Abstract
Multidrug resistance (MDR) is a major obstacle in the therapy of infectious diseases and cancer. One of the major mechanisms of MDR is the overexpression of efflux pumps (EPs) that are responsible for extruding antimicrobial and anticancer agents. EPs have additional roles of detoxification that may aid the development of bacterial infection and the progression of cancer. Therefore, targeting EPs may be an attractive strategy to treat bacterial infections and cancer. The development and discovery of a new drug require a long timeline and may come with high development costs. A potential alternative to reduce the time and costs of drug development is to repurpose already existing drugs. Antidepressants and antipsychotic agents are widely used in clinical practice in the treatment of psychiatric disorders and some somatic diseases. Antidepressants and antipsychotics have demonstrated various beneficial activities that may be utilized in the treatment of infections and cancer. This review aims to provide a brief overview of antibacterial and anticancer effects of selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs) and phenothiazine antipsychotics, while focusing on EPs. However, it should be noted that the antimicrobial activity of a traditionally non-antibiotic drug may have clinical implications regarding dysbiosis and bacterial MDR.
Keywords: drug repurposing, MDR efflux pumps, multidrug resistance, antidepressant, antipsychotic, selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), phenothiazines
1. Introduction
Drug repurposing (or drug repositioning, reprofiling, or re-tasking) is a strategy to find new applications for approved drugs that are outside the scope of the original medical indication [1,2]. The strategy of drug repositioning or repurposing rules out the structural modification of the drugs, it means a new indication regarding the biological properties or therapeutic use such as a new formulation, a new dose or via a new route of administration [2]. There are several advantages of drug repurposing, such as a lower risk of failure and better safety profile, shorter drug development procedures because of the already completed preclinical tests, and lower financial costs [2]. The repositioning of drugs could be an attractive alternative to treat human diseases such as cancer, infectious diseases, neurodegenerative and autoimmune diseases [3]. The approach has to face several challenges, including the lack of knowledge on regulatory requirements, financial and resource difficulties, new problems in clinical trials resulting in missing or inadequate proof, intellectual property issues that can hamper the commercialization of the given drug, and market analysis [4].
Microorganisms naturally develop antimicrobial resistance as a result of interactions with their environment [5]. The number of infections caused by multidrug resistant (MDR) bacteria is increasing as a consequence of selection pressure from the widespread use of antibiotics [6]. However, the MDR phenotype is not unique to microbes, since MDR cancer is a serious hindrance to oncological treatment leading to relapses and recurrences [7]. There are similarities in the drug resistance mechanisms of bacteria and cancer cells. Drug resistance in bacteria and cancer may be intrinsic or acquired, and may involve the inactivation of the drug, overexpression of efflux pumps (EPs), and modification of drug targets [8,9]. The role of EPs in bacteria and cancer is more than just detoxification. In bacteria, EPs may mediate cell–cell communication and biofilm formation, thus enabling bacteria to adapt to various environmental stimuli and contributing to the virulence of bacteria [10]. In cancer, EPs may promote cancer growth not only by extruding anticancer drugs but also by other mechanisms that are related to cell proliferation and metastasis [11].
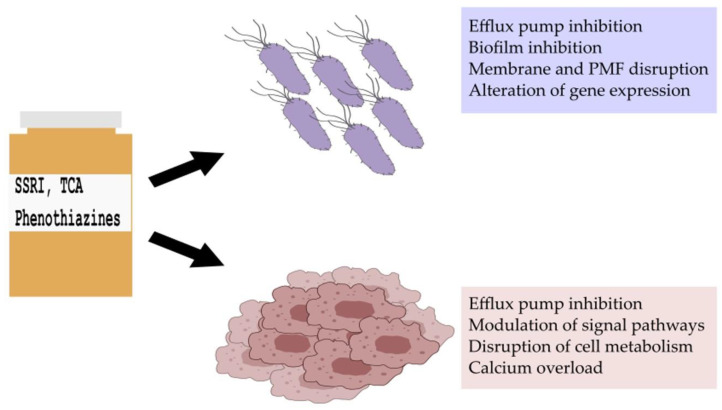
Antidepressants, especially selective serotonin reuptake inhibitors (SSRIs), are widely prescribed in clinical practice to treat mood and anxiety disorders. In cancer patients, besides treating symptoms of depression and anxiety, they can be used as adjuvants in the therapy of neuropathic pain; additionally, SSRIs can alleviate hot flashes caused by hormone therapy [12]. Antipsychotics in oncology are also essential in supportive therapy for managing cancer-related delirium and other psychotic disorders [12]. Moreover, they may be preferred to benzodiazepines to treat acute anxiety and insomnia, since benzodiazepines may be delirogenic and cause respiratory depression [12]. The antiemetic effects of antipsychotics can be beneficial in the treatment of chemotherapy-induced nausea and the side effects of increased appetite and weight gain can help in cancer-related anorexia [12]. Numerous studies have emerged indicating that psychiatric drugs may have anti-inflammatory, antimicrobial, anticancer properties, and they may be attractive candidates to combat bacterial multidrug resistance and cancer (Figure 1) [13,14,15]. In this review, we aim to present an overview of the antibacterial, anticancer, and efflux pump modulating activity of SSRIs, tricyclic antidepressants (TCAs), and phenothiazine-type antipsychotics (Figure 2 and Figure 3).
Figure 1.
Antibacterial, anticancer, and efflux pump-modulating activity of selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and phenothiazine-type antipsychotics. PMF: proton motive force.
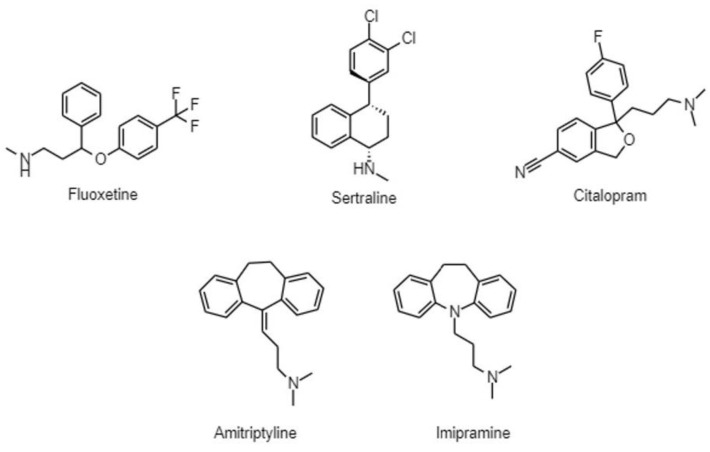
Figure 2.
Chemical structures of antidepressants.
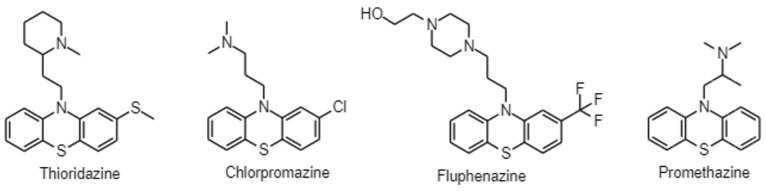
Figure 3.
Chemical structure of phenothiazines.
2. Antibacterial Resistance
Drug resistance is a natural process and is based on the interaction of organisms with their environment [16]. However, during the last decades, the occurrence of drug-resistant microbial infections has increased dramatically which can be explained by the global spread of drug resistant microbes (bacteria, fungi, viruses, and parasites) and the extensive use and misuse of antimicrobial agents. The spread of antibiotic resistance is related to high mortality rates and high costs regarding the therapy and hospitalization of patients [17].
Concerning the antibiotic resistance of bacteria, there are natural (intrinsic) and acquired (extrinsic) resistance mechanisms. In the former case, the cells can be considered to be resistant even before encountering the harmful agents and this resistance is a trait of a species or genus. In contrast, the acquired resistance arises from mutations and horizontal gene transfer [16].
There are numerous antibiotic resistance mechanisms such as restricted penetration, drug efflux, target modification, destruction/modification of the antibiotics, target switching, and target sequestration [18]. Out of these mechanisms, the overexpression of the efflux pump can result in higher virulence of bacteria because these pumps can contribute to bacterial communication (quorum sensing) and biofilm formation [10].
2.1. Bacterial Efflux Pumps
Bacterial efflux pumps have the capacity to regulate the internal environment by removing harmful agents, metabolites as well as cell–cell communication signal molecules. The expression of efflux pumps is precisely regulated by different local and global transcriptional regulators suggesting that these pumps have essential physiological functions. Concerning the physiological functions, efflux pumps are crucial in stress adaptation, pathogenesis, and virulence of bacteria. The over-expression of multidrug efflux pumps has been increasingly found to be associated with clinically relevant drug resistance [19]. There are six EP families based on their membrane topology, energy coupling mechanisms as well as substrate specificities. These EP families are as follows: ATP-binding cassette (ABC) family, multidrug and toxic compound extrusion (MATE) family, small multidrug resistance (SMR) family, major facilitator superfamily (MFS), resistance nodulation division (RND) family, and proteobacterial antimicrobial compound efflux (PACE) family [19].
2.2. Bacterial Efflux Pump Inhibitors (EPIs)
The abolishment of efflux mechanisms could be achieved in different ways: (1) the downregulation of the expression of EP genes, (2) the re-design of antibiotics and development of new antibiotics, (3) the inhibition of the assembly of functional pumps, (4) the inhibition of the substrate binding by competitive or non-competitive inhibitors, (5) blocking the outer membrane channel, as well as (6) interference with the energy supply of pumps [20].
Efflux pump inhibitors alone could sensitize bacteria to antibiotics before antibiotic treatment or in combination they could increase the susceptibility of resistant strains by increasing the intracellular concentration of antibiotics. EPIs alone or in combination with conventional antibiotics are able to block multidrug efflux systems. The first EPI compound MC-207,110 (Phe-Arg-β-napthylamide or PAβN) was described in 2001 that could inhibit the clinically relevant efflux pumps of Pseudomonas aeruginosa; furthermore, it could inhibit other RND-type pumps of Gram-negative bacteria [21].
Classification of EPIs is a difficult task because some inhibitors are pump specific, while others are not and have multiple targets in the bacterial cells. Based on their origin, bacterial EPIs can be classified into two major groups: natural bioactive agents and synthetic compounds [22]. However, drug development is a time-consuming and expensive way to find new EPIs. An auspicious strategy could be the drug repurposing approach that applies existing drugs in a new indication, in this case, to treat infections. This perspective has numerous advantages such as lower costs and a short development period [21].
2.2.1. Selective Serotonin Reuptake Inhibitors (SSRIs) and Serotonin and Norepinephrine Reuptake Inhibitors (SNRIs)
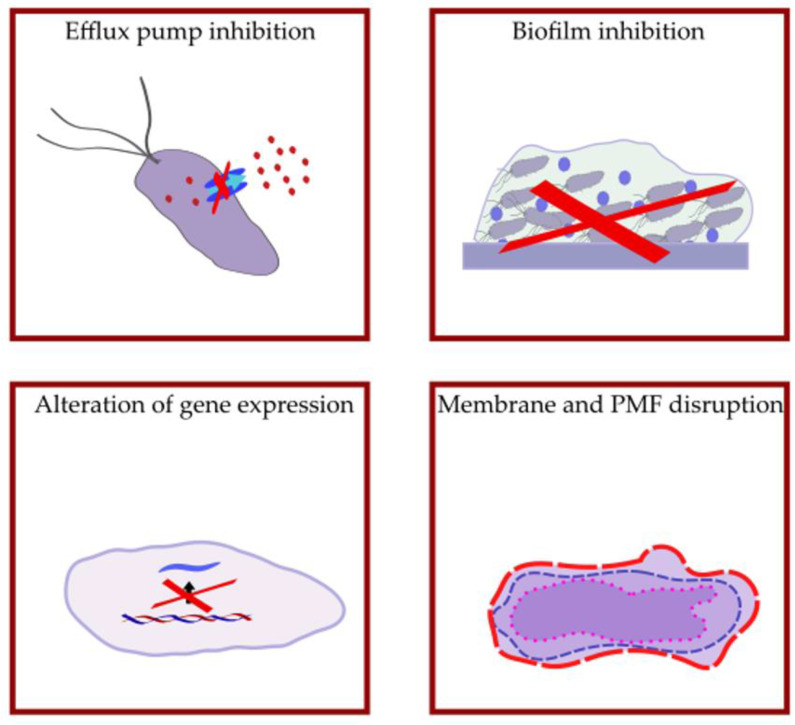
It has been demonstrated that selective serotonin reuptake inhibitors (SSRIs) possess antimicrobial activity [23]. According to Kaatz et al., phenylpiperidine-SSRIs exerted mild intrinsic antimicrobial activity against Staphylococcus aureus, P. aeruginosa, and Escherichia coli expressing MDR efflux pumps. One isomer of paroxetine showed EPI activity against MFS-(NorA and TetK) and RND-class (AcrB) efflux pumps (Figure 4) [24,25].
Figure 4.
Antibacterial activity of selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and phenothiazine-type antipsychotics. PMF: proton motive force.
Sertraline is able to inhibit bacterial growth and the activity of efflux pumps in E. coli; furthermore, increased expression of marA and acrB was detected after sertraline treatment. It can be concluded that sertraline could influence the activity of efflux pumps and the expression of MDR and efflux-related genes; however, the plasma concentration in the patients is below the concentrations that is required for efflux pump inhibition (10–150 ng/mL in patients versus 30.6 μg/mL in the assay) [26].
Fluoxetine treatment was effective against the biofilm formation of the urinary tract pathogen Proteus mirabilis. It is known that efflux pumps are essential for biofilm production, and they can promote the colonization of P. mirabilis. Molecular docking results confirmed that fluoxetine attaches to the channel region of the biofilm-associated Bcr/CflA transporter (Figure 4) [27].
Citalopram acted synergistically with antibiotics against Gram-positive and Gram-negative bacterial strains. Resistance of E. coli to cefixime and P. aeruginosa to cloxacillin was reversed in the presence of citalopram by disk diffusion method. A total of 310 µg/mL of citalopram combined with levofloxacin, moxifloxacin, and gentamicin enhanced the activity of these antibiotics against S. aureus [28].
Fluvoxamine exerted potent antibacterial activity against E. coli, Klebsiella pneumoniae, Acinetobacter baumanii, and Staphylococcus epidermidis by disc diffusion assay as described in the study of Kalaycı et al., who tested the antimicrobial activity of various psychotropic drugs against bacteria [29]. However, it should be pointed out that the chronic use of antidepressant medication can influence the gut microbiota leading to potential adverse effects [30].
The widely used antidepressant duloxetine acted synergistically with chloramphenicol in E. coli contributing to the evolution and spread resistant E. coli clones. The expression levels of acrA, acrB, and marA genes were upregulated 1.7–2.2-fold by combined duloxetine and chloramphenicol treatment compared to the exposure to the single drugs. With the enhancement of the AcrAB-TolC system, multidrug resistant E. coli clones can evolve and cause the emergence of resistant strains in the gut. Since the gut microbiota may have been exposed to non-antibiotic pharmaceuticals and antibiotics for a longer period of time, bacteria can develop resistance towards these agents in vivo [31].
Using the disk diffusion technique, 600 µg/mL of venlafaxine improved the activity of ciprofloxacin, levofloxacin, norfloxacin, and moxifloxacin against P. aeruginosa. It is suspected that venlafaxine can inhibit bacterial efflux pumps and can compete with the substrates of these pumps [28].
2.2.2. Tricyclic Antidepressants (TCA)
The interaction of amitriptyline and norfloxacin was synergistic against Salmonella Typhimurium [32]. Furthermore, it has been confirmed that amitriptyline can potentiate the activity of AcrB substrates. In combination with antibiotics, amitriptyline increased the activity of chloramphenicol, nalidixic acid, and tetracycline against S. Typhimurium overexpressing the AcrAB-TolC efflux system. In addition, besides its intrinsic antimicrobial activity, amitriptyline also augmented the susceptibility of A. baumannii to chloramphenicol. It was confirmed that amitriptyline is an AcrB substrate, and it is able to bind to residues of AcrB that are crucial for substrate recognition and/or transport via AcrAB-TolC system [33]. Imipramine had a slight antibacterial effect against methicillin-susceptible and -resistant S. aureus; however, the concentrations applied in the assay cannot be used in patients (the therapeutic concentration is 175–300 ng/mL versus the bacterial MIC of 128 μg/mL) [34]. The tricyclic psychopharmacons imipramine and desipramine possessing non-planar tricyclic skeletons become relatively inactive on saturation of the ring system regarding their antibacterial effect. Furthermore, imipramine and desipramine were able to eliminate the F’lac plasmid from E. coli K12 strain and in this aspect, these compounds could reverse the spread of resistance mediated by plasmids [35].
2.2.3. Phenothiazines
Many of the phenothiazines possess wide-ranging antibacterial activity against Mycobacteria, some Gram-positive and Gram-negative bacteria [36]. Regarding the mode of action, phenothiazines can target the bacterial membrane, the nucleic acids, and the energy supply of the pumps. Since these compounds may also be AcrB substrates, their interaction is not selective [36]. The application of a subinhibitory concentration of a non-antibiotic substance such as phenothiazines can enhance the susceptibility of bacteria towards antibiotics indicating that efflux mechanisms are involved in this phenomenon (Figure 4) [37].
Thioridazine (TZ), chlorpromazine (CPZ), and fluphenazine reduced NorA-mediated ethidium bromide efflux by at least half indicating that these phenothiazines are able to inhibit NorA of S. aureus and other non-NorA-mediated resistance mechanisms in a dose-dependent manner [38]. In addition, it was confirmed that in the case of MRSA, the susceptibility to oxacillin was influenced by CPZ or TZ probably due to efflux-related mechanisms [39]. In the case of Burkholderia pseudomallei, the intrinsic resistance to aminoglycosides and macrolides is due to the RND efflux pumps BpeAB-OprB and AmrAB-OprA [40], which are secondary pumps deriving their energy from the proton motive force (PMF). The combined administration of chlorpromazine or prochlorperazine with erythromycin could inhibit the efflux of erythromycin by disrupting the proton gradient required for the function of the efflux pumps. These results may confirm the potential use of phenothiazines as EPIs and resistance modifiers in B. pseudomallei; however, the high concentrations (250 μM to 1 mM or 79.715 μg/mL to 318.86 μg/mL) applied in the experiments cannot be achieved in patients (therapeutic concentration: 30–300 ng/mL) [41]. Chlorpromazine treatment provoked an increase in the expression of ramA, and a reduction in the expression of acrB in Salmonella Typhimurium [32].
TZ and CPZ increased the intracellular accumulation of the efflux pump substrate ethidium bromide in Mycobacterium smegmatis and Mycobacterium avium. It has been confirmed that thioridazine could inhibit the intrinsic efflux pump system of M. avium causing erythromycin resistance [42]. CPZ and TZ exerted a direct inhibitory effect on respiration in Mycobacterium tuberculosis as shown by transcriptional responses detected by microarray [43]. Following TZ treatment, the gene of the efflux pump Mmr was upregulated in M. tuberculosis [44]. It was confirmed that CPZ being an AcrB substrate could enhance the intracellular accumulation of ethidium bromide and norfloxacin in S. Typhimurium. According to molecular docking studies, CPZ has a binding site beneath the CH3 channel of the AcrB pump and the binding of CPZ can interfere with some conformational states of AcrB during substrate efflux indicating that CPZ could be an inhibitor of the pump [33].
CPZ could inhibit the chloramphenicol resistance in Enterobacter aerogenes isolates reversing the resistance of these strains that was indicated for example by a 32-fold reduction in the original MIC of chloramphenicol in the presence of CPZ [45].
The EPI properties of promethazine (PMZ) are pH-dependent because the EPI activity of PMZ was less effective at acidic pH compared to a neutral pH in E. coli K12 AG100 because the proton motive force can drive the AcrAB-TolC system more efficiently at acidic pH. After 18 h of PMZ treatment, the efflux pump genes acrA and acrB were upregulated at pH 5 and pH 7 confirming the defense mechanism of bacteria in order to remove the noxious agents from the cells (Figure 4) [46]. In Burkholderia pseudomallei, PMZ increased the susceptibility to erythromycin, trimethoprim/sulfamethoxazole, gentamicin, and ciprofloxacin. In addition, PMZ could alter the structure of bacterial biofilm improving the penetration of antibiotics by damaging the biofilm structure (Figure 4) [47].
2.3. Potential Risks: Dysbiosis and Antibiotic Resistance
SSRIs and several other psychotropic drugs possess antibiotic effects, that can have direct consequences for the composition and stability of the gut microbiome [23,48]. Some SSRIs may exert antimicrobial activity against gut microbes for several hours each day, such as sertraline, fluoxetine, paroxetine, and fluvoxamine, and the different species and strains differ in their susceptibility to SSRIs. It was confirmed that sertraline and fluoxetine may exert stronger inhibitory effects on Gram-positive bacteria than on Gram-negative bacteria [49]. It is obvious that SSRIs influence the balance of the gut microbiome and microbial defense system including bacterial efflux pump systems, motility of microbes; furthermore, these drugs may have a synergistic interaction with other drugs provoking dysbiosis [48].
Psychoactive drugs can trigger the SOS response in Gram-negative bacteria influencing the virulence of these strains [50]. It should be pointed out that SSRIs are detectable in wastewater samples, and in surface waters [51,52]; furthermore, the accumulation of SSRIs in animals can appear in the food chain as demonstrated in fishes [52].
3. Anticancer Activity of SSRIs, TCAs, and Phenothiazines
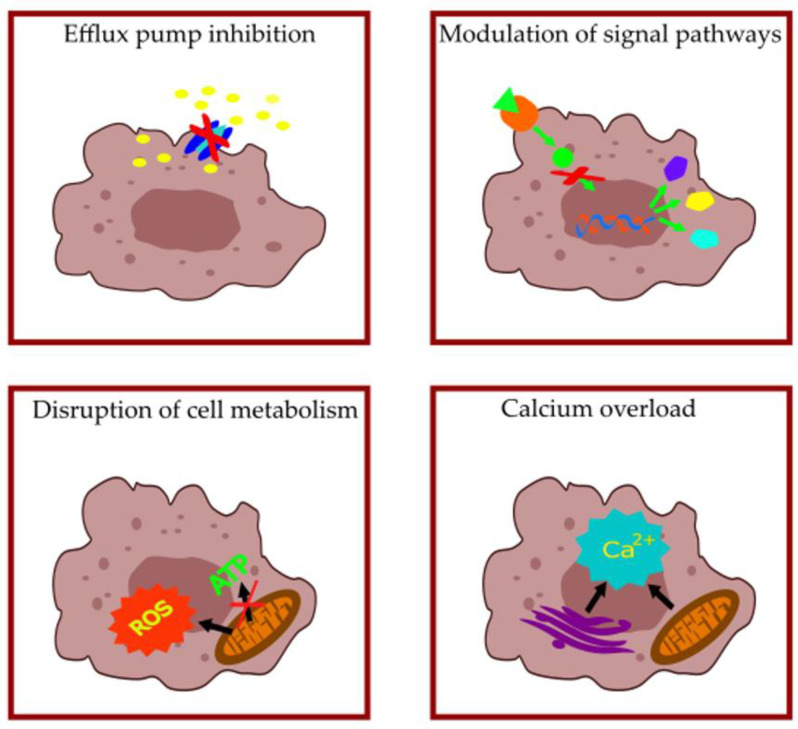
SSRIs, TCAs, and phenothiazines achieve their anticancer activity through various mechanisms, including the modulation of different signaling pathways (e.g., NFκB, AKT, mTOR, β-catenin), altering membrane permeability and cellular metabolism, inducing endoplasmic reticulum (ER) stress and modulating autophagy, influencing intracellular Ca2+, ultimately resulting in sensitization to apoptotic cell death and antimetastatic effects (Table 1, Table 2 and Table 3, Figure 5).
Table 1.
Selective serotonin reuptake inhibitors (SSRIs) with anticancer properties.
| Compound | Mechanism of Action | Cell Line | Reference |
|---|---|---|---|
| Citalopram Escitalopram |
Modulation of NFκB-signaling, ROS formation | HepG2 hepatocellular carcinoma cell line, A549 and H460 non-small lung cancer cell lines | [53,54] |
| Disruption of mitochondrial membrane potential | Burkitt lymphoma cell lines | [55] | |
| Fluoxetine | Modulation of NFκB-signaling | CL1-5-F4 human lung adenocarcinoma cell line | [56] |
| OVCAR-3 human epithelial ovarian cancer cell line | [57] | ||
| Modulation of AKT/mTOR pathway | A549 and H460 non-small lung cancer cell lines | [58] | |
| Disruption of mitochondrial membrane potential | HT29 and CaCo-2 human colon adenocarcinoma cell lines, Burkitt lymphoma cell lines | [56,59] | |
| Mitochondrial Ca2+-overload | HeLa human cervical carcinoma cell line | [60] | |
| Paroxetine | Modulation of AKT-signaling | HCT116 and HT29 human colon adenocarcinoma | [61] |
| Disruption of mitochondrial membrane potential | Burkitt lymphoma cell lines | [55] | |
| Sertraline | Modulation of mTOR signaling | MCF-7 human breast adenocarcinoma cell line | [62] |
| SGC-7901/DDP gastric cancer cell line | [63] | ||
| Modulation of AKT signaling | A375 human melanoma cell line | [64] | |
| Ca2+-overload, ROS formation | Prostate cancer cell lines | [65,66] | |
| MG63 human osteosarcoma cell line | [67] |
Table 2.
Tricyclic antidepressants (TCAs) with anticancer properties.
| Compound | Mechanism of Action | Cell Line | Reference |
|---|---|---|---|
| Amitryptiline | Modulation of NFκB-signaling | T98G human glioblastoma multiforme cell line | [68] |
| Inhibition of mitochondrial respiration | IPSB-18 anaplastic astrocytoma-derived cell line | [69] | |
| SK-MEL28, SK-ML2 and patient-derived melanoma cell lines | [70] | ||
| Nortryptiline | Inhibition of mitochondrial respiration | SK-MEL28, SK-ML2 and patient-derived melanoma cell lines | [70] |
| Imipramine | Modulation of NFκB-signaling | T98G human glioblastoma multiforme cell line | [68] |
| CL1-5-F4 human lung adenocarcinoma cell line | [71] | ||
| Modulation of AKT/mTOR pathway | U-87MG glioma cells | [72] | |
| Modulation of AKT- and NFκB-signaling | PC-3 human prostate cancer cell line | [73] | |
| Inhibition of mitochondrial respiration | IPSB-18 anaplastic astrocytoma-derived cell line | [69] | |
| Clomipramine | Inhibition of mitochondrial respiration | IPSB-18 anaplastic astrocytoma-derived cell line | [69] |
| SK-MEL28, SK-ML2 and patient-derived melanoma cell lines | [70] | ||
| Desipramine | Ca2+-overload | Hep3B hepatocellular carcinoma | [74] |
| MG63 human osteosarcoma cell line | [75] |
Table 3.
Phenothiazines with anticancer properties.
| Compound | Mechanism of Action | Cell Line | Reference |
|---|---|---|---|
| Thioridazine | Modulation of mTOR-signaling | ECA-109 and TE-1 esophageal squamous cell carcinoma | [76] |
| Human cervical and endometrial cancer cell lines | [77] | ||
| Modulation of AKT-signaling | A549 stem cell-like non-small lung cancer cell lines | [78] | |
| HepG2 hepatocellular carcinoma cell line | [79] | ||
| Caki human renal carcinoma cell line | [80] | ||
| A2780 and SKOV3 human ovarian cancer cell lines | [81] | ||
| 4T1 and MDA-MB-231 breast cancer cell lines | [82] | ||
| Disruption of mitochondrial membrane potential | A549 and A549/DDP human non-small lung cancer cell lines, SKOV3 and SKOV3/DDP ovarian cancer cell lines | [83] | |
| HeLa cervical cancer line | [84] | ||
| HCT116 human colon cancer cell line | [85] | ||
| NCI-N87 and AGS gastric cancer cell lines | [86] | ||
| Ca2+-overload | HepG2 hepatocellular carcinoma cell line | [87] | |
| K-562 chronic myelogenous leukemia cell | [88] | ||
| Chlorpromazine | Modulation of mTOR-signaling | U-87MG human glioma cells | [89] |
| HSC-3 and Ca9-22 human oral cancer cells | [90] | ||
| Promethazine | Modulation of AKT-signaling | HT29 and SW480 human colorectal carcinoma cell lines | [91] |
| K-562 chronic myelogenous leukemia cell | [92] | ||
| Fluphenazine | Modulation of mTOR-signaling | MDA-MB-231 human breast cancer cell line | [93] |
| Modulation of AKT-signaling | OVCAR-3 ovarian cancer cell line | [94] |
Figure 5.
Main mechanisms of anticancer activity of selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and phenothiazine-type antipsychotics.
3.1. Modulation of Signal Pathways
NFκB signaling has a complex role in inflammation and cellular proliferation, since it may have proinflammatory, tumorigenic and anti-inflammatory, anti-tumorigenic roles. However, the modulation of the NFκB pathway may be an effective therapeutic target in some cancers [95]. AKT is a proto-oncogene overexpressed in different cancers, it has a regulatory function in cell proliferation and apoptosis. The downstream effector of AKT mTOR has many different roles in cellular functions, it is involved in glycolytic and lipid metabolism, suppression of autophagy, growth factor receptor signaling and neoangiogenesis, and promoting tumor growth and metastasis formation. Inhibiting the aforementioned pathways is a promising approach in cancer therapy [96,97]. Numerous studies have shown the modulation of these pathways in different cancerous cell lines when treated with SSRIs, TCAs, and phenothiazines, leading to apoptosis induction, autophagy modulation, and antimigratory effects (Table 1, Table 2 and Table 3). Sertraline and thioridazine were also able to inhibit translationally controlled tumor protein (TCTP), a regulator of cell proliferation associated with the mTOR pathway [98,99,100]. Additionally, sertraline, amitryptiline, and TZ inhibited β-catenin signaling, which has many protumorigenic roles and is linked to cancer stem cells [101,102,103].
3.2. Effects on Cellular Metabolism
The effect of antidepressants and antipsychotics on mitochondria and cellular metabolism is one of the key mechanisms by which they exert their anticancer effects. The administration of SSRIs, TCAs, phenothiazines promotes ROS generation and leads to mitochondrial dysfunction by disrupting mitochondrial membrane potential, which results in disrupted cellular respiration and cytochrome c leaking, ultimately causing apoptosis (Table 1, Table 2 and Table 3). Furthermore, fluoxetine has also inhibited sphingomyelin phosphodiesterase 1, causing sphingomyelin accumulation and cell death in glioblastoma cell lines [104]. In BRAF/MEK inhibitor-tolerant melanoma persister cells fatty acid oxidation is essential to fuel cellular metabolism, this may be exploited by TZ, since it can suppress peroxisomal β-oxidation [105]. Interestingly, paroxetine could reduce doxorubicin-mediated cardiotoxicity in male Wistar rats that is related to free radical formation as a result of doxorubicin metabolism [106].
3.3. Ca2+ Overload
Ca2+ is a key second messenger responsible for essential cellular processes in both normal cells and cancerous cells, such as metabolism, proliferation, and cell death. Uniquely intracellular Ca2+ level, unlike other second messengers, is regulated by compartmentalization orchestrated by Ca2+ channels and transporters of the cell membrane and Ca2+ storing organelles, namely the ER and mitochondrium [107]. SSRIs, TCAs, and phenothiazines may increase cytosolic free Ca2+ by liberating Ca2+ from intracellular stores or causing Ca2+ influx from extracellular space that may induce mitochondrial dysfunction, ER stress, and apoptosis in various cancerous cell lines (Table 1, Table 2 and Table 3).
3.4. Drug Efflux Pumps in Cancer
One of the main functions of ABC-transporters is to protect the organism against potentially harmful molecules and thus ABC-transporters are mainly expressed at barrier surfaces, e.g., blood–brain barrier, gastrointestinal and hepatobiliary tract, renal tubules [108]. In cancer, ABC-transporters, besides extruding chemotherapeutic drugs, may be involved in apoptosis regulation, cell migration [109,110]. Some substrates of ABC-transporters, such as cyclic nucleotides, platelet-activating factors, prostaglandins, leukotrienes, and cholesterols, are also significant regarding tumor metabolism and progression [11]. Seven subfamilies belong to the ABC superfamily; among them, three members of subfamilies are commonly involved in MDR cancer: P-glycoprotein (P-gp), multidrug resistance protein 1 (MRP 1 or ABCC1), and breast cancer resistance protein (BRCP or ABCG2) [111].
P-gp or ABCB1 was thought to be the most important mechanism responsible for MDR. According to the cancer stem cell (CSC) concept, there are CSC subpopulations in the cancerous tissue that are inherently resistant to chemotherapeutics by their ability to express efflux pumps, repair their DNA, and remain quiescent [112,113]. P-gp is coded by the MDR1 gene; furthermore, the transcriptional regulation of MDR1 gene expression is mediated by various signal pathways. The activation of NFκB, AKT, and Wnt/β-catenin signaling may enhance the transcription of MDR1 gene which may lead to the increased expression of P-gp. Therefore, targeting these pathways may be an effective approach to increase the sensitivity of MDR cancer [114]. Another effective approach may be targeting tumor metabolism, since cancerous cells adapt their metabolism to fuel their metabolic needs for rapid proliferation. In cancerous cells, a high rate of glycolysis can be observed to provide precursors for the anabolic pathways, still ATP, which is needed for proliferation and ATP-consuming efflux pumps, is mainly generated by mitochondria. In addition, mitochondrial respiration is essential for generating reactive oxygen species (ROS) for cell proliferation [115]. To counterbalance the increased ROS, cancer cells have higher levels of ROS-scavengers; however, this balance is fragile and modulating ROS may be an effective approach to combat cancer and drug resistance [116,117].
3.5. Targeting Efflux Pumps in Cancer with SSRIs, TCAs, and Phenothiazines
Antidepressant and antipsychotic drugs may be promising candidates against P-gp-expressing cancers, since they may reduce the transcription of the MDR1 gene by inhibiting the signal pathways that regulate its transcription and the inhibition of mitochondrial respiration may deprive tumor cells of ATP, which is essential for ABC-transporters. Several in vitro and in vivo studies also suggest that antidepressants and antipsychotics are direct inhibitors or substrates for P-gp (Figure 1, Figure 2 and Figure 3) [118,119,120].
Fluoxetine, a widely used SSRI antidepressant with a broad safety range, is emerging as a new chemosensitizer in preclinical models. In vivo studies showed prolonged survival of nude mice bearing P-gp expressing resistant MCF-7/ADM breast cancer and HCT-15 colon cancer, when treated with fluoxetine and doxorubicin [121,122]. Fluoxetine treatment resulted in the downregulation of P-gp protein expression. When combined with paclitaxel, it downregulated the MDR1 gene expression in MCF7/ADM human breast cancer cell line; moreover, cytosolic glutathione S-transferase was also downregulated, which is a commonly expressed antioxidant system in resistant cancer [123,124]. Sertraline, another SSRI with halogenic atoms, demonstrated promising efflux pump inhibitory activity in P-gp expressing OVCAR-8 human ovarian cancer in vitro and in vivo xenograft model, combined with doxorubicin or pegylated liposomal doxorubicin [125].
Thioridazine, a first-generation phenothiazine antipsychotic that was widely used in psychotic disorders, could sensitize antimitotic drug resistant KBV20C oral cancer cells to vinblastine, although its potential use in cancer therapy might be limited by its cardiac toxicity [126,127]. On the same cell line fluphenazine, another phenothiazine antipsychotic included on the WHO Model List of Essential Medicines, achieved similar chemosensitizing effects [128,129]. Fluphenazine also decreased the expression of MDR1 (ABCB1) and COX2 genes in doxorubicin-resistant LoVo/Dx colon adenocarcinoma cell lines. Moreover, the combination of fluphenazine and simvastatin could enhance the COX-2 inhibitory activity and sensitivity to doxorubicin [130]. Promethazine, a phenothiazine derivative, mainly used as an antihistamine and antiemetic agent, exhibited chemosensitizing activity in MCF7 human breast cancer drug resistant sublines when combined with vincristine or doxorubicin by modulating P-gp activity and decreasing the expression of MDR1 and MRP1 genes [131,132].
4. Conclusions
The rising microbial drug resistance is a public health threat worldwide and multidrug resistance in cancer is a major barrier to chemotherapeutic treatment. Drug repositioning may be an attractive strategy to combat multidrug resistance in bacteria and cancer, since it may be a shorter procedure and less costly than traditional drug development. Efflux pump-mediated resistance is one of the major mechanisms contributing to MDR, therefore targeting EPs may be an attractive approach that may improve the efficacy of chemotherapeutics in MDR bacteria and cancer. Furthermore, inhibiting efflux pumps may decrease bacterial virulence, and in cancer, it may inhibit metastasis formation and decrease relapses, since EPs are also involved in other processes than detoxification.
Numerous studies have emerged describing the antibacterial and anticancer effects of antidepressant and antipsychotic drugs. Many of the SSRIs and some TCAs showed promising activity regarding efflux pump inhibition in various Gram-positive and Gram-negative bacteria. Phenothiazine antipsychotics have also shown anti-mycobacterial activity and EP inhibition in mycobacteria. Regarding anticancer activity of SSRI, TCA, and phenothiazine antipsychotics, several in vitro and in vivo studies showed anticancer activity, which is associated with signal pathway modulation, cellular metabolism alteration, Ca2+-overload. Additionally, this may also lead to EP modulation, although several antidepressants and antipsychotics are direct EP inhibitors or substrates of EPs. Exploring EP inhibitor activity of antidepressants and antipsychotics in MDR cancers may be important since they are already used for supportive therapy in cancer patients.
While repurposing antidepressants and antipsychotics as antibacterial or anticancer agents may be an attractive idea, there are some risks that should be taken into consideration. In some cases, to achieve the antibacterial, anticancer, or EP inhibitory effects higher doses should be given. SSRIs have a wide therapeutic window, but the application of TCAs and phenothiazine antipsychotics may be limited due to their cardiac toxicity. Another question that should be taken into account regarding the antibacterial activity of antidepressants and antipsychotics is how they can contribute to the rapid spread of drug-resistant bacteria. Mental health disorders, e.g., depression, are of great importance. This may be explained by increasing economic and social inequalities and public health emergencies, thus the demand for antidepressants is increasing, which may also increase the selection pressure on bacteria [133,134]. Another problem is that some patients with depression and schizophrenic patients require lifelong treatment with psychiatric drugs. This medication may alter the gut microbiota also contributing to the development of metabolic side effects of these drugs, resulting in increased risk for metabolic diseases [135]. This may suggest that there might be a need to use pro- or prebiotics, when taking traditionally non-antibiotic drugs with antibacterial effects [136].
Author Contributions
Conceptualization, B.R., G.S., writing—original draft preparation, B.R., G.S., writing—review and editing, B.R., G.S., visualization, B.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
B.R. was supported by the Szeged Foundation for Cancer Research (Szegedi Rákkutatásért Alapítvány). G.S. was supported by the János Bolyai Research Scholarship (BO/00158/22/5) of the Hungarian Academy of Sciences.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ashburn T.T., Thor K.B. Drug Repositioning: Identifying and Developing New Uses for Existing Drugs. Nat. Rev. Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 2.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 3.Dinić J., Efferth T., García-Sosa A.T., Grahovac J., Padrón J.M., Pajeva I., Rizzolio F., Saponara S., Spengler G., Tsakovska I. Repurposing Old Drugs to Fight Multidrug Resistant Cancers. Drug Resist. Update. 2020;52:100713. doi: 10.1016/j.drup.2020.100713. [DOI] [PubMed] [Google Scholar]
- 4.Parvathaneni V., Kulkarni N.S., Muth A., Gupta V. Drug Repurposing: A Promising Tool to Accelerate the Drug Discovery Process. Drug Discov. Today. 2019;24:2076–2085. doi: 10.1016/j.drudis.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michael C.A., Dominey-Howes D., Labbate M. The Antimicrobial Resistance Crisis: Causes, Consequences, and Management. Front. Public Health. 2014;2:145. doi: 10.3389/fpubh.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolaou M., Pavlopoulou A., Georgakilas A.G., Kyrodimos E. The Challenge of Drug Resistance in Cancer Treatment: A Current Overview. Clin. Exp. Metastasis. 2018;35:309–318. doi: 10.1007/s10585-018-9903-0. [DOI] [PubMed] [Google Scholar]
- 8.Reygaert W.C. Department of Biomedical Sciences, Oakland University William Beaumont School of Medicine, Rochester, MI, USA An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018;4:482–501. doi: 10.3934/microbiol.2018.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansoori B., Mohammadi A., Davudian S., Shirjang S., Baradaran B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017;7:339–348. doi: 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alav I., Sutton J.M., Rahman K.M. Role of Bacterial Efflux Pumps in Biofilm Formation. J. Antimicrob. Chemother. 2018;73:2003–2020. doi: 10.1093/jac/dky042. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher J.I., Haber M., Henderson M.J., Norris M.D. ABC Transporters in Cancer: More than Just Drug Efflux Pumps. Nat. Rev. Cancer. 2010;10:147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 12.Thekdi S.M., Trinidad A., Roth A. Psychopharmacology in Cancer. Curr. Psychiatry Rep. 2015;17:529. doi: 10.1007/s11920-014-0529-x. [DOI] [PubMed] [Google Scholar]
- 13.Huang J., Zhao D., Liu Z., Liu F. Repurposing Psychiatric Drugs as Anti-Cancer Agents. Cancer Lett. 2018;419:257–265. doi: 10.1016/j.canlet.2018.01.058. [DOI] [PubMed] [Google Scholar]
- 14.Golden S.R., Rosenstein D.L., Belhorn T., Blatt J. Repurposing Psychotropic Agents for Viral Disorders: Beyond Covid. Assay Drug Dev. Technol. 2021;19:373–385. doi: 10.1089/adt.2021.014. [DOI] [PubMed] [Google Scholar]
- 15.Caldara M., Marmiroli N. Antimicrobial Properties of Antidepressants and Antipsychotics—Possibilities and Implications. Pharmaceuticals. 2021;14:915. doi: 10.3390/ph14090915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munita J.M., Arias C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016;4:464–473. doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanwar J., Das S., Fatima Z., Hameed S. Multidrug Resistance: An Emerging Crisis. Interdiscip. Perspect. Infect. Dis. 2014;2014:541340. doi: 10.1155/2014/541340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis K. The Science of Antibiotic Discovery. Cell. 2020;181:29–45. doi: 10.1016/j.cell.2020.02.056. [DOI] [PubMed] [Google Scholar]
- 19.Seukep A.J., Mbuntcha H.G., Kuete V., Chu Y., Fan E., Guo M.-Q. What Approaches to Thwart Bacterial Efflux Pumps-Mediated Resistance? Antibiotics. 2022;11:1287. doi: 10.3390/antibiotics11101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahamoud A., Chevalier J., Alibert-Franco S., Kern W.V., Pagès J.-M. Antibiotic Efflux Pumps in Gram-Negative Bacteria: The Inhibitor Response Strategy. J. Antimicrob. Chemother. 2007;59:1223–1229. doi: 10.1093/jac/dkl493. [DOI] [PubMed] [Google Scholar]
- 21.Rangel-Vega A., Bernstein L.R., Mandujano-Tinoco E.A., García-Contreras S.J., García-Contreras R. Drug Repurposing as an Alternative for the Treatment of Recalcitrant Bacterial Infections. Front. Microbiol. 2015;6:282. doi: 10.3389/fmicb.2015.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spengler G., Kincses A., Gajdács M., Amaral L. New Roads Leading to Old Destinations: Efflux Pumps as Targets to Reverse Multidrug Resistance in Bacteria. Molecules. 2017;22:468. doi: 10.3390/molecules22030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz-Bellido J.L., Munoz-Criado S., Garcìa-Rodrìguez J.A. Antimicrobial Activity of Psychotropic Drugs. Int. J. Antimicrob. Agents. 2000;14:177–180. doi: 10.1016/S0924-8579(99)00154-5. [DOI] [PubMed] [Google Scholar]
- 24.Kaatz G. Phenylpiperidine Selective Serotonin Reuptake Inhibitors Interfere with Multidrug Efflux Pump Activity in Staphylococcus Aureus. Int. J. Antimicrob. Agents. 2003;22:254–261. doi: 10.1016/S0924-8579(03)00220-6. [DOI] [PubMed] [Google Scholar]
- 25.German N., Kaatz G.W., Kerns R.J. Synthesis and Evaluation of PSSRI-Based Inhibitors of Staphylococcus Aureus Multidrug Efflux Pumps. Bioorg. Med. Chem. Lett. 2008;18:1368–1373. doi: 10.1016/j.bmcl.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Bohnert J.A., Szymaniak-Vits M., Schuster S., Kern W.V. Efflux Inhibition by Selective Serotonin Reuptake Inhibitors in Escherichia Coli. J. Antimicrob. Chemother. 2011;66:2057–2060. doi: 10.1093/jac/dkr258. [DOI] [PubMed] [Google Scholar]
- 27.Nzakizwanayo J., Scavone P., Jamshidi S., Hawthorne J.A., Pelling H., Dedi C., Salvage J.P., Hind C.K., Guppy F.M., Barnes L.M., et al. Fluoxetine and Thioridazine Inhibit Efflux and Attenuate Crystalline Biofilm Formation by Proteus Mirabilis. Sci. Rep. 2017;7:12222. doi: 10.1038/s41598-017-12445-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhammad A., Fazal S., Jawad A., Arif K., Farhat U., Abdul S., Nawazish-i-Husain S., Ihsan U., Sajid H. Citalopram and venlafaxine differentially augments antimicrobial properties of antibiotics. Acta Pol. Pharm. 2015;72:1269–1278. [Google Scholar]
- 29.Kalaycı S., Demirci S., Sahin F. Antimicrobial Properties of Various Psychotropic Drugs Against Broad Range Microorganisms. Curr. Psychopharmacol. 2015;3:195–202. doi: 10.2174/2211556004666150520230121. [DOI] [Google Scholar]
- 30.Cussotto S., Strain C.R., Fouhy F., Strain R.G., Peterson V.L., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Differential Effects of Psychotropic Drugs on Microbiome Composition and Gastrointestinal Function. Psychopharmacology. 2019;236:1671–1685. doi: 10.1007/s00213-018-5006-5. [DOI] [PubMed] [Google Scholar]
- 31.Shi D., Hao H., Wei Z., Yang D., Yin J., Li H., Chen Z., Yang Z., Chen T., Zhou S., et al. Combined Exposure to Non-Antibiotic Pharmaceutics and Antibiotics in the Gut Synergistically Promote the Development of Multi-Drug-Resistance in Escherichia Coli. Gut Microbes. 2022;14:2018901. doi: 10.1080/19490976.2021.2018901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey A.M., Paulsen I.T., Piddock L.J.V. RamA Confers Multidrug Resistance in Salmonella Enterica via Increased Expression of AcrB, Which Is Inhibited by Chlorpromazine. Antimicrob. Agents Chemother. 2008;52:3604–3611. doi: 10.1128/AAC.00661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimsey E.M., Fais C., Marshall R.L., Ricci V., Ciusa M.L., Stone J.W., Ivens A., Malloci G., Ruggerone P., Vargiu A.V., et al. Chlorpromazine and Amitriptyline Are Substrates and Inhibitors of the AcrB Multidrug Efflux Pump. mBio. 2020;11:e00465-20. doi: 10.1128/mBio.00465-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendricks O. The In-Vitro Antimicrobial Effect of Non-Antibiotics and Putative Inhibitors of Efflux Pumps on Pseudomonas Aeruginosa and Staphylococcus Aureus. Int. J. Antimicrob. Agents. 2003;22:262–264. doi: 10.1016/S0924-8579(03)00205-X. [DOI] [PubMed] [Google Scholar]
- 35.Spengler G., Molnar A., Schelz Z., Amaral L., Sharples D., Molnar J. The Mechanism of Plasmid Curing in Bacteria. Curr. Drug Targets. 2006;7:823–841. doi: 10.2174/138945006777709601. [DOI] [PubMed] [Google Scholar]
- 36.Grimsey E.M., Piddock L.J.V. Do Phenothiazines Possess Antimicrobial and Efflux Inhibitory Properties? FEMS Microbiol. Rev. 2019;43:577–590. doi: 10.1093/femsre/fuz017. [DOI] [PubMed] [Google Scholar]
- 37.Adkin P., Hitchcock A., Smith L.J., Walsh S.E. Priming with Biocides: A Pathway to Antibiotic Resistance? J. Appl. Microbiol. 2022;133:830–841. doi: 10.1111/jam.15564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaatz G.W., Moudgal V.V., Seo S.M., Kristiansen J.E. Phenothiazines and Thioxanthenes Inhibit Multidrug Efflux Pump Activity in Staphylococcus Aureus. Antimicrob. Agents Chemother. 2003;47:719–726. doi: 10.1128/AAC.47.2.719-726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kristiansen M. Phenothiazines Alter Resistance of Methicillin-Resistant Strains of Staphylococcus Aureus (MRSA) to Oxacillin in Vitro. Int. J. Antimicrob. Agents. 2003;22:250–253. doi: 10.1016/S0924-8579(03)00200-0. [DOI] [PubMed] [Google Scholar]
- 40.Chan Y.Y., Tan T.M.C., Ong Y.M., Chua K.L. BpeAB-OprB, a Multidrug Efflux Pump in Burkholderia Pseudomallei. Antimicrob. Agents Chemother. 2004;48:1128–1135. doi: 10.1128/AAC.48.4.1128-1135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan Y.Y., Ong Y.M., Chua K.L. Synergistic Interaction between Phenothiazines and Antimicrobial Agents against Burkholderia Pseudomallei. Antimicrob. Agents Chemother. 2007;51:623–630. doi: 10.1128/AAC.01033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues L., Wagner D., Viveiros M., Sampaio D., Couto I., Vavra M., Kern W.V., Amaral L. Thioridazine and Chlorpromazine Inhibition of Ethidium Bromide Efflux in Mycobacterium Avium and Mycobacterium Smegmatis. J. Antimicrob. Chemother. 2008;61:1076–1082. doi: 10.1093/jac/dkn070. [DOI] [PubMed] [Google Scholar]
- 43.Boshoff H.I.M., Myers T.G., Copp B.R., McNeil M.R., Wilson M.A., Barry C.E. The Transcriptional Responses of Mycobacterium Tuberculosis to Inhibitors of Metabolism. J. Biol. Chem. 2004;279:40174–40184. doi: 10.1074/jbc.M406796200. [DOI] [PubMed] [Google Scholar]
- 44.Dutta N.K., Mehra S., Kaushal D. A Mycobacterium Tuberculosis Sigma Factor Network Responds to Cell-Envelope Damage by the Promising Anti-Mycobacterial Thioridazine. PLoS ONE. 2010;5:e10069. doi: 10.1371/journal.pone.0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCusker M.P., Ferreira D.A., Cooney D., Alves B.M., Fanning S., Pagès J.-M., Martins M., Davin-Regli A. Modulation of Antimicrobial Resistance in Clinical Isolates of Enterobacter Aerogenes: A Strategy Combining Antibiotics and Chemosensitisers. J. Glob. Antimicrob. Resist. 2019;16:187–198. doi: 10.1016/j.jgar.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Nové M., Kincses A., Molnár J., Amaral L., Spengler G. The Role of Efflux Pumps and Environmental PH in Bacterial Multidrug Resistance. In Vivo. 2020;34:65–71. doi: 10.21873/invivo.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sidrim J.J.C., Vasconcelos D.C., Riello G.B., Guedes G.M.d.M., Serpa R., Bandeira T.d.J.P.G., Monteiro A.J., Cordeiro R.d.A., Castelo-Branco D.d.S.C.M., Rocha M.F.G., et al. Promethazine Improves Antibiotic Efficacy and Disrupts Biofilms of Burkholderia Pseudomallei. Biofouling. 2017;33:88–97. doi: 10.1080/08927014.2016.1262846. [DOI] [PubMed] [Google Scholar]
- 48.Sjöstedt P., Enander J., Isung J. Serotonin Reuptake Inhibitors and the Gut Microbiome: Significance of the Gut Microbiome in Relation to Mechanism of Action, Treatment Response, Side Effects, and Tachyphylaxis. Front. Psychiatry. 2021;12:682868. doi: 10.3389/fpsyt.2021.682868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGovern A.S., Hamlin A.S., Winter G. A Review of the Antimicrobial Side of Antidepressants and Its Putative Implications on the Gut Microbiome. Aust. N. Zeal. J. Psychiatry. 2019;53:1151–1166. doi: 10.1177/0004867419877954. [DOI] [PubMed] [Google Scholar]
- 50.Crane J.K., Salehi M., Alvarado C.L. Psychoactive Drugs Induce the SOS Response and Shiga Toxin Production in Escherichia Coli. Toxins. 2021;13:437. doi: 10.3390/toxins13070437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasskog T., Anderssen T., Pedersen-Bjergaard S., Kallenborn R., Jensen E. Occurrence of Selective Serotonin Reuptake Inhibitors in Sewage and Receiving Waters at Spitsbergen and in Norway. J. Chromatogr. A. 2008;1185:194–205. doi: 10.1016/j.chroma.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 52.Arnnok P., Singh R.R., Burakham R., Pérez-Fuentetaja A., Aga D.S. Selective Uptake and Bioaccumulation of Antidepressants in Fish from Effluent-Impacted Niagara River. Environ. Sci. Technol. 2017;51:10652–10662. doi: 10.1021/acs.est.7b02912. [DOI] [PubMed] [Google Scholar]
- 53.Ahmadian E., Eftekhari A., Babaei H., Nayebi A.M., Eghbal M.A. Anti-Cancer Effects of Citalopram on Hepatocellular Carcinoma Cells Occur via Cytochrome C Release and the Activation of NF-κB. Anticancer Agents Med. Chem. 2017;17:1570–1577. doi: 10.2174/1871520617666170327155930. [DOI] [PubMed] [Google Scholar]
- 54.Yuan I., Horng C., Chen V., Chen C., Chen L., Hsu T., Tzang B. Escitalopram Oxalate Inhibits Proliferation and Migration and Induces Apoptosis in Non-Small Cell Lung Cancer Cells. Oncol. Lett. 2017;15:3376–3382. doi: 10.3892/ol.2017.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serafeim A., Holder M.J., Grafton G., Chamba A., Drayson M.T., Luong Q.T., Bunce C.M., Gregory C.D., Barnes N.M., Gordon J. Selective Serotonin Reuptake Inhibitors Directly Signal for Apoptosis in Biopsy-like Burkitt Lymphoma Cells. Blood. 2003;101:3212–3219. doi: 10.1182/blood-2002-07-2044. [DOI] [PubMed] [Google Scholar]
- 56.Wu J.-Y., Lin S.-S., Hsu F.-T., Chung J.-G. Fluoxetine Inhibits DNA Repair and NF-ĸB-Modulated Metastatic Potential in Non-Small Cell Lung Cancer. Anticancer Res. 2018;38:5201–5210. doi: 10.21873/anticanres.12843. [DOI] [PubMed] [Google Scholar]
- 57.Lee C.S., Kim Y.J., Jang E.R., Kim W., Myung S.C. Fluoxetine Induces Apoptosis in Ovarian Carcinoma Cell Line OVCAR-3 Through Reactive Oxygen Species-Dependent Activation of Nuclear Factor-κB. Basic Clin. Pharmacol. Toxicol. 2009;106:446–453. doi: 10.1111/j.1742-7843.2009.00509.x. [DOI] [PubMed] [Google Scholar]
- 58.Shao S., Zhuang X., Zhang L., Qiao T. Antidepressants Fluoxetine Mediates Endoplasmic Reticulum Stress and Autophagy of Non–Small Cell Lung Cancer Cells Through the ATF4-AKT-MTOR Signaling Pathway. Front. Pharmacol. 2022;13:904701. doi: 10.3389/fphar.2022.904701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kannen V., Garcia S.B., Silva W.A., Gasser M., Mönch R., Alho E.J.L., Heinsen H., Scholz C.-J., Friedrich M., Heinze K.G., et al. Oncostatic Effects of Fluoxetine in Experimental Colon Cancer Models. Cell. Signal. 2015;27:1781–1788. doi: 10.1016/j.cellsig.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 60.Charles E., Hammadi M., Kischel P., Delcroix V., Demaurex N., Castelbou C., Vacher A.-M., Devin A., Ducret T., Nunes P., et al. The Antidepressant Fluoxetine Induces Necrosis by Energy Depletion and Mitochondrial Calcium Overload. Oncotarget. 2017;8:3181–3196. doi: 10.18632/oncotarget.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jang W.-J., Jung S.K., Vo T.T.L., Jeong C.-H. Anticancer Activity of Paroxetine in Human Colon Cancer Cells: Involvement of MET and ERBB3. J. Cell Mol. Med. 2019;23:1106–1115. doi: 10.1111/jcmm.14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin C.-J., Robert F., Sukarieh R., Michnick S., Pelletier J. The Antidepressant Sertraline Inhibits Translation Initiation by Curtailing Mammalian Target of Rapamycin Signaling. Cancer Res. 2010;70:3199–3208. doi: 10.1158/0008-5472.CAN-09-4072. [DOI] [PubMed] [Google Scholar]
- 63.Mu C., Peng R.-K., Guo C.-L., Li A., Yang X.-M., Zeng R., Li Y.-L., Gu J., Ouyang Q. Discovery of Sertraline and Its Derivatives Able to Combat Drug-Resistant Gastric Cancer Cell via Inducing Apoptosis. Bioorg. Med. Chem. Lett. 2021;41:127997. doi: 10.1016/j.bmcl.2021.127997. [DOI] [PubMed] [Google Scholar]
- 64.Reddy K.K., Lefkove B., Chen L.B., Govindarajan B., Carracedo A., Velasco G., Carrillo C.O., Bhandarkar S.S., Owens M.J., Mechta-Grigoriou F., et al. The Antidepressant Sertraline Downregulates Akt and Has Activity against Melanoma Cells. Pigment. Cell Melanoma Res. 2008;21:451–456. doi: 10.1111/j.1755-148X.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 65.Huang J.-K., Chang H.-T., Chou C.-T., Shu S.-S., Kuo C.-C., Tsai J.-Y., Liao W.-C., Wang J.-L., Lin K.-L., Lu Y.-C., et al. The Mechanism of Sertraline-Induced [Ca2+]i Rise in Human PC3 Prostate Cancer Cells. Basic Clin. Pharmacol. Toxicol. 2011;109:103–110. doi: 10.1111/j.1742-7843.2011.00690.x. [DOI] [PubMed] [Google Scholar]
- 66.Chinnapaka S., Bakthavachalam V., Munirathinam G. Repurposing Antidepressant Sertraline as a Pharmacological Drug to Target Prostate Cancer Stem Cells: Dual Activation of Apoptosis and Autophagy Signaling by Deregulating Redox Balance. Am. J. Cancer Res. 2020;10:2043–2065. [PMC free article] [PubMed] [Google Scholar]
- 67.Lin K.-L., Chi C.-C., Lu T., Tseng L.-L., Wang J.-L., Lu Y.-C., Jan C.-R. Effect of Sertraline on [Ca 2+]i and Viability of Human MG63 Osteosarcoma Cells. Drug Chem. Toxicol. 2013;36:231–240. doi: 10.3109/01480545.2012.710625. [DOI] [PubMed] [Google Scholar]
- 68.Bielecka-Wajdman A.M., Ludyga T., Machnik G., Gołyszny M., Obuchowicz E. Tricyclic Antidepressants Modulate Stressed Mitochondria in Glioblastoma Multiforme Cells. Cancer Control. 2018;25:107327481879859. doi: 10.1177/1073274818798594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Higgins S.C., Pilkington G.J. The in Vitro Effects of Tricyclic Drugs and Dexamethasone on Cellular Respiration of Malignant Glioma. Anticancer Res. 2010;30:391–397. [PubMed] [Google Scholar]
- 70.Parker K.A., Glaysher S., Hurren J., Knight L.A., McCormick D., Suovouri A., Amberger-Murphy V., Pilkington G.J., Cree I.A. The Effect of Tricyclic Antidepressants on Cutaneous Melanoma Cell Lines and Primary Cell Cultures. Anti-Cancer Drugs. 2012;23:65–69. doi: 10.1097/CAD.0b013e32834b1894. [DOI] [PubMed] [Google Scholar]
- 71.Yueh P.-F., Lee Y.-H., Chiang I.-T., Chen W.-T., Lan K.-L., Chen C.-H., Hsu F.-T. Suppression of EGFR/PKC-δ/NF-ΚB Signaling Associated with Imipramine-Inhibited Progression of Non-Small Cell Lung Cancer. Front. Oncol. 2021;11:735183. doi: 10.3389/fonc.2021.735183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jeon S.-H., Kim S.H., Kim Y., Kim Y.S., Lim Y., Lee Y.H., Shin S.Y. The Tricyclic Antidepressant Imipramine Induces Autophagic Cell Death in U-87MG Glioma Cells. Biochem. Biophys. Res. Commun. 2011;413:311–317. doi: 10.1016/j.bbrc.2011.08.093. [DOI] [PubMed] [Google Scholar]
- 73.Lim E.Y., Park J., Kim Y.T., Kim M.J. Imipramine Inhibits Migration and Invasion in Metastatic Castration-Resistant Prostate Cancer PC-3 Cells via AKT-Mediated NF-ΚB Signaling Pathway. Molecules. 2020;25:4619. doi: 10.3390/molecules25204619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang D.K., Kim S.-J. Desipramine Induces Apoptosis in Hepatocellular Carcinoma Cells. Oncol. Rep. 2017;38:1029–1034. doi: 10.3892/or.2017.5723. [DOI] [PubMed] [Google Scholar]
- 75.Jan C.-R., Lu Y.-C., Tseng L.-L., Jiann B.-P., Chang H.-T., Wang J.-L., Chen W.-C., Huang J.-K. Effect of the Antidepressant Desipramine on Cytosolic Ca2+ Movement and Proliferation in Human Osteosarcoma Cells. Pharmacology. 2003;69:190–196. doi: 10.1159/000073663. [DOI] [PubMed] [Google Scholar]
- 76.Li H., Juan L., Xia L., Wang Y., Bao Y., Sun G. Thioridazine Sensitizes Esophageal Carcinoma Cell Lines to Radiotherapy-Induced Apoptosis In Vitro and In Vivo. Med. Sci. Monit. 2016;22:2624–2634. doi: 10.12659/MSM.899950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang S., Dong S.M., Kim B.-R., Park M.S., Trink B., Byun H.-J., Rho S.B. Thioridazine Induces Apoptosis by Targeting the PI3K/Akt/MTOR Pathway in Cervical and Endometrial Cancer Cells. Apoptosis. 2012;17:989–997. doi: 10.1007/s10495-012-0717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen J., Ma B., Zhang X., Sun X., Han J., Wang Y., Chu L., Xu H., Yang Y. Thioridazine Has Potent Antitumor Effects on Lung Cancer Stem-like Cells. Oncol. Lett. 2017;13:1563–1568. doi: 10.3892/ol.2017.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ibrahim N.E.-S., Morsy H., Abdelgwad M. The Comparative Effect of Nisin and Thioridazine as Potential Anticancer Agents on Hepatocellular Carcinoma. Rep. Biochem. Mol. Biol. 2021;9:452–462. doi: 10.52547/rbmb.9.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Min K., Seo B.R., Bae Y.C., Yoo Y.H., Kwon T.K. Antipsychotic Agent Thioridazine Sensitizes Renal Carcinoma Caki Cells to TRAIL-Induced Apoptosis through Reactive Oxygen Species-Mediated Inhibition of Akt Signaling and Downregulation of Mcl-1 and c-FLIP(L) Cell Death Dis. 2014;5:e1063. doi: 10.1038/cddis.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yong M., Yu T., Tian S., Liu S., Xu J., Hu J., Hu L. DR2 Blocker Thioridazine: A Promising Drug for Ovarian Cancer Therapy. Oncol. Lett. 2017;14:8171–8177. doi: 10.3892/ol.2017.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song Y., Li L., Chen J., Chen H., Cui B., Feng Y., Zhang P., Zhang Q., Xia Y., Luo M. Thioridazine Hydrochloride: An Antipsychotic Agent That Inhibits Tumor Growth and Lung Metastasis in Triple-Negative Breast Cancer via Inducing G0/G1 Arrest and Apoptosis. Cell Cycle. 2020;19:3521–3533. doi: 10.1080/15384101.2020.1850969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qian G., Dai L., Yu T. Thioridazine Sensitizes Cisplatin Against Chemoresistant Human Lung and Ovary Cancer Cells. DNA Cell Biol. 2019;38:718–724. doi: 10.1089/dna.2019.4715. [DOI] [PubMed] [Google Scholar]
- 84.Seervi M., Rani A., Sharma A.K., Santhosh Kumar T.R. ROS Mediated ER Stress Induces Bax-Bak Dependent and Independent Apoptosis in Response to Thioridazine. Biomed. Pharmacother. 2018;106:200–209. doi: 10.1016/j.biopha.2018.06.123. [DOI] [PubMed] [Google Scholar]
- 85.Zhang C., Gong P., Liu P., Zhou N., Zhou Y., Wang Y. Thioridazine Elicits Potent Antitumor Effects in Colorectal Cancer Stem Cells. Oncol. Rep. 2017;37:1168–1174. doi: 10.3892/or.2016.5313. [DOI] [PubMed] [Google Scholar]
- 86.Mu J., Xu H., Yang Y., Huang W., Xiao J., Li M., Tan Z., Ding Q., Zhang L., Lu J., et al. Thioridazine, an Antipsychotic Drug, Elicits Potent Antitumor Effects in Gastric Cancer. Oncol. Rep. 2014;31:2107–2114. doi: 10.3892/or.2014.3068. [DOI] [PubMed] [Google Scholar]
- 87.Chen I.-S., Liang W.-Z., Wang J.-L., Kuo C.-C., Hao L.-J., Chou C.-T., Jan C.-R. Exploration of Thioridazine-Induced Ca 2+ Signaling and Non-Ca 2+ -Triggered Cell Death in HepG2 Human Hepatocellular Carcinoma Cells. Chin. J. Physiol. 2020;63:187. doi: 10.4103/CJP.CJP_45_20. [DOI] [PubMed] [Google Scholar]
- 88.Moraes V.W.R., Santos V.M., Suarez E.R., Ferraz L.S., Lopes R.d.M., Mognol G.P., Campeiro J.D., Machado-Neto J.A., Nascimento F.D., Hayashi M.A.F., et al. Targeting Ca2+ and Mitochondrial Homeostasis by Antipsychotic Thioridazine in Leukemia Cells. Life. 2022;12:1477. doi: 10.3390/life12101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shin S.Y., Lee K.S., Choi Y.-K., Lim H.J., Lee H.G., Lim Y., Lee Y.H. The Antipsychotic Agent Chlorpromazine Induces Autophagic Cell Death by Inhibiting the Akt/MTOR Pathway in Human U-87MG Glioma Cells. Carcinogenesis. 2013;34:2080–2089. doi: 10.1093/carcin/bgt169. [DOI] [PubMed] [Google Scholar]
- 90.Jhou A.-J., Chang H.-C., Hung C.-C., Lin H.-C., Lee Y.-C., Liu W., Han K.-F., Lai Y.-W., Lin M.-Y., Lee C.-H. Chlorpromazine, an Antipsychotic Agent, Induces G2/M Phase Arrest and Apoptosis via Regulation of the PI3K/AKT/MTOR-Mediated Autophagy Pathways in Human Oral Cancer. Biochem. Pharmacol. 2021;184:114403. doi: 10.1016/j.bcp.2020.114403. [DOI] [PubMed] [Google Scholar]
- 91.Tan X., Gong L., Li X., Zhang X., Sun J., Luo X., Wang Q., Chen J., Xie L., Han S. Promethazine Inhibits Proliferation and Promotes Apoptosis in Colorectal Cancer Cells by Suppressing the PI3K/AKT Pathway. Biomed. Pharmacother. 2021;143:112174. doi: 10.1016/j.biopha.2021.112174. [DOI] [PubMed] [Google Scholar]
- 92.Medeiros H.C.D., Colturato-Kido C., Ferraz L.S., Costa C.A., Moraes V.W.R., Paredes-Gamero E.J., Tersariol I.L.S., Rodrigues T. AMPK Activation Induced by Promethazine Increases NOXA Expression and Beclin-1 Phosphorylation and Drives Autophagy-Associated Apoptosis in Chronic Myeloid Leukemia. Chem. Biol. Interact. 2020;315:108888. doi: 10.1016/j.cbi.2019.108888. [DOI] [PubMed] [Google Scholar]
- 93.Goyette M.-A., Cusseddu R., Elkholi I., Abu-Thuraia A., El-Hachem N., Haibe-Kains B., Gratton J.-P., Côté J.-F. AXL Knockdown Gene Signature Reveals a Drug Repurposing Opportunity for a Class of Antipsychotics to Reduce Growth and Metastasis of Triple-Negative Breast Cancer. Oncotarget. 2019;10:2055–2067. doi: 10.18632/oncotarget.26725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi J.H., Yang Y.R., Lee S.K., Kim S.-H., Kim Y.-H., Cha J.-Y., Oh S.-W., Ha J.-R., Ryu S.H., Suh P.-G. Potential Inhibition of PDK1/Akt Signaling by Phenothiazines Suppresses Cancer Cell Proliferation and Survival. Ann. N. Y. Acad. Sci. 2008;1138:393–403. doi: 10.1196/annals.1414.041. [DOI] [PubMed] [Google Scholar]
- 95.Xia L., Tan S., Zhou Y., Lin J., Wang H., Oyang L., Tian Y., Liu L., Su M., Wang H., et al. Role of the NFκB-Signaling Pathway in Cancer. Onco Targets Ther. 2018;11:2063–2073. doi: 10.2147/OTT.S161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song M., Bode A.M., Dong Z., Lee M.-H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019;79:1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 97.Hua H., Kong Q., Zhang H., Wang J., Luo T., Jiang Y. Targeting MTOR for Cancer Therapy. J. Hematol. Oncol. 2019;12:71. doi: 10.1186/s13045-019-0754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koziol M.J., Gurdon J.B. TCTP in Development and Cancer. Biochem. Res. Int. 2012;2012:1–9. doi: 10.1155/2012/105203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boia-Ferreira M., Basílio A.B., Hamasaki A.E., Matsubara F.H., Appel M.H., da Costa C.R.V., Amson R., Telerman A., Chaim O.M., Veiga S.S., et al. TCTP as a Therapeutic Target in Melanoma Treatment. Br. J. Cancer. 2017;117:656–665. doi: 10.1038/bjc.2017.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amson R., Auclair C., André F., Karp J., Telerman A. Targeting TCTP with sertraline and thioridazine in cancer treatment. In: Telerman A., Amson R., editors. TCTP/tpt1—Remodeling Signaling from Stem Cell to Disease. Volume 64. Springer International Publishing; Cham, Switzerland: 2017. pp. 283–290. Results and Problems in Cell Differentiation. [DOI] [PubMed] [Google Scholar]
- 101.Duarte D., Rêma A., Amorim I., Vale N. Drug Combinations: A New Strategy to Extend Drug Repurposing and Epithelial-Mesenchymal Transition in Breast and Colon Cancer Cells. Biomolecules. 2022;12:190. doi: 10.3390/biom12020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Evason K.J., Francisco M.T., Juric V., Balakrishnan S., Lopez Pazmino M.d.P., Gordan J.D., Kakar S., Spitsbergen J., Goga A., Stainier D.Y.R. Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish. PLoS Genet. 2015;11:e1005305. doi: 10.1371/journal.pgen.1005305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng H.-W., Liang Y.-H., Kuo Y.-L., Chuu C.-P., Lin C.-Y., Lee M.-H., Wu A.T.H., Yeh C.-T., Chen E.I.-T., Whang-Peng J., et al. Identification of Thioridazine, an Antipsychotic Drug, as an Antiglioblastoma and Anticancer Stem Cell Agent Using Public Gene Expression Data. Cell Death Dis. 2015;6:e1753. doi: 10.1038/cddis.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bi J., Khan A., Tang J., Armando A.M., Wu S., Zhang W., Gimple R.C., Reed A., Jing H., Koga T., et al. Targeting Glioblastoma Signaling and Metabolism with a Re-Purposed Brain-Penetrant Drug. Cell Rep. 2021;37:109957. doi: 10.1016/j.celrep.2021.109957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen S., Faouzi S., Souquere S., Roy S., Routier E., Libenciuc C., André F., Pierron G., Scoazec J.-Y., Robert C. Melanoma Persister Cells Are Tolerant to BRAF/MEK Inhibitors via ACOX1-Mediated Fatty Acid Oxidation. Cell Rep. 2020;33:108421. doi: 10.1016/j.celrep.2020.108421. [DOI] [PubMed] [Google Scholar]
- 106.Kosić M., Nešić Z., Glumac S., Vasić M., Pajović V., Savić B., Japundžić-Žigon N. Paroxetine Mitigates Cardiac Remodelling by Doxorubicin and Increases Survival. Biomed. Pharmacother. 2022;145:112411. doi: 10.1016/j.biopha.2021.112411. [DOI] [PubMed] [Google Scholar]
- 107.Bruce J.I.E., James A.D. Targeting the Calcium Signalling Machinery in Cancer. Cancers. 2020;12:2351. doi: 10.3390/cancers12092351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vasiliou V., Vasiliou K., Nebert D.W. Human ATP-Binding Cassette (ABC) Transporter Family. Hum. Genom. 2008;3:281. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zu Y., Yang Z., Tang S., Han Y., Ma J. Effects of P-Glycoprotein and Its Inhibitors on Apoptosis in K562 Cells. Molecules. 2014;19:13061–13075. doi: 10.3390/molecules190913061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nanayakkara A.K., Follit C.A., Chen G., Williams N.S., Vogel P.D., Wise J.G. Targeted Inhibitors of P-Glycoprotein Increase Chemotherapeutic-Induced Mortality of Multidrug Resistant Tumor Cells. Sci. Rep. 2018;8:967. doi: 10.1038/s41598-018-19325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dean M., Rzhetsky A., Allikmets R. The Human ATP-Binding Cassette (ABC) Transporter Superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 112.Mohammad I.S., He W., Yin L. Understanding of Human ATP Binding Cassette Superfamily and Novel Multidrug Resistance Modulators to Overcome MDR. Biomed. Pharmacother. 2018;100:335–348. doi: 10.1016/j.biopha.2018.02.038. [DOI] [PubMed] [Google Scholar]
- 113.Dean M., Fojo T., Bates S. Tumour Stem Cells and Drug Resistance. Nat. Rev. Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 114.Katayama K., Noguchi K., Sugimoto Y. Regulations of P-Glycoprotein/ABCB1/ MDR1 in Human Cancer Cells. New J. Sci. 2014;2014:476974. doi: 10.1155/2014/476974. [DOI] [Google Scholar]
- 115.DeBerardinis R.J., Chandel N.S. Fundamentals of Cancer Metabolism. Sci. Adv. 2016;2:e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liou G.-Y., Storz P. Reactive Oxygen Species in Cancer. Free Radic. Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cai Y., Lu J., Miao Z., Lin L., Ding J. Reactive Oxygen Species Contribute to Cell Killing and P-Glycoprotein Downregulation by Salvicine in Multidrug Resistant K562/A02 Cells. Cancer Biol. Ther. 2007;6:1794–1799. doi: 10.4161/cbt.6.11.4860. [DOI] [PubMed] [Google Scholar]
- 118.O’Brien F.E., Dinan T.G., Griffin B.T., Cryan J.F. Interactions between Antidepressants and P-Glycoprotein at the Blood-Brain Barrier: Clinical Significance of in Vitro and in Vivo Findings: Antidepressant-P-Gp Interactions at the BBB. Br. J. Pharmacol. 2012;165:289–312. doi: 10.1111/j.1476-5381.2011.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.El Ela A.A., Härtter S., Schmitt U., Hiemke C., Spahn-Langguth H., Langguth P. Identification of P-Glycoprotein Substrates and Inhibitors among Psychoactive Compounds—Implications for Pharmacokinetics of Selected Substrates. J. Pharm. Pharmacol. 2010;56:967–975. doi: 10.1211/0022357043969. [DOI] [PubMed] [Google Scholar]
- 120.Mishra R., Sareen S., Sharma B., Goyal S., Kaur G., Bhardwaj S., Siddiqui A.A., Husain A., Singla R.K., Rashid M., et al. Phenothiazines and Related Drugs as Multi Drug Resistance Reversal Agents in Cancer Chemotherapy Mediated by P-Glycoprotein. Curr. Cancer Ther. Rev. 2017;13:28–42. doi: 10.2174/1573394713666170524122904. [DOI] [Google Scholar]
- 121.Peer D., Dekel Y., Melikhov D., Margalit R. Fluoxetine Inhibits Multidrug Resistance Extrusion Pumps and Enhances Responses to Chemotherapy in Syngeneic and in Human Xenograft Mouse Tumor Models. Cancer Res. 2004;64:7562–7569. doi: 10.1158/0008-5472.CAN-03-4046. [DOI] [PubMed] [Google Scholar]
- 122.Argov M., Kashi R., Peer D., Margalit R. Treatment of Resistant Human Colon Cancer Xenografts by a Fluoxetine–Doxorubicin Combination Enhances Therapeutic Responses Comparable to an Aggressive Bevacizumab Regimen. Cancer Lett. 2009;274:118–125. doi: 10.1016/j.canlet.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y., Zhou T., Duan J., Xiao Z., Li G., Xu F. Inhibition of P-Glycoprotein and Glutathione S-Transferase-Pi Mediated Resistance by Fluoxetine in MCF-7/ADM Cells. Biomed. Pharmacother. 2013;67:757–762. doi: 10.1016/j.biopha.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 124.Duarte D., Nunes M., Ricardo S., Vale N. Combination of Antimalarial and CNS Drugs with Antineoplastic Agents in MCF-7 Breast and HT-29 Colon Cancer Cells: Biosafety Evaluation and Mechanism of Action. Biomolecules. 2022;12:1490. doi: 10.3390/biom12101490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Drinberg V., Bitcover R., Rajchenbach W., Peer D. Modulating Cancer Multidrug Resistance by Sertraline in Combination with a Nanomedicine. Cancer Lett. 2014;354:290–298. doi: 10.1016/j.canlet.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 126.Choi A.-R., Kim J.-H., Yoon S. Thioridazine Specifically Sensitizes Drug-Resistant Cancer Cells through Highly Increase in Apoptosis and P-gp Inhibition. Tumor Biol. 2014;35:9831–9838. doi: 10.1007/s13277-014-2278-1. [DOI] [PubMed] [Google Scholar]
- 127.Salvo F., Pariente A., Shakir S., Robinson P., Arnaud M., Thomas S., Raschi E., Fourrier-Réglat A., Moore N., Sturkenboom M., et al. Sudden Cardiac and Sudden Unexpected Death Related to Antipsychotics: A Meta-Analysis of Observational Studies. Clin. Pharmacol. Ther. 2016;99:306–314. doi: 10.1002/cpt.250. [DOI] [PubMed] [Google Scholar]
- 128.WHO . World Health Organization Model List of Essential Medicines—22nd List. World Health Organization; Geneva, Switzerland: 2021. [Google Scholar]
- 129.Cheon J.H., Lee B.M., Kim H.S., Yoon S. Highly Halaven-Resistant KBV20C Cancer Cells Can Be Sensitized by Co-Treatment with Fluphenazine. Anticancer Res. 2016;36:5867–5874. doi: 10.21873/anticanres.11172. [DOI] [PubMed] [Google Scholar]
- 130.Środa-Pomianek K., Michalak K., Palko-Łabuz A., Uryga A., Świątek P., Majkowski M., Wesołowska O. The Combined Use of Phenothiazines and Statins Strongly Affects Doxorubicin-Resistance, Apoptosis, and Cox-2 Activity in Colon Cancer Cells. Int. J. Mol. Sci. 2019;20:955. doi: 10.3390/ijms20040955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kars M.D., İşeri O.D., Gunduz U., Molnar J. Reversal of Multidrug Resistance by Synthetic and Natural Compounds in Drug-Resistant MCF-7 Cell Lines. Chemotherapy. 2008;54:194–200. doi: 10.1159/000140462. [DOI] [PubMed] [Google Scholar]
- 132.Dönmez Y., Akhmetova L., İşeri Ö.D., Kars M.D., Gündüz U. Effect of MDR Modulators Verapamil and Promethazine on Gene Expression Levels of MDR1 and MRP1 in Doxorubicin-Resistant MCF-7 Cells. Cancer Chemother. Pharm. 2011;67:823–828. doi: 10.1007/s00280-010-1385-y. [DOI] [PubMed] [Google Scholar]
- 133.WHO . World Mental Health Report: Transforming Mental Health for All. World Health Organization; Geneva, Switzerland: 2022. [Google Scholar]
- 134.Moreno-Agostino D., Wu Y.-T., Daskalopoulou C., Hasan M.T., Huisman M., Prina M. Global Trends in the Prevalence and Incidence of Depression:A Systematic Review and Meta-Analysis. J. Affect. Disord. 2021;281:235–243. doi: 10.1016/j.jad.2020.12.035. [DOI] [PubMed] [Google Scholar]
- 135.Bretler T., Weisberg H., Koren O., Neuman H. The Effects of Antipsychotic Medications on Microbiome and Weight Gain in Children and Adolescents. BMC Med. 2019;17:112. doi: 10.1186/s12916-019-1346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Munawar N., Ahsan K., Muhammad K., Ahmad A., Anwar M.A., Shah I., Al Ameri A.K., Al Mughairbi F. Hidden Role of Gut Microbiome Dysbiosis in Schizophrenia: Antipsychotics or Psychobiotics as Therapeutics? Int. J. Mol. Sci. 2021;22:7671. doi: 10.3390/ijms22147671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.