Abstract
Oxidative stress arises when the generation of reactive oxygen species or reactive nitrogen species overwhelms antioxidant systems. Developing kidneys are vulnerable to oxidative stress, resulting in adult kidney disease. Oxidative stress in fetuses and neonates can be evaluated by assessing various biomarkers. Using animal models, our knowledge of oxidative-stress-related renal programming, the molecular mechanisms underlying renal programming, and preventive interventions to avert kidney disease has grown enormously. This comprehensive review provides an overview of the impact of perinatal oxidative stress on renal programming, the implications of antioxidant strategies on the prevention of kidney disease, and the gap between animal models and clinical reality.
Keywords: oxidative stress, antioxidant, kidney disease, nitric oxide, asymmetric dimethylarginine, reactive oxygen species, melatonin, fetal programming
1. Introduction
Imbalances between reactive oxygen species or reactive nitrogen species (ROS and RNS, respectively) and innate antioxidant systems result in oxidative stress [1]. During pregnancy, ROS and RNS have dual roles in fetal development [2]. Normally, a moderate increase in ROS and RNS levels is essential for placental angiogenesis, cell differentiation, and fetal organogenesis. In contrast, the overproduction of ROS and RNS, as observed in compromised pregnancies, is associated with adverse pregnancy and fetal outcomes [3]. In addition, a surplus of ROS reduces nitric oxide (NO) bioavailability. NO is recognized as a key regulator of both maternal and fetal homeostasis during gestation [4].
After birth, newborns are highly vulnerable to ROS- and RNS-induced oxidative damage [5]. A newborn encounters the transition from a hypoxic intrauterine environment to a postnatal oxygen-rich environment with an approximately five-fold increase in oxygen exposure. Notably, preterm babies have increased susceptibility to increased oxidative stress conditions (e.g., infection and inflammation), in addition to their antioxidant defenses being impaired [6].
During development, the kidneys are vulnerable to oxidative stress and other environmental insults that impair nephrogenesis [7]. In humans, kidney development starts at week three and is completed at week 36 of pregnancy [8]. An exponential increase in nephrons occurs at 18–32 weeks of pregnancy. Nephron development is complete at the end of gestation [9]. Thus, preterm birth is associated with a reduction in nephron numbers and increased risk of kidney disease [9]. Impaired nephrogenesis results in low nephron endowment and a spectrum of defects in the kidneys and urinary tract [10].
To date, little information is available about the influence of perinatal oxidative stress on the development of kidney disease in humans. Unlike humans, nephrogenesis in rats lasts after birth and finishes at 1–2 weeks postnatally [11]. Developing kidneys are mostly vulnerable to suboptimal pre-, peri-, and early postnatal conditions, resulting in alterations in structure and function, namely renal programming [12]. A growing body of evidence from animal models has offered greater understanding of the link between oxidative stress and the development of kidney disease [7,13,14]. On the contrary, data from animal studies indicated that the perinatal use of antioxidants was able to reverse programming processes and prevent kidney diseases of developmental origins [15].
Although substantial progress has been achieved in developing various animal models to study oxidative-stress-related renal programming, the need for meaningful translation into clinical practice is still a research priority. Hence, the present review seeks to highlight the best available evidence on the interplay between perinatal oxidative stress and renal programming. We attempt to discuss the impact of oxidative stress on fetuses and neonates, its associations with common mechanisms behind renal programming, and the potential of antioxidant strategies for the prevention of kidney disease.
2. Oxidative Stress and Fetal Programming
2.1. ROS, RNS, and NO
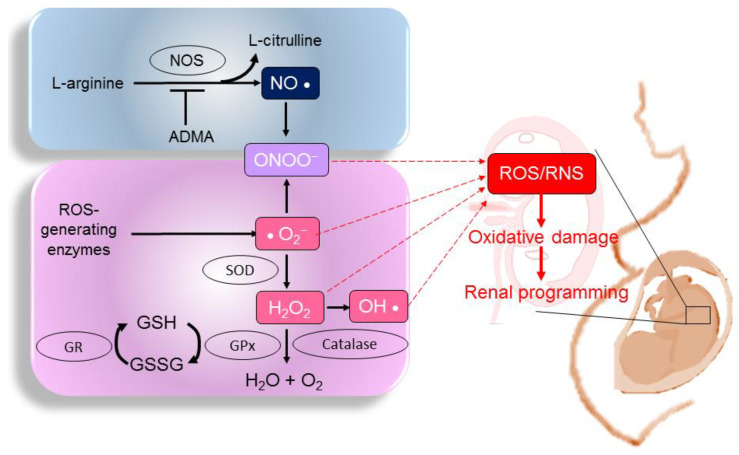
The disequilibrium of the pro-oxidant–antioxidant balance leads to oxidative stress. ROS and RNS can be radical or non-radical compounds. Examples of ROS include free radicals, such as superoxide anions (•O2−) and hydroxyl radicals (•OH), as well as non-radicals, such as hydrogen peroxide (H2O2) [16]. Nitrogen-containing oxidants, such as nitric oxide (•NO), nitrogen dioxide (NO2), and peroxynitrite (ONOO−), are called RNS [17]. On the other hand, the excess of ROS or RNS can be neutralized by antioxidant systems including enzymatic components (e.g., superoxide dismutase (SOD) and non-enzymatic antioxidants (e.g., glutathione) [18]. NO is generated by NO synthases (NOSs) [19]. Asymmetric and symmetric dimethylarginine (ADMA and SDMA, respectively) can uncouple NOSs to generate peroxynitrite, further reducing NO bioavailability and enhancing oxidative stress [20]. As ROS, RNS, and NO are essential for pregnancy, maintenance of their balance is crucial for the normal development of a fetus. The ROS- and RNS-generating pathways, the NO pathway, and antioxidant systems in a fetus, as well as their interconnections with renal programming, are illustrated in Figure 1.
Figure 1.
Diagram illustrating the pathways that generate reactive oxygen species (ROS) and reactive nitrogen species (RNS), the nitric oxide (NO) pathway, and antioxidant systems in a fetus. The overproduction of ROS or RNS under adverse intrauterine conditions overwhelms the antioxidant system, resulting in oxidative damage and, thereby, compromising renal development. NOS: nitric oxide synthase; ADMA: asymmetric dimethylarginine; ONOO−: peroxynitrite; SOD: superoxide dismutase; GPx: glutathione peroxidase; GR: glutathione reductase; GSH: reduced glutathione; GSSH: oxidized glutathione; H2O2: hydrogen peroxide; OH•: hydroxyl radical.
2.2. Studies in Humans: Oxidative Stress in Fetuses and Neonates
A fetus obtains sufficient amounts of oxygen to meet its growth and metabolic needs. During the first trimester, the fetal oxygen requirement is low. Nevertheless, increasing oxygen levels are required for the establishment of fetal–placental circulation and the rapid fetal weight gain in the second and third trimesters [21]. Although a moderate physiological level of ROS is crucial to maintain a healthy pregnancy [2,3], prior work has indicated that increased oxidative stress exists in a variety of complications in pregnancy. These include, but are not limit to, gestational diabetes [22], preeclampsia [23], preterm birth [24], placenta dysfunction [25], maternal obesity [26], preterm premature rupture of membranes [27], and intrauterine growth retardation (IUGR) [28]. Fetuses with these complications during pregnancy have long-term consequences in their health later in life.
Preterm babies are particularly vulnerable to oxidative damage due to the immaturity of antioxidant systems, high non-protein-bound iron (NPBI) levels, and the high energy requirements for growth [5]. These instances of oxidative damage include the oxidation of biological molecules, such as lipids, proteins, and DNA. The plasma of preterm babies showed reduced antioxidant capacity characterized by low levels of SOD, CAT, GPX, copper, vitamin E, selenium, ceruloplasmin, zinc, etc. [29]. Oxidative stress is identified as one of the main causes responsible for several complications of prematurity, including necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), intraventricular hemorrhage (IVH), respiratory distress syndrome (RDS), kidney disease, etc. [5].
2.3. Biomarkers of Oxidative Stress in Clinical Practice
Oxidative stress in fetuses and neonates has been evaluated by assessing products of lipid peroxidation in the serum or amniotic fluid, such as malondialdehyde (MDA), F2-isoprostanes (F2-IsoPs), 4-hydroxy-2-nonenal (4-HNE), and thiobarbituric-acid-reactive substances (TBARSs) [30,31]. In addition, oxidative-stress-related protein damage can be measured by advanced oxidation protein products (AOPPs) in the serum or cord blood [30,32]. Regarding DNA damage, 8-hydroxy-2′-deoxyguanosine (8-OHdG) is a commonly used biomarker, as it is an oxidized nucleoside released upon the repair of damaged DNA [33]. 8-OHdG is excreted in urine without further metabolism; therefore, urinary 8-OHdG is employed as a biomarker of oxidative stress in newborn medicine [34]. The ratio of reduced to oxidized glutathione (GSH/GSSG) is another biomarker employed [35], as it represents a dynamic balance between oxidants and antioxidants. Moreover, measurements of antioxidant status that include the total antioxidant capacity (TAC) and antioxidant enzymes (e.g., SOD and catalase) can also be utilized as oxidative stress biomarkers [36].
Regarding RNS, plasma and cerebrospinal fluid levels of 3-nitrotyrosine have been applied as markers for peroxynitrite in neonates [37,38]. ADMA and the NO metabolites of nitrite and nitrate have been measured in the plasma and urine [39,40]. Preeclampsia is connected to low NO bioavailability, represented by the L-arginine-to-ADMA ratio [41]. Thus far, NO can be detected in vivo using various methods, such as chemiluminescence, fluorescence, and electron spin resonance spectroscopy. Nevertheless, NO measurements by these methods are still limited in neonatal medicine.
2.4. What Is Missing from Human Studies?
At full-term birth, neonates generally possess a complete endowment of nephrons. Nevertheless, nephron numbers may be reduced in infants who are born preterm due to compromised pregnancy, inadequacy of postnatal nutrition, intrauterine growth retardation (IUGR), and treatment with certain medications (e.g., gentamicin) after birth [42]. Low nephron numbers play a part in glomerular hypertension and hyperperfusion injury, consequently provoking a vicious cycle of more nephron loss later in life [43]. Importantly, low nephron endowment presumably enacts a first hit to the kidneys, which makes the remaining glomeruli more vulnerable to developing CKD when facing second-hit kidney injuries [44].
To date, nephron numbers cannot be calculated in vivo. Despite average nephron numbers reported at about 1 million in each kidney based on prior studies of kidney autopsies, human nephron numbers are highly variable (10-fold difference) [8]. In human studies, there remain unmet needs to elucidate the molecular mechanisms behind perinatal oxidative-stress-induced kidney disease and to develop interventions necessary to prove causation. Clinically, kidney biopsies are a technically difficult procedure in children, especially in neonates. It should be noted that it remains largely unknown whether there is a link between kidney pathologies and circulating oxidative stress biomarkers in fetuses and neonates. This is the reason why much of our knowledge of oxidative-stress-related renal programming, the molecular mechanisms underlying renal programming, and preventive interventions to avert kidney disease mainly originate from animal studies.
3. Animal Models of Oxidative-Stress-Related Renal Programming
Through the use of animal models, our understanding of the molecular mechanisms behind renal programming has grown enormously in recent years [7,12]. Core mechanisms include, but are not limited to, oxidative stress, NO deficiency, low nephron number, aberrant activation of the renin–angiotensin system, dysregulated nutrient-sensing signals, and gut microbiota dysbiosis [7,12,45,46]. The tight interconnections between oxidative stress and other common mechanisms behind renal programming mean that oxidative stress plays a prominent role.
Table 1 provides a summary of animal models of oxidative-stress-related renal programming [33,35,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77]. The current review is chiefly restricted to adverse environmental cues beginning in gestation and lactation. A wide range of environmental cues can lead to oxidative-stress-related renal programming, including imbalanced maternal nutrition [47,48,49,50,51,52,53,54,55,56,57,58,59], maternal disorders [60,61,62,63,64,65,66,67,68,69], environmental chemical and toxin exposure [70,71,72,73], and medication use [74,75,76,77].
Table 1.
Animal models of oxidative-stress-related renal programming.
| Animal Models | Species/ Gender |
Age at Evaluation | Mechanisms of Oxidative Stress | Renal Outcomes | Ref. |
|---|---|---|---|---|---|
| Maternal nutritional insults | |||||
| Maternal caloric restriction diet | SD rat/M | 12 weeks | ↑ ADMA, ↓ NO, ↑ renal 8-OHdG expression | ↓nephron number, glomerular hypertrophy, ↑ tubulointerstitial injury, hypertension | [47,48] |
| Maternal protein restriction diet | Wistar rat/M | 12 weeks | ↑ F2-isoprostane, ↓ glutathione | ↑BP | [49] |
| Maternal high-fructose diet | SD rat/M | 12 weeks | ↓ NO, ↑renal 8-OHdG expression | ↑BP | [50] |
| Maternal plus post-weaning high-fructose diet | SD rat/M | 12 weeks | ↑ renal 8-OHdG expression | ↑BP | [51] |
| Maternal methyl-deficient diet | SD rat/M | 12 weeks | ↑ renal 8-OHdG expression | ↑BP | [52] |
| Maternal high-methyl-donor diet | SD rat/M | 12 weeks | ↑ renal 8-OHdG expression | ↑BP | [52] |
| Maternal iron deficiency | SD rat/M | 16 weeks | ↑ renal 8-OHdG expression | ↑BP | [53] |
| Maternal high-fat and high-cholesterol diet | SD rat/M and F | 90 days | ↓ SOD activity in M, ↑ renal MDA level in F | ↑BP | [54] |
| Maternal plus post-weaning high-fat diet | SD rat/M and F | 16 weeks | ↓ NO, ↑renal 8-OHdG expression | ↑BP, ↑kidney injury in M | [55,56] |
| Maternal high-fat and high-cholesterol diet | SD rat/M | 18 weeks | ↑renal MDA, ↓antioxidant enzymatic activity | hypertension, impaired renal function | [57] |
| Maternal high-fat diet | C57BL/6 mice/M | 9 weeks | ↑renal 8-OHdG expression | ↑renal hypertrophy, ↑albuminuria | [58] |
| Maternal high-fat diet | C57BL/6 mice/M | 32 weeks | ↑ renal 3-NT, ↑ renal NOX2 expression | ↑renal global DNA methylation, ↑albuminuria, ↑glomerulosclerosis | [59] |
| Maternal disorders | |||||
| Maternal L-NAME administration | SD rat/M | 12 weeks | ↑ renal F2-isoprostane | ↑BP | [60] |
| Maternal ADMA administration | SD rat/M | 12 weeks | ↓ NO | ↑BP | [61] |
| Streptozotocin-induced diabetes | SD rat/M | 12 weeks | ↓ NO, ↑ ADMA | ↓nephron number,↑ tuburointerstitial injury | [62] |
| Streptozotocin-induced diabetes | SD rat/M | 12 weeks | ↑ renal TBARS, ↑3-NT | ↑BP, discurbed acute renal hemodynamics | [63] |
| Maternal suramin administration | SD rat/M | 12 weeks | ↓ NO, ↑ ADMA | ↑BP | [64] |
| Maternal adenine-induced CKD | SD rat/M | 12 weeks | ↓ NO, ↑ ADMA, ↑ renal 8-OHdG expression, | ↑BP, ↑renal hypertrophy | [65,66] |
| Reduced uterine perfusion | SD rat/M | 16 weeks | ↑ urinary F2-isoprostane level and renal NADPH-oxidase-dependent superoxide | ↑BP | [67] |
| Maternal angiotensin II administration | Wistar rat/M | 18 week | ↑ renal ROS | ↑BP, ↑tuburointerstitial injury | [68] |
| Prenatal LPS Exposure |
Wistar rat/M | 28 weeks | ↑ renal MDA | ↑BP | [69] |
| Toxins | |||||
| Prenatal bisphenol A exposure plus high-fat diet | SD rat/M | 16 weeks | ↑ ADMA, ↓ NO, ↑renal 8-OHdG expression | ↑BP | [70] |
| Prenatal dexamethasone plus TCDD exposure | SD rat/M | 16 weeks | ↑ renal 8-OHdG expression, ↑ ADMA | ↑BP | [71] |
| Maternal di-n-butyl phthalate exposure | SD rat/M and F | 18 months | ↑ renal ROS | Renal dysplasia,↑ tuburointerstitial injury | [72] |
| Matenal smoking exposure | Balb/c mice/M | 13 weeks | ↑ renal ROS | ↓nephron number,↑albuminuria | [73] |
| Medication and Drugs | |||||
| Dexamethasone administration during lactation | Wistar rat/M and F | 12 weeks | ↑renal MDA level, ↓SOD and catalase activity | ↑Tubular necrosis, renal dysfunction | [74] |
| Prenatal dexamethasone exposure | SD rat/M | 16 weeks | ↓ renal NO | ↑BP | [75] |
| Prenatal dexamethasone exposure plus postnatal high-fat intake | SD rat/M | 16 weeks | ↑ renal 8-OHdG expression, ↓ NO | ↑BP | [76] |
| Prenatal betamethasone exposure | Sheep/M and F | 18 months | ↓ NO,↑ ROS | ↑BP | [77] |
ADMA: asymmetric dimethylarginine; MDA: malondialdehyde; 8-OHdG: 8-hydroxy-2’–deoxyguanosine; 3-NT: 3-nitrotyrosine; 4-NHE: 4-hydroxynonenal; TBARS: thiobarbituric acid; NO: nitric oxide; ROS: reactive oxygen species; CKD: chronic kidney disease; LPS: lipopolysaccharide; SD: Sprague–Dawley; TCDD: 2,3,7,8-tetrachlorodibenzo-p-dioxin; L-NAME: L-NG-nitro arginine methyl ester; BP: blood pressure; M: male; F: female; ↑: increase; ↓: decrease.
3.1. Maternal Insults
Table 1 illustrates that nutritional imbalance is the most common insult that induces renal programming. Types of maternal nutritional insults can be grouped into different models that aim to reduce calorie intake [47,48], reduce protein intake [49], increase fructose intake [50,51], manipulate methyl donor [52] or iron intake [53], and increase fat intake [54,55,56,57,58,59]. In addition, maternal disorders, such as NO deficiency [60,61], diabetes [62,63], preeclampsia [64], CKD [65,66], reduced uterine perfusion [67], hypertension [68], and inflammation [69], have all been reported to impair nephrogenesis, resulting in renal programming. Environmental toxins, such as bisphenol A [70], 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) [71], di-n-butyl phthalate [72], and smoking [73], also contribute to renal programming. Moreover, medications, such as glucocorticoid, are able to induce renal programming [74,75,76,77].
As all nutrients during pregnancy have essential roles in fetal development, the excessive or insufficient intakes of certain nutrients have been employed to establish animal model for studying renal programing. As shown in Table 1, different maternal nutritional insults could induce the same phenotype of hypertension, suggesting there might be common mechanisms involved in nutritional programming [78]. Conversely, the BP of offspring exposed to high-fat maternal diets could vary according to age, sex, fatty acid composition, and strain [79]. In addition, the data from Table 1 indicate that high-fat maternal diets could induce renal programming related to various sources and mechanisms of oxidative stress. Accordingly, a deeper understanding of oxidative-stress-induced nutritional programming may help to limit or avoid specific foods during pregnancy and develop effective nutritional interventions for clinical practice.
As shown in Table 1, rats are the preferred animals used to study renal programming, followed by mice and sheep. Unlike humans, kidney development in rats lasts until 1–2 week after birth [11]. Adverse environmental conditions, not only during gestation but also lactation, can affect kidney development, consequently leading to kidney disease later in life. As each rat month is roughly equivalent to three human years in adulthood [80], Table 1 illustrates the ages at evaluation, allowing calculations for reference to human ages.
Table 1 shows that the most common outcome of renal programming evaluation is hypertension. Although several environmental cues have been connected to low nephron endowment [81], the interconnection between low nephron number and oxidative stress has only been reported in models of streptozotocin-induced diabetes [62], caloric restriction [47], and maternal smoking exposure [73]. In addition to reduced nephron number, renal hypertrophy [47,48,58], glomerulosclerosis [59], tubulointerstitial injury [47,48,62,68,72,74], renal dysfunction [57,74], and albuminuria [58,59,73] are major adverse renal outcomes associated with renal programming (Table 1).
3.2. Oxidative-Stress-Mediated Mechanisms
As a fetus has low antioxidant capacity, a surplus of ROS or RNS under adverse intrauterine conditions can overwhelm antioxidants, resulting in oxidative damage and, thereby, compromising fetal development [2,3]. Cumulative evidence supports the key role of oxidative stress implicated in fetal programming. ROS can mediate several key epigenetic processes, such as DNA methylation, histone modifications, and micro-RNAs (mRNAs) [82]. It is noteworthy that these epigenetic modifications of genes are considered crucial mechanisms for fetal programming [83].
NO is also involved in epigenetic regulation and fetal programming [84,85]. ADMA can reduce NO production and increase ROS [20]. In our prior work, ADMA-treated embryonic kidneys exhibited reductions in nephron numbers in a dose-dependent manner [86]. We also evaluated a transcriptome analysis of developing kidneys in response to ADMA. Embryonic kidneys grown in 10 µM ADMA were isolated for a next-generation RNA sequencing (NGS) analysis, and 1221 differentially expressed genes (DEGs; 735 up- and 486 down-regulated) were identified [86]. In a model of maternal NO inhibition by NG-nitro-L-arginine-methyl ester (L-NAME), a total of 2289 DEGs (1259 up- and 1030 down-regulated) were identified in neonatal kidneys [60]. Among these DEGs, several genes were related to kidney development and epigenetic regulation. These observations suggest that a link between oxidative stress and epigenetic gene regulation during pregnancy could represent a strong contribution to renal programming and kidney disease risk in offspring later in life.
Renal programming can be attributed to several oxidative-stress-mediated mechanisms, including increased ROS-producing enzyme expression [59], increased ROS [68,72,73,77], increased peroxynitrite [59,63], decreased antioxidant capabilities [54,57,74], increased ADMA [47,48,62,64,65,66,70,71], reduced NO bioavailability [47,48,50,61,62,64,65,66,70,75,76,77], and increased oxidative damage [47,48,49,50,51,52,53,54,55,56,57,58,60,63,69,70,71,74,76].
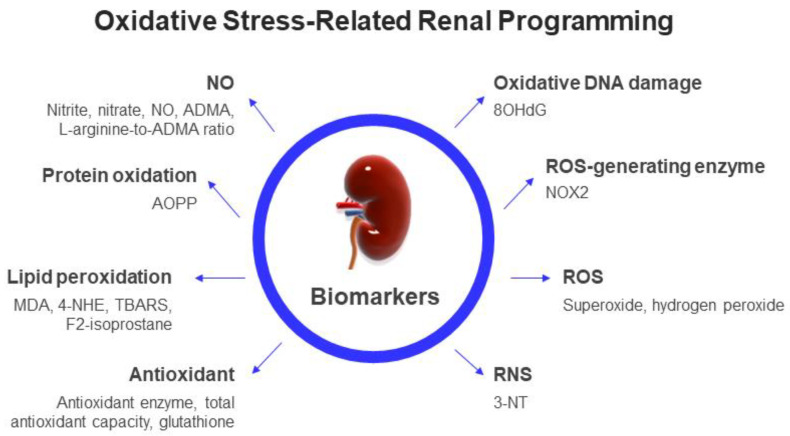
As delineated earlier, several biomarkers of lipid peroxidation have been demonstrated in neonates, such as MDA, F2-IsoPs, and TBARS. Table 1 reveals that these biomarkers of lipid peroxidation are elevated in offspring kidneys in different rodent models of renal programming [49,54,57,60,63,66,69,74]. In addition, 8-OHdG, a frequently studied oxidative DNA damage marker, is highly expressed in rat offspring kidneys and is correlated to adverse renal outcomes [47,48,50,51,52,53,55,56,58,65,66,70,71,76]. As ROS is difficult to determine in human kidneys, animal studies have provided evidence that increased renal ROS is associated with adverse renal outcomes in models of reduced uterine perfusion [73], maternal angiotensin II administration [68], maternal DEHP exposure [72], and maternal smoking exposure [73]. Renal 3-NT level can also be used to detect peroxynitrite in rat models of renal programming [59,63]. Moreover, numerous studies in Table 1 indicate that an impaired ADMA/NO pathway contributes to oxidative-stress-induced renal programming [47,48,62,64,65,66,70,71,75,76,77]. In summary, these observations support the idea that oxidative-stress-induced renal programming contributes to adverse renal outcomes later in life. The impact of oxidative stress on renal programming can be evaluated by biomarkers that quantify the levels of ROS, RNS, NO, antioxidants, and oxidation by-products from DNA, protein, and lipid damage, as illustrated in Figure 2.
Figure 2.
Schema summarizing the potential biomarkers regarding oxidative-stress-related renal programming in clinical and experimental studies. NO: nitric oxide; ADMA: asymmetric dimethylarginine; AOPPs: advanced oxidation protein products; 4-HNE: 4-hydroxy-2-nonenal; MDA: malondialdehyde; TBARS: thiobarbituric acid; 8-OHdG: 8-hydroxy-2′-deoxyguanosine; 3-NT: 3-nitrotyrosine.
4. Antioxidant Strategies for Kidney Health
As mentioned above, perinatal oxidative stress plays a pivotal role in renal programming, resulting in adult-onset kidney diseases. It is reasonably assumed that a surplus of ROS or RNS may be amenable to antioxidant therapies, which, if administered in early life, may avert the development of kidney diseases. Even though the role of oxidative stress in the pathogenesis of renal programming is undoubted, the positive effects of antioxidant therapies on kidney diseases remain inconclusive clinically [87,88], as well as in fetuses and neonates [89,90,91]. While the majority of human trials have not confirmed any evidence of kidney benefits from antioxidant supplementation, we recognize the potential benefit of antioxidant therapies through current evidence in preclinical animal models and limited human studies.
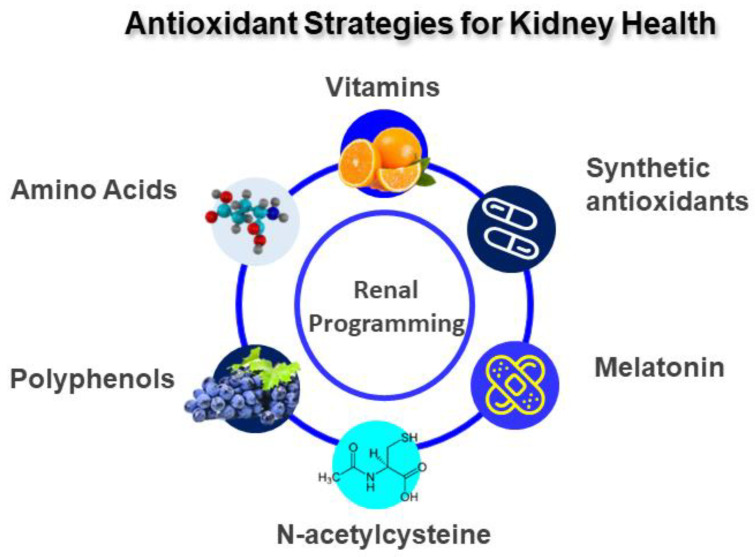
Antioxidants can be grouped as enzymatic or non-enzymatic and natural or synthetic. They are categorized by mechanism of action as either targeting ROS or NO. As reviewed elsewhere [7], data from animal studies indicate that the uses of several natural antioxidants, including vitamins, amino acids, melatonin, and polyphenol, during pregnancy and lactation have shown to benefits to kidney health and prevent renal programming. Sources of natural antioxidants are mainly plants, i.e., vegetables, fruits, seeds, and nuts, which are rich in vitamins, polyphenol, carotenoids, and glutathione. Along with natural antioxidants, some synthetic antioxidants have also been implemented in animal models of renal programming.
As mentioned earlier, nutritional programming is emerging as a critical mechanism contributing to oxidative-stress-related renal programming. It is noteworthy that nutritional programming can also be advantageous [92]. Several nutritional interventions with antioxidant or anti-inflammatory diets have been proved effective in preventing the development of adult-onset kidney diseases with the use of animal models [7] (Figure 3). These are discussed below.
Figure 3.
Diagram outlining a potential antioxidant strategy to prevent adult-onset kidney diseases.
4.1. Vitamins
Vitamins A, C, and E, as well as selenium, folic acid, etc., exhibit advantageous effects on kidney health [93]. The most frequently used antioxidant supplements are vitamins C and E. Vitamin C, a water-soluble antioxidant, is a scavenger of free radicals and a reducing agent [94]. Vitamin E, a lipid-soluble antioxidant, inhibits several oxidative enzymes to reduce ROS production [95].
Vitamin C or E supplementation alone during pregnancy protects maternal lipopolysaccharide (LPS)-exposure-primed offspring hypertension, a major phenotype of renal programming [96,97]. Additionally, the combined supplementation of vitamins C and E with selenium and folic acid averted offspring hypertension in a rat model of maternal caloric restriction [98].
Though several vitamins exhibit beneficial effects on oxidative-stress-related kidney diseases [93], little attention has been paid to determining their protective actions starting at the fetus and neonate stages. As mentioned earlier, the disturbance of epigenetic regulation can lead to oxidative stress, linking to renal programming. Although vitamins B6, vitamin B12, and folate contribute to DNA methylation and have recognized roles as methyl donors [99], it would be interesting to know whether perinatal use of these vitamins can prevent renal programming via the regulation of epigenetics.
On the other hand, a meta-analysis recruiting 56 clinical trials concluded that high doses of vitamin A, β-carotene, and vitamin E appeared to increase mortality [100]. It is noteworthy that excessive dietary vitamin A intake was linked to human birth defects [101]. Perinatal vitamin supplements should only be administered in cases of deficiency, not as a usual intake. The contamination of vitamin supplements is another concerning problem being discussed. With the particular vulnerability of a developing fetus, attention to the detrimental effects of heavy metals and toxic elements contaminating vitamins consumed in pregnancy is imperative [102].
4.2. Amino Acids
The moderation of dietary amino acid consumption has therapeutic and protective effects on kidney diseases [103]. Several amino acids are known to possess antioxidant properties [104].
l-arginine is a substrate for the NOS production of NO, and l-citrulline is a precursor of l-arginine [105,106]. Considering that NO deficiency is a major pathogenetic mechanism behind renal programming, perinatal use of these two amino acids has been assessed to protect offspring against adult kidney diseases [105,106].
Human kidneys can covert l-citrulline to l-arginine [106]. Oral l-citrulline supplementation enables bypassing hepatic metabolism to enhance l-arginine production and raise NO levels [106]. Currently, maternal l-citrulline supplementation has been reported to enhance NO bioavailability and protect adult rat offspring against renal programming in oxidative-stress-related models of streptozotocin-induced diabetes [62], maternal caloric restriction [47], and prenatal dexamethasone exposure [75].
Additionally, L-tryptophan and L-cysteine have also been assessed as reprogramming interventions to target oxidative stress in maternal CKD-primed renal programming models [65,107]. Despite other amino acids, such as L-taurine and branched-chain amino acids, showing beneficial potential for kidney diseases [108], whether their protective effects are attributed to the reduction of oxidative stress awaits further clarification.
4.3. Melatonin
Melatonin is an endogenous tryptophan-derived indolamine with multiple biofunctions [109]. Melatonin plays an essential role in pregnancy and fetal development [110]. Melatonin and its metabolites are able to scavenge ROS and RNS, upregulate antioxidant enzymes, and increase NO bioavailability [111,112]. Hence, it has been clinically applied as an antioxidant therapy in pregnant women and neonates [113,114].
Several human studies reported that melatonin treatment ameliorated oxidative stress in newborns with asphyxia, sepsis, or other conditions with overproduction of ROS [114]. Moreover, the urinary excretion of melatonin’s metabolite could be used as a biomarker for babies with IUGR, suggesting the impact of the melatonin pathway in fetal programming [115].
Data from animal studies indicated that perinatal melatonin treatment could serve as a preventive intervention for many adult-onset diseases, including kidney diseases [116]. Maternal melatonin treatment has shown kidney benefits in several models of oxidative stress programming, such as caloric restriction [48], methyl donor diet [52], maternal l-NAME exposure [60], and high fructose intake [117]. When targeting oxidative stress, the protective effects of melatonin include reduced lipid peroxidation [60], ADMA [48], and 8-OHdG expression [52], as well as enhanced NO [117].
Although melatonin has a favorable safety profile in the pediatric population [113,114,117], the use of melatonin in pregnant women is not yet recommended [118]. As such, perinatal use of melatonin as a preventive strategy for kidney health, especially in fetuses and neonates, still awaits further clinical translation.
4.4. Polyphenols
Polyphenols are well-known phytochemical antioxidants [119]. Resveratrol exerts antioxidant properties by acting as a metal chelator, a free-radical scavenger, an NOS activator, and a stimulator of antioxidant enzymes [119]. Accordingly, polyphenols have been utilized to improve kidney health [120,121]. Although polyphenols have been reported as a prophylactic therapy for neonatal hypoxia–ischemia [122], there is a relative scarcity of human studies to support its benefits on fetal and neonatal kidney health.
Polyphenols can be grouped as flavonoids and nonflavonoids [119]. As a flavonoid antioxidant, the use of quercetin in gestation was noted to protect adult rat progeny against high-fat maternal-diet-induced renal programming and hypertension [123]. Another example is epigallocatechin gallate. Its use in gestation and lactation moderated prenatal dexamethasone-exposure-primed hypertension in a rat model [124].
Resveratrol is a naturally occurring nonflavonoid polyphenol [125]. Its antioxidant effects include scavenging ROS and RNS, enhancing antioxidant enzymes, increasing glutathione levels, upregulating NOS expression, etc. [126]. Several rat models of renal programming, such as high-fructose diet [51], maternal ADMA administration [61], adenine-induced CKD [66], bisphenol A exposure [70], and TCDD exposure [71], have shown beneficial effects of resveratrol on renal outcomes in adult progeny. For example, perinatal resveratrol therapy could protect offspring against renal programming, accompanied by reducing renal 8-OHdG expression and increasing NO [66].
One major limitation of the clinical utility of polyphenols is low bioavailability [127]. Taking into account the interindividual variability and complexity of polyphenol pharmacokinetics, future investigations are essential to better clarify the impacts of various polyphenols on kidney health, especially in fetal and neonatal medicine.
4.5. N-Acetylcysteine
N-acetylcysteine (NAC) is a well-known plant antioxidant [128]. In addition, NAC is a precursor to glutathione and an L-cysteine analogue that can be used for hydrogen sulfide (H2S) synthesis [129]. The therapeutic role of NAC in neonatal kidney disease has been shown in a rat sepsis model [130] and a porcine neonatal asphyxia model [131], despite limited human studies in this regard.
A prior study showed that perinatal NAC therapy protected rat offspring against maternal L-NAME-administration-induced renal programming, coinciding with the enhancement of renal H2S-generating enzyme expression and activity [60]. In another prenatal dexamethasone and postnatal high-fat diet model [76], the protective effect of NAC against oxidative stress was associated with increased plasma glutathione level and the upregulation of H2S-producing enzymes. Moreover, perinatal NAC therapy could avert maternal suramin-administration-induced hypertension and oxidative stress in adult rat progeny, which was involving increases of glutathione production, restoration of NO, and augmentation of the H2S pathway [64].
4.6. Synthetic Antioxidants
Along with natural antioxidants, a few synthetic antioxidants have been utilized in kidney diseases [87,88]. MitoQ, a coenzyme Q10 analogue, could reduce oxidative stress by the suppression of superoxide production and lipid peroxidation [132]. A prior study demonstrated that perinatal MitoQ treatment averted mouse adult offspring from hypertension and reduced nephron numbers and kidney injuries in a maternal smoking model [80]. Another example is dimethyl fumarate (DMF), a classical activator of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) [133]. In an antenatal dexamethasone exposure and postnatal high-fat diet model, the protective actions of DMF therapy were relevant for the reduction of oxidative stress, which was represented by reductions in ADMA and 8-OHdG, as well as increasing NO [134].
Some synthetic antioxidants classified as SOD mimetics show therapeutic potential for many disorders related to oxidative stress [135]. While the gestational use of the SOD mimetic tempol was noted to reduce proteinuria and BP in adult spontaneously hypertensive rat offspring [136], none of these synthetic antioxidants have been introduced into clinical practice in neonatal medicine.
5. The Gap between Animal Models and Clinical Reality
In patients with CKD, oxidative stress is present in the early stages of CKD and is more exacerbated in the end stages of kidney disease. Accordingly, the exogenous intake of antioxidants has repeatedly been shown to suppress oxidative stress in CKD patients [137,138]. However, none of these antioxidants are recommended in therapeutic guidelines for CKD. While preclinical studies using animals have highlighted antioxidant strategies as an attractive approach to kidney health, their efficacy still awaits validation in clinical reality. It is, however, important to know the correct antioxidant and the correct therapeutic dose to obtain direct benefits for the human body and to not only show beneficial effects in animal studies. Further studies in large cohorts of pregnant women are required to establish causality between perinatal antioxidant supplementation and clinical hard endpoints of renal outcomes in their children.
In view of the difficulties of recruiting pregnant women and neonates for human research, the use of breastmilk as an antioxidant strategy might be a good start. It is well known that breastmilk has a powerful antioxidant composition [139]. Given that the World Health Organization recommends exclusive breastfeeding for the first 6 months [140], the antioxidant protection provided by breastfeeding against renal programming is a significant issue that warrants further study.
Another concern is the safety of antioxidant supplements. Several antioxidants might provoke oxidative stress due to their pro-oxidative properties [141]. For example, vitamin E is known not only as a potent antioxidant, but also as a harmful pro-oxidant. If there is not enough vitamin C for its regeneration, vitamin E becomes a radical when reacting with ROS [142]. Additionally, controversy around antioxidants is due to their capacity to act as pro-oxidants depending on concentration. Therefore, there is only scientific evidence that antioxidants should be supplemented solely in cases where oxidative stress is identified.
Oxidative damage in kidneys can be determined in animal models, while human studies are limited in this regard, especially in fetuses and neonates. Accordingly, antioxidant therapies may cause unexpected damage to health, as they might reach healthy tissues that have not experienced oxidative stress damage, as well as the targeted organs of the kidneys. The balance between antioxidants and ROS or RNS should be optimal, as antioxidant extremes, namely antioxidative stress, are all damaging [143].
Regardless of recent advances in developing biomarkers of oxidative stress, most of these have not yet been assessed in the context of the early prediction of adult-onset kidney diseases. Currently, an ideal oxidative stress biomarker for kidney disease does not exist, and overlaps between biomarkers are a reality [144]. A panel of biomarkers that covers the pathogenic process of kidney disease identified in animal studies might optimize the specific value of each biomarker [145]. Therefore, the introduction of a panel of oxidative stress biomarkers correlating with the extent of kidney damage for the early identification of at-risk fetuses and neonates is a practical way to bridge the gap between animal models and clinical practice. Considering the rapid development of liquid biopsy technology with respect to kidney diseases [146], the application of liquid biopsies in the rapid diagnosis of oxidative-stress-related kidney disease should become more prominent.
6. Conclusions and Future Perspectives
Kidney health can be improved via oxidative-stress-targeting strategies, from pregnancy to the infantile stage [45]. First, promoting an optimal prenatal environment to minimize early-life risk factors may not only promote optimal fetal development, but may even avert oxidative-stress-mediated damage to developing kidneys. Second, several antioxidant strategies have revealed promising data in animal models, and their efficacy needs future translation into human investigations. More importantly, additional studies are required to determine the correct antioxidant with the correct dosage to avert oxidative-stress-induced renal programming. Lastly, since oxidative stress is the major pathogenic mechanism behind renal programming, the development and validation of reliable oxidative stress biomarkers correlating with kidney damage and the early identification of at-risk fetuses and neonates is urgently required in pediatric care.
Author Contributions
Conceptualization, Y.-L.T. and C.-N.H.; writing—original draft, Y.-L.T. and C.-N.H.; data curation, Y.-L.T. and C.-N.H.; writing—review and editing, Y.-L.T. and C.-N.H.; funding acquisition, Y.-L.T. and C.-N.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan, under grants CMRPG8M0721, CORPG8M0201, CORPG8M0151, and CORPG8M0081.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rahal A., Kumar A., Singh V., Yadav B., Tiwari R., Chakraborty S., Dhama K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dennery P.A. Oxidative stress in development: Nature or nurture? Free Radic. Biol. Med. 2010;49:1147–1151. doi: 10.1016/j.freeradbiomed.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Thompson L.P., Al-Hasan Y. Impact of oxidative stress in fetal programming. J. Pregnancy. 2012;2012:582748. doi: 10.1155/2012/582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zullino S., Buzzella F., Simoncini T. Nitric oxide and the biology of pregnancy. Vascul. Pharmacol. 2018;110:71–74. doi: 10.1016/j.vph.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Lembo C., Buonocore G., Perrone S. Oxidative Stress in Preterm Newborns. Antioxidants. 2021;10:1672. doi: 10.3390/antiox10111672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore T.A., Ahmad I.M., Zimmerman M.C. Oxidative stress and preterm birth: An integrative review. Biol. Res. Nurs. 2018;20:497–512. doi: 10.1177/1099800418791028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu C.N., Tain Y.L. Developmental Origins of Kidney Disease: Why Oxidative Stress Matters? Antioxidants. 2021;10:33. doi: 10.3390/antiox10010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Little M.H., McMahon A.P. Mammalian kidney development: Principles, progress, and projections. Cold Spring Harb. Perspect. Biol. 2012;4:a008300. doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luyckx V.A., Brenner B.M. The clinical importance of nephron mass. J. Am. Soc. Nephrol. 2010;21:898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 10.Murugapoopathy V., Gupta I.R. A Primer on Congenital Anomalies of the Kidneys and Urinary Tracts (CAKUT) Clin. J. Am. Soc. Nephrol. 2020;15:723–731. doi: 10.2215/CJN.12581019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartman H.A., Lai H.L., Patterson L.T. Cessation of renal morphogenesis in mice. Dev. Biol. 2007;310:379–387. doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kett M.M., Denton K.M. Renal programming: Cause for concern? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300:R791–R803. doi: 10.1152/ajpregu.00791.2010. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox C.S. Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 14.Hsu C.N., Tain Y.L. Early-Life Programming and Reprogramming of Adult Kidney Disease and Hypertension: The Interplay between Maternal Nutrition and Oxidative Stress. Int. J. Mol. Sci. 2020;21:3572. doi: 10.3390/ijms21103572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tain Y.L., Chan S.H.H., Chan J.Y.H. Biochemical basis for pharmacological intervention as a reprogramming strategy against hypertension and kidney disease of developmental origin. Biochem. Pharmacol. 2018;153:82–90. doi: 10.1016/j.bcp.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox C.S. Reactive oxygen species: Roles in blood pressure and kidney function. Curr. Hypertens. Rep. 2002;4:160–166. doi: 10.1007/s11906-002-0041-2. [DOI] [PubMed] [Google Scholar]
- 17.Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA. 2018;115:5839–5848. doi: 10.1073/pnas.1804932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins C., Wilson R., Roberts J., Miller H., McKillop J.H., Walker J.J. Antioxidants: Their role in pregnancy and miscarriage. Antioxid. Redox Signal. 2000;2:623–628. doi: 10.1089/15230860050192369. [DOI] [PubMed] [Google Scholar]
- 19.Kone B.C. Nitric oxide synthesis in the kidney: Isoforms, biosynthesis, and functions in health. Semin. Nephrol. 2004;24:299–315. doi: 10.1016/j.semnephrol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Tain Y.L., Hsu C.N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA) Toxins. 2017;9:92. doi: 10.3390/toxins9030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter A.M. Placental oxygen consumption. Part I. In vivo studies—A review. Placenta. 2000;21:S31–S37. doi: 10.1053/plac.1999.0513. [DOI] [PubMed] [Google Scholar]
- 22.Arya S., Ye C., Connelly P.W., Hanley A.J., Sermer M., Zinman B., Retnakaran R. Asymmetric dimethylarginine and arginine metabolites in women with and without a history of gestational diabetes. J. Diabetes Complicat. 2017;31:964–970. doi: 10.1016/j.jdiacomp.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Pettersson A., Hedner T., Milsom I. Increased circulating concentrations of asymmetric dimethyl arginine (ADMA), an endogenous inhibitor of nitric oxide synthesis, in preeclampsia. Acta Obstet. Gynecol. Scand. 1998;77:808–813. [PubMed] [Google Scholar]
- 24.de Almeida V.O., Pereira R.A., Amantéa S.L., Rhoden C.R., Colvero M.O. Neonatal diseases and oxidative stress in premature infants: An integrative review. J. Pediatr. (Rio. J.) 2022;98:455–462. doi: 10.1016/j.jped.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoots M.H., Gordijn S.J., Scherjon S.A., van Goor H., Hillebrands J.L. Oxidative stress in placental pathology. Placenta. 2018;69:153–161. doi: 10.1016/j.placenta.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Negro S., Boutsikou T., Briana D.D., Tataranno M.L., Longini M., Proietti F., Bazzini F., Dani C., Malamitsi-Puchner A., Buonocore G., et al. Maternal obesity and perinatal oxidative stress: The strength of the association. J. Biol. Regul. Homeost. Agents. 2017;31:221–227. [PubMed] [Google Scholar]
- 27.Longini M., Perrone S., Vezzosi P., Marzocchi B., Kenanidis A., Centini G., Rosignoli L., Buonocore G. Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clin. Biochem. 2007;40:793–797. doi: 10.1016/j.clinbiochem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Kamath U., Rao G., Kamath S.U., Rai L. Maternal and fetal indicators of oxidative stress during intrauterine growth retardation (IUGR) Indian J. Clin. Biochem. 2006;21:111–115. doi: 10.1007/BF02913077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bracci R., Buonocore G. The antioxidant status of erythrocytes in preterm and term infants. Semin. Neonatol. 1998;3:191–197. doi: 10.1016/S1084-2756(98)80004-3. [DOI] [Google Scholar]
- 30.Perrone S., Laschi E., Buonocore G. Biomarkers of oxidative stress in the fetus and in the newborn. Free Radic. Biol. Med. 2019;142:23–31. doi: 10.1016/j.freeradbiomed.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 31.Longini M., Perrone S., Kenanidis A., Vezzosi P., Marzocchi B., Petraglia F. Isoprostanes in amniotic fluid: A predictive marker for fetal growth restriction in pregnancy. Free Radic. Biol. Med. 2005;38:1537–1541. doi: 10.1016/j.freeradbiomed.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Longini M., Belvisi E., Proietti F., Bazzini F., Buonocore G., Perrone S. Oxidative Stress Biomarkers: Establishment of Reference Values for Isoprostanes, AOPP, and NPBI in Cord Blood. Mediat. Inflamm. 2017;2017:1758432. doi: 10.1155/2017/1758432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilger A., Rüdiger H.W. 8-Hydroxy-2′-deoxyguanosine as a marker of oxidative DNA damage related to occupational and environmental exposures. Int. Arch. Occup. Environ. Health. 2006;80:1–15. doi: 10.1007/s00420-006-0106-7. [DOI] [PubMed] [Google Scholar]
- 34.Murata T., Kyozuka H., Fukuda T., Endo Y., Kanno A., Yasuda S., Yamaguchi A., Sato A., Ogata Y., Shinoki K., et al. Japan Environment and Children’s Study (JECS) Group. Urinary 8-hydroxy-2′-deoxyguanosine levels and small-for-gestational age infants: A prospective cohort study from the Japan Environment and Children’s Study. BMJ Open. 2021;11:e054156. doi: 10.1136/bmjopen-2021-054156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Fiore J.M., Vento M. Intermittent hypoxemia and oxidative stress in preterm infants. Respir. Physiol. Neurobiol. 2019;266:121–129. doi: 10.1016/j.resp.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dizdar E.A., Uras N., Oguz S., Erdeve O., Sari F.N., Aydemir C., Dilmen U. Total antioxidant capacity and total oxidant status after surfactant treatment in preterm infants with respiratory distress syndrome. Ann. Clin. Biochem. 2011;48:462–467. doi: 10.1258/acb.2011.010285. [DOI] [PubMed] [Google Scholar]
- 37.Gücüyener K., Ergenekon E., Demiryürek T., Erbaş D., Oztürk G., Koç E., Atalay Y. Cerebrospinal fluid levels of nitric oxide and nitrotyrosine in neonates with mild hypoxic-ischemic encephalopathy. J. Child Neurol. 2002;17:815–818. doi: 10.1177/08830738020170111101. [DOI] [PubMed] [Google Scholar]
- 38.Banks B.A., Ischiropoulos H., McClelland M., Ballard P.L., Ballard R.A. Plasma 3-nitrotyrosine is elevated in premature infants who develop bronchopulmonary dysplasia. Pediatrics. 1998;101:870–874. doi: 10.1542/peds.101.5.870. [DOI] [PubMed] [Google Scholar]
- 39.Tsukahara H., Ohta N., Tokuriki S., Nishijima K., Kotsuji F., Kawakami H., Ohta N., Sekine K., Nagasaka H., Mayumi M. Determination of asymmetric dimethylarginine, an endogenous nitric oxide synthase inhibitor, in umbilical blood. Metabolism. 2008;57:215–220. doi: 10.1016/j.metabol.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Lücke T., Kanzelmeyer N., Kemper M.J., Tsikas D., Das A.M. Developmental changes in the L-arginine/nitric oxide pathway from infancy to adulthood: Plasma asymmetric dimethylarginine levels decrease with age. Clin. Chem. Lab Med. 2007;45:1525–2530. doi: 10.1515/CCLM.2007.300. [DOI] [PubMed] [Google Scholar]
- 41.Tashie W., Fondjo L.A., Owiredu W.K.B.A., Ephraim R.K.D., Asare L., Adu-Gyamfi E.A., Seidu L. Altered Bioavailability of Nitric Oxide and L-Arginine Is a Key Determinant of Endothelial Dysfunction in Preeclampsia. BioMed Res. Int. 2020;2020:3251956. doi: 10.1155/2020/3251956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luyckx V.A., Brenner B.M. Birth weight, malnutrition and kidney-associated outcomes—A global concern. Nat. Rev. Nephrol. 2015;11:135–149. doi: 10.1038/nrneph.2014.251. [DOI] [PubMed] [Google Scholar]
- 43.Bertram J.F., Douglas-Denton R.N., Diouf B., Hughson M.D., Hoy W.E. Human nephron number: Implications for health and disease. Pediatr. Nephrol. 2011;26:1529–1533. doi: 10.1007/s00467-011-1843-8. [DOI] [PubMed] [Google Scholar]
- 44.Nenov V.D., Taal M.W., Sakharova O.V., Brenner B.M. Multi-hit nature of chronic renal disease. Curr. Opin. Nephrol. Hypertens. 2000;9:85–97. doi: 10.1097/00041552-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Hsu C.N., Tain Y.L. The First Thousand Days: Kidney Health and Beyond. Healthcare. 2021;9:1332. doi: 10.3390/healthcare9101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu C.N., Tain Y.L. Chronic Kidney Disease and Gut Microbiota: What Is Their Connection in Early Life? Int. J. Mol. Sci. 2022;23:3954. doi: 10.3390/ijms23073954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tain Y.L., Hsieh C.S., Lin I.C., Chen C.C., Sheen J.M., Huang L.T. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide. 2010;23:34–41. doi: 10.1016/j.niox.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Tain Y.L., Huang L.T., Hsu C.N., Lee C.T. Melatonin therapy prevents programmed hypertension and nitric oxide deficiency in offspring exposed to maternal caloric restriction. Oxid. Med. Cell Longev. 2014;2014:283180. doi: 10.1155/2014/283180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cambonie G., Comte B., Yzydorczyk C., Ntimbane T., Germain N., Lê N.L., Pladys P., Gauthier C., Lahaie I., Abran D., et al. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1236–R1245. doi: 10.1152/ajpregu.00227.2006. [DOI] [PubMed] [Google Scholar]
- 50.Tain Y.L., Lee W.C., Wu K.L.H., Leu S., Chan J.Y.H. Targeting arachidonic acid pathway to prevent programmed hypertension in maternal fructose-fed male adult rat offspring. J. Nutr. Biochem. 2016;38:86–92. doi: 10.1016/j.jnutbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Tain Y.L., Lee W.C., Wu K.L.H., Leu S., Chan J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018;30:e1800066. doi: 10.1002/mnfr.201800066. [DOI] [PubMed] [Google Scholar]
- 52.Tain Y.L., Chan J.Y.H., Lee C.T., Hsu C.N. Maternal Melatonin Therapy Attenuates Methyl-Donor Diet-Induced Programmed Hypertension in Male Adult Rat Offspring. Nutrients. 2018;10:1407. doi: 10.3390/nu10101407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang Y.H., Chen W.H., Su C.H., Yu H.R., Tain Y.L., Huang L.T., Sheen J.M. Maternal Iron Deficiency Programs Rat Offspring Hypertension in Relation to Renin-Angiotensin System and Oxidative Stress. Int. J. Mol. Sci. 2022;23:8294. doi: 10.3390/ijms23158294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Do Nascimento L.C.P., Neto J.P.R.C., de Andrade Braga V., Lagranha C.J., de Brito Alves J.L. Maternal exposure to high-fat and high-cholesterol diet induces arterial hypertension and oxidative stress along the gut-kidney axis in rat offspring. Life Sci. 2020;261:118367. doi: 10.1016/j.lfs.2020.118367. [DOI] [PubMed] [Google Scholar]
- 55.Tain Y.L., Lin Y.J., Sheen J.M., Yu H.R., Tiao M.M., Chen C.C., Tsai C.C., Huang L.T., Hsu C.N. High Fat Diets Sex-Specifically Affect the Renal Transcriptome and Program Obesity, Kidney Injury, and Hypertension in the Offspring. Nutrients. 2017;9:357. doi: 10.3390/nu9040357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai W.L., Hsu C.N., Tain Y.L. Whether AICAR in Pregnancy or Lactation Prevents Hypertension Programmed by High Saturated Fat Diet: A Pilot Study. Nutrients. 2020;12:448. doi: 10.3390/nu12020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.do Nascimento L.C.P., de Souza E.L., de Luna Freire M.O., de Andrade Braga V., de Albuqeurque T.M.R., Lagranha C.J., de Brito Alves J.L. Limosilactobacillus fermentum prevent gut-kidney oxidative damage and the rise in blood pressure in male rat offspring exposed to a maternal high-fat diet. J. Dev. Orig. Health Dis. 2022;19:1–8. doi: 10.1017/S2040174422000198. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen L.T., Mak C.H., Chen H., Zaky A.A., Wong M.G., Pollock C.A., Saad S. SIRT1 Attenuates Kidney Disorders in Male Offspring Due to Maternal High-Fat Diet. Nutrients. 2019;11:146. doi: 10.3390/nu11010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larkin B.P., Saad S., Glastras S.J., Nguyen L.T., Hou M., Chen H., Wang R., Pollock C.A. Low-dose hydralazine during gestation reduces renal fibrosis in rodent offspring exposed to maternal high fat diet. PLoS ONE. 2021;16:e0248854. doi: 10.1371/journal.pone.0248854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tain Y.L., Lee C.T., Chan J.Y., Hsu C.N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal N(G)-Nitro-L-arginine methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 2016;215:636. doi: 10.1016/j.ajog.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 61.Hsu C.N., Hou C.Y., Chang-Chien G.P., Lin S., Chan J.Y.H., Lee C.T., Tain Y.L. Maternal resveratrol therapy protected adult rat offspring against hypertension programmed by combined exposures to asymmetric dimethylarginine and trimethylamine-N oxide. J. Nutr. Biochem. 2021;93:108630. doi: 10.1016/j.jnutbio.2021.108630. [DOI] [PubMed] [Google Scholar]
- 62.Tain Y.L., Lee W.C., Hsu C.N., Lee W.C., Huang L.T., Lee C.T., Lin C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS ONE. 2013;8:e55420. doi: 10.1371/journal.pone.0055420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martínez Gascón L.E., Ortiz M.C., Galindo M., Sanchez J.M., Sancho-Rodriguez N., Albaladejo Otón M.D., Rodriguez Mulero M.D., Rodriguez F. Role of heme oxygenase in the regulation of the renal hemodynamics in a model of sex dependent programmed hypertension by maternal diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022;322:R181–R191. doi: 10.1152/ajpregu.00213.2021. [DOI] [PubMed] [Google Scholar]
- 64.Tain Y.L., Hsu C.N., Lee C.T., Lin Y.J., Tsai C.C. N-Acetylcysteine prevents programmed hypertension in male rat offspring born to suramin-treated mothers. Biol. Reprod. 2016;95:8. doi: 10.1095/biolreprod.116.139766. [DOI] [PubMed] [Google Scholar]
- 65.Hsu C.N., Yang H.W., Hou C.Y., Chang-Chien G.P., Lin S., Tain Y.L. Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites. Int. J. Mol. Sci. 2020;21:7237. doi: 10.3390/ijms21197237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsu C.N., Hou C.Y., Chang-Chien G.P., Lin S., Yang H.W., Tain Y.L. Perinatal Resveratrol Therapy Prevents Hypertension Programmed by Maternal Chronic Kidney Disease in Adult Male Offspring: Implications of the Gut Microbiome and Their Metabolites. Biomedicines. 2020;8:567. doi: 10.3390/biomedicines8120567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ojeda N.B., Hennington B.S., Williamson D.T., Hill M.L., Betson N.E., Sartori-Valinotti J.C., Reckelhoff J.F., Royals T.P., Alexander B.T. Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension. 2012;60:114–122. doi: 10.1161/HYPERTENSIONAHA.112.192955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Svitok P., Okuliarova M., Varga I., Zeman M. Renal impairment induced by prenatal exposure to angiotensin II in male rat offspring. Exp. Biol. Med. 2019;244:923–931. doi: 10.1177/1535370219851110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vieira L.D., Farias J.S., de Queiroz D.B., Cabral E.V., Lima-Filho M.M., Sant’Helena B.R.M., Aires R.S., Ribeiro V.S., SantosRocha J., Xavier F.E., et al. Oxidative stress induced by prenatal LPS leads to endothelial dysfunction and renal haemodynamic changes through angiotensin II/NADPH oxidase pathway: Prevention by early treatment with α-tocopherol. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:3577–3587. doi: 10.1016/j.bbadis.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 70.Hsu C.N., Lin Y.J., Tain Y.L. Maternal exposure to bisphenol A combined with high-fat diet-induced programmed hypertension in adult male rat offspring: Effects of resveratrol. Int. J. Mol. Sci. 2019;20:4382. doi: 10.3390/ijms20184382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsu C.N., Lin Y.J., Lu P.C., Tain Y.L. Maternal resveratrol therapy protects male rat offspring against programmed hypertension induced by TCDD and dexamethasone exposures: Is it relevant to aryl hydrocarbon receptor? Int. J. Mol. Sci. 2018;19:2459. doi: 10.3390/ijms19082459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu Y.P., Chen L., Wang X.J., Jiang Q.H., Bei X.Y., Sun W.L., Xia S.J., Jiang J.T. Maternal exposure to di-n-butyl phthalate (DBP) induces renal fibrosis in adult rat offspring. Oncotarget. 2017;8:31101–31111. doi: 10.18632/oncotarget.16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sukjamnong S., Chan Y.L., Zakarya R., Nguyen L.T., Anwer A.G., Zaky A.A., Santiyanont R., Oliver B.G., Goldys E., Pollock C.A., et al. MitoQ supplementation prevent long-term impact of maternal smoking on renal development, oxidative stress and mitochondrial density in male mice offspring. Sci. Rep. 2018;8:6631. doi: 10.1038/s41598-018-24949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeje S.O., Akindele O.O., Ushie G., Rajil Y. Changes in kidney function and oxidative stress biomarkers in offspring from dams treated with dexamethasone during lactation in Wistar rats. Afr. J. Med. Med. Sci. 2016;45:237–242. [PubMed] [Google Scholar]
- 75.Tain Y.L., Sheen J.M., Chen C.C., Yu H.R., Tiao M.M., Kuo H.C., Huang L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014;48:580–586. doi: 10.3109/10715762.2014.895341. [DOI] [PubMed] [Google Scholar]
- 76.Tai I.H., Sheen J.M., Lin Y.J., Yu H.R., Tiao M.M., Chen C.C., Huang L.T., Tain Y.L. Maternal N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and prevents programmed hypertension in male offspring exposed to prenatal dexamethasone and postnatal high-fat diet. Nitric Oxide. 2016;53:6–12. doi: 10.1016/j.niox.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 77.Gwathmey T.M., Shaltout H.A., Rose J.C., Diz D.I., Chappell M.C. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension. 2011;57:620–626. doi: 10.1161/HYPERTENSIONAHA.110.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Langley-Evans S.C. Nutritional programming of disease: Unravelling the mechanism. J. Anat. 2009;215:36–51. doi: 10.1111/j.1469-7580.2008.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams L., Seki Y., Vuguin P.M., Charron M.J. Animal models of in utero exposure to a high fat diet: A review. Biochim. Biophys. Acta. 2014;1842:507–519. doi: 10.1016/j.bbadis.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sengupta P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- 81.Tain Y.L., Hsu C.N. Developmental Origins of Chronic Kidney Disease: Should We Focus on Early Life? Int. J. Mol. Sci. 2017;18:381. doi: 10.3390/ijms18020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhat A.V., Hora S., Pal A., Jha S., Taneja R. Stressing the (Epi) Genome: Dealing with Reactive Oxygen Species in Cancer. Antioxid. Redox Signal. 2018;29:1273–1292. doi: 10.1089/ars.2017.7158. [DOI] [PubMed] [Google Scholar]
- 83.Bianco-Miotto T., Craig J.M., Gasser Y.P., van Dijk S.J., Ozanne S.E. Epigenetics and DOHaD: From basics to birth and beyond. J. Dev. Orig. Health Dis. 2017;8:513–519. doi: 10.1017/S2040174417000733. [DOI] [PubMed] [Google Scholar]
- 84.Vasudevan D., Bovee R.C., Thomas D.D. Nitric oxide, the new architect of epigenetic landscapes. Nitric Oxide. 2016;59:54–62. doi: 10.1016/j.niox.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 85.Huang L.T., Hsieh C.S., Chang K.A., Tain Y.L. Roles of nitric oxide and asymmetric dimethylarginine in pregnancy and fetal programming. Int. J. Mol. Sci. 2012;13:14606–14622. doi: 10.3390/ijms131114606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tain Y.L., Huang L.T., Chan J.Y., Lee C.T. Transcriptome analysis in rat kidneys: Importance of genes involved in programmed hypertension. Int. J. Mol. Sci. 2015;16:4744–4758. doi: 10.3390/ijms16034744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berger R.G., Lunkenbein S., Ströhle A., Hahn A. Antioxidants in food: Mere myth or magic medicine? Crit. Rev. Food Sci. Nutr. 2012;52:162–171. doi: 10.1080/10408398.2010.499481. [DOI] [PubMed] [Google Scholar]
- 88.Jun M., Venkataraman V., Razavian M., Cooper B., Zoungas S., Ninomiya T., Webster A.C., Perkovic V. Antioxidants for chronic kidney disease. Cochrane Database Syst. Rev. 2012;10:CD008176. doi: 10.1002/14651858.CD008176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buonocore G., Groenendaal F. Anti-oxidant strategies. Semin Fetal Neonatal Med. 2007;12:287–295. doi: 10.1016/j.siny.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 90.Perez M., Robbins M.E., Revhaug C., Saugstad O.D. Oxygen radical disease in the newborn, revisited: Oxidative stress and disease in the newborn period. Free Radic. Biol. Med. 2019;142:61–72. doi: 10.1016/j.freeradbiomed.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee J.W., Davis J.M. Future applications of antioxidants in premature infants. Curr. Opin. Pediatr. 2011;23:161–166. doi: 10.1097/MOP.0b013e3283423e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hsu C.N., Tain Y.L. The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients. 2019;11:894. doi: 10.3390/nu11040894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rapa S.F., Di Iorio B.R., Campiglia P., Heidland A., Marzocco S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019;21:263. doi: 10.3390/ijms21010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Said H.M., Nexo E. Gastrointestinal Handling of Water-Soluble Vitamins. Compr. Physiol. 2018;8:1291–1311. doi: 10.1002/cphy.c170054. [DOI] [PubMed] [Google Scholar]
- 95.Niki E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014;66:3–12. doi: 10.1016/j.freeradbiomed.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 96.Wang J., Yin N., Deng Y., Wei Y., Huang Y., Pu X., Li L., Zheng Y., Guo J., Yu J., et al. Ascorbic Acid Protects against Hypertension through Downregulation of ACE1 Gene Expression Mediated by Histone Deacetylation in Prenatal Inflammation-Induced Offspring. Sci. Rep. 2016;6:39469. doi: 10.1038/srep39469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Farias J.S., Santos K.M., Lima N.K.S., Cabral E.V., Aires R.S., Veras A.C., Paixão A.D., Vieira L.D. Maternal endotoxemia induces renal collagen deposition in adult offspring: Role of NADPH oxidase/TGF-β1/MMP-2 signaling pathway. Arch. Biochem. Biophys. 2020;684:108306. doi: 10.1016/j.abb.2020.108306. [DOI] [PubMed] [Google Scholar]
- 98.Franco Mdo C., Ponzio B.F., Gomes G.N., Gil F.Z., Tostes R., Carvalho M.H., Fortes Z.B. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009;85:327–333. doi: 10.1016/j.lfs.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 99.Li K., Wahlqvist M.L., Li D. Nutrition, One-Carbon Metabolism and Neural Tube Defects: A Review. Nutrients. 2016;8:741. doi: 10.3390/nu8110741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012;3:CD007176. doi: 10.1002/14651858.CD007176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Azaïs-Braesco V., Pascal G. Vitamin A in pregnancy: Requirements and safety limits. Am. J. Clin. Nutr. 2000;71:1325S–1333S. doi: 10.1093/ajcn/71.5.1325s. [DOI] [PubMed] [Google Scholar]
- 102.Schwalfenberg G., Rodushkin I., Genuis S.J. Heavy metal contamination of prenatal vitamins. Toxicol. Rep. 2018;5:390–395. doi: 10.1016/j.toxrep.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li X., Zheng S., Wu G. Amino Acid Metabolism in the Kidneys: Nutritional and Physiological Significance. Adv. Exp. Med. Biol. 2020;1265:71–95. doi: 10.1007/978-3-030-45328-2_5. [DOI] [PubMed] [Google Scholar]
- 104.Ali S.S., Ahsan H., Zia M.K., Siddiqui T., Khan F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020;44:e13145. doi: 10.1111/jfbc.13145. [DOI] [PubMed] [Google Scholar]
- 105.Hsu C.N., Tain Y.L. Regulation of nitric oxide production in the developmental programming of hypertension and kidney disease. Int. J. Mol. Sci. 2019;20:681. doi: 10.3390/ijms20030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cynober L., Moinard C., De Bandt J.P. The 2009 ESPEN Sir David Cuthbertson. Citrulline: A new major signaling molecule or just another player in the pharmaconutrition game? Clin. Nutr. 2010;29:545–551. doi: 10.1016/j.clnu.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 107.Hsu C.N., Hou C.Y., Chang-Chien G.P., Lin S., Tain Y.L. Dietary Supplementation with Cysteine during Pregnancy Rescues Maternal Chronic Kidney Disease-Induced Hypertension in Male Rat Offspring: The Impact of Hydrogen Sulfide and Microbiota-Derived Tryptophan Metabolites. Antioxidants. 2022;11:483. doi: 10.3390/antiox11030483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hsu C.N., Tain Y.L. Early Origins of Hypertension: Should Prevention Start Before Birth Using Natural Antioxidants? Antioxidants. 2020;9:1034. doi: 10.3390/antiox9111034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hardeland R., Tan D.X., Reiter R.J. Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 2009;47:109–126. doi: 10.1111/j.1600-079X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 110.Tamura H., Nakamura Y., Terron M.P., Flores L.J., Manchester L.C., Tan D.X., Sugino N., Reiter R.J. Melatonin and pregnancy in the human. Reprod. Toxicol. 2008;25:291–303. doi: 10.1016/j.reprotox.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 111.Reiter R.J., Tan D.X., Terron M.P., Flores L.J., Czarnocki Z. Melatonin and its metabolites: New findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 2007;54:1–9. doi: 10.18388/abp.2007_3264. [DOI] [PubMed] [Google Scholar]
- 112.Reiter R.J., Mayo J.C., Tan D.X., Sainz R.M., Alatorre-Jimenez M., Qin L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 113.Chen Y.C., Tain Y.L., Sheen J.M., Huang L.T. Melatonin utility in neonates and children. J. Formos. Med. Assoc. 2012;111:57–66. doi: 10.1016/j.jfma.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 114.Aversa S., Pellegrino S., Barberi I., Reiter R.J., Gitto E. Potential utility of melatonin as an antioxidant during pregnancy and in the perinatal period. J. Matern. Fetal Neonatal Med. 2012;25:207–221. doi: 10.3109/14767058.2011.573827. [DOI] [PubMed] [Google Scholar]
- 115.Marseglia L., D’Angelo G., Manti S., Reiter R.J., Gitto E. Potential utility of melatonin in preeclampsia, intrauterine fetal growth retardation, and perinatal asphyxia. Reprod. Sci. 2016;23:970–977. doi: 10.1177/1933719115612132. [DOI] [PubMed] [Google Scholar]
- 116.Tain Y.L., Huang L.T., Hsu C.N. Developmental Programming of Adult Disease: Reprogramming by Melatonin? Int. J. Mol. Sci. 2017;18:426. doi: 10.3390/ijms18020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tain Y.L., Leu S., Wu K.L., Lee W.C., Chan J.Y. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. J. Pineal Res. 2014;57:80–89. doi: 10.1111/jpi.12145. [DOI] [PubMed] [Google Scholar]
- 118.Andersen L.P., Gögenur I., Rosenberg J., Reiter R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016;36:169–175. doi: 10.1007/s40261-015-0368-5. [DOI] [PubMed] [Google Scholar]
- 119.Durazzo A., Lucarini M., Souto E.B., Cicala C., Caiazzo E., Izzo A.A., Novellino E., Santini A. Polyphenols: A concise over-view on the chemistry, occurrence, and human health. Phytother. Res. 2019;33:2221–2243. doi: 10.1002/ptr.6419. [DOI] [PubMed] [Google Scholar]
- 120.Bao H., Peng A. The Green Tea Polyphenol (-)-epigallocatechin-3-gallate and its beneficial roles in chronic kidney disease. J. Transl. Int. Med. 2016;4:99–103. doi: 10.1515/jtim-2016-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guerreiro Í., Ferreira-Pêgo C., Carregosa D., Santos C.N., Menezes R., Fernandes A.S., Costa J.G. Polyphenols and Their Metabolites in Renal Diseases: An Overview. Foods. 2022;11:1060. doi: 10.3390/foods11071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roumes H., Goudeneche P., Pellerin L., Bouzier-Sore A.K. Resveratrol and Some of Its Derivatives as Promising Prophylactic Treatments for Neonatal Hypoxia-Ischemia. Nutrients. 2022;14:3793. doi: 10.3390/nu14183793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu Z., Zhao J., Xu H., Lyv Y., Feng X., Fang Y., Xu Y. Maternal quercetin administration during gestation and lactation decrease endoplasmic reticulum stress and related inflammation in the adult offspring of obese female rats. Eur. J. Nutr. 2014;53:1669–1683. doi: 10.1007/s00394-014-0673-4. [DOI] [PubMed] [Google Scholar]
- 124.Lamothe J., Khurana S., Tharmalingam S., Williamson C., Byrne C.J., Lees S.J., Khaper N., Kumar A., Tai T.C. Oxidative Stress Mediates the Fetal Programming of Hypertension by Glucocorticoids. Antioxidants. 2021;10:531. doi: 10.3390/antiox10040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Singh A.P., Singh R., Verma S.S., Rai V., Kaschula C.H., Maiti P., Gupta S.C. Health benefits of resveratrol: Evidence from 926 clinical studies. Med. Res. Rev. 2019;39:1851–1891. doi: 10.1002/med.21565. [DOI] [PubMed] [Google Scholar]
- 126.Truong V.L., Jun M., Jeong W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors. 2018;44:36–49. doi: 10.1002/biof.1399. [DOI] [PubMed] [Google Scholar]
- 127.Walle T., Hsieh F., DeLegge M.H., Oatis J.E., Jr., Walle U.K. High absorption but very low bioavailability of oral resveratrol 957 in humans. Drug Metab. Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 128.Salamon S., Kramar B., Marolt T.P., Poljšak B., Milisav I. Medical and Dietary Uses of N-Acetylcysteine. Antioxidants. 2019;8:111. doi: 10.3390/antiox8050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide. 2014;41:4–10. doi: 10.1016/j.niox.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 130.Plotnikov E.Y., Pavlenko T.A., Pevzner I.B., Zorova L.D., Manskikh V.N., Silachev D.N. The role of oxidative stress in acute renal injury of newborn rats exposed to hypoxia and endotoxin. FEBS J. 2017;284:3069–3078. doi: 10.1111/febs.14177. [DOI] [PubMed] [Google Scholar]
- 131.Johnson S.T., Bigam D.L., Emara M., Obaid L., Slack G., Korbutt G. N-acetylcysteine improves the hemodynamics and oxidative stress in hypoxic newborn pigs reoxygenated with 100% oxygen. Shock. 2007;28:484–490. doi: 10.1097/shk.0b013e31804f775d. [DOI] [PubMed] [Google Scholar]
- 132.James A.M., Smith R.A., Murphy M.P. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Arch. Biochem. Biophys. 2004;423:47–56. doi: 10.1016/j.abb.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 133.Tintoré M., Sastre-Garriga J. Multiple sclerosis: Dimethyl fumarate is coming of age. Nat. Rev. Neurol. 2016;12:436–437. doi: 10.1038/nrneurol.2016.106. [DOI] [PubMed] [Google Scholar]
- 134.Hsu C.N., Lin Y.J., Yu H.R., Lin I.C., Sheen J.M., Huang L.T., Tain Y.L. Protection of Male Rat Offspring against Hypertension Programmed by Prenatal Dexamethasone Administration and Postnatal High-Fat Diet with the Nrf2 Activator Dimethyl Fumarate during Pregnancy. Int. J. Mol. Sci. 2019;20:3957. doi: 10.3390/ijms20163957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rosa A.C., Corsi D., Cavi N., Bruni N., Dosio F. Superoxide Dismutase Administration: A Review of Proposed Human Uses. Molecules. 2021;26:1844. doi: 10.3390/molecules26071844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koeners M.P., Braam B., Joles J.A. Perinatal inhibition of NF-kappaB has long-term antihypertensive effects in spontaneously hypertensive rats. J. Hypertens. 2011;29:1160–1166. doi: 10.1097/HJH.0b013e3283468344. [DOI] [PubMed] [Google Scholar]
- 137.Liakopoulos V., Roumeliotis S., Bozikas A., Eleftheriadis T., Dounousi E. Antioxidant Supplementation in Renal Replacement Therapy Patients: Is There Evidence? Oxidative Med. Cell. Longev. 2019;2019:9109473. doi: 10.1155/2019/9109473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roumeliotis S., Roumeliotis A., Dounousi E., Eleftheriadis T., Liakopoulos V. Dietary Antioxidant Supplements and Uric Acid in Chronic Kidney Disease: A Review. Nutrients. 2019;11:1911. doi: 10.3390/nu11081911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yuksel S., Yigit A.A., Cinar M., Atmaca N., Onaran Y. Oxidant and Antioxidant Status of Human Breast Milk during Lactation Period. Dairy Sci. Technol. 2015;95:295–302. doi: 10.1007/s13594-015-0211-z. [DOI] [Google Scholar]
- 140.Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 141.Sotler R., Poljšak B., Dahmane R., Jukić T., Pavan Jukić D., Rotim C., Trebše P., Starc A. Prooxidant activities of antioxidants and their impact on health. Acta Clin. Croat. 2019;58:726–736. doi: 10.20471/acc.2019.58.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carlisle D.L., Pritchard D.E., Singh J., Owens B.M., Blankenship L.J., Orenstein J.M., Patierno S.R. Apoptosis and P53 induction in human lung fibroblasts exposed to chromium (VI): Effect of ascorbate and tocopherol. Toxicol. Sci. 2000;55:60–68. doi: 10.1093/toxsci/55.1.60. [DOI] [PubMed] [Google Scholar]
- 143.Halliwell B. The antioxidant paradox. Lancet. 2000;355:1179–1180. doi: 10.1016/S0140-6736(00)02075-4. [DOI] [PubMed] [Google Scholar]
- 144.Okamura D.M., Himmelfarb J. Tipping the redox balance of oxidative stress in fibrogenic pathways in chronic kidney disease. Pediatr. Nephrol. 2009;24:2309–2319. doi: 10.1007/s00467-009-1199-5. [DOI] [PubMed] [Google Scholar]
- 145.Schena F.P., Cox S.N. Biomarkers and Precision Medicine in IgA Nephropathy. Semin. Nephrol. 2018;38:521–530. doi: 10.1016/j.semnephrol.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 146.Jin C., Wu P., Li L., Xu W., Qian H. Exosomes: Emerging Therapy Delivery Tools and Biomarkers for Kidney Diseases. Stem Cells Int. 2021;2021:7844455. doi: 10.1155/2021/7844455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.