Abstract
Background: The number of geriatric traumatic brain injury (TBI) patients is increasing every year due to the population’s aging in most of the developed countries. Unfortunately, there is no widely recognized tool for specifically evaluating the prognosis of geriatric TBI patients. We designed this study to compare the prognostic value of different machine learning algorithm-based predictive models for geriatric TBI. Methods: TBI patients aged ≥65 from the Medical Information Mart for Intensive Care-III (MIMIC-III) database were eligible for this study. To develop and validate machine learning algorithm-based prognostic models, included patients were divided into a training set and a testing set, with a ratio of 7:3. The predictive value of different machine learning based models was evaluated by calculating the area under the receiver operating characteristic curve, sensitivity, specificity, accuracy and F score. Results: A total of 1123 geriatric TBI patients were included, with a mortality of 24.8%. Non-survivors had higher age (82.2 vs. 80.7, p = 0.010) and lower Glasgow Coma Scale (14 vs. 7, p < 0.001) than survivors. The rate of mechanical ventilation was significantly higher (67.6% vs. 25.9%, p < 0.001) in non-survivors while the rate of neurosurgical operation did not differ between survivors and non-survivors (24.3% vs. 23.0%, p = 0.735). Among different machine learning algorithms, Adaboost (AUC: 0.799) and Random Forest (AUC: 0.795) performed slightly better than the logistic regression (AUC: 0.792) on predicting mortality in geriatric TBI patients in the testing set. Conclusion: Adaboost, Random Forest and logistic regression all performed well in predicting mortality of geriatric TBI patients. Prognostication tools utilizing these algorithms are helpful for physicians to evaluate the risk of poor outcomes in geriatric TBI patients and adopt personalized therapeutic options for them.
Keywords: traumatic brain injury, geriatric, machine learning, prognosis, prediction
1. Introduction
Population aging is a challenge in most of the developed countries. Estimated by the American Census Bureau, the elderly population (age ≥ 65 years) in the United States will increase to 80 million by 2050 [1]. The elderly population in Japan and South Korea has, respectively, reached to 27.7% and 13.8% in 2017 [2,3]. And the trend of population aging will remain or even be enhanced in the next decades. With the increase of the elderly population, the number of elderly traumatic brain injury (TBI) patients is also gradually increasing. It has been reported that emergency department visits and hospitalizations for TBI in elderly people of United States increased by 46% and 34%, respectively [4]. A report analyzed from the Japan Neurotrauma Data Bank Project 2015 indicated that 53.6% of registered TBI patients were elderly (age ≥ 65 years) and that most severe TBI patients were elderly [5]. Additionally, impaired performance of muscle strength, balance and agility caused by aging render older adults more likely to fall than young people [6]. Actually, more than half of TBI incidents among the elderly are attributable to ground-level falls [7].
Previous studies have shown that age is an independent risk factor of TBI prognosis [8,9]. And elderly TBI patients commonly suffer more complications and unfavorable outcomes than do non-elderly TBI patients [10,11]. Research from different countries has reported that the mortality rate of geriatric TBI ranged from 6.4% to 67.2% [3,12,13,14,15]. Although some elderly TBI patients do not suffer death in the short term, these TBI survivors survive with prominent physical and cognitive deficits [16]. Additionally, TBI survivors commonly develop psychiatric disorders and tend to be at higher risk of dementia and Alzheimer’s [17,18,19]. These disabilities and sequelas would continuously affect quality of life, and they bring a heavy economic burden for geriatric TBI patients [20,21]. Therefore, evaluating the prognosis of geriatric TBI patients early on could guide doctors in making individualized treatments and rehabilitation strategies for improving the prognosis, quality of life and reducing the medical expenditure.
Many previous studies have developed prognostic models for geriatric TBI utilizing conventional logistic regression [8,22,23,24]. Some risk factors for poor prognosis have been found, such as age, Charlson Comorbidity Index, Glasgow Coma Scale (GCS), Injury Severity Score (ISS), systolic blood pressure, intraventricular hemorrhage, and neurosurgical intervention [8,22,23,24]. However, there are no studies using machine learning algorithms to evaluate the prognosis of geriatric TBI. Compared with the conventional logistic regression, machine learning algorithms may perform better in analyzing nonlinear correlations and handling massive high-dimensional datasets. We designed this study to explore the prognostic value of different machine learning algorithm-based models for predicting mortality in geriatric TBI patients.
2. Materials and Methods
2.1. Patients
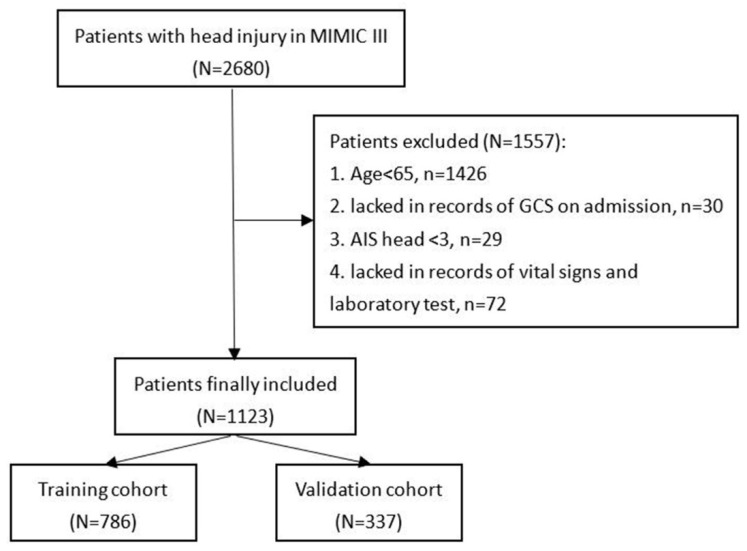
Patients included in this study were found in the Medical Information Mart for Intensive Care-III (MIMIC-III) database designed and produced by the computational physiology laboratory of Massachusetts Institute of Technology (MIT) (Cambridge, MA). This freely available database collects the information of patients admitted to Beth Israel Deaconess Medical Center (BIDMC) (Boston, MA) between 2001 and 2012 and obtains pre-approval from the institutional review boards of MIT and BIDMC. All patients included in the MIMIC-III were deidentified and anonymized in consideration of privacy protection. We included patients with head injury from the MIMIC-III based on ICD-9 codes (80000–80199; 80300–80499; 8500–85419). Then, patients were excluded according to the following criteria: (1) TBI patients with age < 65; (2) patients who lacked records of GCS on admission; (3) Abbreviated Injury Score (AIS) head < 3; or (4) patients who lacked records of vital signs and laboratory test (Figure 1). After screening, 1123 patients were finally included in the study.
Figure 1.
Flowchart of patients’ inclusion.
2.2. Data Collection
Age, gender, and comorbidities, including diabetes mellitus and hypertension were collected. Records of vital signs on admission, including systolic blood pressure, diastolic blood pressure, heart rate, respiratory rate, body temperature, and pulse oxygen saturation (SpO2) were extracted. Clinical scores including GCS, AIS of face, head, chest, abdomen, surface, and limb, and ISS were included [25,26]. Anatomical intracranial injury locations including epidural hematoma, subdural hematoma, subarachnoid hemorrhage, and intracerebral hemorrhage were classified based on ICD-9 codes. The results of laboratory tests analyzed from the first blood sample after admission were extracted, including white blood cell, platelet, red blood cell, red cell distribution width, hemoglobin, glucose, blood urea nitrogen, serum creatinine, sodium, potassium, phosphorus, calcium, magnesium, chloride, anion gap, prothrombin time, and international normalized ratio. Medical interventions including mechanical ventilation and neurosurgical operation were included. The primary outcome of this study was 30-day mortality. All above mentioned variables were extracted from the MIMIC-III through Navicat Premium 12 using Structure Query Language.
2.3. Statistical Analysis
The normality of included variables was confirmed by the Kolmogorov–Smirnov test. Normal distributed and non-normal distributed variables were presented as mean ± standard deviation and median (interquartile range), respectively. Categorical variables were shown as counts (percentage). Differences between the two groups of normal distributed and non-normal distributed variables were verified by Student’s t-test and the Mann–Whitney U test, respectively. A chi-square test or Fisher exact test was conducted to analyze the difference between two groups of categorical variables. To develop and validate machine learning algorithms-based models, all TBI patients were randomly divided between a training set (70%) and a testing set (30%). Logistic regression and six machine learning algorithms, including decision tree, Random Forest, support vector machine (SVM), Naïve Bayes, Adaboost and XGboost, were utilized to train predictive models for a 30-day mortality in training dataset. Variables with p < 0.05 in univariate logistic regression analysis were included into multivariate logistic regression analysis in the training set. The receiver operating characteristic (ROC) curve was drawn and the area under the ROC curve (AUC) was calculated to compare predictive performance of different machine learning algorithms-based models. Additionally, sensitivity, specificity, accuracy and F1 score (F1 score is calculated as the harmonic average of the precision rate and recall rate) were also calculated to evaluate the performance of these models.
All analyses were performed using R software (version 3.6.1; R Foundation, R Core Team, Vienna, Austria). R packages used for machine learning included ‘rpart’, ‘rpart.plot’, ‘party’, ‘randomForest’, ‘e1071′, ‘adabag’, and ‘xgboost’.
3. Results
3.1. Baseline Characteristics of Included TBI Patients
1123 TBI patients from the MIMIC-III were ultimately included, with a 30-day mortality of 24.8% (Table 1). Compared with survivors, non-survivors had higher age (p = 0.010) but lower incidence of hypertension (p = 0.007). Non-survivors had lower systolic blood pressure (p = 0.014), lower body temperature (p < 0.001) and higher SpO2 (p < 0.001) than survivors. Pupillary nonreactivity was more frequently observed in non-survivors (p < 0.001). Non-survivors had lower GCS (p < 0.001), higher AIS head (p < 0.001), AIS chest (p = 0.029), ISS (p < 0.001) and higher incidence of epidural hematoma (p = 0.001), and subarachnoid hemorrhage (p = 0.048). Results of laboratory tests showed that white blood cell (p < 0.001), red cell distribution width (p < 0.001), glucose (p < 0.001), blood urea nitrogen (p < 0.001), serum creatinine (p < 0.001), anion gap (p = 0.002), prothrombin time (p < 0.001), and international normalized ratio (p < 0.001) were higher in non-survivors, while platelet (p = 0.030), hemoglobin (p = 0.010), and calcium (p = 0.002) were lower in non-survivors. Finally, the usage incidence of mechanical ventilation was significantly higher in non-survivors (p < 0.001). Non-survivors had shorter length of hospital stay than survivors (p < 0.001).
Table 1.
Baseline characteristics of geriatric TBI patients in MIMIC-III.
| Variables | Overall Patients (n = 1123) |
Survivors (n = 845, 75.2%) |
Non-Survivors (n = 278, 24.8%) |
p |
|---|---|---|---|---|
| Age (year) | 81.0 (74.6–86.6) | 80.7 (74.0–85.9) | 82.2 (76.5–87.9) | 0.010 |
| Male gender (%) | 571 (50.8%) | 426 (50.4%) | 145 (52.2%) | 0.663 |
| Diabetes (%) | 258 (23.0%) | 185 (21.9%) | 73 (26.3%) | 0.156 |
| Hypertension (%) | 630 (56.1%) | 494 (58.5%) | 136 (48.9%) | 0.007 |
| Systolic blood pressure (mmHg) | 137 (121–152) | 138 (123–153) | 135 (114–150) | 0.014 |
| Diastolic blood pressure (mmHg) | 65 (53–76) | 65 (54–76) | 63 (52–75) | 0.099 |
| Heart rate (s−1) | 80 (70–91) | 80 (70–91) | 81 (70–93) | 0.171 |
| Respiratory rate (s−1) | 18 (15–20) | 18 (15–20) | 18 (15–21) | 0.400 |
| Body temperature (℉) | 97.9 (96.9–99.0) | 98.0 (97.1–99.0) | 97.6 (96.4–98.7) | <0.001 |
| SpO2 (%) | 98 (96–100) | 98 (96–100) | 99 (97–100) | <0.001 |
| Pupillary nonreactivity (size, %) | <0.001 | |||
| None | 969 (86.3%) | 773 (91.5%) | 196 (70.5%) | |
| One size | 64 (5.7%) | 42 (5.0%) | 22 (7.9%) | |
| Two size | 90 (8.0%) | 30 (3.6%) | 60 (21.6%) | |
| GCS | 14 (7–15) | 14 (10–15) | 7 (5–13) | <0.001 |
| AIS face | 0 | 0 | 0 | 0.315 |
| AIS head | 4 (3–4) | 4 (3–4) | 4 (4–5) | <0.001 |
| AIS chest | 0 | 0 | 0 | 0.029 |
| AIS abdomen | 0 | 0 | 0 | 0.870 |
| AIS surface | 0 | 0 | 0 | 0.158 |
| AIS limb | 0 | 0 | 0 | 0.728 |
| ISS | 16 (16–20) | 16 (16–17) | 16 (16–25) | <0.001 |
| Epidural hematoma (%) | 165 (14.7%) | 106 (12.5%) | 59 (21.2%) | 0.001 |
| Subdural hematoma (%) | 696 (62.0%) | 533 (63.1%) | 163 (58.6%) | 0.210 |
| Subarachnoid hemorrhage (%) | 403 (35.9%) | 289 (34.2%) | 114 (41.0%) | 0.048 |
| Intracerebral hemorrhage (%) | 185 (16.5%) | 135 (16.0%) | 50 (18.0%) | 0.490 |
| White blood cell (109/L) | 10.80 (8.10–14.10) | 10.30 (7.70–13.40) | 12.65 (9.53–16.43) | <0.001 |
| Platelet (109/L) | 216 (173–267) | 220 (176–269) | 206 (165–260) | 0.030 |
| Red blood cell (109/L) | 3.96 (3.58–4.36) | 3.97 (3.59–4.37) | 3.90 (3.42–4.34) | 0.100 |
| Red cell distribution width (%) | 13.9 (13.2–14.9) | 13.8 (13.2–14.7) | 14.1 (13.4–15.3) | <0.001 |
| Hemoglobin (g/dL) | 12.2 (10.9–13.4) | 12.3 (11.1–13.5) | 12.0 (10.5–13.2) | 0.010 |
| Glucose (mg/dL) | 137 (113–173) | 132 (110–163) | 160 (128–192) | <0.001 |
| Blood urea nitrogen (mg/dL) | 21 (16–28) | 21 (16–27) | 23 (17–32) | <0.001 |
| Serum creatinine (mg/dL) | 1.00 (0.80–1.30) | 1.00 (0.80–1.20) | 1.10 (0.90–1.40) | <0.001 |
| Sodium (mmol/L) | 139 (137–141) | 139 (137–141) | 139 (137–142) | 0.384 |
| Potassium (mmol/L) | 4.00 (3.70–4.50) | 4.00 (3.70–4.40) | 4.00 (3.60–4.50) | 0.548 |
| Phosphorus (mmol/L) | 3.20 (2.70–3.70) | 3.20 (2.80–3.70) | 3.20 (2.70–3.80) | 0.853 |
| Calcium (mmol/L) | 8.50 (7.35–9.00) | 8.50 (7.70–9.10) | 8.30 (1.18–9.00) | 0.002 |
| Magnesium (mmol/L) | 1.90 (1.70–2.10) | 1.90 (1.70–2.10) | 1.90 (1.60–2.10) | 0.519 |
| Chloride (mmol/L) | 103 (100–106) | 103 (100–106) | 103 (100–107) | 0.093 |
| Anion gap (mmol/L) | 15 (13–17) | 15 (13–17) | 15 (14–17) | 0.002 |
| Prothrombin time (s) | 13.10 (12.40–15.00) | 13.00 (12.30–14.70) | 13.40 (12.62–15.85) | <0.001 |
| International normalized ratio | 1.10 (1.00–1.40) | 1.10 (1.00–1.30) | 1.20 (1.10–1.50) | <0.001 |
| Mechanical ventilation (%) | 407 (36.2%) | 219 (25.9%) | 188 (67.6%) | <0.001 |
| Neurosurgical operation (%) | 269 (24.0%) | 205 (24.3%) | 64 (23.0%) | 0.735 |
| 30-day mortality (%) | 278 (24.8%) | 0 (0.0%) | 278 (100.0%) | <0.001 |
| Length of hospital stay (day) | 7 (4–12) | 7 (4–12) | 6 (3–10) | <0.001 |
SpO2, pulse oxygen saturation; GCS, Glasgow Coma Scale; AIS, Abbreviated Injury Score; ISS, Injury Severity Score. The bold value indicated p < 0.05.
3.2. Performance of Machine Learning Algorithms for Predicting Mortality in Geriatric TBI Patients
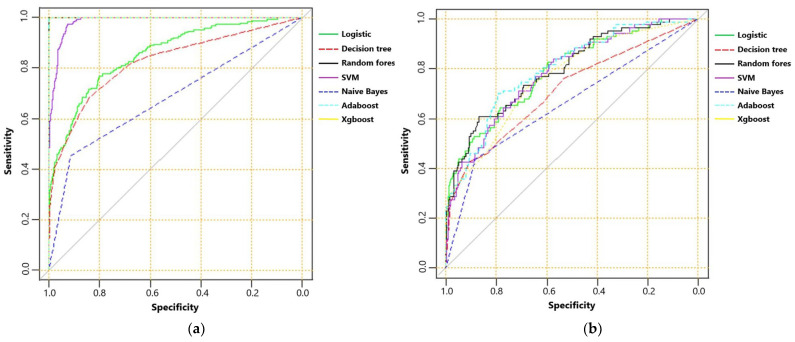
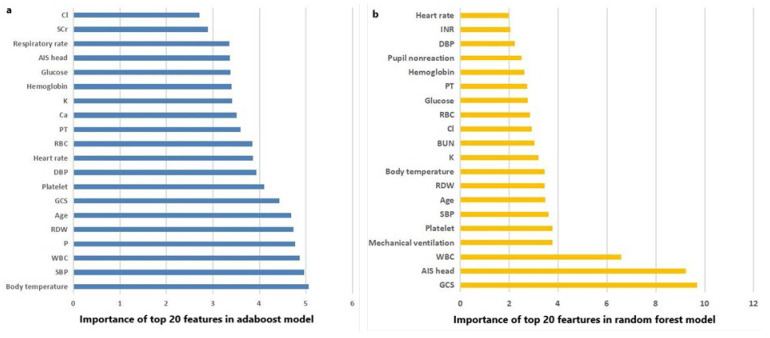
The AUC, sensitivity, specificity, accuracy and F score of machine learning algorithms for predicting mortality in the training set and the testing set are presented in Table 2. In the training set, Random Forest, Adaboost and XGboost reached the highest AUC of 1.000. In testing set, however, Adaboost, Random Forest and logistic regression ranked first, second and third, with AUC of 0.799, 0.795 and 0.792, respectively. ROC curves of machine learning algorithms for predicting mortality in the training set and the testing set are shown as Figure 2a,b. The importance of the top-20 features for predicting mortality in training set is shown in Figure 3a,b. The three most important features in Adaboost were body temperature, systolic blood pressure and white blood cell, sequentially. The three most important features in the Random Forest were GCS, AIS head and white blood cell, sequentially. The details of each variable in the logistic regression-based model was presented as Table 3.
Table 2.
Performance of machine learning algorithms for predicting 30-day mortality in the training set and the testing set.
| Training Set | AUC | 95% CI | Sensitivity | Specificity | Accuracy | F Score |
|---|---|---|---|---|---|---|
| Decision tree | 0.825 | 0.789–0.861 | 0.686 | 0.840 | 0.803 | 0.628 |
| Random Forest | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| SVM | 0.985 | 0.979–0.991 | 0.974 | 0.928 | 0.938 | 0.884 |
| Naïve Bayes | 0.684 | 0.647–0.721 | 0.455 | 0.913 | 0.802 | 0.527 |
| Logistic | 0.859 | 0.828–0.890 | 0.77 | 0.802 | 0.793 | 0.643 |
| Adaboost | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| XGboost | 1.000 | 1.000 | 1.000 | 0.998 | 1.000 | 1.000 |
| Testing set | AUC | 95% CI | Sensitivity | Specificity | Accuracy | F score |
| Decision Tree | 0.712 | 0.647–0.777 | 0.425 | 0.908 | 0.783 | 0.503 |
| Random Forest | 0.795 | 0.739–0.851 | 0.609 | 0.868 | 0.801 | 0.613 |
| SVM | 0.785 | 0.730–0.840 | 0.713 | 0.712 | 0.712 | 0.561 |
| Naïve Bayes | 0.658 | 0.602–0.715 | 0.437 | 0.880 | 0.766 | 0.490 |
| Logistic | 0.792 | 0.736–0.848 | 0.644 | 0.784 | 0.745 | 0.561 |
| Adaboost | 0.799 | 0.746–0.853 | 0.701 | 0.792 | 0.769 | 0.610 |
| XGboost | 0.766 | 0.709–0.823 | 0.724 | 0.680 | 0.691 | 0.548 |
AUC, area under the receiver operating characteristic curve; SVM, support vector machine; Adaboost, adaptive boost; XGboost, extreme gradient boost.
Figure 2.
(a) The receiver operating characteristic curve of machine learning algorithms for predicting mortality in training set; and (b) receiver operating characteristic curve of machine learning algorithms for predicting mortality in testing set.
Figure 3.
(a) The importance of the top 20 features in the Adaboost model; and (b) the importance of the top 20 features in the Random Forest model.
Table 3.
Univariate and multivariate logistic regression analysis of risk factors for 30-day mortality in training set.
| Variables | Univariate Logistic Regression Analysis | Multivariate Logistic Regression Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% Cl | p | OR | 95% Cl | p | |
| Age | 1.031 | 1.009–1.054 | 0.006 | 1.054 | 1.023–1.087 | 0.001 |
| Male gender | 1.044 | 0.753–1.447 | 0.796 | |||
| Diabetes | 1.370 | 0.940–1.996 | 0.101 | |||
| Hypertension | 0.774 | 0.558–1.074 | 0.125 | |||
| Systolic blood pressure | 0.992 | 0.985–0.998 | 0.013 | 1.002 | 0.994–1.010 | 0.667 |
| Diastolic blood pressure | 0.991 | 0.982–1.001 | 0.077 | |||
| Heart rate | 1.007 | 0.997–1.016 | 0.171 | |||
| Respiratory rate | 0.991 | 0.960–1.024 | 0.595 | |||
| Body temperature | 0.783 | 0.709–0.866 | <0.001 | 0.825 | 0.728–0.934 | 0.002 |
| SpO2 | 1.052 | 0.993–1.115 | 0.084 | |||
| Pupillary nonreactivity | <0.001 | 0.001 | ||||
| None | 1.000 | [Reference] | 1.000 | [Reference] | ||
| One size | 1.930 | 0.995–3.743 | 0.052 | 1.509 | 0.668–3.410 | 0.322 |
| Two size | 9.797 | 5.546–17.305 | <0.001 | 3.745 | 1.818–7.716 | <0.001 |
| GCS | 0.786 | 0.754–0.820 | <0.001 | 0.888 | 0.831–0.948 | <0.001 |
| AIS face | 0.899 | 0.700–1.154 | 0.403 | |||
| AIS head | 2.767 | 1.993–3.841 | <0.001 | 2.383 | 1.309–4.339 | 0.004 |
| AIS chest | 1.202 | 1.044–1.384 | 0.010 | 1.071 | 0.776–1.477 | 0.678 |
| AIS abdomen | 1.041 | 0.767–1.414 | 0.796 | |||
| AIS surface | 0.669 | 0.291–1.542 | 0.346 | |||
| AIS limb | 1.057 | 0.869–1.287 | 0.579 | |||
| ISS | 1.069 | 1.045–1.094 | <0.001 | 0.980 | 0.919–1.044 | 0.530 |
| Epidural hematoma | 1.776 | 1.154–2.734 | 0.009 | 1.419 | 0.788–2.556 | 0.244 |
| Subdural hematoma | 0.791 | 0.567–1.103 | 0.166 | |||
| Subarachnoid hemorrhage | 1.204 | 0.859–1.687 | 0.282 | |||
| Intracerebral hemorrhage | 1.317 | 0.871–1.992 | 0.192 | |||
| White blood cell | 1.097 | 1.063–1.133 | <0.001 | 1.077 | 1.039–1.117 | <0.001 |
| Platelet | 1.000 | 0.998–1.002 | 0.897 | |||
| Red blood cell | 0.843 | 0.658–1.080 | 0.176 | |||
| Red cell distribution width | 1.121 | 1.022–1.230 | 0.015 | 1.118 | 0.981–1.273 | 0.093 |
| Hemoglobin | 0.903 | 0.832–0.981 | 0.015 | 0.916 | 0.816–1.029 | 0.139 |
| Glucose | 1.006 | 1.003–1.008 | <0.001 | 1.002 | 0.999–1.005 | 0.239 |
| Blood urea nitrogen | 1.013 | 1.002–1.024 | 0.026 | 1.002 | 0.986–1.018 | 0.831 |
| Serum creatinine | 1.183 | 0.979–1.429 | 0.081 | |||
| Sodium | 1.020 | 0.983–1.059 | 0.302 | |||
| Potassium | 0.955 | 0.751–1.215 | 0.710 | |||
| Phosphorus | 1.011 | 0.833–1.228 | 0.909 | |||
| Calcium | 0.946 | 0.902–0.993 | 0.025 | 1.113 | 1.038–1.193 | 0.003 |
| Magnesium | 0.916 | 0.541–1.553 | 0.745 | |||
| Chloride | 1.048 | 1.016–1.082 | 0.003 | 1.025 | 0.983–1.067 | 0.245 |
| Anion gap | 1.054 | 1.002–1.109 | 0.041 | 1.048 | 0.975–1.125 | 0.201 |
| Prothrombin time | 1.018 | 0.997–1.038 | 0.089 | |||
| International normalized ratio | 1.185 | 1.000–1.404 | 0.050 | |||
| Mechanical ventilation | 5.768 | 4.053–8.208 | <0.001 | 3.542 | 2.012–6.238 | <0.001 |
| Neurosurgical operation | 0.992 | 0.676–1.457 | 0.969 | |||
OR, odds ratio; CI, confidence interval; SpO2, pulse oxygen saturation; GCS, Glasgow Coma Scale; AIS, Abbreviated Injury Score; ISS, Injury Severity Score. The bold value indicated p < 0.05.
4. Discussion
The 30-day mortality of included geriatric TBI patients in this study was 24.8%, which was similar to previously reported incidence ranging from 6.4% to 67.2% [3,12,13,14,15]. The significant mortality difference in different studies may be attributable to differences of injury severity, therapeutic options, and age distribution. A total of eight factors were found to be independently associated with mortality by the logistic regression, including age, body temperature, pupillary nonreactivity, GCS, AIS head, white blood cell, calcium, and mechanical ventilation, all of which have been confirmed as risk factors for poor prognosis in TBI.
Many previous studies have verified that increasing age was actually the strongest predictor of poor outcome in TBI [27,28,29]. The increase in age may indicate worse nutritional status, extracranial organ function, cerebrovascular autoregulation and higher likelihood of infectious complication, or secondary brain injury. The pupillary nonreactivity implying impaired function of medulla oblongata and midbrain, has been confirmed as an important and convenient index to evaluate the prognosis of TBI [30,31,32]. Although the GCS has been utilized to evaluate the condition of brain injury patients for decades, it shows unstable performance under several situations, including drinking, seizure, and being sedated. Especially, geriatric patients commonly suffer complications with cerebrovascular disease, dementia, and impaired hearing, which could limit the reliability of GCS evaluation [33]. The median GCS of our included geriatric TBI patients was 14, with a lower and upper quartile of 7 and 15, which indicates that most of included geriatric patients suffered mild to moderate TBI. This fact may reflect the characteristic of fall injury among geriatric patients, which is significantly different from the traffic-accident-induced injury prevalent in young adults presenting with lower GCS. Another risk factor for mortality discovered by logistic regression was mechanical ventilation. The incidence of receiving mechanical ventilation in non-survivors was 67.6%, which was significantly higher than the 25.9% of survivors. Mechanical ventilation is commonly used to assist breathing for TBI patients with respiratory failure, pulmonary infection, or chest trauma. These patients have worse organ function, higher injury severity and higher risk of a poor outcome.
Finally, abnormal body temperature is prevalent in TBI patients [34]. One previous study found that both elevated temperature and low temperature immediately after prehospital transport were independently associated with higher mortality and with increased length of hospital stay [35]. Elevated temperature after TBI may be caused by a series of factors, such as infection and overactivated sympathetic activity, which may be both associated with poor prognosis.
In addition to factors discovered by the logistic regression, Random Forest and Adaboost algorithms also confirmed several other important factors, including systolic blood pressure, diastolic blood pressure, red cell distribution width, and platelet, based on their contribution degrees to the prediction. The hypotension and even shock status reflected by low blood pressure undoubtedly promote the deterioration of organ function and unfavorable outcomes. Additionally, unstable control of blood pressure and high blood pressure variability would cause the deviation from optimal cerebral perfusion pressure [36]. As a key component of the coagulation system, the platelet has been testified regulating neuroinflammation and restoring blood brain barrier integrity after TBI [37]. Furthermore, platelet dysfunction has been confirmed as one of coagulopathy etiologies after TBI and associated with poor outcomes [38,39]. Finally, previous studies showed red cell distribution width to platelet ratio is a reliable prognostic marker of TBI [40,41].
In our study, the neurosurgical operation did not show an independent association with the mortality of TBI patients analyzed by the logistic regression. Additionally, it did not rank within the top 20 regarding the feature importance of Adaboost and Random Forest. Actually, it is still debated whether conservative or aggressive treatment should be provided for geriatric TBI patients. Although many centers have adopted the conservative treatment for geriatric TBI in the past years, increasing evidence supports the benefit of surgical operation for geriatric TBI. One Japanese study found surgical operation was associated with better functional outcomes and lower mortality of geriatric TBI patients with subdural hematoma and GCS ≥ 6 [8]. The effect of surgical management upon geriatric TBI may depend on many factors, such as injury severity, emergence of symptoms, size and location of hematoma mass, surgical options, physical state and comorbidities of patients. It would be worthwhile to design and perform randomized controlled trials to explore the benefit of surgical management for specific geriatric TBI patients in the future.
It is generally recognized that the prognosis of geriatric TBI is poorer than in young adults with TBI. But there is insufficient literature and studies specially focusing on multiple fields of geriatric TBI patients, including risk evaluation, treatments, prognosis and rehabilitation. Up to now, there has not been a widely acknowledged prognostic risk assessment tool for the geriatric TBI. Previous studies have explored the prognostic value of International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) score and Corticosteroid Randomization after Significant Head Injury (CRASH) score in geriatric TBI patients [33,42,43,44]. One of them found IMPACT showed moderate discrimination and slight overestimation of the actual outcome for geriatric TBI [42]. And another confirmed that CRASH was an effective prognostic tool for geriatric TBI and it showed no difference of performance between geriatric patients and young patients [44]. However, the small sample size and the highly specialized TBI population of these studies limit the reliability of conclusions. Some studies have utilized logistic regression to develop prognostication tools specific to geriatric TBI, based on multiple factors such as age, GCS, hypotension, Charlson Comorbidity Index and ISS [8,22,28]. Previous studies found machine learning algorithms-based models performed well on the prediction of prognosis in many kinds of neurosurgical patients, such as aneurysmal subarachnoid hemorrhage, and intracerebral hemorrhage [45,46,47]. Additionally, some studies exploring the prognostic value of machine learning in pediatric TBI found machine learning performed better than conventional statistical models and CT scores in predicting outcomes [48,49], while there is still no study exploring the prognostic value of machine learning algorithms in geriatric TBI patients. The results of our study show that machine learning algorithms did not perform worse than the logistic regression, and even show slightly higher accuracy than the logistic regression. The greater statistical difference needs to be verified in a study with a larger sample size. Adaboost and Random Forest showed the best accuracy among several machine learning algorithms adopted in our study. Based on the bagging method, Random Forest is a classifier containing multiple decision trees. Its output category is determined by the mode of individual trees’ category output. There are several advantages of Random Forest, including high accuracy, fast running speed on large datasets, and maintained accuracy in the case of a large part of missing data [50,51]. The Adaboost algorithm is an effective and practical boosting algorithm. Its algorithmic principle is to select weak classifiers with the smallest weight coefficient from the trained weak classifiers by adjusting the sample weight and the weight of the weak classifier, and then combine the two into a final strong classifier [52].
This study has several limitations. Firstly, TBI patients analyzed in this study were identified in the MIMIC-III, which is a freely available intensive care database produced by a hospital in Boston, United States with large sample size. Geriatric TBI patients from this database are mainly classified into mild to moderate brain injury with GCS quartiles of 7 and 15. Therefore, selection bias could not be avoided and future studies mainly including moderate to severe geriatric TBI patients conducted in other medical centers may offer external support to our findings. Secondly, the prognosis is different between mild and moderate to severe TBI patients. Developing machine learning based prognostic models for these two groups of TBI respectively may be more individualized and accurate. Thirdly, though many clinical factors and laboratory indexes have been brought into this study, there are still some risk factors of poor prognosis that have not been collected, such as antiplatelet drugs, anticoagulants and comorbidities excepting for diabetes mellitus and hypertension. Fourthly, several previously developed scores were not recorded and compared with our predictive models such as International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT), Corticosteroid Randomization after Significant Head Injury (CRASH) and Marshall CT score. Finally, the only outcome of this study was 30-day mortality, we did not collect functional outcome and cognitive status which were important measures for evaluating prognosis of geriatric patients due to the nature of the database study.
5. Conclusions
Adaboost and Random Forest performed slightly better than the logistic regression on predicting mortality of geriatric TBI patients. Future works could be focused on developing practical application software utilizing these algorithms in portable electronic equipment to quickly evaluate prognosis of geriatric TBI.
Author Contributions
Conceptualization, R.W. and X.Z.; methodology, R.W.; software, R.W.; formal analysis, Y.L. and J.Z.; data curation, R.W. and H.B.; writing—original draft preparation, R.W.; writing—review and editing, M.H. and J.X.; supervision, J.X.; project administration, J.X. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study did not need IRB approval. Because data were collected from the free MIMIC database with deidentified data. The MIMIC database was designed and produced by the Beth Israel Deaconess Medical Center (BIDMC). This database was approved by the institutional review boards of Massachusetts Institute of Technology and BIDMC. All patients included in this public database were anonymized and de-identified for protecting individual privacy. This study was conducted in accordance with the ethical standards of the Helsinki Declaration.
Informed Consent Statement
Patient consent was waived because this is a retrospective study.
Data Availability Statement
The datasets used for the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was funded by the 1·3·5 project for disciplines of excellence—Clinical Research Incubation Project, West China Hospital, Sichuan University (2020HXFH036), the Knowledge Innovation Program of the Chinese Academy of Sciences (JH2022007), the General Program of the National Natural Science Foundation of China (82173175), the Sichuan Science and Technology Program (2021YFS0184, 2021YFS0082), and the Post-Doctor Research Project, Sichuan University (2021SCU12027).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Stein D.M., Kozar R.A., Livingston D.H., Luchette F., Adams S.D., Agrawal V., Arbabi S., Ballou J., Barraco R.D., Bernard A.C., et al. Geriatric traumatic brain injury-What we know and what we don’t. J. Trauma Acute Care Surg. 2018;85:788–798. doi: 10.1097/TA.0000000000001910. [DOI] [PubMed] [Google Scholar]
- 2.Suehiro E., Fujiyama Y., Kiyohira M., Haji K., Ishihara H., Nomura S., Suzuki M. Risk of Deterioration of Geriatric Traumatic Brain Injury in Patients Treated with Antithrombotic Drugs. World Neurosurg. 2019;127:e1221–e1227. doi: 10.1016/j.wneu.2019.04.108. [DOI] [PubMed] [Google Scholar]
- 3.Eom K.S. Epidemiology and Outcomes of Traumatic Brain Injury in Elderly Population: A Multicenter Analysis Using Korean Neuro-Trauma Data Bank System 2010–2014. J. Korean Neurosurg. Soc. 2019;62:243–255. doi: 10.3340/jkns.2018.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson H.J., Weir S., Rivara F.P., Wang J., Sullivan S.D., Salkever D., MacKenzie E.J. Utilization and costs of health care after geriatric traumatic brain injury. J. Neurotrauma. 2012;29:1864–1871. doi: 10.1089/neu.2011.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suehiro E., Suzuki M. (4) Pitfalls in the Treatment of Geriatric Traumatic Brain Injury. No Shinkei Geka Neurol. Surg. 2018;46:1127–1135. doi: 10.11477/mf.1436203880. [DOI] [PubMed] [Google Scholar]
- 6.Thompson H.J., McCormick W.C., Kagan S.H. Traumatic brain injury in older adults: Epidemiology, outcomes, and future implications. J. Am. Geriatr. Soc. 2006;54:1590–1595. doi: 10.1111/j.1532-5415.2006.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Traumatic Brain Injury and Concussion. [(accessed on 21 December 2017)]; Updated 6 July 2017. Available online: https://www.cdc.gov/traumaticbraininjury/data/dist_death.html.
- 8.Shimoda K., Maeda T., Tado M., Yoshino A., Katayama Y., Bullock M.R. Outcome and surgical management for geriatric traumatic brain injury: Analysis of 888 cases registered in the Japan Neurotrauma Data Bank. World Neurosurg. 2014;82:1300–1306. doi: 10.1016/j.wneu.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Mushkudiani N.A., Engel D.C., Steyerberg E.W., Butcher I., Lu J., Marmarou A., Slieker F., McHugh G.S., Murray G.D., Maas A.I. Prognostic value of demographic characteristics in traumatic brain injury: Results from the IMPACT study. J. Neurotrauma. 2007;24:259–269. doi: 10.1089/neu.2006.0028. [DOI] [PubMed] [Google Scholar]
- 10.Toida C., Muguruma T., Gakumazawa M., Shinohara M., Abe T., Takeuchi I., Morimura N. Age- and Severity-Related In-Hospital Mortality Trends and Risks of Severe Traumatic Brain Injury in Japan: A Nationwide 10-Year Retrospective Study. J. Clin. Med. 2021;10:1072. doi: 10.3390/jcm10051072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosomi S., Kitamura T., Sobue T., Ogura H., Shimazu T. Sex and age differences in isolated traumatic brain injury: A retrospective observational study. BMC Neurol. 2021;21:261. doi: 10.1186/s12883-021-02305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedetto N., Gambacciani C., Montemurro N., Morganti R., Perrini P. Surgical management of acute subdural haematomas in elderly: Report of a single center experience. Br. J. Neurosurg. 2017;31:244–248. doi: 10.1080/02688697.2016.1244249. [DOI] [PubMed] [Google Scholar]
- 13.Won S.Y., Dubinski D., Brawanski N., Strzelczyk A., Seifert V., Freiman T.M., Konczalla J. Significant increase in acute subdural hematoma in octo- and nonagenarians: Surgical treatment, functional outcome, and predictors in this patient cohort. Neurosurg. Focus. 2017;43:E10. doi: 10.3171/2017.7.FOCUS17417. [DOI] [PubMed] [Google Scholar]
- 14.Fu W.W., Fu T.S., Jing R., McFaull S.R., Cusimano M.D. Predictors of falls and mortality among elderly adults with traumatic brain injury: A nationwide, population-based study. PLoS ONE. 2017;12:e0175868. doi: 10.1371/journal.pone.0175868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosomi S., Sobue T., Kitamura T., Ogura H., Shimazu T. Nationwide improvements in geriatric mortality due to traumatic brain injury in Japan. BMC Emerg. Med. 2022;22:24. doi: 10.1186/s12873-022-00577-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eapen B.C., Allred D.B., O’Rourke J., Cifu D.X. Rehabilitation of moderate-to-severe traumatic brain injury. Semin. Neurol. 2015;35:e1–e3. doi: 10.1055/s-0035-1549094. [DOI] [PubMed] [Google Scholar]
- 17.Gu D., Ou S., Liu G. Traumatic Brain Injury and Risk of Dementia and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology. 2022;56:4–16. doi: 10.1159/000520966. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z., Wang Z.H., Liu X., Zhang Z., Gu X., Yu S.P., Keene C.D., Cheng L., Ye K. Traumatic brain injury triggers APP and Tau cleavage by delta-secretase, mediating Alzheimer’s disease pathology. Prog. Neurobiol. 2020;185:101730. doi: 10.1016/j.pneurobio.2019.101730. [DOI] [PubMed] [Google Scholar]
- 19.Howlett J.R., Nelson L.D., Stein M.B. Mental Health Consequences of Traumatic Brain Injury. Biol. Psychiatry. 2022;91:413–420. doi: 10.1016/j.biopsych.2021.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faul M., Wald M.M., Rutland-Brown W., Sullivent E.E., Sattin R.W. Using a cost-benefit analysis to estimate outcomes of a clinical treatment guideline: Testing the Brain Trauma Foundation guidelines for the treatment of severe traumatic brain injury. J. Trauma. 2007;63:1271–1278. doi: 10.1097/TA.0b013e3181493080. [DOI] [PubMed] [Google Scholar]
- 21.Prang K.H., Ruseckaite R., Collie A. Healthcare and disability service utilization in the 5-year period following transport-related traumatic brain injury. Brain Inj. 2012;26:1611–1620. doi: 10.3109/02699052.2012.698790. [DOI] [PubMed] [Google Scholar]
- 22.Okazaki T., Hifumi T., Kawakita K., Nakashima R., Matsumoto A., Shishido H., Ogawa D., Okauchi M., Shindo A., Kawanishi M., et al. Association Between Comorbidities, Nutritional Status, and Anticlotting Drugs and Neurologic Outcomes in Geriatric Patients with Traumatic Brain Injury. World Neurosurg. 2016;93:336–340. doi: 10.1016/j.wneu.2016.06.070. [DOI] [PubMed] [Google Scholar]
- 23.Yokobori S., Yamaguchi M., Igarashi Y., Hironaka K., Onda H., Kuwamoto K., Araki T., Fuse A., Yokota H. Outcome and Refractory Factor of Intensive Treatment for Geriatric Traumatic Brain Injury: Analysis of 1165 Cases Registered in the Japan Neurotrauma Data Bank. World Neurosurg. 2016;86:127–133.e121. doi: 10.1016/j.wneu.2015.09.105. [DOI] [PubMed] [Google Scholar]
- 24.Utomo W.K., Gabbe B.J., Simpson P.M., Cameron P.A. Predictors of in-hospital mortality and 6-month functional outcomes in older adults after moderate to severe traumatic brain injury. Injury. 2009;40:973–977. doi: 10.1016/j.injury.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 25.Baker S.P., O’Neill B., Haddon W., Jr., Long W.B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma. 1974;14:187–196. doi: 10.1097/00005373-197403000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Civil I.D., Schwab C.W. The Abbreviated Injury Scale, 1985 revision: A condensed chart for clinical use. J. Trauma. 1988;28:87–90. doi: 10.1097/00005373-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Hukkelhoven C.W., Steyerberg E.W., Rampen A.J., Farace E., Habbema J.D., Marshall L.F., Murray G.D., Maas A.I. Patient age and outcome following severe traumatic brain injury: An analysis of 5600 patients. J. Neurosurg. 2003;99:666–673. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- 28.Mosenthal A.C., Lavery R.F., Addis M., Kaul S., Ross S., Marburger R., Deitch E.A., Livingston D.H. Isolated traumatic brain injury: Age is an independent predictor of mortality and early outcome. J. Trauma. 2002;52:907–911. doi: 10.1097/00005373-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Tokutomi T., Miyagi T., Ogawa T., Ono J., Kawamata T., Sakamoto T., Shigemori M., Nakamura N. Age-associated increases in poor outcomes after traumatic brain injury: A report from the Japan Neurotrauma Data Bank. J. Neurotrauma. 2008;25:1407–1414. doi: 10.1089/neu.2008.0577. [DOI] [PubMed] [Google Scholar]
- 30.Marmarou A., Lu J., Butcher I., McHugh G.S., Murray G.D., Steyerberg E.W., Mushkudiani N.A., Choi S., Maas A.I. Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrollment: An IMPACT analysis. J. Neurotrauma. 2007;24:270–280. doi: 10.1089/neu.2006.0029. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann M., Lefering R., Rueger J.M., Kolb J.P., Izbicki J.R., Ruecker A.H., Rupprecht M., Lehmann W. Pupil evaluation in addition to Glasgow Coma Scale components in prediction of traumatic brain injury and mortality. Br. J. Surg. 2012;99((Suppl. 1)):122–130. doi: 10.1002/bjs.7707. [DOI] [PubMed] [Google Scholar]
- 32.Emami P., Czorlich P., Fritzsche F.S., Westphal M., Rueger J.M., Lefering R., Hoffmann M. Impact of Glasgow Coma Scale score and pupil parameters on mortality rate and outcome in pediatric and adult severe traumatic brain injury: A retrospective, multicenter cohort study. J. Neurosurg. 2017;126:760–767. doi: 10.3171/2016.1.JNS152385. [DOI] [PubMed] [Google Scholar]
- 33.Staples J.A., Wang J., Zaros M.C., Jurkovich G.J., Rivara F.P. The application of IMPACT prognostic models to elderly adults with traumatic brain injury: A population-based observational cohort study. Brain Inj. 2016;30:899–907. doi: 10.3109/02699052.2016.1146964. [DOI] [PubMed] [Google Scholar]
- 34.Saxena M.K., Taylor C., Hammond N., Young P., Mysore J., Billot L., Myburgh A., Myburgh J. A multicentre audit of temperature patterns after traumatic brain injury. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2015;17:129–134. [PubMed] [Google Scholar]
- 35.Gaither J.B., Chikani V., Stolz U., Viscusi C., Denninghoff K., Barnhart B., Mullins T., Rice A.D., Mhayamaguru M., Smith J.J., et al. Body Temperature after EMS Transport: Association with Traumatic Brain Injury Outcomes. Prehosp. Emerg. Care. 2017;21:575–582. doi: 10.1080/10903127.2017.1308609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wettervik T.S., Howells T., Lewén A., Enblad P. Blood Pressure Variability and Optimal Cerebral Perfusion Pressure-New Therapeutic Targets in Traumatic Brain Injury. Neurosurgery. 2020;86:E300–E309. doi: 10.1093/neuros/nyz515. [DOI] [PubMed] [Google Scholar]
- 37.Gao C., Wang H., Wang T., Luo C., Wang Z., Zhang M., Chen X., Tao L. Platelet regulates neuroinflammation and restores blood-brain barrier integrity in a mouse model of traumatic brain injury. J. Neurochem. 2020;154:190–204. doi: 10.1111/jnc.14983. [DOI] [PubMed] [Google Scholar]
- 38.Riojas C.M., Ekaney M.L., Ross S.W., Cunningham K.W., Furay E.J., Brown C.V.R., Evans S.L. Platelet Dysfunction after Traumatic Brain Injury: A Review. J. Neurotrauma. 2021;38:819–829. doi: 10.1089/neu.2020.7301. [DOI] [PubMed] [Google Scholar]
- 39.Guillotte A.R., Herbert J.P., Madsen R., Hammer R.D., Litofsky N.S. Effects of platelet dysfunction and platelet transfusion on outcomes in traumatic brain injury patients. Brain Inj. 2018;32:1849–1857. doi: 10.1080/02699052.2018.1536805. [DOI] [PubMed] [Google Scholar]
- 40.Ge X., Zhu L., Li W., Sun J., Chen F., Li Y., Lei P., Zhang J. Red Cell Distribution Width to Platelet Count Ratio: A Promising Routinely Available Indicator of Mortality for Acute Traumatic Brain Injury. J. Neurotrauma. 2021;39:159–171. doi: 10.1089/neu.2020.7481. [DOI] [PubMed] [Google Scholar]
- 41.Wang R., He M., Zhang J., Wang S., Xu J. A Prognostic Model Incorporating Red Cell Distribution Width to Platelet Ratio for Patients with Traumatic Brain Injury. Ther. Clin. Risk Manag. 2021;17:1239–1248. doi: 10.2147/TCRM.S337040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan X., Zhao K., Wang S., Zhang H., Zeng L., Wang Y., Han L., Beejadhursing R., Shu K., Lei T. Is It Reliable to Predict the Outcome of Elderly Patients with Severe Traumatic Brain Injury Using the IMPACT Prognostic Calculator? World Neurosurg. 2017;103:584–590. doi: 10.1016/j.wneu.2017.04.069. [DOI] [PubMed] [Google Scholar]
- 43.Moorthy D.G.S.R.K., Rajesh K., Priya S.M., Abhinov T., Prasad K.J.D. Prediction of Outcome Based on Trauma and Injury Severity Score, IMPACT and CRASH Prognostic Models in Moderate-to-Severe Traumatic Brain Injury in the Elderly. Asian J. Neurosurg. 2021;16:500–506. doi: 10.4103/ajns.AJNS_512_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staples J.A., Wang J., Mills B., Temkin N., Zaros M.C., Jurkovich G.J., Rivara F.P. The Application of the CRASH-CT Prognostic Model for Older Adults with Traumatic Brain Injury: A Population-Based Observational Cohort Study. J. Head Trauma Rehabil. 2016;31:E8–E14. doi: 10.1097/HTR.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 45.Deng J., He Z. Characterizing Risk of In-Hospital Mortality Following Subarachnoid Hemorrhage Using Machine Learning: A Retrospective Study. Front. Surg. 2022;9:891984. doi: 10.3389/fsurg.2022.891984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maldaner N., Zeitlberger A.M., Sosnova M., Goldberg J., Fung C., Bervini D., May A., Bijlenga P., Schaller K., Roethlisberger M., et al. Development of a Complication- and Treatment-Aware Prediction Model for Favorable Functional Outcome in Aneurysmal Subarachnoid Hemorrhage Based on Machine Learning. Neurosurgery. 2021;88:E150–E157. doi: 10.1093/neuros/nyaa401. [DOI] [PubMed] [Google Scholar]
- 47.Trevisi G., Caccavella V.M., Scerrati A., Signorelli F., Salamone G.G., Orsini K., Fasciani C., D’Arrigo S., Auricchio A.M., D’Onofrio G., et al. Machine learning model prediction of 6-month functional outcome in elderly patients with intracerebral hemorrhage. Neurosurg. Rev. 2022;45:2857–2867. doi: 10.1007/s10143-022-01802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hale A.T., Stonko D.P., Brown A., Lim J., Voce D.J., Gannon S.R., Le T.M., Shannon C.N. Machine-learning analysis outperforms conventional statistical models and CT classification systems in predicting 6-month outcomes in pediatric patients sustaining traumatic brain injury. Neurosurg. Focus. 2018;45:E2. doi: 10.3171/2018.8.FOCUS17773. [DOI] [PubMed] [Google Scholar]
- 49.Daley M., Cameron S., Ganesan S.L., Patel M.A., Stewart T.C., Miller M.R., Alharfi I., Fraser D.D. Pediatric severe traumatic brain injury mortality prediction determined with machine learning-based modeling. Injury. 2022;53:992–998. doi: 10.1016/j.injury.2022.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Breiman L. Random Forests. Mach. Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 51.Denil M., Matheson D., Freitas N.D. Narrowing the Gap: Random Forests in Theory and in Practice; Proceedings of the 31st International Conference on Machine Learning; Beijing, China. 21–26 June 2014; pp. 665–673. [Google Scholar]
- 52.Shakeel P.M., Tolba A., Al-Makhadmeh Z., Jaber M.M. Automatic detection of lung cancer from biomedical data set using discrete AdaBoost optimized ensemble learning generalized neural networks. Neural Comput. Appl. 2019;33:777–790. doi: 10.1007/s00521-018-03972-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used for the current study are available from the corresponding author on reasonable request.