Key Points
Question
Does torsemide reduce all-cause mortality compared with furosemide in patients with heart failure following hospitalization?
Findings
In this randomized clinical trial of 2859 patients, 26.1% of patients randomized to torsemide and 26.2% randomized to furosemide died over a median follow-up of 17.4 months without a significant difference between groups.
Meaning
Among patients discharged after hospitalization for heart failure, torsemide compared with furosemide did not result in a significant difference in all-cause mortality over 12 months; however, interpretation of these findings is limited by loss to follow-up and participant crossover and nonadherence.
Abstract
Importance
Although furosemide is the most commonly used loop diuretic in patients with heart failure, some studies suggest a potential benefit for torsemide.
Objective
To determine whether torsemide results in decreased mortality compared with furosemide among patients hospitalized for heart failure.
Design, Setting, and Participants
TRANSFORM-HF was an open-label, pragmatic randomized trial that recruited 2859 participants hospitalized with heart failure (regardless of ejection fraction) at 60 hospitals in the United States. Recruitment occurred from June 2018 through March 2022, with follow-up through 30 months for death and 12 months for hospitalizations. The final date for follow-up data collection was July 2022.
Interventions
Loop diuretic strategy of torsemide (n = 1431) or furosemide (n = 1428) with investigator-selected dosage.
Main Outcomes and Measures
The primary outcome was all-cause mortality in a time-to-event analysis. There were 5 secondary outcomes with all-cause mortality or all-cause hospitalization and total hospitalizations assessed over 12 months being highest in the hierarchy. The prespecified primary hypothesis was that torsemide would reduce all-cause mortality by 20% compared with furosemide.
Results
TRANSFORM-HF randomized 2859 participants with a median age of 65 years (IQR, 56-75), 36.9% were women, and 33.9% were Black. Over a median follow-up of 17.4 months, a total of 113 patients (53 [3.7%] in the torsemide group and 60 [4.2%] in the furosemide group) withdrew consent from the trial prior to completion. Death occurred in 373 of 1431 patients (26.1%) in the torsemide group and 374 of 1428 patients (26.2%) in the furosemide group (hazard ratio, 1.02 [95% CI, 0.89-1.18]). Over 12 months following randomization, all-cause mortality or all-cause hospitalization occurred in 677 patients (47.3%) in the torsemide group and 704 patients (49.3%) in the furosemide group (hazard ratio, 0.92 [95% CI, 0.83-1.02]). There were 940 total hospitalizations among 536 participants in the torsemide group and 987 total hospitalizations among 577 participants in the furosemide group (rate ratio, 0.94 [95% CI, 0.84-1.07]). Results were similar across prespecified subgroups, including among patients with reduced, mildly reduced, or preserved ejection fraction.
Conclusions and Relevance
Among patients discharged after hospitalization for heart failure, torsemide compared with furosemide did not result in a significant difference in all-cause mortality over 12 months. However, interpretation of these findings is limited by loss to follow-up and participant crossover and nonadherence.
Trial Registration
ClinicalTrials.gov Identifier: NCT03296813
This clinical trial compares the efficacy of torsemide vs furosemide in decreasing the risk of death from any cause among patients hospitalized for heart failure (regardless of ejection fraction).
Introduction
Heart failure is a major and growing public health problem worldwide.1 Many patients with heart failure experience symptoms of congestion and volume overload including dyspnea and edema.2 The majority of patients with symptomatic heart failure are prescribed loop diuretics for the treatment of congestion.3,4,5,6 Guidelines indicate that the use of diuretics is a cornerstone of a successful approach to the treatment of congestion in heart failure.7
Furosemide is the most commonly used loop diuretic for heart failure.5,8 However, preclinical and clinical data suggest potential benefits of torsemide compared with furosemide. Torsemide has increased bioavailability and a longer half-life than furosemide.3 Torsemide may also have beneficial effects on myocardial fibrosis, aldosterone production, sympathetic activation, ventricular remodeling, and natriuretic peptides.9,10 Several small studies of torsemide vs furosemide and meta-analyses suggest a decrease in morbidity and potentially mortality with torsemide compared with furosemide.8,11,12,13 However, in light of the lack of an adequately powered clinical outcomes study, there is insufficient evidence to recommend torsemide over furosemide.
The TRANSFORM-HF (Torsemide Comparison With Furosemide for Management of Heart Failure) Trial was designed to compare the effect of torsemide with furosemide in patients hospitalized with heart failure. The trial was an open-label, pragmatic, randomized, comparative-effectiveness study to assess whether a strategy of torsemide vs furosemide on discharge from the hospital would result in a lower risk of death from any cause among patients with heart failure (regardless of ejection fraction).14 The study incorporated pragmatic elements to perform a real-world trial of loop diuretic strategies in routine heart failure care.15 The primary hypothesis was that torsemide would reduce all-cause mortality by 20% compared with furosemide.
Methods
Trial Design and Oversight
The trial design and operations have been previously described.14 In brief, the event-driven trial was conducted in 60 hospitals in the United States. Patients were recruited during hospitalization with heart failure. The trial protocol and statistical analysis plan are available in Supplement 1 and Supplement 2, respectively. The protocol was designed by academic investigators at Duke Clinical Research Institute, Yale School of Medicine, and the University of Michigan in collaboration with the National Heart, Lung, and Blood Institute. An independent data and safety monitoring board (DSMB) approved the trial protocol and monitored patient safety throughout the trial. The trial was approved by the Duke University institutional review board (IRB) as well as a central IRB or local site IRBs. All patients provided written informed consent before enrollment.
Trial Participants
Eligible patients were hospitalized for heart failure and could have either de novo heart failure or worsening of chronic heart failure as defined by the inclusion and exclusion criteria in the eMethods in Supplement 3. In brief, participants had either a left ventricular ejection fraction of 40% or less within 24 months or an elevated natriuretic peptide level during the index hospitalization as measured by the local laboratory. Participants needed to have a plan for daily outpatient oral loop diuretic with anticipated long-term use. Patients were recruited during hospitalization up until the time of discharge. Patients with end-stage kidney disease requiring dialysis or a history of heart transplant or left ventricular assist device were excluded. The inclusion of race and ethnicity data was aligned with National Institutes of Health guidance. Participants made the determination based on fixed categories, which allowed multiple responses and “other.”
Randomization
Treatment assignment was generated using a simple randomization scheme (ie, no stratification) given the open-label nature of the intervention to limit the potential bias due to predictable treatment assignment.
Interventions
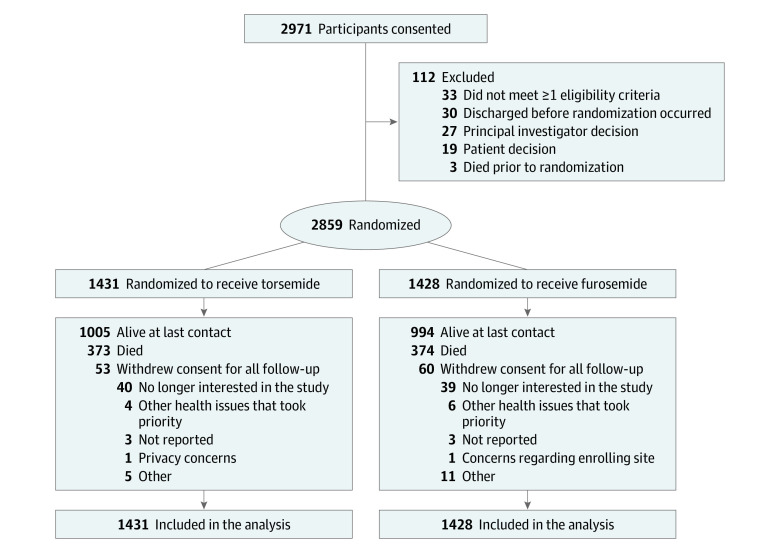
After providing informed consent, patients were randomly assigned in a 1:1 ratio to a treatment strategy of torsemide or furosemide prior to hospital discharge (Figure 1). Dose and frequency of the randomized therapy during hospitalization and at discharge were determined by the treating clinician with the following conversion provided as a guide: 1 mg of torsemide to 2 to 4 mg of oral furosemide. Flexibility in dosing 2 to 4 mg of furosemide vs 1 mg of torsemide was chosen given the low quality and somewhat contradictory data available regarding dose conversion of these diuretics. Changes in dose and frequency of the randomized therapy after discharge were at the discretion of the patient’s usual outpatient clinicians. Participants received medication as per routine care with open-label prescription. Participants were provided medication adherence and educational materials to support adherence to the randomized therapy.
Figure 1. Participant Flow in the TRANSFORM-HF Randomized Clinical Trial.
For patients randomized twice, the second randomizations were completely removed from this diagram. TRANSFORM-HF indicates Torsemide Comparison With Furosemide for Management of Heart Failure.
Follow-up
After this initial contact, no further study-specific patient contact was required at the site level. The trial used centralized follow-up via the Duke Clinical Research Institute call center. Participants had telephone interviews at 30 days, 6 months, and 12 months following discharge. To provide estimates on long-term treatment adherence and to support adequate event accrual, the first 1500 participants had additional follow-up: the first 500 participants received telephone calls every 6 months through 30 months, participants 501 through 1000 received calls through 24 months, and participants 1001 through 1500 received telephone calls through 18 months.
At each telephone interview, participants (or approved proxies) provided information on adherence to the randomized therapy, vital status, hospitalization events, and patient-reported measures. Hospitalization events were verified by the call center using hospitalization records when possible. The National Death Index (NDI) was searched at regular intervals to confirm deaths and supplement vital status data obtained by the call center as previously detailed.16
The trial was event driven and designed to continue until at least 721 primary end points events (all-cause mortality) were observed with an initially projected enrollment of approximately 6000 patients. Interim data analyses for efficacy were conducted by the DSMB due to the large sample size. The DSMB met approximately every 6 months to review study progress.
Trial Outcomes
The primary effectiveness outcome was all-cause mortality assessed in a time-to-event analysis. All-cause mortality was selected based on prior data supporting a reduction in death with torsemide and the need to minimize bias in the setting of an unblinded trial. The prespecified subgroups were age (<65, ≥65; <75, ≥75 years), sex, race and ethnicity (Asian, Black, White, other), ejection fraction (≤40%, 41%-49%, ≥50%), loop diuretic treatment prior to index hospitalization, New York Heart Association class at randomization (I/II vs III/IV), systolic blood pressure (</≥ median), estimated glomerular filtration rate categories (<30, ≥30 to <60, ≥60 mL/min/1.73 m2), diabetes (yes/no), mineralocorticoid receptor antagonist use at randomization (yes/no), academic/university hospital (yes/no), and duration of heart failure. There were 5 secondary end points, with 3 of them being clinical outcomes with a hierarchy of all-cause mortality or all-cause hospitalization over 12 months, total hospitalizations assessed over 12 months, and all-cause mortality or all-cause hospitalization assessed over 30 days. All-cause hospitalization was evaluated as opposed to cardiovascular or heart failure hospitalization to assess the total readmission burden. The 2 remaining secondary outcomes were quality-of-life end points at 12 months and will be reported separately.
Data Sources
Outcomes were ascertained from multiple data sources, including patient (or proxy) report at scheduled trial encounters, queries of medical records, obituaries, grave markers, and the NDI. NDI assessments were performed through December 2021. Details on the process for triggering and verifying outcomes and censoring rules are described in Supplement 2. In brief, the NDI is the most complete death data set available in the United States.16 The implication is that NDI deaths are actual deaths and the absence of an NDI death means that a patient may be considered alive at the end of the reporting year. Therefore, use of the NDI supported very low rates of missing data for the primary end point.
Sample Size Calculation
The trial protocol and statistical analysis plan in Supplement 1 and Supplement 2, respectively, provide details on the sample size calculation. In brief, the planned study population for this trial was broader than most prior heart failure clinical trials. As such, it was difficult to anticipate the expected event rates. However, prior observational data demonstrated 1-year mortality rates following heart failure hospitalization of more than 30%.17 A meta-analysis of studies assessing mortality with torsemide vs furosemide demonstrated a nominal reduction in death events of more than 20% with torsemide.8 Therefore, the required number of primary end point events (ie, all-cause mortality) necessary to obtain power ranging from 80% to 90% with hazard ratios ranging from 0.75 to 0.85 was evaluated (Supplement 2). At least 721 primary end point events were needed to have 85% power or more to detect a hazard ratio of 0.80 assuming 1:1 randomization, a 2-side type I error of .05, and a test statistic based on the log-rank test. It was initially estimated that up to 6000 participants would be needed to accrue the necessary event count. Power calculations did not account for inclusion of covariates in the primary outcome model.
Statistical Analysis
The full analysis set included all randomized patients and was the primary analysis population. Comparisons based on randomized treatment assignments were performed. Descriptive summaries of baseline variables were generated for each randomized treatment group. Continuous variables were presented as medians with IQRs or means with the SDs, and discrete variables were summarized with the use of frequencies and percentages.
For the primary end point of all-cause mortality, a Cox proportional hazards regression model was used to assess outcome differences between the 2 treatment groups and compute a hazard ratio and 95% CI. Prespecified covariates in the primary model included randomized treatment, age, sex, ejection fraction category (≤40%, 41%-49%, ≥50%), and loop diuretic treatment before index hospital admission. Additional post hoc analyses were performed to (1) include site as a random effect in the adjusted model, (2) report the unadjusted Cox model data with robust variance estimators, and (3) assess the implications of the COVID-19 pandemic via a time-dependent covariate before or after the US national emergency data of March 13, 2020. All events post randomization (including in-hospital deaths) were included in the analysis. The proportional hazards assumption was assessed using the ZPH test option in the SAS Cox PH regression model statement (PROC PHREG), which is based on weighted Schoenfeld residuals. There was no violation in proportional hazards. As detailed in the statistical analysis plan (section 12.1, Supplement 2), censoring based on the NDI follow-up period was the primary censoring definition. Regarding the baseline covariates in the outcome model, no patients were excluded due to missing data. Age and sex were available for all patients and for ejection fraction and prior loop diuretic categories, an “unknown category” was used so there were no missing data.
With regard to secondary end points, analyses of the composite of all-cause mortality or all-cause hospitalization at 30 days and 12 months was by time-to-event as for the primary end point analysis. The frequency of primary all-cause rehospitalization events was analyzed by negative binomial regression with relative risks and 95% CIs provided. To address competing risk, a post hoc analysis was performed with multivariable Fine and Gray competing risk models for all-cause hospitalizations through 12 months in the full analysis data set as well as on-treatment at discharge and 30 days.
Prespecified supportive analyses were based on the subset of participants discharged alive taking the assigned medication (as-treated at discharge) and as-treated at day 30 as detailed in Supplement 2.
All analyses were performed with the use of SAS software version 9.4 (SAS Institute). For the primary analysis, a 2-sided P value less than .05 was considered statistically significant. For all other analyses, including secondary analyses and subgroup analyses, a P value less than .005 was considered statistically significant to improve the reproducibility of study results.18 P values are only reported until the last comparison for which the P value is significant. Thus, P values for the first nonsignificant comparison and for all comparisons thereafter are not reported. The secondary end points in section 4.2 of the statistical analysis plan (Supplement 2) are listed in the order of importance and testing. The widths of the 95% CIs are not adjusted for multiplicity and the intervals should not be used in place of hypothesis testing. The interpretation of these confidence intervals avoids the language of definitive conclusions used to report statistically significant findings as assessed by formal hypothesis testing.
Results
Patients and Follow-up
Recruitment began in June 2018. Following a routine DSMB meeting on February 18, 2022, the DSMB recommended stopping recruitment because the sample size was sufficient to answer the primary research question. The trial sponsor (National Heart, Lung, and Blood Institute) reviewed and accepted these recommendations with determination that the trial should execute an orderly closeout.
Recruitment ended March 4, 2022, with 2859 randomized participants (1431 to torsemide and 1428 to furosemide). Following the recommendation of the DSMB and sponsor to conclude the trial, participants recruited in the past 12 months received an end-of-study contact from the call center by May 15, 2022. The final date for follow-up data collection was July 29, 2022.
The characteristics of the patients at baseline were similar in the 2 groups (Table 1). The median age of participants was 65 years (IQR, 56-75); 36.9% of participants were women and 33.9% were Black. In the subset with reduced ejection fraction heart failure (≤40%, n = 1836), baseline β-blocker use was 81.5%, angiotensin-converting enzyme/angiotensin receptor blocker or angiotensin receptor-neprilysin inhibitor (ARNI) use was 67.5% (25.2% ARNI use), mineralocorticoid receptor antagonist use was 44.3%, and sodium-glucose cotransporter 2 (SGLT2) inhibitor use was 7.8%. A total of 113 patients (53 [3.7%] in the torsemide group and 60 [4.2%] in the furosemide group) withdrew consent from the trial prior to completion (Figure 1). The median duration of follow-up for all-cause mortality was 17.4 months (IQR, 8.0-29.0) and was similar in the 2 groups.
Table 1. Baseline Characteristics of TRANSFORM-HF Participants by Treatment Group.
| Characteristic | No. (%)a | |
|---|---|---|
| Torsemide (n = 1431) | Furosemide (n = 1428) | |
| Age, y | ||
| Mean (SD) | 64.0 (14.0) | 65.0 (14.0) |
| Median (IQR) | 65.0 (55.0-74.0) | 65.5 (56.0-75.0) |
| Sex | ||
| Female | 498 (34.8) | 557 (39.0) |
| Male | 933 (65.2) | 871 (61.0) |
| Raceb | ||
| American Indian or Alaska Native | 9 (0.6) | 3 (0.2) |
| Asian | 37 (2.6) | 26 (1.8) |
| Black or African American | 474 (33.1) | 494 (34.6) |
| Native Hawaiian or Pacific Islander | 13 (0.9) | 7 (0.5) |
| White | 831 (58.1) | 837 (58.6) |
| Other | 44 (3.1) | 35 (2.5) |
| Multiple | 21 (1.5) | 23 (1.6) |
| Not reported | 2 (0.1) | 3 (0.2) |
| Hispanic ethnicity, No./total (%) | 75/1430 (5.2) | 80/1425 (5.6) |
| Newly diagnosed heart failure | 428 (29.9) | 410 (28.7) |
| Heart failure hospitalization in past year, No./total (%) | 524/1415 (37.0) | 476/1414 (33.7) |
| Left ventricular ejection fraction, No./total (%), % | ||
| ≥50 | 318/1334 (23.8) | 330/1301 (25.4) |
| 41-49 | 81/1334 (6.1) | 70/1301 (5.4) |
| ≤40 | 935/1334 (70.1) | 901/1301 (69.3) |
| Prior loop diuretic (before randomization) | 964 (67.4) | 956 (66.9) |
| Furosemide | 754 (52.7) | 778 (54.5) |
| Torsemide | 146 (10.2) | 113 (7.9) |
| Bumetanide | 64 (4.5) | 65 (4.6) |
| Ischemic etiology | 427 (29.8) | 381 (26.7) |
| Comorbidities | ||
| Atrial fibrillation/flutter | 625/1419 (44.0) | 649/1420 (45.7) |
| Diabetes | 688 (48.1) | 676 (47.3) |
| Chronic kidney disease | 497 (34.7) | 512 (35.9) |
| Vital signs | ||
| Systolic blood pressure, mm Hg | 118 (19) | 119 (20) |
| Heart rate, /min | 81 (16) | 80 (16) |
| No. | 1430 | 1427 |
| Body mass indexc | 32.3 (9.7) | 32.0 (9.3) |
| Baseline laboratories, median (IQR) | ||
| NT-proBNP, pg/mL | 3994 (1938-8850) | 3833 (1936-7807) |
| No. | 680 | 696 |
| BNP, pg/dL | 982 (468-1790) | 921 (480-1865) |
| No. | 703 | 678 |
| Estimated GFR, mean (SD), mL/min/1.73 m2 | 59.1 (25.0) | 59.7 (26.0) |
| No. | 1429 | 1425 |
| Devices and medications | ||
| β-Blocker | 1140 (79.7) | 1106 (77.5) |
| ACE inhibitor or ARB | 640 (44.7) | 603 (42.2) |
| Mineralocorticoid receptor antagonist | 524 (36.6) | 498 (34.9) |
| Sacubitril-valsartan | 264 (18.4) | 272 (19.0) |
| SGLT2 inhibitor | 89/1383 (6.4) | 81/1375 (5.9) |
| Implantable cardioverter–defibrillator | 293/1428 (20.5) | 298/1426 (20.9) |
| Cardiac resynchronization therapy | 119/1430 (8.3) | 105/1427 (7.4) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; GFR, glomerular filtration rate; NT-proBNP, N-terminal pro–brain natriuretic peptide; SGLT2, sodium glucose cotransporter 2.
Values shown as No. (%) or mean (SD), unless otherwise specified.
The inclusion of race and ethnicity data was aligned with National Institutes of Health guidance. Participants made the determination based on fixed categories, which allowed multiple responses and “other.”
Calculated as weight in kilograms divided by height in meters squared.
Primary Outcome
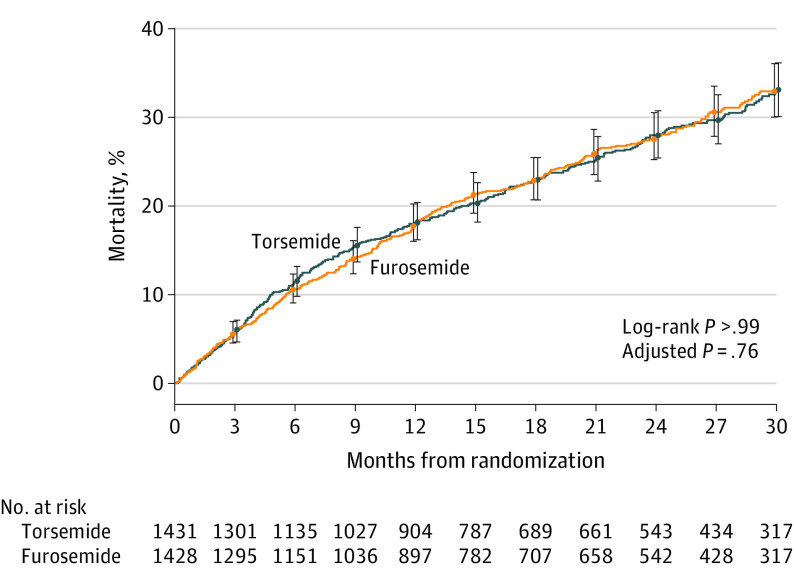
Death occurred in 373 of 1431 patients (26.1%) in the torsemide group and 374 of 1428 patients (26.2%) in the furosemide group (hazard ratio, 1.02 [95% CI, 0.89-1.18]; P = .76) (Figure 2 and Table 2). There were 11 deaths during index hospitalization (7 in the torsemide group and 4 in the furosemide group). The effect of torsemide on the primary outcome was consistent across prespecified subgroups (Figure 3). Prespecified sensitivity analyses in the as-treated population were consistent with the primary analysis (eTable 1 in Supplement 3) and baseline characteristics by adherence are reported in eTable 2 in Supplement 3. Post hoc analyses that included site as a random effect in the adjusted model, the unadjusted Cox model data with robust variance estimators, and a COVID-19 assessment were consistent with the primary results (eTable 3 and eTable 4 in Supplement 3).
Figure 2. Primary Outcome of All-Cause Mortality.
The cumulative incidence of the primary outcome in the 2 groups is shown. The whiskers represent 95% CIs at the months specified. Variables for the adjusted P value are listed in Table 2.
Table 2. Primary and Secondary Outcomes.
| Variable | Torsemide (n = 1431) | Furosemide (n = 1428) | Risk reduction (95% CI)a | HR (95% CI)b | P valueb | ||
|---|---|---|---|---|---|---|---|
| No. (%) | Events per 100 patient-years | No. (%) | Events per 100 patient-year | ||||
| Primary outcome | |||||||
| All-cause mortality | 373 (26.1) | 17.0 | 374 (26.2) | 17.0 | 0.12 (−2.85 to 3.14) | 1.02 (0.89 to 1.18) | .76 |
| Secondary outcomes | |||||||
| All-cause mortality or all-cause hospitalization (over 12 mo) | 677 (47.3) | 99.2 | 704 (49.3) | 107.6 | 1.99 (−1.79 to 5.56) | 0.92 (0.83 to 1.02) | |
| Total hospitalizations (over 12 mo) | 940 | 106.3 | 987 | 111.9 | RR, 0.94 (0.84 to 1.07) | ||
| All-cause mortality or all-cause hospitalization (over 30 d) | 149 (10.4) | 147.2 | 157 (11.0) | 157.5 | 0.58 (−1.80 to 2.75) | 0.94 (0.75 to 1.18) | |
Abbreviations: HR, hazard ratio; RR, rate ratio.
Risk reduction = furosemide % minus torsemide %, and the 95% CI based on 2.5th and 97.5th percentiles of the risk reductions from 10 000 bootstrap samples of each treatment group.
HRs, 95% CIs, and P values are based on a Cox proportional hazards regression model including the assigned treatment (torsemide vs furosemide as the reference group), as well as age, sex, baseline ejection fraction (<40%, 41%-49%, >50%, unknown), and loop diuretic treatment prior to index hospital admission as covariates.
Figure 3. Primary Outcome in Prespecified Subgroups.
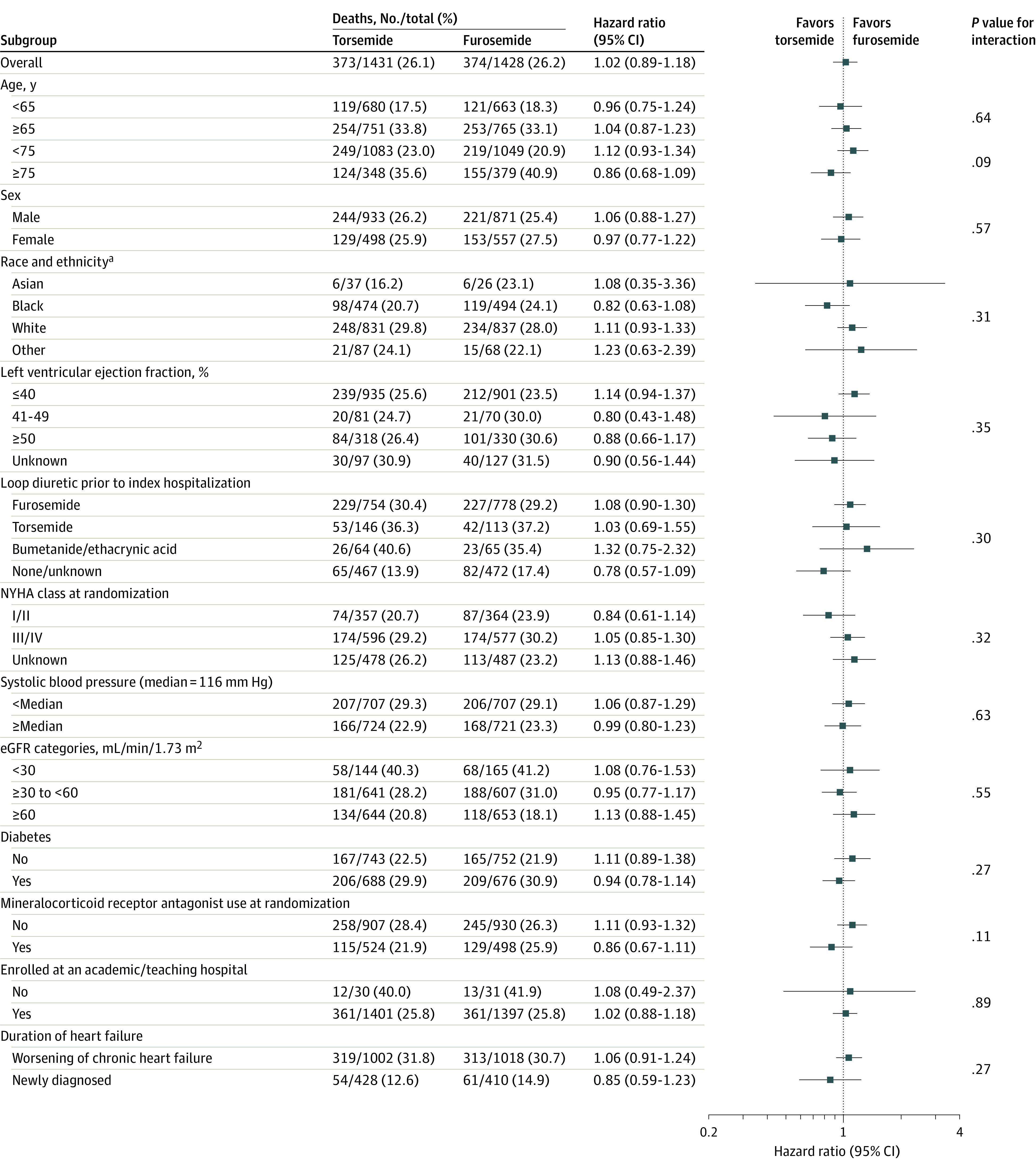
Results of the primary outcome of the trial—all-cause mortality—are shown according to subgroups that were prespecified in the protocol.
eGFR indicates estimated glomerular filtration rate and NYHA class, New York Heart Association symptom class at time of randomization.
aAmerican Indian, Native Hawaiian, and “multiple” categories were not included due to very small sample sizes.
Secondary Outcomes
All-cause mortality or all-cause hospitalization occurred in 677 patients (47.3%) in the torsemide group and 704 patients (49.3%) in the furosemide group (hazard ratio, 0.92 [95% CI, 0.83-1.02]) (eFigure in Supplement 3; Table 2). There were 940 total hospitalizations among 536 participants (37.5%) in the torsemide group and 987 total hospitalizations among 577 participants (40.4%) in the furosemide group (rate ratio, 0.94 [95% CI, 0.84-1.07]) (Table 2). The post hoc analysis to address competing risk for all-cause hospitalizations through 12 months demonstrated a hazard ratio of 0.88 (95% CI, 0.78-0.99) with torsemide compared with furosemide with consistent results in the on-treatment groups (eTable 5 in Supplement 3).
Adherence to Trial Medication
Of the patients with known prescription status at discharge from the index hospitalization (2755/2859, 96.4%), 2491 participants (90.4%) were receiving the assigned loop diuretic. At hospital discharge, we observed 7.0% crossover from torsemide to furosemide and 3.8% crossover from furosemide to torsemide (5.4% overall). In terms of loop diuretic discontinuation, 2.8% of patients were not discharged taking any loop diuretic. At 30 days and 6 months, 7.0% and 9.5% were not taking any loop diuretic, respectively. eTable 6 in Supplement 3 provides details of follow-up loop diuretic status.
Loop Diuretic Dosing
At index hospitalization discharge, the mean (SD) loop diuretic dose in furosemide equivalents (using a 2:1 conversion for furosemide to torsemide) was 79.3 (63.3) mg and was similar in both groups for those individuals prescribed the assigned loop diuretic (79.1 [56.4] mg of furosemide vs 79.5 [69.8] mg of torsemide). At 1 month (data available in 2047 participants, excluding deaths and unknown diuretic status), in those prescribed the assigned loop diuretic, the mean (SD) dose was 73.1 (63.4) mg, with a lower dose in the furosemide group than the torsemide group (68.4 [50.2] mg vs 77.8 [74.5] mg).
Discussion
Among patients discharged after hospitalization for heart failure, torsemide did not result in a significant difference in all-cause mortality compared with furosemide. However, interpretation of these findings is limited by loss to follow-up and participant crossover and nonadherence.
While prior mechanistic studies, observational analyses, and meta-analyses suggested advantages with torsemide, this study did not demonstrate a treatment benefit compared with furosemide. There was no evidence that torsemide’s favorable bioavailability or purported antifibrotic effects translated into improved outcomes for patients recently hospitalized with heart failure. The results were consistent across the various end points and subgroups including those of different demographic profiles (eg, age, sex, race and ethnicity) and ejection fraction phenotypes and in those whose index hospitalization was with newly diagnosed heart failure as compared with worsening chronic heart failure. However, the nonspecific all-cause outcomes may have been too imprecise for measuring subtle differences between the treatment groups. Furthermore, given that approximately 30% of the participants had newly diagnosed heart failure, postbaseline changes in guideline-directed medical therapy (eg, addition of ARNI, β-blocker, SGLT2 inhibitor, and mineralocorticoid receptor antagonist) may have affected clinical outcomes. In particular, the uptake of newer therapies, such as ARNI and SGLT2 inhibitor, over the course of the trial warrants consideration because these not only reduce clinical events, but potentially also diuretic requirements. Whether other patient populations, such as those diagnosed in the outpatient setting and/or without prior hospitalization, have differential benefit with these therapies was not assessed.
Crossover and loop diuretic discontinuation may have diminished the ability to distinguish the hypothesized between-group difference. Loop diuretics were prescribed as part of the routine strategy of care in the trial with mechanisms in place to support adherence to the randomized therapy, yet crossover was observed. Higher crossover in the torsemide group than furosemide group may relate to reversion to prior loop diuretic, differences in cost between the agents, patient or clinician preference, or perceived adverse effects. Despite the inclusion criterion of anticipated need for long-term loop diuretic therapy, diuretic discontinuation during follow-up was higher than expected for a hospitalized population. The as-treated analyses at discharge and 30 days supported the primary trial results. However, given challenges contacting patients at follow-up, there was missing data for follow-up diuretic status, which limits the interpretation.
There were significant differences in the loop diuretic dosing with regard to furosemide equivalents during follow-up. While dosing was similar at index hospital discharge, in patients with dosing data available at 1 month, dose was 10% to 15% greater in the torsemide than furosemide group (based on a 2:1 conversion) for participants continuing to take the randomized therapy. Given the uncertainty regarding the correct dose conversion, the protocol allowed flexibility with a 2:1 to 4:1 furosemide to torsemide conversion. If the true conversion is closer to 4:1, it may be that dosing was higher in the torsemide group. Future work will explore different dose conversions and time-varying analyses to better understand the implications of differences in dose.
The broad eligibility criteria, site selection, and streamlined study protocol embedded within routine care supported inclusion of diverse participants. By having centralized follow-up without site-specific visits, this supported inclusion of patients who historically have been less well represented in trials. In the study, 36.9% of trial participants were women and 33.9% were Black. Prior trials of patients with heart failure typically recruited less than 30% women19 and among trials reporting race, persons who were Black, Indigenous, or of racial or ethnic minority groups represented only 18.7% of study populations.20 The pragmatic elements lowered traditional barriers for patient and site participation in clinical trials and supported robust enrollment rates (even during the COVID-19 pandemic) with results that are generalizable to practice. A mean recruitment rate of more than 2 patients per site per month prior to the pandemic and more than 1 patient per site per month during the pandemic (following initial lockdown) highlight advantages of trials incorporating centralized follow-up mechanisms to reduce the burden on enrolling sites and patients.
The event-driven trial was initially projected to target the recruitment of 6000 participants. However, the broad eligibility criteria and in-hospital recruitment preceding the vulnerable period post discharge21 supported a higher-than-anticipated event rate. The trial reached the target event count of 721 death events (747 observed deaths) with a sample size approximately half that initially planned. The event rate of 17.0 per 100 patient-years was similar to that observed in the recent clinical outcome trial of vericiguat following a recent worsening heart failure event (placebo all-cause mortality rate of 16.9 per 100 patient-years in the VICTORIA trial).22 This study enrolled a high-risk phenotype with a median N-terminal pro–brain natriuretic peptide level of 3913 pg/mL as compared with 4812 pg/mL in the PIONEER-HF trial,23 2816 pg/mL in the VICTORIA trial,22 and 1437 pg/mL in the DAPA-HF trial.24 The high baseline N-terminal pro–brain natriuretic peptide in this study is notable given that the trial included patients with preserved ejection fraction where natriuretic peptide levels are comparatively lower than in reduced ejection fraction.
Limitations
Several important limitations should be acknowledged. First, while we achieved the target event count, the sample size was approximately half that originally planned. Subgroup analyses were therefore limited by the more modest patient numbers. Patient withdrawals were higher than in some prior heart failure trials, likely related in part to the reduced intensity of site contact.
Second, the all-cause outcomes may have been too imprecise for measuring subtle differences between the treatment groups in heart failure–specific outcomes. The end point classification differed from traditional clinical outcome adjudication with cause-specific end points given the pragmatic nature of the trial. The treatment effect assumed in trial planning was informed by available meta-analyses, which may have not fully incorporated the beneficial effects of guideline-directed medical therapy (particularly for reduced ejection fraction heart failure).
Third, while clinical events were systematically evaluated, the pragmatic design did not allow for assessment of subtle relative benefits (or harms) of these generically available therapies such as worsening kidney function, electrolyte abnormalities, or nonhospitalization events (eg, emergency department visits, outpatient intravenous diuretics, thiazide use).
Fourth, loop diuretic discontinuation and crossovers occurred during follow-up and are informative in this comparative strategy study, yet they may bias toward neutral results. Given the open-label design, it is plausible that patient or clinician bias about differential benefits of the loop diuretics may have led to switching over time.
Fifth, loop diuretic dose was left to clinician discretion, which may have influenced results. Future work will characterize how nonadherence and dose titration may have affected these findings including via the evaluation of varying definitions of “as-treated” and incorporation of time-varying factors.
Sixth, the recruitment of patients with heart failure with preserved ejection fraction and individuals of Hispanic ethnicity was lower than anticipated.
Conclusions
Among patients discharged after hospitalization for heart failure, torsemide compared with furosemide did not result in a significant difference in all-cause mortality over 12 months. However, interpretation of these findings is limited by loss to follow-up and participant crossover and nonadherence.
Trial Protocol
Statistical Analysis Plan
eMethods. Inclusion and Exclusion Criteria
eFigure. All-Cause Mortality or All-Cause Hospitalization
eTable 1. Pre-specified On-Treatment Sensitivity Analyses Assessing the Primary Endpoint and All-Cause Mortality or All-Cause Hospitalization
eTable 2. Baseline Characteristics of TRANSFORM-HF Participants by On-Treatment Status at 30 Days
eTable 3. Additional Post-Hoc Cox Proportional Hazards Models of the Time to Event Endpoints
eTable 4. Additional Post-Hoc Sensitivity Analysis for COVID-19 Pandemic
eTable 5. Additional Post-Hoc Analysis of All-Cause Hospitalizations Through 12 Months With a Competing Risk Model
eTable 6. Details of Loop Diuretic Status at Study Follow-up as Acquired via the DCRI Call Center
Nonauthor Collaborator Group. TRANSFORM-HF Investigators
Data Sharing Statement
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153-e639. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E. Heart failure. JACC Heart Fail. 2013;1(1):1-20. [DOI] [PubMed] [Google Scholar]
- 3.Ellison DH, Felker GM. Diuretic treatment in heart failure. N Engl J Med. 2017;377(20):1964-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faris RF, Flather M, Purcell H, Poole-Wilson PA, Coats AJ. Diuretics for heart failure. Cochrane Database Syst Rev. 2012;(2):CD003838. [DOI] [PubMed] [Google Scholar]
- 5.Khan MS, Greene SJ, Hellkamp AS, et al. Diuretic changes, health care resource utilization, and clinical outcomes for heart failure with reduced ejection fraction: from the Change the Management of Patients With Heart Failure Registry. Circ Heart Fail. 2021;14(11):e008351. [DOI] [PubMed] [Google Scholar]
- 6.Greene SJ, Triana TS, Ionescu-Ittu R, et al. In-hospital therapy for heart failure with reduced ejection fraction in the United States. JACC Heart Fail. 2020;8(11):943-953. [DOI] [PubMed] [Google Scholar]
- 7.Writing Committee Members; ACC/AHA Joint Committee Members . 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Card Fail. 2022;28(5):e1-e167. [DOI] [PubMed] [Google Scholar]
- 8.Bikdeli B, Strait KM, Dharmarajan K, et al. Dominance of furosemide for loop diuretic therapy in heart failure: time to revisit the alternatives? J Am Coll Cardiol. 2013;61(14):1549-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buggey J, Mentz RJ, Pitt B, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J. 2015;169(3):323-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters AE, Mentz RJ, DeWald TA, Greene SJ. An evaluation of torsemide in patients with heart failure and renal disease. Expert Rev Cardiovasc Ther. 2022;20(1):5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray MD, Deer MM, Ferguson JA, et al. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111(7):513-520. [DOI] [PubMed] [Google Scholar]
- 12.Cosín J, Díez J; TORIC investigators . Torasemide in chronic heart failure: results of the TORIC Study. Eur J Heart Fail. 2002;4(4):507-513. [DOI] [PubMed] [Google Scholar]
- 13.DiNicolantonio JJ. Should torsemide be the loop diuretic of choice in systolic heart failure? Future Cardiol. 2012;8(5):707-728. [DOI] [PubMed] [Google Scholar]
- 14.Greene SJ, Velazquez EJ, Anstrom KJ, et al. ; TRANSFORM-HF Investigators . Pragmatic design of randomized clinical trials for heart failure: rationale and design of the TRANSFORM-HF Trial. JACC Heart Fail. 2021;9(5):325-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene SJ, Velazquez EJ, Anstrom KJ, Eisenstein EL, Mentz RJ. Reply: pragmatic clinical trials: the big picture. JACC Heart Fail. 2021;9(8):606-608. [DOI] [PubMed] [Google Scholar]
- 16.Eisenstein EL, Prather K, Greene SJ, et al. Death: the simple clinical trial endpoint. Stud Health Technol Inform. 2019;257:86-91. [PMC free article] [PubMed] [Google Scholar]
- 17.Rathore SS, Foody JM, Wang Y, et al. Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. JAMA. 2003;289(19):2517-2524. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin DJ, Berger JO, Johannesson M, et al. Redefine statistical significance. Nat Hum Behav. 2018;2(1):6-10. [DOI] [PubMed] [Google Scholar]
- 19.Reza N, Gruen J, Bozkurt B. Representation of women in heart failure clinical trials: barriers to enrollment and strategies to close the gap. Am Heart J Plus. 2022;13:100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei S, Le N, Zhu JW, et al. Factors associated with racial and ethnic diversity among heart failure trial participants: a systematic bibliometric review. Circ Heart Fail. 2022;15(3):e008685. [DOI] [PubMed] [Google Scholar]
- 21.Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol. 2015;12(4):220-229. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong PW, Pieske B, Anstrom KJ, et al. ; VICTORIA Study Group . Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382(20):1883-1893. [DOI] [PubMed] [Google Scholar]
- 23.Velazquez EJ, Morrow DA, DeVore AD, et al. ; PIONEER-HF Investigators . Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380(6):539-548. [DOI] [PubMed] [Google Scholar]
- 24.McMurray JJV, Solomon SD, Inzucchi SE, et al. ; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods. Inclusion and Exclusion Criteria
eFigure. All-Cause Mortality or All-Cause Hospitalization
eTable 1. Pre-specified On-Treatment Sensitivity Analyses Assessing the Primary Endpoint and All-Cause Mortality or All-Cause Hospitalization
eTable 2. Baseline Characteristics of TRANSFORM-HF Participants by On-Treatment Status at 30 Days
eTable 3. Additional Post-Hoc Cox Proportional Hazards Models of the Time to Event Endpoints
eTable 4. Additional Post-Hoc Sensitivity Analysis for COVID-19 Pandemic
eTable 5. Additional Post-Hoc Analysis of All-Cause Hospitalizations Through 12 Months With a Competing Risk Model
eTable 6. Details of Loop Diuretic Status at Study Follow-up as Acquired via the DCRI Call Center
Nonauthor Collaborator Group. TRANSFORM-HF Investigators
Data Sharing Statement