Abstract
Background and aim
The etiopathogenesis of inflammatory bowel disease (IBD) is associated with different factors such as genetic, infectious, immunological, and environmental, including modification of the gut microbiota. IBD′s conventional pharmacological therapeutic approaches have become a challenge due to side effects, complications from prolonged use, and higher costs. Kefir fermented milk beverage is a functional food that has demonstrated multiple beneficial effects including anti-inflammatory and antioxidant activity. Alternative therapeutic strategies have been used for IBD as more natural products with low-cost and easy acquisition. The aim of this study is to evaluate the anti-inflammatory effects of kefir fermented milk beverage on sodium dextran sulfate (DSS)-induced colitis in rats.
Methods
We used 4 groups to perform this study: baseline control (BC), kefir control (KC), 5% untreated DSS-induced colitis (DSS), and 5% DSS-induced colitis treated with kefir (DSSK). The animals received fermented kefir milk beverage ad libitum for six days and the disease activity index was recorded daily. Colon samples were processed for Transmission Electron Microscopy and histopathological evaluation. We analyzed short fatty chain acids through the fecal sample using gas chromatography.
Results
Kefir supplementation was able to reduce the clinical activity index and inflammatory process evidenced by decreased neutrophil accumulation, decreased reticulum edema, and increased autophagosomes. Also, showed a trend to increase the levels of acetate and propionate.
Conclusions
Our results suggest that kefir fermented milk beverage may have an anti-inflammatory effect minimizing the intestinal damage of DSS-induced colitis.
Keywords: Inflammatory bowel disease, Ulcerative colitis, Probiotics, Kefir, Short-chain fatty acids
Graphical abstract

Inflammatory bowel disease, Ulcerative colitis, Probiotics, Kefir, short-chain fatty acids.
1. Introduction
The major forms of inflammatory bowel disease (IBD) are Crohn's disease (CD) and ulcerative colitis (UC), which affect the gastrointestinal tract in a chronic, non-curable, and recurrent process, with a higher predominance among young (15–30 years old), and Caucasian individuals [1, 2]. In the last decades, there has been a global expansion of IBD cases becoming serious endangers of human health with a significant social and financial impact on individuals, families, and society [1, 2, 3, 4].
Etiopathogenesis is associated wit, h different factors such as genetic, infectious, immunological, and environmental, including modification of the gut microbiota [5, 6]. Individuals with IBD have decreased numbers of bifidobacteria and lactobacilli, and anaerobic bacteria, compromising the intestinal barrier homeostasis affecting the innate, and adaptive immune response causing tissue damage [5, 6, 7, 8].
IBD′s conventional treatments focus on the control of symptoms through pharmacotherapy (including aminosalicylates, corticosteroids, immunomodulators, and biologics) and/or surgical resection in some cases [1, 2]. However, the complications of prolonged use, side effects, and adverse reactions remain a challenge [9, 10, 11]. Also, a considerable fraction of patients do not respond or lose response during the treatment [9, 10, 11, 12].
Emerging therapeutic approaches, such as the use of probiotic strains for the improvement of intestinal microecology have been more attention from researchers as an adjuvant therapeutic strategy in IBD [12, 13]. In this view, there is an increasing interest in beneficial microorganisms present in fermented food given its ability to resist the digestive system and act in the intestine, modifying the composition of gut microbiota, improving the control of intestinal permeability, and increasing its barrier function [4, 14, 15, 16].
Originally from the Caucasian, Tibetan, and Mongolian mountains, kefir comprises a complex microbial consortium of mainly lactic acid and acetic acid bacteria and yeasts, with great functional potential, low-cost, easy to handle, and accessible to the general population [17, 18]. Besides improving gut microbiota composition [19], kefir fermented milk beverage shows many different properties including anti-inflammatory [20, 21, 22], antioxidant [23, 24], antitumor [25], anti-allergenic [26], and immune-modulatory activities [27]. Furthermore, kefir's ability to heal wounds may result from its antimicrobial and anti-inflammatory activities, which can act synergistically to contribute to healing [19, 20, 21, 22].
The immune-modulatory properties of kefir may result from the direct action of the microbiota or may be indirect, through different bioactive compounds produced during the fermentation process [28] like short-chain fatty acids (SCFAs) that represents a rich source of energy for colonocytes [29]. The highest concentrations of acetate, butyrate, and propionate in the colonic lumen suggest a direct action on the epithelium and immune cells promoting health effects [30].
In a prospective, randomized, open-label, and controlled clinical trial study conducted on 45 patients with IBD, was verified that the supplementation with 400 mL/day of kefir decreased abdominal discomfort providing welfare [31]. The role of kefir was also investigated in both TNBS and DSS models of colitis demonstrating some possible mechanisms of action such as anti-inflammatory effects and regulation of intestinal barrier function [32, 33].
In this context, it is necessary to develop alternative therapeutic strategies for IBD with more natural and effective products, associated with low-cost and easy acquisition. Therefore, this study aimed to evaluate the anti-inflammatory effects of kefir fermented milk beverage on sodium dextran sulfate (DSS)-induced colitis in rats.
2. Materials and methods
2.1. Experimental animals
Male Wistar rats (8–10 weeks old, weighing between 180 and 250 g) were obtained from the animal facility of the Center for Development of Experimental Models for Medicine and Biology (CEDEME) at the Federal University of São Paulo - Escola Paulista de Medicina (UNIFESP-EPM). The animals were housed in groups of four per cage in a light and temperature-controlled room (12 h light/dark cicles and 23 °C) and were given free access to a standard chow diet and water. All procedures were conducted respecting the ethical precepts of experimental animals and the study was approved by the Commission for the Use of Animals in Research (CEUA 3627290119 and 6581021019) of UNIFESP/EPM.
2.2. Fermented kefir milk beverage
Fermented milk beverage was prepared from 20 mg of freeze-dried kefir culture (Danisco Biolacta, Olsztyn, Poland), containing 1010 colony-forming units per gram (CFU/g) of lactic acid bacteria and 104–107 CFU/g of yeast including Lactobacillus sp., Lactococcus lactis subsp., Streptococcus termophilus, Leuconostoc sp. grains, and yeast of kefir. To this mixture was added 100 mL of pre-reconstituted whole powder milk (Ninho, Nestlé, SP) in 10% of distilled water and incubated at 85 °C for 15 min (TM5 Thermomix, Vorwerk & Co. KG, Germany). Fermentation was performed for 16 h at 23 °C and was monitored by the Cinac System (Cynetiqued’ acidification, Yseabaert, Frepillon, France) until it reached pH 4.6. After that, we stopped the fermentation by cooling the vials in the ice bath, and the product was stored under refrigeration at 4 °C [34]. The kefir was prepared once a week, thus ensuring the freshness of the product. All procedures were performed at the Functional Fermented Food Laboratory, Faculty of Pharmaceutical Sciences, Universidade de São Paulo, São Paulo, SP, Brazil.
2.3. Colitis induction and treatment methods
The animals were randomized into four groups with eight rats in each group: baseline control (BC), kefir control (KC), 5% untreated DSS-induced colitis (DSS), and 5% DSS-induced colitis treated with kefir (DSSK). Colitis was induced by administration of 5% Dextran Sulfate Sodium (DSS) (MP Biomedicals), diluted in drinking water, and offered ad libitum for five days. On the third day, the bottles with DSS solution were changed to maintain drug efficacy. The DSS solution was changed for drinking water after 5 days.
The fermented kefir milk beverage was offered ad libitum for six days for rats of KC and DSSK groups. The DSSK group started the supplementation with kefir on the third day of the experiment.
2.4. General observations and disease activity index
After colitis induction, the disease activity index (DAI) (percentage of weight loss, bleeding, and stool consistency) was recorded daily using the score of Sanchez-Fidalgo et al. [35]. In addition, the consumption of food and water was evaluated daily.
2.5. Euthanasia and sample collection
The euthanasia of all groups happened on day nine of the experiment with a lethal dose of halothane (Tanohalo®). After 10 min of the total loss of reflexes, a cardiac puncture was performed to confirm the death, followed by the opening of the abdominal cavity, with the removal of the entire colon, and washing with sterile phosphate-buffered saline (PBS). The entire colon of each animal was recorded. Representative sections of the distal colon (two from each group) in the size of 1mm × 1mm × 2 mm were fixed in 2% formaldehyde + 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 2 h at 4 °C and subsequently fixed in 2% osmium tetroxide in 0.1 M sodium cacodylate buffer for the same period, for posteriorly ultrastructural analysis. For histopathological analysis, samples from each animal′s distal colon (of each animal per group) were fixed in 10% buffered formalin and embedded in paraffin. Sample feces were frozen in liquid nitrogen and stored at -80°C for posterior short-chain fatty acids (SCFAs) analysis.
2.6. Histopathological analysis
Histological sections of 3–4 μm were stained with hematoxylin and eosin for histopathological evaluation of colonic damage by light microscopy. Parameters such as inflammation extent, regeneration, and crypt damage were graded according to Dieleman et al. [36]. The images were captured in the Zeiss Axioskop 40 optical microscope at 50X magnification. The images were obtained using the Zeiss AxioCam MRc5 camera attached to the optical microscope and photographed using Zeiss AxioVision Software. The scale bar was inserted by the same software.
2.7. Goblet cell index
We used the histochemical technique of Alcian Blue (AB) to evaluate the goblet cell. The slides were deparaffinized in xylene and alcohol baths, then the dye Alcian Blue (Ventana Medical Systems, Arizona, USA) was added to the sections for 20 min with subsequent rinsing in distilled water. Hematoxylin was applied to the sections for 30 s followed by rinsing in tap water, alcohol, and xylene baths, and then mounted on Entellan (Merck, Darmstadt, Germany). For each animal, about 15 fields were captured in 5 distinct sections, from which approximately 1000 consecutive cells were quantified, AB positive or not, with the aid of the Olympus BX40 optical microscope, using 20x objective. The ratio between the number of goblet cells and the number of enterocytes in the mucosa was defined as the goblet cell index (CI), as follows: CI = (No. of goblet cells/the total number of mucosal cells) x 100.
2.8. Analysis of ultrastructural changes by transmission electron microscopy (TEM)
Tissue specimen previously fixed in 2% formaldehyde + 2.5% glutaraldehyde, and in 2% osmium tetroxide were washed in PBS, dehydrated in ethanol, embedded in a mounting medium at 37 °C for 3 h, and finally polymerized at 60 °C for 36 h. Propylene oxide is used in the last dehydration step because it is purer than ethanol and attracts less water and is also used in the dilution of the resin, which is hydrophobic. The EPON resin was used for electron microscopy. Sections were made in an ultramicrotome. The semi-fine sections (300–500 μm) for area delimitation. The slides were put under light microscopy and hot stained with 1% toluidine blue. To obtain the ultra-thin sections (70 nm) fenestrated copper metal grids covered with plastic film and contrasted with uranyl acetate staining and lead citrate were added. The subsequent analyses were performed using a transmission electron microscope, model EX II 1200 (JEOL, Japan) coupled to a digital camera system GATAN Orius (USA).
2.9. Analysis of short-chain fatty acids (SCFAs)
Sample preparation and SCFAs measurement were performed as described in the previous protocol [37] with adaptations. Commercially available acetic, propionic, and butyric acids of analytical grade were used as standards. To quantify SCFAs, a standard curve for the concentration range of 0.156–10 mmol/L was constructed using rat feces as the matrix. Briefly, feces samples were weighed into microtubes (approximately 30 mg per sample) and homogenized with 100 μL of distilled water. Subsequently, 40 mg of sodium chloride, 50 μL of 1 M hydrochloric acid, and 300 μL of n-butanol were added. The microtubes were vortexed for 2 min and centrifuged at 18,000×g for 15 min. The organic phase was transferred to chromatographic vials and analyzed using a GC-FID 2010 (Shimadzu) equipped with AOC-20i Autosampler and using a 30 m × 0.25 mm I.D. fused silica capillary Rtx-wax column (Restek Corp.) coated with 0.25 μm polyethylene glycol. Samples (1 μL) were injected at 260 °C using a 20:1 split ratio. High-grade pure nitrogen was used as gas carried at 1 mL/min constant flow. Data were expressed as μM.
2.10. Statistical analysis
Data were reported as mean ± SEM. Statistical analysis was performed by one-way analysis of variance (ANOVA), followed by Tukey post hoc test using Graph Pad Prism (version 6.0). The significance level adopted was p < 0.05 (α = 5%).
3. Results
3.1. Effects of kefir on disease activity index
The results of DAI demonstrated that colitis was successfully induced by DSS. The rats had abdominal distension, bleeding diarrhea, and massive weight loss. The supplementation with kefir significantly decreased clinical activity (p=0.0013) and promotes weight gain. Rats of baseline and kefir control groups did not show symptoms of colitis and had an increased weight body (Figure 1).
Figure 1.
A) and B) Effects of kefir on disease activity index and body weight: aBasal control (BC), bkefir control (KC), and cDSS-induced colitis supplemented with kefir (DSSK) vs DSS control group (p=0.0013); C) Consumption of water and D) Consumption of food: non-significant. ANOVA – Tukey′s test (8 rats per group).
The consumption of kefir was 25 mL/day/rat in both control and treated groups (KC and DSSK, respectively). There were no significant differences regarding the consumption of water between the groups, however, was observed a slight increase in water consumption in the DSS group (50 mL/day/rat) in comparison with BC and KC groups (40 mL/day/rat). There was a small decrease in water consumption after the inclusion of kefir in the DSS-induced colitis group (DSSK) (Figure 1).
Regarding food consumption, the BC group maintained a mean consumption of 40 g/day/rat. There was an oscillation in food consumption in other groups (KC, DSS, and DSSK groups, respectively).
3.2. Effects of kefir on macroscopic and microscopic alterations
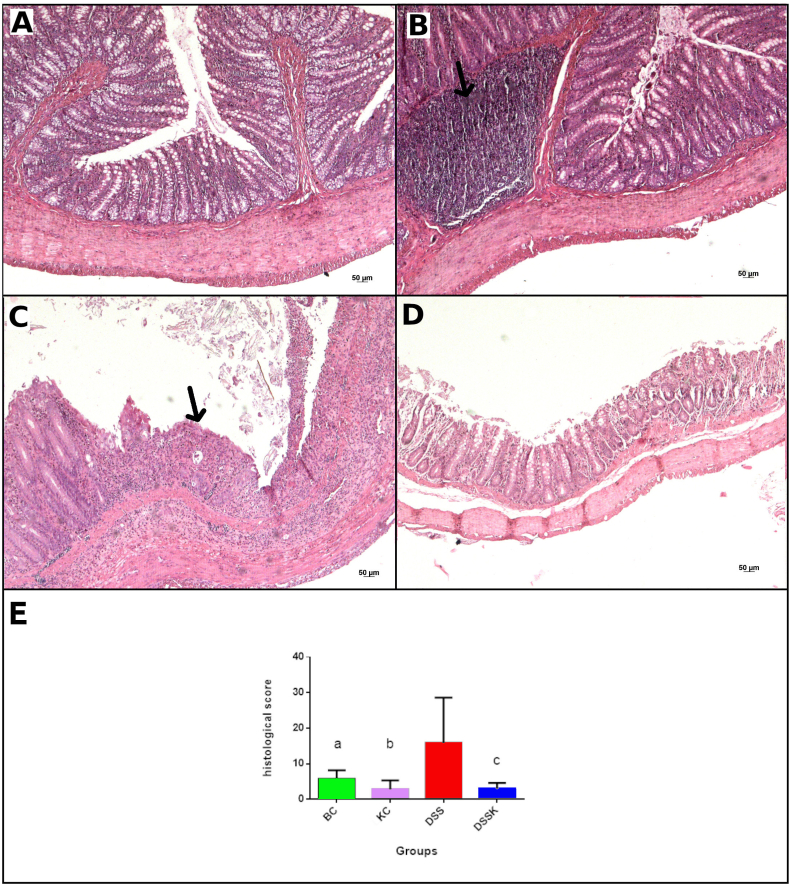
Macroscopically, the colon rats in the DSS group showed edema and increased colon wall thickness. The BC and KC did not show macroscopic alterations. The DSSK group showed mild edema (Figure 2). Regarding histological abnormalities, the DSS group showed mostly changes such as loss of cellular architecture, ulceration, intense inflammatory infiltrate, exudate, intraluminal fibrin, and in some cases, necrotic mucosa. Supplementation with kefir was markedly able to decrease the inflammatory process and preserve the intestinal epithelium (p=0.0013) (Figure 3).
Figure 2.
Effects of kefir on macroscopic colon appearance. A) Basal control and B) kefir control: no macroscopic alterations; C) DSS-induced colitis: edema and colon wall thickness; and D) DSS-induced colitis supplemented with kefir: mild edema.
Figure 3.
Effects of kefir on microscopic alterations (Zeiss optical microscope – 50x magnification) – Hematoxylin-eosin. A) Basal control and B) kefir control: normal morphology (arrow: Peyer′s patches); C) DSS-induced colitis: ulceration area (arrow) permeated by preserved tissue; D) DSS-induced colitis supplemented with kefir: preserved cellular architecture, and E) Histological score: aBasal control (BC), bkefir control (KC), and cDSS-induced colitis supplemented with kefir (DSSK) vs DSS control group (p=0.0013). ANOVA – Tukey′s test (8 rats per group).
3.3. Effects of kefir on goblet cells index
As expected, the BC and KC groups maintained the preservation of goblet cells in all the tissue evaluated compared to the DSS group and those treated with kefir (p < 0.0001). There were no significant differences in goblet cell index between the DSS and DSSK groups, however, rats of the DSSK group showed a trend of increase in goblet cells (Figure 4).
Figure 4.
Effects of kefir on goblet cell index (Zeiss optical microscope – 50x magnification) – Alcian Blue staining. A) Basal control and B) kefir control: preserved goblet cells (arrow); C) DSS-induced colitis: depletion of goblet cells (arrow); D) DSS-induced colitis supplemented with kefir: preserved goblet cells, and E) Goblet cells index: aBasal control (BC) and bkefir control (KC) vs DSS control group (DSS) (p < 0.0001). ANOVA – Tukey′s test (5 rats per group).
3.4. Effects of kefir on ultrastructural changes
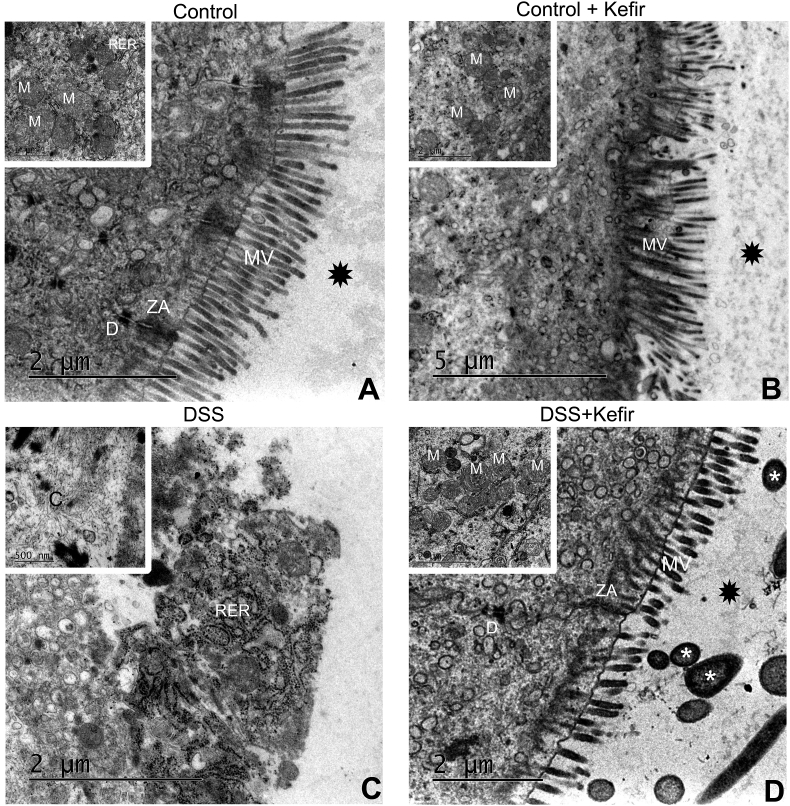
The rats of BC and KC groups did not present ultrastructural alterations, the tight junctions, absorptive epithelium, and other structures being easily seen. Rats of the DSS group exhibited loss of absorptive epithelium and tight junctions. Also, there was edema of the reticular system and mitochondrial crest. Fibrin deposits were observed indicating tissue healing. On the other hand, rats with DSS-induced colitis supplemented with kefir showed both absorptive epithelium and tight junctions preserved. In addition, was verified decreased reticulum edema and mitochondrial crest and an increase in autophagosomes (Figure 5).
Figure 5.
Representative transmission electron micrographs. A) Basal control and B) Kefir control - display normal morphological of mucosal villus, zonula adherens preserved, normal rugous endoplasmic reticulum, preserved desmosome, and preserved mitochondria; C) DSS-induced colitis display edema of rugous endoplasmic reticulum, swollen mitochondria, loss of mucosal villus and zonula adherens; and D) DSS-induced colitis supplemented with kefir: a normal mucosal villus, zonula adherens preserved, normal rugous endoplasmic reticulum, preserved desmosome, preserved mitochondria, and formation of autophagosomes. Abbreviations: MV – mucosal villus, ZA – zonula adherens, RER – rugous endoplasmic reticulum, D – desmosome, and M − mitochondria. Symbols: • - mucus; * - bacterium.
3.5. Effects of kefir on SCFAs production
There were no significant differences in SCFAs measurement between the groups. All SCFAs were decreased in DSS group rats, especially butyrate. With exception of butyrate, the supplementation with kefir on rats with DSS-induced colitis possibly influenced the levels of acetate and propionate (Figure 6).
Figure 6.
Effects of kefir on SCFAs measurement in feces. A) Acetate levels; B) Propionate levels; C) Butyrate levels; and D) The total SCFAs levels. *p < 0.0001. ANOVA – Tukey′s test (6 rats per group).
4. Discussion
The necessity of developing an alternative therapy for IBD is a new reality. The theoretical basis of intestinal microbiota disorder awakens the researchers for search potentially effective treatment methods for IBD by improving the intestinal microbiome [1, 12, 13]. In this perspective, Lactobacillus and Bifidobacteria are important species of lactic acid bacteria used as probiotics with evidence of their importance in human health [1, 12, 13, 14]. Recently, exposure of T cells from biopsy specimens of patients with active IBD to lactobacillus treatment reduced the secretion of pro-inflammatory cytokines and modulated T lymphocyte cell proliferation [38]. Similarly, biopsies exposed to the probiotic Lactococcus lactis exhibited reduced secretion of TNF-α and IL-23 [39]. According to Braat et al. the consumption of Lactobacillus lactis for one-week reduced C-reactive protein (CRP) levels in CD patients [40].
Comprising mainly lactic acid and acetic acid bacteria, and yeasts, kefir fermented food is gaining popularity in the Latin American population because it can be purchased by donation, which significantly reduces production and consumption costs [17, 18, 41]. In this view, we evaluated the effects of kefir fermented milk beverage on sodium dextran sulfate (DSS)-induced colitis in rats. Our results demonstrated that the supplementation with kefir fermented milk beverage significantly decreased the colitis clinical activity and microscopic damage, corroborating with other studies. Sevencan et al. (2019) demonstrated that kefir supplementation at a concentration of 10% decreased episodes of diarrhea and rectal bleeding as well as the inflammatory process, preserving cell architecture on TNBS-induced colitis in rats. On the other hand, the concentration of 30% exacerbated both clinical disease activity and tissue damage [42].
In the study conducted by Senol et al. (2015) the administration of the same Lactic kefir culture (Danisco Biolacta, Olsztyn, Poland) resulted in decreased clinical activity and inflammatory process, as well TNF-α levels of 3% in DSS-induced colitis in Wistar rats [43]. Chen et al. (2012), demonstrated that administration of 108 CFU/d of Lactobacillus kefiranofaciens decreased rectal bleeding and the inflammatory process, increased IL-10 production, and suppressed pro-inflammatory cytokines via the TLR2 pathway, suggesting the restoration of the epithelial barrier [44]. Similarly, Shin et al. (2020) reported that the administration of Lactobacillus brevis (Bmb6) exerted positive effects on DSS-induced colitis by suppressing pro-inflammatory cytokines, enhancing ZO-1 expression, and preserving the intestinal epithelium possibly by an increase in the goblet cells number [45].
Indeed, the intestinal barrier function plays an important role in IBD pathogenesis. Damage to intestinal epithelial cells compromises the integrity and loosens tight junctions (TJs) facilitating direct interaction between pro-inflammatory antigenic components in the lumen and the intestinal epithelium [46, 47]. Our findings in the electron microscopy revealed the integrity of the TJs and the absorptive epithelium in rats with DSS-induced colitis supplemented with kefir. Furthermore, considering the importance of goblet cells for the continuous production of mucus, as a mechanism of epithelial cells protection, we evaluated goblet cells index, however, there were no significant differences.
Still, regarding our electron microscopy findings, it is important to emphasize that we observed an increase of autophagosome in rats with DSS-induced colitis supplemented with kefir. To the best of our knowledge, this is the first paper to report this finding. According to the literature, autophagy is an essential mechanism of cell survival, involving and degrading its constituents such as misfolded proteins, and excess and/or damaged organelles [48, 49, 50, 51, 52, 53]. Nighot et al. (2015) demonstrated that induction of autophagy in cell lines promoted increased barrier function of tight junctions by reducing paracellular permeability of ions and small solutes through claudin-2 degradation [51]. Thus, our findings suggest that the supplementation with kefir may have promoted epithelial barrier restoration, but further studies are required to verify our hypothesis.
Kefir has beneficial properties with the ability to increase the diversity of the intestinal microbiota by the colonization of SCFAs-producing bacteria [[54], [55], 56]. Production of SCFAs is very important because they provide an energy source for colonocytes, help to reduce inflammation, inhibit pathogen growth, and modulate the immune system [57, 58, 59, 60]. Therefore, the measurement of SCFAs also was investigated in this study, however, no statistical difference between the groups analyzed was significant. We observed a trend towards the increasing of acetate and propionate levels in the feces of rats with DSS supplemented with kefir, which may have helped to reduce the inflammatory process, facilitating the restoration of the epithelial barrier, but further studies are required to verify our hypothesis.
Tong et al. (2016) observed that pre-administration and co-treatment with propionate were shown to be effective in reducing the expression levels of IL-1β, IL-6, and TNF-α as well as in decreasing neutrophil recruitment on DSS-induced colitis [61]. We also verify a decrease in neutrophil recruitment.
Butyrate is also considered an important source of energy for colonocytes, playing a significant role in epithelial homeostasis [62]. It has an important contribution to immune tolerance, increasing the intestinal regulatory T cells (Tregs) and macrophage activity modulation [63, 64] dendritic cells [65], and lymphocytes [64]. Sabatino et al. (2005) administered 4 g butyrate/day for 8 weeks by oral via CD patients, which induced clinical improvement and remission in 53% of them [66]. In a study by Kaiko et al. (2016) butyrate administration led to reduced epithelial proliferation in crypts adjacent to ulcers in mice with DSS-induced colitis [67]. Different from our expectations, our results showed a decreased level of butyrate. There are two possible explanations for these results. Firstly, the number of samples analyzed may not have been sufficient, and secondly, the kefir culture used in this experiment may not have enough butyrate-producing strains.
The study′s main limitations were the small number of samples and the short treatment time. Possibly, a further protocol with many samples and a long time of treatment as well the more specific molecular methods may better elucidate the role of kefir. Thus, it is essential to emphasize that the different manufacturing conditions of kefir, production methods such as fermentation time and temperature, type of milk used, and origin of the grains can influence the chemical and microbiological composition of the fermented milk, can change the characteristics of microorganisms, and affect their health effects [19]. Besides, it is important to emphasize that probiotics cannot cure the disease but can prolong the remission period, thus increasing patients' quality of life [7]. Taken altogether, kefir can be a good option in IBD treatment and should be better investigated.
5. Conclusion
In conclusion, our results suggest that kefir fermented milk beverage may have an anti-inflammatory effect minimizing the intestinal damage of DSS-induced colitis. However, more studies are needed to prove its effectiveness as well as better strain composition and dosage recommendations.
Declarations
Author contribution statement
Karina Nascimento da Silva, MSc; Ana Paula Ribeiro Paiotti, Ph.D; Sender Jankiel Miszputen, MD, Ph.D; Orlando Ambrogini Junior, MD, Ph.D: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Aline Garnevi Fávero, MSc; William Ribeiro, Ph.D; Caroline Marcantonio Ferreira, Ph.D; Patrícia Sartorelli, Ph.D; Leonardo Cardili, MD, MSc; Cristina Stewart Bogsan, Ph.D; Joice Naiara Bertaglia Pereira, Ph.D; Rita de Cássia Sinigaglia;
Andréa Cristina de Moraes Malinverni, Ph.D: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Prof Ana Paula Ribeiro Paiotti was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [001].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
Acknowledgments
The authors wish to express their gratitude to Universidade Federal de São Paulo – Escola Paulista de Medicina – UNIFESP/EPM, São Paulo, SP, Brazil: Laboratory of Experimental and Molecular Pathology for the assistance in the execution of the experimental design; and to the Institute of Environmental, Chemistry and Pharmaceutical Sciences, Department of Pharmaceutics Sciences - Universidade Federal de São Paulo, Diadema, SP, Brazil by the assistance on the gas chromatography execution.
References
- 1.Cai Z., Wang S., Li J. Treatment of inflammatory bowel disease: a comprehensive review. Front. Med. 2021;20(8):765474. doi: 10.3389/fmed.2021.765474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parra R.S., Chebli J.M., M Amarante H., et al. Quality of life, work productivity impairment and healthcare resources in inflammatory bowel diseases in Brazil. World J. Gastroenterol. 2019;25(38):5862–5882. doi: 10.3748/wjg.v25.i38.5862. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan G.G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 4.Peluzio M.C.G., Moura e Dias M., Martinez J.A., Milagro F.I. Kefir and intestinal microbiota modulation: implications in human health. Front. Nutr. 2021;22(8):638740. doi: 10.3389/fnut.2021.638740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ungaro R., Mehandru S., Allen P.B., Peyrin-Biroulet L., Colombel J.F. Ulcerative colitis. Lancet. 2017;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ocansey D.K.W., Zhang L., Wang Y., Yan Y., Qian H., Zhang X., et al. Exosomemediated effects and applications in inflammatory bowel disease. Biol. Rev. Camb. Phil. Soc. 2020;95:1287–1307. doi: 10.1111/brv.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ocansey D.K.W., Wang L., Wang J., Yan Y., Qian H., Zhang X., et al. Mesenchymal stem cell-gut microbiota interaction in the repair of inflammatory bowel disease: an enhanced therapeutic effect. Clin. Transl. Med. 2019;8:31. doi: 10.1186/s40169-019-0251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Alencar Junior H., Paiotti A.P.R., de Araújo Filho H., et al. The relationship between the commensal microbiota levels and Crohn′s disease activity. JGH Open. 2020;4(5):784–789. doi: 10.1002/jgh3.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currò D., Ianiro G., Pecere S., Bibbò S., Cammarota G. Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. Br. J. Pharmacol. 2017;174(11):1426–1449. doi: 10.1111/bph.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celiberto L.S., Graef F.A., Healey G.R., Bosman E.S., Jacobson K., M Sly L., et al. Inflammatory bowel disease and immunonutrition: novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology. 2018;155(1):36–52. doi: 10.1111/imm.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan I., Ullah N., Zha L., Bai Y., Khan A., Zhao T., et al. Alteration of gut microbiota in inflammatory bowel disease (IBD): cause or consequence? IBD Treat. Targ. Gut Microb. Pathog. 2019;8(3):1–28. doi: 10.3390/pathogens8030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mijan M.A., Lim B.O. Diets, functional foods, and nutraceuticals as alternative therapies for inflammatory bowel disease: present status and future trends. World J. Gastroenterol. 2018;24(25):2673–2685. doi: 10.3748/wjg.v24.i25.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saez-Lara M.J., Gomez-Llorente C., Plaza-Diaz J., Gil A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: a systematic review of randomized human clinical trials. BioMed Res. Int. 2015;2015:1–15. doi: 10.1155/2015/505878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kok C.R., Hutkins R. Yogurt and other fermented foods as sources of health promoting bacteria. Nutr. Rev. 2018;76:4–15. doi: 10.1093/nutrit/nuy056. [DOI] [PubMed] [Google Scholar]
- 15.Sanlier N., Gökcen B.B., Sezgin A.C. Health benefits of fermented foods. Crit. Ver. Food. Sci. Nutr. 2019;59:506–527. doi: 10.1080/10408398.2017.1383355. [DOI] [PubMed] [Google Scholar]
- 16.Bell V., Ferrão J., Pimentel L., Pintado M., Fernandes T. One health, fermented foods and gut microbiota. Foods. 2018;7:195. doi: 10.3390/foods7120195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabak B., Dobson A.D. An introduction to the traditional fermented foods and beverages of Turkey. Crit. Rev. Food Sci. Nutr. 2011;51:248–260. doi: 10.1080/10408390903569640. [DOI] [PubMed] [Google Scholar]
- 18.Rosa D.D., Dias M.M.S., Grzes’kowiak L.M., Reis S.A., Conceição L.L., Peluzio M.C.G. Milk kefir: nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017;30:82–96. doi: 10.1017/S0954422416000275. [DOI] [PubMed] [Google Scholar]
- 19.Iraporda C., Romanin D.E., Rumbo M., Garrote G.L., Abraham A.G. The role of lactate on the immunomodulatory properties of the nonbacterial fraction of kefir. Int. Food Res. 2014;62:247–253. [Google Scholar]
- 20.Huseini H.F., Rahimzadeh G., Fazeli M.R., et al. Evaluation of wound healing activities of kefir products. Burns. 2012;38:719–723. doi: 10.1016/j.burns.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Furuno T., Nakanishi M. Kefiran suppresses antigen-induced mast cell activation. Biol. Pharm. Bull. 2012;35(2):178–183. doi: 10.1248/bpb.35.178. [DOI] [PubMed] [Google Scholar]
- 22.Purchiaroni F., Tortora A., Gabrielli M., et al. The role of intestinal microbiota and the immune syste. Eur. Rev. Med. Pharmacol. Sci. 2013;17:323–333. [PubMed] [Google Scholar]
- 23.R Punaro G., Maciel F.R., Rodrigues A.M., Rogero M.M., Bogsan C.S., Oliveira M.N., Ihara S.S., Araujo S.R., Sanches T.R., Andrade L.C., Higa E.M. Kefir administration reduced progression of renal injury in STZ-diabetic rats by lowering oxidative stress. Nitric Oxide. 2014;37:53–60. doi: 10.1016/j.niox.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Maciel F.R., Punaro G.R., Rodrigues A.M., Bogsan C.S., Rogero M.M., Oliveira M.N., Mouro M.G., Higa E.M. Immunomodulation and nitric oxide restoration by a probiotic and its activity in gut and peritoneal macrophages in diabetic rats. Clin. Nutr. 2016;35(5):1066–1072. doi: 10.1016/j.clnu.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Ghoneum M., Gimzewski J. Apoptotic effect of a novel kefir product, PFT, on multidrug-resistant myeloid leukemia cells via a hole-piercing mechanism. Int. J. Oncol. 2014;44(3):830–837. doi: 10.3892/ijo.2014.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjögren Y.M., Jenmalm M.C., Böttcher M.F., Björkstén B., Sverremark-Ekström E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin. Exp. Allergy. 2009;39(4):518–526. doi: 10.1111/j.1365-2222.2008.03156.x. [DOI] [PubMed] [Google Scholar]
- 27.Medrano M., Racedo S.M., Rolny I.S., Abraham A.G., Pérez P.F. Oral administration of kefiran induces changes in the balance of immune cells in a murine model. J. Agric. Food Chem. 2011;59(10):5299–5304. doi: 10.1021/jf1049968. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J., Liu X., Jiang H., et al. Analysis of the microflora in Tibetan kefir grains using denaturing gradient gel electrophoresis. Food Microbiol. 2009;26:770–775. doi: 10.1016/j.fm.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Gasaly N., Hermoso M.A., Gotteland M. Butyrate and fine-tuning of colonic homeostasis: implications for inflammatory bowel diseases. Int. J. Mol. Sci. 2020;22:3061. doi: 10.3390/ijms22063061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivaprakasam S., D Prasad P., Singh N. Benefits of Short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016;164:144–151. doi: 10.1016/j.pharmthera.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yılmaz I., Dolar M.E., Özpınar H. Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: a randomized controlled trial. Turk. J. Gastroenterol. 2019;30(3):242–253. doi: 10.5152/tjg.2018.18227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo M.K., Park E.J., Ko S.Y., Choi E.W., Kim S. Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2,4,6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. J. Dairy Sci. 2018;101:8662–8671. doi: 10.3168/jds.2018-15014. [DOI] [PubMed] [Google Scholar]
- 33.Kang E.A., Choi H.I., Hong S.W., et al. Extracellular vesicles derived from kefir grain lactobacillus ameliorate intestinal inflammation via regulation of proinflammatory pathway and tight junction integrity. Biomedicines. 2020;8:522. doi: 10.3390/biomedicines8110522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendes E., B Casaro M., Fukumori C., Ribeiro W.R., Dos Santos A.L., Sartorelli P., Lazarini M., Bogsan C.S.B., Oliveira M.A., Ferreira C.M. Preventive oral kefir supplementation protects mice from ovariectomy-induced exacerbated allergic airway inflammation. Benef. Microbes. 2021;12(2):187–197. doi: 10.3920/BM2020.0112. [DOI] [PubMed] [Google Scholar]
- 35.Sánchez-Fidalgo S., Cárdeno A., Sánchez-Hidalgo M., Aparicio-Soto M., de la Lastra C.A. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. JNB (J. Nutr. Biochem.) 2013;24(7):1401–1413. doi: 10.1016/j.jnutbio.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Dieleman L.A., Palmen M.J., Akol H., Bloemena E., Pena A.S., Meuwissen S.G., et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998;114(3):385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribeiro W.R., Vinolo M.A.R., Calixto L.A., Ferreira C.M. Use of gas chromatography to quantify short chain fatty acids in the serum, colonic luminal content and feces of mice. Bio Protoc. 2018;8(22) doi: 10.21769/BioProtoc.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curciarello R., Canziani K.E., Salto I., Romero E Barbiera, Rocca A., Doldan I., Peton E., Brayer S., Sambuelli A.M., Goncalves S., Tirado P., Correa G.J., Yantorno M., Garbi L., Docena G.H., Serradell M.L.Á., Muglia C.I. Probiotic lactobacilli isolated from kefir promote down-regulation of inflammatory lamina propria T cells from patients with active IBD. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.658026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simčič S., Berlec A., Stopinšek S., Štrukelj B., Orel R. Engineered and wild-type L. lactis promote anti-inflammatory cytokine signalling in inflammatory bowel disease patient's mucosa. World J. Microbiol. Biotechnol. 2019;35(3):45. doi: 10.1007/s11274-019-2615-z. [DOI] [PubMed] [Google Scholar]
- 40.Braat H., Rottiers P., Huyghebaert N., et al. IL-10 producing Lactococcus lactis for the treatment of crohn’s disease. Inflamm. Bowel Dis. 2006;12:26–27. [Google Scholar]
- 41.Sottoriva H.M., Melo D.R., Matias T.C.F., Furioso A.A., Santos L.P., Alves G. Characteristics and properties of kefir. Arquivo Ciências Vet Zool UNIPAR. 2018;21:141–142. [Google Scholar]
- 42.Sevencan N.O., Isler M., Kapucuoglu F.N., Senol A., Kayhan B., Kiztanir S., Kockar M.C. Dose dependent effects of kefir on colitis induced by trinitrobenzene sulfonic acid in rats. Food Sci. Nutr. 2019;7:110–3118. doi: 10.1002/fsn3.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senol A., Isler M., Kockar M.C. Kefir treatment ameliorates dextran sulfate sodium-induced colitis in rats, Department of Gastroenterology, Suleyman Demirel University School of. World J. Gastroenterol. 2015;21(46):13020–13029. doi: 10.3748/wjg.v21.i46.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y.P., Hsiao P.J., Hong W.S., Dai T.Y., Chen M.J. Lactobacillus kefiranofaciens M1 isolated from milk kefir grains ameliorates experimental colitis in vitro and in vivo. J. Dairy Sci. 2012;95(1):63–74. doi: 10.3168/jds.2011-4696. [DOI] [PubMed] [Google Scholar]
- 45.Shin M.Y., Yong C.C., Oh S. Regulatory effect of lactobacillus brevis Bmb6 on gut barrier functions in experimental colitis. Foods. 2020;9(7):864. doi: 10.3390/foods9070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martini E., Krug S.M., Siegmund B., Neurath M.F., Becker C. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2017;4:33–46. doi: 10.1016/j.jcmgh.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merga Y., Campbell B.J., Rhodes J.M. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Dig. Dis. 2014;32:475–483. doi: 10.1159/000358156. [DOI] [PubMed] [Google Scholar]
- 48.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Groulx J.F., Khalfaoui T., Benoit Y.D., Bernatchez G., Carrier J.C., Basora N., Beaulieu J.F. Autophagy is active in normal colon mucosa. Autophagy. 2012;8(6):893–902. doi: 10.4161/auto.19738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakiyama T., Musch M.W., Ropeleski M.J., Tsubouchi H., Chang E.B. Glutamine increases autophagy under Basal and stressed conditions in intestinal epithelial cells. Gastroenterology. 2009;136(3):924–932. doi: 10.1053/j.gastro.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nighot P.K., Hu C.A., Ma T.Y. Autophagy enhances intestinal epithelial tight junction barrier function by targeting claudin-2 protein degradation. J. Biol. Chem. 2015;290(11):7234–7246. doi: 10.1074/jbc.M114.597492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Constante M., Fragoso G., Lupien-Meilleur J., Calvé A., Santos M.M. Iron supplements modulate colon microbiota composition and potentiate the protective effects of probiotics in dextran sodium sulfate-induced colitis. Inflamm. Bowel Dis. 2017;23:753–766. doi: 10.1097/MIB.0000000000001089. [DOI] [PubMed] [Google Scholar]
- 53.Kühbacher T., Ott S.J., Helwig U., Mimura T., Rizzello F., Kleessen B., Gionchetti P., Blaut M., Campieri M., Fölsch U.R., Kamm M.A., Schreiber S. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. 2006;55:833–841. doi: 10.1136/gut.2005.078303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ewaschuk J.B., Walker J.W., Diaz H., Madsen K.L. Bioproduction of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J. Nutr. 2006;136:1483–1487. doi: 10.1093/jn/136.6.1483. [DOI] [PubMed] [Google Scholar]
- 55.Collado M.C., Surono I.S., Meriluoto J., Salminen S. Potential probiotic characteristics of Lactobacillus and Enterococcus strains isolated from traditional dadih fermented milk against pathogen intestinal colonization. J. Food Protect. 2007;70:700–705. doi: 10.4315/0362-028x-70.3.700. [DOI] [PubMed] [Google Scholar]
- 56.Hamet M.F., Medrano M., Pérez P.F., Abraham A.G. Oral administration of kefiran exerts a bifidogenic effect on BALB/c mice intestinal microbiota. Benef. Microbes. 2016;7(2):237–246. doi: 10.3920/BM2015.0103. [DOI] [PubMed] [Google Scholar]
- 57.Tedelind S., Westberg F., Kjerrulf M., Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007;13:2826–2832. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cavaglieri C.R., Nishiyama A., Fernandes L.C., Curi R., Miles E.A., Calder P.C. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003;73(13):1683–1690. doi: 10.1016/s0024-3205(03)00490-9. [DOI] [PubMed] [Google Scholar]
- 59.Segain J., de la Bletiere D.R., Bourreille A., Leray V., Gervois N., Rosales C., Ferrier L., Bonnet C., Blottiere H., Galmiche J. Butyrate inhibits inflammatory responses through NFκB inhibition: implications for Crohn's disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lührs H., Kudlich T., Neumann M., Schauber J., Melcher R., Gostner A., Scheppach W., Menzel T.P. Butyrate-enhanced TNFalpha-induced apoptosis is associated with inhibition of NF-kappaB. Anticancer Res. 2002;22(3):1561–1568. PMID: 12168837. [PubMed] [Google Scholar]
- 61.Tong L.C., Wang Y., Wang Z.B., Liu W.Y., Sun S., Li L., Su D.F., Zhang L.C. Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front. Pharmacol. 2016;7:253. doi: 10.3389/fphar.2016.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daly K., Cuff M.A., Fung F., Shirazi-Beechey S.P. The importance of colonic butyrate transport to the regulation of genes associated with colonic tissue homoeostasis. Biochem. Soc. Trans. 2005;33(4):733–735. doi: 10.1042/BST0330733. [DOI] [PubMed] [Google Scholar]
- 63.Furusawa Y. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 64.Kaisar M.M.M., Pelgrom L.R., van der Ham A.J., Yazdanbakhsh M., Everts B. Butyrate conditions human dendritic cells to prime type 1 regulatory T cells via both histone deacetylase inhibition and G protein-coupled receptor 109A signaling. Front. Immunol. 2017;8:1429. doi: 10.3389/fimmu.2017.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh N., Gurav A., Sivaprakasam S., Brady E., Padia R., Shi H., Thangaraju M., Prasad P.D., Manicassamy S., Munn D.H., et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Di Sabatino A., Morera R., Ciccocioppo R., Cazzola P., Gotti S., Tinozzi F.P., Tinozzi S., Corazza G.R. Oral butyrate for mildly to moderately active Crohn's disease. Aliment. Pharmacol. Ther. 2005;22(9):789–794. doi: 10.1111/j.1365-2036.2005.02639.x. [DOI] [PubMed] [Google Scholar]
- 67.Kaiko G.E., Ryu S.H., Koues O.I., Collins P.L., Solnica-Krezel L., Pearce E.J., L Pearce E., Oltz E.M., Stappenbeck T.S. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell. 2016;165(7):1708–1720. doi: 10.1016/j.cell.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.