Key Points
Question
Is a polygenic risk score (PRS) derived from European ancestry data associated with Alzheimer disease (AD) dementia risk in non-European populations?
Findings
In this cohort study of genome data from European and Asian databases of patients with AD, a PRS derived from a genome-wide study of individuals with European ancestry was associated with high genetic risk for AD dementia in 1634 Korean individuals. Furthermore, the PRS was associated with amnestic mild cognitive impairment, earlier symptom onset of AD dementia, and amyloid β deposition.
Meaning
These findings emphasize the transancestry transferability and clinical value of PRSs and reinforce the need for enriching diversity in genetic studies of AD.
This cohort study evaluates the ability of a polygenic risk score for Alzheimer disease (AD) created from a genome-wide association study of European populations to assess risk of AD dementia and related outcomes among patients in Korea.
Abstract
Importance
Polygenic risk scores (PRSs), which aggregate the genetic effects of single-nucleotide variants identified in genome-wide association studies (GWASs), can help distinguish individuals at a high genetic risk for Alzheimer disease (AD). However, genetic studies have predominantly focused on populations of European ancestry.
Objective
To evaluate the transferability of a PRS for AD in the Korean population using summary statistics from a prior GWAS of European populations.
Design, Setting, and Participants
This cohort study developed a PRS based on the summary statistics of a large-scale GWAS of a European population (the International Genomics of Alzheimer Project; 21 982 AD cases and 41 944 controls). This PRS was tested for an association with AD dementia and its related phenotypes in 1634 Korean individuals, who were recruited from 2013 to 2019. The association of a PRS based on a GWAS of a Japanese population (the National Center for Geriatrics and Gerontology; 3962 AD cases and 4074 controls) and a transancestry meta-analysis of European and Japanese GWASs was also evaluated. Data were analyzed from December 2020 to June 2021.
Main Outcomes and Measures
Risk of AD dementia, amnestic mild cognitive impairment (aMCI), earlier symptom onset, and amyloid β deposition (Aβ).
Results
A total of 1634 Korean patients (969 women [59.3%]), including 716 individuals (43.6%) with AD dementia, 222 (13.6%) with aMCI, and 699 (42.8%) cognitively unimpaired controls, were analyzed in this study. The mean (SD) age of the participants was 71.6 (9.0) years. Higher PRS was associated with a higher risk of AD dementia independent of APOE ɛ4 status in the Korean population (OR, 1.95; 95% CI, 1.40-2.72; P < .001). Furthermore, PRS was associated with aMCI, earlier symptom onset, and Aβ deposition independent of APOE ɛ4 status. The PRS based on a transancestry meta-analysis of data sets comprising 2 distinct ancestries showed a slightly improved accuracy.
Conclusions and Relevance
In this cohort study, a PRS derived from a European GWAS identified individuals at a high risk for AD dementia in the Korean population. These findings emphasize the transancestry transferability and clinical value of PRSs and suggest the importance of enriching diversity in genetic studies of AD.
Introduction
Alzheimer disease (AD) is the main cause of dementia, affecting approximately 50 million individuals worldwide, and the number is expected to triple by 2050 owing to population aging.1 This is particularly problematic in East Asia, where the population is aging rapidly. It is estimated that nearly a quarter of patients with dementia live in East Asia, and the number is expected to double over the next 20 years.2
The pathological process of AD begins long before the onset of clinical dementia. Therefore, identifying individuals at a high risk for developing AD is of utmost importance for potential preventive and therapeutic strategies.3 Genetic information can be used to identify individuals at a high risk for AD because the heritability of AD is estimated to be 60% to 80%.4 Previous studies have demonstrated that polygenic risk scores (PRSs), which aggregate the genetic effects of single-nucleotide variants (SNVs) identified in genome-wide association studies (GWASs), can help distinguish individuals at a high genetic risk for AD.5
However, previous genetic studies were conducted predominantly in populations of European ancestry. Thus, the generalizability of a PRS to non-European populations remains unknown.6 A 2019 study examined the risk assessment capability of European ancestry–derived PRSs in samples of non-European ancestry with various phenotypes.7 The PRS for AD derived from European populations was also tested in non-Hispanic Black8,9 and Caribbean Hispanic individuals.10 However, the performance of PRSs for AD in Asian populations has not yet been evaluated.
Our study aimed to evaluate the transferability of a PRS for AD in the Korean population using summary statistics from a prior large-scale GWAS of European populations.11 Moreover, we applied our PRS to determine whether it is associated with risk of amnestic mild cognitive impairment (aMCI), earlier symptom onset, or amyloid β (Aβ) deposition. We also evaluated the PRS based on a GWAS of a Japanese population12 and a transancestry meta-analysis of European and Japanese GWASs.
Methods
All participants provided written informed consent in the primary Korean data set, and the study was approved by the institutional review board of each center. This study followed the reporting requirements of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.
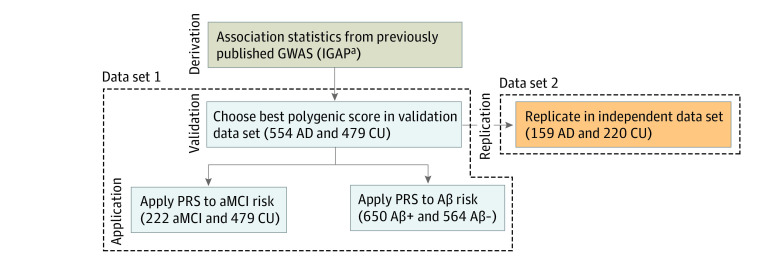
Data Set 1
A total of 1255 participants of Korean ancestry were recruited from 14 referral hospitals in the Republic of Korea from January 2013 to July 2019 (Figure 1). Among them, 954 participants were recruited from the Samsung Medical Center, 202 from a multicenter study of the Korean Brain Aging Study for Early Diagnosis and Prediction of AD,13 and 99 from a multicenter clinical research platform study based on the dementia cohort. We included participants who were diagnosed with AD dementia or aMCI or those who were cognitively unimpaired (CU) based on detailed neuropsychological test results.14,15,16 We used the participants’ diagnoses at the latest assessment point. AD dementia was defined in accordance with the core clinical criteria for probable AD dementia according to the National Institute on Aging-Alzheimer Association.15 aMCI was defined in accordance with the following criteria, modified from Peterson’s criteria17: (1) normal activities of daily living performance, (2) objective memory impairment on a verbal or visual memory test below the 16th percentile of age- and education-matched norms, and (3) no dementia.
Figure 1. Study Data Sets and Analysis Steps.
Aβ indicates amyloid β; AD, Alzheimer disease; aMCI, amnestic mild cognitive impairment; CU, cognitively unimpaired; GWAS, genome-wide association study; IGAP, International Genomics of Alzheimer Project.
aSummary statistics were obtained from the European GWAS (IGAP).
Participants were excluded when they had (1) a causative genetic mutation for AD in known genes, such as PSEN1, PSEN2, or APP; (2) structural abnormalities detected on brain magnetic resonance imaging, such as severe cerebral ischemia, territorial infarction, or brain tumors; or (3) other medical or psychiatric diseases that may cause cognitive impairment.
Data Set 2
For the replication data set, 379 participants of Korean ancestry were recruited from 20 referral hospitals in the Republic of Korea. Of these, 125 participants were from the biobank of the Chronic Cerebrovascular Disease Consortium, recruited from 2016 to 2018. This was part of the ongoing BICWALZS study (Biobank Innovation for Chronic Cerebrovascular Disease With Alzheimer’s Disease Study) and data from the Center for Convergence Research of Neurological Disorders.18 The remaining 254 participants were recruited from the PREMIER (Precision Medicine Platform for Mild Cognitive Impairment Based on Multi-omics, Imaging and Evidence-based Research & Business Development) study. We included participants who were diagnosed with AD dementia or CU according to the same criteria in data set 1.
Genotyping and Imputation
DNA samples were genotyped using the Asian screening array (ASA) chip (Illumina). A subset of 125 samples was genotyped using a customized Korea Biobank array (KBA) chip (Affymetrix).19 Quality control for SNV data was conducted using the PLINK software and imputation was conducted using the Minimac4 software at the University of Michigan Imputation Server (eMethods in the Supplement).
Amyloid Positron Emission Tomography (PET)
A subset of 1214 participants in data set 1 underwent either 18F-florbetaben or 18F-flutemetamol PET (eMethods in the Supplement).20 Aβ positivity was determined by visual assessments.
GWAS Summary Statistics
To investigate the transferability of the PRS in the Korean population, we utilized the summary statistics generated from the European International Genomics of Alzheimer Project (IGAP) GWAS (11 480 632 SNVs from 21 982 AD cases and 41 944 controls)11 and East Asian–based National Center for Geriatrics and Gerontology (NCGG) Japanese GWAS (4 852 957 SNVs from 3962 AD cases and 4074 controls).12 Furthermore, we derived the PRS using transancestry meta-GWAS results (12 519 321 SNVs) obtained from an inverse variance-weighted fixed-effects meta-analysis of the European and Japanese GWAS results using METAL.21
PRS Generation
Based on previous study data,5,22,23 we excluded 3877 SNVs surrounding APOE (chromosome 19, 44 400 to 46 500 kb, GRCh37/hg19) to derive the PRS independent of the APOE region (eMethods in the Supplement). PRSice-2 software version 2.3.3 (GNU General Public License) was used to generate the PRS for AD dementia using prior GWAS summary statistics (European GWAS, Japanese GWAS, or meta-analysis).
Validation and Replication of the PRS for AD
After calculating each participant’s PRS, we performed a logistic regression analysis to determine whether the PRS derived from the summary statistics for the AD risk based on European populations was associated with AD dementia diagnosis in the data set 1 and 2 after adjusting for age, sex, education year, APOE ɛ4 carrier status, and the first 4 principal components of genetic ancestry. To verify whether the association of the PRS with AD dementia diagnosis varied by the APOE ɛ4 carrier status, we performed the same analysis after stratifying the participants into APOE ɛ4 carriers and noncarriers. In addition, we developed the PRS based on previous Japanese GWAS and transancestry meta-GWAS results and evaluated the association of PRS with AD.
Application of the PRS in Various Phenotypes
A multivariable logistic regression analysis was conducted for the participants with aMCI to evaluate whether the PRS is associated with aMCI independent of age, sex, education year, APOE ɛ4 carrier status, and the first 4 principal components of genetic ancestry.
We stratified the participants based on quartiles of the PRS and evaluated whether the PRS can be used for risk stratification in addition to APOE ɛ4 genotyping. We also evaluated whether the participants with a high PRS showed earlier development of AD than did those with a low PRS. We performed a Cox regression analysis with age at AD onset and age at the last clinical visit as time variables and the diagnosis of AD as a status variable.
Furthermore, using a subset of 1214 participants who also underwent Aβ PET, we also performed a logistic regression analysis to evaluate whether the PRS is associated with Aβ positivity. We adjusted for the effect of age at which Aβ PET was performed, sex, education year, and APOE ɛ4 carrier status.
Statistical Analysis
For demographic and clinical characteristics, categorical and continuous variables were presented as totals and mean averages, respectively. The χ2 test was used for categorical variables and analysis of variance for continuous variables. Cochran-Armitage tests were used to determine P values for trend. We reported 2-tailed P values and defined P < .05 as statistically significant. All statistical analyses and result visualization were performed using PLINK version 1.90,24 R version 3.6.1 (R Project for Statistical Computing), and MATLAB.
Results
Participants
Data set 1 included a total of 1255 participants with a mean (SD) age of 72.2 (8.9) years (739 women [58.9%]) (Table 1). Data set 2 included 379 participants with a mean (SD) age of 69.8 (9.3) years (230 women [60.7%]). In the principal component analysis (PCA) with data from the 1000 Genomes Project, there was an ethnic overlap of our data set with those of other East Asian populations. In the East Asian population, mean (5-SD) of PC1 and PC2 were 0.152 (0.001) and 0.034 (0.005) respectively. In our study cohorts, mean (5-SD) of PC1 and PC2 were 0.151 (0.001) and 0.032 (0.005), respectively. However, there was no stratification by genotyping arrays (ASA and KBA) (eFigure 1 in the Supplement). In addition, the PRS distributions among the study participants were not significantly different according to the genotyping arrays. In patients with AD dementia, mean (standard error [SE]) of PRS was 0.269 (0.016) and 0.285 (0.051) for those using ASA and KBA chips respectively (P = .33). In patient who were CU, mean (SE) of PRS was 0.158 (0.016) and 0.212 (0.053) for those using ASA and KBA chips respectively (P = .77) (eFigure 2 in the Supplement).
Table 1. Demographic and Clinical Characteristics of the Study Data Sets.
| Characteristics | Patients, No. (%) | ||||
|---|---|---|---|---|---|
| Data set 1 (n = 1255) | Data set 2 (n = 379) | ||||
| CU (n = 479) | AD dementia (n = 554) | aMCI (n = 222) | CU (n = 220) | AD dementia (n = 159) | |
| Age, mean (SD), y | 70.7 (7.6) | 73.1 (10.0) | 73.0 (8.2) | 67.8 (9.2) | 72.6 (8.6) |
| Sex | |||||
| Women | 282 (58.9) | 348 (62.8) | 109 (49.1) | 139 (63.2) | 91 (57.2) |
| Men | 197 (41.1) | 206 (37.2) | 113 (50.9) | 81 (26.8) | 68 (42.8) |
| Education, mean (SD), y | 11.2 (4.9) | 10.4 (5.0) | 11.9 (4.7) | 11.3 (4.6) | 9.7 (5.3) |
| APOE ε4 carrier | 118 (24.6) | 314 (56.7) | 79 (35.6) | 55 (25.0) | 74 (46.5) |
Abbreviations: AD, Alzheimer disease; aMCI, amnestic mild cognitive impairment; CU, cognitively unimpaired.
Optimal PRS Generation for the Korean Population
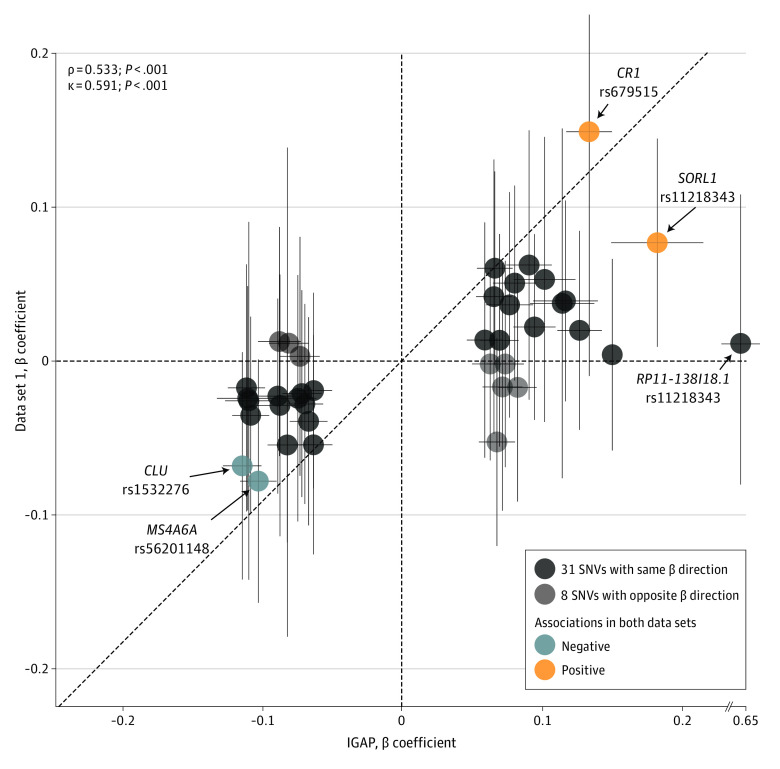
To determine the best parameters (P value threshold cut-off and linkage disequilibrium [LD]-based clumping value) for PRS calculation, we used PRSice-2 using the European GWAS (IGAP) summary statistics. Among various thresholds, we observed the highest Nagelkerke R2 value (0.020) when the P and LD values were 4.15 × 10−6 and 0.1, respectively (eFigure 3A in the Supplement). From these thresholds, 39 SNVs were selected, and their β coefficients were used to create the PRS (eTable 1 in the Supplement). We observed a significant correlation between the β coefficients of the 39 SNVs calculated from the European GWAS (IGAP) and those from data set 1 (Spearman correlation = 0.533; P < .001) (Figure 2).
Figure 2. Scatter Plot of β Coefficients of 39 SNVs.
GWAS indicates genome-wide association study; IGAP, International Genomics of Alzheimer Project; SNV, single-nucleotide variant. Correlations were determined using Spearman correlation (ρ) and Cohen κ coefficient tests between the European GWAS (IGAP) and the data set 1. Detailed information of SNVs shown in eTable 1 in the Supplement.
Association of the PRS With AD Dementia, aMCI, and Aβ Deposition
A higher PRS was associated with an increased risk of AD dementia after adjusting for the effect of age, sex, education, and APOE ɛ4 status (odds ratio [OR], 1.95; 95% CI, 1.40-2.72; P < .001) (Table 2). Furthermore, PRS was also associated with the AD dementia risk in both APOE ɛ4 carriers (OR, 2.73; 95% CI, 1.53-4.97; P = .001) and noncarriers (OR, 1.70; 95% CI, 1.14-2.59; P = .01). These results were replicated in data set 2 (eg, AD dementia diagnosis: OR, 1.85; 95% CI, 1.05-3.32) (Table 2). Similarly, we observed that a higher PRS was significantly associated with an increased risk of aMCI (OR, 1.74; 95% CI, 1.16-2.64; P = .008) and Aβ deposition in the brain (OR, 1.81; 95% CI, 1.32-2.48; P < .001).
Table 2. Association of the PRS With AD Dementia, aMCI, and Aβ Deposition.
| Measure | OR (95% CI) | P value |
|---|---|---|
| AD dementia diagnosisa | ||
| Data set 1 | 1.95 (1.40-2.72) | <.001 |
| Data set 2 | 1.85 (1.05-3.32) | .04 |
| aMCI diagnosisb | 1.74 (1.16-2.64) | .008 |
| Aβ PET depositionc | 1.81 (1.32-2.48) | <.001 |
Abbreviations: Aβ, amyloid β; AD, Alzheimer disease; aMCI, amnestic mild cognitive impairment; CU, cognitively unimpaired; OR, odds ratio; PC, principal component; PET, positron emission tomography; PRS, polygenic risk score.
Diagnosis (CU = 0, AD dementia = 1) = sex + age + education year + PC1-4 + APOE ε4 carrier (0 or 1) + PRS.
Diagnosis (CU = 0, aMCI = 1) = sex + age + education year + PC1-4 + APOE ε4 carrier (0 or 1) + PRS.
Aβ deposition (negative = 0, positive = 1) = sex + age + education year + PC1-4 + APOE ε4 carrier (0 or 1) + PRS.
Utility of the PRS in Risk Stratification of AD Dementia–Related Outcomes
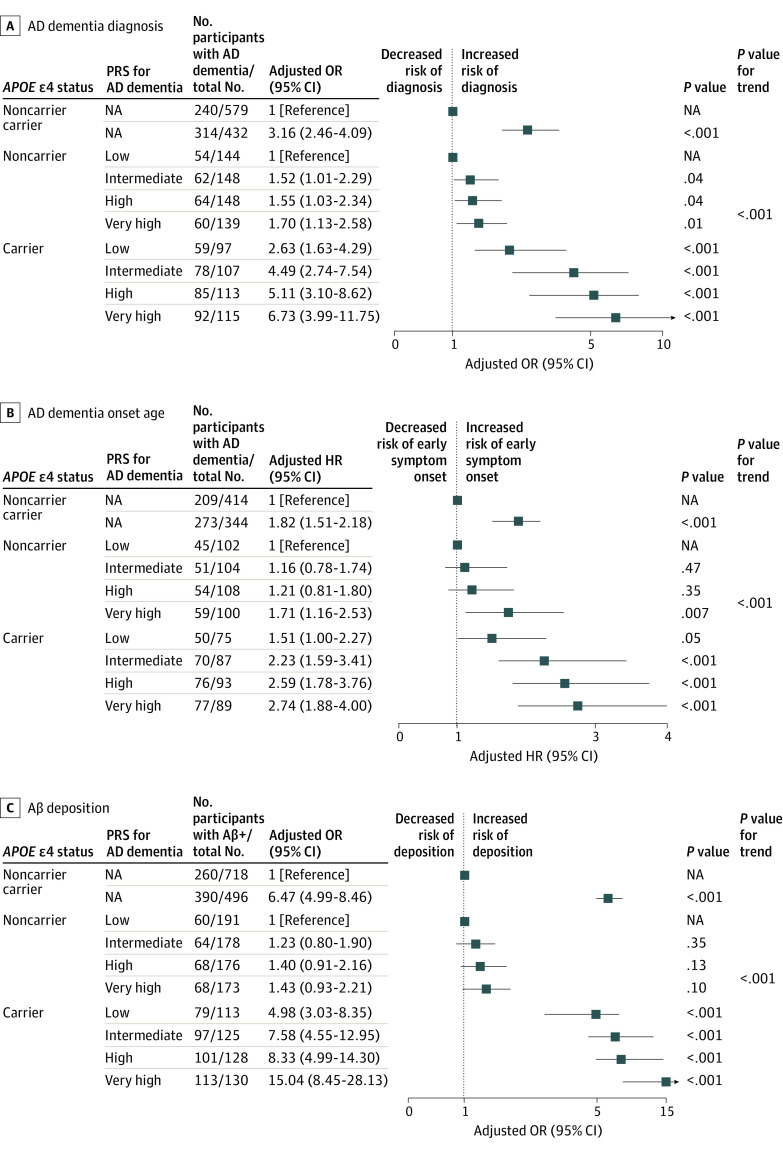
To evaluate the AD dementia risk using the PRS, we stratified the participants according to PRS quartiles. There were significant differences among the PRS risk groups in amyloid positivity, diagnosis, and age at symptom onset. Particularly, the mean age at symptom onset was approximately 3.7 years younger in the very high PRS group than in the low PRS group (mean [SD] of age at symptom onset: low PRS group, 69.0 [9.9] vs very high PRS group, 65.3 [9.7]) (eTable 2 in the Supplement). When we combined PRS and APOE ε4 status, we observed a stepwise increase in the risk of AD dementia, earlier age at symptom onset, and Aβ deposition according to the PRS quartile in both APOE ε4 carriers and noncarriers (Figure 3). Notably, compared with APOE ε4 noncarriers in the low PRS group, the APOE ε4 carriers in the very high PRS group showed a 6.73-fold (95% CI, 3.99-11.75), 2.74-fold (95% CI, 1.88-4.00), and 15.04-fold (95% CI, 8.45-28.13) higher risk for AD dementia, earlier age at symptom onset, and Aβ deposition, respectively.
Figure 3. Forest Plots of AD Dementia–Related Outcomes According to the PRS Group and APOE ε4 Status.
Aβ indicates amyloid β; AD, Alzheimer disease; HR, hazard ratio; OR, odds ratio; PRS, polygenic risk score. Adjustments made for the effect of sex, age, education year, and the first 4 principal components from genotyping data. P values for trend were determined using Cochran-Armitage tests.
Transferability of the PRS Based on the Japanese GWAS and Meta-analysis GWAS Data
We further evaluated the PRS derived from the Japanese GWAS (3962 AD cases and 4074 controls). Across the various thresholds (P and LD values), the highest Nagelkerke R2 value for PRS was 0.006 (P = .03) when the P and LD values were 5.00 × 10−8 and 0.1, respectively, which was smaller than PRS based on the European GWAS (Nagelkerke R2 = 0.020; P < .001). Next, we performed transancestry meta-GWAS from European and Japanese GWAS (eFigure 4 in the Supplement). When we developed PRS from the transancestry meta-GWAS, the transancestry PRS achieved the highest performance (Nagelkerke R2 = 0.023; P < .001) among other PRSs (eFigure 3, eTables 3 and 5 in the Supplement). In contrast to European population–based and transancestry PRS, Japanese population–based PRS showed the highest performance when using a single SNV (eTable 4 in the Supplement) and its estimation of AD dementia risk was not replicated in the data set 2 (eTable 5 in the Supplement).
Discussion
In this study, we demonstrated that a PRS derived from a prior GWAS of European ancestry was associated with AD dementia risk independent of APOE ɛ4 status in the Korean population. Furthermore, the PRS was associated with aMCI, earlier symptom onset of AD dementia, and Aβ deposition independent of APOE ɛ4 status.
Our results support the potential utility of a prior large-scale GWAS of one population in developing a PRS in another population. Consistent with our findings, previous studies have shown that a PRS for AD derived from European GWASs accurately estimated the dementia risk among non-Hispanic Black8,9 and Caribbean Hispanic individuals.10 In our multivariable logistic model, PRS was associated with the AD dementia risk independent of the APOE ɛ4 carrier status, which was replicated in the independent data set. We observed a stepwise increase in the risk of AD dementia with increased PRS quantiles (Figure 3). In our Korean population data set, the APOE ɛ4 status showed the highest effect size among the factors, including the PRS, confirming that the APOE ɛ4 status is an important risk factor for AD dementia across various ancestries.25 However, risk stratification based on the APOE ɛ4 status alone might be insufficient because individuals are classified into only 3 genotypes (APOE ɛ4 noncarrier, heterozygous carrier, and homozygous carrier), and this status does not provide sufficient explanation of the phenotypic variance of AD. In this regard, aside from the APOE genotype, PRS may further explain the phenotypic variance and represent the polygenicity of AD dementia. Notably, compared with APOE ε4 noncarriers in the low PRS group, the APOE ε4 carriers in the very high PRS group showed a 6.73-fold (95% CI, 3.99-11.75) higher risk for AD dementia. Therefore, PRS is expected to be a useful risk tool for assessing risk in addition to the APOE ɛ4 status in precision medicine.
We demonstrated that higher PRS was associated with increased risk of aMCI. This is consistent with previous findings that PRS for AD was associated with MCI.26,27 In addition, we observed that patients with higher PRS were more likely to develop AD dementia symptoms at a younger age. The mean age at symptom onset was approximately 3.7 years younger in the very high PRS group compared with the low PRS group. It is well known that APOE ɛ4 is associated with earlier symptom onset of AD dementia. Our results showed that PRS further indicates an acceleration in the age at symptom onset beyond the effect of APOE ɛ4. A previous study also showed that PRS derived from 23 genetic variants was associated with the age at symptom onset of AD dementia.28
Furthermore, we found a significant association between PRS and Aβ positivity independent of the APOE ɛ4 carrier status (Figure 3). When we stratified participants according to APOE genotype and PRS, we found that compared with APOE ε4 noncarriers in the low PRS group, the APOE ε4 carriers in the very high PRS group showed a 15.04-fold (95% CI, 8.45-28.13) higher risk for Aβ deposition (Figure 3). This is in line with previous findings,22,29,30,31,32,33,34 which showed that the PRS was associated with AD pathologies (Aβ deposition, τ, and neurodegeneration). Identifying patients with Aβ deposition is crucial in predicting prognosis and selecting patients for clinical trials of anti-Aβ therapy.35 Currently available diagnostic tools for measuring Aβ deposition are either invasive (cerebrospinal fluid examination) or expensive (PET).36 Our findings highlight that the genetic data (PRS and APOE ɛ4 status) obtained from less invasive methods (blood or saliva specimen evaluation) can be used to prescreen individuals for Aβ positivity. These findings indicate the potential use of the PRS to promote early intervention by early identification of individuals at an increased risk for AD dementia.
The performance of the PRS was low when it was developed based on a prior GWAS of the Japanese population (eFigure 3 in the Supplement), despite the closer genetic relatedness of Korean individuals with Japanese populations than with European populations, as shown in the 1000 Genomes Project data set (eFigure 1 in the Supplement). We speculated that this low performance could be attributed to the difference in the sample size of the GWAS (8036 Japanese patients vs 63 926 European patients). The GWAS with a larger sample size identified more SNVs, estimated more accurate β coefficients of each SNV, and further improved the performance of PRS compared with its counterpart. When we used the data from the transancestry meta-analysis of European and Japanese GWAS, the transancestry PRS achieved the highest Nagelkerke R2 value for AD dementia in the Korean population, indicating the importance of ancestral background as well. As an additional point of view, several studies have shown that different LD patterns could affect the transferability when a risk is assessed by tagged SNVs from different ancestral backgrounds.37,38,39,40 Thus, the sample size and ancestral background of prior GWASs are both important factors in developing PRS.
Limitations
This study has several limitations. First, the sample size of the Korean population was relatively small compared with that of the European population, which limited the statistical power to compare the effects of each variant between populations. Although this study was performed in thoroughly phenotyped subjects using clinical and neuroimaging data, our findings should be replicated in larger independent data sets. Second, the findings of this study were limited to the Korean population. Subsequent studies including other East Asian populations, such as Chinese or Japanese populations, may further strengthen the evidence for transancestry transferability of PRS in the East Asian population.
Conclusions
This cohort study found that a PRS derived from a European GWAS was associated with AD dementia independent of APOE ɛ4 status in the Korean population. Furthermore, it was associated with aMCI, earlier symptom onset of AD dementia, and Aβ deposition independent of APOE ɛ4 status. Our findings emphasize the ancestral transferability and clinical value of the PRS and further emphasizes the need for enriching diversity in genetic studies of AD.
eMethods.
eTable 1. Thirty-nine SNVs Used in the Best-fit PRS
eTable 2. Demographics of the Participants According to the PRS Quantiles
eTable 3. Characteristics of SNVs Selected for Transancestry PRS
eTable 4. Characteristics of SNVs Selected for Japanese-based PRS
eTable 5. Predictive Accuracy of PRSs Derived From European, Japanese, and Transancestry Meta-GWAS
eFigure 1. Principal Component Analysis Comparison of the Korean Study Cohorts and the 1000 Genomes Project Populations
eFigure 2. PRS Distributions Among the Study Participants According to the Genotyping Arrays
eFigure 3. Distribution Plots for Nagelkerke’s R2 Values of Each Population-based PRS Across the SNV Selection Thresholds
eFigure 4. Quantile-quantile Plot and Miami Plot for the Transancestry Meta-GWAS
eReferences.
References
- 1.Alzheimer’s Association . 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321-387. doi: 10.1016/j.jalz.2019.01.010 [DOI] [Google Scholar]
- 2.Wu YT, Brayne C, Matthews FE. Prevalence of dementia in East Asia: a synthetic review of time trends. Int J Geriatr Psychiatry. 2015;30(8):793-801. doi: 10.1002/gps.4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63(2):168-174. doi: 10.1001/archpsyc.63.2.168 [DOI] [PubMed] [Google Scholar]
- 5.Escott-Price V, Sims R, Bannister C, et al. ; GERAD/PERADES; IGAP consortia . Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain. 2015;138(Pt 12):3673-3684. doi: 10.1093/brain/awv268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet. 2019;51(4):584-591. doi: 10.1038/s41588-019-0379-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan L, Shen H, Gelaye B, et al. Analysis of polygenic risk score usage and performance in diverse human populations. Nat Commun. 2019;10(1):3328. doi: 10.1038/s41467-019-11112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marden JR, Walter S, Tchetgen Tchetgen EJ, Kawachi I, Glymour MM. Validation of a polygenic risk score for dementia in black and white individuals. Brain Behav. 2014;4(5):687-697. doi: 10.1002/brb3.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marden JR, Mayeda ER, Walter S, et al. Using an Alzheimer’s Disease polygenic risk score to predict memory decline in Black and White Americans over 14 years of follow-up. Alzheimer Dis Assoc Disord. 2016;30(3):195-202 . doi: 10.1097/WAD.0000000000000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sariya S, Felsky D, Reyes-Dumeyer D, et al. Polygenic risk score for Alzheimer’s disease in Caribbean Hispanics. Ann Neurol. 2021;90(3):366-376. doi: 10.1002/ana.26131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunkle BW, Grenier-Boley B, Sims R, et al. ; Alzheimer Disease Genetics Consortium (ADGC); European Alzheimer’s Disease Initiative (EADI); Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium (CHARGE); Genetic and Environmental Risk in AD/Defining Genetic, Polygenic and Environmental Risk for Alzheimer’s Disease Consortium (GERAD/PERADES) . Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019;51(3):414-430. doi: 10.1038/s41588-019-0358-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shigemizu D, Mitsumori R, Akiyama S, et al. Ethnic and trans-ethnic genome-wide association studies identify new loci influencing Japanese Alzheimer’s disease risk. Transl Psychiatry. 2021;11(1):151. doi: 10.1038/s41398-021-01272-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang J, Jeong JH, Yoon SJ, et al. Clinical and biomarker characteristics according to clinical spectrum of Alzheimer’s disease (AD) in the validation cohort of Korean Brain Aging Study for the Early Diagnosis and Prediction of AD. J Clin Med. 2019;8(3):341. doi: 10.3390/jcm8030341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee DY, Lee KU, Lee JH, et al. A normative study of the CERAD neuropsychological assessment battery in the Korean elderly. J Int Neuropsychol Soc. 2004;10(1):72-81. doi: 10.1017/S1355617704101094 [DOI] [PubMed] [Google Scholar]
- 15.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang SH, Park YH, Lee D, et al. The cortical neuroanatomy related to specific neuropsychological deficits in Alzheimer’s continuum. Dement Neurocogn Disord. 2019;18(3):77-95. doi: 10.12779/dnd.2019.18.3.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen RC. Clinical practice: mild cognitive impairment. N Engl J Med. 2011;364(23):2227-2234. doi: 10.1056/NEJMcp0910237 [DOI] [PubMed] [Google Scholar]
- 18.Roh HW, Kim N-R, Lee D-G, et al. Baseline Clinical and Biomarker Characteristics of Biobank Innovations for Chronic Cerebrovascular Disease With Alzheimer's Disease Study: BICWALZS. Psychiatry Investig. 2022;19(2):100-109. doi: 10.30773/pi.2021.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon S, Kim YJ, Han S, et al. The Korea Biobank Array: design and identification of coding variants associated with blood biochemical traits. Sci Rep. 2019;9(1):1382. doi: 10.1038/s41598-018-37832-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H-R, Jung S-H, Kim J, et al. Identifying novel genetic variants for brain amyloid deposition: a genome-wide association study in the Korean population. Alzheimers Res Ther. 2021;13(1):117. doi: 10.1186/s13195-021-00854-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190-2191. doi: 10.1093/bioinformatics/btq340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonenko G, Shoai M, Bellou E, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Genetic risk for Alzheimer disease is distinct from genetic risk for amyloid deposition. Ann Neurol. 2019;86(3):427-435. doi: 10.1002/ana.25530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonenko G, Baker E, Stevenson-Hoare J, et al. Identifying individuals with high risk of Alzheimer’s disease using polygenic risk scores. Nat Commun. 2021;12(1):4506. doi: 10.1038/s41467-021-24082-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a toolset for whole-genome association and population-based linkage analysis. American Journal of Human Genetics. 2007;81(3):559-575. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrer LA, Cupples LA, Haines JL, et al. ; APOE and Alzheimer Disease Meta Analysis Consortium . Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278(16):1349-1356. doi: 10.1001/jama.1997.03550160069041 [DOI] [PubMed] [Google Scholar]
- 26.Adams HH, de Bruijn RF, Hofman A, et al. Genetic risk of neurodegenerative diseases is associated with mild cognitive impairment and conversion to dementia. Alzheimers Dement. 2015;11(11):1277-1285. doi: 10.1016/j.jalz.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 27.Logue MW, Panizzon MS, Elman JA, et al. Use of an Alzheimer’s disease polygenic risk score to identify mild cognitive impairment in adults in their 50s. Mol Psychiatry. 2019;24(3):421-430. doi: 10.1038/s41380-018-0030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Lee SJ, Wolters FJ, Ikram MK, et al. The effect of APOE and other common genetic variants on the onset of Alzheimer’s disease and dementia: a community-based cohort study. Lancet Neurol. 2018;17(5):434-444. doi: 10.1016/S1474-4422(18)30053-X [DOI] [PubMed] [Google Scholar]
- 29.Zettergren A, Najar J, Kern S, et al. Association between polygenic risk score of Alzheimer’s disease and CSF amyloid beta 42 in a cohort of 70-year-olds from the general population: genetics/endophenotypes. Alzheimers Dement. 2020;16(suppl_S3):e042616. doi: 10.1002/alz.042616 [DOI] [Google Scholar]
- 30.Tan CH, Bonham LW, Fan CC, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Polygenic hazard score, amyloid deposition and Alzheimer’s neurodegeneration. Brain. 2019;142(2):460-470. doi: 10.1093/brain/awy327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darst BF, Koscik RL, Racine AM, et al. Pathway-specific polygenic risk scores as predictors of amyloid-β deposition and cognitive function in a sample at increased risk for Alzheimer’s disease. J Alzheimers Dis. 2017;55(2):473-484. doi: 10.3233/JAD-160195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge T, Sabuncu MR, Smoller JW, Sperling RA, Mormino EC; Alzheimer’s Disease Neuroimaging Initiative . Dissociable influences of APOE ε4 and polygenic risk of AD dementia on amyloid and cognition. Neurology. 2018;90(18):e1605-e1612. doi: 10.1212/WNL.0000000000005415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan CH, Fan CC, Mormino EC, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Polygenic hazard score: an enrichment marker for Alzheimer’s associated amyloid and tau deposition. Acta Neuropathol. 2018;135(1):85-93. doi: 10.1007/s00401-017-1789-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altmann A, Scelsi MA, Shoai M, et al. A comprehensive analysis of methods for assessing polygenic burden on Alzheimer’s disease pathology and risk beyond APOE. Brain Commun. 2020;2(1):fcz047. doi: 10.1093/braincomms/fcz047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jack CR Jr, Bennett DA, Blennow K, et al. ; Contributors . NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fargo KN, Carrillo MC, Weiner MW, Potter WZ, Khachaturian Z. The crisis in recruitment for clinical trials in Alzheimer’s and dementia: an action plan for solutions. Alzheimers Dement. 2016;12(11):1113-1115. doi: 10.1016/j.jalz.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 37.Cavazos TB, Witte JS. Inclusion of variants discovered from diverse populations improves polygenic risk score transferability. HGG Adv. 2021;2(1):100017. doi: 10.1016/j.xhgg.2020.100017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathieson I. The omnigenic model and polygenic prediction of complex traits. Am J Hum Genet. 2021;108(9):1558-1563. doi: 10.1016/j.ajhg.2021.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sirugo G, Williams SM, Tishkoff SA. The missing diversity in human genetic studies. Cell. 2019;177(1):26-31. doi: 10.1016/j.cell.2019.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu CC, Yu K, Ketkar S, Templeton AR, Rao DC. On transferability of genome-wide tagSNPs. Genet Epidemiol. 2008;32(2):89-97. doi: 10.1002/gepi.20269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Thirty-nine SNVs Used in the Best-fit PRS
eTable 2. Demographics of the Participants According to the PRS Quantiles
eTable 3. Characteristics of SNVs Selected for Transancestry PRS
eTable 4. Characteristics of SNVs Selected for Japanese-based PRS
eTable 5. Predictive Accuracy of PRSs Derived From European, Japanese, and Transancestry Meta-GWAS
eFigure 1. Principal Component Analysis Comparison of the Korean Study Cohorts and the 1000 Genomes Project Populations
eFigure 2. PRS Distributions Among the Study Participants According to the Genotyping Arrays
eFigure 3. Distribution Plots for Nagelkerke’s R2 Values of Each Population-based PRS Across the SNV Selection Thresholds
eFigure 4. Quantile-quantile Plot and Miami Plot for the Transancestry Meta-GWAS
eReferences.