Abstract
TolC and its homologues are outer membrane proteins that are essential for the transport of many molecules across the cell envelope. In this study we characterized the gene encoding Vibrio cholerae TolC. V. cholerae tolC mutants failed to secrete the RTX cytotoxin, were hypersensitive to antimicrobial agents, and were deficient in intestinal colonization.
Cholera is an acute intestinal infection that is characterized by a profuse watery diarrhea which can quickly lead to dehydration and death. The causal agent for the disease cholera is Vibrio cholerae, a gram-negative bacterium often found in aquatic environments. Cholera is acquired by the ingestion of water and food contaminated with V. cholerae. Upon entering the host, V. cholerae colonizes the small intestine where it produces several virulence factors. Cholera is endemic in many parts of the developing world, including India, Asia, Africa, the Mediterranean, South and Central America, and Mexico. Epidemic cholera can occur when conditions of poor sanitation, crowding, war, and famine are present. In 1998, a total of 293,121 cases of cholera resulting in 10,586 deaths were reported to the World Health Organization (36).
The expression of many V. cholerae virulence factors is coordinately regulated in response to environmental factors via the ToxR, TcpP and ToxT regulatory proteins (21, 24). ToxR functions in conjunction with TcpP and ToxT to activate the expression of toxin-coregulated pilus (TCP), cholera toxin, accessory colonization factor, and additional virulence genes. ToxR, independent of TcpP and ToxT, inversely regulates the production of the OmpT and OmpU outer membrane porin proteins (3, 6). ToxR regulation of the OmpU and OmpT porins has been implicated in V. cholerae pathogenicity and bile resistance (27, 28). This result suggests that regulation of outer membrane permeability may be an important component in the pathogenicity and intrinsic antimicrobial resistance of V. cholerae.
In addition to decreased outer membrane permeability, the intrinsic antimicrobial resistance of many gram-negative bacteria is mediated by the expression of active efflux mechanisms (19). The combined activity of these two mechanisms functions to decrease the steady-state level of antimicrobial compounds within the cell (19) and thus effect alterations in antimicrobial sensitivity. Several different families of active efflux systems are involved in antimicrobial resistance in gram-negative bacteria (29). These include members of the resistance nodulation division (RND), major facilitator (MF), and ATP binding cassette (ABC) families (29). The RND family multiple drug efflux systems are of particular interest because of their unusually broad substrate specificity. Individual RND efflux pumps, such as the Escherichia coli acrAB (18) and Pseudomonas aeruginosa mexAB (26) systems, have been shown to efflux numerous chemically unrelated antimicrobial compounds, including dyes, detergents, and antibiotics.
Common among the gram-negative RND, MF, and ABC transport systems is the requirement for a TolC homologue to function as their outer membrane pore protein (23). In E. coli, for example, TolC is essential for type I protein secretion (i.e., RTX toxin [1]), colicin V export (9), multiple drug export (19), and colicin E1 uptake (22). The crystal structure for TolC was recently solved (15). TolC was found to form a channel, composed of an outer membrane-spanning β-barrel domain and a periplasm-spanning α-helical domain, to the external environment (15).
Homologues of E. coli TolC have been identified in numerous gram-negative bacteria (23). Whereas the transport systems in E. coli have evolved to share a single tolC allele as their outer membrane component, individual transport systems in many other bacterial species encode their own cognate TolC homologue. For example, the P. aeruginosa mexAB-oprM (16), mexCD-oprJ (25), and mexEF-oprN operons (14) each encode their own cognate outer membrane pore proteins (oprM, oprJ, and oprN, respectively) that are TolC homologues. Similar systems can be found in Helicobacter pylori where each RND system contains a gene encoding a distinct outer membrane protein (2). In these bacterial species, the functions attributed to TolC in E. coli are not linked to a single tolC allele but are distributed among the various tolC homologues and their cognate transport system.
In this study we sought to identify and characterize the role of the V. cholerae tolC gene in antimicrobial resistance and pathogenesis. Potential tolC candidate genes were identified by TBLASTX search of the V. cholerae genome (13) with E. coli TolC (accession number AAC76071). The results of this search identified five open reading frames (ORFs VC1409, VC1565, VC1606, VC1621, and VC2436) whose translated products possessed amino acid sequence similarity to TolC (Table 1). All the identified ORFs were localized on the large chromosome. Amino acid sequence alignments of the translated products of each of the V. cholerae ORFs with E. coli TolC revealed that VC2436 possessed the highest level of sequence similarity to E. coli TolC (Table 1).
TABLE 1.
V. cholerae tolC homologues
| ORF | Location (nt) | % Similaritya |
|---|---|---|
| VC1409 | 1501627 → 1503078 | 44.7 |
| VC1565 | 1676745 → 1678001 | 53.2 |
| VC1606 | 1720423 → 1721850 | 50.5 |
| VC1621 | 1741764 → 1743098 | 54.4 |
| VC2436 | 2610550 → 2611863 | 70.6 |
Percent similarity of the translated product to E. coli TolC (accession no. AAC76071).
Analysis of the regions flanking the five V. cholerae ORFs suggested that four of the five ORFs were linked to potential transport systems. ORFs VC1565 and VC1606 were linked to putative membrane fusion and ATP binding proteins—hallmarks of bacterial transport systems (data not shown). VC1621 was linked to a region of DNA containing strong similarity to RTX family toxin genes (data not shown), and VC1409 was linked to the recently described vceAB efflux system (5). Analysis of the vceAB locus revealed that the DNA regions flanking the vceAB genes, where VC1409 is located in N16961, are required for efflux activity (5). Based on these results we inferred that VC1409 functions as the outer membrane pore protein for the vceAB efflux system and did not consider VC1409 as a TolC candidate.
Insertion mutations were introduced into each of the remaining four ORFs in V. cholerae N16961Sm. Approximately 500-bp internal DNA fragments of each putative V. cholerae tolC homologue were amplified by PCR from genomic DNA of N16961Sm using the following PCR primer pairs (5′ 224 3′): VC1565F (TGG CCC AAC TTG AAC GTA ACC) and VC1565R (GTT TCG TTG ATC GCC GTC TT), VC1606F (AGT GTT GGC CAA AGA GGT GC) and VC1606R (GGA ATA TTC ACA CCA ACG CCA), VC1621F (CAG CAT ATC GTG CAT CGA GG) and VC1621R (GTG GGT CAA GAA CCT GTG AGC), and VC2436F (CCA TCA CGT CTT GCT CAC TCA) and VC2436R (GCA CGT GAC AAC ATT TCG CT). The resulting PCR amplification products were cloned into pCR2.1 using the TOPO DNA cloning kit from Invitrogen (Carlsbad, Calif.). The cloned internal fragments were subsequently excised from pCR2.1 with EcoRI and cloned into the EcoRI site of the suicide vector pGP704 (21) to generate pM1565, pM1606, pM1621, and pM2436. These plasmids were then mated into N16961Sm, and exconjugants were selected for resistance to ampicillin and streptomycin. Disruption of each of the specific V. cholerae tolC homologues in selected exconjugants was confirmed by PCR.
We hypothesized that null mutants in V. cholerae would be hypersensitive to multiple antimicrobial agents. Therefore, the resulting insertion mutants were individually analyzed for changes in antimicrobial susceptibility using a disk diffusion assay. To accomplish this, overnight cultures of each V. cholerae strain were independently diluted to approximately 104 CFU/ml and individually used to inoculate a lawn of cells onto the surface of Luria-Bertani (LB) agar plates. Paper disks impregnated with various detergents or antibiotics were then placed on the inoculated agar plates. Antibiotic-impregnated disks were used as supplied from the manufacturer (6 mm in diameter; BBL, Cockeysville, Md.). For the detergent-containing disks, paper disks (6 mm in diameter; BBL) were individually impregnated with 6 mg of deoxycholate or 4 mg of bile acids. Subsequently, the plates were incubated at 37°C for 14 to 18 h before zones of bacterial growth inhibition surrounding the paper disks were measured.
The antimicrobial compounds tested were previously shown to be substrates for efflux in E. coli and P. aeruginosa (16, 18) and are the same as those listed in Table 2. The results of these experiments revealed that mutation of VC2436 resulted in hypersensitivity to detergents and antibiotics while strains containing mutations in ORFs VC1565, VC1606, and VC1621 were unaffected in their susceptibility to any of these antimicrobial compounds (data not shown). Based on these results plus the sequence similarity of VC2436 to E. coli tolC and the complementation results (see below), we conclude that VC2436 encodes V. cholerae tolC and hereafter refer to VC2436 as tolC.
TABLE 2.
Antimicrobial susceptibility of V. cholerae strains
| Compound | Growth inhibition zone
(mm)a for:
|
|
|---|---|---|
| N16961Sm | N16961Sm-ΔtolC | |
| Bile acids | 6 (± 0.0) | 27 (± 1.2) |
| Chloramphenicol | 20.0 (± 1.2) | 20.0 (± 2.3) |
| Erythromycin | 19.8 (± 1.0) | 27.0 (± 1.2) |
| Gentamicin | 18.0 (± 0.0) | 18.0 (± 0.0) |
| Nalidixic acid | 28.8 (± 0.5) | 29.5 (± 2.4) |
| Novobiocin | 22.5 (± 0.6) | 25.0 (± 0.8) |
| Polymyxin B | 6.0 (± 0.0) | 9.0 (± 0.0) |
| Tetracycline | 26.0 (± 0.0) | 27.0 (± 1.2) |
Disk diameter is 6 mm. Standard deviation is indicated in parentheses.
Sequence analysis revealed that V. cholerae tolC encoded a 438-amino acid protein with a calculated molecular mass of 47.7 kDa. This protein is similar in size to E. coli TolC, which is composed of 495 amino acids and has a calculated molecular mass of 53.9 kDa. As for TolC homologues from other bacterial species, a general secretory pathway-dependent signal sequence was present (SignalIP probability of 1.00) with a predicted cleavage site located between amino acids 22 and 23 (SignalIP probability of 0.998).
During the course of the above experiments, we noticed that the tolC insertion mutant, in contrast to the other mutants, spontaneously lost the integrated plasmid at a high frequency. Correlated with plasmid loss was a reversion to wild-type deoxycholate resistance (data not shown). We therefore decided to construct a stable in-frame deletion of tolC for further analysis.
V. cholerae N16961Sm-ΔtolC was constructed by crossover PCR as follows. Oligonucleotide PCR primer pairs ΔtolC-F1/ΔtolC-R2 and ΔtolC-F2/ΔtolC-R1 (5′ 224 3′: ΔtolC-F1, GCA GGC AGC AGA GCA TTC A; ΔtolC-F2, TAG GAC CGA TGG ATG TCA ACG CAG GCC TA; ΔtolC-R1, GAC TTT GAA CGC TAT CGT G; and ΔtolC-R2, GTT GAC ATC CAT CGG TCC TAT TCC TGA CG) were used in separate PCR amplifications with N16961Sm chromosomal DNA as a template. The resulting PCR amplification products were purified, and 2 μl of each purified amplification product was subsequently used together in a second PCR (without added oligonucleotide PCR primers). A 2-μl aliquot of the second PCR amplification mixture was then used as the template in a third PCR amplification with use of the flanking ΔtolC-F1/ΔtolC-R1 oligonucleotide PCR primers. The PCR amplification product resulting from the third PCR amplification was cloned into pCR2.1 as described above. The cloned fragment was then collected from pCR2.1 as an EcoRI fragment and cloned into the EcoRI site of pWM91 (20). The pWM91-based plasmid was subsequently mated to N16961Sm, and exconjugants were selected for resistance to ampicillin and streptomycin. Several clones were subsequently patched onto LB agar and allowed to grow for 3 days, after which the colonies were resuspended in water and plated onto LB-no salt agar containing 5% sucrose to select for excision of the integrated plasmid. Several sucrose-resistant colonies were selected and screened by PCR for deletion of tolC using the ΔtolC-F1/ΔtolC-R1 oligonucleotide PCR primers.
Analysis of N16961Sm-ΔtolC by the disk diffusion assay confirmed our initial results obtained with the tolC insertion mutant. Interestingly, N16961Sm-ΔtolC was extremely sensitive to bile acids (Table 2). N16961Sm-ΔtolC also showed elevated sensitivity to the hydrophobic antibiotics erythromycin and novobiocin, but not to chloramphenicol, nalidixic acid, or tetracycline (Table 2); these antibiotics are common substrates for tolC-dependent efflux systems in other bacteria.
Bile resistance is likely to be an important adaptation for intestinal colonization (28). Bile is a detergent-like substance whose primary antimicrobial constituents consist of the detergents cholate and deoxycholate (32). Bile is secreted into the small intestines and functions to solubilize ingested dietary lipids (32). The concentrations of cholate and deoxycholate in the proximal duodenum have been reported to be in excess of 20 mM (7, 12). Analysis of N16961Sm-ΔtolC revealed that the in vitro N16961Sm-ΔtolC mean bactericidal concentration for bile was less than the reported in vivo bile concentration in the small intestine (Table 3). This observation may explain the colonization defect of the tolC mutant (see below).
TABLE 3.
V. cholerae tolC is required for bile resistance
| Compound | MBC (%)a for:
|
|
|---|---|---|
| N16961Sm | N16961Sm-ΔtolC | |
| Bile | <10 | 0.78 |
| Cholate | <10 | 0.78 |
| Deoxycholate | <10 | 0.78 |
| SDSb | <4 | 0.0097 |
MBC, mimimal bactericidal concentration.
SDS, sodium dodecyl sulfate.
We tested whether ectopic expression of V. cholerae ΔtolC could complement both an E. coli tolC mutant and N16961-ΔtolC to the wild-type level of deoxycholate resistance. V. cholerae tolC was cloned into the arabinose-inducible pBAD18 expression vector (11) as follows. Oligonucleotide PCR primers tolCF-NheI and tolCR-SphI (5′ 224 3′: tolCF-NheI, CCG CTA GCA TCA CGT CAG GAA TAG G, and tolCR-SphI, TTG CAT GCA GCT CAA AAG AGA TGG) were used to amplify tolC from N16961Sm chromosomal DNA. The resulting PCR product was digested with NheI and SphI restriction endonucleases and cloned into similarly digested pBAD18 to generate pBAD-tolC. pBAD-tolC was then electroporated into N16961Sm-ΔtolC and E. coli AG100(tolC). The resulting strains were subsequently analyzed for changes in antimicrobial susceptibility by the disk diffusion assay. The disk diffusion assays were performed as described above except for the presence of ampicillin to maintain the plasmid and either no arabinose or 0.2% arabinose for regulating expression from the arabinose promoter.
In N16961Sm-ΔtolC and E. coli AG100(tolC), ectopic expression of tolC from the arabinose promoter (in the presence of 0.2% arabinose) in pBAD-tolC complemented these strains to wild-type levels of deoxycholate resistance. This result supports the hypothesis that the antimicrobial hypersensitivity phenotype of N16961Sm-ΔtolC resulted from the mutation of tolC and that the V. cholerae tolC is functionally equivalent to E. coli tolC.
Salmonella enterica serovar Enteritidis tolC mutants have elevated sensitivity to the bactericidal effects of human serum (30, 33). Therefore, we tested whether N16961Sm-ΔtolC was altered in its susceptibility to human serum. Overnight cultures of N16961Sm and N16961Sm-ΔtolC were used to separately inoculate 5 ml of fresh LB broth (1:100 dilution), which was then incubated with shaking at 37°C for 3 h (late logarithmic phase). Then, 1-ml aliquots of each strain were removed, and the cells were collected by centrifugation, washed twice in phosphate-buffered saline (PBS), and suspended in 0.5 ml of PBS. One hundred microliters of each washed and diluted strain was then individually mixed with either 100 μl of human serum or 100 μl of PBS. The tubes were then placed on a rotary shaker at 37°C for 90 min, and aliquots from each reaction were serially diluted and plated onto LB agar for enumeration. Separate incubation of N16961Sm and N16961Sm-ΔtolC with human serum resulted in an identical 5-log reduction in the number of recovered cells for each strain; this finding suggests that there is no alteration in serum sensitivity associated with mutation of tolC.
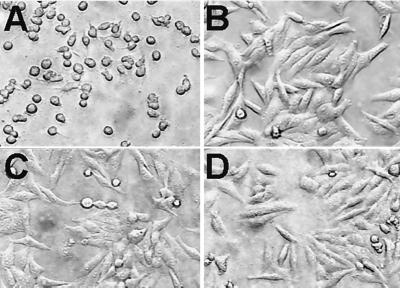
The V. cholerae RTX locus lacks a TolC homologue for transport of the RtxA cytotoxin across the outer membrane (17). Therefore, we tested whether any of the insertion mutants constructed for this study failed to secrete the RtxA cytotoxin. Cytotoxicity assays were performed as previously described (8, 17). Separate coincubation of HEp-2 cells with each of the four V. cholerae mutants revealed that mutation of tolC (VC2436) abolished in vitro cytotoxic activity (Fig. 1), while mutations in ORF VC1565, VC1606, or VC1621 had no effect on production of cytotoxic activity (data not shown). This observation suggests that tolC is required for RTX secretion and that there is little or no functional complementation among the remaining TolC homologues for RTX secretion.
FIG. 1.
Cytotoxicity of V. cholerae N16961Sm-tolC::pM2436 on tissue cultured HEp-2 cells. Subconfluent HEp-2 monolayers were exposed for 90 min to PBS-washed V. cholerae cells at a multiplicity of infection of 100. V. cholerae strains added were N16961Sm (A), mock (PBS) (B), N16961Sm-tolC::pM2436 (C), and N16961Sm-ΔrtxA (D).
The role of tolC in colonization was determined by a growth competition assay in the infant mouse small intestine colonization model (4). These experiments compared the in vivo growth phenotype of strains N16961Sm-ΔlacZ (wild type), N16961Sm-ΔrtxA, and N16961Sm-ΔtolC. Approximately 1.6 × 105 CFU each of strains N16961Sm-ΔtolC or N16961Sm-ΔrtxA plus N16961Sm-ΔlacZ were inoculated intragastrically into infant mice (input ratio of 1:1). Following 24 h of growth in the mice, we were unable to recover any N16961Sm-ΔtolC clones while recovering an average of 6.7 × 105 CFU/mouse of strain N16961Sm-ΔlacZ (total of five mice; standard deviation = 3.25 × 105 CFU). In contrast, N16961Sm-ΔrtxA was unaffected in its ability to colonize. The in vivo attenuated growth phenotype of strain N16961Sm-ΔtolC was not observed in vitro. When N16961Sm-ΔtolC and N16961Sm-ΔlacZ were separately inoculated in vitro into LB broth their growth rates were identical (data not shown). When the two strains were inoculated together in an in vitro competition assay, N16961Sm-ΔlacZ grew slightly better than N16961Sm-ΔtolC (data not shown).
One possible explanation for the observed in vivo competition results is that tolC is required for production of TCP, an essential colonization factor (34). Therefore TCP-specific transduction assays were used to test for alterations in TCP production in a tolC-negative background. Since only classical strains of V. cholerae produce TCP under normal laboratory conditions (growth in LB broth at pH 6.5 and 30°C), a tolC insertion mutation was introduced into the classical V. cholerae O395 strain by integration of pM2436 into the O395 tolC locus to generate strain O395-tolC. Phage transduction was carried out as described previously (35) and revealed that the transduction frequency for O395-tolC was identical to its parent strain (data not shown). This finding suggests that the tolC mutation has no effect on TCP production in vitro. Further supporting this finding, the TCP-dependent phenotype of autoagglutination was observed for O395-tolC under conditions optimal for TCP production.
The role of tolC in bacterial pathogenesis is not well understood. N16961Sm-ΔtolC was highly attenuated in in vivo growth competition assays. Although the growth of N16961Sm-ΔtolC was slightly attenuated in in vitro growth competition experiments, the in vitro growth deficiency was not sufficient to explain the large in vivo growth deficiency. Two possibilities that we have ruled out are the effects of the tolC mutation on RtxA secretion and TCP production. An alternative hypothesis is that the extreme bile acid sensitivity of N16961Sm-ΔtolC is responsible for the in vivo growth deficiency.
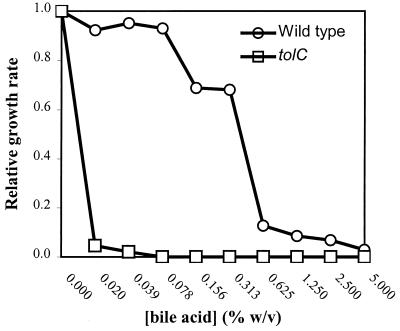
We hypothesize that ingested N16961Sm-ΔtolC encounters bile acid concentrations in the duodenum that are growth inhibitory and probably bactericidal. To test this hypothesis we compared the relative growth rates of N16961Sm-ΔtolC and its isogenic parent strain in various concentrations of bile acids (Fig. 2). This analysis revealed that the growth rate of N16961Sm-ΔtolC is dramatically reduced at bile acid concentrations as low as <0.02% (≈0.5 mM) (Fig. 2). This result supports our hypothesis and suggests that it is unlikely that N16961Sm-ΔtolC could tolerate the endogenous concentration of bile acids in the small intestine.
FIG. 2.
Effect of bile salts on relative growth rates of N16961Sm and N16961-ΔtolC. The relative exponential growth rates of N16961Sm (open circles) and N16961-ΔtolC (open squares) at 37°C in LB broth containing various amounts of bile salts are shown. The reported growth rates were normalized to the growth rates of the respective strains in the absence of bile salts.
The most likely mechanism by which TolC effects antimicrobial resistance (e.g., bile resistance) is mediated by members of the RND family of bacterial efflux systems (19). A cursory examination of the V. cholerae genome revealed the presence of six RND family efflux systems (data not shown). Interestingly, there were no TolC homologues linked to the RND efflux systems (nor were there any other linked ORFs resembling outer membrane proteins). Our results are consistent with the hypothesis that one or more of these RND efflux systems function as an antimicrobial efflux pump with tolC functioning as its cognate outer membrane channel.
This report adds to the accumulating data that suggest that bile resistance is an important virulence determinant in V. cholerae. This finding is evidenced by the overlapping mechanisms involved in bile resistance (e.g., the vceAB efflux system [5], the OmpT/OmpU porins [27, 28], and the work presented here) and the observation that bile is an effector molecule for the induction of ToxR (10) and a modulator of toxT activity (31).
Although the data presented here support our conclusions for the in vivo role of tolC in bile resistance and colonization, we cannot rule out the possibility that the tolC mutation results in other alterations that affect colonization, such as the production of other unknown adhesins, toxins, or other proteins important for colonization.
Considering the pleiotropic nature of the tolC mutant, assigning specific functional roles to tolC will require further studies aimed at identifying individual TolC-dependent transport systems and determining their contribution to the in vitro antimicrobial resistance and in vivo colonization phenotype of V. cholerae. Likely candidates for further analysis include genes encoding the V. cholerae RND, MF efflux systems, and ABC transporters.
Acknowledgments
J.E.B. thanks B. Guo for help with the mouse experiments, K. Fullner for help with the RTX assays and for providing the in vivo data for V. cholerae-ΔrtxA, C. Walchle for help in constructing V. cholerae-ΔrtxA, and the many members of the Mekalanos laboratory for their helpful suggestions and critical reading of the manuscript.
This research was supported by grant AI-18045 from the National Institutes of Health. J.E.B. was supported by a Postdoctoral Fellowship from the Cystic Fibrosis Foundation.
REFERENCES
- 1.Bhakdi S, Mackman N, Menestrina G, Gray L, Hugo F, Seeger W, Holland I B. The hemolysin of Escherichia coli. Eur J Epidemiol. 1988;4:135–143. doi: 10.1007/BF00144740. [DOI] [PubMed] [Google Scholar]
- 2.Bina J E, Alm R A, Uria-Nickelsen M, Thomas S R, Trust T J, Hancock R E. Helicobacter pyloriuptake and efflux: basis for intrinsic susceptibility to antibiotics in vitro. Antimicrob Agents Chemother. 2000;44:248–254. doi: 10.1128/aac.44.2.248-254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakrabarti S R, Chaudhuri K, Sen K, Das J. Porins of Vibrio cholerae: purification and characterization of OmpU. J Bacteriol. 1996;178:524–530. doi: 10.1128/jb.178.2.524-530.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang S L, Taylor R K, Koomey M, Mekalanos J J. Single amino acid substitutions in the N terminus of Vibrio choleraeTcpA affect colonization, autoagglutination, and serum resistance. Mol Microbiol. 1995;17:1133–1142. doi: 10.1111/j.1365-2958.1995.mmi_17061133.x. [DOI] [PubMed] [Google Scholar]
- 5.Colmer J A, Fralick J A, Hamood A N. Isolation and characterization of a putative multidrug resistance pump from Vibrio cholerae. Mol Microbiol. 1998;27:63–72. doi: 10.1046/j.1365-2958.1998.00657.x. [DOI] [PubMed] [Google Scholar]
- 6.Crawford J A, Kaper J B, DiRita V J. Analysis of ToxR-dependent transcription activation of ompU, the gene encoding a major envelope protein in Vibrio cholerae. Mol Microbiol. 1998;29:235–246. doi: 10.1046/j.1365-2958.1998.00925.x. [DOI] [PubMed] [Google Scholar]
- 7.de Kok T M, van Faassen A, Glinghammar B, Pachen D M, Eng M, Rafter J J, Baeten C G, Engels L G, Kleinjans J C. Bile acid concentrations, cytotoxicity, and pH of fecal water from patients with colorectal adenomas. Dig Dis Sci. 1999;44:2218–2225. doi: 10.1023/a:1026644418142. [DOI] [PubMed] [Google Scholar]
- 8.Fullner K J, Mekalanos J J. In vivo covalent cross-linking of cellular actin by the Vibrio choleraeRTX toxin. EMBO J. 2000;19:5315–5323. doi: 10.1093/emboj/19.20.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilson L, Mahanty H K, Kolter R. Genetic analysis of an MDR-like export system: the secretion of colicin V. EMBO J. 1990;9:3875–3894. doi: 10.1002/j.1460-2075.1990.tb07606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Chowdhury R. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect Immun. 1997;65:1131–1134. doi: 10.1128/iai.65.3.1131-1134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzman L M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt P R. The roles of bile acids during the process of normal fat and cholesterol absorption. Arch Intern Med. 1972;130:574–583. [PubMed] [Google Scholar]
- 13.Heidelberg J E E, Nelson W C, Clayton R A, Ginn M L, Dodson R J, Haft D H, Hickey E K, Peterson J D, Umayam L, Gill S R, Nelson K E, Read T D, Tettelin H, Richardson D, Ermolaeva M D, Vamathevan J, Bass S, Qin H, Dragoi I, Sellers P, McDonald L, Utterback T, Fleishmann R D, Nierman W C, White O, Salzberg S L, Smith H O, Colwell R R, Mekalanos J J, Venter J C, Fraser C M. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohler T, Michea-Hamzehpour M, Henze U, Gotoh N, Curty L K, Pechere J C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 15.Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–919. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- 16.Li X Z, Nikaido H, Poole K. Role of mexA-mexB-oprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–1953. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin W, Fullner K J, Clayton R, Sexton J A, Rogers M B, Calia K E, Calderwood S B, Fraser C, Mekalanos J J. Identification of a Vibrio choleraeRTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci USA. 1999;96:1071–1076. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 19.Ma D, Cook D N, Hearst J E, Nikaido H. Efflux pumps and drug resistance in gram-negative bacteria. Trends Microbiol. 1994;2:489–493. doi: 10.1016/0966-842x(94)90654-8. [DOI] [PubMed] [Google Scholar]
- 20.Metcalf W W, Jiang W, Daniels L L, Kim S K, Haldimann A, Wanner B L. Conditionally replicative and conjugative plasmids carrying lacZalpha for cloning, mutagenesis, and allele replacement in bacteria. Plasmid. 1996;35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 21.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otsuji N, Soejima T, Maki S, Shinagawa H. Cloning of colicin E1 tolerant tolC (mtcB) gene of Escherichia coliK12 and identification of its gene product. Mol Gen Genet. 1982;187:30–36. doi: 10.1007/BF00384379. [DOI] [PubMed] [Google Scholar]
- 23.Paulsen I T, Park J H, Choi P S, Saier M H J. A family of Gram-negative bacterial outer membrane factors that function in the export of proteins, carbohydrates, drugs and heavy metals from Gram-negative bacteria. FEMS Microbiol Lett. 1997;156:1–8. doi: 10.1111/j.1574-6968.1997.tb12697.x. [DOI] [PubMed] [Google Scholar]
- 24.Peterson K M, Mekalanos J J. Characterization of the Vibrio choleraeToxR regulon: identification of novel genes involved in intestinal colonization. Infect Immum. 1988;56:2822–2829. doi: 10.1128/iai.56.11.2822-2829.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J, Li X Z, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 26.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Provenzano D, Klose K E. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio choleraebile resistance, virulence factor expression, and intestinal colonization. Proc Natl Acad Sci USA. 2000;97:10220–10224. doi: 10.1073/pnas.170219997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Provenzano D, Schuhmacher D A, Barker J L, Klose K E. The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio choleraeand other pathogenic Vibrio species. Infect Immun. 2000;68:1491–1497. doi: 10.1128/iai.68.3.1491-1497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saier M H J, Paulsen I T, Sliwinski M K, Pao S S, Skurray R A, Nikaido H. Evolutionary origins of multidrug and drug-specific efflux pumps in bacteria. FASEB J. 1998;12:265–274. doi: 10.1096/fasebj.12.3.265. [DOI] [PubMed] [Google Scholar]
- 30.Schnaitman C A, Klena J D. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol Rev. 1993;57:655–682. doi: 10.1128/mr.57.3.655-682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuhmacher D A, Klose K E. Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae. J Bacteriol. 1999;181:1508–1514. doi: 10.1128/jb.181.5.1508-1514.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shlygin G K. The physiology of intestinal digestion. Prog Food Nutr Sci. 1977;2:249–306. [PubMed] [Google Scholar]
- 33.Stone B J, Miller V L. Salmonella enteritidis has a homologue of tolCthat is required for virulence in BALB/c mice. Mol Microbiol. 1995;17:701–712. doi: 10.1111/j.1365-2958.1995.mmi_17040701.x. [DOI] [PubMed] [Google Scholar]
- 34.Thelin K H, Taylor R K. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio choleraeO1 El Tor biotype and O139 strains. Infect Immum. 1996;64:2853–2856. doi: 10.1128/iai.64.7.2853-2856.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1944. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. Cholera 1998. Wkly Epidemiol Rec. 1999;74:257–264. [PubMed] [Google Scholar]