Abstract
Mosquito transgenesis and gene-drive technologies provide the basis for developing promising new tools for vector-borne disease prevention by either suppressing wild mosquito populations or reducing their capacity from transmitting pathogens. Many studies of the regulatory DNA and promoters of genes with robust sex-, tissue- and stage-specific expression profiles have supported the development of new tools and strategies that could bring mosquito-borne diseases under control. Although the list of regulatory elements available is significant, only a limited set of those can reliably drive spatial–temporal expression. Here, we review the advances in our ability to express beneficial and other genes in mosquitoes, and highlight the information needed for the development of new mosquito-control and anti-disease strategies.
Keywords: regulatory DNA, gene expression, marker genes, mosquito transgenesis
1. Introduction
Mosquito-borne diseases are one of the greatest challenges to global health [1]. Anopheles mosquitoes are the main vectors of human malaria parasites; Aedes species are major transmitters of arboviruses, including dengue, chikungunya, and Zika; and Culex mosquitoes are prominent vectors of viruses that cause encephalitic infections, including West Nile virus, and nematode parasites that cause lymphatic filariasis. Classical disease control methods, including repellents and bed nets, target bite prevention and mosquito elimination, typically using chemical insecticides [2]. However, disease incidences remain high, and resistance to commonly used insecticides is increasingly present in wild mosquito populations [3]. In order to supplement insecticide-based control strategies, the use of genetically engineered mosquitoes has been proposed to provide next-generation tools for disease prevention, and these include genetic-based vector population elimination or a reduced pathogen transmission capacity [4,5].
Advances in the knowledge of vector–pathogen interactions and mosquito biology, combined with the development of genomic data and sophisticated tools for genetic editing, provide opportunities to improve transgenic technologies in major mosquito vectors. Transgenesis experiments are essential to investigate endogenous gene function and to introduce exogenous DNA products desired to mitigate pathogen transmission [4,6]. Current gene drive research is centered mainly on drives based on the CRISPR-Cas9 genome-editing toolset, and the proposed strategies use pre-characterized promoter and terminator elements, each driving tissue-specific transgene expression as required for different functions in the germline and in various somatic tissues. However, compared to the knowledge accumulated on transcriptional regulation in the vinegar fly, Drosophila melanogaster, little is known about the regulatory genome of mosquitoes. In fact, the regulatory networks of most mosquito genes remain understudied from a mechanistic perspective [7].
Computational predictions and comparative genomics tools have assisted in the identification of cis-acting regulatory regions and transcriptional enhancers in mosquitoes [8,9]. Additionally, the functional fragments of gene control sequences have been defined primarily through transposon-mediated transgenesis experiments [10,11,12]. Nevertheless, a comprehensive understanding of mosquito regulatory biology requires combining and cross validating data generated using direct, indirect and in silico approaches. Recent efforts are in place to further characterize mosquito gene regulatory networks in vivo and provide new insights into mechanisms controlling mosquito functional gene expression [7,13].
Here we review the various promoters that have been shown to drive transgene expression in mosquitoes and their utility for biotechnology-based control approaches, as well as the valuable insights into mosquito regulatory biology they provide.
2. Genetic Engineering Toolbox
The ability to manipulate gene expression in specific tissues at specific times in development is key to understanding mosquito biology and developing genetic means for vector control [14]. This work is facilitated in mosquitoes through the use of bi-partite expression systems such as the Gal4-UAS system [10,15,16]. For example, a recent contribution using this system expanded the genetic tools available to study gene function in hemocytes by characterizing the gene expression pattern driven by the Drosophila hml promoter in An. gambiae adult females [17]. Similarly, in an effort to obtain multi-tissue ubiquitous-like expression of transgenes, promoters of highly conserved ‘housekeeping’ genes such as polyubiquitin [18] have been investigated in mosquitoes, with successful validation of constitutive transcriptional activities [19,20]. Exogenously derived promoters and control sequences from genes such as heat-shock protein 70, actin5c and ubiquitin from D. melanogaster, and the baculovirus immediate-early (IE1), have also been useful in mosquito transgenesis [21,22,23,24,25,26].
Sustained, easily scored marker gene expression is desirable during screening for transgenic individuals when making new or maintaining previously established lines. This has been traditionally achieved by the use of a variety of viral and insect promoters, due to their lack of organism and tissue-specificity, to direct the expression of a fluorescent protein-encoding or other visible marker gene. The most frequently used and best characterized promoters come from D. melanogaster or baculoviruses and have been used successfully to express both exogenous and modified endogenous genes in mosquitoes or mosquito cell lines [12,25,26,27,28,29,30,31,32,33,34]. Later, genomic sequences derived from regions adjacent to the 5′-end (upstream) of endogenous heat shock protein-encoding genes were shown to be capable of driving marker expression in Ae. aegypti, both transiently in cells and embryos and through the stable integration of transgenes [35,36]. Although the most commonly used promoter for marker visualization is 3xP3 [37,38], which is remarkably visible in the optic nerves of larvae and pupae [39], identification of positive individuals can be difficult in weaker phenotypes or in later stages of development due to dark eye pigmentation [40]. In such cases, marker genes driven by strong constitutive promoters can be advantageous, particularly if there is a need to reliably identify transgenic mosquitoes in a wider range of stages [41], such as in field applications.
Initial work to define and characterize mosquito promoters in vivo relied on Class II transposable elements (transposons) to integrate modified endogenous and exogenous gene constructs stably and heritably into vector genomes. Class II elements are DNA-based and comprise a gene (and regulatory elements) encoding a transposase enzyme flanked by inverted repeat sequences of varying complexity and length (length and sequence are characteristic of each family of transposons). Complete (autonomous) elements are able to mobilize (excise and integrate) through either a conservative (no net increase in copy number) or replicative (increase in copy number) mode, thereby changing their linkage relationships in the genome [41]. Following the inability to adapt the P element, first discovered in D. melanogaster, to mosquito species, new discoveries identified a number of elements, Hermes, Mos 1 mariner, Minos, and ultimately, piggyBac, that work well in both anopheline and culicine mosquitoes [42].
More recently, high-efficiency genome engineering applications, such as those based on CRISPR-Cas9 technologies, have supplanted most applications of transposable elements. Their ability to target the integration of DNA to a preselected site in the genome can be used to control or mitigate variations of transgene performance resulting from position-site effects often encountered when using transposable elements [42]. The Cas9-based systems require a ubiquitously expressed guide RNA (gRNA) sequence to direct nuclease cleavage activity at the preselected site as a first step in integration or modification. In mosquitoes, RNA Polymerase III (Pol III) promoters have been used for genetic control strategies that depend on gRNA or RNAi [43] expression. In particular, the U6 RNA polymerase III gene promoters are ideal for non-coding RNA expression due to their nucleus-associated transcription without the 5′- and 3′-end mRNA modification associated with Polymerase II gene expression. Endogenous U6 regulatory sequences have been used to drive gRNA expression across different mosquito species, with varying degrees of activity [40,44,45,46]. To complement the tools used for efficient transgene transmission in mosquitoes, we discuss below the multiple sequences used to drive expression of Cas9 in the germline, a capability essential for gene drive development.
3. Germline-Specific Promoters
Two general genetic strategies for mosquito population management have been envisioned: population suppression, and population replacement with individuals that are refractory to disease transmission. Several gene-drive based versions of these strategies require the use of regulatory sequences that drive expression primarily or exclusively in the germline, particularly the ones that utilize engineered site-specific homing endonucleases. When expression of an endonuclease such as Cas9 is activated in the male and/or female germline in a hemizygote, cleavage of the target site on the wild-type chromosome followed by homology-directed DNA repair results in an increase in the frequency of the drive transgene in the population [47].
In mosquitoes, expression of transgenes in germline-specific patterns were achieved using notable regulatory sequences. The regulatory regions of the β2-tubulin gene have been utilized to drive testis-specific marker expression [48,49]. The germline-specific regulatory promoter and untranslated regions from the vasa, nanos, and zero population growth (zpg) genes have been used to direct expression of the Cas9 nuclease in male and female germ cells as components of gene-drive systems [40,50,51,52,53]. In addition, sex-specific expression of fluorescent markers can be exploited for an efficient high-throughput sex separation during mosquito rearing. This strategy has been pursued using the β2 tubulin promoter in Ae. aegypti [48] and An. stephensi [49], and in An. gambiae, where the use of the doublesex (dsx) promoter [54] permits early larval separation due to its selective expression-driven pattern in male larvae at early developmental stages [55].
Considering gene drives, different Cas9 expression constructs support the conclusion that promoter-dependent Cas9 transcript localization may play a critical role in drive integration outcomes [56,57]. It has been demonstrated that successful drive conversion in the male germline occurs without subsequent formation of resistance alleles in the embryo due to paternally deposited Cas9 [57]. In contrast, in zygotes with maternal deposition of Cas9/gRNA complexes, the physical distance between the paternal and maternal chromosomes within the embryo may prevent homology-directed repair and result in a potentially drive-resistant allele [40,58]. Furthermore, lower levels of paternally transmitted Cas9 in the embryo can minimize off-target and toxicity effects. Additionally, the nanos promoter significantly lowered somatic Cas9 expression compared to the vasa promoter, supporting the conclusion that it is a better choice in drive strategies where gene disruption in somatic cells could have fitness costs [59]. Recently, an examination of transcript distribution patterns of Cas9 transgenes driven by the vasa or nanos promoters in the germline of transgenic Anopheles mosquitoes showed an overall strong concordance between promoter-driven Cas9 and endogenous gene expression patterns for both drive systems in males, but also distinct colocalization patterns for the two drives in female reproductive tissues [53]. Despite the imperfect overlap of transgene vs. endogenous transcript patterns, transgenic nanos-Cas9 mosquitoes display highly efficient drive performance [58,60].
The zpg control DNA sequences have suitable performances in An. gambiae drive studies [61,62], potentially due to reduced Cas9 expression levels [53]. In Ae. aegypti, a comprehensive assessment revealed transcriptional regulatory regions able to drive female germline-specific, and female and male germline-specific expression, with overlapping but also distinct transcriptional patterns [63]. Using these, different Cas9 strains engineered achieved great mutagenic efficiency and specificity [34]. Additionally, a study of Ae. aegypti developmental transcriptomes [64] led to the identification a novel female germline and early zygote promoter from the transcription factor bZip1 [65]. It was demonstrated that transgenic lines in which the bZip1 promoter expresses a fluorescent marker protein follow the same pattern of expression as the endogenous gene, although the genomic fragment chosen appears to be strongly repressed by position effects.
Hence, the use of different germline-specific control DNA sequences has provided initial resources toward understanding the basis for differing drive properties and the identification of regulatory elements that will be instrumental in furthering our understanding of mosquito biology and control.
4. Population Suppression
Genetics-based population suppression can be achieved through mass-release of mosquitoes carrying dominant-lethal, sex-conversion, or female reproductive damaging transgenes. Dominant-lethal and female reproductive damaging approaches usually require spatially restricted expression of a deleterious gene product under the control of specific regulatory elements to achieve their designed outcomes. Additionally, to maintain breeding populations of transgenic strains, these lethal or damaging effector genes must be under conditional regulation so that lethality or sterility only occurs under non-permissive conditions [66]. The promoter and control DNA derived from the flight muscle-specific Actin-4 gene in Ae. aegypti [67] and an antibiotic-repressible lethal factor were used to create a female-specific flightless phenotype strain [68]. This approach was also shown to work in a related species, Ae. albopictus [69] and was successfully adapted to An. stephensi [70], with the use of orthologue endogenous regulatory sequences. Moreover, while early studies in Cx. quinquefasciatus transgenesis proved the functional conservation of the flight muscle D. melanogaster act88F promoter [71], its use to drive effector transgene expression has not yet been accomplished for this species.
Some insect promoters can be exploited for developing mosquito control strategies to reduce vector populations by female-to-male sex conversion, or to aid in sterile insect techniques that require releasing only non-biting males. For example, the ectopic expression of the Y chromosome-linked signal gene Yob under the control of the germline promoter vasa generated a partial female lethal phenotype in An. gambiae [72]. However, complete penetrance of the lethal phenotype may require the use of promoters that are more active during the early zygotic stage. This has been achieved in An. stephensi, where a male-only phenotype was achieved by expression of an autosomally integrated construct consisting of the male-determining gene, Guy1, driven by its own endogenous promoter [73]. Additionally, successful conversions of females into fertile males with all male-specific sexually dimorphic features were achieved using the native promoter of the male-determining factor Nix in Ae. aegypti and Ae. albopictus [74,75].
A newly developed molecular tool expanded the flexibility of suppression technologies by engineering a paralysis-inducing neurotoxic synthetic effector designed to be secreted by the adult fat body following a blood-meal, under the control of the vitellogenin (Vg) promoter in Ae. aegypti [76]. This makes it possible to dissociate the temporal and spatial expression patterns of an effector, and allow the use of a wider panel of endogenous regulatory components for building genetic lethal systems.
5. Population Modification
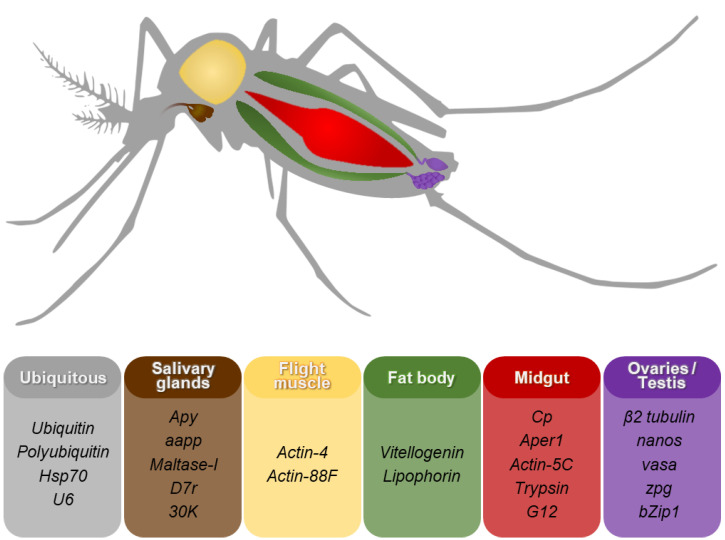
Mosquito genomic studies have long focused on the design of engineered genes under the control of promoter-regulatory DNA to drive site-specific expression in infection-relevant tissues (‘compartments’ [77]). In addition to spatial considerations, the time of transgene-mediated protein synthesis relative to pathogen arrival in each of these compartments was considered. This is a favorable design feature in engineering mosquitoes to minimize potential transgenesis-related fitness costs by restricting the expression of transgenes to the infection-relevant sex, developmental stage, and mosquito body compartments in which the pathogens are found. Many endogenous promoters have been used to drive transgene expression in mosquitoes and examples of these are listed in Figure 1.
Figure 1.
Genes whose promoters and 5′- and 3′ end DNA sequences are used in genetic engineering of mosquitoes. The promoters and control DNA of a number of genes have been used to drive expression of genetic components or effector molecules in different tissues of the mosquito. Details and references on the promoters listed are included in the text. Abbreviations: aapp, anopheline antiplatelet gene; Aper1, adult peritrophic matrix gene; Apy, apyrase; Cp, zinc carboxypeptidase A1; D7r, D7-related gene; Hsp70, heat-shock protein 70.
As the midgut is the first tissue encountered by newly introduced parasites and arboviruses, the regulatory DNA of midgut-specific genes, particularly those that are expressed at high levels in response to a blood-meal, are ideal candidates for directing the expression of effector genes in this compartment. The most widely characterized regulatory regions are from digestive enzyme-encoding genes such as trypsin [78,79,80,81] and carboxypeptidase (Cp) [82]. The control DNA sequences of other genes can be useful for strict female-specificity, such as the An. gambiae G12 gene [81], or being abundantly expressed in the midgut even prior to a blood meal, such as peritrophin (Aper1) and actin5C [12,83]. A number of robust anti-pathogen strategies have been developed using Cp gene ortholog promoters in Anopheles species [84,85] and Aedes aegypti [86]. However, the use of this promoter also has been associated with lowered fitness of transgenic mosquitoes, linked to the action of the transgenes themselves. Mosquitoes with Cp-driven Akt signaling have an impacted lifespan, likely due to leaky expression at the non-blood-fed stage [87], and certain exogenous antimicrobial peptides can exert internal damage to the midgut or cause undesired physiological effects [85,88].
During subsequent stages of infection, the pathogens traverse the midgut wall (intracellularly and/or extracellularly) and migrate through the open circulatory system (hemocoel) to the mosquito salivary glands (Plasmodium parasites and arboviruses) or proboscis (filarial nematodes). Hence, effector gene expression targeted to the mosquito hemocoel can impact pathogen migration. The Vg gene cis-acting DNA sequences are the most widely used [23,26,89,90,91] to induce late-digestion and sex-specific expression of desired gene products in the fat body for secretion into the hemolymph. This gene has a restricted temporal profile of expression that peaks around 24 h after a blood meal and returns to basal level by 48 h. However, for sustained Vg-driven expression, the promoter can be re-activated by additional blood meal(s) [92]. Similar to Cp-induced expression, different combinations of molecules and Vg expression systems have contrasting impacts on mosquito survival and consequent transgene integration into populations. For example, expression of the peptide SM1 driven by the Vg promoter imposes a significant fitness load to transgenic mosquitoes [93], but the same does not occur on individuals with the Cp control DNA driving expression of SM1 [88]. Nonetheless, a number of highly effective transgenic lines that target multiple infection stages through multi-effector expression using both Cp and Vg do not show impaired life spans [85,92]. Additionally, heterologous [94] or mosquito promoter regions can be used to drive salivary gland-specific transgene expression, including those from Maltase-I, D7r and apyrase [21,95,96,97], and the anopheline antiplatelet gene (aapp) [98,99,100]. Transgene products were expressed in Ae. aegypti under the control of a functional bi-directional 30K gene promoter, significantly reducing Dengue virus titers in mosquito salivary glands [101]. The promoter region of aapp also has been used to induce production, secretion, and host inoculation of a malarial protein through An. stephensi saliva [102].
Finally, mosquito promoters of immune-modulated genes are potentially useful for being sensitive to pathogen presence in the system [103]. In addition, the importance of cis-acting mutations on detoxification enzyme genes for insecticide resistance in mosquitoes is widely accepted [104,105,106] and promoters identified as having neural expression patterns could be used for the functional analysis of SNPs within insecticide-resistant alleles. Given the availability of mosquito genomes and increased transcriptome data, a great number of promoters can be predicted for their ability to drive transgenes in mosquitoes. Additionally, sophisticated genetic tools for expression analysis allow cross-species computational enhancer prediction [13,107,108]. However, these regulatory elements need to be tested before being used to create genetically engineered mosquitoes. A novel artificial-intron-based strategy for mosquito transgenesis supports the co-option of regulatory elements of endogenous loci directly without prior labor-intensive promoter characterization [109] and is a viable approach to satisfy the need of promoters for many infection-relevant tissues.
6. Conclusions
Methods to produce transgenic mosquitoes have been available for over 20 years [22,110,111,112]. A number of possible promoters and 5′- and 3′-end DNA sequences to drive the expression of transgenes and effector molecules whose products hinder mosquito population survival or pathogen development have been discovered, yet only a handful of these pre-characterized promoter elements are used routinely for generating transgenic lines intended for population suppression or modification strategies (Table 1). These regulatory sequences can be classified into two groups (Figure 1). The first comprises the ubiquitously expressed promoters, and RNA Pol III promoters (U6) used to generate guide RNA (gRNA)-expressing lines. The second are the tissue-specific promoters, which can drive expression in the fat body, midgut, salivary glands, hemocytes, and testis and/or ovaries, the latter being used to generate Cas9-expressing lines.
Table 1.
Transgenic mosquitoes exhibiting pathogen refractoriness or lethal/sterile phenotypes.
| Mosquito Species | Phenotype | Promoter | References |
|---|---|---|---|
| An. stephensi | Pathogen refractoriness |
Cp | [84,86,87,88,92,113,114,115,116] |
| Aper1 | [83,117] | ||
| Vg | [84,85,92,116] | ||
| aapp | [98,99,100] | ||
| Lethality/Sterility | Act-4 | [70] | |
| Guy1 | [73] | ||
| An. gambiae | Pathogen refractoriness |
Cp | [118,119,120] |
| Vg | [121,122,123] | ||
| Lethality/Sterility | β2 tub | [124] | |
| Yob | [72] | ||
| Ae. aegypti | Pathogen refractoriness |
Cp | [86,125,126,127] |
| PUb | [26,127] | ||
| 30K | [101] | ||
| Vg | [23] | ||
| Lethality/Sterility | Vg | [76] | |
| Act-4 | [68] | ||
| Nix | [74] | ||
| Ae. albopictus | Lethality/Sterility | Act-4 | [69] |
| Nix | [75] |
Cp, zinc carboxypeptidase A1; Aper1, peritrophin; Vg, vitellogenin; aapp, anopheline antiplatelet protein; Act-4, actin-4; β2 tub, β2 tubulin; PUb, polyubiquitin.
Given that the success of transgenic mosquito vector control approaches relies on well-targeted gene expression, the identification and characterization of a diverse set of mosquito promoters and transcriptional enhancers are required for technological progress [13]. An increased knowledge of the expression systems currently used also can help establish dosage-response curves of different types of effectors that may require distinct levels of effectively expressed proteins. Furthermore, the importance of characterizing mosquito regulatory systems goes beyond their use for biotechnology-based approaches, as different sequences acting in each of the life cycle stages of the insect or disease agent can provide valuable insights into mosquito biology and pathogen interaction [128].
Finally, it is important to acknowledge that the application of new genetic engineering technology is challenging because an accepted standard for moving it from the laboratory to the field may not exist or have been tested yet [77]. Pathways for moving gene-drive population suppression and modification mosquitoes to the field are being charted as the work progresses and the science is often ahead of community-based efforts to certify best practices. In response, investigators, scientific advisory groups, and potential stakeholders have offered analyses of challenges and issued guidelines for moving the science forward [129,130,131]. Accepted guiding principles include that the work be conducted in phases in which stringent criteria must be met before moving from one phase to the next. The World Health Organization (WHO) proposed early on a framework for testing genetically engineered mosquitoes and defined four phases: Phase 1 tests are discovery stages physically confined to laboratories and insectaries; Phase 2 moves the strains to development and are carried out in small-scale physically and/or ecologically contained field tests; Phase 3 continues development in a series of open release trials that increase in size, length, and complexity at one or more sites; and Phase 4 moves the technology to a wider application as a malaria control tool in the delivery stage [129]. Specific strains are evaluated and subjected to rigorous ‘go/no go’ criteria in each phase. Later efforts acknowledged the special challenges posed by the gene-drive system [132,133,134]. We encourage all scientists working with these technologies to adopt the principles outlined in these frameworks and make the essential efforts to engage potential stakeholders and end-users [135,136].
Acknowledgments
The authors thank the members of the James laboratory and the many collaborators for sharing insights and reagents. A.A.J. is a Donald Bren Professor at the University of California, Irvine.
Author Contributions
Conceptualization, V.B.-R. and A.A.J.; methodology, V.B.-R. and A.A.J.; validation, V.B.-R. and A.A.J.; formal analysis, V.B.-R. and A.A.J.; investigation, V.B.-R. and A.A.J.; resources, A.A.J.; data curation, V.B.-R. and A.A.J.; writing—original draft preparation, V.B.-R. and A.A.J.; writing—review and editing, V.B.-R. and A.A.J.; visualization, V.B.-R.; supervision, A.A.J.; project administration, A.A.J.; funding acquisition, A.A.J. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Original research in the James laboratory is funded by the University of California Irvine Malaria Initiative and the Bill & Melinda Gates Foundation (INV-043645).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO . Framework for a National Vector Control Needs Assessment. World Health Organization; Geneva, Switzerland: 2017. [Google Scholar]
- 2.WHO . Global Vector Control Response 2017–2030. World Health Organization; Geneva, Switzerland: 2017. [Google Scholar]
- 3.Ranson H., Lissenden N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation That Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016;32:187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Caragata E.P., Dong S., Dong Y., Simões M.L., Tikhe C.V., Dimopoulos G. Prospects and Pitfalls: Next-Generation Tools to Control Mosquito-Transmitted Disease. Annu. Rev. Microbiol. 2020;74:455–475. doi: 10.1146/annurev-micro-011320-025557. [DOI] [PubMed] [Google Scholar]
- 5.Dong S., Dong Y., Simões M.L., Dimopoulos G. Mosquito Transgenesis for Malaria Control. Trends Parasitol. 2021;38:54–66. doi: 10.1016/j.pt.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Terenius O., Marinotti O., Sieglaff D., James A.A. Molecular Genetic Manipulation of Vector Mosquitoes. Cell Host Microbe. 2008;4:417–423. doi: 10.1016/j.chom.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz J.L., Ranford-Cartwright L.C., Gómez-Díaz E. The Regulatory Genome of the Malaria Vector Anopheles gambiae: Integrating Chromatin Accessibility and Gene Expression. NAR Genom. Bioinform. 2021;3:lqaa113. doi: 10.1093/nargab/lqaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sieglaff D.H., Dunn W.A., Xie X.S., Megy K., Marinotti O., James A.A. Comparative Genomics Allows the Discovery of Cis-Regulatory Elements in Mosquitoes. Proc. Natl. Acad. Sci. USA. 2009;106:3053–3058. doi: 10.1073/pnas.0813264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holm I., Nardini L., Pain A., Bischoff E., Anderson C.E., Zongo S., Guelbeogo W.M., Sagnon N., Gohl D.M., Nowling R.J., et al. Comprehensive Genomic Discovery of Non-Coding Transcriptional Enhancers in the African Malaria Vector Anopheles coluzzii. Front. Genet. 2022;12:2716. doi: 10.3389/fgene.2021.785934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brochta D.A., Pilitt K.L., Harrell R.A., Aluvihare C., Alford R.T. Gal4-Based Enhancer-Trapping in the Malaria Mosquito Anopheles stephensi. G3 Genes Genomes Genet. 2012;2:1305–1315. doi: 10.1534/g3.112.003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid W., Pilitt K., Alford R., Cervantes-Medina A., Yu H., Aluvihare C., Harrell R., O’Brochta D.A. An Anopheles stephensi Promoter-Trap: Augmenting Genome Annotation and Functional Genomics. G3 Genes Genomes Genet. 2018;8:3119–3130. doi: 10.1534/g3.118.200347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volohonsky G., Terenzi O., Soichot J., Naujoks D.A., Nolan T., Windbichler N., Kapps D., Smidler A.L., Vittu A., Costa G., et al. Tools for Anopheles gambiae Transgenesis. G3 Genes Genomes Genet. 2015;5:1151–1163. doi: 10.1534/g3.115.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schember I., Halfon M.S. Identification of New Anopheles gambiae Transcriptional Enhancers Using a Cross-Species Prediction Approach. Insect Mol. Biol. 2021;30:410–419. doi: 10.1111/imb.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews B.J., Vosshall L.B. How to Turn an Organism into a Model Organism in 10 ‘Easy’ Steps. J. Exp. Biol. 2020;223:jeb218198. doi: 10.1242/jeb.218198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kokoza V.A., Raikhel A.S. Targeted Gene Expression in the Transgenic Aedes aegypti Using the Binary Gal4-UAS System. Insect Biochem. Mol. Biol. 2011;41:637–644. doi: 10.1016/j.ibmb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynd A., Lycett G.J. Development of the Bi-Partite Gal4-UAS System in the African Malaria Mosquito, Anopheles gambiae. PLoS ONE. 2012;7:e31552. doi: 10.1371/journal.pone.0031552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pondeville E., Puchot N., Parvy J.P., Carissimo G., Poidevin M., Waterhouse R.M., Marois E., Bourgouin C. Hemocyte-Targeted Gene Expression in the Female Malaria Mosquito Using the Hemolectin Promoter from Drosophila. Insect Biochem. Mol. Biol. 2020;120:103339. doi: 10.1016/j.ibmb.2020.103339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beard C.B., Cornel A.J., Collins F.H. The Polyubiquitin Gene of the Mosquito Anopheles gambiae: Structure and Expression. Insect Mol. Biol. 1996;5:109–117. doi: 10.1111/j.1365-2583.1996.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 19.Anderson M.A.E., Gross T.L., Myles K.M., Adelman Z.N. Validation of Novel Promoter Sequences Derived from Two Endogenous Ubiquitin Genes in Transgenic Aedes aegypti. Insect Mol. Biol. 2010;19:441–449. doi: 10.1111/j.1365-2583.2010.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adolfi A., Pondeville E., Lynd A., Bourgouin C., Lycett G.J. Multi-Tissue GAL4-Mediated Gene Expression in All Anopheles gambiae Life Stages Using an Endogenous Polyubiquitin Promoter. Insect Biochem. Mol. Biol. 2018;96:1–9. doi: 10.1016/j.ibmb.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Coates C.J., Jasinskiene N., Pott G.B., James A.A. Promoter-Directed Expression of Recombinant Fire-Fly Luciferase in the Salivary Glands of Hermes-Transformed Aedes aegypti. Gene. 1999;226:317–325. doi: 10.1016/S0378-1119(98)00557-5. [DOI] [PubMed] [Google Scholar]
- 22.Jasinskiene N., Coates C.J., Benedict M.Q., Cornel A.J., Rafferty C.S., James A.A., Collins F.H. Stable Transformation of the Yellow Fever Mosquito, Aedes aegypti, with the Hermes Element from the Housefly. Proc. Natl. Acad. Sci. USA. 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X.G., Marinotti O., Whitman L., Jasinskiene N., James A.A. The Anopheles gambiae Vitellogenin Gene (VGT2) Promoter Directs Persistent Accumulation of a Reporter Gene Product in Transgenic Anopheles stephensi Following Multiple Bloodmeals. Am. J. Trop. Med. Hyg. 2007;76:1118–1124. doi: 10.4269/ajtmh.2007.76.1118. [DOI] [PubMed] [Google Scholar]
- 24.Pinkerton A.C., Michel K., O’Brochta D.A., Atkinson P.W. Green Fluorescent Protein as a Genetic Marker in Transgenic Aedes aegypti. Insect Mol. Biol. 2000;9:1–10. doi: 10.1046/j.1365-2583.2000.00133.x. [DOI] [PubMed] [Google Scholar]
- 25.Besansky N.J., Mukabayire O., Benedict M.Q., Salazar Rafferty C., Mills Hamm D., McNitt L. The Anopheles gambiae Tryptophan Oxygenase Gene Expressed from a Baculovirus Promoter Complements Drosophila melanogaster Vermilion. Insect Biochem. Mol. Biol. 1997;27:803–805. doi: 10.1016/S0965-1748(97)00040-4. [DOI] [PubMed] [Google Scholar]
- 26.Jasinskiene N., Coleman J., Ashikyan A., Salampessy M., Marinotti O., James A.A. Genetic Control of Malaria Parasite Transmission: Threshold Levels for Infection in an Avian Model System. Am. J. Trop. Med. Hyg. 2007;76:1072–1078. doi: 10.4269/ajtmh.2007.76.1072. [DOI] [PubMed] [Google Scholar]
- 27.Huynh C.Q., Zieler H. Construction of Modular and Versatile Plasmid Vectors for the High-Level Expression of Single or Multiple Genes in Insects and Insect Cell Lines. J. Mol. Biol. 1999;288:13–20. doi: 10.1006/jmbi.1999.2674. [DOI] [PubMed] [Google Scholar]
- 28.Naik N.G., Lo Y.W., Wu T.Y., Lin C.C., Kuo S.C., Chao Y.C. Baculovirus as an Efficient Vector for Gene Delivery into Mosquitoes. Sci. Rep. 2018;8:17778. doi: 10.1038/s41598-018-35463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu C.C.N., Fallon A.M. Evaluation of a Heterologous Metallothionein Gene Promoter in Transfected Mosquito Cells. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997;116:353–358. doi: 10.1016/S0305-0491(96)00265-9. [DOI] [PubMed] [Google Scholar]
- 30.Berger E.M., Marino G., Torrey D. Expression of Drosophila Hsp 70-CAT Hybrid Gene in Aedes Cells Induced by Heat Shock. Somat. Cell Mol. Genet. 1985;11:371–377. doi: 10.1007/BF01534414. [DOI] [PubMed] [Google Scholar]
- 31.Kovach M.J., Carlson J.O., Beaty B.J. A Drosophila Metallothionein Promoter Is Inducible in Mosquito Cells. Insect Mol. Biol. 1992;1:37–43. doi: 10.1111/j.1365-2583.1993.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 32.Gray C.E., Coates C.J. High-Level Gene Expression in Aedes albopictus Cells Using a Baculovirus Hr3 Enhancer and IE1 Transactivator. BMC Mol. Biol. 2004;5:8. doi: 10.1186/1471-2199-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y.-G., Eggleston P. Comparative Analysis of Promoters for Transient Gene Expression in Cultured Mosquito Cells. Insect Mol. Biol. 1999;8:31–38. doi: 10.1046/j.1365-2583.1999.810031.x. [DOI] [PubMed] [Google Scholar]
- 34.Li M., Bui M., Yang T., Bowman C.S., White B.J., Akbari O.S. Germline Cas9 Expression Yields Highly Efficient Genome Engineering in a Major Worldwide Disease Vector, Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2017;114:E10540–E10549. doi: 10.1073/pnas.1711538114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carpenetti T.L.G., Aryan A., Myles K.M., Adelman Z.N. Robust Heat-Inducible Gene Expression by Two Endogenous Hsp70-Derived Promoters in Transgenic Aedes aegypti. Insect Mol. Biol. 2012;21:97–106. doi: 10.1111/j.1365-2583.2011.01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webster S.H., Scott M.J. The Aedes aegypti (Diptera: Culicidae) Hsp83 Gene Promoter Drives Strong Ubiquitous DsRed and ZsGreen Marker Expression in Transgenic Mosquitoes. J. Med. Entomol. 2021;58:2533–2537. doi: 10.1093/jme/tjab128. [DOI] [PubMed] [Google Scholar]
- 37.Horn C., Wimmer E.A. A Versatile Vector Set for Animal Transgenesis. Dev. Genes Evol. 2000;210:630–637. doi: 10.1007/s004270000110. [DOI] [PubMed] [Google Scholar]
- 38.Berghammer A.J., Klingler M., Wimmer E.A. A Universal Marker for Transgenic Insects. Nature. 1999;402:370–371. doi: 10.1038/46463. [DOI] [PubMed] [Google Scholar]
- 39.Kokoza V., Ahmed A., Wimmer E.A., Raikhel A.S. Efficient Transformation of the Yellow Fever Mosquito Aedes aegypti Using the PiggyBac Transposable Element Vector PBac[3xP3-EGFP Afm] Insect Biochem. Mol. Biol. 2001;31:1137–1143. doi: 10.1016/S0965-1748(01)00120-5. [DOI] [PubMed] [Google Scholar]
- 40.Gantz V.M., Jasinskiene N., Tatarenkova O., Fazekas A., Macias V.M., Bier E., James A.A. Highly Efficient Cas9-Mediated Gene Drive for Population Modification of the Malaria Vector Mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. USA. 2015;112:E6736–E6743. doi: 10.1073/pnas.1521077112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atkinson P.W., James A.A. Germline transformants spreading out to many insect species. Adv Genet. 2002;47:49–86. doi: 10.1016/s0065-2660(02)47002-2. [DOI] [PubMed] [Google Scholar]
- 42.Bottino-Rojas V., James A.A. Mosquito Transposon-Mediated Transgenesis. In: Duvall L., Matthews B., editors. Mosquitoes: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2022. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konet D.S., Anderson J., Piper J., Akkina R., Suchman E., Carlson J. Short-Hairpin RNA Expressed from Polymerase III Promoters Mediates RNA Interference in Mosquito Cells. Insect Mol. Biol. 2007;16:199–206. doi: 10.1111/j.1365-2583.2006.00714.x. [DOI] [PubMed] [Google Scholar]
- 44.Anderson M.A.E., Purcell J., Verkuijl S.A.N., Norman V.C., Leftwich P.T., Harvey-Samuel T., Alphey L.S. Expanding the CRISPR Toolbox in Culicine Mosquitoes: In Vitro Validation of Pol III Promoters. ACS Synth. Biol. 2020;9:678–681. doi: 10.1021/acssynbio.9b00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M., Yang T., Kandul N.P., Bui M., Gamez S., Raban R., Bennett J., Sánchez C.H.M., Lanzaro G.C., Schmidt H., et al. Development of a Confinable Gene Drive System in the Human Disease Vector Aedes aegypti. eLife. 2020;9:e5170. doi: 10.7554/eLife.51701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng X., López Del Amo V., Mameli E., Lee M., Bishop A.L., Perrimon N., Gantz V.M. Optimized CRISPR Tools and Site-Directed Transgenesis towards Gene Drive Development in Culex quinquefasciatus Mosquitoes. Nat. Commun. 2021;12:2960. doi: 10.1038/s41467-021-23239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Champer J. Transgenic Insects—Techniques and Applications. 2nd ed. CABI Books; CABI International; Wallingford, UK: 2022. Drosophila melanogaster as a Model for Gene Drive Systems; pp. 200–223. [DOI] [Google Scholar]
- 48.Smith R.C., Walter M.F., Hice R.H., O’Brochta D.A., Atkinson P.W. Testis-Specific Expression of the Β2 Tubulin Promoter of Aedes aegypti and Its Application as a Genetic Sex-Separation Marker. Insect Mol. Biol. 2007;16:61–71. doi: 10.1111/j.1365-2583.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 49.Catteruccia F., Benton J.P., Crisanti A. An Anopheles Transgenic Sexing Strain for Vector Control. Nat. Biotechnol. 2005;23:1414–1417. doi: 10.1038/nbt1152. [DOI] [PubMed] [Google Scholar]
- 50.Calvo E., Walter M., Adelman Z.N., Jimenez A., Onal S., Marinotti O., James A.A. Nanos (Nos) Genes of the Vector Mosquitoes, Anopheles gambiae, Anopheles stephensi and Aedes aegypti. Insect Biochem. Mol. Biol. 2005;35:789–798. doi: 10.1016/j.ibmb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Adelman Z.N., Jasinskiene N., Onal S., Juhn J., Ashikyan A., Salampessy M., MacCauley T., James A.A. Nanos Gene Control DNA Mediates Developmentally Regulated Transposition in the Yellow Fever Mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2007;104:9970–9975. doi: 10.1073/pnas.0701515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papathanos P.A., Windbichler N., Menichelli M., Burt A., Crisanti A. The Vasa Regulatory Region Mediates Germline Expression and Maternal Transmission of Proteins in the Malaria Mosquito Anopheles gambiae: A Versatile Tool for Genetic Control Strategies. BMC Mol. Biol. 2009;10:65. doi: 10.1186/1471-2199-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terradas G., Hermann A., James A.A., McGinnis W., Bier E. High-Resolution in Situ Analysis of Cas9 Germline Transcript Distributions in Gene-Drive Anopheles Mosquitoes. G3 Genes Genomes Genet. 2022;12:jkab369. doi: 10.1093/g3journal/jkab369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magnusson K., Mendes A.M., Windbichler N., Papathanos P.A., Nolan T., Dottorini T., Rizzi E., Christophides G.K., Crisanti A. Transcription Regulation of Sex-Biased Genes during Ontogeny in the Malaria Vector Anopheles gambiae. PLoS ONE. 2011;6:e21572. doi: 10.1371/journal.pone.0021572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marois E., Scali C., Soichot J., Kappler C., Levashina E.A., Catteruccia F. High-Throughput Sorting of Mosquito Larvae for Laboratory Studies and for Future Vector Control Interventions. Malar. J. 2012;11:302. doi: 10.1186/1475-2875-11-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Port F., Chen H.-M., Lee T., Bullock S.L. Optimized CRISPR/Cas Tools for Efficient Germline and Somatic Genome Engineering in Drosophila. Proc. Natl. Acad. Sci. USA. 2014;111:E2967–E2976. doi: 10.1073/pnas.1405500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Champer J., Reeves R., Oh S.Y., Liu C., Liu J., Clark A.G., Messer P.W. Novel CRISPR/Cas9 Gene Drive Constructs Reveal Insights into Mechanisms of Resistance Allele Formation and Drive Efficiency in Genetically Diverse Populations. PLoS Genet. 2017;13:e1006796. doi: 10.1371/journal.pgen.1006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carballar-Lejarazú R., Tushar T., Pham T.B., James A.A. Cas9-Mediated Maternal Effect and Derived Resistance Alleles in a Gene-Drive Strain of the African Malaria Vector Mosquito, Anopheles gambiae. Genetics. 2022;221:iyac055. doi: 10.1093/genetics/iyac055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Champer J., Liu J., Oh S.Y., Reeves R., Luthra A., Oakes N., Clark A.G., Messer P.W. Reducing Resistance Allele Formation in CRISPR Gene Drive. Proc. Natl. Acad. Sci. USA. 2018;115:5522–5527. doi: 10.1073/pnas.1720354115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carballar-Lejarazú R., Ogaugwu C., Tushar T., Kelsey A., Pham T.B., Murphy J., Schmidt H., Lee Y., Lanzaro G.C., James A.A. Next-Generation Gene Drive for Population Modification of the Malaria Vector Mosquito, Anopheles gambiae. Proc. Natl. Acad. Sci. USA. 2020;117:22805–22814. doi: 10.1073/pnas.2010214117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kyrou K., Hammond A.M., Galizi R., Kranjc N., Burt A., Beaghton A.K., Nolan T., Crisanti A. A CRISPR–Cas9 Gene Drive Targeting Doublesex Causes Complete Population Suppression in Caged Anopheles gambiae Mosquitoes. Nat. Biotechnol. 2018;36:1062–1071. doi: 10.1038/nbt.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hammond A., Karlsson X., Morianou I., Kyrou K., Beaghton A., Gribble M., Kranjc N., Galizi R., Burt A., Crisanti A., et al. Regulating the Expression of Gene Drives Is Key to Increasing Their Invasive Potential and the Mitigation of Resistance. PLoS Genet. 2021;17:e1009321. doi: 10.1371/journal.pgen.1009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akbari O.S., Papathanos P.A., Sandler J.E., Kennedy K., Hay B.A. Identification of Germline Transcriptional Regulatory Elements in Aedes aegypti. Sci. Rep. 2015;4:3954. doi: 10.1038/srep03954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akbari O.S., Antoshechkin I., Amrhein H., Williams B., Diloreto R., Sandler J., Hay B.A. The Developmental Transcriptome of the Mosquito Aedes aegypti, an Invasive Species and Major Arbovirus Vector. G3. 2013;3:1493–1509. doi: 10.1534/g3.113.006742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kojin B.B., Biedler J.K., Tu Z., Adelman Z.N. Characterization of a Female Germline and Early Zygote Promoter from the Transcription Factor BZip1 in the Dengue Mosquito Aedes aegypti. Parasites Vectors. 2020;13:353. doi: 10.1186/s13071-020-04216-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Brochta D.A., Handler A.M. Perspectives on the State of Insect Transgenics. In: Aksoy S., editor. Transgenesis and the Management of Vector-Borne Disease. Springer; Berlin/Heidelberg, Germany: 2008. pp. 1–18. (Advances in Experimental Medicine and Biology, Volume 627). [DOI] [PubMed] [Google Scholar]
- 67.Muñoz D., Jimenez A., Marinotti O., James A.A. The AeAct-4 Gene Is Expressed in the Developing Flight Muscles of Female Aedes aegypti. Insect Mol. Biol. 2004;13:563–568. doi: 10.1111/j.0962-1075.2004.00519.x. [DOI] [PubMed] [Google Scholar]
- 68.Fu G., Lees R.S., Nimmo D., Aw D., Jin L., Gray P., Berendonk T.U., White-Cooper H., Scaife S., Phuc H.K., et al. Female-Specific Flightless Phenotype for Mosquito Control. Proc. Natl. Acad. Sci. USA. 2010;107:4550–4554. doi: 10.1073/pnas.1000251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Labbé G.M.C., Scaife S., Morgan S.A., Curtis Z.H., Alphey L. Female-Specific Flightless (FsRIDL) Phenotype for Control of Aedes albopictus. PLoS Negl. Trop. Dis. 2012;6:e1724. doi: 10.1371/journal.pntd.0001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marinotti O., Jasinskiene N., Fazekas A., Scaife S., Fu G., Mattingly S.T., Chow K., Brown D.M., Alphey L., James A.A. Development of a Population Suppression Strain of the Human Malaria Vector Mosquito, Anopheles stephensi. Malar. J. 2013;12:142. doi: 10.1186/1475-2875-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allen M.L., Christensen B.M. Flight Muscle-Specific Expression of Act88F: GFP in Transgenic Culex quinquefasciatus Say (Diptera: Culicidae) Parasitol. Int. 2004;53:307–314. doi: 10.1016/j.parint.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Krzywinska E., Krzywinski J. Effects of Stable Ectopic Expression of the Primary Sex Determination Gene Yob in the Mosquito Anopheles gambiae. Parasites Vectors. 2018;11:648. doi: 10.1186/s13071-018-3211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Criscione F., Qi Y., Tu Z. GUY1 Confers Complete Female Lethality and Is a Strong Candidate for a Male-Determining Factor in Anopheles stephensi. eLife. 2016;5:e19281. doi: 10.7554/eLife.19281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aryan A., Anderson M.A.E., Biedler J.K., Qi Y., Overcash J.M., Naumenko A.N., Sharakhova M.V., Mao C., Adelman Z.N., Tu Z. Nix Alone Is Sufficient to Convert Female Aedes aegypti into Fertile Males and Myo-Sex Is Needed for Male Flight. Proc. Natl. Acad. Sci. USA. 2020;117:17702–17709. doi: 10.1073/pnas.2001132117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao Y., Jin B., Liu P., Xiao X., Cai L., Xie Z., Kong L., Liu T., Yang W., Wu Y., et al. The AalNix3&4 Isoform Is Required and Sufficient to Convert Aedes albopictus Females into Males. PLoS Genet. 2022;18:e1010280. doi: 10.1371/journal.pgen.1010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haghighat-Khah R.E., Harvey-Samuel T., Basu S., StJohn O., Scaife S., Verkuijl S., Lovett E., Alphey L. Engineered Action at a Distance: Blood-Meal-Inducible Paralysis in Aedes aegypti. PLoS Negl. Trop. Dis. 2019;13:e0007579. doi: 10.1371/journal.pntd.0007579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carballar-Lejarazú R., James A.A. Population Modification of Anopheline Species to Control Malaria Transmission. Pathog. Glob. Health. 2017;111:424–435. doi: 10.1080/20477724.2018.1427192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giannoni F., Müller H.M., Vizioli J., Catteruccia F., Kafatos F.C., Crisanti A. Nuclear Factors Bind to a Conserved DNA Element That Modulates Transcription of Anopheles gambiae Trypsin Genes. J. Biol. Chem. 2001;276:700–707. doi: 10.1074/jbc.M005540200. [DOI] [PubMed] [Google Scholar]
- 79.Müller H.M., Catteruccia F., Vizioli J., della Torre A., Crisanti A. Constitutive and Blood Meal-Induced Trypsin Genes in Anopheles gambiae. Exp. Parasitol. 1995;81:371–385. doi: 10.1006/expr.1995.1128. [DOI] [PubMed] [Google Scholar]
- 80.Skavdis G., Sidén-Kiamos I., Müller H.M., Crisanti A., Louis C. Conserved Function of Anopheles gambiae Midgut-Specific Promoters in the Fruitfly. EMBO J. 1996;15:344–350. doi: 10.1002/j.1460-2075.1996.tb00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nolan T., Petris E., Müller H.-M., Cronin A., Catteruccia F., Crisanti A. Analysis of Two Novel Midgut-Specific Promoters Driving Transgene Expression in Anopheles stephensi Mosquitoes. PLoS ONE. 2011;6:e16471. doi: 10.1371/journal.pone.0016471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moreira L.A., Edwards M.J., Adhami F., Jasinskiene N., James A.A., Jacobs-Lorena M. Robust Gut-Specific Gene Expression in Transgenic Aedes aegypti Mosquitoes. Proc. Natl. Acad. Sci. USA. 2000;97:10895–10898. doi: 10.1073/pnas.97.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abraham E.G., Donnelly-Doman M., Fujioka H., Ghosh A., Moreira L., Jacobs-Lorena M. Driving Midgut-Specific Expression and Secretion of a Foreign Protein in Transgenic Mosquitoes with AgAper1 Regulatory Elements. Insect Mol. Biol. 2005;14:271–279. doi: 10.1111/j.1365-2583.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- 84.Isaacs A.T., Li F., Jasinskiene N., Chen X., Nirmala X., Marinotti O., Vinetz J.M., James A.A. Engineered Resistance to Plasmodium falciparum Development in Transgenic Anopheles stephensi. PLoS Pathog. 2011;7:e1002017. doi: 10.1371/journal.ppat.1002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong Y., Simões M.L., Dimopoulos G. Versatile Transgenic Multistage Effector-Gene Combinations for Plasmodium falciparum Suppression in Anopheles. Sci. Adv. 2020;6:eaay5898. doi: 10.1126/sciadv.aay5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buchman A., Gamez S., Li M., Antoshechkin I., Li H.-H., Wang H.-W., Chen C.-H., Klein M.J., Duchemin J.-B., Crowe J.E., et al. Broad Dengue Neutralization in Mosquitoes Expressing an Engineered Antibody. PLoS Pathog. 2020;16:e1008103. doi: 10.1371/journal.ppat.1008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corby-Harris V., Drexler A., de Jong L.W., Antonova Y., Pakpour N., Ziegler R., Ramberg F., Lewis E.E., Brown J.M., Luckhart S., et al. Activation of Akt Signaling Reduces the Prevalence and Intensity of Malaria Parasite Infection and Lifespan in Anopheles stephensi Mosquitoes. PLoS Pathog. 2010;6:e1001003. doi: 10.1371/annotation/738ac91f-8c41-4bf5-9a39-bddf0b777a89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moreira L.A., Wang J., Collins F.H., Jacobs-Lorena M. Fitness of Anopheline Mosquitoes Expressing Transgenes That Inhibit Plasmodium Development. Genetics. 2004;166:1337–1341. doi: 10.1534/genetics.166.3.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nirmala X., Marinotti O., Sandoval J.M., Phin S., Gakhar S., Jasinskiene N., James A.A. Functional Characterization of the Promoter of the Vitellogenin Gene, AsVg1, of the Malaria Vector, Anopheles stephensi. Insect Biochem. Mol. Biol. 2006;36:694–700. doi: 10.1016/j.ibmb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 90.Kokoza V., Ahmed A., Cho W.-L., Jasinskiene N., James A.A., Raikhel A. Engineering Blood Meal-Activated Systemic Immunity in the Yellow Fever Mosquito, Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2000;97:9144–9149. doi: 10.1073/pnas.160258197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen S., Rasgon J.L. Culex tarsalis Vitellogenin Gene Promoters Investigated in Silico and in Vivo Using Transgenic Drosophila melanogaster. PLoS ONE. 2014;9:e88994. doi: 10.1371/journal.pone.0088994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Isaacs A.T., Jasinskiene N., Tretiakov M., Thiery I., Zettor A., Bourgouin C., James A.A. Transgenic Anopheles stephensi Coexpressing Single-Chain Antibodies Resist Plasmodium falciparum Development. Proc. Natl. Acad. Sci. USA. 2012;109:4–9. doi: 10.1073/pnas.1207738109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li C., Marrelli M.T., Yan G., Jacobs-Lorena M. Fitness of Transgenic Anopheles stephensi Mosquitoes Expressing the SM1 Peptide under the Control of a Vitellogenin Promoter. J. Hered. 2008;99:275–282. doi: 10.1093/jhered/esn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morris A.C., Pott G.B., Chen J., James A.A. Transient Expression of a Promoter-Reporter Construct in Differentiated Adult Salivary Glands and Embryos of the Mosquito Aedes aegypti. Am. J. Trop. Med. Hyg. 1995;52:456–460. doi: 10.4269/ajtmh.1995.52.456. [DOI] [PubMed] [Google Scholar]
- 95.Lombardo F., Nolan T., Lycett G., Lanfrancotti A., Stich N., Catteruccia F., Louis C., Coluzzi M., Arcà B., Arca B. An Anopheles gambiae Salivary Gland Promoter Analysis in Drosophila melanogaster and Anopheles stephensi. Insect Mol. Biol. 2005;14:207–216. doi: 10.1111/j.1365-2583.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- 96.Lombardo F., Lycett G.J., Lanfrancotti A., Coluzzi M., Arcà B. Analysis of Apyrase 5′ Upstream Region Validates Improved Anopheles gambiae Transformation Technique. BMC Res. Notes. 2009;2:24. doi: 10.1186/1756-0500-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lombardo F., Di Cristina M., Spanos L., Louis C., Coluzzi M., Arcà B. Promoter Sequences of the Putative Anopheles gambiae Apyrase Confer Salivary Gland Expression in Drosophila melanogaster. J. Biol. Chem. 2000;275:23861–23868. doi: 10.1074/jbc.M909547199. [DOI] [PubMed] [Google Scholar]
- 98.Yoshida S., Watanabe H. Robust Salivary Gland-Specific Transgene Expression in Anopheles stephensi Mosquito. Insect Mol. Biol. 2006;15:403–410. doi: 10.1111/j.1365-2583.2006.00645.x. [DOI] [PubMed] [Google Scholar]
- 99.Sumitani M., Kasashima K., Yamamoto D.S., Yagi K., Yuda M., Matsuoka H., Yoshida S. Reduction of Malaria Transmission by Transgenic Mosquitoes Expressing an Antisporozoite Antibody in Their Salivary Glands. Insect Mol. Biol. 2013;22:41–51. doi: 10.1111/j.1365-2583.2012.01168.x. [DOI] [PubMed] [Google Scholar]
- 100.Yamamoto D.S., Sumitani M., Kasashima K., Sezutsu H., Matsuoka H. Inhibition of Malaria Infection in Transgenic Anopheline Mosquitoes Lacking Salivary Gland Cells. PLoS Pathog. 2016;12:e1005872. doi: 10.1371/journal.ppat.1005872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mathur G., Sanchez-Vargas I., Alvarez D., Olson K.E., Marinotti O., James A.A. Transgene-Mediated Suppression of Dengue Viruses in the Salivary Glands of the Yellow Fever Mosquito, Aedes aegypti. Insect Mol. Biol. 2010;19:753–763. doi: 10.1111/j.1365-2583.2010.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matsuoka H. Production of a Transgenic Mosquito, as a Flying Syringe, to Deliver Protective Vaccine via Saliva. MW J. 2013;4:1–3. doi: 10.5281/zenodo.10894636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dimopoulos G., Christophides G.K., Meister S., Schultz J., White K.P., Barillas-Mury C., Kafatos F.C. Genome Expression Analysis of Anopheles gambiae: Responses to Injury, Bacterial Challenge, and Malaria Infection. Proc. Natl. Acad. Sci. USA. 2002;99:8814–8819. doi: 10.1073/pnas.092274999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hawkes N.J., Hemingway J. Analysis of the Promoters for the β-Esterase Genes Associated with Insecticide Resistance in the Mosquito Culex quinquefasciatus. Biochim. Biophys. Acta Gene Struct. Expr. 2002;1574:51–62. doi: 10.1016/S0167-4781(01)00344-X. [DOI] [PubMed] [Google Scholar]
- 105.Ding Y., Hawkes N., Meredith J., Eggleston P., Hemingway J., Ranson H. Characterization of the Promoters of Epsilon Glutathione Transferases in the Mosquito Anopheles gambiae and Their Response to Oxidative Stress. Biochem. J. 2005;387:879–888. doi: 10.1042/BJ20041850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Itokawa K., Komagata O., Kasai S., Tomita T. A Single Nucleotide Change in a Core Promoter Is Involved in the Progressive Overexpression of the Duplicated CYP9M10 Haplotype Lineage in Culex quinquefasciatus. Insect Biochem. Mol. Biol. 2015;66:96–102. doi: 10.1016/j.ibmb.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 107.Mysore K., Li P., Duman-Scheel M. Identification of Aedes aegypti cis-regulatory elements that promote gene expression in olfactory receptor neurons of distantly related dipteran insects. Parasites Vectors. 2018;11:406. doi: 10.1186/s13071-018-2982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Behura S.K., Sarro J., Li P., Mysore K., Severson D.W., Emrich S.J., Duman-Scheel M. High-throughput cis-regulatory element discovery in the vector mosquito Aedes aegypti. BMC Genom. 2016;17:341. doi: 10.1186/s12864-016-2468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoermann A., Tapanelli S., Capriotti P., Del Corsano G., Masters E.K.G., Habtewold T., Christophides G.K., Windbichler N. Converting Endogenous Genes of the Malaria Mosquito into Simple Non-Autonomous Gene Drives for Population Replacement. eLife. 2021;10:e58791. doi: 10.7554/eLife.58791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nolan T., Bower T.M., Brown A.E., Crisanti A., Catteruccia F. PiggyBac-Mediated Germline Transformation of the Malaria Mosquito Anopheles stephensi Using the Red Fluorescent Protein DsRED as a Selectable Marker. J. Biol. Chem. 2002;277:8759–8762. doi: 10.1074/jbc.C100766200. [DOI] [PubMed] [Google Scholar]
- 111.Grossman G.L., Rafferty C.S., Clayton J.R., Stevens T.K., Mukabayire O., Benedict M.Q. Germline Transformation of the Malaria Vector, Anopheles gambiae, with the PiggyBac Transposable Element. Insect Mol. Biol. 2001;10:597–604. doi: 10.1046/j.0962-1075.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- 112.Allen M.L., O’Bhochta D.A., Atkinson P.W., Levesque C.S. Stable, Germ-Line Transformation of Culex quinquefasciatus (Diptera: Culicidae) J. Med. Entomol. 2001;38:701–710. doi: 10.1603/0022-2585-38.5.701. [DOI] [PubMed] [Google Scholar]
- 113.Ito J., Ghosh A., Moreira L.A., Wimmer E.A., Jacobs-Lorena M. Transgenic Anopheline Mosquitoes Impaired in Transmission of a Malaria Parasite. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- 114.Moreira L.A., Ito J., Ghosh A., Devenport M., Zieler H., Abraham E.G., Crisanti A., Nolan T., Catteruccia F., Jacobs-Lorena M. Bee Venom Phospholipase Inhibits Malaria Parasite Development in Transgenic Mosquitoes. J. Biol. Chem. 2002;277:40839–40843. doi: 10.1074/jbc.M206647200. [DOI] [PubMed] [Google Scholar]
- 115.Yoshida S., Shimada Y., Kondoh D., Kouzuma Y., Ghosh A.K., Jacobs-Lorena M., Sinden R.E. Hemolytic C-Type Lectin CEL-III from Sea Cucumber Expressed in Transgenic Mosquitoes Impairs Malaria Parasite Development. PLoS Pathog. 2007;3:1962–1970. doi: 10.1371/journal.ppat.0030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dong Y., Das S., Cirimotich C., Souza-Neto J.A., McLean K.J., Dimopoulos G. Engineered Anopheles Immunity to Plasmodium Infection. PLoS Pathog. 2011;7:e1002458. doi: 10.1371/journal.ppat.1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pascini T.V., Jeong Y.J., Huang W., Pala Z.R., Sá J.M., Wells M.B., Kizito C., Sweeney B., Alves e Silva T.L., Andrew D.J., et al. Transgenic Anopheles Mosquitoes Expressing Human PAI-1 Impair Malaria Transmission. Nat. Commun. 2022;13:3949. doi: 10.1038/s41467-022-30606-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Meredith J.M., Basu S., Nimmo D.D., Larget-Thiery I., Warr E.L., Underhill A., McArthur C.C., Carter V., Hurd H., Bourgouin C., et al. Site-Specific Integration and Expression of an Anti-Malarial Gene in Transgenic Anopheles gambiae Significantly Reduces Plasmodium Infections. PLoS ONE. 2011;6:e14587. doi: 10.1371/journal.pone.0014587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoermann A.A., Habtewold T., Selvaraj P., Del G. Gene Drive Mosquitoes Can Aid Malaria Elimination by Retarding Plasmodium Sporogonic Development. Sci. Adv. 2022;8:eabo1733. doi: 10.1126/sciadv.abo1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dong S., Fu X., Dong Y., Simões M.L., Zhu J., Dimopoulos G. Broad Spectrum Immunomodulatory Effects of Anopheles gambiae MicroRNAs and Their Use for Transgenic Suppression of Plasmodium. PLoS Pathog. 2020;16:e1008453. doi: 10.1371/journal.ppat.1008453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Volohonsky G., Hopp A.K., Saenger M., Soichot J., Scholze H., Boch J., Blandin S.A., Marois E. Transgenic Expression of the Anti-Parasitic Factor TEP1 in the Malaria Mosquito Anopheles gambiae. PLoS Pathog. 2017;13:e1006113. doi: 10.1371/journal.ppat.1006113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Simões M.L., Dong Y., Hammond A., Hall A., Crisanti A., Nolan T., Dimopoulos G. The Anopheles FBN9 Immune Factor Mediates Plasmodium Species-Specific Defense through Transgenic Fat Body Expression. Dev. Comp. Immunol. 2017;67:257–265. doi: 10.1016/j.dci.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 123.Windbichler N., Papathanos P.A., Crisanti A. Targeting the X Chromosome during Spermatogenesis Induces Y Chromosome Transmission Ratio Distortion and Early Dominant Embryo Lethality in Anopheles gambiae. PLoS Genet. 2008;4:e1000291. doi: 10.1371/journal.pgen.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu W.L., Hsu C.W., Chan S.P., Yen P.S., Su M.P., Li J.C., Li H.H., Cheng L., Tang C.K., Ko S.H., et al. Transgenic Refractory Aedes aegypti Lines Are Resistant to Multiple Serotypes of Dengue Virus. Sci. Rep. 2021;11:23865. doi: 10.1038/s41598-021-03229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Williams A.E., Sanchez-Vargas I., Reid W.R., Lin J., Franz A.W.E., Olson K.E. The Antiviral Small-Interfering RNA Pathway Induces Zika Virus Resistance in Transgenic Aedes aegypti. Viruses. 2020;12:1231. doi: 10.3390/v12111231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ramyasoma H.P.B.K.D., Dassanayake R.S., Hapugoda M., Capurro M.L., Silva Gunawardene Y.I.N. Multiple Dengue Virus Serotypes Resistant Transgenic Aedes aegypti Fitness Evaluated under Laboratory Conditions. RNA Biol. 2020;17:918–929. doi: 10.1080/15476286.2020.1735210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yen P.-S., James A., Li J.-C., Chen C.-H., Failloux A.-B. Synthetic MiRNAs Induce Dual Arboviral-Resistance Phenotypes in the Vector Mosquito Aedes aegypti. Commun. Biol. 2018;1:11. doi: 10.1038/s42003-017-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Raikhel A.S., Kokoza V.A., Zhu J., Martin D., Wang S.F., Li C., Sun G., Ahmed A., Dittmer N., Attardo G. Molecular Biology of Mosquito Vitellogenesis: From Basic Studies to Genetic Engineering of Antipathogen Immunity. Insect Biochem. Mol. Biol. 2002;32:1275–1286. doi: 10.1016/S0965-1748(02)00090-5. [DOI] [PubMed] [Google Scholar]
- 129.James A.A., James S., Mumford J., Toure Y. Progress and Prospects for the Use of Genetically Modified Mosquitoes to Inhibit Disease Transmission. Report on Planning Meeting 1: Technical Consultation on Current Status and Planning for Future Development of Genetically Modified Mosquitoes for Malaria and Dengue Control. WHO/TDR Publications; Geneva, Switzerland: 2010. [DOI] [Google Scholar]
- 130.Benedict M., Bonsall M., James A.A., James S., Lavery J., Mumford J., Quemada H., Rose R., Thompson P., Toure Y., et al. Guidance Framework for Testing of Genetically Modified Mosquitoes. WHO/TDR Publications; Geneva, Switzerland: 2014. [Google Scholar]
- 131.Wilson A.L., Boelaert M., Kleinschmidt I., Pinder M., Scott T.W., Tusting L.S., Lindsay S.W. Evidence-based vector control? Improving the quality of vector control trials. Trends Parasitol. 2015;31:380–390. doi: 10.1016/j.pt.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 132.National Academies of Sciences, Engineering, and Medicine . Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values. National Academies Press (US); Washington, DC, USA: 2016. [DOI] [PubMed] [Google Scholar]
- 133.Adelman Z., Akbari O., Bauer J., Bier E., Bloss C., Carter S.R., Callender C., Denis A.C., Cowhey P., Dass B., et al. Rules of the road for insect gene drive research and testing. Nat. Biotechnol. 2017;35:716–718. doi: 10.1038/nbt.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.James S., Collins F.H., Welkhoff P.A., Emerson C., Godfray C.H., Gottlieb M.J., Greenwood B., Lindsay S.W., Mbogo C.M., Okumu F.O., et al. Pathway to Deployment of Gene Drive Mosquitoes as a Potential Biocontrol Tool for Elimination of Malaria in Sub-Saharan Africa: Recommendations of a Scientific Working Group. Am. J. Trop. Med. Hyg. 2018;98:1–49. doi: 10.4269/ajtmh.18-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kormos A., Lanzaro G.C., Bier E., Dimopoulos G., Marshall J.M., Pinto J., dos Santos A.A., Bacar A., Rompão H.S.P.S., James A.A. Application of the relationship-based model to community and regulatory engagement for field trials of genetically engineered mosquitoes for malaria control. J. Am. Soc. Trop. Med. Hyg. 2020;104:805–811. doi: 10.4269/ajtmh.20-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kormos A., Lanzaro G.C., Bier E., Santos V., Nazaré L., Pinto J., Dos Santos A.A., James A.A. Ethical Considerations for Gene Drive: Challenges of Balancing Inclusion, Power and Perspectives. Front. Bioeng. Biotechnol. 2022;10:826727. doi: 10.3389/fbioe.2022.826727. [DOI] [PMC free article] [PubMed] [Google Scholar]