Abstract
Brain-computer interface (BCI) can be used as a real-time bidirectional information gateway between the brain and machines. In particular, rapid progress in invasive BCI, propelled by recent developments in electrode materials, miniature and power-efficient electronics, and neural signal decoding technologies has attracted wide attention. In this review, we first introduce the concepts of neuronal signal decoding and encoding that are fundamental for information exchanges in BCI. Then, we review the history and recent advances in invasive BCI, particularly through studies using neural signals for controlling external devices on one hand, and modulating brain activity on the other hand. Specifically, regarding modulating brain activity, we focus on two types of techniques, applying electrical stimulation to cortical and deep brain tissues, respectively. Finally, we discuss the related ethical issues concerning the clinical application of this emerging technology.
Keywords: invasive, BCI, decode, encode, modulate, ICMS, DBS
1. Introduction
Brain–computer interface (BCI) or brain–machine interface (BMI) is a gateway for information communication between the brain and computers that does not depend on the regular input and output pathways composed of sensory organs, peripheral nerves, and muscles [1]. It aims at restoring or enhancing brain functions by establishing an effective channel of information transmission between external devices, e.g., computers, and the nervous system.
Depending on how to recode neural signals, BCI is divided into two categories: non-invasive BCI and invasive BCI [2]. Non-invasive BCIs collect information about brain activity without requiring brain surgery by utilizing methods such as electroencephalography (EEG), magnetoencephalography (MEG), functional magnetic resonance imaging (fMRI), and functional near-infrared spectroscopy (fNIRS). The most popular non-invasive BCI technique is EEG, which records electrical brain activity signals using scalp electrodes. In contrast, invasive BCI records activity via surgically implanted electrodes close to the target neurons in the cortex or/and deep brain structures using, for example, microelectrode array (MEA), electrocorticography (ECoG) electrodes, stereo-electroencephalography (sEEG) electrodes, and deep brain stimulation (DBS) electrodes. MEA, which is typically placed within the gray matter of the cerebral cortex, is capable of detecting neuronal action potentials (also known as spikes) generated from a single or multiple neurons. ECoG electrodes (placed either below the dura matter, i.e., subdural, or above it, i.e., epidural) and sEEG electrodes record local field potentials (LFP), while DBS electrodes deliver electrical currents to activate or inactivate neuronal populations close by [3].
Invasive BCI, in comparison to non-invasive BCI, has essential advantages: (1) it can obtain neural signals with a much higher spatial and temporal resolution [4], e.g., to record activities from individual neurons or to modulate the activities of a small population of neurons; (2) it has a higher signal-to-noise ratio (SNR) and is more robust against electrical noise interferences or movement artifacts; and (3) its electrodes can be placed very close to or directly in the target cortical areas or subcortical structures, which is of paramount importance for developing BCI capable of decoding particular information and modulating specific brain functions. Of course, the disadvantages or limitations of the current invasive BCI technology are equally obvious. First, the implantation of electrodes directly into neural tissues requires a surgical operation that is invasive by itself and increases the risk of complications. Second, the system, once being implanted, is difficult to fix hardware problems or to update any component, therefore requiring much higher reliability and reducing a certain degree of flexibility. Finally, due to the complexity of the surgical procedure itself and the necessary care afterwards, invasive BCI is expensive, which needs to be addressed in order to increase its accessibility. Overall, the advantages of invasive BCI are more fundamental and its limitations may well be overcome through technological innovations. Thus, invasive BCI has great development potential and is a very promising technology for both basic neuroscience research and clinical application, particularly for modulating brain activity.
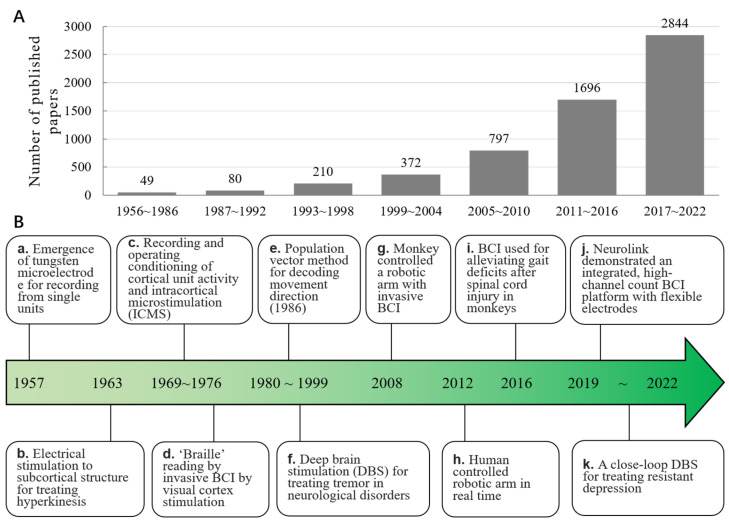
Performing a straightforward search in the MEDLINE (PubMed) database reveals a rapid expansion of the literature on invasive BCI, particularly in the past two decades. With a specific search formula: (((((brain-computer interface [Title/Abstract]) OR (brain machine interface [Title/Abstract])) AND (intracranial [Title/Abstract])) OR (invasive [Title/Abstract])) AND (cortex [Title/Abstract])) OR (deep brain [Title/Abstract]), 20,263 outcomes are available for the years 1956 to 2002. The results are shown in Figure 1a, indicating an obvious upward trend of published papers related to cortical and deep brain BCI since the 1950s. In Figure 1b, we summarize a timeline of important milestones and representative developments in this field.
Figure 1.
The progression of invasive BCI. (A). The number of published papers over time. A rapid growth in the number of papers in recent (20) years demonstrates the rapidly increasing interest in invasive BCI technology. (B). The historical timeline for major breakthroughs and representative developments in invasive BCI. The references cited in a–k correspond to the following: a [5], b [6], c [7,8], d [9], e [10], f [11,12,13,14], g [15], h [16], i [17], j [18], and k [19].
Based on the direction of information flow between the brain and machines, invasive BCI can be further categorized into two classes: controlling external devices using recorded brain signals, sometimes called “control by the brain”, and modulating neural activities based on external signals, or sometimes called “modulation of the brain”. To achieve accurate “control by the brain” or “modulation of the brain”, it is fundamental to decode and encode neural activity correctly for understanding the meaning of brain signals, and how to modulate them for specific purposes. For example, by decoding the signals generated from brain activity, one can control external devices, including a cursor [20,21,22], robotic arm [16], etc., which has a great value for neural rehabilitation or functional compensation for patients with movement deficits due to stroke, or injury to the brain or the spinal cord [23]. On the other hand, by properly encoding external information to modulate brain activity, one can develop effective therapeutic techniques to deal with brain diseases, such as Parkinson’s disease, depression, etc., and reconstruct sensory function for people with visual or hearing disabilities.
For modulating brain activity by invasive BCI, according to the implant location of the electrodes, the technologies can be classed as cortical stimulation (focus on the cortex) and deep brain stimulation (focus on the deep brain tissues), which have been called intracortical microstimulation (ICMS) [24] and deep brain stimulation (DBS) [25], respectively. To date, ICMS has been mainly used to perform sensory feedback with high spatiotemporal precision [26,27,28], while DBS has been applied primarily to treat neurologic and neuropsychiatric disorders [29,30,31].
This review is aimed at helping researchers interested in BCI, particularly clinicians, to understand how invasive BCI can be used for modulating brain activities. To this end, we first introduce the concept of neural decoding and encoding, then discuss the progress in their applications by using invasive BCI, especially in modulating brain activity based on both ICMS and DBS. Moreover, the promising directions of invasive BCI, especially the closed-loop, real-time bidirectional BCI [32,33], are analyzed. Finally, the ethical issues related to clinical applications of invasive BCI are discussed.
2. The Framework of Neural Decoding and Encoding
Encoding and decoding are essential to understand how the brain works and interacts with outside environments, which is fundamental for accurate “control by the brain” or “modulation of the brain” by BCI. After Edgar Adrian successfully recorded the spikes produced by sensory nerves and reckoned that the firing rate of neurons could be an expression of external information [34], the study of neural decoding and encoding has become an essential field in neuroscience (see [35,36] for recent examples). In a nutshell, neural decoding and encoding are similar to looking up information in a dictionary. Decoding corresponds to retrieving the meaning of a specific word, while encoding corresponds to finding a specific word according to its meaning.
Specifically, the encoding of neural information typically refers to the representation of external stimuli by the activities of neurons. Decoding of neural information is to infer the characteristics of either external stimuli information or controlling commands from the observed neural activities. Decoding and encoding are closely connected and can be quantitatively formulated by the Bayesian theory. Let represent the occurring probability of a specific external stimulus represent the probability of observing a specific neural activity pattern. Thus, the likelihood of detecting activity pattern r when the stimulus s presents can be denoted as , known as conditional probability. Based on the Bayesian theory, we have
| (1) |
To solve the neural encoding problem is to calculate , which can be computed as
| (2) |
To solve the neural decoding problem is to calculate , which can be computed as
| (3) |
The above-mentioned analysis can be directly extended to calculate the distribution of neural activities, namely:
| (4) |
where sn represents the nth external stimulus, and the external stimuli, namely:
| (5) |
where ri represents the ith neural activity pattern.
Based on the encoding–decoding framework described above, modulating neural activities for a specific purpose, for instance, hacking the brain to mistakenly perceive a specific stimulus s which does not actually exist, is to modulate the activity pattern to resemble r, which satisfies
| (6) |
Similarly, to decode the meaning of activity pattern r is to find the stimulus s that satisfies
| (7) |
3. Application of Decoding for Controlling External Devices
Various invasive BCIs have been developed to control a robotic arm by decoding the neural activity of the primary motor cortex (M1) [16]. It is a promising way to help disabled persons improve their quality of life by inserting electrodes close to the neurons of brain tissues where the spikes of neurons’ firing can be recorded with ~1 ms resolution. Different from turning on/off external devices [37], it is desirable that “free manipulation” of robotic arms via invasive BCIs can be achieved, for which appropriate decoding methods are the key. Many decoding methods are effective in predicting arm movements, such as population vector algorithms [38], optimal linear estimators [39], sliced inverse regression [40], the Bayesian decoder [41] and Kalman filter [42], which are used to predict the movement direction, speed, and trajectory. Besides decoding accuracy, another important factor needs to be considered: the ability to control robotic arms in real-time.
The real-time control was developed by decoding M1 activities in monkeys and using them to control a cursor movement without preliminary training [43,44]. Schwartz et al. optimized the decoding of the monkey’s M1 to achieve more precise manipulation of the robotic arms, which included movement in the 3D space and gripping force [15]. In humans, a tetraplegic patient controlled the cursor movement on a screen in real time with the assistance of invasive BCI [45], which was proved chronically safe [46]. In 2012, a patient with tetraplegia successfully drank coffee by using an invasive BCI to control the robotic arm, which was a breakthrough in clinical application [16]. Recently the invasive BCI has been used to help patients use a bimanual robotic limbs system [47]. Although a robotic arm controlled by invasive BCIs will be a replacement for rehabilitation of the lost motor function in the future, it is necessary to combine it with perceptual feedback for better control [48]. Invasive BCIs with perceptual feedback and bidirectional, closed-loop motor control will be progressed using ICMS and DBS technologies.
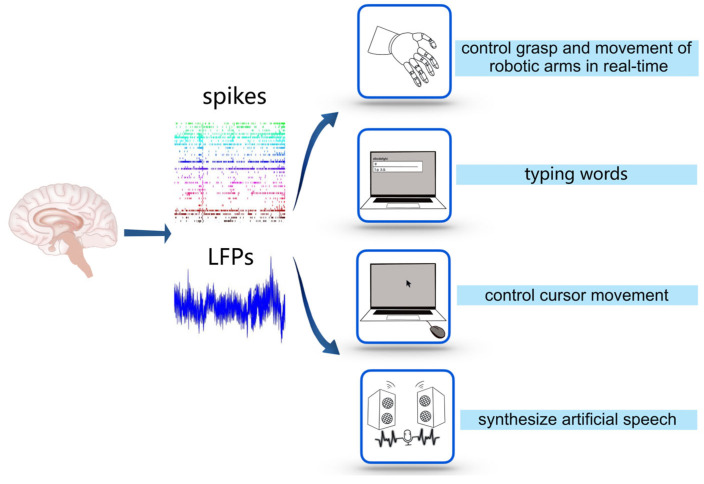
Recently, invasive BCIs have been developed to accomplish other functions along with controlling robotic arms (see Figure 2). Capogrosso et al. used an invasive BCI to alleviate the gait deficits of macaques with a spinal cord injury by decoding the motor states from M1 and transmitting them to an electrical stimulator in the spinal cord [17]. Gopala et al. used an invasive BCI to synthesize the correct artificial speech when the subjects imitated sentences silently by recurrent neural networks to decode the neural activity of the cortex into a representation of articulatory movement and transform it into speech acoustics (see Figure 2) [49]. Francis et al. decoded the intention of a patient who was trying to write letters from the neural activity of the motor cortex by the recurrent neural network and converted it into text in real time by implanting microelectrode arrays into the motor cortex. Using this invasive BCI, patients achieved a typing speed of 90 characters/min, with an online original accuracy of 94.1% and offline automatic correction accuracy of more than 99% [50], while the typing speed on typical smartphones is about 115 characters/min (see Figure 2). Dekleva et al. developed a new decoding approach for cursor point-and-click based on identifying hand grasp, which provides a high-performance cursor control [51] (see Figure 2). It is noteworthy that, in recent decoding studies, the methods based on artificial neural networks have attracted much attention [52,53], as they provide a promising way to combine deep learning with BCI to produce accurate estimation/prediction using spikes [54,55,56,57] or intracortical LFPs [58,59,60,61].
Figure 2.
Applications of decoding. The application of decoding is a process from recording electrical signals of brain activity, such as spikes and LFPs, to using them to control external devices.
4. Modulating Cortical Activity by ICMS
Ever since Wilder Penfield mapped the “cortical homunculus” in the motor cortex using electrical stimulation [62], many scholars have used ICMS technology to investigate more precise motor cortex and sensory cortex maps [63,64,65] and have pointed out a clear direction to regulate visual–motor function. As a method for injecting information into the cortex, Salzman et al. used ICMS to stimulate selective neurons in the middle temporal visual area (MT or V5) to change the visual motor information processing in macaques, making their eyes more oriented towards the motion encoded by the stimulated neurons [66]. Fujii et al. used ICMS to stimulate the frontal eye field (FEF) of macaques to interfere with the gaze task. They found that stimulating unilateral FEF and bilateral FEF induced a reverse saccade and a two-step saccade, respectively [67]. Overall, ICMS has been proven as an effective method to understand the functional role of cortical activity and establish a basis for manipulating sensory perception through modulating cortical activity by invasive BCIs.
4.1. ICMS for Restoration of Tactile Feedback
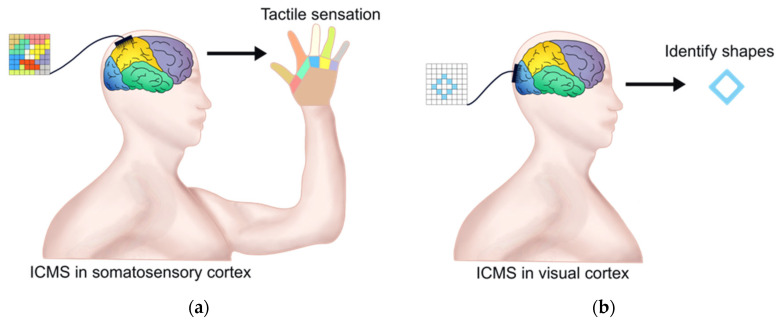
The fundamental research on the primary somatosensory cortex (S1) by ICMS is vital for invasive BCIs with perceptual feedback. Romo et al. trained two macaques to distinguish between two mechanical flutter stimuli applied sequentially to the fingertips. Microelectrodes were inserted into clusters of quickly adapting (QA) neurons of the S1, and the first or both stimuli were then substituted with trains of current pulses during the discrimination task. It was found that microstimulation can be used to elicit a memorizable and discriminable analog range of percepts, and the activation of the QA circuit of S1 was sufficient to initiate all the subsequent neural processes associated with flutter discrimination [68]. Recently, Flesher et al. used ICMS to evoke the hand sensation of patients with long-term spinal cord injuries. By applying ICMS in the hand area of the somatosensory cortex, they successfully evoked the pressure sensitivity of all parts of the fingers and maintained stability for a long time (see Figure 3a). In addition, adjusting the stimulus amplitude classified the perceptual intensity of the stimuli, indicating that ICMS could be used to convey information about the contact position and pressure required for hand movement [69]. In addition, chronic ICMS may be a viable means of transmitting sensory feedback into neural prostheses without inducing significant damage [70].
Figure 3.
ICMS in restoring tactile sensation and vision. (a) ICMS with the microelectrode array in the primary somatosensory cortex can evoke tactile sensation of hand. The left colorful square represents the microelectrode array and the color is the ICMS area relative to tactile sensation of the hand area with the same color; (b) ICMS with microelectrode array in the visual cortex can help patients to identify some letters and object boundaries. The left square represents the microelectrode array and the blue rhombus within it represents the ICMS area, which relates to the optical illusion.
Using ICMS, an invasive BCI could be a brain–machine–brain interface (BMBI) [71], which controls the exploratory arrival movement of the actuator and facilitates artificial tactile feedback. In 2009, Doherty et al. trained macaques to move the cursor on the screen by hand, then to control the virtual robotic arms through the neural activity in M1 and dorsal premotor cortex (PMd) and established a correlation between touching virtual objects and ICMS in S1. The macaques successfully underwent BMBI to find and distinguish three visual objects and successfully identified each object through the virtual robotic arm [48]. Richard et al. also demonstrated that BMBI with ICMS in S1 can provide tactile sensation [72]. Recently, Flesher et al. supplemented vision with tactile perception evoked using a bidirectional BCI that records neural activity from the motor cortex and generates tactile sensations through intracortical microstimulation of the somatosensory cortex, enabling subjects with tetraplegia to substantially improve performance with a robotic limb [73]. For amputees, a neural–machine-interface prosthesis with sensory feedback directly on a missing limb in real-time is widely used [74,75,76,77,78].
4.2. ICMS for Restoration of Visual Sense
Along with restoring tactile feedback by ICMS in S1, some scholars attempted to modulate the visual cortical activity to restore the visual sense. In 1968, Brindley and Lewin implanted an electrode array in the occipital lobe of the right hemisphere of a blind patient, and she could then feel light in the left half of the field of vision after ICMS [79]. Dobelle et al. found that ICMS could produce a sense of visualizing light spots (phosphenes), which appeared immediately at the beginning of stimulation and disappeared immediately after cessation of stimulation [80]. Schmidt et al. found that the visual sensation produced by ICMS was more evident in the report of a blind patient, and even color could be produced [81]. Edward et al. used ICMS in the primary visual cortex (V1) of macaques, revealing that the size of the phosphene depended on the current transmitted to V1 and the retinal cortical magnification factor [82]. These findings are valuable in the direction of the application of ICMS in the visual cortex for regaining sight and developing visual prostheses [83].
Recently, Chen et al. successfully realized the modulation of the activity of the visual cortex through invasive BCI. They implanted Utah electrodes (a total of 1024 channels) in the V1 and V4 regions of the visual cortex of monkeys and used ICMS to trigger optical illusions by injecting currents through hundreds of electrodes. Meanwhile, the positions of these electrodes were mapped with the receptive fields of stimulated neurons. Finally, they encoded the patterns composed of several optical illusions and simultaneously stimulated multiple visual cortical neurons through ICMS. Monkeys could immediately recognize them as simple shapes, letters, etc. [84]. Recently, a 96-channel microelectrode array was implanted into the visual cortex of a 57-year-old patient with complete blindness for 6 months. The research team developed an invasive BCI with ICMS in the occipital cortex and successfully helped the patient to identify some letters and object boundaries (see Figure 3b) [85]. These results confirmed the potential of invasive BCI for restoring functional vision.
5. Modulating Brain Activity by DBS
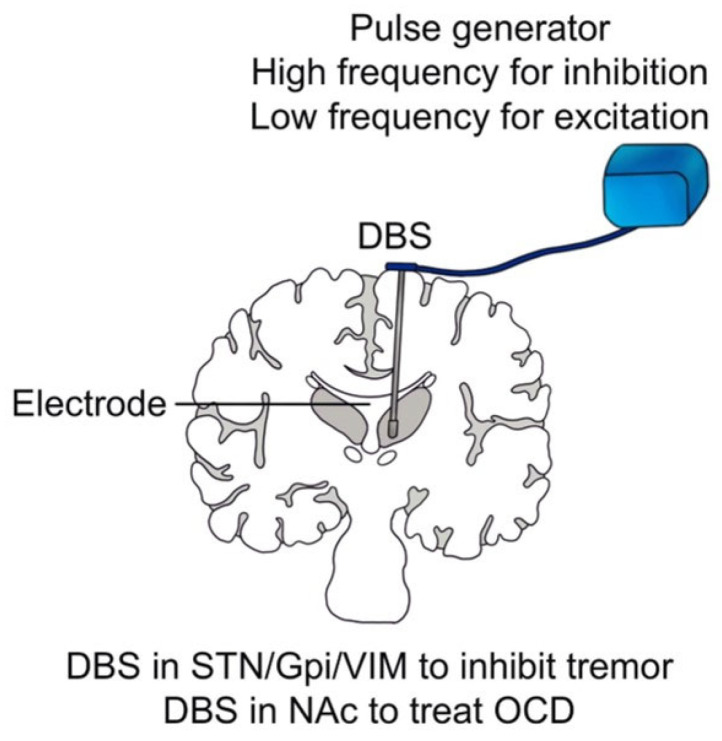
Conventionally, DBS is composed of an electrode and a pulse generator whose stimulation parameters, including amplitude, frequency, pulse duration, and pulse width, are controlled by a computer (see Figure 4). However, unlike ICMS, whose effective stimulation current ranges from 40 to 180 μA to target a relatively small number of neurons, the effective stimulation current of DBS ranges from 1 to 40 mA [86], aiming at manipulating a large group of neurons. Scholars have mainly used DBS to treat neurologic and neuropsychiatric conditions, including essential tremor (ET), dystonia, Parkinson’s disease (PD), and refractory obsessive-compulsive disorder (OCD) [87], which have been approved by the Food and Drug Administration (FDA).
Figure 4.
Framework and clinical application of DBS. Framework and clinical application of DBS. The blue bag represents the pulse generator and the stick in brain tissue represents electrode of DBS.
5.1. DBS for Neurological Disorders
Brice et al. found that the DBS for contralateral midbrain and basal ganglia could inhibit intentional tremor in three patients, and then they implanted permanent electrodes in two patients. During the half-year follow-up, the therapeutic effect of the DBS remained without changing the electrical threshold [11]. Benabid et al. relieved tremors in 26 patients with PD and 6 patients with ET through high-frequency (130 Hz) stimulation of the ventral intermediate nucleus (VIM) [88]. They found that VIM-DBS could effectively inhibit tremors and replace thalamic surgeries in the treatment of PD and ET [12]. They also noted that DBS for the subthalamic nucleus (STN) could reduce the dosage of administrated medicines by 30–50%, and DBS for the globus pallidus internus (GPi) could inhibit abnormal involuntary movements [13]. Recent studies have also shown that the GPi-DBS could effectively treat dystonia [89], Huntington’s disease [90], and Tourette syndrome [91]. Although the therapeutic effect of DBS on tremors is noticeable, the underlying mechanism has remained elusive. Benazzouz et al. found that high-frequency stimulation of the STN reduced the excitatory glutamate output, resulting in the inactivation of the reticular neurons of black substance, which may be the mechanism of inhibiting tremors by DBS [92]. Boraud et al. found that high-frequency stimulation of GPi could significantly reduce the high discharge rate of GPi, thereby alleviating the tetany and motor symptoms of PD [14]. Some researchers also pointed out that high-frequency stimulation of GPi may interrupt the abnormal pattern of thalamic discharge [93]. Common targets of DBS for tremors include STN, GPi, and VIM (see Figure 4) [94,95]. Besides, DBS has been recently applied to treat refractory epilepsy [96,97].
5.2. DBS for Neuropsychiatric Disorders
In recent years, DBS has also been used for treatment-resistant depression (TRD), addiction, anorexia, OCD, schizophrenia, and other mental diseases, which has shown good efficacy when targeting the nucleus accumbens (NAc) [98,99,100,101]. Recent research has shown that DBS in the internal capsule [102] and caudate nucleus [103] effectively treat OCD (see Figure 4). In addition, DBS in the amygdala can effectively alleviate post-traumatic stress disorder [104,105,106]. Castillo et al. found that DBS in the globus pallidus externus can effectively treat insomnia in patients with PD [107]. Recently, Scangos et al. have effectively treated severe depression through a closed-loop invasive BCI. In this study, researchers first identified specific biomarkers of depression and potential treatment sites by observing deep brain signals. Then, a closed-loop BCI system was developed by using the biomarkers detected in the brain signals to modulate the stimulators chronically implanted in the deep brain area, achieving controlled regulation of the biomarkers. The depressive symptoms of the patient were relieved during the one-year period of the treatment [19]. It is noteworthy that developing closed-loop invasive BCI will be one of the leading research directions for the clinical treatment of some neuropsychiatric disorders [108].
6. Technological Challenges and Future Directions
Although this review focuses on invasive BCI techniques, it is noteworthy that cortical and deep brain regions can also be stimulated at the macroscopic level using non-invasive methods, such as transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), and transcranial focused ultrasound (tFUS), in order to treat neurodegenerative diseases and neuropsychiatric disorders [109,110,111,112]. A new method of stimulating cortical regions at the microscopic level is optogenetics. It works by utilizing light to activate neurons that have been either genetically manipulated or infected by specific viruses to express light-sensitive ion channels, which is currently only used in animal research [113,114].
ICMS and DBS, particularly DBS technology, have been used with success to treat certain neuropsychiatric and neurological disorders. However, there are still two main significant technical obstacles to be overcome for the development of invasive BCIs: (1) to minimize tissue damage due to electrode implantation, (2) to prolong the periods in which electrodes can effectively work in vivo. Currently, invasive BCI, especially ICMS-based technology, still needs complicated craniotomy to temporally remove a relatively large piece of skull, which adds more risks to both the surgery itself and later recovery. Novel implantation techniques that can minimize the wound and simplify the procedure will be very helpful. In addition, widely-used silicon-based electrodes irritate the surrounding tissues after implantation. In the longer term, this can cause cellular responses that change the local environments, resulting in (1) encapsulation of the electrodes by glia cells and deterioration of the recorded signals, and (2) neural cell death close to the implantation site [3]. Materials that are more biocompatible will be necessary for the development of electrodes in the future, e.g., the flexible electrode which reduces damage to brain tissue and foreign body reactions [115]. Neuralink recently demonstrated flexible electrodes named “treads”, which are inserted in the cortex through specially designed surgical robots [18]. Another interesting electrode introduced recently is called a “stentrode”, which is delivered endovascularly to be close to the target area to record signals [21]. Similar techniques may also be used for DBS [116].
7. Ethical Issues of Invasive BCI
Clinical research on invasive BCI is currently being used to treat diseases that cannot be treated with drugs, such as paralysis, muscular dystrophy, and others, by allowing patients to control assistive devices, regain important daily motor functions or lost sensations. It can also be used as a complementary treatment to drug-based therapies for neurological and neuropsychiatric disorders. Although invasive BCI has enormous potential in clinical applications, its surgical complications, such as bleeding, immunologic rejection, infection, and mechanical failure [117], need to be treated with more caution. In particular, the precise localization that is important for electrode implantation and the comprehensive understanding of neural circuits, which is important to modulate brain activity through invasive BCI, are still potential problems that may induce side effects over time. In addition, other factors, such as the psychological acceptance of the patient for invasive BCI, privacy, etc., need to be weighed. The following notes should be considered in the clinical application of invasive BCI: 1. The specific medical benefits, such as diagnosis, cure, palliative treatment, or prevention. 2. Possible damage and side effects. 3. Assistance to the patients to better understand invasive BCI and improve their psychological acceptance. 4. Assistance to the patients to more accurately understand the prognosis after BCI treatment. 5. Providing solutions for the failure of invasive BCI.
Currently, invasive BCI is in the early stages, especially in modulating brain activity. In the future, with the development of decoding and encoding research, electrodes, implantation methods, and other technologies, as well as the mature encoding and decoding technologies of brain activity, invasive BCI can effectively read and even “write” information to the brain. It will face the risks of privacy disclosure and malicious interference [118]. Therefore, designing a series of hardware and software is essential to protect the safety and security of invasive BCI. As suggested by Zeng et al., it should be people-oriented and protect user privacy. Comprehensive and strict standards should be established for identity authentication, information encryption, and system protection. Besides, it is crucial to prohibit abuse and misuse, and develop intrusion detection systems as well as the corresponding protection mechanisms [119]. The formulation of ethical principles is the key to the responsible development of invasive BCI in clinical applications.
Author Contributions
Review conception: Z.-P.Z.; manuscript writing: Z.-P.Z. and S.Y.; J.-W.G.; C.N., C.-T.J., S.-H.C. and K.-X.T. discussed and contributed to improving the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data were used to support this study.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported in part by the National Key Research and Development Program of China under Grant 2021ZD0200402, and the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS) under Grant XDB32040200.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wolpaw J.R., Birbaumer N., Heetderks W.J., McFarland D.J., Peckham P.H., Schalk G., Donchin E., Quatrano L.A., Robinson C.J., Vaughan T.M. Brain-computer interface technology: A review of the first international meeting. IEEE Trans. Rehabil. Eng. 2000;8:164–173. doi: 10.1109/TRE.2000.847807. [DOI] [PubMed] [Google Scholar]
- 2.Lebedev M.A., Nicolelis M.A. Brain-Machine Interfaces: From Basic Science to Neuroprostheses and Neurorehabilitation. Physiol. Rev. 2017;97:767–837. doi: 10.1152/physrev.00027.2016. [DOI] [PubMed] [Google Scholar]
- 3.Moran D. Evolution of brain-computer interface: Action potentials, local field potentials and electrocorticograms. Curr. Opin. Neurobiol. 2010;20:741–745. doi: 10.1016/j.conb.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saha S., Mamun K.A., Ahmed K., Mostafa R., Naik G.R., Darvishi S., Khandoker A.H., Baumert M. Progress in Brain Computer Interface: Challenges and Opportunities. Front. Syst. Neurosci. 2021;15:578875. doi: 10.3389/fnsys.2021.578875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubel D.H. Tungsten Microelectrode for Recording from Single Units. Science. 1957;125:549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- 6.Bekhtereva N.P., Grachev K.V., Orlova A.N., Iatsuksl Utilization of multiple electrodes implanted in the subcortical structure of the human brain for the treatment of hyperkinesis. Zh. Nevropatol. Psikhiatr. Im. S. S. Korsakova. 1963;63:3–8. [PubMed] [Google Scholar]
- 7.Fetz E.E. Operant conditioning of cortical unit activity. Science. 1969;163:955–958. doi: 10.1126/science.163.3870.955. [DOI] [PubMed] [Google Scholar]
- 8.Asanuma H., Arnold A., Zarzecki P. Further study on the excitation of pyramidal tract cells by intracortical microstimulation. Exp. Brain Res. 1976;26:443–461. doi: 10.1007/BF00238820. [DOI] [PubMed] [Google Scholar]
- 9.Dobelle W.H., Mladejovsky M.G., Evans J.R., Roberts T.S., Girvin J.P. “Braille” reading by a blind volunteer by visual cortex stimulation. Nature. 1976;259:111–112. doi: 10.1038/259111a0. [DOI] [PubMed] [Google Scholar]
- 10.Georgopoulos A.P., Schwartz A.B., Kettner R.E. Neuronal population coding of movement direction. Science. 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- 11.Brice J., McLellan L. Suppression of intention tremor by contingent deep-brain stimulation. Lancet. 1980;1:1221–1222. doi: 10.1016/S0140-6736(80)91680-3. [DOI] [PubMed] [Google Scholar]
- 12.Benabid A.L., Pollak P., Gao D., Hoffmann D., Limousin P., Gay E., Payen I., Benazzouz A. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J. Neurosurg. 1996;84:203–214. doi: 10.3171/jns.1996.84.2.0203. [DOI] [PubMed] [Google Scholar]
- 13.Benabid A.L., Benazzouz A., Hoffmann D., Limousin P., Krack P., Pollak P. Long-term electrical inhibition of deep brain targets in movement disorders. Mov. Disord. 1998;13((Suppl. 3)):119–125. doi: 10.1002/mds.870131321. [DOI] [PubMed] [Google Scholar]
- 14.Boraud T., Bezard E., Bioulac B., Gross C. High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci. Lett. 1996;215:17–20. doi: 10.1016/S0304-3940(96)12943-8. [DOI] [PubMed] [Google Scholar]
- 15.Velliste M., Perel S., Spalding M.C., Whitford A.S., Schwartz A.B. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098–1101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- 16.Hochberg L.R., Bacher D., Jarosiewicz B., Masse N.Y., Simeral J.D., Vogel J., Haddadin S., Liu J., Cash S.S., van der Smagt P., et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capogrosso M., Milekovic T., Borton D., Wagner F., Moraud E.M., Mignardot J.B., Buse N., Gandar J., Barraud Q., Xing D., et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 2016;539:284–288. doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musk E., Neuralink An Integrated Brain-Machine Interface Platform with Thousands of Channels. J. Med. Internet Res. 2019;21:e16194. doi: 10.2196/16194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scangos K.W., Khambhati A.N., Daly P.M., Makhoul G.S., Sugrue L.P., Zamanian H., Liu T.X., Rao V.R., Sellers K.K., Dawes H.E., et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat. Med. 2021;27:1696–1700. doi: 10.1038/s41591-021-01480-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacher D., Jarosiewicz B., Masse N.Y., Stavisky S.D., Simeral J.D., Newell K., Oakley E.M., Cash S.S., Friehs G., Hochberg L.R. Neural Point-and-Click Communication by a Person with Incomplete Locked-In Syndrome. Neurorehabil. Neural Repair. 2015;29:462–471. doi: 10.1177/1545968314554624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oxley T.J., Yoo P.E., Rind G.S., Ronayne S.M., Lee C.M.S., Bird C., Hampshire V., Sharma R.P., Morokoff A., Williams D.L., et al. Motor neuroprosthesis implanted with neurointerventional surgery improves capacity for activities of daily living tasks in severe paralysis: First in-human experience. J. Neurointerv. Surg. 2021;13:102–108. doi: 10.1136/neurintsurg-2020-016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandman D.M., Hosman T., Saab J., Burkhart M.C., Shanahan B.E., Ciancibello J.G., Sarma A.A., Milstein D.J., Vargas-Irwin C.E., Franco B., et al. Rapid calibration of an intracortical brain-computer interface for people with tetraplegia. J. Neural Eng. 2018;15:026007. doi: 10.1088/1741-2552/aa9ee7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zabcikova M., Koudelkova Z., Jasek R., Lorenzo Navarro J.J. Recent advances and current trends in brain-computer interface research and their applications. Int. J. Dev. Neurosci. 2021;82:107–123. doi: 10.1002/jdn.10166. [DOI] [PubMed] [Google Scholar]
- 24.Vidal G.W., Rynes M.L., Kelliher Z., Goodwin S.J. Review of Brain-Machine Interfaces Used in Neural Prosthetics with New Perspective on Somatosensory Feedback through Method of Signal Breakdown. Scientifica. 2016;2016:8956432. doi: 10.1155/2016/8956432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camacho-Conde J.A., Gonzalez-Bermudez M.D.R., Carretero-Rey M., Khan Z.U. Brain stimulation: A therapeutic approach for the treatment of neurological disorders. CNS Neurosci. Ther. 2022;28:5–18. doi: 10.1111/cns.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osborn L.E., Christie B.P., McMullen D.P., Nickl R.W., Thompson M.C., Pawar A.S., Thomas T.M., Alejandro Anaya M., Crone N.E., Wester B.A., et al. Intracortical microstimulation of somatosensory cortex enables object identification through perceived sensations; Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) Mexico; 1–5 November 2021; pp. 6259–6262. [DOI] [PubMed] [Google Scholar]
- 27.Hughes C.L., Flesher S.N., Weiss J.M., Downey J.E., Boninger M., Collinger J.L., Gaunt R.A. Neural stimulation and recording performance in human sensorimotor cortex over 1500 days. J. Neural Eng. 2021;18:045012. doi: 10.1088/1741-2552/ac18ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bashford L., Rosenthal I., Kellis S., Pejsa K., Kramer D., Lee B., Liu C., Andersen R.A. The Neurophysiological Representation of Imagined Somatosensory Percepts in Human Cortex. J. Neurosci. 2021;41:2177–2185. doi: 10.1523/JNEUROSCI.2460-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bucur M., Papagno C. Deep Brain Stimulation in Parkinson Disease: A Meta-analysis of the Long-term Neuropsychological Outcomes. Neuropsychol. Rev. 2022;103:956–967. doi: 10.1007/s11065-022-09540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lisoni J., Barlati S., Deste G., Ceraso A., Nibbio G., Baldacci G., Vita A. Efficacy and tolerability of Brain Stimulation interventions in Borderline Personality Disorder: State of the art and future perspectives—A systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2022;116:110537. doi: 10.1016/j.pnpbp.2022.110537. [DOI] [PubMed] [Google Scholar]
- 31.Chandra V., Hilliard J.D., Foote K.D. Deep brain stimulation for the treatment of tremor. J. Neurol. Sci. 2022;435:120190. doi: 10.1016/j.jns.2022.120190. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X., Ma Z., Zheng H., Li T., Chen K., Wang X., Liu C., Xu L., Wu X., Lin D., et al. The combination of brain-computer interfaces and artificial intelligence: Applications and challenges. Ann. Transl. Med. 2020;8:712. doi: 10.21037/atm.2019.11.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes C., Herrera A., Gaunt R., Collinger J. Bidirectional brain-computer interfaces. Handb. Clin. Neurol. 2020;168:163–181. doi: 10.1016/B978-0-444-63934-9.00013-5. [DOI] [PubMed] [Google Scholar]
- 34.Adrian E.D. The impulses produced by sensory nerve endings: Part I. J. Physiol. 1926;61:49–72. doi: 10.1113/jphysiol.1926.sp002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long K.H., Lieber J.D., Bensmaia S.J. Texture is encoded in precise temporal spiking patterns in primate somatosensory cortex. Nat. Commun. 2022;13:1311. doi: 10.1038/s41467-022-28873-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Q., Shen J., Ran X., Tang H., Pan G., Liu J.K. Robust Transcoding Sensory Information with Neural Spikes. IEEE Trans. Neural Netw. Learn. Syst. 2021;33:1935–1946. doi: 10.1109/TNNLS.2021.3107449. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy P.R., Bakay R.A. Restoration of neural output from a paralyzed patient by a direct brain connection. Neuroreport. 1998;9:1707–1711. doi: 10.1097/00001756-199806010-00007. [DOI] [PubMed] [Google Scholar]
- 38.Kettner R.E., Schwartz A.B., Georgopoulos A.P. Primate motor cortex and free arm movements to visual targets in three-dimensional space. III. Positional gradients and population coding of movement direction from various movement origins. J. Neurosci. 1988;8:2938–2947. doi: 10.1523/JNEUROSCI.08-08-02938.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salinas E., Abbott L.F. Vector reconstruction from firing rates. J. Comput. Neurosci. 1994;1:89–107. doi: 10.1007/BF00962720. [DOI] [PubMed] [Google Scholar]
- 40.Yang S.H., Chen Y.Y., Lin S.H., Liao L.D., Lu H.H., Wang C.F., Chen P.C., Lo Y.C., Phan T.D., Chao H.Y., et al. A Sliced Inverse Regression (SIR) Decoding the Forelimb Movement from Neuronal Spikes in the Rat Motor Cortex. Front. Neurosci. 2016;10:556. doi: 10.3389/fnins.2016.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu B.M., Kemere C., Santhanam G., Afshar A., Ryu S.I., Meng T.H., Sahani M., Shenoy K.V. Mixture of trajectory models for neural decoding of goal-directed movements. J. Neurophysiol. 2007;97:3763–3780. doi: 10.1152/jn.00482.2006. [DOI] [PubMed] [Google Scholar]
- 42.Shenoy K.V., Carmena J.M. Combining decoder design and neural adaptation in brain-machine interfaces. Neuron. 2014;84:665–680. doi: 10.1016/j.neuron.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 43.Chapin J.K., Moxon K.A., Markowitz R.S., Nicolelis M.A. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat. Neurosci. 1999;2:664–670. doi: 10.1038/10223. [DOI] [PubMed] [Google Scholar]
- 44.Serruya M.D., Hatsopoulos N.G., Paninski L., Fellows M.R., Donoghue J.P. Instant neural control of a movement signal. Nature. 2002;416:141–142. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- 45.Donoghue J.P., Nurmikko A., Black M., Hochberg L.R. Assistive technology and robotic control using motor cortex ensemble-based neural interface systems in humans with tetraplegia. J. Physiol. 2007;579:603–611. doi: 10.1113/jphysiol.2006.127209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simeral J.D., Kim S.P., Black M.J., Donoghue J.P., Hochberg L.R. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J. Neural Eng. 2011;8:025027. doi: 10.1088/1741-2560/8/2/025027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Handelman D.A., Osborn L.E., Thomas T.M., Badger A.R., Thompson M., Nickl R.W., Anaya M.A., Wormley J.M., Cantarero G.L., McMullen D., et al. Shared Control of Bimanual Robotic Limbs with a Brain-Machine Interface for Self-Feeding. Front. Neurorobot. 2022;16:918001. doi: 10.3389/fnbot.2022.918001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Doherty J., Lebedev M., Hanson T., Fitzsimmons N., Nicolelis M. A brain-machine interface instructed by direct intracortical microstimulation. Front. Integr. Neurosci. 2009;3:20. doi: 10.3389/neuro.07.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anumanchipalli G.K., Chartier J., Chang E.F. Speech synthesis from neural decoding of spoken sentences. Nature. 2019;568:493–498. doi: 10.1038/s41586-019-1119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willett F.R., Avansino D.T., Hochberg L.R., Henderson J.M., Shenoy K.V. High-performance brain-to-text communication via handwriting. Nature. 2021;593:249–254. doi: 10.1038/s41586-021-03506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dekleva B.M., Weiss J.M., Boninger M.L., Collinger J.L. Generalizable cursor click decoding using grasp-related neural transients. J. Neural Eng. 2021;18:0460e9. doi: 10.1088/1741-2552/ac16b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoo J., Shoaran M. Neural interface systems with on-device computing: Machine learning and neuromorphic architectures. Curr. Opin. Biotechnol. 2021;72:95–101. doi: 10.1016/j.copbio.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 53.Cao C., Liu F., Tan H., Song D., Shu W., Li W., Zhou Y., Bo X., Xie Z. Deep Learning and Its Applications in Biomedicine. Genom. Proteom. Bioinform. 2018;16:17–32. doi: 10.1016/j.gpb.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghanbari A., Lee C.M., Read H.L., Stevenson I.H. Modeling stimulus-dependent variability improves decoding of population neural responses. J. Neural Eng. 2019;16:066018. doi: 10.1088/1741-2552/ab3a68. [DOI] [PubMed] [Google Scholar]
- 55.Hosokawa T., Xu M., Katori Y., Yamada M., Aihara K., Tsutsui K.I. Monkey Prefrontal Single-Unit Activity Reflecting Category-Based Logical Thinking Process and Its Neural Network Model. J. Neurosci. 2022;42:6380–6391. doi: 10.1523/JNEUROSCI.2286-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hagen E., Magnusson S.H., Ness T.V., Halnes G., Babu P.N., Linssen C., Morrison A., Einevoll G.T. Brain signal predictions from multi-scale networks using a linearized framework. PLoS Comput. Biol. 2022;18:e1010353. doi: 10.1371/journal.pcbi.1010353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamazaki K., Vo-Ho V.K., Bulsara D., Le N. Spiking Neural Networks and Their Applications: A Review. Brain Sci. 2022;12:863. doi: 10.3390/brainsci12070863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heydari Beni N., Foodeh R., Shalchyan V., Daliri M.R. Force decoding using local field potentials in primary motor cortex: PLS or Kalman filter regression? Australas. Phys. Eng. Sci. Med. 2020;43:175–186. doi: 10.1007/s13246-019-00833-7. [DOI] [PubMed] [Google Scholar]
- 59.Asgharpour M., Foodeh R., Daliri M.R. Regularized Kalman filter for brain-computer interfaces using local field potential signals. J. Neurosci. Methods. 2021;350:109022. doi: 10.1016/j.jneumeth.2020.109022. [DOI] [PubMed] [Google Scholar]
- 60.Liu D., Xu X., Li D., Li J., Yu X., Ling Z., Hong B. Intracranial brain-computer interface spelling using localized visual motion response. Neuroimage. 2022;258:119363. doi: 10.1016/j.neuroimage.2022.119363. [DOI] [PubMed] [Google Scholar]
- 61.Kashefi M., Daliri M.R. A stack LSTM structure for decoding continuous force from local field potential signal of primary motor cortex (M1) BMC Bioinform. 2021;22:26. doi: 10.1186/s12859-020-03953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Penfield W., Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. doi: 10.1093/brain/60.4.389. [DOI] [Google Scholar]
- 63.Murphy J.T., Kwan H.C., Wong Y.C. Differential effects of reciprocal wrist torques on responses of somatotopically identified neurons of precentral cortex in awake primates. Brain Res. 1979;172:329–337. doi: 10.1016/0006-8993(79)90542-0. [DOI] [PubMed] [Google Scholar]
- 64.Sessle B.J., Wiesendanger M. Structural and functional definition of the motor cortex in the monkey (Macaca fascicularis) J. Physiol. 1982;323:245–265. doi: 10.1113/jphysiol.1982.sp014071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waters R.S., Samulack D.D., Dykes R.W., McKinley P.A. Topographic organization of baboon primary motor cortex: Face, hand, forelimb, and shoulder representation. Somatosens. Mot. Res. 1990;7:485–514. doi: 10.3109/08990229009144721. [DOI] [PubMed] [Google Scholar]
- 66.Salzman C.D., Murasugi C.M., Britten K.H., Newsome W.T. Microstimulation in visual area MT: Effects on direction discrimination performance. J. Neurosci. 1992;12:2331–2355. doi: 10.1523/JNEUROSCI.12-06-02331.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fujii N., Mushiake H., Tanji J. Intracortical microstimulation of bilateral frontal eye field. J. Neurophysiol. 1998;79:2240–2244. doi: 10.1152/jn.1998.79.4.2240. [DOI] [PubMed] [Google Scholar]
- 68.Romo R., Hernandez A., Zainos A., Brody C.D., Lemus L. Sensing without touching: Psychophysical performance based on cortical microstimulation. Neuron. 2000;26:273–278. doi: 10.1016/S0896-6273(00)81156-3. [DOI] [PubMed] [Google Scholar]
- 69.Flesher S.N., Collinger J.L., Foldes S.T., Weiss J.M., Downey J.E., Tyler-Kabara E.C., Bensmaia S.J., Schwartz A.B., Boninger M.L., Gaunt R.A. Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med. 2016;8:361ra141. doi: 10.1126/scitranslmed.aaf8083. [DOI] [PubMed] [Google Scholar]
- 70.Rajan A.T., Boback J.L., Dammann J.F., Tenore F.V., Wester B.A., Otto K.J., Gaunt R.A., Bensmaia S.J. The effects of chronic intracortical microstimulation on neural tissue and fine motor behavior. J. Neural Eng. 2015;12:066018. doi: 10.1088/1741-2560/12/6/066018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Doherty J.E., Lebedev M.A., Ifft P.J., Zhuang K.Z., Shokur S., Bleuler H., Nicolelis M.A. Active tactile exploration using a brain-machine-brain interface. Nature. 2011;479:228–231. doi: 10.1038/nature10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klaes C., Shi Y., Kellis S., Minxha J., Revechkis B., Andersen R.A. A cognitive neuroprosthetic that uses cortical stimulation for somatosensory feedback. J. Neural Eng. 2014;11:056024. doi: 10.1088/1741-2560/11/5/056024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flesher S.N., Downey J.E., Weiss J.M., Hughes C.L., Herrera A.J., Tyler-Kabara E.C., Boninger M.L., Collinger J.L., Gaunt R.A. A brain-computer interface that evokes tactile sensations improves robotic arm control. Science. 2021;372:831–836. doi: 10.1126/science.abd0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raspopovic S., Capogrosso M., Petrini F.M., Bonizzato M., Rigosa J., Di Pino G., Carpaneto J., Controzzi M., Boretius T., Fernandez E., et al. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci. Transl. Med. 2014;6:222ra219. doi: 10.1126/scitranslmed.3006820. [DOI] [PubMed] [Google Scholar]
- 75.D’Anna E., Valle G., Mazzoni A., Strauss I., Iberite F., Patton J., Petrini F.M., Raspopovic S., Granata G., Di Iorio R., et al. A closed-loop hand prosthesis with simultaneous intraneural tactile and position feedback. Sci. Robot. 2019;4:eaau8892. doi: 10.1126/scirobotics.aau8892. [DOI] [PubMed] [Google Scholar]
- 76.Zollo L., Di Pino G., Ciancio A.L., Ranieri F., Cordella F., Gentile C., Noce E., Romeo R.A., Bellingegni A.D., Vadala G., et al. Restoring Tactile sensations via neural interfaces for real-time force-and-slippage closed-loop control of bionic hands. Sci. Robot. 2019;4:eaau9924. doi: 10.1126/scirobotics.aau9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marasco P.D., Hebert J.S., Sensinger J.W., Beckler D.T., Thumser Z.C., Shehata A.W., Williams H.E., Wilson K.R. Neurorobotic fusion of prosthetic touch, kinesthesia, and movement in bionic upper limbs promotes intrinsic brain behaviors. Sci. Robot. 2021;6:eabf3368. doi: 10.1126/scirobotics.abf3368. [DOI] [PubMed] [Google Scholar]
- 78.Schofield J.S., Shell C.E., Beckler D.T., Thumser Z.C., Marasco P.D. Long-Term Home-Use of Sensory-Motor-Integrated Bidirectional Bionic Prosthetic Arms Promotes Functional, Perceptual, and Cognitive Changes. Front. Neurosci. 2020;14:120. doi: 10.3389/fnins.2020.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brindley G.S., Lewin W.S. The sensations produced by electrical stimulation of the visual cortex. J. Physiol. 1968;196:479–493. doi: 10.1113/jphysiol.1968.sp008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dobelle W.H., Mladejovsky M.G. Phosphenes produced by electrical stimulation of human occipital cortex, and their application to the development of a prosthesis for the blind. J. Physiol. 1974;243:553–576. doi: 10.1113/jphysiol.1974.sp010766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmidt E.M., Bak M.J., Hambrecht F.T., Kufta C.V., O’Rourke D.K., Vallabhanath P. Feasibility of a visual prosthesis for the blind based on intracortical microstimulation of the visual cortex. Pt 2Brain. 1996;119:507–522. doi: 10.1093/brain/119.2.507. [DOI] [PubMed] [Google Scholar]
- 82.Tehovnik E.J., Slocum W.M. Phosphene induction by microstimulation of macaque V1. Brain Res. Rev. 2007;53:337–343. doi: 10.1016/j.brainresrev.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tehovnik E.J., Slocum W.M., Smirnakis S.M., Tolias A.S. Microstimulation of visual cortex to restore vision. Prog. Brain Res. 2009;175:347–375. doi: 10.1016/S0079-6123(09)17524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen X., Wang F., Fernandez E., Roelfsema P.R. Shape perception via a high-channel-count neuroprosthesis in monkey visual cortex. Science. 2020;370:1191–1196. doi: 10.1126/science.abd7435. [DOI] [PubMed] [Google Scholar]
- 85.Fernandez E., Alfaro A., Soto-Sanchez C., Gonzalez-Lopez P., Lozano A.M., Pena S., Grima M.D., Rodil A., Gomez B., Chen X., et al. Visual percepts evoked with an intracortical 96-channel microelectrode array inserted in human occipital cortex. J. Clin. Investig. 2021;131:e151331. doi: 10.1172/JCI151331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hanson T., Fitzsimmons N., O’Doherty J.E. Technology for Multielectrode MicroStimulation of Brain Tissue. In: Nicolelis M.A.L., editor. Methods for Neural Ensemble Recordings. 2nd ed. CRC Press; Boca Raton, FL, USA: 2008. Frontiers in Neuroscience. [PubMed] [Google Scholar]
- 87.Dougherty D.D. Deep Brain Stimulation: Clinical Applications. Psychiatr. Clin. N. Am. 2018;41:385–394. doi: 10.1016/j.psc.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 88.Benabid A.L., Pollak P., Gervason C., Hoffmann D., Gao D.M., Hommel M., Perret J.E., de Rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. doi: 10.1016/0140-6736(91)91175-T. [DOI] [PubMed] [Google Scholar]
- 89.Rajan R., Garg K., Saini A., Radhakrishnan D.M., Carecchio M., Bk B., Singh M., Srivastava A.K. GPi-DBS for KMT2B-Associated Dystonia: Systematic Review and Meta-Analysis. Mov. Disord. Clin. Pract. 2022;9:31–37. doi: 10.1002/mdc3.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gonzalez V., Cif L., Biolsi B., Garcia-Ptacek S., Seychelles A., Sanrey E., Descours I., Coubes C., de Moura A.M., Corlobe A., et al. Deep brain stimulation for Huntington’s disease: Long-term results of a prospective open-label study. J. Neurosurg. 2014;121:114–122. doi: 10.3171/2014.2.JNS131722. [DOI] [PubMed] [Google Scholar]
- 91.Smeets A., Duits A.A., Plantinga B.R., Leentjens A.F.G., Oosterloo M., Visser-Vandewalle V., Temel Y., Ackermans L. Deep Brain Stimulation of the internal globus pallidus in refractory Tourette Syndrome. Clin. Neurol. Neurosurg. 2016;142:54–59. doi: 10.1016/j.clineuro.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 92.Benazzouz A., Gao D.M., Ni Z.G., Piallat B., Bouali-Benazzouz R., Benabid A.L. Effect of high-frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat. Neuroscience. 2000;99:289–295. doi: 10.1016/S0306-4522(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 93.Anderson M.E., Postupna N., Ruffo M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J. Neurophysiol. 2003;89:1150–1160. doi: 10.1152/jn.00475.2002. [DOI] [PubMed] [Google Scholar]
- 94.Fariba K.A., Gupta V. Deep Brain Stimulation. StatPearls; Treasure Island, FL, USA: 2022. [PubMed] [Google Scholar]
- 95.Shetty N. Essential Tremor-Do We Have Better Therapeutics? A Review of Recent Advances and Future Directions. Curr. Neurol. Neurosci. Rep. 2022;22:197–208. doi: 10.1007/s11910-022-01185-8. [DOI] [PubMed] [Google Scholar]
- 96.Velasco F., Saucedo-Alvarado P.E., Vazquez-Barron D., Trejo D., Velasco A.L. Deep Brain Stimulation for Refractory Temporal Lobe Epilepsy. Current Status and Future Trends. Front. Neurol. 2022;13:796846. doi: 10.3389/fneur.2022.796846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Touma L., Dansereau B., Chan A.Y., Jette N., Kwon C.S., Braun K.P.J., Friedman D., Jehi L., Rolston J.D., Vadera S., et al. Neurostimulation in People with Drug-Resistant Epilepsy: Systematic Review and Meta-Analysis from the ILAE Surgical Therapies Commission. Epilepsia. 2022;63:1314–1329. doi: 10.1111/epi.17243. [DOI] [PubMed] [Google Scholar]
- 98.Bewernick B.H., Kayser S., Sturm V., Schlaepfer T.E. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: Evidence for sustained efficacy. Neuropsychopharmacology. 2012;37:1975–1985. doi: 10.1038/npp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Denys D., Mantione M., Figee M., van den Munckhof P., Koerselman F., Westenberg H., Bosch A., Schuurman R. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2010;67:1061–1068. doi: 10.1001/archgenpsychiatry.2010.122. [DOI] [PubMed] [Google Scholar]
- 100.Wu H., Van Dyck-Lippens P.J., Santegoeds R., van Kuyck K., Gabriels L., Lin G., Pan G., Li Y., Li D., Zhan S., et al. Deep-brain stimulation for anorexia nervosa. World Neurosurg. 2013;80:S29e21-10. doi: 10.1016/j.wneu.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 101.Corripio I., Roldan A., Sarro S., McKenna P.J., Alonso-Solis A., Rabella M., Diaz A., Puigdemont D., Perez-Sola V., Alvarez E., et al. Deep brain stimulation in treatment resistant schizophrenia: A pilot randomized cross-over clinical trial. eBioMedicine. 2020;51:102568. doi: 10.1016/j.ebiom.2019.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huys D., Kohl S., Baldermann J.C., Timmermann L., Sturm V., Visser-Vandewalle V., Kuhn J. Open-label trial of anterior limb of internal capsule-nucleus accumbens deep brain stimulation for obsessive-compulsive disorder: Insights gained. J. Neurol. Neurosurg. Psychiatry. 2019;90:805–812. doi: 10.1136/jnnp-2018-318996. [DOI] [PubMed] [Google Scholar]
- 103.Welter M.L., Alves Dos Santos J.F., Clair A.H., Lau B., Diallo H.M., Fernandez-Vidal S., Belaid H., Pelissolo A., Domenech P., Karachi C., et al. Deep Brain Stimulation of the Subthalamic, Accumbens, or Caudate Nuclei for Patients with Severe Obsessive-Compulsive Disorder: A Randomized Crossover Controlled Study. Biol. Psychiatry. 2021;90:e45–e47. doi: 10.1016/j.biopsych.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 104.Langevin J.P., Chen J.W., Koek R.J., Sultzer D.L., Mandelkern M.A., Schwartz H.N., Krahl S.E. Deep Brain Stimulation of the Basolateral Amygdala: Targeting Technique and Electrodiagnostic Findings. Brain Sci. 2016;6:28. doi: 10.3390/brainsci6030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Van Rooij S.J.H., Sippel L.M., McDonald W.M., Holtzheimer P.E. Defining focal brain stimulation targets for PTSD using neuroimaging. Depress. Anxiety. 2021;38:768–785. doi: 10.1002/da.23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meeres J., Hariz M. Deep Brain Stimulation for Post-Traumatic Stress Disorder: A Review of the Experimental and Clinical Literature. Stereotact. Funct. Neurosurg. 2022;100:1–13. doi: 10.1159/000521130. [DOI] [PubMed] [Google Scholar]
- 107.Castillo P.R., Middlebrooks E.H., Grewal S.S., Okromelidze L., Meschia J.F., Quinones-Hinojosa A., Uitti R.J., Wharen R.E., Jr. Globus Pallidus Externus Deep Brain Stimulation Treats Insomnia in a Patient with Parkinson Disease. Mayo Clin. Proc. 2020;95:419–422. doi: 10.1016/j.mayocp.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 108.Marceglia S., Guidetti M., Harmsen I.E., Loh A., Meoni S., Foffani G., Lozano A.M., Volkmann J., Moro E., Priori A. Deep brain stimulation: Is it time to change gears by closing the loop? J. Neural Eng. 2021;18:046002. doi: 10.1088/1741-2552/ac3267. [DOI] [PubMed] [Google Scholar]
- 109.Sigrist C., Vockel J., MacMaster F.P., Farzan F., Croarkin P.E., Galletly C., Kaess M., Bender S., Koenig J. Transcranial magnetic stimulation in the treatment of adolescent depression: A systematic review and meta-analysis of aggregated and individual-patient data from uncontrolled studies. Eur. Child Adolesc. Psychiatry. 2022;31:1501–1525. doi: 10.1007/s00787-022-02021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mosilhy E.A., Alshial E.E., Eltaras M.M., Rahman M.M.A., Helmy H.I., Elazoul A.H., Hamdy O., Mohammed H.S. Non-invasive transcranial brain modulation for neurological disorders treatment: A narrative review. Life Sci. 2022;307:120869. doi: 10.1016/j.lfs.2022.120869. [DOI] [PubMed] [Google Scholar]
- 111.Zhang T., Pan N., Wang Y., Liu C., Hu S. Transcranial Focused Ultrasound Neuromodulation: A Review of the Excitatory and Inhibitory Effects on Brain Activity in Human and Animals. Front. Hum. Neurosci. 2021;15:749162. doi: 10.3389/fnhum.2021.749162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Simko P., Kent J.A., Rektorova I. Is non-invasive brain stimulation effective for cognitive enhancement in Alzheimer’s disease? An updated meta-analysis. Clin. Neurophysiol. 2022;144:23–40. doi: 10.1016/j.clinph.2022.09.010. [DOI] [PubMed] [Google Scholar]
- 113.Kumar S., Khammash M. Platforms for Optogenetic Stimulation and Feedback Control. Front. Bioeng. Biotechnol. 2022;10:918917. doi: 10.3389/fbioe.2022.918917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lan T.H., He L., Huang Y., Zhou Y. Optogenetics for transcriptional programming and genetic engineering. Trends Genet. 2022;38:1253–1270. doi: 10.1016/j.tig.2022.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mian S.Y., Honey J.R., Carnicer-Lombarte A., Barone D.G. Large Animal Studies to Reduce the Foreign Body Reaction in Brain-Computer Interfaces: A Systematic Review. Biosensors. 2021;11:275. doi: 10.3390/bios11080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Neudorfer C., Bhatia K., Boutet A., Germann J., Elias G.J., Loh A., Paff M., Krings T., Lozano A.M. Endovascular deep brain stimulation: Investigating the relationship between vascular structures and deep brain stimulation targets. Brain Stimul. 2020;13:1668–1677. doi: 10.1016/j.brs.2020.09.016. [DOI] [PubMed] [Google Scholar]
- 117.Cabral A.M., Pereira A.A., Vieira M.F., Pessoa B.L., de Oliveira Andrade A. Prevalence of distinct types of hardware failures related to deep brain stimulation. Neurosurg. Rev. 2021;45:1123–1134. doi: 10.1007/s10143-021-01673-4. [DOI] [PubMed] [Google Scholar]
- 118.Zhang X., Wu D. On the Vulnerability of CNN Classifiers in EEG-Based BCIs. IEEE Trans. Neural Syst. Rehabil. Eng. 2019;27:814–825. doi: 10.1109/TNSRE.2019.2908955. [DOI] [PubMed] [Google Scholar]
- 119.Zeng Y., Sun K., Lu E. Declaration on the ethics of brain–computer interfaces and augment intelligence. AI Ethics. 2021;1:209–211. doi: 10.1007/s43681-020-00036-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.