Key Points
Question
What are the frequency, clinical features, and early outcomes associated with myopericarditis after COVID-19 mRNA vaccination in adolescents and young adults?
Findings
In this systematic review and meta-analysis of 23 studies, including 854 patients aged 12 to 20 years with vaccine-associated myopericarditis, the incidence of myopericarditis was higher in males after the second dose. Although 15.6% of patients had left ventricular (LV) systolic dysfunction, only 1.3% had severe LV systolic dysfunction (ejection fraction <35%); late gadolinium enhancement was found in 87.2% and 23.2% required intensive care unit admission; however, no in-hospital mortality was observed.
Meaning
These findings suggest largely favorable outcomes of COVID-19 vaccine-associated myopericarditis in adolescents and young adults.
This systematic review and meta-analysis investigates the clinical features and early outcomes associated with myopericarditis after COVID-19 mRNA vaccination in a heterogeneous population of adolescents and young adults.
Abstract
Importance
Published data on COVID-19 mRNA vaccine–associated myopericarditis in adolescents and young adults have been derived from small case series, national population-based studies, or passive reporting systems. Pooled evidence from a larger, international cohort is scarce.
Objective
To investigate the clinical features and early outcomes associated with myopericarditis after COVID-19 mRNA vaccination in a heterogeneous population of adolescents and young adults.
Data Sources
PubMed and EMBASE were searched through August 2022. Language restrictions were not applied.
Study Selection
Observational studies and case series describing COVID-19 vaccine–associated myopericarditis in adolescents and young adults aged 12 to 20 years and reporting clinical characteristics and early outcomes were included.
Data Extraction and Synthesis
Two independent investigators extracted relevant data from each study. One-group meta-analysis in a random effects model was performed. The Preferred Reporting Items for Systematic Reviews and Meta-analysis and Meta-analysis of Observational Studies in Epidemiology reporting guidelines were followed.
Main Outcomes and Measures
The primary outcomes were clinical features and early outcomes for COVID-19 mRNA vaccine–associated myopericarditis, including incident rate, cardiac findings, hospitalization, intensive care unit (ICU) admission, and in-hospital mortality.
Results
A total of 23 observational studies were identified, including 854 individuals (mean age, 15.9 [95% CI, 15.5-16.2] years) with COVID-19 vaccine–associated myopericarditis. Male sex was predominant, at 90.3% (95% CI, 87.3%-93.2%) of individuals. The incident rate was higher after the second dose than the first dose, with 74.4% (95% CI, 58.2%-90.5%) of events occurring after the second dose. Most patients (84.4% [95% CI, 80.5%-88.3%] of patients) had preserved left ventricular (LV) function. Of the 15.6% (95% CI, 11.7%-19.5%) of patients with LV systolic dysfunction (LV ejection fraction [LVEF] <55%), most (14.1% [95% CI, 10.2%-18.1%]) were mild (ie, LVEF 45%-54%), and only 1.3% (95% CI, 0%-2.6%) of patients had severe LV systolic dysfunction (ie, LVEF<35%). Interestingly, cardiac magnetic resonance imaging revealed late gadolinium enhancement in 87.2% (95% CI, 79.8%-94.7%) of patients. Although 92.6% (95% CI, 87.8%-97.3%) of patients were hospitalized and 23.2% (95% CI, 11.7%-34.7%) of patients required ICU admission, inotropes were used in only 1.3% (95% CI, 0%-2.7%) of patients, no patients died or required mechanical support, and the hospital length of stay was 2.8 (95% CI, 2.1-3.5) days.
Conclusions and Relevance
This systematic review and meta-analysis found low incidence rate and largely favorable early outcomes of COVID-19 mRNA vaccine–associated myopericarditis in adolescents and young adults from a wide range of populations. These findings are reassuring but continued follow-up is warranted.
Introduction
The COVID-19 global pandemic began in December 2019.1 The introduction of the messenger RNA (mRNA) vaccine against SARS-CoV-2 has resulted in a significant decline in COVID-19–related morbidity and mortality all over the world.2,3,4 COVID-19 mRNA vaccines are the current standard approach to contain the pandemic and the Emergency Use Authorizations for these vaccines were recently extended to children aged 6 months and older in the US. Since the emergency use was authorized, the association of the mRNA-based COVID-19 vaccine with myopericarditis, which is a rare but serious adverse event, has been reported.5,6 Cases of myopericarditis following COVID-19 mRNA vaccination have been reported worldwide, especially in adolescents and young adults. In addition, in June 2021, the Centers for Disease Control and Prevention (CDC) observed a rate of postvaccine myopericarditis that was higher in young males after the second mRNA vaccine doses.7 Previous studies reported largely favorable outcomes in adults with myocarditis following COVID-19 mRNA vaccination, demonstrating resolution of clinical symptoms, preservation of cardiac function, and no complications.8,9,10,11 However, data on the clinical features and outcomes of myopericarditis after COVID-19 vaccination in adolescents and young adults are scarce compared with adults and often consist of small case series. Most large-scale studies on COVID-19 vaccine–associated myopericarditis among adolescents and young adults are derived from national population-based studies that contain homogeneous populations or from surveillance networks that rely on passive reporting. In this study, we conducted a systematic review and meta-analysis to investigate the clinical spectrum and outcomes of COVID-19 vaccine–associated myopericarditis in adolescents and young adults from an international population.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline12 and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline.13 The protocol was registered in PROSPERO (CRD42022335550).
Information Sources and Search Strategy
All observational studies and case series including adolescents and young adults with myopericarditis after COVID-19 vaccination were identified using a 2-level strategy. First, databases, including PubMed and EMBASE, were searched through August 25, 2022. Search terms included COVID-19 OR SARS-CoV-2, vaccination OR vaccine, myocarditis OR myopericarditis, and adolescent OR children OR child OR pediatric OR young adult OR young adults. The search strategies specific to each database are shown in eTable 1 and eTable 2 in the Supplement. Second, we performed an additional manual search of secondary sources, such as references of initially identified studies, reviews, and commentaries, to collect relevant articles comprehensively. No restrictions on language, publication date, or publication status were applied.
Eligibility Criteria
Included studies met the following criteria: (1) observational studies or case series published in a peer-reviewed journal, (2) the study population was adolescents and young adults (aged 12-20 years) with myopericarditis after COVID-19 vaccination, and (3) the study reported the clinical characteristics and outcomes of myopericarditis following COVID-19 vaccination. Case reports that only included 1 patient with vaccine-associated myopericarditis were excluded.
Data Extraction
Two independent authors (J.Y. and K.M.) reviewed the search results separately to select the studies based on the inclusion and exclusion criteria and assessed the eligibility for each study. After screening the articles based on title and abstract, the full texts of potentially eligible studies were retrieved for further review.
Risk of Bias Assessment
The risk of bias in the observational studies was evaluated using the assessment of risk of bias in prevalence studies.14 Furthermore, the risk of bias for prevalence studies as well as case series was assessed using the Joanna Briggs Institute guidance for the appropriate checklist.15 The overall quality of each study was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations approach.16
Data Items
The following information was extracted: author, year of publication, country of the study, sample size, age, sex, and race and ethnicity. We assessed race and ethnicity because the racial and ethnic differences in the frequency and outcomes of myopericarditis after COVID-19 mRNA vaccination are unclear. Race and ethnicity were categorized as African American, American Indian or Alaskan Native, Asian, Hispanic, White, and other, which included individuals reported as other race and ethnicity in the reviewed studies. Regarding the COVID-19 vaccine, the type of vaccine, dose of vaccine, and symptoms were collected, including BNT162b2 (Pfizer-BioNTech) vaccine and mRNA-1273 (Moderna). Furthermore, the symptoms, outcomes (hospitalization, intensive care unit [ICU] admission, and death), treatment, laboratory values, electrocardiogram, echocardiogram, and cardiac magnetic resonance (CMR) findings of vaccine-associated myopericarditis were extracted. In addition, the incidence rate of vaccine-associated myopericarditis was collected in each study. The primary outcomes of this study were the clinical features and early outcomes of myopericarditis in adolescents and young adults following COVID-19 vaccination, including incidence, cardiac findings, in-hospital mortality, hospitalization, ICU admission, and treatments. The definition of myopericarditis followed each study. Left ventricular (LV) systolic dysfunction was defined as LV ejection fraction (LVEF) less than 55%; mild LV systolic function, as LVEF 45% to 54%; moderate, as LVEF 35% to 44%; and severe, as LVEF less than 35%.
Statistical Analysis
We performed 1-group meta-analysis in a random effects model using the DerSimonian-Laird method for continuous values and Wald method for discrete values with the OpenMetaAnalyst version 12.11.14 (Brown University), in which pooled estimates of baseline characteristics were calculated as the inverse variance-weighted mean with 95% CIs. Case series were excluded from the meta-analysis because case series are prone to selection bias, limiting generalizability to larger patient populations, and the data in case series can be exceptionally divergent. Measuring the degree of heterogeneity attributable to actual between-study differences was used to quantify heterogeneity, with I2 greater than 50% indicating substantial heterogeneity. Publication bias was assessed by Egger test and funnel plot of the clinical characteristics and early outcomes of COVID-19 vaccine–associated myopericarditis in each study.17 The Duval and Tweedy trim-and-fill method was also performed.18 All analyses were conducted using Comprehensive Meta-Analysis version 2 (Biostat). There was no transformation that was conducted for meta-analysis of proportions. We performed subgroup analyses specifically including the studies with only patients with myocarditis after COVID-19 mRNA vaccination. P values were 2-sided, and statistical significance was set at P < .05. Statistical analyses were conducted on September 10, 2022.
Results
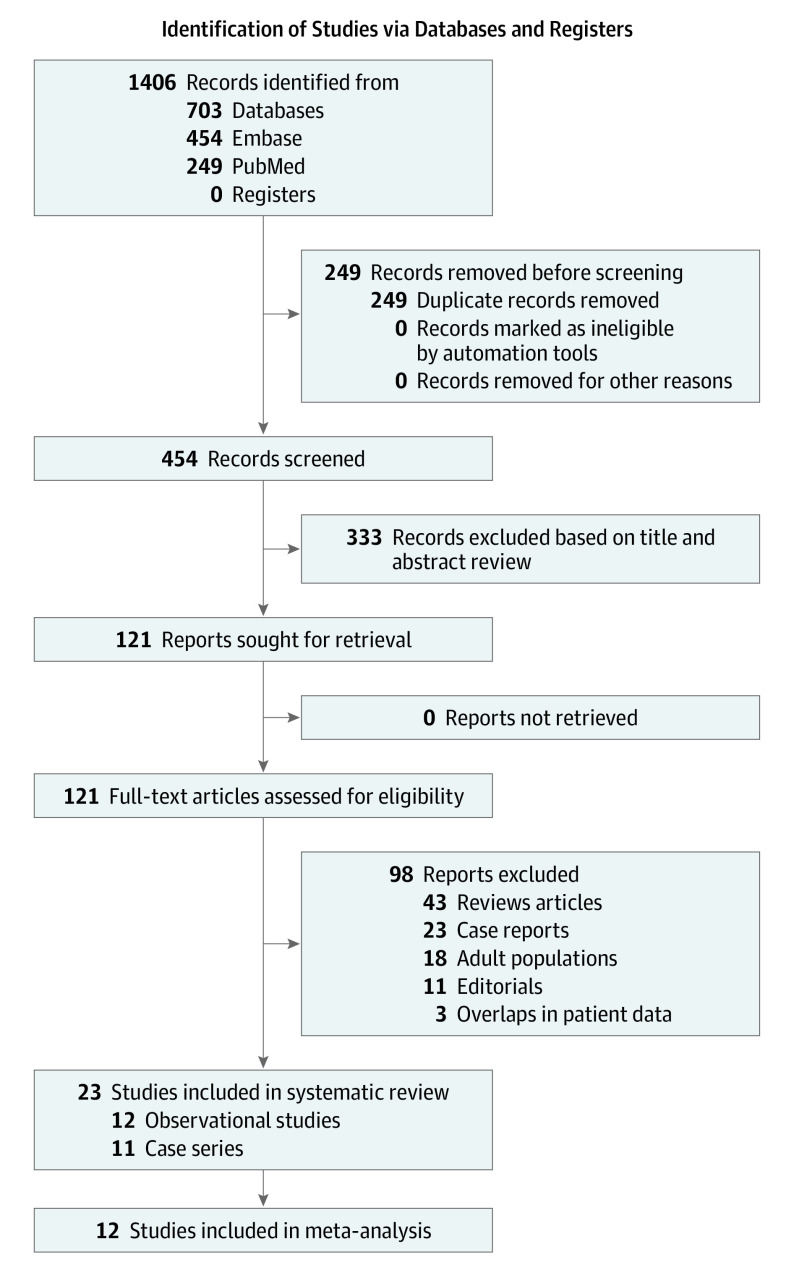
We identified 454 articles by the initial database search and subsequent manual search. After removing 333 records based on the title and abstract, we retrieved 121 articles for full-text review. Of those, 98 articles were excluded based on the article type (clinical guidelines, consensus documents and conference proceedings, reviews), conference abstracts, population (adult patients with COVID-19, individuals without myopericarditis after the vaccines), and irrelevant topic. Furthermore, 3 articles were excluded because they were likely to have overlapping patient data with other studies based on the author list, institution, country, and study period. A total of 23 studies19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41 met the inclusion criteria and were analyzed for the systemic review and meta-analysis (Figure 1).
Figure 1. Flowchart of Study Selection.
Of 454 identified articles, 23 studies19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41 reporting clinical characteristics and outcomes of myopericarditis following COVID-19 vaccination in adolescents and young adults were included for a systematic review and meta-analysis.
There were 12 retrospective or prospective cohort studies19,20,21,22,23,24,25,26,27,28,29,30 and 11 case series.31,32,33,34,35,36,37,38,39,40,41 Across all 23 studies, we included a total of 854 patients with myopericarditis following COVID-19 vaccination. There were 6 observational studies from the US (494 patients),20,22,24,27,28,30 2 from Israel (43 patients),21,25 and 1 each from Hong Kong (15 patients),19 South Korea (40 patients),29 Denmark (15 patients),26 and Europe (193 patients).23 There were 5 case series from the US (21 patients),33,34,35,36,37 3 from Poland (10 patients)31,38,39 and 1 each from Italy (5 patients),32 Germany (2 patients),41 and Iraq (3 patients).40
Baseline Characteristics
The baseline characteristics are summarized in Table 1, with characteristics in the observations studies provided eTable 3 in the Supplement , and characteristics in the case series provided in eTable 4 in the Supplement. The pooled estimates from 1-group meta-analysis in a random-effects model are presented in Table 2 and eFigure 1 in the Supplement. The pooled estimate of the mean age was 15.9 (95% CI, 15.5-16.2) years, and there was male preponderance, at 90.3% (95% CI, 87.3%-93.2%) of the population. The proportion of prior SARS-CoV-2 infection was 3.8% (95% CI, 1.1%-6.4%) of patients. There were no patients with prior history of myopericarditis or underlying cardiovascular disease, including cardiomyopathy. Notably, myopericarditis occurred more commonly after the second dose (74.4% [95% CI, 58.2%-90.5%] of patients) than after the first dose (20.7% [95% CI, 58.2%-90.5%] of patients). Among patients with vaccine-associated myopericarditis, 97.5% (95% CI, 95.7%-99.2%) received the BNT162b2 vaccine and 2.2% (95% CI, 0.6%-3.7%) received the mRNA-1273 vaccine. The incidence rate of myopericarditis was higher after the second dose (12.7-118.7 per million persons) than the first dose (0.6-10.0 per million persons) (eTable 3 in the Supplement). The pooled estimate of the mean interval from vaccination to the onset of myopericarditis was 2.6 (95% CI, 1.9-3.3) days. The definition of myopericarditis in each study is summarized in eTable 5 in the Supplement. One study22 used both the CDC criteria for myopericarditis and the Lake Louise CMR criteria for patients who had CMR data.42,43 In 2 studies,20,28 the CDC criteria were used. One study19 used only International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), and another24 used only the Lake Louise criteria. In 3 studies,25,29,30 vaccine-associated myocarditis was diagnosed clinically.
Table 1. Characteristics of the Studies Included in the Meta-analysis.
| Source | Country | Cohort size, No. | Age, mean (SD), y | Patients, No. (%) | Onset after vaccination, mean (SD), d | LV systolic dysfunction, No. (%)a | LGE | Outcomes, No. (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | BNT162b2 | mRNA-1273 | First dose | Second dose | Mild | Moderate | Severe | Hospitalization | Hospital LOS, mean (SD), d | ICU admission | Inotropes | ECMO | Death | |||||||
| Li et al,19 2022 | Hong Kong | 43 | 14.9 (1.5) | NA | NA | 43 (100) | 0 | 7 (16.3) | 36 (83.7) | NA | NA | NA | NA | NA | 43 (100) | NA | NA | NA | NA | NA | |
| Krug et al,20 2022 | US | 253 | NA | 23 (9.1) | 230 (90.9) | 253 (100) | 0 | 129 (51.0) | 124 (49.0) | NA | NA | NA | NA | NA | 220 (87) | NA | NA | NA | NA | NA | |
| Mevorach et al,21 2022 | Israel | 13 | NA | 1 (0.8) | 12 (92.3) | 13 (100) | 0 | 1 (7.7) | 12 (92.3) | NA | NA | NA | NA | NA | 13 (100) | 3.1 | NA | 0 | NA | NA | |
| Truong et al,22 2022 | US | 139 | 15.8 (14.5-17.0)b | 13 (9.4) | 126 (90.6) | 131 (94.2) | 5 (3.6) | 12 (8.6) | 128 (91.4) | 2 (1-3)b | 22 (16.0) | 2 (1.4) | 2 (1.4) | 74 (76.3) | NA | 2 (2-3)b | 26 (18.7) | 2 (1.4) | 0 | 0 | |
| Foltran et al,23 2022 | 148 countries | 193 | 15.9 (1.3) | 21 (10.9) | 172 (89.1) | 185 (95.9) | 8 (4.1) | 31 (16.1) | 58 (30.1) | 3 | NA | NA | NA | NA | 172 (89.1) | NA | NA | NA | NA | NA | |
| Jain et al,24 2021 | US | 63 | 15.6 (1.8) | 5 (7.9) | 58 (92.1) | 59 (93.7) | 4 (6.3) | 1 (1.6) | 62 (98.4) | 2.1 (1.3) | 9 (14.0) | 0 | 0 | 49 (88.0) | NA | 3 (1.4) | 27 (42.9) | 0 | 0 | 0 | |
| Amir et al,25 2022 | Israel | 15 | 17 (1.0) | 0 | 15 (100) | 15 (100) | 0 | 1 (6.7) | 14 (92.3) | 4.4 (6.7) | 2 (13.3) | 0 | 0 | 14 (93.0) | 13 (86.7) | 4.8 (1.6) | 7 (46.7) | 0 | NA | NA | |
| Nygaard et al,26 2022 | Denmark | 15 | 16 (1.2) | 2 (13.3) | 13 (86.7) | 15 (100) | 0 | 8 (53.3) | 7 (46.7) | NA | 2 (13.0) | 0 | 1 (6.7) | NA | 15 (100) | 3.7 (2.1) | 1 (6.7) | NA | NA | NA | |
| Chelala et al,27 2022 | US | 5 | 17.2 (1.0) | 0 | 5 (100) | 4 (80.0) | 1 (20.0) | 0 | 5 (100) | 3.6 (0.5) | 1 (20.0) | 0 | 0 | 5 (100) | NA | 4.4 (2.3) | NA | NA | NA | 0 | |
| Das et al,28 2021 | US | 25 | 15.3 (1.4) | 3 (12.0) | 22 (88.0) | 25 (100) | 0 | 3 (12.0) | 22 (88.0) | 3.1 (3.6) | 2 (8.0) | 0 | 0 | 15 (93.8) | 22 (88.0) | 2.6 (1.2) | NA | NA | NA | 0 | |
| Roh et al,29 2022 | South Korea | 40 | 16 (14.5-17)b | 14 (35.0) | 26 (65.0) | 40 (100) | 0 | 25 (62.5) | 15 (37.5) | 2 (1-5)b | 6 (15.0) | 0 | 0 | NA | NA | 1 (0-3)b | 5 (12.5) | 1 (2.5) | 0 | 0 | |
| Patel et al,30 2022 | US | 9 | 15.5 (14.5-16.6)b | 0 | 9 (100) | NA | NA | 1 (11.1) | 8 (88.9) | NA | NA | NA | NA | NA | 9 (100) | 2 (1-3)b | 2 (22.2) | NA | 0 | 0 | |
Abbreviations: ECMO, extracorporeal membrane oxygenation; EF, ejection fraction; ICU, intensive care unit; LGE, late gadolinium enhancement; LV, left ventricular; LOS, length of stay; NA, not available.
LV systolic dysfunction was defined as LV ejection fraction (LVEF) less than 55%; mild LV systolic function, as LVEF 45% to 54%; moderate, as LVEF 35% to 44%; and severe, as LVEF less than 35%.
Presented as median (IQR).
Table 2. Pooled Estimates of Characteristics and Outcomes of Myopericarditis After COVID-19 Vaccination in Adolescents and Young Adults.
| Characteristic | Pooled estimates, % (95% CI) | I2, % | P value for heterogeneity |
|---|---|---|---|
| Age, y | 15.9 (15.5-16.2) | 83.6 | <.001 |
| Sex | |||
| Female | 9.7 (6.8-12.7) | 33.6 | .13 |
| Male | 90.3 (87.3-93.2) | 33.6 | .13 |
| History of COVID-19 | 3.8 (1.1-6.4) | 11.5 | .34 |
| Dose of vaccination | |||
| First | 20.7 (1.0-31.8) | 95.7 | <.001 |
| Second | 74.4 (58.2-90.5) | 97.9 | <.001 |
| Type of vaccine | |||
| BNT162b2 (Pfizer-BioNTech) | 97.5 (95.7-99.2) | 52.8 | .02 |
| mRNA-1273 (Moderna) | 2.2 (0.6-3.7) | 44.7 | .06 |
| Time from vaccination to symptom onset, mean (95% CI), d | 2.6 (1.9-3.3) | 89.0 | <.001 |
| Symptoms | |||
| Chest pain | 83.7 (72.7-94.6) | 98.6 | <.001 |
| Fever | 44.5 (16.9-72.0) | 98.1 | <.001 |
| Headache | 33.3 (8.6-58.0) | 98.0 | <.001 |
| Dyspnea or respiratory distress | 25.2 (17.2-33.1) | 51.2 | .07 |
| Myalgia | 17.8 (2.7-33.3) | 90.9 | <.001 |
| Treatment | |||
| NSAIDs | 81.8 (75.3-88.3) | 45.4 | .09 |
| Glucocorticoid | 13.8 (6.7-20.9) | 67.5 | .005 |
| IVIG | 12.0 (3.8-20.2) | 80.1 | <.001 |
| Colchicine | 7.3 (4.1-10.4) | 0 | .62 |
| Elevated troponin I | 84.5 (75.1-94.5) | 97.5 | <.001 |
| Electrocardiography findings | |||
| ST-segment elevation or changes | 53.0 (34.6-71.3) | 91.5 | <.001 |
| T-wave changes | 14.5 (5.1-24.0) | 0 | .80 |
| Nonsustained VT | 5.3 (2.5-8.1) | 0 | .75 |
| Echocardiography findings | |||
| LVEF, mean (95% CI), % | 62.1 (59.1-65.1) | 71.2 | .03 |
| LV systolic dysfunctiona | |||
| Any | 15.6 (11.7-19.5) | 0 | .90 |
| Mild | 14.1 (10.2-18.1) | 0 | .94 |
| Moderate | 1.3 (0-2.6) | 0 | .98 |
| Severe | 1.3 (0-2.6) | 0 | .96 |
| Pericardial effusion | 5.1 (0.6-9.6) | 45.2 | .11 |
| Cardiac magnetic resonance findings | |||
| Presence of LGE | 87.2 (79.8-94.7) | 52.2 | .08 |
| Myocardial edema | 58.0 (33.5-82.5) | 92.3 | <.001 |
| Outcome | |||
| Hospitalization | 92.6 (87.8-97.3) | 74.0 | <.001 |
| ICU admission | 23.2 (11.7-34.7) | 79.4 | <.001 |
| Inotropes | 1.3 (0-2.7) | 0 | .93 |
| Hospital length of stay, d | 2.8 (2.1-3.5) | 92.9 | <.001 |
Abbreviations: ICU, intensive care unit; IVIG, intravenous immune globulin; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricle ejection fraction; NSAIDS, nonsteroidal anti-inflammatory drugs; VT, ventricular tachycardia.
LV systolic dysfunction was defined as LVEF less than 55%; mild LV systolic function, as LVEF 45% to 54%; moderate, as LVEF 35% to 44%; and severe, as LVEF less than 35%.
Clinical Features of Myopericarditis Following COVID-19 Vaccination
The most common presenting symptoms of myopericarditis were chest pain (83.7% [95% CI, 72.7%-94.6%] of patients), fever (44.5% [95% CI, 16.9%-72.0%] of patients), headache (33.3% [95% CI, 8.6%-58.0%] of patients) and dyspnea or respiratory distress (25.2% [95% CI, 17.2%-33.1%] of patients) (Table 2; eFigure 1 in the Supplement). The most common medication used for treatment was a nonsteroidal anti-inflammatory drug (81.8% [95% CI, 75.3%-88.3%] of patients), followed by glucocorticoid (13.8% [95% CI, 6.7%-20.9%] of patients) and intravenous immune globulin (12.0% [95% CI, 3.8%-20.2%] of patients). Colchicine was also used in 7.3% (95% CI, 4.1%-10.4%) of patients.
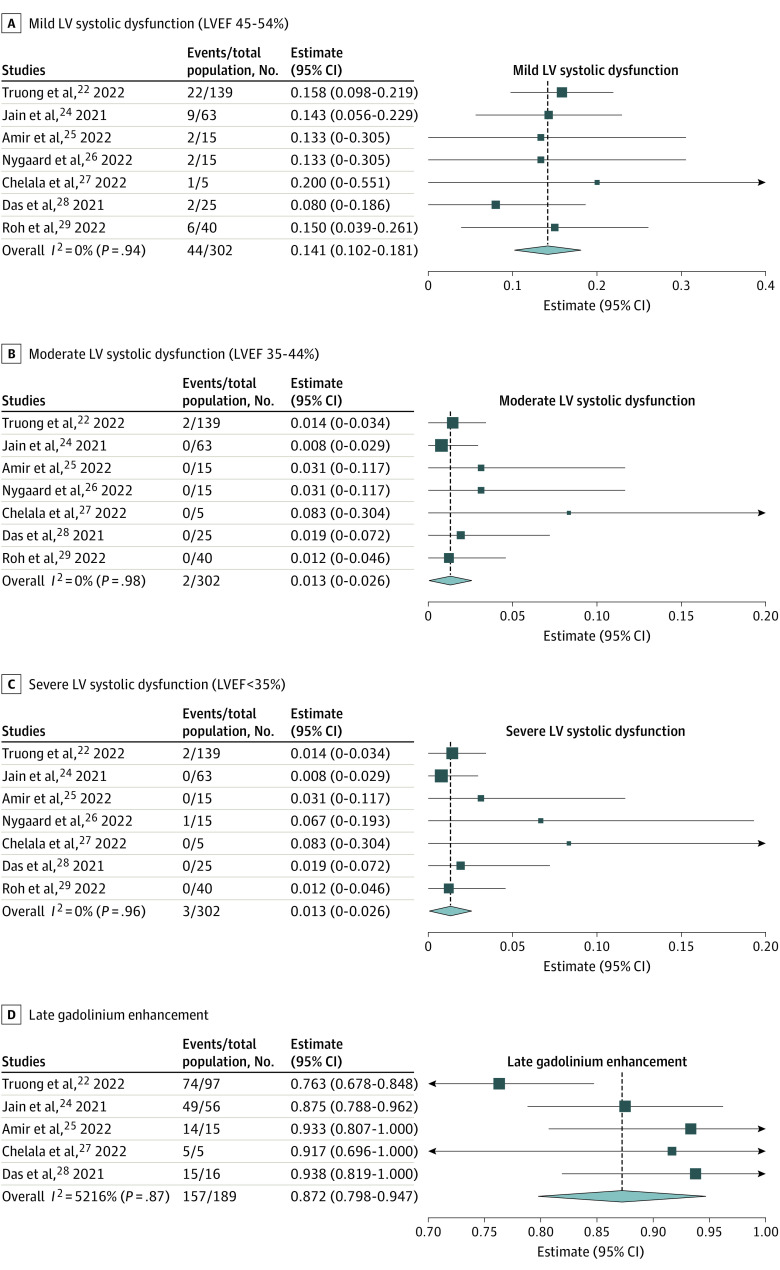
Troponin level was elevated in 84.5% (95% CI, 75.1%-94.5%) of patients (Table 2). Electrocardiography findings showed ST-segment elevation or depression in 53.0% (95% CI, 34.6%-71.3%) of patients, T-wave changes in 14.5% (95% CI, 5.1%-24.0%) of patients, and nonsustained ventricular tachycardia in 5.3% (95% CI, 2.5%-8.1%) of patients. As for echocardiography findings, mean LVEF was 62.1% (95% CI, 59.1%-65.1%), and 15.6% (95% CI, 11.7%-19.5%) of patients demonstrated LV systolic dysfunction (eFigure 1 in the Supplement). Notably, most patients with LV systolic dysfunction had mild dysfunction (14.1% [95% CI, 10.2%-18.1%] of patients), 1.3% (95% CI, 0%-2.6%) of patients had moderate dysfunction, and 1.3% (95% CI, 0%-2.6%) of patients had severe dysfunction (Figure 2). Pericardial effusion was seen in 5.1% (95% CI, 0.6%-9.6%) of patients. CMR was performed in 199 of 262 patients (80.7%) and the timing of CMR ranged between 3 to 28 days after the onset of myopericarditis. CMR revealed late gadolinium enhancement (LGE) in 87.2% (95% CI, 79.8%-94.7%) of patients (Figure 2). Myocardial edema, defined as abnormally high signal intensity on T2-weighted imaging or prolonged T2 relaxation time on T2 mapping, was seen in 58.0% (95% CI, 33.5%-82.5%) of patients (Table 2; eFigure 1 in the Supplement).
Figure 2. Forest Plots Showing the Prevalence of Echocardiogram and Cardiac Magnetic Resonance Findings.
The pooled estimates of echocardiogram and CMR findings of myopericarditis after COVID-19 mRNA vaccination in adolescents and young adults were calculated using 1-group meta-analysis in random-effects model. LV indicates left ventricular; LVEF, left ventricular ejection fraction; diamond, total estimate with 95% CI.
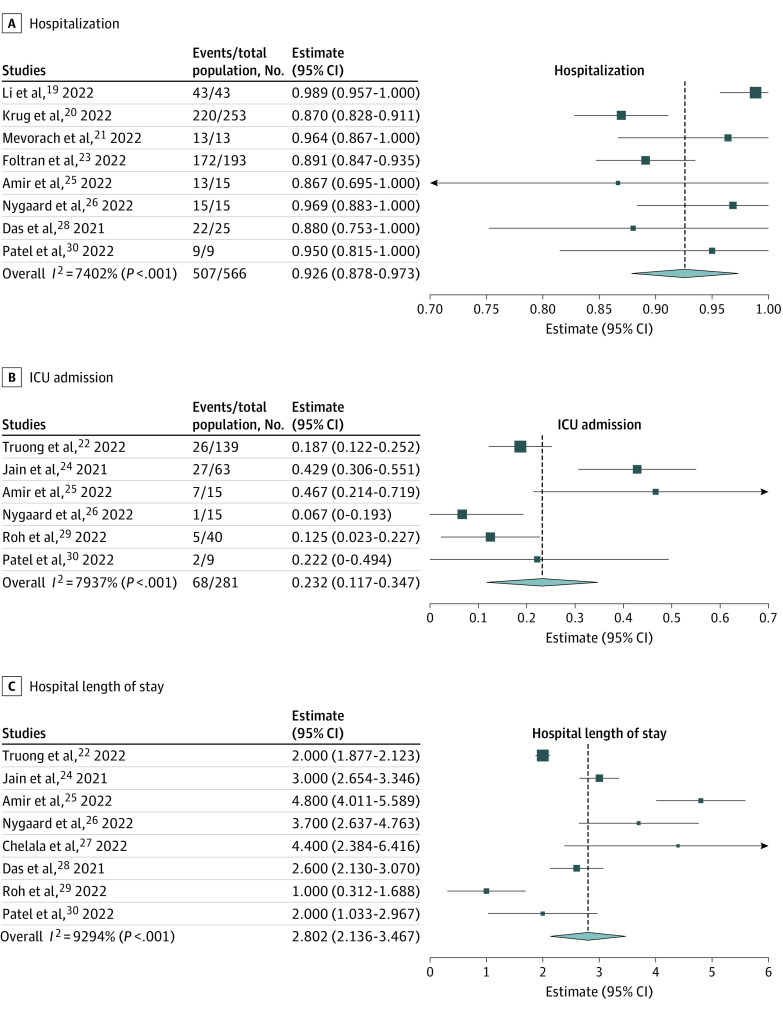
Early Outcomes
Overall, 92.6% (95% CI, 87.8%-97.3%) of patients were hospitalized and 23.2% (95% CI, 11.7%-34.7%) of patients required ICU admission, mainly for arrhythmia monitoring; however, inotropic support was used in only 1.3% (95% CI, 0%-2.7%) of patients (Table 2 and Figure 3; eFigure 1 in the Supplement). No patients received extracorporeal membrane oxygenation and no deaths were observed. In all the studies available, the pooled estimate of the hospital length of stay was 2.8 (95% CI, 2.1-3.5) days. Although the data are limited regarding LV systolic dysfunction at follow-up periods, some available cases showed improvement in LVEF from 45% at baseline to 50% to 54% at 3 months. The subgroup analyses including studies with only patients with myocarditis showed largely similar results (eTable 6 and eFigure 2 in the Supplement).
Figure 3. Forest Plots Showing the Pooled Estimates of Early Outcomes.
The pooled estimates of the prevalence of early outcomes of myopericarditis after COVID-19 mRNA vaccination in adolescents and young adults were calculated using 1-group meta-analysis in random-effects model. ICU indicates intensive care unit; diamond, total estimate with 95% CI.
Bias Assessments
The risk of bias assessments for prevalence studies as well as case series are summarized in eTable 7, eTable 8, and eFigure 3 in the Supplement. The overall quality of each study is summarized in eTable 9 in the Supplement and the overall quality of evidence of most studies were graded low or moderate level of certainty.
Egger test revealed funnel plot asymmetry for hospitalization, which raised the possibility of publication bias (eTable 10 and eFigure 4 in the Supplement). However, the imputed 3 studies using the trim-and-fill method produced a symmetrical funnel plot (eFigure 5 in the Supplement), and the pooled analysis incorporating the 3 hypothetical studies did not substantively alter the results of the primary meta-analysis (eTable 11 in the Supplement).
Discussion
This systematic review and meta-analysis comprehensively summarized the available published literature and assessed the current situation regarding myopericarditis after COVID-19 vaccination in adolescents and young adults across a wide range of populations. There are 4 main findings of our study. First, COVID-19 vaccine–associated myopericarditis was predominantly observed in males after the second dose. Second, LV systolic dysfunction was identified in 15.6% of the patients; however, only 1.3% of patients had severe LV systolic dysfunction. Third, CMR showed LGE in 87.2% of patients. Fourth, although more than 90.0% of patients were hospitalized and 23.2% of patients were admitted to the ICU, inotropes were used in only 1.3% of patients, the duration of hospitalization was 2.8 days, and no patients died or required mechanical support during the hospitalization.
These findings are consistent with results from previous studies in adults, in which clinical course of COVID-19 vaccine–associated myopericarditis was typically mild, with complete resolution of symptoms and LV systolic dysfunction at presentation normalized within a few days.44 Furthermore, recent systematic reviews have summarized the rate and clinical characteristics of myopericarditis after COVID-19 vaccination in children and adolescents (age <19 years).45,46 They reported that echocardiographic findings were often normal, including pericardial effusion and borderline or mild depressed LV systolic function, and most patients recovered, with very few deaths reported. In contrast, compared with these reviews, the novelty of our study lies on providing the pooled estimates across the published observational studies with a wide range of international population using 1-group meta-analysis. In particular, more detailed and specific data focusing on the clinical characteristics and outcomes, such as LV systolic dysfunction (mild, moderate, or severe), presence of LGE, myocardial edema, hospitalization, ICU admission, and hospital length of stay would be useful for a broad range of physicians as well as parents.
The overall incidence of COVID-19 mRNA vaccine–associated myocarditis was reported to be low, estimated as 0.3 to 5.0 cases per 100 000 vaccinated people in case series studies from the US and Israel.47,48,49,50 Myocarditis occurred primarily after the second vaccination in young males (1 case per 12 361 individuals in male adolescents vs 1 case per 144 439 individuals in female adolescents; 0.56 cases per 100 000 individuals after the first dose vs 8.09 cases per 100 000 individuals after the second dose in males).48 Within the ages of 12 to 17 years, males were 7.2 times more likely to develop myocarditis following COVID-19 vaccination compared with females and myocarditis incidence was 6.8 times higher after the second dose than the first dose.51 Our results are consistent with these reports, suggesting that myocarditis incidence after COVID-19 mRNA vaccination is rare and occurred mainly after the second dose in males. The incidence of myocarditis appears to be different among mRNA vaccines. In our study, almost all the cases of myocarditis were seen after the BNT162b2 vaccine. In contrast, a higher risk of myocarditis with mRNA-1273 compared with BNT162b2 has been observed in large observational studies.52,53,54
Importantly, the risk of developing myocarditis after SARS-CoV-2 infection is significantly higher than after COVID-19 mRNA vaccination. The incidence of myocarditis after SARS-CoV-2 infection is higher than after COVID-19 mRNA vaccination (11.0 events per 100 000 persons vs 3.2 events per 100 000 persons).55 Furthermore, compared with cardiac complications associated with COVID-19, our study revealed largely favorable early outcomes of vaccine-associated myopericarditis.56 Accordingly, the benefits of the mRNA COVID-19 vaccination are deemed to outweigh the potential risks. Despite the lack of severe complications commonly associated with COVID-19 mRNA vaccination, vaccine hesitancy remains high, and some parents still hesitate to vaccinate their children against COVID-19.57 Our findings corroborate the relatively low risks and good early outcomes for COVID-19 vaccine–associated myopericarditis across a wide population from multiple counties, improving understanding of myopericarditis following COVID-19 mRNA vaccination among adolescents and young adults and decision-making for parents with vaccine hesitancy.
The use of CMR is important in the noninvasive diagnosis and risk stratification of myocarditis. In particular, T2-weighted imaging is routinely performed to detect acute myocardial inflammation or edema. Similarly, LGE is widely used to detect necrosis and fibrosis and is incorporated into the original and revised Lake Louise criteria for diagnosis of acute myocarditis. In our study, the Lake Louise Criteria was used, and CMR was performed for making the diagnosis of myocarditis. However, a large proportion of patients with subclinical myocarditis might not have been diagnosed with myocarditis, leading to much lower hospitalization and ICU admission rates than currently reported. This would strengthen the argument that most cases of myocarditis are mild and possibly even underdiagnosed. Interestingly, our study found LGE in 87.2% of adolescents and young adults, while the clinical course was mild and no in-hospital mortality was observed. Although a previous study also detected LGE in 88.3% of patients (aged 14-70 years) with myocarditis after COVID-19 mRNA vaccination, all patients recovered and were discharged.9 Unfortunately, the degree and extent of LGE was not reported, and follow-up studies with CMR have not been published, to our knowledge. The persistence of LGE indicates the potential myocardial fibrosis and could be a risk factor for adverse cardiac events, including arrhythmias, cardiac dysfunction, or recurrent myocarditis, in patients with myocarditis due to other causes.58 While this is similar to other, non–vaccine-associated myocarditis, a clinical follow-up of cardiovascular events in patients with vaccine-associated myocarditis is essential. Further studies are needed to investigate the association of CMR findings and long-term outcomes.
Limitations
This study had several limitations to be noted. First, the available studies were observational studies or case series, subject to methodological biases or publication biases. Second, each study contained a small number of patients, potentially leading to substantial heterogeneity. Additionally, we excluded case reports, which may have included severe cases not reported in large observational studies. Furthermore, the retrospective designs of the included studies might have underestimated the complications. Third, it is difficult to identify a small number of overlaps in patient data between multicenter studies from the same countries, although we removed 1 study from Hong Kong and 2 single-center studies from the US that were likely to have overlapping patient data with other studies. Fourth, the lack of universal case inclusion criteria or diagnostic tests could lead to a misdiagnosis or underreporting of vaccine-associated myocarditis. Fifth, several variables were not available. For example, whereas an 8-week or longer interval has been associated with a lower risk of myopericarditis associated with COVID-19 mRNA vaccines in a population-based cohort study,59 these variables were not obtainable across the included studies; the influence of the interval in adolescents and young adults remains uncertain. Sixth, our study did not find the association of symptoms with unfavorable outcomes in adolescents and young adults. Although a previous study reported that adult patients with gastrointestinal symptoms received more intensive care, risk factors of poor prognosis remain elusive.60 Seventh, in this study, most of the types of vaccine were BNT162b2 vaccines, limiting the generalizability of findings to other COVID-19 vaccines, including the mRNA-1273 vaccine.
Conclusions
The findings of this systematic review and meta-analysis pooling data from multiple countries demonstrate low incidence rate and largely favorable early outcomes of COVID-19 vaccine–associated myopericarditis in adolescents and young adults from a wide range of populations. While mortality data are reassuring, a significant number of patients were reported to have acute LGE. Our findings could help improve understanding of myopericarditis among adolescents and young adults and decision-making for parents.
eTable 1. The Search Strategy of MEDLINE via PubMed
eTable 2. The Search Strategy of EMBASE
eTable 3. Characteristics of Observational Studies Included
eTable 4. Characteristics of Case Series Included
eTable 5. Definition of Myocarditis
eTable 6. Pooled Estimates for Clinical Characteristics and Outcomes From Subgroup Analyses Including Studies With Patients With Myocarditis
eTable 7. Joanna Briggs Institute Critical Appraisal Checklist for Prevalence Studies
eTable 8. Joanna Briggs Institute Critical Appraisal Checklist for Case Series
eTable 9. GRADE Evidence Profile for the Included Studies
eTable 10. Results of Egger Linear Regression Tests of the Clinical Presentation and Outcomes
eTable 11. Results of Point Estimate of the Clinical Presentation and Outcomes Using the Trim-and-Fill Analysis
eFigure 1. Forest Plots Showing the Pooled Estimates of Clinical Characteristics and Early Outcomes
eFigure 2. Forest Plots of Subgroup Analysis Including Studies With Only Patients With Myocarditis
eFigure 3. Risk of Bias Summary
eFigure 4. Funnel Plots of the Clinical Characteristics and Outcomes
eFigure 5. Adjusted Funnel Plots of the Clinical Characteristics and Outcomes From the Trim-and-Fill Analysis
References
- 1.World Health Organization . Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. Accessed February 2, 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 2.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819-1829. doi: 10.1016/S0140-6736(21)00947-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lv G, Yuan J, Xiong X, Li M. Mortality rate and characteristics of deaths following COVID-19 vaccination. Front Med (Lausanne). 2021;8:670370. doi: 10.3389/fmed.2021.670370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373(1088):n1088. doi: 10.1136/bmj.n1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuehn BM. Myocarditis adverse event less common after COVID-19 vaccine booster. JAMA. 2022;327(14):1324. doi: 10.1001/jama.2022.4582 [DOI] [PubMed] [Google Scholar]
- 6.Times of Israel Staff . Israel said probing link between Pfizer shot and heart problem in men under 30. Times of Israel. April 23, 2021. Accessed June 20, 2021. https://www.timesofisrael.com/israel-said-probing-link-between-pfizer-shot-and-heart-problem-in-men-under-30/
- 7.Shimabukuro T. COVID-19 vaccine safety updates. 2021. Accessed June 23, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-COVID-Shimabukuro-508.pdf
- 8.Salah HM, Mehta JL. COVID-19 vaccine and myocarditis. Am J Cardiol. 2021;157:146-148. doi: 10.1016/j.amjcard.2021.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matta A, Kunadharaju R, Osman M, et al. Clinical presentation and outcomes of myocarditis post mRNA vaccination: a meta-analysis and systematic review. Cureus. 2021;13(11):e19240. doi: 10.7759/cureus.19240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordero A, Cazorla D, Escribano D, et al. Myocarditis after RNA-based vaccines for coronavirus. Int J Cardiol. 2022;353:131-134. doi: 10.1016/j.ijcard.2022.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellos I, Karageorgiou V, Viskin D. Myocarditis following mRNA COVID-19 vaccination: a pooled analysis. Vaccine. 2022;40(12):1768-1774. doi: 10.1016/j.vaccine.2022.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, et al. ; Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group . Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 14.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934-939. doi: 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 15.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147-153. doi: 10.1097/XEB.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 19.Li X, Lai FTT, Chua GT, et al. Myocarditis following COVID-19 BNT162b2 vaccination among adolescents in Hong Kong. JAMA Pediatr. 2022;176(6):612-614. doi: 10.1001/jamapediatrics.2022.0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krug A, Stevenson J, Høeg TB. BNT162b2 vaccine-associated myo/pericarditis in adolescents: a stratified risk-benefit analysis. Eur J Clin Invest. 2022;52(5):e13759. doi: 10.1111/eci.13759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 vaccination in Israeli adolescents. N Engl J Med. 2022;386(10):998-999. doi: 10.1056/NEJMc2116999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truong DT, Dionne A, Muniz JC, et al. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults: suspected myocarditis after COVID-19 vaccination. Circulation. 2022;145(5):345-356. doi: 10.1161/CIRCULATIONAHA.121.056583 [DOI] [PubMed] [Google Scholar]
- 23.Foltran D, Delmas C, Flumian C, et al. Myocarditis and pericarditis in adolescents after first and second doses of mRNA COVID-19 vaccines. Eur Heart J Qual Care Clin Outcomes. 2022;8(2):99-103. doi: 10.1093/ehjqcco/qcab090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain SS, Steele JM, Fonseca B, et al. COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics. 2021;148(5):e2021053427. doi: 10.1542/peds.2021-053427 [DOI] [PubMed] [Google Scholar]
- 25.Amir G, Rotstein A, Razon Y, et al. CMR imaging 6 months after myocarditis associated with the BNT162b2 mRNA COVID-19 vaccine. Pediatr Cardiol. 2022;43(7):1522-1529. doi: 10.1007/s00246-022-02878-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nygaard U, Holm M, Bohnstedt C, et al. Population-based incidence of myopericarditis after COVID-19 vaccination in Danish adolescents. Pediatr Infect Dis J. 2022;41(1):e25-e28. doi: 10.1097/INF.0000000000003389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chelala L, Jeudy J, Hossain R, Rosenthal G, Pietris N, White CS. Cardiac MRI findings of myocarditis after COVID-19 mRNA vaccination in adolescents. AJR Am J Roentgenol. 2022;218(4):651-657. doi: 10.2214/AJR.21.26853 [DOI] [PubMed] [Google Scholar]
- 28.Das BB, Kohli U, Ramachandran P, et al. Myopericarditis after messenger RNA coronavirus disease 2019 vaccination in adolescents 12 to 18 years of age. J Pediatr. 2021;238:26-32.e1. doi: 10.1016/j.jpeds.2021.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roh DE, Na H, Kwon JE, Choi I, Kim YH, Cho HJ. Chest pain and suspected myocarditis related to COVID-19 vaccination in adolescents—a case series. Children (Basel). 2022;9(5):693. doi: 10.3390/children9050693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel T, Kelleman M, West Z, et al. Comparison of multisystem inflammatory syndrome in children-related myocarditis, classic viral myocarditis, and COVID-19 vaccine-related myocarditis in children. J Am Heart Assoc. 2022;11(9):e024393. doi: 10.1161/JAHA.121.024393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puchalski M, Kamińska H, Bartoszek M, Brzewski M, Werner B. COVID-19-vaccination–induced myocarditis in teenagers: case series with further follow-up. Int J Environ Res Public Health. 2022;19(6):3456. doi: 10.3390/ijerph19063456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manfredi R, Bianco F, Bucciarelli V, et al. Clinical profiles and CMR findings of young adults and pediatrics with acute myocarditis following mRNA COVID-19 vaccination: a case series. Vaccines (Basel). 2022;10(2):169. doi: 10.3390/vaccines10020169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ambati S, Colon M, Mihic M, Sanchez J, Bakar A. Acute myopericarditis after COVID-19 vaccine in teenagers. Case Rep Cardiol. 2021;2021:8268755. doi: 10.1155/2021/8268755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tano E, San Martin S, Girgis S, Martinez-Fernandez Y, Sanchez Vegas C. Perimyocarditis in adolescents after Pfizer-BioNTech COVID-19 vaccine. J Pediatric Infect Dis Soc. 2021;10(10):962-966. doi: 10.1093/jpids/piab060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J, Brekke DR, Bratincsak A. Self-limited myocarditis presenting with chest pain and ST segment elevation in adolescents after vaccination with the BNT162b2 mRNA vaccine. Cardiol Young. 2022;32(1):146-149. doi: 10.1017/S1047951121002547 [DOI] [PubMed] [Google Scholar]
- 36.Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148(3):e2021052478. doi: 10.1542/peds.2021-052478 [DOI] [PubMed] [Google Scholar]
- 37.Starekova J, Bluemke DA, Bradham WS, Grist TM, Schiebler ML, Reeder SB. Myocarditis associated with mRNA COVID-19 vaccination. Radiology. 2021;301(2):E409-E411. doi: 10.1148/radiol.2021211430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Łaźniak-Pfajfer A, Surmacz R, Rajewska-Tabor J, Pyda M, Lesiak M, Bobkowski W. Myocarditis associated with COVID-19 vaccination in three male teenagers. Pol Arch Intern Med. 2022;132(2):16160. doi: 10.20452/pamw.16160 [DOI] [PubMed] [Google Scholar]
- 39.Meyer-Szary J, Bazgier M, Lubocka P, Dorniak K, Sabiniewicz R. Cardiac magnetic resonance characteristics of acute myocarditis occurring after mRNA-based COVID-19 vaccines immunization. Cardiol J. 2022;29(1):160-162. doi: 10.5603/CJ.a2021.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed SK. Myocarditis after BNT162b2 and mRNA-1273 COVID-19 vaccination: A report of 7 cases. Ann Med Surg (Lond). 2022;77:103657. doi: 10.1016/j.amsu.2022.103657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freise NF, Kivel M, Grebe O, et al. Acute cardiac side effects after COVID-19 mRNA vaccination: a case series. Eur J Med Res. 2022;27(1):80. doi: 10.1186/s40001-022-00695-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedrich MG, Sechtem U, Schulz-Menger J, et al. ; International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis . Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53(17):1475-1487. doi: 10.1016/j.jacc.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158-3176. doi: 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 44.Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi: 10.1038/s41569-021-00662-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morello R, Pepe M, Martino L, et al. COVID-19 review shows that benefits of vaccinating children and adolescents appear to outweigh risks of post-vaccination myopericarditis. Acta Paediatr. 2022;111(10):1846-1852. doi: 10.1111/apa.16462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chou OHI, Mui J, Chung CT, et al. ; Cardiovascular Analytics Group, the International Health Informatics Study Network . COVID-19 vaccination and carditis in children and adolescents: a systematic review and meta-analysis. Clin Res Cardiol. 2022;111(10):1161-1173. doi: 10.1007/s00392-022-02070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Witberg G, Barda N, Hoss S, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi: 10.1056/NEJMoa2110737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med. 2021;385(23):2140-2149. doi: 10.1056/NEJMoa2109730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326(14):1390-1399. doi: 10.1001/jama.2021.15072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi: 10.1001/jamacardio.2021.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471-484. doi: 10.1161/CIRCULATIONAHA.121.056135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi: 10.1001/jama.2021.24110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karlstad Ø, Hovi P, Husby A, et al. SARS-CoV-2 vaccination and myocarditis in a Nordic cohort study of 23 million residents. JAMA Cardiol. 2022;7(6):600-612. doi: 10.1001/jamacardio.2022.0583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Husby A, Hansen JV, Fosbøl E, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021;375:e068665. doi: 10.1136/bmj-2021-068665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078-1090. doi: 10.1056/NEJMoa2110475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feldstein LR, Tenforde MW, Friedman KG, et al. ; Overcoming COVID-19 Investigators . Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074-1087. doi: 10.1001/jama.2021.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Temsah MH, Alhuzaimi AN, Aljamaan F, et al. Parental attitudes and hesitancy about COVID-19 vs. routine childhood vaccinations: a national survey. Front Public Health. 2021;9:752323. doi: 10.3389/fpubh.2021.752323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gräni C, Eichhorn C, Bière L, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70(16):1964-1976. doi: 10.1016/j.jacc.2017.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccination by vaccine product, schedule, and interdose interval among adolescents and adults in Ontario, Canada. JAMA Netw Open. 2022;5(6):e2218505. doi: 10.1001/jamanetworkopen.2022.18505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woo W, Kim AY, Yon DK, et al. Clinical characteristics and prognostic factors of myocarditis associated with the mRNA COVID-19 vaccine. J Med Virol. 2022;94(4):1566-1580. doi: 10.1002/jmv.27501 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. The Search Strategy of MEDLINE via PubMed
eTable 2. The Search Strategy of EMBASE
eTable 3. Characteristics of Observational Studies Included
eTable 4. Characteristics of Case Series Included
eTable 5. Definition of Myocarditis
eTable 6. Pooled Estimates for Clinical Characteristics and Outcomes From Subgroup Analyses Including Studies With Patients With Myocarditis
eTable 7. Joanna Briggs Institute Critical Appraisal Checklist for Prevalence Studies
eTable 8. Joanna Briggs Institute Critical Appraisal Checklist for Case Series
eTable 9. GRADE Evidence Profile for the Included Studies
eTable 10. Results of Egger Linear Regression Tests of the Clinical Presentation and Outcomes
eTable 11. Results of Point Estimate of the Clinical Presentation and Outcomes Using the Trim-and-Fill Analysis
eFigure 1. Forest Plots Showing the Pooled Estimates of Clinical Characteristics and Early Outcomes
eFigure 2. Forest Plots of Subgroup Analysis Including Studies With Only Patients With Myocarditis
eFigure 3. Risk of Bias Summary
eFigure 4. Funnel Plots of the Clinical Characteristics and Outcomes
eFigure 5. Adjusted Funnel Plots of the Clinical Characteristics and Outcomes From the Trim-and-Fill Analysis