] [PubMed] [Google Scholar]
- 42.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, Mamas MA. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes 2017;10:e003497. [DOI] [PubMed] [Google Scholar]
- 43.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 44.Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging 2000;21:49–55. [DOI] [PubMed] [Google Scholar]
- 45.Ninomiya T, Ohara T, Hirakawa Y, Yoshida D, Doi Y, Hata J, Kanba S, Iwaki T, Kiyohara Y. Midlife and late-life blood pressure and dementia in Japanese elderly: the Hisayama study. Hypertension 2011;58:22–8. [DOI] [PubMed] [Google Scholar]
- 46.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011;77:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord 2009;28:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396:413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mendez MF. Early-Onset Alzheimer Disease. Neurol Clin 2017;35:263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farina MP, Hayward MD, Kim JK, Crimmins EM. Racial and Educational Disparities in Dementia and Dementia-Free Life Expectancy. J Gerontol B Psychol Sci Soc Sci 2020;75:e105–e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holdt Somer SJ, Sinkey RG, Bryant AS. Epidemiology of racial/ethnic disparities in severe maternal morbidity and mortality. Semin Perinatol 2017;41:258–65. [DOI] [PubMed] [Google Scholar]
- 52.Johnson JD, Louis JM. Does race or ethnicity play a role in the origin, pathophysiology, and outcomes of preeclampsia? An expert review of the literature. Am J Obstet Gynecol 2022;226:S876–S885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data extracted from included studies, data used for all analysis, and analytic code available upon request from Corresponding Author.


