This cohort study investigates whether lipoprotein(a) values are associated with lower-limb amputation or revascularization.
Key Points
Question
What is the association between lipoprotein(a) (Lp[a]) levels and major adverse limb events, defined as major amputation, peripheral artery endovascular revascularization, or peripheral artery surgical revascularization?
Finding
In this cohort study of 16 513 patients, patients with high Lp(a) levels had a major adverse limb events–free survival that was 57% shorter than in patients with normal Lp(a) levels. Patients with very high Lp(a) levels had a major adverse limb event–free survival that was 83% shorter than that of patients with normal levels.
Meaning
Findings of this study suggest that higher Lp(a) levels are independently associated with an increased risk of major adverse limb events and that Lp(a) measurement might help improve lower-limb vascular risk assessment.
Abstract
Importance
High lipoprotein(a) (Lp[a]) levels are involved in the development of cardiovascular events, particularly in myocardial infarction, stroke, and peripheral artery disease. Studies assessing the Lp(a) levels associated with adverse lower-limb events are lacking.
Objective
To assess the association between Lp(a) levels and incidence of major adverse limb events in unselected hospitalized patients.
Design, Setting, and Participants
This large retrospective monocentric cohort study was conducted from January 1, 2000, to December 31, 2020. Data were derived from the clinical information system of the Hôpital Européen Georges-Pompidou, a Paris-based university hospital. Patients who underwent at least 1 Lp(a) measurement at the center during the study period were included. Patients who had no follow-up data or who had the first Lp(a) measurement after the study outcome had occurred were excluded. Data analyses were performed from May 2021 to January 2022.
Main Outcomes and Measures
The primary outcome was the first inpatient major adverse limb event, defined as a major amputation, peripheral endovascular revascularization, or peripheral surgical revascularization, during follow-up. Secondary outcomes included individual components of the primary outcome. Lipoprotein(a) levels were categorized as follows: normal (<50 mg/dL), high (50 to <134 mg/dL), and very high (≥134 mg/dL); to convert Lp(a) values to milligrams per liter, multiply by 0.1.
Results
A total of 16 513 patients (median [IQR] age, 58.2 [49.0-66.7] years; 9774 men [59.2%]) were included in the cohort. The median (IQR) Lp(a) level was 24 (10.0-60.0) mg/dL. The 1-year incidence of major adverse limb event was 2.44% in the overall population and 4.54% among patients with very high Lp(a) levels. High (adjusted accelerated failure time [AFT] exponential estimate: 0.43; 95% CI, 0.24-0.78; Benjamini-Hochberg–corrected P = .01) and very high (adjusted AFT exponential estimate: 0.17; 95% CI, 0.07-0.40; Benjamini-Hochberg–corrected P < .001) Lp(a) levels were independently associated with an increased risk of major adverse limb event.
Conclusions and Relevance
Results of this study showed that higher Lp(a) levels were independently associated with an increased risk of a major adverse limb event in hospitalized patients. The Lp(a) measurement needs to be taken into account to improve lower-limb vascular risk assessment.
Introduction
Peripheral artery disease (PAD) is an atherosclerotic disease of the lower-limb arteries affecting more than 200 million people worldwide.1,2 Moreover, PAD remains underdiagnosed and untreated due to the asymptomatic onset of the disease.3 Patients with PAD can develop limb symptoms, ranging from claudication to critical limb-threatening ischemia, leading to a major adverse limb event, such as peripheral artery revascularization and lower-limb amputation.1,4,5,6 In addition to the usual cardiovascular risk factors, such as diabetes, arterial hypertension, and smoking, one of the most important indicators of major adverse limb event is a previous peripheral revascularization itself, highlighting the importance of preventing the occurrence of major adverse limb events.7,8,9
Lipoprotein(a) (Lp[a]) is a low-density lipoprotein (LDL)-like particle synthesized in the liver and is composed of an apolipoprotein B100 covalently bound to the apolipoprotein(a), a glycoprotein not found in native LDL.10 High Lp(a) levels are well described in the literature as being involved in the development of cardiovascular events,11,12,13,14,15,16,17,18,19,20,21 such as myocardial infarction (MI) and stroke. Underlying pathophysiological mechanisms have not yet been fully elucidated and have a converging association with the development of atherosclerosis and thrombosis.22,23,24,25,26,27 More than 90% of Lp(a) plasmatic levels are genetically determined, with low influence from the environment or nutrition,10 and statins have a neutral or modest Lp(a)-increasing outcome.28 New treatments are currently being developed to specifically reduce this target.29 Thus far, Lp(a) levels are assessed to better stratify patients at intermediate cardiovascular risk.30 Furthermore, elevated Lp(a) levels are associated with an increased risk of PAD, but studies investigating the role of Lp(a) in lower-limb amputation or revascularization in a large population are lacking.31,32,33,34 To better understand the potential value of Lp(a)-lowering therapy in PAD, we assessed the association between Lp(a) levels and the incidence of major adverse limb events in unselected hospitalized patients.
Methods
This study received approval from the CERAPHP.5 Institutional Review Board. Because the study was retrospective and observational, consent from patients was not required by French law. The study was performed in accordance with the Declaration of Helsinki.35 We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.36
Data Collection and Study Population
Data were derived from the clinical information system of the Hôpital Européen Georges-Pompidou (HEGP), a Paris-based university hospital.37 Since its opening in 2000, HEGP has used a certified clinical information system,38 combined with an i2b2 clinical data warehouse, to facilitate the reuse of health care data.39,40 All clinical data were coded using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), and all procedures performed during the hospital stay were coded using CCAM (Classification Commune des Actes Médicaux), the French common classification of medical procedures.41,42
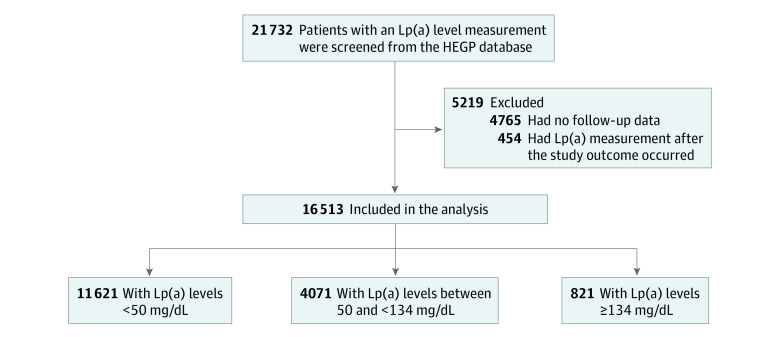
All patients (n = 21 732) who underwent at least 1 Lp(a) measurement at HEGP between January 1, 2000, and December 31, 2020, were screened. The routine lipid panel testing at HEGP always includes Lp(a) measurement. Patients who had no follow-up data (n = 4765) or who underwent the first Lp(a) measurement after the primary outcome had occurred (n = 454) were excluded (Figure 1). We considered the date of the first Lp(a) measurement to be the inclusion date and the date of the final hospital contact or date of the outcome occurrence to be the last follow-up date.
Figure 1. Flowchart of Study Population.
HEGP indicates Hôpital Européen Georges-Pompidou; Lp(a), lipoprotein(a).
Lipoprotein(a) Measurement
All venous blood samples were collected during the hospital stay. Quantitation of Lp(a) levels was performed by nephelometry technique using rabbit polyclonal anti–Lp(a) antibody. All measurements were carried out using an immunochemistry system (IMMAGE 800; Beckman Coulter). The Lp(a) measurements were reported in milligrams per deciliter; to convert to milligrams per liter, multiply by 0.1. Measurement of this assay ranges from 2 to 640 mg/dL.
Outcomes and Covariates
Age, sex, hospitalization data, and biological variables were extracted from electronic medical records using the HEGP clinical information system. Race and ethnicity data were not collected because ethnic statistics are forbidden in France by the Data Protection Act of January 6, 1978. Comorbidities were identified from the ICD-10 codes related to the hospitalization at the inclusion date. The primary outcome was the first inpatient major adverse limb event, defined as a major amputation, peripheral endovascular revascularization, or peripheral surgical revascularization, during follow-up. We defined major amputation as a lower-limb amputation event above the forefoot. Peripheral endovascular and peripheral surgical procedures included endovascular and surgical revascularization, respectively, of lower-limb arteries or aorta. Secondary outcomes were the individual components of the primary outcome. Corresponding CCAM procedure codes and ICD-10 comorbidity codes are presented in eMethods 1 and 2 in Supplement 1. Lipid-lowering treatment data were not available for patients who were admitted for 1-day hospitalization. Distribution of patients by hospitalization unit and Lp(a) levels at inclusion are shown in eFigure 5 in Supplement 1.
Statistical Analysis
Continuous variables were described as medians (IQRs), and binary variables were expressed as numbers (proportions). Qualitative variables were compared using Fisher exact tests, and binary variables were compared using Kruskal-Wallis tests. We computed Lp(a) level categories less than 50 mg/dL as the upper limit for a normal level, according to the 2018 American College of Cardiology and American Heart Association Guideline on the Management of Blood Cholesterol43 and the European Atherosclerosis Society consensus statement about Lp(a).44 The 95th percentile of Lp(a) level (ie, ≥134 mg/dL in the present cohort) was used to define a very high level, as proposed in the literature.45,46,47,48 Lp(a) levels between 50 and less than 134 mg/dL were used to define a high level. Lp(a), LDL-cholesterol, and serum creatinine levels were log-transformed in the multivariate regression analyses due to the positively skewed distribution.
We used Kaplan-Meier curves to generate outcome-free survival curves and compute 1-year, 5-year, and 10-year cumulative incidence rates of outcomes. Given that the Schoenfeld residuals analyses showed a violation of the proportional hazards assumption required for Cox proportional hazards regression models,49 we used accelerated failure time (AFT) regression models to assess the association between Lp(a) levels and outcomes. Accelerated failure time models assume that an explanatory variable acts multiplicatively on the speed at which an event occurs for a patient.50,51 Among log-normal, log-logistic, Weibull, exponential, gaussian, and logistic distributions, we chose the log-normal distribution, which provided the best model fit according to the Akaike information criterion. The exponentiated AFT regression coefficient represents acceleration factors. An AFT exponential estimate of 1.0 shows no association between the explanatory variable and the outcome. Values lower than 1.0 show an earlier occurrence of the event, and values higher than 1.0 show delayed occurrence of the event. We fit multivariate AFT models to analyze the association between primary or secondary outcomes and log-transformed Lp(a) levels or Lp(a) threshold. Sex, age, diabetes, arterial hypertension, LDL-cholesterol level, smoking status, serum creatinine level, and dialysis were considered as potential confounders, according to the literature, and were included in the regression models.3,52,53 All AFT models with included variables and coefficient results are shown in eTables 5-8 in Supplement 1.
We performed sensitivity analyses with the primary outcome models, adding lipid-lowering drug status for patients with available data (eTable 1 in Supplement 1). The time effect variable was defined as the binary variable inclusion time before or after the median inclusion time of follow-up for this cohort, which was set to September 29, 2011 (eTable 2 in Supplement 1). Multiple imputation with chained equations to handle missing data was performed at baseline using the MICE package54 in R, version 3.5.0 (R Foundation for Statistical Computing). Estimates were pooled across 10 imputed data sets.
To reduce α risk inflation, we used the Benjamini-Hochberg false discovery rate approach55 to obtain corrected P values for all tests involving the primary outcome, including sensitivity analyses (eTable 9 in Supplement 1). Two-sided testing was used, with Benjamini-Hochberg–corrected P < .05 considered to be statistically significant. All analyses were performed using R, version 3.6.0, for Mac (R Foundation for Statistical Computing). Data analyses were performed from May 2021 to January 2022.
Results
Patient Population and Baseline Characteristics
A total of 16 513 patients were included. These patients had a median (IQR) age of 58.2 (49.0-66.7) years and included 6739 women (40.8%) and 9774 men (59.2%). Among these patients, 10 502 (63.6%) had dyslipidemia; 9062 (54.9%) had arterial hypertension; 2146 (13.0%) had diabetes; and approximately one-third were either former smokers (2770 [16.8%]) or current smokers (2394 [14.5%]) (Table 1).
Table 1. Characteristics of Study Participants and by Lp(a) Level Categoriesa.
| No. (%) | P value | ||||
|---|---|---|---|---|---|
| Overall (n = 16 513) | Normal level (n = 11 621) | High level (n = 4071) | Very high level (n = 821) | ||
| Age, median (IQR), y | 58.2 (49.0-66.7) | 58.0 (48.6-66.7) | 58.3 (49.6-66.7) | 60.1 (51.9-67.8) | <.001 |
| Female sex | 6739 (40.8) | 4528 (39.0) | 1790 (44.0) | 421 (51.3) | <.001 |
| Male sex | 9774 (59.2) | 7093 (61.0) | 2281 (56.0) | 400 (48.7) | <.001 |
| PAD | 1139 (6.9) | 726 (6.2) | 313 (7.7) | 100 (12.2) | <.001 |
| History of ischemic heart disease | 2387 (14.5) | 1548 (13.3) | 681 (16.7) | 158 (19.2) | <.001 |
| History of stroke | 204 (1.2) | 122 (1.0) | 65 (1.6) | 17 (2.1) | .002 |
| Arterial hypertension | 9062 (54.9) | 6310 (54.3) | 2255 (55.4) | 497 (60.5) | .002 |
| Cigarette use | |||||
| Never | 11 349 (68.7) | 7824 (67.3) | 2899 (71.2) | 626 (76.2) | <.001 |
| Former | 2770 (16.8) | 2017 (17.4) | 647 (15.9) | 106 (12.9) | |
| Current | 2394 (14.5) | 1780 (15.3) | 525 (12.9) | 89 (10.8) | |
| Dyslipidemia | 10 502 (63.6) | 7126 (61.3) | 2810 (69.0) | 566 (68.9) | <.001 |
| Diabetes | 2146 (13.0) | 1480 (12.7) | 521 (12.8) | 145 (17.7) | <.001 |
| Dialysis | 75 (0.5) | 52 (0.4) | 17 (0.4) | 6 (0.7) | .47 |
| Lp(a) | |||||
| Median (IQR), mg/dL | 24 (10-60) | 15 (7-27) | 78 (62-10) | 168 (150-202) | |
| By level category | 16 513 (100.0) | 11 621 (70.4) | 4071 (24.7) | 821 (5.0) | |
| Cholesterol level, median (IQR), mg/dL | |||||
| LDL | 118.33 (92.42-146.56) | 117.56 (91.65-144.62) | 120.26 (94.35-150.43) | 120.65 (93.19-156.23) | <.001 |
| HDL | 48.72 (39.83-59.94) | 48.34 (39.06-59.55) | 49.50 (40.99-60.71) | 52.20 (42.54-64.19) | <.001 |
| Total | 194.51 (165.12-227.38) | 193.35 (163.96-225.06) | 197.60 (167.44-231.63) | 201.08 (170.92-242.07) | <.001 |
| Triglyceride level, median (IQR), mg/dL | 106.19 (76.11-153.10) | 107.08 (76.11-155.75) | 104.42 (75.22-146.90) | 107.08 (78.76-156.64) | .002 |
| Serum creatinine level, median (IQR), mg/dL | 0.90 (0.76-1.10) | 0.90 (0.76-1.09) | 0.92 (0.76-1.12) | 0.96 (0.77-1.26) | <.001 |
| Measured GFR, median (IQR), mL/min/1.73 m2 | 80 (66-93) | 80 (67-93) | 78 (64-92) | 72 (53-89) | <.001 |
| Proteinuria, median (IQR), g/L | 0.12 (0.08-0.18) | 0.12 (0.08-0.18) | 0.12 (0.08-0.18) | 0.12 (0.07-0.23) | .24 |
| Conjugated bilirubin level, median (IQR), mg/dL | 0.23 (0.18-0.29) | 0.23 (0.18-0.29) | 0.23 (0.18-0.29) | 0.18 (0.12-0.23) | .71 |
| Total bilirubin level, median (IQR), mg/dL | 0.70 (0.53-0.88) | 0.70 (0.53-0.94) | 0.70 (0.53-0.88) | 0.64 (0.18-0.29) | <.001 |
| γ-glutamyltransferase level, median (IQR), U/L | 22 (14-37) | 22 (13-38) | 22 (14-37) | 23 (14-40) | .51 |
| Alkaline phosphatase level, median (IQR), U/L | 60 (50-74) | 60 (49-73) | 62 (51-76) | 67 (54-83) | <.001 |
| Alanine aminotransferase level, median (IQR), U/L | 21 (15-30) | 21 (15-31) | 21 (15-29) | 18 (13-26) | <.001 |
| Aspartate aminotransferase level, median (IQR), U/L | 21 (17-26) | 21 (17-26) | 21 (17-26) | 21 (17-26) | .64 |
| Serum albumin, median (IQR), g/dL | 3.8 (3.4-4.1) | 3.8 (3.5-4.1) | 3.7 (3.2-4.0) | 3.7 (3.0-3.9) | <.001 |
| Lipid-lowering drugs, No./total No. (%) | 1520/2852 (53.3) | 944/1901 (49.7) | 435/749 (58.1) | 141/202 (69.8) | <.001 |
| Follow-up duration, median (IQR), y | 3.74 (1.07-7.30) | 3.80 (1.11-7.35) | 3.64 (1.01-7.27) | 3.29 (1.08-6.74) | .02 |
Abbreviations: GFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); PAD, peripheral artery disease.
SI conversion factors: To convert alkaline phosphatase to microkatal per liter, multiply by 0.0167; ALT and AST to microkatal per liter, multiply by 0.0167; bilirubin to micromoles per liter, multiply by 17.104; γ-glutamyltransferase to microkatal per liter, multiply by 0.0167; LDL, HDL, and total cholesterol to millimoles per liter, multiply by 0.0259; Lp(a) to milligrams per liter, multiply by 0.1; serum albumin to grams per liter, multiply by 10; serum creatinine to micromoles per liter, multiply by 88.4; triglycerides to millimoles per liter, multiply by 0.0113.
Continuous variables were described as medians (IQRs), and binary variables were expressed as numbers (proportions). Qualitative variables were compared using Fisher tests, and binary variables were compared using Kruskal-Wallis tests. Lipoprotein(a) level categories were as follows: normal (<50 mg/dL), high (50 to <134 mg/dL), and very high (≥134 mg/dL).
Lipoprotein(a) levels ranged from 2 to 540 mg/dL, and the median (IQR) Lp(a) level was 24.0 (10.0-60.0) mg/dL. A total of 11 621 patients (70.4%) were within the normal level (<50 mg/dL), 4071 patients (24.7%) were within the high level (50 to <134 mg/dL), and 821 patients (5.0%) were within the very high level (≥134 mg/dL). Distribution is shown in eFigure 1 in Supplement 1. Compared with patients with high or normal Lp(a) level categories, patients with a very high level were less frequently men (56.0% or 61.0% vs 48.7%; P < .001). Patients with a very high level had more comorbidities, notably PAD (12.2% vs 7.7% or 6.2%; P < .001) and ischemic heart disease (19.2% vs 16.7% or 13.3%; P < .001) compared with those with high or normal Lp(a) levels. Concomitantly, patients with a very high level had higher total cholesterol, HDL-cholesterol, or LDL-cholesterol levels than those within high or normal Lp(a) levels (Table 1). Further details regarding subgroup characteristics are available in eTables 3 and 4 in Supplement 1.
Association of High and Very High Lp(a) Levels With Major Adverse Limb Event
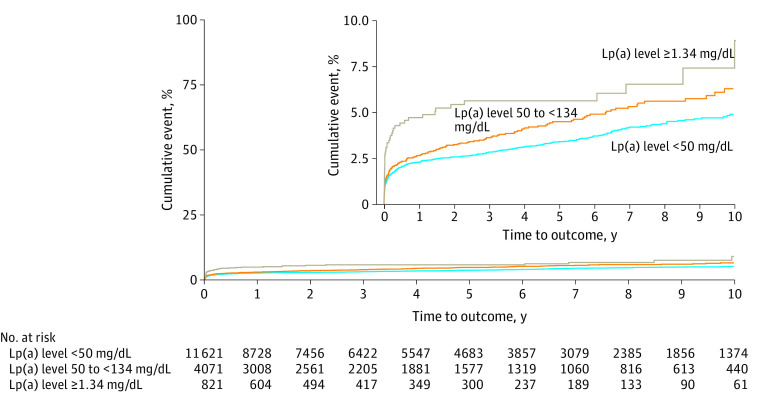
Cumulative incidence of major adverse limb event and its individual components are presented in Table 2. A total of 572 major adverse limb events occurred during a median (IQR) follow-up period of 3.74 (1.07-7.30) years. The cumulative incidence rates were 2.44% (95% CI, 2.20%-2.68%) for 1 year, 3.70% (95% CI, 3.37%-4.03%) for 5 years, and 5.33% (95% CI, 4.80%-5.85%) for 10 years. The 1-year incidence rate of major adverse limb event was 4.54% (95% CI, 3.08%-5.98%) among patients with very high Lp(a) levels, and the 1-year major amputation rate overall was 0.40% (95% CI, 0.30%-0.50%). Figure 2 shows Kaplan-Meier survival curves according to the Lp(a) level categories. Patients with very high Lp(a) level had a 5-year cumulative incidence of 5.44% (95% CI, 3.79%-7.07%) vs 4.43% (95% CI, 3.68%-5.16%) for those with high level or 3.33% (95% CI, 2.96%-3.70%) for those with normal level.
Table 2. Cumulative Incidence of Primary and Secondary Outcomes by Lp(a) Level Categoriesa.
| Lp(a) level category | Patients, No. | 1-y Event rate | 5-y Event rate | 10-y Event rate | |||
|---|---|---|---|---|---|---|---|
| Event, No. | Rate (95% CI), % | Event, No. | Rate (95% CI), % | Event, No. | Rate (95% CI), % | ||
| Major adverse limb event | |||||||
| Overall | 16 513 | 383 | 2.44 (2.20-2.68) | 504 | 3.70 (3.37-4.03) | 572 | 5.33 (4.80-5.85) |
| Normal | 11 621 | 247 | 2.24 (1.96-2.51) | 320 | 3.33 (2.96-3.70) | 366 | 4.83 (4.24-5.41) |
| High | 4071 | 100 | 2.60 (2.09-3.10) | 143 | 4.43 (3.68-5.16) | 161 | 6.21 (5.06-7.34) |
| Very high | 821 | 36 | 4.54 (3.08-5.98) | 41 | 5.44 (3.79-7.07) | 45 | 8.67 (4.72-12.45) |
| Major amputation | |||||||
| Overall | 16 513 | 61 | 0.40 (0.30-0.50) | 80 | 0.59 (0.46-0.73) | 90 | 0.84 (0.63-1.04) |
| Normal | 11 621 | 38 | 0.35 (0.24-0.47) | 49 | 0.5 (0.36-0.65) | 56 | 0.76 (0.51-1.00) |
| High | 4071 | 19 | 0.50 (0.28-0.73) | 24 | 0.76 (0.44-1.07) | 27 | 1.00 (0.57-1.42) |
| Very high | 821 | 4 | 0.56 (0.01-1.11) | 7 | 1.10 (0.28-1.93) | 7 | 1.10 (0.28-1.93) |
| Endovascular revascularization | |||||||
| Overall | 16 513 | 288 | 1.83 (1.62-2.04) | 377 | 2.78 (2.49-3.07) | 429 | 4.08 (3.6-4.54) |
| Normal | 11 621 | 180 | 1.63 (1.39-1.87) | 233 | 2.44 (2.12-2.76) | 267 | 3.56 (3.05-4.07) |
| High | 4071 | 76 | 1.97 (1.53-2.41) | 108 | 3.36 (2.71-4.01) | 124 | 5.16 (4.02-6.30) |
| Very high | 821 | 32 | 4.03 (2.65-5.38) | 36 | 4.82 (3.24-6.38) | 38 | 6.19 (3.66-8.65) |
| Surgical revascularization | |||||||
| Overall | 16 513 | 114 | 0.74 (0.60-0.88) | 162 | 1.23 (1.04-1.43) | 198 | 2.11 (1.75-2.47) |
| Normal | 11 621 | 77 | 0.71 (0.55-0.87) | 109 | 1.17 (0.94-1.39) | 133 | 1.98 (1.57-2.38) |
| High | 4071 | 29 | 0.76 (0.48-1.03) | 44 | 1.41 (0.98-1.84) | 52 | 2.18 (1.46-2.89) |
| Very high | 821 | 8 | 1.09 (0.33-1.85) | 9 | 1.28 (0.44-2.12) | 13 | 4.15 (0.68-7.51) |
Abbreviation: Lp(a), lipoprotein(a).
Lipoprotein(a) level categories were as follows: normal (<50 mg/dL), high (50 to <134 mg/dL), and very high (≥134 mg/dL).
Figure 2. Kaplan-Meier Survival Curves of the Cumulative Incidence of Major Adverse Limb Event (MALE) by Lipoprotein(a) (Lp[a]) Level Categories.
The inset shows the detail on an enlarged y-axis.
Unadjusted and adjusted AFT analysis showed that Lp(a) was associated with increased incidence of a major adverse limb event (adjusted AFT exponential estimate: 0.57; 95% CI, 0.46-0.71; Benjamini-Hochberg–corrected P < .001) (Table 3). Sensitivity analyses confirmed this result, by adjusting on lipid-lowering drug (adjusted AFT exponential estimate: 0.69; 95% CI, 0.53-0.89; Benjamini-Hochberg–corrected P < .001); history of ischemic heart disease (adjusted AFT exponential estimate: 0.59; 95% CI, 0.47-0.73; Benjamini-Hochberg–corrected P < .001); history of stroke (adjusted AFT exponential estimate: 0.57; 95% CI, 0.46-0.71; Benjamini-Hochberg–corrected P < .001); or PAD, a major factor associated with major adverse limb event (adjusted AFT exponential estimate: 0.82; 95% CI, 0.69-0.97; Benjamini-Hochberg–corrected P = .03).
Table 3. AFT Survival Model With Lognormal Distribution for the Full Cohorta.
| Outcome | Variable | Nonadjusted AFT model exponential estimate (95% CI) | P value | Adjusted AFT model exponential estimate (95% CI)b | P value | ||
|---|---|---|---|---|---|---|---|
| Uncorrected | Benjamini-Hochberg–corrected | Uncorrected | Benjamini-Hochberg–corrected | ||||
| Major adverse limb event | Log[Lp(a)] | 0.61 (0.48-0.76) | <.001 | <.001 | 0.57 (0.46-0.71) | <.001 | <.001 |
| High Lp(a)c | 0.50 (0.27-0.93) | .03 | .04 | 0.43 (0.24-0.78) | .005 | .008 | |
| Very High Lp(a)c | 0.18 (0.07-0.44) | <.001 | <.001 | 0.17 (0.07-0.40) | <.001 | <.001 | |
| Major amputation | Log[Lp(a)] | 0.56 (0.35-0.91) | 0.62 (0.40-0.98) | ||||
| High Lp(a)c | 0.42 (0.12-1.41) | 0.41 (0.13-1.32) | |||||
| Very High Lp(a)c | 0.22 (0.03-1.96) | 0.41 (0.05-3.40) | |||||
| Endovascular revascularization | Log[Lp(a)] | 0.49 (0.37-0.65) | 0.49 (0.37-0.63) | ||||
| High Lp(a)c | 0.41 (0.20-0.84) | 0.36 (0.18-0.70) | |||||
| Very High Lp(a)c | 0.07 (0.02-0.24) | 0.09 (0.03-0.29) | |||||
| Surgical revascularization | Log[Lp(a)] | 0.80 (0.60-1.07) | 0.72 (0.54-0.95) | ||||
| High Lp(a)c | 0.74 (0.34-1.61) | 0.61 (0.28-1.30) | |||||
| Very high Lp(a)c | 0.45 (0.11-1.89) | 0.35 (0.09-1.45) | |||||
| Major adverse limb event | |||||||
| Sensitivity analyses with lipid-lowering drug when prescription was available (n = 2852) as adjustment variable | Log[Lp(a)] | 0.69 (0.53-0.89) | .004 | .007 | |||
| Sensitivity analyses with PAD added as adjustment variable | Log[Lp(a)] | 0.82 (0.69-0.97) | .02 | .03 | |||
| Sensitivity analyses with history of ischemic heart disease added as adjustment variable | Log[Lp(a)] | 0.59 (0.47-0.73) | <.001 | <.001 | |||
| Sensitivity analyses with history of stroke added as adjustment variable | Log[Lp(a)] | 0.57 (0.46-0.71) | <.001 | <.001 | |||
Abbreviations: AFT, accelerated failure time; Lp(a), lipoprotein(a); PAD, peripheral artery disease.
Some cells were purposely left blank to indicate that tests were not performed to avoid α risk inflation, as stated in Methods.
AFT regressions were adjusted on sex, age, diabetes, arterial hypertension, low-density lipoprotein cholesterol, smoking status, serum creatinine levels, and dialysis. AFT regression subanalyses with lipid-lowering drugs available included the same covariables, with the lipid-lowering drugs status added.
Compared with normal Lp(a) level category as the reference.
Regarding Lp(a) level categories, patients within the very high level had a major adverse limb event–free survival time that was 83% shorter than that of patients within the normal level over a median (IQR) follow-up period of 3.29 (1.08-6.74) years (adjusted AFT exponential estimate: 0.17; 95% CI, 0.07-0.40; Benjamini-Hochberg–corrected P < .001). Accordingly, patients within the high Lp(a) level had a major adverse limb event–free survival time that was 57% shorter than that of patients within the normal level over a median (IQR) follow-up period of 3.64 (1.01-7.27) years (adjusted AFT exponential estimate: 0.43; 95% CI, 0.24-0.78; Benjamini-Hochberg–corrected P = .01).
Regarding secondary outcomes, 90 major amputations, 429 endovascular revascularizations, and 198 surgical revascularizations occurred during follow-up. Adjusted AFT analysis suggested that Lp(a) was associated with major amputation (adjusted AFT exponential estimate: 0.62; 95% CI, 0.40-0.98), endovascular revascularization (adjusted AFT exponential estimate: 0.49; 95% CI, 0.37-0.63), and surgical revascularization (adjusted AFT exponential estimate: 0.72; 95% CI, 0.54-0.95). On the other hand, none of the Lp(a) level categories was associated with major amputation or surgical revascularization (Table 3). Nevertheless, patients with very high (adjusted AFT exponential estimate: 0.09; 95% CI, 0.03-0.29) or high Lp(a) levels (adjusted AFT exponential estimate: 0.36; 95% CI, 0.18-0.70) tended to have more endovascular revascularization than patients with a normal Lp(a) level. Kaplan-meier survival curves of secondary outcomes incidence are presented in eFigures 2-4 in Supplement 1.
Discussion
In this large, retrospective cohort study using administrative data from a single center in France, we found that the Lp(a) level was independently associated with an increased incidence of major adverse limb event. High Lp(a) level (values between 50 and <134 mg/dL) was associated with an increased risk of major adverse limb event compared with a normal Lp(a) level (<50 mg/dL), corresponding to a 0.43 times shorter time to event (or 57% shorter major adverse limb event–free survival over the median follow-up period of 3.64 years). Very high Lp(a) level (≥134 mg/dL) was associated with an increased risk of major adverse limb event compared with a normal Lp(a) level, corresponding to a 0.17 times shorter time to event (or 83% shorter major adverse limb event–free survival over the median follow-up period of 3.29 years).
Lipoprotein(a) level increase is known to be associated with an increased occurrence of cardiovascular events.11,12,13,14,15,16,17,18,19,20,21 Many studies have investigated the association between MI and Lp(a) and reported an increased risk in patients with elevated Lp(a) values.11,12,13,56,57 Previous studies have suggested that Lp(a) could also be associated with stroke.14,15,16 Regarding PAD, the InCHIANTI (Invecchiare in Chianti) study involving 1002 Italian patients showed a cross-sectional association between Lp(a) levels and PAD prevalence.58 Similarly, in the Edinburgh Artery Study, elevated Lp(a) values corresponding to an increased intertertile range were significantly associated with PAD, with an adjusted hazard ratio (HR) of 1.22 (95% CI, 1.04-1.44).59 Moreover, the prospective European Prospective Investigation into Cancer–Norfolk study including 18 720 patients found an adjusted HR for PAD of 1.37 (95% CI, 1.25-1.50) for a 2.7-fold increase in Lp(a) levels.57 Recently, an Australian study including 1472 patients with PAD, which was defined by intermittent claudication, abdominal aortic aneurysm, or critical limb ischemia, found that Lp(a) levels higher than 30 mg/dL were associated with more requirements for any PAD intervention (lower-limb peripheral revascularization, abdominal aortic aneurysm repair, other aneurysm repair, or carotid artery revascularization), with an HR of 1.33 (95% CI, 1.06-1.66).33
Though data are scarce on the elevated Lp(a) values associated with the incidence of major adverse limb event,31,32 we believe we provided such data in this study, which assessed the association between Lp(a) and major adverse limb event in, to our knowledge, the largest cohort of unselected inpatient population. An analysis of the Spanish FRENA registry reported that among the 1503 stable outpatients with coronary, cerebrovascular, or peripheral artery disease, patients with Lp(a) levels between 30 and 50 mg/dL had significantly more limb amputations than those with Lp(a) levels less than 30 mg/dL (HR, 3.18; 95% CI, 1.36-7.44) and even more amputations were required for those with Lp(a) levels higher than 50 mg/dL (HR, 22.7; 95% CI, 9.38-54.9).34 A prespecified analysis of the ODYSSEY OUTCOMES (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) randomized clinical trial conducted in 18 924 patients with recent acute coronary syndrome found in the placebo group a significant link between Lp(a) levels and risk of PAD events, which were defined as any critical limb ischemia, limb revascularization, or amputation for ischemia.60 Two other studies found an association between elevated Lp(a) levels and major adverse limb event incidence among patients with documented PAD who underwent iliofemoral endarterectomy or endovascular therapy.61,62
These previous findings are consistent with the results of the current study, wherein Lp(a) values between 50 mg/dL and less than 134 mg/dL or 134 mg/dL or greater were associated with a major adverse limb event–free survival time that was 57% or 83% shorter, respectively, than the time for a Lp(a) level less than 50 mg/dL. In this cohort, the 1-year major adverse limb event rate was 2.44%, and the 1-year major amputation rate was 0.40%. In the COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) randomized, double-blind placebo-controlled trial assessing the efficacy of low-dose rivaroxaban and aspirin combination or rivaroxaban alone compared with aspirin alone, the incidence of major adverse limb event was 1.2% per year, and the incidence of major amputation was 0.4% per year in the aspirin with placebo group.8,63 In the EUCLID (Examining Use of Ticagrelor in PAD) trial comparing clopidogrel with ticagrelor in patients with symptomatic PAD, the incidence of major adverse limb event was 0.77 per 100 person-year, and the incidence of major amputation was 0.41 per 100 person-year.64,65 In the COMPASS trial, however, the major adverse limb event outcome was defined as acute limb ischemia, chronic limb ischemia, and major amputation, whereas in the EUCLID trial, the definition included only acute limb ischemia and major amputation. We could not differentiate between acute and chronic revascularizations due to the coding modalities of the HEGP medicoadministrative database, and thus we could not distinguish acute limb ischemia from chronic limb-threatening ischemia interventions. Despite this situation, the major adverse limb event rate seemed higher than rates observed in other studies probably due to a recruitment bias from the HEGP. However, the major amputation rate was consistent with rates observed in previous trials.
The Lp(a) level thresholds we selected may be debated. We chose less than 50 mg/dL as the upper limit of normal level to be in line with the 2018 American College of Cardiology and American Heart Association Guideline on the Management of Blood Cholesterol and the European Atherosclerosis Society consensus statement about Lp(a),44 in which a level greater than 50 mg/dL was considered to be a factor associated with enhanced risk of atherosclerotic cardiovascular disease. For the choice of the threshold to define extreme Lp(a) levels, the 2019 European Society of Cardiology/European Atherosclerosis Society Guidelines for the Management of Dyslipidaemias recommended considering 1 lifetime Lp(a) measurement to detect patients with extreme Lp(a) levels (>180 mg/dL).66 This guideline was based on a Mendelian randomization study suggesting that extreme Lp(a) levels increased atherosclerotic cardiovascular disease risk similarly to the levels in people with heterozygous familial hypercholesterolemia.67 Because a recent study did not have enough power to assess the association between extreme Lp(a) levels and the need for a peripheral artery revascularization,33 we took advantage of the large size of the present cohort to establish the 95th percentile Lp(a) level (≥134 mg/dL) as the very high level category, which was proposed in several studies.45,46,47,48 The very high level category was associated with major adverse limb event risk in this cohort. Moreover, the association between Lp(a) and major adverse limb event incidence remained significant after adjustment for history of ischemic heart disease or history of stroke in the sensitivity analyses, and with PAD being the main factor associated with major adverse limb event. Beyond indicating the Lp(a) level associated with MI and coronary heart disease,47 these results reinforce the potential association of Lp(a) with improved lower-limb vascular risk.
The pathophysiological mechanisms underlying the atherogenicity of Lp(a) remain poorly elucidated. However, 3 central components can be described: prothrombotic (plasminogen-like), proatherogenic (LDL-like), and proinflammatory10,22 properties. First, due to the homologic structure of apolipoprotein(a) with plasminogen, high Lp(a) levels interfere with the intrinsic fibrinolysis, promoting thrombosis and increasing cardiovascular events.23,24 Second, as for LDL cholesterol, it has been shown in atheromatous coronary and carotid lesions that there is an accumulation of Lp(a) through its LDL particle components, suggesting a deposit on atherosclerotic lesions and an increased risk of plaque rupture.25,26 In addition, Lp(a) can have a proinflammatory role in the endothelium via the oxidized phospholipids bound to the apolipoprotein(a).27 A recent secondary analysis of the ACCELERATE (Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition With Evacetrapib in Patients at a High Risk for Vascular Outcomes) trial investigating the effects of evacetrapib on patients with high cardiovascular risk reported that elevated Lp(a) levels were associated with cardiovascular death, MI, or stroke only when high-sensitivity C-reactive protein levels were greater than 2 mg/mL.68 As much as an elevated Lp(a) level seems associated with the development of coronary atherosclerosis through Lp(a) genetic polymorphism, according to large Mendelian randomization studies,12,13 it remains to be demonstrated in lower-limb atherosclerosis development. Attention should also be paid to the potential benefits of Lp(a)-lowering treatment for lower-limb vascular risk. To this point, few treatments are available for investigation. A prespecified analysis of the FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) trial showed that PCSK9 (proprotein convertase subtilisin/kexin 9) inhibition with evolocumab significantly reduced the Lp(a) level by approximately 27% among patients who received treatment and decreased the risk of coronary heart disease, death, MI, or urgent revascularization by 23% in patients with Lp(a) levels higher than 15.4 mg/dL.69 Moreover, a recent phase 2 trial assessing hepatocyte-directed antisense oligonucleotide inhibiting apolipoprotein(a) production showed a dose-dependent reduction of Lp(a) levels among patients with established cardiovascular disease and elevated Lp(a) levels,29 but the benefits for cardiovascular outcomes are still unknown.
Limitations
This study presents several limitations. First, it is a monocentric cohort analysis with a potential attrition bias given that some patients may have been admitted to another hospital for a qualifying major adverse limb event composite outcome, such as a lower-limb revascularization or major amputation. Thus, we underestimated the true incidence rate, which reinforced the power of the associations we could have found. Second, some medical information was extracted from a medicoadministrative database using ICD-10 diagnosis codes. Consequently, some data were lacking that described patients’ arterial status more precisely, such as functional symptoms, ankle brachial index measurements, ultrasonography results, or radiological anatomic patterns of arterial lesions. In particular, we could not ensure the vascular nature of coded amputations. Moreover, ICD-10 diagnosis codes might include inaccuracies or underestimation because they are coded for the French Hospital Discharge Database, a system initially used for hospital pricing. However, external validity of the methods comes from other published cardiovascular epidemiological studies on hospital inpatients in France.70,71,72,73,74 As a retrospective cohort analysis, this study might have residual confounding issues, and there is a possibility that unmeasured variables could explain the association between Lp(a) and major limb events. We tried to limit those biases by conducting an adjusted AFT regression analysis.
Third, this study covered 2 decades, during which endovascular interventional radiology made substantial progress, and its use spread widely.75,76 For this reason, we performed a sensitivity analysis on the primary outcome by considering a time effect variable, and we found that the sensitivity analysis results were consistent with those of the primary analysis.
Conclusions
In this cohort study, Lp(a) level was associated with an increased risk of lower-limb artery revascularization or major amputation among a large cohort of unselected hospitalized patients. Lipoprotein(a) needs to be considered to improve not only the cardiovascular risk but also the lower-limb vascular risk assessment.
eTable 1. Sensitivity Analyses With the Primary Outcome Models, Adding Hypolipemiant Drug Status on Patients With Available Data
eTable 2. Sensitivity Analyses With the Primary Outcome Models, Adding Time Effect Variable
eTable 3. Sensitivity Analyses With the Primary Outcome Models, Adding PAD, History of Ischaemic Heart Disease and History of Stroke
eTable 4. Baseline Characteristics According to Subgroups
eTable 5. Accelerated Failure Time (AFT) Models With MALE as the Outcome Variable
eTable 6. Accelerated Failure Time (AFT) Models With Major Amputation as the Outcome Variable
eTable 7. Accelerated Failure Time (AFT) Models With Peripheral Endovascular Revascularization as the Outcome Variable
eTable 8. Accelerated Failure Time (AFT) Models With Peripheral Surgical Revascularization as the Outcome Variable
eTable 9. P-values Included in the Benjamini-Hochberg False Discovery Rate Approach
eFigure 1. Frequency Distribution of Lipoprotein(a) [Lp(a)] in All Patients
eFigure 2. Kaplan-Meier Survival Curves of the Cumulative Incidence of Major Amputation by Lp(a) Levels
eFigure 3. Kaplan-Meier Survival Curves of the Cumulative Incidence of Peripheral Endovascular Revascularization by Lp(a) Levels
eFigure 4. Kaplan-Meier Survival Curves of the Cumulative Incidence of Peripheral Surgical Revascularization by Lp(a) Levels
eFigure 5. Distribution of Patients by Hospitalization Unit and Lp(a) Levels at Inclusion
eMethods 1. List of ICD-10 Used for Comorbidities Definitions
eMethods 2. List of CCAM (French Common Classification of Medical Procedures) Used for Outcomes Definitions
Data Sharing Statement
References
- 1.Criqui MH, Matsushita K, Aboyans V, et al. ; American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Stroke Council . Lower extremity peripheral artery disease: contemporary epidemiology, management gaps, and future directions: a scientific statement from the American Heart Association. Circulation. 2021;144(9):e171-e191. doi: 10.1161/CIR.0000000000001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowkes FGR, Aboyans V, Fowkes FJI, McDermott MM, Sampson UKA, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017;14(3):156-170. doi: 10.1038/nrcardio.2016.179 [DOI] [PubMed] [Google Scholar]
- 3.Nehler MR, Duval S, Diao L, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60(3):686-95.e2. doi: 10.1016/j.jvs.2014.03.290 [DOI] [PubMed] [Google Scholar]
- 4.Aboyans V, Ricco JB, Bartelink MEL, et al. ; ESC Scientific Document Group . 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO) The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39(9):763-816. doi: 10.1093/eurheartj/ehx095 [DOI] [PubMed] [Google Scholar]
- 5.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(12):e686-e725. doi: 10.1161/CIR.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conte MS, Bradbury AW, Kolh P, et al. ; GVG Writing Group . Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69(6S):e2245720. doi: 10.1016/j.jvs.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biscetti F, Cecchini AL, Rando MM, et al. Principal predictors of major adverse limb events in diabetic peripheral artery disease: a narrative review. Atherosclerosis Plus. 2021;46:1-14. doi: 10.1016/j.athplu.2021.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand SS, Caron F, Eikelboom JW, et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS trial. J Am Coll Cardiol. 2018;71(20):2306-2315. doi: 10.1016/j.jacc.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 9.Hess CN, Wang TY, Weleski Fu J, et al. Long-term outcomes and associations with major adverse limb events after peripheral artery revascularization. J Am Coll Cardiol. 2020;75(5):498-508. doi: 10.1016/j.jacc.2019.11.050 [DOI] [PubMed] [Google Scholar]
- 10.Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69(6):692-711. doi: 10.1016/j.jacc.2016.11.042 [DOI] [PubMed] [Google Scholar]
- 11.Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117(2):176-184. doi: 10.1161/CIRCULATIONAHA.107.715698 [DOI] [PubMed] [Google Scholar]
- 12.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331-2339. doi: 10.1001/jama.2009.801 [DOI] [PubMed] [Google Scholar]
- 13.Clarke R, Peden JF, Hopewell JC, et al. ; PROCARDIS Consortium . Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518-2528. doi: 10.1056/NEJMoa0902604 [DOI] [PubMed] [Google Scholar]
- 14.Langsted A, Nordestgaard BG, Kamstrup PR. Elevated lipoprotein(a) and risk of ischemic stroke. J Am Coll Cardiol. 2019;74(1):54-66. doi: 10.1016/j.jacc.2019.03.524 [DOI] [PubMed] [Google Scholar]
- 15.Virani SS, Brautbar A, Davis BC, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125(2):241-249. doi: 10.1161/CIRCULATIONAHA.111.045120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nave AH, Lange KS, Leonards CO, et al. Lipoprotein (a) as a risk factor for ischemic stroke: a meta-analysis. Atherosclerosis. 2015;242(2):496-503. doi: 10.1016/j.atherosclerosis.2015.08.021 [DOI] [PubMed] [Google Scholar]
- 17.Widmann MD, Sumpio BE. Lipoprotein (a): a risk factor for peripheral vascular disease. Ann Vasc Surg. 1993;7(5):446-451. doi: 10.1007/BF02002128 [DOI] [PubMed] [Google Scholar]
- 18.Valentine RJ, Grayburn PA, Vega GL, Grundy SM. Lp(a) lipoprotein is an independent, discriminating risk factor for premature peripheral atherosclerosis among White men. Arch Intern Med. 1994;154(7):801-806. doi: 10.1001/archinte.1994.00420070129015 [DOI] [PubMed] [Google Scholar]
- 19.Aboyans V, Criqui MH, Denenberg JO, Knoke JD, Ridker PM, Fronek A. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113(22):2623-2629. doi: 10.1161/CIRCULATIONAHA.105.608679 [DOI] [PubMed] [Google Scholar]
- 20.Cheng SW, Ting AC, Wong J. Lipoprotein (a) and its relationship to risk factors and severity of atherosclerotic peripheral vascular disease. Eur J Vasc Endovasc Surg. 1997;14(1):17-23. doi: 10.1016/S1078-5884(97)80220-1 [DOI] [PubMed] [Google Scholar]
- 21.Laschkolnig A, Kollerits B, Lamina C, et al. Lipoprotein (a) concentrations, apolipoprotein (a) phenotypes, and peripheral arterial disease in three independent cohorts. Cardiovasc Res. 2014;103(1):28-36. doi: 10.1093/cvr/cvu107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spence JD, Koschinsky M. Mechanisms of lipoprotein(a) pathogenicity: prothrombotic, proatherosclerotic, or both? Arterioscler Thromb Vasc Biol. 2012;32(7):1550-1551. doi: 10.1161/ATVBAHA.112.251306 [DOI] [PubMed] [Google Scholar]
- 23.Martínez C, Rivera J, Loyau S, et al. Binding of recombinant apolipoprotein(a) to human platelets and effect on platelet aggregation. Thromb Haemost. 2001;85(4):686-693. doi: 10.1055/s-0037-1615654 [DOI] [PubMed] [Google Scholar]
- 24.Discepolo W, Wun T, Berglund L. Lipoprotein(a) and thrombocytes: potential mechanisms underlying cardiovascular risk. Pathophysiol Haemost Thromb. 2006;35(3-4):314-321. doi: 10.1159/000093224 [DOI] [PubMed] [Google Scholar]
- 25.Rath M, Niendorf A, Reblin T, Dietel M, Krebber HJ, Beisiegel U. Detection and quantification of lipoprotein(a) in the arterial wall of 107 coronary bypass patients. Arteriosclerosis. 1989;9(5):579-592. doi: 10.1161/01.ATV.9.5.579 [DOI] [PubMed] [Google Scholar]
- 26.van Dijk RA, Kolodgie F, Ravandi A, et al. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J Lipid Res. 2012;53(12):2773-2790. doi: 10.1194/jlr.P030890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnitzler JG, Hoogeveen RM, Ali L, et al. Atherogenic lipoprotein(a) increases vascular glycolysis, thereby facilitating inflammation and leukocyte extravasation. Circ Res. 2020;126(10):1346-1359. doi: 10.1161/CIRCRESAHA.119.316206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsimikas S, Gordts PLSM, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020;41(24):2275-2284. doi: 10.1093/eurheartj/ehz310 [DOI] [PubMed] [Google Scholar]
- 29.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, et al. ; AKCEA-APO(a)-LRx Study Investigators . Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382(3):244-255. doi: 10.1056/NEJMoa1905239 [DOI] [PubMed] [Google Scholar]
- 30.Willeit P, Kiechl S, Kronenberg F, et al. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J Am Coll Cardiol. 2014;64(9):851-860. doi: 10.1016/j.jacc.2014.03.061 [DOI] [PubMed] [Google Scholar]
- 31.Giovanetti F, Gargiulo M, Laghi L, et al. Lipoprotein(a) and other serum lipid subfractions influencing primary patency after infrainguinal percutaneous transluminal angioplasty. J Endovasc Ther. 2009;16(3):389-396. doi: 10.1583/09-2733.1 [DOI] [PubMed] [Google Scholar]
- 32.Hishikari K, Hikita H, Nakamura S, et al. Usefulness of lipoprotein(a) for predicting clinical outcomes after endovascular therapy for aortoiliac atherosclerotic lesions. J Endovasc Ther. 2017;24(6):793-799. doi: 10.1177/1526602817728068 [DOI] [PubMed] [Google Scholar]
- 33.Golledge J, Rowbotham S, Velu R, et al. Association of serum lipoprotein (a) with the requirement for a peripheral artery operation and the incidence of major adverse cardiovascular events in people with peripheral artery disease. J Am Heart Assoc. 2020;9(6):e015355. doi: 10.1161/JAHA.119.015355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez Muñoz-Torrero JF, Rico-Martín S, Álvarez LR, Aguilar E, Alcalá JN, Monreal M; FRENA Investigators . Lipoprotein (a) levels and outcomes in stable outpatients with symptomatic artery disease. Atherosclerosis. 2018;276:10-14. doi: 10.1016/j.atherosclerosis.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 35.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 36.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 37.Degoulet P, Marin L, Lavril M, et al. The HEGP component-based clinical information system. Int J Med Inform. 2003;69(2-3):115-126. doi: 10.1016/S1386-5056(02)00101-6 [DOI] [PubMed] [Google Scholar]
- 38.HIMSS. Electronic Medical Record Adoption Model (EMRAM). January 20, 2021. Accessed September 26, 2021. https://www.himss.org/what-we-do-solutions/digital-health-transformation/maturity-models/electronic-medical-record-adoption-model-emram
- 39.Zapletal E, Rodon N, Grabar N, Degoulet P. Methodology of integration of a clinical data warehouse with a clinical information system: the HEGP case. Stud Health Technol Inform. 2010;160(Pt 1):193-197. [PubMed] [Google Scholar]
- 40.Jannot AS, Zapletal E, Avillach P, Mamzer MF, Burgun A, Degoulet P. The Georges Pompidou University Hospital clinical data warehouse: a 8-years follow-up experience. Int J Med Inform. 2017;102:21-28. doi: 10.1016/j.ijmedinf.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 41.Iezzoni LI. Assessing quality using administrative data. Ann Intern Med. 1997;127(8 Pt 2):666-674. doi: 10.7326/0003-4819-127-8_Part_2-199710151-00048 [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization . International Statistical Classification of Diseases and Related Health Problems 10th Revision. Accessed September 26, 2021. https://icd.who.int/browse10/2016/en
- 43.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168-3209. doi: 10.1016/j.jacc.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 44.Nordestgaard BG, Chapman MJ, Ray K, et al. ; European Atherosclerosis Society Consensus Panel . Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844-2853. doi: 10.1093/eurheartj/ehq386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varvel S, McConnell JP, Tsimikas S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532 359 patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36(11):2239-2245. doi: 10.1161/ATVBAHA.116.308011 [DOI] [PubMed] [Google Scholar]
- 46.Arsenault BJ, Pelletier W, Kaiser Y, et al. Association of long-term exposure to elevated lipoprotein(a) levels with parental life span, chronic disease-free survival, and mortality risk: a Mendelian randomization analysis. JAMA Netw Open. 2020;3(2):e200129. doi: 10.1001/jamanetworkopen.2020.0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol. 2013;61(11):1146-1156. doi: 10.1016/j.jacc.2012.12.023 [DOI] [PubMed] [Google Scholar]
- 48.O’Donoghue ML, Morrow DA, Tsimikas S, et al. Lipoprotein(a) for risk assessment in patients with established coronary artery disease. J Am Coll Cardiol. 2014;63(6):520-527. doi: 10.1016/j.jacc.2013.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239-241. doi: 10.1093/biomet/69.1.239 [DOI] [Google Scholar]
- 50.Wei LJ. The accelerated failure time model: a useful alternative to the Cox regression model in survival analysis. Stat Med. 1992;11(14-15):1871-1879. doi: 10.1002/sim.4780111409 [DOI] [PubMed] [Google Scholar]
- 51.Harrell FE. Parametric survival models. In: Harrell Jr Frank E, ed. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Springer Series in Statistics. Springer International Publishing; 2015:423-451. doi: 10.1007/978-3-319-19425-7_18 [DOI] [Google Scholar]
- 52.Weissler EH, Clare RM, Lokhnygina Y, et al. Predicting major adverse limb events in individuals with type 2 diabetes: insights from the EXSCEL trial. Diabet Med. 2021;38(10):e14552. doi: 10.1111/dme.14552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dua A, Lee CJ. Epidemiology of peripheral arterial disease and critical limb ischemia. Tech Vasc Interv Radiol. 2016;19(2):91-95. doi: 10.1053/j.tvir.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. 2016;4(2):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 56.Erqou S, Kaptoge S, Perry PL, et al. ; Emerging Risk Factors Collaboration . Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412-423. doi: 10.1001/jama.2009.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gurdasani D, Sjouke B, Tsimikas S, et al. Lipoprotein(a) and risk of coronary, cerebrovascular, and peripheral artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2012;32(12):3058-3065. doi: 10.1161/ATVBAHA.112.255521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volpato S, Vigna GB, McDermott MM, et al. Lipoprotein(a), inflammation, and peripheral arterial disease in a community-based sample of older men and women (the InCHIANTI study). Am J Cardiol. 2010;105(12):1825-1830. doi: 10.1016/j.amjcard.2010.01.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GDO, Fowkes FGR. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur Heart J. 2007;28(3):354-362. doi: 10.1093/eurheartj/ehl441 [DOI] [PubMed] [Google Scholar]
- 60.Schwartz GG, Steg PG, Szarek M, et al. ; ODYSSEY OUTCOMES Committees and Investigators* . Peripheral artery disease and venous thromboembolic events after acute coronary syndrome: role of lipoprotein(a) and modification by alirocumab: prespecified analysis of the ODYSSEY OUTCOMES randomized clinical trial. Circulation. 2020;141(20):1608-1617. doi: 10.1161/CIRCULATIONAHA.120.046524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomoi Y, Takahara M, Soga Y, et al. Impact of high lipoprotein(a) levels on clinical outcomes following peripheral endovascular therapy. JACC Cardiovasc Interv. 2022;15(14):1466-1476. doi: 10.1016/j.jcin.2022.05.050 [DOI] [PubMed] [Google Scholar]
- 62.Verwer MC, Waissi F, Mekke JM, et al. High lipoprotein(a) is associated with major adverse limb events after femoral artery endarterectomy. Atherosclerosis. 2022;349:196-203. doi: 10.1016/j.atherosclerosis.2021.11.019 [DOI] [PubMed] [Google Scholar]
- 63.Anand SS, Bosch J, Eikelboom JW, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10117):219-229. doi: 10.1016/S0140-6736(17)32409-1 [DOI] [PubMed] [Google Scholar]
- 64.Hiatt WR, Fowkes FGR, Heizer G, et al. ; EUCLID Trial Steering Committee and Investigators . Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017;376(1):32-40. doi: 10.1056/NEJMoa1611688 [DOI] [PubMed] [Google Scholar]
- 65.Baumgartner I, Norgren L, Fowkes FGR, et al. ; Executive Committee and Investigators of the EUCLID Trial . Cardiovascular outcomes after lower extremity endovascular or surgical revascularization: the EUCLID trial. J Am Coll Cardiol. 2018;72(14):1563-1572. doi: 10.1016/j.jacc.2018.07.046 [DOI] [PubMed] [Google Scholar]
- 66.Mach F, Baigent C, Catapano AL, et al. ; ESC Scientific Document Group . 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 67.Burgess S, Ference BA, Staley JR, et al. ; European Prospective Investigation Into Cancer and Nutrition–Cardiovascular Disease (EPIC-CVD) Consortium . Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a Mendelian randomization analysis. JAMA Cardiol. 2018;3(7):619-627. doi: 10.1001/jamacardio.2018.1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Puri R, Nissen SE, Arsenault BJ, et al. Effect of C-reactive protein on lipoprotein(a)-associated cardiovascular risk in optimally treated patients with high-risk vascular disease: a prespecified secondary analysis of the ACCELERATE trial. JAMA Cardiol. 2020;5(10):1136-1143. doi: 10.1001/jamacardio.2020.2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Donoghue ML, Fazio S, Giugliano RP, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139(12):1483-1492. doi: 10.1161/CIRCULATIONAHA.118.037184 [DOI] [PubMed] [Google Scholar]
- 70.Maura G, Blotière PO, Bouillon K, et al. Comparison of the short-term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity-matched cohort study. Circulation. 2015;132(13):1252-1260. doi: 10.1161/CIRCULATIONAHA.115.015710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lainay C, Benzenine E, Durier J, et al. Hospitalization within the first year after stroke: the Dijon stroke registry. Stroke. 2015;46(1):190-196. doi: 10.1161/STROKEAHA.114.007429 [DOI] [PubMed] [Google Scholar]
- 72.Lorgis L, Cottenet J, Molins G, et al. Outcomes after acute myocardial infarction in HIV-infected patients: analysis of data from a French nationwide hospital medical information database. Circulation. 2013;127(17):1767-1774. doi: 10.1161/CIRCULATIONAHA.113.001874 [DOI] [PubMed] [Google Scholar]
- 73.Goueslard K, Cottenet J, Mariet AS, et al. Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovasc Diabetol. 2016;15(1):15. doi: 10.1186/s12933-016-0338-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Béjot Y, Benzenine E, Lorgis L, et al. Comparative analysis of patients with acute coronary and cerebrovascular syndromes from the national French hospitalization health care system database. Neuroepidemiology. 2011;37(3-4):143-152. doi: 10.1159/000331908 [DOI] [PubMed] [Google Scholar]
- 75.Loffroy R, Qanadli SD. Editorial: modern endovascular therapy for peripheral arterial disease. Front Cardiovasc Med. 2021;8:651489. doi: 10.3389/fcvm.2021.651489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qanadli SD. Research in vascular medicine: where we are and where we are going. Front Cardiovasc Med. 2020;7:45. doi: 10.3389/fcvm.2020.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Sensitivity Analyses With the Primary Outcome Models, Adding Hypolipemiant Drug Status on Patients With Available Data
eTable 2. Sensitivity Analyses With the Primary Outcome Models, Adding Time Effect Variable
eTable 3. Sensitivity Analyses With the Primary Outcome Models, Adding PAD, History of Ischaemic Heart Disease and History of Stroke
eTable 4. Baseline Characteristics According to Subgroups
eTable 5. Accelerated Failure Time (AFT) Models With MALE as the Outcome Variable
eTable 6. Accelerated Failure Time (AFT) Models With Major Amputation as the Outcome Variable
eTable 7. Accelerated Failure Time (AFT) Models With Peripheral Endovascular Revascularization as the Outcome Variable
eTable 8. Accelerated Failure Time (AFT) Models With Peripheral Surgical Revascularization as the Outcome Variable
eTable 9. P-values Included in the Benjamini-Hochberg False Discovery Rate Approach
eFigure 1. Frequency Distribution of Lipoprotein(a) [Lp(a)] in All Patients
eFigure 2. Kaplan-Meier Survival Curves of the Cumulative Incidence of Major Amputation by Lp(a) Levels
eFigure 3. Kaplan-Meier Survival Curves of the Cumulative Incidence of Peripheral Endovascular Revascularization by Lp(a) Levels
eFigure 4. Kaplan-Meier Survival Curves of the Cumulative Incidence of Peripheral Surgical Revascularization by Lp(a) Levels
eFigure 5. Distribution of Patients by Hospitalization Unit and Lp(a) Levels at Inclusion
eMethods 1. List of ICD-10 Used for Comorbidities Definitions
eMethods 2. List of CCAM (French Common Classification of Medical Procedures) Used for Outcomes Definitions
Data Sharing Statement