This randomized clinical trial investigates the effect of a machine learning recommender messaging system and viral peer recruitment tool kit on a smoking-cessation intervention.
Key Points
Question
Can a machine learning recommender system and viral peer recruitment improve a digital intervention for smoking cessation?
Findings
In this randomized clinical trial of 1487 people who smoked, those with access to viral peer-recruitment tools had significantly improved smoking cessation outcomes (44.8%) compared with those with no such access (30.8%). There were no differences in cessation outcomes between a machine learning recommender and standard motivational messaging intervention.
Meaning
These findings suggest that viral peer-recruitment tools may improve the dissemination and effectiveness of digital smoking-cessation interventions.
Abstract
Importance
Novel data science and marketing methods of smoking-cessation intervention have not been adequately evaluated.
Objective
To compare machine learning recommender (ML recommender) computer tailoring of motivational text messages vs a standard motivational text–based intervention (standard messaging) and a viral peer-recruitment tool kit (viral tool kit) for recruiting friends and family vs no tool kit in a smoking-cessation intervention.
Design, Setting, and Participants
This 2 ×2 factorial randomized clinical trial with partial allocation, conducted between July 2017 and September 2019 within an online tobacco intervention, recruited current smokers aged 18 years and older who spoke English from the US via the internet and peer referral. Data were analyzed from March through May 2022.
Interventions
Participants registering for the online intervention were randomly assigned to the ML recommender or standard messaging groups followed by partially random allocation to access to viral tool kit or no viral tool kit groups. The ML recommender provided ongoing refinement of message selection based on user feedback and comparison with a growing database of other users, while the standard system selected messages based on participant baseline readiness to quit.
Main Outcomes and Measures
Our primary outcome was self-reported 7-day point prevalence smoking cessation at 6 months.
Results
Of 1487 participants who smoked (444 aged 19-34 years [29.9%], 508 aged 35-54 years [34.1%], 535 aged ≥55 years [36.0%]; 1101 [74.0%] females; 189 Black [12.7%] and 1101 White [78.5%]; 106 Hispanic [7.1%]), 741 individuals were randomly assigned to the ML recommender group and 746 individuals to the standard messaging group; viral tool kit access was provided to 745 participants, and 742 participants received no such access. There was no significant difference in 6-month smoking cessation between ML recommender (146 of 412 participants [35.4%] with outcome data) and standard messaging (156 of 389 participants [40.1%] with outcome data) groups (adjusted odds ratio, 0.81; 95% CI, 0.61-1.08). Smoking cessation was significantly higher in viral tool kit (177 of 395 participants [44.8%] with outcome data) vs no viral tool kit (125 of 406 participants [30.8%] with outcome data) groups (adjusted odds ratio, 1.48; 95% CI, 1.11-1.98).
Conclusions and Relevance
In this study, machine learning–based selection did not improve performance compared with standard message selection, while viral marketing did improve cessation outcomes. These results suggest that in addition to increasing dissemination, viral recruitment may have important implications for improving effectiveness of smoking-cessation interventions.
Trial Registration
ClinicalTrials.gov Identifier: NCT03224520
Introduction
More than 7 million yearly deaths are attributed to smoking globally, including 480 000 deaths in the US.1 In-person smoking-cessation programs have limited reach due to logistical, geographic, and time constraints.2 Deciding to use these intensive interventions is a major step for people who smoke given that most such individuals (70%-80%) are not ready to quit.3 Smoking-cessation health communication programs accessible via the internet and mobile devices offer a light-touch intervention and fewer barriers to entry. Compared with usual care or attention controls, these programs increase quit rates.4,5 Because of this potential, many real-world implementations have been created (eg, the US government SmokeFree website6) as alternate resources for people who smoke.7 Leaders have called for further innovations to improve the reach and effectiveness of digital interventions to increase their impact.8
The promise of big data analytics for behavioral science has been recognized.9 However, this promise has not been realized in computer-tailored health communication, a widely used method in health communication programs.10 Computer tailoring focuses on selecting the best message for an individual. Standard computer tailoring uses selected variables from patient baseline profiles matched to specific if-then rules to select messages for subsets of patients.10,11 Companies like Amazon use a special class of algorithms (recommender systems based on artificial intelligence and machine learning) to select messages for individuals.12,13 Recommender systems can be programmed to adapt to ongoing individual and group user feedback to improve tailoring over time. We developed a similar machine learning–based recommender system for computer tailoring, and our pilot studies demonstrated modest improvement in smoking cessation rates.14,15,16
Currently, viral peer marketing (ie, providing participants with a tool kit to recruit friends or family members) is a preferred method for finding new research participants for online studies given that potential new recruits are more likely to trust a referral from a friend.17 In our prior pilot study, we found that in approximately 1 year (July 2013 to September 2014), viral peer recruitment was associated with a 4-fold increase in the number of individuals in our sample using the digital tobacco intervention.18 Many participants noted that having the opportunity and tools to recruit peers was beneficial to their own quit smoking efforts (75.4%) and motivated them to get support from those around them.18
Our pilot data suggested that viral peer marketing and recommender system functions could synergistically affect each other to improve the reach and effectiveness of our intervention. Although the primary function of the viral peer marketing (viral tool kit) is to increase the reach, it may lead to increased smoking cessation, with the peer recruiter serving as a positive role model to those recruited.19,20 In addition to promoting cessation, the recommender system (ML recommender) may lead to more engaged participants (due to the increased relevance of the messages), potentially motivating them to use the viral marketing tools, increasing the reach of the digital intervention. Thus, we designed our hybrid dissemination and effectiveness trial as a 2 × 2 factorial randomized large trial to test the viral tool kit and ML recommender independently and their interaction effect on reach and smoking cessation.21
Methods
This randomized clinical trial is reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline and template for intervention description and replication checklist. The study was approved by the University of Massachusetts Chan Medical School Institutional Review Board, and participants provided informed consent online.
Study Design
Our protocol for this study is described elsewhere.21 Briefly, all participants registering on the digital tobacco intervention site between August 2017 and March 2019 were first randomly assigned to ML recommender or standard messaging groups. They were then allocated to receive the viral tool kit based on their recruitment source, using a partial randomization approach. Participants recruited directly to the study (via search engine advertisements and ResearchMatch, a free and secure online tool developed by Vanderbilt University)22 were randomized to receive or not receive the viral tool kit, while those who self-reported recruitment by their peers were assigned to receive access to the viral tool kit. Our respective comparisons were a standard motivational messaging intervention (standard messaging) and no access to the viral peer marketing tool kit (no viral tool kit). This study presents 3 hypotheses related to our smoking-cessation outcomes. In the first hypothesis (H1), we tested whether quit rates among individuals exposed to the fully enhanced group (ML recommender and viral tool kit) were greater than those assigned to the other 3 groups: ML recommender and no tool kit, standard messaging and viral tool kit, and standard messaging and no tool kit. Our second hypothesis (H2) compared quit rates between individuals who were exposed to the viral tool kit with any type of messaging intervention. The third hypothesis (H3) compared quit rates of the ML recommender and standard messaging groups with any type of viral peer-recruitment tool set access. These hypotheses were tested within the Decide2Quit (University of Massachusetts)23,24 digital tobacco intervention that included self-management functions, online community, and peer support accessed through the website interface. The trial protocol and statistical analysis plan are provided in Supplement 1.
Participants and Recruitment
Participants were current smokers aged 18 years and older who spoke English. Participants were recruited from the US online (via Google and Facebook), via SmokeFree6 and ResearchMatch, and using peer recruitment.25 To recruit online, we developed and posted online advertisements customized to appear to smokers searching for quit smoking–related search terms online. We posted a summary of our project with contact information on the SmokeFree website’s “Join a Research Study” webpage. Volunteers on ResearchMatch were contacted by our research team if they matched our eligibility criteria and agreed to be contacted. Lastly, we tested peer recruitment (access to the viral tool kit) for increasing access to the digital tobacco intervention site as a recruitment source.
Randomization
We randomized participants using a table conducted in random blocks of different sizes (8 and 12 participants) to ensure balance among groups and reduce predictability of the allocation process. Randomization occurred in 2 stages at the time of initial registration. Participants were first randomized to receive messages from the ML recommender or standard messaging communication systems. This was followed by an allocation to receive or not receive access to the viral tool kit depending on the recruitment source captured via a self-report question presented to participants immediately after they consented to the study. The question was “How did you hear about Decide2Quit?” and answer choices included a friend or family member, online ad (Facebook or Google), ResearchMatch, or the SmokeFree government site. All peer-recruited participants (those who indicated that they were referred by friends or family) received access to the viral tool kit to reduce cross-contamination given that these peer-recruited participants could communicate with their peers about the viral tool kit. Participants who indicated other sources were randomly allocated to receive or not receive the viral tool kit. Study staff were blinded to allocation during initial baseline assessment and follow-up.
Interventions
Computer-Tailored Health Communication Systems
Participants received 2 email messages weekly in the first 4 weeks after registration. They then received 1 email message per week for the remaining 4 months until they reached 6 months from their registration date. Because our goal was to evaluate the selection method, both systems selected from the same database of messages. The messaging database included messages that were developed in a prior study26 and written by experts (eg, behaviorists, physicians, and nurses) and peers, informed by current guidelines and social cognitive theory.3,27 However, the ML recommender and standard messaging systems differed in how messages were selected for participants. The standard messaging system selected messages for participants based on their baseline readiness to quit (described subsequently).10,11 The ML recommender system used feedback from participants to improve message selection. In this study, we used participant feedback from prior data collection from 900 then-current or former smokers and participants in this study.28,29
Access to Viral Peer-Recruitment Tool Kit
We provided peer-recruitment tools, including a social media website plug-in that allowed participants to send private recruitment messages to friends or family and email referrals.18 We encouraged users to recruit friends or family members who were smokers during registration. We also sent 1 weekly motivational email encouraging peer recruitment throughout the study period. Participants additionally had access to an online training video and a recruitment tracker. However, participants were allowed to recruit without using our tools given that our goal was to increase recruitment of participants via peer recruitment. Unlike some other studies,30,31 we did not incentivize participants to recruit others to the study in an effort to mimic real-world settings.
Data Collection
Participants received $25 incentives at each data collection time (baseline registration, 1 week, 1 month, and 6 months). We collected self-reported baseline data and sociodemographic information online as a 2-part survey (at registration and 1 week) to reduce participant burden. We sent up to 4 email reminders over 2 weeks to complete the surveys online. If participants failed to respond to our email messages for the 6-month survey, we contacted them to complete the survey over the phone. Data collection was completed in September 2019.
Measures
At baseline, participants self-reported their age category, sex, race, ethnicity, education level (high school or less, some college or technical school, or college graduate), financial status (difficulty paying for medical care), and current number of cigarettes smoked per day. Given that there are known differences in smoking status and cessation by racial and ethnic background and because we analyzed at these levels in other studies, we collected race and ethnicity data for this study. Available categories for race were African American, American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander, and White; we combined American Indian or Alaska Native, Asian, and Native Hawaiian or other Pacific Islander as other race due to small sample sizes. For ethnicity, participants could self-report as Hispanic or Latino or not Hispanic or Latino. We assessed readiness to quit smoking by asking, “People often go through different steps when quitting smoking. Where are you in the process?” (available responses were “I’m not thinking about quitting,” “I set a quit date,” and “I quit today or I’ve already quit”). Our primary outcome was 6-month 7-day point prevalence abstinence assessed using the question “Do you currently smoke cigarettes (smoked even 1 puff in the last 7 days)?”32 This self-report outcome and time point are valid, have been commonly used in other cessation trials,33,34,35 and are acceptable to researchers.36 For participants who self-reported quitting, we offered an additional $50 incentive if they were willing to submit a NicAlert nicotine saliva test (Nymox).37,38,39 For participants willing to do so, we mailed strips with clear instructions and a link to an online location for participants to upload images of their results. The nicotine saliva test is a semiquantitative method that uses a dipstick to measure the level of cotinine in a sample of saliva. The results were read as 0 to 6, and as recommended, any value of 1 or greater was considered to indicate tobacco use.40,41,42 A secondary outcome of risk reduction was assessed as the difference between the number of cigarettes smoked per day at 6-month follow-up compared with baseline.
Sample Size
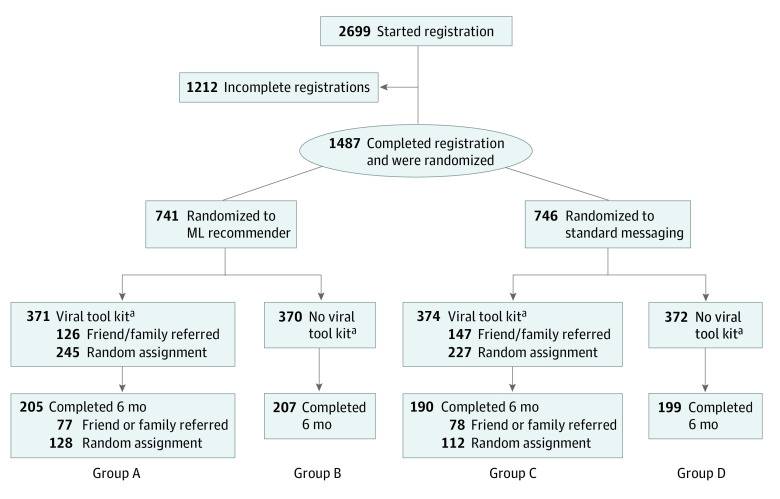
Assuming a comparison cessation rate of 15%43 and a 2-sided significance level of 0.05, a sample size of 300 participants in each group would achieve 80% power to detect a difference of 9% in quit rates between any 2 groups (quit rate in intervention = 24%) based on a Z-test with pooled variance. Therefore, the targeted sample size was 300 participants for each of 4 allocation groups (Figure).
Figure. Study Flowchart.
ML recommender indicates machine learning recommender computer-tailored motivational texting intervention; standard messaging, standard motivational texting intervention; viral tool kit, viral peer-recruitment tool kit. Group A was the fully enhanced group, exposed to the ML recommender and viral tool kit. Group B was exposed to the ML recommender with no viral tool kit. Group C was exposed to standard messaging with viral tool kit. Group D was exposed to standard messaging with no viral tool kit.
aPartial randomization: individuals referred by family or friends were allocated to the viral tool kit group, while those recruited from other sources were randomized.
Statistical Analysis
Statistical analyses were performed using Stata/IC statistical software version 15.1 (StataCorp). For cessation analysis, we used the 7-day point prevalence cessation that was self-reported by participants at 6 months as the outcome variable and used logistic regression and intent-to-treat analysis (ie, participants were analyzed according to their original study groups assigned by randomization). The 3 subhypotheses of H1 were analyzed using logistic regression for a 2 × 2 factorial design with an interaction term. We analyzed H2 and H3 by testing with indicator variables for combined group membership, as described subsequently in more detail. We created unadjusted models and models that were adjusted for sex and education. Each hypothesis was examined by a complete case analysis (primary analysis) and 2 sensitivity analyses: penalty imputation, in which participants with missing outcomes were regarded as smoking, and multiple imputation of missing outcomes. For complete case and penalty imputation analyses, we report unadjusted and adjusted regression results accounting for unbalanced covariates introduced by peer recruitment. For multiple imputation, we stratified the imputation of missing outcome values by study groups and generated 100 imputation sets. The imputation model built on covariates that were unbalanced due to missing data or predictive for outcome values. The same covariates were used to adjust the analysis model.
H1 comprised 3 subhypotheses. The first H1 subhypothesis compared participants exposed to the ML recommender and viral tool kit interventions (group A) vs those exposed only to the ML recommender intervention (group B). The second H1 subhypothesis compared participants in group A vs those who were exposed only to the viral tool kit and standard messaging (group C). The third H1 subhypothesis compared individuals in group A vs those who were exposed to standard messaging but not the viral tool kit (group D) (Figure).
H2 compared 2 combined groups (individuals exposed to the ML recommender intervention regardless of exposure to the viral tool kit [A and B]) vs all others (C and D). This hypothesis also compared 2 additional combined groups (individuals exposed to the viral tool kit regardless of exposure to the ML recommender [A and C]) vs all others (B and D) (Figure).
Although the number of nicotine tests was small, we examined test results for all hypotheses by categorizing tests by smoker vs nonsmoker groups and used the χ2 statistic to test for differences. We also tested all 3 hypotheses with risk reduction analysis as our outcome. We limited the maximum number of cigarettes smoked per day at baseline to 75 cigarettes given that there were 3 outliers reporting more than 100 cigarettes per day. If individuals reported not smoking at 6 months, we set their number of cigarettes per day at 6 months to 0. We used a t test to compare the difference in mean risk reduction between study groups. Risk reduction analysis was conducted only on complete case data. A 2-sided P < .05 was considered statistically significant. Data were analyzed from March through May 2022.
Results
Among 1487 participants (444 aged 19-34 years [29.9%], 508 aged 35-54 years [34.1%], 535 aged ≥55 years [36.0%]; 1101 [74.0%] females; 189 Black [12.7%] and 1101 White [78.5%]; 106 Hispanic [7.1%]) (Table 1), 741 individuals were randomly assigned to the ML recommender group and 746 participants to the standard messaging group (Figure). Viral tool kit access was provided to 745 participants, and 742 participants received no such access. Among all randomized individuals, 801 participants completed their 6-month survey and 686 participants did not, for a retention rate of 53.4% and a lost to follow-up rate of 46.1%. We found significant differences at baseline in sex or education across groups.
Table 1. Baseline Participant Characteristics by Study Group.
| Characteristic | Participants, No. (%) (N = 1487)a | |||
|---|---|---|---|---|
| Group A (n = 371) | Group B (n = 370) | Group C (n = 374) | Group D (n = 372) | |
| Age group, y | ||||
| 19-34 | 109 (29.4) | 98 (26.5) | 127 (34.0) | 110 (29.6) |
| 35-54 | 129 (34.8) | 132 (35.7) | 130 (34.8) | 117 (31.5) |
| ≥55 | 133 (35.9) | 140 (37.8) | 117 (31.3) | 145 (39.0) |
| Sex | ||||
| Female | 259 (69.8) | 281 (76.0) | 270 (72.2) | 291 (78.2) |
| Male | 112 (30.2) | 89 (24.1) | 104 (27.8) | 81 (21.8) |
| Race | ||||
| African American or Black | 49 (13.2) | 49 (13.2) | 47 (12.6) | 44 (11.8) |
| White | 299 (80.6) | 298 (80.5) | 294 (78.6) | 300 (80.7) |
| Otherb | 23 (6.2) | 23 (6.2) | 33 (8.8) | 28 (7.5) |
| Ethnicity | ||||
| Total with data, No. | 354 | 349 | 347 | 347 |
| Hispanic or Latino | 22 (6.2) | 22 (6.3) | 27 (7.8) | 35 (10.1) |
| Not Hispanic or Latino | 332 (93.8) | 327 (93.7) | 320 (92.2) | 312 (89.9) |
| Education level | ||||
| Total with data, No. | 245 | 257 | 246 | 239 |
| ≤High school | 73 (29.8) | 69 (26.9) | 95 (38.6) | 62 (25.9) |
| Some college or technical school | 108 (44.1) | 111 (43.2) | 98 (39.8) | 109 (45.6) |
| College graduate | 64 (26.1) | 77 (30.0) | 53 (22.0) | 68 (28.0) |
| Difficulty paying for medical care | ||||
| Total with data, No. | 246 | 257 | 249 | 239 |
| Very hard or hard | 90 (36.6) | 83 (32.3) | 82 (32.9) | 75 (31.4) |
| Somewhat hard | 77 (31.3) | 86 (33.5) | 88 (35.3) | 99 (41.4) |
| Not very hard | 79 (32.1) | 88 (34.2) | 79 (31.7) | 65 (27.2) |
| Readiness to quit smoking | ||||
| Total with data, No. | 368 | 365 | 372 | 371 |
| I am not thinking about quitting | 17 (4.6) | 10 (2.7) | 17 (4.6) | 18 (4.9) |
| I am thinking of quitting, or I have set a quit date | 303 (82.3) | 299 (81.9) | 294 (79.0) | 286 (77.1) |
| I quit today or have already quit | 49 (13.0) | 56 (15.3) | 61 (16.4) | 67 (18.1) |
| Cigarettes smoked, No./dc | ||||
| 0-5 | 53 (14.3) | 39 (10.5) | 55 (14.8) | 48 (12.9) |
| 6-20 | 253 (68.2) | 248 (67.0) | 246 (66.0) | 238 (64.0) |
| ≥21 | 65 (17.5) | 83 (22.4) | 72 (19.3) | 86 (23.1) |
Group A was the fully enhanced group, exposed to the machine learning recommender computer-tailored motivational texting intervention (ML recommender) and viral peer-recruitment tool kit (viral tool kit). Group B was exposed to the ML recommender with no viral tool kit. Group C was exposed to the standard motivational texting intervention (standard messaging) with viral tool kit. Group D was exposed to standard messaging with no viral tool kit.
American Indian or Alaska Native, Asian, and Native Hawaiian or other Pacific Islander individuals were combined as other race due to low sample sizes.
Maximum number of baseline cigarettes per day was set at 75 cigarettes given that there were 3 outliers reporting more than 100 cigarettes/d.
In H1, the fully enhanced group (A) had significantly increased quit rates compared with the group exposed to the ML recommender with no access to the viral tool kit (B) (89 of 205 participants [43.4%] vs 57 of 207 participants [27.5%] with outcome data; adjusted odds ratio [aOR], 1.91; 95% CI, 1.20-3.03) (Table 2). In H2, we found significant baseline differences between individuals exposed to the viral tool kit (groups B and D) and those not receiving the viral tool kit (groups A and C) in sex (529 [71.0%] female vs 572 [77.1%] female; P = .007) and education level (eg, 117 college graduates [23.8%] vs 145 college graduates [29.2%]; P = .02) (eTable 1 in Supplement 2). We additionally found that individuals with access to the viral tool kit (groups A and C) had significant improvements in the 6-month 7-day point prevalence smoking-cessation outcome compared with those without the viral tool kit (groups B and D) (177 of 395 participants [44.8%] vs 125 of 406 participants [30.8%] with outcome data; aOR, 1.48; 95% CI, 1.11-1.98) (Table 2). There was a significant decrease in the mean (SD) number of cigarettes smoked per day between these groups (−10.2 [10.8] cigarettes/d vs −8.6 [10.8] cigarettes/d; P = .04).
Table 2. Smoking Cessation Rates at 6 mo.
| Group comparisona | Participants, No./total No. (%) (n = 801)b | Unadjusted model | Model adjusted for covariatesc | ||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Hypothesis 1 | |||||
| Subhypothesis 1 | |||||
| Group A | 89/205 (43.4) | 2.02 (1.34-3.05) | .001 | 1.91 (1.20-3.03) | .005 |
| Group B | 57/207 (27.5) | 1 [Reference] | 1 [Reference] | ||
| Subhypothesis 2 | |||||
| Group A | 89/205 (43.4) | 0.89 (0.60-1.32) | .56 | 0.82 (0.52-1.28) | .39 |
| Group C | 88/190 (46.3) | 1 [Reference] | 1 [Reference] | ||
| Subhypothesis 3 | |||||
| Group A | 89/205 (43.4) | 1.48 (0.99-2.21) | .06 | 1.24 (0.79-1.95) | .41 |
| Group D | 68/199 (34.2) | 1 [Reference] | 1 [Reference] | ||
| Hypothesis 2 | |||||
| Groups A and C (viral tool kit) | 177/395 (44.8) | 1.54 (1.19-1.99) | .001 | 1.48 (1.11-1.98) | .01 |
| Groups B and D (no viral tool kit) | 125/406 (30.8) | 1 [Reference] | 1 [Reference] | ||
| Hypothesis 3 | |||||
| Groups A and B (ML recommender) | 146/412 (35.4) | 0.93 (0.71-1.20) | .56 | 0.81 (0.61-1.08) | .16 |
| Groups C and D (standard messaging) | 156/389 (40.1) | 1 [Reference] | 1 [Reference] | ||
Abbreviations: ML recommender, machine learning recommender computer-tailored motivational texting intervention; OR, odds ratio; standard messaging, standard motivational texting intervention; viral tool kit, viral peer-recruitment tool kit.
Group A was the fully enhanced group, exposed to the ML recommender and viral tool kit. Group B was exposed to the ML recommender with no viral tool kit. Group C was exposed to standard messaging with viral tool kit. Group D was exposed to standard messaging with no viral tool kit.
Analyses are among participants with complete data at 6 months.
Multivariable logistic regression was adjusted for sex and education level.
In H3, there were no baseline differences between individuals exposed to the ML recommender (groups A and B) and those exposed to standard messaging (groups C and D) (eTable 2 in Supplement 2). There was no significant difference in 6-month smoking cessation between ML recommender (146 of 412 participants [35.4%] with outcome data) and standard messaging (156 of 389 participants [40.1%] with outcome data) groups (aOR, 0.81; 95% CI, 0.61-1.08) (Table 2) or mean (SD) decrease in cigarettes smoked per day (−8.9 [10.5] cigarettes/d vs −9.9 [10.8] cigarettes/d; P = .20).
In 2 sensitivity analyses (penalty and multiple imputation) on 6-month smoking cessation, results were consistent with the complete case analysis for all hypotheses (eTables 3 and 4 in Supplement 2). For example, there was no significant difference in 6-month smoking cessation between the ML recommender (146 participants [19.7%]) and standard messaging (156 participants [20.9%]) groups when treating missing outcomes as smoking (eTable 3 in Supplement 2). Our secondary analysis compared smoking cessation and decrease in number of cigarettes smoked per day at 6 months by self-reported referral source. Among 155 participants with complete data who self-reported being recruited by friends or family members, 94 participants quit smoking (60.6%) compared with 83 of 240 participants with complete data who were recruited from other sources (34.6%; aOR, 2.56; 95% CI, 1.66-3.96) (Table 3). Participants who self-reported being recruited by friends or family members had a mean (SD) decrease of −12.1 (9.4) cigarettes/d, which was significantly different from the mean (SD) decrease of −8.7 (10.9) cigarettes/d in those who were recruited from other sources (P < .001).
Table 3. Sensitivity Analysis of Fully Randomized Group vs Groups Partially Allocated With or Without Viral Tool Kit.
| Groupa | Randomization or allocation | No./total No. (%) (N = 1487) | Unadjusted model | Model adjusted for covariates b | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| Viral tool kit groups (A and C) | Fully randomized participantsc | 83/240 (34.6) | 1.19 (0.85-1.67) | .32 | 1.26 (0.86-1.86) | .23 |
| Allocated participantsd | 94/155 (60.6) | 3.46 (2.36-5.09) | <.001 | 2.56 (1.66-3.96) | <.001 | |
| No viral tool kit groups (B and D) | All participants fully randomized | 125/406 (30.8) | 1 [Reference] | NA | 1 [Reference] | NA |
Abbreviations: ML recommender, machine learning recommender computer-tailored motivational texting intervention; NA, not applicable; OR, odds ratio; standard messaging, standard motivational texting intervention; viral tool kit, viral peer-recruitment tool kit.
Group A was the fully enhanced group, exposed to the ML recommender and viral tool kit. Group B was exposed to the ML recommender with no viral tool kit. Group C was exposed to standard messaging with viral tool kit. Group D was exposed to standard messaging with no viral tool kit.
Multivariable logistic regression, adjusted for age, sex, and education level reported through the baseline survey, was used for analyses for the complete study sample and study sample with smoking data missing. The analysis model for multiple imputation was adjusted by age, race, sex, education level, and smoking status and number of cigarettes per day measured at baseline.
Self-reported as recruited by methods other than the viral peer recruitment, including social media ads, web browser ads, ResearchMatch, and the government SmokeFree website.25
Self-reported as recruited via viral peer recruitment.
Discussion
This randomized clinical trial examined 6-month smoking-cessation data among 1487 people enrolled in a digital smoking-cessation trial. Much of the effect in smoking cessation in our study may have been due to access to the viral peer recruitment tool kit, as our H2 results suggested. Viral peer recruitment draws strength from the constructs of relatedness as proposed in self-determination theory.44 Relatedness among individuals, or the desire to feel connected to others, can support behavior change and may explain the significant difference in H2 results.44,45 For the recruiter, the act of recruiting others may have promoted accountability to act as a role model and hence promoted cessation. In our pilot study,18 many participants reported that the act of peer recruitment motivated them to quit smoking. Conversely, being recruited by a family or peer may also motivate the individual to quit smoking (because the individual does not want to disappoint the friend or family member). Our secondary analyses for this hypothesis revealed that participants who self-reported that they were peer-recruited were more likely to quit than those who self-reported that they were recruited by other means. When individuals engage in activities that are social in nature, such as providing social support, perceptions of relatedness play an important role in motivation and increasing engagement.46
There could be several reasons for the H3 null finding. We were comparing the recommender system against a robust standard messaging system that was proven effective in a prior trial.15 These systems selected messages from the same database; thus, the only difference was the method of message selection. Our recommender system was programmed with minimal data from 900 participants, while the recommender systems used by large companies have been programmed on large amounts of user data, and this difference may have had an effect on our outcomes.
Limitations
This study has several limitations. We did not adjust for multiple comparisons47 given that our hypotheses were planned before data collection and were supported by robust pilot data, thus lessening the probability of false positive findings. Testing peer recruitment for recruiting and effectiveness resulted in inclusion of nonrandomized elements in the peer-recruitment study. Because of privacy concerns, we were not able to track which participants recruited their peers, reducing our ability to conduct further assessment of how peer recruitment affected participants who recruited peers. Although our retention rate of 52.7% at 6 months was comparable with those of many published trials48,49,50,51,52 that recruited via social media and we conducted sensitivity analyses using multiple imputation, our results could be affected by nonresponse bias. We also based our 6-month smoking-cessation data on self-report. Although we collected biochemical verification for some participants, the data were not sufficient to perform subanalyses. Biochemical verification is mainly used to monitor for differential misclassification by randomization group.40 Studies that are in person and intense generally have more misclassification because of the personal connection between the smoker and the study team. Less misclassification occurs in low-intensity, light-touch studies in which comparisons are similar, as they were our study. Our missing data rate was similar among all groups.
Conclusions
This randomized clinical trial may enhance understanding of novel viral recruitment methods and recommender systems for smoking cessation. Our results suggest that viral recruitment may be beneficial not only for spreading the intervention, but also for motivating smokers to quit smoking. This may open new opportunities to design digital interventions for smoking cessation as team efforts and build collaborative tools for people who smoke to not only refer, but also engage with one another throughout the intervention. Our results further suggest that through digital methods, we may have the potential to reach a larger proportion of individuals with effective methods, resulting in a greater impact. Although the recommender system in this study did not outperform the standard messaging system, the benefit of the system’s ability to continuously learn from its users may be best realized in a large and long-term implementation in which the system has sufficient duration to collect substantial amounts of feedback data.
Trial Protocol
eTable 1. Comparison of Baseline Characteristics Between Individuals With and Without Viral Tool Kit
eTable 2. Comparison of Baseline Characteristics Between Individuals Randomized to ML Recommender and Standard Messaging
eTable 3. Smoking-Cessation Rates at 6 Months for Each 3 Hypotheses With Missing Outcomes Treated as Smoking
eTable 4. Smoking-Cessation Rates at 6 mo for 3 Hypotheses With Missing Outcomes Treated Using Multiple Imputation
Data Sharing Statement
References
- 1.Office on Smoking and Health . Smoking and tobacco use: fast facts and fact sheets. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/. Centers for Disease Control and Prevention. Accessed April 27, 2015.
- 2.Hendricks PS, Cases MG, Thorne CB, et al. Hospitalized smokers’ expectancies for electronic cigarettes versus tobacco cigarettes. Addict Behav. 2015;41:106-111. doi: 10.1016/j.addbeh.2014.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiore C, Jaén CR, Baker TB. Treating Tobacco Use And Dependence: 2008 Update. US Department of Health and Human Services; 2008. Accessed December 1, 2022. https://www.ncbi.nlm.nih.gov/books/NBK63952/ [Google Scholar]
- 4.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y, Dobson R. Mobile phone text messaging and app-based interventions for smoking cessation. Cochrane Database Syst Rev. 2019;10(10):CD006611. doi: 10.1002/14651858.CD006611.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amiri S, Khan MAB. Digital interventions for smoking abstinence: a systematic review and meta-analysis of randomized control trials. J Addict Dis. 2022:1-25. doi: 10.1080/10550887.2022.2058300 [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health . SmokeFree. Accessed December 1, 2022. https://smokefree.gov/
- 7.Bol N, Smit ES, Lustria MLA. Tailored health communication: opportunities and challenges in the digital era. Digit Health. 2020;6:2055207620958913. doi: 10.1177/2055207620958913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mermelstein R, Fiore MC, Bernstein SL, et al. Tobacco control research priorities for the next decade: working group recommendations for 2016-2025. National Cancer Institute; 2015. Accessed December 1, 2022. https://cancercontrol.cancer.gov/sites/default/files/2020-06/nci-tobacco-control-research-priorities-report.pdf [Google Scholar]
- 9.Mahmoodi J, Leckelt M, van Zalk MWH, Geukes K, Back MD. Big data approaches in social and behavioral science: four key trade-offs and a call for integration. Curr Opin Behav Sci. 2017;18:57-62. doi: 10.1016/j.cobeha.2017.07.001 [DOI] [Google Scholar]
- 10.Kreuter M, Farrell D, Olevitch L, Brennan L. Tailoring Health Messages: Customizing Communication With Computer Technology. Lawrence Erlbaum Associates Publishers; 2000. Accessed December 1, 2022. https://psycnet.apa.org/record/1999-04356-000 [Google Scholar]
- 11.Sadasivam RS, Cutrona SL, Kinney RL, et al. Collective-intelligence recommender systems: advancing computer tailoring for health behavior change into the 21st century. J Med internet Res. 2016;18(3):e42. doi: 10.2196/jmir.4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aridor G, Goncalves D, Sikdar S. Deconstructing the filter bubble: user decision-making and recommender systems. Paper presented at: 14th ACM Conference on Recommender Systems; September 22-26, 2020; virtual. [Google Scholar]
- 13.Triantafyllidis AK, Tsanas A. Applications of machine learning in real-life digital health interventions: review of the literature. J Med internet Res. 2019;21(4):e12286. doi: 10.2196/12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marlin BM, Adams RJ, Sadasivam R, Houston TK. Towards collaborative filtering recommender systems for tailored health communications. Paper presented at: American Medical Informatics Association Annual Symposium; November 16-20, 2013; Washington, District of Columbia. [PMC free article] [PubMed] [Google Scholar]
- 15.Sadasivam RS, Borglund EM, Adams R, Marlin BM, Houston TK. Impact of a collective intelligence tailored messaging system on smoking cessation: the PERSPECT randomized experiment. J Med internet Res. 2016;18(11):e285. doi: 10.2196/jmir.6465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams RJ, Sadasivam RS, Balakrishnan K, Kinney RL, Houston TK, Marlin BM. PERSPECT: collaborative filtering for tailored health communications. Paper presented at: Proceedings of the 8th ACM Conference on Recommender systems; October 6-10, 2014; Foster City, California. [Google Scholar]
- 17.Carroll B. The Hidden Power of Your Customers: 4 Keys to Growing Your Business Through Existing Customers. Wiley; 2011. [Google Scholar]
- 18.Sadasivam RS, Cutrona SL, Luger TM, et al. Share2Quit: online social network peer marketing of tobacco cessation systems. Nicotine Tob Res. 2017;19(3):314-323. doi: 10.1093/ntr/ntw187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKeganey SPN. The rise and rise of peer education approaches. Drugs (Abingdon Engl). 2000;7(3):293-310. doi: 10.1080/09687630050109961 [DOI] [Google Scholar]
- 20.Georgie J M, Sean H, Deborah M C, Matthew H, Rona C. Peer-led interventions to prevent tobacco, alcohol and/or drug use among young people aged 11-21 years: a systematic review and meta-analysis. Addiction. 2016;111(3):391-407. doi: 10.1111/add.13224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faro JM, Orvek EA, Blok AC, et al. Dissemination and effectiveness of the peer marketing and messaging of a web-assisted tobacco intervention: protocol for a hybrid effectiveness trial. JMIR Res Protoc. 2019;8(7):e14814. doi: 10.2196/14814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanderbilt University Medical Center . ResearchMatch. Accessed December 7, 2022. https://www.researchmatch.org/
- 23.Centers for Disease Control and Prevention (CDC) . Quitting smoking among adults—United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60(44):1513-1519. [PubMed] [Google Scholar]
- 24.University of Massachusetts . Decide2Quit. Accessed December 1, 2022. https://www.decide2quit.org/default.aspx
- 25.Faro JM, Nagawa CS, Orvek EA, et al. Comparing recruitment strategies for a digital smoking cessation intervention: technology-assisted peer recruitment, social media, ResearchMatch, and smokefree.gov. Contemp Clin Trials. 2021;103:106314. doi: 10.1016/j.cct.2021.106314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coley HL, Sadasivam RS, Williams JH, et al. ; National Dental PBRN and QUITPRIMO Collaborative Group . Crowdsourced peer- versus expert-written smoking-cessation messages. Am J Prev Med. 2013;45(5):543-550. doi: 10.1016/j.amepre.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandura A; National Institute of Mental Health . Social Foundations of Thought and Action: a Social Cognitive Theory. Prentice-Hall; 1986. [Google Scholar]
- 28.Segaran T. Programming Collective Intelligence: Building Smart Web 2.0 Applications. O'Reilly Media; 2007. [Google Scholar]
- 29.Ricci F, Rokach L, Shapira B. Introduction to recommender systems handbook. In: Ricci F, Rokach L, Shapira B, Kantor PB, eds. Recommender Systems Handbook. Springer; 2011:1-35. doi: 10.1007/978-0-387-85820-3_1 [DOI] [Google Scholar]
- 30.Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300(22):2631-2637. doi: 10.1001/jama.2008.804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore DM, Cui Z, Lachowsky N, et al. ; Momentum Health Study team . HIV community viral load and factors associated with elevated viremia among a community-based sample of men who have sex with men in Vancouver, Canada. J Acquir Immune Defic Syndr. 2016;72(1):87-95. doi: 10.1097/QAI.0000000000000934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jarvis MJT-PH, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health. 1987;77(11):1435-1438. doi: 10.2105/AJPH.77.11.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baliunas D, Ivanova A, Tanzini E, Dragonetti R, Selby P. Impact of comprehensive smoking cessation training of practitioners on patients’ 6-month quit outcome. Can J Public Health. 2020;111(5):766-774. doi: 10.17269/s41997-020-00318-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong SL, Shields M, Leatherdale S, Malaison E, Hammond D. Assessment of validity of self-reported smoking status. Health Rep. 2012;23(1):47-53. [PubMed] [Google Scholar]
- 35.Hughes JR, Carpenter MJ, Naud S. Do point prevalence and prolonged abstinence measures produce similar results in smoking cessation studies: a systematic review. Nicotine Tob Res. 2010;12(7):756-762. doi: 10.1093/ntr/ntq078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheung KL, de Ruijter D, Hiligsmann M, et al. Exploring consensus on how to measure smoking cessation: a Delphi study. BMC Public Health. 2017;17(1):890. doi: 10.1186/s12889-017-4902-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaalema DE, Higgins ST, Bradstreet MP, Heil SH, Bernstein IM. Using NicAlert strips to verify smoking status among pregnant cigarette smokers. Drug Alcohol Depend. 2011;119(1-2):130-133. doi: 10.1016/j.drugalcdep.2011.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marrone GF, Paulpillai M, Evans RJ, Singleton EG, Heishman SJ. Breath carbon monoxide and semiquantitative saliva cotinine as biomarkers for smoking. Hum Psychopharmacol. 2010;25(1):80-83. doi: 10.1002/hup.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raja M, Garg A, Yadav P, Jha K, Handa S. Diagnostic methods for detection of cotinine level in tobacco users: a review. J Clin Diagn Res. 2016;10(3):ZE04-ZE06. doi: 10.7860/JCDR/2016/17360.7423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabe-Hesketh S, Skrondal A, Pickles A. Reliable estimation of generalized linear mixed models using adaptive quadrature. Stata J. 2002;2(1):1-21. doi: 10.1177/1536867X0200200101 [DOI] [Google Scholar]
- 41.Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychol Bull. 1992;111(1):23-41. doi: 10.1037/0033-2909.111.1.23 [DOI] [PubMed] [Google Scholar]
- 42.Cooke F, Bullen C, Whittaker R, McRobbie H, Chen MH, Walker N. Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine Tob Res. 2008;10(4):607-612. doi: 10.1080/14622200801978680 [DOI] [PubMed] [Google Scholar]
- 43.Carpenter MJ, Hughes JR, Gray KM, Wahlquist AE, Saladin ME, Alberg AJ. Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: a randomized clinical trial. Arch Intern Med. 2011;171(21):1901-1907. doi: 10.1001/archinternmed.2011.492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55(1):68-78. doi: 10.1037/0003-066X.55.1.68 [DOI] [PubMed] [Google Scholar]
- 45.Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self-determination of behavior. Psychol Inq. 2000;11(4):227-268. doi: 10.1207/S15327965PLI1104_01 [DOI] [Google Scholar]
- 46.Vallerand RJ. Deci and Ryan’s self-determination theory: a view from the hierarchical model of intrinsic and extrinsic motivation. Psychol Inq. 2000;11(4):312-318. [Google Scholar]
- 47.Juszczak E, Altman DG, Hopewell S, Schulz K. Reporting of multi-arm parallel-group randomized trials: extension of the CONSORT 2010 statement. JAMA. 2019;321(16):1610-1620. doi: 10.1001/jama.2019.3087 [DOI] [PubMed] [Google Scholar]
- 48.Houston TK, Sadasivam RS, Allison JJ, et al. Evaluating the QUIT-PRIMO clinical practice ePortal to increase smoker engagement with online cessation interventions: a national hybrid type 2 implementation study. Implement Sci. 2015;10:154. doi: 10.1186/s13012-015-0336-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danaher BG, Seeley JR. Methodological issues in research on web-based behavioral interventions. Ann Behav Med. 2009;38(1):28-39. doi: 10.1007/s12160-009-9129-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson DB, Partin MR, Fu SS, Joseph AM, An LC. Why assigning ongoing tobacco use is not necessarily a conservative approach to handling missing tobacco cessation outcomes. Nicotine Tob Res. 2009;11(1):77-83. doi: 10.1093/ntr/ntn013 [DOI] [PubMed] [Google Scholar]
- 51.Smolkowski K, Danaher BG, Seeley JR, Kosty DB, Severson HH. Modeling missing binary outcome data in a successful web-based smokeless tobacco cessation program. Addiction. 2010;105(6):1005-1015. doi: 10.1111/j.1360-0443.2009.02896.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saul JE, Amato MS, Cha S, Graham AL. Engagement and attrition in internet smoking cessation interventions: insights from a cross-sectional survey of “one-hit-wonders”. Internet Interv. 2016;5:23-29. doi: 10.1016/j.invent.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Comparison of Baseline Characteristics Between Individuals With and Without Viral Tool Kit
eTable 2. Comparison of Baseline Characteristics Between Individuals Randomized to ML Recommender and Standard Messaging
eTable 3. Smoking-Cessation Rates at 6 Months for Each 3 Hypotheses With Missing Outcomes Treated as Smoking
eTable 4. Smoking-Cessation Rates at 6 mo for 3 Hypotheses With Missing Outcomes Treated Using Multiple Imputation
Data Sharing Statement