Abstract
Candida albicans is an opportunistic pathogen, which primarily affects neonates and immunocompromised individuals. The pathogen can invade the central nervous system, resulting in meningitis. At present, the pathogenesis of C. albicans meningitis is unclear. We used an in vitro model of the human blood-brain barrier to investigate the interaction(s) of C. albicans with human brain microvascular endothelial cells (BMEC). Binding of C. albicans to human BMEC was time and inoculum dependent. Invasion of C. albicans into human BMEC was demonstrated by using an enzyme-linked immunosorbent assay based on fluorescent staining of C. albicans with calcoflour. In contrast, avirulent Candida mutant strains and nonpathogenic yeast Saccharomyces cerevisiae were not able to bind and invade human BMEC. Morphological studies revealed that on association with human BMEC, C. albicans formed germ tubes and was able to bud intracellularly. Transmission electron microscopy showed various stages of C. albicans interactions with human BMEC, e.g., pseudopod-like structures on human BMEC membrane and intracellular vacuole-like structures retaining C. albicans. Of interest, C. albicans was able to bud and develop pseudohyphae inside human BMEC without apparent morphological changes of the host cells. In addition, C. albicans penetrates through human BMEC monolayers without a detectable change in transendothelial electrical resistance and inulin permeability. This is the first demonstration that C. albicans is able to adhere, invade, and transcytose across human BMEC without affecting monolayer integrity. A complete understanding of the interaction(s) of C. albicans with human BMEC should contribute to the understanding of the pathogenic mechanism(s) of C. albicans meningitis.
Candida albicans is the most frequently isolated fungal pathogen in humans with yeast infections, and the infection ranges from superficial to systemic (41). During the last two decades, human yeast infections have increased dramatically due to a variety of factors, e.g., the widespread use of broad-spectrum antibiotics, the expanded use of immunosuppressive drugs, the use of intravascular devices, and a longer survival of neonates and immunocompromised individuals. Life-threatening complications occur when C. albicans invades the central nervous system (CNS), causing meningitis (47). Candidal meningitis is common in immature infants (12). Other risk factors of candidal meningitis include malignant neoplasia, neutropenia, and hyperalimentation (46).
To invade the CNS and cause meningitis, C. albicans must cross the blood brain barrier (BBB). The functional site of the BBB is the endothelial lining of the brain capillaries. Brain microvascular endothelial cells (BMEC) differ from systemic vascular endothelium (35). BMEC are continuous, are joined by tight junctions, display a high transendothelial electrical resistance, and have few endocytotic vesicles, thereby limiting the transcellular flux. However, essential nutrients, e.g., glucose, amino acids, and proteins, are able to cross into the CNS by using specific membrane transporter systems (7). In contrast, systemic endothelial cells are either fenestrated (in liver) or discontinuous, display a low resistance, and allow a free exchange of proteins and nutrients. C. albicans interacts with a variety of cells, e.g., epithelium and human umbilical vein endothelial cells (HUVEC) (10, 24) revealing multiple virulence factors, e.g., adhesins, dimorphism, phenotypic switching, secretion of proteinases, and phospholipases. In addition, several ligands and receptors involved in the adherence of C. albicans to epithelial cells and subendothelial cell matrix components have been identified (10, 24, 30, 40). At present, no information is available concerning how C. albicans interacts with the BBB endothelium and causes meningitis. Our group has developed an in vitro model of the BBB with BMEC derived from human, bovine and rat brains and has used this model to characterize the interactions of various prokaryotic and viral pathogens with BMEC (3, 13, 22, 32).
We applied our in vitro model of the BBB to examine whether C. albicans adheres to and invades human BMEC and crosses the human BBB endothelium. We developed a DimScan immunfluorescence method to detect the internalization efficiency of C. albicans invasion of human BMEC. Transcytosis studies demonstrated that C. albicans was able to penetrate the human BMEC monolayer. In addition, light and transmission electron microscopic methods were used to characterize C. albicans invasion of human BMEC. We show for the first time that C. albicans is able to bind to, invade, and cross the in vitro BBB model. Our results provide evidence that C. albicans traversal occurs with no effect on the integrity of the human BMEC monolayer.
MATERIALS AND METHODS
Yeast strains.
C. albicans strain CAI4 (Δura3::imm434/Δura3::imm434) (2) was kindly provided by J. Pla and C. Nombela, University of Complutense, Madrid, Spain; strains CAF2 (isogenic to CAI4 but containing one functional URA3 gene) and CHK21 (Δcahk1) (with both CaHK1 genes deleted from CAI4) were provided by Mikio Arisawa, Nippon Roche K.K. Research Center, Kanagawa, Japan. Strains ATCC 10261 (B311, serotype A) and hOG301 (a constitutively filamentous mutant) (48) were kindly provided by J. F. Ernst. Saccharomyces cerevisiae BJ5459 was from the Yeast Stock Center (Berkeley, Calif.), and strain EBY100 was purchased from Invitrogen, Inc. Yeast cells were grown aerobically at 30°C in rich YPD broth (1% yeast extract, 2% peptone, 2% dextrose) or Sabouraud medium (Difco Laboratories, Detroit, Mich.). Yeast cells were harvested at log phase, washed with phosphate-buffered saline (PBS), and resuspended in the experimental medium Hams' F12-M199 (1:1) supplemented with 5% heat-inactivated fetal bovine serum (FBS).
Isolation, characterization and culture of human BMEC.
Human BMEC were isolated from human brain specimens obtained postmortem or by surgical resection during surgery for seizure disorder and characterized as previously described (39). Briefly, brain specimens were cut into small pieces and homogenized in Dulbecco's minimal essential medium (DMEM) containing 2% FBS (DMEM-S) using a Dounce homogenizer with a loose fitting. The homogenate was centrifuged in 15% dextran in DMEM-S for 10 min at 10,000 × g. The pellet containing crude microvessels was further digested for 1 h at 37°C in a solution containing 1 mg of collagenase-dispase per ml in DMEM-S. Microvascular capillaries were isolated by absorption to a column of glass beads (0.25 to 0.3 mm) and washing off the beads. Immunocytochemical staining indicated that >95% of the isolated cell fraction displayed brain endothelial cell characteristics. Very few astrocytes, microglia and pericytes were found. In cases where the nonendothelial cell population was less than 5%, we used fluorescence-activated cell sorting for further purification, and >99% pure endothelial cells were obtained, as described previously (39).
The isolated human BMEC were plated on rat tail collagen-fibronectin-coated dishes or glass coverslips and cultured in RPMI 1640-based medium supplemented with growth factors, 10% heat inactivated FBS, 10% NuSerum, heparin (5 U/ml), l-glutamine (2 mM), sodium pyrvuate (1 mM), nonessential amino acids, vitamins, penicillin, and streptomycin (100 U/ml). Human BMEC were positive for factor VIII-Rag, took up fluorescently labeled acetylated low-density lipoprotein, and expressed γ-glutamyl transpeptidase, thus demonstrating their brain endothelial cell characteristics. Human BMEC cultures were maintained in RPMI-based medium at 37°C in a humid atmosphere of 5% CO2 as previously described (39). These brain endothelial characteristics were present in culture up to passages 8 to 10. In this study, primary human BMEC were used up to passage 10.
C. albicans adhesion to and invasion of human BMEC.
Adhesion and invasion assays were performed as previously described (3, 22, 42), with the following modifications. Human BMEC or HUVEC were grown to confluency in collagen-coated 24-well tissue culture plates (Costar), an approximate inoculum of 0.5 × 104, 1 × 105, or 1 × 106 C. albicans cells in 0.5 ml of experimental medium was added (multiplicity of infection, 101 to 103) and the mixture was incubated for 2 h at 37°C. Human BMEC were extensively washed with experimental medium to remove extracellular C. albicans. Subsequently, the human BMEC were lysed with 0.5% Triton X-100, diluted, and plated onto a blood or YPD agar plate and the colonies were counted. The results are presented as percent adhesion: [(number of C. albicans recovered)/(number of C. albicans inoculated)] × 100%. Noninvasive Saccharomyces cerevisiae BJ5459 or EBY100 was used as a negative control,
To determine the invasion of C. albicans, a modified method of Borg-von Zepelin and Wagner (5) was used. Human BMEC were plated onto collagen-coated 96-well microtiter plates. At confluency, yeast cells (C. albicans or S. cerevisiae) were incubated with human BMEC for 4 h at 37°C. After incubation, human BMEC were washed four times to remove nonadherent yeast cells. In one set of samples, extracellular yeast cells were directly stained with calcofluor (CWF) (20 μg/ml) for 15 min on ice. In a second set of samples, total cell-associated yeast was stained with CWF as follows: human BMEC were fixed and permeabilized with 0.5% Triton X-100–2% paraformaldehyde–0.25% glutaraldehyde in PBS and then stained with CWF. CWF stains the yeast cell wall components chitin and glucan but has no apparent reactivity with human BMEC. CWF cannot penetrate viable human BMEC unless the human BMEC have been fixed and the cell membrane has been permeabilized. The binding of CWF to chitin occurred almost instantaneously and was not affected by the permeabilization treatment. Thus, without fixation and permeation, CWF stains extracellular yeast cells, while with fixation and permeation, CWF stains both extracellular and intracellular yeast cells. The difference in fluorescence readings indicates the relative amount of invaded (or intracellular) yeast cells. CWF fluorescence was quantitated in 96-well plates using a semiautomated digital imaging microscopy scanning system (DIM-SCAN) with excitation at 350 nm and emission at 405 nm (33). The results of yeast adherence and invasion were expressed as relative fluorescence. The viability of human BMEC was assessed by examination of cellular morphology and by trypan blue exclusion. Yeast cells are relatively big compared to other pathogens such as Escherichia coli. Another way to examine the internal and external yeast cells is to count the cells located inside and outside the human BMEC by high-power microscopy. The overall results of microscopic counting were consistent with those of our DIM-SCAM method, but the latter method is less subjective and therefore is suitable for counting the cells in the large number of samples in our experiments.
Morphological examination of the C. albicans-human BMEC interaction. (i) Light microscopy.
Human BMEC were cultured on collagen-coated eight-well chamber slides, incubated with inocula of 105 or 106 C. albicans/ml for the indicated period, fixed in 2% paraformaldehyde, counterstained with methyl green, mounted, and viewed in a Nikon Diaphot light microscope.
(ii) ApopTag staining of human BMEC.
Human BMEC were grown on collagen-coated eight-well chamber slides. At confluence, C. albicans was added to human BMEC (inocula, 105 and 106/ml) and the mixture was incubated for up to 48 h at 37°C. Subsequently, human BMEC were washed three times with experimental medium and fixed with 1% paraformaldehyde in PBS at 4°C (39). Apoptotic cells were detected with the ApopTag staining kit (Intergen, Purchase, N.Y.) as specified by the manufacturer. Human BMEC were counterstained with methyl green, mounted, and viewed and photographed in Nikon Diaphot microscope (39).
(iii) TEM.
Transmission electron microscopy (TEM) was performed on human BMEC that had been incubated with C. albicans for 4 h at 37°C, washed four times with experimental medium, fixed with 4% paraformaldehyde–2.5% glutaraldehyde in PBS for 10 min at 4°C, and gently scraped from the plastic surface. The cell suspension was then centrifuged for 5 min at 2,000 × g and processed for TEM. Briefly, a human BMEC pellet was postfixed with 2% OsO4 for 1 h, dehydrated through graded ethanol solutions and propylene oxide, and embedded in Epon. Ultrathin sections were cut, stained with uranyl acetate and lead citrate, and examined under a Philips CM transmission electron microscope.
Transcytosis of C. albicans across human BMEC.
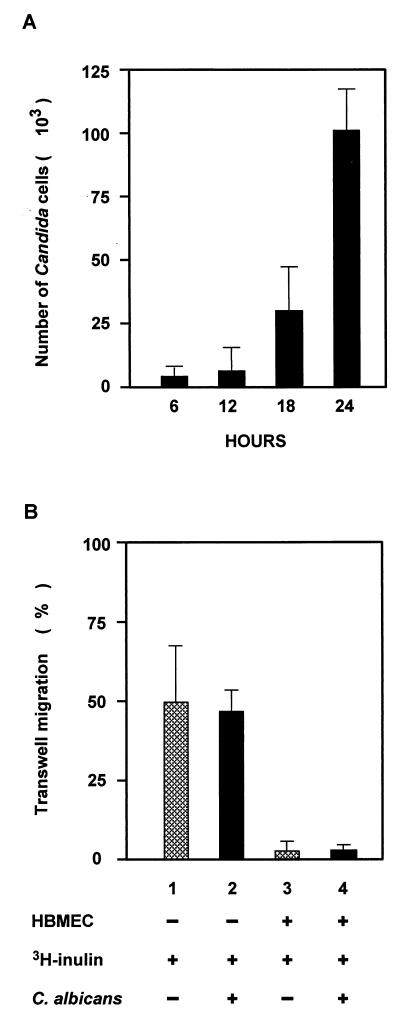
Human BMEC were cultured on 6.5-mm-diameter, collagen-coated Transwell polycarbonate tissue culture inserts with a pore size of 8 μm (Corning Costar Corp., Cambridge, Mass.) for at least 5 days as previously described (3, 28, 32, 48). This in vitro model of the BBB allows separate access to the upper chamber (blood side) and lower chamber (brain side) and permits mimicking of C. albicans penetration into the brain. Human BMEC are polarized and exhibit a transendothelial electric resistance (TEER) of at least 300 Ω/cm2 (28, 32, 48) as measured with an Endohm volt/ohm meter in conjunction with an Endohm chamber (WPI) as previously described (3). Under similar conditions, the TEER for HUVEC was approximately 17 Ω/cm2. On the morning of the assay, the human BMEC monolayers were washed, experimental medium was added, 106 yeast cells were added to the upper chamber (total volume, 200 μl), and the monolayers were incubated at 37°C. At 6, 12, 18, and 24 h, samples of 100 μl were taken from the lower chamber (an equivalent volume of medium was immediately added, maintaining a total bottom volume of 1 ml) and plated for CFU determination. The integrity of the human BMEC monolayer was assessed by measuring (i) passive diffusion of [3H]inulin (molecular weight, 4,000) and (b) TEER. The results are expressed as the percentage of initial inoculum transcytosed. Consistent with previous observations (48), Candida cells did not divide rapidly in the human BMEC culture medium but appeared to form short pseudohyphae consisting of four to six elongated yeast cells. The cell numbers indicated in Fig. 5A were determined based on the viable-cell counts on the YPD plates. Noninvasive S. cerevisiae BJ5459 or EBY100 was used as a negative control. The pH of the incubation medium was monitored with colorpHast pH indicator sticks (EM Reagents, Darmstadt, Germany) and did not change during the time course studied.
FIG. 5.
Transcytosis of C. albicans and S. cerevisiae across human BMEC. (A) C. albicans (106 cells) was added to the upper compartment of human BMEC monolayers grown on Transwell filters. Samples were collected from the bottom chambers at the indicated times, and the results are depicted as total CFU recovered (mean and standard deviation). C. albicans crosses the human BMEC monolayer in increased numbers in a time-dependent manner. (B) Transwell filters without human BMEC did not form a barrier to the inert diffusion marker [3H]inulin with or without C. albicans (lanes 1 and 2), whereas human BMEC formed a tight barrier for [3H]inulin with and without C. albicans cells (lanes 3 and 4). Lanes 1, migration of [3H]inulin across the Transwell filter without a human BMEC barrier; 2, migration of [3H]inulin in the presence of Candida cells across the Transwell filter without a human BMEC barrier; 3; Transwell filters with a human BMEC barrier and [3H]inulin; 4, Transwell filters with a human BMEC barrier, [3H]inulin, and Candida cells. Incubation of human BMEC with C. albicans for 24 h did not change the permeability for [3H]inulin.  , without C. albicans; ■, C. albicans.
, without C. albicans; ■, C. albicans.
RESULTS
Binding to and invasion of human BMEC by C. albicans.
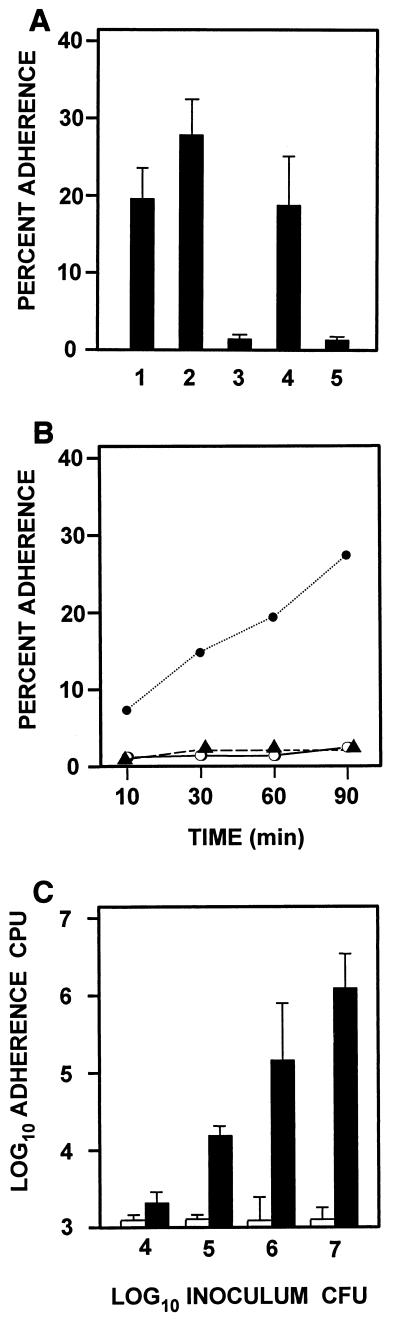
To invade host cells, C. albicans cells are likely to first adhere to them; therefore, the association of C. albicans with human BMEC was studied. Several Candida strains were tested for their ability to adhere to and associate with human BMEC (Fig. 1A). Strains CAI4 and CAF2 are isogenic, except that one copy of the URA3 selection marker was inserted in CAF2. Both strains bound human BMEC effectively (Fig. 1A, lanes 1 and 2). However, the CaHK1 null mutant strain CHK21, which is avirulent (9, 45), was not able to adhere to or associate with HBMEC (lane 3). Another pair of strains, ATTC 10261 and hOG301, was also used for comparison (lanes 4 and 5). Strain hOG301 grows constitutively in filamentous form, predominantly as pseudohyphae. It does not bind to bovine aortic endothelial cells (48). Strain ATTC 10261 was used in parallel as the wild-type strain in these studies. Our results for human BMEC adhesion and association were consistent with their reported binding capabilities. Therefore, the commonly used CAI4 strain was selected for further studies. Association of C. albicans with human BMEC was time and dose dependent (Fig. 1B and C). Approximately 30% of the inoculum of C. albicans was associated with human BMEC after a 90-min incubation period. An increase in inoculum size of C. albicans also proportionally increased the association with human BMEC. In contrast to the robust adherence of virulent C. albicans, the association of avirulent hOG301 or nonpathogenic S. cerevisiae with human BMEC was negligible and did not increase with increased time and inoculum. This suggests that the adherence of C. albicans to human BMEC may be relevant to the pathogenesis of Candida meningitis.
FIG. 1.
Binding of yeast cells to cultured human BMEC as a function of incubation time and inoculum. (A) Human BMEC were incubated with 106 yeast cells/ml for 90 min. The following C. albicans strains were used: CAI4 (lane 1), CAF2 (lane 2), CHK21 (lane 3), ATCC 10261 (lane 4), and hOG301 (lane 5). (B) Human BMEC were incubated for the indicated time with 106 cells of C. albicans strains CAI4 (●) or hOG301 (▴) or S. cerevisiae (○) per ml. (C) Effect of the inoculum size of C. albicans (solid bars) on S cerevisiae (open bar) on adherence to human BMEC. The indicated yeast inoculum was incubated with human BMEC for 90 min, and associated yeasts (viable colonies) were determined. Data are expressed as a percentage of the inoculum. Results are expressed as the mean and standard deviation of triplicates.
Adhesion to and invasion of human BMEC by C. albicans.
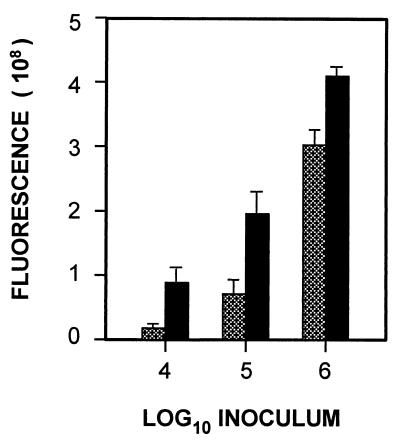
The above cell association assay gives the total number of viable yeasts associated with human BMEC both extracellularly and intracellularly but does not distinguish between adhesion and invasion. To establish the relation between adhesion to and invasion of human BMEC by C. albicans, we used a fluorescence enzyme-linked immunosorbent assay (ELISA) based on the staining of yeast cell wall components, e.g., chitin and glucan, by CWF and the absence of human BMEC staining by CWF. This method was used to estimate the relative numbers of extracellular and intracellular yeast. Figure 2 summarizes the inoculum-dependent adhesion and invasion. The extracellular C. albicans (e.g., without permeabilization of human BMEC) increased when the inoculum of C. albicans was increased (Fig. 2). A concomitant increase in the number of stained C. albicans cells upon human BMEC permeabilization was observed, which represents the total cell-associated C. albicans. The difference between these two values represents internalized (or invaded) C. albicans. At 4 h of incubation, the intracellular C. albicans was approximately 75, 50, and 25% of the C. albicans inoculum of 104, 105, and 106 yeast cells, respectively. These findings suggest C. albicans can adhere to and invade human BMEC based on the fluorescence ELISA method.
FIG. 2.
Adherence to and invasion of human BMEC by C. albicans. The indicated inoculum of yeast was incubated with human BMEC. Adherent yeast was determined by fluorescence ELISA and expressed as relative fluorescence. Hatched bars indicate extracellular binding (nonpermeabilized samples); solid bars indicate total cell-associated yeast (permeabilized samples). Results are expressed as the mean and standard deviation of triplicates.
Morphological studies of C. albicans in human BMEC.
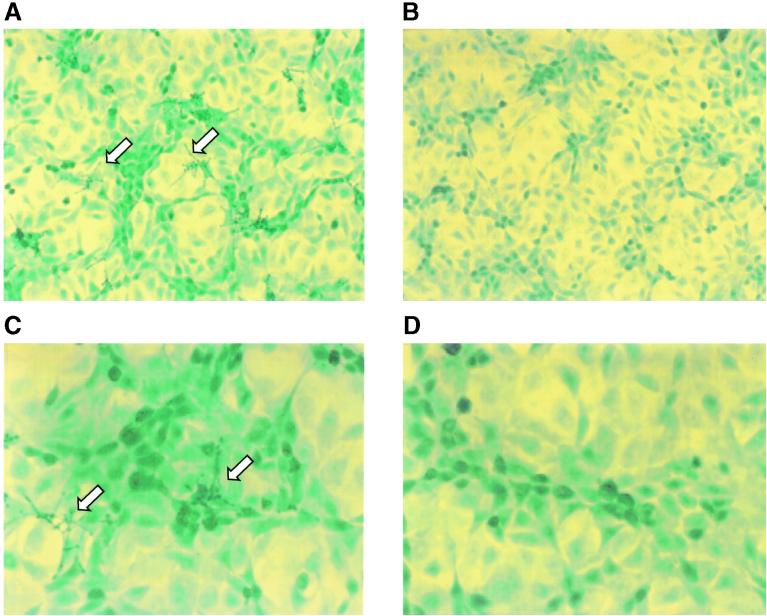
Compared to other pathogenic microorganisms (e.g., E. coli), C. albicans is relatively large (2 to 5 μm in diameter), and germ tubes will make it considerably larger. Therefore, it is of considerable interest to morphologically characterize the interaction of C. albicans with its host, human BMEC. Light microscopic studies revealed that C. albicans attached to human BMEC and developed an arboraceous scattering along the top edge of human BMEC (Fig. 3A and C). Incubation of the noninvasive S. cerevisiae with human BMEC under the same experimental conditions did not result in an association with human BMEC (Fig. 3B and D). We observed that the human BMEC monolayer showed a slightly more clustered appearance after incubation with C. albicans than after incubation with S. cerevisiae. However, no dramatic morphological changes (e.g., rounding up and lifting of human BMEC) were observed (Fig. 3). In a few cases (after a 4-h incubation with the highest inoculum [106 Candida cells per ml]), a slightly stressed human BMEC phenotype was observed, e.g., clustered appearance and some retracting of cells; however, even then, trypan blue was excluded. In addition, ApopTag staining revealed essentially no signs of DNA breakage in human BMEC incubated with C. albicans.
FIG. 3.
Surface colonization of human BMEC by C. albicans. Human BMEC was stained with ApopTag and counterstained with methyl green. (A) Association of C. albicans with human BMEC. Arrows indicate a representative extended germ tube and arborization of Candida colonies that scatter on the top edge of human BMEC. (B) No binding of S. cerevisiae to human BMEC. (C) Evidence of apoptosis (arrows) in a small population of human BMEC (∼1%) exposed to C. albicans. (D) No apoptosis in human BMEC exposed to S. cerevisiae. Also note that human BMEC are slightly more clustered when exposed to C. albicans. Magnifications, ×200 (panels A and B) and ×400 (panels C and D).
TEM.
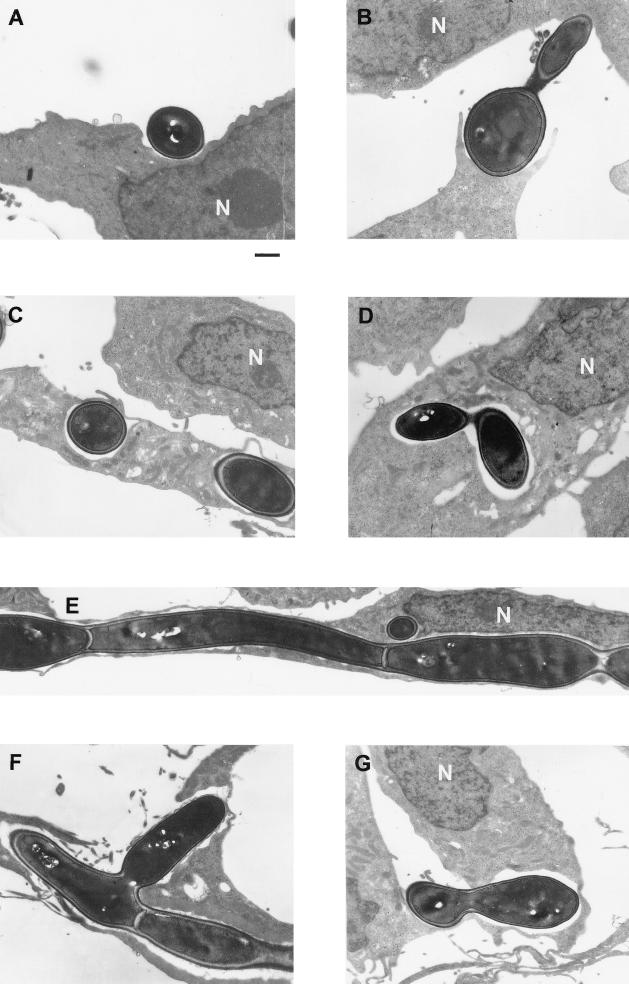
To further characterize the interaction between C. albicans and human BMEC at the ultrastructural level, transmission electron microscopy was used. Figure 4A shows a single C. albicans cell adhering to the surface of human BMEC. Figure 4B shows pseudopod-like human BMEC structures embracing C. albicans, suggestive of a partial ingestion, while its germ tube touches another human BMEC. This suggests that both the yeast and germ tube forms of C. albicans may have the cell wall ligand(s) for human BMEC surface components. Internalization of two single Candida cells into human BMEC is shown in Fig. 4C. Despite the relatively large size of C. albicans, the yeast cells were completely engulfed by the human BMEC membrane into a phagocyte-like vacuole. In addition, budded yeast cells were observed in intracellular vacuoles, suggesting that internalized yeast cells were able to develop pseudohyphae or to replicate (Fig. 4D). Interestingly, C. albicans occupied a majority of the cytoplasmic space, since we observed extending C. albicans yeast cells along inside one endothelial cell (Fig. 4E) and C. albicans budding laterally (Fig. 4F). In most cases, the thin human BMEC membrane could be observed surrounding the Candida, indicating complete internalization. Only in few cases was C. albicans observed in a vacuole open to the seemingly abluminal side of human BMEC (Fig. 4G), suggesting that (i) the human BMEC was disrupted during sample preparation or (ii) yeast organisms opened and crossed the human BMEC monolayer. Light microscopic studies suggested that C. albicans did not induce any apparent morphological damage or apoptosis in human BMEC. This was also confirmed at the ultrastructural level; e.g., human BMEC containing C. albicans exhibited no obvious ultrastructural changes reminiscent of damage or apoptosis in nuclear, mitochondrial, and endoplasmic reticular morphology compared to control human BMEC with no C. albicans. This suggests that human BMEC were able to tolerate intracellular growth of C. albicans during the time frame studied.
FIG. 4.
TEM study of C. albicans binding to and invasion of human BMEC. (A) Extracellular C. albicans attached to human BMEC. (B) Pseudopod-like human BMEC structure around a C. albicans germ tube that is attached to adjacent human BMEC. (C) C. albicans located in a human BMEC vacuole. (D) Replicating C. albicans in a human BMEC vacuole. (E) Dividing and extending germ tube of C. albicans cells in a single human BMEC. The cell wall segment can be observed between different yeast cells. (G) Dividing C. albicans cell protruding inside and/or outside of the human BMEC. N, nucleus. Bar, 1 μm.
Transcytosis of C. albicans across human BMEC.
TEM studies suggested that C. albicans was able to cross human BMEC. To further determine whether C. albicans was able to cross human BMEC from the apical to the basolateral side, we used our in vitro model of the human BBB, which has been successfully used by us and others for studying transcytosis of microbes (3, 28, 32, 43, 48). In the experiment in Fig. 5A, the C. albicans cells were measured at different time intervals. The doubling time of Candida in culture medium has been well documented (48). Consistent with their studies, the doubling time of Candida was approximately 6 to 8 h in the culture medium (DMEM). Thus, Candida cells did not divide rapidly in the human BMEC culture medium but appeared to develop short pseudophyphae consisting of elongated yeast cells. The cell number indicated in Fig. 5A is based on the viable-cell counts on the YPD plates.
The effect of C. albicans traversal on the human BMEC barrier function was evaluated using [3H]inulin. We first tested whether the Transwell filter presented a barrier for either [3H]inulin or C. albicans traversal. As shown in Fig. 5B, 50% of the added dose of [3H]inulin crossed the Transwell filter in the absence or presence of C. albicans. In separate experiments, it was shown that 50% of the inoculum of C. albicans crossed to the lower chamber within 4 h (data not shown). This indicates that the 8-μm pore size of the Transwell filters does not form a barrier for either [3H]inulin or C. albicans. The presence of a human BMEC monolayer on the filter inserts did present a barrier since less than 4% of the added [3H]inulin was recovered from the lower compartment (Fig. 5B, lane 3). Figure 5A shows that C. albicans was able to penetrate human BMEC and to cross into the bottom compartment in a time-dependent manner. After 18 to 24 h of incubation, up to 3 × 104 and 1 × 105 cells were recorded in the bottom compartment of the Transwell filters. [3H]inulin was added to the upper compartment at the end of the 24-h incubation. The permeability to [3H]inulin was not altered by the traversal of C. albicans (Fig. 5B, lane 4).
Moreover, the TEER was not affected by crossing of C. albicans within the 24-h period. Saccharomyces was used as a noninvasive yeast control, and no crossing of human BMEC was observed. No differences in lactate dehydrogenase release, as a functional measure of cellular damage, was found between the control and Candida-incubated HBMEC (data not shown). Taken together, the results indicate that pathogenic C. albicans was able to cross a human BMEC monolayer without affecting its monolayer integrity (permeability, TEER, and lactate dehydrogerase release) whereas nonpathogenic Saccharomyces was not able to transcytose. This observation, along with our TEM findings, suggests a transcellular (versus paracellular) mechanism of C. albicans traversal across the BBB.
DISCUSSION
Meningitis occurs in humans infected with C. albicans, indicating that this microorganism is able to breach the BBB (12, 46). Despite intensive studies on the pathogenicity of C albicans, the mechanism by which this organism invades the CNS and causes meningitis is little understood. Since the brain microvascular endothelium is the major site of the BBB, C. albicans must be able to cross human BMEC to cause CNS infection. There are several possible ways by which C. albicans could penetrate the endothelial barrier: (i) via a paracellular route by crossing between the adjacent endothelial cells, (ii) via direct invasion of the endothelial cells and subsequent release from the basolateral side, (iii) indirectly via migration of the yeast infected monocytes across the endothelial barrier, or (iv) via destruction of the endothelial barrier by affecting the integrity of the BBB endothelium. To our knowledge, no information is available concerning which mechanism(s) is operative in the traversal of C. albicans across the BBB. In the present study, our investigation of the interaction of Candida cells with human BMEC showed for the first time that C. albicans adheres to, invades, and transcytose across human BMEC monolayers without altering their integrity.
In vitro systems to study the invasion of human BMEC by bacterial pathogens, using a gentamicin protection assay, have been previously described by our group (3, 22, 32, 39). Unfortunately, a similar antimicrobial protection assay cannot be applied to yeast systems because no specific and effective antifungal drugs to kill extracellular yeasts are available (42, 43). To distinguish between adhesion to and invasion of human BMEC by C. albicans, we developed an ELISA-based fluorescence assay to determine relative invasion and total cell-associated yeast by using the properties of the dye CWF (5). CWF is a small fluorescent dye, which can specifically stain yeast cell wall components, e.g. chitin and glucans, which are absent from mammalian host cells. In addition, CWF does not penetrate live mammalian cells but is able to enter fixed and permeabilized host cells effectively and consequently stain intracellular yeast. We showed that CWF could be used effectively to stain yeast without appearing to stain the human BMEC. The relative contributions of adhesion and invasion can therefore be determined from the differences in the fluorescence reading between permeabilized (total cell-associated yeast) and nonpermeabilized (extracellular yeast) host cells. Our data indicate that Candida can adhere to and invade human BMEC very robustly. We consistently observed that approximately 30 to 50% of the total cell-associated C. albicans represents invaded organisms. In contrast, no such binding and invasion was observed for S. cerevisiae.
The initial step in establishing contact with host cells is the adherence of Candida to the host cell membrane, which is likely to play a critical role in withstanding blood flow in vivo. Our morphological studies showed that C. albicans often attached to the apical surface of human BMEC in clusters with long germ tubes protruding upward. This so-called arborization of C. albicans colonies seems a unique feature of this organism. Invasion of human BMEC by Candida had no noticeable effect on cells and monolayer integrity, since BMEC morphology, ApopTag staining, inulin permeability, and TEER gave results similar to those for the uninfected control cells. In addition, trypan blue was excluded by monolayers incubated with C. albicans. In contrast, penetration of C. albicans into HUVEC was found to induce cellular injury of the HUVEC (14, 16) but caused no visible changes in the architecture of the HUVEC microtubules. Studies are in progress to examine these apparent differences between human BMEC and HUVEC in response to C. albicans.
The morphological characteristics of C. albicans invading human BMEC were further studied by TEM. Invasion of human BMEC by C. albicans involved protruding contacts and membrane engulfment, similar to the zipper mechanism of the host cell surface around C. albicans trapped in intracellular vacuoles (19). Other possible mechanisms of pathogenic invasion, e.g., membrane ruffling (4, 15), pedestal formation, or coated-pit formation (37), were not observed in the invasion of human BMEC by C. albicans in these studies, although we could not rule out the possibility that they could be involved under different physiological or pathogenic conditions. Intracellular survival is a challenge for most pathogens, yet C. albicans seems to adapt well in human BMEC. TEM studies showed that the intracellular C. albicans organisms were located within a vacuole-like structure and were able to bud and develop psuedohyphae within vacuoles. These findings differ from those for meningitis-causing bacteria, such as E. coli and group B streptococci, where there is no evidence of intracellular multiplication within human BMEC. In contrast, we showed that Citrobacter was able to replicate within human BMEC vacuoles (3). Compared to bacterial pathogens, Candida may have some advantage(s) in surviving in host cells by being able to bud or form pseudohyphae intracellularly in vacuoles. The optimal pH for yeast growth is around 5, which is close to the pH in endosomal vacuoles, and this may contribute to the survival and budding of C. albicans within human BMEC.
From our binding and invasion assays and our TEM and transcytosis data, it became clear that C. albicans was able to invade human BMEC from the apical side, cross the BMEC, and exit from the basolateral side. The nature of the trafficking mechanisms(s) is unclear, but the exit of C. albicans cells from the basolateral side could occur by exocytosis of budded yeast or by growth of germ tubes across the human BMEC monolayer. Although we occasionally observed some internal Candida cells associated with disrupted human BMEC membrane, we could not conclude that the disrupted membrane was due to (i) sample preparation or (ii) lysis by Candida cells. Further study is required to clarify this observation. Interestingly, transcytosis of C. albicans occurred with no effect on the integrity of the human BMEC monolayer. In accordance with our observation, a previous study using bovine aortic endothelial cells (AEC) (48) showed that C. albicans cells were in yeast form in the initial phases of association with bovine AEC. Fungal cells were rarely observed between endothelial cells, and evading cells were shown to form hyphal extensions. However, the bovine AEC monolayer gradually loses its integrity during C. albicans transmigration (48). Together, these results suggest that the primary route of Candida traversal across the endothelial cells is a transcellular pathway but the effect of the Candida transcytosis on the endothelial cell integrity may differ between human BMEC and bovine AEC.
At present, it is unclear what structures of C. albicans contribute to binding, invasion, intracellular replication, and transcytosis in human BMEC. Multiple fungal factors have been implicated in the pathogenesis of C. albicans infections. These include genes for adherence capacity (ALS1-9, HWP1, INT1, etc.) (17, 18, 20, 21, 38), phenotypic switching (EFG1, CPH1, TUP1, etc.) (6, 28), transition between blastospores and hyphal forms (CEK1, COS1/NIK1, HK1, SSK1, HOG1, RSR1, CLN1, CST20, HST7, CLA4, etc.) (1, 2, 8, 9, 11, 25, 26, 29, 44, 45), hydrolytic enzymes (PLB1) (27, 34) and secreted aspartyl proteinases, SAP1-SAP9 (23, 31, 36). We have so far tested two mutants, hOG301 and CHK21 (Δcahk1). Our preliminary findings indicate that these mutants have lost their ability to adhere to or associate with human BMEC (Fig. 1A). It is not known whether the defect(s) is due to their primary function (loss of invasive ability) or to a secondary effect (morphological aberration, etc.). Additional studies are needed to find which structures of C. albicans contribute to their interactions with human BMEC. In summary, we showed that C. albicans adheres to and invades human BMEC and is able to bud or form pseudohyphae inside human BMEC and traverse the BMEC without affecting the integrity of the monolayer. The mechanism of crossing human BMEC monolayer is likely to involve a transcellular pathway.
ACKNOWLEDGMENTS
We thank J. Pla and C. Nombela for providing strain CAI4, J. F. Ernst for providing strains ATCC 10261 and hOG301, and Mikio Arisawa for providing strains CAF2 and CHK21 (Δcahk1). We also thank Carol Miller for providing postmortem biopsy specimens from the Alzheimer's Disease Research Center Neuropathology Core, USC School of Medicine, Los Angeles, Calif., which is funded by P50-AG05142. We also thank Peter Jordan for assistance in the DIM-SCAN analysis.
This research was supported by a grant to the Neil Bogart Memorial Laboratories by the T. J. Martell Foundation, by the American Heart Association AHA0150094N (A.J.), and by NIH grants R29 AI40635 (S.H.H.) and RO1 NS26310-13 (K.S.K.).
REFERENCES
- 1.Alex L A, Korch C, Selitrennikoff C P, Simon M I. COS1, a two-component histidine kinase that is involved in hyphal development in the opportunistic pathogen Candida albicans. Proc Natl Acad Sci USA. 1998;95:7069–7073. doi: 10.1073/pnas.95.12.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso-Monge R, Navarro-Garciá F, Molero G, Diez-Orejas R, Gustin M, Pla J, Sanchez M, Nombela C. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J Bacteriol. 1999;181:3058–3068. doi: 10.1128/jb.181.10.3058-3068.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badger J L, Stins M F, Kim K S. Citrobacter freundii invades and replicates in human brain microvascular endothelial cells. Infect Immun. 1999;67:4208–4215. doi: 10.1128/iai.67.8.4208-4215.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar-Sagi D, Feramisco J R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- 5.Borg-von Zepelin M, Wagner T. Fluorescence assay for the detection of adherent Candida yeasts to target cells in microtest plates. Mycoses. 1995;38:339–347. doi: 10.1111/j.1439-0507.1995.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 6.Braun B R, Johnson A D. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- 7.Broadwell R D, Baker-Cairns B J, Friden P M, Oliver C, Villegas J C. Transcytosis of protein through the mammalian cerebral epithelium and endothelium. III. Receptor-mediated transcytosis through the blood-brain barrier of blood-borne transferrin and antibody against the transferrin receptor. Exp Neurol. 1996;142:47–65. doi: 10.1006/exnr.1996.0178. [DOI] [PubMed] [Google Scholar]
- 8.Calera J A, Zhao X J, Calderone R. Defective hyphal development and avirulence caused by a deletion of the SSK1 response regulator gene in Candida albicans. Infect Immun. 2000;68:518–525. doi: 10.1128/iai.68.2.518-525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calera J A, Zhao X J, De Bernardis F, Sheridan M, Calderone R. Avirulence of Candida albicans CaHK1 mutants in a murine model of hematogenously disseminated candidiasis. Infect Immun. 1999;67:4280–4284. doi: 10.1128/iai.67.8.4280-4284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaffin W L, Lopez-Ribot J L, Casanova M, Gozalbo D, Martinez J P. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol Mol Biol Rev. 1998;62:130–180. doi: 10.1128/mmbr.62.1.130-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csank C, Schroppel K, Leberer E, Harcus D, Mohamed O, Meloche S, Thomas D Y, Whiteway M. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun. 1998;66:2713–2721. doi: 10.1128/iai.66.6.2713-2721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies P A, Rudd P T. Neonatal meningitis. In: Davis P A, editor. Clinics in developmental medicine. United Kingdom: The Laveham Press Ltd.; 1994. pp. 1–75. [Google Scholar]
- 13.Fiala M, Looney D J, Stins M, Way D D, Zhang L, Gan X, Chiappelli F, Schweitzer E S, Shapshak P, Weinand M, Graves M C, Witte M, Kim K S. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol Med. 1997;3:553–564. [PMC free article] [PubMed] [Google Scholar]
- 14.Filler S G, Swerdloff J N, Hobbs C, Luckett P M. Penetration and damage of endothelial cells by Candida albicans. Infect Immun. 1995;63:976–983. doi: 10.1128/iai.63.3.976-983.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis C L, Ryan T A, Jones B D, Smith S J, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- 16.Fratti R A, Belanger P H, Ghannoum M A, Edwards J E, Jr, Filler S G. Endothelial cell injury caused by Candida albicans is dependent on iron. Infect Immun. 1998;66:191–196. doi: 10.1128/iai.66.1.191-196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu Y, Rieg G, Fonzi W A, Belanger P H, Edwards J E J, Filler S G. Expression of the Candida albicans gene ALS1 in Saccharomyces cerevisiae induces adherence to endothelial and epithelial cells. Infect Immun. 1998;66:1783–1786. doi: 10.1128/iai.66.4.1783-1786.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gale C A, Bendel C M, McClellan M, Hauser M, Becker J M, Berman J, Hostetter M K. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science. 1998;279:1355–1358. doi: 10.1126/science.279.5355.1355. [DOI] [PubMed] [Google Scholar]
- 19.Griffin F M J, Griffin J A, Leider J E, Silverstein S C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975;142:1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hostetter M K. Linkage of adhesion, morphogenesis, and virulence in Candida albicans. J Lab Clin Med. 1998;132:258–263. doi: 10.1016/s0022-2143(98)90038-5. [DOI] [PubMed] [Google Scholar]
- 21.Hoyer L L, Payne T L, Hecht J E. Identification of Candida albicans ALS2 and ALS4 and localization of als proteins to the fungal cell surface. J Bacteriol. 1998;180:5334–5343. doi: 10.1128/jb.180.20.5334-5343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang S H, Wass C, Fu Q, Prasadarao N V, Stins M, Kim K S. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect Immun. 1995;63:4470–4475. doi: 10.1128/iai.63.11.4470-4475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim A S, Filler S G, Sanglard D, Edwards J E, Jr, Hube B. Secreted aspartyl proteinases and interactions of Candida albicans with human endothelial cells. Infect Immun. 1998;66:3003–3005. doi: 10.1128/iai.66.6.3003-3005.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy M J, Calderone R A, Cutler J E, Kanabe T, Riesselman M H, Robert R, Senet J M, Annaix V, Bouali A, Mahaza C. Molecular basis of Candida albicans adhesion. J Med Vet Mycol. 1992;30(Suppl. 1):95–122. [PubMed] [Google Scholar]
- 25.Kohler J R, Fink G R. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc Natl Acad Sci USA. 1996;93:13223–13228. doi: 10.1073/pnas.93.23.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leberer E, Ziegelbauer K, Schmidt A, Harcus D, Dignard D, Ash J, Johnson L, Thomas D Y. Virulence and hyphal formation of Candida albicans require the Ste20p-like protein kinase CaCla4p. Curr Biol. 1997;7:539–546. doi: 10.1016/s0960-9822(06)00252-1. [DOI] [PubMed] [Google Scholar]
- 27.Leidich S D, Ibrahim A S, Fu Y, Koul A, Jessup C, Vitullo J, Fonzi W, Mirbod F, Nakashima S, Nozawa Y, Ghannoum M A. Cloning and disruption of caPLB1, a phospholipase B gene involved in the pathogenicity of Candida albicans. J Biol Chem. 1998;273:26078–26086. doi: 10.1074/jbc.273.40.26078. [DOI] [PubMed] [Google Scholar]
- 28.Lo H J, Kohler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 29.Loeb J D, Sepulveda-Becerra M, Hazan I, Liu H. A G1 cyclin is necessary for maintenance of filamentous growth in Candida albicans. Mol Cell Biol. 1999;19:4019–4027. doi: 10.1128/mcb.19.6.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell A P. Dimorphism and virulence in Candida albicans. Curr Opin Microbiol. 1998;1:687–692. doi: 10.1016/s1369-5274(98)80116-1. [DOI] [PubMed] [Google Scholar]
- 31.Naglik J R, Newport G, White T C, Fernandes-Naglik L L, Greenspan J S, Greenspan D, Sweet S P, Challacombe S J, Agabian N. In vivo analysis of secreted aspartyl proteinase expression in human oral candidiasis. InfectImmun. 1999;67:2482–2490. doi: 10.1128/iai.67.5.2482-2490.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nizet V, Kim K S, Stins M, Jonas M, Chi E Y, Nguyen D, Rubens C E. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 1997;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proffitt R T, Tran J V, Reynolds C P. A fluorescence digital image microscopy system for quantifying relative cell numbers in tissue culture plates. Cytometry. 1996;24:204–213. doi: 10.1002/(SICI)1097-0320(19960701)24:3<204::AID-CYTO3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 34.Rodier M H, el Moudni B, Kauffmann-Lacroix C, Daniault G, Jacquemin J L. A Candida albicans metallopeptidase degrades constitutive proteins of extracellular matrix. FEMS MicrobiolLett. 1999;177:205–210. doi: 10.1111/j.1574-6968.1999.tb13733.x. [DOI] [PubMed] [Google Scholar]
- 35.Rubin L L, Staddon J M. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 36.Sanglard D, Hube B, Monod M, Odds F C, Gow N A. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect Immun. 1997;65:3539–3546. doi: 10.1128/iai.65.9.3539-3546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmid S L, Damke H. Coated vesicles: a diversity of form and function. FASEB J. 1995;9:1445–1453. doi: 10.1096/fasebj.9.14.7589986. [DOI] [PubMed] [Google Scholar]
- 38.Staab J F, Bradway S D, Fidel P L, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283:1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- 39.Stins M F, Gilles F, Kim K S. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol. 1997;76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 40.Sundstrom P. Adhesins in Candida albicans. Curr Opin Microbiol. 1999;2:353–357. doi: 10.1016/S1369-5274(99)80062-9. [DOI] [PubMed] [Google Scholar]
- 41.Tuomanen E. Entry of pathogens into the central nervous system. FEMS Microbiol Rev. 1996;18:289–299. doi: 10.1111/j.1574-6976.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 42.van Putten P J, Paul S M. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry into human mucosal cells. EMBO J. 1995;14:2144–2154. doi: 10.1002/j.1460-2075.1995.tb07208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyrick P B, Knight S T, Gerbig D G J, Raulston J E, Davis C H, Paul T R, Malamud D. The microbicidal agent C31G inhibits Chlamydia trachomatis infectivity in vitro. Antimicrob Agents Chemother. 1997;41:1335–1344. doi: 10.1128/aac.41.6.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yaar L, Mevarech M, Koltin Y. A Candida albicans RAS-related gene (CaRSR1) is involved in budding, cell morphogenesis and hypha development. Microbiology. 1997;143:3033–3044. doi: 10.1099/00221287-143-9-3033. [DOI] [PubMed] [Google Scholar]
- 45.Yamada-Okabe T, Mio T, Ono N, Kashima Y, Matsui M, Arisawa M, Yamada-Okabe H. Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J Bacteriol. 1999;181:7243–7247. doi: 10.1128/jb.181.23.7243-7247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yogev R. Meningtitis. In: Jenson H B, Baltimore R S, Markowitz W A, editors. Pediatric infectious diseases: principles and practice. Norwalk, Conn: Appleton & Lange; 1995. [Google Scholar]
- 47.Zhang J R, Tuomanen E. Molecular and cellular mechanisms for microbial entry into the CNS. J Neurovirol. 1999;5:591–603. doi: 10.3109/13550289909021288. [DOI] [PubMed] [Google Scholar]
- 48.Zink S, Nass T, Rosen P, Ernst J F. Migration of the fungal pathogen Candida albicans across endothelial monolayers. Infect Immun. 1996;64:5085–5091. doi: 10.1128/iai.64.12.5085-5091.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]