Abstract
Cholera toxin (CT) and the type II heat-labile enterotoxins (HLT) LT-IIa and LT-IIb act as potent systemic and mucosal adjuvants and induce distinct T-helper (Th)-cell cytokine profiles. In the present study, CT and the type II HLT were found to differentially affect cytokine production by anti-CD3-stimulated human peripheral blood mononuclear cells (PBMC), and the cellular mechanisms responsible were investigated. CT suppressed interleukin-2 (IL-2), tumor necrosis factor alpha (TNF-α), and IL-12 production by PBMC cultures more than either LT-IIa or LT-IIb. CT but not LT-IIa or LT-IIb reduced the expression of CD4+ T-cell surface activation markers (CD25 and CD69) and subsequent proliferative responses of anti-CD3-stimulated T cells. CT but not LT-IIa or LT-IIb significantly reduced the expression of CD40 ligand (CD40L) on CD4+ T cells. In a coculture system, CT-treated CD4+ T cells induced significantly less TNF-α and IL-12 p70 production by both autologous monocytes and monocyte-derived dendritic cells than either LT-IIa- or LT-IIb-treated CD4+ T cells. These findings demonstrate that CT, LT-IIa, and LT-IIb differentially affect CD40-CD40L interactions between antigen-presenting cells and T cells and help explain the distinct cytokine profiles observed with type I and type II HLT when used as mucosal adjuvants.
The prototypical type I heat-labile enterotoxin (HLT) cholera toxin (CT) and the type II HLT from Escherichia coli (LT-IIa and LT-IIb) are AB5 toxins that consist of an ADP-ribosylating A subunit noncovalently associated with a pentameric B subunit (12, 13, 30). Despite their inherent toxicity, the HLT have become important adjuvants for enhancing both mucosal and systemic immune responses. Previous studies addressing the adjuvant properties of HLT have demonstrated their ability to abrogate oral tolerance, as well as enhance both local and systemic antibody (Ab) responses to coadministered antigen (Ag) (6, 9, 17). Additionally, after mucosal administration, both type I and type II HLT have been shown to enhance T-helper (Th) cytokine production from both systemic and mucosal lymphoid compartments; however, there appear to be marked differences in both the Th1 and Th2 cytokine profiles induced by these enterotoxins (21, 34). Several studies have shown that CT induces a predominant Th2 response with increased production of interleukin-4 (IL-4), IL-5, and IL-10 and subsequent elevated levels of antigen-specific immunoglobulin G-1 (IgG1) Ab (21, 34, 37, 39). With the aid of IL-4−/− knockout mice, it was further demonstrated that the adjuvanticity of CT is highly dependent upon Th2-associated cytokines (20). Compared to CT, the type II HLT LT-IIa and LT-IIb have been shown to induce a more balanced Ag-specific Th1 and Th2 cytokine profile and IgG subclass response (21). However, the mechanism responsible for these observed differences remains to be elucidated.
An important factor during the initial phase of an immune response that determines whether Th cells will develop into Th1 or Th2 effector cells depends upon the presence of IL-12 and IL-4, respectively. CD40 ligand (CD40L), or CD154, is a type II transmembrane protein that is transiently expressed on CD4+ T cells and recognizes CD40 on B cells, monocytes/macrophages, and dendritic cells (1). CD40-CD40L interactions have been demonstrated to be important for the induction of IL-12 from antigen-presenting cells (APC) (11, 29). IL-12, which consists of a p40 and p35 chain linked via a disulfide bond, promotes the differentiation of naive CD4+ T cells into Th1 effector cells while suppressing the development of Th2-type responses (15, 19, 27). Consistent with these findings, CD40L−/− mice have been shown to have defective Th1 responses while concomitantly exhibiting elevated IL-4 production compared to wild-type mice (14). Thus, the regulation of CD40-CD40L interactions appears to play an important role in determining the function of Th cells.
In the present study, we have focused on whether CT and the type II enterotoxins differentially affect CD40L expression on CD4+ T cells and the subsequent CD40-CD40L-dependent IL-12 production from APC. We found that CT but not LT-IIa or LT-IIb significantly inhibited T-cell activation and the upregulation of CD40L expression on CD4+ T cells after anti-CD3 stimulation. Using a coculture system, CT-, LT-IIa-, and LT-IIb-treated CD4+ T cells differentially affected CD40-CD40L-dependent tumor necrosis factor alpha (TNF-α) and IL-12 production by both autologous monocytes and monocyte-derived dendritic cells.
MATERIALS AND METHODS
Reagents.
LT-IIa and LT-IIb holotoxins were derived from an E. coli XL-1 Blue (Stratagene) strain transformed with plasmid pTDC200 or pTDC101, respectively (8). Growth and purification of LT-IIa and LT-IIb were done as previously described (21). CT was purchased from List Biological Laboratories. Human anti-CD3 and neutralizing human anti-CD40L Abs were obtained from Pharmingen (San Diego, Calif.). Recombinant human IL-4, gamma interferon (IFN-γ), and granulocyte-macrophage colony-stimulating factor (GM-CSF) were purchased from R & D Systems (Minneapolis, Minn.).
Isolation and stimulation of human PBMC, CD4+ T cells, monocytes, and dendritic cells.
Human peripheral blood mononuclear cells (PBMC) were obtained from healthy donors and isolated from heparinized venous blood by isolating the buffy coat and eliminating red blood cell (RBC) contamination by histopaque (SG-1.077) density gradients. After washing in phosphate-buffered saline (PBS) containing 1% fetal calf serum (FCS) and 2 mM EDTA, PBMC were resuspended at a concentration of 2 × 106 cells/ml in complete culture medium (RPMI 1640; Cellgro Mediatech, Washington, D.C.) containing 10% FCS, 1% l-glutamine, and 10 mM HEPES plus 10 U of penicillin, 100 μg of streptomycin, and 50 μg of gentamicin per ml and then stimulated with soluble anti-CD3 (1 μg/ml) in the presence or absence of the desired holotoxin (1 to 1,000 ng/ml). Cell supernatants were collected after incubation for 48 to 72 h and stored at −20°C until assayed for cytokine production.
CD4+ T cells were purified from isolated human PBMC with the aid of a CD4+ T-cell indirect magnetic labeling kit containing monoclonal hapten-conjugated CD8, CD11b, CD16, CD19, CD36, and CD56 Abs (Miltenyi Biotec). As determined by flow cytometry, this procedure routinely yielded >95% CD4+ T cells. CD4+ T cells were preincubated with CT, LT-IIa, or LT-IIb (1 to 1,000 ng/ml) for 1 h and then stimulated with plate-bound anti-CD3 (5 μg/ml). CD4+ T cells were then stained for CD25, CD69, or CD40L expression using Abs obtained from Pharmingen and analyzed by flow cytometry.
To determine the functional consequence of CD40L expression on CT-, LT-IIa-, and LT-IIb-treated CD4+ T cells, after anti-CD-3 stimulation for 6 h, CD4+ T cells were fixed with 1% paraformaldehyde, washed extensively in complete RPMI, and cocultured at a concentration of 2 × 106 cells/ml with autologous monocytes or monocyte-derived dendritic cells (106 cells/ml) in the presence or absence of anti-CD40L or an isotype-matched control Ab.
Monocytes were isolated from PBMC by depletion of nonmonocyte cells, which was performed with the aid of an indirect magnetic isolation kit using monoclonal hapten-conjugated CD3, CD7, CD19, CD45RA, CD56, and IgE Abs (Miltenyi Biotec). This procedure routinely resulted in >90% pure CD14+ cells, as shown by flow cytometry.
Dendritic cells were derived from monocytes purified by negative selection as described above. Briefly, monocytes were cultured for 7 days in the presence of 100 ng of IL-4 and GM-CSF per ml, and nonadherent cells were harvested. The majority of the resulting cells (>80%) excluded trypan blue, and >60% expressed CD1a and exhibited characteristic dendrite formation.
Cytokine analysis.
Cell culture supernatants were assayed for cytokine concentration by enzyme-linked immunosorbent assay (ELISA) by using reagents for human IL-2, IL-4, IL-10, IL-12, IFN-γ, and TNF-α, obtained from R&D Systems. Briefly, flat-bottomed 96-well microtiter plates (Nunc) were coated with mouse monoclonal Abs (MAbs) anti-IL-2, anti-IL-4, anti-IL-10, anti-IL-12, anti-IFN-γ, or anti-TNF-α at 1 μg/ml in PBS and incubated overnight at 4°C. Plates were washed with PBS-Tween (PBS-Tw) and blocked to limit nonspecific binding with 10% FCS in PBS for 1 h at 37°C. After the plates were washed, supernatants were serially diluted in 1% bovine serum albumin (BSA) in PBS and added to the wells. Standard curves were generated using serial dilutions of recombinant IL-2 (2,000 pg/ml), IL-4 (2,000 pg/ml), IL-10 (2,000 pg/ml), IL-12 (2,000 pg/ml), IFN-γ (2,000 pg/ml), or TNF-α (1,000 pg/ml). Plates were incubated at 4°C overnight, followed by washing with PBS-Tw. Appropriate secondary Abs, consisting of either biotin-or peroxidase-labeled goat Abs, were added to plates. In assays using biotinylated Abs, a 1:1,000 dilution of horseradish peroxidase-conjugated streptavidin containing 1% BSA in PBS-Tw was added to the appropriate wells, and plates were incubated at room temperature for 2 h. The reaction was developed for 20 min with o-phenylenediamine-H2O2 substrate and stopped with 1 M H2SO4. The color reaction was measured at 490 nm.
Flow cytometry.
PBMC or CD4+ T cells were cultured at a concentration of 2 × 106 cells/ml in complete culture medium and stimulated with soluble (1 μg/ml) or plate-bound (5 μg/ml) anti-CD3 in the presence or absence of CT, LT-IIa, or LT-IIb (1 to 1,000 ng/ml). Cells were harvested, centrifuged, and resuspended in fluorescence-activated cell sorting (FACS) buffer (PBS containing 3% FCS and 0.1% NaN3) for 15 min and then centrifuged. Cells were then resuspended in FACS buffer, stained with CD25-fluorescein isothiocyanate (FITC), CD69-phycoerythrin (PE), CD1a-PE, or C40L-PE, and costained with CD4-APC (Pharmingen). After a 30-min incubation at 4°C, cells were washed in FACS buffer and resuspended in 1% paraformaldehyde. Fluorochrome-labeled cells were analyzed by flow cytometry using a FACStar flow cytometer (Becton Dickinson, Mountain View, Calif.).
RESULTS
CT, LT-IIa, and LT-IIb differentially affect cytokine production from anti-CD3-stimulated PBMC.
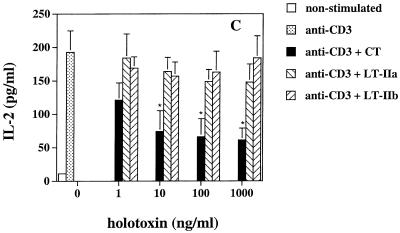
We previously found that CT and the type II HLT have different effects on the production of Th1 and Th2 cytokines from mouse lymphocytes (21). In order to determine if similar effects occurred in human PBMC, we analyzed the cytokine profile induced in these cells by CT, LT-IIa, and LT-IIb when activated by soluble anti-CD3 MAb. Cell supernatants were collected 48 to 72 h after stimulation and analyzed for cytokine production by ELISA. No significant differences in the level of IL-4 or IL-10 were observed between CT-, LT-IIa-, or LT-IIb-treated PBMC cultures (Fig. 1A and B). In contrast, CT-treated PBMC exhibited a pronounced reduction in IL-2, TNF-α, and IL-12 p70 production compared to PBMC treated with LT-IIa or LT-IIb (Fig. 1C to E). These data agree with our previous findings on the effects of type I and type II HLT on the immune response in mice and demonstrate that CT and the type II HLT differentially affect Th cytokine production from anti-CD3-treated human PBMC.
FIG. 1.
(A) IL-4, (B) IL-10, (C) IL-2, (D) TNF-α, and (E) IL-12 p70 production by PBMC cultured with soluble anti-CD3 (1 μg/ml) in the presence and absence of various concentrations of CT, LT-IIa, and LT-IIb. After 48 to 72 h, cell supernatants were analyzed for cytokine concentrations. The data represent the mean ± standard deviation (SD) of cultures derived from four donors. ∗, statistically significant difference at P < 0.05 comparing CT- to LT-IIa- or LT-IIb-treated cultures.
CT, LT-IIa, and LT-IIb differentially affect activation and proliferation of anti-CD3-stimulated PBMC.
We next determined whether CT and the type II HLT could be influencing cytokine production from APC by affecting T-cell activation. PBMC were cultured with soluble anti-CD3 for 24 h in the presence or absence of holotoxin, and then CD4+ T cells were analyzed by flow cytometry for T-cell activation markers. Anti-CD3 stimulation significantly increased CD25 and CD69 expression on CD4+ T cells compared to untreated controls (Table 1). However, when PBMC were stimulated with anti-CD3 in the presence of CT, a significantly lower mean level of both CD25 and CD69 expression was seen on CD4+ T cells compared to controls, whereas LT-IIa and LT-IIb exhibited minimal inhibitory effects on the expression of CD25 and CD69 (Table 1). Similarly, CT inhibited the proliferation of anti-CD3-stimulated PBMC, while LT-IIa and LT-IIb had no significant effect on proliferation (Table 1). Thus, there are significant differences in the ability of CT, LT-IIa, and LT-IIb to suppress T-cell activation and subsequent proliferation.
TABLE 1.
Effects of CT, LT-IIa, and LT-IIb on T-cell activation and proliferation
| Stimulant | Toxin (100 ng/ml) | Mean surface expression (% positive)a ± SD
|
Mean [3H]thymidine incorporationb (cpm) ± SD | |
|---|---|---|---|---|
| CD25 | CD69 | |||
| None | None | 3.48 ± 1.51 | 2.96 ± 2.68 | 421 ± 189 |
| Anti-CD3 | None | 30.77 ± 5.94 | 26.55 ± 4.29 | 32,747 ± 7,459 |
| CT | 6.83 ± 3.22* | 8.47 ± 5.63* | 7,992 ± 2,359* | |
| LT-IIa | 25.55 ± 6.02 | 21.49 ± 5.95 | 28,956 ± 5,625 | |
| LT-IIb | 23.44 ± 5.63 | 21.88 ± 3.31 | 26,331 ± 6,523 | |
Cell surface expression of CD25 and CD69 was analyzed by FACS following a 24-h incubation. Positive staining for IgG1 isotype control (CD25 and CD69) was 0.59% ± 0.14%. Data represent the means of cultures derived from four donors. *, statistically significant difference at P < 0.05 compared to anti-CD3-treated PBMC.
[3H]thymidine was added during the last 18 h of a 72-h culture.
Effects of CT, LT-IIa, and LT-IIb on CD40L expression.
We next questioned whether the observed differences in the effects of CT and the type II HLT on activation and cytokine production by anti-CD3-stimulated PBMC were related to an alteration in the upregulation of CD40L. If so, this could influence IL-12 production by APC through interaction with CD40. Since CD40L is expressed predominantly on CD4+ T cells, PBMC were untreated or treated with CT, LT-IIa, or LT-IIb and stimulated with anti-CD3 for 2 to 24 h, and CD4+ T cells were analyzed for CD40L expression by flow cytometry. Anti-CD3 stimulation of PBMC resulted in a marked upregulation of CD40L on CD4+ T cells from 2 to 6 h, but CT treatment resulted in >50% reduction (P < 0.05) in the mean level of CD40L expression at all time points (Table 2). In contrast, the addition of LT-IIa or LT-IIb to anti-CD3-stimulated PBMC minimally affected the mean level of CD40L expression compared to anti-CD3 controls (Table 2).
TABLE 2.
Effects of CT, LT-IIa, and LT-IIb on CD40L expression by human PBMCa
| Stimulant | Toxin (100 ng/ml) | Mean % CD40L-positive cells ± SD at:
|
|||
|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | 24 h | ||
| None | None | 1.81 ± 0.93 | 2.24 ± 1.27 | 1.16 ± 0.64 | 1.32 ± 0.47 |
| Anti-CD3 | None | 28.39 ± 7.49 | 37.41 ± 9.21 | 33.18 ± 8.77 | 5.35 ± 3.88 |
| CT | 11.06 ± 3.94* | 15.41 ± 5.57* | 14.22 ± 4.69* | 2.88 ± 1.31 | |
| LT-IIa | 27.61 ± 6.08 | 35.68 ± 4.49 | 32.15 ± 6.77 | 5.19 ± 3.35 | |
| LT-IIb | 23.83 ± 4.12 | 33.19 ± 6.27 | 31.59 ± 4.13 | 4.93 ± 2.17 | |
The data represent the means of cultures derived from four donors. *, significant difference at P < 0.05 compared to anti-CD3-treated PBMC. Percent positive staining for IgG1 isotype control (CD25 and CD69) was 0.59% ± 0.14%.
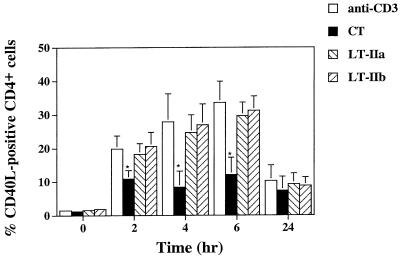
To determine if the observed difference in CD40L expression was due to a direct effect of CT, LT-IIa or LT-IIb on CD4+ T cells, purified CD4+ T cells were stimulated with plate-bound anti-CD3 with or without the toxins and then analyzed by flow cytometry for CD40L expression. Anti-CD3-stimulated CD4+ T cells again showed a marked upregulation of CD40L that reached maximal levels at 6 h, and the addition of CT resulted in a significant (∼50%) reduction (P < 0.05) in CD40L expression at 2, 4, and 6 h (Fig. 2). In contrast, CD4+ T cells cultured with LT-IIa or LT-IIb exhibited <15% reduction (P > 0.05) in the mean level of CD40L expression between 2 and 24 h (Fig. 2). No significant differences were observed in cell viability as revealed by trypan blue dye exclusion between CT-, LT-IIa-, and LT-IIb-treated CD4+ T cells (data not shown). Thus, CT has a direct suppressive effect on the upregulation of CD40L in CD4+ T cells.
FIG. 2.
Expression of CD40L on CD4+ T cells cultured with or without CT, LT-IIa, or LT-IIb (100 ng/ml) in the presence of plate-bound anti-CD3 (5 μg/ml). CD40L expression on nonstimulated CD4+ T cells was less than 2.4% at all time points tested (data not shown). Data represent the mean ± SD of cultures derived from four donors. ∗, statistically significant difference at P < 0.05 compared to anti-CD3-treated CD4+ T cells.
CT- but not LT-IIa- or LT-IIb-treated CD4+ T cells suppressed CD40-CD40L-dependent TNF-α and IL-12 p70 production by monocytes and dendritic cells.
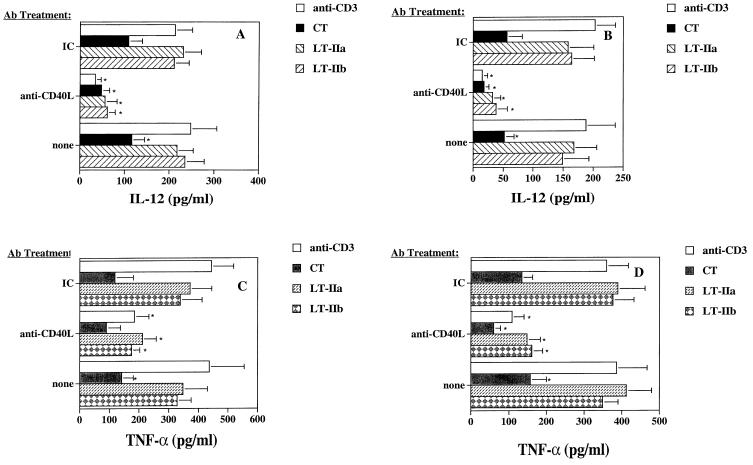
Because of the importance of CD40-CD40L interactions in the production of IL-12, we examined the functional significance of the effects of the toxins on CD40L expression by coculturing toxin-treated CD4+ T cells with APC and analyzing TNF-α and IL-12 p70 production. For this purpose, CD4+ T cells were stimulated with plate-bound anti-CD3 in the presence or absence of the toxins for 6 h, washed, fixed with paraformaldehyde, and then cocultured with autologous monocytes or monocyte-derived dendritic cells. After 48 h of incubation, cell supernatants were analyzed for TNF-α and IL-12 p70 production by ELISA. To determine whether CD40L was involved in the production of these cytokines, the coculture was also carried out in the presence of anti-CD40L MAb or an isotype control Ab. Treatment of anti-CD3-stimulated CD4+ T cells with CT resulted in a significant reduction in TNF-α and IL-12 production by monocytes and dendritic cells, whereas neither LT-IIa- nor LT-IIb-treatment of CD4+ T cells resulted in any significant reduction in the synthesis of IL-12 production by the APC (Fig. 3A and B). The addition of anti-CD40L MAb to CT-treated cocultures resulted in a further reduction in TNF-α and IL-12 production (Fig. 3A to D). Thus, although CT-treated cocultures produced significantly less IL-12 from monocytes and dendritic cells compared to anti-CD3 controls, CT did not completely abrogate CD40L-dependent IL-12 p70 or TNF-α production (Fig. 3). The addition of anti-CD40L MAb to LT-IIa- or LT-IIb-treated cocultures significantly inhibited both TNF-α and IL-12 production from autologous monocytes and dendritic cells (Fig. 3A to D). Thus, the action of CT on CD4+ T cells led to the suppression of CD40-dependent TNF-α and IL-12 production in both monocytes and dendritic cells. In contrast, LT-IIa- and LT-IIb-treated CD4+ T cells were more able to stimulate cytokine production from APC through CD40-dependent interactions.
FIG. 3.
Production of IL-12 from (A) monocytes and (B) monocyte-derived dendritic cells and TNF-α from (C) monocytes and (D) monocyte-derived dendritic cells cocultured for 48 h with paraformaldehyde-fixed CD4+ T cells that were previously activated by plate-bound anti-CD3 in the presence or absence of CT, LT-IIa, or LT-IIb. Cultures were also performed in the presence of anti-CD40L Ab or an isotype-matched control Ab (IC). Data represent the arithmetic mean ± SD of cultures derived from four donors. ∗, significant difference at P < 0.05 compared to anti-CD3-treated CD4+ T cells.
DISCUSSION
We previously reported that CT, LT-IIa, and LT-IIb exhibited potent but distinct adjuvant responses in mice (7, 21) and that CT and the type II HLT induced discrete Th cytokine production and IgG subclass responses. In the present study, we show that CT and the type II HLT have similar differential effects on anti-CD3-stimulated human PBMC and use this system to investigate the mechanisms responsible for these differences. We found that CT, LT-IIa, and LT-IIb differentially affected CD4+ T-cell activation and CD40-dependent cytokine production: CT, but not LT-IIa or LT-IIb, significantly suppressed the level of CD40L expression on anti-CD3-stimulated CD4+ T cells. Furthermore, CT-treated CD4+ T cells induced significantly less CD40-dependent IL-12 p70 production in both monocytes and monocyte-derived dendritic cells than either LT-IIa- or LT-IIb-treated CD4+ T cells.
Analysis of holotoxin-treated PBMC suggested that CT exhibited distinct suppressive effects on CD4+ T cells that were not apparent with LT-IIa- or LT-IIb-treated cultures. Our observations that CT reduced T-cell activation markers on anti-CD3-stimulated CD4+ T cells are in agreement with previous studies (33), but we found that neither LT-IIa nor LT-IIb significantly suppressed CD25 or CD69 expression on CD4+ T cells. These differences were further supported by the significant reduction in T-cell proliferation and CD40L expression in CT-treated cultures. The upregulation of CD40L on human T cells has been shown to depend on both IL-2 and IL-12 production (25). Moreover, previously activated T cells could upregulate CD40L in the presence of IL-2 and without anti-CD3 stimulation. Thus, the differential inhibition of CD25 as well as IL-2 and IL-12 production by CT and the type II HLT may, in part, explain the observed levels of CD40L expression in CT-, LT-IIa-, and LT-IIb-treated cultures.
The differentiation of naive Th cells into Th1 and Th2 cells is governed by IL-12 and IL-4, respectively, and their ability to activate specific signal transducer and activator of transcription (STAT) molecules (15, 16, 24, 26, 27, 32, 35). The ability of CT to act as a mucosal adjuvant has been well studied, and it has typically been classified as an adjuvant inducing Th2-associated immune responses. Several studies, including data presented here, demonstrate that CT can directly or indirectly downregulate IL-12 production from APC (5, 38). Conversely, while CT does possess inhibitory properties for the induction of Th1 cytokines, its mucosal adjuvanticity has been shown to depend strongly upon Th2 cytokines, especially IL-4 (20). Moreover, CT appears to directly inhibit Th1 clones but does not have these inhibitory effects on Th2 clones producing IL-4 (22). The data presented in this study define an additional pathway by which HLT affect IL-12 production from APC. The type II HLT did not suppress the upregulation of CD40L or CD40-dependent IL-12 production from autologous APC compared to the levels observed with CT-treated cultures. Th cells able to differentiate into Th1 or Th2 cells have been found to polarize predominantly into the Th2 phenotype when primed under conditions containing both IL-4 and IL-12 compared to conditions containing only IL-12 (23).. However, in the presence of a higher IL-12 dose, the number of IL-4-producing cells observed when priming in the presence of IL-4 and IL-12 was reduced by almost half, while there was a concomitant rise in the number of INF-γ-producing cells. Considering that anti-CD3-stimulated PBMC cultures produced similar levels of IL-4, the ability of CT and the type II HLT to alter the levels of IL-12 produced during Th-cell activation and priming further explains the distinct Th profiles observed when these HLT are used as adjuvants.
The ability of APC and T cells to deliver mutual costimulatory signals during an immune response is necessary for the development of effector cells. Among these circumstances, the CD40-CD40L pathway has diverse effects (1, 11, 36). Upregulation of CD40L upon T cell activation has been implicated as a primary mechanism responsible for the induction of IL-12 and Th1 responses (29, 31). Th1 responses are deficient in the absence of CD40-CD40L interactions, and CD40L-deficient T cells are defective in IFN-γ production, while conversely exhibiting higher IL-4 production than normal cells (14). Moreover, the inability of CD40L-deficient T cells to differentiate into Th1 effector cells was due to a lack of IL-12 production from APC. These findings are consistent with our present observations concerning the abilities of CT, LT-IIa, and LT-IIb to differentially affect CD40L expression on CD4+ T cells and subsequent Th1 and Th2 cytokine production.
Dendritic cells are believed to be the major APC involved in the primary immune response. Mature dendritic cells have been shown to express an array of costimulatory molecules and thus are potent stimulators of both T and B lymphocytes (2). An important final step in the development of dendritic cells from an immature phagocytic cell to a mature cell capable of priming naive T cells involves signaling through CD40 (10, 18), and the CD40-CD40L interaction promotes the ability of dendritic cells to efficiently stimulate CD8+ cytotoxic T-lymphocyte (CTL) responses (4, 28). Despite previous studies demonstrating the ability of CT to induce a predominantly Th2-associated immune response, as well as its potent inhibitory effects on IL-12 production from APC, several groups have demonstrated the ability of CT to induce IL-12-dependent CTL responses in mice (3). Thus, although CT has been shown to reduce IL-12 production from monocytes and dendritic cells as well as suppress CD40L-dependent IL-12 production from APC, CT did not abrogate IL-12 production. We found that CT-treated CD4+ T cells expressed significantly higher levels of CD40L than nonstimulated CD4+ cultures and these cells were still capable of inducing IL-12 p70 production from both monocytes and monocyte-derived dendritic cells. However, due to the differences in CD40L expression and IL-12 production induced by CT and the type II HLT, their use as adjuvants may permit the selective generation of predominantly Ab-mediated or cell-mediated immunity.
In summary, our present study indicates that CT, LT-IIa, and LT-IIb induced different Th cell profiles from anti-CD3-stimulated PBMC cultures. By directly comparing these HLT, we were able to demonstrate that CT, LT-IIa, and LT-IIb have unique immunomodulatory effects on CD4+ T cells that accounted in part for their observed cytokine differences.
ACKNOWLEDGMENTS
We thank Dana Stinson for excellent technical assistance.
This work was supported in part by grants DE 06746 and T32-AI07051.
REFERENCES
- 1.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi J P, van Kooten C, Liu Y J, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Belyakov I M, Derby M A, Ahlers J D, Kelsall B L, Earl P, Moss B, Strober W, Berzofsky J A. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc Natl Acad Sci USA. 1998;95:1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett S R, Carbone F R, Karamalis F, Flavell R A, Miller J F, Heath W R. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 5.Braun M C, Jianping H, Wu C-Y, Kelsall B L. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor β1 and β2 chain expression. J Exp Med. 1999;189:541–552. doi: 10.1084/jem.189.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clements J D, Hartzog N M, Lyon F L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 7.Connell T D, Cornelia S, Metzger D, Evans R T. Immunostimulatory activity of LT-IIa, a type II heat-labile enterotoxin of Escherichia coli. Immunol Lett. 1998;62:117–120. doi: 10.1016/s0165-2478(98)00038-8. [DOI] [PubMed] [Google Scholar]
- 8.Connell T D, Holmes R K. Molecular genetic analysis of ganglioside GD1b-binding activity of Escherichia coli type IIa heat-labile enterotoxin by use of random and site-directed mutagenesis. Infect Immun. 1992;60:63–70. doi: 10.1128/iai.60.1.63-70.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elson C O. Mucosal vaccines. San Diego, Calif: Academic Press; 1996. Cholera toxin as a mucosal adjuvant; pp. 59–72. [Google Scholar]
- 10.Flores-Romo L, Bjorck P, Duvert V, Van Kooten C, Saeland S, Banchereau J. CD40 ligation on human cord blood CD34+ hematopoietic progenitors induces their proliferation and differentiation into functional dendritic cells. J Exp Med. 1997;185:341–349. doi: 10.1084/jem.185.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foy T M, Aruffo A, Bajorath J, Buhlmann J E, Noelle R J. Immune regulation by CD40 and its ligand gp39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 12.Gill D M, Clements J D, Robertson D C, Finkelstein R A. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect Immun. 1981;33:677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes R K, Jobling M G, Connell T D. Cholera toxin and related enterotoxins of gram negative bacteria. In: Moss J, Iglewski B, Vaughn M, Tu A T, editors. Handbook of natural toxins. New York, N.Y: Marcel Dekker, Inc; 1995. pp. 225–255. [Google Scholar]
- 14.Howland K C, Ausubel L J, London C A, Abbas A K. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J Immunol. 2000;164:4465–4470. doi: 10.4049/jimmunol.164.9.4465. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan M H, Schindler U, Smiley S T, Grusby M J. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 17.Lycke N. The mechanism of cholera toxin adjuvanticity. Res Immunol. 1997;148:504–520. doi: 10.1016/s0923-2494(98)80144-2. [DOI] [PubMed] [Google Scholar]
- 18.Mackey M F, Gunn J R, Maliszewski C, Kikutani H, Noelle R J, Barth R J. Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J Immunol. 1998;161:2094–2098. [PubMed] [Google Scholar]
- 19.Manetti R, Parronchi P, Giudiz M G, Piccinni M P, Maggi E, Trinchieri G, Romagnani S. Natural kill cell stimulatory factor (interleukin12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marinaro M, Staats H F, Hiroi T, Jackson R J, Coste M, Boyaka P, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, Fujihashi K, McGhee J R. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 21.Martin M, Metzger D J, Michalek S M, Connell T D, Russell M W. Comparative analysis of the mucosal adjuvanticity of the type II heat-labile enterotoxins, LT-IIa and LT-IIb. Infect Immun. 2000;68:281–287. doi: 10.1128/iai.68.1.281-287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munoz E, Zubiaga A M, Merrow M, Sauter N P, Huber B T. Cholera toxin discriminates between T helper 1 and 2 cells in T cell receptor-mediated activation: role of cAMP in T cell proliferation. J Exp Med. 1990;172:95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishikomori R, Ehrhardt R O, Strober W. T helper type 2 cell differentiation occurs in the presence of interleukin-12 receptor β2 chain expression and signaling. J Exp Med. 2000;191:847–858. doi: 10.1084/jem.191.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul W E. Interleukin 4/B cell stimulatory factor 1: one lymphokine, many functions. FASEB J. 1987;1:456–461. doi: 10.1096/fasebj.1.6.3315808. [DOI] [PubMed] [Google Scholar]
- 25.Peng X, Remacle J E, Kasran A, Huylebroeck D, Ceuppens J L. IL-12 up-regulates CD40 ligand (CD154) expression on human T cells. J Immunol. 1998;160:1166–1172. [PubMed] [Google Scholar]
- 26.Rogge L, D'Ambrosio D, Biffi M, Penna G, Minetti L J, Presky D H, Adorini L, Sinigaglia F. The role of Stat4 in species-specific regulation of Th cell development by type I IFNs. J Immunol. 1998;161:6567–6574. [PubMed] [Google Scholar]
- 27.Schmitt E, Hoehn P, Germann T, Rude E. Differential effects of interleukin-12 on the development of naive mouse CD4+ T cells. Eur J Immunol. 1994;24:343–347. doi: 10.1002/eji.1830240211. [DOI] [PubMed] [Google Scholar]
- 28.Schoenberger S P, Toes R E, van der Voort E I, Offringa R, Melief C J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 29.Shu U, Kiniwa M, You Wu C, Maliszewski C, Vezzio N, Hakimi J, Gately M, Delespesse G. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur J Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 30.Spangler B D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stuber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swain S L, Weinberg A D, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 33.Szamel M, Ebel U, Uciechowski P, Kaever V, Resch K. T cell antigen receptor dependent signalling in human lymphocytes: cholera toxin inhibits interleukin-2 receptor expression but not interleukin-2 synthesis by preventing the activation of a protein kinase C isotype, PKC-α. Biochim Biophys Acta. 1997;1356:237–248. doi: 10.1016/s0167-4889(96)00174-7. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi I, Marinaro M, Kiyono H, Jackson R J, Nakagawa I, Fujihashi K, Hamada S, Clements J D, Bost K L, McGhee J R. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J Infect Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- 35.Trinchieri G. Immunobiology of interleukin-12. Immunol Res. 1998;17:269–278. doi: 10.1007/BF02786451. [DOI] [PubMed] [Google Scholar]
- 36.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukocyte Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 37.Xu-Amano J, Kiyono H, Jackson R L, Staats H F, Fujihashi K, Burrows P D, Elson C O, Pillai S, McGhee J R. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa-associated tissues. J Exp Med. 1993;178:1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto M, Kiyono H, Yamamoto S, Batanero E, Kweon M-N, Otake S, Azuma M, Takeda Y, McGhee J R. Direct effects on antigen-presenting cells and T lymphocytes explain the adjuvanticity of a nontoxic cholera toxin mutant. J Immunol. 1999;162:7015–7021. [PubMed] [Google Scholar]
- 39.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Fujihashi K, Van Ginkel F W, Noda M, Takeda Y, McGhee J R. A nontoxic mutant of cholera toxin elicits TH2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]