This systematic review and meta-analysis evaluates data for patients with hearing loss from studies of the association of hearing interventions with cognitive function, cognitive decline, cognitive impairment, and dementia.
Key Points
Question
Do hearing aids and cochlear implants decrease the risk of subsequent cognitive decline in individuals with hearing loss?
Findings
In this systematic review and multiadjusted observational meta-analysis including 137 484 participants, the use of hearing restorative devices was associated with a 19% decrease in hazards of long-term cognitive decline such as incident dementia over a duration ranging from 2 to 25 years. Usage of these devices was also associated with a 3% improvement in cognitive test scores in the short term.
Meaning
In this meta-analysis, the usage of hearing aids and cochlear implants is associated with a decreased risk of subsequent cognitive decline; physicians should strongly encourage their patients with hearing loss to adopt such devices.
Abstract
Importance
Hearing loss is associated with cognitive decline. However, it is unclear if hearing restorative devices may have a beneficial effect on cognition.
Objective
To evaluate the associations of hearing aids and cochlear implants with cognitive decline and dementia.
Data Sources
PubMed, Embase, and Cochrane databases for studies published from inception to July 23, 2021.
Study Selection
Randomized clinical trials or observational studies published as full-length articles in peer-reviewed journals relating to the effect of hearing interventions on cognitive function, cognitive decline, cognitive impairment, and dementia in patients with hearing loss.
Data Extraction and Synthesis
The review was conducted in accordance with Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) reporting guidelines. Two authors independently searched the PubMed, Embase, and Cochrane databases for studies relating to the effect of hearing interventions on cognitive decline and dementia in patients with hearing loss.
Main Outcomes and Measures
Maximally adjusted hazard ratios (HRs) were used for dichotomous outcomes and ratio of means for continuous outcomes. Sources of heterogeneity were investigated using sensitivity and subgroup analyses, and publication bias was assessed using visual inspection, the Egger test, and trim and fill.
Results
A total of 3243 studies were screened; 31 studies (25 observational studies, 6 trials) with 137 484 participants were included, of which 19 (15 observational studies, 4 trials) were included in quantitative analyses. Meta-analysis of 8 studies, which had 126 903 participants, had a follow-up duration ranging from 2 to 25 years, and studied long-term associations between hearing aid use and cognitive decline, showed significantly lower hazards of any cognitive decline among hearing aid users compared with participants with uncorrected hearing loss (HR, 0.81; 95% CI, 0.76-0.87; I2 = 0%). Additionally, meta-analysis of 11 studies with 568 participants studying the association between hearing restoration and short-term cognitive test score changes revealed a 3% improvement in short-term cognitive test scores after the use of hearing aids (ratio of means, 1.03; 95% CI, 1.02-1.04, I2 = 0%).
Conclusions and Relevance
In this meta-analysis, the usage of hearing restorative devices by participants with hearing loss was associated with a 19% decrease in hazards of long-term cognitive decline. Furthermore, usage of these devices was significantly associated with a 3% improvement in cognitive test scores that assessed general cognition in the short term. A cognitive benefit of hearing restorative devices should be further investigated in randomized trials.
Introduction
The incidence of dementia is expected to triple by 2050 to cross 150 million cases worldwide and result in up to $50 billion in economic losses by 2030.1 Despite the high burden of disease, no exact cure currently exists to treat dementia, making addressing and targeting preventable risk factors a crucial factor in addressing dementia and cognitive impairment. Recently, hearing loss has been identified as among the top modifiable risk factors for dementia, accounting for a 9% in risk reduction.2 This is significant because not only is hearing loss highly prevalent in the community, affecting approximately 20% to 26% of adults aged 45 years and increasing to 63% in adults older than 70 years, it is also often treatable with hearing aids.3,4
Hearing restorative devices, such as cochlear implants and behind-the-ear or in-the-ear hearing aids, are electronic devices to correct hearing loss. Some studies suggested fitting hearing aids may prevent incident cognitive impairment by addressing hearing loss.5 Some observational studies have suggested hearing aids may attenuate the onset of dementia,6 possibly through decreasing cognitive load or correcting sensory deprivation in those with hearing loss. However, not all observational studies showed benefits from hearing aid use, which may be attributed to a lack of consistency in wearing hearing devices in social situations or late implementation of these devices.7,8,9
To date, no meta-analysis has pooled the available evidence on the cognitive benefit of hearing restorative devices. Because some studies may have an inadequate sample size, a pooled analysis may help increase the statistical power. Hence, this study aims to analyze both cognitive scores and longitudinal data to determine the long-term associations of hearing restorative devices with cognitive impairment and incident dementia.
Methods
This review was registered with PROSPERO (CRD42021281359) and was done in accordance with the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) guidelines (eTable 1 in the Supplement).10 Two authors (B.S.Y.Y., H.J.J.M.D.S.) searched 3 databases, PubMed, Embase, and the Cochrane Library, for retrospective and prospective cohort studies, cross-sectional studies, and randomized clinical trials relating to the effect of hearing interventions on cognitive function, cognitive decline, cognitive impairment, and dementia in patients with hearing loss from each database’s inception to July 23, 2021. The full search strategy is included in the eTable 2 in the Supplement. Two authors (B.S.Y.Y., H.J.J.M.D.S.) independently screened abstracts and titles followed by full texts to check the eligibility for inclusion, with disputes being resolved through consensus from a third independent author (E.M.S.T.). Article screening was done using the online platform Rayyan.11
Inclusion and Exclusion Criteria
We included studies with a population of adults at least 18 years old with hearing loss confirmed via pure-tone audiometry. The intervention was use of hearing restorative devices, including hearing aids and cochlear implants. The comparator was adult participants with uncorrected hearing loss. The main outcomes were either (1) dementia diagnosed based on accepted clinical diagnostic criteria (eg, Diagnostic Statistical Manual of Mental Disorders [DSM] criteria) or (2) measurement of general cognitive function via Mini-Mental State Examination (MMSE) and/or Montreal Cognitive Assessment (MoCA) or other commonly used cognitive test, or (3) cognitive impairment, diagnosed based on accepted clinical diagnostic criteria (eg, DSM criteria) or through standardized screening questionnaires (eg, MMSE, MoCA). We included randomized clinical trials or observational studies published as full-length articles in peer-reviewed journals. We excluded reviews, meta-analyses, studies that used proprietary tests not extensively validated by other studies, and studies published in any language other than English.
Risk-of-Bias Assessment
Two reviewers (B.S.Y.Y., H.J.J.M.D.S.) assessed the risk of bias of included studies using the Newcastle-Ottawa Quality Assessment Scale, and disagreements were resolved by consensus or appeal to a third author (E.M.S.T.).12 Studies scoring 7 to 9 points, 4 to 6 points, and 3 or fewer points were at low, moderate, and high risk of bias, respectively. The Newcastle-Ottawa Scale is a 9-point/star scale based on an 8-item checklist that consists of 3 domains: selection, comparability, and outcome. For each item in the checklist assessed, predetermined responses that were indicative of low risk of bias were awarded a star (eTable 3 in the Supplement).
Data Extraction
Relevant data from included articles were extracted by a pair of independent authors (B.S.Y.Y., H.J.J.M.D.S.) into a standardized extraction template. Patient data and characteristics were extracted, including first author, year published, study design, setting, country, sample size, duration of follow-up, percentage male, mean/median age, intervention (eg, the type of hearing restorative device used), hazard ratios (HRs) of dementia or mild cognitive impairment between hearing restorative device users and nonusers, mean cognitive test scores of hearing restorative device users before and after the use of the device, covariates adjusted for, statistical methods, and key findings. This process was verified by at least 1 other reviewer. A summary of characteristics of included studies can be found in eTable 4 in the Supplement.
Publication Bias Assessment and Overall Quality of Evidence
Publication bias was assessed by visual inspection for asymmetry in their respective funnel plots, and by performing an Egger test.13 The quality appraisal scores for included articles are shown in eTable 5 in the Supplement. Overall quality of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework.14
Statistical Analysis
Where there were sufficient data, we pooled maximally covariate-adjusted HRs via a mixed-effects model to compute the overall hazards of cognitive decline of participants who used hearing restorative devices, compared with those who did not. We also conducted subgroup analyses with the study population according to type of cognitive impairment, type of cognitive test used, type of hearing restorative device, and quality of studies and by continent, whereby we first computed a study-level estimate by pooling the different subgroup estimates using a fixed-effects model. Then, we pooled the different study estimates in a random-effects model to compute the overall summary estimate. For continuous outcome measures such as cognitive test scores, we pooled the ratio of means (ROM) of cognitive test scores that assessed general cognition before and after the use of hearing restorative devices. For dichotomous outcome measures such as dementia, we pooled maximally adjusted HRs.
Statistical heterogeneity was assessed via I2 and Cochran Q test values, where an I2 value of more than 50% and a Cochran Q test with a P ≤ .10 was considered significant for heterogeneity.15 Statistical significance was considered for outcomes with a P ≤ .05. All analyses were done using R version 4.0.5.16
Results
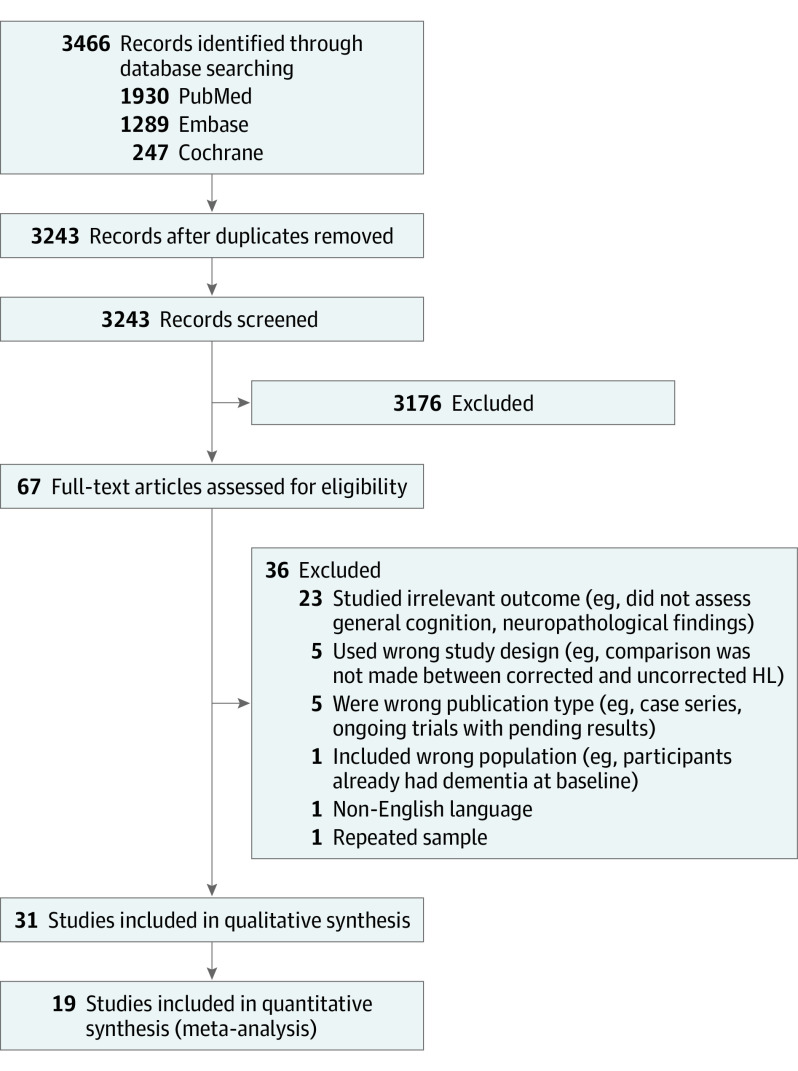
Our study selection process is summarized in Figure 1. Our systematic search generated 3243 records after removing duplicates, where 67 records had their full texts assessed for eligibility. Thirty-one studies with 137 484 participants were included in our final review, and 19 were included in our quantitative analyses. Of the 31 studies, 16 were prospective cohorts, 6 were retrospective cohorts, 3 were cross-sectional studies, 2 were nonrandomized clinical trials, 2 were single-group trials, and 2 were randomized clinical trials (with only short-term data available). Twenty-one and 10 studies had a moderate and low risk of bias, respectively, when assessed using the Newcastle-Ottawa Scale. Thirteen studies were conducted in Europe,6,7,8,17,18,19,20,21,22,23,24,25,26 12 studies were conducted in North America,27,28,29,30,31,32,33,34,35,36,37 3 studies were conducted in Asia,38,39,40 and 2 studies were conducted in Australasia.41,42 One study consisted of a multinational cohort (eTable 4 in the Supplement).43 Overall quality of evidence was low when assessed using GRADE (eTable 5 in the Supplement).
Figure 1. PRISMA Flow Diagram of the Study Selection Process.
HL indicates hearing loss; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Severity of Hearing Loss
Fourteen studies only involved participants with minimally moderate hearing loss. Five studies used self-reported hearing loss. Of the articles that included the degree of hearing loss, 7 studies stratified the number of patients based on mild, moderate, severe, and profound hearing loss; 5 studies reported the mean pure-tone audiometry definition; and 4 studies reported the minimum pure-tone audiometry results of included patients.
Cognitive Outcomes Assessed and Types of Hearing Restorative Devices
Five studies assessed the effect of hearing restorative devices on the outcome of dementia, with 1 of them looking at the conversion from mild cognitive impairment to dementia. In these studies, dementia was ascertained through physician diagnosis, International Classification of Diseases codes, or DSM criteria. Three studies looked at cognitive impairment, which was assessed dichotomously through a cutoff cognitive test score. For the remaining 23 studies, changes in general cognitive function were assessed through cognitive test scores such as MMSE (6 studies), MoCA (4 studies), Repeatable Battery for the Assessment of Neuropsychological Status (RBANS, 3 studies), and Digit Symbol Substitution Test (DSST, 3 studies). Eleven studies defined hearing restoration as the use of cochlear implants, while the remaining 20 studies defined it as the use of hearing aids.
Longitudinal Association Between Hearing Aid Use and Long-term Incidence of Cognitive Decline
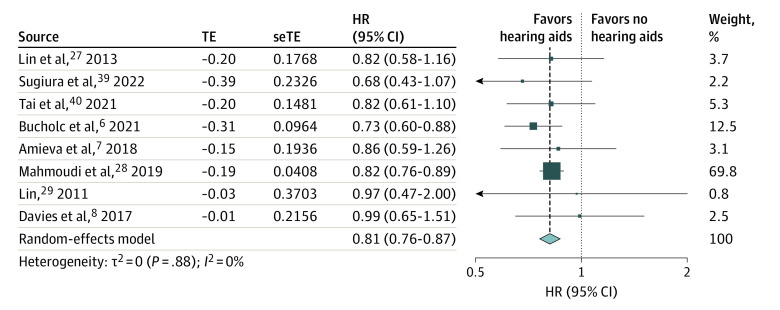
Meta-analysis of the longitudinal association of hearing aid use and cognitive decline included 8 studies with a follow-up duration ranging from 2 years to 25 years. There were no included studies that used cochlear implants for the longitudinal association between hearing aids and cognitive decline. A pooled analysis of 126 903 participants showed significantly lower hazards of any cognitive decline among hearing aid users compared with participants with uncorrected hearing loss (HR, 0.81; 95% CI, 0.76-0.87; I2 = 0%) (Figure 2). This association was adjusted for potential confounders such as age and gender (all 8 studies), education (6 studies), socioeconomic status (3 studies), and comorbidities such as hypertension (4 studies).
Figure 2. Longitudinal Association of Hearing Aid Use and Any Cognitive Decline.
The size of each box reflects the relative weight apportioned to the study; the diamond indicates the estimated pooled hazard ratio (HR) for each random-effects meta-analysis. seTE indicates standard error of treatment estimate; TE, estimated treatment effect.
Subgroup Analyses Stratified by Type of Cognitive Decline, Continent, and Quality of Studies
Stratifying the analysis into different subgroups by type of cognitive decline, studies were separated into 3 groups: incident cognitive impairment (3 studies), the development of dementia from mild cognitive impairment (1 study), and incident dementia (4 studies) (eFigure 1 in the Supplement). In all 3 subgroups, hearing aid users were associated with a significantly lower hazard of cognitive impairment (HR, 0.79; 95% CI, 0.65-0.97; I2 = 0%), conversion from mild cognitive impairment to dementia (HR, 0.73; 95% CI, 0.60-0.88), and incident dementia (HR, 0.83; 95% CI, 0.77-0.90; I2 = 0%), compared with that of the control group. Statistical tests of heterogeneity were not significant in the subgroups (I2 = 0%).
Furthermore, the analysis was also stratified by geographic continent into 3 subgroups, North America (3 studies), Asia (2 studies), and Europe (3 studies). Hearing aid users were associated with significantly lower hazards of any type of cognitive decline across all continents, including North America (HR, 0.83; 95% CI, 0.76-0.89; I2 = 0%), Asia (HR, 0.78; 95% CI, 0.61-0.99; I2 = 0%), and Europe (HR, 0.78; 95% CI, 0.67-0.92; I2 = 0%). There was no detected statistical heterogeneity in any of the subgroups (I2 = 0%) (eFigure 2 in the Supplement).
Stratifying the analysis by the quality of studies, the studies were separated into 2 subgroups, moderate risk of bias (2 studies) and low risk of bias (6 studies). There were no low-quality studies included in this meta-analysis. Our findings were not significant in either the moderate risk of bias (HR, 0.83; 95% CI, 0.77-0.90; I2 = 0%) or low risk of bias (HR, 0.77; 95% CI, 0.68-0.88; I2 = 0%) subgroup (eFigure 3 in the Supplement). There was no statistical heterogeneity in either subgroup (I2 = 0%).
Publication Bias
While visual inspection suggested possible funnel plot asymmetry, an Egger test did not indicate the presence of funnel plot asymmetry (intercept = 0.052; 95% CI, –0.73 to 0.84; P = .90). Trim and fill imputed zero additional studies (eFigure 4 in the Supplement). Further cumulative meta-analyses and leave-1-out analyses showed a stable and consistent effect size (eFigures 5 and 6 in the Supplement).
Association Between Hearing Restoration and Changes in Short-term Cognitive Test Scores
A summary of results is presented in Figure 3. Eleven studies were analyzed to demonstrate the pooled ROM of cognitive test scores before and after the use of hearing restorative devices. A pooled analysis of 568 participants revealed a 3% improvement in cognitive test scores after the use of hearing restorative devices (ROM, 1.03; 95% CI, 1.02-1.04; I2 = 0%). Short-term follow-up duration for these studies ranged from 3 months to a year. There was no statistical heterogeneity (I2 = 0%).
Figure 3. Pooled Ratio of Means (ROM) of Cognitive Test Scores Before and After the Use of Hearing Restorative Devices.
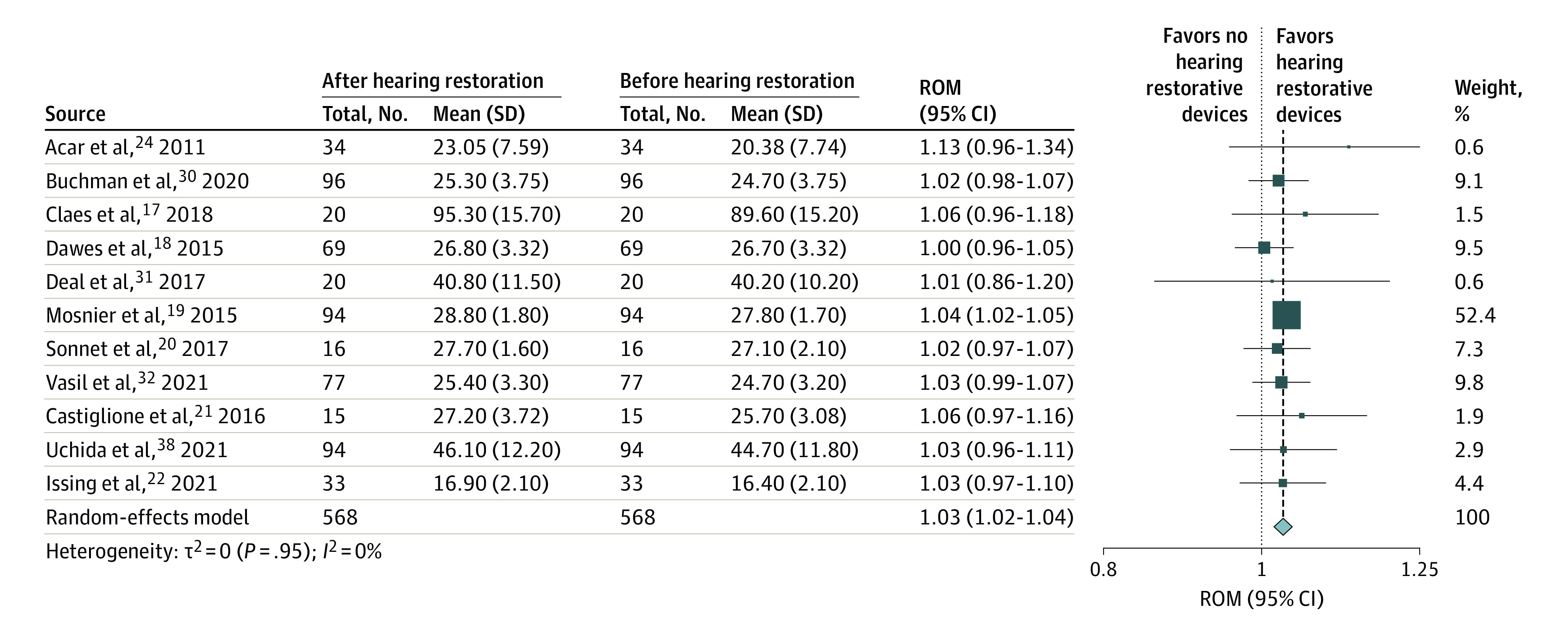
The size of each box reflects the relative weight apportioned to each study; the diamond indicates the estimated pooled ROM for each random-effects meta-analysis.
Subgroup Analyses
We conducted 4 different subgroup analyses by type of cognitive test, type of hearing restorative device, and continent. Stratifying the analysis by type of cognitive test, the studies were separated into 3 subgroups, MMSE (4 studies, n = 213), MoCA (3 studies, n = 188), and DSST (2 studies, n = 114). The improvement in cognitive test scores remained significant in the MMSE (ROM, 1.03; 95% CI, 1.01-1.05; I2 = 5%) and MoCA (ROM, 1.03; 95% CI, 1.00-1.06; I2 = 0%) subgroups but did not reach statistical significance in the DSST (ROM, 1.03; 95% CI, 0.96-1.10; I2 = 0%) subgroup (eFigure 7 in the Supplement). When analyzed by type of hearing restorative device, the studies were separated into 2 subgroups, cochlear implants (7 studies, n = 351) and hearing aids (4 studies, n = 217). Our findings remained significant in the cochlear implant (ROM, 1.03; 95% CI, 1.02-1.05) subgroup but became nonsignificant in that of hearing aids (ROM, 1.02; 95% CI, 0.98-1.05) (eFigure 8 in the Supplement).
When stratifying the results by continent, the studies were separated into 3 subgroups, Asia (2 studies, n = 128), North America (3 studies, n = 193), and Europe (6 studies, n = 247). The trend of improved cognitive test scores remained consistent in the North American (ROM, 1.03; 95% CI, 1.00-1.06) and European (ROM, 1.03; 95% CI, 1.02-1.05) subgroups, although statistical significance was not achieved in the Asian subgroup (eFigure 9 in the Supplement). Stratifying the analysis by the quality of studies, the studies were separated into 2 subgroups, moderate risk of bias (9 studies, n = 454) and low risk of bias (2 studies, n = 114). There were no low-quality studies included in this meta-analysis. Our findings were not significant in either the moderate risk of bias (ROM, 1.02; 95% CI, 1.00-1.04; I2 = 0%) or low risk of bias (ROM, 1.04; 95% CI, 1.02-1.05; I2 = 0%) subgroup (eFigure 10 in the Supplement). There was no statistical heterogeneity in all subgroups (I2 = 0%).
Publication Bias and Other Analyses
Visual inspection suggested possible funnel plot asymmetry. However, an Egger test did not indicate the presence of funnel plot asymmetry (intercept = 0.152; 95% CI, –0.52 to 0.83; P = .67). Trim and fill imputed 2 additional studies with minimal changes to the pooled effect size (ROM, 1.03; 95% CI, 1.02 to 1.04) (eFigure 11 in the Supplement).
Systematic Review
There were a few additional studies excluded from our meta-analysis because of incompatible data such as regression coefficients and the lack of raw cognitive test scores before and after the hearing intervention. There were 9 studies that supported the conclusions of our meta-analysis. Brewster et al33 conducted a small pilot randomized clinical trial (n = 13) that showed an improvement in RBANS for the component of immediate memory after 12 weeks of hearing aid use. Furthermore, Cuoco et al23 found that patients with mild hearing loss fitted with hearing aids had significant improvements on the clock drawing test compared with patients without hearing aids after 6 months. Deal et al34 revealed greater declines in memory and global function between those with moderate/severe hearing impairment; declines among those without hearing aids were the greatest, and the follow-up was 20 years. Additionally, Glick and Sharma35 performed neuroelectrical studies where hearing aid treatment was associated with reversal in abnormal cortical reorganization and with gains in speech perception and cognitive performance after 6 months. Lin29 also elucidated that patients with greater hearing loss scored lower on the DSST, and usage of hearing restorative devices was positively associated with cognitive functioning. Furthermore, Mertens et al43 found cochlear implantation improved overall cognitive functioning and attention after 14 months of use scored on RBANS. Separately, Qian et al37 highlighted that hearing aid use was associated with better MMSE scores. Lastly, Sarant et al41 found that the use of cochlear implants after 5 years resulted in an improvement in average executive function. In a separate study by the same author and colleagues,42 cognitive test scores also improved significantly after 18 months of hearing aid use, especially in female individuals.
On the other hand, 3 studies we found provided evidence against the conclusions in our meta-analysis. A small cross-sectional study by Kramer et al36 found that patients fitted with cochlear implants did not have significantly different cognitive function, after adjustment, compared with those with uncorrected profound hearing loss. Similarly, Öberg et al25 found no significant differences in cognitive function between patients with hearing aids and patients with hearing difficulties without hearing aids. Additionally, van Hooren et al26 also found hearing aid use did not significantly improve cognitive performance after 1 year when measured. More details on these studies can be found in eTables 3 and 4 in the Supplement.
Discussion
In this systematic review and meta-analysis of 31 studies comprising 137 484 participants, the use of hearing restorative devices in participants with hearing loss was found to be significantly associated with a 19% reduction in hazards of any cognitive decline, compared with their counterparts with uncorrected hearing loss, adjusting for possible confounders, including age and gender, education, socioeconomic status, and comorbidities. Importantly, this benefit is evident for both normal baseline cognition and baseline mild cognitive impairment. Furthermore, the use of these devices was significantly associated with a 3% improvement in cognitive test scores assessing general cognition. These findings were robust to subgroup analyses, quantitative assessments of publication bias, as well as cumulative and leave-1-out meta-analyses.
To the best of our knowledge, this is the first comprehensive quantitative synthesis looking at associations between hearing restoration and cognitive decline. While previous meta-analyses have established a significantly increased odds of dementia and cognitive impairment among participants with hearing loss,44 this study further adds value by suggesting that correcting for this sensory deficit is associated with a slower decline in cognition.
The exact mechanisms underlying the cognitive benefits of hearing aids and cochlear implants have not been elucidated, although there are several postulated theories regarding the relationship between hearing loss and dementia that have been extensively discussed.45,46,47 First, the common-cause hypothesis suggests both hearing loss and cognitive decline occur independently as a result of a common mechanism such age-related neurodegenerative processes involving vascular burden, oxidative stress, and genetics.45,46,48 However, the results of our study seem to refute this hypothesis, as it proposes that hearing aids would not be able to correct hearing loss as auditory function deteriorates alongside cognitive function and would be unaffected by interventions. Instead we have shown that there is a statistically significant benefit associated with hearing interventions, which could correct or prevent cognitive decline to a certain degree.
Multiple other hypotheses for the link between hearing loss and dementia provide possible support for the cognitive benefits of hearing aids. The cognitive load hypothesis suggests individuals with hearing loss may allocate high amounts of cognitive resources for auditory perceptual processing as they perform effortful listening, as evidenced by poorer downstream recall and secondary task performance in patients with hearing loss.49,50 Hence, fewer cognitive resources may be allocated for executive function and other cognitive tasks, including memory encoding. Thus, hearing restorative devices may reduce cognitive burden from listening, redirecting cognitive resources back to cognitive tasks.6 Furthermore, the sensory deficit hypothesis suggests that lack of sensory input may lead to structural alterations, including atrophy. Imaging studies found that reduced volumes in the primary auditory cortex, whole brain, and especially the right temporal lobe were predicted by hearing impairment,51,52 and this atrophy affects cognitive ability that originates from similarly affected cortical areas. Allowing hearing restorative devices to provide sensory stimulation before prolonged deprivation may cause cortical changes that could prevent cognitive deterioration. Further, hearing loss may be associated with social isolation,53,54 possibly due to difficulties in following conversation that result from hearing loss, causing individuals to withdraw from social activities.47,55 Multiple prospective studies have demonstrated strong relationships between social isolation and dementia.56,57,58 Hearing restorative devices could improve social connectedness by empowering individuals to participate in social activities that require hearing and communication, and some studies have suggested hearing interventions reduce loneliness.55,59 Thus, hearing aid use may prevent social isolation and its resultant development of cognitive impairment,5 although further studies are required to analyze this association.60
Hence, hearing loss in dementia is likely to be multifactorial, and a combination of these theories most likely contributes to the benefit seen from hearing aids and cochlear implants. Furthermore, our results suggest with appropriate follow-up time, hearing interventions are effective. A recent study showed subclinical hearing loss was related to cognitive function, suggesting the importance of early intervention.61 This adds on to the Lancet Commission,2 suggesting that hearing loss may be a risk factor for dementia in middle-aged adults. Because this is an emerging area of study where we are increasingly able to recognize subclinical hearing loss, further studies should explore whether patients who are unaware of their hearing loss could benefit from hearing restorative devices.
Our subgroup analysis found that the results remained significant in the North American and European subgroups of the cochlear implant group but was not statistically significant in the Asian subgroup. However, it should be noted that these results should be interpreted with caution, as subgroup analysis results are observational and require studies with larger power to evaluate the effect of hearing restorative devices in the subgroups. The difference in statistical significance may be attributed to a smaller sample size and the fewer included studies in these subgroups, rather than an absence of effect, and differences are unlikely to be clinically meaningful. Hence, having more studies in Asian subgroups or using the DSST would be important to strengthen our results and calls for more studies to provide power to these various subgroups.
Strengths and Limitations
The strengths of this study lie in the large number of systematically included studies from different countries and the lack of evidence of publication bias, which enhances the credibility of our results. Additionally, we studied a myriad of cognitive domains and different cognitive tests, providing a comprehensive scope of studies to analyze the relationship between hearing intervention use and cognitive function. However, the limitations of our study should be addressed. Most importantly, we were also unable to compare the severity of hearing loss in patients. Hence, we were unable to determine if the benefit of using hearing restorative devices is seen across the spectrum of hearing loss or to different degrees of deafness. Consequently, some studies included were also of low quality on the GRADE score. Additionally, we could not perform meta-regression for other confounding variables such as patients’ ethnicity and education level because data were only available in limited studies.
Nevertheless, our meta-analysis findings were thorough, which were complemented by a comprehensive series of sensitivity analyses, stratified based on the type of cognitive test used, type of hearing restorative device, continent, and type of cognitive decline. To minimize clinical heterogeneity, we only opted for tests that assessed general cognitive function, such as MMSE and MoCA, in our prespecified protocol. Consequently, our study may be limited in showing which areas of cognition hearing interventions truly affect. Also, there could be residual confounding present, especially in studies with very short follow-up times. The supposed improvement in cognitive test scores is confounded by the fact that participants can simply hear the instructions of the test better after hearing restoration. Studies that present longer follow-up times may also be more useful in evaluating the association of hearing intervention on dementia.
Conclusions
In this multiadjusted observational meta-analysis of 31 observational studies comprising 137 484 participants, the use of hearing restorative devices by participants with hearing loss was found to be significantly associated with a 19% decrease in the hazards of long-term cognitive decline. Furthermore, the use of these devices was also significantly associated with a 3% improvement in short-term cognitive test scores that assessed general cognition. This study adds to the growing evidence base and serves as an impetus for clinicians treating patients with hearing loss to persuade them to adopt hearing restorative devices, to mitigate their risk of cognitive decline such as dementia.
eTable 1. PRISMA checklist
eTable 2. Search strategy
eTable 3. Risk of bias assessment using Newcastle-Ottawa Scale
eTable 4. Characteristics of included studies
eTable 5. Evaluation of quality of pooled evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework
eFigure 1. Forest plot showing the longitudinal association of hearing aid use and any cognitive decline, stratified by the type of cognitive decline
eFigure 2. Forest plot showing the longitudinal association of hearing aid use and any cognitive decline, stratified by continent
eFigure 3. Forest plot showing the pooled ratio of means of cognitive test scores before and after the use of hearing restorative devices, stratified by the quality of studies
eFigure 4. Contour-enhanced funnel plot showing the longitudinal association of hearing aid use and any cognitive decline, with missing studies imputed via the trim-and-fill method
eFigure 5. Cumulative meta-analysis, by publication year, showing the longitudinal association of hearing aid use and any cognitive decline
eFigure 6. Leave-out-one meta-analysis, showing the longitudinal association of hearing aid use and any cognitive decline
eFigure 7. Forest plot showing the pooled ratio of means of cognitive test scores before and after the use of hearing restorative devices, stratified by the cognitive test used
eFigure 8. Forest plot showing the pooled ratio of means of cognitive test scores before and after the use of hearing restorative devices, stratified by the type of device
eFigure 9. Forest plot showing the pooled ratio of means of cognitive test scores before and after the use of hearing restorative devices, stratified by continent
eFigure 10. Forest plot showing the longitudinal association of hearing aid use and any cognitive decline, stratified by the quality of studies
eFigure 11. Contour-enhanced funnel plot for the ratio of means of cognitive test scores before and after the use of hearing restorative devices, with missing studies imputed via the trim-and-fill method
References
- 1.GBD 2019 Dementia Forecasting Collaborators . Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105-e125. doi: 10.1016/S2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673-2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 3.Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66(5):582-590. doi: 10.1093/gerona/glr002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scholes S, Biddulph J, Davis A, Mindell JS. Socioeconomic differences in hearing among middle-aged and older adults: cross-sectional analyses using the Health Survey for England. BMJ Open. 2018;8(2):e019615. doi: 10.1136/bmjopen-2017-019615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregory S, Billings J, Wilson D, Livingston G, Schilder AG, Costafreda SG. Experiences of hearing aid use among patients with mild cognitive impairment and Alzheimer’s disease dementia: a qualitative study. SAGE Open Med. 2020;8:2050312120904572. doi: 10.1177/2050312120904572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucholc M, McClean PL, Bauermeister S, et al. Association of the use of hearing aids with the conversion from mild cognitive impairment to dementia and progression of dementia: a longitudinal retrospective study. Alzheimers Dement (N Y). 2021;7(1):e12122. doi: 10.1002/trc2.12122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amieva H, Ouvrard C, Meillon C, Rullier L, Dartigues JF. Death, depression, disability, and dementia associated with self-reported hearing problems: a 25-year study. J Gerontol A Biol Sci Med Sci. 2018;73(10):1383-1389. doi: 10.1093/gerona/glx250 [DOI] [PubMed] [Google Scholar]
- 8.Davies HR, Cadar D, Herbert A, Orrell M, Steptoe A. Hearing impairment and incident dementia: findings from the English Longitudinal Study of Ageing. J Am Geriatr Soc. 2017;65(9):2074-2081. doi: 10.1111/jgs.14986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen MF, Bonnefoy M, Adrait A, et al. ; ADPHA study group . Efficacy of hearing aids on the cognitive status of patients with Alzheimer’s disease and hearing loss: a multicenter controlled randomized trial. J Alzheimers Dis. 2017;58(1):123-137. doi: 10.3233/JAD-160793 [DOI] [PubMed] [Google Scholar]
- 10.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan, a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90(8):1067-1076. doi: 10.1097/ACM.0000000000000786 [DOI] [PubMed] [Google Scholar]
- 13.Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74(3):785-794. doi: 10.1111/biom.12817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334(7584):94-96. doi: 10.1136/bmj.39057.406644.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153-160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claes AJ, Van de Heyning P, Gilles A, Van Rompaey V, Mertens G. Cognitive performance of severely hearing-impaired older adults before and after cochlear implantation: preliminary results of a prospective, longitudinal cohort study using the RBANS-H. Otol Neurotol. 2018;39(9):e765-e773. doi: 10.1097/MAO.0000000000001936 [DOI] [PubMed] [Google Scholar]
- 18.Dawes P, Cruickshanks KJ, Fischer ME, Klein BE, Klein R, Nondahl DM. Hearing-aid use and long-term health outcomes: hearing handicap, mental health, social engagement, cognitive function, physical health, and mortality. Int J Audiol. 2015;54(11):838-844. doi: 10.3109/14992027.2015.1059503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosnier I, Bebear JP, Marx M, et al. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol Head Neck Surg. 2015;141(5):442-450. doi: 10.1001/jamaoto.2015.129 [DOI] [PubMed] [Google Scholar]
- 20.Sonnet MH, Montaut-Verient B, Niemier JY, Hoen M, Ribeyre L, Parietti-Winkler C. Cognitive abilities and quality of life after cochlear implantation in the elderly. Otol Neurotol. 2017;38(8):e296-e301. doi: 10.1097/MAO.0000000000001503 [DOI] [PubMed] [Google Scholar]
- 21.Castiglione A, Benatti A, Velardita C, et al. Aging, cognitive decline and hearing loss: effects of auditory rehabilitation and training with hearing aids and cochlear implants on cognitive function and depression among older adults. Audiol Neurootol. 2016;21(suppl 1):21-28. doi: 10.1159/000448350 [DOI] [PubMed] [Google Scholar]
- 22.Issing C, Baumann U, Pantel J, Stöver T. Impact of hearing rehabilitation using cochlear implants on cognitive function in older patients. Otol Neurotol. 2021;42(8):1136-1141. doi: 10.1097/MAO.0000000000003153 [DOI] [PubMed] [Google Scholar]
- 23.Cuoco S, Cappiello A, Scarpa A, et al. Neuropsychological profile of hearing-impaired patients and the effect of hearing aid on cognitive functions: an exploratory study. Sci Rep. 2021;11(1):9384. doi: 10.1038/s41598-021-88487-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acar B, Yurekli MF, Babademez MA, Karabulut H, Karasen RM. Effects of hearing aids on cognitive functions and depressive signs in elderly people. Arch Gerontol Geriatr. 2011;52(3):250-252. doi: 10.1016/j.archger.2010.04.013 [DOI] [PubMed] [Google Scholar]
- 25.Öberg M, Marcusson J, Nägga K, Wressle E. Hearing difficulties, uptake, and outcomes of hearing aids in people 85 years of age. Int J Audiol. 2012;51(2):108-115. doi: 10.3109/14992027.2011.622301 [DOI] [PubMed] [Google Scholar]
- 26.van Hooren SA, Anteunis LJ, Valentijn SA, et al. Does cognitive function in older adults with hearing impairment improve by hearing aid use? Int J Audiol. 2005;44(5):265-271. doi: 10.1080/14992020500060370 [DOI] [PubMed] [Google Scholar]
- 27.Lin FR, Yaffe K, Xia J, et al. ; Health ABC Study Group . Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293-299. doi: 10.1001/jamainternmed.2013.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmoudi E, Basu T, Langa K, et al. Can hearing aids delay time to diagnosis of dementia, depression, or falls in older adults? J Am Geriatr Soc. 2019;67(11):2362-2369. doi: 10.1111/jgs.16109 [DOI] [PubMed] [Google Scholar]
- 29.Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66(10):1131-1136. doi: 10.1093/gerona/glr115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchman CA, Herzog JA, McJunkin JL, et al. ; CI532 Study Group . Assessment of speech understanding after cochlear implantation in adult hearing aid users: a nonrandomized controlled trial. JAMA Otolaryngol Head Neck Surg. 2020;146(10):916-924. doi: 10.1001/jamaoto.2020.1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deal JA, Albert MS, Arnold M, et al. A randomized feasibility pilot trial of hearing treatment for reducing cognitive decline: results from the Aging and Cognitive Health Evaluation in Elders pilot study. Alzheimers Dement (N Y). 2017;3(3):410-415. doi: 10.1016/j.trci.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasil KJ, Ray C, Lewis J, Stefancin E, Tamati TN, Moberly AC. How does cochlear implantation lead to improvements on a cognitive screening measure? J Speech Lang Hear Res. 2021;64(3):1053-1061. doi: 10.1044/2020_JSLHR-20-00195 [DOI] [PubMed] [Google Scholar]
- 33.Brewster KK, Pavlicova M, Stein A, et al. A pilot randomized controlled trial of hearing aids to improve mood and cognition in older adults. Int J Geriatr Psychiatry. 2020;35(8):842-850. doi: 10.1002/gps.5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deal JA, Sharrett AR, Albert MS, et al. Hearing impairment and cognitive decline: a pilot study conducted within the Atherosclerosis Risk in Communities neurocognitive study. Am J Epidemiol. 2015;181(9):680-690. doi: 10.1093/aje/kwu333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glick HA, Sharma A. Cortical neuroplasticity and cognitive function in early-stage, mild-moderate hearing loss: evidence of neurocognitive benefit from hearing aid use. Front Neurosci. 2020;14:93. doi: 10.3389/fnins.2020.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kramer S, Vasil KJ, Adunka OF, Pisoni DB, Moberly AC. Cognitive functions in adult cochlear implant users, cochlear implant candidates, and normal-hearing listeners. Laryngoscope Investig Otolaryngol. 2018;3(4):304-310. doi: 10.1002/lio2.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian ZJ, Wattamwar K, Caruana FF, et al. Hearing aid use is associated with better Mini-Mental State Exam performance. Am J Geriatr Psychiatry. 2016;24(9):694-702. doi: 10.1016/j.jagp.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 38.Uchida Y, Mise K, Suzuki D, et al. A multi-institutional study of older hearing aids beginners: a prospective single-arm observation on executive function and social interaction. J Am Med Dir Assoc. 2021;22(6):1168-1174. doi: 10.1016/j.jamda.2021.02.035 [DOI] [PubMed] [Google Scholar]
- 39.Sugiura S, Uchida Y, Nishita Y, et al. Prevalence of usage of hearing aids and its association with cognitive impairment in Japanese community-dwelling elders with hearing loss. Auris Nasus Larynx. 2022;49(1):18-25. doi: 10.1016/j.anl.2021.03.017 [DOI] [PubMed] [Google Scholar]
- 40.Tai CJ, Tseng TG, Hsiao YH, et al. Effects of hearing impairment and hearing aid use on the incidence of cognitive impairment among community-dwelling older adults: evidence from the Taiwan Longitudinal Study on Aging (TLSA). BMC Geriatr. 2021;21(1):76. doi: 10.1186/s12877-021-02012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarant J, Harris D, Busby P, et al. The effect of cochlear implants on cognitive function in older adults: initial baseline and 18-month follow up results for a prospective international longitudinal study. Front Neurosci. 2019;13:789. doi: 10.3389/fnins.2019.00789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarant J, Harris D, Busby P, et al. The effect of hearing aid use on cognition in older adults: can we delay decline or even improve cognitive function? J Clin Med. 2020;9(1):E254. doi: 10.3390/jcm9010254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mertens G, Andries E, Claes AJ, et al. Cognitive improvement after cochlear implantation in older adults with severe or profound hearing impairment: a prospective, longitudinal, controlled, multicenter study. Ear Hear. 2021;42(3):606-614. doi: 10.1097/AUD.0000000000000962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2018;144(2):115-126. doi: 10.1001/jamaoto.2017.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev. 2015;23(pt B):154-166. doi: 10.1016/j.arr.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 46.Uchida Y, Sugiura S, Nishita Y, Saji N, Sone M, Ueda H. Age-related hearing loss and cognitive decline: the potential mechanisms linking the two. Auris Nasus Larynx. 2019;46(1):1-9. doi: 10.1016/j.anl.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 47.Griffiths TD, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108(3):401-412. doi: 10.1016/j.neuron.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Humes LE, Busey TA, Craig J, Kewley-Port D. Are age-related changes in cognitive function driven by age-related changes in sensory processing? Atten Percept Psychophys. 2013;75(3):508-524. doi: 10.3758/s13414-012-0406-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging. 2009;24(3):761-766. doi: 10.1037/a0014802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCoy SL, Tun PA, Cox LC, Colangelo M, Stewart RA, Wingfield A. Hearing loss and perceptual effort: downstream effects on older adults’ memory for speech. Q J Exp Psychol A. 2005;58(1):22-33. doi: 10.1080/02724980443000151 [DOI] [PubMed] [Google Scholar]
- 51.Peelle JE, Troiani V, Grossman M, Wingfield A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. 2011;31(35):12638-12643. doi: 10.1523/JNEUROSCI.2559-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin FR, Ferrucci L, An Y, et al. Association of hearing impairment with brain volume changes in older adults. Neuroimage. 2014;90:84-92. doi: 10.1016/j.neuroimage.2013.12.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shukla A, Harper M, Pedersen E, et al. Hearing loss, loneliness, and social isolation: a systematic review. Otolaryngol Head Neck Surg. 2020;162(5):622-633. doi: 10.1177/0194599820910377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg. 2014;150(3):378-384. doi: 10.1177/0194599813518021 [DOI] [PubMed] [Google Scholar]
- 55.Hughes SE, Hutchings HA, Rapport FL, McMahon CM, Boisvert I. Social connectedness and perceived listening effort in adult cochlear implant users: a grounded theory to establish content validity for a new patient-reported outcome measure. Ear Hear. 2018;39(5):922-934. doi: 10.1097/AUD.0000000000000553 [DOI] [PubMed] [Google Scholar]
- 56.Rafnsson SB, Orrell M, d’Orsi E, Hogervorst E, Steptoe A. Loneliness, social integration, and incident dementia over 6 years: prospective findings from the English Longitudinal Study of Ageing. J Gerontol B Psychol Sci Soc Sci. 2020;75(1):114-124. doi: 10.1093/geronb/gbx087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355(9212):1315-1319. doi: 10.1016/S0140-6736(00)02113-9 [DOI] [PubMed] [Google Scholar]
- 58.Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322-2326. doi: 10.1212/01.WNL.0000147473.04043.B3 [DOI] [PubMed] [Google Scholar]
- 59.Weinstein BE, Sirow LW, Moser S. Relating hearing aid use to social and emotional loneliness in older adults. Am J Audiol. 2016;25(1):54-61. doi: 10.1044/2015_AJA-15-0055 [DOI] [PubMed] [Google Scholar]
- 60.Ellis S, Sheik Ali S, Ahmed W. A review of the impact of hearing interventions on social isolation and loneliness in older people with hearing loss. Eur Arch Otorhinolaryngol. 2021;278(12):4653-4661. doi: 10.1007/s00405-021-06847-w [DOI] [PubMed] [Google Scholar]
- 61.Golub JS, Brickman AM, Ciarleglio AJ, Schupf N, Luchsinger JA. Association of subclinical hearing loss with cognitive performance. JAMA Otolaryngol Head Neck Surg. 2020;146(1):57-67. doi: 10.1001/jamaoto.2019.3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. PRISMA checklist
eTable 2. Search strategy
eTable 3. Risk of bias assessment using Newcastle-Ottawa Scale
eTable 4. Characteristics of included studies
eTable 5. Evaluation of quality of pooled evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework
eFigure 1. Forest plot showing the longitudinal association of hearing aid use and any cognitive decline, stratified by the type of cognitive decline
eFigure 2. Forest plot showing the longitudinal association of hearing aid use and any cognitive decline, stratified by continent
eFigure 3. Forest plot showing the pooled ratio of means of cognitive test scores before and after the use of hearing restorative devices, stratified by the quality of studies
eFigure 4. Contour-enhanced funnel plot showing the longitudinal association of hearing aid use and any cognitive decline, with missing studies imputed via the trim-and-fill method
eFigure 5. Cumulative meta-analysis, by publication year, showing the longitudinal association of hearing aid use and any cognitive decline
eFigure 6. Leave-out-one meta-analysis, showing the longitudinal association of hearing aid use and any cognitive decline
eFigure 7. Forest plot showing the pooled ratio of means of cognitive test scores before and after the use of hearing restorative devices, stratified by the cognitive test used
eFigure 8. Forest plot showing the pooled ratio of means of cognitive test scores before and after the use of hearing restorative devices, stratified by the type of device
eFigure 9. Forest plot showing the pooled ratio of means of cognitive test scores before and after the use of hearing restorative devices, stratified by continent
eFigure 10. Forest plot showing the longitudinal association of hearing aid use and any cognitive decline, stratified by the quality of studies
eFigure 11. Contour-enhanced funnel plot for the ratio of means of cognitive test scores before and after the use of hearing restorative devices, with missing studies imputed via the trim-and-fill method