Abstract
Objectives
Antibiotic prescribing in primary care contributes significantly to antibiotic overuse. Nudge interventions alter the decision-making environment to achieve behaviour change without restricting options. Our objectives were to conduct a systematic review to describe the types of nudge interventions used to reduce unnecessary antibiotic prescribing in primary care, their key features, and their effects on antibiotic prescribing overall.
Methods
Medline, Embase and grey literature were searched for randomised trials or regression discontinuity studies in April 2021. Risk of bias was assessed independently by two researchers using the Cochrane Effective Practice and Organisation of Care group’s tool. Results were synthesised to report the percentage of studies demonstrating a reduction in overall antibiotic prescribing for different types of nudges. Effects of social norm nudges were examined for features that may enhance effectiveness.
Results
Nineteen studies were included, testing 23 nudge interventions. Four studies were rated as having a high risk of bias, nine as moderate risk of bias and six as at low risk. Overall, 78.3% (n=18, 95% CI 58.1 to 90.3) of the nudges evaluated resulted in a reduction in overall antibiotic prescribing. Social norm feedback was the most frequently applied nudge (n=17), with 76.5% (n=13; 95% CI 52.7 to 90.4) of these studies reporting a reduction. Other nudges applied were changing option consequences (n=3; with 2 reporting a reduction), providing reminders (n=2; 2 reporting a reduction) and facilitating commitment (n=1; reporting a reduction). Successful social norm nudges typically either included an injunctive norm, compared prescribing to physicians with the lowest prescribers or targeted high prescribers.
Conclusions
Nudge interventions are effective for improving antibiotic prescribing in primary care. Expanding the use of nudge interventions beyond social norm nudges could reap further improvements in antibiotic prescribing practices. Policy-makers and managers need to be mindful of how social norm nudges are implemented to enhance intervention effects.
Keywords: Quality in health care, Public health, PRIMARY CARE, Respiratory infections, INFECTIOUS DISEASES
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study employed a broad search strategy and the assessment of whether an intervention was a nudge was conducted at the full-text stage.
Implementation features of social norm nudges were extracted from the studies.
We were not able to synthesise results with meta-analysis due to the differences in outcome measures reported.
Introduction
Antimicrobial resistance is one of the most pressing challenges to global health.1 Overuse and inappropriate use of antibiotics is a major contributor to the rise of antimicrobial resistance, and yet, between 2000 and 2010 global antibiotic consumption rose by 35%.2 Concerningly, global per-capita consumption of antibiotics flagged by the WHO as having high resistance potential (Watch category)3 rose by 90.9% between 2000 and 2015.4 Primary care accounts for the majority of antibiotic use, and rates of inappropriate use are estimated to be high.5–7 For example, the majority of upper respiratory tract infections (RTIs) do not benefit from antibiotic treatment, particularly when weighed against the rates of adverse effects, however, antibiotics continue to be prescribed.5 8 9
Efforts to reduce antibiotic prescribing in primary care have predominantly focused on the use of point-of-care testing, shared decision-making and education strategies aimed at physicians and patients.10–12 While some of these intervention strategies have been successful in improving antibiotic prescribing, they can be resource intensive, and in some cases only provide marginal reductions in antibiotic prescribing.10–12 Furthermore, these intervention strategies rarely take into account of how cues in the environment, unrelated to clinician knowledge or access to resources such as information or tests, can influence decision-making.
The field of behavioural economics has generated a collection of approaches, called ‘nudges’, that involve subtle changes in the decision-making environment, or choice architecture, to guide people towards a specific decision or behaviour. Nudge interventions are typically simple and low-cost interventions, and thus are attractive to healthcare managers and policy makers. Furthermore, they do not restrict choices or penalise ‘unfavourable’ choices, thus preserving an individual’s autonomy in the decision-making process. Examples of nudge interventions include changing default settings, changing option consequences and providing reminders during the decision-making process.
Nudge interventions have similarities to traditional behaviour change techniques applied in health services and public health.13 14 For example, audit and feedback has long been applied in health service interventions and has similarities to social norm feedback nudges. However, audit and feedback may not necessarily include a comparison to the performance of peers, the essential component that would make it a nudge.15 16 Furthermore, social norm feedback nudges tend to target ‘underperformers’, as evidence from psychology has demonstrated a ‘boomerang’ effect; that is, high performers drop their performance toward the group mean (beyond that expected due to regression toward the mean).17 However, audit and feedback interventions used in health services may not take performance into account when deciding on who should receive feedback. Thus, there can be nuanced differences in the techniques from each of these paradigms.
Nudge interventions have been successfully implemented in fields other than health,18 and the evidence base for their use in influencing consumers’ health-related behaviours is growing.19 20 However, while the use of nudge interventions in specific areas of health services and to influence clinical decision making is increasing,18 21 there is emerging evidence that the effect of nudges can vary depending on the context in which they are applied, as well as the type of nudge implemented.22 23 Against this background, our aim was to explore the use of nudge interventions and their effectiveness to improve antibiotic prescribing in primary care, and to draw out lessons to inform future directions for nudge intervention design and testing in healthcare. Our specific objectives were to describe the types of nudge interventions trialled to date, their key features and their effects on the rates of antibiotic prescribing overall, in order to elucidate ‘what kind of nudges work best in this … setting?’.22
Methods
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (online supplemental file 1).24
bmjopen-2022-062688supp001.pdf (62.6KB, pdf)
Information sources and search strategy
The databases MEDLINE (via Ovid and PubMed) and Embase were searched for original research articles reporting on randomised trials or regression discontinuity studies of interventions to improve antibiotic prescribing in primary care, published in English in the last 20 years. Though the behavioural economics term ‘nudge’ was proposed in 2008, many of the interventions now termed ‘nudges’ have been applied to influence behaviour prior to the emergence of this term. Therefore, we did not exclude articles published before 2008 if the interventions met the criteria for a nudge intervention, and our search strategy did not include ‘nudges’ as a theme. Instead, our search strategy covered three themes: antibiotics AND primary care AND intervention study designs. The reference lists of included studies were hand searched for relevant citations. Websites of government nudge units and other organisations working to apply and test nudge theory were also searched for grey literature of relevance. Searches were carried out in April 2021. The full search strategy is presented in online supplemental file 2.
bmjopen-2022-062688supp002.pdf (63.7KB, pdf)
Eligibility criteria
Studies conducted in primary care facilities, general and family practices were included. Studies in hospital wards or in long-term care were excluded. The intervention tested had to fall under the broad definition of a nudge proposed by Thaler and Sunstein: ‘A nudge … is any aspect of the choice architecture that alters people’s behaviour in a predictable way without forbidding any options or significantly changing their economic incentives. To count as a mere nudge, the intervention must be easy and cheap to avoid’.25 For further guidance on whether the intervention used qualified as a nudge, we used a taxonomy of choice architecture techniques which focuses on interventions rather than the underlying cognitive processes of the interventions.26 Interventions involving education, providing physicians with access to guidelines, passive decision support tools the clinician had to actively decide to use, and audit and feedback interventions with no social norm comparison were excluded. Studies evaluating multifaceted interventions that included a nudge strategy were also excluded as they did not allow evaluation of the impact of the nudge intervention alone. Studies had to evaluate the impact of the intervention on antibiotic prescribing rates or rates of appropriate antibiotic prescribing to be eligible. Randomised controlled trials and regression discontinuity studies were included. Regression discontinuity studies allow assessment of causality in studies where a cut-off point is used to allocate an intervention. This is of particular relevance to social norm nudges, where, for example, the bottom 10% performers are targeted by an intervention. Studies have shown that regression discontinuity studies have similar effect estimates to randomised trials, though they require a large sample size.27 28 Interrupted time-series, controlled before-after, cross-sectional and before-after studies were excluded as they are at higher risk of bias.
Study selection
Titles and abstracts of citations returned from the searches were independently reviewed by at least two reviewers. At this stage, the reviewers assessed study setting, study design and outcomes for eligibility. The full-text of all selected citations were then reviewed independently by two of three authors against all eligibility criteria, including an assessment of whether the intervention qualified as a nudge using the definitions outlined above. Discrepancies between reviewers were resolved through discussions until consensus was reached.
Data collection and data items
Data extraction and categorisation of interventions were carried out independently by two reviewers for each study. We extracted data on study characteristics (country, study years, sample size), nudge intervention description, types of infections targeted (eg, all, RTIs, urinary tract infections (UTIs)), outcomes and study results. When studies reported more than one outcome, we extracted results for the outcome measuring changes in overall antibiotic use, appropriate antibiotic use and any outcome defined as the primary outcome of the study. When a study trialled more than one nudge intervention, we extracted intervention data on the impact of each nudge individually.
Nudge interventions were classified using a taxonomy of choice architecture techniques (table 1),26 and we refer to these as nudge intervention categories.
Table 1.
Taxonomy of choice architecture techniques with implementation examples26
| Category | Technique | Technique examples |
| A. Decision information | A1. Translate information |
|
| A2. Make information visible |
|
|
| A3. Provide social reference point |
|
|
| B. Decision structure | B1. Change choice defaults |
|
| B2. Change option-related effort |
|
|
| B3. Change range or composition of options |
|
|
| B4. Change option consequences |
|
|
| C. Decision assistance | C1. Provide reminders |
|
| C2. Facilitate commitment |
|
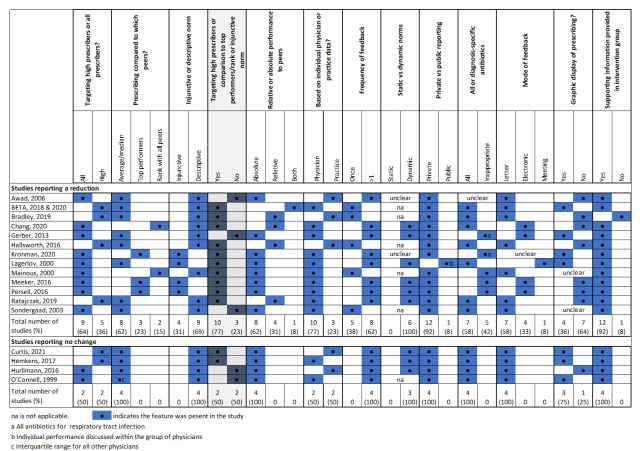
Social norm feedback nudge interventions are a frequent behaviour change technique in healthcare, often termed audit and feedback. Social norm feedback involves providing people with feedback on their performance relative to their peers. However, this can be implemented in a variety of way. For example, the comparison can be descriptive or injunctive, that is, associating a judgement to the performance. Psychology and health research has shown that certain features may enhance social norm feedback interventions,15–17 29 and thus, we extracted details of how social norm nudges were implemented (box 1), with the aim that this may further elucidate the important features of effective social norm nudges to reduce antibiotic prescribing in primary care.
Box 1. Social norm feedback nudge features extracted from studies.
Target of intervention: high antibiotic prescribers or all physicians.
The comparison group (average of group, top performers or rank within peers).
Use of injunctive or descriptive norm.
Frequency of feedback.
For studies with more than one round of feedback: whether the norm for comparison was static or dynamic (ie, did it change as the outcome change?).
Use of a static norm or dynamic norm (ie, one that changes with group performance).
Whether feedback was based on prescribing data for practices or individual physicians.
Whether the reported performance was relative or absolute.
Was the antibiotic use reported on for all antibiotics or for diagnoses where antibiotic use is inappropriate.
The mode of intervention delivery (eg, letter, email, meeting).
Whether a graphic representation of data was included.
Whether supporting information was provided to aid behaviour change.
Assessment of risk of bias in included studies
The risk of bias of each study was assessed using the Cochrane Effective Practice and Organisation of Care group’s tool for studies with a separate control group.30 Each study was independently assessed by two authors against each of the nine criteria assigning a score of either low risk, high risk or unclear risk of bias. Discrepancies were resolved through discussion. A summary assessment of the overall risk of bias was allocated to each study as follows: low risk of bias when all criteria were scored ‘low’, medium risk of bias when one or two criteria were scored ‘unclear’ or ‘high’ risk and high risk when more than two criteria scored ‘unclear’ or ‘high’.31
Synthesis of results
Inconsistencies in the outcomes and data reported in the studies precluded meta-analysis. Thus, we applied vote counting to summarise results for each category of nudge intervention and for features of social norm feedback nudges.32 Vote counting allows a comparison of the number of effects reporting a benefit to the number that showed no benefit. It is the recommended method by Cochrane for summarising studies when meta-analysis or other quantitative methods are not able to be applied.32 For each nudge intervention, we recorded whether the study demonstrated a reduction or no change in overall antibiotic prescribing compared with controls. As per the Cochrane Handbook, the statistical significance of the effect was not taken into account, so as not to erroneously conclude that underpowered studies had no effect. For studies with multiple study outcomes, we only considered the effect on overall antibiotic prescribing. The percentage of interventions with a reduction in overall antibiotic prescribing was calculated for all nudge interventions and social norm feedback nudges. Sensitivity analyses were conducted removing studies with a high risk of bias. CIs for proportions were calculated using the Wilson method. Effect sizes from studies were summarised narratively by reporting the range of change for overall antibiotic prescribing outcomes.
We used harvest plots to graphically summarise the vote counting results.33 In a harvest plot, each mark represents a study or intervention. We used the position of the mark to indicate whether the intervention effect (reduction or no change in overall antibiotic prescribing) and the size of the mark to indicate the risk of bias of the study (low-risk studies having a larger mark). Harvest plots were created for all nudge interventions by nudge category, and for social norm nudges by whether the intervention targeted high antibiotic prescribers or all prescribers, whether the comparison group was the average or above average performers and whether the feedback was a descriptive or injunctive norm. These features were chosen as there is evidence from the psychology literature that they play an important role in intervention effects and avoid possible negative impacts, such as the boomerang effect. Thus, the stratification of the social norm nudge interventions by these features aimed to examine if there was evidence these features were important for intervention effects in the context of antibiotic prescribing in primary care. Finally, results from studies that directly compared different nudge interventions, social norm nudge implementation strategies, or examined intervention effects over time or on different subgroups, were described narratively.
Public and patient involvement
Patients or the public were not involved.
Results
Nineteen studies were assessed as eligible for inclusion (figure 1).34–49 Table 2 presents the study characteristics. One study was a pilot study46 of a larger trial,44 but was included as a separate study as it was conducted in a different population. The majority of studies were conducted in Europe (n=8),36 38–41 47 48 six in the USA,37 42–44 46 two in Australia,35 45 two in China49 and one in Sudan.34 Seventeen studies were randomised controlled trials and two were regression discontinuity studies.36 47 Interventions were aimed at improving antibiotic use for all types of infections in nine studies,34–36 38 39 45 47 RTIs in eight studies,37 42–44 46 48 49 UTIs in one study41 and both RTIs and UTIs in one study.40
Figure 1.
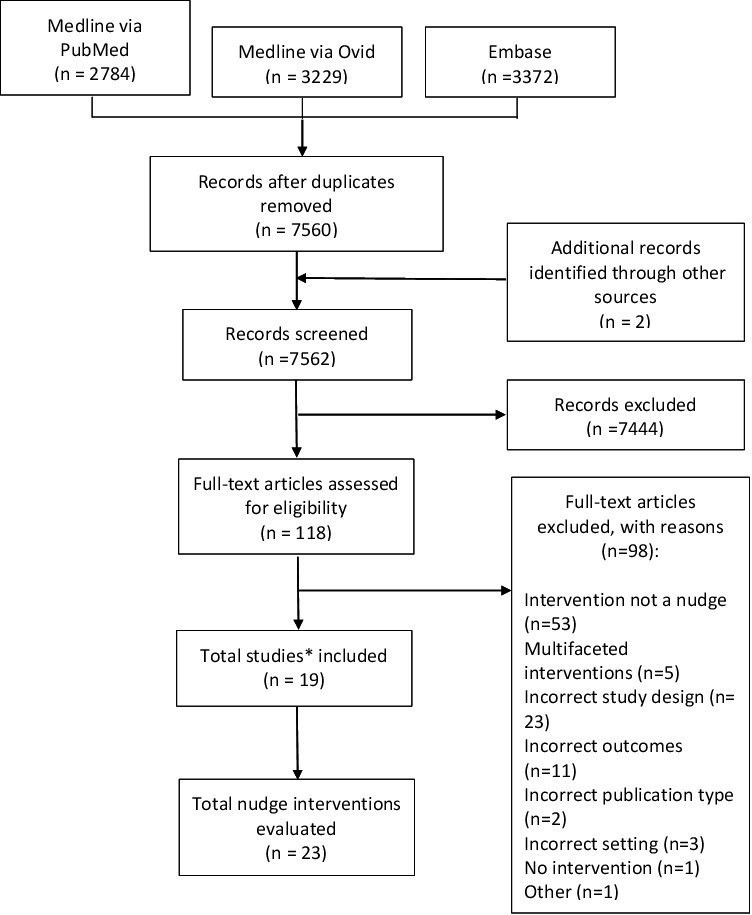
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart of search and screening results. *One study had two publications.
Table 2.
Characteristics of studies evaluating nudge interventions to improve antibiotic prescribing in primary care
| Author | Country | Sample size | Infections targeted | Nudge intervention/s | Outcomes of interest | Overall risk of bias* |
| Awad et al34 | Sudan | 20 practices | All | Social norm feedback | No. of consultations with AB; No. of consultations with an inappropriate AB† |
High |
| BETA35 50 | Australia | 6608 physicians | All | Social norm feedback | No. of ABs per 1000 consultations | Moderate |
| Bradley et al36 | Northern Ireland | 331 practices | All | Social norm feedback | No. of ABs per 1000 registered population | Moderate |
| Chang56 | China | 163 physicians | All | Social norm feedback | No. of AB prescriptions per 100 prescriptions | Moderate |
| Curtis et al51 | England | 1401 practices | All | Social norm feedback | % broad spectrum AB of all AB | Low |
| Gerber et al37 | USA | 162 physicians | RTI | Social norm feedback | % broad spectrum ABs among children with AB prescription; ABs for viral RTI |
High |
| Hallsworth et al38 | England | 1581 practices | All | Social norm feedback | No. of ABs per 1000 registered population | Low |
| Hemkens et al39 | Switzerland | 2900 physicians | All | Social norm feedback | Antibiotic DDD per 1000 consultations | Low |
| Hürlimann et al40 | Switzerland | 136 practices | RTI; UTI | Social norm feedback | % AB prescriptions for upper RTIs; % penicillins for RTI; % trimethoprim/sulfamethoxazole for UTI |
Moderate |
| Kronman et al52 | USA | 57 physicians | RTI | Social norm feedback | % of RTI with AB prescribed | Low |
| Lagerløv et al41 | Norway | 199 physicians | UTI | Social norm feedback | % inappropriate ABs for UTI | High |
| Mainous et al42 | USA | 216 physicians | RTI | Social norm feedback | % inappropriate AB treatments | Low |
| Meeker et al43 | USA | 14 physicians | RTI | Public commitment | No. of ABs per 100 AB inappropriate RTIs | Moderate |
| Meeker et al44 | USA | 244 physicians | RTI | Social norm feedback, accountable justification, suggested alternatives | No. of ABs per 100 AB inappropriate RTIs | Moderate |
| O'Connell et al45 | Australia | 2440 physicians | All | Social norm feedback | No. of ABs per 100 consultations | Moderate |
| Persell et al46 | USA | 28 physicians | RTI | Social norm feedback, accountable justification, suggested alternatives | No. of ABs per 100 RTIs; No. of ABs per 100 AB inappropriate RTIs |
High |
| Ratajczak et al47 | England | 6995 practices | All | Social norm feedback | No. of ABs per 1000 registered population | Moderate |
| Søndergaard et al48 | Denmark | 299 physicians | RTI | Social norm feedback | No. of ABs per 1000 registered population | Moderate |
| Yang et al49 | China | 20 practices (54 physicians) | RTI | Public reporting | % of RTI consultations with AB; % of RTI consultations with >1 AB |
Low |
*Risk of bias assessed using the Cochrane Effective Practice and Organisation of Care group’s tool for studies with a control group. Overall rating assigned ‘low’ when all criteria were ‘low’ risk; ‘medium’ when 1–2 criteria were scored ‘unclear’ or ‘high’ risk; and ‘high’ when >2 criteria were scored ‘unclear’ or ‘high’ risk.
†Inappropriate with respect to antibiotic, doses and/or duration.
AB, antibiotic; DDD, defined daily doses; RTI, respiratory tract infection; UTI, urinary tract infection.
Risk of bias in included studies
Four studies were rated as having a high overall risk of bias,34 37 41 46 nine as moderate risk of bias,35 36 40 43–45 47 48 and six as at low risk of bias (table 2).38 39 42 49 Overall scores of meeting risk of bias criteria ranged from 4/9 to 9/9 across studies. No single criterion was more frequently at high or unclear risk of bias across studies. Online supplemental file 3 shows the risk of bias assessment against each of the criteria for each study.
bmjopen-2022-062688supp003.pdf (75.4KB, pdf)
Description of nudge interventions
Seventeen studies evaluated one type of nudge intervention and two evaluated three types of nudge interventions each,43 44 with a total of 23 nudge interventions evaluated. Three studies compared different features of social norm nudges.35 42
Social norm feedback nudges (‘Decision information’ category of nudge interventions) were the most common intervention (n=17) evaluated.34–42 44–48 Implementation of social norm feedback varied between studies (figure 2). Social norm feedback was most commonly: sent to all prescribers (n=11)34 37 40–42 45 46 48 as opposed to the highest prescribers only35 36 38 39 47 50 51; and compared prescribing to the group average (n=12).34–36 38 40 41 45 47 48 50 Only four studies used an injunctive norm, which also provided positive reinforcement to those performing well.41 44 46 52 Feedback was typically provided more than once (n=11)34 37 39–41 44–46; based on prescribing data for individual physicians (n=12)35 37 39 41 42 44–48; and distributed via letters (n=11).34–36 38–40 42 45 47 48 Studies also cited application of other behavioural techniques or considerations in the design of their social norm feedback, such as the inclusion of actionable advice, and addressing the feedback letter from a high profile or respected individuals.
Figure 2.
Implementation features of social norm feedback nudge interventions.
Three interventions used nudge techniques from the ‘Decision structure’ category involving changing option consequences (table 3).44 46 49 Three interventions used techniques from the ‘Decision assistance’ category (table 3) involving providing reminders via suggested alternatives to antibiotic use (n=2)44 46 and a statement of public commitment to reducing antibiotic use in RTIs (n=1).43
Table 3.
Description of nudge and direction of effect on overall antibiotic prescribing in primary care (other than social norm feedback)
| Nudge category/ author | Type of nudge | Mode | Description | Intervention effect* |
| Decision structure—change option consequences | ||||
| Meeker et al44 | Accountable justification | Electronic health record | At time of prescribing an antibiotic, physicians were asked to justify their treatment decision in a mandatory free text field. The prompt informed physicians the justification would be visible in the patient’s record | Reduction |
| Persell et al46 | Accountable justification | Electronic health record | At time of prescribing an antibiotic, physicians were asked to justify their treatment decision in a mandatory free text field. The prompt informed physicians the justification would be visible in the patient’s record | No change |
| Yang et al49 | Public reporting | Posters and reports | Posters with antibiotic prescribing data were publicly displayed in the primary care clinics and reports with the data were sent to clinic managers and local health authorities | Reduction |
| Decision assistance—provide reminders | ||||
| Meeker et al44 | Suggested alternatives | Electronic health record | At time of prescribing an antibiotic, a pop-up screen stated antibiotics are generally not indicated for the diagnosis and showed a list of alternative treatments | Reduction |
| Persell et al46 | Suggested alternatives | Electronic health record | At time of prescribing an antibiotic, a computerised order set appeared with treatment alternatives and education materials for the patient | Reduction |
| Decision assistance—facilitate commitment | ||||
| Meeker et al43 | Public commitment | Poster | A poster-sized letter signed by physicians and posted in examination rooms indicating commitment to reducing antibiotics for RTIs | Reduction |
*Results of vote counting assessment based on nudge effect on overall antibiotic prescribing.
RTI, respiratory tract infection.
Effect of nudge interventions on overall antibiotic prescribing rates
Of the 23 nudge interventions evaluated, 78.3% (n=18, 95% CI 58.1 to 90.3) showed a reduction in overall antibiotic prescribing rates. Removing studies with a high risk of bias, the percentage of studies showing a reduction in overall antibiotic prescribing was 76.5% (n=13, 95% CI 52.7 to 90.4). Figure 3 shows the distribution of intervention effects by the type of nudge strategy evaluated.
Figure 3.
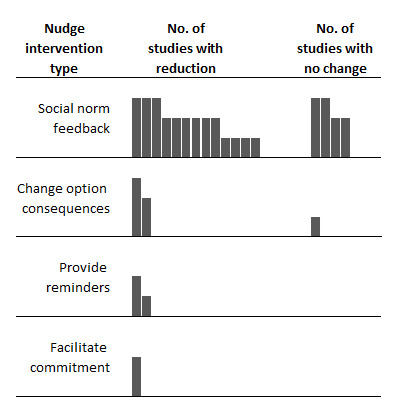
Harvest plot of effects of nudge interventions targeting antibiotic prescribing in primary care on overall antibiotic prescribing. Each mark or column represents one nudge intervention. Column height represents the risk of bias in the study: tallest columns are studies with low risk of bias; medium columns are moderate risk of bias; short columns are high risk of bias.
Of the 17 studies evaluating social norm feedback nudges, 76.5% (n=13, 95% CI 52.7 to 90.4) reported a reduction in overall antibiotic prescribing (figure 3). Removing studies with a high risk of bias, this percentage was 69.2% (n=9, 95% CI 42.4 to 87.3). Figure 4 shows social norm nudges stratified by whether they targeted only high prescribers or all prescribers, the comparison group and the use of an injunctive or descriptive norm. All but two (83%) studies targeting high prescribers or using an injunctive norm or a comparison to low prescribers reported a reduction in overall prescribing. Whereas 60% of studies without these features reported a reduction in antibiotic prescribing.
Figure 4.
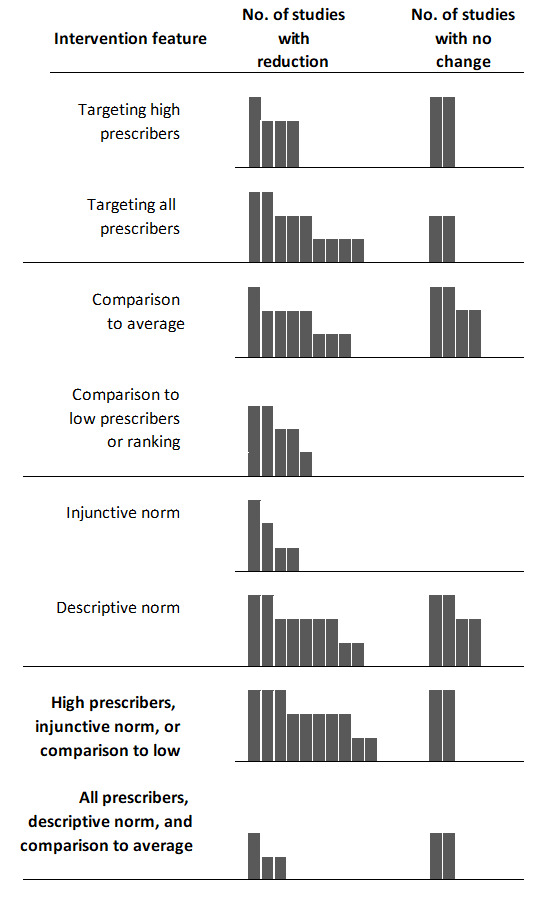
Harvest plot of effects of social norm feedback nudge interventions on overall antibiotic prescribing by implementation features. Each mark or column represents one nudge intervention. Column height represents the risk of bias of the study: tallest columns are studies with low risk of bias; medium columns are moderate risk of bias; short columns are high risk of bias.
Effect size of nudge interventions on antibiotic prescribing rates
The effect sizes of social norm feedback interventions on the number of antibiotics/1000 consultations (n=3) ranged from no change45 to a reduction of 13.6% (95% CI 16.6 to 10.6) at 6 months postintervention35; and the number of antibiotic prescriptions/1000 registered population (n=5) from no change51 to an approximate 5% reduction (−58.7/1000 population (95% CI 116.7 to 0.7)) 12-month postintervention.36
Studies measuring antibiotic prescribing for specific infection types reported absolute difference effect sizes of −1.2% (95% CI −10.5 to 8.2),40 −1.7% (p=0.93)37 and −5.2% (95% CI −6.9 to –1.6)44 in the proportion of upper RTI treated with an antibiotic; a relative decrease of 9.6% (p=0.0004)41 in inappropriate antibiotic for UTIs, and lower odds of antibiotic prescribing for RTI (OR: 0.73 (95% CI 0.53 to 0.995)).46
The effect sizes of the two studies of accountable justification interventions ranged from no change46 to a reduction of 7.0 percentage points (95% CI 9.1 to 2.9)44 in the number of antibiotics/100 antibiotic inappropriate infections. One study of public reporting showed a 1.93 percentage point reduction (95% CI −6.61 to 2.75) in the percentage of RTI consultations with an antibiotic, and a 6.97 percentage point (95% CI −13.9 to 0.00) reduction in the percentage of RTI consultations with>1 antibiotic.
Online supplemental file 4 provides details of the effects of interventions on outcomes.
bmjopen-2022-062688supp004.pdf (101KB, pdf)
Studies comparing the effects of different nudge interventions
Two studies compared the impact of three different types of nudge interventions on antibiotic prescribing for RTIs.44 46 One study (with a moderate risk of bias) examined the impact of nudges on RTI where an antibiotic was not indicated, that is, antibiotic inappropriate RTIs.44 This study reported a reduction in the prescribing of antibiotics for antibiotic inappropriate RTIs in the physician groups receiving social norm feedback and accountable justification nudges, and a non-significant reduction in the physician group receiving a suggested alternatives nudge intervention.44 The second study (high risk of bias) compared the same three nudge interventions, and reported a reduction in antibiotic prescribing for all RTIs for the social norm feedback and suggested alternative nudges, but not in the groups receiving the accountable justification nudges.46
Online supplemental file 4 provides details of the impact of interventions on outcomes and their vote counting results.
Social norm nudge effects over time and following repeat messaging
Two studies examined the effect of a single social norm nudge letter sent to high antibiotic prescribing physicians over time and both reported a diminishing effect on prescribing rates compared with controls over time.35 36 In one study, the effect of the intervention was examined over 12 months after the letter was sent.36 While there was a significant reduction in antibiotic prescribing compared with controls in the 12 months after the intervention, the effect diminished over time, such that the reductions in antibiotic prescribing rates in the second, third and fourth quarters after the intervention were not statistically significant. The second study also reported a diminishing effect of the social norm nudge letter over a 12-month period, but the reduction continued to remain significant at 12 months after the intervention.35 50
Two studies examined the impact of repeat social norm feedback interventions over time.39 47 In the first study, the effect of quarterly social norm feedback sent to the top 50% of antibiotic prescribers was assessed for 2 years.39 While there was no difference in overall antibiotic prescribing rates in the first and second years of the intervention, there was a significant reduction in the antibiotic prescribing for children and adolescents in the first year (−8.6%) and young to middle-aged adults in the second year of the intervention (−4.6%).
In the second study, a social norm nudge was first used in 2014 targeting the top 20% antibiotic prescribers, and due to its success was repeated annually since.47 The study evaluated whether the intervention reduced antibiotic prescribing by physicians who had previously received the letter and those that had not. The top 10% of prescribers did not reduce their prescribing whether or not they had previously been sent a letter. However, the top 11%–20% antibiotic prescribers reduced their antibiotic prescribing even when they had previously been sent a letter. The authors speculated that the failure of the top 10% to reduce antibiotic prescribing may have been due to the more forceful message in the communication they received (ie, that the great majority (90%) of practices prescribed fewer antibiotics), resulting in negative attitudes to the message and a lower behavioural intention to reduce prescribing.
Discussion
In this systematic review we have compiled the evidence on the effectiveness of nudge interventions in reducing antibiotic prescribing in primary care. Overall, 78.3% of studies reported a reduction in antibiotic prescribing. Social norm feedback was the most frequently evaluated nudge, and the evidence suggests that comparisons should include an aspirational target, injunctive norm or target high prescribers to enhance intervention effects. However, future research should explore the types of features that will further enhance social norm feedback nudges in this context. Only four studies examined nudge strategies other than social norm nudges, such as changing option consequences, providing reminders and facilitating commitment, thus further research is also needed to evaluate other nudge strategies despite promising results thus far of their effectiveness.
The studies included in this review trialled five different nudges (social norm feedback, accountable justification, public reporting, suggested alternatives and public commitment) from four of the nine subcategories of choice architecture techniques described by Münscher et al.26 Two other broad reviews of nudges targeting health providers reported identifying a similar number of nudges employed in their included studies, but the types of nudges applied differed to those that we identified.21 53 For example, changing choice defaults is a frequently applied nudge to guide healthcare provider behaviour, but was not used to influence antibiotic prescribing in our review.21 53 Another example of a nudge not applied in studies in our review, but used in other contexts targeting health providers is changing the framing of information.21 53 Thus, there is scope for implementing and evaluating other nudge techniques in the primary care setting to improve antibiotic use. This is important since it is currently not clear whether the same nudge applied over more than 1 year will continue to have sustained impact.
We attempted to elucidate whether features of social norm feedback nudges have a role in their effectiveness. For example, the behavioural economics literature suggests that social norm nudges should only be provided to poor performers (ie, high antibiotic prescribers in our case).25 This is because of the ‘boomerang effect’ that may occur in individuals performing above average when they are provided social norm feedback confirming their above average performance, that is, they reduce their performance. The studies in our review most frequently provided the social norm feedback to all prescribers (not only high prescribers) and all but one of these studies showed a reduction in overall antibiotic prescribing. However, the studies providing feedback to all prescribers also predominantly provided feedback more than once, which may have played a role in the reduction in antibiotic prescribing. Other factors that may have played a role in the prevention of a ‘boomerang’ effect in low prescribers, was the way the use of an injunctive norm and the comparison group used in the feedback. For example, one study informed the physicians with the lowest prescribing that they were a ‘top performer’, whereas the remaining physicians were informed they were ‘not a top performer’.44 The psychology literature supports the use of an injunctive norm when providing feedback, that is, conveying social approval or disapproval, as a way to eliminate the ‘boomerang’ effect.17 The study also compared physicians’ performance to the mean of the lowest decile prescribers, rather than the group mean.44 Our results showed that comparison of performance to the group mean, use of a descriptive norm and targeting all prescribers produced mixed results with three of five studies reporting a reduction in antibiotic prescribing. Thus, our results support the use of injunctive norms, comparisons to the lowest prescribers or targeting the highest prescribers.
The frequency of feedback may also play a role in social norm nudge effects. In the study described above that informed prescribers they were a ‘top performer’ or ‘not a top performer’, feedback was provided on a monthly basis, which allowed physicians to assess the degree to which they had changed their antibiotic prescribing.44 This is a different approach to studies that targeted only the high prescribers, that is, poor performers. These studies tended to provide the feedback once, informing the physicians that they prescribed at a higher rate than, for example, 80% of their peers.35 36 38 47 50 However, care should be taken when deciding on the comparison group, as if becoming a ‘top performer’ is perceived as unattainable, this can be demotivating. This can occur when the comparison norm is dynamic, that is, changes according the group’s behaviour, which was the case in all our studies that provided feedback more than once (figure 2). For example, if the comparison group is consistently the top 10%, 90% of people will never reach the target. One study included in our review reported that the top 10% of prescribers did not change their prescribing behaviour following the social norm nudge, despite an overall reduction following the intervention.47 The authors speculated this may be due to the message not motivating behaviour change. Furthermore, individuals need to trust the data being presented is an accurate representation of their performance, and in the case of antibiotic prescribing, adequately accounts for the clinical features of the populations they treat. Thus, it is crucial for there to be an understanding of factors that may affect the intervention during intervention design so as to maximise impact.23
It has been suggested that we can also learn from nudges that fail.14 54 There were four studies that implemented a social norm feedback nudge that had no effect on overall antibiotic prescribing.39 40 45 51 All four studies had two intervention features in common. First, the peer comparison used was the mean prescribing rate of the group or in the case of one study the IQR of the group. For those prescribers that were at the mean prescribing level or marginally below it, this may not have provided enough motivation to change their behaviour. Furthermore, as mentioned above, the ‘boomerang effect’ may occur in individuals performing above average. Second, the feedback in the four evaluations of social norm nudges that did not reduce overall prescribing was not provided from a high-profile or respected figure, which may have reduced the salience of the message.
The literature on audit and feedback interventions in healthcare provides insights into what features make these interventions more effective, and complement those from the behavioural economics and psychology literature.16 A Cochrane review found that feedback is more likely to be effective when: baseline performance is low; the source is a supervisor or colleague; the frequency is more than once; it is delivered both verbally and in written formats; and when feedback includes both targets and an action plan.15 Many of these features were included in the social norm nudges we identified in this review. For example, most of the social norm nudges included information on appropriate antibiotic prescribing in primary care. Thus, synthesising such evidence from behavioural economics and psychology is likely to enhance the effectiveness of these interventions.
This systematic review has a number of strengths. First, our search strategy was inclusive of all studies evaluating interventions to improve antibiotic prescribing in primary care. The selection of studies based on the type of intervention occurred at the full-text screening stage to ensure that studies not explicitly stating they used nudge techniques were included. Second, we used a comprehensive taxonomy of behavioural architecture techniques,26 rather than attempting to ascertain whether the underlying cognitive processes addressed by the intervention had the features of a nudge. However, there are a number of limitations. We were unable to perform a meta-analysis or summarise the results quantitatively due to the heterogenous reporting of study outcomes. Furthermore, though we aimed to examine the features of social norm nudges that may enhance their effectiveness, the variation with which these nudges were implemented across a small number of studies prevented firm conclusions being drawn. The need for further research to improve the effectiveness of social norm nudges, also sometimes called audit and feedback interventions, in healthcare is recognised. Nonetheless, this review has provided practical insights into the use of nudge interventions to reduce antibiotic use in primary care, and highlighted areas for further research.
Conclusions
Health systems worldwide continue to struggle to consistently deliver evidence-based care.55 Nudges can be used in lieu of, or to augment, more traditional efforts such as education (targeting clinicians, as well as the public), financial incentives, promotion of guidelines and changing models of care. Evaluation of nudges applied in healthcare will play a key role in identifying interventions suitable for use in different contexts, including primary care, and in further developing applications of nudge strategies to improve the delivery of effective healthcare services.
Supplementary Material
Footnotes
Twitter: @magdazr, @lli_sydney, @JWestbrook91
Contributors: MZR conceived the study with JW and BRN. MZR and GG designed the search strategy and GG ran the searches. MZR, GG and AN screened articles for inclusion with input from BRN. MZR, GG and AN conducted data extraction and quality assessments. LL and KS provided support for the compilation of results. MZR compiled results and wrote the initial manuscript draft. All authors contributed to the editorial process of the manuscript and approved the final manuscript. MZR has responsibility for the overall content of the manuscript as the guarantor.
Funding: This study was funded by a National Health and Medical Research Council (NHMRC) Partnership Project (2006957). MZR is supported by an NHMRC Early Career Fellowship (APP1143941). JW is supported by an NHMRC Elizabeth Blackburn Leadership Investigator Grant (APP1174021). The funding body had no role in the design of the study, data collection, analysis, interpretation or writing of the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Jee Y, Carlson J, Rafai E, et al. Antimicrobial resistance: a threat to global health. Lancet Infect Dis 2018;18:939–40. 10.1016/S1473-3099(18)30471-7 [DOI] [PubMed] [Google Scholar]
- 2.Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014;14:742–50. 10.1016/S1473-3099(14)70780-7 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Model List of Essential Medicines, 21st List. Geneva: WHO, 2019. [Google Scholar]
- 4.Klein EY, Milkowska-Shibata M, Tseng KK, et al. Assessment of who antibiotic consumption and access targets in 76 countries, 2000-15: an analysis of pharmaceutical sales data. Lancet Infect Dis 2021;21:107–15. 10.1016/S1473-3099(20)30332-7 [DOI] [PubMed] [Google Scholar]
- 5.Arnolda G, Hibbert P, Ting HP, et al. Assessing the appropriateness of paediatric antibiotic overuse in Australian children: a population-based sample survey. BMC Pediatr 2020;20:185. 10.1186/s12887-020-02052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pouwels KB, Dolk FCK, Smith DRM, et al. Actual versus ‘ideal’ antibiotic prescribing for common conditions in English primary care. J Antimicrob Chemother 2018;73:19–26. 10.1093/jac/dkx502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hay AD. Antibiotic prescribing in primary care. BMJ 2019;364:l780. 10.1136/bmj.l780 [DOI] [PubMed] [Google Scholar]
- 8.Leis JA, Born KB, Theriault G, et al. Using antibiotics wisely for respiratory tract infection in the era of covid-19. BMJ 2020;20:m4125. 10.1136/bmj.m4125 [DOI] [PubMed] [Google Scholar]
- 9.Lemiengre M, et al. Antibiotics for sinus infection of short duration in adults. Cochrane Database Syst Rev 10.1002/14651858.CD006089.pub5 [DOI] [Google Scholar]
- 10.Köchling A, Löffler C, Reinsch S, et al. Reduction of antibiotic prescriptions for acute respiratory tract infections in primary care: a systematic review. Implement Sci 2018;13:47. 10.1186/s13012-018-0732-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonkin-Crine SK, Tan PS, van Hecke O, et al. Clinician-targeted interventions to influence antibiotic prescribing behaviour for acute respiratory infections in primary care: an overview of systematic reviews. Cochrane Database Syst Rev 2017;9:CD012252. 10.1002/14651858.CD012252.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vodicka TA, Thompson M, Lucas P, et al. Reducing antibiotic prescribing for children with respiratory tract infections in primary care: a systematic review. Br J Gen Pract 2013;63:e445–54. 10.3399/bjgp13X669167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6:42. 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osman M, McLachlan S, Fenton N, et al. Learning from behavioural changes that fail. Trends Cogn Sci 2020;24:969–80. 10.1016/j.tics.2020.09.009 [DOI] [PubMed] [Google Scholar]
- 15.Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012;6).:CD000259. 10.1002/14651858.CD000259.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivers NM, Sales A, Colquhoun H, et al. No more 'business as usual' with audit and feedback interventions: towards an agenda for a reinvigorated intervention. Implement Sci 2014;9:14. 10.1186/1748-5908-9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz PW, Nolan JM, Cialdini RB, et al. The constructive, destructive, and reconstructive power of social norms. Psychol Sci 2007;18:429–34. 10.1111/j.1467-9280.2007.01917.x [DOI] [PubMed] [Google Scholar]
- 18.Hummel D, Maedche A. How effective is nudging? A quantitative review on the effect sizes and limits of empirical nudging studies. J Behav Exp Econ 2019;80:47–58. 10.1016/j.socec.2019.03.005 [DOI] [Google Scholar]
- 19.Adam A, Jensen JD. What is the effectiveness of obesity related interventions at retail grocery stores and supermarkets? -A systematic review. BMC Public Health 2016;16:1247. 10.1186/s12889-016-3985-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucher T, Collins C, Rollo ME, et al. Nudging consumers towards healthier choices: a systematic review of positional influences on food choice. Br J Nutr 2016;115:2252–63. 10.1017/S0007114516001653 [DOI] [PubMed] [Google Scholar]
- 21.Yoong SL, Hall A, Stacey F, et al. Nudge strategies to improve healthcare providers' implementation of evidence-based guidelines, policies and practices: a systematic review of trials included within Cochrane systematic reviews. Implement Sci 2020;15:50. 10.1186/s13012-020-01011-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox CR, Doctor JN, Goldstein NJ, et al. Details matter: predicting when nudging clinicians will succeed or fail. BMJ 2020;370:m3256. 10.1136/bmj.m3256 [DOI] [PubMed] [Google Scholar]
- 23.de Ridder D, Kroese F, van Gestel L. Nudgeability: mapping conditions of susceptibility to Nudge influence. Perspect Psychol Sci 2022;17:346–59. 10.1177/1745691621995183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thaler R, Sunstein C. Nudge: Improving decisions about health, wealth, and happiness. US: Penguin Putnam Inc, 2009. [Google Scholar]
- 26.Münscher R, Vetter M, Scheuerle T. A review and taxonomy of choice architecture techniques. J Behav Decis Mak 2016;29:511–24. 10.1002/bdm.1897 [DOI] [Google Scholar]
- 27.Hagemeier A, Samel C, Hellmich M. The regression discontinuity design: methods and implementation with a worked example in health services research. Z Evid Fortbild Qual Gesundhwes 2022;172:71–7. 10.1016/j.zefq.2022.04.014 [DOI] [PubMed] [Google Scholar]
- 28.Tang Y, Cook TD. Statistical power for the comparative regression discontinuity design with a pretest no-treatment control function: theory and evidence from the National head start impact study. Eval Rev 2018;42:71–110. 10.1177/0193841X18776117 [DOI] [PubMed] [Google Scholar]
- 29.Colquhoun HL, Carroll K, Eva KW, et al. Informing the research agenda for optimizing audit and feedback interventions: results of a prioritization exercise. BMC Med Res Methodol 2021;21:20. 10.1186/s12874-020-01195-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cochrane Effective Practice and Organisation of Care . Epoc resources for review authors: suggested risk of bias criteria for EPOC reviews, 2017. [Google Scholar]
- 31.Raban MZ, Gasparini C, Li L, et al. Effectiveness of interventions targeting antibiotic use in long-term aged care facilities: a systematic review and meta-analysis. BMJ Open 2020;10:e028494. 10.1136/bmjopen-2018-028494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins J, et al. Cochrane. In: Cochrane Handbook for systematic reviews of interventions version 6.2, 2021. [Google Scholar]
- 33.Ogilvie D, Fayter D, Petticrew M, et al. The harvest plot: a method for synthesising evidence about the differential effects of interventions. BMC Med Res Methodol 2008;8:8. 10.1186/1471-2288-8-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Awad AI, Eltayeb IB, Baraka OZ. Changing antibiotics prescribing practices in health centers of Khartoum state, Sudan. Eur J Clin Pharmacol 2006;62:135–42. 10.1007/s00228-005-0089-4 [DOI] [PubMed] [Google Scholar]
- 35.Behavioural Economics Team of the Australian Goverment (BETA) . Nudge vs Superbugs: A behavioural economics trial to reduce the overprescribing of antibiotics. Canberra: Department of the Prime Minister and Cabinet, 2018. [Google Scholar]
- 36.Bradley DT, Allen SE, Quinn H, et al. Social norm feedback reduces primary care antibiotic prescribing in a regression discontinuity study. J Antimicrob Chemother 2019;74:2797–802. 10.1093/jac/dkz222 [DOI] [PubMed] [Google Scholar]
- 37.Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA 2013;309:2345. 10.1001/jama.2013.6287 [DOI] [PubMed] [Google Scholar]
- 38.Hallsworth M, Chadborn T, Sallis A, et al. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet 2016;387:1743–52. 10.1016/S0140-6736(16)00215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hemkens LG, Saccilotto R, Reyes SL, et al. Personalized prescription feedback using routinely collected data to reduce antibiotic use in primary care: a randomized clinical trial. JAMA Intern Med 10.1001/jamainternmed.2016.8040 [DOI] [PubMed] [Google Scholar]
- 40.Hürlimann D, Limacher A, Schabel M, et al. Improvement of antibiotic prescription in outpatient care: a cluster-randomized intervention study using a sentinel surveillance network of physicians. J Antimicrob Chemother 2015;70:602–8. 10.1093/jac/dku394 [DOI] [PubMed] [Google Scholar]
- 41.Lagerløv P, Loeb M, Andrew M, et al. Improving doctors' prescribing behaviour through reflection on guidelines and prescription feedback: a randomised controlled study. Qual Health Care 2000;9:159–65. 10.1136/qhc.9.3.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mainous AG, Hueston WJ, Love MM, et al. An evaluation of statewide strategies to reduce antibiotic overuse. Fam Med 2000;32:22–9. [PubMed] [Google Scholar]
- 43.Meeker D, Knight TK, Friedberg MW, et al. Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med 2014;174:425–31. 10.1001/jamainternmed.2013.14191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA 2016;315:562–70. 10.1001/jama.2016.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Connell DL, Henry D, Tomlins R. Randomised controlled trial of effect of feedback on general practitioners' prescribing in Australia. BMJ 1999;318:507–11. 10.1136/bmj.318.7182.507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Persell SD, Doctor JN, Friedberg MW, et al. Behavioral interventions to reduce inappropriate antibiotic prescribing: a randomized pilot trial. BMC Infect Dis 2016;16:373. 10.1186/s12879-016-1715-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ratajczak M, Gold N, Hailstone S, et al. The effectiveness of repeating a social norm feedback intervention to high prescribers of antibiotics in general practice: a national regression discontinuity design. J Antimicrob Chemother 2019;74:3603–10. 10.1093/jac/dkz392 [DOI] [PubMed] [Google Scholar]
- 48.Søndergaard J, Andersen M, Støvring H, et al. Mailed prescriber feedback in addition to a clinical guideline has no impact: a randomised, controlled trial. Scand J Prim Health Care 2003;21:47–51. 10.1080/02813430310000564 [DOI] [PubMed] [Google Scholar]
- 49.Yang L, Liu C, Wang L, et al. Public reporting improves antibiotic prescribing for upper respiratory tract infections in primary care: a matched-pair cluster-randomized trial in China. Health Res Policy Syst 2014;12:61. 10.1186/1478-4505-12-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Behavioural Economics Team of the Australian Goverment (BETA) . Nudge vs Superbugs. Canberra: Department of the Prime Minister and Cabinet, 2020. [Google Scholar]
- 51.Curtis HJ, Bacon S, Croker R, et al. Evaluating the impact of a very low-cost intervention to increase practices' engagement with data and change prescribing behaviour: a randomized trial in English primary care. Fam Pract 2021;38:373–80. 10.1093/fampra/cmaa128 [DOI] [PubMed] [Google Scholar]
- 52.Kronman MP, Gerber JS, Grundmeier RW, et al. Reducing antibiotic prescribing in primary care for respiratory illness. Pediatrics 2020;146. 10.1542/peds.2020-0038. [Epub ahead of print: 03 08 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Last BS, Buttenheim AM, Timon CE, et al. Systematic review of clinician-directed nudges in healthcare contexts. BMJ Open 2021;11:e048801. 10.1136/bmjopen-2021-048801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sunstein CR. Nudges that fail. Behav Public Policy 2017;1:4–25. 10.1017/bpp.2016.3 [DOI] [Google Scholar]
- 55.Braithwaite J, Glasziou P, Westbrook J. The three numbers you need to know about healthcare: the 60-30-10 challenge. BMC Med 2020;18:102. 10.1186/s12916-020-01563-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang Y, Sangthong R, McNeil EB, et al. Effect of a computer network-based feedback program on antibiotic prescription rates of primary care physicians: a cluster randomized crossover-controlled trial. J Infect Public Health 2020;13:1297–303. 10.1016/j.jiph.2020.05.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062688supp001.pdf (62.6KB, pdf)
bmjopen-2022-062688supp002.pdf (63.7KB, pdf)
bmjopen-2022-062688supp003.pdf (75.4KB, pdf)
bmjopen-2022-062688supp004.pdf (101KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.