Key Points
Question
Can a prognostic tool for the occurrence of cerebral palsy (CP) in all term neonates be developed from easily obtainable variables pertaining to the pregnancy, delivery, and neonate?
Findings
This case-control study of potential CP risk factors including 1265 individuals with CP and 1985 controls demonstrated that risk factors are additive. The prognostic tool had a sensitivity and specificity for CP of 56% and 82%, respectively, which identified twice as many children with CP than if just encephalopathy was considered.
Meaning
In this study, a simple prognostic tool using 12 variables identified term infants at risk of developing CP who may lack a classic risk factor, such as encephalopathy.
This case-control study evaluates a cerebral palsy prognostic tool that can be applied to all term neonates to identify those at increased risk of developing cerebral palsy.
Abstract
Importance
Cerebral palsy (CP) is the most common abnormality of motor development and causes lifelong impairment. Early diagnosis and therapy can improve outcomes, but early identification of infants at risk remains challenging.
Objective
To develop a CP prognostic tool that can be applied to all term neonates to identify those at increased risk of developing CP.
Design, Setting, and Participants
This case-control study used data from the Canadian Cerebral Palsy Registry (data collected from January 2003 to December 2019) for children with CP and the Alberta Pregnancy Outcomes and Nutrition study (mothers enrolled from May 2009 to September 2012; data extracted in 2020) for controls. There were 2771 children with CP and 2131 controls evaluated; 941 and 144, respectively, were removed for gestational age less than 37 weeks at birth, 565 with CP removed for incomplete data, and 2 controls removed for a diagnosis of CP. Data were analyzed from April to August 2022.
Exposures
Potential risk factors were selected a priori based on the literature, including maternal, intrapartum, and infant characteristics.
Main Outcomes and Measures
Diagnosis of CP, defined as a disorder of motor function due to a nonprogressive brain abnormality before age 1 year and classified by Gross Motor Function Classification System levels I to V.
Results
Of 3250 included individuals, 1752 (53.9%) were male, and the median (IQR) gestational age at birth was 39 (38-40) weeks. Encephalopathy was present in 335 of 1184 infants with CP (28%) and 0 controls. The final prediction model included 12 variables and correctly classified 75% of infants, with a sensitivity of 56% (95% CI, 52-60) and specificity of 82% (95% CI, 81-84). The C statistic was 0.74 (95% CI, 71-76). Risk factors were found to be additive. A proposed threshold for screening is probability greater than 0.3, with a sensitivity of 65% (95% CI, 61-68) and specificity of 71% (95% CI, 69-73). The prognostic tool identified 2.4-fold more children with CP than would have presented with encephalopathy (odds ratio, 13.8; 95% CI, 8.87-22.65; P < .001).
Conclusions and Relevance
In this case-control study, a prognostic model using 12 clinical variables improved the prediction of CP compared with clinical presentation with encephalopathy. This tool can be applied to all term newborns to help select infants for closer surveillance or further diagnostic tests, which could improve outcomes through early intervention.
Introduction
Cerebral palsy (CP) is a common and lifelong abnormality of motor development due to a congenital or acquired abnormality of the developing brain in the antenatal and/or neonatal period. Despite advances in obstetrical and neonatal care in past decades, the birth prevalence in wealthy countries has been relatively stable at 1 to 4 births per 1000, although registries in Australia and Norway have demonstrated encouraging recent reductions. The diagnosis often takes months or years to establish because of the relative paucity of voluntary movements in early infancy and the slow evolution of abnormal tone that was historically a core feature for accurate diagnosis. This delay in diagnosis may prevent intervention with therapies that capitalize on early inherent neuroplasticity to improve outcomes. There are current efforts to improve early prediction of CP in high-risk neonates with combinations of motor scores, such as the General Movements Assessment (GMA), and magnetic resonance imaging features. The implementation of these sophisticated evaluation tools is dependent on recognizing infants at risk. Infants considered at risk are typically graduates of the neonatal intensive care unit who were premature, had neonatal encephalopathy, or neurological risk factors, such as birth defects or growth abnormalities; however, approximately 50% of children diagnosed with CP do not have these risk factors.
CP is challenging to predict because the diagnosis does not represent a single disease entity but rather a heterogenous group of conditions that result in motor impairment. In addition, many practitioners may believe prematurity or difficult delivery are the primary risk factors and may not consider the diagnosis in a child born healthy at term, yet children born at term represent 60% of those with CP, and intrapartum asphyxia is thought to be causative in as few as 10%. Numerous studies and reviews have highlighted a variety of risk factors that can work in concert to cause CP, but to our knowledge, there is no published standardized strategy to evaluate these risk factors. Dramatic clinical presentations, such as neonatal encephalopathy, can be used to identify select populations of high-risk infants; however, in half of children with CP, there is no such history, and CP may not be recognized until the second year of life. Later recognition of CP delays therapies that may be more effective when performed earlier. There is currently no method to screen the population of newborn term infants to identify those at risk of developing CP who may benefit from early diagnosis and therapy.
We used a case-control design capitalizing on a national CP registry cohort to develop a regression model to identify risk factors that are associated with CP and readily available from the pregnancy, delivery, and neonatal course history. With this model, we developed a prognostic tool and threshold at which all infants can be screened, particularly those who appear healthy at birth. We hypothesized that such a tool could expand the early recognition of CP compared with neonates presenting with encephalopathy.
Methods
Population and Data Sources
CP cases were from the Canadian Cerebral Palsy Registry, a prospective Canadian registry established in 2003 in Quebec and now including pediatric centers in British Columbia, Alberta, Ontario, Newfoundland, and Nova Scotia. Cases included all births up to and including those in 2019. CP was diagnosed as previously described. Details such as CP type and Gross Motor Function Classification System (GMFCS) were abstracted. Race and ethnicity data were collected from each parent from a drop-down menu. Written informed consent was obtained from each participant’s responsible caregiver. Ethical approval was obtained by each participating institution from their research ethics boards with overall approval for the Canadian Cerebral Palsy Registry provided by the Research Institute of the McGill University Health Center. This study followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline.
Healthy control data were obtained from the Alberta Pregnancy Outcomes and Nutrition (APrON) longitudinal study, established in 2009 to 2012 as a population-based prospective cohort of 2189 pregnant Alberta women. This longitudinal cohort study collected pregnancy, delivery, and neonatal data and continues to observe child outcomes; the present study used data up to age 3 years. We recently demonstrated the ability of Alberta Pregnancy Outcomes and Nutrition data to serve as a control sample in risk factor studies of perinatal brain injury. The participants provided their written informed consent to participate. Ethics approval was obtained by the University of Calgary Conjoint Health Research Ethics Board and the University of Alberta Health Research Ethics Board.
Criteria for Inclusion and Exclusion
Registries were reviewed for eligible cases and controls. Participants were selected based on the following inclusion criteria: (1) birth between 2003 (Quebec) and 2007 (other provinces) and March 2020 and (2) term birth at 37 weeks’ gestation or more. Control cases additionally required normal motor development at age 3 years to rule out delayed presentation of CP. Participants were excluded if caregiver consent was absent or if more than 50% of participant data fields were missing.
Predictor Variables
Medical definitions of clinical variables were according to the National Institutes of Health Common Data Elements and consisted of maternal/pregnancy, intrapartum, and infant characteristics (eTable 1 in Supplement 1). Only variables that were consistently defined between databases were considered. Selection of specific variables was based on the literature.
Statistical Analysis
Descriptive statistics, including counts with percentages and medians with IQRs, were used. Missing data were addressed by examining patterns and then using multiple imputation (multivariable normal distribution; eTable 2 in Supplement 1). Variables with more than 50% missing data were excluded as model predictors. In addition, variables for inclusion in modeling had at least 5 participants for each variable in each group. Univariable analyses using Fisher exact test were done for binary variables and Wilcoxon rank sum test for continuous variables. McNemar χ2 test was used to compare the proportion of those with CP detected by the prediction model at a threshold of 0.3 compared with the proportion with encephalopathy.
Model Development
The multivariable logistic regression model was built using multiple imputation with all potential variables in Table 1 and simplified by removing variables that did not modify the outcome of CP to generate a simpler prognostic tool. The coefficients from this model were used to generate the prognostic tool. Results were expressed as odds ratios and 95% CIs. For each variable in the model, the standardized dominance statistic was generated along with the ranking of the variable’s importance in the model (Table 1) to demonstrate the relative importance of each variable.
Table 1. Study Group Characteristics, Including Missing Data, and Univariable and Multivariable Analysisa.
| Characteristic | Controls (n = 1985) | Children with CP (n = 1265) | Univariable analysis | Multivariable analysis (45 multiple imputations) | ||||
|---|---|---|---|---|---|---|---|---|
| No./total No. (%) | Missing, No. (%) | No./total No. (%) | Missing, No. (%) | OR (95% CI) | P value | OR (95% CI) | Standardized dominance statistic (ranking) | |
| Pregnancy and maternal characteristics | ||||||||
| Maternal age, median (IQR), y | 31 (29-34) | 47 (2.4) | 30 (26-33) | 19 (1.5) | NA | NA | NA | NA |
| No. of pregnancies, median (IQR) | 2 (1-3) | 0 | 2 (1-3) | 20 (1.6) | 1.2 (1.2-1.3) | <.001 | 1.4 (1.3-1.5) | 0.041 (6) |
| History of miscarriage | 464/1985 (23.4) | 0 | 308/1232 (25) | 33 (2.5) | 1.1 (0.9-1.3) | .30 | NS | NA |
| No. of miscarriages, median (IQR) | 0 (0-0) | 0 | 0 (0-0.5) | 33 (2.6) | 1.1 (1.0-1.2) | .25 | 0.8 (0.6-0.9) | 0.0075 (13) |
| Tobacco use | 110/1835 (6.0) | 150 (7.6) | 202/1128 (17.9) | 137 (10.8) | 3.1 (2.4-4.0) | <.001 | 2.3 (1.7-3.0) | 0.078 (4) |
| Alcohol use | 130/1802 (7.2) | 183 (9.3) | 143/1120 (12.8) | 145 (11.5) | 1.7 (1.3-2.2) | <.001 | NS | NA |
| Drug use | 14/1843 (0.8) | 142 (7.2) | 132/1224 (10.8) | 41 (3.2) | 15.8 (9.0-29.8) | <.001 | 10.4 (6.1-18.0) | 0.15 (3) |
| Diabetes | 104/1983 (5.2) | 2 (0.1) | 74/12330 (6.0) | 35 (2.8) | 2.4 (1.7-3.3) | <.001 | 2.1 (1.5-3.0) | 0.039 (7) |
| Preeclampsia | 13/1983 (0.7) | 2 (0.1) | 46/1173 (3.9) | 92 (7.3) | 6.2 (3.3-12.6) | <.001 | 4.0 (2.0-8.0) | 0.037 (9) |
| Labor and delivery characteristics | ||||||||
| Prolonged rupture of membranes (>18 h) | 234/1976 (11.8) | 9 (0.5) | 90/1172 (7.7) | 93 (7.4) | 0.6 (0.5-0.8) | <.001 | 0.5 (0.4-0.7) | 0.053 (5) |
| Chorioamnionitis | 8/1985 (0.4) | 0 | 79/808 (9.8) | 457 (36.1) | 26.8 (12.9-64.4) | <.001 | 15.4 (6.9-39.1) | 0.21 (2) |
| 5-min Apgar score, median (IQR) | 9 (9-9) | 4 (0.2) | 9 (7-9) | 106 (8.4) | 0.6 (0.6-0.7) | <.001 | 0.6 (0.6-0.7) | 0.31 (1) |
| Umbilical cord pH, median (IQR) | 7.3 (7.2-7.3) | 294 (14.8) | 7.3 (7.1-7.3) | 455 (36.0) | 0 (0-0.6) | <.001 | NS | NA |
| Maternal fever in labor | 77/1985 (3.9) | 0 | 87/1049 (8.3) | 216 (17.1) | 2.2 (1.6-3.1) | <.0001 | NS | NA |
| Emergency caesarian delivery | 244/1985 (12.3) | 0 | 300/461 (65) | 804 (63.6) | 13.3 (10.5-16.9) | <.0001 | NA | NA |
| Infant characteristics | ||||||||
| Male sex | 1033/1985 (52) | 0 | 719/1265 (56.8) | 0 | 1.2 (1.1-1.4) | .007 | 1.2 (1.0-1.5) | 0.011 (12) |
| Gestational age, median (IQR), wk | 39.4 (38.7-40.1) | 49 (2.4) | 39 (38-40) | 37 (2.9) | 0.8 (0.8-0.9) | <.001 | 0.9 (0.8-1.0) | 0.037 (8) |
| Birth weight, median (IQR), kg | 3.4 (3.1-3.69) | 2 (0.1) | 3.3 (2.98-3.65) | 46 (3.6) | 0.7 (0.6-0.8) | <.001 | 0.1 (0-0.6) | 0.17 (10) |
| Birth weight, kg2 | NA | NA | NA | NA | NA | NA | 1.3 (1.04-1.66) | 0.014 (11) |
| Small for gestational age | 95/1934 (4.9) | 51 (2.6) | 126/1227 (10.3) | 38 (3.0) | 2.2 (1.7-3.0) | <.001 | NS | NA |
| Encephalopathy | 0/1985 (0) | 0 | 335/1184 (28.3) | 81 (6.4) | 786.4 (139-31160) | <.001 | NA | NA |
| Seizure | NA | 1987 (100) | 289/1204 (24) | 61 (4.8) | NA | NA | NA | NA |
Abbreviations: NA, not applicable; NS, not significant; OR, odds ratio.
The multivariable analysis represents the prediction model by multiple imputation. The standardized dominance statistic represents the relative contribution of each variable to the model.
The fit of the model to the complete data was evaluated with 2 methods: resampling and random split-sample development and validation. Resampling was accomplished by bootstrapping with 1500 participants and 100 repetitions. The model performance was evaluated by generating a probability of CP for each study participant. The distribution of probability for CP vs controls was compared using Wilcoxon rank sum tests. Sensitivity and specificity were determined for a range of probability thresholds to identify potential thresholds for CP screening. A C statistic (a measure of goodness of fit, ie, the probability that a randomly selected patient has a higher score than a control; values greater than 0.7 indicate a good model and greater than 0.8 indicate a strong model) and calibration plot was generated. The fit was retested on only participants without encephalopathy to test a real-world sample where infants with encephalopathy would already be identified for screening in neonatal intensive care unit follow-up clinics. Test of proportions was used to compare participants with encephalopathy and CP at various probability thresholds. To further validate the model, it was retested by CP severity as either ambulatory CP (GMFCS level I to III) or nonambulatory (GMFCS level IV to V) to assess for a dose response whereby higher probability of CP was more likely in severe CP than mild. To improve generalizability of the results, the relative risk (RR) of CP was determined at various probability thresholds to adjust for the high proportion of CP in the study group. Analyses were conducted using Stata version 15 (eTable 3 in Supplement 1).
Results
Population Characteristics
The final study population consisted of 1265 children with CP and 1985 controls, after the removal of premature infants born younger than 37 weeks’ gestation (941 and 144, respectively), participants with less than 50% complete data (565 children with CP), and 2 participants from the control group who were diagnosed with CP. Clinical characteristics are displayed in Table 1. Of 3250 included individuals, 1752 (53.9%) were male, and the median (IQR) gestational age at birth was 39 (38-40) weeks. There were significantly more boys in the CP group (719 of 1265 [57%]) compared with controls (1033 of 1985 [52%]; P = .008). CP severity was ambulatory (GMFCS level I to III) in 813 children (71.9%) and nonambulatory (GMFCS level IV to V) in 317 children (28.1%). Neonatal encephalopathy was present in 335 of 1184 children with CP (28.3%) and 0 controls.
Model Development
Gestational age and infant weight were best fitted with a quadratic (U-shaped) relationship and were evaluated as such in the model (eFigure 1 in Supplement 1). Missing data were nonrandom in distribution (eFigure 2 in Supplement 1). Multiple imputation using multivariable normal distribution was performed to generate the prognostic model using 45 imputations (eTable 2 in Supplement 1) on 3250 participants (1265 with CP and 1985 controls). The results are summarized in Table 1.
The initial model included 11 binary variables (tobacco use, alcohol use, drug use, diabetes, preeclampsia, clinical chorioamnionitis, prolonged rupture of membranes, maternal fever, male sex, small for gestational age, and history of spontaneous abortion) as well as 6 continuous variables (number of pregnancies, number of miscarriages, 5-minute Apgar score, birth weight, umbilical cord pH, and gestational age). Gestational age was assessed as both a linear and quadratic relationship, and there was no difference in the fit of the model, so linear was used (eFigure 1 in Supplement 1). Prolonged rupture of membranes greater than 18 hours was found to be protective for CP, as was number of miscarriages. Maternal age was excluded after it was determined the control group significantly differed from the general Canadian population (eFigure 3 in Supplement 1). Emergency caesarian delivery was excluded for excessive missing values. Variables removed due to lack of modification were maternal fever, small for gestational age, alcohol use, umbilical cord pH, and history of miscarriages. Encephalopathy and seizures were excluded as there were no controls with encephalopathy, and information about seizures was not gathered from controls.
Final Model
The final model developed using multiple imputation consisted of 7 binary variables (tobacco use, drug use, diabetes, preeclampsia, chorioamnionitis, prolonged rupture of membranes, and male sex) and 5 continuous variables (number of pregnancies, number of miscarriages, 5-minute Apgar score, weight (and a weight2 term), and gestational age in weeks. The equation is provided in eTable 4 in Supplement 1. An Excel version of the model is provided in Supplement 2.
Fitting the Model
The model developed by multiple imputation was then applied to participants with complete data for the model variables (724 with CP and 1784 controls). Both resampling and random split-sampling methods yielded similar models, and the bootstrapping model was selected to maximize power. The overall sensitivity of the model was 56% (95% CI, 52-60) with a specificity of 82% (95% CI, 81-84), correctly classifying 1875 of 2508 participants (74.8%), with a C statistic of 0.74 (95% CI, 0.72-0.76) (eFigure 4A in Supplement 1). The positive predictive value was 56% (95% CI, 53-60) and the negative predictive value was 82% (95% CI, 80-84). When CP severity was considered, for ambulatory CP (GMFCS level I to III), the sensitivity was 54% (95% CI, 49-58) and specificity was 79% (95% CI, 77-81), with a C statistic of 0.71 (95% CI, 0.69-0.75) (eFigure 4B in Supplement 1). For nonambulatory CP (GMFCS level IV to V), the sensitivity was 65% (95% CI, 58-71), specificity was 86% (95% CI, 84-88), 1669 of 1989 participants (83.9%) were correctly classified, and the C statistic was 0.81 (95% CI, 0.77-0.85) (eFigure 4C in Supplement 1). There were more cases of ambulatory CP at lower prediction thresholds and more cases of nonambulatory CP at higher thresholds (eFigure 4D in Supplement 1).
Model Performance
The model was applied to each participant in the study as described above to generate an individual probability of CP (Figure 1A). A calibration plot was generated, demonstrating a slope of 1.016, an intercept of −0.29, and C statistic of 0.74 (95% CI, 0.72-0.76) (Figure 1B). The median (IQR; range) probability of CP for the control group was 0.25 (0.19-0.32; 0.06-0.98), and the median (IQR; range) probability for the CP group was 0.4 (0.25-0.79; 0.1-1) (P < .001). To determine thresholds for the prognostic tool, the sensitivity and specificity were calculated for a range of probabilities (Figure 1C). At a probability threshold of 0.3, the sensitivity was 65% (95% CI, 61-68) and the specificity was 71% (95% CI, 69-73). The positive predictive value was 47% (95% CI, 44-50) and the negative predictive value was 83% (95% CI, 81-85).
Figure 1. Using the Prognostic Tool Developed Using Multiple Imputation on 3250 Participants on a Sample of 2509 With Complete Data to Determine Potential Thresholds and Assess Model Performance.
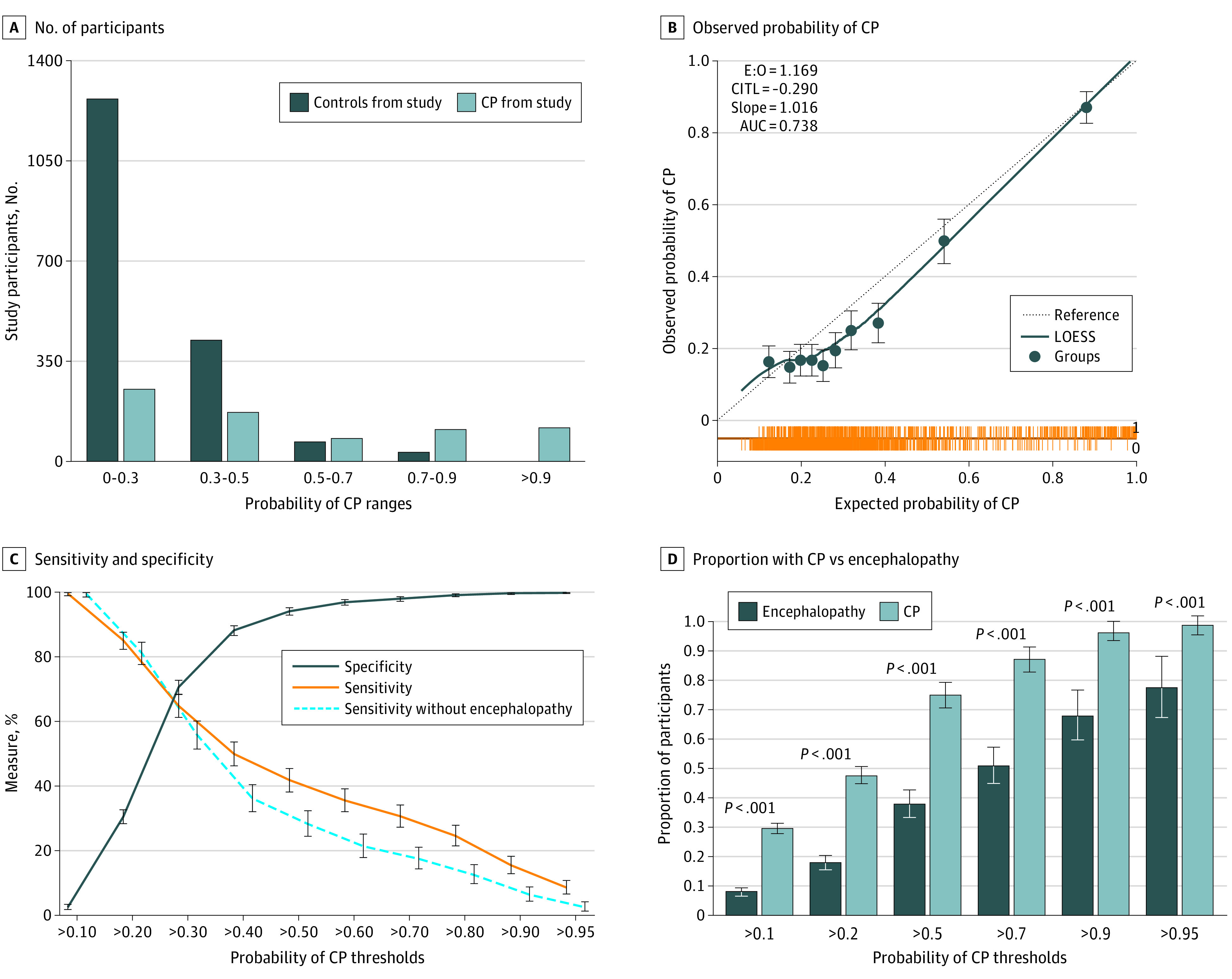
A, The prediction tool was applied to the cerebral palsy (CP) group (n = 724) and the control group (n = 1785) with complete data for the variables of interest, demonstrating the number of participants at each probability prediction range. B, Calibration plot comparing observed proportion with CP with the probability of CP generated by the model, in 10 equal groups. The slope evaluates the spread of estimated risk, target value is 1, and slope greater than 1 suggests estimates are too moderate. The locally estimated scatterplot smoothed (LOESS) flexible calibration curve shows the association between estimated risk of CP (x-axis) and observed proportion of CP (y-axis) (dotted line). The spike plot (orange line) demonstrates distribution of participants for controls (CP = 0) and those with CP (CP = 1). C, The sensitivity and specificity of the prognostic tool at each potential probability threshold. D, Comparison of proportion with CP and proportion with encephalopathy at each potential prediction tool threshold. P values for 2-sample test of proportion are shown. AUC indicates area under the curve (ie, C statistic); CITL, calibration in the large/intercept (average predicted risk compared with overall event rate; target value = 0; if positive, there is underestimation and if negative, there is overestimation); E:O, ratio of expected to observed. Error bars indicate 95% CIs.
The proportion with CP was compared with the proportion with encephalopathy at various thresholds (Figure 1D). At a probability threshold of 0.3, the model detected 466 of 716 participants with CP (65.0%), while only 197 of 716 (27.5%) presented with encephalopathy, an increased detection of 2.4-fold (odds ratio, 13.8; 95% CI, 8.87-22.65; P < .001). When children with a history of neonatal encephalopathy were excluded, the sensitivity of the model was 57% (95% CI, 53-62), the specificity was 73% (95% CI, 71-75), the positive predictive value was 38% (95% CI, 35-42), the negative predictive value was 85% (95% CI, 85%-87%), and the C statistic was 0.7 (95% CI, 0.69-0.74), with 1598 of 2303 participants (69.4%) correctly classified (Figure 1C; eTable 5 and eFigure 5 in Supplement 1).
Additive Risk Factors
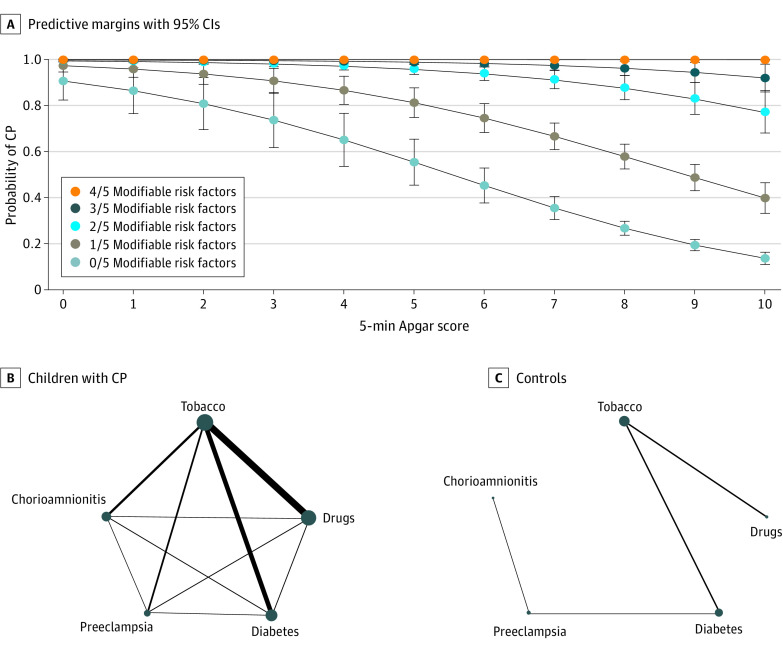
The 5 significant potentially modifiable binary risk factors (tobacco use, drug exposure, diabetes, preeclampsia, and chorioamnionitis) were complete in 914 CP cases and 1836 controls. At least 1 of these risk factors was present in 446 of 914 with CP (48.8%) and 209 of 1836 (11.4%), and at least 2 were found in 101 with CP (11.1%) and 10 controls (0.5%). Children with CP had significantly more of these modifiable risk factors than controls (Table 2). Using the model, it was found at Apgar scores less than 3, multiple risk factors did not further increase the probability of CP; however, at higher Apgar scores, each additional risk factor increased the probability (Figure 2A). Many of these risk factors were found to cooccur, for example, tobacco and drug exposure (Figure 2B).
Table 2. Comparison of the Proportion With Any Number of the 5 Modifiable Risk Factors in the Prediction Toola.
| Group | No. (%) | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Controls | 1627 (89) | 198 (11) | 10 (0.5) | 0 | 0 |
| Children with CP | 468 (61) | 230 (30) | 56 (7) | 12 (0.5) | 1 (0.1) |
Abbreviation: CP, cerebral palsy.
The 5 modifiable risk factors include tobacco use, drug use, preeclampsia, diabetes, and chorioamnionitis.
Figure 2. Cerebral Palsy (CP) Risk Factors Are Additive.
A, Graphic representation of the prognostic tool model with predictive margins and 95% CIs and probability of CP vs 5-minute Apgar score by the 5 potentially modifiable binary risk factors: tobacco use, drug use, preeclampsia, diabetes, and chorioamnionitis. B, Depiction of association between the 5 potentially modifiable binary risk factors; circle diameter represents the proportion with each risk factor (denominator is participants with complete data for the 5 variables of interest for each CP and control group), and line width represents the proportion who share the 2 connected risk factors. Error bars indicate 95% CIs.
Model Interpretation
The proposed interpretation of the model (ie, the prognostic tool) is shown in Figure 3. To adjust for the high proportion of CP in our study (1265 of 3250 [38.9%]), RR and number needed to assess were calculated (eTable 6 in Supplement 1). It is expected that 71% of newborns would fall in the low-risk range of probability less than 0.3, which has an RR of 0.5 compared with the estimated baseline population prevalence of 1 in 500. For the expected 5.9% of infants who score probability greater than 0.5, the RR of CP was significantly elevated. The expected prevalence of CP ranged from 1 in 1005 for the lowest risk (probability less than 0.3) to 1 in 8 for those with a probability greater than 0.9.
Figure 3. Proposed Prognostic Tool Interface and Interpretation for a Range of Predicted Cerebral Palsy (CP) Probability Results.
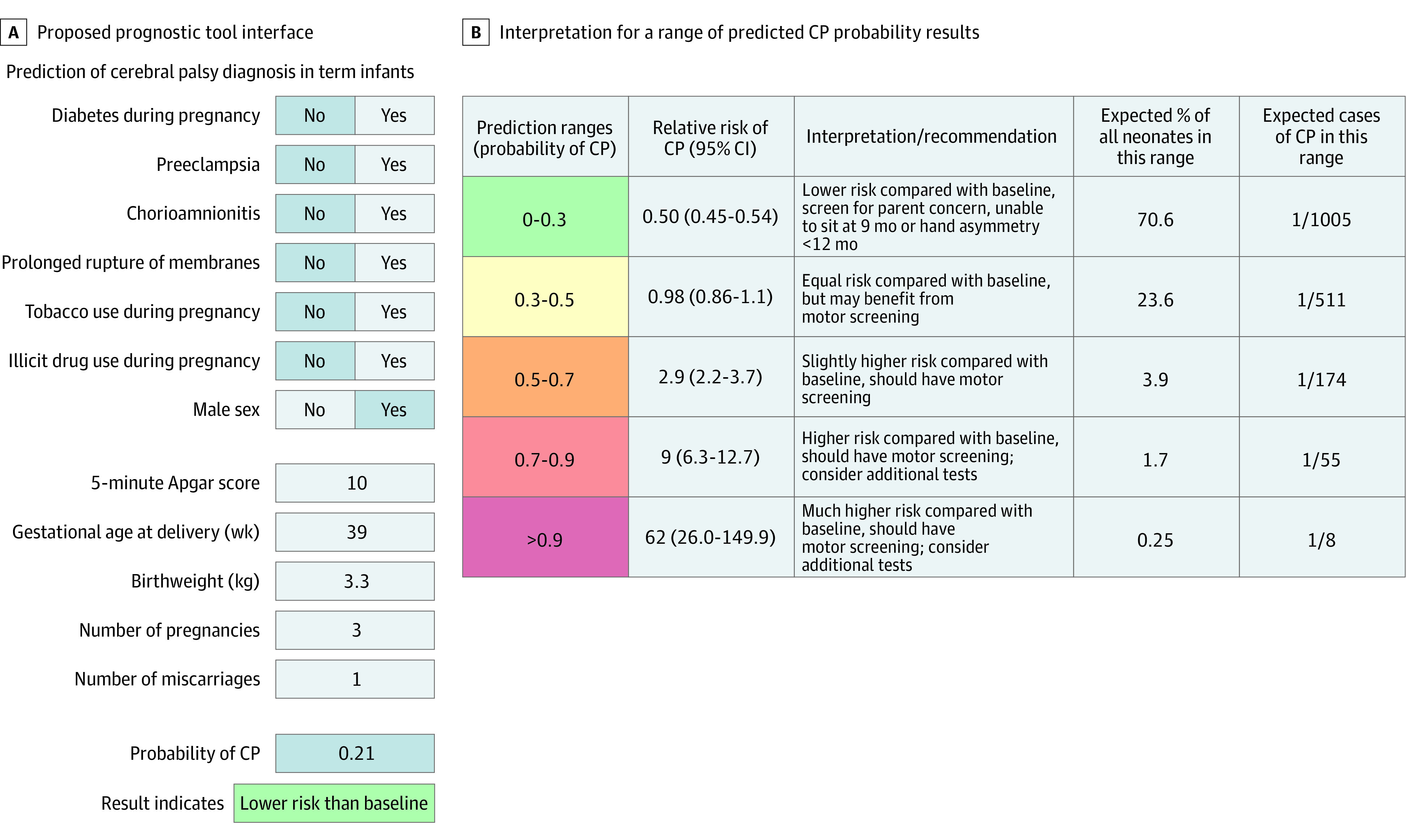
A, Example of possible prognostic screening tool, with an example patient and result. B, Relative risk, expected percentage, and expected cases calculated using model sensitivity and specificity at each range applied to a hypothetical population of neonates with a birth prevalence of CP of 1 in 500 (eTable 6 in Supplement 1).
Discussion
We developed a prognostic tool based on 7 binary variables (tobacco use, illicit drug use, diabetes, preeclampsia, clinical chorioamnionitis, prolonged rupture of membranes, and male sex) and 5 continuous variables (number of pregnancies, number of miscarriages, 5-minute Apgar score, infant weight, and gestational age) to be used as bedside tool to predict the probability of developing CP in a term newborn. To our knowledge, this is the first attempt to integrate these risk factors into a tool that can be applied to all term infants and the first time that the link between Apgar Score and additive risk factors has been demonstrated. At various thresholds, this tool identified twice as many children who went on to be diagnosed with CP compared with those who presented acutely with encephalopathy. An important result of the prognostic tool is the confirmation that CP in term infants is related to risk factors beyond those associated with intrapartum asphyxia. Furthermore, the moderate sensitivity of the prognostic tool (65% at the proposed threshold) supports that there are heterogenous etiologies underlying CP, and no single test will capture all patients. Similar to previous studies, we found that 30% of children with CP did not have pregnancy, delivery, and neonatal risk factors to aid in the prediction of CP diagnosis, and this group requires further characterization, particularly with regard to genetic diagnoses.
Proposed Use of the Prognostic Tool
We have presented interpretation of the tool at a range of thresholds, as is recommended, with suggestions about possible interventions at each range. We propose the results of this tool be integrated into an algorithm such as the one proposed by Novak et al, especially for the half of infants with CP in whom pregnancy and birth appeared unremarkable, which may allow for earlier identification similar to infants who present with “prematurity, atypical intrauterine growth, encephalopathy, genetic abnormalities, and seizures.” The main intervention suggested is enhanced routine clinical surveillance by general practitioners, sensitized to appreciate motor abnormalities in infants (such as augmenting routine well baby visits at 6 weeks and 3, 6, and 12 months with focused examination for CP, using a tool such as the Hammersmith Infant Neurological Examination), so the additional burden of false-positives is relatively low and does not involve additional invasive or expensive tests. Infants with an abnormal examination or who score in the higher-risk range (probability greater than 0.7 and RR greater than 9) may benefit from referral for early expert evaluation, such as a GMA, since at this range, the number needed to screen is 55 infants for each case of CP, and at a probability greater than 0.9, the number needed to screen is 8. Children scoring in this high-risk range are comparable with a very high-risk group, such as neonatal encephalopathy, where a 2021 study showed a CP prevalence of 12%. Available resources will differ widely in various settings; hence, we suggest a stepwise approach, with most infants with a probability greater than 0.3 (30% of all infants) screened at routine well baby visits by their primary clinician, with referral for more limited resources, such as GMA, reserved for those with abnormal examination findings or higher probability (probability greater than 0.7 [2% of all infants] or greater than 0.9 [0.25% of all infants]), depending on resources. Translation of this knowledge can be accomplished by building the prognostication tool into existing health-region newborn screening tools to be completed by public health nurses and nurse practitioners as part of routine care. Evaluation by an examination, such as Hammersmith Infant Neurological Examination, by trained nurses with referral as needed could help prevent additional burden for family physicians.
Additive Risk Factors
We found at higher Apgar scores, multiple risk factors were additive. This is particularly important when considering modifiable risk factors; for example, for a pregnancy with preeclampsia but otherwise low risk, the probability of CP would be 0.53 (95% CI, 0.2-0.7), but the same pregnancy with tobacco exposure has an probability of CP of 0.72 (95% CI, 0.46-0.88), moving them from average risk to higher risk. These findings highlight the importance of risk factor mitigation efforts, such as supporting smoking cessation during pregnancy.
Significant Individual Risk Factors
An important finding was that some risk factors independently increase the probability of CP, and any infant with these would be recommended for enhanced surveillance by their primary care clinician, with a low threshold to refer for advanced testing, such as the GMA. These included 5-minute Apgar score less than 6, chorioamnionitis, illicit drug use during the pregnancy, and encephalopathy and/or seizures (encephalopathy/seizures were not included in the model, as they were not present in controls). Many of the infants presenting with encephalopathy/seizures will already be identified for close developmental follow-up, and the sensitivity of the prognostic tool is slightly reduced at intermediate probabilities (probability greater than 0.3) without these patients (Figure 1C).
Chorioamnionitis has previously been associated with an increased risk of CP in infants of all gestational ages in this registry. Meta-analysis has shown a 140% increase in CP in infants with chorioamnionitis compared with controls, and in our study, we found a very strong effect of chorioamnionitis (odds ratio, 15.4; 95% CI, 6.9-39.1) in multivariable regression. The mechanism presumably involves inflammation of the uterine environment, which may evolve to a potentially deleterious fetal inflammatory response syndrome. Chorioamnionitis is often suspected to be the culprit in preterm birth, particularly in the setting of prolonged rupture of membranes. Interestingly, in the present sample of term infants, prolonged rupture of membranes had a protective effect for CP because of a significantly higher proportion of controls with prolonged rupture but with less fever and chorioamnionitis, which may reflect the effect of routine administration of antibiotics and increased fetal surveillance. This is indirect encouragement that standardized protocols to prevent chorioamnionitis in prolonged rupture of membranes may be effective but are inconsistently applied.
Illicit substance exposure was an independent risk factor for CP. Intrauterine exposure to illicit drugs, such as barbiturates, cannabis, and stimulants, has been associated with a higher prevalence of CP in a large study of infants older than 32 weeks’ gestation. Support for pregnant people struggling with substance use is a crucial ongoing need.
Low 5-minute Apgar scores were associated with increased probability of CP. A large study in Sweden similarly found adjusted hazard ratios of CP with 5-minute Apgar score ranging from 1.9 for a score of 9 to 278 for a score of zero. Apgar scores less than 5 are typically thought to represent an acute intrapartum event, which may in some cases be sufficient to cause CP, while Apgar scores in the middle range may represent a more mild intrapartum events that contribute to a multiple-hit model of CP.
Generalizability
The control group have slightly lower proportions of tobacco use, alcohol use, drug use, and chorioamnionitis compared with the general Canadian population (eTable 7 in Supplement 1). However, among the CP group, the proportions with these variables were higher than the general population, notably drug use and chorioamnionitis, supporting that the significant differences found between the controls and cases may represent a biological phenomenon rather than bias due to an excessively healthy control group. External validation of the prognostic tool is required to address this.
Limitations
Our study has limitations. The high proportion of participants with CP in our study lead to a ceiling effect when calculating the RR of CP at the highest probabilities, and thus RR was calculated by extrapolating to a hypothetical population. The control group had some challenges with regard to generalizability, specifically being older, better educated, and a higher socioeconomic status and higher proportion of White race compared with the average Canadian population. This was mitigated in part by omitting maternal age in our model as well as through our statistical techniques; however, this may have contributed to highlighting the apparent importance of lifestyle risk factors, such as tobacco and drug use. The issue of recall bias is present in the CP group, as parents of children with adverse outcomes are more likely to recall risk factors in the pregnancy; however, this bias may be countered with the desirability bias of nondisclosure of stigmatized behaviors, such as illicit drug use.
Conclusions
In this case-control study, a prognostic model using 12 clinical variables expanded the prediction of CP compared with clinical presentation with encephalopathy. Some risk factors were independently associated with CP, while others were additive. This study capitalizes on a large, robust national database of children diagnosed with CP in the magnetic resonance imaging era to explore the prognostic tool at various outcome ranges. This tool may be used at a population level for the early detection of CP and may allow for infants with uneventful pregnancies or deliveries to be identified for early interventions.
eTable 1. Variable Definitions and Coding
eTable 2. Commands and Output for Multiple Imputation
eTable 3. TRIPOD Checklist
eTable 4. Prognostic Tool Equation
eTable 5. Sensitivity, Specificity, PPV, and NPV for Various Threshold CP Probability Results from the Prediction Model
eTable 6. Method Used to Calculate the Relative Risk Based on a Hypothetical Population With a CP Prevalence of 1 in 500
eTable 7. Comparison of Study Group, Controls, and General Canadian Population for Variables Potentially Affected by Various Types of Bias
eFigure 1. Quadratic and Linear Variables
eFigure 2. Missing Values
eFigure 3. Maternal Age in Controls, Canadian Population, and CCPR
eFigure 4. Fit of the Regression Model From eTable 3 For All Participants With Complete Data, Ambulatory and Nonambulatory CP
eFigure 5. Prognostic Tool Performance With Children With a History of Neonatal Encephalopathy Removed
Prediction Tool
Group Information. Canadian Cerebral Palsy Registry
References
- 1.Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8-14. [PubMed] [Google Scholar]
- 2.Badawi N, Mcintyre S, Hunt RW. Perinatal care with a view to preventing cerebral palsy. Dev Med Child Neurol. 2021;63(2):156-161. doi: 10.1111/dmcn.14754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oskoui M, Coutinho F, Dykeman J, Jetté N, Pringsheim T. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2013;55(6):509-519. doi: 10.1111/dmcn.12080 [DOI] [PubMed] [Google Scholar]
- 4.Hollung SJ, Vik T, Lydersen S, Bakken IJ, Andersen GL. Decreasing prevalence and severity of cerebral palsy in Norway among children born 1999 to 2010 concomitant with improvements in perinatal health. Eur J Paediatr Neurol. 2018;22(5):814-821. doi: 10.1016/j.ejpn.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 5.Smithers-Sheedy H, Waight E, Goldsmith S, et al. ; Australian Cerebral Palsy Register Group . Declining trends in birth prevalence and severity of singletons with cerebral palsy of prenatal or perinatal origin in Australia: a population-based observational study. Dev Med Child Neurol. 2022;64(9):1114-1122. doi: 10.1111/dmcn.15195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boychuck Z, Bussières A, Goldschleger J, Majnemer A; Prompt Group . Age at referral for diagnosis and rehabilitation services for cerebral palsy: a scoping review. Dev Med Child Neurol. 2019;61(8):908-914. doi: 10.1111/dmcn.14034 [DOI] [PubMed] [Google Scholar]
- 7.Novak I, Morgan C, Adde L, et al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr. 2017;171(9):897-907. doi: 10.1001/jamapediatrics.2017.1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spittle AJ, Morgan C, Olsen JE, Novak I, Cheong JLY. Early diagnosis and treatment of cerebral palsy in children with a history of preterm birth. Clin Perinatol. 2018;45(3):409-420. doi: 10.1016/j.clp.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 9.Hadders-Algra M. Early diagnosis and early intervention in cerebral palsy. Front Neurol. 2014;5(suppl):185. doi: 10.3389/fneur.2014.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glass HC, Li Y, Gardner M, et al. Early identification of cerebral palsy using neonatal MRI and General Movements Assessment in a cohort of high-risk term neonates. Pediatr Neurol. 2021;118:20-25. doi: 10.1016/j.pediatrneurol.2021.02.003 [DOI] [PubMed] [Google Scholar]
- 11.Boychuck Z, Andersen J, Bussières A, et al. ; Prompt Group . International expert recommendations of clinical features to prompt referral for diagnostic assessment of cerebral palsy. Dev Med Child Neurol. 2020;62(1):89-96. doi: 10.1111/dmcn.14252 [DOI] [PubMed] [Google Scholar]
- 12.Shevell M, Dagenais L, Oskoui M. The epidemiology of cerebral palsy: new perspectives from a Canadian registry. Semin Pediatr Neurol. 2013;20(2):60-64. doi: 10.1016/j.spen.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 13.Moreno-De-Luca A, Ledbetter DH, Martin CL. Genetic insights into the causes and classification of cerebral palsies. Lancet Neurol. 2012;11(3):283-292. doi: 10.1016/S1474-4422(11)70287-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson KB. Causative factors in cerebral palsy. Clin Obstet Gynecol. 2008;51(4):749-762. doi: 10.1097/GRF.0b013e318187087c [DOI] [PubMed] [Google Scholar]
- 15.Papavasileiou A, Petra M. Risk factors for developing cerebral palsy. In: Miller F, Bachrach S, Lennon N, O’Neil M, eds. Cerebral Palsy. Springer; 2020:111-128. doi: 10.1007/978-3-319-74558-9_219 [DOI] [Google Scholar]
- 16.McIntyre S, Morgan C, Walker K, Novak I. Cerebral palsy—don’t delay. Dev Disabil Res Rev. 2011;17(2):114-129. doi: 10.1002/ddrr.1106 [DOI] [PubMed] [Google Scholar]
- 17.Vitagliano M, Dunbar M, Dyck Holzinger S, et al. Perinatal arterial ischemic stroke and periventricular venous infarction in infants with unilateral cerebral palsy. Dev Med Child Neurol. 2022;64(1):56-62. doi: 10.1111/dmcn.15000 [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Pediatrics . Neonatal Encephalopathy and Neurologic Outcome, Second Edition: Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Pediatrics. 2014;133(5):e1482-e1488. doi: 10.1542/peds.2014-0724 [DOI] [PubMed] [Google Scholar]
- 19.Letourneau N, Aghajafari F, Bell RC, et al. ; APrON Study Team . The Alberta Pregnancy Outcomes and Nutrition (APrON) longitudinal study: cohort profile and key findings from the first three years. BMJ Open. 2022;12(2):e047503. doi: 10.1136/bmjopen-2020-047503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava R, Dunbar M, Shevell M, et al. Development and validation of a prediction model for perinatal arterial ischemic stroke in term neonates. JAMA Netw Open. 2022;5(6):e2219203. doi: 10.1001/jamanetworkopen.2022.19203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen ML, Rackauskaite G, Greisen G, et al. Declining prevalence of cerebral palsy in children born at term in Denmark. Dev Med Child Neurol. 2022;64(6):715-722. doi: 10.1111/dmcn.15136 [DOI] [PubMed] [Google Scholar]
- 22.Luchman JN. Determining relative importance in Stata using dominance analysis: domin and domme. Stata J. 2021;21(2):510-538. doi: 10.1177/1536867X211025837 [DOI] [Google Scholar]
- 23.Palisano R, Rosenbaum P, Bartlett D, Livingston MH. Gross Motor Function Classification System Expanded and Revised. CanChild Centre for Childhood; 2007. [DOI] [PubMed] [Google Scholar]
- 24.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMC Med. 2015;13(1):1. doi: 10.1186/s12916-014-0241-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ensor J, Snell KIE, Martin EC. PMCALPLOT: Stata module to produce calibration plot of prediction model performance. Accessed April 23, 2022. https://ideas.repec.org/c/boc/bocode/s458486.html
- 26.Nijman S, Leeuwenberg AM, Beekers I, et al. Missing data is poorly handled and reported in prediction model studies using machine learning: a literature review. J Clin Epidemiol. 2022;142:218-229. doi: 10.1016/j.jclinepi.2021.11.023 [DOI] [PubMed] [Google Scholar]
- 27.Van Calster B, McLernon DJ, van Smeden M, Wynants L, Steyerberg EW; Topic Group ‘Evaluating diagnostic tests and prediction models’ of the STRATOS initiative . Calibration: the Achilles heel of predictive analytics. BMC Med. 2019;17(1):230. doi: 10.1186/s12916-019-1466-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35(29):1925-1931. doi: 10.1093/eurheartj/ehu207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fahey MC, Maclennan AH, Kretzschmar D, Gecz J, Kruer MC. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59(5):462-469. doi: 10.1111/dmcn.13363 [DOI] [PubMed] [Google Scholar]
- 30.Costeff H. Estimated frequency of genetic and nongenetic causes of congenital idiopathic cerebral palsy in west Sweden. Ann Hum Genet. 2004;68(pt 5):515-520. doi: 10.1046/j.1529-8817.2004.00105.x [DOI] [PubMed] [Google Scholar]
- 31.Wynants L, van Smeden M, McLernon DJ, Timmerman D, Steyerberg EW, Van Calster B; Topic Group ‘Evaluating Diagnostic Tests and Prediction Models’ of the STRATOS initiative . Three myths about risk thresholds for prediction models. BMC Med. 2019;17(1):192. doi: 10.1186/s12916-019-1425-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shevell A, Wintermark P, Benini R, Shevell M, Oskoui M. Chorioamnionitis and cerebral palsy: lessons from a patient registry. Eur J Paediatr Neurol. 2014;18(3):301-307. doi: 10.1016/j.ejpn.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 33.Shatrov JG, Birch SCM, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet Gynecol. 2010;116(2, pt 1):387-392. doi: 10.1097/AOG.0b013e3181e90046 [DOI] [PubMed] [Google Scholar]
- 34.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179(1):194-202. doi: 10.1016/S0002-9378(98)70272-8 [DOI] [PubMed] [Google Scholar]
- 35.Leviton A, Fichorova RN, O’Shea TM, et al. ; ELGAN Study Investigators . Two-hit model of brain damage in the very preterm newborn: small for gestational age and postnatal systemic inflammation. Pediatr Res. 2013;73(3):362-370. doi: 10.1038/pr.2012.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charpentier C, McDonald S, Elwood C, et al. A survey on variation in diagnosis and treatment of chorioamnionitis in tertiary centres in Canada. J Obstet Gynaecol Can. 2022;44(1):28-33. doi: 10.1016/j.jogc.2021.06.003 [DOI] [PubMed] [Google Scholar]
- 37.Benninger KL, Purnell J, Conroy S, et al. ; NCH Early Developmental Group . Intrauterine drug exposure as a risk factor for cerebral palsy. Dev Med Child Neurol. 2022;64(4):453-461. doi: 10.1111/dmcn.15050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finnegan L. Licit and Illicit Drug Use During Pregnancy: Maternal, Neonatal and Early Childhood Consequences. Substance Abuse In Canada; 2013. [Google Scholar]
- 39.Persson M, Razaz N, Tedroff K, Joseph KS, Cnattingius S. Five and 10 minute Apgar scores and risks of cerebral palsy and epilepsy: population based cohort study in Sweden. BMJ. 2018;360:k207. doi: 10.1136/bmj.k207 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Variable Definitions and Coding
eTable 2. Commands and Output for Multiple Imputation
eTable 3. TRIPOD Checklist
eTable 4. Prognostic Tool Equation
eTable 5. Sensitivity, Specificity, PPV, and NPV for Various Threshold CP Probability Results from the Prediction Model
eTable 6. Method Used to Calculate the Relative Risk Based on a Hypothetical Population With a CP Prevalence of 1 in 500
eTable 7. Comparison of Study Group, Controls, and General Canadian Population for Variables Potentially Affected by Various Types of Bias
eFigure 1. Quadratic and Linear Variables
eFigure 2. Missing Values
eFigure 3. Maternal Age in Controls, Canadian Population, and CCPR
eFigure 4. Fit of the Regression Model From eTable 3 For All Participants With Complete Data, Ambulatory and Nonambulatory CP
eFigure 5. Prognostic Tool Performance With Children With a History of Neonatal Encephalopathy Removed
Prediction Tool
Group Information. Canadian Cerebral Palsy Registry